SUMMARY
The persistent mal-attachment of microtubules to chromosomes at kinetochores is a major mechanism of chromosomal instability (CIN) [1, 2]. In normal diploid cells, mal-attachments arise spontaneously and are efficiently corrected to preserve genomic stability [3]. However, it is unknown if cancer cells with CIN possess the ability to efficiently correct attachment errors. Here we show that kinetochore-microtubule attachments in cancer cells with CIN are inherently more stable than those in normal diploid RPE-1 cells. The observed differences in attachment stability account for the persistence of mal-attachments into anaphase, where they cause chromosome mis-segregation. Furthermore, increasing the stability of kinetochore-microtubule attachments in normal diploid RPE-1 cells, either by depleting the tumor suppressor protein, APC, or the kinesin-13 protein, MCAK, is sufficient to promote chromosome segregation defects to levels comparable to those in cancer cells with CIN. Collectively, these data identify that cancer cells have a diminished capacity to correct erroneous kinetochore-microtubule attachments and accounts for the widespread occurrence of CIN in tumors [4].
RESULTS
The origin and contribution of CIN in cancer have long been a mystery [4, 5]. CIN is characteristic of many solid tumors and is a major cause of aneuploidy [6]. It positively correlates with poor prognosis and drug resistance, presumably, by promoting genetic plasticity [7–9] and tumor cell heterogeneity [10]. The most common pathway leading to chromosome mis-segregation and CIN in cancer cells is lagging chromosomes [1, 2]. Chromosomes lag at anaphase due to mal-orientation, where kinetochores simultaneously bind to microtubules emanating from opposite spindle poles (merotely) [11]. These attachments errors are not detected by the spindle assembly checkpoint (SAC) [11]. The prevalence of merotelic attachments is governed by the rate at which they form and the rate at which they are corrected. In normal diploid cells, merotelic attachments commonly form in early mitosis due to stochastic kinetochore-microtubule interactions and are corrected by the release of maloriented microtubules prior to anaphase onset [3]. The correction process is enabled by the dynamic kinetochore-microtubule interface where microtubules continuously attach and detach from kinetochores at rates that govern the overall stability of the attachment [12]. Thus, high levels of merotelic attachments in cancer cells with CIN may be caused by either increased generation or reduced elimination of these erroneous attachments. It was recently shown that transient defects in spindle geometry in cancer cells, such as those caused by supernumerary centrosomes, elevate the incidence of merotelic attachments indicating that some cancer cells with CIN have excessive rates of formation of attachment errors [13, 14]. However, it is unknown whether cancer cells have an inherent defect in the ability to correct merotelic attachment errors once they arise. Uncovering such defects would present new targets to suppress CIN and inspire novel strategies to prevent tumor progression and metastasis.
Cancer cells have hyperstable kinetochore-microtubules
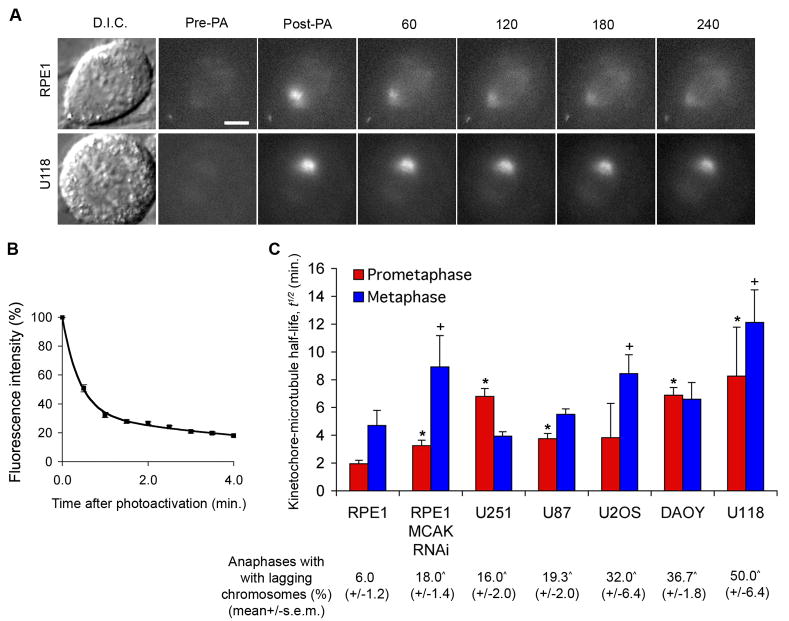
To assess the efficiency with which cancer cells with CIN correct merotelic attachments we measured the dynamics of kinetochore-microtubules (kMT) in cells expressing photactivatable GFP-tubulin (PA-GFP-tubulin). We used fluorescence dissipation after photoactivation (FDAPA) of spindle microtubules to evaluate the stability of kinetochore-microtubule attachments by measuring the half-life, t1/2 of kinetochore-microtubules [2, 12, 15] (Figure 1A and experimental procedures). We had previously performed this analysis in only one cancer cell line that exhibits CIN (U2OS) [2] and here we extend our analysis to evaluate a variety of other cancer cells with CIN that were able to tolerate stable expression of PA-GFP-tubulin (U251, U118, U87, and DAOY). We chose these cell lines because they have been karyotypically defined as grossly aneuploid (not shown), represent three different tissues of origin (U2OS is an osteosarcoma-derived cell line, U251, U118 and U87 are glioblastoma-derived cell lines, whereas DAOY is a medulloblastoma-derived cell line), and have each been established in culture for many years. We chose RPE-1 cells as a chromosomally stable diploid cell line for comparison to these CIN cell lines. RPE-1 cells are non-transformed, chromosomally stable, and routinely used in the mitosis field to represent normal cells [13, 16]. Stable expression of PA-GFP-tubulin does not affect the basal rates of lagging chromosomes [2]. Fluorescence decay fit a double-exponential decay curve (r2>0.99) with the fast and slow decaying fluorescence corresponding to unstable (non-kinetochore-associated) and stable (kinetochore-associated) microtubule populations, respectively [2, 12] (Figure 1B).
Figure 1. Deviant kinetochore-microtubule dynamics in cancer cells.
(A) Examples of D.I.C. and time-lapse fluorescent images of spindles of RPE-1 and U118 cells before (Pre-PA) and at the indicated times (s) after activation (Post-PA) of GFP-tubulin fluorescence. Scale bar, 5 μm. (B) Example of normalized fluorescence intensity over time after photoactivating spindles of RPE1 cells at metaphase. Datapoints represent mean ± s.e.m., n = 11 cells. (C) Kinetochore-microtubule half-life (min.) in cancer cell lines at prometaphase (blue) and metaphase (red). Percent of cells at anaphase with lagging chromosomes for each cell line is denoted below the x-axis. Error bars in the graph represent standard-error derived from the exponential decay curve of the photoactivated fluorescence, (r2>0.99). *p < 0.05, †p<0.05, t-test, when compared to RPE-1 values at prometaphase and metaphase, respectively, n = 9–19 cells. ^p < 0.05, t-test, when compared to control RPE-1 values, n = 150 cells, 3 experiments.
Strikingly, the half-life of kinetochore-microtubules was significantly higher in all CIN cell lines examined than in non-transformed diploid RPE-1 cells at either prometaphase, metaphase, or both (Figure 1A and C). This indicates elevated kinetochore-microtubule attachment stability in cancer cells with CIN. Accordingly, the frequencies of lagging chromosomes observed in these cells strongly correlated with the extent of overall attachment stability (Figure 1C). Indeed, increased stability of kinetochore-microtubule attachments in either prometaphase or metaphase correlated with elevated frequencies of lagging chromosomes in anaphase indicating that a global change in kinetochore-microtubule attachment stability in either phase of mitosis can be sufficient to elevate rates of lagging chromosomes. All cells examined produced a robust arrest when treated with the microtubule depolymerizing drug nocodazole for 16 hours indicating that they have a functional SAC (Figure S1). While some cell lines had increased rates of monopolar and multipolar spindles in prometaphase (Table S1), these spindle defects did not always correlate with the observed rates of lagging chromosomes (for example compare RPE-1 with U2OS in Table S1). This indicates that spindle geometry defects are insufficient to account for the elevated frequency of lagging chromosomes in all cancer cell lines with CIN. These data also indicate that the prevalence of lagging chromosomes in cancer cell lines with CIN may arise as a result of global increase in the stability of kinetochore-microtubule attachments (both proper and improper) which would then delay the release of improperly attached microtubules.
Increased kinetochore-microtubule stability accounts for reduced correction of merotelic attachments
To assess if the observed differences in overall kinetochore-microtubule stability between normal cells and cancer cells with CIN account for physiologically relevant differences in the ability of cells to correct merotelic attachments, we combined our data with quantitative electron microscopy of kinetochore-microtubule attachments in PtK1 cells [17]. Although they are derived from a different species, these cells exhibit similar kinetochore-microtubule dynamics to RPE-1 cells at similar temperatures [12] (kinetochore-microtubule-t1/2 = 5.3 ± 0.8 min. and 4.7 ± 1.05 for PtK1 and RPE1 cells respectively, p = 1.7, t-test). We estimate that, in RPE-1 cells at prometaphase and metaphase, individual microtubules are released from kinetochores every ~12 and ~20 seconds, respectively (Figure S2A; Supplementary text). This means that the turnover of an entire kinetochore-fiber (all microtubules attached to an individual kinetochore) takes place within ~3 minutes at prometaphase and ~7 minutes at metaphase and that the mean-lifetime of individual kinetochore-microtubule attachments (including proper and improper attachments) are unlikely to exceed these durations (Figure S2B). Conversely, in cancer cells with CIN, kinetochore-microtubules turnover at much slower rates. For instance, in U118 cells, single microtubules are released at metaphase on average once every ~40 seconds resulting in the turnover of the entire kinetochore-fiber in ~18 minutes. Thus, the expected mean-lifetime of merotelic attachments is much higher than that in normal cells (Figure S2B), and reaches the time that RPE-1 cells complete the transition from nuclear envelope breakdown to anaphase onset (~19 minutes) [16]. Previous work showed that prolonging metaphase only slightly reduced the number of merotelic kinetochores, indicating that merotelic attachments continuously form during mitosis [18]. Therefore, elevated mean-lifetimes of individual attachment errors can account for reduced correction efficiencies and for increased rates of chromosome mis-segregation in cancer cells with CIN. This is consistent with observations that chromosomally stable cells exhibit fewer lagging chromosomes than cancer cells with CIN when both are subjected to the same drug treatments that artificially increase kinetochore-microtubule attachment errors [1, 2, 13]. It is important to note that at the turnover rates measured for both RPE1 as well as the different cancer cell lines, we estimate that kinetochore-microtubule occupancy would not reach saturation, similar to direct observations in PtK1 cells.
Stabilizing kinetochore-microtubule attachments in diploid RPE-1 cells causes chromosome segregation defects
Stabilizing kinetochore-microtubule attachments in cancer cells has been shown to increase the inherent frequency of lagging chromosomes [2, 19]. However it is yet to be determined if increasing attachment stability is, by itself, sufficient to induce chromosome segregation defects in otherwise chromosomally stable and diploid human cells. To test this, we used RNA interference to deplete RPE-1 cells of the microtubule depolymerizing kinesin, MCAK (Figure S3A). MCAK depletion does not affect spindle geometry [2]. However, it led to a significant increase in kinetochore-microtubule stability in RPE-1 cells making their kinetochore-microtubule attachments as stable as those in cancer cells with CIN (Figure 1C). Accordingly, depletion of MCAK from RPE-1 cells led to frequencies of lagging chromosomes that were comparable to those in cancer cells with CIN (Figure 1C). This indicates that increasing kinetochore-microtubule attachment stability, alone, is sufficient to generate chromosome segregation defects similar to those observed in cancer cells with CIN and supports our proposition that deviant kinetochore-microtubule dynamics underlie CIN in many cancer cells.
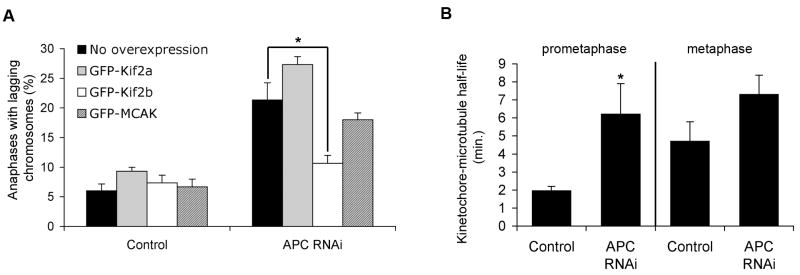
Many alterations in cancer cells have been shown to lead to CIN [20–24]. However, the mechanism for how those changes cause persistent chromosome mis-segregation is poorly understood. To test if kinetochore-microtubule stability is perturbed by the disruption of genes whose loss-of-function is associated with CIN in cancers, we depleted the adenomatous polyposis coli (APC) tumor suppressor protein from non-transformed diploid RPE-1 cells (Figure S3B). APC is a kinetochore localized microtubule-associated protein whose function is frequently disrupted in colorectal cancers with CIN [20, 25–27], making it a good candidate to test our hypothesis. In line with previous reports [25, 26], its depletion led to significant increase in the frequencies of lagging chromosomes in RPE1 cells at anaphase (Figure 2A). Interestingly, APC-deficient RPE-1 cells also showed a significant increase in kinetochore-microtubule half-life during prometaphase, indicative of hyperstable kinetochore-microtubule attachments (Figure 2B). There were no noticeable defects in spindle geometry or the SAC in these cells (not shown). We reasoned that if APC depletion induces chromosome segregation defects by stabilizing kinetochore-microtubule attachments, then independently destabilizing these attachments should suppress frequencies of lagging chromosomes in APC-deficient cells. We previously showed that overexpression of the microtubule depolymerizing kinesins, Kif2b or MCAK, in cancer cells with CIN destabilizes kinetochore-microtubule attachments, reduces the frequencies of lagging chromosomes, and suppresses CIN [2]. Accordingly, overexpression of GFP-Kif2b, but not GFP-MCAK, GFP-Kif2a, or GFP-tubulin significantly suppressed the frequencies of lagging chromosomes in APC-deficient RPE-1 cells (Figure 2A). It is unclear why overexpression of GFP-MCAK was unable to rescue the lagging chromosomes observed upon APC-depletion. One possibility is that this reflects a higher inherent microtubule-depolymerizing activity of Kif2b relative to MCAK. Nevertheless, this result shows that hyperstable kinetochore-microtubule attachments are a primary mechanism of chromosome mis-segregation in APC-deficient RPE-1 cells.
Figure 2. APC loss leads to hyperstable kinetochore-microtubule attachments.
(A) Percent of cells at anaphase with lagging chromosomes for control and APC-depleted RPE-1 cells overexpressing nothing, GFP-Kif2a, GFP-Kif2b, or GFP-MCAK. Bars represent mean ± s.e.m., *p<0.05, t-test, n = 150 cells, 3 experiments. (B) Kinetochore-microtubule half-life (min.) in control and APC-depleted RPE-1 cells at prometaphase and metaphase. Error bars represent standard-error derived from the exponential decay curve of the photoactivated fluorescence (r2>0.99). *p < 0.05, t-test, n = 8–19 cells.
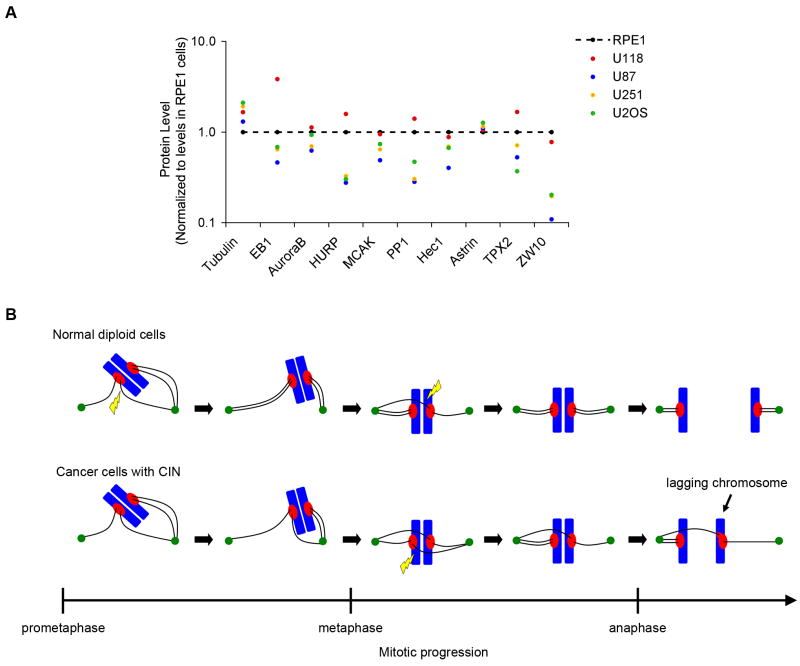
To further investigate the defects that may influence kinetochore-microtubule attachment dynamics, we determined the relative abundance of several spindle proteins. Mitotic cells were collected following treatment with low doses of nocodazole, and quantitative immunoblotting used to measure protein abundance relative to actin as an iternal control. Mitotically arrested cancer cell lines with CIN show significant disparity in levels of various spindle proteins when normalized to the levels in chromosomally stable, diploid RPE-1 cells (Figure 3A). For example, U118 cells have elevated levels of EB1 but near normal levels of ZW10, but U87 cells have modestly reduced levels of EB1 and very low levels of ZW10. These data do not discriminate between bystander perturbations and disruptions that are causally related to CIN, and variation in protein level would be observed regardless of which cell is used for normalization. However, relative to RPE-1 cells, these results show that there is large variation in the abundance of various spindle-associated proteins in mitosis, and altered expresssion in any of these proteins may contribute to changes in the dynamics of kinetochore-microtubule attachments.
Figure 3. Variable levels of spindle proteins in mitotic cancer cells.
(A) Fold-changes in levels of various proteins in cancer cell lines that were arrested in mitosis in the presence of nocodazole for 16 hrs. Values are normalized to those of RPE-1 cells using actin as a loading control. Quantitative immunoblotting (2–3 blots for each protein) was used to estimate the average levels of the different proteins using specific antibodies. (B) Increased overall stability of microtubule (black lines) attachments to chromosomes (blue) underlies CIN in cancer cells. In normal cells, microtubules are frequently released (yellow lightning bolt) from kinetochores (red) prior to anaphase to promote correction of erroneous attachments and prevent chromosome mis-segregation. Slow release rates in cancer cells with CIN increases the likelihood that mal-attachments will persist until anaphase causing lagging chromosomes and chromosome mis-segregation.
DISCUSSION
CIN is commonly caused by kinetochore-microtubule attachment errors [1, 2] whose occurrence is dictated by both the rate of their formation and the rate of their correction. Our data demonstrate that cancer cells with CIN have an inherent defect in the rate of correction of kinetochore-microtubule attachment errors. The rate-limiting step in correcting these inappropriate attachments is the release of maloriented microtubules [28]. Whereas our data do not exclude models invoking the selective recruitment of the correction machinery to kinetochores with erroneous attachments [29], they support the proposition that excessive overall stability of kinetochore-microtubule attachments in cancer cells reduces their correction efficiency, compromising faithful chromosome segregation (Figure 3B). Since kinetochores have a bias to capture microtubules emanating from the spindle pole they face [28], once merotelically attached microtubules are released, they are likely to be replaced with properly oriented ones. We cannot currently resolve if properly and improperly oriented kinetochore microtubules have different turnover rates, but our data show that global increases in kinetochore-microtubule attachment stability is sufficient to generate chromosome segregation errors associated with CIN in otherwise normal diploid RPE-1 cells. This indicates that indiscriminate changes (increases or decreases) in overall kinetochore-microtubule attachment stability directly impacts chromosome segregation fidelity. These data also explain the occurrence of CIN in cancer cells with normal numbers of centrosomes [13, 14] and why normal diploid RPE-1 cells still exhibit fewer lagging chromosomes than cancer cells with CIN when both are treated with drugs that artificially increase merotelic attachments [1, 2]. It was recently shown that the presence of extra centrosomes contributes to CIN in cancer cells by increasing the rate of formation of kinetochore-microtubule attachment errors [13, 14], and additional chromosome mis-segregation would be expected if inherent defects in error correction are combined with increased rates of formation of merotelic attachments through acquisition of extra centrosomes. Yet, these data provide a compelling account for the widespread occurrence of CIN in tumors regardless of the number of centrosomes present [4, 5] and explains why promoting kinetochore-microtubule dynamics leads to the suppression of CIN in four independent cancer cell lines that were derived from three different types of human tumors (U2OS [2], MCF-7 [2], U251, and U118, data not shown).
The prevelance of merotelic kinechores is higher in prometaphase than metaphase [3]. This implies that the majority of correction of erroneous kinetochore-microtubule attachments occurs during early mitosis and it explains why increasing the overall stability of kinetochore-microtubule dynamics in early mitosis is most detrimental to chromosome segregation fidelity [2]. However, extending the duration of metaphase only slightly reduces the numbers of merotelically attached kinetochores [18]. Moreover, increasing kinetochore-microtubule attachment stability selectively during metaphase leads to a significant increase in the frequencies of lagging chromosomes (Figure 1C) [2]. Thus, the formation of merotelic attachments is ongoing, even during metaphase, and the dynamics of kinetochore-microtubule attachments must remain regulated throughout mitosis. This explains why cancer cells displaying excessively stable kinetochore microtubule attachments only in metaphase are CIN.
Relatively minor perturbations in kinetochore-microtubule attachment dynamics may be sufficient to exceed the maximum threshold of attachment stability required for faithful chromosome segregation. These perturbations can arise through mutations in single genes whose products play important roles in kinetochore function (e.g. APC) or through global imbalance in levels of proteins involved in regulating microtubule dynamics by epigenetic mechanisms or following spontaneous chromosome mis-segregation. It may not be feasible to identify a single culprit responsible for CIN since imbalances in any one protein might be sufficient to disturb normal kinetochore-microtubule dynamics and perturbations in levels of some proteins may buffer effects of perturbations in others. Nevertheless, a mechanism that generates CIN through imbalances in proteins that regulate kinetochore-microtubule attachment dynamics would fit with the observation that CIN appears genetically dominant in cell fusion assays [6]. Moreover, persistent chromosome mis-segregation would trigger further mis-regulation of kinetochore-microtubule dynamics in such a way that CIN may be a self-propagating process.
EXPERIMENTAL PROCEDURES
GFP-Tubulin photoactivation
As previously described [2, 12, 15], mitotic cells were identified by D.I.C. microscopy and several pulses from a micropoint laser (Photonic Instruments, St Charles, IL) were used to photoactivate an area of < 2 μm2 of GFP within the spindle as previously described. Images were acquired with a Hamamatsu Orca II camera binned 2 × 2 with a 63X, 1.4 NA objective on a Zeiss Axioplan 2 microscope. Fluorescent image stacks in the z-axis were collected containing 3 images, each separated by 1-μm before and <1s after photoactivation. Similar image stacks were subsequently collected every 30s and measurements performed using the single focal plane where the fluorescence was most in focus. D.I.C. microscopy was then used to verify that cells did not undergo anaphase.
Photoactivation analysis
FDAPA analysis was performed primarily as described previously [2, 12, 15]. Briefly, pixel intensities were measured within a square area (12 × 12 pixels) surrounding the region with the brightest fluorescence. Pixel intensities from an equally sized area from the opposite half-spindle were subtracted. Correction for photobleaching was made by normalizing to values obtained from photoactivated taxol-stabilized spindles where the photoactivatable region clearly did not dissipate. Bleaching-induced decrease in average fluorescence after 30-captured images was 35%. For each cell, fluorescence values were normalized to the first time-point after photoactivation. Normalized fluorescence was then averaged for multiple cells at each time-point. A double exponential regression analysis was used to fit the data to the following equation: F (t)= A1e−k1t + A2 e−k2t, where F(t) is measured photoactivated fluorescence at time t, A1 and A2 represent less (non-kinetochore-associated) and more (kinetochore-associated) stable microtubule populations with decay rate constants of k1 and k2, respectively.
Cell culture
Cells were maintained at 37 °C in a 5% CO2 atmosphere in Dulbecco’s modified medium (DMEM) or McCoy’s medium containing 10% fetal bovine serum, 50 IU/ml penicillin, and 50-μg/ml streptomycin. For plasmid selection cells were maintained in 0.5–1.0 mg/ml of G418 (geneticin).
Antibodies
MCAK-specific antibody [30], Tubulin-specific mAb DM1α (Sigma-Aldrich, St. Louis, MO), CREST-specific antibody (provided by Kevin Sullivan), APC-specific antibody (provided by Yashi Ahmed), Actin-specific mAb (provided by Harry Higgs), EB1-specific antibody (Santa Cruz Biologicals), aurora B-specific antibody (Novus Biologicals), HURP-specific antibody (Bethyl Labs), Protein phosphatases 1 (PP1)-specific antibody (Santa Cruz Biologicals), Hec1-specific antibody (Novus Biologicals), astrin-specific antibody [31], TPX2-specific antibody [32], ZW10-specific antibody (provided by Conly Rieder).
RNA interference and plasmid transfection
Published sequences were used to deplete MCAK [30] and a pre-validated RNAi sequence used to deplete APC was purchased from Ambion. 70–200 nM dsRNA (Ambion) were transfected into cells using Oligofectamine reagent (Invitrogen) as previously described [30] and cells were analyzed ~70 hours after transfection. Plasmids encoding GFP-tagged Kif2a, Kif2b and MCAK were provided by Linda Wordeman (U. Washington). Cells were transfected with plasmid DNA using the lipofectamine reagent (Invitrogen).
Indirect immunofluorescence microscopy
Cell fixation and antibody staining, used to determine spindle morphology used in table S1 and score lagging chromosomes in Figure 1, were performed as previously described [30]. Cells were fixed with either ice-cold methanol or with 1% gluteraldehyde when staining for microtubules, DNA, or CREST (kinetochores). Cells were scored using a Zeiss Axioplan 2 microscope with 63X and 100X, 1.4 NA objectives or a Nikon TE-2000E microscope with a 60x, 1.4 NA objective. To score lagging chromosomes in anaphase, we stained microtubules with a tubulin-specific antibody, kinetochores with a CREST-specific antibody and DNA with DAPI.
Drug treatments
For experiments in Supplementary Figure 1 and Figure 3, 0.1 μg/ml of nocodazole was used and cells were arrested for ~16 hours.
Supplementary Material
Acknowledgments
We thank Yashi Ahmed, Harry Higgs, Kevin Sullivan, and Conly Rieder for providing reagents. We thank Sarah Thompson and other members of the Compton lab for technical assistance and helpful discussions. This work was supported by a National Institutes of Health grant GM51542 to D.A.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thompson SL, Compton DA. Examining the link between chromosomal instability and aneuploidy in human cells. J Cell Biol. 2008;180:665–672. doi: 10.1083/jcb.200712029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakhoum SF, Thompson SL, Manning AL, Compton DA. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nature Cell Biol. 2009;11:27–35. doi: 10.1038/ncb1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cimini D, Moree B, Canman JC, Salmon ED. Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J Cell Sci. 2003;116:4213–4225. doi: 10.1242/jcs.00716. [DOI] [PubMed] [Google Scholar]
- 4.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 5.Storchova Z, Pellman D. From polyploidy to aneuploidy, genome instability and cancer. Nature Rev Mol Cell Biol. 2004;5:45–54. doi: 10.1038/nrm1276. [DOI] [PubMed] [Google Scholar]
- 6.Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386:623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- 7.Walther A, Houlston R, Tomlinson I. Association between chromosomal instability and prognosis in colorectal cancer: a meta-analysis. Gut. 2008;57:941–950. doi: 10.1136/gut.2007.135004. [DOI] [PubMed] [Google Scholar]
- 8.Gao CF, Furge K, Koeman J, Dykema K, Su YL, Cutler ML, Werts A, Haak P, Woude GFV. Chromosome instability, chromosome transcriptome, and clonal evolution of tumor cell populations. Proc Natl Acad Sci, USA. 2007;104:8995–9000. doi: 10.1073/pnas.0700631104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuukasjarvi T, Karhu R, Tanner M, Kahkonen M, Schaffer A, Nupponen N, Pennanen S, Kallioniemi A, Kallioniemi OP, Isola J. Genetic heterogeneity and clonal evolution underlying development of asynchronous metastasis in human breast cancer. Cancer Res. 1997;57:1597–1604. [PubMed] [Google Scholar]
- 10.Nowell PC. Clonal Evolution of Tumor-Cell Populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 11.Cimini D. Merotelic kinetochore orientation, aneuploidy, and cancer. Bioch Biophys Acta. 2008;1786:32–40. doi: 10.1016/j.bbcan.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Zhai Y, Kronebusch PJ, Borisy GG. Kinetochore microtubule dynamics and the metaphase-anaphase transition. J Cell Biol. 1995;131:721–734. doi: 10.1083/jcb.131.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganem NJ, Godinho SA, Pellman D. A Mechanism Linking Extra Centrosomes to Chromosomal Instability. Nature. 2009;460:278–282. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silkworth WT, Nardi IK, Scholl LM, Cimini D. Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. Plos One. 2009;4:e6564. doi: 10.1371/journal.pone.0006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cimini D, Wan XH, Hirel CB, Salmon ED. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr Biol. 2006;16:1711–1718. doi: 10.1016/j.cub.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 16.Yang Z, Loncarek J, Khodjakov A, Rieder CL. Extra centrosomes and/or chromosomes prolong mitosis in human cells. Nature Cell Biol. 2008;10:748–751. doi: 10.1038/ncb1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McEwen BF, Heagle AB, Cassels GO, Buttle KF, Rieder CL. Kinetochore fiber maturation in PtK1 cells and its implications for the mechanisms of chromosome congression and anaphase onset. J Cell Biol. 1997;137:1567–1580. doi: 10.1083/jcb.137.7.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cimini D, Moree CB, Maddox PS, Degrassi F, Salmon ED. Prolonging metaphase decreases kinetochore merotelic orientations. Mol Biol Cell. 2001;12:315a. [Google Scholar]
- 19.DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 2006;127:969–982. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 20.Green RA, Kaplan KB. Chromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC. J Cell Biol. 2003;163:949–961. doi: 10.1083/jcb.200307070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajagopalan H, Jallepalli PV, Rago C, Velculescu VE, Kinzler KW, Vogelstein B, Lengauer C. Inactivation of hCDC4 can cause chromosomal instability. Nature. 2004;428:77–81. doi: 10.1038/nature02313. [DOI] [PubMed] [Google Scholar]
- 22.Joukov V, Groen AC, Prokhorova T, Gerson R, White E, Rodriguez A, Walter JC, Livingston DM. The BRCA1/BARD1 heterodimer modulates ran-dependent mitotic spindle assembly. Cell. 2006;127:539–552. doi: 10.1016/j.cell.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 23.Cahill DP, Lengauer C, Yu J, Riggins GJ, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Cummins JM, Shen D, Cahill DP, Jallepalli PV, Wang TL, Parsons DW, Traverso G, Awad M, Silliman N, Ptak J, Szabo S, Willson JK, Markowitz SD, Goldberg ML, Karess R, Kinzler KW, Vogelstein B, Velculescu VE, Lengauer C. Three classes of genes mutated in colorectal cancers with chromosomal instability. Cancer Res. 2004;64:2998–3001. doi: 10.1158/0008-5472.can-04-0587. [DOI] [PubMed] [Google Scholar]
- 25.Fodde R, Kuipers J, Rosenberg C, Smits R, Kielman M, Gaspar C, van Es JH, Breukel C, Wiegant J, Giles RH, Clevers H. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nature Cell Biol. 2001;3:433–438. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan KB, Burds AA, Swedlow JR, Bekir SS, Sorger PK, Nathke IS. A role for the Adenomatous Polyposis Coli protein in chromosome segregation. Nature Cell Biol. 2001;3:429–432. doi: 10.1038/35070123. [DOI] [PubMed] [Google Scholar]
- 27.Dikovskaya D, Schiffmann D, Newton IP, Oakley A, Kroboth K, Sansom O, Jamieson TJ, Meniel V, Clarke A, Nathke IS. Loss of APC induces polyploidy as a result of a combination of defects in mitosis and apoptosis. J Cell Biol. 2007;176:183–195. doi: 10.1083/jcb.200610099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicklas RB, Ward SC. Elements of error correction in mitosis: microtubule capture, release, and tension. J Cell Biol. 1994;126:1241–1253. doi: 10.1083/jcb.126.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knowlton AL, Lan W, Stukenberg PT. Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Curr Biol. 2006;16:1705–1710. doi: 10.1016/j.cub.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 30.Manning AL, Ganem NJ, Bakhoum SF, Wagenbach M, Wordeman L, Compton DA. The kinesin-13 proteins Kif2a, Kif2b, and Kif2c/MCAK have distinct roles during mitosis in human cells. Mol Biol Cell. 2007;18:2970–2979. doi: 10.1091/mbc.E07-02-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mack GJ, Compton DA. Analysis of mitotic microtubule-associated proteins using mass spectrometry identifies astrin, a spindle-associated protein. Proc Natl Acad Sci, USA. 2001;98:14434–14439. doi: 10.1073/pnas.261371298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garrett S, Auer K, Compton DA, Kapoor TM. hTPX2 is required for normal spindle morphology and centrosome integrity during vertebrate cell division. Curr Biol. 2002;12:2055–2059. doi: 10.1016/s0960-9822(02)01277-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.