Abstract
Several neurotransmitters act through G-protein coupled receptors (GPCR) to evoke a “slow” excitation of neurons1, 2. These include peptides, such as substance P (SP) and neurotensin (NT), as well as acetylcholine and noradrenaline. Unlike the fast (~ ms) ionotropic actions of small molecule neurotransmitters, the slow excitation is not well understood at the molecular level, but can be mainly attributed to suppressing K+ currents and/or activating a non-selective cation channel3–9. The molecular identity of this cation channel has yet to be determined; similarly how the channel is activated and its relative contribution to neuronal excitability induced by the neuropeptides are unknown. Here, we show that, in the hippocampal and ventral tegmental area neurons, SP and NT activate a channel complex containing NALCN and a large novel protein UNC-80. The activation by SP through NK1R (a GPCR for SP) is via a unique mechanism: it does not require G-protein activation but is dependent on Src family kinases (SFKs). These findings identify NALCN as the cation channel activated by SP receptor, and suggest that UNC-80 and SFKs, rather than a G-protein, are involved in the coupling from receptor to channel.
NALCN is a neuronal cation channel carrying a small background leak Na+ current at the resting membrane potential10. When overexpressed in HEK293T fibroblast cells, it generates a Na+-permeable cation channel that is voltage-independent, non-inactivating, tetrodotoxin (TTX)-resistant and Gd3+-blockable10. It is not known whether, like background K+ channels, NALCN is also regulated by neuromodulators, but the biophysical and pharmacological properties of the NALCN currents (INALCN) closely resemble those of the SP-activated cation channel currents (ISP) studied in several brain regions11–14.
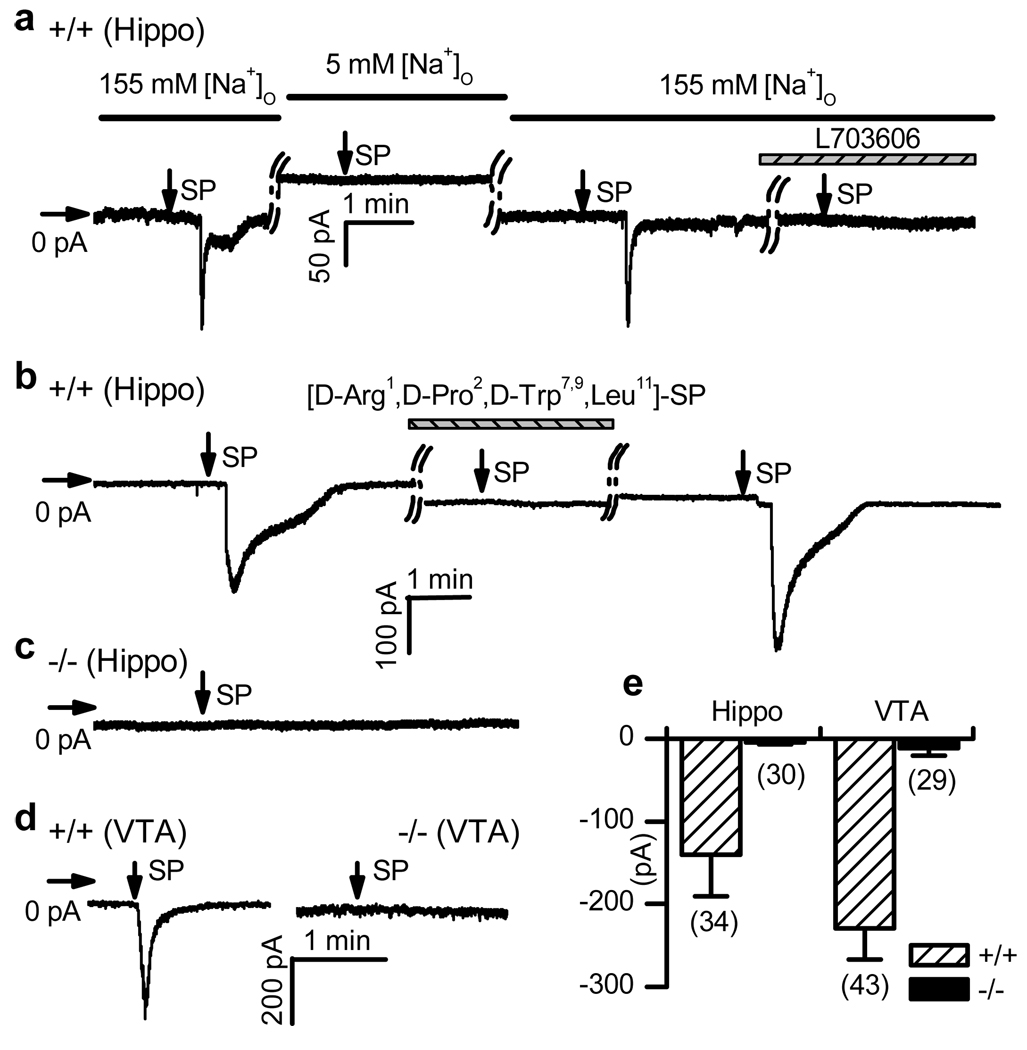
To test the possibility that ISP requires NALCN, we recorded ISP in wild-type and Nalcn knockout10 (Nalcn−/−) neurons via patch clamp with measures taken to minimize K+ channel effects and to block voltage-gated Na+ channel and synaptic currents. In 16 of 34 wild-type hippocampal pyramidal neurons held at −67 mV, an inward current (> 50 pA) developed within 1.1 ± 0.2 min (range: 0.4 – 2.9 min) after a pulse of SP was delivered through a puffer pipette placed ~ 20 µm from the cell body (Fig. 1a, e). The time courses of ISP vary between neurons, but are comparable to previous recordings11, 12, 15 and are in agreement with the “slow” nature of the signal transduction pathway (see below). ISP was blocked by pre-incubating cells with a competitive peptide (n = 3, Fig. 1b) or an NK1R antagonist (L703606; n = 14; Fig. 1a), suggesting involvement of NK1R or another member of the tachykinin receptor family of GPCRs. Reducing bath concentration of Na+ from 155 mM to 5 mM largely abolished the ISP (Fig. 1a), suggesting that Na+ was the major charge carrier of the current at this holding potential. ISP has also been recorded in the rat ventral tegmental area (VTA)9. Of 43 putative dopaminergic (DA) neurons cultured from the VTA of wild-type mice (see Methods), 32 had an ISP > 50 pA (Fig. 1d). In contrast to the wild-type, none of the 30 hippocampal (Fig. 1c, e) and 29 VTA (Fig. 1d) neurons from Nalcn−/− mutant mice showed a significant ISP (Fig. 1e).
Figure 1. NALCN is required for ISP.
Currents (at −67 mV) were recorded from wild-type (+/+) or Nalcn knockout (−/−) hippocampal (Hippo) and VTA neurons. Horizontal and vertical arrows indicate 0 current level and SP application (10 seconds of puffing), respectively. a, ISP developed in bath containing 155 mM Na+, but not when Na+ was lowered to 5 mM (replaced with NMDG). Incubation with L703606 (10 µM, 6 min) blocked ISP. b, Blockade by a peptide NK1R antagonist (10 µM, 6 min incubation). c, Nalcn−/− hippocampal neuron. d, VTA neurons. e, Summary of the ISP sizes. Numbers of cells are in parentheses. Error bars, mean ± s.e.m.
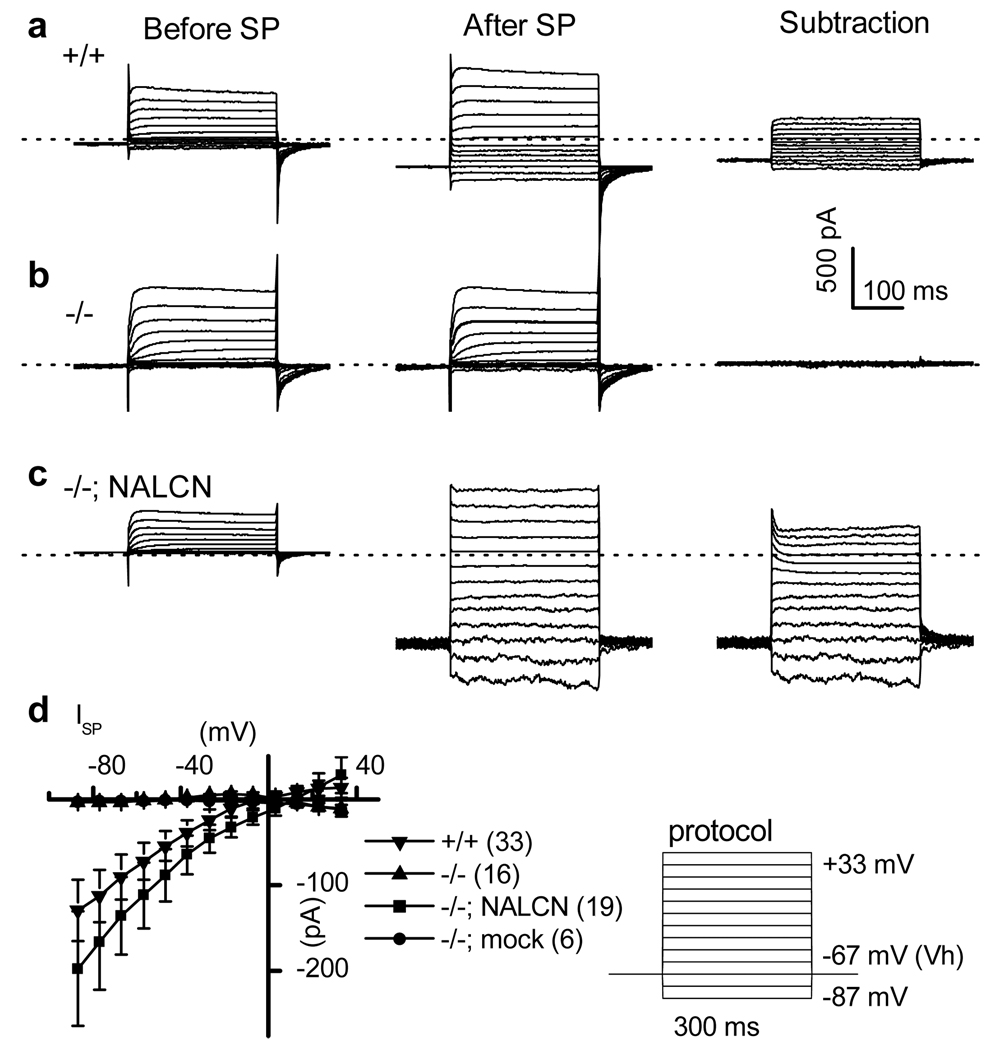
Like INALCN10, the SP-activated currents did not inactivate within 300 ms at any voltage (Fig. 2a). The averaged current (I)-voltage (V) relationship was linear at negative membrane potentials, suggesting little voltage-dependence of conductance in this range (Fig. 2d). The overall properties of the ISP in cultured mouse hippocampal neurons were similar to those of the ISP recorded from numerous other neuronal preparations, primarily from brain slices12, 13, 16. Due to low current amplitudes, potential space clamping problems in neuronal recordings, and possible contamination by voltage-gated currents at positive potentials, a detailed biophysical characterization could not be performed in the neurons.
Figure 2. Characterization of the ISP in hippocampal neurons.
a–c, Net SP-activated currents at various voltages (right) were obtained by subtracting the currents before (left) from after (middle) SP bath application (1 µM) in wild-type (+/+) (a), mutant (−/−) (b), and mutant transfected with NALCN (−/−; NALCN) (c). Dotted lines indicate 0 current level. d, Averaged ISP amplitudes. The lines from mutant (−/−) and mutant transfected with empty vector (−/−; mock) overlap. Right panel shows the voltage step protocols used. Error bars, mean ± s.e.m.
ISP could be restored in the Nalcn−/− neurons by transfecting a NALCN cDNA (Fig. 2c, d). The restored ISP (Supplementary Fig. 2b, d), like that from wild-type neurons (Supplementary Fig. 2a, d), was blocked by a trivalent NALCN blocker Gd3+ (10 µM). The current, however, became resistant to 10 µM Gd3+ (Supplementary Fig. 2c, d) when restored with a Gd3+-resistant NALCN pore mutant (EEKA) that has a single amino acid change (E to A) in the ion filter region (Supplementary Fig. 1). Taken together, the similarity of ISP and INALCN, the absence of ISP in Nalcn−/− neurons and presence with NALCN cDNA transfection, and the alteration of ISP pharmacology by the EEKA pore mutant strongly suggest that NALCN forms the pore conduit carrying the ISP.
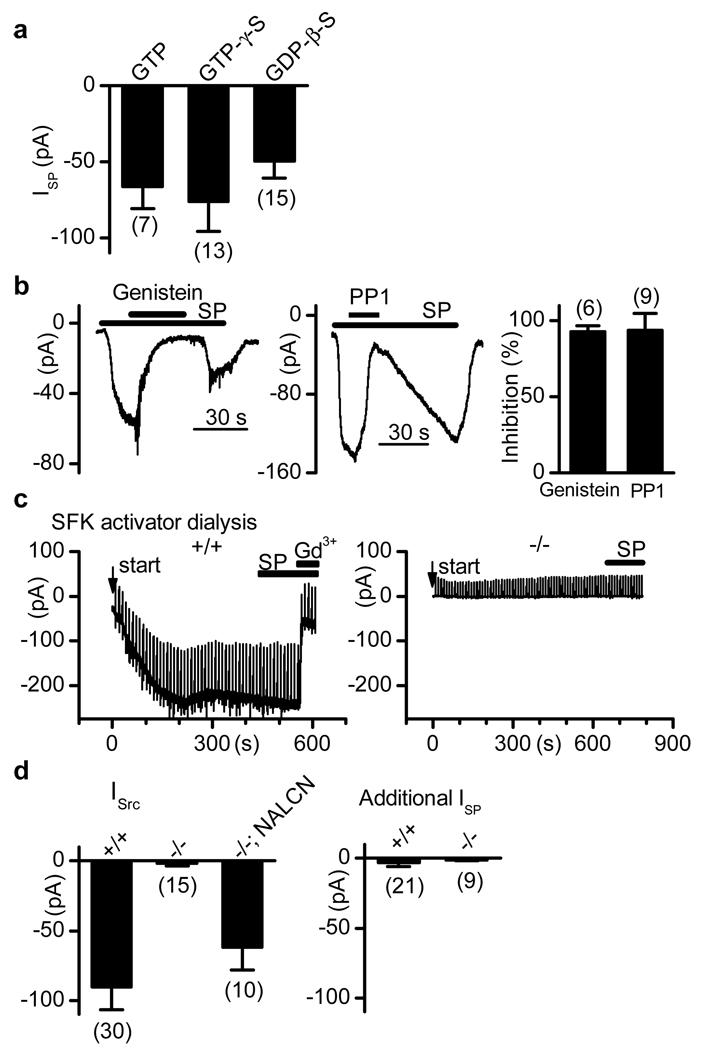
To determine the role of G-proteins (the immediate downstream effectors of GPCR NK1R) in the NALCN activation by SP, we “locked” G-proteins in active or inactive states with non-hydrolyzable analogs of GTP (GTP-γ-S) or GDP (GDP-β-S), respectively, applied via patch pipettes. Surprisingly, SP still induced inward currents of comparable sizes (Fig. 3a). Thus, the activation of NALCN by SP through GPCRs is likely via an unconventional mechanism that does not require G-protein stimulation.
Figure 3. ISP is G protein-independent but requires SFKs.
Hippocampal neurons were used. a, ISP (at −67 mV) from recordings with GTP-, GTP-γ-S - and GDP-β-S –containing pipette solutions. b, Inhibition of ISP (at −67 mV) by genistein (30 µM; left) and PP1 (20 µM; middle). Right, summary. c, In wild-type neurons (left), a Gd3+-blockable current developed upon intracellular dialysis (start time indicated by arrow) with pipette solution containing an SFK activator (1 µM; a ramp from −67 to −47 mV in 1.4 s was given every 10 s to monitor input resistance). After the current reached a plateau (size defined as ISrc for d), SP did not induce an additional current (ISP for d). Right, Nalcn−/− neuron. d, Summary of ISrc (left) and additional currents activated by SP after the dialysis-induced currents plateaued (right). Error bars, mean ± s.e.m.
Some GPCRs may also activate the Src family of tyrosine kinases (SFKs), which is a more recently discovered GPCR signaling cascade that regulates downstream factors such as mitogen-activated protein kinase and gene expression17, 18. Bath application of genistein (a phosphotyrosine kinase inhibitor) or PP1 (an SFK inhibitor) abolished ISP (Fig. 3b), suggesting that SFKs are required for the activation of NALCN by SP.
Similar to previous findings19, intracellular dialysis with an SFK activator via patch pipette induced a gradual increase of inward current (defined as ISrc; Fig. 3c, left panel). After the current plateaued, SP no longer activated an additional inward current (ISP; Fig. 3c, d), suggesting that SP and SFKs activate a common channel. Similar to ISP, the ISrc was blocked by Gd3+ (Fig. 3c, left). In contrast to wild-types, Nalcn−/− neurons lacked ISrc (Fig. 3c,d); this current was largely restored by transfection with NALCN cDNA (Fig. 3d). These data suggest that SFK activation is both necessary and sufficient for ISP. Given that many other ion channels can also be regulated by SFKs20, the lack of ISrc in the Nalcn−/− neurons was surprising, but seems to suggest that NALCN is a major cation channel target for SFKs near the resting membrane potential and may also be modulated by the diverse array of stimuli (e.g. neurotransmitters, growth factors, cytokines, cell stretch and adhesion molecules) that lead to SFK activation21, 22.
Other neuropeptides such as NT also elicit slow depolarization of neurons and activate a cation current (INT) similar to ISP9. In the wild-type VTA neurons, NT puffing (10 µM) elicited an inward current (−90.0 ± 25.3 pA at −67 mV; n = 29) that was blocked by an NT receptor antagonist SR48692 and the SFK inhibitor PP1 (not shown). In contrast, neurons from Nalcn−/− mice lacked an INT (Supplementary Fig. 3), suggesting that the INT is also through NALCN.
To determine the role of the SP- and NT- activated NALCN currents in modulating neuronal excitability, we recorded action potentials in the VTA DA neurons. Firing frequencies were significantly increased by 1 µM SP (Supplementary Fig. 4a, c) or 1 µM NT (Supplementary Fig. 4b, c) in the wild-type, but not in the Nalcn−/− neurons. The defect in the mutant was partially restored by transfecting with NALCN cDNA (Supplementary Fig. 4c). We conclude that the NALCN channel plays a major role in the potentiation of neuronal excitability by the neuropeptides in these neurons. Under other conditions or in other cells, the peptides may also excite neurons by suppressing K+ currents or by activating some of the TRP family members6, 8, 9, 23–26.
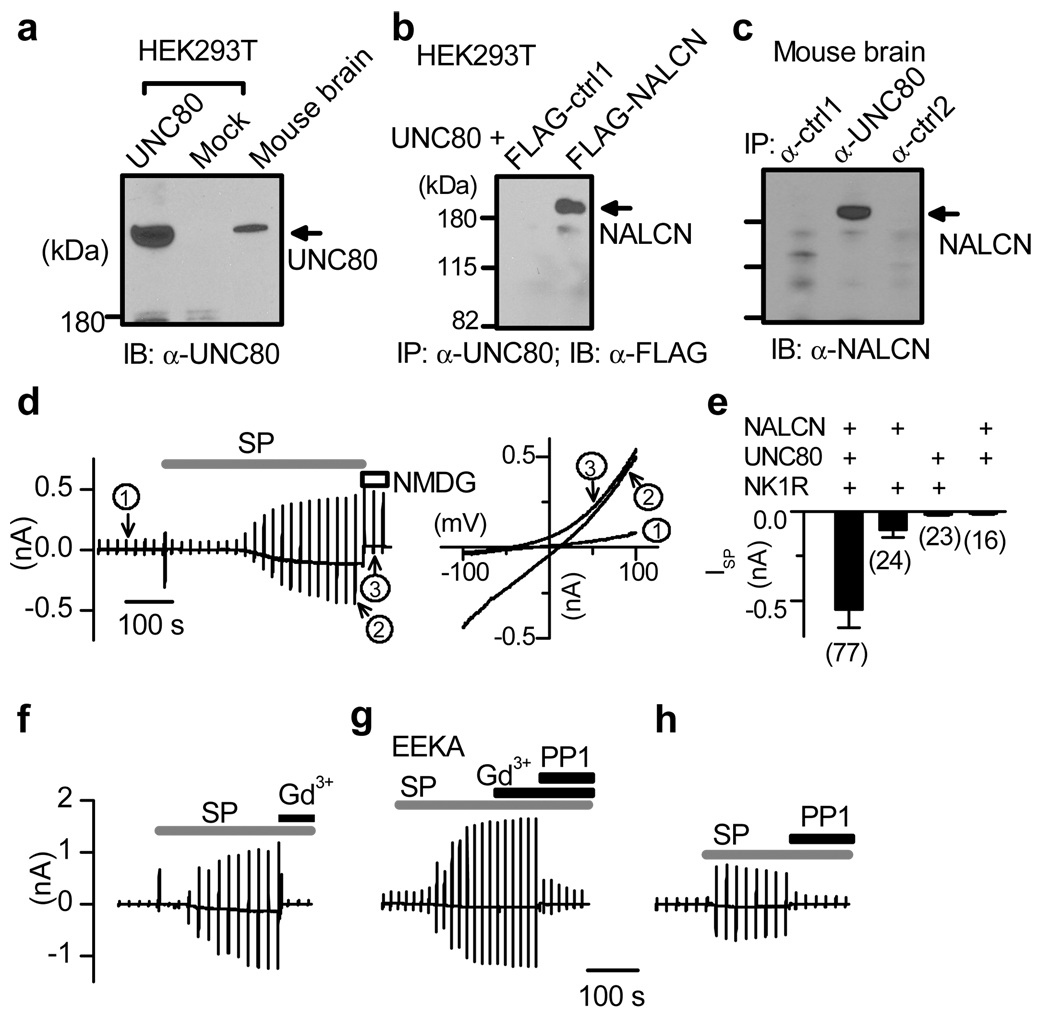
Unlike in neurons, little robust ISP was observed from HEK293T fibroblast cells co-transfected with NK1R and NALCN (see Fig. 4e), possibly because of a lack of key component for the channel activation. In Drosohpila melanogaster and C. elegans, Nalcn genetically interacts with others such as unc-79 and unc-8027–29. To investigate whether UNC-80 forms a physical complex with NALCN in the brain, we cloned a mammalian UNC-80 homolog (hereafter called mUNC80) from mouse brain (Supplementary Fig. 5). The full-length mUNC80 is predicted to encode a 371 kDa protein (Fig. 4a) with no obvious domains of known function. It has high (96%) and moderate (~30%) identities with its human and invertebrate homologs, respectively. NALCN and mUNC80 form a complex in mouse brain (Fig. 4c), and in transfected HEK293T cells (Fig. 4b). In addition, both are tyrosine-phosphorylated and such phosphorylation can be inhibited by PP1 (Supplementary Fig. 6).
Figure 4. ISP reconstituted in HEK293T cells.
a, Immunoblot (IB) with lysates from transfected HEK293T cells and brain. b, c, Immunoprecipitation (IP) showing protein complex between mUNC80 and NALCN in HEK293T transfected as indicated (b) and in brain (c). Ctrl1 and ctrl2 were two unrelated proteins used as controls. Recordings in d–h were done using ramp protocols (Vh = −20 mV; −100 to +100 mV in 1 s, every 20 s). d, Recordings from a cell transfected with NK1R, mUNC80 and NALCN. Currents at three time points are expanded in the right panel (circled 1, before SP; 2, after SP; 3, after Na+ and K+ in the bath were replaced with NMDG). e, Summary of ISP sizes (at −100 mV) from cells transfected with combinations as indicated. f, g, Recordings showing that ISP was blocked by Gd3+ (f) but became resistant to Gd3+ when NALCN was replaced by the EEKA pore mutant (g). h, Inhibition of ISP by PP1 (20 µM). Error bars, mean ± s.e.m.
In HEK293T cells with moderate levels of co-expression of NK1R, mUNC80 and NALCN (see Methods), SP activated a current (−550.6 ± 102.9 pA at −100 mV; 0 to −4904 pA; Fig. 4d, e) with a linear I/V relationship (Fig. 4d, right, curve 2). Like ISP in neurons, the current was blocked by the NALCN blocker Gd3+ (Fig. 4f), but became resistant to Gd3+ when NALCN was replaced with the EEKA pore mutant (Fig. 4g). Inclusion of GDP-β-S in the pipette did not prevent current activation (−914.0 ± 317.1 pA, n = 10), suggesting independence of G-protein activation.
Consistent with a requirement of SFKs, the current was suppressed by SFK inhibitors (Fig. 4g, h, Supplementary Fig. 7) and an anti-phospho-SFK antibody (Supplementary Fig. 8). Furthermore, when a constitutively active Src (with Y529 mutated to F) was co-transfected, a basal current was increased from −104.1 ± 22.1 pA (empty vector to replace Src Y529F in transfection; n = 22) to −434.3 ± 119.9 pA (n = 21), and application of SP no longer activated significant ISP in the Src Y529F co-transfected cells (−64.6 ± 33.1 pA, n = 13; compared with −416.2 ± 89.6 pA in the empty vector cotransfection control, n = 13) (Supplementary Fig. 9). Finally, inclusion of a recombinant active Src protein in the pipette led to a gradual increase of inward current (Supplementary Fig. 10), suggesting that, like in neurons (Fig. 3c), SFK is sufficient to activate the current.
The simplest interpretation of our work is that the SP- and NT- activated cation current is through a channel complex consisting of NALCN and mUNC80. Our preliminary studies found that UNC-79 is also associated with mUNC80 and NALCN in the brain (not shown). Further studies need to investigate if UNC-79 affects ISP’s properties such as the activation time courses. Similarly, the mechanisms underlying the involvement of SFKs in the coupling between receptor and channel remain to be uncovered. Due to the large driving force of Na+ at resting membrane potential, INALCN should be a potent excitatory current in neurons. The channel is voltage-independent and non-inactivating, and is thus ideal for regulating neuronal excitability by neuromodulators.
Method Summary
For recording the basal current in NALCN-overexpressing HEK293T cells10 (Supplementary Fig. 1), only cells expressing the highest amount of NALCN (top ~5%; judged by the levels of GFP expression from the pTracer vector containing both GFP and NALCN under separate promoters) were used. Receptor-activated currents (Fig. 4, Supplementary Figs. 7–10) were recorded from cells with modest level of expression (top ~40%).
METHODS
Cloning of mUNC80 and antibody generation
The mouse mUNC80 was cloned from single-strand brain cDNA as four fragments using RT-PCR with primers designed from partial sequences in NCBI databases. Multiple clones were sequenced from each fragment and clones without mutation were used to assemble the full-length in vector pcDNA3.1(+). The start of the ORF was unanimously identified by the presence of an in-frame stop codon in the 5’UTR. The anti-NALCN antibody was generated in rabbit with a GST-fusion protein containing the last 80 amino acids of NALCN. The mUNC80 polyclonal antibody was generated against the carboxyl-terminus (Supplementary Fig. 5). Both antibodies were affinity-purified. Immunoprecipitation and Western blotting followed previously described methods30.
Neuronal culture and transfection
Hippocampal neurons were cultured from P0 pups on glia pre-plated 35-mm dishes and coverslips as previously described10 and were used between DIV7 and 18. The protocol for VTA neuron culture was modified from one established in rat31. Briefly, the VTA was dissected from P0 pups and digested with papain (12 units/ml) for 30 min with occasional mixing; digestion was stopped with 10% serum. Tissue was then triturated and plated in culture medium (Neurobasal-A) supplemented with 2% B-27, 0.5× Pen/Strep and 1 mM Glutamax. Due to the small size of P0 mice, the VTA neuron culture also likely contained neurons from adjacent brain areas. Putative dopaminergic neurons were confirmed by tyrosine hydroxylase staining and morphologically identified31–33. VTA neurons of ages DIV18 to 30 were used for patch clamp. For transfection with Lipofectamine 2000 (Invitrogen), younger neurons (hippocampal, DIV5–7; VTA, DIV 6–7) were used because of higher efficiency. Transfected neurons were used 48–60 hrs later.
Patch clamp analysis
All experiments were performed at room temperature (20–25°C). For recording the basal leak current in HEK293T cells transfected with NALCN alone10 (Supplementary Fig. 1), cells were transfected with 3 µg NALCN (in a pTracer vector expressing GFP under a separate promoter). Only the most fluorescent cells (~ 5% of all the green ones) that presumably expressed the highest level of NALCN were selected. For recording SP-activated currents, NALCN (0.5 µg) was co-transfected with mUNC80 (0.5 µg, in pcDNA3.1(+) vector) and human NK1R (2 µg, in pcDNA3 vector), unless otherwise stated. An empty vector was added to ensure that the same amount of DNA was transfected when one or more constructs were not included. Cells with an above average level of fluorescence (~ 40% of the total number of green cells) were selected for analysis. It’s possible that some of the basal “leak” currents in neurons and overexpressing HEK293T cells are a result of basal level of receptor activation and tyrosine kinase activity in the cells. Cells with non-specific leak (e.g., due to cell damage) were identified by replacing Na+ and K+ with NMDG or by application of blockers. In NK1R-mUNC80-NALCN transfected cells, ~70% (53 of 77) of those analyzed had SP-activated currents > 100 pA (at −100 mV). The absence of currents in the rest presumably reflects the efficiency of having all three proteins (two of which are very large) expressed in the same cell, or the varying levels of endogenous signaling molecules. For current amplitude averages, all cells (including the ones without current) were included. HEK293T cells cultured with several batches of sera did not yield robust currents unless they were serum-starved (with Opti-MEM medium) for 9–16 hrs before recording (not shown). Data from these batches of transfections were not included for analysis.
Standard pipette solutions used for HEK293T cells contained (mM): 150 Cs, 120 Mes, 10 NaCl, 10 EGTA, 2 MgATP, 4 CaCl2, 0.3 Na2GTP and 10 HEPES (pH 7.4, Osm ~300 mOsm/L). Bath was a Tyrode’s solution containing 150 NaCl, 3.5 KCl, 1 MgCl2, 1.2 CaCl2, 10 HEPES, 20 glucose (pH 7.4 with NaOH; final Na+ 155 mM; Osm ~ 320 mOsm/L). Some cells were patched with pipette solutions containing no GTP; no differences were observed, consistent with ISP’s independence of G-protein activation. GDP-β-S -containing pipette solutions contained 1 mM GDP-β-S and no GTP. As a control, intracellular dialysis with GDP-β-S -containing pipette solutions blocked the activation of TRPC6 current by carbochol, which is G-protein dependent, in HEK293T cells co-transfected with TRPC6 and m3AChR3 (not shown). When pipette solutions containing anti-phospho-SFK (from Millipore) or recombinant active Src protein (from Stressgen) were used, heat-inactivated (100°C for 30 min) -proteins were used as control. Storage buffer of the recombinant protein was exchanged to pipette solution with a dilution of ~ 30,000 times by spinning 3 times in a concentrator (Microcon-50, Millipore).
Pyramidal hippocampal neurons and presumably dopaminergic VTA neurons used in patch clamp recordings were morphologically identified31–33. Unless otherwise stated, pipette solutions used for neuronal ISP and INT recordings contained (in mM): 120 CsCl, 4 EGTA, 2 CaCl2, 2 MgCl2, 10 HEPES, 4 Mg-ATP, 0.3 Tris-GTP and 14 phosphocreatine (di-tris salt) (pH adjusted to 7.4 with CsOH; final Cs+ 143 mM; free [Ca2+] ~60 nM; 300 mOsm/L). When GTP-γ-S (1.5 mM) or GDP-β-S (1 mM) was included in pipette solution, GTP was omitted and cells were dialyzed for 6–9 min before stimulus application. In experiments with SFK activator-containing pipette solution, 1 µM SFK activator (a tyrosine-phosphorylated peptide that binds to the SH2 domain of Src kinases, from Santa Cruz Biotechnology Inc., Cat. # sc-3052) was added. TTX (0.8 or 1 µM) was added in the bath of Tyrode’s solution. For whole bath SP application, concentrated SP (5 mM) was diluted to 50 µM with bath solution and pipetted into the bath to generate a final concentration of ~1 µM. Currents were continuously recorded for 10–20 minutes upon SP application and the peak currents were used to plot the I/V curves. In puffer applications, diluted SP (10 µM) was pressure applied using a pneumatic picopump for 10 seconds with a glass pipette (3~5 µm opening) placed ~ 20 µm away from the neuron.
For current clamp with the VTA neurons (Supplementary Fig. 4), Tyrode’s solution was used as the bath; pipette solution contained (in mM): 135 K-Asp, 5 NaCl, 5 KCl, 1 MgCl2, 1 EGTA, 10 HEPES, 4 Mg-ATP, 0.3 Tris-GTP, 14 phosphocreatine (di-tris) (pH adjusted to 7.4 with KOH; total K+ 147 mM). Neurons were isolated during current clamp with APV (10 µM), bicuculline (20 µM) and CNQX (20 µM). Some cultured neurons from both the wild-type and mutant showed spontaneous firing at 0 holding current. For others, small current (wild-type, −2.2 + 4.6 pA, n = 29; mutant, +10.2 + 4.4 pA, n = 19) were injected to artificially elicit repetitive firing. Firing frequencies were calculated from time windows (5 min) before and 30 sec after SP or NT application. Liquid junction potentials (estimated using the Clampex software) were corrected offline.
Statistical analyses
Analyses were done using Clampfit, Sigma Plot and Origin. Data are presented as mean ± s.e.m.
Supplementary Material
Acknowledgements
We thank Drs. D. Clapham, C. Deutsch, I. Medina, B. Novarro, M. Schmidt, and H. Xu for critically reading earlier versions of the manuscript, J. Xia for help on experiments, H. Yu and L. Yue for cDNA constructs, and Sanofi-Aventis for the gift of SR48692. This work was supported, in part, by funding from American Heart Association, NIH and the University of Pennsylvania Research Foundation.
Footnotes
Author Contributions. BL did recordings from neurons (Fig. 1, Fig. 2, Fig. 3, Supplementary Fig. 2) and all the HEK293T cells (Fig. 4, Supplementary Figs. 1, 7–10). YS contributed to neuronal recordings (Fig. 1, Fig. 2, Supplementary Figs. 3 and 4). SD contributed to work in Fig. 2. HW, YW and JL did the protein work (Fig. 4, Supplementary Fig. 6). DR started the project, designed experiments, and developed the cDNA constructs. BL and DR wrote the paper.
Author Information. The sequence of mUNC80 is deposited in GenBank under accession number FJ210934. Reprints and permissions information is available at npg.nature.com/reprintsandpermissions.
The authors declare that they have no competing financial interests.
References
- 1.Kandel ER, Schwartz JH, Jessell TM. In: Principles of Neural Science. McGraw-Hill, editor. McGraw-Hill; 2000. [Google Scholar]
- 2.Hille B. In: Ion Channels of Excitable Membranes. Sinauer, editor. Sunderland, MA: 2001. [Google Scholar]
- 3.Kuba K, Koketsu K. Synaptic events in sympathetic ganglia. Prog Neurobiol. 1978;11:77–169. doi: 10.1016/0301-0082(78)90010-2. [DOI] [PubMed] [Google Scholar]
- 4.Jan Y, Jan L, Kuffler S. Further evidence for peptidergic transmission in smypathetic ganglia. Proc Natl Acad Sci U S A. 1980;77:5008–5012. doi: 10.1073/pnas.77.8.5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuffler S, Sejnowski T. Muscarinic and peptidergic excitation of bull-frog sympathetic neurons. J Physiol. 1983;341:257–278. doi: 10.1113/jphysiol.1983.sp014805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanfield PR, Nakajima Y, Yamaguchi K. Substance P raises neuronal membrane excitability by reducing inward rectification. Nature. 1985;315:498–501. doi: 10.1038/315498a0. [DOI] [PubMed] [Google Scholar]
- 7.Shen KZ, North RA. Muscarine increases cation conductance and decreases potassium conductance in rat locus coeruleus neurones. J Physiol. 1992;455:471–485. doi: 10.1113/jphysiol.1992.sp019312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen KZ, Surprenant A. Common ionic mechanisms of excitation by substance P and other transmitters in guinea-pig submucosal neurones. J Physiol. 1993;462:483–501. doi: 10.1113/jphysiol.1993.sp019565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farkas RH, Chien PY, Nakajima S, Nakajima Y. Properties of a slow nonselective cation conductance modulated by neurotensin and other neurotransmitters in midbrain dopaminergic neurons. J Neurophysiol. 1996;76:1968–1981. doi: 10.1152/jn.1996.76.3.1968. [DOI] [PubMed] [Google Scholar]
- 10.Lu B, et al. The neuronal NALCN channel contributes resting sodium permeability and is required for normal respiratory rhythm. Cell. 2007;129:371–383. doi: 10.1016/j.cell.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 11.Shen KZ, North RA. Substance P opens cation channels and closes potassium channels in rat locus coeruleus neurons. Neuroscience. 1992;50:345–353. doi: 10.1016/0306-4522(92)90428-5. [DOI] [PubMed] [Google Scholar]
- 12.Aosaki T, Kawaguchi Y. Actions of substance P on rat neostriatal neurons in vitro. J Neurosci. 1996;16:5141–5153. doi: 10.1523/JNEUROSCI.16-16-05141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue K, Nakazawa K, Inoue K, Fujimori K. Nonselective cation channels coupled with tachykinin receptors in rat sensory neurons. J Neurophysiol. 1995;73:736–742. doi: 10.1152/jn.1995.73.2.736. [DOI] [PubMed] [Google Scholar]
- 14.Pena F, Ramirez JM. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J Neurosci. 2004;24:7549–7556. doi: 10.1523/JNEUROSCI.1871-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones SW. Muscarinic and peptidergic excitation of bull-frog sympathetic neurones. J Physiol. 1985;366:63–87. doi: 10.1113/jphysiol.1985.sp015785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otsuka M, Yoshioka K. Neurotransmitter functions of mammalian tachykinins. Physiol Rev. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- 17.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 18.DeFea KA, et al. The proliferative and antiapoptotic effects of substance P are facilitated by formation of β-arrestin-dependent scaffolding complex. Proc Natl Acad Sci U S A. 2000;97:11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heuss C, Scanziani M, Gahwiler BH, Gerber U. G-protein-indenpendent signaling mediated by metabotropic glutamate receptors. Nat Neurosci. 1999;2:1070–1077. doi: 10.1038/15996. [DOI] [PubMed] [Google Scholar]
- 20.Davis MJ, et al. Regulation of ion channels by protein tyrosine phosphorylation. Am J Physiol Heart Circ Physiol. 2001;281:H1835–H1862. doi: 10.1152/ajpheart.2001.281.5.H1835. [DOI] [PubMed] [Google Scholar]
- 21.Salters MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 22.Heuss C, Gerber U. G-protein-independent signaling by G-protein-coupled receptors. Trends Neurosci. 2000;23:469–475. doi: 10.1016/s0166-2236(00)01643-x. [DOI] [PubMed] [Google Scholar]
- 23.Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 24.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 25.Oh EJ, Gover TD, Cordoba-Rodriguez R, Weinreich D. Substance P evokes cation currents through TRP channels in HEK293 cells. J Neurophysiol. 2003;90:2069–2073. doi: 10.1152/jn.00026.2003. [DOI] [PubMed] [Google Scholar]
- 26.Bley KR, Tsien RW. Inhibition of Ca2+ and K+ channels in sympathetic neurons by neuropeptides and other ganglionic transmitters. Neuron. 1990;4:379–391. doi: 10.1016/0896-6273(90)90050-p. [DOI] [PubMed] [Google Scholar]
- 27.Jospin M, et al. UNC-80 and the NCA ion channels contribute to endocytosis defects in synaptojanin mutants. Curr Biol. 2007;17:1595–1600. doi: 10.1016/j.cub.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 28.Yeh E, et al. A Putative cation Channel, NCA-1, and a novel Protein, UNC-80, transmit neuronal activity in C. elegans. PloS Biol. 2008;6:e55. doi: 10.1371/journal.pbio.0060055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Humphrey JA, et al. A putative cation channel and its novel regulator: cross-species conservation of effects on general anesthesia. Curr Biol. 2007;17:624–629. doi: 10.1016/j.cub.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Xia J, Cho KH, Clapham DE, Ren D. Catsper β: A novel transmembrane protein in the catsper channel complex. J Biol Chem. 2007;282:18945–18952. doi: 10.1074/jbc.M701083200. [DOI] [PubMed] [Google Scholar]
- 31.Masuko S, Nakajima S, Nakajima Y. Dissociated high-purity dopaminergic neuron cultures from the substantia nigra and the ventral tegmental area of the postnatal rat. Neuroscience. 1992;49:347–364. doi: 10.1016/0306-4522(92)90101-7. [DOI] [PubMed] [Google Scholar]
- 32.Rayport S, et al. Identified postnatal mesolimbic dopamine neurons in culture: morphology and electrophysiology. J Neurosci. 1992;12 doi: 10.1523/JNEUROSCI.12-11-04264.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grace AA, Onn S-P. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9:3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.