Abstract
Recent studies have suggested that Nkx6.2/Gtx and Nkx2.2 homeodomain transcription factors are involved in the regulation of oligodendrocyte maturation and/or myelination which occur predominantly in postnatal stages. However, their cellular specificity in postnatal central nervous system has not been characterized and their dynamic expressional relationship during oligodendrocyte lineage progression has not been determined. Here we report that both Nkx2.2 and Nkx6.2 are selectively expressed in Olig2+ cells of oligodendrocyte lineage in postnatal spinal cords. While Nkx6.2 is specifically expressed in the APC+ mature oligodendrocytes, Nkx2.2 is initially expressed in differentiating oligodendrocyte precursor cells (OPCs) but quickly down-regulated as OPCs undergo terminal differentiation. Intriguingly, Nkx2.2 expression is up-regulated in mature myelinating oligodendrocytes at later stages. The co-expression of Nkx2.2 and Nkx6.2 transcription factors in myelinating oligodendrocytes suggests their functional interactions in the regulation of myelin sheath formation and/or maintenance.
Keywords: gene expression, homeobox gene, transcription factor, spinal cord, Oligodendrocyte
INTRODUCTION
Recent studies have revealed that transcription factors play important roles in the fate specification and differentiation of oligodendrocyte lineage in the developing central nervous system (CNS). Oligodendrocyte progenitor cells (OPCs or OLPs), regardless of their developmental origins, initially express the bHLH transcription factor Olig2 (Lu et al., 2000; Takeyabashi et al., 2000; Zhou et al., 2000). Genetic and molecular studies have implicated Olig2 as the key determinant of oligodendrocyte lineage specification (Lu et al., 2002; Takeyabashi et al., 2002; Zhou and Anderson, 2002). Soon after OPCs are generated from the ventricular zone, they are rapidly dispersed into the surrounding regions where they undergo further proliferation and differentiation. Along with OPC migration and differentiation, they gradually acquire the expression of other oligodendrocyte lineage-specific transcription factors, notably Sox10 and Nkx2.2 (Stolt et al., 2002; Xu et al., 2000; Soula et al., 2001). In rodents, Sox10 expression can be detected in early OPCs, whereas Nkx2.2 expression is acquired later by OPCs after they migrate into the white matter (Fu et al., 2002; Liu et al., 2003). Sox10 and Nkx2.2 appear to have similar functions in the control of oligodendrocyte differentiation, and targeted mutations of these two genes caused similar phenotypes with retarded oligodendrocyte maturation and myelin gene expression (Qi et al., 2001; Stolt et al., 2002).
Despite the significant progress in our understanding of the transcriptional control of oligodendrocyte specification and differentiation, transcription factors that regulate the myelination process of oligodendrocytes remain to be defined. There is emerging evidence that Nkx6.2/Gtx homeodomain transcription factor is involved in this developmental process. It was previously reported that Nkx6.2 was expressed in differentiating oligodendrocytes (Awatramani et al., 1997), and possibly in astrocytes as well (Komuro et al., 1993). The multiple Nkx6.2 binding sites in the MBP and PLP promoters suggested that it may be involved in the regulation of myelin-specific gene expression (Awatramani et al., 1997). Contrary to this suggestion, oligodendrocyte differentiation and myelin gene expression are normal in Nkx6.2 null mutants (Cai et al., 2001). Subsequent studies demonstrated that Nkx6.2 mutation leads to small myelination defects in association with mild neurological and behavioral disorders (Southwood et al., 2004).
However, it has not been determined whether Nkx6.2 expression is restricted to cells of oligodendrocyte lineage and it is not clear whether Nkx6.2 collaborates with other related transcription factors such as Nkx2.2 to regulate the complicated process of myelin sheath formation. In this study, we performed detailed studies on the dynamic expression of Nkx6.2 in postnatal CNS, and demonstrated that Nkx6.2 is specifically expressed in cells of oligodendrocyte lineage, specifically in APC+/cytoplasmic Olig1+ mature oligodendrocytes. Interestingly, after a transient and rapid down-regulation in differentiated oligodendrocytes in early postnatal spinal cord, Nkx2.2 expression is restored in the Nkx6.2+ mature oligodendrocytes at later stages. While the expression of Nkx2.2 in early differentiating OPCs controls oligodendrocyte differentiation, the late phase of Nkx2.2 expression in mature oligodendrocytes suggests its possible role in the transcriptional regulation of myelin sheath formation and/or maintenance of myelin structures.
MATERIALS AND METHODS
Genotyping of Nkx2.2 and Nkx6.2 mutant mice
The Nkx2.2 or Nkx6.2 homozygous null mice and their wild-type littermates were obtained by the interbreeding of heterozygous animals. Genomic DNA extracted from mouse tails was used for genotyping by Southern analysis or by PCR. Genotyping of Nkx2.2 and Nkx6.2 loci was described in Sussel et al., (1998) and Cai et al., (2001), respectively.
Immunofluorescent staining
Postnatal mice from day 0 (P0) to the adult were fixed by cardiac perfusion with 4% paraformaldehyde (PFA). Brain and spinal cord tissues were dissected out and submerged in 4% PFA at 4°C overnight. Following fixation, tissues were transferred to 20% sucrose in PBS overnight, embedded in OCT media and then sectioned (16 µm thickness) on a cryostat. Double and triple immunofluorescent procedures were modified from the previous description in Xu et al., (2000). The dilution ratio of antibodies is as follows: anti-mouse A2B5 (1:50), anti- mouse PDGFRα (1:300, BD), anti-NG2 (1:1500, Chemicon), anti- mouse O4 (1:1000, Chemicon), anti-mouse Nkx2.2 (1:50, DSHB; 1:5000, gift from Dr. Ericson), anti- mouse Nkx6.2 (1:6000, gift from Dr. Ericson), anti- mouse Olig2 (1:3000, gift from Drs. Stiles and Alberta), anti- mouse Olig1 (1:100, gift from Drs. Stiles and Alberta), anti- mouse Sox10 (1:3000, gift from Dr. Wegner), anti-mouse APC/CC1 (1:50, Oncogene), anti-mouse MAG (1:300, Chemicon), anti-mouse MBP (1:5000, Chemicon), anti-mouse NeuN (1:1000, Chemicon), anti-rat GFAP (1:300, Chemicon), anti-mouse Glast (1:500, Abcam) and anti-mouse S100B (1:1000, BD).
OPC culture and differentiation assays
OPCs were obtained from newborn rat brain tissues by the simple shaking method as described previously (McCarthy and de Vellis, 1980). Briefly, the dissected forebrain tissues were minced into 1-mm3 pieces and incubated in Hanks’ balanced salt solution containing 0.1% papain, 0.1% neutral protease, and 0.01% DNase for 30 minutes at 37°C. The digestion was stopped by the addition of an equal volume of Dulbecco’s modified Eagle’s medium (DMEM) containing 20% fetal bovine serum (FBS). The medium was changed after 24 hr of plating until the cells reached confluence after 8–12 days. At this stage, a loosely adherent superficial layer of cells that represents OPCs and microglia lying on a confluent astrocyte layer was removed by vigorous shaking of the cultures at 200rpm for 60min, followed by a change to fresh medium. Top-dwelling OPCs were dislodged by overnight (18–22 h) shaking of the cultures at 200 rpm and placed into uncoated tissue culture dishes for 60 min to allow adherence of any residual microglia. The suspension was filtered through 70-um nylon mesh and pelleted at 800 rpm for 5 min. The pellets were re-suspended in BDM/CYS+ medium (DMEM, 5 µg/ml insulin, 50 µg/ml apo-transferrin, 1mg/ml BSA, 30 ng/ml sodium selenite, 200 µM L-cysteine, 10 ng/ml D-biotin, and 100 µg/ml penicillin-streptomycin-glutamine, 10 nM hydrocortisone). In these cell preparations, about 90% of the bound cells expressed A2B5 and were therefore suitable the subsequent differentiation experiments. OPC cells were subsequently cultured in fresh growth medium containing PDGF-AA (10 ng/ml) and FGF-2 (10 ng/ml) every other day. For differentiation assays, cells were switched to MBP differentiation medium containing T3 (15 ng/ml) and CNTF (100 ng/ml or 4 ng/ml) for another week.
Myelination assays in OPC-DRG COCULTURE
For co-culture studies, O4+ oligodendrocytes were immunopanned from adult spinal cord tissues using O4 antibody as described in the previous study (Cheng et al., 2007). DRG neurons were isolated from E15 Sprague-Dawley rat embryos (Charles River Laboratories). Briefly, the DRGs were treated with 0.25% trypsin (Invitrogen) for 8 mins followed by neutralization with 40% FBS. After washing twice with 10% FBS in L-15, DRG explants were resuspended in DRG-growth medium.
In myelination assays, O4+ OPCs were added to DRG neuron cultures in OPC-growth medium (DMEM/F12, 1×N2, 1×B27, 1×P/S, 20 ng/ml FGF-2, 10 ng/ml PDGF-AA, and 30 ng/ml Insulin). On the following day, the cultures were changed to the differentiation medium (DMEM/F12, 1×N2, 1×B27, 1×P/S, 30 ng/ml insulin) for one week to induce adult OPC differentiation. Immunostaining was performed in 2-week-old cultures with antibodies against MBP and Nkx2.2.
RESULTS
Selective expression of Nkx2.2 and Nkx6.2 in the postnatal spinal cord
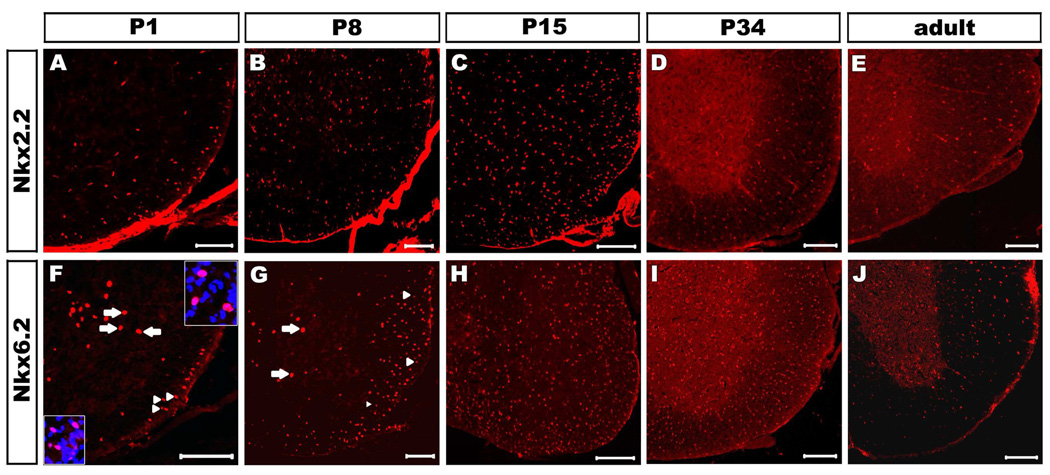
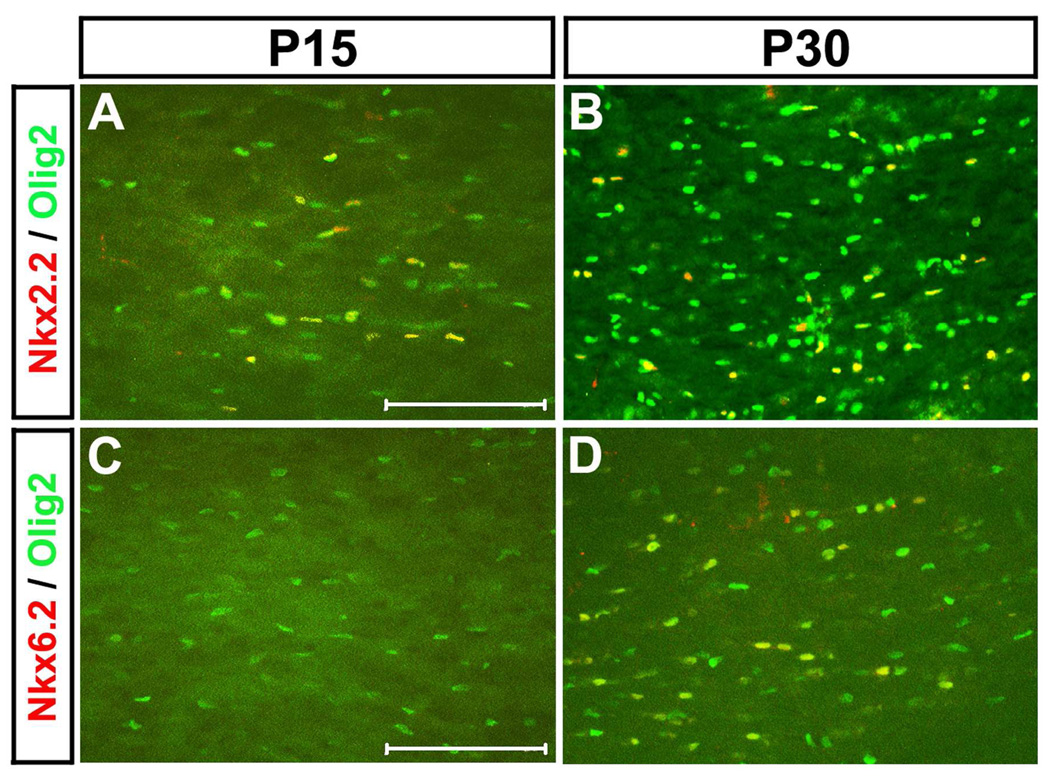
To investigate the possible functional collaboration between Nkx2.2 and Nkx6.2 during oligodendrocyte myelination stages, we compared the Nkx2.2 and Nkx6.2 immunostaining in the mouse spinal cord at various developmental stages. Nkx2.2 expression in the ventral white matter was first detected at E17.5 with a rapid increase afterwards (Fig. 1 A–E). At postnatal day 8 (P8), Nkx2.2-expressing cells were found throughout the entire spinal cord, including both grey and white matter (Fig. 1 B). The robust expression was continuous to P30 before it started to be down-regulated to a lower level thereafter (Fig. 1 C–E, data not shown). In contrast, Nkx6.2 expression was not observed in the white matter before birth (P0) (Fig S1). However, a small number of Nkx6.2+ small nuclei started to show up in the ventral white matter from P1 to P4 (arrowheads in Fig 1F, 1G; Fig S1). At these stages, a few Nkx6.2+ large nuclei were also observed in the ventral gray matter (Fig. 1 F and G, arrows; Fig S2), and these cells are likely to represent the V1 ventral interneurons (Vallstedt et al., 2001) because they were co-labeled with pan-neuronal marker NeuN (Fig S2). Interestingly, the number of Nkx6.2+ cells in the white matter were rapidly up-regulated from P4 to P15 in a ventral-to-dorsal gradient (Fig 1 F–H, arrowheads; Fig S1), peaked at around P34 and then gradually declined in P68 adult spinal cord (Fig 1 I, J, Fig S1). At the same time, the number of Nkx6.2+ small nuclei in the gray matter was also increased with time after P8 (Fig.1 G, arrowheads). Similarly, up-regulation of Nkx6.2 after Nkx2.2 was also observed in the white matter of the cerebral cortex at postnatal stages. While Nkx2.2 expression could be detected Olig2+ oligodendrocytes in P15 corpus callosum, Nkx6.2 expression in Olig2+ cells was not seen until P30 (Fig 2).
Figure 1.
Nkx2.2 and Nkx6.2 expression in postnatal mouse spinal cords. Transverse sections from P1 (A, F), P8 (B, G), P15 (C, H), P34 (D, I) and P68 (E, J) wild-type spinal cords were subject to immunofluorescent staining with anti-Nkx2.2 (A-E) or anti-Nkx6.2 (F-J). Only the ventrolateral spinal cord positions were shown. The Nkx6.2+ cells with large nuclei in the grey matter (top-right inset in F) and those with small nuclei in the white matter (bottom-left inset in F) are represented by arrows and arrowheads, respectively. Scale bar: 100µm.
Figure 2.
Nkx2.2 and Nkx6.2 expression in postnatal mouse brains. Transverse sections from P15 (A, C) and P30 (B, D) wild-type corpus callosum regions were subject to double immunofluorescent staining with anti-Nkx2.2 and anti-Olig2 (A, B) or anti-Nkx6.2 and anti-Olig2 (C, D). Scale bar: 100 µm.
Nkx6.2 is selectively expressed in mature differentiated oligodendrocytes
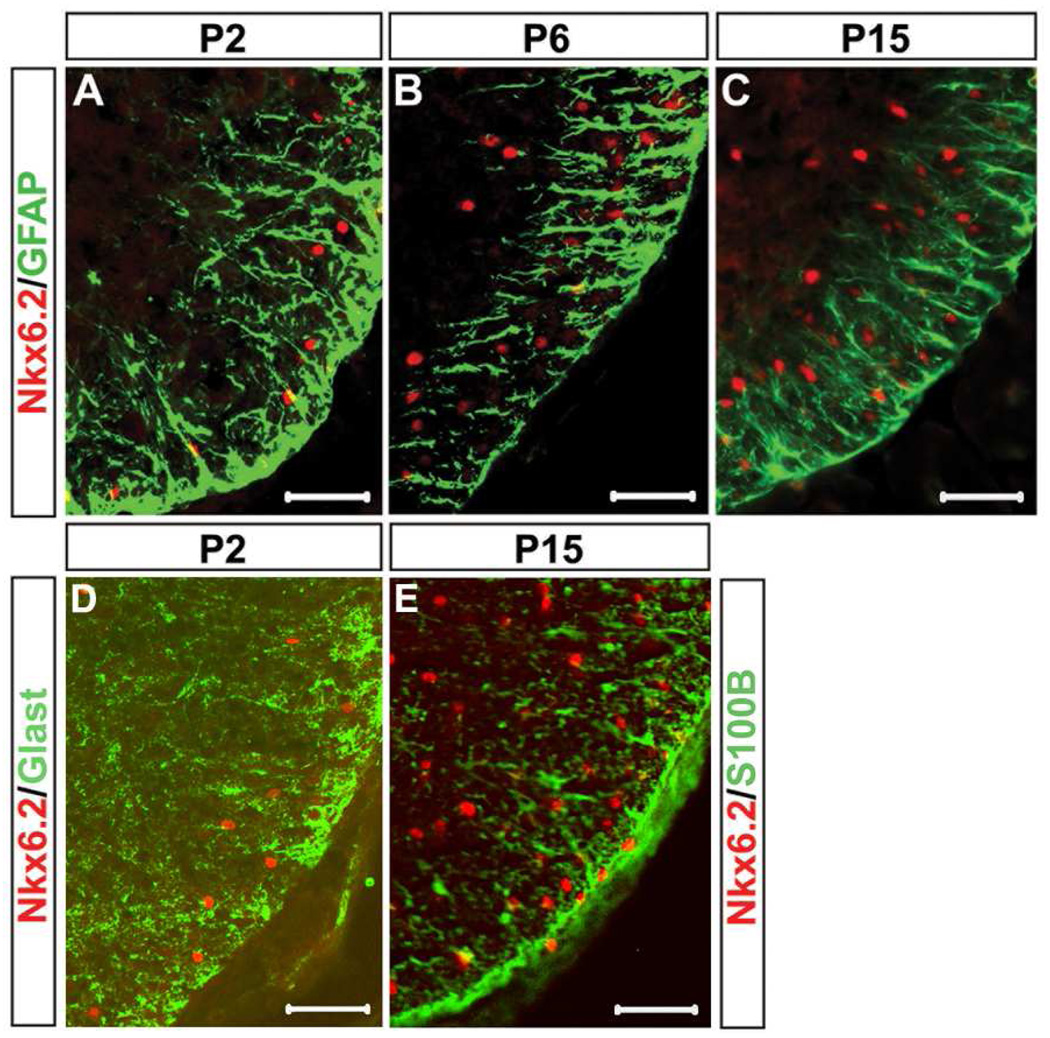
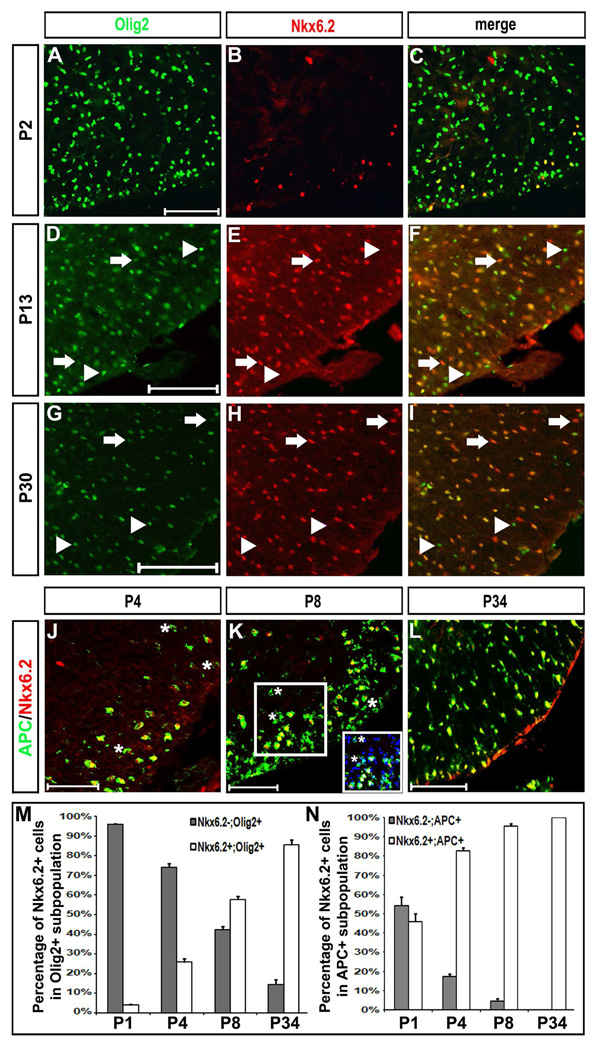
Previous in vitro studies suggested that Nkx6.2 homeodomain factor was expressed in both astrocytes and oligodendrocytes (Komuro et al., 1993). To examine whether Nkx6.2 is selectively expressed in oligodendrocyte lineage in vivo, we performed anti-Nkx6.2 double immunofluorescent staining in conjunction with the anti-GFAP and anti-Olig2 antibodies in postnatal mouse spinal cords. Nkx6.2 expression in the white matter was never observed in GFAP+, Glast+ or S100β+ astroglial cells after birth (Fig 3,). Olig2 is expressed in both OPC cells and mature oligodendrocytes, and can be used as a specific and reliable marker for oligodendrocyte lineage identification in the spinal cord (Lu et al., 2000; Takeyabashi et al., 2000; Zhou et al., 2000, Arnett et al., 2004). At early postnatal stages (P2), an increasing percentage of Olig2+ cells expressed Nkx6.2 (Fig 4 A–C, 4M); and all Nkx6.2+ nuclei co-expressed Olig2 except for a few large Nkx6.2+ interneurons in the ventral gray matter (Fig 4 A–C; Fig S2). However, at later stages, an increasing percentage of Nkx6.2+ cells exhibited weak expression of Olig2 (Fig. 4 D–I, arrows in F and I), suggesting that Olig2 expression is down-regulated in myelinating oligodendrocytes. Meanwhile, many Olig2+ cells, especially those in the gray matter region, did not co-express Nkx6.2 (Fig. 4 F and I, arrowheads) at all postnatal stages examined. These Olig2+/Nkx6.2- cells are likely to represent undifferentiated OPCs.
Figure 3.
Non-overlapping of Nkx6.2 and astrocytic markers in postnatal mouse spinal cords. Cross sections from P2 (A and D), P6 (B) and P15 (C and E) wild-type pups were subject to double immunofluorescent staining with anti-Nkx6.2 and anti-GFAP (A-C), anti-Glast (D), or anti-S100B (E). Only the ventrolateral spinal cord positions were shown. Scale bar: 50 µm.
Figure 4.
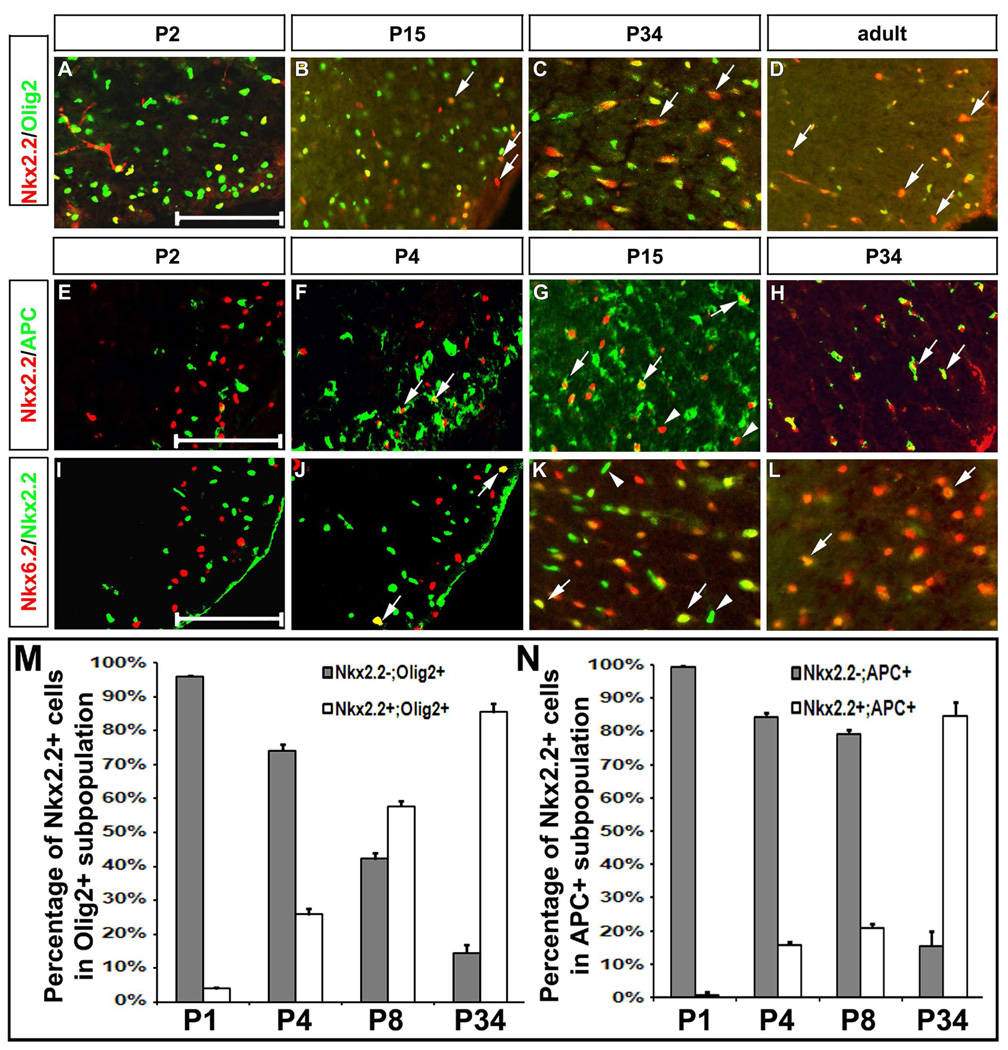
Co-expression of Nkx6.2 with Olig2 and APC in spinal cord oligodendrocytes. Spinal cord sections from P2 (A–C), P4 (J), P8 (K), P13 (D–F), P30 (G–I) and P34 (L) wild-type pups were subject to double immunofluorescent staining with anti-Nkx6.2 and anti-Olig2 (A–I), or anti-Nkx6.2 and anti-APC (J–L). Only the ventrolateral spinal cord positions were shown. The Nkx6.2+++/Olig2+ and Olig2+++/Nkx6.2- single positive cells were represented by arrows and arrowheads, respectively. The percentage of Nkx6.2+ and Nkx6.2- cells in APC+ or Olig2+ population from P1 to P34 were shown in M and N. The Nkx6.2+ cells in C and J and represent the ventral interneurons. The APC+/Nkx6.2- cells were indicated by asterisks. Inset in K showed the APC/Nkx6.2/Dapi triple labeling from the outlined region. Scale bar: 100 µm.
The observations that Nkx6.2+ cells emerge in the white matter region later than Nkx2.2 have raised the possibility that Nkx6.2 is selectively expressed in myelinating mature oligodendrocytes after birth. To examine this possibility, we performed the anti-Nkx6.2 and anti-APC double immunostaining in postnatal spinal cord tissues. The antibody to APC protein (CC-1) specifically labels differentiated oligodendrocytes (Bhat et al., 1996; Lu et al., 2000). At all postnatal stages examined, nearly all Nkx6.2+ cells were immunoreactive to APC in the cytoplasm in both the gray and white matter regions (Fig. 4 J–L, data not shown). Interestingly, between P4 and P8, some APC+ cells in the white matter were not co-labeled with anti-Nkx6.2 antibody (Fig. 4 J, K, N; represented by asterisks in the insets), suggesting that APC expression in differentiated oligodendrocytes occurs slightly earlier than Nkx6.2. Based on these observations, we concluded that Nkx6.2 is selectively expressed in mature differentiated oligodendrocytes. In support of this notion, Nkx6.2+ oligodendrocytes co-expressed other mature markers MBP and MAG (Fig S3 C and D, arrows), but not OPC markers PDGFRα and NG2 (Fig S3 A and B, arrowheads).
Nkx2.2 is transiently down-regulated in the differentiating oligodendrocytes but restored in Nkx6.2+ differentiated oligodendrocytes
In the developing mouse spinal cord, Nkx2.2 was previously shown to be up-regulated in Olig2+ differentiating immature oligodendrocytes at neonatal stages (Qi et al., 2001; Fu et al., 2002). To determine whether Nkx2.2 expression persisted in myelinating mature oligodendrocytes, we carried out Nkx2.2 double immunostaining with other oligodendrocyte lineage-specific markers in the spinal cord at various postnatal stages. At P2, all Nkx2.2+ oligodendrocytes co-expressed Olig2 transcription factor (Fig. 5 A). Surprisingly, at later stages, an increasing number of Nkx2.2+ cells started to lose Olig2 expression (Fig. 5 B–D, arrows; 5M). This result suggested the gradual down-regulation of Olig2 expression in mature oligodendrocytes at late postnatal stages.
Figure 5.
Expression of Nkx2.2 in the oligodendroglial cells of postnatal spinal cords. Spinal cord sections at the thoracic level were prepared from P2 (A, E, I), P4 (F, J), P15 (B, G, K), P34 (C, H, L), and adult (D) wild-type mice. A-D. Immunofluorescence with anti-Nkx2.2 (red) and anti-Olig2 (green). Arrows indicated the Nkx2.2+/Olig2- or weak Olig2+ cells. E-H. Double immunostaining with anti-Nkx2.2 (red) and anti-APC (green). I-L. Double immunolabeling with anti-Nkx6.2 (red) and anti-Nkx2.2 (Green). Arrows in F–H and J–L represented the Nkx2.2+/APC+ and Nkx6.2+/Nkx2.2+ (either strong or weak) cells, respectively. Arrowheads in G and K indicated the Nkx2.2+/APC- or Nkx6.2- cells. The percentage of Nkx2.2+ and Nkx2.2- cells in APC+ or Olig2+ population from P1 to P34 were shown in M and N. Scale bar: 100 µm.
Double labeling experiments also revealed that there was no overlapping expression of Nkx2.2 with APC or Nkx6.2 at P2. At this stage, Nkx2.2 and Nkx6.2/APC were expressed in distinct populations of cells in the ventral white matter (Fig. 5 E, I). Two days later, Nkx2.2 expression started to be detected in a few APC+ and Nkx6.2+ oligodendrocytes (Fig. 5 F and J, arrows; Fig 6. D–E, arrowheads). At P15, a larger number of APC+ and Nkx6.2+ oligodendrocytes co-expressed Nkx2.2 (Fig. 5 G and K, arrows), despite the presence of many Nkx2.2+/APC- and Nkx2.2+/Nkx6.2- cells (Fig. 5 G and K, arrowheads). By P30, Nkx2.2 expression was observed in the majority of APC+/Nkx6.2+ cells (Fig. 5 H, L, N). In adult spinal cord (P68), virtually all Nkx6.2+ oligodendrocytes co-expressed the Nkx2.2 transcription factor (data not shown).
Figure 6.
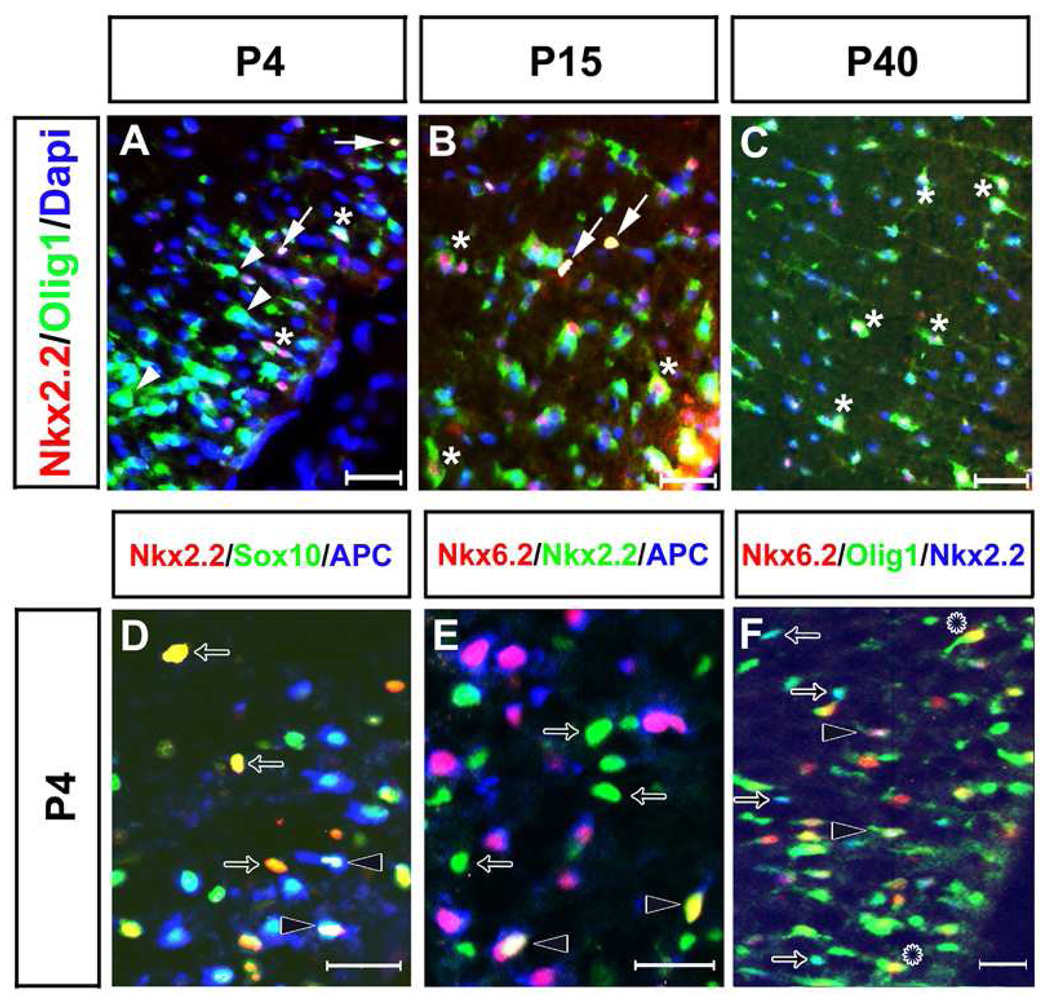
Definition of Nkx2.2 and Nkx6.2 expression by triple immunostaining with stage-specific markers in oligodendrocytes. Spinal cord sections were collected from P4 (A, D-F), P15 (B), and P40 (C) wild-type pups. Only the ventrolateral spinal cord positions were shown. A-C. Immunofluorescent staining with anti-Nkx2.2 (red), anti-Olig1 (green) and Dapi (blue). Arrows delineated the Nkx2.2+/nuclear Olig1+. The Nkx2.2-/cytoplasmic Olig1+ and Nkx2.2+/cytoplasmic Olig1+ cells were represented by arrowheads and asterisks, respectively. D. Triple labeling with anti-Nkx2.2 (red), anti-Sox10 (green), and anti-APC (blue). E. Immunofluorescence with anti-Nkx6.2 (red), anti-Nkx2.2 (green), and anti-APC (blue). F. Triple staining with anti-Nkx6.2 (red), anti-Olig1 (green) and anti-Nkx2.2 (blue). Arrows represented either Nkx2.2+/Sox10+ cells in D or Nkx2.2+/nuclear Olig1+ in F. Open arrows represented Nkx2.2+/Sox10+/APC- cells in D, Nkx6.2-/Nkx2.2+/APC- in E, or Nkx6.2-/Nkx2.2+/nuclear Olig1+ in F. Leis in F delineated the Nkx6.2+/cytoplasmic Olig1+ cells. Scale bar: 50 µm.
Recent studies also showed that Olig1 protein is localized in the nucleus in immature oligodendrocytes but translocated to the cytoplasm in mature oligodendrocytes (Arnett et al., 2004; Kitada and Rowitch, 2006). Consistently, at P4, most Nkx2.2+ cells had nuclear Olig1 staining and a few had weak cytoplasmic Olig1 staining (Fig. 6 A, F, arrows). In mature oligodendrocytes with strong cytoplasmic Olig1 staining, Nkx2.2 expression was not detected, suggesting that Nkx2.2 is rapidly down-regulated in differentiating oligodendrocytes when Olig1 underwent cytoplasmic translocation (Fig. 6 A, F, arrowheads). In support, Nkx2.2 expression was only detected in newly differentiated oligodendrocytes with weak MAG expression, but not in mature oligodendrocytes with strong MAG expression in both mouse and chicken spinal cord (Fig S4). At later stages (P15 and P40), while some Nkx2.2+ cells with nuclear or weak cytoplasmic Olig1 staining remained to be observed, weak Nkx2.2 expression could be detected in oligodendrocytes with strong cytoplasmic Olig1 expression (Fig. 6 B, C), suggesting that mature oligodendrocytes regained Nkx2.2 expression at later postnatal stages.
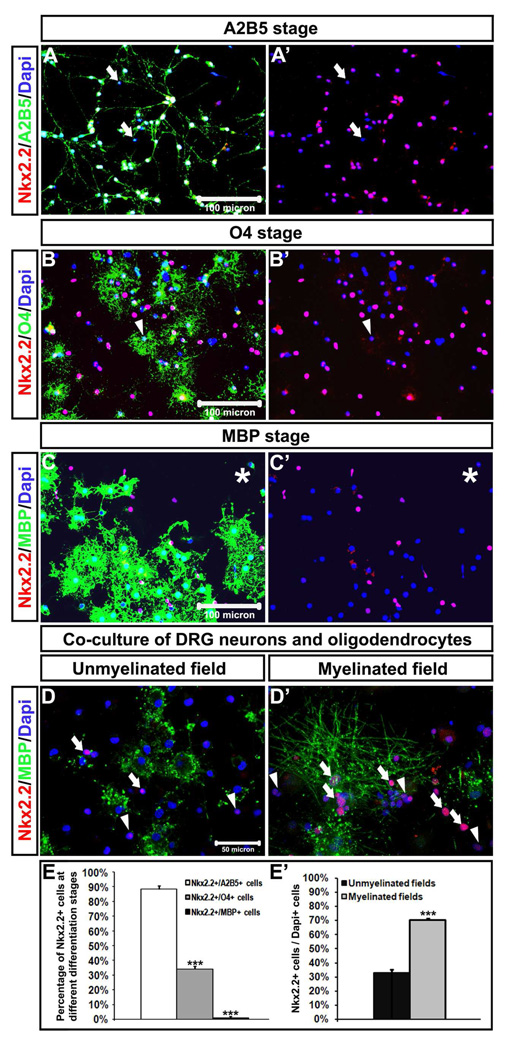
To further verify the transient down-regulation of Nkx2.2 expression in newly differentiated oligodendrocytes, we first purified OPC cells from rat brain or spinal cord tissues and cultured them in differentiation medium for various time lengths prior to gene expression assays. Double immunostaining revealed that about 90% A2B5+ OPC cells expressed Nkx2.2 (Fig 7A, A’, E), about 35% O4+ pro-oligodendrocytes expressed Nkx2.2 (Fig 7 B, B’, E), whereas fewer than 2% MBP+ oligodendrocytes co-expressed Nkx2.2 (Fig 7 C, C’, E). However, when oligodendrocytes formed myelin sheaths around DRG axons, a higher percentage of oligodendrocytes in DRG-OPC co-culture co-expressed Nkx2.2 protein (Fig 7D, D’, E’). Together, our in vitro studies demonstrated that Nkx2.2 expression was progressively down-regulated as OPCs underwent terminal differentiation, but up-regulated again in oligodendrocytes during myelination stage. These findings are consistent with the notion of biphasic Nkx2.2 expression during oligodendrocyte differentiation and myelination stages.
Figure 7.
Up-regulation of Nkx2.2 in OPCs and pro-oligodendrocytes but down-regulation in mature oligodendrocytes in vitro. Immunofluorescence with anti-Nkx2.2 (red) and A2B5 (green), O4 (green) or anti-MBP (green) were performed in disassociated oligodendrocytes at A2B5 (A, A’), O4 (B, B’), and MBP (C,C’) stages, or co-culture of DRG neurons and oligodendrocytes (D, D’). The percentage of Nkx2.2+ cells oligodendrocyte population at different differentiation stages was summarized in E and E’. ***: p<0.001. Arrows represented either Nkx2.2-/Dapi+ nuclei in A and A’ or Nkx2.2+ /Dapi+ nuclei in D and D’. Arrowheads in B, B’, D, and D’ or stars in C and C’ indicated Nkx2.2+ (weak)/Dapi+ cells.
Nkx6.2 expression in the white matter is reduced in Nkx2.2 mutant spinal cords
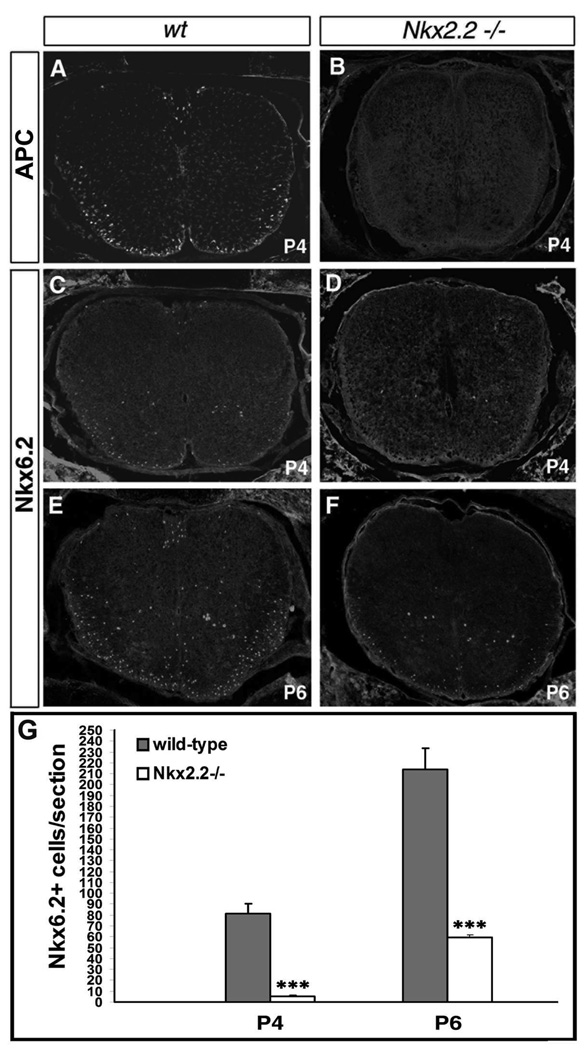
The non-overlapping expression of Nkx2.2 and Nkx6.2 in Olig2+ cells in P2 spinal cord could be explained by the transient down-regulation of Nkx2.2 in differentiated Nkx6.2+/APC+ oligodendrocytes. However, it remains possible that they are initially expressed in two distinct oligodendrocyte subpopulations (Kitada and Rowitch 2006) which differentiate independently at early postnatal stages, but gain the expression of each other at later stages. To distinguish these two possibilities, we examined Nkx6.2 and APC expression in the early postnatal spinal cords of Nkx2.2 null mutants, in which oligodendrocyte differentiation is severely inhibited (Qi et al., 2001). At P4, APC expression was detected in wild-type but not mutant tissues (Fig. 8 A–B). Likewise, Nkx6.2 expression was also absent in the white matter region of the mutants (Fig. 8 C, D and G), although a small number of Nkx6.2+ oligodendrocytes started to emerge in P6 mutant spinal cord (Fig. 8 E–G). Contrarily, Nkx2.2 and APC expression are normal in Nkx6.2 mutant spinal cord (data not shown). The absence of Nkx6.2 and APC may be the consequence of inhibited oligodendrocyte differentiation due to Nkx2.2 disruption, providing evidence that Nkx2.2+ and Nkx6.2+ oligodendrocyte cells are from the same lineage and that Nkx6.2 expression is dependent on Nkx2.2. This observation is consistent with the concept that Nkx2.2 expression is transiently down-regulated in newly differentiated oligodendrocytes before they progress into Nkx6.2+/APC+ myelinating oligodendrocytes.
Figure 8.
Delayed expression of Nkx6.2 and APC in Nkx2.2 mutant spinal cords. Cross sections of cervical spinal cord from wild-type (A, C, E) and Nkx2.2 mutant (B, D, F) pups at P4 (A-D) and P6 (E, F) were labeled with anti-APC (A, B) and anti-Nkx6.2 (C-F) antibodies. G. Nkx6.2+ cells per section in P4 and P6 wild-type and Nkx2.2 null mutant spinal cords (n=3). ***: p<0.001.
DISCUSSION
In this study, we characterized the spatiotemporal pattern of Nkx6.2 and Nkx2.2 expression in postnatal spinal cords in relation to other stage-specific oligodendrocyte lineage markers. Detailed analyses revealed that both Nkx genes are selectively expressed in oligodendrocyte lineage. Specifically, Nkx6.2 is expressed in mature oligodendrocytes, whereas Nkx2.2 is initially expressed in differentiating OPCs and then transiently down-regulated in newly differentiated oligodendrocytes before it is re-expressed in myelinating oligodendrocytes.
Specific expression of Nkx6.2 in mature oligodendrocytes downstream of Nkx2.2 in postnatal spinal cord
During the early neural development, Nkx6.2 is transiently transcribed in p1 progenitor domain of the ventral spinal cord and later in the V1 interneurons as well (Cai et al., 1999; Vallstedt et al., 2001; Fig 1). At later stages, Nkx6.2 expression is down-regulated in V1 neurons and neural progenitor cells but up-regulated in glial cells; however, its expression specificity in glial lineage has not been fully characterized. It was reported that Nkx6.2/Gtx was expressed in astrocytes (Kumoro et al., 1993), differentiating or differentiated oligodendrocytes (Awatramani et al., 1997; Southwood et al., 2004). In this study, we provided evidence that Nkx6.2 is exclusively expressed in mature oligodendrocytes in postnatal spinal cord (Fig 1 and 4) and brain (Fig 2), as all Nkx6.2+ cells co-expressed the mature marker APC (Fig 4) and had strong cytoplasmic Olig1 staining (Fig 6). Nkx6.2 starts to be expressed in the white matter region of spinal cord at postnatal day 0 (Fig. S1), about two days later than Nkx2.2 whose expression can be detected in the white matter as early as E17.5 (Qi et al., 2001; Fu et al., 2002). Previous studies showed that Nkx2.2 is selectively expressed in differentiating OPC cells, and its activity is required for the timely differentiation of oligodendrocytes (Qi et al., 2001). Surprisingly, there was no overlapping expression of Nkx2.2 and Nkx6.2 during the early stages of oligodendrocyte differentiation, and Nkx2.2 expression was not detected in Nkx6.2+ oligodendrocytes prior to P4 when oligodendrocytes undergo rapid differentiation (Fig 5). One possibility for the complete lack of overlapping expression of these two genes is that they are initially expressed by two distinct oligodendrocyte populations or lineages which develop independently at early postnatal stages, as suggested by Kitada et al., (2006). However, the dramatic inhibition of Nkx6.2 expression in Nkx2.2 mutant spinal cord (Fig 8) strongly argues against this possibility and supports the alternative idea that Nkx2.2 is quickly down-regulated once OPCs become fully differentiated into Nkx6.2+/APC+ mature oligodendrocytes. Consistent with this notion, there was no co-expression of Nkx2.2 with APC in newly differentiated oligodendrocytes at P4 (Fig 6), although expression of these two mature markers is also dependent on Nkx2.2 activity. Additional evidence for the down-regulation of Nkx2.2 in mature oligodendrocytes came from the observation that at P4, Nkx2.2 expression is co-localized with nuclear Olig1 staining, but not with strong cytoplasmic Olig1 staining (Fig 6A–C). Furthermore, Nkx2.2 expression was detected in newly differentiated oligodendrocytes with a weak MAG expression, but not in mature oligodendrocytes with strong MAG expression in both chicken and mouse spinal cord tissues (Fig S4).
Re-expression of Nkx2.2 in myelinating oligodendrocytes
Starting at P4, Nkx2.2 expression can be detected in an increasing number of differentiated APC+/Nkx6.2+ oligodendrocytes with cytoplasmic Olig1 localization (Fig 5, 6). The expression of Nkx2.2 in mature oligodendrocytes is relatively weak compared to its initial expression in immature oligodendrocytes, and persists in adult spinal cord (Fig 1, 5, 6; Kitada et al., 2006). Since Nkx6.2+ mature oligodendrocytes do not co-express Nkx2.2 prior to P4, it is most likely that the Nkx6.2-expressing mature oligodendrocytes regain Nkx2.2 expression.
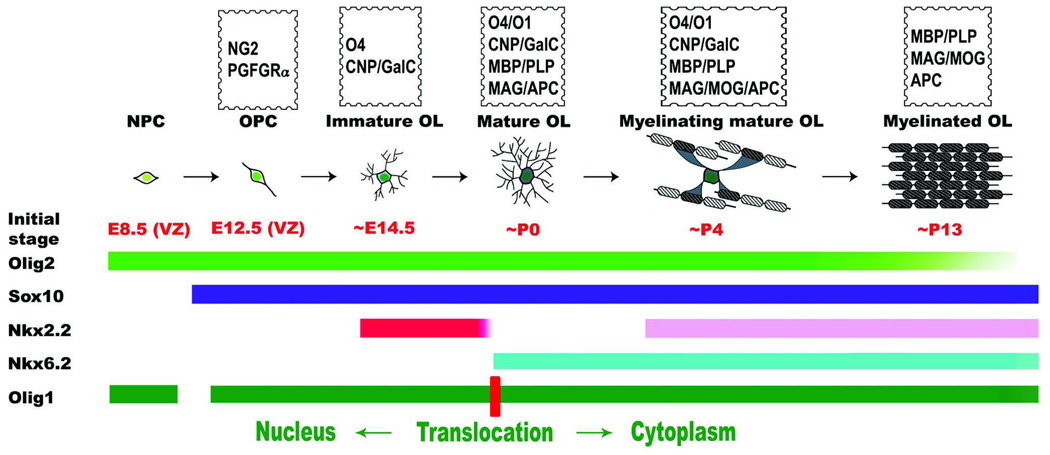
The up-regulation of Nkx2.2 in differentiated oligodendrocytes suggests that there is a biphasic Nkx2.2 expression in oligodendrocytes lineage in the postnatal spinal cord. At perinatal stages (prior to P4), Nkx2.2 expression is restricted to differentiating OPCs. Between P4 and P30, Nkx2.2 is expressed by both differentiating OPCs and mature oligodendrocytes. In adult spinal cord, Nkx2.2 is co-expressed with Nkx6.2 and APC in mature myelinating oligodendrocytes. The temporal sequence of Nkx2.2 and Nkx6.2 expression in relation to other oligodendrocyte stage-specific markers is summarized in Figure 9. In the postnatal spinal cord, the Nkx2.2+/Nkx6.2- cells represent the differentiating immature population, the Nkx2.2-/Nkx6.2+ cells represent the newly differentiated but non-myelinating oligodendrocytes, whereas the Nkx2.2+/Nkx6.2+ cells represent mature myelinating oligodendrocytes. It should be noted that there is heterogeneity among OPC cells in the timing of their differentiation and thus the expression of Nkx2.2, Nkx6.2 and other maturation-related genes. Once OPC cells enter the maturation and myelination program, they undergo biphasic pattern of Nkx2.2 expression. The heterogeneity of oligodendrocyte differentiation may offer an alternative explanation for the existence of different oligodendrocyte populations that express distinct combinations of transcription factors at postnatal stages (Kitada et al., 2006).
Figure 9.
Schematic illustration of oligodendrocyte development and stage-specific expression of transcription factors during oligodendrogial lineage progression. NPC, neural progenitor cells; OPC, oligodendrocyte progenitor cell; OL, oligodendrocyte; VZ, ventricular zone.
The biphasic Nkx2.2 expression during oligodendrocyte lineage progression appears to be associated with its distinct functions in oligodendrocyte development. The early Nkx2.2 expression in OPC cells is required for initiating oligodendrocyte differentiation program (Qi et al., 2001; Sun et al., 2001; Zhou et al., 2001; Sugimori et al., 2008), possibly by suppressing the expression of some unknown inhibitors (e.g. Id or Hes genes) in OPCs. As oligodendrocytes further differentiate along and produce myelin proteins, Nkx2.2 expression is rapidly down-regulated. The functional significance for the rapid down-regulation of Nkx2.2 in differentiated oligodendrocytes is not clear. Given that Nkx2.2 functions as a transcription repressor (Muhr et al., 2001) and has an inhibitory effect on the expression of MBP gene in vitro (Wei et al., 2005), Nkx2.2 down-regulation may be necessary to allow for the rapid transcription of myelin genes during active myelination stage.
The late phase of Nkx2.2 expression in mature oligodendrocytes may be involved in regulation of myelin sheath formation and/or maintain myelin structures in collaboration with Nkx6.2. In support of this hypothesis, Nkx2.2 heterozygosity in Nkx6.2 mutant background (Nkx2.2+/-Nkx6.2-/-) resulted in severe neurological defects and abnormal ultra-structure of myelin sheaths (unpublished observations). Alternatively, it is also possible that Nkx2.2 expression in mature myelinating oligodendrocytes may function as a negative regulator to prevent excessive production of myelin proteins. In other words, Nkx2.2 and Sox10 may act concertly to fine tune the expression of myelin genes in myelinating OLs.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Drs. John Rubenstein and Lori Sussel for Nkx2.2 mutant mice, Drs. Chuck Stiles and John Alberta for anti-Olig1 and Olig2 antibodies, Dr. Johan Ericson for anti-Nkx6.2 and anti-Nkx2.2 antibodies, and Dr. Michael Wegner for anti-Sox10 antibody. We thank Chi Minh Tuong for tissue preparation. This work is supported by NMSS R3247 (M Qiu), NIH R01 NS37717 (M Qiu) and in part by NIH COBRE grant 2P20 RR017702 (RM Greene). Dr. Jun Cai is COBRE supported junior investigator.
REFERENCES
- Arnett HA, Fancy SPJ, Alberta JA, Zhao C, Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJM, Stiles CD. bHLH Transcription Factor Olig1 Is Required to Repair Demyelinated Lesions in the CNS. Science. 2004;306(5704):2111–2115. doi: 10.1126/science.1103709. [DOI] [PubMed] [Google Scholar]
- Awatramani R, Scherer S, Grinspan J, Collarini E, Skoff R, O'Hagan D, Garbern J, Kamholz J. Evidence That the Homeodomain Protein Gtx Is Involved in the Regulation of Oligodendrocyte Myelination. J Neurosci. 1997;17(17):6657–6668. doi: 10.1523/JNEUROSCI.17-17-06657.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat RV, Axt KJ, Fosnaugh JS, Smith KJ, Johnson KA, Hill DE, Kinzler KW, Baraban JM. Expression of the APC tumor suppressor protein in oligodendroglia. Glia. 1996;17:169–174. doi: 10.1002/(SICI)1098-1136(199606)17:2<169::AID-GLIA8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Cai J, St Amand T, Yin H, Guo H, Li G, Zhang Y, Chen Y, Qiu M. Expression and regulation of the chicken Nkx-6.2 homeobox gene suggest its possible involvement in the ventral neural patterning and cell fate specification. Developmental Dynamics. 1999;216:459–468. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<459::AID-DVDY14>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Cai J, Qi Y, Wu R, Modderman G, Fu H, Liu R, Qiu M. Mice Lacking the Nkx6.2 (Gtx) Homeodomain Transcription Factor Develop and Reproduce Normally. Mol Cell Biol. 2001;21(13):4399–4403. doi: 10.1128/MCB.21.13.4399-4403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Wang Y, He Q, Qiu M, Whittemore SR, Cao Q. Bone morphogenetic protein signaling and olig1/2 interact to regulate the differentiation and maturation of adult oligodendrocyte precursor cells. Stem Cells. 2007;25(12):3204–3214. doi: 10.1634/stemcells.2007-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Qi Y, Tan M, Cai J, Takebayashi H, Nakafuku M, Richardson W, Qiu M. Dual origin of spinal oligodendrocyte progenitors and evidence for the cooperative role of Olig2 and Nkx2.2 in the control of oligodendrocyte differentiation. Development. 2002;129(3):681–693. doi: 10.1242/dev.129.3.681. [DOI] [PubMed] [Google Scholar]
- Kitada M, Rowitch DH. Transcription factor co-expression patterns indicate heterogeneity of oligodendroglial subpopulations in adult spinal cord. Glia. 2006;54(1):35–46. doi: 10.1002/glia.20354. [DOI] [PubMed] [Google Scholar]
- Komuro I, Schalling M, Jahn L, Bodmer R, Jenkins N, Copeland N, Izumo S. Gtx: a novel murine homeobox-containing gene, expressed specifically in glial cells of the brain and germ cells of testis, has a transcriptional repressor activity in vitro for a serum-inducible promoter. EMBO J. 1993;12:1387–1401. doi: 10.1002/j.1460-2075.1993.tb05783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Cai J, Hu X, Tan M, Qi Y, German M, Rubenstein J, Sander M, Qiu M. Region-specific and stage-dependent regulation of Olig gene expression and oligodendrogenesis by Nkx6.1 homeodomain transcription factor. Development. 2003;130(25):6221–6231. doi: 10.1242/dev.00868. [DOI] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. Common Developmental Requirement for Olig Function Indicates a Motor Neuron/Oligodendrocyte Connection. Cell. 2002;109(1):75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Lu QR, Yuk D-i, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH. Sonic Hedgehog-Regulated Oligodendrocyte Lineage Genes Encoding bHLH Proteins in the Mammalian Central Nervous System. Neuron. 2000;25(2):317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhr J, Andersson E, Persson M, Jessell TM, Ericson J. Groucho-Mediated Transcriptional Repression Establishes Progenitor Cell Pattern and Neuronal Fate in the Ventral Neural Tube. Cell. 2001;104(6):861–873. doi: 10.1016/s0092-8674(01)00283-5. [DOI] [PubMed] [Google Scholar]
- Qi Y, Cai J, Wu Y, Wu R, Lee J, Fu H, Rao M, Sussel L, Rubenstein J, Qiu M. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development. 2001;128(14):2723–2733. doi: 10.1242/dev.128.14.2723. [DOI] [PubMed] [Google Scholar]
- Soula C, Danesin C, Kan P, Grob M, Poncet C, Cochard P. Distinct sites of origin of oligodendrocytes and somatic motoneurons in the chick spinal cord: oligodendrocytes arise from Nkx2.2-expressing progenitors by a Shh-dependent mechanism. Development. 2001;128(8):1369–1379. doi: 10.1242/dev.128.8.1369. [DOI] [PubMed] [Google Scholar]
- Southwood C, He C, Garbern J, Kamholz J, Arroyo E, Gow A. CNS Myelin Paranodes Require Nkx6-2 Homeoprotein Transcriptional Activity for Normal Structure. J Neurosci. 2004;24(50):11215–11225. doi: 10.1523/JNEUROSCI.3479-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt CC, Rehberg S, Ader M, Lommes P, Riethmacher D, Schachner M, Bartsch U, Wegner M. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 2002;16(2):165–170. doi: 10.1101/gad.215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimori M, Nagao M, Parras CM, Nakatani H, Lebel M, Guillemot F, Nakafuku M. Ascl1 is required for oligodendrocyte development in the spinal cord. Development. 2008;135(7):1271–1281. doi: 10.1242/dev.015370. [DOI] [PubMed] [Google Scholar]
- Sun T, Echelard Y, Lu R, Yuk D-i, Kaing S, Stiles CD, Rowitch DH. Olig bHLH proteins interact with homeodomain proteins to regulate cell fate acquisition in progenitors of the ventral neural tube. Current Biology. 2001;11(18):1413–1420. doi: 10.1016/s0960-9822(01)00441-9. [DOI] [PubMed] [Google Scholar]
- Sussel L, Kalamaras J, Hartigan-O'Connor DJ, Meneses J, Pedersen RA, Rubenstein JL, German MS. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. 1998;125:2213–2221. doi: 10.1242/dev.125.12.2213. [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Nabeshima Y, Yoshida S, Chisaka O, Ikenaka K, Nabeshima Y-i. The Basic Helix-Loop-Helix Factor Olig2 Is Essential for the Development of Motoneuron and Oligodendrocyte Lineages. Current Biology. 2002;12(13):1157–1163. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Yoshida S, Sugimori M, Kosako H, Kominami R, Nakafuku M, Nabeshima Y-i. Dynamic expression of basic helix-loop-helix Olig family members: implication of Olig2 in neuron and oligodendrocyte differentiation and identification of a new member, Olig3. Mechanisms of Development. 2000;99(1–2):143–148. doi: 10.1016/s0925-4773(00)00466-4. [DOI] [PubMed] [Google Scholar]
- Vallstedt A, Muhr J, Pattyn A, Pierani A, Mendelsohn M, Sander M, Jessell TM, Ericson J. Different Levels of Repressor Activity Assign Redundant and Specific Roles to Nkx6 Genes in Motor Neuron and Interneuron Specification. Neuron. 2001;31(5):743–755. doi: 10.1016/s0896-6273(01)00412-3. [DOI] [PubMed] [Google Scholar]
- Wei Q, Miskimins WK, Miskimins R. Stage-specific Expression of Myelin Basic Protein in Oligodendrocytes Involves Nkx2.2-mediated Repression That Is Relieved by the Sp1 Transcription Factor. J Biol Chem. 2005;280(16):16284–16294. doi: 10.1074/jbc.M500491200. [DOI] [PubMed] [Google Scholar]
- Xu X, Cai J, Fu H, Wu R, Qi Y, Modderman G, Liu R, Qiu M. Selective Expression of Nkx-2.2 Transcription Factor in Chicken Oligodendrocyte Progenitors and Implications for the Embryonic Origin of Oligodendrocytes. Molecular and Cellular Neuroscience. 2000;16(6):740–753. doi: 10.1006/mcne.2000.0916. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Anderson DJ. The bHLH Transcription Factors OLIG2 and OLIG1 Couple Neuronal and Glial Subtype Specification. Cell. 2002;109(1):61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Choi G, Anderson DJ. The bHLH Transcription Factor Olig2 Promotes Oligodendrocyte Differentiation in Collaboration with Nkx2.2. Neuron. 2001;31(5):791–807. doi: 10.1016/s0896-6273(01)00414-7. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wang S, Anderson DJ. Identification of a Novel Family of Oligodendrocyte Lineage-Specific Basic Helix-Loop-Helix Transcription Factors. Neuron. 2000;25(2):331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.