INTRODUCTION: KAPOSI’S SARCOMA AND KSHV/HHV-8
In 1872, the famed Hungarian dermatologist Moritz Kaposi characterized an “idiopathic multiple pigmented sarcoma” which is now known as Kaposi’s sarcoma (KS).424 For most of its history, KS has been a rare, slowly progressing neoplasm affecting mainly elderly men of Mediterranean and Eastern European descent. KS was not typically fatal, and patients tended to live ten or more years with the condition, and die of other unrelated ailments.201 An abrupt increase in the number of cases of KS among previously healthy young homosexual men was first reported in 1981, ushering in a new era of aggressive, rapidly fatal KS.40,155,180,210,401 Since approximately 30% of AIDS patients presented with KS as their initial symptom of HIV infection, KS evolved into a defining characteristic of one of the most devastating infectious diseases in history.35,177
The progressive depletion of cell-mediated immunity and subsequent loss of immune function caused by HIV infection predisposes patients to an array of unusual malignancies.177,381 AIDS patients do not have higher incidence of the more common tumors of the breast, colon, or lung than the general population; rather they exhibit a greatly increased frequency of cancers induced by oncogenic viruses such as Epstein-Barr virus (EBV), human papilloma virus (HPV), and Kaposi’s sarcoma-associated virus (KSHV), also known as human herpesvirus 8 (HHV-8).177,381 Impaired immune surveillance promotes a permissive environment for uncontrolled viral replication, the spread of virus to surrounding cells and tissues, and contributes to the multistep process of tumorigenesis.99,428 In the case of AIDS-KS, interactions between immunosuppression, HIV, and KSHV are required for malignant progression. Although HIV infection is neither necessary nor sufficient for the development of KS, it is associated with a much higher frequency of KS and alteration of its natural course.99 The HIV epidemic in Africa along with the high prevalence of endemic KSHV infection in the general population has led to an alarming number of cases of KS and KSHV-associated diseases on that continent alone.118 With the recent advent of HAART (highly active antiretroviral therapy), the overall incidence of AIDS-associated neoplasms has declined;478 however, factors such as lack of access to treatment, noncompliance with treatment regimens, and the development of drug resistance all contribute to an elevated risk for KS in HIV-infected patients46,204,492 and ensure that KS will present a continuing major health problem for years to come.381 Understanding of the molecular biology of KSHV alone and in the context of HIV infection is crucial for the development of therapies to treat and prevent the associated pathological processes.
Discovery
In the 1920s, it was observed that KS occurred more frequently in East and Central Africa. The uneven geographical distribution led to the hypothesis that KS might be caused by an infectious agent.173,328 In 1990, a landmark epidemiological study from Beral et al. reported that KS was 20,000 times more likely to occur in people with HIV than in the general population.36 KS was more common in those who had acquired HIV sexually than in those who had acquired it via other routes. The incidence of KS was not related to age or race, but showed a definite geographical distribution, with the highest prevalence in the areas that were the initial foci of the AIDS epidemic.36
The accumulation of the epidemiological evidence suggested the involvement of a sexually transmissible agent in the development of KS, which in western countries had spread mainly among homosexual men. Several groups attempted to identify the unknown agent, and in 1994, Yuan Chang and Patrick Moore used representational difference analysis to identify two fragments of a previously unknown herpesvirus in a biopsy sample from an AIDS-KS patient,89 instigating a new era in KS research.
Diseases Associated with KSHV
Since its discovery, extensive studies have demonstrated an etiologic role for KSHV in the development of KS.79,113,129,302 The involvement of KSHV in the pathogenesis of KS is now widely accepted based on the following criteria: (1) KSHV genomes are detectable in all clinical forms of KS (classic, endemic, iatrogenic, and AIDS related);44,89,91,134,253,301,383 (2) KSHV infection is highly associated with subjects at high risk for developing KS such as HIV-infected gay men;167,168,229,403 (3) KSHV infection rate in the general population correlates with KS incidence rate in different geographic regions, e.g., high in some African regions, intermediate in Mediterranean and Eastern European regions, and low in North America;168 (4) KSHV is detected in the endothelial spindle cells, the neoplastic component of KS lesions;43,132 and (5) KSHV DNA sequences in peripheral blood and seroconversion to KSHV are detected prior to the development of KS.167,168,291,304,325,326,337,355,360 In addition to its association with KS, KSHV has also been implicated as the causative agent of two other AIDS-associated malignancies: primary effusion lymphoma (PEL)80,89 and the plasma cell variant of multicentric Castleman’s disease (MCD)133,415 and may be pathologically involved with other disorders resulting from dysregulation of the immune system.129,384
Kaposi’s Sarcoma
Four clinical forms of KS have been described. Classic KS is an indolent tumor of elderly men, most often found in Mediterranean, Eastern European, and Near East regions. Endemic KS is prevalent in Central Africa, where it is one of the most frequently occurring tumors. Iatrogenic KS has been identified in transplant recipients undergoing immunosuppressive therapy. In western nations, KS became a hallmark of the AIDS epidemic.77 The occurrence of KS in young male homosexuals/bisexuals was first reported in 1981.40,155,180,210,401 Epidemic AIDS-KS is the most common neoplastic manifestation of AIDS in the United States and Europe280 and is one of the diagnostic criteria for AIDS.1 In contrast to the indolent course of classic KS, AIDS-related KS takes a much more aggressive course.36 AIDS-related KS tends to disseminate widely to mucous membranes and the visceral organs.1,424 Despite the different clinical manifestations of KS, the histology of lesions from skin, lymph nodes, respiratory tract, and intestines are very similar.1 KS is a vascular tumor consisting of interweaving bands of spindle cells, irregular slit-like channels embedded with reticular and collagen fibers, and inflammatory infiltrates of mononuclear cells and plasma cells. The neoplastic components of the KS lesion are the so-called spindle cells due to their characteristic abnormal elongated shapes.99 The tumor is highly vascular, containing abnormally dense and irregular blood vessels, which leak red blood cells into the surrounding tissue and give the tumor its dark color.
Cutaneous lesions are divided into patch, plaque, and nodular stages. The patch stage demonstrates a proliferation of irregularly branching blood vessels, which may be grouped around normal-appearing blood vessels. Perivascular infiltrating lymphocytes and multiple plasma cells are typically present. The irregular vessels are small, flat and widely spaced and comprised apparently normal endothelial cells.1
The plaque stage is characterized by the appearance of spindle cells forming bundles in the vascular spaces. Mitoses and nuclear abnormalities are more prominent in both the spindle cells and the endothelial cells. Extravasation of red blood cells and macrophages is often evident.
As the vascular and spindle cells continue to proliferate, the characteristic nodular phase of classic KS develops. The nodular lesions are composed of well-defined, densely packed aggregates of spindle cells and vascular spaces arranged into bundles. The vascular slits are often distended by red blood cells. Mitotic figures, extravasated red blood cells, hemosiderin, and hemosiderophages are often present.1
KS lesions are composed of a mixed-cell infiltrate with hyperplastic spindle-shaped cells that resemble cytokine-activated ECs and monocytes/macrophages by their expression of tissue-specific markers (CD34, vascular-endothelial cadherin, endothelial leukocyte adhesion molecule type 1, CD4, CD14, CD68, and PECAM-1).207,444,472 Immunohistology, in situ hybridization, and in situ PCR studies have shown that the majority of spindle endothelial cells in advanced KS lesions as well as atypical endothelial cells in early lesions are latently infected by KSHV.43,132,336,353,497 Less than 3% of the KSHV-infected cells in KS lesions have been found to express viral proteins characteristic of viral lytic replication.101,227,336,420 The low rate of spontaneous lytic replication is postulated to have a role in KS development through an autocrine and paracrine mechanism, as well as ensuring the continued maintenance of KSHV infection.276
Though the classic form of KS is usually localized to the lower extremities, AIDS-KS commonly involves many other parts of the body. The skin of the face, the extremities, torso, and mucous membranes of the oral cavity are often affected. In one study, 45% of patients presented with one or more lesions along the gastrointestinal tract.99
Primary Effusion Lymphoma
PEL, also called body cavity-based lymphoma, is a rare lymphoma commonly found in HIV-infected patients.79 This type of lymphoma is characterized as a malignant effusion in the peritoneal, pleural, or pericardial space, usually without a tumor mass.69,79 The lymphoma cells are usually monoclonal and of B-cell origin, but display only a few markers of B-cell differentiation i.e., CD20, as well as several activation markers such as CD30, CD38, CD71, and epithelial membrane antigen.68,161 PEL cells typically express a 420 kDa isoform of CD138/syndecan-1, suggesting they are in a preterminal stage of B-cell differentiation close to that of plasma cells.66,162 KSHV is invariably detected in PEL, and is considered to be a diagnostic criterion for this type of lymphoma.79,161 KSHV is detected as either monoclonal or oligoclonal episomes in PEL samples.222 Similar to that seen in KS lesions, the pattern of KSHV gene expression in PEL is mainly latent.132,227,336,362 The majority of cells express the viral latent proteins LANA-1 (ORF73), vCyclin (ORF72), vFLIP (ORF71), kaposin (ORFK12), and LANA-2 (ORFK10.5) with a small percentage (2–5%) expressing vIL-6 (ORFK2).132,227,336,362 Proteins of the viral lytic cycle are detected in less than 1% of the tumor cells. Though generally considered to be a lytic protein, vIL-6 expression in PEL may be independent of the lytic replication cascade of gene expression.92
Multicentric Castleman’s Disease
MCD is a localized lymphoproliferative condition characterized by expanded germinal centers with B-cell proliferation and vascular proliferation. The plasma cell variant of MCD is more commonly seen in AIDS patients and transplant recipients. MCD is frequently but not invariantly associated with KSHV infection.11,109,133,415 Immunohistochemical analysis of biopsy specimens has shown that 10–50% of B-cells surrounding the follicular centers of MCD are positive for LANA-1.131,132,227,336 Of the LANA-1 positive B cells, about 5–25% also express the KSHV proteins vIL-6 and vIRF-1 (ORFK9).227,336 In addition, a small proportion of the mantle zone cells of MCD also express viral proteins associated with lytic replication,227,336 suggesting that in MCD, KSHV adopts a less restricted pattern of gene expression compared to that seen in either KS or PEL.
Other Possible KSHV-Associated Disorders
Since the isolation of KSHV DNA from KS lesions in 1994, KSHV has been postulated to be involved in several other disease states resulting from immune dysfunction.
Primary pulmonary hypertension is a progressive disorder characterized by elevated mean arterial pressure that may lead to right ventricular failure, and complex, lumen-occluding vascular lesions resulting from dysregulated endothelial cell proliferation. A study published in 2003 reported a possible association between KSHV infection and the nonfamilial form of this disorder, but follow-up studies were not able to confirm this association.108,250
Hemophagocytic syndrome, also called macrophage activation syndrome and hemophagocytic lymphohistiocytosis, is a reactive disorder of the mononuclear phagocytic system that is characterized by benign generalized histiocyte proliferation with profound hemophagocytosis resulting in the destruction of the formed elements of the blood. The acquired form of this syndrome is associated with underlying disease such as immunodeficiency, and can be triggered by infection or medication.142 KSHV infection may be able to trigger episodes of this syndrome, but it is not the only viral cause.3,384
Limited evidence indicates a possible role for KSHV infection in other diseases, including pemphigus, salivary gland tumors, bullous phemigoid, nonneoplastic lymphadenopathies, sarcoidosis, Kikuchi’s disease, and multiple myeloma.3
Interaction of HIV and KSHV
As KSHV is a necessary but not sufficient etiological factor for KS, only a small proportion of infected people ever develop KS or KSHV-induced lymphomas. Cofactors such as HIV infection and iatrogenic immunosuppression dramatically increase the risk of developing a KSHV-related malignancy in infected individuals. Among KSHV-infected individuals, the risk of KS is much higher in those with HIV-1 infection than among those with other types of immunosuppression, suggesting a direct action of HIV-1 on KSHV replication and the development of tumorigenesis. These two viruses may interact at the molecular level in coinfected patients, resulting in increased HIV-1 viral load.293 Studies have demonstrated that coinfection with KSHV can modulate HIV-1 replication.73–76 This occurs either through direct interaction between these two viruses or through secondary effects resulting from the release of cellular factors in response to infection. The KSHV ORF50-encoded reactivation and transcriptional activator (RTA) interacts synergistically with HIV-1 Tat protein in the transactivation of HIV-1 LTR, leading to increased cellular susceptibility to HIV infection.76 LANA-1 interacts with Tat and activates HIV-1 LTR.211 Expression of RTA increases the efficiency of HIV infection in different cell types.75 This potentially could result in enhanced HIV spread within the infected organism and faster progression of the disease. On the other hand, HIV-1 infection leads to reactivation of latent KSHV genomes, through Tat.292 HIV-1-encoded Vpr proteins increase intracellular KSHV-gene expression.206 Consequently, HIV infection can promote KS progression. In AIDS-KS, HIV and its Tat protein induce inflammatory cytokines, which can further promote the pathogenesis of KS.137,140 In fact, HIV induced oncostatin-M (OSM) and interferon (IFN)-γ to promote KSHV lytic replication.294 It was shown that HIV infection of PEL cells triggered KSHV reactivation292 while KSHV enhanced HIV replication in acutely infected cells and induced reactivation in latently infected cells.73 HIV-1 Tat also enhanced KSHV infectivity, probably by concentrating virions on cell surface.16
In order to elucidate the basis for the increased frequency, enhanced aggressiveness, and disease progression seen in AIDS-KS, understanding of the molecular biology of KSHV is required. The remainder of this chapter focuses on describing the structure and genetics of the virus, infection systems and animal models used to study the virus, the viral lifecycle, impact on host cellular pathways, potential oncogenic mechanisms, and specific gene functions.
BIOLOGY OF KSHV
Virion Structure and Assembly
Herpesviruses are members of the Herpesviridae family. This family comprises three subfamilies, alpha-, beta-, and gammaherpesvirus. KSHV belongs to the γ2-herpesvirus group.288 KSHV and other members of the herpesvirus family share a characteristic architecture in which the double-stranded DNA genome is surrounded by an icosahedral protein capsid, a thick tegument layer, and a lipid bilayer envelope.481 Mature KSHV has at least 24 virion-associated proteins. These include five capsid proteins, eight envelope glycoproteins, six tegument proteins, and five proteins whose locations in the virion have not yet been defined.323,499 The 3D structure of KSHV capsids has been investigated by cryo-electron microscopy.481 The studies show that KSHV has the same T = 16 triangulation number and much the same capsid architecture as herpes simplex virus (HSV) and cytomegalovirus despite limited sequence similarity between corresponding capsid proteins.481 The principal constituents of the capsids are a major capsid protein (ORF25), two triplex proteins (ORF62 and ORF26), and a small capsid protein of ~19 kDa (ORF65).323 Viral assembly within infected cells has yet to be studied.
Genome Structure and Organization
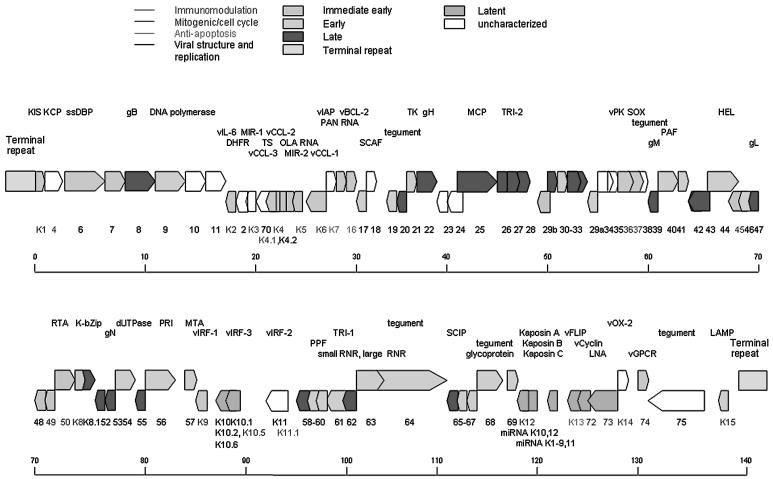
KSHV contains a large double-stranded DNA which is a closed circular episome in the nucleus during latency but is linear during lytic replication. Over 90 genes/ORFs are encoded by a 140 kb long unique region (LUR) with 53.5% G+C content, which is flanked by 20–35 kb terminal repeat regions composed of 801 bp terminal repeat units with 84.5% G+C content (Fig. 1). The KSHV genome shares the seven block organization of other herpesviruses with KSHV unique ORFs present between blocks. These unique genes, some of which are homologs to human genes, were designated names with a “K” prefix followed by number.367 Because of the KSHV genome complexity, the exact number of genes in the genome remains unknown. It is possible that new genes have yet to be discovered. Organization of KSHV genome is closely colinear to that of herpesvirus saimiri (HVS) and rhesus macaque rhadinovirus (RRV) genomes. HVS is the prototypical gammaherpesvirus of the Rhadinovirus genus.144 Sequence analysis indicates HVS shares significant homology with KSHV.367 RRV, a simian gamma-2 herpesvirus closely related to KSHV, replicates lytically in cultured rhesus monkey fibroblasts and establishes persistence in B cells. The similarity between the genomes of RRV and KSHV is high, and almost all of the ORFs/genes in KSHV have at least one homolog in RRV.10,388
Figure 1.
KSHV genome and genes. The names of genes are labeled in color according to gene function (immunomodulation in blue, mitogenic and cell cycle in purple, antiapoptosis in green, and viral structure and replication in black). The blocks representing the ORFs are also labeled in color according to gene class (immediate-early in orange, early in light blue, late in red, latent in green, and unclassified in white). (See Color Plates)
Genetic Manipulation of KSHV Genome
Study of KSHV infection and functions of individual genes have been hampered by the lack of viral mutants. For KSHV regulatory genes whose functions have been characterized, most have been examined in cell culture after gene cloning; however, their biological functions in KSHV infection remain largely unclear.129,302 The construction of viral mutants is the most straightforward way to examine the functions of individual viral genes, and has been widely used for other herpesviruses. In recent years, the adoption of the bacteria artificial chromosome (BAC) system has accelerated the study of functions of herpesvirus genes because the genome can be easily modified either by transposon or ET cloning in E. coli once the genome is cloned as a BAC. Transfection of the cloned viral DNA into permissive mammalian cells leads to the production of infectious virions without the need for further genetic repair and homologous recombination in the transfected cells.
Recently, Zhou et al. successfully cloned the full-length KSHV genome as a BAC in E. coli, and reconstituted it in 293 cells.498 The sequences of BAC, GFP and hygromycin-resistance cassette were inserted into the loci between ORF18 and ORF19 by homologous recombination without disrupting the expression of these two genes. The recombinant virus could be induced into productive lytic replication producing infectious virions that are capable of infecting 293 and primary human endothelial cells at high efficiency.166,498 To elucidate gene function in the context of KSHV infection, the ET cloning system for BAC mutation was developed to generate mutants. The first mutant of BAC36 was achieved by replacing vIRF with a kanamycin-resistance cassette.498 Later K8.1, RTA, gB, and MTA mutants of BAC36 were made using the same strategy and their functions have been further addressed.194,242,277,485 Transposition is a powerful tool for modifying DNA molecules and a Tn5-based mutagenesis library of BAC36 has been constructed, in which one mutant with Tn5-disrupted LANA-1 has been used to dissect the function of LANA-1.487
Host Range and Cell Tropism
As with all human herpesviruses, KSHV may infect a large proportion of the human population and remain in a latent state throughout most of the life of the host. Although a number of cell types are known to harbor the latent virus, the true latent reservoir(s) remain to be defined. Upon reactivation by immunosuppression, such as in HIV infection, the latent virus switches to lytic replication, producing new virus particles that infect and trigger the proliferation of endothelial cells involved in KS. Alternatively, the latent cells might home to the endothelium and proliferate into spindle cells. An early study demonstrated 78 times higher number of KS-like spindle cells detected in the peripheral blood of HIV-1-infected individuals compared to normal controls.58 These peripheral blood-derived spindle cells (PBsc) were shown to express a variety of endothelial cell markers, such as Ulex europaeus I lectin, EN4, EN2/3, EN7/44, CD13, CD34, CD36, CD54, ELAM-1, and HLA-DR. Consistent with these early observations, recent studies suggested that KSHV could infect human fetal mesenchymal stem cells (MSCs) and CD34+ hematopoietic progenitor cells (HPCs) and the virus may be disseminated following differentiation of infected HPCs into B cell and monocyte lineages.338,483 Thus, MSCs and CD34+ HPCs may be reservoirs for KSHV infection and provide continuous sources of virally infected cells in vivo. Several studies also suggest that the lymphoid system could be the reservoir of latently infected cells from which KSHV reactivates under conditions of immunosuppression.38,327 Similar to EBV, KSHV displays a tropism for B lymphocytes, since viral DNA has been detected in purified CD19+ B cells but not in CD8+ cells from the peripheral blood of patients with both KS and HIV infection.38,252,327
The origin of KS tumor cells has been a matter of controversy and many mesenchymal cell types have been proposed.34,402 The recent finding of several endothelial markers (including CD31, CD34, CD36, and KDR) on KS tumor cells has supported the theory that endothelial cells are the cell of origin of KS.472 More support comes from several studies reporting spindle-shape transformation of different types of human endothelial cells following infection with KSHV.106,166 However, the lack of Pal-E and eNOS expression has brought into question the endothelial origin of this tumor.473 Recently, several new endothelial markers have been identified and characterized. The expression of two such proteins, VEGFR3 (VEGF-C receptor, flt-4) and podoplanin (a membrane glycoprotein), have been found to be restricted to lymphatic endothelium.472 KS tumor cells express these two markers of lymphatic endothelium as well as the general endothelial marker CD31, strongly suggesting that KS is derived from lymphatic endothelium.350,472
The most common cell type isolated from AIDS-KS has been the spindle-shaped endothelial-like cells that proliferate in response to inflammatory cytokines and have a limited replicating life span.281,329,372,373 The spindle cells isolated from KS lesions of HIV-1-infected individuals are generally hyperplastic nontransformed cells that proliferate in culture with inflammatory cytokines. These cells initially contain KSHV that is rapidly lost as the cells are passaged in culture. Cell lines composed of transformed cells have also been isolated from advanced KS lesions. KS cell lines have been isolated from KS lesions (KS SLK and KS IMM) from two HIV-1 noninfected renal transplant recipients.9,251 These cell lines, unlike AIDS-KS spindle cells, are transformed cells that grow in the absence of inflammatory cytokines, contain cytogenetic abnormalities, and induce durable tumor lesions when inoculated into nude mice.223,373 Similar to the hyperplastic KS spindle cells, the KS transformed cells have also lost the KSHV genomes. Taken together, these findings indicate that early stage KS is a hyperplasia resulting from proliferation of spindle-shaped endothelial-like cells in response to KSHV-induced angiogenesis and inflammation. However, the spindle-shaped cells, most of which have latent KSHV, may eventually become transformed malignant tumor cells during advanced KS. In support of this concept, it has been experimentally demonstrated that primary human endothelial cells and keratinocytes could be immortalized upon de novo KSHV infection.78,147 Ectopic expression of a number of KSHV genes also leads to immortalization and/or transformation of various cells, further supporting that KSHV is an oncogenic virus.22,165,311,429
In cell culture, KSHV is capable of infecting a diverse range of human and animal cell types/lines including primary CD19+ B cells, HPV-transformed human brain endothelial cells BB18 and 181GB1-4, primary neonatal capillary endothelial cells, human embryonic kidney 293 cells, Ln-Cap cells, human lung carcinoma A549 cells, CHELI (Chediak–Higashi syndrome) cells, squamous cell carcinoma SCC15 cells, human fibroblast cells, human bladder carcinoma T24 cells, human prostate carcinoma DU145 cells, human cervical carcinoma HeLa cells, baby hamster kidney BHK-21 cells, owl monkey kidney OMK637 cells, green monkey fibroblasts (Vero) cells, green monkey kidney CV-1 cells, SLK cells (KS-spindle cells), and murine fibroblast 3T3 cells.31,357
KSHV Infection Systems
Because of the direct involvement of endothelial cells in KS tumors, many groups have focused on infection of endothelial cells and several reports have documented KSHV infection of human primary endothelial cells.106,147,166,246,306 In the initial report, KSHV infected only a small number of cells in primary human bone marrow microvascular endothelial cell cultures and primary human umbilical vein endothelial cell (HUVEC) cultures. The KSHV-infected cells acquired a spindle-shape morphology and were maintained for >12 months while the control cultures underwent senescence within 3 weeks of culture.147 It has been proposed that the small number of infected cells provide a paracrine effect to sustain the cultures. Subsequent study showed that KSHV could infect primary human dermal microvascular endothelial cell (DMVEC) cultures and form colonies or plaques of spindle-shaped cells.106 Again, the primary-infection efficiency in this system was low, even though the virus eventually infected the entire culture after 2–3 weeks. Paradoxically, to sustain long-term KSHV infection, it was necessary to periodically replenish the cultures with uninfected endothelial cells at a ratio of ten portions of normal cells to one portion of infected cells. To facilitate the manipulation of primary endothelial cells, HPV E6, E7-immortalized DMVEC cultures were used as targets for KSHV infection.306 In this system, the primary-infection efficiency remained low even though the virus also eventually spread to the entire culture, which could now be stably maintained. In contrast, KSHV infection of telomerase-immortalized microvascular endothelial cells was extremely efficient, reaching the entire culture within 2–3 days of infection; however, the infected cells were unable to sustain persistent KSHV infection, and the cultures quickly lost the virus after several passages.246 The limitations, such as low primary-infection efficiency and/or failure of long-term sustainability for virus growth, of the above-mentioned systems have restricted their use for KSHV characterization, especially virus–cell interactions at the initial stage of infection. Furthermore, even in systems that can sustain persistent KSHV infection, the cultures are predominantly in the viral latent phase.106 Active viral lytic replication was not observed in any stages of infection in these systems. In contrast to the above systems, efficient infection of HUVEC cultures by recombinant KSHV BAC36 is permissive for lytic replication at the early stage of infection, producing large amounts of infectious virion.166 Infected cultures form bundles of spindle-shaped cells, which are reminiscent of KS vascular structures, and establish latency at a late stage of infection. The latently infected cells can be induced into lytic replication. Thus, this system can be used for examining KHSV latent and lytic replication, as well as productive primary infection.166
Animal Models
Animal models have been developed to investigate the in vivo behavior of KSHV-related malignancies. In a number of studies, tumors were induced by injecting KSHV-infected PEL cells into mice.42,343 In a report, BCBL-1 cells were injected alone or with human peripheral blood mononuclear cells (PBMC) into SCID mice.343 The lymphomas, which developed at or near the site of injection, appeared to derive exclusively from the injected BCBL-1 cells and not from the injected human PBMC. The tumors induced a marked murine angiogenic response, but known angiogenic cytokines were not detected in the BCBL-1 cells.343 In a similar more comprehensive study, Boshoff et al. found that injection of either singly (KSHV+) or dually (KSHV+/EBV+) infected PEL cell lines into Nod/SCID mice resulted in a significant pathogenic difference.42 PEL-like effusions were observed in the mice following intraperitoneal (i.p.) injection of both types of PEL cells, whereas only the dually infected, but not singly infected, PEL produced an effusion following intravenous injection. Singly infected PELs express an array of cell surface homing receptors very different from most lymphomas, with the potential for both positive and negative effects on effusion formation. Dually infected PEL cells expressed adhesion molecules that were very similar to EBV-positive Burkitt’s lymphoma (BL) cells. These differences might explain the differential metastasis to solid tumors (as in BLs) between the PEL types.42 Another study hypothesized that PEL cells secrete VEGF to promote the vascular permeability of peritoneal vessels, leading to effusion rather than to neovascularization of tumors. The ability of various lymphoma lines (including both PELs and BLs) to form effusions following i.p. inoculation correlated directly with their respective magnitudes of VEGF release. Coinjection of antibodies specific for VEGF, but not control antibodies, blocked effusion formation by the PELs.17 A de novo infection model of SCID/hu mice by KSHV demonstrated that the virus remained confined to the grafted CD19+ B cells, and did not spread to mouse tissue based on the detection of the viral DNA and mRNA.126 In a similar model, six of eight mice developed KS-like lesions with angiogenesis.151 Similar to human infection, keratinocytes in the epidermis and spindle cells in the dermis supported a largely latent infection, with rare cells expressing lytic genes. The lack of universal infection suggests that the skin model may allow an analysis of viral and host determinants of permissiveness.126 A study published in 2004 describes the attempt to develop a primate model by infecting rhesus macaque with KSHV, either with or without SIV, the simian form of HIV.358 Unfortunately, only a very low amount of viral DNA was detectable, and after 27 months postinoculation, KSHV infection did not result in any observable pathology in either SIV-negative or SIV-positive animals. Two macaque homologs of KSHV, retroperitoneal fibromatosis-associated herpesviruses (RFHV), were identified in retroperitoneal fibromatosis, a malignancy closely resembling KS.366 Unfortunately, attempts to grow these viruses have not been successful so far. The recently identified RRV is closely related to KSHV,10,388 and when coinfected with SIV, rhesus macaques develop a B-cell hyperplasia similar to MCD occurring in humans with HIV/KSHV.477
THE LIFECYCLE OF KSHV
Like all other herpesviruses, KSHV exhibits two distinct phases of infection. Lytic or productive infection results in the replication of viral DNA, the production of infectious virions, and the death of the host cell. During latent infection, the viral genome is maintained as a circular episome within the host cell nucleus and only a fraction of the viral genes are expressed. The viral episome replicates each time the host cell divides, using existing cellular replication machinery. Although infectious virions are not produced during latency, the viral genome retains the ability to be reactivated into lytic replication.129 The molecular mechanisms involved in latency and lytic reactivation are described in details in the following sections.
Mechanisms of KSHV Latency
Expression of KSHV Latent Genes/Transcripts
Latent infection by KSHV involves the expression of only a few of the 90 KSHV genes. Specifically, all KSHV latently infected cells express vFLIP, vCyclin, and LANA-1.132,217,377 Interestingly, vFLIP, vCyclin, and LANA-1 are located adjacent to one another in the KSHV genome.367 They belong to a multicistronic transcriptional unit, known as the latency transcript (LT) cluster.125 The LT cluster is transcribed from a constitutively active promoter (LTc) which is initiated at nucleotide 127,886, giving rise to a unspliced 5.8-kb mRNA and an alternative splicing a 5.4-kb mRNA, both containing vFLIP, vCyclin, and LANA-1, and a 1.7-kb transcript containing vFLIP and vCyclin (Fig. 1). It is likely that LANA-1 is the principal translation product of the longer mRNAs, whereas both vCyclin and vFLIP are synthesized from the shorter transcript, the latter by way of an internal ribosome entry site upstream of vFLIP.37,272 These three genes are separated from the K12 gene, which is expressed at low levels during latency, by an ~4.5 kb KSHV sequence that lacks any significant ORFs, representing the largest coding gap within the unique region of the KSHV genome.367 Surprisingly, 10 of the 12 KSHV microRNAs (miRNAs) identified so far are located within this coding gap, whereas the miR-K10 is found within K12,63,342,374 and the miR-K12 is located within the 3′-UTR, 265 nt away from the end of the transcript.186 All 12 KSHV miRNAs are oriented in the same genomic direction.
MicroRNAs are endogenous approximately 22 nucleotide RNAs that play important gene regulatory roles by pairing to the messages of protein coding genes to specify mRNA cleavage or by repressing productive translation.29 miRNAs’ functions ranging from control of cell proliferation, cell death and metabolism, to cancer. Recent discovery of virus-encoded miRNAs indicates that viruses also use this fundamental mode of gene regulation.316 The first reported virus-encoded miRNAs were the five viral miRNAs expressed in EBV-infected cells. Among the various families of viruses, the herpesvirus family stands out in establishing long-standing latent infection as a major part in the viral life cycle. It is possible that miRNAs may play a critical role in the establishment and/or maintenance of latent infection initiated by herpesviruses. In fact, the KSHV microRNAs are all expressed in latently infected cells and largely unaffected after induction of lytic replication.63,342,374 Computationally predicted targets for KSHV miRNAs include viral genes such as ORF23, 27, 31, 52, 49, 61, 68, K7, K13, and K14, and several B-cell-specific genes involved in apoptosis and signaling.62 Recently, another latent promoter was characterized which is embedded within the ORF71-73 transcription unit specifying transcripts that encode ORF71/72 or K12 (Fig. 2). This promoter is initiated at 123,751 and polyadenylated at 122,070 or 117,436.62,340 Multiple novel transcripts initiated from LTc and terminated at either 122,070 or 117,436 have also been identified. These transcripts may also be the source of miRNAs.340 It is believed that the transcripts initiating at the two latent promoters present in the KSHV latency-associated region can undergo two entirely distinct fates, i.e., processing to give a kaposin mRNA and viral microRNAs on the one hand or expression as KSHV vFLIP, vCyclin, or LANA-1 mRNAs on the other, depending on whether the viral polyadenylation site located at position 122,070 is ignored or recognized, respectively.62
Figure 2.
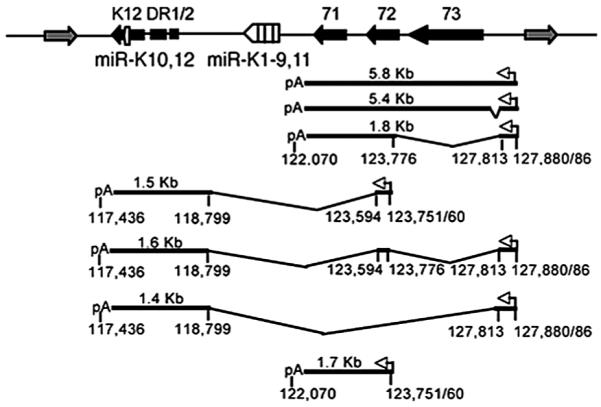
Transcription of the latency-associated region of the KSHV genome. The KSHV latency-associated region encodes four proteins, indicated by black boxes, as well as 12 miRNAs (white boxes) and is flanked by lytic genes (gray boxes). Two latent promoters (white arrows), splice sites, and poly(A) addition sites are indicated. Sequence coordinates are derived from the prototype BC-1 KSHV genome (GenBank accession number NC_003409). Figure is modified from reference 73.
The KSHV K15 is another gene that has been detected in latently infected cells; however, the expression levels of this transcript increase following induction of lytic replication.103 The K15 gene is encoded by the last ORF in the KSHV genome, adjacent to the terminal repeats, and is transcribed in a leftward orientation away from the terminal repeat region.103 The K15 gene contains eight exons which undergo alternative splicing, yielding several isoforms with a predicted common carboxyl-terminal cytoplasmic tail and different numbers of transmembrane domains.103
ORFK10.5 encodes latency-associated nuclear antigen 2 (LANA-2/vIRF-3), which is expressed in KSHV-infected hematopoietic tissues, including PEL and MCD but not KS lesions. LANA-2 is abundantly expressed in the nuclei of cultured KSHV-infected PEL cells. Transcription of K10.5 in PEL cell cultures is neither inhibited by DNA polymerase inhibitors nor significantly induced by phorbol ester treatment.362
Mechanism of KSHV Episomal Persistence
LANA-1, originally named latent nuclear antigen (LNA), is the dominant protein expressed during latency.167 LANA-1 is a multifunctional multidomain protein of 1,162 amino acids in length, 222–234 kDa in size, with a characteristic speckled or punctate nuclear localization.167,168,229 LANA-1 has three domains: (1) a 329 aa N-terminal rich in serine/threonine, proline, and basic residues; (2) a highly polymorphic 534 aa internal repeat domain (IRD) rich in glutamic acid, aspartic acid, glutamine, and leucine and contains a putative leucine zipper motif; and (3) a 227 aa C-terminal domain rich in charged and hydrophobic residues.169,495 Because the KSHV viral genome does not encode any of its own centromeres, it must have an alternative method for maintaining and replicating its episome from generation to generation. In KSHV, LANA-1 is responsible for episomal maintenance, replication, and segregation into daughter cells.
In order to carry out these functions, LANA-1 tethers the KSHV episome to the chromosome.25,27,112,285,398,433 In KSHV-infected cells, LANA-1 colocalizes with KSHV and binds episomes.25,238,441 Because of this colocalization, LANA-1 was implicated as an important element in episomal persistence and there is much in vitro evidence to support this conclusion. LANA-1 expression is sufficient for maintenance, replication, and segregation of plasmids containing terminal repeat units.170,184,205 LANA-1 is sufficient and necessary for viral latent DNA replication and efficient episomal segregation during mitosis, which assures that an equal number of KSHV episomes is distributed to each daughter cell.25,184,205 Transposon-mediated disruption of LANA-1 protein expression in recombinant KSHV BAC36 renders the virus incapable of establishing latency and leads to the loss of the episome, demonstrating that LANA-1 is essential for these functions in vivo.487
LANA-1 utilizes protein–protein interactions with the folding regions of core histones H2A and H2B to facilitate episomal tethering to the nucleosome during mitosis and interphase.27 Both the N- and C-terminal domains of LANA-1 interact with the host chromosomes; however, the C-terminal cannot maintain the KSHV episome in N-terminal mutants.26,27,243,345,398 It is most likely that LANA-1 binds to two unique sites in the long terminal repeat of the KSHV episome with its C-terminal and the chromosomes with the N-terminal while the C-terminal plays a supportive role in binding KSHV episomes to host chromosomes. Nevertheless, the C-terminal is important for LANA-1 oligomerization and evidence suggests that oligomerization is important for efficient tethering of the KSHV episome.385,447,452 The LANA-1 C-terminus also interacts with a number of chromosome binding and origin recognition complex (ORCs) proteins such as Brd2/RING3, CBP, ORC2, and HBO1 to create an optimal microenvironment for episomal replication.423,447 When the expression levels of these proteins are knocked down by siRNAs, the efficiency of episomal replication is also reduced, indicating that the C-terminal is likely essential for ensuring episomal replication.423 Thus, in addition to tethering the episome to the chromosomes, LANA-1 ably hijacks cellular proteins to aid the virus in episomal replication.
Mechanisms of KSHV Reactivation
Expression of KSHV Lytic Genes
A hallmark of herpesvirus life cycle is the expression cascade of genes that can be divided into four categories based on their expression kinetics: latent, immediateearly (IE), early, and late genes. The IE genes are expressed immediately after primary infection or upon reactivation from latency, and do not require de novo protein synthesis. IE genes generally encode for regulatory proteins and are critical for initiating viral transcription. RTA is the gene product of the major IE transcript of KSHV, which is necessary and sufficient to drive the switch from latency to lytic gene expression and the production of viral progeny in infected cells. KSHV may also encode several other IE gene products, including ORF45, ORF29b, and ORF4.2.245 ORF45 is present in purified KSHV virions and appears to be a tegument protein.499,502 ORF45 was characterized as a phosphorylated protein and may interact with IFN regulatory factor 7 (IRF-7) and inhibit virus-mediated induction of IFN-α/β.501
Early gene products are made after the IE genes, but prior to viral DNA synthesis; therefore, their expression is not affected after the inhibition of viral DNA replication by agents such as phosphonoacetic acid (PAA). Several KSHV early genes have also been identified; they include K8 (K-bZIP, also known as RAP), vIRF-1, K1, K3, and K5 (MIR-1 and MIR-2, modulator of immune recognition proteins 1 and 2), ORF57 (a functional homologue of the EBV MTA gene), vIL-6, viral CC chemokine ligands (vCCLs), polyadenylated nuclear (PAN) RNA, vBcl-2, ORF49, K12, K15, viral G protein-coupled receptor (vGPCR), viral dihydrofolate reductase (vDHFR), and thymidylate synthase.47,48,96,178,195,257,300, 340,361,377,431,439,466,467,476
Late genes whose expression is abolished by PAA usually do not appear until 30 h after induction.431,500 Examples of these proteins include envelope glycoproteins gB, K8.1, and a small viral capsid antigen encoded by ORF65.181,377,431
Molecular Mechanisms Involved in KSHV Reactivation
The switch from latent to lytic infection of KSHV is initiated by a number of stimuli that induce the expression of the key lytic switch protein, RTA. The expression of RTA is necessary and sufficient to trigger the full lytic program resulting in the ordered expression of viral proteins, release of viral progeny, and host cell death.275,276,430 The expression of RTA precedes the expression of all other cycloheximide-resistant IE genes and cycloheximide-sensitive early genes. In addition, activation of RTA leads to the complete replication of nascent, infectious virus particles with kinetics that is consistent with stimulation by chemical inducers.181
Following primary infection, KSHV generally establishes latent infection.31 During latency, only a few genes are transcribed, while the expression of RTA is tightly repressed. However, cloned promoter region of RTA shows a high basal activity,95,121,370 indicating that epigenetic change and chromatin remodeling of KSHV genome may be involved in this repression. Epigenetic changes such as DNA methylation act to regulate gene expression in normal mammalian development as well as in cancer through transcriptional silencing of critical growth regulators.30 With approximately 70% of the CpG sites in the human genome methylated, it is clear that the cellular environment is predisposed toward methylation, and a herpesvirus infecting a host cell must contend with this environment. In fact, before the discovery of KSHV in 199485 the genomes of the known gammaherpesviruses (including EBV and various murine, bovine and simian family members) were all shown to be CpG suppressed, suggesting they too have been subjected to heavy methylation.226 The finding by Chen et al.95 suggested a mechanism that hypermethylation in the RTA promoter may regulate its expression and subsequently KSHV reactivation from latency. Other chromatin modifications, such as histone deacetylation and alterations of chromatin-binding proteins, affect local chromatin structure and, in concert with DNA methylation, may also regulate RTA gene transcription.273 It has been suggested that methylation of the RTA promoter region during latency promotes the association of transcriptional repressors and histone deacetylase (HDAC). Lytic replication of KSHV can be triggered by chemical agents including butyrate and 12-O-tetradecanoylphorbol-13-acetate (TPA). Butyrate, a known activator of lytic replication, is an inhibitor of HDAC, and conversely, TPA is an inducer of histone acetylases (HAT). Therefore, both inducers of KSHV lytic replication are affecting the acetylation state of the RTA promoter that is in turn dependent on methylation. Such findings imply that the control of latency and of lytic switching is a function of chromosomal architecture, and will involve the interplay between viral RTA and host factors that regulate chromatin methylation and acetylation.474
RTA can also autoactivate its own expression.121,370,387 A striking feature of RTA-mediated lytic gene expression was that RTA induced KSHV gene expression in a more powerful and efficient manner than TPA stimulation, indicating that RTA plays a central, leading role in KSHV lytic gene expression.318
After activation, RTA is recruited to its responsive elements through direct interaction and transactivation with RBP-Jκ, a notch signal pathway transcription factor, to activate viral lytic gene expression.83,84,199,262,263,474 Interestingly, RTA also contributes to the establishment of KSHV latency by activating LANA-1 expression through the notch signaling pathway RBP-Jκ. On the other hand, LANA-1 can inhibit viral lytic replication by inhibiting expression as well as antagonizing the function of RTA.248 The interaction between RTA and LANA-1 provides a feedback mechanism by which these two proteins can regulate each other and is likely to be a key event in the establishment of KSHV latency.247,249 RTA may also recruit CBP, the SWI/SNF chromatin remodeling complex, and the TRAP/mediator coactivator to its down-stream viral promoters, and that this recruitment is essential for RTA-dependent viral gene expression.190
RTA mRNAs and Protein Structure
Multiple transcripts were found to be encoded by the ORF50 region; the main transcript is a 3.6 kb mRNA that encodes the entire RTA. In addition, a downstream gene, K8 was also found to be encoded within the same RNA species. RTA protein is mainly encoded by ORF50 but obtains an additional 60 amino acids for its N-terminal through a splicing event which shifts its start code across ORF49.96,430 The transcript for RTA can potentially encode for a protein of 691 amino acids, and is predicted to have a molecular mass of 73.7 kDa. However, the expressed protein when analyzed by Western-blotting appears to be about 110 kDa, suggesting that RTA could be modified post-translationally by phosphorylation or by other mechanisms.275 RTA lacks any significant homology with cellular proteins but is functionally and genetically homologous the RTA proteins from EBV, HVS, RRV, and MHV68.116,179,268,482 The RTA protein consists of an N-terminal DNA binding domain, a central dimerization domain, a C-terminal acidic activation domain, and two nuclear localization signals (NLS).96,275 The DNA-binding domain of RTA is located at the amino terminus from aa 1 to 530. A deletion mutant of the activation domain sequences (aa 531–691), containing only the DNA binding domain, has been shown to be a trans-dominant-negative mutant that maintains DNA-binding activity for RTA responsive promoters but no longer activates lytic gene expression.275 The activation domain is located at the carboxyl terminus of the protein (aa 486–691) which is highly acidic and contains numerous charged amino acids.275 This region also contains four repeated units of a highly hydrophobic domain with sequence homology to other transcriptional factors such as VP16 domain A.275 These characteristics suggest that KSHV RTA is a member of a family of transcriptional factors.474
Downstream Targets of RTA
RTA has been shown to regulate and transactivate a number of downstream viral genes that function in lytic replication, including K1, K3, K5, DNA polymerase, vIL-6, vIRFs, vGPCR, K12, K15, etc.47,48,120,121,178,195,275,370,414,445,476 RTA activates downstream KSHV target genes by at least two mechanisms: direct recognition of RTA response elements (RRE) in the promoters of its target genes and interaction with cellular or viral proteins bound to the promoters.83,413 For example, RTA directly binds to the promoters of PAN and K12 but does not bind to ORF57 or vCCL-1 promoters. Conversely, RTA transactivates the promoters of ORF57 and vCCL-1 through the binding of a cellular factor, RBP-Jκ protein.88
There is no defined consensus sequence for direct RTA binding and there is no significant homology present in the RTA-responsive viral promoters. However, a comparison of the K8 RRE with other viral RRE revealed a pattern of multiple A/T triplets spaced with a periodicity of 10 or 20 bp.264 The diversity of RTA binding pattern implies that the activation of RTA target gene promoters may result from RTA interaction with other cellular or viral proteins that mediate the DNA-transcriptional complex interaction. One such cellular factor could be NF-κB.363 Other cellular factors involved in RTA transactivation may include TATA-binding protein such as the case in the K1 promoter.48 RTA may regulate vOX-2 (K14) and vGPCR genes through an IFN-stimulated response element (ISRE)-like sequence (K14 ISRE) in the promoter region.493
Cellular Factors Involved in RTA-Mediated KSHV Reactivation
Identification of cellular proteins that coordinate with RTA in transactivation and characterization of the mechanisms whereby such host–viral protein complexes mediate the switch from KSHV latency to lytic replication is an important step in understanding the virus life cycle and pathogenesis. Increased viral lytic reactivation has been observed following exposure of latently infected PEL cells to agents such as IL-6, IFN-γ, hypoxia, other viral agents, n-butyrate, ionomycin, 5-azacytidine, and TPA.86,92,95,117,123,198,292,295,303,359,412,451,504 Cellular pathways involved in the induction of viral lytic reactivation include the NF-κB pathway, and the protein kinase C (PKC) δ, and MEK/ERK, JNK, and p38 MAPK pathways.107,123,333,392 AP-1, the cellular activator protein complex composed of c-Jun and c-Fos heterodimers, mediates the transcription of MAPK target genes.463 The RTA promoter contains a consensus AP-1 binding site,463 and the OriLyts (origins of lytic replication) also contain several putative AP-1 binding sites.19 The presence of AP-1 binding sites in these regions allows the virus to respond to cellular conditions that may not be favorable for latency. NF-κB has been shown to be constitutively active in PEL cells231 and promotes their survival. The involvement of NF-κB in lytic reactivation is controversial.56,392 One study reported that inhibition of NF-κB led to increased viral lytic protein synthesis in KSHV-infected epithelial cells and PEL cells,56 however, another study demonstrated a requirement for NF-κB activation for the production of infectious virions.392 In the latter study, virion production was not diminished by suppression of NF-κB, but infectivity of the virions was decreased, suggesting NF-κB may be involved in multiple aspects of lytic reactivation and viral production. In addition, cellular pathways contributing to epigenetic effects such as DNA methylation and histone acetylation may also participate in the reactivation of KSHV.95,273
The direct interaction of (CREB)-binding protein (CBP) and p300 with KSHV RTA in the activation of lytic replication has recently been reported by Hwang et al.191,209 HDAC was shown to repress RTA activity by binding directly to the proline-rich sequences in the RTA central domain aa 301–449. Specific inhibition of HDAC by trichostatin A (TSA) derepressed the inhibition of RTA and stimulated RTA-directed gene expression. KSHV RTA strongly induces CD21 and CD23a expression through RBP-Jκ binding sites and regulates RBP-Jκ-mediated cellular gene expression, which ultimately provides a favorable milieu for viral reproduction in the infected host.84 RTA was also shown to interact with other cellular factors such as RAP, C/EBPα, CBP, STATs, RBP-Jκ, SWI/SNF, TRAP230, PKC, MAP kinase, AP-1, NF-κB190,191,193,209, 258,265,423,462,464,480,484,488 and transactivates target genes through Oct-1 and Sp-1.370,445
Recently, Wang et al. reported that IRF-7 negatively regulates KSHV lytic reactivation by competing with RTA for binding to the RTA response element in the ORF57 promoter to down regulate RTA-induced gene expression.458 Yu et al. reported that IRF-7 is targeted for degradation through an ubiquitination-dependent fashion, and RTA itself acts as a ubiquitin E3 ligase.491 Previously, Zhu et al. reported that ORF45 blocks the phosphorylation and nuclear translocation of IRF-7 and efficiently inhibits the activation of IFN-α/β genes during viral infection.501
KSHV Primary Infection
Characterization of KSHV primary infection relies heavily on the development of systems with high infection efficiency. KSHV isolated from PEL and sometimes KS lesions is able to infect various cell types but with limited primary infection efficiency or unsustainable infection culture (see Sect. A). Of all the systems examined so far, KSHV eventually establishes latency after primary infection. In some studies, KSHV was found to immediately enter latency. However, other studies have indicated that KSHV has an early full productive replication phase during infection in at least some cell types or infection conditions.124,149,166 In fact, in the first description of KSHV infection and transmission system in 293 cells, cytopathic effect (CPE) was observed after primary infection, an indication of lytic replication.152 In both MVDEC and HUVEC, KSHV linear genomes and lytic transcripts were detected several days after primary infection, again indicating productive primary infection.124 Strikingly, efficient infection of HUVEC by recombinant KSHV BAC36 is lytic replication-permissive at the early stage of infection, producing large amount of infectious virions.166 Examination of the expression of lytic proteins and transcripts further confirmed KSHV productive lytic replication in this system.166,489 BAC36 infection of HUVEC displayed two phases: an early productive phase, in which the virus actively replicates producing large number of virions, and concomitantly resulting in massive cell death; and a latent phase, in which the virus switches into latent infection in the surviving cells.166 Similar results were also observed in a separate study.149 The different results from these studies point to the variations of cell types in supporting KSHV productive primary infection. The determination of the cell types and/or conditions that can support KSHV productive primary infection and the delineation of the underlying molecular basis could help understand the mechanism controlling KSHV replication and latency.
Attachment, Entry, and Cellular Receptors
Enveloped viruses infect host cells in two steps. The first step is attachment, or binding, of the virus particle to host cell receptors. This step is mediated by the interaction of viral proteins with cell surface molecules such as glycosaminoglycans (i.e., heparan sulfate).416 Attachment allows other viral proteins to contact cellular coreceptors, which will then stimulate the second step, entry, by either a fusion event between viral envelope and cell membrane, or receptor-mediated endocytosis.416
Similar to other herpesviruses, KSHV expresses several transmembrane glycoproteins that are virion associated, and involved in the attachment and entry of target cells. KSHV glycoproteins gB (ORF8), gH (ORF22), gL (ORF47), gM (ORF39), and gN (ORF53) are all conserved among the herpesviruses. In addition, KSHV encodes several unique glycoproteins K1, K8.1A, K8.1B, and vOX-2 that share no significant homology with glycoproteins of other herpesviruses.82,105,240,242,261,277
The ability of KSHV to infect a variety of cell types in vivo and in vitro7,31,164,303,357 indicates that it must be able to recognize either ubiquitously expressed cell surface receptors, or more than one type of receptor. To date, KHV has been shown to attach to the cell surface molecules heparan sulfate, integrin α3β1, and DCSIGN.6–8,39,354,454 K8.1A and gB have both been shown to interact with the ubiquitously expressed heparan sulfate.6,8,39,454 Heparan sulfate is a linear carbohydrate that is localized at the extracellular cell surface, typically covalently bound to a core proteoglycan imbedded in the cell membrane. Heparan sulfate proteoglycans participate in many biological processes including cell–matrix interactions, activation of chemokines, enzymes, and growth factors,440 and is well established to be important for the cell attachment of many other herpesviruses.416
Although conserved among herpesviruses, only KSHV gB contains an RGD motif, which is the minimal peptide region known to interact with integrins in the cell membrane.5,7,455 KSHV gB specifically binds integrin α3β1 through its unique RGD motif.7,494 Integrins are a large family of heterodimeric receptors that contain two transmembrane glycoprotein subunits, α and β. There are 24α and 9β subunits identified so far, with more than 24 known combinations of these subunits.172,346 Each cell expresses several combinations of αβ integrins, and each combination has its own binding specificity and signaling properties.172,346,380,382 Integrin α3β1 is a receptor for laminin 5 and fibronectin, and is expressed at high levels in endothelial cells.220,479,494
DC-SIGN (dendritic cell-specific ICAM-3-grabbing nonintegrin) is a type II C-type lectin that is found on myeloid dendritic cells in the dermis, mucosa, lymph nodes, lung, and thymus, and IL-4-stimulated monocyte-derived dendritic cells.356,409,443 It is also expressed on lung alveolar macrophages,409 placenta,408 inflammatory lesions411 and IL-13-stimulated, monocyte derived macrophages,94,409–411 DC-SIGN acts as a pathogen recognition receptor in these cells of the immune system, activating macrophages and dendritic cells to ingest and process pathogens for antigen presentation to T cells.94,171 KSHV may have evolved the ability to exploit DC-SIGN in order to infect dendritic cells and macrophages, and interfere with their antigen presentation functions.354
Following attachment to the cell, an enveloped virus such as KSHV can gain entry to the cell by either direct fusion of its envelope with the plasma membrane or by endocytosis, followed by fusion with the endosomal membrane. Evidence for both routes of entry has been published, and KSHV may use more than one mechanism, depending upon the cell type and which receptors are expressed.5,124,212,341
Endocytosis provides rapid and convenient transport of virion across the plasma membrane, through the cytoplasm, and delivery of the viral cargo to the perinuclear region. Endocytosis can occur through four major mechanisms: clathrincoated vesicles, the caveolar pathway, macropinocytosis, and a poorly characterized nonclathrin, noncaveolar dependent form of endocytosis.232,289,400 KSHV is able to infect human fibroblasts via endocytosis as demonstrated by the presence of virus particles inside endocytic vesicles within 5 min following attachment.5 The presence of transferrin within the vesicles implicates clathrin-mediated endocytosis as the means used by the virus to enter human fibroblasts. However, it has been demonstrated for other herpesviruses, i.e., HSV-1, that entry via endocytosis results in nonproductive infection with minimal infection.237
Fusion of the viral envelope at the plasma membrane has been well established for other members of the herpesvirus family.416 Some studies indicate that KSHV fuses with the plasma membrane to enter target cells in 293 cells and MVDEC.124,212,341 Expression of KSHV envelope glycoproteins gB, gH, and gL in mammalian cells induced cell–cell fusion.341 Expression of the cellular membrane protein xCT facilitates the fusion of KSHV-negative cells with KSHV-positive cells BCBL-1 that have been stimulated to express viral lytic proteins, specifically glycoproteins at the cell plasma membrane.225 Transfection of xCT into cell lines that normally are resistant to KSHV infection rendered them permissive to fusion and infection by KSHV. The xCT protein is a transmembrane protein that is upregulated in response to stress induced by the production of reactive oxygen species.351,379 Exposure of endothelial cells to ROS may enhance the infectivity of KSHV.459 In addition, the HIV protein Tat has been shown to stimulate the expression of xCT,51 and Tat can also enhance the infectivity of KSHV.16
Limited evidence demonstrates that KSHV adheres to the general dogma of herpesvirus family members once inside the host cell. In MVDEC, following envelope fusion with either the plasma membrane or the endosomal membrane, the viral tegument proteins and nucleocapsid are released into the cytoplasm.124 The nucleocapsid is then degraded, and the tightly packaged linear viral genome decondenses and is delivered to the nucleus. Within 8 h post infection, the viral genome circularizes, which is the typical conformation of the genome during latency. However by 72 h post infection, both circular and linear genomes can be detected, indicating a mixed population of latent and lytic infection.124 In fibroblasts, nuclear trafficking appears to be mediated by microtubules and the associated dynein motors.321 These observations remain to be confirmed in endothelial cells.
Regulation of Cellular Signaling Pathways and Expression of Viral and Cellular Genes
The events that lead to successful infection by KSHV, i.e., attachment, entry, nuclear trafficking, and expression of viral genes, cannot occur without careful manipulation of preexisting host cell signaling pathways and machinery, as well as suppression or evasion of host defenses.
The interaction of glycoprotein gB (discussed above) with cellular integrin α3β1 activates focal adhesion kinase (FAK) and the MEK-ERK1/2 pathway.7,393,394 Primary infection of HUVEC cells causes a conversion from the normal flat “cobblestone” cell morphology to a “spindle cell” typical of the cells seen in KS lesions.166 Spindle-cell conversion occurs as early as 6 h post infection and requires viral modulation of host cytoskeletal apparatus. Glycoprotein gB mediates this extensive cytoskeletal rearrangement by modulating the FAK-Src-PI3-kinase-RhoGTPase pathway.320,393 It has also been reported that glycoprotein gB can activate VEGFR-3 on the microvascular endothelium and trigger a migratory and proliferative response in these cells.494 Primary KSHV infection also activates and is dependent on the JNK and p38 MAPK pathways in addition to the MEK-ERK1/2 pathway.333,484 The activation of the multiple MAPK pathways is instrumental in the activation of the transcription factor AP-1. AP-1 regulates the expression of a variety of cellular genes, including IL-6, and in fact is required for the transcription of KSHV genes. KSHV induction of AP-1 could also contribute to a variety of KSHV-induced malignant phenotypes such as cellular proliferation, angiogenesis, inflammatory cytokine production, and dissemination of tumor cells.484 During primary infection, KSHV-encoded host-modulating genes are expressed which could impact the expression of host genes.241,489 Analysis of cellular transcripts during primary infection reveals that KSHV is able to significantly upregulate expression of cellular genes that are implicated in cell growth and survival, inflammation and angiogenesis, and immune responses.322
Viral Gene Expression During Primary Infection
The expression profiles of KSHV transcripts during KSHV primary infection depend on the infection systems. In the productive HUVEC primary infection system, the expression of latency-associated genes is generally expressed first preceding the cascade of lytic genes and onset of lytic replication. The transcription of lytic genes peaks around 54 h post infection followed the production of infectious virions.166,489 While after 54 h post infection, the expression of lytic genes declines, latency-associated transcripts continue to increase, indicating that following the permissive phase of lytic replication, surviving cells express the latency gene cluster, specifically ORF71/ORF72/ORF73, and have the tendency to switch to latency.489 Similarly, KSHV-encoded genes with host modulating functions, including mitogenic and cell cycle-regulatory, immune-modulating, and antiapoptotic genes, were expressed before those encoding viral structure and replication genes, and sustained at high levels throughout the infection, suggesting KSHV manipulation of host environment to facilitate infection.489 In the default latency infection systems of fibroblasts and MVDEC, the latent genes are also expressed throughout the infection process; however, lytic genes such as RTA are only transiently expressed, which is consistent with the lack of productive viral replication in this system. Nevertheless, the early expression of lytic genes involved in immunomodulation and resistance to apoptosis also suggests KSHV manipulation of host defenses to facilitate infection.241
MECHANISMS OF KSHV-INDUCED PATHOGENESIS
Introduction: KSHV Infection Promotes Oncogenesis
Although it remains controversial whether KS is a malignant neoplasm, at least the late stage of KS lesions are true clonal cancers, probably evolved from a constant reactive, inflammatory/angioproliferative process in immune compromised patients.77,138,139 Given the association of KSHV with two different human malignancies (KS and PEL), KSHV is considered to be a human oncogenic virus. Distinctive from other oncogenic viruses, KSHV is a complex DNA virus that not only has the ability to promote cellular growth and survival for tumor development but also can provoke deregulated angiogenesis, inflammation, and modulate the patient’s immune system in favor of tumor growth. Nevertheless, not all individuals harboring KSHV develop KS. The presence of KSHV DNA in some healthy individuals indicates that KSHV alone may not be sufficient to cause clinical KS. Since KS is most commonly found in immunosuppressed individuals, it has been suggested that immune deficiency is an important factor in the pathogenesis of AIDS-KS and that HIV infection may be an important cofactor in disease progression.54,138,139,176
The majority of spindle-shaped cells in KS lesions are latently infected by KSHV indicating that latent infection plays an essential role in KSHV-induced malignancy and pathogenesis.122,141,422 Nevertheless, a small subset of KS cells also undergoes spontaneous viral lytic replication indicating that KSHV lytic replication might also be important for KS development. There is strong correlation between viral load and progression of KS tumor.55,115,135,146,347 Several drugs that target herpesvirus replication have been shown to be effective to inhibit KS tumor growth.45,221,296,365 A number of viral lytic genes have been linked to KSHV-induced malignancy and pathogenesis.77 The production of infectious virions should lead to new infection, which could also produce virus-encoded cytokines and induce cellular cytokines as stated in Sect. C.
Promotion of Cellular Growth and Survival
Although the pathogenesis of KSHV-induced malignancies is still not completely understood, it appears that the virus targets multiple pathways to promote cell proliferation and survival to facilitate tumor development. Several studies have shown that genetic instability is present in KS tumors and PEL.72,119,160,163,348 KSHV infection is sufficient to induce chromosome instability,334 for which LANA-1 and vCyclin might be partially responsible.399,450 Like other oncogenic DNA viruses, KSHV targets both p53 and pRb tumor suppressor pathways. At least five KSHV genes, LANA-1, RTA, K-bZIP, LANA-2, and vIRF-1, interact with and suppress the functions of p53 and pRb.154,192,317,352,362,391,397 Loss or dysfunction of tumor suppressor genes will inhibit the host cell’s ability to repair damaged DNA, eliminate p53-dependent cell death, and dictate cell cycle for uncontrolled cell proliferation, hence contributing to KSHV-induced oncogenesis. Furthermore, KSHV can regulate cell cycle progression through vCyclin.90,260 Expressed in latently infected cells and in both KS and PEL cells, vCyclin can interact with and phosphorylate cyclin-dependent kinase 6 (Cdk6) to promote cell cycle progression from G1 to S-phase and accelerate cellular proliferation.90,100,175,224,260,376,432
KSHV uses multiple mechanisms to promote cell survival. The KSHV genome encodes several virus homologues of human antiapoptosis proteins. For instance, vBcl-2 is a KSHV homolog of human antiapoptosis protein Bcl-2.98,378 KSHV also encodes several cellular IRF homologues vIRFs that inhibit apoptosis.60,148,165,233,259,274,317,390,391 In addition, the KSHV K7 gene encodes a viral inhibitor of apoptosis (vIAP) survivin-ΔEx3 to inhibit apoptosis.456 Another unique mechanism that KSHV utilizes to promote tumor growth and cell survival is through activation of the NF-κB pathway. The NF-κB pathway is constitutively active in both KS and PEL,21,284 and treatment with inhibitors of NF-κB pathway lead to a complete regression of PEL tumors in a mouse model.230 Two KSHV genes, vGPCR and vFLIP, have been shown to enhance cell growth and survival by activating NF-κB pathway.93,145,270,284,386 vGPCR seems to play an important role in promoting endothelial cell proliferation and transformation.18,22,114,182,298,310,339,386,404,405 Endothelial cells ectopically expressing vGPCR have constitutively active VEGF receptors and can proliferate to form both foci in culture and tumors in nude mice independently of VEGF stimulation.23,182 KSHV-encoded vIL-6 also promotes cell survival.15,202,236,297,308,324,332,453 Finally, it is believed that a variety of cellular growth factors and inflammatory cytokines secreted by KSHV-infected endothelial cells and tumor-interacting stromal cells play a pivotal role in the development of KS. It is well documented that KSHV infection induces the secretion of various growth factors such as IL-6, IL-8, Gro-α, VEGF, and bFGF,71,78,282,322,457,484 which could promote cell proliferation through autocrine and/or paracrine signaling. It is important to emphasize that the interaction between HIV and KSHV in AIDS patients plays a significant role in tumor growth. The aggressiveness of AIDS-related KS implicates HIV-1 infection as an important cofactor in rapid KS progression; indeed, the time of KSHV seroconversion until the onset of KS may be years to decades in classic KS while it is only several months in most of the AIDS-related KS cases.167,168,291,325,337,355,360 HIV-1 not only activates lytic replication of KSHV206 but also induces secretion of a number of cytokines and growth factor from infected macrophages,189,215,256 further promoting tumor cell proliferation and survival, as well as tumor angiogenesis.
Regulation of Angiogenesis
KS tumors are highly angiogenic with abnormally dense and irregular blood vessels. The importance of angiogenesis in KS tumor development is further highlighted by the fact that early stage KS might not yet be a true tumor but a neoplasm of proliferative spindle-shaped cells driven by angiogenesis and inflammation. Angiogenesis is the formation of new blood vessels from existing capillary beds, which, with the exception of wound healing and female reproduction, is a rare event in adults.70,150 Pathological angiogenesis, however, correlates with tumor growth and metastasis.150 Angiogenesis is a complicated process that involves a number of different angiogenic factors. Some angiogenic factors initiate blood vessel remodeling by disrupting the existing blood vessels, while others are responsible for promoting migration, adhesion, and proliferation of endothelial cells for new blood vessel growth and maturation.150
The mechanisms of KSHV-induced angiogenesis remain to be further elucidated. KSHV-induced angiogenic factors and inflammatory cytokines likely play essential roles. In a SCID mouse model with human skin grafts, it was demonstrated that VEGF is essential for the inoculated early-stage KS cells to grow into KS-like tumors.373 Many of the cytokines, including VEGF, bFGF, IL-6, IL-8, GRO-α, TNF-β, and ephrin B2, induced by KSHV infection are angiogenic.282,283,322,457,484 Higher levels of serum VEGF were also seen in HIV-1 infected persons with KS compared with HIV-1 infected persons without KS,469 and higher mRNA levels of VEGF and angiopoietins, including both Ang-1 and Ang-2, were also detected at higher levels in KS lesions compared to the adjacent normal tissues.57 KSHV infection induces expression of cyclooxygenase-2 (COX-2)322 and heme oxygenase-1,286 both of which are important players in angiogenesis. In addition to the host factors, KSHV vIL-6 expressed during early infection has been shown to stimulate hematopoiesis, plasmacytosis, and angiogenesis.15,269,290 A number of KSHV proteins vIL-6, vGPCR, vCCL-1, and vCCL-II have been shown to promote angiogenesis by inducing VEGF expression through different signaling pathways.15,41,407,425 vGPCR alone can activate PKC, protein kinase B (PKB), Akt, NF-κB, and MAP kinases to regulate the expression of growth factors and cytokines.18,22,114,298,310,339,386,404 Transgenic mice expressing vGPCR display multi-focal and angioproliferative KS-like lesions.189,218,486
Immune Modulation
Like other viruses, KSHV must evade the host innate immunity that involves type 1 IFNs, phagocytes, natural killer (NK) cells, and complement, as well as the adaptive immune responses that include humoral and cell-mediated immunity. Viral evasion of host immunity not only safeguards virus persistence but also contributes to viral oncogenesis and pathogenesis. KSHV utilizes two major strategies to achieve successful infections. The first is the so-called passive strategy. After a successful entry into host cell, KSHV establishes latency, during which only a minimum number of virus genes are expressed, thus reducing the number of antigens that are exposed to the immune systems. Secondly, during lytic replication or de novo infection, when most viral proteins are expressed and are susceptible to immune surveillance, the virus utilizes an active strategy that involves a number of its own unique genes to modulate the host immune response. Thus, KSHV has evolved multiple mechanisms to not only evade the host immune response but also manipulate existing cytokine regulatory networks to facilitate viral replication and maintenance. In addition to exploiting the cytokine networks, KSHV also expresses genes that modulate the T-cell response, B-cell activation, as well as regulate the complement cascade.
KSHV has a large and complicated genome. It encodes not only proteins necessary for the reproduction and persistence of the virus but also pathogenic factors to promote tumor cell proliferation and survival, regulate angiogenesis, and modulate host immune response in favor of tumor growth (Fig. 1). The function and roles of each of these factors in relation to KSHV-induced malignancy and pathogenesis are further discussed in details.
Functions of KSHV Genes
LANA-1/LNA
In addition to its role in episomal persistence (maintenance, replication, and segregation), LANA-1 is a promiscuous protein that has many cellular interaction partners and modulates p53, STAT3, Rb, and GSK-3β pathways with integral roles in apoptosis, immune response, development, cell growth, proliferation, and transformation. LANA-1 acts directly or indirectly as a general transcriptional repressor or occasionally as a transcriptional activator. LANA-1 modulation of cellular functions might ultimately contribute to viral latency in KSHV-infected cells.
Much work has been done to determine the role of LANA-1 in KSHV-mediated transformation. In conjunction with the expression of Hras, LANA-1 transforms primary rat fibroblasts.352 Overexpression of LANA-1 increases cellular proliferation and lifespan of primary HUVEC.399,468 Nevertheless, overexpression of LANA-1 cannot induce anchorage independent growth, a hallmark of cellular transformation, in NIH3T3 cells, indicating that other viral genes are likely necessary for KSHV-induced malignant transformation.468
The mechanism of LANA-1-mediated cellular transformation has been extensively investigated. LANA-1 targets the Rb/E2F pathway and alleviates Rb-mediated transcriptional repression of the E2F pathway.352 LANA-1 upregulates the expression of telomerase by interacting with the Sp1 transcription factor.446 In latently infected PEL cells, LANA-1 blocks p53-mediated apoptosis by binding to and inhibiting its transcriptional activity.154 In KS tumors, apoptosis is not correlated with high expression levels of p53, rather, it is negatively correlated with the expression of LANA-1.228 LANA-1 inactivation of p53 activity has also been associated with genomic instability.399 HeLa and Rat-1 cell lines stably expressing LANA-1 have increased multinucleation, micronuclei, mitotic bridges, and abnormal formation of centrosomes.399
β-Catenin is overexpressed in PEL cells and KS tumors.156–158 Normally, β-catenin is degraded in the cytoplasm by its negative regulator GSK3β. LANA-1 causes the excess accumulation of β-catenin by binding and relocating GSK3β to the nucleus where it can no longer regulate β-catenin. Excess β-catenin accumulates and ultimately translocates to the nucleus where it interacts with Tcf/Lef transcription factors to regulate the expression of target genes. β-Catenin targets many genes that have important roles in cell cycle progression, cell growth, and cell proliferation.28,50 Notable β-catenin target genes are myc, jun, and cellular cyclin D1.158
Overexpression of LANA-1 induces the expression of IL-6 by activating the AP-1 pathway.12 One consequence of the autocrine and paracrine effects of IL-6 signaling is activation of the STAT3 signaling pathway. LANA-1 itself also binds STAT3 in KSHV-infected PEL cells and augments STAT3-induced transcription in reporter assays.315
LANA-1 positively and negatively regulates transcription through a variety of mechanisms. In in vitro experiments, the N- and C-terminals of LANA-1 repress transcription when fused to a heterologous DNA binding domain.385 Recruitment and interaction with members of the mSin3 transcriptional repressor complex is one mechanism by which LANA-1 represses transcription.244 LANA-1 represses the ATF4/CREB transcriptional activator without interfering with its DNA binding ability.266 LANA-1 inhibits the CBP histone acetyltransferase activity and represses CBP transcriptional activation by preventing its dimerization with c-Fos.244 LANA-1 interacts with a potent transcriptional repressor KLIP1 and relieves its transcriptional repression effect.335,496 LANA-1 activates the AP-1 pathway by binding to Jun and facilitating the formation of Jun–Fos heterodimers.13 LANA-1 also upregulates Id-1, which is a member of a family of basic helix–loop–helix containing proteins that have key roles in inhibiting cell differentiation, cell cycle regulation, and tumorigenesis.438 Id proteins are expressed at high levels in KS tumor cells, but their roles in KSHV-mediated tumorigenesis are not well understood because LANA-1 induced increases in Id protein expression has no discernable effect on the proliferative capacity of cells.438
LANA-1-mediated transcriptional regulation could contribute to viral latency. LANA-1 form complexes with RBP-Jκ to repress RTA expression.247 In addition, LANA-1 interacts with RTA and antagonizes its activation of the RTA promoter.247,248 LANA-1 also inhibits transcription of nonlatency-associated genes through its interaction with SUV39H1 and recruitment of HP-1.371 SUV39H1 is a histone-H3 methyltransferase. Upon methylation of histone-H3 by SUV39H1, HP-1 is recruited and forms condensed, transcriptionally inactive heterochromatin. Recruitment of these two factors by LANA-1 prompts condensation of the KSHV episome and consequently, transcriptional repression of all viral genes except those within the latency locus.371 Finally, LANA-1 autoregulates its promoter and induces transcription of itself.219
vCyclin
The decision to enter into the cell cycle is made in G0/G1 when cells are receptive to growth and differentiation signals.208 Cdks act in combination with cellular cyclins A, D, and E to push cells through the cell cycle.260 The KSHV vCyclin gene encodes a 257 amino acid protein with a molecular weight of 29 kDa. vCyclin has 32% identity and 54% similarity to mammalian cyclin D2 with the homology extending over the full length of the protein.260
vCyclin strongly binds to Cdk6 and weakly binds to Cdk2, Cdk3, Cdk4, and Cdk5; however, only the interaction with Cdk6 has functional significance.136,260,279,330 Cdk6 is the catalytic subunit of the protein complex.216 vCyclin binds tightly to Cdk6 to form a potent phosphorylation complex that phosphorylates many more targets, including p27KIP1, Id-2, Cdc25a, histone H1, Cdc6, Orc1, and Bcl-2260,279,449 than the Cdk6–cellular cyclin D2 protein complex.260 In addition, the vCyclin–Cdk6 protein complex is resistant to many cell cycle checkpoints, which contributes to increased rates of cell proliferation.330 These two characteristics have important implications for KSHV-infected cells. Increased cellular proliferation, apoptosis,330,450 and chromosomal abnormalities450 are three main consequences of overexpressing vCyclin.136,175,279,376,432 The vCyclin–Cdk6 complex phosphorylates and inactivates Bcl-2, thus contributing to vCyclin-mediated apoptosis.330,331
Rb serves as an important cell cycle checkpoint protein by binding to the transactivation domain of E2F transcription factors to inhibit their functions and prevent cell cycle progression.130,200 Rb interacts with a number of chromosome remodeling proteins like HDAC and the SWI/SNF complex to regulate gene expression.239 Cdk6–vCyclin phosphorylates and inactivates Rb. Consequently, KSHV-infected cells avoid Rb-induced cell cycle arrest.90,175,260
p27KIP1 is another important regulator of cell cycle. p27KIP1 mutation or inactivity is often associated with poor prognosis in tumor progression. vCyclin can overcome p27KIP1-mediated cell block.136,175,279,376,432 When vCyclin is overexpressed in cells, the vCyclin–Cdk6 complex interacts with and phosphorylates p27KIP1 on Thr187 inducing its degradation via the proteasome dependent pathway;136,376 however, the situation may be more complicated in vivo. Unexpectedly, p27KIP1 is more highly expressed in PEL cell lines when compared to other lymphomas.67,216,376 In PEL cells, the Cdk6–vCyclin complex prevents p27KIP1 from carrying out its function in two ways. First, it forms a stable complex with p27KIP1, thereby sequestering it and preventing it from carrying out its normal function.216 Second, the Cdk6–vCyclin complex phosphorylates p27KIP1 on Ser10, which causes p27KIP1 to undergo relocation to the cytoplasm. Upon lytic reactivation, though, the Cdk6–vCyclin complex does induce degradation of p27KIP1 via phosphorylation of Thr187.376 In addition to resistance to p27KIP1 inhibition, Cdk6–vCyclin resistance to cell cycle blocks by checkpoint proteins p16(Ink4a) and p21Cip1 has also been shown.279
K13/vFLIP
K13 encodes viral FLICE (FADD[Fas-associated death domain]-like IL-1betaconverting enzyme)-inhibitory protein (vFLIP), which is a viral homologue of cellular FLIP proteins.128 vFLIP utilizes its death effector domain and TRAF-binding domains to perform two principal functions, inhibition of apoptosis and constitutive activation of the NF-κB pathway. vFLIP is coded by either tricistronic transcripts or a bicistronic transcript that share with to other latent genes LANA-1 and vCyclin.125,185,434 The translation of the vFLIP protein is mostly initiated from IRES present in the vCyclin coding region, although a minor amount comes from reinitiation of translation.185,272
In the cytosol, vFLIP interacts with procaspase 8 to reduce the cleavage of procaspase 8 into its active subunits.32 vFLIP also inhibits caspase 3 and caspase 9 like activities in Fas-triggered A20 cells.32 In addition to its role in inhibiting Fas-induced apoptosis, vFLIP constitutively activates both canonical and noncanonical NF-κB pathways. vFLIP utilizes its TRAF-interacting domain to interact with the IκB kinase complex via TRAF2.188 IκB sequesters inactive NF-κB in the cytoplasm.20,24 Activation by cytokines induces recruitment of the IκB kinase complex, composed of IKKα, IKKβ, and IKKγ, which phosphorylates and inactivates IκB.20,24 This IκB inactivation allows NF-κB to translocate to the nucleus and stimulate the expression of NF-κB response genes. In PEL cells, vFLIP interacts with the IκB kinase complex and RIP, a protein kinase that recruits and activates MAPKKK proteins that in turn activate the IκB kinase complex.145,270 Hsp90 is an important member of this protein complex. Treatment of PEL cells with Hsp90 inhibitor ablates NF-κB activation.145
The implications of vFLIP-induced constitutive activation of the NF-κB pathway are significant. NF-κB activates many mitogenic pathways. Constitutive activation of the NF-κB pathways has been observed in KSHV PEL cells.187,270,284 RNAi-mediated knock-down of vFLIP reduced NF-κB activity by approximately 80% and sensitized PEL cells to external apoptotic signals, subsequently increasing apoptosis levels.187 The transforming ability of vFLIP derives from activation of the NF-κB pathway. Single gene expression studies of vFLIP in a Rat-1 cell line demonstrate that stable vFLIP expression increases cell growth and proliferation, but also facilitates loss of contact inhibition, anchorage independent growth in soft agar assays and induction of tumors in nude mice.429 A20 cells, a murine B-lymphoma cell line, transduced with vFLIP underwent cell proliferation despite constant stimulation with death signals.128 Ninety percent of immunocompetent mice injected with these vFLIP transduced A20 cells developed aggressive tumors. As mentioned above, IL-6 is a necessary growth factor for KSHV PEL cells. vFLIP upregulates the expression of cellular IL-6 by activating the JNK/AP-1 pathway.14 In situ hybridization analysis shows that vFLIP transcripts express at low levels in early KS lesions, but at dramatically higher rates in late stage KS tumors.427 vFLIP expression is inversely correlated with apoptosis.426
K12/Kaposin
Along with the proteins of the latency locus, K12 is expressed in most KSHV-infected spindle tumor cells.235 Moreover, K12 expression is found in all stages of KSHV tumorigenesis. In advanced spindle cell tumors, K12 expression is detected in approximately 85% of the spindle cells.257,311 K12 expression during latency varies from tumor to tumor, and cell line to cell line;257 however, its expression is strongly upregulated in the viral lytic cycle.87,413 Initial characterization of the K12 transcript identified a 700 bp gene product that coded for a protein with a predicted molecular weight of 6 kDa, now known as Kaposin.368 Later, detailed transcript analysis determined that the most common mRNA transcript encoded by the K12 locus is actually 1.5–2.3 kb in size.235,368 Consequently, the translation program for K12 was determined to be more complex than originally thought, utilizing canonical and alternative translation initiation codons to encode three proteins instead of one.368 These proteins were assigned the names, Kaposin A, Kaposin B, and Kaposin C. While functional characterization of Kaposin A and Kaposin B has been carried out, to date no function has been ascribed to Kaposin C.
Most of the initial characterization on K12 was done with the predominant protein product, Kaposin A.235 Kaposin A has a predicted molecular weight of 6 kDa; however, it is often detected on Western blots as 16–18 kDa bands and sometimes higher,235,312 hinting that the protein undergoes extensive posttranslational modification.312 Kaposin A is a highly hydrophobic type II transmembrane protein and is expressed on the cell surface.235,311 Initial characterization revealed the transformation nature of Kaposin A. In NIH3T3 and Rat-3 cell lines, overexpression of Kaposin A caused increased foci formation, loss of stress fibers, and loss of contact inhibition.235,311 When focally transformed Rat-3 cell lines were injected into nude mice; highly vascular and undifferentiated fibrosarcomas formed within 1–2 weeks.311 Though the level of transforming activity found in the Rat-3 assay was less than what is observed in typical cellular and retroviral oncogenes, it was similar to levels found in other herpesviruses.311 Kaposin A might mediate mitogenic pathways to achieve cellular transformation. Kaposin A activates the ERK-1/2 MAPK pathway and downstream AP-1235 through its interaction with cytohesin-1, a guanine nucleotide exchange factor that regulates the function of β-integrins. Cells transformed by Kaposin A show elevated activities of cellular serine-threonine kinases such as PKC, CAM kinase I, and MLCK.312 Kaposin A possesses a LXXLL nuclear receptor-binding motif. Mutations in this LXXLL motif ablated Kaposin A’s transforming ability.312
Most cells that express Kaposin A also express Kaposin B. Kaposin B is a 38 kDa protein442 coded by a sequence upstream of the Kaposin A translation initiation site. The Kaposin B transcript utilizes the alternative start codon, CUG, and encodes a series of 23 nucleotide GC rich repeats that vary in different KSHV isolates.257,368 Kaposin B upregulates cytokine expression by blocking the degradation of their mRNA transcripts.287 Kaposin B binds to and activates MK2 resulting in the inhibition of U-rich elements (AREs)-mediated mRNA degradation.287
K15
K15 has a complex expression profile.103 Isolates from BCBL-1 cells show differentially spliced protein products ranging from 281 to 489 aa in length, and 23–55 kDa in size that localize to the endoplasmic reticulum, mitochondria, and plasma membrane surface.103,395 K15 can also undergo proteolytic cleavage.395 K15 is weakly expressed during latency, but is strongly upregulated in viral lytic cycle.476 K15 promoter is activated by RTA.103,174,476 Although there is no sequence homology, K15 has the same structural characteristics as EBV LMP2A including a highly hydrophobic N-terminus, a variable number of transmembrane domains, and a C-terminus containing motifs with high sequence identity to putative SH2 and SH3 src kinase binding domains.52,103,476 Unlike LMP2A, the cytoplasmic domain of K15 does not possess signal transduction capability even though the domain is readily phoshphorylated103; however, like LMP2A, it inhibits B-cell receptor (BCR) activation.103 K15 has been shown to localize in the lipid rafts, common carriers of signal transducers like BCR.52 The K15 C-terminus binds TRAF-1, TRAF-2, and TRAF-3.52,174 K15 interaction with TRAF-2 strongly activates the NF-κB pathway and the AP-1 transcription factor through the JNK1 and ERK MAP kinase.52 All K15 spliced variants have a common C-terminus and high sequence variation in the N-terminal hydrophobic regions.52,103,476 Even though the C-terminus cannot function as a signal transducer, phosphorylation in its SH2 domain has been detected.52
Viral Interferon Regulatory Factors
KSHV encodes three homologues of human IFN regulatory factors (vIRFs) including vIRF, vIRF-2 encoded by K11.1, and vIRF-3 or LANA-2.61,165,300,362 Cellular IRFs are known to participate in IFN signal transduction. The diverse cellular effects of IFN, including tumor suppression through induction of p21 and histone H4 gene, induction of apoptosis, upregulation of MHC class I and II antigen expression, and regulation of the natural killer cell development, are mediated directly by IRFs or indirectly through interaction with various cellular factors including STAT proteins, p300/CBP, and NF-κB.203,278,307 The most common IRF-targeting promoter sites are ISRE, GAS, PRD-I, PRD-II, PRD-III, PRD-IV, and NF-κB-binding sites. Some IRFs positively regulate the transcription of IFN-responsive genes53,196,344,369 while others negatively regulate IFN signaling.470,471 For example, IRF-1 and IRF-2 compete for the identical ISRE, accounting for their antagonistic activities.196 IRF-1 positively reinforces IFN-β signal transduction, whereas IRF-2 is a negative regulator. Furthermore, IRF-1 is a tumor suppressor while IRF-2 is an oncogene.196,436,437 IRF-1 overexpression promotes cell cycle arrest through p53-independent pathways by direct induction of p21.435,436 IRF-2 overexpression inhibits these IFN-mediated effects leading to cellular transformation and tumor formation in nude mice.197 vIRF is the first KSHV oncogene identified.165 Overexpression of vIRF in rodent fibroblast cells causes cellular transformation and downregulation of p21 and MHC class I antigen through the inhibition of cellular IFN signal transduction pathways.148,165,259,503 In this regard, vIRF behaves similarly to IRF-2. vIRF has also been shown to be a transcriptional regulator and regulates the expression of other KSHV genes.259,364 vIRF probably achieves these functions through interactions with cellular IRF-1, IRF-3, ICSBP, p300, and CBP.60,165,258,267,319,389 It appears that vIRF represses the IFN antiviral response by blocking IRF-3 recruitment of the CBP/p300 coactivators.267 Furthermore, vIRF interacts with p53, and inhibits p53-dependent apoptosis.317,391 vIRF also autoactivates its own expression through two cis-elements, named Vac1 and Vac2, in the vIRF promoter that do not contain any ISRE-like sequences and are unresponsive to induction with IFN-α/β.465 Thus, vIRF might be able to target other viral and cellular genes in an IFN-independent mechanism. vIRF is an early lytic gene expressed in KS lesions.127,467 Although the expression of vIRF is significantly increased by TPA, low level of vIRF expression is also detected in uninduced KSHV-infected cell lines which may be either due to the presence of a minor latent transcript or an undefined expression mechanism of regulation that is not tied to viral lytic replication.96,467 The control of vIRF expression during latency is mostly mediated by a transcriptional silencer Tis in the vIRF promoter.466 Similar mechanisms of regulation may also be present for other KSHV lytic genes in addition to LANA-1-mediated gene repression.
vIRF-2 encodes a protein of 18 kDa. Similar to vIRF, vIRF-2 does not bind to ISRE, rather it binds to the NF-κB-binding site.61 vIRF-2 interacts with IRF-1, IRF-2, ICSBP, RelA, and p300 but not IRF-3 in vitro. Consequently, vIRF-2 inhibits the IRF-1- or IRF-3-mediated transcriptional activation of IFN-α promoter as well as RelA-stimulation of HIV LTR.61 vIRF-2 inhibits the antiviral effect of IFN by interacting with (double-stranded RNA-activated protein kinase) PKR and inhibiting its autophosphorylation, and the phosphorylation of PKR substrates histone 2A and eukaryotic translation initiation factor 2α.59 Interestingly, vIRF-2 is expressed at low levels in BCBL-1 cells and is not induced by TPA, suggesting that it may be a latent gene.59 The expression profile of vIRF-2 in KS lesions is unknown.
LANA-2 is abundantly expressed in the nuclei of PEL cells and has been described as a latent gene. However, because its expression levels can be greatly increased following TPA treatment, and is not expressed in KS tumors, whether it is truly latent remains controversial.274,362 LANA-2 interacts with p53 to mediate gene transcription, and recruits IRF3 and IRF7 to inhibit IFN signaling.274,362
vGPCR
vGPCR is a 7-transmembrane, IL-8 receptor homologue that constitutively signals several pathways downstream of multiple G protein subunits in a phospholipase C-and phosphatidylinositol 3-kinase (PI-3K)-dependent manner.65 Signaling through PKC, PKB, Akt, NF-κB, and MAPK pathways increases expression of genes involved in cellular proliferation, cell survival, and transformation, tendering vGPCR as one of the potential key factors involved in KSHV-induced malignancies.18,22,114,183,298,299,310,339,386,404,406,407 vGPCR increases expression of a number of autocrine and paracrine cytokines and growth factors such as IL-1β, IL-6, IL-8, GM-CSF, TNF-α, bFGF, Gro-α, VEGF, and MCP-1, which might be essential for early KS cell proliferation and KS tumor angiogenesis and inflammation.22,299,339,386,396,407 Thus, vGPCR may contribute to KS development via an autocrine and paracrine mechanism albeit it is a viral lytic gene. Expression of vGPCR in endothelial cells leads to constitutive activation of VEGF receptor and cell immortalization,23,183,406 and transgenic mice expressing vGPCR developed highly vascular “KS-like” lesions.218,486 Interestingly, vGPCR may also regulate the expression of both latent and lytic genes.64,421
vIL-6
vIL-6 is a viral early gene. As a homologue of cellular IL-6, vIL-6 can stimulate multiple cellular pathways to promote cellular proliferation, cell survival, and extrahepatic acute-phase response through engagement of the gp130 coreceptor independently of IL-6 receptor gp80.104,202,236,297,300,308,324,332,453 vIL-6 also plays an important role in KSHV immune modulation, protecting PEL cells from IFN-α-induced antiviral response.92 In addition, vIL-6 induces the secretion of cellular IL-6 and VEGF to support cell growth of IL-6-dependent cells, and contribute to hematopoiesis, tumorigenesis, and angiogenesis.15,153,159,269,305
K1 (KIS)
K1, the first ORF in the KSHV genome, encodes a type 1 membrane glycoprotein that is significantly induced during viral lytic replication.254,448 K1 can activate B cells and induce inflammatory cytokines to regulate immune responses.254 K1 promotes cell growth and causes immortalization of primary endothelial cells.255,460 Expression of K1 in rodent fibroblasts produces morphological changes and foci formation, characteristics of cellular transformation.255 K1 transgenic mice develop sarcomas and plasmablastic lymphomas and display constitutive activation of NF-κB, Oct-2, and Lyn.349 In addition, it has been shown that K1 can induce matrix metalloproteinase-9 and VEGF in endothelial cells,457,461 two factors implicated in angiogenesis and cell invasion.
K7/vIAP
vIAP is a homolog of the cellular protein survivin-ΔEx3 and is not found in any other herpesviruses. Both the viral and cellular survivin-ΔEx3 contain a conserved BH2 domain and a partial IAP repeat domain.456 vIAP is a 19–21 kDa glycoprotein that localizes to the host cell mitochondrial membrane and inhibits apoptosis induced by the Fas and TRAIL pathways, Bax, TNF-α and cycloheximide, staurosporine, and ceramide.143,456,490 vIAP protects KSHV-infected cells from apoptotic death in two distinct ways. First, it enhances cytosolic Ca2+ influx and prevents mitochondrial damage.143 Second, it acts as protein bridge between the host mitochondrial protein Bcl-2 and caspase 3 to effectively suppress caspase 3 function.456
MIR-1/K3 and MIR-2/K5
Two similar proteins MIR-1 and MIR-2 have been shown to actively prevent the expression of cell surface MHC Class I antigen that serve to alert the cytolytic T-lymphocytes (CTLs), the main protagonists of virus elimination.110,111,214 Most virus-specific CTLs are CD8+ T cells that recognize cytosolic, usually endogenously synthesized, viral antigens in association with Class I MHC on any nucleated cell. MIR-1 and MIR-2, expressed during lytic replication and localized at the endoplasmic reticulum (ER), negatively modulate the CTLs-dependent immune response by triggering endocytosis of the surface MHC Class I molecules for degradation.110,214 This function of MIR-2 is specific for human leukocyte antigen (HLA)-A and HLA-B, which are responsible for presenting antigens to CTLs, and to a lesser extent, HLA-E, but not HLA-C.110,214 In contrast to MIR-2, MIR-1 downregulates HLA-A, -B, -C, and -E.214 Consequently, KSHV-infected cells expressing reduced levels of MHC Class I molecules, are deficient in antigen processing and presentation, and are therefore less susceptible to CTL killing.49 In addition, MIR-2 also reduces the expression of molecules involved in T-cell activation, reducing the visibility of KSHV-infected B cell to T-helper cells, thereby decreasing T-cell responses, cytokine release and the production of co-stimulatory signals for CTL generation.111 Both MIR-1 and MIR-2 may also help KSHV evade natural killer cell immune responses, which cause immediate killing of virus-infected cells through exocytosis of perforin- and granzyme-containing granules and secreting cytokines, mainly IFN-γ, to activate CTLs and macrophages.213,375
vCCL-1, vCCL-2, and vCCL-3
vCCLs are three KSHV lytic proteins formerly known as vMIPs. vCCL-1 (K6), vCCL-2 (K4), and vCCL-3 (K4.1) share 25–40% homology with human MIP-1α (macrophage inflammatory protein1α) aβCC chemokine.300,324 Chemokines are molecules that interact with G-protein coupled chemokine receptors and recruit leukocytes to sites of inflammation, and also are involved in angiogenesis, haematopoiesis, and lymphocyte development.4,102,271,313,314 All three vCCLs are able to induce angiogenesis in the chorioalantonic membranes of chicken eggs41 suggesting that they may act synergistically with host factors such as VEGF, bFGF, and IL-6 to promote the abnormal angiogenesis in KS lesions. vCCL-1 is able to bind to a broad range of chemokine receptors including CCR1, CCR2, CCR5, CXCR4, and CX3CR1.234 vCCL-1 may act as a competitive antagonist with the host chemokines, since it does not induce a Ca2+ influx after binding these receptors. In vitro, vCCL-1 blocked the chemoattractive properties of host CC and CXC chemokines,41,97,234,300 while in a rat model, vCCL-1 suppressed the host inflammatory response and decreased the infiltration of inflammatory leukocytes.97 vCCL-1 and vCCL-2 bind to CCR-8, and vCCL-1 is able to induce the secretion of VEGF-A in PEL cells.269 Both vCCL-1 and vCCL-2 are able to block dexamethasone-induced apoptosis in PEL cells.269 vCCL-3 binds to CCR-8 and CCR-4, and has been shown to be selectively chemoattractant for Th2 cells.425 KSHV vCCLs can polarize the adaptive immune response toward a predominantly Th2-type (i.e., humoral) response at sites of KSHV infection, potentially reducing the efficacy of the antiviral response.
KCP (ORF4)
Complement bridges both innate and adaptive immune responses and humoral and cell-mediated immunity. The ORF4-encoded complement-control protein (KCP) inhibits the activation of the complement cascade.309,417–419 KCP is also associated with the envelope of purified KSHV virions where it potentially protects them from complement-mediated immune response.418
K14 (vOX2)
vOX2 is a homolog of the cellular protein OX2, a glycosylated cell surface protein and member of the immunoglobulin superfamily that restricts cytokine production in a paracrine fashion. vOX2 has structure similar to cellular OX2; however, in contrast to cellular OX2, it potently activates inflammatory cytokine production.105 vOX2 induces the production of IL-1β, TNF-α, and IL-6 from various cell types, including monocytes/macrophages, and dendritic cells, and cooperates with IFN-γ in a paracrine manner to induce cytokine production in a B-cell line.105
vBcl-2 (ORF16)
vBcl-2 is a viral lytic protein expressed in both spindle cells and monocytes in KS lesions.378,475 vBcl-2 only displays 15–20% amino acid identity to cellular Bcl-2, but it contains the critical BH1 and BH2 domains required to heterodimerize with cellular Bcl-2, and potently suppress caspase 3 death effector functions.98,378 vBcl-2 is an even more potent suppressor of apoptosis than its cellular homolog, Bcl-2. vBcl-2 has been shown to prevent apoptosis induced by vCyclin, and unlike cellular Bcl-2, vBcl-2 cannot be converted to a pro-apoptotic form by caspase-mediated cleavage.33,98
SUMMARY
KSHV has been established as the causative agent of KS, PEL, and MCD, malignancies occurring more frequently in AIDS patients. The aggressive nature of KSHV in the context of HIV infection suggests that interactions between the two viruses enhance pathogenesis. KSHV latent infection and lytic reactivation are characterized by distinct gene expression profiles, and both latency and lytic reactivation seem to be required for malignant progression. As a sophisticated oncogenic virus, KSHV has evolved to possess a formidable repertoire of potent mechanisms that enable it to target and manipulate host cell pathways, leading to increased cell proliferation, increased cell survival, dysregulated angiogenesis, evasion of immunity, and malignant progression in the immunocompromised host.
Worldwide, approximately 40.3 million people are currently living with HIV infection.2 Of these, a significant number are coinfected with KSHV.118,129,381 The complex interplay between the two viruses dramatically elevates the risk for development of KSHV-induced malignancies, KS, PEL, and MCD. Although HAART significantly reduces HIV viral load, the entire T-cell repertoire and immune function may not be completely restored.99 In fact, clinically significant immune deficiency is not necessary for the induction of KSHV-related malignancy.81 Because of variables such as lack of access to therapy, noncompliance with prescribed treatment, failure to respond to treatment and the development of drug-resistant strains of HIV, KSHV-induced malignancies will continue to present a major health concern.
REFERENCES
- 1.Classification system for human T-lymphotropic virus type III/lymphadenopathy-associated virus infections. JAMA. 1986;256:20–1. 24–5. Leads from the MMWR. [PubMed] [Google Scholar]
- 2.UNAIDS Reference Group on estimates, modelling and projections – statement on the use of the BED assay for the estimation of HIV-1 incidence for surveillance or epidemic monitoring. Wkly Epidemiol Rec. 2006;81:40. [PubMed] [Google Scholar]
- 3.Ablashi DV, Chatlynne LG, Whitman JE, Jr., Cesarman E. Spectrum of Kaposi’s sarcoma-associated herpesvirus, or human herpesvirus 8, diseases. Clin Microbiol Rev. 1997;15:439–64. doi: 10.1128/CMR.15.3.439-464.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams DH, Lloyd AR. Chemokines: leukocyte recruitment and activation cytokines. Lancet. 1997;349:490–5. doi: 10.1016/s0140-6736(96)07524-1. [DOI] [PubMed] [Google Scholar]
- 5.Akula SM, Naranatt PP, Walia NS, Wang FZ, Fegley B, Chandran B. Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis. J Virol. 2003;77:7978–90. doi: 10.1128/JVI.77.14.7978-7990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akula SM, Pramod NP, Wang FZ, Chandran B. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology. 2001;284:235–49. doi: 10.1006/viro.2001.0921. [DOI] [PubMed] [Google Scholar]
- 7.Akula SM, Pramod NP, Wang FZ, Chandran B. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell. 2002;108:407–19. doi: 10.1016/s0092-8674(02)00628-1. [DOI] [PubMed] [Google Scholar]
- 8.Akula SM, Wang FZ, Vieira J, Chandran B. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology. 2001;282:245–55. doi: 10.1006/viro.2000.0851. [DOI] [PubMed] [Google Scholar]
- 9.Albini A, Paglieri I, Orengo G, Carlone S, Aluigi MG, DeMarchi R, Matteucci C, Mantovani A, Carozzi F, Donini S, Benelli R. The beta-core fragment of human chorionic gonadotrophin inhibits growth of Kaposi’s sarcoma-derived cells and a new immortalized Kaposi’s sarcoma cell line. AIDS. 1997;11:713–21. doi: 10.1097/00002030-199706000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Alexander L, Denekamp L, Knapp A, Auerbach MR, Damania B, Desrosiers RC. The primary sequence of rhesus monkey rhadinovirus isolate 26–95: sequence similarities to Kaposi’s sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J Virol. 2000;74:3388–98. doi: 10.1128/jvi.74.7.3388-3398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amin HM, Medeiros LJ, Manning JT, Jones D. Dissolution of the lymphoid follicle is a feature of the HHV8+ variant of plasma cell Castleman’s disease. Am J Surg Pathol. 2003;27:91–100. doi: 10.1097/00000478-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 12.An J, Lichtenstein AK, Brent G, Rettig MB. The Kaposi sarcoma-associated herpesvirus (KSHV) induces cellular interleukin 6 expression: role of the KSHV latency-associated nuclear antigen and the AP1 response element. Blood. 2002;99:649–54. doi: 10.1182/blood.v99.2.649. [DOI] [PubMed] [Google Scholar]
- 13.An J, Sun Y, Rettig MB. Transcriptional coactivation of c-Jun by the KSHV-encoded LANA. Blood. 2004;103:222–8. doi: 10.1182/blood-2003-05-1538. [DOI] [PubMed] [Google Scholar]
- 14.An J, Sun Y, Sun R, Rettig MB. Kaposi’s sarcoma-associated herpesvirus encoded vFLIP induces cellular IL-6 expression: the role of the NF-kappaB and JNK/AP1 pathways. Oncogene. 2003;22:3371–85. doi: 10.1038/sj.onc.1206407. [DOI] [PubMed] [Google Scholar]
- 15.Aoki Y, Jaffe ES, Chang Y, Jones K, Teruya-Feldstein J, Moore PS, Tosato G. Angiogenesis and hematopoiesis induced by Kaposi’s sarcoma-associated herpesvirus-encoded interleukin-6. Blood. 1999;93:4034–43. [PubMed] [Google Scholar]
- 16.Aoki Y, Tosato G. HIV-1 Tat enhances Kaposi sarcoma-associated herpesvirus (KSHV) infectivity. Blood. 2004;104:810–4. doi: 10.1182/blood-2003-07-2533. [DOI] [PubMed] [Google Scholar]
- 17.Aoki Y, Tosato G. Role of vascular endothelial growth factor/vascular permeability factor in the pathogenesis of Kaposi’s sarcoma-associated herpesvirus-infected primary effusion lymphomas. Blood. 1999;94:4247–54. [PubMed] [Google Scholar]
- 18.Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn MC, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature. 1997;385:347–50. doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- 19.AuCoin DP, Colletti KS, Cei SA, Papouskova I, Tarrant M, Pari GS. Amplification of the Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 lytic origin of DNA replication is dependent upon a cis-acting AT-rich region and an ORF50 response element and the trans-acting factors ORF50 (K-Rta) and K8 (K-bZIP) Virology. 2004;318:542–55. doi: 10.1016/j.virol.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 21.Bailer RT, Ng-Bautista CL, Ness GM, Mallery SR. Expression of interleukin-6 receptors and NF-kappa B in AIDS-related Kaposi sarcoma cell strains. Lymphology. 1997;30:63–76. [PubMed] [Google Scholar]
- 22.Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, Gutkind JS, Asch AS, Cesarman E, Gershengorn MC. G-protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–9. doi: 10.1038/34193. E. A. and Mesri. [DOI] [PubMed] [Google Scholar]
- 23.Bais C, Van Geelen A, Eroles P, Mutlu A, Chiozzini C, Dias S, Silverstein RL, Rafii S, Mesri EA. Kaposi’s sarcoma associated herpesvirus G protein-coupled receptor immortalizes human endothelial cells by activation of the VEGF receptor-2/KDR. Cancer Cell. 2003;3:131–43. doi: 10.1016/s1535-6108(03)00024-2. [DOI] [PubMed] [Google Scholar]
- 24.Baldwin AS., Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 25.Ballestas ME, Chatis PA, Kaye KM. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science. 1999;284:641–4. doi: 10.1126/science.284.5414.641. [DOI] [PubMed] [Google Scholar]
- 26.Barbera AJ, Ballestas ME, Kaye KM. The Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen 1 N terminus is essential for chromosome association, DNA replication, and episome persistence. J Virol. 2004;78:294–301. doi: 10.1128/JVI.78.1.294-301.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbera AJ, Chodaparambil JV, Kelley-Clarke B, Joukov V, Walter JC, Luger K, Kaye KM. The nucleosomal surface as a docking station for Kaposi’s sarcoma herpesvirus LANA. Science. 2006;311:856–61. doi: 10.1126/science.1120541. [DOI] [PubMed] [Google Scholar]
- 28.Barker N, Morin PJ, Clevers H. The Yin-Yang of TCF/beta-catenin signaling. Adv Cancer Res. 2000;77:1–24. doi: 10.1016/s0065-230x(08)60783-6. [DOI] [PubMed] [Google Scholar]
- 29.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 30.Baylin SB. DNA methylation and gene silencing in cancer. Nat Clin Pract Oncol. 2005;2(Suppl 1):S4–11. doi: 10.1038/ncponc0354. [DOI] [PubMed] [Google Scholar]
- 31.Bechtel JT, Liang Y, Hvidding J, Ganem D. Host range of Kaposi’s sarcoma-associated herpesvirus in cultured cells. J Virol. 2003;77:6474–81. doi: 10.1128/JVI.77.11.6474-6481.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belanger C, Gravel A, Tomoiu A, Janelle ME, Gosselin J, Tremblay MJ, Flamand L. Human herpesvirus 8 viral FLICE-inhibitory protein inhibits Fas-mediated apoptosis through binding and prevention of procaspase-8 maturation. J Hum Virol. 2001;4:62–73. [PubMed] [Google Scholar]
- 33.Bellows DS, Chau BN, Lee P, Lazebnik Y, Burns WH, Hardwick JM. Antiapoptotic herpesvirus Bcl-2 homologs escape caspase-mediated conversion to proapoptotic proteins. J Virol. 2000;74:5024–31. doi: 10.1128/jvi.74.11.5024-5031.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benelli R, Repetto L, Carlone S, Parravicini C, Albini A. Establishment and characterization of two new Kaposi’s sarcoma cell cultures from an AIDS and a non-AIDS patient. Res Virol. 1994;145:251–9. doi: 10.1016/s0923-2516(07)80030-6. [DOI] [PubMed] [Google Scholar]
- 35.Beral V. Epidemiology of Kaposi’s sarcoma. Cancer Surv. 1991;10:5–22. [PubMed] [Google Scholar]
- 36.Beral V, Peterman TA, Berkelman RL, Jaffe HW. Kaposi’s sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123–8. doi: 10.1016/0140-6736(90)90001-l. [DOI] [PubMed] [Google Scholar]
- 37.Bieleski L, Talbot SJ. Kaposi’s sarcoma-associated herpesvirus vCyclin open reading frame contains an internal ribosome entry site. J Virol. 2001;75:1864–9. doi: 10.1128/JVI.75.4.1864-1869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bigoni B, Dolcetti R, de Lellis L, Carbone A, Boiocchi M, Cassai E, Di Luca D. Human herpesvirus 8 is present in the lymphoid system of healthy persons and can reactivate in the course of AIDS. J Infect Dis. 1996;173:542–9. doi: 10.1093/infdis/173.3.542. [DOI] [PubMed] [Google Scholar]
- 39.Birkmann A, Mahr K, Ensser A, Yaguboglu S, Titgemeyer F, Fleckenstein B, Neipel F. Cell surface heparan sulfate is a receptor for human herpesvirus 8 and interacts with envelope glycoprotein K8.1. J Virol. 2001;75:11583–93. doi: 10.1128/JVI.75.23.11583-11593.2001. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borkovic SP, Schwartz RA. Kaposi’s sarcoma presenting in the homosexual man – a new and striking phenomenon! Ariz Med. 1981;38:902–4. [PubMed] [Google Scholar]
- 41.Boshoff C, Endo Y, Collins PD, Takeuchi Y, Reeves JD, Schweickart VL, Siani MA, Sasaki T, Williams TJ, Gray PW, Moore PS, Chang Y, Weiss RA. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278:290–4. doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]
- 42.Boshoff C, Gao SJ, Healy LE, Matthews S, Thomas AJ, Coignet L, Warnke RA, Strauchen JA, Matutes E, Kamel OW, Moore PS, Weiss RA, Chang Y. Establishing a KSHV+ cell line (BCP-1) from peripheral blood and characterizing its growth in Nod/SCID mice. Blood. 1998;91:1671–9. [PubMed] [Google Scholar]
- 43.Boshoff C, Schulz TF, Kennedy MM, Graham AK, Fisher C, Thomas A, McGee JO, Weiss RA, O’Leary JJ. Kaposi’s sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat Med. 1995;1:1274–8. doi: 10.1038/nm1295-1274. [DOI] [PubMed] [Google Scholar]
- 44.Boshoff C, Whitby D, Hatziioannou T, Fisher C, van der Walt J, Hatzakis A, Weiss R, Schulz T. Kaposi’s-sarcoma-associated herpesvirus in HIV-negative Kaposi’s sarcoma. Lancet. 1995;345:1043–4. doi: 10.1016/s0140-6736(95)90780-7. [DOI] [PubMed] [Google Scholar]
- 45.Boulanger E. Human herpesvirus 8 (HHV8). II. Pathogenic role and sensitivity to antiviral drugs. Ann Biol Clin (Paris) 1999;57:19–28. [PubMed] [Google Scholar]
- 46.Bower M, Fox P, Fife K, Gill J, Nelson M, Gazzard B. Highly active anti-retroviral therapy (HAART) prolongs time to treatment failure in Kaposi’s sarcoma. AIDS. 1999;13:2105–11. doi: 10.1097/00002030-199910220-00014. [DOI] [PubMed] [Google Scholar]
- 47.Bowser BS, DeWire SM, Damania B. Transcriptional regulation of the K1 gene product of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2002;76:12574–83. doi: 10.1128/JVI.76.24.12574-12583.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bowser BS, Morris S, Song MJ, Sun R, Damania B. Characterization of Kaposi’s sarcoma-associated herpesvirus (KSHV) K1 promoter activation by Rta. Virology. 2006 doi: 10.1016/j.virol.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Brander C, Suscovich T, Lee Y, Nguyen PT, O’Connor P, Seebach J, Jones NG, van Gorder M, Walker BD, Scadden DT. Impaired CTL recognition of cells latently infected with Kaposi’s sarcoma-associated herpesvirus. J Immunol. 165:2077–83. doi: 10.4049/jimmunol.165.4.2077. [DOI] [PubMed] [Google Scholar]
- 50.Brantjes H, Barker N, van Es J, Clevers H. TCF: Lady Justice casting the final verdict on the outcome of Wnt signaling. Biol Chem. 2002;383:255–61. doi: 10.1515/BC.2002.027. [DOI] [PubMed] [Google Scholar]
- 51.Bridges CC, Hu H, Miyauchi S, Siddaramappa UN, Ganapathy ME, Ignatowicz L, Maddox DM, Smith SB, Ganapathy V. Induction of cystine-glutamate transporter xc-by human immunodeficiency virus type 1 transactivator protein tat in retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2004;45:2906–14. doi: 10.1167/iovs.03-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brinkmann MM, Glenn M, Rainbow L, Kieser A, Henke-Gendo C, Schulz TF. Activation of mitogen-activated protein kinase and NF-kappaB pathways by a Kaposi’s sarcoma-associated herpesvirus K15 membrane protein. J Virol. 2003;77:9346–58. doi: 10.1128/JVI.77.17.9346-9358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Briscoe J, Rogers NC, Witthuhn BA, Watling D, Harpur AG, Wilks AF, Stark GR, Ihle JN, Kerr IM. Kinase-negative mutants of JAK1 can sustain interferon-gamma-inducible gene expression but not an antiviral state. EMBO J. 1996;15:799–809. [PMC free article] [PubMed] [Google Scholar]
- 54.Brooks LA, Wilson AJ, Crook T. Kaposi’s sarcoma-associated herpesvirus (KSHV)/human herpesvirus 8 (HHV8)–a new human tumor virus. J Pathol. 1997;182:262–5. doi: 10.1002/(SICI)1096-9896(199707)182:3<262::AID-PATH836>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 55.Brown EE, Whitby D, Vitale F, Fei PC, Del Carpio C, Marshall V, Alberg AJ, Serraino D, Messina A, Gafa L, Goedert JJ. Correlates of Human Herpesvirus-8 DNA detection among adults in Italy without Kaposi sarcoma. Int J Epidemiol. 2005;34:1110–7. doi: 10.1093/ije/dyi131. [DOI] [PubMed] [Google Scholar]
- 56.Brown HJ, Song MJ, Deng H, Wu TT, Cheng G, Sun R. NF-kappaB inhibits gammaherpesvirus lytic replication. J Virol. 2003;77:8532–40. doi: 10.1128/JVI.77.15.8532-8540.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown LF, Dezube BJ, Tognazzi K, Dvorak HF, Yancopoulos GD. Expression of Tie1, Tie2, and angiopoietins 1, 2, and 4 in Kaposi’s sarcoma and cutaneous angiosarcoma. Am J Pathol. 2000;156:2179–83. doi: 10.1016/S0002-9440(10)65088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Browning PJ, Sechler JM, Kaplan M, Washington RH, Gendelman R, Yarchoan R, Ensoli B, Gallo RC. Identification and culture of Kaposi’s sarcoma-like spindle cells from the peripheral blood of human immunodeficiency virus-1-infected individuals and normal controls. Blood. 1994;84:2711–20. [PubMed] [Google Scholar]
- 59.Burysek L, Pitha PM. Latently expressed human herpesvirus 8-encoded interferon regulatory factor 2 inhibits double-stranded RNA-activated protein kinase. J Virol. 2001;75:2345–52. doi: 10.1128/JVI.75.5.2345-2352.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burysek L, Yeow WS, Lubyova B, Kellum M, Schafer SL, Huang YQ, Pitha PM. Functional analysis of human herpesvirus 8-encoded viral interferon regulatory factor 1 and its association with cellular interferon regulatory factors and p300. J Virol. 1999;73:7334–42. doi: 10.1128/jvi.73.9.7334-7342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burysek L, Yeow WS, Pitha PM. Unique properties of a second human herpesvirus 8-encoded interferon regulatory factor (vIRF-2) J Hum Virol. 1999;2:19–32. [PubMed] [Google Scholar]
- 62.Cai X, Cullen BR. Transcriptional origin of Kaposi’s sarcoma-associated herpesvirus microRNAs. J Virol. 2006;80:2234–42. doi: 10.1128/JVI.80.5.2234-2242.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cai X, Lu S, Zhang Z, Gonzalez CM, Damania B, Cullen BR. Kaposi’s sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proc Natl Acad Sci USA. 2005;102:5570–5. doi: 10.1073/pnas.0408192102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cannon M, Cesarman E, Boshoff C. KSHV G protein-coupled receptor inhibits lytic gene transcription in primary-effusion lymphoma cells via p21-mediated inhibition of Cdk2. Blood. 2006;107:277–84. doi: 10.1182/blood-2005-06-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cannon ML, Cesarman E. The KSHV G protein-coupled receptor signals via multiple pathways to induce transcription factor activation in primary effusion lymphoma cells. Oncogene. 2004;23:514–23. doi: 10.1038/sj.onc.1207021. [DOI] [PubMed] [Google Scholar]
- 66.Carbone A, Cilia AM, Gloghini A, Canzonieri V, Pastore C, Todesco M, Cozzi M, Perin T, Volpe R, Pinto A, Gaidano G. Establishment of HHV-8-positive and HHV-8-negative lymphoma cell lines from primary lymphomatous effusions. Int J Cancer. 1997;73:562–9. doi: 10.1002/(sici)1097-0215(19971114)73:4<562::aid-ijc18>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 67.Carbone A, Cilia AM, Gloghini A, Capello D, Fassone L, Perin T, Rossi D, Canzonieri V, De Paoli P, Vaccher E, Tirelli U, Volpe R, Gaidano G. Characterization of a novel HHV-8-positive cell line reveals implications for the pathogenesis and cell cycle control of primary effusion lymphoma. Leukemia. 2000;14:1301–9. doi: 10.1038/sj.leu.2401802. [DOI] [PubMed] [Google Scholar]
- 68.Carbone A, Cilia AM, Gloghini A, Capello D, Todesco M, Quattrone S, Volpe R, Gaidano G. Establishment and characterization of EBV-positive and EBV-negative primary effusion lymphoma cell lines harboring human herpesvirus type-8. Br J Haematol. 1998;102:1081–9. doi: 10.1046/j.1365-2141.1998.00877.x. [DOI] [PubMed] [Google Scholar]
- 69.Carbone A, Gaidano G. HHV-8-positive body-cavity-based lymphoma: a novel lymphoma entity. Br J Haematol. 1997;97:515–22. doi: 10.1046/j.1365-2141.1997.00064.x. [DOI] [PubMed] [Google Scholar]
- 70.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 71.Carroll PA, Brazeau E, Lagunoff M. Kaposi’s sarcoma-associated herpesvirus infection of blood endothelial cells induces lymphatic differentiation. Virology. 2004;328:7–18. doi: 10.1016/j.virol.2004.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Casalone R, Albini A, Righi R, Granata P, Toniolo A. Nonrandom chromosome changes in Kaposi sarcoma: cytogenetic and FISH results in a new cell line (KS-IMM) and literature review. Cancer Genet Cytogenet. 2001;124:16–9. doi: 10.1016/s0165-4608(00)00241-7. [DOI] [PubMed] [Google Scholar]
- 73.Caselli E, Galvan M, Cassai E, Caruso A, Sighinolfi L, Di Luca D. Human herpesvirus 8 enhances human immunodeficiency virus replication in acutely infected cells and induces reactivation in latently infected cells. Blood. 2005;106:2790–7. doi: 10.1182/blood-2005-04-1390. [DOI] [PubMed] [Google Scholar]
- 74.Caselli E, Galvan M, Cassai E, Di Luca D. Transient expression of human herpesvirus-8 (Kaposi’s sarcoma-associated herpesvirus) ORF50 enhances HIV-1 replication. Intervirology. 2003;46:141–9. doi: 10.1159/000071454. [DOI] [PubMed] [Google Scholar]
- 75.Caselli E, Galvan M, Santoni F, Rotola A, Caruso A, Cassai E, Luca DD. Human herpesvirus-8 (Kaposi’s sarcoma-associated virus) ORF50 increases in vitro cell susceptibility to human immunodeficiency virus type 1 infection. J Gen Virol. 84:1123–31. doi: 10.1099/vir.0.18799-0. [DOI] [PubMed] [Google Scholar]
- 76.Caselli E, Menegazzi P, Bracci A, Galvan M, Cassai E, Di Luca D. Human herpesvirus-8 (Kaposi’s sarcoma-associated herpesvirus) ORF50 interacts synergistically with the tat gene product in transactivating the human immunodeficiency virus type 1 LTR. J Gen Virol. 2001;82:1965–70. doi: 10.1099/0022-1317-82-8-1965. 2001. [DOI] [PubMed] [Google Scholar]
- 77.Cathomas G. Kaposi’s sarcoma-associated herpesvirus (KSHV)/human herpesvirus 8 (HHV-8) as a tumor virus. Herpes. 2003;10:72–7. [PubMed] [Google Scholar]
- 78.Cerimele F, Curreli F, Ely S, Friedman-Kien AE, Cesarman E, Flore O. Kaposi’s sarcoma-associated herpesvirus can productively infect primary human keratinocytes and alter their growth properties. J Virol. 2001;75:2435–43. doi: 10.1128/JVI.75.5.2435-2443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cesarman E. The role of Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) in lymphoproliferative diseases. Recent Results Cancer Res. 2002;159:27–37. doi: 10.1007/978-3-642-56352-2_4. [DOI] [PubMed] [Google Scholar]
- 80.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–91. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 81.Chan J, Kravcik S, Angel JB. Development of Kaposi’s sarcoma despite sustained suppression of HIV plasma viremia. J Acquir Immune Defic Syndr. 1999;22:209–10. doi: 10.1097/00126334-199910010-00017. [DOI] [PubMed] [Google Scholar]
- 82.Chandran B, Bloomer C, Chan SR, Zhu L, Goldstein E, Horvat R. Human herpesvirus-8 ORF K8.1 gene encodes immunogenic glycoproteins generated by spliced transcripts. Virology. 1998;249:140–9. doi: 10.1006/viro.1998.9316. [DOI] [PubMed] [Google Scholar]
- 83.Chang H, Dittmer DP, Chul SY, Hong Y, Jung JU. Role of Notch signal transduction in Kaposi’s sarcoma-associated herpesvirus gene expression. J Virol. 2005;79:14371–82. doi: 10.1128/JVI.79.22.14371-14382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chang H, Gwack Y, Kingston D, Souvlis J, Liang X, Means RE, Cesarman E, Hutt-Fletcher L, Jung JU. Activation of CD21 and CD23 gene expression by Kaposi’s sarcoma-associated herpesvirus RTA. J Virol. 2005;79:4651–63. doi: 10.1128/JVI.79.8.4651-4663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang HK, Gendelman R, Lisziewicz J, Gallo RC, Ensoli B. Block of HIV-1 infection by a combination of antisense tat RNA and TAR decoys: a strategy for control of HIV-1. Gene Ther. 1994;1:208–16. [PubMed] [Google Scholar]
- 86.Chang J, Renne R, Dittmer D, Ganem D. Inflammatory cytokines and the reactivation of Kaposi’s sarcoma-associated herpesvirus lytic replication. Virology. 2000;266:17–25. doi: 10.1006/viro.1999.0077. [DOI] [PubMed] [Google Scholar]
- 87.Chang PJ, Shedd D, Gradoville L, Cho MS, Chen LW, Chang J, Miller G. Open reading frame 50 protein of Kaposi’s sarcoma-associated herpesvirus directly activates the viral PAN and K12 genes by binding to related response elements. J Virol. 2002;76:3168–78. doi: 10.1128/JVI.76.7.3168-3178.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang PJ, Shedd D, Miller G. Two subclasses of Kaposi’s sarcoma-associated herpesvirus lytic cycle promoters distinguished by open reading frame 50 mutant proteins that are deficient in binding to DNA. J Virol. 2005;79:8750–63. doi: 10.1128/JVI.79.14.8750-8763.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–9. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 90.Chang Y, Moore PS, Talbot SJ, Boshoff CH, Zarkowska T, Godden K, Paterson H, Weiss RA, Mittnacht S. Cyclin encoded by KS herpesvirus. Nature. 1996;382:410. doi: 10.1038/382410a0. [DOI] [PubMed] [Google Scholar]
- 91.Chang Y, Ziegler J, Wabinga H, Katangole-Mbidde E, Boshoff C, Schulz T, Whitby D, Maddalena D, Jaffe HW, Weiss RA, Moore PS, Uganda Kaposi’s Sarcoma Study Group Kaposi’s sarcoma-associated herpesvirus and Kaposi’s sarcoma in Africa. Arch Intern Med. 1996;156:202–4. [PubMed] [Google Scholar]
- 92.Chatterjee M, Osborne J, Bestetti G, Chang Y, Moore PS. Viral IL-6-induced cell proliferation and immune evasion of interferon activity. Science. 2002;298:1432–5. doi: 10.1126/science.1074883. [DOI] [PubMed] [Google Scholar]
- 93.Chaudhary PM, Jasmin A, Eby MT, Hood L. Modulation of the NF-kappa B pathway by virally encoded death effector domains-containing proteins. Oncogene. 1999;18:5738–46. doi: 10.1038/sj.onc.1202976. [DOI] [PubMed] [Google Scholar]
- 94.Chehimi J, Luo Q, Azzoni L, Shawver L, Ngoubilly N, June R, Jerandi G, Farabaugh M, Montaner LJ. HIV-1 transmission and cytokine-induced expression of DC-SIGN in human monocyte-derived macrophages. J Leukoc Biol. 2003;74:757–63. doi: 10.1189/jlb.0503231. [DOI] [PubMed] [Google Scholar]
- 95.Chen J, Ueda K, Sakakibara S, Okuno T, Parravicini C, Corbellino M, Yamanishi K. Activation of latent Kaposi’s sarcoma-associated herpesvirus by demethylation of the promoter of the lytic transactivator. Proc Natl Acad Sci USA. 2001;98:4119–24. doi: 10.1073/pnas.051004198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen J, Ueda K, Sakakibara S, Okuno T, Yamanishi K. Transcriptional regulation of the Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor gene. J Virol. 2000;74:8623–34. doi: 10.1128/jvi.74.18.8623-8634.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen S, Bacon KB, Li L, Garcia GE, Xia Y, Lo D, Thompson DA, Siani MA, Yamamoto T, Harrison JK, Feng L. In vivo inhibition of CC and CX3C chemokine-induced leukocyte infiltration and attenuation of glomerulonephritis in Wistar-Kyoto (WKY) rats by vMIP-II. J Exp Med. 1998;188:193–8. doi: 10.1084/jem.188.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cheng EH, Nicholas J, Bellows DS, Hayward GS, Guo HG, Reitz MS, Hardwick JM. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc Natl Acad Sci USA. 1997;94:690–4. doi: 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cheung TW. AIDS-related cancer in the era of highly active antiretroviral therapy (HAART): a model of the interplay of the immune system, virus, and cancer.“On the offensive–the Trojan Horse is being destroyed”–Part A: Kaposi’s sarcoma. Cancer Invest. 2004;22:774–86. doi: 10.1081/cnv-200032788. [DOI] [PubMed] [Google Scholar]
- 100.Child ES, Mann DJ. Novel properties of the cyclin encoded by Human Herpesvirus 8 that facilitate exit from quiescence. Oncogene. 2001;20:3311–22. doi: 10.1038/sj.onc.1204447. [DOI] [PubMed] [Google Scholar]
- 101.Chiou CJ, Poole LJ, Kim PS, Ciufo DM, Cannon JS, ap Rhys CM, Alcendor DJ, Zong JC, Ambinder RF, Hayward GS. Patterns of gene expression and a transactivation function exhibited by the vGCR (ORF74) chemokine receptor protein of Kaposi’s sarcoma-associated herpesvirus. J Virol. 76:3421–39. doi: 10.1128/JVI.76.7.3421-3439.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Choi J, Means RE, Damania B, Jung JU. Molecular piracy of Kaposi’s sarcoma associated herpesvirus. Cytokine Growth Factor Rev. 2001;12:245–57. doi: 10.1016/s1359-6101(00)00029-0. [DOI] [PubMed] [Google Scholar]
- 103.Choi JK, Lee BS, Shim SN, Li M, Jung JU. Identification of the novel K15 gene at the rightmost end of the Kaposi’s sarcoma-associated herpesvirus genome. J Virol. 2000;74:436–46. [PMC free article] [PubMed] [Google Scholar]
- 104.Chow D, He X, Snow AL, Rose-John S, Garcia KC. Structure of an extracellular gp130 cytokine receptor signaling complex. Science. 2001;291:2150–5. doi: 10.1126/science.1058308. [DOI] [PubMed] [Google Scholar]
- 105.Chung YH, Means RE, Choi JK, Lee BS, Jung JU. Kaposi’s sarcoma-associated herpesvirus OX2 glycoprotein activates myeloid-lineage cells to induce inflammatory cytokine production. J Virol. 2002;76:4688–98. doi: 10.1128/JVI.76.10.4688-4698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ciufo DM, Cannon JS, Poole LJ, Wu FY, Murray P, Ambinder RF, Hayward GS. Spindle cell conversion by Kaposi’s sarcoma-associated herpesvirus: formation of colonies and plaques with mixed lytic and latent gene expression in infected primary dermal microvascular endothelial cell cultures. J Virol. 2001;75:5614–26. doi: 10.1128/JVI.75.12.5614-5626.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cohen A, Brodie C, Sarid R. An essential role of ERK signaling in TPA-induced reactivation of Kaposi’s sarcoma-associated herpesvirus. J Gen Virol. 2006;87:795–802. doi: 10.1099/vir.0.81619-0. [DOI] [PubMed] [Google Scholar]
- 108.Cool CD, Rai PR, Yeager ME, Hernandez-Saavedra D, Serls AE, Bull TM, Geraci MW, Brown KK, Routes JM, Tuder RM, Voelkel NF. Expression of human herpesvirus 8 in primary pulmonary hypertension. N Engl J Med. 2003;349:1113–22. doi: 10.1056/NEJMoa035115. [DOI] [PubMed] [Google Scholar]
- 109.Corbellino M, Poirel L, Aubin JT, Paulli M, Magrini U, Bestetti G, Galli M, Parravicini C. The role of human herpesvirus 8 and Epstein-Barr virus in the pathogenesis of giant lymph node hyperplasia (Castleman’s disease) Clin Infect Dis. 1996;22:1120–1. doi: 10.1093/clinids/22.6.1120. [DOI] [PubMed] [Google Scholar]
- 110.Coscoy L, Ganem D. Kaposi’s sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc Natl Acad Sci USA. 2000;97:8051–6. doi: 10.1073/pnas.140129797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Coscoy L, Ganem D. A viral protein that selectively downregulates ICAM-1 and B7–2 and modulates T cell costimulation. J Clin Invest. 2001;107:1599–606. doi: 10.1172/JCI12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cotter MA, 2nd, Robertson ES. The latency-associated nuclear antigen tethers the Kaposi’s sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology. 1999;264:254–64. doi: 10.1006/viro.1999.9999. [DOI] [PubMed] [Google Scholar]
- 113.Cotter MA, 2nd, Robertson ES. Molecular biology of Kaposi’s sarcoma-associated herpesvirus. Front Biosci. 2002;7:d358–75. doi: 10.2741/cotter. [DOI] [PubMed] [Google Scholar]
- 114.Couty JP, Geras-Raaka E, Weksler BB, Gershengorn MC. Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor signals through multiple pathways in endothelial cells. J Biol Chem. 2001;276:33805–11. doi: 10.1074/jbc.M104631200. [DOI] [PubMed] [Google Scholar]
- 115.Dagna L, Broccolo F, Paties CT, Ferrarini M, Sarmati L, Praderio L, Sabbadini MG, Lusso P, Malnati MS. A relapsing inflammatory syndrome and active human herpesvirus 8 infection. N Engl J Med. 2005;353:156–63. doi: 10.1056/NEJMoa042850. [DOI] [PubMed] [Google Scholar]
- 116.Damania B, Jeong JH, Bowser BS, DeWire SM, Staudt MR, Dittmer DP. Comparison of the Rta/Orf50 transactivator proteins of gamma-2-herpesviruses. J Virol. 2004;78:5491–9. doi: 10.1128/JVI.78.10.5491-5499.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Davis DA, Rinderknecht AS, Zoeteweij JP, Aoki Y, Read-Connole EL, Tosato G, Blauvelt A, Yarchoan R. Hypoxia induces lytic replication of Kaposi sarcoma-associated herpesvirus. Blood. 2001;97:3244–50. doi: 10.1182/blood.v97.10.3244. [DOI] [PubMed] [Google Scholar]
- 118.Dedicoat M, Newton R. Review of the distribution of Kaposi’s sarcoma-associated herpesvirus (KSHV) in Africa in relation to the incidence of Kaposi’s sarcoma. Br J Cancer. 2003;88:1–3. doi: 10.1038/sj.bjc.6600745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Delli Bovi P, Donti E, Knowles DM, 2nd, Friedman-Kien A, Luciw PA, Dina D, Dalla-Favera R, Basilico C. Presence of chromosomal abnormalities and lack of AIDS retrovirus DNA sequences in AIDS-associated Kaposi’s sarcoma. Cancer Res. 1986;46:6333–8. [PubMed] [Google Scholar]
- 120.Deng H, Chu JT, Rettig MB, Martinez-Maza O, Sun R. Rta of the human herpesvirus 8/Kaposi sarcoma-associated herpesvirus up-regulates human interleukin-6 gene expression. Blood. 2002;100:1919–21. doi: 10.1182/blood-2002-01-0015. [DOI] [PubMed] [Google Scholar]
- 121.Deng H, Young A, Sun R. Auto-activation of the rta gene of human herpesvirus-8/Kaposi’s sarcoma-associated herpesvirus. J Gen Virol. 2000;81:3043–8. doi: 10.1099/0022-1317-81-12-3043. [DOI] [PubMed] [Google Scholar]
- 122.Deng JH, Zhang YJ, Wang XP, Gao SJ. Lytic replication-defective Kaposi’s sarcoma-associated herpesvirus: potential role in infection and malignant transformation. J Virol. 2004;78:11108–20. doi: 10.1128/JVI.78.20.11108-11120.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Deutsch E, Cohen A, Kazimirsky G, Dovrat S, Rubinfeld H, Brodie C, Sarid R. Role of protein kinase C delta in reactivation of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2004;78:10187–92. doi: 10.1128/JVI.78.18.10187-10192.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dezube BJ, Zambela M, Sage DR, Wang JF, Fingeroth JD. Characterization of Kaposi sarcoma-associated herpesvirus/human herpesvirus-8 infection of human vascular endothelial cells: early events. Blood. 2002;100:888–96. doi: 10.1182/blood.v100.3.888. [DOI] [PubMed] [Google Scholar]
- 125.Dittmer D, Lagunoff M, Renne R, Staskus K, Haase A, Ganem D. A cluster of latently expressed genes in Kaposi’s sarcoma-associated herpesvirus. J Virol. 1998;72:8309–15. doi: 10.1128/jvi.72.10.8309-8315.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dittmer D, Stoddart C, Renne R, Linquist-Stepps V, Moreno ME, Bare C, McCune JM, Ganem D. Experimental transmission of Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) to SCID-hu Thy/Liv mice. J Exp Med. 1999;190:1857–68. doi: 10.1084/jem.190.12.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dittmer DP. Transcription profile of Kaposi’s sarcoma-associated herpesvirus in primary Kaposi’s sarcoma lesions as determined by real-time PCR arrays. Cancer Res. 2003;63:2010–5. [PubMed] [Google Scholar]
- 128.Djerbi M, Screpanti V, Catrina AI, Bogen B, Biberfeld P, Grandien A. The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J Exp Med. 190:1025–32. doi: 10.1084/jem.190.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dourmishev LA, Dourmishev AL, Palmeri D, Schwartz RA, Lukac DM. Molecular genetics of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus-8) epidemiology and pathogenesis. Microbiol Mol Biol Rev. 2003;67:175–212. doi: 10.1128/MMBR.67.2.175-212.2003. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dunaief JL, Strober BE, Guha S, Khavari PA, Alin K, Luban J, Begemann M, Crabtree GR, Goff SP. The retinoblastoma protein and BRG1 form a complex and cooperate to induce cell cycle arrest. Cell. 1994;79:119–30. doi: 10.1016/0092-8674(94)90405-7. [DOI] [PubMed] [Google Scholar]
- 131.Dupin N, Diss TL, Kellam P, Tulliez M, Du MQ, Sicard D, Weiss RA, Isaacson PG, Boshoff C. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood. 2000;95:1406–12. [PubMed] [Google Scholar]
- 132.Dupin N, Fisher C, Kellam P, Ariad S, Tulliez M, Franck N, van Marck E, Salmon D, Gorin I, Escande JP, Weiss RA, Alitalo K, Boshoff C. Distribution of human herpesvirus-8 latently infected cells in Kaposi’s sarcoma, multicentric Castleman’s disease, and primary effusion lymphoma. Proc Natl Acad Sci USA. 1999;96:4546–51. doi: 10.1073/pnas.96.8.4546. C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dupin N, Gorin I, Deleuze J, Agut H, Huraux JM, Escande JP. Herpes-like DNA sequences, AIDS-related tumors, and Castleman’s disease. N Engl J Med. 1995;333:798. author reply 798–9. [PubMed] [Google Scholar]
- 134.Dupin N, Grandadam M, Calvez V, Gorin I, Aubin JT, Havard S, Lamy F, Leibowitch M, Huraux JM, Escande JP, et al. Herpesvirus-like DNA sequences in patients with Mediterranean Kaposi’s sarcoma. Lancet. 1995;345:761–2. doi: 10.1016/s0140-6736(95)90642-8. [DOI] [PubMed] [Google Scholar]
- 135.Duprez R, Kassa-Kelembho E, Plancoulaine S, Briere J, Fossi M, Kobangue L, Minsart P, Huerre M, Gessain A. Human herpesvirus 8 serological markers and viral load in patients with AIDS-associated Kaposi’s sarcoma in Central African Republic. J Clin Microbiol. 2005;43:4840–3. doi: 10.1128/JCM.43.9.4840-4843.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ellis M, Chew YP, Fallis L, Freddersdorf S, Boshoff C, Weiss RA, Lu X, Mittnacht S. Degradation of p27(Kip) cdk inhibitor triggered by Kaposi’s sarcoma virus cyclin–cdk6 complex. EMBO J. 1999;18:644–53. doi: 10.1093/emboj/18.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ensoli B, Sgadari C, Barillari G, Sirianni MC, Sturzl M, Monini P. Biology of Kaposi’s sarcoma. Eur J Cancer. 2001;37:1251–69. doi: 10.1016/s0959-8049(01)00121-6. [DOI] [PubMed] [Google Scholar]
- 138.Ensoli B, Sirianni MC. Kaposi’s sarcoma pathogenesis: a link between immunology and tumor biology. Crit Rev Oncog. 1998;9:107–24. doi: 10.1615/critrevoncog.v9.i2.20. [DOI] [PubMed] [Google Scholar]
- 139.Ensoli B, Sturzl M. Kaposi’s sarcoma: a result of the interplay among inflammatory cytokines, angiogenic factors and viral agents. Cytokine Growth Factor Rev. 1998;9:63–83. doi: 10.1016/s1359-6101(97)00037-3. [DOI] [PubMed] [Google Scholar]
- 140.Ensoli B, Sturzl M, Monini P. Reactivation and role of HHV-8 in Kaposi’s sarcoma initiation. Adv Cancer Res. 2001;81:161–200. doi: 10.1016/s0065-230x(01)81005-8. [DOI] [PubMed] [Google Scholar]
- 141.Fakhari FD, Jeong JH, Kanan Y, Dittmer DP. The latency-associated nuclear antigen of Kaposi sarcoma-associated herpesvirus induces B cell hyperplasia and lymphoma. J Clin Invest. 2006;116:735–42. doi: 10.1172/JCI26190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fardet L, Blum L, Kerob D, Agbalika F, Galicier L, Dupuy A, Lafaurie M, Meignin V, Morel P, Lebbe C. Human herpesvirus 8-associated hemophagocytic lymphohistiocytosis in human immunodeficiency virus-infected patients. Clin Infect Dis. 37:285–91. doi: 10.1086/375224. [DOI] [PubMed] [Google Scholar]
- 143.Feng P, Park J, Lee BS, Lee SH, Bram RJ, Jung JU. Kaposi’s sarcoma-associated herpesvirus mitochondrial K7 protein targets a cellular calcium-modulating cyclophilin ligand to modulate intracellular calcium concentration and inhibit apoptosis. J Virol. 2002;76:11491–504. doi: 10.1128/JVI.76.22.11491-11504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fickenscher H, Fleckenstein B. Herpesvirus saimiri. Philos Trans R Soc Lond B Biol Sci. 2001;356:545–67. doi: 10.1098/rstb.2000.0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Field N, Low W, Daniels M, Howell S, Daviet L, Boshoff C, Collins M. KSHV vFLIP binds to IKK-gamma to activate IKK. J Cell Sci. 2003;116:3721–8. doi: 10.1242/jcs.00691. [DOI] [PubMed] [Google Scholar]
- 146.Fife K, Howard MR, Gracie F, Phillips RH, Bower M. Activity of thalidomide in AIDS-related Kaposi’s sarcoma and correlation with HHV8 titer. Int J STD AIDS. 1998;9:751–5. doi: 10.1258/0956462981921512. [DOI] [PubMed] [Google Scholar]
- 147.Flore O, Rafii S, Ely S, O’Leary JJ, Hyjek EM, Cesarman E. Transformation of primary human endothelial cells by Kaposi’s sarcoma-associated herpesvirus. Nature. 1998;394:588–92. doi: 10.1038/29093. [DOI] [PubMed] [Google Scholar]
- 148.Flowers CC, Flowers SP, Nabel GJ. Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor confers resistance to the antiproliferative effect of interferon-alpha. Mol Med. 1998;4:402–12. [PMC free article] [PubMed] [Google Scholar]
- 149.Foglieni C, Scabini S, Belloni D, Broccolo F, Lusso P, Malnati MS, Ferrero E. Productive infection of HUVEC by HHV-8 is associated with changes compatible with angiogenic transformations. Eur J Histochem. 2005;49:273–84. doi: 10.4081/954. [DOI] [PubMed] [Google Scholar]
- 150.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 151.Foreman KE, Friborg J, Chandran B, Katano H, Sata T, Mercader M, Nabel GJ, Nickoloff BJ. Injection of human herpesvirus-8 in human skin engrafted on SCID mice induces Kaposi’s sarcoma-like lesions. J Dermatol Sci. 2001;26:182–93. doi: 10.1016/s0923-1811(01)00087-1. [DOI] [PubMed] [Google Scholar]
- 152.Foreman KE, Friborg J, Jr., Kong WP, Woffendin C, Polverini PJ, Nickoloff BJ, Nabel GJ. Propagation of a human herpesvirus from AIDS-associated Kaposi’s sarcoma. N Engl J Med. 336:163–71. doi: 10.1056/NEJM199701163360302. [DOI] [PubMed] [Google Scholar]
- 153.Foussat A, Wijdenes J, Bouchet L, Gaidano G, Neipel F, Balabanian K, Galanaud P, Couderc J, Emilie D. Human interleukin-6 is in vivo an autocrine growth factor for human herpesvirus-8-infected malignant B lymphocytes. Eur Cytokine Netw. 1999;10:501–8. D. [PubMed] [Google Scholar]
- 154.Friborg J, Jr., Kong W, Hottiger MO, Nabel GJ. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature. 1999;402:889–94. doi: 10.1038/47266. [DOI] [PubMed] [Google Scholar]
- 155.Friedman-Kien AE. Disseminated Kaposi’s sarcoma syndrome in young homosexual men. J Am Acad Dermatol. 1981;5:468–71. doi: 10.1016/s0190-9622(81)80010-2. [DOI] [PubMed] [Google Scholar]
- 156.Fujimuro M, Hayward SD. The latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus manipulates the activity of glycogen synthase kinase-3beta. J Virol. 2003;77:8019–30. doi: 10.1128/JVI.77.14.8019-8030.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fujimuro M, Liu J, Zhu J, Yokosawa H, Hayward SD. Regulation of the interaction between glycogen synthase kinase 3 and the Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen. J Virol. 2005;79:10429–41. doi: 10.1128/JVI.79.16.10429-10441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fujimuro M, Wu FY, ApRhys C, Kajumbula H, Young DB, Hayward GS, Hayward SD. A novel viral mechanism for dysregulation of beta-catenin in Kaposi’s sarcoma-associated herpesvirus latency. Nat Med. 2003;9:300–6. doi: 10.1038/nm829. [DOI] [PubMed] [Google Scholar]
- 159.Gage JR, Breen EC, Echeverri A, Magpantay L, Kishimoto T, Miles S, Martinez-Maza O. Human herpesvirus 8-encoded interleukin 6 activates HIV-1 in the U1 monocytic cell line. AIDS. 1999;13:1851–5. doi: 10.1097/00002030-199910010-00006. [DOI] [PubMed] [Google Scholar]
- 160.Gaidano G, Capello D, Pastore C, Antinori A, Gloghini A, Carbone A, Larocca LM, Saglio G. Analysis of human herpesvirus type 8 infection in AIDS-related and AIDS-unrelated primary central nervous system lymphoma. J Infect Dis. 1997;175:1193–7. doi: 10.1086/593456. [DOI] [PubMed] [Google Scholar]
- 161.Gaidano G, Carbone A. Primary effusion lymphoma: a liquid phase lymphoma of fluid-filled body cavities. Adv Cancer Res. 2001;80:115–46. doi: 10.1016/s0065-230x(01)80014-2. [DOI] [PubMed] [Google Scholar]
- 162.Gaidano G, Cechova K, Chang Y, Moore PS, Knowles DM, Dalla-Favera R. Establishment of AIDS-related lymphoma cell lines from lymphomatous effusions. Leukemia. 1996;10:1237–40. [PubMed] [Google Scholar]
- 163.Gaidano G, Pastore C, Gloghini A, Capello D, Tirelli U, Saglio G, Carbone A. Microsatellite instability in KSHV/HHV-8 positive body-cavity-based lymphoma. Hum Pathol. 1997;28:748–50. doi: 10.1016/s0046-8177(97)90187-8. [DOI] [PubMed] [Google Scholar]
- 164.Ganem D. Human herpesvirus 8 and its role in the genesis of Kaposi’s sarcoma. Curr Clin Top Infect Dis. 1998;18:237–51. [PubMed] [Google Scholar]
- 165.Gao SJ, Boshoff C, Jayachandra S, Weiss RA, Chang Y, Moore PS. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene. 1997;15:1979–85. doi: 10.1038/sj.onc.1201571. [DOI] [PubMed] [Google Scholar]
- 166.Gao SJ, Deng JH, Zhou FC. Productive lytic replication of a recombinant Kaposi’s sarcoma-associated herpesvirus in efficient primary infection of primary human endothelial cells. J Virol. 2003;77:9738–49. doi: 10.1128/JVI.77.18.9738-9749.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gao SJ, Kingsley L, Hoover DR, Spira TJ, Rinaldo CR, Saah A, Phair J, Detels R, Parry P, Chang Y, Moore PS. Seroconversion to antibodies against Kaposi’s sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi’s sarcoma. N Engl J Med. 1996;335:233–41. doi: 10.1056/NEJM199607253350403. [DOI] [PubMed] [Google Scholar]
- 168.Gao SJ, Kingsley L, Li M, Zheng W, Parravicini C, Ziegler J, Newton R, Rinaldo CR, Saah A, Phair J, Detels R, Chang Y, Moore PS. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2:925–8. doi: 10.1038/nm0896-925. [DOI] [PubMed] [Google Scholar]
- 169.Gao SJ, Zhang YJ, Deng JH, Rabkin CS, Flore O, Jenson HB. Molecular polymorphism of Kaposi’s sarcoma-associated herpesvirus (Human herpesvirus 8) latent nuclear antigen: evidence for a large repertoire of viral genotypes and dual infection with different viral genotypes. J Infect Dis. 1999;180:1466–76. doi: 10.1086/315098. [DOI] [PubMed] [Google Scholar]
- 170.Garber AC, Shu MA, Hu J, Renne R. DNA binding and modulation of gene expression by the latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2001;75:7882–92. doi: 10.1128/JVI.75.17.7882-7892.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Geijtenbeek TB, van Kooyk Y. Pathogens target DC-SIGN to influence their fate DC-SIGN functions as a pathogen receptor with broad specificity. Apmis. 2003;111:698–714. doi: 10.1034/j.1600-0463.2003.11107803.x. [DOI] [PubMed] [Google Scholar]
- 172.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–32. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 173.Giraldo G, Beth E, Haguenau F. Herpes-type virus particles in tissue culture of Kaposi’s sarcoma from different geographic regions. J Natl Cancer Inst. 1972;49:1509–26. doi: 10.1093/jnci/49.6.1509. [DOI] [PubMed] [Google Scholar]
- 174.Glenn M, Rainbow L, Aurade F, Davison A, Schulz TF. Identification of a spliced gene from Kaposi’s sarcoma-associated herpesvirus encoding a protein with similarities to latent membrane proteins 1 and 2A of Epstein-Barr virus. J Virol. 1999;73:6953–63. doi: 10.1128/jvi.73.8.6953-6963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Godden-Kent D, Talbot SJ, Boshoff C, Chang Y, Moore P, Weiss RA, Mittnacht S. The cyclin encoded by Kaposi’s sarcoma-associated herpesvirus stimulates cdk6 to phosphorylate the retinoblastoma protein and histone H1. J Virol. 1997;71:4193–8. doi: 10.1128/jvi.71.6.4193-4198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Goedert JJ. The epidemiology of acquired immunodeficiency syndrome malignancies. Semin Oncol. 2000;27:390–401. [PubMed] [Google Scholar]
- 177.Goedert JJ, Cote TR, Virgo P, Scoppa SM, Kingma DW, Gail MH, Jaffe ES, Biggar RJ. Spectrum of AIDS-associated malignant disorders. Lancet. 1998;351:1833–9. doi: 10.1016/s0140-6736(97)09028-4. [DOI] [PubMed] [Google Scholar]
- 178.Gonzalez CM, Wong EL, Bowser BS, Hong GK, Kenney S, Damania B. Identification and characterization of the Orf49 protein of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2006;80:3062–70. doi: 10.1128/JVI.80.6.3062-3070.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Goodwin DJ, Walters MS, Smith PG, Thurau M, Fickenscher H, Whitehouse A. Herpesvirus saimiri open reading frame 50 (Rta) protein reactivates the lytic replication cycle in a persistently infected A549 cell line. J Virol. 2001;75:4008–13. doi: 10.1128/JVI.75.8.4008-4013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gottlieb GJ, Ragaz A, Vogel JV, Friedman-Kien A, Rywlin AM, Weiner EA, Ackerman AB. A preliminary communication on extensively disseminated Kaposi’s sarcoma in young homosexual men. Am J Dermatopathol. 1981;3:111–4. doi: 10.1097/00000372-198100320-00002. [DOI] [PubMed] [Google Scholar]
- 181.Gradoville L, Gerlach J, Grogan E, Shedd D, Nikiforow S, Metroka C, Miller G. Kaposi’s sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J Virol. 2000;74:6207–12. doi: 10.1128/jvi.74.13.6207-6212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Grisotto MG, Garin A, Martin AP, Jensen KK, Chan P, Sealfon SC, Lira SA. The human herpesvirus 8 chemokine receptor vGPCR triggers autonomous proliferation of endothelial cells. J Clin Invest. 2006 doi: 10.1172/JCI26666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Grisotto MG, Garin A, Martin AP, Jensen KK, Chan P, Sealfon SC, Lira SA. The human herpesvirus 8 chemokine receptor vGPCR triggers autonomous proliferation of endothelial cells. J Clin Invest. 2006;116:1264–73. doi: 10.1172/JCI26666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Grundhoff A, Ganem D. The latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus permits replication of terminal repeat-containing plasmids. J Virol. 2003;77:2779–83. doi: 10.1128/JVI.77.4.2779-2783.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Grundhoff A, Ganem D. Mechanisms governing expression of the v-FLIP gene of Kaposi’s sarcoma-associated herpesvirus. J Virol. 75:1857–63. doi: 10.1128/JVI.75.4.1857-1863.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Grundhoff A, Sullivan CS, Ganem D. A combined computational and microarray-based approach identifies novel microRNAs encoded by human gamma-herpesviruses. RNA. 2006 doi: 10.1261/rna.2326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Guasparri I, Keller SA, Cesarman E. KSHV vFLIP is essential for the survival of infected lymphoma cells. J Exp Med. 199:993–1003. doi: 10.1084/jem.20031467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 188.Guasparri I, Wu H, Cesarman E. The KSHV oncoprotein vFLIP contains a TRAF-interacting motif and requires TRAF2 and TRAF3 for signaling. EMBO Rep. 2006;7:114–9. doi: 10.1038/sj.embor.7400580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Guo HG, Pati S, Sadowska M, Charurat M, Reitz M. Tumorigenesis by human herpesvirus 8 vGPCR is accelerated by human immunodeficiency virus type 1 Tat. J Virol. 2004;78:9336–42. doi: 10.1128/JVI.78.17.9336-9342.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gwack Y, Baek HJ, Nakamura H, Lee SH, Meisterernst M, Roeder RG, Jung JU. Principal role of TRAP/mediator and SWI/SNF complexes in Kaposi’s sarcoma-associated herpesvirus RTA-mediated lytic reactivation. Mol Cell Biol. 2003;23:2055–67. doi: 10.1128/MCB.23.6.2055-2067.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gwack Y, Byun H, Hwang S, Lim C, Choe J. CREB-binding protein and histone deacetylase regulate the transcriptional activity of Kaposi’s sarcoma-associated herpesvirus open reading frame 50. J Virol. 2001;75:1909–17. doi: 10.1128/JVI.75.4.1909-1917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gwack Y, Hwang S, Byun H, Lim C, Kim JW, Choi EJ, Choe J. Kaposi’s sarcoma-associated herpesvirus open reading frame 50 represses p53-induced transcriptional activity and apoptosis. J Virol. 2001;75:6245–8. doi: 10.1128/JVI.75.13.6245-6248.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gwack Y, Nakamura H, Lee SH, Souvlis J, Yustein JT, Gygi S, Kung HJ, Jung JU. Poly(ADP-ribose) polymerase 1 and Ste20-like kinase hKFC act as transcriptional repressors for gamma-2 herpesvirus lytic replication. Mol Cell Biol. 2003;23:8282–94. doi: 10.1128/MCB.23.22.8282-8294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Han Z, Swaminathan S. Kaposi’s sarcoma-associated herpesvirus lytic gene ORF57 is essential for infectious virion production. J Virol. 2006;80:5251–60. doi: 10.1128/JVI.02570-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Haque M, Chen J, Ueda K, Mori Y, Nakano K, Hirata Y, Kanamori S, Uchiyama Y, Inagi R, Okuno T, Yamanishi K. Identification and analysis of the K5 gene of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2000;74:2867–75. doi: 10.1128/jvi.74.6.2867-2875.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Harada H, Fujita T, Miyamoto M, Kimura Y, Maruyama M, Furia A, Miyata T, Taniguchi T. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell. 1989;58:729–39. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- 197.Harada H, Kitagawa M, Tanaka N, Yamamoto H, Harada K, Ishihara M, Taniguchi T. Anti-oncogenic and oncogenic potentials of interferon regulatory factors-1 and -2. Science. 1993;259:971–4. doi: 10.1126/science.8438157. [DOI] [PubMed] [Google Scholar]
- 198.Harrington W, Jr., Sieczkowski L, Sosa C, Chan-a-Sue S, Cai JP, Cabral L, Wood C. Activation of HHV-8 by HIV-1 tat. Lancet. 1997;349:774–5. doi: 10.1016/s0140-6736(05)60199-7. [DOI] [PubMed] [Google Scholar]
- 199.Hayward SD. Viral interactions with the Notch pathway. Semin Cancer Biol. 2004;14:387–96. doi: 10.1016/j.semcancer.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 200.Helin K, Harlow E, Fattaey A. Inhibition of E2F-1 transactivation by direct binding of the retinoblastoma protein. Mol Cell Biol. 1993;13:6501–8. doi: 10.1128/mcb.13.10.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hengge UR, Ruzicka T, Tyring SK, Stuschke M, Roggendorf M, Schwartz RA, Seeber S. Update on Kaposi’s sarcoma and other HHV8 associated diseases. Part 1: epidemiology, environmental predispositions, clinical manifestations, and therapy. Lancet Infect Dis. 2002;2:281–92. doi: 10.1016/s1473-3099(02)00263-3. [DOI] [PubMed] [Google Scholar]
- 202.Hideshima T, Chauhan D, Teoh G, Raje N, Treon SP, Tai YT, Shima Y, Anderson KC. Characterization of signaling cascades triggered by human interleukin-6 versus Kaposi’s sarcoma-associated herpes virus-encoded viral interleukin 6. Clin Cancer Res. 2000;6:1180–9. [PubMed] [Google Scholar]
- 203.Hiscott J, Grandvaux N, Sharma S, Tenoever BR, Servant MJ, Lin R. Convergence of the NF-kappaB and interferon signaling pathways in the regulation of antiviral defense and apoptosis. Ann N Y Acad Sci. 2003;1010:237–48. doi: 10.1196/annals.1299.042. [DOI] [PubMed] [Google Scholar]
- 204.Holkova B, Takeshita K, Cheng DM, Volm M, Wasserheit C, Demopoulos R, Chanan-Khan A. Effect of highly active antiretroviral therapy on survival in patients with AIDS-associated pulmonary Kaposi’s sarcoma treated with chemotherapy. J Clin Oncol. 2001;19:3848–51. doi: 10.1200/JCO.2001.19.18.3848. [DOI] [PubMed] [Google Scholar]
- 205.Hu J, Garber AC, Renne R. The latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus supports latent DNA replication in dividing cells. J Virol. 2002;76:11677–87. doi: 10.1128/JVI.76.22.11677-11687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Huang LM, Chao MF, Chen MY, Shih H, Chiang YP, Chuang CY, Lee CY. Reciprocal regulatory interaction between human herpesvirus 8 and human immunodeficiency virus type 1. J Biol Chem. 2001;276:13427–32. doi: 10.1074/jbc.M011314200. [DOI] [PubMed] [Google Scholar]
- 207.Huang YQ, Friedman-Kien AE, Li JJ, Nickoloff BJ. Cultured Kaposi’s sarcoma cell lines express factor XIIIa, CD14, and VCAM-1, but not factor VIII or ELAM-1. Arch Dermatol. 1993;129:1291–6. [PubMed] [Google Scholar]
- 208.Hunter T, Pines J. Cyclins and cancer. Cell. 1991;66:1071–4. doi: 10.1016/0092-8674(91)90028-w. [DOI] [PubMed] [Google Scholar]
- 209.Hwang S, Gwack Y, Byun H, Lim C, Choe J. The Kaposi’s sarcoma-associated herpesvirus K8 protein interacts with CREB-binding protein (CBP) and represses CBP-mediated transcription. J Virol. 2001;75:9509–16. doi: 10.1128/JVI.75.19.9509-9516.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hymes KB, Cheung T, Greene JB, Prose NS, Marcus A, Ballard H, William DC, Laubenstein LJ. Kaposi’s sarcoma in homosexual men-a report of eight cases. Lancet. 1981;2:598–600. doi: 10.1016/s0140-6736(81)92740-9. [DOI] [PubMed] [Google Scholar]
- 211.Hyun TS, Subramanian C, Cotter MA, 2nd, Thomas RA, Robertson ES. Latency-associated nuclear antigen encoded by Kaposi’s sarcoma-associated herpesvirus interacts with Tat and activates the long terminal repeat of human immunodeficiency virus type 1 in human cells. J Virol. 2001;75:8761–71. doi: 10.1128/JVI.75.18.8761-8771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Inoue N, Winter J, Lal RB, Offermann MK, Koyano S. Characterization of entry mechanisms of human herpesvirus 8 by using an Rta-dependent reporter cell line. J Virol. 2003;77:8147–52. doi: 10.1128/JVI.77.14.8147-8152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ishido S, Choi JK, Lee BS, Wang C, DeMaria M, Johnson RP, Cohen GB, Jung JU. Inhibition of natural killer cell-mediated cytotoxicity by Kaposi’s sarcoma-associated herpesvirus K5 protein. Immunity. 2000;13:365–74. doi: 10.1016/s1074-7613(00)00036-4. [DOI] [PubMed] [Google Scholar]
- 214.Ishido S, Wang C, Lee BS, Cohen GB, Jung JU. Downregulation of major histocompatibility complex class I molecules by Kaposi’s sarcoma-associated herpesvirus K3 and K5 proteins. J Virol. 2000;74:5300–9. doi: 10.1128/jvi.74.11.5300-5309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jacobson LP, Jenkins FJ, Springer G, Munoz A, Shah KV, Phair J, Zhang Z, Armenian H. Interaction of human immunodeficiency virus type 1 and human herpesvirus type 8 infections on the incidence of Kaposi’s sarcoma. J Infect Dis. 2000;181:1940–9. doi: 10.1086/315503. [DOI] [PubMed] [Google Scholar]
- 216.Jarviluoma A, Koopal S, Rasanen S, Makela TP, Ojala PM. KSHV viral cyclin binds to p27KIP1 in primary effusion lymphomas. Blood. 2004;104:3349–54. doi: 10.1182/blood-2004-05-1798. [DOI] [PubMed] [Google Scholar]
- 217.Jenner RG, Alba MM, Boshoff C, Kellam P. Kaposi’s sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J Virol. 2001;75:891–902. doi: 10.1128/JVI.75.2.891-902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jensen KK, Manfra DJ, Grisotto MG, Martin AP, Vassileva G, Kelley K, Schwartz TW, Lira SA. The human herpes virus 8-encoded chemokine receptor is required for angioproliferation in a murine model of Kaposi’s sarcoma. J Immunol. 2005;174:3686–94. doi: 10.4049/jimmunol.174.6.3686. [DOI] [PubMed] [Google Scholar]
- 219.Jeong JH, Orvis J, Kim JW, McMurtrey CP, Renne R, Dittmer DP. Regulation and autoregulation of the promoter for the latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus. J Biol Chem. 2004;279:16822–31. doi: 10.1074/jbc.M312801200. [DOI] [PubMed] [Google Scholar]
- 220.Johansson S, Svineng G, Wennerberg K, Armulik A, Lohikangas L. Fibronectin-integrin interactions. Front Biosci. 1997;2:d126–46. doi: 10.2741/a178. [DOI] [PubMed] [Google Scholar]
- 221.Jones JL, Hanson DL, Chu SY, Ward JW, Jaffe HW. AIDS-associated Kaposi’s sarcoma. Science. 1995;267:1078–9. doi: 10.1126/science.7855583. [DOI] [PubMed] [Google Scholar]
- 222.Judde JG, Lacoste V, Briere J, Kassa-Kelembho E, Clyti E, Couppie P, Buchrieser C, Tulliez M, Morvan J, Gessain A. Monoclonality or oligoclonality of human herpesvirus 8 terminal repeat sequences in Kaposi’s sarcoma and other diseases. J Natl Cancer Inst. 2000;92:729–36. doi: 10.1093/jnci/92.9.729. [DOI] [PubMed] [Google Scholar]
- 223.Juhasz I, Albelda SM, Elder DE, Murphy GF, Adachi K, Herlyn D, Valyi-Nagy IT, Herlyn M. Growth and invasion of human melanomas in human skin grafted to immunodeficient mice. Am J Pathol. 1993;143:528–37. [PMC free article] [PubMed] [Google Scholar]
- 224.Kaldis P, Ojala PM, Tong L, Makela TP, Solomon MJ. CAK-independent activation of CDK6 by a viral cyclin. Mol Biol Cell. 2001;12:3987–99. doi: 10.1091/mbc.12.12.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kaleeba JA, Berger EA. Kaposi’s sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science. 2006;311:1921–4. doi: 10.1126/science.1120878. [DOI] [PubMed] [Google Scholar]
- 226.Karlin S, Mocarski ES, Schachtel GA. Molecular evolution of herpesviruses: genomic and protein sequence comparisons. J Virol. 1994;68:1886–902. doi: 10.1128/jvi.68.3.1886-1902.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Katano H, Sato Y, Kurata T, Mori S, Sata T. Expression and localization of human herpesvirus 8-encoded proteins in primary effusion lymphoma, Kaposi’s sarcoma, and multicentric Castleman’s disease. Virology. 2000;269:335–44. doi: 10.1006/viro.2000.0196. [DOI] [PubMed] [Google Scholar]
- 228.Katano H, Sato Y, Sata T. Expression of p53 and human herpesvirus-8 (HHV-8)-encoded latency-associated nuclear antigen with inhibition of apoptosis in HHV-8-associated malignancies. Cancer. 2001;92:3076–84. doi: 10.1002/1097-0142(20011215)92:12<3076::aid-cncr10117>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 229.Kedes DH, Operskalski E, Busch M, Kohn R, Flood J, Ganem D. The seroepidemiology of human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat Med. 1996;2:918–24. doi: 10.1038/nm0896-918. [DOI] [PubMed] [Google Scholar]
- 230.Keller SA, Hernandez-Hopkins D, Vider J, Ponomarev V, Hyjek E, Schattner EJ, Cesarman E. NF-kappaB is essential for the progression of KSHV- and EBV-infected lymphomas in vivo. Blood. 2006;107:3295–302. doi: 10.1182/blood-2005-07-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Keller SA, Schattner EJ, Cesarman E. Inhibition of NF-kappaB induces apoptosis of KSHV-infected primary effusion lymphoma cells. Blood. 2000;96:2537–42. [PubMed] [Google Scholar]
- 232.Kirchhausen T. Three ways to make a vesicle. Nat Rev Mol Cell Biol. 2000;1:187–98. doi: 10.1038/35043117. [DOI] [PubMed] [Google Scholar]
- 233.Kirchhoff S, Sebens T, Baumann S, Krueger A, Zawatzky R, Li-Weber M, Meinl E, Neipel F, Fleckenstein B, Krammer PH. Viral IFN-regulatory factors inhibit activation-induced cell death via two positive regulatory IFN-regulatory factor 1-dependent domains in the CD95 ligand promoter. J Immunol. 2002;168:1226–34. doi: 10.4049/jimmunol.168.3.1226. [DOI] [PubMed] [Google Scholar]
- 234.Kledal TN, Rosenkilde MM, Coulin F, Simmons G, Johnsen AH, Alouani S, Power CA, Luttichau HR, Gerstoft J, Clapham PR, Clark-Lewis I, Wells TN, Schwartz TW. A broad-spectrum chemokine antagonist encoded by Kaposi’s sarcoma-associated herpesvirus. Science. 1997;277:1656–9. doi: 10.1126/science.277.5332.1656. [DOI] [PubMed] [Google Scholar]
- 235.Kliche S, Nagel W, Kremmer E, Atzler C, Ege A, Knorr T, Koszinowski U, Kolanus W, Haas J. Signaling by human herpesvirus 8 kaposin A through direct membrane recruitment of cytohesin-1. Mol Cell. 2001;7:833–43. doi: 10.1016/s1097-2765(01)00227-1. [DOI] [PubMed] [Google Scholar]
- 236.Klouche M, Brockmeyer N, Knabbe C, Rose-John S. Human herpesvirus 8-derived viral IL-6 induces PTX3 expression in Kaposi’s sarcoma cells. AIDS. 2002;16:F9–18. doi: 10.1097/00002030-200205240-00001. [DOI] [PubMed] [Google Scholar]
- 237.Knipe DM, Howley Peter, M. Fields Virology. Lippincott Williams and Wilkins; 2001. [Google Scholar]
- 238.Komatsu T, Ballestas ME, Barbera AJ, Kelley-Clarke B, Kaye KM. KSHV LANA1 binds DNA as an oligomer and residues N-terminal to the oligomerization domain are essential for DNA binding, replication, and episome persistence. Virology. 2004;319:225–36. doi: 10.1016/j.virol.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 239.Kouzarides T. Histone acetylases and deacetylases in cell proliferation. Curr Opin Genet Dev. 1999;9:40–8. doi: 10.1016/s0959-437x(99)80006-9. [DOI] [PubMed] [Google Scholar]
- 240.Koyano S, Mar EC, Stamey FR, Inoue N. Glycoproteins M and N of human herpesvirus 8 form a complex and inhibit cell fusion. J Gen Virol. 84:1485–91. doi: 10.1099/vir.0.18941-0. [DOI] [PubMed] [Google Scholar]
- 241.Krishnan HH, Naranatt PP, Smith MS, Zeng L, Bloomer C, Chandran B. Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi’s sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression. J Virol. 2004;78:3601–20. doi: 10.1128/JVI.78.7.3601-3620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Krishnan HH, Sharma-Walia N, Zeng L, Gao SJ, Chandran B. Envelope glycoprotein gB of Kaposi’s sarcoma-associated herpesvirus is essential for egress from infected cells. J Virol. 2005;79:10952–67. doi: 10.1128/JVI.79.17.10952-10967.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Krithivas A, Fujimuro M, Weidner M, Young DB, Hayward SD. Protein interactions targeting the latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus to cell chromosomes. J Virol. 2002;76:11596–604. doi: 10.1128/JVI.76.22.11596-11604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Krithivas A, Young DB, Liao G, Greene D, Hayward SD. Human herpesvirus 8 LANA interacts with proteins of the mSin3 corepressor complex and negatively regulates Epstein-Barr virus gene expression in dually infected PEL cells. J Virol. 2000;74:9637–45. doi: 10.1128/jvi.74.20.9637-9645.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lacoste V, de la Fuente C, Kashanchi F, Pumfery A. Kaposi’s sarcoma-associated herpesvirus immediate early gene activity. Front Biosci. 2004;9:2245–72. doi: 10.2741/1394. [DOI] [PubMed] [Google Scholar]
- 246.Lagunoff M, Bechtel J, Venetsanakos E, Roy AM, Abbey N, Herndier B, McMahon M, Ganem D. De novo infection and serial transmission of Kaposi’s sarcoma-associated herpesvirus in cultured endothelial cells. J Virol. 2002;76:2440–8. doi: 10.1128/jvi.76.5.2440-2448.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lan K, Kuppers DA, Robertson ES. Kaposi’s sarcoma-associated herpesvirus reactivation is regulated by interaction of latency-associated nuclear antigen with recombination signal sequence-binding protein Jkappa, the major downstream effector of the Notch signaling pathway. J Virol. 2005;79:3468–78. doi: 10.1128/JVI.79.6.3468-3478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lan K, Kuppers DA, Verma SC, Robertson ES. Kaposi’s sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen inhibits lytic replication by targeting Rta: a potential mechanism for virus-mediated control of latency. J Virol. 2004;78:6585–94. doi: 10.1128/JVI.78.12.6585-6594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lan K, Kuppers DA, Verma SC, Sharma N, Murakami M, Robertson ES. Induction of Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen by the lytic transactivator RTA: a novel mechanism for establishment of latency. J Virol. 2005;79:7453–65. doi: 10.1128/JVI.79.12.7453-7465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Laney AS, De Marco T, Peters JS, Malloy M, Teehankee C, Moore PS, Chang Y. Kaposi sarcoma-associated herpesvirus and primary and secondary pulmonary hypertension. Chest. 2005;127:762–7. doi: 10.1378/chest.127.3.762. [DOI] [PubMed] [Google Scholar]
- 251.Lang ME, Lottersberger C, Roth B, Bock G, Recheis H, Sgonc R, Sturzl M, Albini A, Tschachler E, Zangerle R, Donini S, Feichtinger H, Schwarz S. Induction of apoptosis in Kaposi’s sarcoma spindle cell cultures by the subunits of human chorionic gonadotropin. AIDS. 1997;11:1333–40. [PubMed] [Google Scholar]
- 252.Lazzi S, Bellan C, Amato T, Palummo N, Cardone C, D’Amuri A, De Luca F, Beyanga M, Facchetti F, Tosi P, Leoncini L. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 infection in reactive lymphoid tissues: a model for KSHV/HHV-8-related lymphomas? Hum Pathol. 2006;37:23–31. doi: 10.1016/j.humpath.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 253.Lebbe C, de Cremoux P, Rybojad M, Costa da Cunha C, Morel P, Calvo F. Kaposi’s sarcoma and new herpesvirus. Lancet. 1995;345:1180. doi: 10.1016/s0140-6736(95)91011-5. [DOI] [PubMed] [Google Scholar]
- 254.Lee BS, Lee SH, Feng P, Chang H, Cho NH, Jung JU. Characterization of the Kaposi’s sarcoma-associated herpesvirus K1 signalosome. J Virol. 2005;79:12173–84. doi: 10.1128/JVI.79.19.12173-12184.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Lee H, Veazey R, Williams K, Li M, Guo J, Neipel F, Fleckenstein B, Lackner A, Desrosiers RC, Jung JU. Deregulation of cell growth by the K1 gene of Kaposi’s sarcoma-associated herpesvirus. Nat Med. 1998;4:435–40. doi: 10.1038/nm0498-435. [DOI] [PubMed] [Google Scholar]
- 256.Lennette ET, Busch MP, Hecht FM. Potential herpesvirus interaction during HIV type 1 primary infection. AIDS Res Hum Retroviruses. 21:869–75. doi: 10.1089/aid.2005.21.869. J. A. and Levy. [DOI] [PubMed] [Google Scholar]
- 257.Li H, Komatsu T, Dezube BJ, Kaye KM. The Kaposi’s sarcoma-associated herpesvirus K12 transcript from a primary effusion lymphoma contains complex repeat elements, is spliced, and initiates from a novel promoter. J Virol. 2002;76:11880–8. doi: 10.1128/JVI.76.23.11880-11888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Li M, Damania B, Alvarez X, Ogryzko V, Ozato K, Jung JU. Inhibition of p300 histone acetyltransferase by viral interferon regulatory factor. Mol Cell Biol. 2000;20:8254–63. doi: 10.1128/mcb.20.21.8254-8263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Li M, Lee H, Guo J, Neipel F, Fleckenstein B, Ozato K, Jung JU. Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor. J Virol. 1998;72:5433–40. doi: 10.1128/jvi.72.7.5433-5440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Li M, Lee H, Yoon DW, Albrecht JC, Fleckenstein B, Neipel F, Jung JU. Kaposi’s sarcoma-associated herpesvirus encodes a functional cyclin. J Virol. 1997;71:1984–91. doi: 10.1128/jvi.71.3.1984-1991.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Li M, MacKey J, Czajak SC, Desrosiers RC, Lackner AA, Jung JU. Identification and characterization of Kaposi’s sarcoma-associated herpesvirus K8.1 virion glycoprotein. J Virol. 1999;73:1341–9. doi: 10.1128/jvi.73.2.1341-1349.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Liang Y, Chang J, Lynch SJ, Lukac DM, Ganem D. The lytic switch protein of KSHV activates gene expression via functional interaction with RBP-Jkappa (CSL), the target of the Notch signaling pathway. Genes Dev. 2002;16:1977–89. doi: 10.1101/gad.996502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Liang Y, Ganem D. Lytic but not latent infection by Kaposi’s sarcoma-associated herpesvirus requires host CSL protein, the mediator of Notch signaling. Proc Natl Acad Sci USA. 2003;100:8490–5. doi: 10.1073/pnas.1432843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Liao W, Tang Y, Kuo YL, Liu BY, Xu CJ, Giam CZ. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 transcriptional activator Rta is an oligomeric DNA-binding protein that interacts with tandem arrays of phased A/T-trinucleotide motifs. J Virol. 2003;77:9399–411. doi: 10.1128/JVI.77.17.9399-9411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Lim C, Gwack Y, Hwang S, Kim S, Choe J. The transcriptional activity of cAMP response element-binding protein-binding protein is modulated by the latency associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus. J Biol Chem. 2001;276:31016–22. doi: 10.1074/jbc.M102431200. [DOI] [PubMed] [Google Scholar]
- 266.Lim C, Sohn H, Gwack Y, Choe J. Latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus-8) binds ATF4/CREB2 and inhibits its transcriptional activation activity. J Gen Virol. 2000;81:2645–52. doi: 10.1099/0022-1317-81-11-2645. [DOI] [PubMed] [Google Scholar]
- 267.Lin R, Genin P, Mamane Y, Sgarbanti M, Battistini A, Harrington WJ, Jr., Barber GN, Hiscott J. HHV-8 encoded vIRF-1 represses the interferon antiviral response by blocking IRF-3 recruitment of the CBP/p300 coactivators. Oncogene. 2001;20:800–11. doi: 10.1038/sj.onc.1204163. [DOI] [PubMed] [Google Scholar]
- 268.Lin SF, Robinson DR, Oh J, Jung JU, Luciw PA, Kung HJ. Identification of the bZIP and Rta homologues in the genome of rhesus monkey rhadinovirus. Virology. 2002;298:181–8. doi: 10.1006/viro.2002.1490. [DOI] [PubMed] [Google Scholar]
- 269.Liu C, Okruzhnov Y, Li H, Nicholas J. Human herpesvirus 8 (HHV-8)-encoded cytokines induce expression of and autocrine signaling by vascular endothelial growth factor (VEGF) in HHV-8-infected primary-effusion lymphoma cell lines and mediate VEGF-independent antiapoptotic effects. J Virol. 2001;75:10933–40. doi: 10.1128/JVI.75.22.10933-10940.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Liu L, Eby MT, Rathore N, Sinha SK, Kumar A, Chaudhary PM. The human herpes virus 8-encoded viral FLICE inhibitory protein physically associates with and persistently activates the Ikappa B kinase complex. J Biol Chem. 2002;277:13745–51. doi: 10.1074/jbc.M110480200. [DOI] [PubMed] [Google Scholar]
- 271.Locati M, Murphy PM. Chemokines and chemokine receptors: biology and clinical relevance in inflammation and AIDS. Annu Rev Med. 1999;50:425–40. doi: 10.1146/annurev.med.50.1.425. [DOI] [PubMed] [Google Scholar]
- 272.Low W, Harries M, Ye H, Du MQ, Boshoff C, Collins M. Internal ribosome entry site regulates translation of Kaposi’s sarcoma-associated herpesvirus FLICE inhibitory protein. J Virol. 2001;75:2938–45. doi: 10.1128/JVI.75.6.2938-2945.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lu F, Zhou J, Wiedmer A, Madden K, Yuan Y, Lieberman PM. Chromatin remodeling of the Kaposi’s sarcoma-associated herpesvirus ORF50 promoter correlates with reactivation from latency. J Virol. 2003;77:11425–35. doi: 10.1128/JVI.77.21.11425-11435.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Lubyova B, Pitha PM. Characterization of a novel human herpesvirus 8-encoded protein, vIRF-3, that shows homology to viral and cellular interferon regulatory factors. J Virol. 2000;74:8194–201. doi: 10.1128/jvi.74.17.8194-8201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Lukac DM, Kirshner JR, Ganem D. Transcriptional activation by the product of open reading frame 50 of Kaposi’s sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J Virol. 1999;73:9348–61. doi: 10.1128/jvi.73.11.9348-9361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Lukac DM, Renne R, Kirshner JR, Ganem D. Reactivation of Kaposi’s sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology. 1998;252:304–12. doi: 10.1006/viro.1998.9486. [DOI] [PubMed] [Google Scholar]
- 277.Luna RE, Zhou F, Baghian A, Chouljenko V, Forghani B, Gao SJ, Kousoulas KG. Kaposi’s sarcoma-associated herpesvirus glycoprotein K8.1 is dispensable for virus entry. J Virol. 2004;78:6389–98. doi: 10.1128/JVI.78.12.6389-6398.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Malmgaard L. Induction and regulation of IFNs during viral infections. J Interferon Cytokine Res. 2004;24:439–54. doi: 10.1089/1079990041689665. [DOI] [PubMed] [Google Scholar]
- 279.Mann DJ, Child ES, Swanton C, Laman H, Jones N. Modulation of p27(Kip1) levels by the cyclin encoded by Kaposi’s sarcoma-associated herpesvirus. EMBO J. 1999;18:654–63. doi: 10.1093/emboj/18.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Martin RW, 3rd, Hood AF, Farmer ER. Kaposi sarcoma. Medicine (Baltimore) 1993;72:245–61. doi: 10.1097/00005792-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 281.Masood R, Cai J, Zheng T, Smith DL, Naidu Y, Gill PS. Vascular endothelial growth factor/vascular permeability factor is an autocrine growth factor for AIDS-Kaposi sarcoma. Proc Natl Acad Sci USA. 1997;94:979–84. doi: 10.1073/pnas.94.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Masood R, Cesarman E, Smith DL, Gill PS, Flore O. Human herpesvirus-8-transformed endothelial cells have functionally activated vascular endothelial growth factor/vascular endothelial growth factor receptor. Am J Pathol. 2002;160:23–9. doi: 10.1016/S0002-9440(10)64344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Masood R, Xia G, Smith DL, Scalia P, Still JG, Tulpule A, Gill PS. Ephrin B2 expression in Kaposi sarcoma is induced by human herpesvirus type 8: phenotype switch from venous to arterial endothelium. Blood. 2005;105:1310–8. doi: 10.1182/blood-2004-03-0933. [DOI] [PubMed] [Google Scholar]
- 284.Matta H, Chaudhary PM. Activation of alternative NF-kappa B pathway by human herpes virus 8-encoded Fas-associated death domain-like IL-1 beta-converting enzyme inhibitory protein (vFLIP) Proc Natl Acad Sci USA. 2004;101:9399–404. doi: 10.1073/pnas.0308016101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Mattsson K, Kiss C, Platt GM, Simpson GR, Kashuba E, Klein G, Schulz TF, Szekely L. Latent nuclear antigen of Kaposi’s sarcoma herpesvirus/human herpesvirus-8 induces and relocates RING3 to nuclear heterochromatin regions. J Gen Virol. 2002;83:179–88. doi: 10.1099/0022-1317-83-1-179. [DOI] [PubMed] [Google Scholar]
- 286.McAllister SC, Hansen SG, Ruhl RA, Raggo CM, DeFilippis VR, Greenspan D, Fruh K, Moses AV. Kaposi sarcoma-associated herpesvirus (KSHV) induces heme oxygenase-1 expression and activity in KSHV-infected endothelial cells. Blood. 2004;103:3465–73. doi: 10.1182/blood-2003-08-2781. [DOI] [PubMed] [Google Scholar]
- 287.McCormick C, Ganem D. The kaposin B protein of KSHV activates the p38/MK2 pathway and stabilizes cytokine mRNAs. Science. 2005;307:739–41. doi: 10.1126/science.1105779. [DOI] [PubMed] [Google Scholar]
- 288.McGeoch DJ, Davison AJ. The descent of human herpesvirus 8. Semin Cancer Biol. 1999;9:201–9. doi: 10.1006/scbi.1999.0093. [DOI] [PubMed] [Google Scholar]
- 289.McPherson PS, Kay BK, Hussain NK. Signaling on the endocytic pathway. Traffic. 2001;2:375–84. doi: 10.1034/j.1600-0854.2001.002006375.x. [DOI] [PubMed] [Google Scholar]
- 290.Meads MB, Medveczky PG. Kaposi’s sarcoma-associated herpesvirus-encoded viral interleukin-6 is secreted and modified differently than human interleukin-6: evidence for a unique autocrine signaling mechanism. J Biol Chem. 2004;279:51793–803. doi: 10.1074/jbc.M407382200. [DOI] [PubMed] [Google Scholar]
- 291.Melbye M, Cook PM, Hjalgrim H, Begtrup K, Simpson GR, Biggar RJ, Ebbesen P, Schulz TF. Risk factors for Kaposi’s-sarcoma-associated herpesvirus (KSHV/HHV-8) seropositivity in a cohort of homosexual men, 1981–1996. Int J Cancer. 1998;77:543–8. doi: 10.1002/(sici)1097-0215(19980812)77:4<543::aid-ijc12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 292.Merat R, Amara A, Lebbe C, de The H, Morel P, Saib A. HIV-1 infection of primary effusion lymphoma cell line triggers Kaposi’s sarcoma-associated herpesvirus (KSHV) reactivation. Int J Cancer. 2002;97:791–5. doi: 10.1002/ijc.10086. [DOI] [PubMed] [Google Scholar]
- 293.Mercader M, Nickoloff BJ, Foreman KE. Induction of human immunodeficiency virus 1 replication by human herpesvirus 8. Arch Pathol Lab Med. 125:785–9. doi: 10.5858/2001-125-0785-IOHIVR. [DOI] [PubMed] [Google Scholar]
- 294.Mercader M, Taddeo B, Panella JR, Chandran B, Nickoloff BJ, Foreman KE. Induction of HHV-8 lytic cycle replication by inflammatory cytokines produced by HIV-1-infected T cells. Am J Pathol. 2000;156:1961–71. doi: 10.1016/S0002-9440(10)65069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Miller G, Heston L, Grogan E, Gradoville L, Rigsby M, Sun R, Shedd D, Kushnaryov VM, Grossberg S, Chang Y. Selective switch between latency and lytic replication of Kaposi’s sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J Virol. 1997;71:314–24. doi: 10.1128/jvi.71.1.314-324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Mocroft A, Youle M, Gazzard B, Morcinek J, Halai R, Phillips AN, Royal Free/Chelsea and Westminster Hospitals Collaborative Group Anti-herpesvirus treatment and risk of Kaposi’s sarcoma in HIV infection. AIDS. 10:1101–5. [PubMed] [Google Scholar]
- 297.Molden J, Chang Y, You Y, Moore PS, Goldsmith MA. A Kaposi’s sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J Biol Chem. 1997;272:19625–31. doi: 10.1074/jbc.272.31.19625. [DOI] [PubMed] [Google Scholar]
- 298.Montaner S, Sodhi A, Pece S, Mesri EA, Gutkind JS. The Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer Res. 2001;61:2641–8. [PubMed] [Google Scholar]
- 299.Montaner S, Sodhi A, Servitja JM, Ramsdell AK, Barac A, Sawai ET, Gutkind JS. The small GTPase Rac1 links the Kaposi sarcoma-associated herpesvirus vGPCR to cytokine secretion and paracrine neoplasia. Blood. 2004;104:2903–11. doi: 10.1182/blood-2003-12-4436. [DOI] [PubMed] [Google Scholar]
- 300.Moore PS, Boshoff C, Weiss RA, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274:1739–44. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- 301.Moore PS, Chang Y. Detection of herpesvirus-like DNA sequences in Kaposi’s sarcoma in patients with and without HIV infection. N Engl J Med. 1995;332:1181–5. doi: 10.1056/NEJM199505043321801. [DOI] [PubMed] [Google Scholar]
- 302.Moore PS, Chang Y. Molecular virology of Kaposi’s sarcoma-associated herpesvirus. Philos Trans R Soc Lond B Biol Sci. 2001;356:499–516. doi: 10.1098/rstb.2000.0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Moore PS, Gao SJ, Dominguez G, Cesarman E, Lungu O, Knowles DM, Garber R, Pellett PE, McGeoch DJ, Chang Y. Primary characterization of a herpesvirus agent associated with Kaposi’s sarcoma. J Virol. 1996;70:549–58. doi: 10.1128/jvi.70.1.549-558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Moore PS, Kingsley LA, Holmberg SD, Spira T, Gupta P, Hoover DR, Parry JP, Conley LJ, Jaffe HW, Chang Y. Kaposi’s sarcoma-associated herpesvirus infection prior to onset of Kaposi’s sarcoma. AIDS. 1996;10:175–80. doi: 10.1097/00002030-199602000-00007. [DOI] [PubMed] [Google Scholar]
- 305.Mori Y, Nishimoto N, Ohno M, Inagi R, Dhepakson P, Amou K, Yoshizaki K, Yamanishi K. Human herpesvirus 8-encoded interleukin-6 homologue (viral IL-6) induces endogenous human IL-6 secretion. J Med Virol. 2000;61:332–5. doi: 10.1002/1096-9071(200007)61:3<332::aid-jmv8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 306.Moses AV, Fish KN, Ruhl R, Smith PP, Strussenberg JG, Zhu L, Chandran B, Nelson JA. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J Virol. 1999;73:6892–902. doi: 10.1128/jvi.73.8.6892-6902.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Moynagh PN. TLR signaling and activation of IRFs: revisiting old friends from the NF-kappaB pathway. Trends Immunol. 2005;26:469–76. doi: 10.1016/j.it.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 308.Mullberg J, Geib T, Jostock T, Hoischen SH, Vollmer P, Voltz N, Heinz D, Galle PR, Klouche M, Rose-John S. IL-6 receptor independent stimulation of human gp130 by viral IL-6. J Immunol. 2000;164:4672–7. doi: 10.4049/jimmunol.164.9.4672. [DOI] [PubMed] [Google Scholar]
- 309.Mullick J, Bernet J, Singh AK, Lambris JD, Sahu A. Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) open reading frame 4 protein (kaposica) is a functional homolog of complement control proteins. J Virol. 2003;77:3878–81. doi: 10.1128/JVI.77.6.3878-3881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Munshi N, Ganju RK, Avraham S, Mesri EA, Groopman JE. Kaposi’s sarcoma-associated herpesvirus-encoded G protein-coupled receptor activation of c-jun amino-terminal kinase/stress-activated protein kinase and lyn kinase is mediated by related adhesion focal tyrosine kinase/proline-rich tyrosine kinase 2. J Biol Chem. 1999;274:31863–7. doi: 10.1074/jbc.274.45.31863. [DOI] [PubMed] [Google Scholar]
- 311.Muralidhar S, Pumfery AM, Hassani M, Sadaie MR, Kishishita M, Brady JN, Doniger J, Medveczky P, Rosenthal LJ. Identification of kaposin (open reading frame K12) as a human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus) transforming gene. J Virol. 1998;72:4980–8. doi: 10.1128/jvi.72.6.4980-4988.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Muralidhar S, Veytsmann G, Chandran B, Ablashi D, Doniger J, Rosenthal LJ. Characterization of the human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus) oncogene, kaposin (ORF K12) J Clin Virol. 2000;16:203–13. doi: 10.1016/s1386-6532(99)00081-5. [DOI] [PubMed] [Google Scholar]
- 313.Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95:3032–43. [PubMed] [Google Scholar]
- 314.Murdoch C, Finn A. Chemokine receptors and their role in vascular biology. J Vasc Res. 2000;37:1–7. doi: 10.1159/000025707. [DOI] [PubMed] [Google Scholar]
- 315.Muromoto R, Okabe K, Fujimuro M, Sugiyama K, Yokosawa H, Seya T, Matsuda T. Physical and functional interactions between STAT3 and Kaposi’s sarcoma-associated herpesvirus-encoded LANA. FEBS Lett. 2006;580:93–8. doi: 10.1016/j.febslet.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 316.Nair V, Zavolan M. Virus-encoded microRNAs: novel regulators of gene expression. Trends Microbiol. 2006;14:169–75. doi: 10.1016/j.tim.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 317.Nakamura H, Li M, Zarycki J, Jung JU. Inhibition of p53 tumor suppressor by viral interferon regulatory factor. J Virol. 2001;75:7572–82. doi: 10.1128/JVI.75.16.7572-7582.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Nakamura H, Lu M, Gwack Y, Souvlis J, Zeichner SL, Jung JU. Global changes in Kaposi’s sarcoma-associated virus gene expression patterns following expression of a tetracycline-inducible Rta transactivator. J Virol. 2003;77:4205–20. doi: 10.1128/JVI.77.7.4205-4220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Nakano H, Shindo M, Sakon S, Nishinaka S, Mihara M, Yagita H, Okumura K. Differential regulation of IkappaB kinase alpha and beta by two upstream kinases, NF-kappaB-inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proc Natl Acad Sci USA. 1998;95:3537–42. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Naranatt PP, Akula SM, Zien CA, Krishnan HH, Chandran B. Kaposi’s sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-zeta-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J Virol. 2003;77:1524–39. doi: 10.1128/JVI.77.2.1524-1539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Naranatt PP, Krishnan HH, Smith MS, Chandran B. Kaposi’s sarcoma-associated herpesvirus modulates microtubule dynamics via RhoA-GTP-diaphanous 2 signaling and utilizes the dynein motors to deliver its DNA to the nucleus. J Virol. 2005;79:1191–206. doi: 10.1128/JVI.79.2.1191-1206.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Naranatt PP, Krishnan HH, Svojanovsky SR, Bloomer C, Mathur S, Chandran B. Host gene induction and transcriptional reprogramming in Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8)-infected endothelial, fibroblast, and B cells: insights into modulation events early during infection. Cancer Res. 2004;64:72–84. doi: 10.1158/0008-5472.can-03-2767. [DOI] [PubMed] [Google Scholar]
- 323.Nealon K, Newcomb WW, Pray TR, Craik CS, Brown JC, Kedes DH. Lytic replication of Kaposi’s sarcoma-associated herpesvirus results in the formation of multiple capsid species: isolation and molecular characterization of A, B, and C capsids from a gammaherpesvirus. J Virol. 2001;75:2866–78. doi: 10.1128/JVI.75.6.2866-2878.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Nicholas J, Ruvolo VR, Burns WH, Sandford G, Wan X, Ciufo D, Hendrickson SB, Guo HG, Hayward GS, Reitz MS. Kaposi’s sarcoma-associated human herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat Med. 1997;3:287–92. doi: 10.1038/nm0397-287. [DOI] [PubMed] [Google Scholar]
- 325.Nocera A, Corbellino M, Valente U, Barocci S, Torre F, De Palma R, Sementa A, Traverso GB, Icardi A, Fontana I, Arcuri V, Poli F, Cagetti P, Moore P, Parravicini C. Posttransplant human herpes virus 8 infection and seroconversion in a Kaposi’s sarcoma affected kidney recipient transplanted from a human herpes virus 8 positive living related donor. Transplant Proc. 1998;30:2095–6. doi: 10.1016/s0041-1345(98)00550-8. [DOI] [PubMed] [Google Scholar]
- 326.O’Brien TR, Kedes D, Ganem D, Macrae DR, Rosenberg PS, Molden J, Goedert JJ. Evidence for concurrent epidemics of human herpesvirus 8 and human immunodeficiency virus type 1 in US homosexual men: rates, risk factors, and relationship to Kaposi’s sarcoma. J Infect Dis. 180:1010–7. doi: 10.1086/315039. [DOI] [PubMed] [Google Scholar]
- 327.O’Leary JJ, Kennedy M, Luttich K, Uhlmann V, Silva I, Russell J, Sheils O, Ring M, Sweeney M, Kenny C, Bermingham N, Martin C, O’Donovan M, Howells D, Picton S, Lucas SB. Localization of HHV-8 in AIDS related lymphadenopathy. Mol Pathol. 2000;53:43–7. doi: 10.1136/mp.53.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Oettle AG. Geographical and racial differences in the frequency of Kaposi’s sarcoma as evidence of environmental or genetic causes. Acta Unio Int Contra Cancrum. 1962;18:330–63. [PubMed] [Google Scholar]
- 329.Offermann MK. Consideration of host-viral interactions in the pathogenesis of Kaposi’s sarcoma. J Acquir Immune Defic Syndr. 1999;21(Suppl 1):S58–65. [PubMed] [Google Scholar]
- 330.Ojala PM, Tiainen M, Salven P, Veikkola T, Castanos-Velez E, Sarid R, Biberfeld P, Makela TP. Kaposi’s sarcoma-associated herpesvirus-encoded v-cyclin triggers apoptosis in cells with high levels of cyclin-dependent kinase 6. Cancer Res. 1999;59:4984–9. [PubMed] [Google Scholar]
- 331.Ojala PM, Yamamoto K, Castanos-Velez E, Biberfeld P, Korsmeyer SJ, Makela TP. The apoptotic v-cyclin–CDK6 complex phosphorylates and inactivates Bcl-2. Nat Cell Biol. 2000;2:819–25. doi: 10.1038/35041064. [DOI] [PubMed] [Google Scholar]
- 332.Osborne J, Moore PS, Chang Y. KSHV-encoded viral IL-6 activates multiple human IL-6 signaling pathways. Hum Immunol. 1999;60:921–7. doi: 10.1016/s0198-8859(99)00083-x. [DOI] [PubMed] [Google Scholar]
- 333.Pan H, Xie J, Ye F, Gao S-J. Modulation of Kaposi’s sarcoma -associated herpesvirus infection and replication by MEK/ERK, JNK, and p38 multiple mitogen-activated protein kinase pathways during primary infection. J Virol. 80:5371–82. doi: 10.1128/JVI.02299-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Pan H, Zhou F, Gao SJ. Kaposi’s sarcoma-associated herpesvirus induction of chromosome instability in primary human endothelial cells. Cancer Res. 2004;64:4064–8. doi: 10.1158/0008-5472.CAN-04-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Pan HY, Zhang YJ, Wang XP, Deng JH, Zhou FC, Gao SJ. Identification of a novel cellular transcriptional repressor interacting with the latent nuclear antigen of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2003;77:9758–68. doi: 10.1128/JVI.77.18.9758-9768.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Parravicini C, Chandran B, Corbellino M, Berti E, Paulli M, Moore PS, Chang Y. Differential viral protein expression in Kaposi’s sarcoma-associated herpesvirus-infected diseases: Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. Am J Pathol. 2000;156:743–9. doi: 10.1016/S0002-9440(10)64940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Parravicini C, Olsen SJ, Capra M, Poli F, Sirchia G, Gao SJ, Berti E, Nocera A, Rossi E, Bestetti G, Pizzuto M, Galli M, Moroni M, Moore PS, Corbellino M. Risk of Kaposi’s sarcoma-associated herpes virus transmission from donor allografts among Italian posttransplant Kaposi’s sarcoma patients. Blood. 1997;90:2826–9. [PubMed] [Google Scholar]
- 338.Parsons CH, Szomju B, Kedes DH. Susceptibility of human fetal mesenchymal stem cells to Kaposi sarcoma-associated herpesvirus. Blood. 2004;104:2736–8. doi: 10.1182/blood-2004-02-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Pati S, Cavrois M, Guo HG, Foulke JS, Jr., Kim J, Feldman RA, Reitz M. Activation of NF-kappaB by the human herpesvirus 8 chemokine receptor ORF74: evidence for a paracrine model of Kaposi’s sarcoma pathogenesis. J Virol. 2001;75:8660–73. doi: 10.1128/JVI.75.18.8660-8673.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Pearce M, Matsumura S, Wilson AC. Transcripts encoding K12, v-FLIP, v-cyclin, and the microRNA cluster of Kaposi’s sarcoma-associated herpesvirus originate from a common promoter. J Virol. 2005;79:14457–64. doi: 10.1128/JVI.79.22.14457-14464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Pertel PE. Human herpesvirus 8 glycoprotein B (gB), gH, and gL can mediate cell fusion. J Virol. 2002;76:4390–400. doi: 10.1128/JVI.76.9.4390-4400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Pfeffer S, Sewer A, Lagos-Quintana M, Sheridan R, Sander C, Grasser FA, van Dyk LF, Ho CK, Shuman S, Chien M, Russo JJ, Ju J, Randall G, Lindenbach BD, Rice CM, Simon V, Ho DD, Zavolan M, Tuschl T. Identification of microRNAs of the herpesvirus family. Nat Methods. 2005;2:269–76. doi: 10.1038/nmeth746. [DOI] [PubMed] [Google Scholar]
- 343.Picchio G, Sabbe RE, Gulizia RJ, McGrath M, Herndier BG, Mosier DE. The KSHV/HHV8-infected BCBL-1 lymphoma line causes tumors in SCID mice but fails to transmit virus to a human peripheral blood mononuclear cell graft. Virology. 1997;238:22–9. doi: 10.1006/viro.1997.8822. [DOI] [PubMed] [Google Scholar]
- 344.Pine R, Decker T, Kessler DS, Levy DE, Darnell JE., Jr. Purification and cloning of interferon-stimulated gene factor 2 (ISGF2): ISGF2 (IRF-1) can bind to the promoters of both beta interferon- and interferon-stimulated genes but is not a primary transcriptional activator of either. Mol Cell Biol. 1990;10:2448–57. doi: 10.1128/mcb.10.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Piolot T, Tramier M, Coppey M, Nicolas JC, Marechal V. Close but distinct regions of human herpesvirus 8 latency-associated nuclear antigen 1 are responsible for nuclear targeting and binding to human mitotic chromosomes. J Virol. 2001;75:3948–59. doi: 10.1128/JVI.75.8.3948-3959.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem. 2000;275:21785–8. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 347.Polstra AM, Cornelissen M, Goudsmit J, van der Kuyl AC. Retrospective, longitudinal analysis of serum human herpesvirus-8 viral DNA load in AIDS-related Kaposi’s sarcoma patients before and after diagnosis. J Med Virol. 2004;74:390–6. doi: 10.1002/jmv.20192. [DOI] [PubMed] [Google Scholar]
- 348.Popescu NC, Zimonjic DB, Leventon-Kriss S, Bryant JL, Lunardi-Iskandar Y, Gallo RC. Deletion and translocation involving chromosome 3 (p14) in two tumorigenic Kaposi’s sarcoma cell lines. J Natl Cancer Inst. 1996;88:450–5. doi: 10.1093/jnci/88.7.450. [DOI] [PubMed] [Google Scholar]
- 349.Prakash O, Swamy OR, Peng X, Tang ZY, Li L, Larson JE, Cohen JC, Gill J, Farr G, Wang S, Samaniego F. Activation of Src kinase Lyn by the Kaposi sarcoma-associated herpesvirus K1 protein: implications for lymphomagenesis. Blood. 2005;105:3987–94. doi: 10.1182/blood-2004-07-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Pyakurel P, Pak F, Mwakigonja AR, Kaaya E, Heiden T, Biberfeld P. Lymphatic and vascular origin of Kaposi’s sarcoma spindle cells during tumor development. Int J Cancer. 2006 doi: 10.1002/ijc.21969. [DOI] [PubMed] [Google Scholar]
- 351.Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to Maloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol. 2004;78:11926–38. doi: 10.1128/JVI.78.21.11926-11938.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Radkov SA, Kellam P, Boshoff C. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat Med. 6:1121–7. doi: 10.1038/80459. [DOI] [PubMed] [Google Scholar]
- 353.Rainbow L, Platt GM, Simpson GR, Sarid R, Gao SJ, Stoiber H, Herrington CS, Moore PS, Schulz TF. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf 73 and is a component of the latency-associated nuclear antigen. J Virol. 1997;71:5915–21. doi: 10.1128/jvi.71.8.5915-5921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Rappocciolo G, Jenkins FJ, Hensler HR, Piazza P, Jais M, Borowski L, Watkins SC, Rinaldo CR., Jr. DC-SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages. J Immunol. 2006;176:1741–9. doi: 10.4049/jimmunol.176.3.1741. [DOI] [PubMed] [Google Scholar]
- 355.Regamey N, Tamm M, Wernli M, Witschi A, Thiel G, Cathomas G, Erb P. Transmission of human herpesvirus 8 infection from renal-transplant donors to recipients. N Engl J Med. 1998;339:1358–63. doi: 10.1056/NEJM199811053391903. [DOI] [PubMed] [Google Scholar]
- 356.Relloso M, Puig-Kroger A, Pello OM, Rodriguez-Fernandez JL, de la Rosa G, Longo N, Navarro J, Munoz-Fernandez MA, Sanchez-Mateos P, Corbi AL. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-beta, and anti-inflammatory agents. J Immunol. 2002;168:2634–43. doi: 10.4049/jimmunol.168.6.2634. [DOI] [PubMed] [Google Scholar]
- 357.Renne R, Blackbourn D, Whitby D, Levy J, Ganem D. Limited transmission of Kaposi’s sarcoma-associated herpesvirus in cultured cells. J Virol. 72:5182–8. doi: 10.1128/jvi.72.6.5182-5188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Renne R, Dittmer D, Kedes D, Schmidt K, Desrosiers RC, Luciw PA, Ganem D. Experimental transmission of Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) to SIV-positive and SIV-negative rhesus macaques. J Med Primatol. 2004;33:1–9. doi: 10.1046/j.1600-0684.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 359.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2:342–6. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 360.Renwick N, Halaby T, Weverling GJ, Dukers NH, Simpson GR, Coutinho RA, Lange JM, Schulz TF, Goudsmit J. Seroconversion for human herpesvirus 8 during HIV infection is highly predictive of Kaposi’s sarcoma. AIDS. 12:2481–8. doi: 10.1097/00002030-199818000-00018. [DOI] [PubMed] [Google Scholar]
- 361.Rimessi P, Bonaccorsi A, Sturzl M, Fabris M, Brocca-Cofano E, Caputo A, Melucci-Vigo G, Falchi M, Cafaro A, Cassai E, Ensoli B, Monini P. Transcription pattern of human herpesvirus 8 open reading frame K3 in primary effusion lymphoma and Kaposi’s sarcoma. J Virol. 2001;75:7161–74. doi: 10.1128/JVI.75.15.7161-7174.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Rivas C, Thlick AE, Parravicini C, Moore PS, Chang Y. Kaposi’s sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J Virol. 2001;75:429–38. doi: 10.1128/JVI.75.1.429-438.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Roan F, Inoue N, Offermann MK. Activation of cellular and heterologous promoters by the human herpesvirus 8 replication and transcription activator. Virology. 2002;301:293–3040. doi: 10.1006/viro.2002.1582. [DOI] [PubMed] [Google Scholar]
- 364.Roan F, Zimring JC, Goodbourn S, Offermann MK. Transcriptional activation by the human herpesvirus-8-encoded interferon regulatory factor. J Gen Virol. 1999;80(Pt 8):2205–9. doi: 10.1099/0022-1317-80-8-2205. [DOI] [PubMed] [Google Scholar]
- 365.Robles R, Lugo D, Gee L, Jacobson MA. Effect of antiviral drugs used to treat cytomegalovirus end-organ disease on subsequent course of previously diagnosed Kaposi’s sarcoma in patients with AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:34–8. doi: 10.1097/00042560-199901010-00005. [DOI] [PubMed] [Google Scholar]
- 366.Rose TM, Strand KB, Schultz ER, Schaefer G, Rankin GW, Jr., Thouless ME, Tsai CC, Bosch ML. Identification of two homologs of the Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in retroperitoneal fibromatosis of different macaque species. J Virol. 1997;71:4138–44. doi: 10.1128/jvi.71.5.4138-4144.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Russo JJ, Bohenzky RA, Chien MC, Chen J, Yan M, Maddalena D, Parry JP, Peruzzi D, Edelman IS, Chang Y, Moore PS. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–7. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Sadler R, Wu L, Forghani B, Renne R, Zhong W, Herndier B, Ganem D. A complex translational program generates multiple novel proteins from the latently expressed kaposin (K12) locus of Kaposi’s sarcoma-associated herpesvirus. J Virol. 1999;73:5722–30. doi: 10.1128/jvi.73.7.5722-5730.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Sailer A, Nagata K, Naf D, Aebi M, Weissmann C. Interferon regulatory factor-1 (IRF-1) activates the synthetic IRF-1-responsive sequence (GAAAGT)4 in Saccharomyces cerevisiae. Gene Expr. 1992;2:329–37. [PMC free article] [PubMed] [Google Scholar]
- 370.Sakakibara S, Ueda K, Chen J, Okuno T, Yamanishi K. Octamer-binding sequence is a key element for the autoregulation of Kaposi’s sarcoma-associated herpesvirus ORF50/Lyta gene expression. J Virol. 2001;75:6894–900. doi: 10.1128/JVI.75.15.6894-6900.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Sakakibara S, Ueda K, Nishimura K, Do E, Ohsaki E, Okuno T, Yamanishi K. Accumulation of heterochromatin components on the terminal repeat sequence of Kaposi’s sarcoma-associated herpesvirus mediated by the latency-associated nuclear antigen. J Virol. 2004;78:7299–310. doi: 10.1128/JVI.78.14.7299-7310.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Samaniego F, Markham PD, Gallo RC, Ensoli B. Inflammatory cytokines induce AIDS-Kaposi’s sarcoma-derived spindle cells to produce and release basic fibroblast growth factor and enhance Kaposi’s sarcoma-like lesion formation in nude mice. J Immunol. 1995;154:3582–92. [PubMed] [Google Scholar]
- 373.Samaniego F, Young D, Grimes C, Prospero V, Christofidou-Solomidou M, DeLisser HM, Prakash O, Sahin AA, Wang S. Vascular endothelial growth factor and Kaposi’s sarcoma cells in human skin grafts. Cell Growth Differ. 2002;13:387–95. [PubMed] [Google Scholar]
- 374.Samols MA, Hu J, Skalsky RL, Renne R. Cloning and identification of a microRNA cluster within the latency-associated region of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2005;79:9301–5. doi: 10.1128/JVI.79.14.9301-9305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Sanchez DJ, Gumperz JE, Ganem D. Regulation of CD1d expression and function by a herpesvirus infection. J Clin Invest. 2005;115:1369–78. doi: 10.1172/JCI24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Sarek G, Jarviluoma A, Ojala PM. KSHV viral cyclin inactivates p27KIP1 through Ser10 and Thr187 phosphorylation in proliferating primary effusion lymphomas. Blood. 2006;107:725–32. doi: 10.1182/blood-2005-06-2534. [DOI] [PubMed] [Google Scholar]
- 377.Sarid R, Flore O, Bohenzky RA, Chang Y, Moore PS. Transcription mapping of the Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1) J Virol. 1998;72:1005–12. doi: 10.1128/jvi.72.2.1005-1012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Sarid R, Sato T, Bohenzky RA, Russo JJ, Chang Y. Kaposi’s sarcoma-associated herpesvirus encodes a functional bcl-2 homologue. Nat Med. 3:293–8. doi: 10.1038/nm0397-293. [DOI] [PubMed] [Google Scholar]
- 379.Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem. 2002;277:44765–71. doi: 10.1074/jbc.M208704200. [DOI] [PubMed] [Google Scholar]
- 380.Sastry SK, Burridge K. Focal adhesions: a nexus for intracellular signaling and cytoskeletal dynamics. Exp Cell Res. 2000;261:25–36. doi: 10.1006/excr.2000.5043. [DOI] [PubMed] [Google Scholar]
- 381.Scadden DT. AIDS-related malignancies. Annu Rev Med. 2003;54:285–303. doi: 10.1146/annurev.med.54.101601.152143. [DOI] [PubMed] [Google Scholar]
- 382.Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta. 2001;1540:1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- 383.Schalling M, Ekman M, Kaaya EE, Linde A, Biberfeld P. A role for a new herpes virus (KSHV) in different forms of Kaposi’s sarcoma. Nat Med. 1995;1:707–8. doi: 10.1038/nm0795-707. [DOI] [PubMed] [Google Scholar]
- 384.Schulz TF. The pleiotropic effects of Kaposi’s sarcoma herpesvirus. J Pathol. 2006;208:187–98. doi: 10.1002/path.1904. [DOI] [PubMed] [Google Scholar]
- 385.Schwam DR, Luciano RL, Mahajan SS, Wong L, Wilson AC. Carboxy terminus of human herpesvirus 8 latency-associated nuclear antigen mediates dimerization, transcriptional repression, and targeting to nuclear bodies. J Virol. 2000;74:8532–40. doi: 10.1128/jvi.74.18.8532-8540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Schwarz M, Murphy PM. Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor constitutively activates NF-kappa B and induces proinflammatory cytokine and chemokine production via a C-terminal signaling determinant. J Immunol. 2001;167:505–13. doi: 10.4049/jimmunol.167.1.505. [DOI] [PubMed] [Google Scholar]
- 387.Seaman WT, Ye D, Wang RX, Hale EE, Weisse M, Quinlivan EB. Gene expression from the ORF50/K8 region of Kaposi’s sarcoma-associated herpesvirus. Virology. 1999;263:436–49. doi: 10.1006/viro.1999.9963. [DOI] [PubMed] [Google Scholar]
- 388.Searles RP, Bergquam EP, Axthelm MK, Wong SW. Sequence and genomic analysis of a Rhesus macaque rhadinovirus with similarity to Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8. J Virol. 1999;73:3040–53. doi: 10.1128/jvi.73.4.3040-3053.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Seo T, Lee D, Lee B, Chung JH, Choe J. Viral interferon regulatory factor 1 of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) binds to, and inhibits transactivation of, CREB-binding protein. Biochem Biophys Res Commun. 2000;270:23–7. doi: 10.1006/bbrc.2000.2393. [DOI] [PubMed] [Google Scholar]
- 390.Seo T, Lee D, Shim YS, Angell JE, Chidambaram NV, Kalvakolanu DV, Choe J. Viral interferon regulatory factor 1 of Kaposi’s sarcoma-associated herpesvirus interacts with a cell death regulator, GRIM19, and inhibits interferon/retinoic acid-induced cell death. J Virol. 2002;76:8797–807. doi: 10.1128/JVI.76.17.8797-8807.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Seo T, Park J, Lee D, Hwang SG, Choe J. Viral interferon regulatory factor 1 of Kaposi’s sarcoma-associated herpesvirus binds to p53 and represses p53-dependent transcription and apoptosis. J Virol. 2001;75:6193–8. doi: 10.1128/JVI.75.13.6193-6198.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Sgarbanti M, Arguello M, tenOever BR, Battistini A, Lin R, Hiscott J. A requirement for NF-kappaB induction in the production of replication-competent HHV-8 virions. Oncogene. 23:5770–80. doi: 10.1038/sj.onc.1207707. [DOI] [PubMed] [Google Scholar]
- 393.Sharma-Walia N, Krishnan HH, Naranatt PP, Zeng L, Smith MS, Chandran B. RK1/2 and MEK1/2 induced by Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) early during infection of target cells are essential for expression of viral genes and for establishment of infection. J Virol. 2005;79:10308–29. doi: 10.1128/JVI.79.16.10308-10329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Sharma-Walia N, Naranatt PP, Krishnan HH, Zeng L, Chandran B. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-Src-phosphatidylinositol 3-kinase-rho GTPase signal pathways and cytoskeletal rearrangements. J Virol. 2004;78:4207–23. doi: 10.1128/JVI.78.8.4207-4223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Sharp TV, Wang HW, Koumi A, Hollyman D, Endo Y, Ye H, Du MQ, Boshoff C. K15 protein of Kaposi’s sarcoma-associated herpesvirus is latently expressed and binds to HAX-1, a protein with antiapoptotic function. J Virol. 2002;76:802–16. doi: 10.1128/JVI.76.2.802-816.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Shepard LW, Yang M, Xie P, Browning DD, Voyno-Yasenetskaya T, Kozasa T. Constitutive activation of NF-kappa B and secretion of interleukin-8 induced by the G protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus involve G alpha13 and RhoA. J Biol Chem. 2001;276:45979–87. doi: 10.1074/jbc.M104783200. R. D. and Ye. [DOI] [PubMed] [Google Scholar]
- 397.Shin YC, Nakamura H, Liang X, Feng P, Chang H, Kowalik TF, Jung JU. Inhibition of the ATM/p53 signal transduction pathway by Kaposi’s sarcoma-associated herpesvirus interferon regulatory factor 1. J Virol. 2006;80:2257–66. doi: 10.1128/JVI.80.5.2257-2266.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Shinohara H, Fukushi M, Higuchi M, Oie M, Hoshi O, Ushiki T, Hayashi J, Fujii M. Chromosome binding site of latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus is essential for persistent episome maintenance and is functionally replaced by histone H1. J Virol. 2002;76:12917–24. doi: 10.1128/JVI.76.24.12917-12924.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Si H, Robertson ES. Kaposi’s sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen induces chromosomal instability through inhibition of p53 function. J Virol. 2006;80:697–709. doi: 10.1128/JVI.80.2.697-709.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Sieczkarski SB, Whittaker GR. Dissecting virus entry via endocytosis. J Gen Virol. 2002;83:1535–45. doi: 10.1099/0022-1317-83-7-1535. [DOI] [PubMed] [Google Scholar]
- 401.Siegal FP, Lopez C, Hammer GS, Brown AE, Kornfeld SJ, Gold J, Hassett J, Hirschman SZ, Cunningham-Rundles C, Adelsberg BR, et al. Severe acquired immunodeficiency in male homosexuals, manifested by chronic perianal ulcerative herpes simplex lesions. N Engl J Med. 1981;305:1439–44. doi: 10.1056/NEJM198112103052403. [DOI] [PubMed] [Google Scholar]
- 402.Simonart T, Degraef C, Heenen M, Hermans P, Van Vooren JP, Noel JC. Expression of the fibroblast/macrophage marker 1B10 by spindle cells in Kaposi’s sarcoma lesions and by Kaposi’s sarcoma-derived tumor cells. J Cutan Pathol. 2002;29:72–8. doi: 10.1034/j.1600-0560.2002.290202.x. [DOI] [PubMed] [Google Scholar]
- 403.Simpson GR, Schulz TF, Whitby D, Cook PM, Boshoff C, Rainbow L, Howard MR, Gao SJ, Bohenzky RA, Simmonds P, Lee C, de Ruiter A, Hatzakis A, Tedder RS, Weller IV, Weiss RA, Moore PS. Prevalence of Kaposi’s sarcoma associated herpesvirus infection measured by antibodies to recombinant capsid protein and latent immunofluorescence antigen. Lancet. 1996;348:1133–8. doi: 10.1016/S0140-6736(96)07560-5. [DOI] [PubMed] [Google Scholar]
- 404.Smit MJ, Verzijl D, Casarosa P, Navis M, Timmerman H, Leurs R. Kaposi’s sarcoma-associated herpesvirus-encoded G protein-coupled receptor ORF74 constitutively activates p44/p42 MAPK and Akt via G(i) and phospholipase C-dependent signaling pathways. J Virol. 2002;76:1744–52. doi: 10.1128/JVI.76.4.1744-1752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Sodhi A, Montaner S, Gutkind JS. Does dysregulated expression of a deregulated viral GPCR trigger Kaposi’s sarcomagenesis? Faseb J. 2004;18:422–7. doi: 10.1096/fj.03-1035hyp. [DOI] [PubMed] [Google Scholar]
- 406.Sodhi A, Montaner S, Patel V, Gomez-Roman JJ, Li Y, Sausville EA, Sawai ET, Gutkind JS. Akt plays a central role in sarcomagenesis induced by Kaposi’s sarcoma herpesvirus-encoded G protein-coupled receptor. Proc Natl Acad Sci USA. 2004;101:4821–6. doi: 10.1073/pnas.0400835101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Sodhi A, Montaner S, Patel V, Zohar M, Bais C, Mesri EA, Gutkind JS. The Kaposi’s sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1alpha. Cancer Res. 2000;60:4873–80. [PubMed] [Google Scholar]
- 408.Soilleux EJ, Morris LS, Lee B, Pohlmann S, Trowsdale J, Doms RW, Coleman N. Placental expression of DC-SIGN may mediate intrauterine vertical transmission of HIV. J Pathol. 2001;195:586–92. doi: 10.1002/path.1026. [DOI] [PubMed] [Google Scholar]
- 409.Soilleux EJ, Morris LS, Leslie G, Chehimi J, Luo Q, Levroney E, Trowsdale J, Montaner LJ, Doms RW, Weissman D, Coleman N, Lee B. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol. 2002;71:445–57. [PubMed] [Google Scholar]
- 410.Soilleux EJ, Morris LS, Rushbrook S, Lee B, Coleman N. Expression of human immunodeficiency virus (HIV)-binding lectin DC-SIGNR: Consequences for HIV infection and immunity. Hum Pathol. 2002;33:652–9. doi: 10.1053/hupa.2002.124036. [DOI] [PubMed] [Google Scholar]
- 411.Soilleux EJ, Morris LS, Trowsdale J, Coleman N, Boyle JJ. Human atherosclerotic plaques express DC-SIGN, a novel protein found on dendritic cells and macrophages. J Pathol. 2002;198:511–6. doi: 10.1002/path.1205. [DOI] [PubMed] [Google Scholar]
- 412.Song J, Ohkura T, Sugimoto M, Mori Y, Inagi R, Yamanishi K, Yoshizaki K, Nishimoto N. Human interleukin-6 induces human herpesvirus-8 replication in a body cavity-based lymphoma cell line. J Med Virol. 2002;68:404–11. doi: 10.1002/jmv.10218. [DOI] [PubMed] [Google Scholar]
- 413.Song MJ, Deng H, Sun R. Comparative study of regulation of RTA-responsive genes in Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8. J Virol. 2003;77:9451–62. doi: 10.1128/JVI.77.17.9451-9462.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Song MJ, Li X, Brown HJ, Sun R. Characterization of interactions between RTA and the promoter of polyadenylated nuclear RNA in Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8. J Virol. 2002;76:5000–13. doi: 10.1128/JVI.76.10.5000-5013.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d’Agay MF, Clauvel JP, Raphael M, Degos L, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–80. [PubMed] [Google Scholar]
- 416.Spear PG, Longnecker R. Herpesvirus entry: an update. J Virol. 2003;77:10179–85. doi: 10.1128/JVI.77.19.10179-10185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Spiller OB, Blackbourn DJ, Mark L, Proctor DG, Blom AM. Functional activity of the complement regulator encoded by Kaposi’s sarcoma-associated herpesvirus. J Biol Chem. 2003;278:9283–9. doi: 10.1074/jbc.m211579200. [DOI] [PubMed] [Google Scholar]
- 418.Spiller OB, Mark L, Blue CE, Proctor DG, Aitken JA, Blom AM, Blackbourn DJ. Dissecting the regions of virion-associated Kaposi’s sarcoma-associated herpesvirus complement control protein required for complement regulation and cell binding. J Virol. 2006;80:4068–78. doi: 10.1128/JVI.80.8.4068-4078.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Spiller OB, Robinson M, O’Donnell E, Milligan S, Morgan BP, Davison AJ, Blackbourn DJ. Complement regulation by Kaposi’s sarcoma-associated herpesvirus ORF4 protein. J Virol. 2003;77:592–9. doi: 10.1128/JVI.77.1.592-599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Staskus KA, Zhong W, Gebhard K, Herndier B, Wang H, Renne R, Beneke J, Pudney J, Anderson DJ, Ganem D, Haase AT. Kaposi’s sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J Virol. 1997;71:715–9. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Staudt MR, Dittmer DP. Promoter switching allows simultaneous transcription of LANA and K14/vGPCR of Kaposi’s sarcoma-associated herpesvirus. Virology. 2006 doi: 10.1016/j.virol.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 422.Staudt MR, Dittmer DP. Viral latent proteins as targets for Kaposi’s sarcoma and Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) induced lymphoma. Curr Drug Targets Infect Disord. 2003;3:129–35. doi: 10.2174/1568005033481150. [DOI] [PubMed] [Google Scholar]
- 423.Stedman W, Deng Z, Lu F, Lieberman PM. ORC, MCM, and histone hyperacetylation at the Kaposi’s sarcoma-associated herpesvirus latent replication origin. J Virol. 2004;78:12566–75. doi: 10.1128/JVI.78.22.12566-12575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Sternbach G, Varon J. Moritz Kaposi: idiopathic pigmented sarcoma of the skin. J Emerg Med. 1995;13:671–4. doi: 10.1016/0736-4679(95)00077-n. [DOI] [PubMed] [Google Scholar]
- 425.Stine JT, Wood C, Hill M, Epp A, Raport CJ, Schweickart VL, Endo Y, Sasaki T, Simmons G, Boshoff C, Clapham P, Chang Y, Moore P, Gray PW, Chantry D. KSHV-encoded CC chemokine vMIP-III is a CCR4 agonist, stimulates angiogenesis, and selectively chemoattracts TH2 cells. Blood. 2000;95:1151–7. D. [PubMed] [Google Scholar]
- 426.Sturzl M, Hohenadl C, Zietz C, Castanos-Velez E, Wunderlich A, Ascherl G, Biberfeld P, Monini P, Browning J, Ensoli B. Expression of K13/v-FLIP gene of human herpesvirus 8 and apoptosis in Kaposi’s sarcoma spindle cells. J Natl Cancer Inst. 1999;91:1725–33. doi: 10.1093/jnci/91.20.1725. [DOI] [PubMed] [Google Scholar]
- 427.Sturzl M, Wunderlich A, Ascherl G, Hohenadl C, Monini P, Zietz C, Browning PJ, Neipel F, Biberfeld P, Ensoli B. Human herpesvirus-8 (HHV-8) gene expression in Kaposi’s sarcoma (KS) primary lesions: an in situ hybridization study. Leukemia. 1999;13(Suppl 1):S110–2. doi: 10.1038/sj.leu.2401323. [DOI] [PubMed] [Google Scholar]
- 428.Sturzl M, Zietz C, Monini P, Ensoli B. Human herpesvirus-8 and Kaposi’s sarcoma: relationship with the multistep concept of tumorigenesis. Adv Cancer Res. 2001;81:125–59. doi: 10.1016/s0065-230x(01)81004-6. [DOI] [PubMed] [Google Scholar]
- 429.Sun Q, Zachariah S, Chaudhary PM. The human herpes virus 8-encoded viral FLICE-inhibitory protein induces cellular transformation via NF-kappaB activation. J Biol Chem. 2003;278:52437–45. doi: 10.1074/jbc.M304199200. [DOI] [PubMed] [Google Scholar]
- 430.Sun R, Lin SF, Gradoville L, Yuan Y, Zhu F, Miller G. A viral gene that activates lytic cycle expression of Kaposi’s sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1998;95:10866–71. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Sun R, Lin SF, Staskus K, Gradoville L, Grogan E, Haase A, Miller G. Kinetics of Kaposi’s sarcoma-associated herpesvirus gene expression. J Virol. 1999;73:2232–42. doi: 10.1128/jvi.73.3.2232-2242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Swanton C, Mann DJ, Fleckenstein B, Neipel F, Peters G, Jones N. Herpes viral cyclin/Cdk6 complexes evade inhibition by CDK inhibitor proteins. Nature. 390:184–7. doi: 10.1038/36606. [DOI] [PubMed] [Google Scholar]
- 433.Szekely L, Kiss C, Mattsson K, Kashuba E, Pokrovskaja K, Juhasz A, Holmvall P, Klein G. Human herpesvirus-8-encoded LNA-1 accumulates in heterochromatin-associated nuclear bodies. J Gen Virol. 1999;80(Pt 11):2889–900. doi: 10.1099/0022-1317-80-11-2889. [DOI] [PubMed] [Google Scholar]
- 434.Talbot SJ, Weiss RA, Kellam P, Boshoff C. Transcriptional analysis of human herpesvirus-8 open reading frames 71, 72, 73, K14, and 74 in a primary effusion lymphoma cell line. Virology. 1999;257:84–94. doi: 10.1006/viro.1999.9672. [DOI] [PubMed] [Google Scholar]
- 435.Tamura T, Ishihara M, Lamphier MS, Tanaka N, Oishi I, Aizawa S, Matsuyama T, Mak TW, Taki S, Taniguchi T. An IRF-1-dependent pathway of DNA damage-induced apoptosis in mitogen-activated T lymphocytes. Nature. 1995;376:596–9. doi: 10.1038/376596a0. [DOI] [PubMed] [Google Scholar]
- 436.Tanaka N, Ishihara M, Lamphier MS, Nozawa H, Matsuyama T, Mak TW, Aizawa S, Tokino T, Oren M, Taniguchi T. Cooperation of the tumor suppressors IRF-1 and p53 in response to DNA damage. Nature. 1996;382:816–8. doi: 10.1038/382816a0. [DOI] [PubMed] [Google Scholar]
- 437.Tanaka N, Ishihara M, Taniguchi T. Suppression of c-myc or fosB-induced cell transformation by the transcription factor IRF-1. Cancer Lett. 1994;83:191–6. doi: 10.1016/0304-3835(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 438.Tang J, Gordon GM, Muller MG, Dahiya M, Foreman KE. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen induces expression of the helix-loop-helix protein Id-1 in human endothelial cells. J Virol. 2003;77:5975–84. doi: 10.1128/JVI.77.10.5975-5984.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Tang S, Zheng ZM. Kaposi’s sarcoma-associated herpesvirus K8 exon 3 contains three 5′-splice sites and harbors a K8.1 transcription start site. J Biol Chem. 2002;277:14547–56. doi: 10.1074/jbc.M111308200. [DOI] [PubMed] [Google Scholar]
- 440.Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. Faseb J. 2006;20:9–22. doi: 10.1096/fj.05-4682rev. [DOI] [PubMed] [Google Scholar]
- 441.Tetsuka T, Higuchi M, Fukushi M, Watanabe A, Takizawa S, Oie M, Gejyo F, Fujii M. Visualization of a functional KSHV episome-maintenance protein LANA in living cells. Virus Genes. 29:175–82. doi: 10.1023/B:VIRU.0000036377.48454.d8. [DOI] [PubMed] [Google Scholar]
- 442.Tomkowicz B, Singh SP, Cartas M, Srinivasan A. Human herpesvirus-8 encoded Kaposin: subcellular localization using immunofluorescence and biochemical approaches. DNA Cell Biol. 21:151–62. doi: 10.1089/10445490252925413. [DOI] [PubMed] [Google Scholar]
- 443.Turville SG, Cameron PU, Handley A, Lin G, Pohlmann S, Doms RW, Cunningham AL. Diversity of receptors binding HIV on dendritic cell subsets. Nat Immunol. 2002;3:975–83.0. doi: 10.1038/ni841. [DOI] [PubMed] [Google Scholar]
- 444.Uccini S, Ruco LP, Monardo F, Stoppacciaro A, Dejana E, La Parola IL, Cerimele D, Baroni CD. Co-expression of endothelial cell and macrophage antigens in Kaposi’s sarcoma cells. J Pathol. 1994;173:23–31. doi: 10.1002/path.1711730105. [DOI] [PubMed] [Google Scholar]
- 445.Ueda K, Ishikawa K, Nishimura K, Sakakibara S, Do E, Yamanishi K. Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) replication and transcription factor activates the K9 (vIRF) gene through two distinct cis elements by a non-DNA-binding mechanism. J Virol. 2002;76:12044–54. doi: 10.1128/JVI.76.23.12044-12054.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Verma SC, Borah S, Robertson ES. Latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus up-regulates transcription of human telomerase reverse transcriptase promoter through interaction with transcription factor Sp1. J Virol. 2004;78:10348–59. doi: 10.1128/JVI.78.19.10348-10359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Verma SC, Choudhuri T, Kaul R, Robertson ES. Latency-associated nuclear antigen (LANA) of Kaposi’s sarcoma-associated herpesvirus interacts with origin recognition complexes at the LANA binding sequence within the terminal repeats. J Virol. 2006;80:2243–56. doi: 10.1128/JVI.80.5.2243-2256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Verma SC, Lan K, Choudhuri T, Robertson ES. Kaposi’s sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen modulates K1 expression through its cis-acting elements within the terminal repeats. J Virol. 2006;80:3445–58. doi: 10.1128/JVI.80.7.3445-3458.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Verschuren EW, Jones N, Evan GI. The cell cycle and how it is steered by Kaposi’s sarcoma-associated herpesvirus cyclin. J Gen Virol. 2004;85:1347–61. doi: 10.1099/vir.0.79812-0. [DOI] [PubMed] [Google Scholar]
- 450.Verschuren EW, Klefstrom J, Evan GI, Jones N. The oncogenic potential of Kaposi’s sarcoma-associated herpesvirus cyclin is exposed by p53 loss in vitro and in vivo. Cancer Cell. 2002;2:229–41. doi: 10.1016/s1535-6108(02)00123-x. [DOI] [PubMed] [Google Scholar]
- 451.Vieira J, O’Hearn P, Kimball L, Chandran B, Corey L. Activation of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) lytic replication by human cytomegalovirus. J Virol. 2001;75:1378–86. doi: 10.1128/JVI.75.3.1378-1386.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Viejo-Borbolla A, Ottinger M, Bruning E, Burger A, Konig R, Kati E, Sheldon JA, Schulz TF. Brd2/RING3 interacts with a chromatin-binding domain in the Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen 1 (LANA-1) that is required for multiple functions of LANA-1. J Virol. 2005;79:13618–29. doi: 10.1128/JVI.79.21.13618-13629.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Wan X, Wang H, Nicholas J. Human herpesvirus 8 interleukin-6 (vIL-6) signals through gp130 but has structural and receptor-binding properties distinct from those of human IL-6. J Virol. 1999;73:8268–78. doi: 10.1128/jvi.73.10.8268-8278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Wang FZ, Akula SM, Pramod NP, Zeng L, Chandran B. Human herpesvirus 8 envelope glycoprotein K8.1A interaction with the target cells involves heparan sulfate. J Virol. 75:7517–27. doi: 10.1128/JVI.75.16.7517-7527.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Wang FZ, Akula SM, Sharma-Walia N, Zeng L, Chandran B. Human herpesvirus 8 envelope glycoprotein B mediates cell adhesion via its RGD sequence. J Virol. 2003;77:3131–47. doi: 10.1128/JVI.77.5.3131-3147.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Wang HW, Sharp TV, Koumi A, Koentges G, Boshoff C. Characterization of an anti-apoptotic glycoprotein encoded by Kaposi’s sarcoma-associated herpesvirus which resembles a spliced variant of human survivin. EMBO J. 2002;21:2602–15. doi: 10.1093/emboj/21.11.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Wang HW, Trotter MW, Lagos D, Bourboulia D, Henderson S, Makinen T, Elliman S, Flanagan AM, Alitalo K, Boshoff C. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat Genet. 2004;36:687–93. doi: 10.1038/ng1384. [DOI] [PubMed] [Google Scholar]
- 458.Wang J, Zhang J, Zhang L, Harrington W, Jr., West JT, Wood C. Modulation of human herpesvirus 8/Kaposi’s sarcoma-associated herpesvirus replication and transcription activator transactivation by interferon regulatory factor 7. J Virol. 2005;79:2420–31. doi: 10.1128/JVI.79.4.2420-2431.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Wang JF, Zhang X, Groopman JE. Activation of vascular endothelial growth factor receptor-3 and its downstream signaling promote cell survival under oxidative stress. J Biol Chem. 2004;279:27088–97. doi: 10.1074/jbc.M314015200. [DOI] [PubMed] [Google Scholar]
- 460.Wang L, Dittmer DP, Tomlinson CC, Fakhari FD, Damania B. Immortalization of primary endothelial cells by the K1 protein of Kaposi’s sarcoma-associated herpesvirus. Cancer Res. 2006;66:3658–66. doi: 10.1158/0008-5472.CAN-05-3680. [DOI] [PubMed] [Google Scholar]
- 461.Wang L, Wakisaka N, Tomlinson CC, DeWire SM, Krall S, Pagano JS, Damania B. The Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) K1 protein induces expression of angiogenic and invasion factors. Cancer Res. 64:2774–81. doi: 10.1158/0008-5472.can-03-3653. [DOI] [PubMed] [Google Scholar]
- 462.Wang S, Liu S, Wu MH, Geng Y, Wood C. Identification of a cellular protein that interacts and synergizes with the RTA (ORF50) protein of Kaposi’s sarcoma-associated herpesvirus in transcriptional activation. J Virol. 2001;75:11961–73. doi: 10.1128/JVI.75.24.11961-11973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Wang SE, Wu FY, Chen H, Shamay M, Zheng Q, Hayward GS. Early activation of the Kaposi’s sarcoma-associated herpesvirus RTA, RAP, and MTA promoters by the tetradecanoyl phorbol acetate-induced AP1 pathway. J Virol. 2004;78:4248–67. doi: 10.1128/JVI.78.8.4248-4267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Wang SE, Wu FY, Fujimuro M, Zong J, Hayward SD, Hayward GS. Role of CCAAT/enhancer-binding protein alpha (C/EBPalpha) in activation of the Kaposi’s sarcoma-associated herpesvirus (KSHV) lytic-cycle replication-associated protein (RAP) promoter in cooperation with the KSHV replication and transcription activator (RTA) and RAP. J Virol. 2003;77:600–23. doi: 10.1128/JVI.77.1.600-623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Wang XP, Gao SJ. Auto-activation of the transforming viral interferon regulatory factor encoded by Kaposi’s sarcoma-associated herpesvirus (human herpesvirus-8) J Gen Virol. 2003;84:329–36. doi: 10.1099/vir.0.18653-0. [DOI] [PubMed] [Google Scholar]
- 466.Wang XP, Zhang YJ, Deng JH, Pan HY, Zhou FC, Gao SJ. Transcriptional regulation of Kaposi’s sarcoma-associated herpesvirus-encoded oncogene viral interferon regulatory factor by a novel transcriptional silencer, Tis. J Biol Chem. 2002;277:12023–31. doi: 10.1074/jbc.M108026200. [DOI] [PubMed] [Google Scholar]
- 467.Wang XP, Zhang YJ, Deng JH, Pan HY, Zhou FC, Montalvo EA, Gao SJ. Characterization of the promoter region of the viral interferon regulatory factor encoded by Kaposi’s sarcoma-associated herpesvirus. Oncogene. 2001;20:523–30. doi: 10.1038/sj.onc.1204115. [DOI] [PubMed] [Google Scholar]
- 468.Watanabe T, Sugaya M, Atkins AM, Aquilino EA, Yang A, Borris DL, Brady J, Blauvelt A. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen prolongs the life span of primary human umbilical vein endothelial cells. J Virol. 2003;77:6188–96. doi: 10.1128/JVI.77.11.6188-6196.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Weindel K, Marme D, Weich HA. AIDS-associated Kaposi’s sarcoma cells in culture express vascular endothelial growth factor. Biochem Biophys Res Commun. 1992;183:1167–74. doi: 10.1016/s0006-291x(05)80313-4. [DOI] [PubMed] [Google Scholar]
- 470.Weisz A, Kirchhoff S, Levi BZ. IFN consensus sequence binding protein (ICSBP) is a conditional repressor of IFN inducible promoters. Int Immunol. 1994;6:1125–31. doi: 10.1093/intimm/6.8.1125. [DOI] [PubMed] [Google Scholar]
- 471.Weisz A, Marx P, Sharf R, Appella E, Driggers PH, Ozato K, Levi BZ. Human interferon consensus sequence binding protein is a negative regulator of enhancer elements common to interferon-inducible genes. J Biol Chem. 1992;267:25589–96. [PubMed] [Google Scholar]
- 472.Weninger W, Partanen TA, Breiteneder-Geleff S, Mayer C, Kowalski H, Mildner M, Pammer J, Sturzl M, Kerjaschki D, Alitalo K, Tschachler E. Expression of vascular endothelial growth factor receptor-3 and podoplanin suggests a lymphatic endothelial cell origin of Kaposi’s sarcoma tumor cells. Lab Invest. 1999;79:243–51. [PubMed] [Google Scholar]
- 473.Weninger W, Rendl M, Pammer J, Mildner M, Tschugguel W, Schneeberger C, Sturzl M, Tschachler E. Nitric oxide synthases in Kaposi’s sarcoma are expressed predominantly by vessels and tissue macrophages. Lab Invest. 1998;78:949–55. [PubMed] [Google Scholar]
- 474.West JT, Wood C. The role of Kaposi’s sarcoma-associated herpesvirus/human herpesvirus-8 regulator of transcription activation (RTA) in control of gene expression. Oncogene. 2003;22:5150–63. doi: 10.1038/sj.onc.1206555. [DOI] [PubMed] [Google Scholar]
- 475.Widmer I, Wernli M, Bachmann F, Gudat F, Cathomas G, Erb P. Differential expression of viral Bcl-2 encoded by Kaposi’s sarcoma-associated herpesvirus and human Bcl-2 in primary effusion lymphoma cells and Kaposi’s sarcoma lesions. J Virol. 2002;76:2551–6. doi: 10.1128/jvi.76.5.2551-2556.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Wong EL, Damania B. Transcriptional regulation of the Kaposi’s sarcoma-associated herpesvirus K15 gene. J Virol. 2006;80:1385–92. doi: 10.1128/JVI.80.3.1385-1392.2006. B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Wong SW, Bergquam EP, Swanson RM, Lee FW, Shiigi SM, Avery NA, Fanton JW, Axthelm MK. Induction of B cell hyperplasia in simian immunodeficiency virus-infected rhesus macaques with the simian homologue of Kaposi’s sarcoma-associated herpesvirus. J Exp Med. 1999;190:827–40. doi: 10.1084/jem.190.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Wood C, Harrington W., Jr. AIDS and associated malignancies. Cell Res. 2005;15:947–52. doi: 10.1038/sj.cr.7290372. [DOI] [PubMed] [Google Scholar]
- 479.Wu C, Chung AE, McDonald JA. A novel role for alpha 3 beta 1 integrins in extracellular matrix assembly. J Cell Sci. 1995;108(Pt 6):2511–23. doi: 10.1242/jcs.108.6.2511. [DOI] [PubMed] [Google Scholar]
- 480.Wu FY, Tang QQ, Chen H, ApRhys C, Farrell C, Chen J, Fujimuro M, Lane MD, Hayward GS. Lytic replication-associated protein (RAP) encoded by Kaposi sarcoma-associated herpesvirus causes p21CIP-1-mediated G1 cell cycle arrest through CCAAT/enhancer-binding protein-alpha. Proc Natl Acad Sci USA. 2002;99:10683–8. doi: 10.1073/pnas.162352299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Wu L, Lo P, Yu X, Stoops JK, Forghani B, Zhou ZH. Three-dimensional structure of the human herpesvirus 8 capsid. J Virol. 2000;74:9646–54. doi: 10.1128/jvi.74.20.9646-9654.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Wu TT, Usherwood EJ, Stewart JP, Nash AA, Sun R. Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency. J Virol. 2000;74:3659–67. doi: 10.1128/jvi.74.8.3659-3667.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Wu W, Vieira J, Fiore N, Banerjee P, Sieburg M, Rochford R, Harrington W, Jr., Feuer G. KSHV/HHV-8 infection of human hematopoietic progenitor (CD34+) cells: persistence of infection during hematopoiesis in vitro and in vivo. Blood. 2006 doi: 10.1182/blood-2005-04-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Xie J, Pan H, Yoo S, Gao SJ. Kaposi’s sarcoma-associated herpesvirus induction of AP-1 and interleukin 6 during primary infection mediated by multiple mitogen-activated protein kinase pathways. J Virol. 2005;79:15027–37. doi: 10.1128/JVI.79.24.15027-15037.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Xu Y, AuCoin DP, Huete AR, Cei SA, Hanson LJ, Pari GS. A Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 ORF50 deletion mutant is defective for reactivation of latent virus and DNA replication. J Virol. 2005;79:3479–87. doi: 10.1128/JVI.79.6.3479-3487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Yang TY, Chen SC, Leach MW, Manfra D, Homey B, Wiekowski M, Sullivan L, Jenh CH, Narula SK, Chensue SW, Lira SA. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi’s sarcoma. J Exp Med. 2000;191:445–54. doi: 10.1084/jem.191.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Ye FC, Zhou FC, Yoo SM, Xie JP, Browning PJ, Gao SJ. Disruption of Kaposi’s sarcoma-associated herpesvirus latent nuclear antigen leads to abortive episome persistence. J Virol. 78:11121–9. doi: 10.1128/JVI.78.20.11121-11129.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Ye J, Shedd D, Miller G. An Sp1 response element in the Kaposi’s sarcoma-associated herpesvirus open reading frame 50 promoter mediates lytic cycle induction by butyrate. J Virol. 2005;79:1397–408. doi: 10.1128/JVI.79.3.1397-1408.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Yoo SM, Zhou FC, Ye FC, Pan HY, Gao SJ. Early and sustained expression of latent and host modulating genes in coordinated transcriptional program of KSHV productive primary infection of human primary endothelial cells. Virology. 2005;343:47–64. doi: 10.1016/j.virol.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.You RI, Chen MC, Wang HW, Chou YC, Lin CH, Hsieh SL. Inhibition of lymphotoxin-beta receptor-mediated cell death by survivin-DeltaEx3. Cancer Res. 2006;66:3051–61. doi: 10.1158/0008-5472.CAN-05-2479. [DOI] [PubMed] [Google Scholar]
- 491.Yu Y, Wang SE, Hayward GS. The KSHV immediate-early transcription factor RTA encodes ubiquitin E3 ligase activity that targets IRF7 for proteosome-mediated degradation. Immunity. 2005;22:59–70. doi: 10.1016/j.immuni.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 492.Zala C, Ochoa C, Krolewiecki A, Patterson P, Cahn P, Crawford RI, Montaner JS. Highly active antiretroviral therapy does not protect against Kaposi’s sarcoma in HIV-infected individuals. AIDS. 2000;14:2217–8. doi: 10.1097/00002030-200009290-00027. [DOI] [PubMed] [Google Scholar]
- 493.Zhang J, Wang J, Wood C, Xu D, Zhang L. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 replication and transcription activator regulates viral and cellular genes via interferon-stimulated response elements. J Virol. 2005;79:5640–52. doi: 10.1128/JVI.79.9.5640-5652.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Zhang X, Wang JF, Chandran B, Persaud K, Pytowski B, Fingeroth J, Groopman JE. Kaposi’s sarcoma-associated herpesvirus activation of vascular endothelial growth factor receptor 3 alters endothelial function and enhances infection. J Biol Chem. 2005;280:26216–24. doi: 10.1074/jbc.M411392200. [DOI] [PubMed] [Google Scholar]
- 495.Zhang YJ, Deng JH, Rabkin C, Gao SJ. Hot-spot variations of Kaposi’s sarcoma-associated herpesvirus latent nuclear antigen and application in genotyping by PCR-RFLP. J Gen Virol. 2000;81:2049–58. doi: 10.1099/0022-1317-81-8-2049. [DOI] [PubMed] [Google Scholar]
- 496.Zhang YJ, Pan HY, Gao SJ. Reverse transcription slippage over the mRNA secondary structure of the LIP1 gene. Biotechniques. 2001;31:1286, 1288, 1290. doi: 10.2144/01316st02. [DOI] [PubMed] [Google Scholar]
- 497.Zhong W, Wang H, Herndier B, Ganem D. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc Natl Acad Sci USA. 1996;93:6641–6. doi: 10.1073/pnas.93.13.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Zhou FC, Zhang YJ, Deng JH, Wang XP, Pan HY, Hettler E, Gao SJ. Efficient infection by a recombinant Kaposi’s sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis. J Virol. 2002;76:6185–96. doi: 10.1128/JVI.76.12.6185-6196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Zhu FX, Chong JM, Wu L, Yuan Y. Virion proteins of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2005;79:800–11. doi: 10.1128/JVI.79.2.800-811.2005. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Zhu FX, Cusano T, Yuan Y. Identification of the immediate-early transcripts of Kaposi’s sarcoma-associated herpesvirus. J Virol. 1999;73:5556–67. doi: 10.1128/jvi.73.7.5556-5567.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Zhu FX, King SM, Smith EJ, Levy DE, Yuan Y. A Kaposi’s sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proc Natl Acad Sci USA. 2002;99:5573–8. doi: 10.1073/pnas.082420599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Zhu FX, Yuan Y. The ORF45 protein of Kaposi’s sarcoma-associated herpesvirus is associated with purified virions. J Virol. 2003;77:4221–30. doi: 10.1128/JVI.77.7.4221-4230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Zimring JC, Goodbourn S, Offermann MK. Human herpesvirus 8 encodes an interferon regulatory factor (IRF) homolog that represses IRF-1-mediated transcription. J Virol. 1998;72:701–7. doi: 10.1128/jvi.72.1.701-707.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Zoeteweij JP, Moses AV, Rinderknecht AS, Davis DA, Overwijk WW, Yarchoan R, Orenstein JM, Blauvelt A. Targeted inhibition of calcineurin signaling blocks calcium-dependent reactivation of Kaposi sarcoma-associated herpesvirus. Blood. 2001;97:2374–80. doi: 10.1182/blood.v97.8.2374. [DOI] [PubMed] [Google Scholar]