Abstract
Vesicle-associated-membrane protein 8 (VAMP8) is highly expressed in the kidney, but the exact physiological and molecular functions executed by this v-SNARE protein in nephrons remain elusive. Here, we show that the depletion of VAMP8 in mice resulted in hydronephrosis. Furthermore, the level of the vasopressin-responsive water channel aquaporin 2 (AQP2) was increased by three- to fivefold in VAMP8-null mice. Forskolin and [desamino-Cys1, D-Arg8]-vasopressin (DDAVP)-induced AQP2 exocytosis was impaired in VAMP8-null collecting duct cells. VAMP8 was revealed to colocalize with AQP2 on intracellular vesicles and to interact with the plasma membrane t-SNARE proteins syntaxin4 and syntaxin3, suggesting that VAMP8 mediates the regulated fusion of AQP2-positive vesicles with the plasma membrane.
Aquaporins are water channels that can facilitate the movement of water through membranes. They play a crucial role in maintaining body water homeostasis (22, 23). Of the known aquaporins, aquaporin 2 (AQP2) is the major, if not the only, vasopressin-responsive aquaporin. Under physiological conditions, AQP2 exists in a dynamic equilibrium between the plasma membrane and intracellular vesicles. The binding of vasopressin to the G-protein-coupled V2 receptor induces a series of coordinated signaling events in collecting duct cells that eventually lead to elevated levels of surface AQP2 and, consequently, increased water reabsorption. The signaling events include, but may not be limited to, the activation of adenylate cyclase, which elevates the cyclic AMP (cAMP) level, the phosphorylation of AQP2 at S256 by protein kinase A (PKA), the reorganization of the cytoskeleton, and a transient increase in free intracellular Ca2+. In addition to rapidly deploying AQP2 to the plasma membrane from intracellular vesicles, vasopressin can execute a long-term effect on AQP2 by upregulating its gene expression level (7, 22).
The regulated exocytosis of AQP2 ultimately requires the fusion of AQP2-containing vesicles with the plasma membrane, a process known to be driven by SNARE proteins (12, 14). Several SNARE proteins have been identified in kidney collecting duct cells. These include syntaxin2, syntaxin3, and syntaxin4 on the plasma membrane (15), VAMP2 and VAMP3 on intracellular vesicles (5, 20), and SNAP23 on both intracellular vesicles and the plasma membrane (13). Although proposed long ago, and there are some functional studies with cultured cells (10, 24), the roles of these proteins in AQP2 exocytosis have yet to be clarified and confirmed in animal models.
It has been known for more than a decade that the tissue that expresses the most VAMP8 is the kidney (1), but the exact role of VAMP8 in this organ remains elusive. Although initially identified as the endosomal SNARE protein endobrevin (1, 3, 37), VAMP8 recently has been shown to play a major role in the regulated secretory pathway of the exocrine system (8, 34, 35), platelets (26), basophilic cells (16), and mast cells (25, 28, 33). In this study, we show that VAMP8 is present on intracellular AQP2-containing vesicles and appears to play an important role in AQP2 exocytosis.
MATERIALS AND METHODS
Antibodies.
The VAMP8 polyclonal antibody was raised in rabbits with the N-terminal domain of human VAMP8 as the antigen (37). The rabbit anti-syntaxin3 antibody was a gift from Ulrich Blank. The chicken anti-AQP2 antibody was a gift from Mark A. Knepper (5). Other antibodies were purchased from commercial suppliers: monoclonal antibodies against syntaxin8, Vti1a, and Vti1b were from BD Transduction Laboratories; rabbit anti-syntaxin2, anti-syntaxin4, anti-syntaxin13, and anti-SNAP23 were from Synaptic Systems; rabbit anti-AQP1 to anti-AQP9 were from Alpha Diagnostic International, Inc.; rabbit polyclonal antibodies to VAMP2 and VAMP3 were from Abcam; and monoclonal antibody to β-actin was from Sigma Aldrich. The rabbit anti-AQP2 antibody used for Western blotting was from US Biological.
Mice.
The VAMP8 knockout mice have been described previously (34). In this study, we crossed the original VAMP8 knockout mouse line with the cre transgenic strain (29) to remove the neomycin resistance cassette. The neo-deleted VAMP8 knockout line then was bred onto a 129/SvJ background or a C57BL/6 background for five generations. Sex- and age-matched 3- to 5-month-old adult mice were used for experiments. The mouse lines were maintained in a specific-pathogen-free facility. Mice were fed a normal rodent diet or, as otherwise stated, a low-sodium diet (Glen Forest Stockfeeders, Australia).
Histological analysis and immunofluorescence microscopy.
Kidneys were fixed with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) and embedded in paraffin. Sections were stained with hematoxylin and eosin. For immunofluorescence studies, moderately fixed (2% PFA for 30 to 40 min at room temperature) kidneys were embedded in O.C.T. compound (Sakura Finetek). Cryosections were mounted on polylysine-coated slides and stained with primary antibodies and then appropriate fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories). Nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI). In the study of AQP2 translocations in vivo, kidneys were fixed with 4% periodate-lysine paraformaldehyde (PLP) for 3 h at room temperature. Cultured primary cells usually were fixed with 2% PFA for 20 min on ice or with 4% PLP (19) for 30 min at room temperature and then were subjected to immunofluorescence staining.
Immunoelectron microscopy.
The immunogold labeling of AQP2 was carried out according to a protocol that was described previously by others (21).
Immunoblot analysis.
Fresh tissues were homogenized in a modified radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM EDTA, 2 mM dithiothreitol [DTT], 1% deoxycholic acid, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], 1 mM phenylmethylsulfonyl fluoride [PMSF], and complete proteinase inhibitors; Roche Diagnostics). Proteins were transferred onto Hybond-C extramembranes (Amersham) after being separated by SDS-polyacrylamide gels. Protein identities were determined with specific primary antibodies and appropriate peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories). Peroxidase activity was detected with the SuperSignal substrate and enhancer (Pierce) and visualized on X-ray films. Quantitative analysis was carried out with a GS-800 calibrated densitometer from Bio-Rad.
Determination of serum aldosterone levels and plasma rennin activities.
Aldosterone concentrations and renin activities were determined by radioimmunoassay with the COAT-A-COUNT aldosterone kit from Diagnostic Products Corporation (Los Angeles, CA) and the angiotensin I [125I] radioimmunoassay kit from PerkinElmer Life Sciences, Inc. (Boston, MA), respectively. All procedures were carried out according to the manufacturer's instructions.
Urine chemistry.
Samples of urine that had collected in the kidneys of mice for 24 h (designated 24-h urine samples) were taken from 3-month-old female mice with metabolism cages from Tecniplast. To minimize individual variations, urine was collected from groups of six female mice. Total urine protein was detected with the Bio-Rad Protein Assay reagent. Glucose levels were measured with a SmartScan glucose meter (LifeScan, Inc., Milpitas, CA). Other urinary constituents were analyzed at the Department of Laboratory Medicine, National University of Singapore. In the studies of the effect of [desamino-Cys1, d-Arg8]-vasopressin (DDAVP) on urine osmolality, urine was collected by bladder massage. We noticed that there was about 40% difference in osmolality between the 24-h urine sample collected with a cage and the manually collected urine. We believed that this difference resulted from evaporation.
Isolation and culture of mouse collecting duct cells.
We developed a new method for purifying and culturing mouse kidney collecting duct cells based on previously described methods (11, 17, 30). Mouse kidneys were minced into small pieces with a pair of fine scissors. The tissue fragments were washed three times with PBS before being digested at 37°C for 90 to 120 min with 0.2% hyaluronidase (H3884; Sigma) and 0.2% collagenase P (Roche Applied Science) in hypertonic PBS supplemented with 1 mM CaCl2, 1 mM MgCl2, 11 mM glucose, and 150 mM NaCl (∼1 ml of solution per kidney). Cells were dispersed by vigorous pipetting in the middle and at the end of the digestion. The suspension then was diluted five times with PBS and spun for 5 min at 400 rpm (25 × g) on a GS-6R Beckman centrifuge. The pellet was resuspended and centrifuged two more times at 200 rpm (6 × g) for 5 min. The pellet was resuspended and loaded on top of a layer of 20% Percoll (P1644; Sigma) that then was laid on a layer of 35% Percoll. After a spinning at 600 × g (2,000 rpm) for 15 min, a band at the 20 to 35% Percoll interface was recovered and washed with PBS. The purified collecting duct cells were cultured in hypertonic Dulbecco's modified Eagle's medium (DMEM) (2 to 3 ml per kidney) that was supplemented with 2 mM l-glutamine, 1% nonessential amino acids, 5% fetal calf serum, and appropriate antibiotics. The osmolality of the DMEM was adjusted to 600 mOsm with an additional 100 mM NaCl and 100 mM urea. For monitoring AQP2 trafficking, isolated collecting duct cells were grown in hypertonic DMEM for three nights on tissue culture-treated transwell filters with 0.4-μm pores (Costar). The cells then were incubated in 50% DMEM and 50% Ham's F-12 without serum or other supplements for 6 h before being stimulated with 10 μM forskolin in prewarmed 50% DMEM and Ham's F-12. Mouse collecting duct cells are different from rat collecting duct cells in two aspects: (i) dibutyryl cAMP has no effect on AQP2 expression in mouse cells, in which a high osmolality (600 mOsm) is crucial for attenuating the downregulation of AQP2, and (ii) mouse cells grown on coverslips rather than on filters cannot be stimulated by forskolin or DDAVP.
RT-PCR.
For reverse transcription-PCR (RT-PCR), total RNA was extracted from freshly isolated collecting duct cells with the RNeasy midi kit (Qiagen). cDNA was synthesized with an AMV reverse transcriptase form Promega, using oligo(dT)15 as the primer. PCR was performed with the HotStarTaq master mix kit from Qiagen. Primers for individual genes were the following: AQP2 forward (5′-ATGAATCCAGCCCGCTCCCTG-3′), AQP2 reverse (5′-GCAGGCTCTGCGGAGAGTGCA-3′), VAMP2 forward (5′-GTGGTGGACATCATGAGGGTGAA-3′), VAMP2 reverse (5′-TGGGGGAGAGGCCCTTCTTAGG-3′), VAMP3 forward (5′-TAGGGATCAGTGTCCTGGTGATC-3′), VAMP3 reverse (5′-AAGGCACTGTGCAAAATGTGGGG-3′), VAMP8 forward (5′-CCAGTGGGAGTGCCGGAAATGA-3′), VAMP8 reverse (5′-GAGAGGGCTCCTCTTGGCACAT-3′), GAPDH forward (5′-TCACTGGCATGGCCTTCCGTGT-3′), and GAPDH reverse (5′-ATGAGGTCCACCACCCTGTTGC-3′). The cDNA templates were normalized with GAPDH and diluted in a fivefold series. Reactions without reverse transcriptase were used as negative controls.
Surface biotinylation.
Biotinylation was carried out according to protocols described by others (6) and provided by the reagent manufacture (Pierce), with some modifications. All reactions were carried out in PBS. Collecting duct cells were cultured on 6-well Costar filters in hypertonic DMEM for 2 days, transferred to serum-free 50% DMEM plus 50% Ham's F-12, and incubated for 6 to 8 h before being stimulated with 10 μM forskolin in prewarmed serum-free 50% DMEM and 50% Ham's F-12 for 15 min at 37°C. Cells were washed thoroughly with ice-cold PBS before being subjected to oxidation with 10 mM sodium meta-periodate for 30 min on ice. Following three rounds of washing, cell surface glycoproteins were labeled with 5 mM biocytin hydrazide for 30 min on ice. After the biotinylation reagent was washed away, the cells were lysed with 1% Triton X-100 and 0.01% SDS in PBS supplemented with 1 mM PMSF and 10 μg/ml each of leupeptin, chymostatin, and pepstatin. Biotinylated proteins were pulled down with immobilized streptavidin (Pierce) and eluted with SDS gel-loading buffer. The same number of wild-type and VAMP8-null cells were seeded, and the same amount of total lysate protein was used as the input for normal and knockout mice.
In vivo AQP2 translocation.
Forskolin was dissolved in dimethylsulfoxide to 10 mM as a stock solution, which was diluted 10 times in PBS immediately before injection. The diluted forskolin was administrated to 3-month-old mice intravenously at 100 μl per 20 g of body weight. All mice were sacrificed at 15 min after injection. Alternatively, mice were injected with DDAVP at 1 μg per kg and were sacrificed at 30 min after injection. Kidneys were fixed with 4% PLP at room temperature for 3 h before being processed for immunofluorescence staining for AQP2.
GST pulldown analysis.
Glutathione S-transferase (GST) pulldown was performed as previously described (34, 35), except that 1 mM DTT in binding buffer and extraction buffer was replaced with 1 mM N-ethylmaleimide to stabilize SNARE complexes. Proteins were eluted with SDS gel-loading buffer and were identified by Western blot analysis with specific antibodies.
RESULTS
Hydronephrosis in VAMP8-null mice.
Our previous studies show that VAMP8 is required for the regulated exocytosis of the exocrine pancreas and the salivary glands (8, 34, 35). To find out if the impaired secretion of digestive enzymes affects the efficiency of food digestion, we monitored the food consumption of VAMP8 knockout mice and their wild-type littermates. As shown in Table 1, VAMP8-deficient mice ate amounts of food that were similar to, if not slightly more than, that of their normal littermates, although they generally were smaller than their counterparts (34), suggesting that they do not use food as efficiently as their littermates.
TABLE 1.
Food consumption and water intake of adult micea
| Consumption | Male |
Female |
||
|---|---|---|---|---|
| +/+ | −/− | +/+ | −/− | |
| Food (g/mouse/day) | 4.1 ± 0.6 | 4.6 ± 0.8 | 2.5 ± 0.4 | 2.7 ± 0.4 |
| Water (g/mouse/day) | 3.2 ± 0.4 | 6.6 ± 0.3* | 2.2 ± 0.3 | 4.0 ± 0.2* |
Mice were 3 to 5 months old. n > 5. Data shown are means ± standard errors of the means. +/+, wild type; −/−, VAMP8 null. *, P < 0.01.
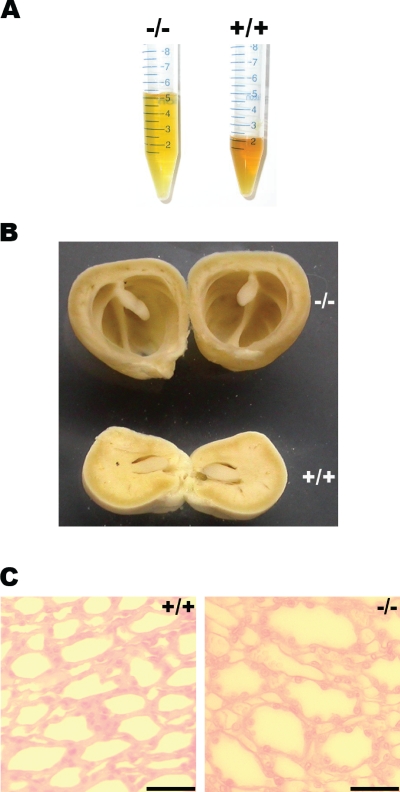
Surprisingly, VAMP8-null mice drank twice as much water as wild-type mice (Table 1), suggesting a water-wasting phenotype in the mutant mice. Consistent with this assumption, the mutant mice produced diluted urine. As shown in Fig. 1A, the urine color of VAMP8-null mice was much lighter than that of normal urine. Clinical chemistry analysis showed that mutant urine was about onefold diluted in terms of ion concentrations and osmolality compared to the wild type (Table 2). Kidney dilation and medulla atrophy were observed in adult VAMP8-deficient mice (Fig. 1B), although the severity of this phenotype varied dramatically with genetic backgrounds: while overt renal degeneration was detected in about one-third of mutant mice on a mixed 129/Sv and C57BL/6 background, less than 10% exhibited moderate atrophy on a pure 129/Sv background. VAMP8-null mice could hardly survive beyond weaning on a pure C57BL/6 background. The few (four) mice that did survive to adulthood on the C57BL/6 background developed severe renal degeneration and died within 10 months of age. Histological analysis revealed the dilation of collecting ducts in papilla (Fig. 1C) in VAMP8-null mice suffering from severe hydronephrosis. To minimize individual and strain variations, we focused our subsequent studies on mice with a pure 129/Sv background. All data presented in this paper, except that in Table 1 and Fig. 1B and C, which were derived from a 129/Sv and C57BL/6 mixed background, pertained to the studies using mice of a pure 129/Sv background.
FIG. 1.
Hydronephrosis in VAMP8-null mice. (A) Twenty-four-hour urine samples from six wild-type (+/+) or six knockout (−/−) female mice. (B) Overview of kidneys that had been fixed with PFA and cross-cut through the middle. +/+, wild type; −/−, VAMP8 null. (C) Hematoxylin- and eosin-stained sections of kidney papilla showing dilation of collecting ducts in VAMP8 knockout mice. Scale bar, 50 μm. +/+, wild type; −/−, VAMP8 null.
TABLE 2.
Urine chemistrya
| Measurement | Mouse type |
|
|---|---|---|
| Wild type | VAMP8 null | |
| Volume (ml) | 2.3 ± 0.2 | 5.4 ± 0.3 |
| Osmolality (mOsm) | 2,804 ± 51 | 1,526 ± 82 |
| Glucose (mg/dl) | 305 ± 57 | 152 ± 12 |
| Protein (mg/ml) | 1.27 ± 0.10 | 0.81 ± 0.06 |
| Creatinine (mM) | 4.2 ± 0.4 | 1.8 ± 0.4 |
| Chloride (mM) | 253 ± 11 | 120 ± 5 |
| Sodium (mM) | 215 ± 32 | 81 ± 13 |
| Potassium (mM) | 291 ± 15 | 139 ± 9 |
| Calcium (mM) | 2.3 ± 0.9 | 0.9 ± 0.3 |
Urine was collected for 24 h from groups of six 3-month-old female mice. Data shown are means ± standard errors of the means. A total of six groups of mice of each genotype were used. P < 0.01 for all the parameters analyzed.
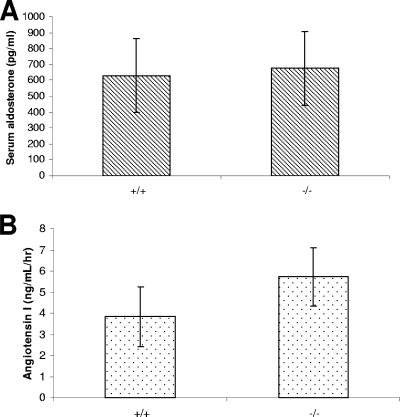
Although polyuria and hydronephrosis can result from defective salt reabsorption (32), this seems unlikely, since the daily ion excretion was similar between normal and mutant mice (Table 2) and the serum osmolality of mutant mice was slightly higher than that of normal mice (330 ± 6 and 322 ± 5 mOsm, respectively; n = 10; P = 0.003). Consistently, the serum aldosterone level of mutant mice was normal (Fig. 2A). Furthermore, feeding the mutant mice a sodium-depleted diet for more than 3 months did not aggravate the phenotype in terms of kidney histology (data not shown). On the contrary, plasma renin activity was elevated by about 50% in VAMP8-null mice (Fig. 2B), which is in line with a water-wasting defect.
FIG. 2.
Serum aldosterone and plasma renin activity. (A) Serum aldosterone levels of adult mice were determined by radioimmunoassay. The values for normal and VAMP8-null mice were 630 ± 232 and 677 ± 232 pg/ml, respectively (n = 6; P = 0.735). (B) Plasma renin activity. The angiotensin I-generating rates for wild-type and VAMP8 knockout mice were 3.84 ± 1.42 and 5.72 ± 1.38 ng/ml/h, respectively (n = 8; P = 0.018 [<0.05]).
Taken together, the data suggest that VAMP8 knockout mice suffer primarily from a water reabsorption defect.
Upregulation of AQP2 in VAMP8 knockout mice.
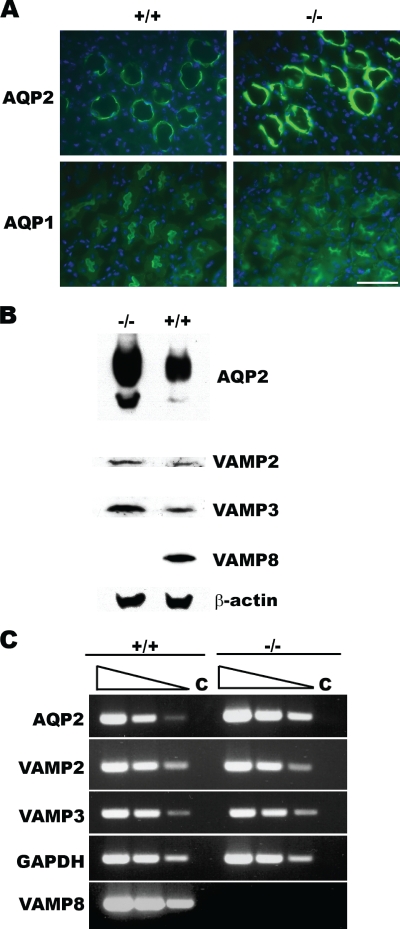
Aquaporins are water channels that have been shown to play a critical role in the rapid transportation of water through the plasma membrane (22, 23). To test if aquaporins were correctly targeted in VAMP8-null kidneys, we screened the expression patterns of nine aquaporins by immunofluorescence staining. While no difference was observed between normal and mutant mice for most aquaporins, AQP2 was significantly increased in VAMP8-null kidney collecting duct cells (Fig. 3A). The upregulation of AQP2 was confirmed by Western blot analysis of freshly isolated collecting duct cells (Fig. 3B). Quantitative analysis with a photodensitometer revealed a three- to fivefold increase of total AQP2 in VAMP8-null cells (4.1 ± 1.2 and 1 U, respectively; n = 4; P < 0.01). Levels of VAMP2 and VAMP3, which are associated with the increase of AQP2, were also increased. Since both VAMP2 and VAMP3 have been shown to be present on AQP2-containing vesicles (5, 20), it is possible that VAMP8-null collecting duct cells accumulate AQP2-positive vesicles. Semiquantitative RT-PCR analysis showed that the level of AQP2 transcripts in VAMP8-null cells was about three to five times higher than those of normal cells. In contrast, VAMP2 and VAMP3 transcripts were not significantly increased in mutant cells, suggesting that, unlike AQP2, these VAMP genes are not upregulated at the transcription level (Fig. 3C).
FIG. 3.
AQP2 was upregulated in VAMP8 knockout mice. (A) Immunofluorescent staining for AQP2 and AQP1 in normal (+/+) and VAMP8-null (−/−) kidney sections. AQP1 was detected at the apical area of proximal tubes, while AQP2 was present in collecting duct cells. Scale bar, 50 μm. (B) Western blot analysis of AQP2 in isolated collecting duct cells. AQP2 and the SNARE proteins VAMP2 and VAMP3 were increased in VAMP8 knockout cells (−/−). (C) Semiquantitative analysis of gene transcription in isolated collecting duct cells by RT-PCR. Total RNA samples extracted from isolated collected duct cells were analyzed by RT-PCR using gene-specific primers. cDNA templates generated by reverse transcription were diluted in a fivefold series (indicated by wedges). Reactions without reverse transcriptase were used as controls (C). +/+, wild type; −/−, VAMP8 null.
Colocalization of VAMP8 and AQP2 on intracellular vesicles.
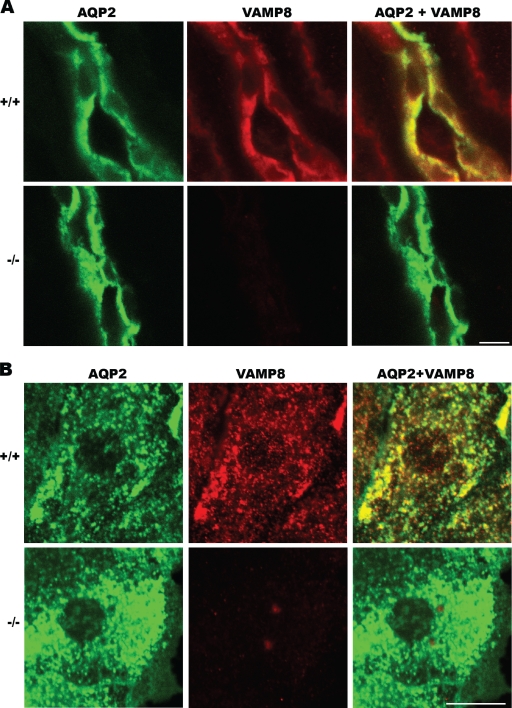
The role of AQP2 in the kidney has been well defined. The increased total amount and surface expression of AQP2 are known to cause water retention under conditions such as pregnancy and congestive heart failure (22). In the VAMP8-null mice, however, the upregulation of AQP2 was associated with water wasting, suggesting that AQP2 exocytosis is impaired in these mice, and the increased mRNA and protein expression is a consequence of a feedback mechanism. This point is underpinned by a similar phenotype in mice bearing an S256L mutation of AQP2 (18), which is known to affect AQP2's phosphorylation and surface expression. It then was critical to determine the subcellular localization of VAMP8 in kidney collecting duct cells to understand its potential role in AQP2 trafficking. Immunofluorescence studies showed that a significant fraction of VAMP8 colocalized with AQP2 on intracellular vesicles (Fig. 4). We suspected that these vesicles were AQP2 storage vesicles, the counterpart of secretory granules in exocrine acinar cells (34, 35).
FIG. 4.
Immunofluorescent staining showing colocalization of AQP2 and VAMP8 in cultured collecting duct cells. (A) Kidney sections from wild-type (+/+) and VAMP8-null (−/−) mice were stained for AQP2 (green) and VAMP8 (red), showing the colocalization of both proteins in collecting duct principal cells. Scale bar, 10 μm. (B) Primary collecting duct cells were isolated from normal (+/+) and VAMP8 knockout (−/−) kidneys and cultured in vitro for 3 days and then subjected to immunofluorescent staining for AQP2 (green) and VAMP8 (red). Scale bar, 10 μm.
VAMP8 was required for exocytosis of AQP2 in vivo.
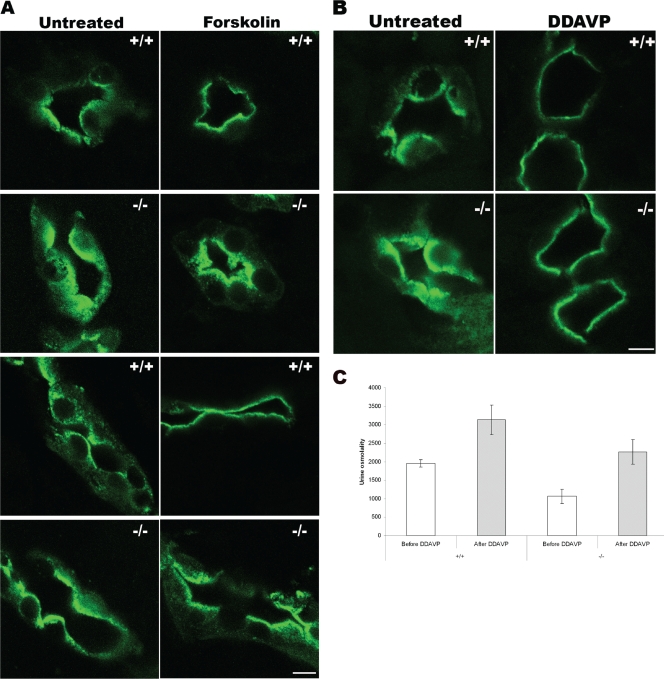
To test if VAMP8 played a role in AQP2 trafficking, we administered forskolin, an adenylyl cyclase activator, to mice by intravenous injection. As shown in Fig. 5A, at 15 min after injection, almost all AQP2 molecules were mobilized to the apical plasma membrane in normal mice. In VAMP8 knockout mice, however, most AQP2 molecules were apparently packed into a wide band underneath the apical plasma membrane rather than inserted into the plasma membrane, indicating a defect at the very last step(s) of AQP2 exocytosis.
FIG. 5.
In vivo AQP2 trafficking. (A) Forskolin-stimulated AQP2 trafficking. Mice were left untreated or were injected with forskolin and sacrificed at 15 min after injection. AQP2 distribution was revealed by immunofluorescent staining. Cross (top)- and longitudinal (bottom) sections of collecting ducts are shown. Scale bar, 10 μm; +/+, wild type; −/−, VAMP8 null. (B) DDAVP-stimulated translocation of AQP2. Mice were left untreated or were injected with DDAVP and sacrificed at 30 min after injection. +/+, wild type; −/−, VAMP8 null; scale bar, 10 μm. (C) Effect of DDAVP on urine osmolality. Urine osmolality of five wild-type (+/+) or VAMP8 knockout (−/−) female mice was determined before and at 2 h after DDAVP injection. Data shown are means ± standard deviations. The values for wild-type mice were 1,955 ± 102 mOsm before and 3,134 ± 396 mOsm after DDAVP treatment. These values for knockout mice were 1,066 ± 187 and 2,262 ± 331 mOsm, respectively.
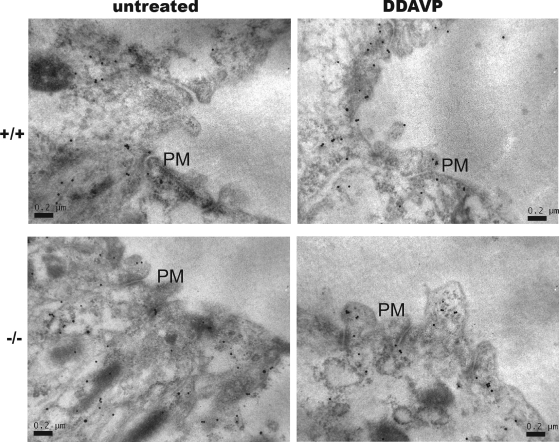
DDAVP is an analog of vasopressin and a strong agonist for the V2 vasopressin receptor. It has been shown to be able to acutely regulate the water permeability of collecting ducts by rapidly mobilizing AQP2 to the plasma membrane from intracellular vesicles. As shown in Fig. 5B, at 30 min after the injection of DDAVP into normal mice, all AQP2 molecules were at the plasma membrane. Surprisingly, VAMP8-null mice showed a very similar response to this drug, except that the AQP2 band was wider than that in normal mice. Since VAMP8-null cells expressed three to five times more AQP2, one might argue that the wider band was a result of more AQP2 molecules. We then decided to address this issue directly using immunoelectron microscopy. As shown in Fig. 6, DDAVP stimulated the mobilization of AQP2 from the cytoplasm toward the apical plasma membrane in both wild-type and knockout mice. While many AQP2 molecules had been inserted into the plasma membrane in normal cells, most AQP2 vesicles in VAMP8-null cells appeared to have accumulated underneath the plasma membrane but failed to fuse with the plasma membrane. This result demonstrates directly that VAMP8 is required for AQP2 exocytosis at the very last step(s).
FIG. 6.
Immunoelectron microscopy study of AQP2 trafficking in vivo. Wild-type (+/+) and VAMP8-null (−/−) mice were left untreated or were injected with DDAVP at 1 ng/kg. Mice were sacrificed at 30 min after DDAVP stimulation, and kidneys were perfusion fixed with 2% PLP for 30 min before being subjected to immunogold labeling for the detection of AQP2. Scale bar, 200 nm. PM, plasma membrane.
We also attempted to determine DDAVP's effects on urine osmolality. As shown in Fig. 5C, the urine osmolality of normal mice was increased from 1,955 ± 102 mOsm to 3,134 ± 396 mOsm at 2 h after DDAVP injection. In VAMP8 knockout mice, DDAVP treatment also caused an increase in urine osmolality (from 1,066 ± 187 to 2,262 ± 331 mOsm), suggesting that the ablation of VAMP8 did not completely block vasopressin-induced AQP2 exocytosis. However, in the presence of DDAVP, the urine osmolality of knockout mice only reached a level close to that of normal mice before DDAVP stimulation, indicating that the majority of AQP2 molecules in VAMP8-null cells were not inserted into the plasma membrane, although they had been mobilized to the apical region immediately underneath the plasma membrane by DDAVP. If urine osmolality is a functional reflection of cell surface AQP2 density, in the context that the total AQP2 level was at least three times higher in VAMP8-null mice than in normal mice, it is reasonable to claim that AQP2 surface deployment was compromised in VAMP8 knockout mice under both physiological and DDAVP treatment conditions, although DDAVP still was able to induce significant AQP2 exocytosis by a VAMP8-independent mechanism. It is likely that VAMP2 and/or VAMP3 partially compensated for the absence of VAMP8. Although DDAVP could rescue the urine-concentrating defect in VAMP8-null mice, the defect was not a consequence of endogenous vasopressin deficiency. Actually, the plasma vasopressin level was approximately doubled in knockout mice (167 ± 20 pg/ml for the knockout and 74 ± 6 pg/ml for the wild type; n = 6; P < 0.01). The elevation of vasopressin probably resulted from a feedback via the renin-angiotensin-vasopressin axis (38).
VAMP8 deficiency impaired AQP2 exocytosis in vitro.
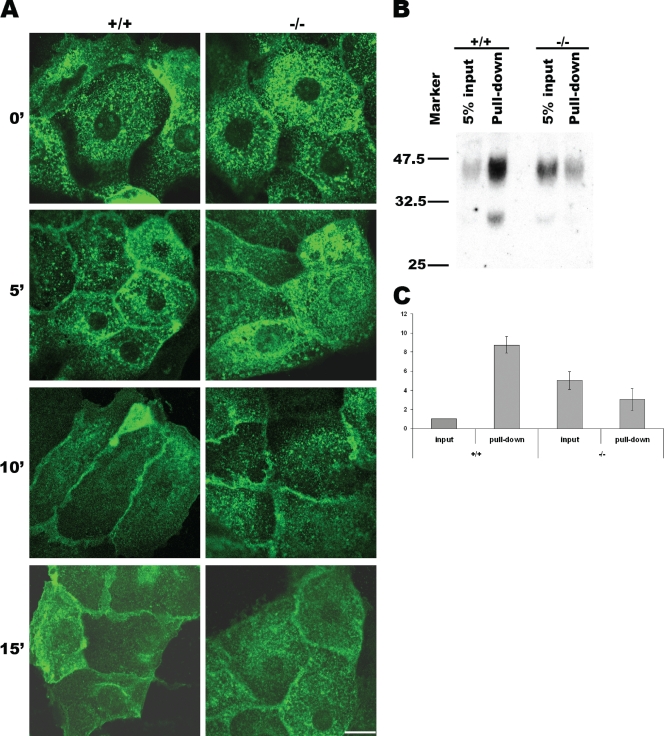
To clearly demonstrate a role of VAMP8 in AQP2 exocytosis in a more direct way, we tested AQP2 trafficking in cultured primary collecting duct cells isolated from normal and VAMP8 knockout mice. As shown in Fig. 7A, before forskolin stimulation, most AQP2 molecules existed on intracellular vesicles in both normal and mutant cells. At 5 min after stimulation, some AQP2 already had been mobilized to the plasma membrane in normal cells, while little was seen on the plasma membrane in mutant cells. At 10 min, most AQP2 molecules in normal cells were apparently associated with the plasma membrane, although they were not evenly distributed throughout the plasma membrane, while the situation in mutant cells was similar to that in normal cells at 5 min, suggesting that AQP2 exocytosis was retarded if not blocked in mutant cells. At 15 min, AQP2 became evenly distributed throughout the plasma membrane in normal cells, while most AQP2 molecules in mutant cells were still spotty, indicating impaired exocytosis.
FIG. 7.
VAMP8 was required for surface expression of AQP2 in cultured collecting duct cells. (A) Immunofluorescent staining showing AQP2 distribution in cultured collecting duct cells at different time points after forskolin stimulation. +/+, wild type; −/−, VAMP8 null; scale bar, 10 μm. (B) Detection of surface AQP2 by biotinylation. Primary collecting duct cells were cultured on filters for 2 days before being stimulated by forskolin for 15 min. Surface glycoproteins were then labeled with biocytin hydrazide and pulled down with streptavidin. AQP2 was detected by Western blot analysis. (C) Quantitative analysis of the 45-kDa band shown in panel B. The +/+ input was arbitrarily defined as 1 U. The +/+ pulldown, the −/− input, and the −/− pulldown values were 8.75 ± 0.87, 5.02 ± 0.93, 3.05 ± 1.14 U, respectively.
To quantitatively analyze the exocytosis of AQP2, we decided to label cell surface AQP2 with biotin. As multitransmembrane AQP2 has limited regions exposed on the cell surface, conventional surface biotinylation reagents (such as sulfo-NHS-biotin and sulfo-NHS-SS-biotin) targeting lysine residues did not work for AQP2. Since AQP2 is glycosylated, we chose to label cell surface glycoproteins with biocytin hydrazide, which targets carbohydrates. Biotinylated proteins were then isolated from cell lysate with immobilized streptavidin. As shown in Fig. 7B, at 15 min after forskolin stimulation, about 40 to 50% of AQP2 in normal cells could be pulled down with streptavidin beads, suggesting that at least 40% of AQP2 molecules were present on the cell surface. In mutant cells, however, less than 5% of AQP2 could be pulled down. The surface AQP2 in VAMP8-null cells was estimated to be about or less than one-third of that in normal cells, although the total amount of AQP2 in mutant cells was about three to five times that in wild-type cells (Fig. 7C). These results suggest that the efficiency of the surface deployment of AQP2 in response to forskolin stimulation in VAMP8-ablated cells was only about 1/9 of normal cells as estimated by this assay. VAMP8 thus plays a major role either in AQP2 exocytosis or in retaining AQP2 in the plasma membrane after exocytosis.
VAMP8 might mediate AQP2 exocytosis by forming complexes with syntaxin3 and/or syntaxin4.
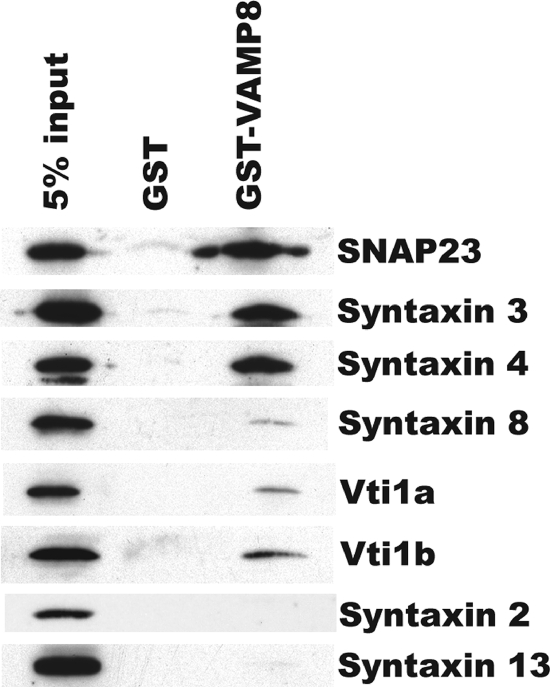
Although it cannot be completely excluded that VAMP8 affects AQP2 exocytosis indirectly, the straightforward interpretation of the results described above is that VAMP8 acts as a v-SNARE of AQP2 storage vesicles and directly mediates the fusion of AQP2-containing vesicles with the plasma membrane. To test if this hypothesis is solid, it is important to determine whether VAMP8 can form SNARE complexes with t-SNAREs on the plasma membrane. Of the known t-SNARE proteins, syntaxin2, syntaxin3, and syntaxin4 have been detected in collecting duct principal cells. Although somewhat controversial, a recent study shows that syntaxin2 and syntaxin3 are on the apical domain, while syntaxin4 is on the basolateral domain of the plasma membrane (15). Using a GST-VAMP8 fusion protein, we could pull down a substantial portion of SNAP23 (>5%) from total membrane extract of freshly isolated collecting duct cells (Fig. 8). Under the same conditions, we could also recover about 5% of syntaxin4 and about 2 to 3% of syntaxin3 but could never pull down detectable syntaxin2. VAMP8 has previously been shown to form a complex with Vti1b, syntaxin7, and syntaxin8 to mediate fusion between endosomes (2, 3, 4). In collecting duct cells, however, we could recover only trace amounts of vti1a, vti1b, and syntaxin8 with the GST-VAMP8 fusion protein. As a negative control, syntaxin13, a recycling endosome SNARE protein, could hardly be recovered (Fig. 8) despite its robust abundance. These results suggest that, as in other tissues, VAMP8 functions primarily in the secretory pathway in collecting duct cells, mediating membrane fusion at the last step(s) of AQP2 exocytosis via interacting with surface SNAREs such as syntaxin3 and/or syntaxin4.
FIG. 8.
GST pulldown experiments showing that VAMP8 might form SNARE complexes with SNAP23, syntaxin4, and syntaxin3 in collecting duct cells.
DISCUSSION
Water reabsorption by kidney collecting ducts is driven by the osmotic difference between the interstitium and the lumen of collecting ducts. Disruption of the osmotic gradient could have a profound effect on water reabsorption, as demonstrated in the NaK2Cl cotransporter knockout mice (32). However, we believe this is not likely the case in our VAMP8 knockout mice, since serum osmolality was slightly but significantly higher in VAMP8-null mice and depleting salt from the diet had no effect on the phenotype. The rate-limiting factor for water reabsorption is surface AQP2 density, which is tightly controlled by vasopressin (22). Although a number of SNARE proteins have been identified in collecting ducts and are considered to be involved in AQP2 exocytosis, their roles have not been functionally confirmed in vivo. For example, VAMP3 has been identified on AQP2 vesicles and claimed to be required for AQP2 exocytosis (24), but deletion of the VAMP3 gene did not affect urine-concentrating ability in mice (our unpublished observation), suggesting either that it does not play a role in AQP2 exocytosis or that other VAMPs can easily take over its role in its absence. In this study, we have provided data showing that VAMP8 is indispensable for AQP2 exocytosis. Although not completely blocked, the regulated surface expression of AQP2 was severely impaired in VAMP8-null cells. We suspect that the water-wasting defect is the major cause of neonatal lethality in VAMP8 knockout mice, although other defects, such as malnutrition, might also make a contribution. This was confirmed by our observation that when water was delivered via a pipe system and thus apparently was slightly less accessible, the majority of VAMP8 knockout mice, which otherwise would survive on a drinking-bottle system, were lost.
The fact that the polyuria defect of VAMP8-null mice was rectified partially by DDAVP administration suggests that there is insufficient vasopressin or the signaling pathway is deranged in these mice. However, the expression level of AQP2 was increased rather than decreased in VAMP8-null mice. Since the disruption of the vasopressin pathway is known to downregulate AQP2 (9, 22), we do not think this pathway was impaired in our mice. Actually, the elevation of endogenous vasopressin in concert with the upregulation of AQP2 argues for an augmentation of vasopressin signaling in VAMP8 knockout mice. Nonetheless, there still were insufficient AQP2 molecules on the cell surface to facilitate efficient water reabsorption. In light of the fact that AQP2 is also upregulated in the S256L mutant mice whose AQP2 exocytosis is blocked (18), we believe that our mice similarly suffered primarily from impaired AQP2 exocytosis. Since the urine-concentrating ability of VAMP8-null mice can be improved with DDAVP, it is reasonable to believe that human suffering from VAMP8 deficiency is treatable with DDAVP.
Generally, the cell has to balance its overall endocytosis and exocytosis activities to maintain the stability of its volume and surface area. Therefore, a defect in either pathway can affect the other indirectly. Although VAMP8 initially was reported to be present on endosomes (1, 37) and some evidence indicates that it is required for epidermal growth factor (2) and GLUT4 endocytosis (36), we believe that the primary defect of VAMP8-null collecting duct cells was an exocytotic rather than endocytotic event. Otherwise, we would have seen the accumulation of AQP2 on the cell surface, as has been reported for the dysfunction of dynamin (31).
The intracellular AQP2-bearing vesicles are heterogeneous. Some newly synthesized AQP2 molecules are packed into vesicles at the trans-Golgi network that eventually will mature for secretion. Some AQP2 molecules might be retrieved from the plasma membrane by endocytosis (7, 22). These endosomal AQP2 molecules could be routed for degradation via the endosome-lysosome pathway or be resecreted to the plasma membrane, probably after a unique process of maturation. We believe that the majority, if not all, of the AQP2 vesicles must somehow acquire VAMP8 before they are competent for regulated secretion. While a role for VAMP8 in the maturation process of AQP2 vesicles cannot be ruled out, our results suggest that it mediates AQP2 exocytosis at the very last step, since it can form complexes with t-SNAREs from the plasma membrane. Usually, AQP2 is deployed to the apical plasma membrane of collecting duct cells. It is possible that VAMP8 mediates apical AQP2 exocytosis by interacting with SNAP23 and syntaxin3, although an unknown syntaxin cannot be excluded. Under some conditions, AQP2 can also be delivered to the basolateral membrane (7). It is likely that VAMP8 participates in the basolateral exocytosis of AQP2 by forming a complex with syntaxin4 and SNAP23.
It is well known that phenotypes of knockout mice can be modified by genetic backgrounds. In this study, we found that the hydronephrosis defect was more severe on the C57BL/6 background than on the 129/Sv background. Not surprisingly, we found that wild-type C57BL/6 mice have a weaker urine-concentrating ability than wild-type 129/Sv mice. The osmolality of the 24-h urine sample from wild-type C57BL/6 female mice was about 1,700 mOsm, close to that of the VAMP8-null mice on the 129/Sv background but much lower than that of the 129/Sv wild-type mice (Table 2). The cause of this difference between C57BL/6 and 129/Sv mice is unknown. Nevertheless, the fact suggests that it is easier to detect a water-wasting defect on the C57BL/6 background than on the 129/Sv background if the genetic alterations do not lead to overt embryonic lethality or animal death after birth. In the case of VAMP8-null mice, the 129/Sv background seems to be more suitable, as it offers a good number of surviving animals with reproducible phenotypes.
Compared to the AQP2 knockout mice (27), the hydronephrosis phenotype of VAMP8-null mice is less severe. Furthermore, the VAMP8 mice can respond to DDAVP stimulation, although not to the same extent as normal mice. These facts suggest that there are other pathways for AQP2 exocytosis. We suspect that other VAMPs, especially VAMP2 and/or VAMP3, which are expressed in collecting duct cells, function in the absence of VAMP8. The relative importance of these VAMP proteins in AQP2 trafficking in vivo will be clarified in future studies.
Acknowledgments
We thank Bor Luen Tang for participation in the early stage of the work and insightful discussions and the staff of the A*STAR Biological Resource Center (BRC) for taking care of the VAMP8 knockout mice. We thank J. F. Pessin for providing the VAMP3 knockout mice, M. A. Knepper for the chicken anti-AQP2 antibody, and U. Blank for the syntaxin3 antibody.
This work is funded by the Agency for Science, Technology and Research (A*Star).
Footnotes
Published ahead of print on 19 October 2009.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Advani, R. J., H. R. Bae, J. B. Bock, D. S. Chao, Y. C. Doung, R. Prekeris, J. S. Yoo, and R. H. Scheller. 1998. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J. Biol. Chem. 273:10317-10324. [DOI] [PubMed] [Google Scholar]
- 2.Antonin, W., C. Holroyd, D. Fasshauer, S. Pabst, G. F. Von Mollard, and R. Jahn. 2000. A SNARE complex mediating fusion of late endosomes defines conserved properties of SNARE structure and function. EMBO J. 19:6453-6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonin, W., C. Holroyd, R. Tikkanen, S. Höning, and R. Jahn. 2000. The R-SNARE endobrevin/VAMP-8 mediates homotypic fusion of early endosomes and late endosomes. Mol. Biol. Cell 11:3289-3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonin, W., D. Fasshauer, S. Becker, R. Jahn, and T. R. Schneider. 2002. Crystal structure of the endosomal SNARE complex reveals common structural principles of all SNAREs. Nat. Struct. Biol. 9:107-111. [DOI] [PubMed] [Google Scholar]
- 5.Barile, M., T. Pisitkun, M. J. Yu, C. L. Chou, M. J. Verbalis, R. F. Shen, and M. A. Knepper. 2005. Large scale protein identification in intracellular aquaporin-2 vesicles from renal inner medullary collecting duct. Mol. Cell Proteomics 4:1095-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brändli, A. W., R. G. Parton, and K. Simons. 1990. Transcytosis in MDCK cells: identification of glycoproteins transported bidirectionally between both plasma membrane domains. J. Cell Biol. 111:2909-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, D. 2003. The ins and outs of aquaporin-2 trafficking. Am. J. Physiol. Renal Physiol. 284:F893-901. [DOI] [PubMed] [Google Scholar]
- 8.Cosen-Binker, L. I., M. G. Binker, C. C. Wang, W. Hong, and H. Y. Gaisano. 2008. VAMP8 is the v-SNARE mediating basolateral exocytosis in alcoholic pancreatitis. J. Clin. Investig. 118:2535-2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiGiovanni, S. R., S. Nielsen, E. I. Christensen, and M. A. Knepper. 1994. Regulation of collecting duct water channel expression by vasopressin in Brattleboro rat. Proc. Natl. Acad. Sci. USA 91:8984-8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gouraud, S., A. Laera, G. Calamita, M. Carmosino, G. Procino, O. Rossetto, R. Mannucci, W. Rosenthal, M. Svelto, and G. Valenti. 2002. Functional involvement of VAMP/synaptobrevin-2 in cAMP-stimulated aquaporin 2 translocation in renal collecting duct cells. J. Cell Sci. 115:3667-3674. [DOI] [PubMed] [Google Scholar]
- 11.Hasler, U., D. Mordasini, M. Bens, M. Bianchi, F. Cluzeand, M. Rousselot, A. Vandewalle, E. Feraille, and P. Y. Martin. 2002. Long term regulation of aquaporin-2 expression in vasopressin-responsive renal collecting duct principal cells. J. Biol. Chem. 277:10379-10386. [DOI] [PubMed] [Google Scholar]
- 12.Hong, W. 2005. SNAREs and traffic. Biochim. Biophys. Acta 1744:493-517. [PubMed] [Google Scholar]
- 13.Inoue, T., S. Nielsen, B. Mandom, J. Terris, B. K. Kishore, and M. A. Knepper. 1998. SNAP-23 in rat kidney: colocalization with aquaporin-2 in collecting duct vesicles. Am. J. Physiol. Renal Physiol. 275:F752-F760. [DOI] [PubMed] [Google Scholar]
- 14.Jahn, R., and R. H. Scheller. 2006. SNAREs—engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 7:631-643. [DOI] [PubMed] [Google Scholar]
- 15.Li, X., S. H. Low, M. Miura, and T. Weimbs. 2002. SNARE expression and localization in renal epithelial cells suggest mechanism for variability of trafficking phenotypes. Am. J. Physiol. Renal Physiol. 283:F1111-1122. [DOI] [PubMed] [Google Scholar]
- 16.Lippert, U., D. M. Ferrari, and R. Jahn. 2007. Endobrevin/VAMP8 mediates exocytotic release of hexosaminidase from rat basophilic leukaemia cells. FEBS Lett. 581:3479-3484. [DOI] [PubMed] [Google Scholar]
- 17.Maric, K., A. Oksche, and W. Rosenthal. 1998. Aquaporin-2 expression in primary cultured rat inner medullary collecting duct cells. Am. J. Physiol. 275:F796-F801. [DOI] [PubMed] [Google Scholar]
- 18.McDill, B. W., S. Z. Li, P. A. Kovach, L. Ding, and F. Chen. 2006. Congenital progressive hydronephrosis (cph) is caused by an S256L mutation in aquaporin-2 that affects its phosphorylation and apical membrane accumulation. Proc. Natl. Acad. Sci. USA 103:6952-6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLean, I. W., and P. K. Nakane. 1974. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J. Histochem. Cytochem. 22:1077-1083. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen, S., D. Marples, H. Birn, M. Mohtashami, N. O. Dalby, M. Trimble, and M. Knepper. 1995. Expression of VAMP-2-like protein in kidney collecting duct intracellular vesicles: colocalization with aquaporin-2 water channels. J. Clin. Investig. 96:1834-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen, S., C. L. Chou, D. Marples, E. I. Christensen, B. K. Kishore, and M. A. Knepper. 1995. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc. Natl. Acad. Sci. USA 92:1013-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen, S., J. Frøkiaer, D. Marples, T. H. Kwon, P. Agre, and M. A. Knepper. 2002. Aquaporins in the kidney: from molecules to medicine. Physiol. Rev. 82:205-244. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen, S., T. H. Kwon, J. Frøkiær, and P. Agre. 2007. Regulation and dysregulation of aquaporins in water balance disorders. J. Intern. Med. 261:53-64. [DOI] [PubMed] [Google Scholar]
- 24.Procino, G., C. Barbieri, G. Tamma, L. De Benedictis, J. E. Pessin, M. Svelto, and G. Valenti. 2008. AQP2 exocytosis in the renal collecting duct—involvement of SNARE isoforms and the regulatory role of Munc18b. J. Cell Sci. 121:2097-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puri, N., and P. A. Roche. 2008. Mast cells possess distinct secretory granule subsets whose exocytosis is regulated by different SNARE isoforms. Proc. Natl. Acad. Sci. USA 105:2580-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren, Q., H. K. Barber, G. L. Crawford, Z. A. Karim, C. Zhao, W. Choi, C. C. Wang, W. Hong, and S. W. Whiteheart. 2007. Endobrevin/VAMP-8 is the primary v-SNARE for the platelet release reaction. Mol. Biol. Cell 18:24-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rojek, A., E. M. Füchtbauer, T. H. Kwon, J. Frøkiaer, and S. Nielsen. 2006. Severe urinary concentrating defect in renal collecting duct-selective AQP2 conditional-knockout mice. Proc. Natl. Acad. Sci. USA 103:6037-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sander, L. E., S. P. Frank, S. Bolat, U. Blank, T. Galli, H. Bigalke, S. C. Bischoff, and A. Lorentz. 2008. Vesicle associated membrane protein (VAMP)-7 and VAMP-8, but not VAMP-2 or VAMP-3, are required for activation-induced degranulation of mature human mast cells. Eur. J. Immunol. 38:855-863. [DOI] [PubMed] [Google Scholar]
- 29.Schwenk, F., U. Baron, and K. Rajewsky. 1995. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 23:5080-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stokes, J. B., C. Grupp, and R. K. Kinne. 1987. Purification of rat papillary collecting duct cells: functional and metabolic assessment. Am. J. Physiol. 253:F251-262. [DOI] [PubMed] [Google Scholar]
- 31.Sun, T. X., A. Van Hoek, Y. Huang, R. Bouley, M. McLaughlin, and D. Brown. 2002. Aquaporin-2 localization in clathrin-coated pits: inhibition of endocytosis by dominant-negative dynamin. Am. J. Physiol. Renal Physiol. 282:F998-1011. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi, N., D. R. Chernavvsky, R. A. Gomez, P. Igarashi, H. J. Gitelman, and O. Smithies. 2000. Uncompensated polyuria in a mouse model of Bartter's syndrome. Proc. Natl. Acad. Sci. USA 97:5434-5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tiwari, N., C. C. Wang, C. Brochetta, G. Ke, F. Vita, Z. Qi, J. Rivera, M. R. Soranzo, G. Zabucchi, W. Hong, and U. Blank. 2008. VAMP-8 segregates mast cell-preformed mediator exocytosis from cytokine trafficking pathways. Blood 111:3665-3674. [DOI] [PubMed] [Google Scholar]
- 34.Wang, C. C., C. P. Ng, L. Lu, V. Atlashkin, W. Zhang, L. F. Seet, and W. Hong. 2004. A role of VAMP8/endobrevin in regulated exocytosis of pancreatic acinar cells. Dev. Cell 7:359-371. [DOI] [PubMed] [Google Scholar]
- 35.Wang, C. C., H. Shi, K. Guo, C. P. Ng, J. Li, B. Q. Gan, H. C. Liew, J. Leinonen, H. Rajaniemi, Z. H. Zhou, Q. Zeng, and W. Hong. 2007. VAMP8/endobrevin as a general vesicular SNARE for regulated exocytosis of the exocrine system. Mol. Biol. Cell 18:1056-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams, D., and J. E. Pessin. 2008. Mapping of R-SNARE function at distinct intracellular GLUT4 trafficking steps in adipocytes. J. Cell Biol. 180:375-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong, S. H., T. Zhang, Y. Xu, V. N. Subramaniam, G. Griffiths, and W. Hong. 1998. Endobrevin, a novel synaptobrevin/VAMP-like protein preferentially associated with the early endosome. Mol. Biol. Cell 9:1549-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zini, S., M. C. Fournie-Zaluski, E. Chauvel, B. P. Roques, P. Corvol, and C. Llorens-Cortes. 1996. Identification of metabolic pathways of brain angiotensin II and III using specific aminopeptidase inhibitors: predominant role of angiotensin III in the control of vasopressin release. Proc. Natl. Acad. Sci. USA 93:11968-11973. [DOI] [PMC free article] [PubMed] [Google Scholar]