Abstract
Trypsin is well known as a pancreatic enzyme that is typically secreted into the intestine to digest proteins. We show in our current study, however, that trypsin is also a key factor in the control of spermatogenesis. A progestin in teleost fish, 17α, 20β-dihydroxy-4-pregnen-3-one (DHP), is an essential component of the spermatogenesis pathway, particularly during the initiation of the first meiotic division. In the course of our investigations into the mechanisms underlying progestin-stimulated spermatogenesis, we identified that eel trypsinogen is upregulated in eel testis by DHP treatment. Trypsinogen is expressed in the Sertoli cells surrounding spermatogonia and in the membranes of spermatids and spermatozoa. Using an in vitro eel testicular culture system, we further analyzed the roles of trypsin in spermatogenesis. The inhibition of trypsin using specific antibodies or serine protease inhibitors was found to compromise DHP-induced spermatogenesis. A low dose of trypsin induces DNA synthesis and the expression of Spo11, a molecular marker of meiosis, in germ cells. By comparison, a higher dose of trypsin partially induced spermiogenesis. Furthermore, trypsin was detectable in the membranes of the spermatozoa and found to be associated with fertilization in fish. Our results thus demonstrate that trypsin and/or a trypsin-like protease is an essential and multifunctional factor in spermatogenesis.
Keywords: fish, germ cell, in vitro culture, teleost, testis
Spermatogenesis is a complex developmental process that begins with the mitotic proliferation of spermatogonia and proceeds through extensive morphological changes that convert haploid spermatids into functional spermatozoa. Since the progression of the cellular stages of spermatogenesis is similar across the vertebrate kingdom, it is very possible that common control mechanisms exist in these species. However, there are known differences in this process between each class of vertebrate. For example, androgens play a major role in spermatogonial pathways in fish, but in mammals, the spermatogonia remain largely unaffected by a loss of androgen production. Moreover, the structures of the testes also differ among vertebrates. In mammals, the seminiferous tubules contain several successive generations of germ cells, whereas fish exhibit a cystic type of spermatogenesis.
Various reproductive styles and gametogenic patterns exist in different teleost species. The Japanese eel, for example, has a specific spermatogenetic pattern and under fresh-water culture conditions, the males of this species show immature testes containing only non-proliferating type A and early type B spermatogonia. It has been reported, however, that injection with human chorionic gonadotropin (hCG) can induce all stages of spermatogenesis in the Japanese eel in vivo (1). Furthermore, the Japanese eel is the only animal in which complete spermatogenesis has been induced by hormonal treatments using an in vitro organ culture system and a germ cell/somatic cell coculture system (2–4). Hence, the eel testis is an excellent model system for studying the regulation of spermatogenesis.
Using the eel model both in vivo and in vitro, we have demonstrated in a number of previous studies that spermatogenesis is controlled by different sex steroid hormones in fish. We found for example that spermatogonial stem cell renewal is regulated by an estrogen, estradiol-17β (E2) (5), that the initiation of spermatogonial proliferation is regulated by an androgen, 11-ketotestosterone (11KT) (3), and that the initiation of meiosis and sperm maturation are regulated by a progestin, 17α, 20β-dihydroxy- 4-pregnen-3-one (DHP) (6). It is generally accepted that the biological activity of steroid hormones is mediated via changes in the expression of steroid target genes. In the eel, E2 activity is mediated by spermatogonial stem cell renewal factor and 11-KT is mediated by Müllerian inhibiting substance (7). In our present study, we first focused on the progestin-regulated control mechanisms governing the initiation of meiosis. During these analyses, we identified trypsin as a multifunctional factor during the male reproductive process. We thus report and discuss the function of trypsin during vertebrate spermatogenesis and fertilization.
Results
Cloning of Trypsinogen from the Eel Testis.
To identify factors that are regulated by DHP, we carried out gene expression cloning using eel testicular fragments that were cultured with (+D) or without (−D) 100 ng/mL DHP for 6 days. After cultivation, poly (A)+ RNAs were extracted from these testicular fragments and a subtracted cDNA library was then constructed from +D and −D cDNA enriched via the RDA procedure. One thousand clones from each of these libraries were subsequently screened by differential hybridization using each enriched cDNA preparation as a probe. We thereby screened 25 non-cross-hybridizing and upregulated cDNA fragments after DHP treatment. Among these, a cDNA fragment corresponding to trypsinogen was identified (GenBank Acc. No. AB519643). Database searches showed that the deduced amino acid sequence of this clone is similar to eel pancreatic trypsinogen (Fig. S1). Eel testicular trypsinogen shares a 92% similarity with the amino acid sequence of eel pancreatic trypsinogen, and both proteins contain two conserved trypsin family serine protease active sites. The deduced amino acid sequence of eel testicular trypsinogen was phylogenetically inferred from that of other vertebrates (Fig. S2). Our analysis clearly shows that eel testicular trypsinogen belongs to the trypsinogen cluster.
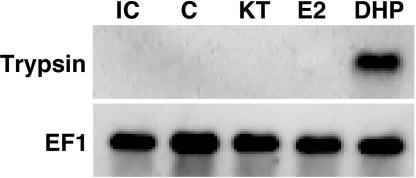
To further elucidate the relationship between testicular trypsinogen expression and sex steroids such as DHP that are associated with the regulation of spermatogenesis, we examines the effects of 11-KT, E2 and DHP upon the trypsinogen mRNA levels in the Japanese eel testis. Eel testicular fragments were cultured for 6 days with these three steroids, and the expression of trypsinogen was then analyzed by Northern blot analysis. The results showed that trypsinogen expression is induced only by DHP stimulation (Fig. 1).
Fig. 1.
Trypsinogen expression in the Japanese eel testis determined by Northern blot analysis of cultured testicular fragments. Pooled testicular fragments from 10 eels were cultured without (C) or with 10 ng/mL of 11-ketotestosterone (KT), estradiol-17β (E2) or 17α,20β-dihydroxy-4-pregnen-3-one (DHP) for 6 days. Lane IC shows the initial control before culturing. Northern blot analysis of EF-1, which serves as reference, is also shown.
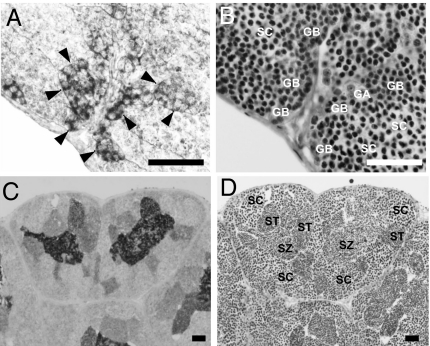
To determine the distribution of trypsinogen in the testis, we performed immunohistochemistry using an eel trypsinogen antibody. Positive staining of the Sertoli cells surrounding late type B spermatogonia (Fig. 2 A and B), and spermatids and spermatozoa (Fig. 2 C and D) was observed. No staining was obtained using preimmune serum as a negative control.
Fig. 2.
Cellular localization of eel trypsinogen in the testis. (A and B) Eel testis at 12 days post-hCG injection. (C and D) Eel testis at 18 days post-hCG injection. (A and C) were assessed by histochemistry using a specific anti-eel trypsinogen antibody. (B and D) show serial sections of A and C, respectively, stained with hematoxylin and eosin. Type A and early type B spermatogonia (GA), late type B spermatogonia (GB), spermatocytes (SC) spermatids (ST), spermatozoa (SZ), and enlarged Sertoli cells (arrowheads) are also shown. (Scale bar, 50 μm.)
Effects of a Trypsinogen Antibody and Serine Protease Inhibitors upon Spermatogenesis.
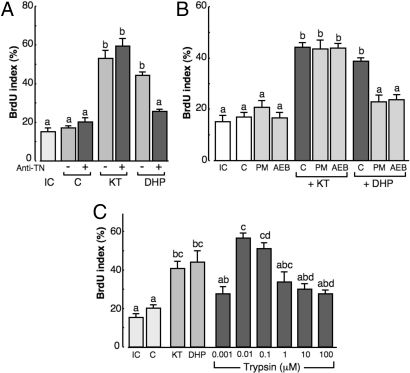
To investigate the relationship between trypsinogen and spermatogenesis, germ cell/somatic cell pellets were cultured with or without an anti-eel trypsinogen antibody and/or DHP (10 ng/mL) or 11-KT (10 ng/mL) for 6 days. To assay spermatogonial DNA synthesis, we monitored BrdU incorporation. As positive controls, treatment with 11-KT or DHP alone significantly stimulated DNA synthesis in spermatogonial cells. The addition of an anti-eel trypsinogen antibody significantly reduced DHP-induced, but not 11-KT-induced, spermatogonial DNA synthesis (Fig. 3A).
Fig. 3.
Effects of trypsin on spermatogenesis in the Japanese eel in vitro. (A) Effect of anti-trypsinogen antibody (Anti-TN) treatment on DHP-induced DNA replication of germ cells in a germ cell/somatic cell coculture system. IC, Initial control; KT, 10 ng/mL; DHP, 10 ng/mL; +, with anti-TN; -, without anti-TN. (B) Effect of serine protease inhibitor treatment on DHP-induced DNA replication of germ cells in a germ cell/somatic cell coculture system. PM, Phenylmethylsulfonyl fluoride (PMSF); AEB, 4-(2-Aminoethyl)-benzenesulfonyl fluoride (AEBSF). (C) Effect of various concentrations of porcine trypsin on germ cell/somatic cell cocultures.
Trypsin is a serine protease and we therefore investigated the effects of the serine protease inhibitors, 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF) and phenylmethylsulfonyl fluoride (PMSF) on spermatogonial DNA synthesis stimulated by DHP. Germ cell/somatic cell pellets were cultured with or without these inhibitors and with DHP (10 ng/mL) or 11-KT (10 ng/mL) for 6 days but only DHP-induced spermatogonial DNA synthesis was reduced (Fig. 3B).
Direct Effects of Trypsin on Eel Spermatogenesis.
The direct effects of trypsin on spermatogenesis were monitored using an eel germ cell/somatic cell coculture system (Fig. 3C). Germ cell/somatic cell pellets were cultured with increasing concentrations of pig-trypsin (1 nM, 10 nM, 100 nM, 1 μM, 10 μM, and 100 μM) or with 10 ng/mL 11-KT or 10 ng/mL DHP as positive controls for 2 days. We then monitored the DNA synthesis of spermatogonia by exposing the pellets to BrdU. At the start of cultivation, all of the germ cells in pellets are undifferentiated spermatogonia with a BrdU index of 15.2 ± 2.42%. The addition of trypsin to the culture medium induces DNA replication with a peak (56.6 ± 2.75%) at 10 nM. Significantly, in the controls without any supplement, the BrdU index did not change from its initial value.
Effects of Trypsin on the Expression of Meiosis-Specific Markers.
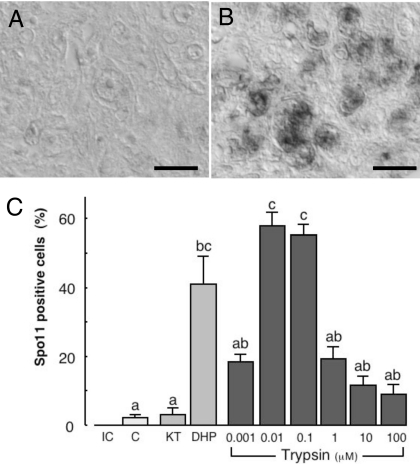
By immunohistochemistry we examined the effects of trypsin on the expression of the meiosis-specific marker, Spo11, in a germ cell/somatic cell coculture system. Before cultivation, there was no detectable Spo11 in the germ cells within the pellets. After 2 days in culture, however, the trypsin treatment was found to induce Spo11 expression in these germ cells with a peak of activity at 10 nM (Fig. 4 A–C).
Fig. 4.
Effects of trypsin upon the induction of meiosis in the Japanese eel in vitro. (A and B) Microphotographs showing anti-eel Spo11 immunoreactive material in germ cell/somatic cell pellets without (A) or with (B) 0.01 μM of trypsin. (Scale bar, 10 μm.) (C) Percentages of Spo11-positive germ cells. The number of immunoreactive germ cells is expressed as a percentage of the total number of germ cells. Results are given as means ± SEM calculated for each of six samples. Values with different letters are significantly different (P < 0.05).
Effects of Trypsin on the Spermatogonial Morphology.
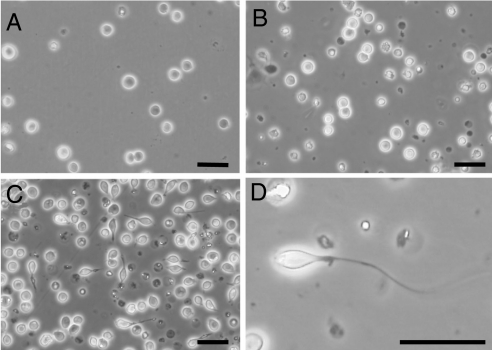
Type A spermatogonia were cultured with various concentrations of trypsin (0.1–100 μM) for 15 days and the appearance of the cells was then evaluated. Before cultivation, type A spermatogonia rounded (Fig. 5A), but after 3 days in culture with 100 μM of trypsin, these cells adopt a spindle-shaped morphology. After 15 days of treatment with 100 μM of trypsin, a flagelliform structure is detectable on one side of these spindle-shaped germ cells (Fig. 5 C and D). In cultures without trypsin or with 0.1, 1, and 10 μM trypsin, no morphological changes were evident in the germ cells (Fig. 5B). Using flow-cytometry, the nuclear phases of these spindle-shaped germ cells were recorded. The resulting flow-cytometric histograms showed 2C and 4C peaks, that is, these cells did not undergo a normal meiotic division.
Fig. 5.
Effects of trypsin on spermiogenesis. Spermatogonia before culture (A), and spermatogonia cultured without (B) or with (C and D) 100 μM trypsin in a spermatogonial germ cell culture for 15 days are shown. D shows a higher magnification image of spermatogonia treated with trypsin. (Scale bar in A–D, 20 μm.)
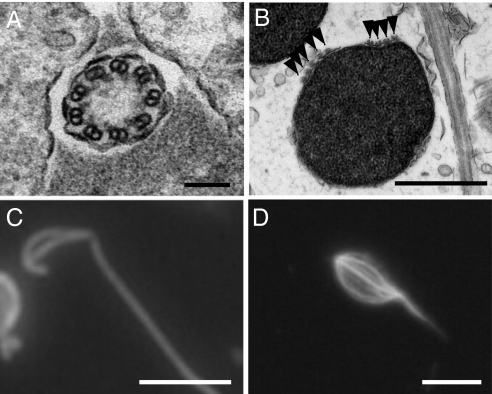
The morphology of the elongated germ cells exposed to trypsin was compared with normal eel spermatozoa by histological observation (Fig. 6 A–C). The flagella of the eel sperm cells exhibit a 9 + 0 axonemal structure (Fig. 6A) and nine pairs of microtubules in each flagellum are divided into four and five pairs, respectively, on the sperm head (Fig. 6B) where they extend to the caput end. Both sets of microtubules and the flagella can be detected by immunocytochemistry using α-tubulin antibodies. In sperm-like cells treated with trypsin, an axonemal structure could not be detected in the flagelliform structure using electron microscopy, whereas flagelliform structures and microtubule-like lines at the cell surface were found to be specifically stained by α-tubulin antibodies (Fig. 6D).
Fig. 6.
Flagella and the manchette-like structures of sperm-like cells are induced by trypsin treatment.(A) Electron microphotograph of a cross section of a flagellum of an eel spermatozoon, showing the 9 + 0 axonemal structure. (Scale bar, 0.1 μm.) (B) Electron microphotograph of cross section of an eel sperm head. Arrowheads indicate the nine sets of microtubules (manchette structure). (Scale bar, 1 μm.) (C) Localization of α-tubulin in a normal eel spermatozoon assessed by immunohistochemistry of whole cells using an anti-α-tubulin antibody. α-tubulin exists in the manchette structure of the sperm head and in the flagellum. (Scale bar, 5 μm.) (D) Site localization of α-tubulin in spermatogonia cultured with 100 μM trypsin assessed by immunohistochemistry of whole cells using an anti-α-tubulin antibody. (Scale bar, 5 μm.)
Trypsin in the Eel Sperm Head.
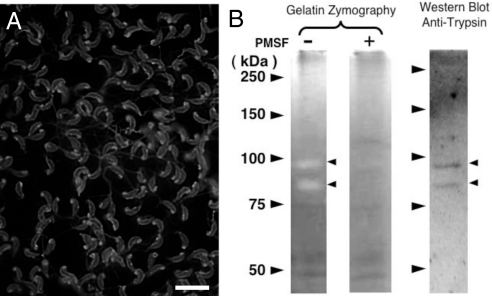
The trypsinogen expression profile in eel spermatozoa was next analyzed. Using a specific antibody, trypsin was detected in the sperm membranes (Fig. 7A) and its activity was assayed using gelatin zymography. In this analysis, 80- and 95-kDa bands with protease activity were detected and these two products could be reduced by PMSF treatment. Furthermore, these two bands corresponded to anti-trypsinogen positive bands detected by Western blot analysis, respectively (Fig. 7B).
Fig. 7.
Localization of trypsin in eel sperm. (A) Immunohistochemical analysis of whole cells using an anti-eel trypsinogen antibody. Trypsin localizes at the sperm membrane and within the mitochondria in the caput end of the sperm head. (Scale bar, 10 μm.) (B) Gelatin zymography assay and Western blot analysis of the eel sperm membrane with an anti-trypsinogen antibody (Anti-TN). PMSF − and + indicate with or without Phenylmethylsulfonyl fluoride, respectively. Arrowheads indicate molecular markers (Left), trypsin activity assayed by zymography, or signal detection by Western blot analysis using the anti-eel trypsinogen antibody.
Relationship Between Sperm Trypsin and Fertilization Pathways.
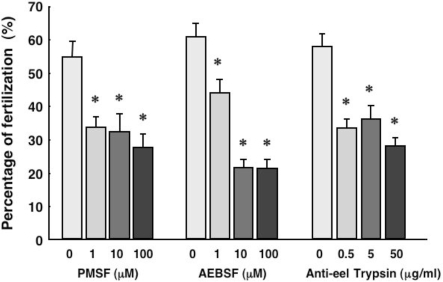
The relationship between fertilization and sperm head trypsin was also analyzed in the eel. Ejaculated eel sperm were incubated in artificial seminal plasma supplemented with the specific serine protease inhibitors PMSF or AEBSF, or with anti-eel trypsin antibodies, for 3 h. Inseminations were then performed using these incubated sperm and normal eel eggs. Four hours after insemination, fertilized eggs were counted and the rate of fertilization was thereby calculated. The results showed that both serine protease inhibitors the trypsin antibodies significantly reduced the fertilization rate (Fig. 8).
Fig. 8.
Functions of the membrane bound trypsin in eel sperm. The effects of serine protease inhibitors or an anti-eel trypsinogen antibody (Anti-TN) upon the fertilization rate of eel eggs were evaluated. Results are given as means ± SEM calculated for each of six samples. AEBSF, 4-(2-aminoethyl)-benzenesulfonyl fluoride. *, significantly different from the control (P < 0.05).
Discussion
Eel trypsinogen cDNAs were cloned by gene expression screening to identify factors that are regulated by the DHP initiator of meiosis during spermatogenesis in the Japanese eel. Trypsinogen is a precursor of trypsin, a member of the serine protease family that is mainly produced in the pancreas as a digestive enzyme. Although it has been reported that serine proteases are present in testicular tissue, their functions have not yet been clarified (8, 9). In our current study, we show that trypsin plays an important role in the regulation of three reproductive events in the male eel; that is, the initiation of meiosis, spermiogenesis, and fertilization.
Previous studies have shown that trypsinogen is expressed in several tumors and cancer cell types (10). It has been reported that trypsin affects the proliferation, invasion, and metastasis of breast cancer cells (11). The matrix metalloproteinases (MMPs) and proteinase-activated receptor 2 (PAR-2) are stimulated by trypsin and both may activate the mitogenic MAPK-ERK pathway via the induction of epidermal growth factor receptor (11). Activation of this pathway also promotes cell division in many cell types. Moreover, another study has investigated the expression of PAR-2, a G-protein-coupled receptor, and the role of trypsin in cell proliferation in human colon cancer cell lines (12). The results of these previous reports indicate that trypsin shows properties as a growth factor and unravel a mechanism whereby serine proteases control the proliferation of colon tumors. In yet another report, it has been demonstrated that trypsin initiates its intracellular role in growth promotion by acting on the cell surface (13). It was suggested from this finding that trypsin acts on intact cells via the insulin receptor as it has been shown to stimulate the phosphorylation of the β-subunit of this receptor. Moreover, trypsin may also directly modify the membrane proteins which form the Ca2+ channel (14, 15). Although further investigations are needed, it may be possible that trypsin may function in spermatogenesis via some of and/or all of these pathways.
In the Japanese eel, spermatogenesis is regulated by three sex steroids; E2, 11-KT, and DHP. E2 regulates spermatogonial renewal proliferation (5, 16), 11-KT regulates spermatogonial proliferation (3), and DHP regulates the initiation of meiosis (6). Each of these steroids can alter the expression of trypsinogen and thus may have a role at each stage of spermatogenesis. In our present study, Northern blot analysis was used to examine the effects of these three steroids on the trypsinogen mRNA levels in an eel testicular organ culture system. The results show that these transcripts respond only to DHP stimulation thus suggesting that trypsinogen functions during the meiotic steps in spermatogenesis. Furthermore, by testicular histochemistry we observed that trypsinogen is expressed in the Sertoli cells surrounding the late type B spermatogonia, but not in the Sertoli cells of the spermatocytes. Strong trypsinogen expression was also observed in earlier late type B spermatogonial generations. In general, the number of mitotic divisions that spermatogonia undergo before entering meiosis is species-specific (17). Previously, we proposed that this number is related to a mediator that regulates entry of Japanese eel spermatogonia into meiosis (18). The results of our histochemical analyses also support the contention that trypsinogen plays a role in meiosis in early stages of late type B spermatogonial development. Hence, we speculate that the mechanism regulating the entry point to meiosis is an early process involving trypsinogen.
The addition of anti-trypsinogen antibodies into the medium of germ cell/somatic cell cocultures significantly abrogates DHP-induced, but not 11-KT-induced, spermatogonial DNA synthesis. The neutralization of trysinogen thus blocks DHP-induced spermatogenesis indicating its essential role in this process. Moreover, since inhibitors of serine proteases prevent DHP-induced pathways in a similar way, we conclude that trypsinogen exerts its functional role in spermatogenesis as trypsin, the mature, biologically active form of this molecule.
Using the eel germ cell/somatic cell coculture system, we could assay the direct effects of trypsinogen upon the initiation of meiosis. The addition of trypsin into the medium of these cultures significantly stimulated DNA replication which in germ cells, occurs during in both mitosis and meiosis. Furthermore, trypsin was also found to significantly induce the expression of Spo11 in germ cells. Spo11 is involved in the formation of DNA double-strand breaks in eukaryotic species during homologous recombination in meiotic prophase (19, 20) and has been recognized as a molecular marker of the initiation of meiosis in germ cells. These data thus indicate that trypsin has an important role in the initiation of meiosis upon DHP stimulation.
In our histochemical analyses using trypsinogen antibodies, the spermatids and spermatozoa were also positively stained and we therefore investigated the relationship between trypsin and late stage spermatogenesis. We found that the shape of type A spermatogonia became “sperm-like”; that is, spindle-shaped with a flagelliform structure, after treatment with 100 μM trypsin. Flow-cytometric histograms of these cells revealed 2C and 4C peaks, that is, these “sperm-like cells” did not undergo meiotic division and exhibited a spermatogonia-type nucleus.
In the Japanese eel, as in other anguillidae (21), the flagella of the sperm cells exhibit a 9 + 0 axonemal structure. The microtubules in each flagellum divide into four and five pairs, respectively, on the sperm head, where they extend to the caput end. This microtubule structure in eel sperm heads is equivalent to the “manchette” structure in mammalian sperm heads (22, 23). The flagellum and the manchette-like structures in mammalian sperm also comprise microtubules (21) consisting of heterodimers of α and β-tubulin (24). Thus, they can be detected by immunocytochemistry using an α-tubulin antibody in the eel. In particular, the manchette-like structures can be observed in two lines on sperm head.
The flagelliform structures produced by the trypsin stimulation of sperm-like cells were not found to exhibit the 9 + 0 axonemal structure by electron microscopic observation. However, the flagelliform structures of these cells, stained by α-tubulin antibodies, and manchette like structures composed of α-tubulin were detectable at the cell surface. These results indicate that trypsin treatment triggers some, but not all aspects of spermiogenesis; that is, the processes by which spermatogonia become spermatozoa and the induction of spermatogonia to develop directly into sperm-like cells. Our current data also suggest that a relatively low concentration of trypsin is sufficient to induce meiosis, while a high concentration of trypsin induces partial spermiogenesis.
In teleost fish, except for some species such as the sturgeon, spermatozoa do not have an acrosome (25, 26), and fertilization is facilitated via the egg's micropyle. Hence, an acrosome reaction is not required during the fertilization of fish eggs. However, there is little information currently available regarding the factors or mechanisms that play a role in the fertilization of fish eggs. Trypsin or trypsin-like proteases in sperm may have a critical role in this regard.
Using a trypsinogen antibody, immunoreactive material was detectable in the sperm membrane that displayed trypsin activity in a gelatin zymography assay. Hence, eel sperm heads have trypsin activity although the protein in question differs from the testicular trypsin that we have cloned, since the molecular mass of sperm head trypsin (80 and 95 kDa) differs from that of testicular type trypsin (27 kDa). Since it has been reported also that membrane-type serine protease exists in elongated spermatids in mammals (27, 28), there is a possibility that sperm head trypsin in eel is similar to this serine protease in mammals.
We wished to investigate also the possibility that sperm head trypsin is related to fertilization and evaluated the relationship between fertilization and sperm head trypsin in the eel. The serine protease inhibitors and the anti-trypsinogen antibody significantly reduced the fertilization rate; hence, sperm head trypsin indeed has an important role in fertilization.
In conclusion, we provide strong evidence that the testicular trypsins are important factors in the control of meiosis, spermiogenesis, and fertilization. In other words, trypsin is a multifunctional factor in male reproduction.
Materials and Methods
cDNA Cloning.
Testicular organ cultures were prepared as described in ref. 1. The testicular fragments were cultured for 6 days with (+D) or without (−D) DHP at a concentration of 100 ng/mL. cDNA subtractions were performed using poly (A)+ RNA extracted from +D and −D cultured testicular fragments as described in ref. 7. After the subtraction enrichment process, enriched upregulated +D cDNA fragments were packaged into λzapII phage particles.
Enriched +D and −D cDNAs were then labeled using PCR DIG labeling Mixplus (Roche). Labeled +D and −D cDNA probes were hybridized separately to duplicate copies of the library. Only those clones that hybridized to the +D probe were collected and purified, and these plasmids were then excision reduced. These partial cDNA fragments were then labeled with DIG-dUTP using PCR and used as probes to screen an intact cDNA library constructed from oligo (dT) primed mRNA extracted from testes at 12 days after hCG injection. Positive clones obtained from this library screening were sequenced by the dideoxy chain termination method using the Dual CyDye Terminator sequencing kit (Amersham Biosciences).
Germ Cell/Somatic Cell Pellet Cultures.
Germ cell/somatic cell pellets were cultured as described in ref. 4. To further understand the function of trypsin during spermatogenesis, these cultures were exposed to porcine trypsin (Nacalai Tesque), Phenylmethylsulfonyl fluoride (PMSF; Nacalai Tesque), 4-(2-Aminoethyl)-benzenesulfonyl fluoride (AEBSF; Nacalai Tesque) or anti-eel trypsinogen antibodies.
Northern Blot Analysis.
Total RNA was extracted using Sepasol RNA I super (Nacalai Tesque) from testicular fragments cultured for 6 days with 10 ng/mL of 11-ketotestosterone (11-KT) or 17α, 20β –dihydroxy -4 –pregnen -3-one (DHP). Testicular fragments before culturing and testicular fragments cultured without steroids were used as the initial control and control, respectively. One mg of poly (A)+RNA purified by Oligotex-dt30 (Takara Bio) was electrophoresed on a 1% agarose denatured gel and blotted onto nylon membranes. The cDNA fragments obtained from cDNA subtraction were labeled using a DIG-dUTP PCR DIG probe synthesis kit (Roche) and served as probes. The cDNA fragment encoding Japanese eel elongation factor1 (EF1) was also labeled for use as an internal standard. After hybridization, the membrane was analyzed using a LAS-1000 mini (Fujifilm).
Immunohistochemistry.
Immunohistochemistry was performed using antibodies raised against eel trypsinogen and eel Spo11. Testicular tissue was fixed in Bouin's solution at 4 °C for 18 h, embedded in paraffin wax, and cut into 5-μm sections. Immunohistochemical analysis was performed using the Histofine SAB-AP kit (Nichirei).
The anti-eel trypsinogen antibody was produced using bacterial recombinant eel trypsinogen. cDNA fragments encoding the mature eel trypsinogen peptide were subcloned into a pQE-32 expression vector (Qiagen) to create a 6× histidine tag on the expressed protein. After bacterial expression, the recombinant protein was purified and refolded. A rabbit was then immunized with 1 mg recombinant eel trypsinogen. The IgG fraction was purified from the respective antiserum by anion exchange chromatography. Western blot analysis of the testes at 12 days after the hCG injection was then carried out using this eel trypsinogen antibody and a single band of approximately 27 kDa was detected (Fig. S3). The production of rabbit polyclonal antibodies against eel Spo11 was performed as described in ref. 20.
Using anti-eel trypsinogen and -eel Spo11 antibodies at a dilution of 1:1,000, immunohistochemistry was also performed. As a negative control, immunohistochemical analysis was performed using preimmune normal rabbit serum (NRS), which did not react with any cells. Using anti-α-tubulin and anti-eel trypsin, whole mount immunohistochemistry of germ cells was also performed. Cells were attached to the polyL-lysine coated glass slides and fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 1 h. After washing with PBS (PBS), the cells were permeabilized for 30 min in 1% Triton X-100 in PBS. The slides were then incubated for 30 min in 5% skimmed milk in PBS to block nonspecific binding sites before immersion for 1 h in PBS containing antibodies against α-tubulin (mouse monoclonal, diluted 1:100) or eel trypsin (diluted 1:1,000). After washing with PBS containing 0.025% Tween 20 (TPBS), the slides were next incubated with FITC-conjugated goat anti-mouse IgG (diluted 1:100) or anti-rabbit IgG (diluted 1:100) for 1 h. Finally, the slides were washed with TPBS and then with PBS and mounted in 50% glycerol in PBS.
Spermatogonial Cell Culture.
Eel testes containing only type A spermatogonia were digested by collagenase/dispase II for 2–3 h at room temperature. The resulting cell suspension was passed successively through meshes with a 75-, 45-, and 20-μm pore size. After washing with eel Ringer's solution, the cells were layered on top of a 13.8% Nycodenz solution and centrifuged for 10 min at 1,300 × g to remove the erythrocytes. The resulting cells were then plated on collagen-coated dishes to remove (adhering) somatic cells. Non-adhering germ cells (1 × 106 cells, mainly type A spermatogonia) were then cultured in L-15 culture medium with or without various concentrations (0.1, 1, 10, and 100 μM) of pig trypsin in non-coated culture dishes (3-cm diameter) at 28 °C for 15 days.
Western Blot Analysis and Gelatin Zymography.
Eel spermatozoa were collected from artificially matured, hCG-treated eels according to described methods in ref. 1. These spermatozoa were then suspended in solubilization buffer (10 mM Tris·HCl, pH 7.8, 0.15 M NaCl, 30 mM n-octyl-b-d-thioglucopyranoside, 1 mM PMSF, and 2 mM EDTA), shaken for 20 min at 4 °C, and then centrifuged at 100,000 × g for 1 h at 4 °C. The resultant supernatants containing solubilized sperm were used as the sperm membrane fractions. SDS/PAGE with gelatin was then performed as described by Gordon and Lilly (30). Gelatin was added to the separating gel to a final concentration of 0.2% (wt/vol). The samples were mixed with an equal volume of sample buffer [125 mM Tris·HCl, 4% (wt/vol) SDS, 20% (vol/vol) glycerol, and 0.05% (wt/vol) bromophenol blue]. After electrophoresis, the gels were equilibrated in 10 mM Tris·HCl, pH 8.0, containing 2.5% (vol/vol) Triton X-100 for 1 h. After washing with 10 mM Tris·HCl, pH 8.0, the gels were incubated with 10 mM Tris·HCl pH 8.0 for 24 h at 37 °C, stained with Coomassie brilliant blue and destained.
Sperm Incubation and Artificial Insemination of Japanese Eel.
Male and female Japanese eels were artificially induced to reach maturity using hormonal treatments (31, 32). Ejaculated milt from five individual male eels was diluted (1:10,000) with artificial seminal plasma for the Japanese eel (33). The diluted milt was then incubated at 4 °C for 2 h with various concentrations of either PMSF (1, 10, and 100 μM), AEBSF (1, 10, and 100 μM) or anti-eel trypsinogen antibody (0.5, 5, and 50 μg/mL). the sperm remain immobilized under these conditions. The fertilization test was then performed as follows. Five hundred micrograms of ovulated eggs were inseminated with 500 μL diluted semen. From 3–4 h after fertilization, the fertilization rate was estimated by counting eggs displaying cleavage under a binocular microscope.
Statistics.
Results are expressed as the means ± SEM. Data analysis was carried out using the Sceirer, Ray, and Hara extension of the Kruskal-Wallis test (a two-way ANOVA design for ranked data), followed by a post-hoc Bonferroni adjustment.
Supplementary Material
Acknowledgments.
We thank Dr. R. W. Schulz (Utrecht University, The Netherlands) for his valuable comments on the manuscript. This work was supported by grants-in-aid for scientific research and for Fellows from the Japan Society for the Promotion of Science, the Global Center of Excellence program, and the Ministry of Education, Culture, Sports, Science, and Technology of the Japanese Government.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907631106/DCSupplemental.
References
- 1.Miura T, Yamauchi K, Nagahama Y, Takahashi H. Induction of spermatogenesis in male Japanese eel, Anguilla japonica, by a single injection of human chorionic gonadotropin. Zool Sci. 1991;8:63–73. [Google Scholar]
- 2.Miura T, Yamauchi K, Takahashi H, Nagahama Y. Human chorionic gonadotropin induced all stages of spermatogenesis in vitro in the male Japanese eel (Anuilla japonica) Dev Biol. 1991;146:258–262. doi: 10.1016/0012-1606(91)90468-i. [DOI] [PubMed] [Google Scholar]
- 3.Miura T, Yamauchi K, Takahashi H, Nagahama Y. Hormonal induction of all stages of spermatogenesis in vitro in the male Japanese eel (Anguilla japonica) Proc Natl Acad Sci USA. 1991;88:5774–5778. doi: 10.1073/pnas.88.13.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miura C, Miura T, Yamashita M, Yamauchi K, Nagahama Y. Hormonal induction of all stages of spermatogenesis in germ-somatic cell coculture from immature Japanese eel testis. Dev Growth Differ. 1996;38:257–262. doi: 10.1046/j.1440-169X.1996.t01-2-00004.x. [DOI] [PubMed] [Google Scholar]
- 5.Miura T, et al. Estradiol-17β stimulated the renewal of spermatogonial stem cell in males. Biochem Biophys Res Commun. 1999;264:230–234. doi: 10.1006/bbrc.1999.1494. [DOI] [PubMed] [Google Scholar]
- 6.Miura T, Higuchi M, Ozaki Y, Ohta T, Miura C. Progestin is an essential factor for the initiation of the meiosis in spermatogenic cell of the eel. Proc Natl Acad Sci USA. 2006;103:7333–7338. doi: 10.1073/pnas.0508419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miura T, Miura C, Konda Y, Yamauchi K. Spermatogenesis-preventing substance in Japanese eel. Development. 2002;129:2689–2697. doi: 10.1242/dev.129.11.2689. [DOI] [PubMed] [Google Scholar]
- 8.Odet F, Verot A, Le Magueresse-Battistoni B. The mouse testis is the source of various serine proteases and serine proteinase inhibitors (SERPINs): Serine proteases and SERPINs identified in Leydig cells are under gonadotropin regulation. Endocrinology. 2006;147:4374–4383. doi: 10.1210/en.2006-0484. [DOI] [PubMed] [Google Scholar]
- 9.Ogiwara K, Takahashi T. Specificity of the medaka enteropeptidase serine protease and its usefulness as a biotechnological tool for fusion-protein cleavage. Proc Natl Acad Sci USA. 2007;104:7021–7026. doi: 10.1073/pnas.0610447104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyata S, et al. Expression of trypsin in human cancer cell lines and cancer tissues and its tight binding to soluble form of Alzheimer amyloid precursor protein in culture. J Biochem. 1999;125:1067–1076. doi: 10.1093/oxfordjournals.jbchem.a022388. [DOI] [PubMed] [Google Scholar]
- 11.Soreide K, Janssen EA, Komer H, Baak JPA. Trypsin in colorectal cancer : Molecular biological mechanisms of proliferation, invasion, and metastasis. J Pathol. 2006;209:147–156. doi: 10.1002/path.1999. [DOI] [PubMed] [Google Scholar]
- 12.Darmoul D, Gratio V, Devaud H, Laburthe M. Protease-activated receptor 2 in colon cancer: Trypsin-induced MAPK phosphorylation and cell proliferation are mediated by epidermal growth factor receptor transactivation. J Biol Chem. 2004;279:20927–20934. doi: 10.1074/jbc.M401430200. [DOI] [PubMed] [Google Scholar]
- 13.Cheng K, Lamer J. Intracellular mediators of insulin action. Ann Rev Physiol. 1985;47:405–424. doi: 10.1146/annurev.ph.47.030185.002201. [DOI] [PubMed] [Google Scholar]
- 14.Hescheler J, Trautwein W. Modification of L-type calcium current by intracellularly applied trypsin in guinea-pig ventricular myocytes. J Physiol. 1988;404:259–274. doi: 10.1113/jphysiol.1988.sp017289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.You Y, Pelzer DJ, Pelzer S. Trypsin and forskolin decrease the sensitivity of L-type calcium current to inhibition by cytoplasmic free calcium in guinea pig heart muscle cells. Biophys J. 1995;69:1838–1846. doi: 10.1016/S0006-3495(95)80054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miura T, Ohta T, Miura C, Yamauchi K. Complementary deoxyribonucleic acid cloning of spermatogonial stem cell renewal factor. Endocrinology. 2003;144:5504–5510. doi: 10.1210/en.2003-0800. [DOI] [PubMed] [Google Scholar]
- 17.Courot M, Reviers M-TH, Ortavant R. In: The Testis. Johnson AD, editor. New York: Academic; 1970. pp. 339–432. [Google Scholar]
- 18.Miura T, Kawamura S, Miura C, Yamauchi K. Impaired spermatogenesis in the Japanese eel, Anguilla japonica: Possibility for the existence of factors that regulate entry of germ cells into meiosis. Dev Growth Differ. 1997;39:685–691. doi: 10.1046/j.1440-169x.1997.t01-5-00004.x. [DOI] [PubMed] [Google Scholar]
- 19.Keeney S, Giroux CN, Kleckner N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 1997;88:375–384. doi: 10.1016/s0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- 20.Ozaki Y, Miura C, Miura T. Molecular cloning and gene expression of Spo11 and Dmc1 during spermatogenesis in the Japanese eel, Anguilla japonica. Comp Biochem Physiol B Biochem Mol Biol. 2006;143:309–314. doi: 10.1016/j.cbpb.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Todd PR. Ultrastructure of spermatozoa and spermiogenesis in New Zealand freshwater eels (Anguillidae) Cell Tissue Res. 1976;171:221–232. doi: 10.1007/BF00219407. [DOI] [PubMed] [Google Scholar]
- 22.Rattner JB, Brinkley BR. Ultrastructure of mammalian spermiogenesis III. the organization and morphogenesis of the manchette during rodent spermiogenesis. J Ultrastruct Res. 1972;41:209–218. doi: 10.1016/s0022-5320(72)90065-2. [DOI] [PubMed] [Google Scholar]
- 23.Clermont Y, Oko R, Hermo L. In: Cell and Molecular Biology of the Testis. Desjardins C, Ewing L L, editors. New York: Oxford Univ Press; 1993. pp. 332–376. [Google Scholar]
- 24.Hechet NB, et al. Localization of a highly divergent mammalian testicular alpha tubulin that is not detectable in brain. Mole Cell Biol. 1988;8:996–1000. doi: 10.1128/mcb.8.2.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grier HJ. Cellular organization of the testis and spermatogenesis in fishes. Am Zool. 1981;21:345–357. [Google Scholar]
- 26.Cherr GN, Clark W H., Jr An acrosome reaction in sperm from white sturgeon, Acipenser transmontanus. J Exp Zool. 1984;232:129–139. [Google Scholar]
- 27.Wong GW, et al. Tryptase 4, a new member of the chromosome 17 family of mouse serine proteases. J Biol Chem. 2001;276:20648–20658. doi: 10.1074/jbc.M010422200. [DOI] [PubMed] [Google Scholar]
- 28.Scarman AL, et al. Organization and chromosomal localization of the murine Testisin gene encoding a serine protease temporally expressed during spermatogenesis. Eur J Biochem. 2001;268:1250–1258. doi: 10.1046/j.1432-1327.2001.01986.x. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura Y, et al. Cloning, expression analysis, and tissue distribution of esp-1/testisin, a membrane-type serine protease from the rat. J Med Invest. 2003;50:78–86. [PubMed] [Google Scholar]
- 30.Gordon LJ, Lilly WW. Quantitative analysis of Schizophyllum commune metalloprotease ScPrB activity in SDS-gelatin PAGE reveals differential mycelial localization of nitrogen limitation-induced autolysis. Curr Microbiol. 1995;30:337–343. [Google Scholar]
- 31.Kagawa H. In: Eel Biology. Aida K, Tsukamoto K, Yamauchi K, editors. Tokyo: Springer-Verlag; 2003. pp. 401–414. [Google Scholar]
- 32.Ohta H, Unuma T. In: Eel Biology. Aida K, Tsukamoto K, Yamauchi K, editors. Tokyo: Springer-Verlag; 1993. pp. 415–423. [Google Scholar]
- 33.Miura T, Kasugai T, Nagahama Y, Yamauchi K. Acquisition of potential for sperm motility in vitro in Japanese eel (Anguilla japonica) Fisheries Sci. 1995;61:533–534. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.