Abstract
Host cells respond to viral infection by the production of type I interferons (IFNs), which induce the expression of antiviral genes. Herpes simplex virus I (HSV-1) encodes many mechanisms that inhibit the type I IFN response, including the ICP27-dependent inhibition of type I IFN signaling. Here we show inhibition of Stat-1 nuclear accumulation in cells that express ICP27. ICP27 expression also induces the secretion of a small, heat-stable type I IFN antagonizing protein that inhibits Stat-1 nuclear accumulation. We show that the inhibition of IFN-induced Stat-1 phosphorylation occurs at or upstream of Jak-1 phosphorylation. Finally, we show that ISG15 expression is induced after IFNα treatment in mock-infected cells, but not cells infected with WT HSV-1 or ICP27− HSV-1. These data suggest that HSV-1 has evolved multiple mechanisms to inhibit IFN signaling not only in infected cells, but also in neighboring cells, thereby allowing for increased viral replication and spread.
Introduction
One of the first lines of defense that is activated upon infection of a host with a pathogen is the interferon (IFN) response. Type I IFNs (α, β, ω, τ) are a family of antiviral cytokines induced in most cell types by viral infection or the presence of double-stranded RNA, and acts in an autocrine and paracrine manner to establish an antiviral state in host cells (Sato et al., 2000). Type II IFN (γ) is a pro-inflammatory cytokine induced in activated T cells and natural killer cells (Schiller et al., 2006). Though there are distinct similarities in the signaling pathways activated by each type of IFN, there are also some key differences. Each family of IFN binds to a distinct heterodimeric receptor (Kotenko et al., 2003; Platanias and Colamonici, 1992; Platanias, Uddin, and Colamonici, 1994; Sheppard and York, 1990), which causes the activation of Janus kinases (Jaks) by phosphorylation. The kinases Jak-1 and Tyk-2 are activated in the case of type I IFN, and Jak-1 and Jak-2 for type II IFN (Darnell, Kerr, and Stark, 1994; David et al., 1993; Platanias, Uddin, and Colamonici, 1994). The Jaks phosphorylate signal transducers and activators of transcription (Stats) -1 and -2, in type I IFN signaling, and only Stat-1 after exposure to IFNγ (Platanias, Uddin, and Colamonici, 1994; Schindler et al., 1992; Uddin, Chamdin, and Platanias, 1995). Once activated by phosphorylation, Stat-1 either homodimerizes (IFNγ) or forms a complex with Stat-2 and with interferon regulatory factor 9 (IFNα/β) (Bandyopadhyay et al., 1995; Kessler et al., 1990; Ramana et al., 2002). These complexes translocate into the nucleus and bind specific DNA elements, interferon stimulated response elements (ISREs, type I signaling) or gamma activated sequences (GASs, type II signaling), to activate transcription of interferon stimulated genes (ISGs). ISGs contribute to the pro-inflammatory or antiviral state and include RNase L, which degrades viral and cellular RNAs (Dong and Silverman, 1995; Kerr and Brown, 1978) and PKR, which inhibits protein synthesis by phosphorylating the translation initiation factor eIF2a (Der et al., 1998; Samuel, 1979a; Samuel, 1979b).
Viruses have evolved mechanisms to evade or counteract the effects of IFNα/β signaling. Several viral proteins, such as the influenza virus NS1 protein and the human papilloma virus (HPV) E6 oncoprotein inhibit expression of type I IFN by blocking the activation or activity of interferon regulatory factor 3 (IRF3), a transcription factor important for type I IFN production (Ronco et al., 1998; Talon et al., 2000). The vaccinia virus protein B18R is secreted from cells and binds IFN in the extracellular space to prevent its binding to cells (Alcamí and Smith, 1995; Colamonici et al., 1995). Other viral proteins, such as cytomegalovirus (CMV) IE1, measles V protein, and dengue virus NS4B, inhibit the signaling pathway itself (Gao et al., 1997; Muñoz-Jordan et al., 2003; Paulus, Krauss, and Nevels, 2006; Yokota et al., 2003).
Herpes simplex virus 1 (HSV-1) is a large, double-stranded DNA virus that productively infects epithelial cells and establishes a latent infection in sensory ganglia for the life of the host (Roizman, Knipe, and Whitley, 2007). In cells that have been exposed to IFNα prior to infection, HSV-1 replication is severely reduced compared with cells infected in the absence of IFN (Altinkilic and Brandner, 1988; Mittnacht et al., 1988; Oberman and Panet, 1988; Pierce et al., 2005). However, cells that are infected with HSV-1 and then treated with IFN show reduced IFN signaling and decreased ISRE reporter gene activity (Chee and Roizman, 2004; Johnson, Song, and Knipe, 2008; Yokota et al., 2001). One anti-IFN activity that has been characterized for HSV-1 is the ICP0-dependent inhibition of IRF-3 stimulated IFNβ expression (Melroe et al., 2007). Second, the HSV-1 late protein γ34.5 binds protein phosphatase 1 to counteract the activity of PKR, by causing the dephosphorylation and reactivation of eIF2a (Chou et al., 1995; He, Gross, and Roizman, 1997; He, Gross, and Roizman, 1998; Leib et al., 2000). We have also shown that HSV-1 ICP27 is necessary and sufficient to inhibit IFNα-induced Stat-1 phosphorylation and nuclear accumulation (Johnson, Song, and Knipe, 2008). The effect was observed by 2 – 4 hpi, so this is likely an early event in HSV infection.
ICP27 is a multifunctional immediate early protein with homologs in all herpesviruses (Roizman, Knipe, and Whitley, 2007) that is essential for transcription of some early and late viral proteins (Jean et al., 2001). Early in infection, it is mostly nuclear, but has been shown to shuttle between the nucleus and cytoplasm later in infection (Clements et al., 2004; Soliman, Sandri-Goldin, and Silverstein, 1997). It has roles in transcriptional regulation through association with RNA polymerase II (Zhou and Knipe, 2002), and translation through association with translation factors eIF3, eIF4g, and PABP (Ellison et al., 2005; Fontaine-Rodriguez and Knipe, 2008; Fontaine-Rodriguez et al., 2004). ICP27 also affects RNA processing through interactions with splicing machinery (Hardwicke and Sandri-Goldin, 1994; Hardy and Sandri-Goldin, 1994; Phelan et al., 1993; Sandri-Goldin and Hibbard, 1996) and regulation of differential polyadenylation (Hann et al., 1998; McGregor et al., 1996; McLauchlan et al., 1992; McLauchlan, Simpson, and Clements, 1989). ICP27 associates with RNA via its RGG box and CR1 regions to stabilize A/U-rich RNAs (Brown et al., 1995; Ingram et al., 1996). In some studies, it has also been implicated in nuclear export of some viral transcripts (Koffa et al., 2001; Mears and Rice, 1998; Pearson, Knipe, and Coen, 2004; Wadd et al., 1999). However, other groups have not seen a difference in RNA export during infection with ICP27 mutant viruses (Ellison et al., 2005; Fontaine-Rodriguez and Knipe, 2008; Pearson, Knipe, and Coen, 2004).
We performed immunofluorescence experiments to determine that ICP27 was necessary and sufficient for inhibition of Stat-1 phosphorylation and nuclear accumulation. In these experiments we also observed that even after IFNα-treatment, many cells that did not stain positive for ICP27 still did not show nuclear accumulation of Stat-1 (Johnson, Song, and Knipe, 2008). It appeared that ICP27 expression was causing a bystander effect in surrounding cells through an unknown mechanism.
There have been several hypotheses about the mechanism(s) by which IFN signaling is inhibited by HSV-1, with possible mechanisms being the HSV-1 virion host shut-off protein (vhs) or the cellular suppressor of cytokine signaling protein SOCS-3 (Chee and Roizman, 2004; Yokota et al., 2005; Yokota et al., 2004). However, the actual mechanism of inhibition is still unknown. In this study, we show that HSV-1 infection inhibits IFN signaling at or before the phosphorylation of Jak-1. In exploring the bystander effect of ICP27 on surrounding cells further, we have have also found that HSV-1 infection and ICP27 transfection cause the secretion of a heat-stable, protease-sensitive soluble factor that inhibits IFNα-induced Stat-1 nuclear accumulation in trans.
Results
HSV-1 infection causes bystander cell inhibition of IFNα-induced Stat-1 nuclear accumulation
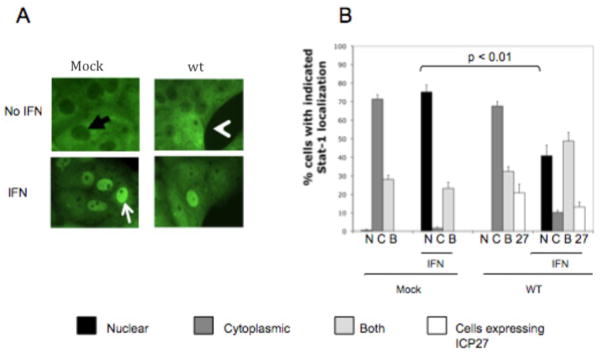
In our previous study, we observed that a number of cells that did not stain for ICP27 showed mostly cytoplasmic distribution of Stat-1 even after treatment with IFNα (Johnson, Song, and Knipe, 2008). To examine the relationship between ICP27 and Stat-1 distribution in HSV-1 infected cells, we mock-infected or infected Vero cells with WT HSV-1 for 10 hours and treated with IFNα at 104 U/mL for 30 minutes prior to fixation. Cells were stained with antibodies to Stat-1 and ICP27, and 200–250 cells per cover slip were scored blindly for Stat-1 localization, as being nuclear, cytoplasmic, or both (Fig. 1A black arrow-cytoplasmic, white arrow-nuclear, white arrow head-both).
Figure 1.
HSV-1 infection inhibits IFNα-induced nuclear accumulation of Stat-1 in surrounding cells. Vero cells were mock infected or infected (MOI = 3) with WT HSV-1 for 10 hours and treated with IFNα for 30 min before fixation, as indicated. Immunofluorescence was done with antibodies towards Stat-1 and ICP27. Stat-1 localization was scored as being predominantly nuclear (A: white arrow), predominantly cytoplasmic (A: black arrow), or both (A: white arrow head). The percent of cells infected was determined by counting cells that stained positive for ICP27 (B). Data shown are from cell counts from replicate cover slips, and statistical analysis was performed with the Student’s T test. The experiment shown is representative of mulitple experiments.
In the absence of IFN, Stat-1 was mostly cytoplasmic in over 70% and both nuclear and cytoplasmic in over 25% of mock-infected cells, but after IFN treatment Stat-1 was redistributed to be approximately 75% nuclear and about 25% cytoplasmic and nuclear (Fig. 1B). After HSV-1 infection, when roughly 20% of cells appeared to be infected (as detected by ICP27 immunofluorescence), Stat-1 was cytoplasmic in nearly 70% of cells and both cytoplasmic and nuclear in about 30% of cells in the absence of IFN (Fig. 1B). After IFNα treatment however, Stat-1 accumulated in the nucleus of only approximately 45% of cells, which is significantly lower than in mock-infected cells (p<0.01).
These data suggested that HSV-1 infection can inhibit type I IFN signaling in more cells than those expressing detectable ICP27, suggesting that IFN signaling was also inhibited in cells neighboring the infected cells.
ICP27 is sufficient for bystander cell inhibition of IFNα-induced Stat-1 nuclear accumulation
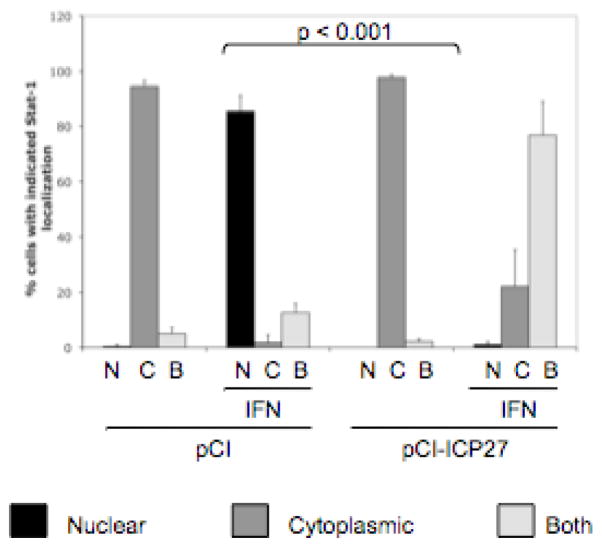
We showed previously that ICP27 is necessary and sufficient to inhibit IFNα-induced Stat-1 nuclear accumulation. To test whether ICP27 was sufficient to cause the bystander cell inhibition of Stat-1 nuclear accumulation, we transfected cells with empty vector or ICP27 expression plasmid. At 24 hours post transfection, we treated cells with IFNα at 104 U/mL for 30 minutes before fixation. Cells were stained for Stat-1 and ICP27 (for transfection efficiency) and 200–250 cells per coverslip were scored for Stat-1 localization.
Consistent with previous results, cells that were transfected with empty vector showed predominantly cytoplasmic staining for Stat-1 (90% of cells, Fig. 2). After treatment with IFNα, over 85% of empty vector-transfected cells showed Stat-1 accumulation in the nucleus (Fig. 2). Cells that were transfected with an ICP27 expression vector plasmid showed an even more dramatic effect than cells infected with WT HSV-1, with nearly 100% cytoplasmic Stat-1 localization in the absence of IFN and only approximately 1% of cells with nuclear staining after IFN treatment (p<0.001, Fig. 2). About 15% of cells stained positive for ICP27 (data not shown). These data suggested that ICP27 expression is sufficient to affect the type I IFN signaling in surrounding cells, although it was possible that the other cells expressed ICP27 at levels below the detection threshold of our antibody.
Figure 2.
ICP27 expression is sufficient to inhibit IFNα-induced Stat-1 nuclear accumulation in surrounding cells. Vero cells were transfected with empty vector (pCI) or an ICP27 expression vector (pCI-ICP27) and treated with IFNα for 30 min before fixation. Immunofluorescence was done with antibodies to ICP27 and Stat-1 and Stat-1 localization was scored as in Fig. 1.
ICP27 causes the release of a heat-stable, protease sensitive IFN-antagonizing factor
Some large DNA viruses encode proteins that are secreted from cells and compete for binding with IFN and IFNAR (Alcamí and Smith, 1995; Colamonici et al., 1995). To determine if the inhibition of Stat-1 nuclear accumulation that we observed was the result of a factor secreted by ICP27-expressing cells, we harvested medium from cells transfected with an empty vector or pCI-ICP27, transferred the medium to new cells and then treated the cells with IFNα, and scored for Stat-1 localization as above. We observed that cells incubated in medium from cells transfected with pCI plasmid had predominantly cytoplasmic Stat-1 in the absence of IFNα (69%, Table 1), and that after IFNα treatment, Stat-1 accumulated in the nucleus in most cells (81%, Table 1). Cells grown in medium from cells transfected with the ICP27 plasmid also showed predominantly cytoplasmic Stat-1 in the absence of IFN (81%, Table 1), but the IFN-induced accumulation of Stat-1 in the nucleus occurred in a significantly smaller percentage of cells (37%) than in cells grown in medium from pCI-transfected cells (p<0.05, Table 1). Therefore, ICP27 expression caused the secretion of a soluble IFN-antagonizing factor.
Table 1.
ICP27 causes the secretion of a heat-stable protease-sensitive IFN antagonist that is 10 – 50 kDa.
| Medium from cells transfecte d with | Treatment | IFN | % Cells with StatI Localization in* | ||
|---|---|---|---|---|---|
| Nucleus | Cytoplasm | Both | |||
| Empty vector | None | − + |
7±1 81±5 |
69±6 3±2 |
24±5 16±4 |
| ICP27 vector | − + |
1±1 37±19, p<0.05+ |
81±2 40±15 |
18±3 21±4 |
|
| Empty vector | 55°C 1 hour, 95°C | − + |
0.5±0.5 75±4 |
68±4 1±1 |
32±3 24±2 |
| ICP27 vector | 10 min | − + |
2±2 41±3, p<0.01+ |
75±6 18±3 |
21±5 41±0.5 |
| Empty vector | 55°C 1 hour, 95°C 10 min with proteinase K | − + |
0.5±0.5 58±7 |
82±2 9±0.5 |
18±2 32±7 |
| ICP27 vector | − + |
0.5±0.5 72±1, p>0.05 |
79±1 2±0.5 |
20±1 26±1 |
|
| Empty vector | Filtered through 50 kDa pores | − + |
1±0.5 84±2 |
70±4 0.5±0.5 |
29±5 16±2 |
| ICP27 vector | − + |
2±1 59±3, p<0.05+ |
69±3 2±0.5 |
28±3 39±3 |
|
| Empty vector | Filtered through 10 kDa pores | − + |
3±1 74±14 |
70±3 4±5 |
31±2 22±6 |
| ICP27 vector | − + |
3±1 77±4, p>0.25 |
67±2 2±0.5 |
31±3 20±4 |
|
Vero cells were transfected with empty vector (pCI) or an ICP27 expression vector (pCI-ICP27). At 24 hours post-transfection, medium from each culture was harvested and left untreated, heated to 55°C for 60 min and to 95°C for 10 min in the absence or presence of proteinase K, or passed through a 50- or 10-kDa filter before being transferred to naïve Vero cells. At 24 hours after media transfer, these cells were treated with IFNα at 104 U/mL for 30 min, as indicated, fixed, and stained for Stat-1. The distribution of Stat-1 localization was determined as in Figure 1.
compared with control medium from cells transfected with empty vector.
To characterize the ICP27-induced secreted factor, we assayed its heat-stability. Vero cells were transfected with empty vector or ICP27 expression vector. At 24 hours post-transfection, we harvested the medium from the transfected cells and incubated it at 55°C for 60 minutes, at 95°C for 10 minutes, cooled it to 37°C, and transferred the medium to new Vero cells. These cells were then treated with IFNα for 30 minutes, fixed, stained with antibodies to Stat-1, and scored for Stat-1 localization as above.
Consistent with previous results, cells incubated in medium from empty vector- or ICP27 expression plasmid-transfected cells showed mostly cytoplasmic Stat-1 localization in the absence of IFN (68% and 75%, respectively, Table 1). However, after IFNα treatment, Stat-1 was nuclear in 75% of cells grown in medium from empty vector-transfected cells, but in only 41% of cells grown in medium from ICP27 plasmid-transfected cells (Table 1), significantly different (p<0.01) from cells treated with control medium.
To further determine the molecular nature of the secreted factor, we incubated the media with Proteinase K, and transferred them to new Vero cells, which were treated with IFNα as indicated (Table 1) for 30 minutes, fixed, stained, and scored for Stat-1 localization. Cells grown in Proteinase K-treated medium from cells transfected with empty vector or ICP27 expression vector showed mostly cytoplasmic Stat-1 localization in the absence of IFN treatment (82% and 79% of cells, respectively, Table 1). Cells grown in Proteinase K-treated medium had mostly nuclear localization after IFNα treatment, regardless of whether the source of the medium was cells transfected with empty vector or ICP27 expression vector (58% and 72%, respectively, Table 1).
To determine the approximate size of the factor released from cells that express ICP27, we transfected cells with empty vector or ICP27 expression plasmid, harvested the medium as above, and passed it through molecular sizing filters with 10 kDa or 50 kDa molecular weight thresholds before overlaying naïve Vero cells. Cells were treated with IFNα, stained, and scored for Stat-1 localization as above.
In the absence of IFN, cells that were grown in medium from cells transfected with either plasmid filtered through the 50 kDa filter showed mostly cytoplasmic Stat-1 localization (70%, Table 1). After IFN treatment, there was a shift to mostly nuclear Stat-1 in cells grown in medium from pCI-transfected cells (84%, Table 1), which was significantly decreased in cells grown in medium from pCI-ICP27-transfected cells (59%, p<0.005, Table 1). Cells grown in medium passed through 10 kDa filter showed mostly cytoplasmic Stat-1 localization in the absence of IFN (70% for medium from pCI-transfected cells, 67% for medium from pCI-ICP27-transfected cells, Table 1). After IFN treatment, Stat-1 accumulated in the nucleus of most cells grown in medium from pCI-transfected cells (74%, Table 1) and cells grown in medium from pCI-ICP27-transfected cells (77%, Table 1).
In total, these results argued that the IFN-antagonizing factor secreted from ICP27-expressing cells was a heat-stable, protease sensitive protein between 10kDa and 50kDa in molecular weight.
Stage of IFN signaling pathway affected by HSV
Type I, but not type II, IFN-induced Stat-1 phosphorylation is inhibited by HSV-1 infection
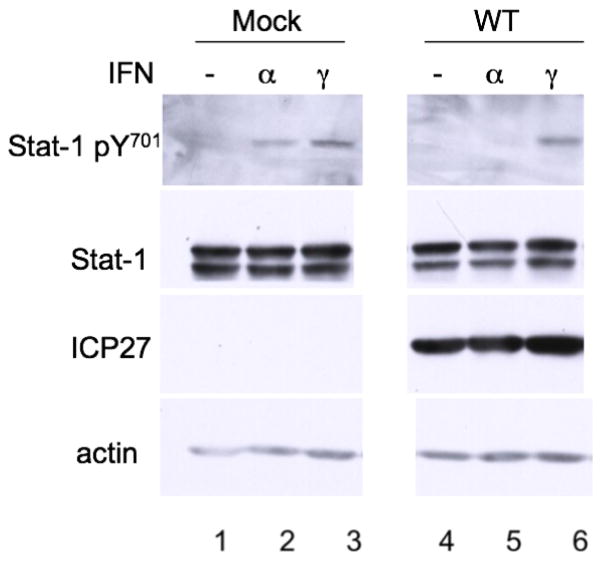
We have shown that ICP27 expression causes the secretion of a soluble protein that inhibits IFNα-induced Stat-1 nuclear localization. However, the stage of the signaling pathway affected was still unknown. Jak-1 and Stat-1 are shared factors between the type I and type II IFN signaling pathways (Samuel, 2001). Type II IFN signaling has been shown to be affected by HSV-1, as detected by reporter gene assay, albeit at significantly reduced levels compared with the inhibition of type I IFN signaling (Yokota et al., 2001). To see if type II IFN-dependent Stat-1 phosphorylation was inhibited under our infection conditions, we mock infected or infected Vero cells for 10 hours and treated them with IFNα or IFNγ at 104 U/mL as indicated, before Western blot analysis with antibodies for pStat-1, Stat-1, ICP27, and actin. Stat-1 was phosphorylated in mock-infected cells following treatment with either type of IFN (Fig. 3, lanes 2 and 3). However, in HSV-1 infected cells, Stat-1 was phosphorylated only after treatment with IFNγ (Fig. 3, lane 6), not with IFNα (Fig. 3, lane 5). These results argued that HSV-1 affects type I but not type II signaling. It is therefore very likely that the inhibition occurs at or before Jak-1 activation.
Figure 3.
Type I but not type II IFN-induced Stat-1 phosphorylation is inhibited by HSV-1. Vero cells were mock infected (lanes 1–3) or infected (MOI = 20) with WT HSV-1 (KOS, lanes 4–6) and treated with IFNα (lanes 2, 5) or IFNγ (lanes 3, 6) for 30 min before harvest. Western blot analysis was done with antibodies to pStat-1, Stat-1, ICP27, and actin.
HSV-1 infection inhibits IFNα-induced ISG15 expression in an ICP27-independent manner
Other studies have shown that HSV-1 inhibited reporter gene activity from constructs containing ISREs and GASs (Yokota et al., 2001). However, there were no published reports of the effects of ICP27 on the expression of endogenous ISG expression. We therefore tested the capacity of HSV-1 to inhibit expression of the ISG15 protein, a ubiquitin-like interferon-stimulated protein that gets conjugated to other proteins with as yet undetermined consequences (Biron and Sen, 2007; Zhao et al., 2004; Zhao et al., 2005).
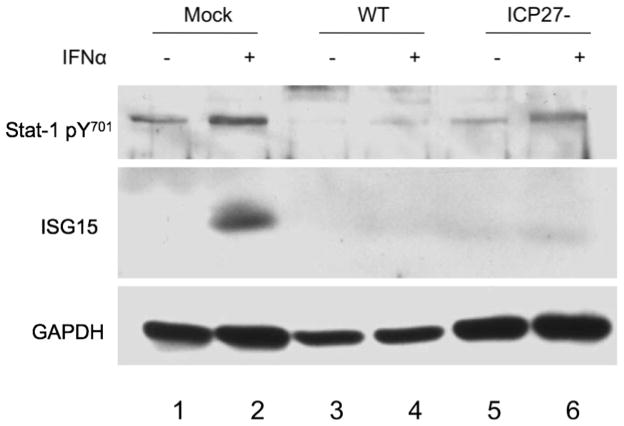
We mock-infected or infected Vero cells with WT HSV-1 or ICP27− virus at an MOI of 20 for 10 hours and treated with IFNα 2 hours before harvest, as indicated (Fig. 4). Western blot analysis was performed with antibodies specific for pStat-1, ISG15, and GAPDH. Phosphorylation of Stat-1 and ISG15 expression were induced in mock-infected cells after treatment with IFNα (Fig. 4 lane 2). Consistent with previous results, cells infected with WT HSV-1, IFNα treatment showed reduced Stat-1 phosphorylation and no evidence of ISG15 expression (Fig. 4, lane 4). Surprisingly, though Stat-1 was phosphorylated after IFNα treatment in cells infected with the ICP27− virus, ISG15 expression was not induced (Fig. 4, lane 6). These data suggested that there are ICP27-dependent and ICP27-independent mechanisms of inhibition of type I IFN signaling.
Figure 4.
HSV-1 inhibits IFNα-induced ISG15 expression in an ICP27-independent manner. Vero cells were mock infected (lanes 1–2) or infected (MOI = 20) with wt HSV-1 (KOS, lanes 3–4), or ICP27− virus (5dl1.2, lanes 5–6) and treated with IFNα (even lanes) for 2 hours before harvest. Western blot analysis was done with antibodies specific for pStat-1, ISG15, and GAPDH.
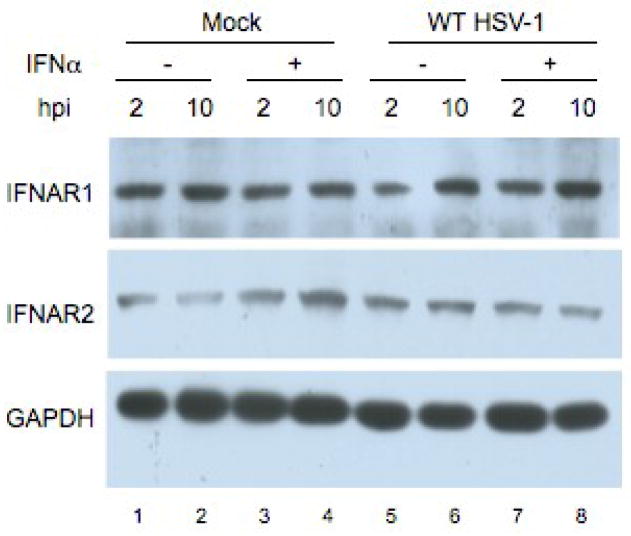
HSV-1 infection does not alter levels of Interferon α/β receptor chain 1 or 2 (IFNAR1, 2)
Because HSV-1 infection inhibited type I, but not type II, IFN signaling, we reasoned that a signaling activity upstream of Jak-1 such as the type I IFN receptor might be affected. Previous reports with vhs mutant viruses showed that, late in infection, there was a slight decrease in the protein levels for the IFNα/β receptor (Chee and Roizman, 2004). However, we and others have observed an effect of HSV-1 on IFN signaling at much earlier times post infection than the IFN receptor levels decreased (Johnson, Song, and Knipe, 2008; Yokota et al., 2001).
To determine if there was a decrease in IFNAR levels in our infection conditions, Vero cells were mock-infected or infected with WT HSV-1 (KOS) at an MOI of 20 for 2 or 10 hours and treated with interferon 30 minutes before harvest. Western blot analysis was done with antibodies specific for IFNAR1, IFNAR2, and GAPDH. There were no consistent changes in IFNAR1 or IFNAR2 levels, regardless of infection or treatment with IFN (Fig. 5). These results suggested that any degradation of IFNAR1 and IFNAR2 at later times post-infection is not the major mechanism of the inhibition of type I IFN signaling.
Figure 5.
Type I IFN receptor levels do not change over the course of infection. Vero cells were mock infected (lanes 1–4) or infected (MOI = 20) with WT HSV–1 (KOS, lanes 5–8) for 2 hours (odd lanes) or 10 hours (even lanes) and treated with IFNα for 30 min before harvest (Lanes 3–4, 7–8). Western blot analysis was done with antibodies specific for IFNAR1, IFNAR2, and GAPDH.
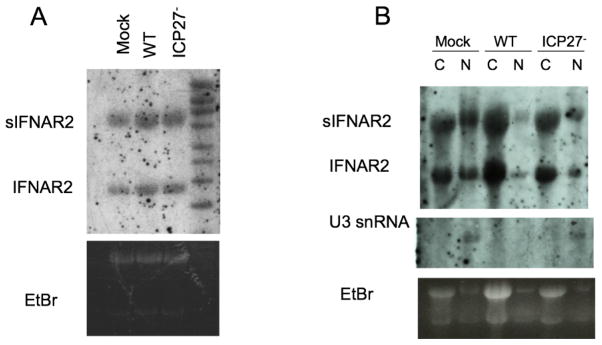
HSV-1 infection does not cause differential splicing or nuclear export of IFNAR2 RNA
We showed above that ICP27 is sufficient to cause release of a soluble heat-stable peptide that inhibits type I IFN signaling. Because ICP27 has been implicated in RNA processing and nuclear export (Hardwicke and Sandri-Goldin, 1994; Hardy and Sandri-Goldin, 1994; Pearson, Knipe, and Coen, 2004; Phelan et al., 1993; Sandri-Goldin and Hibbard, 1996; Sciabica, Dai, and Sandri-Goldin, 2003; Wadd et al., 1999) and has been shown to stabilize IFNβ RNA (Mosca, Pitha, and Hayward, 1992), we hypothesized that ICP27 might affect splicing of the transcript from the IFNAR2 gene. The mRNA for IFNAR2, the major IFN-binding subunit of the receptor (Novick, Cohen, and Rubinstein, 1994), has a splicing variant that encodes a 27 kDa secreted form of the receptor (Hardy et al., 2001; Lutfalla et al., 1995; Novick, Cohen, and Rubinstein, 1994). This secreted IFNAR2 has been shown to inhibit type I IFN signaling in cells that express full-length IFNAR2 (Hardy et al., 2001). Therefore, ICP27 might be affecting the splicing or nuclear export of IFNAR2 RNAs and causing preferential expression of the secreted IFNAR2 protein.
To determine if ICP27 was affecting the splicing of IFNAR2 mRNAs, we mock infected or infected Vero cells with WT or ICP27− HSV-1, and at 10 hours, we isolated RNA and subjected it to Northern blot analysis with a hybridization probe specific for a region of the IFNAR2 transcript that is shared between splice variants (Fig. 6A). There was no apparent difference in the ratio of IFNAR2 RNA splice variant levels between mock, WT, and ICP27− infected cells (Fig. 6A).
Figure 6.
ICP27 does not affect the splicing or nuclear export of IFNAR2 transcripts. Vero cells were mock-infected or infected (MOI = 20) with wt HSV-1 (KOS, A: lane 2, B: lanes 3–4) or ICP27− virus (5dl1.2, A: lane 3, B: lanes 5–6) for 10 hours. RNA was harvested from whole cells (A) or cells fractionated into nuclear and cytoplasmic components (B) and Northern blot analysis was done with probes specific for IFNAR2 and U3 snRNA.
Although there was no difference in the whole cell IFNAR2 mRNA levels, we hypothesized that ICP27 might be causing differential nuclear export of the IFNAR2 mRNA splice variants. Vero cells were mock-infected or infected with WT or ICP27− HSV-1 for 10 hours, and RNA was isolated from nuclear and cytoplasmic fractions. Northern blot analysis was performed as above with probes specific for IFNAR2 mRNA and U3 snRNA, the latter as a control for our fractionation. There was no apparent difference in relative nuclear to cytoplasmic levels of the sIFNAR2 vs IFNAR2 RNA species between mock, WT, or ICP27− infected cells (Fig. 6B). These results argued that the mechanism by which ICP27 induces the secreted inhibitory factor did not involve splicing or nuclear export of IFNAR2 mRNAs.
Discussion
Type I IFN is one of the first lines of defense that a host mounts against viral infection. As such, it is important for the fitness of many viruses that they evade the induction or the effects of type I IFN. Several viruses do this by antagonizing either the activation or activity of IRF-3 to prevent type I IFN expression (Ronco et al., 1998; Talon et al., 2000). In contrast, other viruses inhibit the IFN signaling pathway itself through interactions with and/or degradation of the Jak/Stat factors (Basler et al., 2000; Gao et al., 1997). Thus far, the only virus family found to encode a secreted type I IFN antagonist is Poxviridae. Vaccinia, tanapox, and ectromelia viruses encode secreted IFNα/β receptor homologs that inhibit IFN signaling.
HSV-1 encodes several IFN antagonists, including ICP0, which inhibits IRF-3 nuclear accumulation (Melroe, DeLuca, and Knipe, 2004); ICP27, which inhibits IFNα-induced Stat-1 phosphorylation and nuclear accumulation (Johnson, Song, and Knipe, 2008), and γ34.5, which counteracts the activity of PKR (Chou et al., 1995; He, Gross, and Roizman, 1997; Johnson, Song, and Knipe, 2008; Leib et al., 2000), an ISG that phosphorylates the translation initiation factor eIF2a to inhibit protein synthesis (He, Gross, and Roizman, 1998; Samuel, 1979a; Samuel, 1979b).
HSV-1 infection or ICP27 expression causes the inhibition of type I IFN signaling in surrounding cells
We observed that HSV-1 infection at a fairly low MOI causes inhibition of Stat-1 nuclear accumulation in a larger percentage of cells than are infected, as determined by ICP27 staining. We also show that transfection of cells with a plasmid encoding ICP27 is sufficient for the inhibition of Stat-1 nuclear accumulation in a larger percentage of cells than stain positive for ICP27. This indicates that either ICP27 is present at levels below detection by immunofluorescence in all cells in which we see no nuclear accumulation of Stat-1, or that HSV-1 infection and ICP27 expression cause the secretion of an inhibitory factor that affects the surrounding cells, possibly similar in mechanism to the vaccinia virus B18R protein (Alcamí and Smith, 1995; Colamonici et al., 1995). This effect could have very important implications for viral spread because HSV-1 replication is inhibited in cells that have been pre-exposed to IFNα (Altinkilic and Brandner, 1988; Mittnacht et al., 1988).
ICP27 expression causes the release of a heat-stable, protease-sensitive soluble IFNα antagonist
To differentiate between the two models described above, we transferred medium from cells transfected with empty vector or an ICP27 expression vector to naïve Vero cells. We found that medium harvested from cells transfected with empty vector has no effect on IFNα-induced nuclear accumulation of Stat-1. However, there is inhibition of Stat-1 nuclear accumulation conferred by medium from cells transfected with an ICP27 expression plasmid. We also found that this inhibitory activity is heat-stable and protease sensitive. These results suggested that there is a stable peptide secreted from ICP27-expressing cells that affects the IFN signaling in surrounding cells.
These results also raise the possibility that the secreted factor is either a cellular protein or all or part of ICP27. We were unable to detect ICP27 in the overlaid cells by immunofluorescence or in the medium by Western blot (data not shown). Because the commercially available antibodies to ICP27 are all to N-terminal epitopes, however, it is possible that there is a C-terminal cleavage product of ICP27 that causes the inhibition.
HSV-1 infection inhibits type I but not type II IFN-dependent Stat-1 phosphorylation
Jak-1 and Stat-1 are involved in both type I and type II IFN signaling. We and others have shown that HSV-1 inhibits IFNα-induced Stat-1 phosphorylation (Chee and Roizman, 2004; Mittnacht et al., 1988; Yokota et al., 2001). To determine if Stat-1 or Jak-1 were specifically targeted by the virus, we tested the capacity of HSV-1 to inhibit IFNγ-induced Stat-1 phosphorylation as well, and we found that IFNγ-induced Stat-1 phosphorylation is not inhibited by HSV-1. Because Jak-1 activation is necessary for Stat-1 phosphorylation (Darnell, Kerr, and Stark, 1994; David et al., 1993; Johnson, Song, and Knipe, 2008; Platanias, Uddin, and Colamonici, 1994) these results suggested that the effect of HSV-1 was on a factor or signaling event upstream of Jak-1 activation,. This could include an effect on IFNα, itself, one or both of the receptor proteins, or the interactions between the receptor and the janus kinases.
The lack of inhibition of IFNγ-induced Stat-1 phosphorylation contrasts somewhat with previous studies that show a decrease in IFNα-induced luciferase ivity from an ISRE reporter construct and a less pronounced decrease in IFNγ-induced luciferase from a GAS reporter construct. However, we have also shown that though HSV-1 inhibition of IFNα-induced Stat-1 phosphorylation and nuclear accumulation is ICP27-dependent, there is ICP27-independent inhibition of IFNα-induced ISG15 expression. This suggested the possibility of multiple levels of inhibition of the type I IFN signaling pathway. Another immediate early protein, ICP0 has been shown to be important for the inhibition of expression of some ISGs, including ISG54, ISG56, and ISG15 possibly through its activity as a ubiquitin ligase (Eidson et al., 2002). It is also possible that HSV-1 encodes an activity that inhibits the association of Stat-1 with DNA or with important transcription co-factors, such as CBP/p300. This would result in the inhibition of type I and type II IFN-induced gene expression even if Stat-1 phosphorylation is not affected.
HSV-1 infection has no effect on the levels of the type I IFN receptor subunits
Because of the implications that the step of IFNα signaling that is affected by HSV-1 is upstream of Jak-1 activation, we looked at the stability of the type I IFN receptor. In our hands, both IFNAR1 and IFNAR2 are levels are stable in cells after 10 hours of infection. This argues that HSV-1 is not causing the degradation of IFNAR in time to effect the inhibition of IFNα-dependent Stat-1 phosphorylation. A previous study (Chee and Roizman, 2004) showed a decrease in the levels of one of the receptor proteins, beginning at 8 hpi; however, they infected HeLa cells with HSV-1 strain F, which may cause more protein degradation than the KOS strain. It is possible that HSV-1 strain KOS does cause degradation of IFNAR at later times post-infection. However, it is also possible that the decrease in IFNAR levels seen in that study is due to normal degradation of the protein but decreased synthesis of IFNAR due to the host protein synthesis shut-off functions of vhs and ICP27.
Although the type I receptor levels appear constant through the time post-infection that IFNα-induced Stat-1 phosphorylation is inhibited, it is possible that the subcellular localization of the protein is altered by HSV-1 infection. If, for example, one or both of the receptor subunits were internalized during HSV-1 infection, there would be decreased sensitivity of infected cells to IFNα treatment. HSV-1 has previously been shown to cause internalization of the EGF receptor via interactions between the HSV-1 protein ICP0 and the cellular proteins CIN85 and Cbl (Liang, Kurakin, and Roizman, 2005).
HSV-1 does not affect the splicing or nuclear export of IFNAR2 mRNAs
ICP27 has several functions, including inhibition of splicing, causing differential use of polyadenylation signals, and roles in RNA export from the nucleus (Hann et al., 1998; Hardwicke and Sandri-Goldin, 1994; Hardy and Sandri-Goldin, 1994; Koffa et al., 2001; Liang, Kurakin, and Roizman, 2005; McGregor et al., 1996; McLauchlan et al., 1992; McLauchlan, Simpson, and Clements, 1989; Phelan et al., 1993; Sciabica, Dai, and Sandri-Goldin, 2003; Wadd et al., 1999). ICP27 has recently also been shown to promote production of a secreted form of the HSV-1 glycoprotein gC by causing the transmembrane domain coding sequences to be spliced out of the mRNA (Sedlackova et al., 2008).
The mRNA for IFNAR2 has four known splice variants encoding three proteins with some shared domains (Lutfalla et al., 1995): the full length signaling molecule, a dominant-negative molecule containing the IFN-binding extracellular and transmembrane domains (de Weerd, Samarajiwa, and Hertzog, 2007; Gazziola et al., 2005), and a secreted form with only the IFN-binding ectodomain, which has been shown to inhibit signaling in cells with a full complement of IFNAR2 (de Weerd, Samarajiwa, and Hertzog, 2007; Lutfalla et al., 1995; Novick, Cohen, and Rubinstein, 1994). We hypothesized that ICP27 might affect the splicing or nuclear export of the splice variants of IFNAR2, favoring the expression of the secreted form of the receptor. However, we found no differences in the ratios of secreted IFNAR2 RNA to full-length IFNAR2 RNA between mock-, WT-, and ICP27−- infected cells. We were also unable to find sIFNAR2 by Western blot in the medium from cells transfected with an ICP27 expression vector (data not shown). These results are certainly suggestive that sIFNAR2 is not the secreted factor induced by ICP27 expression that inhibits Stat-1 nuclear accumulation in response to IFNα. However, it is possible that ICP27 causes increased secretion of sIFNAR2 from cells. It is also possible that ICP27 expression causes the expression and secretion of a dominant negative IFN from cells.
These results have very important implications for HSV-1 spread and evasion of innate immunodetection because HSV-1 replication is inhibited in cells that have been exposed to IFN before infection (Hardy et al., 2001; Mittnacht et al., 1988; Oberman and Panet, 1988). This inhibition makes it very difficult for the virus to replicate efficiently, infect new cells, and establish latency in the host. By causing the secretion of a factor that inhibits IFNα signaling in neighboring, uninfected cells, HSV-1 maintains a cellular environment conducive to its own replication and spread. The inhibition of the signaling pathway upstream of Jak-1 activation may be due to the secreted protein competing for binding between IFNα and IFNAR2. This would be consistent with our data showing no difference in IFNAR levels after 10 hours of infection and the inhibition of type I but not type II IFN-induced Stat-1 phosphorylation.
Materials and Methods
Cells and Viruses
Vero cells were maintained in Dulbecco's modified Eagle's medium (DMEM, Gibco-BRL) supplemented with 5% heat-inactivated fetal calf serum (FCS) and 5% heat-inactivated newborn calf serum (BCS).
Viral stocks were grown in the appropriate complementing cell lines (the HSV-1 WT KOS was grown on Vero cells and 5dl1.2 (McCarthy, McMahan, and Schaffer, 1989) was grown on V827 cells. Viral titers were determined by plaque assay in Vero cells or the indicated complementing cell line as described (Knipe and Spang, 1982). For infection experiments, viruses were used at an MOI of 1 or 20 to infect Vero cells, which were incubated at 37°C in PBS with 1% BCS, 0.1% glucose for 1 h before removal of the inoculum and addition of DMEM supplemented with 1% FCS.
Western blots
Mock-infected or infected cells were harvested from 25-cm2 flasks in 400 μL of SDS sample buffer (62.5mM Tris HCl pH 6.8, 20%Glycerol, 2% SDS, 0.1% bromophenol blue, 10mM β-glycerophosphate, 5 mM sodium fluoride, 1mM sodium vanadate, 0.5% β-mercaptoethanol) and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in 12% bis-crosslinked polyacrylamide gels and electrically transferred to nitrocellulose in transfer buffer (25mM Tris, 192mM Glycine, 20% Methanol) overnight at 4°C with the Bio-Rad Transblot system. Membranes were probed with antibodies to Stat-1 (1:1000), pStat-1 (1:500), actin (1:1000), IFNAR1 (1:750), or IFNAR2 (1:500) from Santa Cruz Biotechnology, Inc, ICP27 (H1119 at 1:10,000) from Virusys, and GAPDH (1:40000) from Abcam, in PBST (0.5% Tween 20 in PBS from Gibco), washed twice for 5 min in PBST, and incubated with secondary antibodies conjugated to horseradish peroxidase (HRP) diluted 1:5000–1:20,000 from Santa Cruz Biotechnology, Inc. HRP activity was detected using Western Lightening chemiluminescence reagent (PerkinElmer) or Lumilight Western blotting substrate (Roche) and exposed on X-ray film (Kodak).
Plasmids and transfections
Plasmid pCI was obtained from Promega. Plasmid pCI-ICP27 has been described previously (Olesky et al., 2005). At 24 hours before transfection, Vero cells were seeded into 6-well plates containing glass coverslips for fluorescence experiments. Transfections were carried out using Opti-MEM medium (Gibco) and Genejuice reagent (Novagen) according to the manufacturer's instructions.
Immunofluorescence
Vero cells were seeded for immunofluorescence at 5 × 105 cells/well on glass coverslips in 6-well plates and incubated overnight at 37°C before infection or transfection, after which they were treated as indicated with IFNα (PBL biomedical laboratories). Following incubation for the appropriate time, cells were fixed in 3.7% formaldehyde in phosphate-buffered saline (PBS) for 10 min at room temperature, and cells were permeabilized by incubation in methanol at −20°C for 2 min. Following several washes in PBS, cells were incubated overnight in IF buffer (PBS containing 2.5% goat serum [Sigma]). Dylight-488 conjugated streptavidin (Pierce) or primary antibodies were diluted appropriately (Streptavidin-488 1:50, STAT-1 1:50) and applied to cells in PBS, and the cells were incubated for 45 min at 37°C. Cells were washed twice for 5 min in PBS. Secondary antibodies conjugated to Alexa 594 and Alexa 488 dyes were obtained from Molecular Probes Inc. and were applied at 1:1,000 in PBS for 30 min at 37°C. Cells were then washed twice for 10 min in PBS at room temperature, and coverslips were mounted with Prolong antifade reagent (Molecular Probes, Inc.).
For cell culture medium transfer experiments, medium was removed from transfected Vero cells at 24 hours post transfection, treated as indicated by heating to 55°C for 60 min and then to 95°C for 10 min in the presence or absence of 100μg/mL Proteinase K (Roche), and cooled to 37°C or spun through 10kDa or 50 kDa molecular weight cut off Microcon® filters (Millipore) according to the manufacturer’s instructions before overlaying new Vero cells on coverslips
Slides were viewed with an Axioplan 2 microscope (Zeiss) with a 63x objective and a 10x ocular objective. Images were collected with the Axiovision 4.5 suite of programs (Zeiss) and a Hamamatsu C4742-95-12NR digital camera.
RNA isolation and Northern blot analysis
Total RNA was isolated from Vero cells using TRI reagent (Ambion) according to the manufacturer’s instructions. To separate RNA into nuclear and cytoplasmic fractions, cells were lysed by incubation for 5 min on ice in lysis buffer (50mM Tris-Cl, pH8.0, 100mM NaCl, 5mM MgCl2, 0.5% v/v Nonidet P-40, filter sterilized) and nuclei were removed by centrifugation. RNA was isolated from nuclei using TRI reagent, according to the manufacturer’s instructions. RNA was isolated from the cytoplasm by adding SDS to 0.05% and extracting twice with phenol/chloroform/isoamyl alcohol and once with chloroform/isoamyl alcohol. Cytoplasmic RNA was ethanol precipitated and resuspended in H2O.
For Northern blots, 5μg of RNA of each sample was resolved in an agarose gel using the NorthenMax-Gly™ kit (Ambion) and transferred to BrightStar® Plus positively charged nylon membrane (Ambion) according to the manufacturer’s instructions. DNA probes for IFNAR2 (5′-GCTCATCACTGTGCTCTAAATAAACAGATACACA GTAGTTCGTGTTTGGAA-3′) and U3 (5′-ACCACTCAGACCGCGTTCTCTCCCT CTCACTCCCCAATACGGAGAGAAGAACGA-3′) were obtained from IDT and biotinylated using Brightstar® Psoralen-biotin nonisotopic labeling kit (Ambion) according to the manufacturer’s instructions. Probes were hybridized using UltraHyb® hybridization buffer (Ambion) and blots were washed and RNA detected using the BrightStar® biodetect kit (Ambion) according to the manufacturer’s instructions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcamí A, Smith G. Vaccinia, cowpox, and camelpox viruses encode soluble gamma interferon receptors with novel broad species specificity. J Virol. 1995;69:4633–4639. doi: 10.1128/jvi.69.8.4633-4639.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altinkilic B, Brandner G. Interferon inhibits herpes simplex virus-specific translation: a reinvestigation. Journal of General Virology. 1988;69(Pt 12):3107–3112. doi: 10.1099/0022-1317-69-12-3107. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay SK, Leonard GT, Jr, Bandyopadhyay T, Stark GR, Sen GC. Transcriptional induction by double-stranded RNA is mediated by interferon-stimulated response elements without activation of interferon-stimulated gene factor 3. J Biol Chem. 1995;270:19624–19629. doi: 10.1074/jbc.270.33.19624. [DOI] [PubMed] [Google Scholar]
- Basler CF, Wang X, Mühlberger E, Volchkov V, Paragas J, Klenk HD, García-Sastre A, Palese P. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc Natl Acad Sci U S A. 2000;97:12289–94. doi: 10.1073/pnas.220398297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron C, Sen G. Innate responses to viral infections. In: Knipe DM, Howley PM, editors. Fields Virology. 5. Lippincott, Williams and Wilkins; Philadelphia: 2007. pp. 249–278. [Google Scholar]
- Brown CR, Nakamura MS, Mosca JD, Hayward GS, Straus SE, Perera LP. Herpes simplex virus trans-regulatory protein ICP27 stabilizes and binds to 3′ ends of labile mRNA. J Virol. 1995;69:7187–95. doi: 10.1128/jvi.69.11.7187-7195.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee VA, Roizman B. Herpes simplex virus 1 gene products occlude the interferon signaling pathway at mutliple sites. J Virol. 2004;78:4185–4196. doi: 10.1128/JVI.78.8.4185-4196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J, Chen JJ, Gross M, Roizman B. Association of a M(r) 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2 alpha and premature shutoff of protein synthesis after infection with gamma 134.5-mutants of herpes simplex virus 1. Proc Natl Acad Sci USA. 1995;92(23):10516–20. doi: 10.1073/pnas.92.23.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements CJ, Abdool-Karim Q, Chang ML, Nkowane B, Esparza J. Breaking new ground-are changes in immunization services needed for the introduction of future HIV/AIDS vaccines and other new vaccines targeted at adolescents? Vaccine. 2004;22:2822–6. doi: 10.1016/j.vaccine.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Colamonici O, Domanski P, Sweitzer SM, Larner A, Buller R. Vaccinia virus B18R gene encodes a type I interferon-binding protein that blocks interferon alpha transmembrane signaling. J Bio Chem. 1995;270:15974–8. doi: 10.1074/jbc.270.27.15974. [DOI] [PubMed] [Google Scholar]
- Darnell JJ, Kerr I, Stark G. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- David M, Romero G, Zhang Z, Dixon J, Larner A. In vitro activation of the transcription factor ISGF3 by interferon alpha involves a membrane-associated tyrosine phosphatase and tyrosine kinase. J Bio Chem. 1993;268:6593–6599. [PubMed] [Google Scholar]
- de Weerd N, Samarajiwa S, Hertzog P. Type I interferon receptors: biochemistry and biological functions. J Bio Chem. 2007;282:20053–20057. doi: 10.1074/jbc.R700006200. [DOI] [PubMed] [Google Scholar]
- Der SD, Zhou A, Williams BRG, Silverman RH. Idenification of genes differentially regulated by interferon alpha, beta or gamma using oligonucleotide arrays. Proc Natl Acad Sci U S A. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B, Silverman RH. 2-5A-dependent RNase molecules dimerize during activation by 2-5A. J Biol Chem. 1995;270:4133–7. doi: 10.1074/jbc.270.8.4133. [DOI] [PubMed] [Google Scholar]
- Eidson KM, Hobbs WE, Manning BJ, Carlson P, DeLuca NA. Expression of herpes simplex virus ICP0 inhibits the induction of interferon-stimulated genes by viral Infection. J Virol. 2002;76:2180–2191. doi: 10.1128/jvi.76.5.2180-2191.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison KS, Maranchuk RA, Mottet KL, Smiley JR. Control of VP16 translation by the herpes simplex virus type 1 immediate-early protein ICP27. J Virol. 2005;79:4120–31. doi: 10.1128/JVI.79.7.4120-4131.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine-Rodriguez EC, Knipe DM. Herpes simplex virus ICP27 increases translation of a subset of viral late mRNAs. J Virol. 2008;82(7):3538–45. doi: 10.1128/JVI.02395-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine-Rodriguez EC, Taylor TJ, Olesky M, Knipe DM. Proteomics of herpes simplex virus infected cell protein 27: association with translation initiation factors. Virology. 2004;330:487–92. doi: 10.1016/j.virol.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Gao SJ, Boshoff C, Jayachandra S, Weiss RA, Chang Y, Moore PS. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene. 1997;15(16):1979–85. doi: 10.1038/sj.onc.1201571. [DOI] [PubMed] [Google Scholar]
- Gazziola C, Cordani N, Carta S, De Lorenzo E, Colombatti A, Perris R. The relative endogenous expression levels of the IFNAR2 isoforms influence the cytostatic and pro-apoptotic effect of IFNalpha on pleomorphic sarcoma cells. Int J Oncol. 2005;26:129–40. [PubMed] [Google Scholar]
- Hann LE, Cook WJ, Uprichard SL, Knipe DM, Coen DM. The role of herpes simplex virus ICP27 in the regulation of UL24 gene expression by differential polyadenylation. J Virol. 1998;72:7709–7714. doi: 10.1128/jvi.72.10.7709-7714.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwicke MA, Sandri-Goldin RM. The herpes simplex virus regulatory protein ICP27 contributes to the decrease in cellular mRNA levels during infection. J Virol. 1994;68:4797–4810. doi: 10.1128/jvi.68.8.4797-4810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy M, Owczarek C, Trajanovska S, Liu X, Kola I, Hertzog P. The soluble murine type I interferon receptor Ifnar-2 is present in serum, is independently regulated, and has both agonistic and antagonistic properties. Blood. 2001;97:473–82. doi: 10.1182/blood.v97.2.473. [DOI] [PubMed] [Google Scholar]
- Hardy WR, Sandri-Goldin RM. Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J Virol. 1994;68:7790–7799. doi: 10.1128/jvi.68.12.7790-7799.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Gross M, Roizman B. The gamma (1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1 alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA. 1997;94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Gross M, Roizman B. The gamma134.5 protein of herpes simplex virus 1 has the structural and functional attributes of a protein phosphatase 1 regulatory subunit and is present in a high molecular weight complex with the enzyme in infected cells. Journal of Biological Chemistry. 1998;273:20737–20743. doi: 10.1074/jbc.273.33.20737. [DOI] [PubMed] [Google Scholar]
- Ingram A, Phelan A, Dunlop J, Clements JB. Immediate early protein IE63 of herpes simplex virus type 1 binds RNA directly. Journal of General Virology. 1996;77:1847–1851. doi: 10.1099/0022-1317-77-8-1847. [DOI] [PubMed] [Google Scholar]
- Jean S, LeVan KM, Song B, Levine M, Knipe DM. Herpes simplex virus 1 ICP27 is required for transcription of two viral late (gamma2) genes in infected cells. Virology. 2001;283:273–284. doi: 10.1006/viro.2001.0902. [DOI] [PubMed] [Google Scholar]
- Johnson KE, Song B, Knipe DM. Role for herpes simplex virus 1 ICP27 in the inhibition of type I interferon signaling. Virology. 2008;374:487–94. doi: 10.1016/j.virol.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr IM, Brown RE. pppA2′ p5′ p5′A: An inhibitor of protein synthesis synthesized with an enzyme fraction from interferontuated cells. Proc Natl Acad Sci U S A. 1978;75:256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler DS, Veals SA, Fu XY, Levy DE. Interferon-alpha regulates nuclear translocation and DNA-binding affinity of ISGF3, a multimeric transcriptional activator. Genes Dev. 1990;4:1753–65. doi: 10.1101/gad.4.10.1753. [DOI] [PubMed] [Google Scholar]
- Knipe DM, Spang AE. Definition of a series of stages in the association of two herpesviral proteins with the cell nucleus. J Virol. 1982;43:314–324. doi: 10.1128/jvi.43.1.314-324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffa MD, Clements JB, Izaurralde E, Wadd S, Wilson SA, Mattaj IW, Kuersten S. Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway. EMBO Journal. 2001;20:5769–5778. doi: 10.1093/emboj/20.20.5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotenko S, Gallagher G, Baurin V, Lewis-Antes A, Shen M, Shah N, Langer J, Sheikh F, Dickensheets H, Donnelly R. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nature Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- Leib DA, Machalek MA, Williams BRG, Silverman RH, Virgin HW. Specific phentoypic restoration of an attenuated virus by knockout of a host resistance gene. Proc Natl Acad Sci USA. 2000;97:6097–6101. doi: 10.1073/pnas.100415697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Kurakin A, Roizman B. Herpes simplex virus 1 infected cell protein 0 forms a complex with CIN85 and Cbl and mediates the degradation of EGF receptor from cell surfaces. Proc Natl Acad Sci U S A. 2005;102:5838–43. doi: 10.1073/pnas.0501253102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfalla G, Holland S, Cinato E, Monneron D, Reboul J, Rogers N, Smith J, Stark G, Gardiner K, Mogensen K, et al. Mutant U5A cells are complemented by an interferon-alpha beta receptor subunit generated by alternative processing of a new member of a cytokine receptor gene cluster. EMBO J. 1995;14:5100–5108. doi: 10.1002/j.1460-2075.1995.tb00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy AM, McMahan L, Schaffer PA. Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J Virol. 1989;63:18–27. doi: 10.1128/jvi.63.1.18-27.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor F, Phelan A, Dunlop J, Clements JB. Regulation of herpes simplex virus poly(A) site usage and the action of immediate-early protein IE63 in the early-late switch. J Virol. 1996;70:1931–1940. doi: 10.1128/jvi.70.3.1931-1940.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLauchlan J, Phelan A, Loney C, Sandri-Goldin RM, Clements JB. Herpes simplex virus IE63 acts at the posttranscriptional level to stimulate viral mRNA 3′ processing. J Virol. 1992;66:6939–6945. doi: 10.1128/jvi.66.12.6939-6945.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLauchlan J, Simpson S, Clements JB. Herpes simplex virus induces a processing factor that stimulates poly(A) site usage. Cell. 1989;59:1093–1105. doi: 10.1016/0092-8674(89)90765-4. [DOI] [PubMed] [Google Scholar]
- Mears WE, Rice SA. The herpes simplex virus immediate-early protein ICP27 shuttles between nucleus and cytoplasm. Virology. 1998;242:128–137. doi: 10.1006/viro.1997.9006. [DOI] [PubMed] [Google Scholar]
- Melroe G, DeLuca N, Knipe DM. Herpes simplex virus 1 has multiple mechanisms for blocking virus-induced interferon production. Journal of Virology. 2004;78:8411–20. doi: 10.1128/JVI.78.16.8411-8420.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melroe GT, Silva L, Schaffer PA, Knipe DM. Recruitment of activated IRF-3 and CBP/p300 to herpes simplex virus ICP0 nuclear foci: Potential role in blocking IFN-beta induction. Virology. 2007;360:305–21. doi: 10.1016/j.virol.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittnacht S, Straub P, Kirchner H, Jacobsen H. Interferon treatment inhibits onset of herpes simplex virus immediate-early transcription. Virology. 1988;164:201–210. doi: 10.1016/0042-6822(88)90637-x. [DOI] [PubMed] [Google Scholar]
- Mosca JD, Pitha PM, Hayward GS. Herpes simplex virus infection selectively stimulates accumulation of beta interferon reporter gene mRNA by a posttranscriptional mechanism. J Virol. 1992;66(6):3811–3822. doi: 10.1128/jvi.66.6.3811-3822.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Jordan JL, Sánchez-Burgos GG, Laurent-Rolle M, García-Sastre A. Inhibition of interferon signaling by dengue virus. Proc Natl Acad Sci U S A. 2003;100:14333–8. doi: 10.1073/pnas.2335168100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick D, Cohen B, Rubinstein M. The human interferon alpha/beta receptor: characterization and molecular cloning. Cell. 1994;77:391–400. doi: 10.1016/0092-8674(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Oberman F, Panet A. Inhibition of transcription of herpes simplex virus immediate early genes in interferon-treated human cells. J Gen Virol. 1988;69:1167–1177. doi: 10.1099/0022-1317-69-6-1167. [DOI] [PubMed] [Google Scholar]
- Olesky M, McNamee EE, Zhou C, Taylor TJ, Knipe DM. Evidence for a direct interaction between HSV-1 ICP27 and ICP8 proteins. Virology. 2005;331:94–105. doi: 10.1016/j.virol.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Paulus C, Krauss S, Nevels M. A human cytomegalovirus antagonist of type I IFN-dependent signal transducer and activator of transcription signaling. Proc Natl Acad Sci U S A. 2006;103:3840–5. doi: 10.1073/pnas.0600007103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson A, Knipe DM, Coen DM. ICP27 selectively regulates the cytoplasmic localization of a subset of viral transcripts in herpes simplex virus type 1-infected cells. J Virol. 2004;78:23–32. doi: 10.1128/JVI.78.1.23-32.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan A, Carmo-Fonseca M, McLaughlan J, Lamond AI, Clements JB. A herpes simplex virus type 1 immediate-early gene product, IE63, regulates small nuclear ribonucleoprotein distribution. Proc Natl Acad Sci USA. 1993;90:9056–9060. doi: 10.1073/pnas.90.19.9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce AT, DeSalvo J, Foster TP, Kosinski A, Weller SK, Halford WP. Beta interferon and gamma interferon synergize to block viral DNA and virion synthesis in herpes simplex virus-infected cells. J Gen Virol. 2005;86(Pt 9):2421–32. doi: 10.1099/vir.0.80979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platanias L, Colamonici O. Interferon alpha induces rapid tyrosine phosphorylation of the alpha subunit of its receptor. J Bio Chem. 1992;267:24053–24057. [PubMed] [Google Scholar]
- Platanias L, Uddin S, Colamonici O. Tyrosine phosphorylation of the alpha and beta subunits of the type I interferon receptor. Interferon-beta selectively induces tyrosine phosphorylation of an alpha subunit-associated protein. J Bio Chem. 1994;269:17761–17764. [PubMed] [Google Scholar]
- Ramana CV, Gil MP, Schreiber RD, Stark GR. Stat1-dependent and -independent pathways in IFN-gamma-dependent signaling. Trends Immunol. 2002;23:96–101. doi: 10.1016/s1471-4906(01)02118-4. [DOI] [PubMed] [Google Scholar]
- Roizman B, Knipe DM, Whitley RJ. Herpes Simplex Virus. In: Knipe DM, Howley PM, editors. Fields Virology. 5. Lippincott, Williams and Wilkins; Philadelphia: 2007. pp. 2501–2602. [Google Scholar]
- Ronco LV, Karpova AY, Vidal M, Howley PM. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes & Development. 1998;12(13):2061–72. doi: 10.1101/gad.12.13.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CE. Mechanism of interferon action: phosphorylation of protein synthesis initiation factor eIF-2 in interferon-treated human cells by a ribosome-associated kinase possessing site specificity similar to hemin-regulated rabbit reticulocyte kinase. Proc Natl Acad Sci USA. 1979a;76(2):600–6044. doi: 10.1073/pnas.76.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CE. Phosphorylation of protein synthesis initiation factor eIF-2 in interferon-treated human cells by a ribosome-associated kinase possessing site-specifically similiar to the hemin-regulated rabbit reticulocyte kinase. Proc Natl Acad Sci U S A. 1979b;76:600–604. doi: 10.1073/pnas.76.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri-Goldin RM, Hibbard MK. The herpes simplex virus type 1 regulatory protein ICP27 coimmunoprecipitates with anti-Sm antiserum, and the C terminus appears to be required for this interaction. J Virol. 1996;70:108–118. doi: 10.1128/jvi.70.1.108-118.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, TT Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13:539–548. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- Schiller M, Metze D, Luger TA, Grabbe S, Gunzer M. Immune response modifiers--mode of action. Exp Dermatol. 2006;15:331–41. doi: 10.1111/j.0906-6705.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- Schindler C, Fu X, Improta T, Aebersold R, Darnell JJ. Proteins of transcription factor ISGF-3: one gene encodes the 91-and 84-kDa ISGF-3 proteins that are activated by interferon alpha. Proc Natl Acad Sci USA. 1992;89:7836–7839. doi: 10.1073/pnas.89.16.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciabica KS, Dai QJ, Sandri-Goldin RM. ICP27 interacts with SRPK1 to mediate HSV splicing inhibition by altering SR protein phosphorylation. EMBO J. 2003;22:1608–19. doi: 10.1093/emboj/cdg166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlackova L, Perkins KD, Lengyel J, Strain AK, van Santen VL, Rice SA. Herpes simplex virus type 1 ICP27 regulates expression of a variant, secreted form of glycoprotein C by an intron-retention mechanism. J Virol. 2008;82:7443–55. doi: 10.1128/JVI.00388-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard M, York JJ. Identification of an infectious laryngotracheitis virus equivalent to the herpes simplex virus type 2 major DNA binding protein (ICP8) Acta Virologica. 1990;34(5):443–448. [PubMed] [Google Scholar]
- Soliman TM, Sandri-Goldin RM, Silverstein SJ. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J Virol. 1997;71:9188–9197. doi: 10.1128/jvi.71.12.9188-9197.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talon J, Horvath CM, Polley R, Basler CF, Muster T, Palese P, García-Sastre A. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J Virol. 2000;74:7989–96. doi: 10.1128/jvi.74.17.7989-7996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin S, Chamdin A, Platanias L. Interaction of the transcriptional activator Stat-2 with the type I interferon receptor. J Bio Chem. 1995;270:24627–24630. doi: 10.1074/jbc.270.42.24627. [DOI] [PubMed] [Google Scholar]
- Wadd S, Bryant H, Filhol O, Scott JE, Hsieh TY, Everett RD, Clements JB. The multifunctional herpes simplex virus IE63 protein interacts with heterogeneous ribonucleoprotein K and with casein kinase 2. Journal of Biol Chem. 1999;274:28991–28998. doi: 10.1074/jbc.274.41.28991. [DOI] [PubMed] [Google Scholar]
- Yokota S, Saito H, Kubota T, Yokosawa N, Amano K, Fujii N. Measles virus suppresses interferon-alpha signaling pathway: suppression of Jak1 phosphorylation and association of viral accessory proteins, C and V, with interferon-alpha receptor complex. Virol. 2003;306:135–46. doi: 10.1016/s0042-6822(02)00026-0. [DOI] [PubMed] [Google Scholar]
- Yokota S, Yokosawa N, Kubota T, Suzutani T, Yoshida I, Miura S, Jimbow K, Fujii N. Herpes simplex virus type 1 suppresses the interferon signaling pathway by inhibiting phosphorylation of STATs and janus kinases during an early infection stage. Virology. 2001;286:119–124. doi: 10.1006/viro.2001.0941. [DOI] [PubMed] [Google Scholar]
- Yokota S, Yokosawa N, Okabayashi T, Suzutani T, Fujii N. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 confers efficient viral replication. Virol. 2005;338:173–81. doi: 10.1016/j.virol.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Yokota S, Yokosawa N, Okabayashi T, Suzutani T, Miura S, Jimbow K, Fujii N. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 contributes to inhibition of the interferon signaling pathway. J Virol. 2004;78:6282–6. doi: 10.1128/JVI.78.12.6282-6286.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Beaudenon S, Kelley M, Waddell M, Yuan W, Schulman B, Huibregtse J, Krug R. The UbcH8 ubiquitin E2 enzyme is also the E2 enzyme for ISG15, an IFN-alpha/beta-induced ubiquitin-like protein. Proc Natl Acad Sci USA. 2004;101:7578–7582. doi: 10.1073/pnas.0402528101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Denison C, Huibregtse J, Gygi S, Krug R. Human ISG15 conjugation targets both IFN-induced and constitutively expressed proteins functioning in diverse cellular pathways. Proc Natl Acad Sci U S A. 2005;102:10200–10205. doi: 10.1073/pnas.0504754102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Knipe DM. Association of herpes simplex virus type 1 ICP8 and ICP27 proteins with cellular RNA polymerase II holoenzyme. J Virol. 2002;76:5893–5904. doi: 10.1128/JVI.76.12.5893-5904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]