Abstract
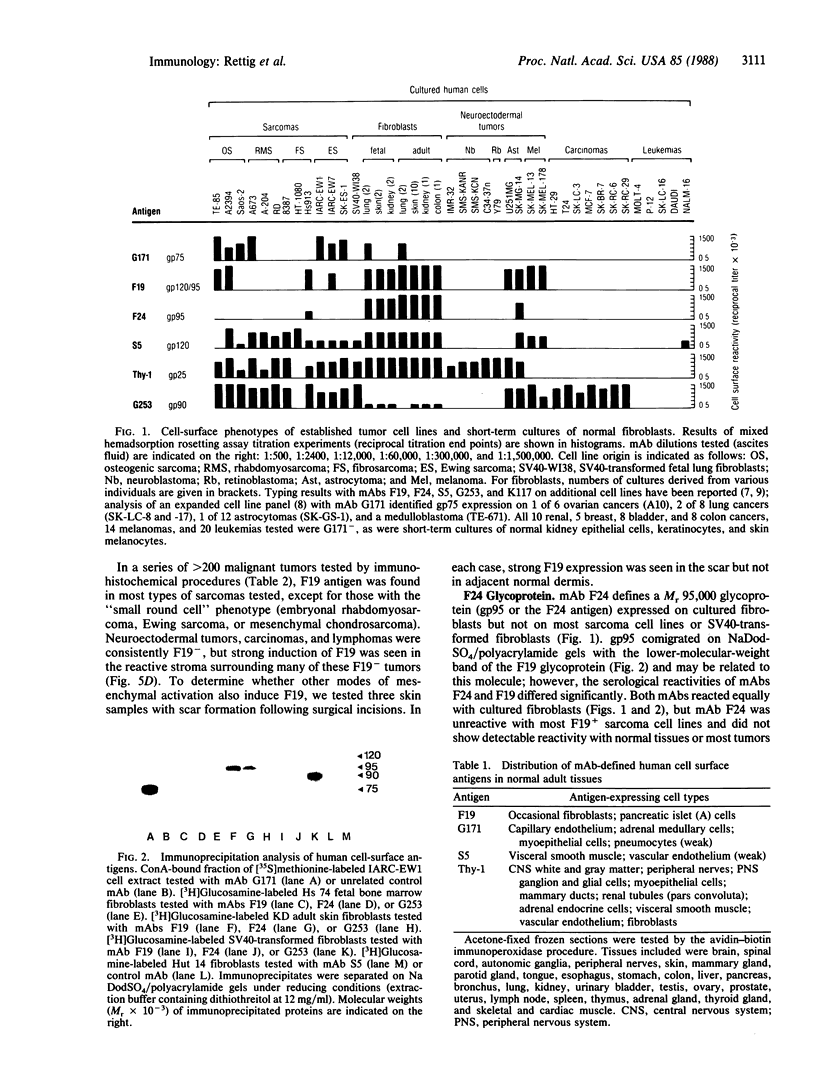
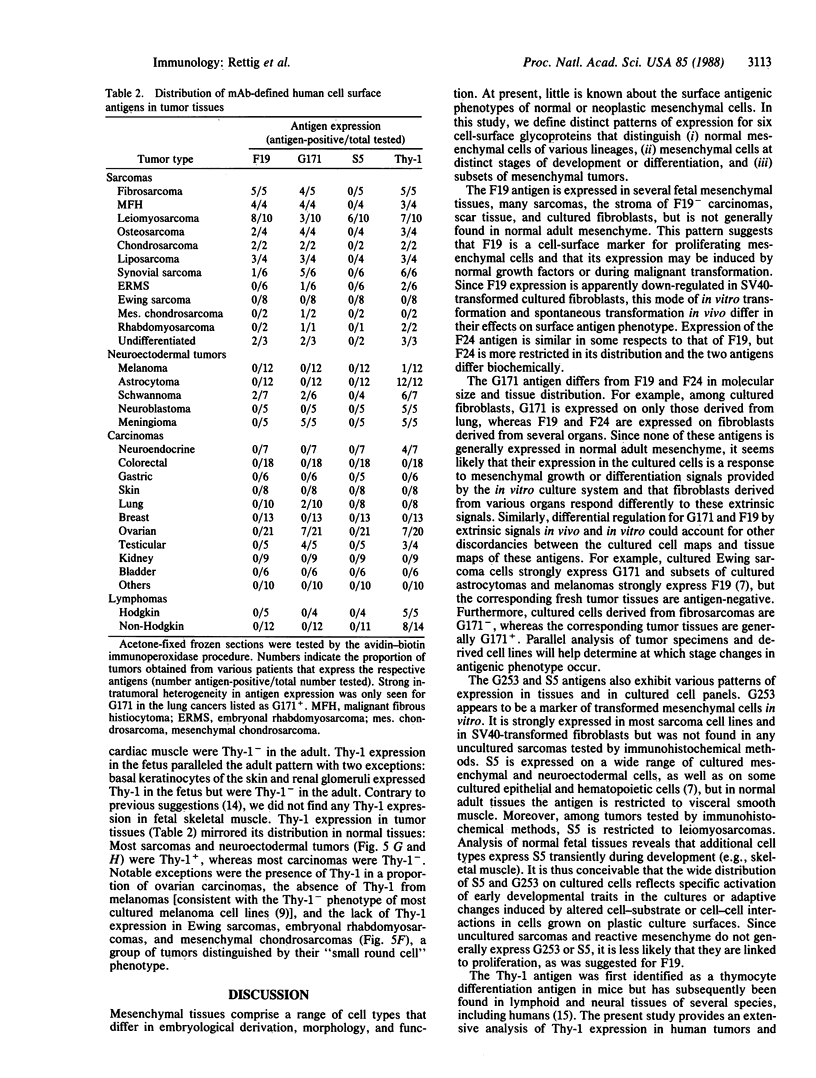
Normal differentiation and malignant transformation of human cells are characterized by specific changes in surface antigen phenotype. In the present study, we have defined six cell-surface antigens of human sarcomas and normal mesenchymal cells, by using mixed hemadsorption assays and immunochemical methods for the analysis of cultured cells and immunohistochemical staining for the analysis of normal tissues and greater than 200 tumor specimens. Differential patterns of F19 (Mr, 120,000/95,000 glycoprotein), F24 (Mr, 95,000 glycoprotein), G171 (Mr, 75,000 glycoprotein), G253 (Mr, 90,000 glycoprotein), S5 (Mr, 120,000 glycoprotein), and Thy-1 (Mr, 25,000 glycoprotein) antigen expression were found to characterize (i) subsets of cultured sarcoma cell lines, (ii) cultured fibroblasts derived from various organs, (iii) normal resting and activated mesenchymal tissues, and (iv) sarcoma and nonmesenchymal tumor tissues. These results provide a basic surface antigenic map for cultured mesenchymal cells and mesenchymal tissues and permit the classification of human sarcomas according to their antigenic phenotypes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chesa P. G., Rettig W. J., Melamed M. R. Expression of cytokeratins in normal and neoplastic colonic epithelial cells. Implications for cellular differentiation and carcinogenesis. Am J Surg Pathol. 1986 Dec;10(12):829–835. doi: 10.1097/00000478-198612000-00001. [DOI] [PubMed] [Google Scholar]
- Foon K. A., Todd R. F., 3rd Immunologic classification of leukemia and lymphoma. Blood. 1986 Jul;68(1):1–31. [PubMed] [Google Scholar]
- Gordon J. W., Chesa P. G., Nishimura H., Rettig W. J., Maccari J. E., Endo T., Seravalli E., Seki T., Silver J. Regulation of Thy-1 gene expression in transgenic mice. Cell. 1987 Jul 31;50(3):445–452. doi: 10.1016/0092-8674(87)90498-3. [DOI] [PubMed] [Google Scholar]
- Houghton A. N., Eisinger M., Albino A. P., Cairncross J. G., Old L. J. Surface antigens of melanocytes and melanomas. Markers of melanocyte differentiation and melanoma subsets. J Exp Med. 1982 Dec 1;156(6):1755–1766. doi: 10.1084/jem.156.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig W. J., Chesa P. G., Beresford H. R., Feickert H. J., Jennings M. T., Cohen J., Oettgen H. F., Old L. J. Differential expression of cell surface antigens and glial fibrillary acidic protein in human astrocytoma subsets. Cancer Res. 1986 Dec;46(12 Pt 1):6406–6412. [PubMed] [Google Scholar]
- Rettig W. J., Chesa P. G., Jennings M. T., Spengler B. A., Melamed M. R., Oettgen H. F., Biedler J. L., Old L. J. Cell surface antigen of human neuroblastomas is related to nuclear antigen of normal cells. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6894–6898. doi: 10.1073/pnas.82.20.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig W. J., Cordon-Cardo C., Ng J. S., Oettgen H. F., Old L. J., Lloyd K. O. High-molecular-weight glycoproteins of human teratocarcinoma defined by monoclonal antibodies to carbohydrate determinants. Cancer Res. 1985 Feb;45(2):815–821. [PubMed] [Google Scholar]
- Rettig W. J., Dracopoli N. C., Chesa P. G., Spengler B. A., Beresford H. R., Davies P., Biedler J. L., Old L. J. Role of human chromosome 11 in determining surface antigenic phenotype of normal and malignant cells. Somatic cell genetic analysis of eight antigens, including putative human Thy-1. J Exp Med. 1985 Nov 1;162(5):1603–1619. doi: 10.1084/jem.162.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig W. J., Murty V. V., Mattes M. J., Chaganti R. S., Old L. J. Extracellular matrix-modulated expression of human cell surface glycoproteins A42 and J143. Intrinsic and extrinsic signals determine antigenic phenotype. J Exp Med. 1986 Nov 1;164(5):1581–1599. doi: 10.1084/jem.164.5.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig W. J., Nishimura H., Yenamandra A. K., Seki T., Obata F., Beresford H. R., Old L. J., Silver J. Differential expression of the human Thy-1 gene in rodent-human somatic cell hybrids [corrected]. J Immunol. 1987 Jun 15;138(12):4484–4489. [PubMed] [Google Scholar]
- Rettig W. J., Spengler B. A., Chesa P. G., Old L. J., Biedler J. L. Coordinate changes in neuronal phenotype and surface antigen expression in human neuroblastoma cell variants. Cancer Res. 1987 Mar 1;47(5):1383–1389. [PubMed] [Google Scholar]
- Sato Y., Mukai K., Watanabe S., Goto M., Shimosato Y. The AMeX method. A simplified technique of tissue processing and paraffin embedding with improved preservation of antigens for immunostaining. Am J Pathol. 1986 Dec;125(3):431–435. [PMC free article] [PubMed] [Google Scholar]
- Walsh F. S., Ritter M. A. Surface antigen differentiation during human myogenesis in culture. Nature. 1981 Jan 1;289(5793):60–64. doi: 10.1038/289060a0. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Gagnon J. Neuronal cell Thy-1 glycoprotein: homology with immunoglobulin. Science. 1982 May 14;216(4547):696–703. doi: 10.1126/science.6177036. [DOI] [PubMed] [Google Scholar]