Abstract
Background
Pathogenic HIV and SIV infections characteristically deplete central memory CD4+ T cells and induce chronic immune activation, but it is controversial whether this also occurs after vaccination with attenuated SIVs and whether depletion or activation of CD4+ T cell play roles in protection against wild-type virus challenge.
Methods
Rhesus macaques were vaccinated with SIVΔnef and quantitative and phenotypic polychromatic flow cytometry analyses were performed on mononuclear cells from blood, lymph nodes and rectal biopsies.
Results
Animals vaccinated with SIVΔnef demonstrated no loss of CD4+ T cells in any tissue, and in fact CCR5+ and CD28+CD95+ central memory CD4+ T cells were significantly increased. In contrast, CD4+ T cell numbers and CCR5 expression significantly declined in unvaccinated controls challenged with SIVmac239. Also, intracellular Ki67 increased acutely as much as 3-fold over baseline in all tissues after SIVΔnef vaccination then declined following primary infection.
Conclusion
We demonstrated in this study that SIVΔnef vaccination did not deplete CD4+ T cells but transiently activated and expanded the memory cell population. However, increases in numbers and activation of memory CD4+ T cells did not appear to influence protective immunity.
Keywords: Simian immunodeficiency virus, live attenuated SIV, T cell activation, Protective immunity
Introduction
HIV infections and pathogenic SIV infections of rhesus macaques are typically characterized by a gradual depletion of CD4+ T cells in the peripheral blood and acutely massive CD4+ T cell loss in the gut mucosa [1–3]. More specifically, the predominant target cells for viral infection and subsequent loss are CD28+ “central” memory CD4+ T cells expressing the HIV/SIV coreceptor CCR5 [2, 4–8]. CD4+ T cell loss is driven, in part, by another feature of progressive HIV/SIV infections, chronic immune activation, which provides a pool of activated CD4+ T cells to serve as substrates for viral replication [9–11].
Currently, vaccinations with live attenuated SIV are the only effective means of inducing sterile protection against lentivirus infections [12–15], and although it is unlikely a live attenuated HIV vaccine will ever be used in practice, this model has provided fundamental insights into the necessary components for protective immunity. Various attenuated SIV strains have been shown to induce potent humoral and cellular immune responses, all of which may contribute to sterilizing immunity [13, 16–20]. Moreover, live attenuated SIVs are replication-competent, and the nef-deficient virus, SIVmac239Δnef, has been shown to infect predominantly CD4+ T cells in vivo [21]. However, it is controversial whether infections with live attenuated SIVs results in a net cellular loss of CD4+ T cells. Veazey et al. [3] showed that while the wild-type virus SIVmac239, from which SIVmac239Δnef is derived, depleted CD4+ T cells in gut mucosa, SIVmac239Δnef did not. Picker et al. [22] showed a similar lack of CD4+ T cell depletion by SIVmac239Δnef in bronchoalveolar lavage. In contrast, some previous studies have demonstrated evidence of CD4+ T cell loss and disease progression in a small number of macaques infected with another attenuated SIV, SIVmac239Δ3 [23–25]. However, cell loss and disease progression were most prominent in neonatal macaques where the immune system is less mature and in macaques immunosuppressed by steroid administration. Furthermore, those studies primarily focused on evaluation of the bulk CD4+ T cell population and lacked the advantage of more modern methods of cellular quantification.
The objective of this study was to enumerate and characterize the activation states of both bulk and CD28+ memory CD4+ T cells during the primary phase of infection with the live attenuated virus, SIVmac239Δnef. We also evaluated what role(s) changes in quantitative and activation states might have on sterile protection of SIVmac239Δnef-vaccinated macaques against SIVmac239 challenge.
Material and methods
Animals and infections
A total of twelve male Indian rhesus macaques (Macaca mulatta) were used in this study. All animals were SIV-negative (naive) at the initiation of the study and were also free of simian retrovirus type D, SIV, simian T-lymphotrophic virus type 1, and herpes B virus. Ten animals were vaccinated intravenously with SIVmac239Δnef as previously described [14, 15] and then intravenously challenged with 10 AID50 of SIVmac239 [26] at either 5 or 15 weeks post-vaccination (five vaccinated macaques and one unvaccinated control at each time point). Both control animals became infected with SIVmac239, while none of the SIVmac239Δnef-vaccinated animals showed any evidence of infection with SIVmac239, nor any evidence of anamnestic responses (R.K.R. and R.P.J., manuscript in preparation). The two vaccine groups (5 and 15 week challenges) are combined herein for selected evaluations of cellular dynamics. All of the macaques were housed at the New England Primate Research Center and maintained in accordance with the guidelines of the Committee on Animals of the Harvard Medical School and the Guide for the Care and Use of Laboratory Animals.
Cell processing
Rhesus macaque PBMCs were isolated from EDTA-treated venous blood by LSM (MP Biomedicals, Solon, OH) density gradient centrifugation and tissue mononuclear cells were isolated from minced peripheral lymph node and rectal biopsies as described previously [27]. Cells were washed and resuspended in PBS supplemented with 2% FCS (Sigma-Aldrich, St. Louis, MO) for subsequent flow cytometric analysis.
Antibodies and flow cytometric analysis
Surface staining was carried out by standard procedures as described previously [28]. Except where noted, all mAb were obtained from BD Biosciences (San Diego, CA) and included: CD3 APC-Cy7 (clone SP34-2), CD4 AmCyan (clone L200, custom) or CD4 PerCP-Cy5.5 (SK3), CD8α Alexa700 (clone RPA-T8), CD28 Texas Red (clone CD28.2 from Beckman-Coulter, Fullerton, CA), CD95 APC (clone DX2), CCR5 PE (clone 3A9), and TCR γδ-FITC (clone B1). Intracellular staining for Ki67 (PE conjugate, clone B56) was performed on mononuclear cells using The Biolegend Fix/Perm kit (Biologend Inc., San Diego, CA) according to the manufacturer’s suggested protocol. All acquisitions were made on an LSR II (BD Biosciences) and analyses were done using FlowJo software (Tree Star Inc., Ashland, OR).
Whole blood lymphocyte count assay
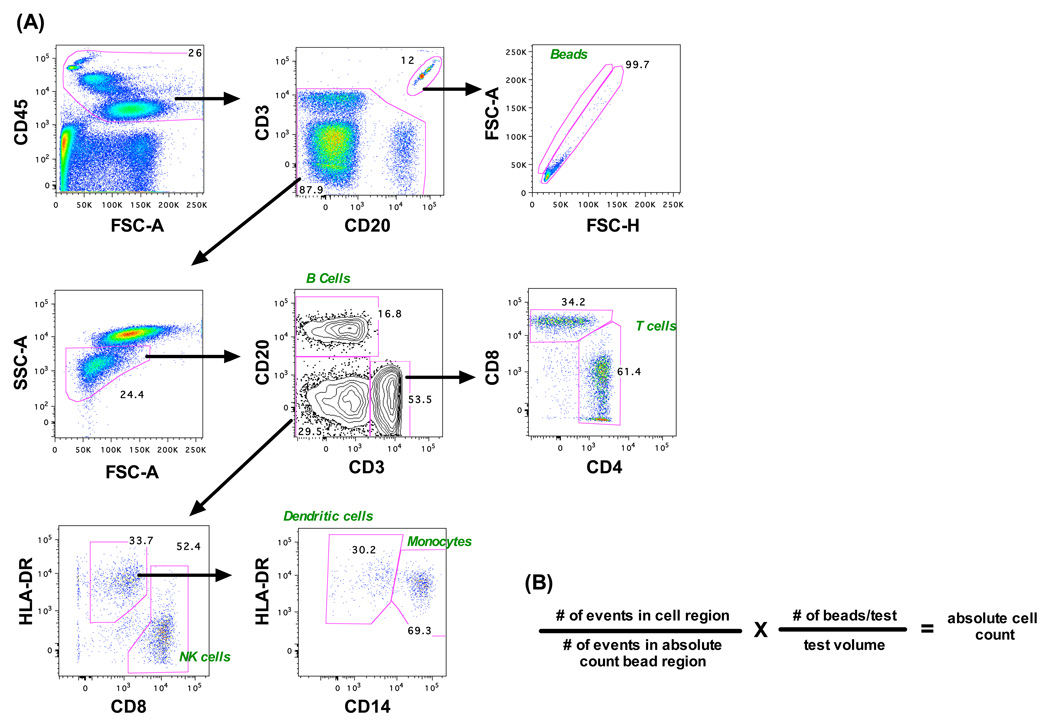
Fifty µl aliquots of whole blood were incubated with 50,050 fluorescent beads and a pool of lyophilized antibodies, or singularly for compensation tubes, for 20 minutes at room temperature and then lysed with FACSLyse (BD Biosciences) for 10 minutes. To preserve cell concentrations, no wash step was used. Acquisitions were performed on an LSR II and 5,000 beads were collected per sample. Analysis was performed using FlowJo software (Fig. 1A) and absolute cell numbers were calculated based on bead-to-cell frequencies (Fig. 1B) [29]. The following antibodies (all from BD) were used for compensation and sample tests: CD3 Alexa 700 (clone SP34-2), CD4 APC-Cy7 (clone L200), CD8 APC (clone SK1), CD14 PE-Cy7 (clone M5E2), CD20 PerCp-Cy5.5 (clone L27), CD45 PE (D058-1283), and HLA-DR FITC (clone L243). For some cell subsets (i.e., CD28+ CD4+ memory cells) absolute counts were determined using this assay in combination with cell frequency data derived from polychromatic flow cytometry.
Figure 1.
Absolute lymphocyte determination by a whole-blood bead-based flow cytometry assay. (A) A representative gating strategy is shown identifying various lymphocyte subsets as well as fluorescent beads. (B) The absolute number of cells per volume of blood is calculated based on the number of events in each cell gate.
Statistical analysis
All statistical and graphical analyses were done using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA). Nonparametric Wilcoxon and Mann-Whitney tests were used where indicated and P < 0.05 were assumed to be significant in all analyses.
Results
Identification and quantification of CD4+ T cells in rhesus macaques
As described in the Material and Methods section and shown in Fig. 1, absolute numbers of CD4+ T cells were enumerated using a whole-blood bead-based assay and cell frequencies were determined using polychromatic flow cytometry (Fig 2A). In normal rhesus macaques (day 0) the median number of total circulating CD4+ T cells was 834 cells/µl of blood (range 345–1415 cells/µl; n = 12) and the median frequency of CD4+ T cells among total T cells was 67% (range, 36–78%). CD4+ T cells were further delineated into naïve, central memory, and effector populations using CD95 (FAS) and the costimulatory molecule CD28 as previously described [30]. Employing this gating strategy, we also defined CD4+ T cell subsets as CD28intCD95−, CD28brightCD95+ and CD28−CD95+ corresponding to naïve, central memory and effector memory cells, respectively (Fig. 2B). The median frequencies of each of these subsets as fractions of the total CD4+ T cell population in normal rhesus macaques were: naïve – 58%, central – 41%, and effector – 1.6% (n =12).
Figure 2.
Phenotypic identification of CD4+ T lymphocyte subsets by polychromatic flow cytometry. Representative gating strategies are shown for identification of various subpopulations of CD4+ T cells in whole PBMC. (A) First, lymphocytes were gated based on forward-versus-side-scatter characteristics and CD4+ αβ T cells were identified as CD4+CD3+CD8− and negative for the γδ T cell receptor. Secondly, (B) naïve and central memory CD4+ T cells were phenotyped as CD28+CD95− and CD28+CD95+, respectively. (C) Frequency of CCR5 expression was quantified on the bulk CD4+ T cell population.
SIVmac239Δnef vaccination does not deplete CD4+ T cells
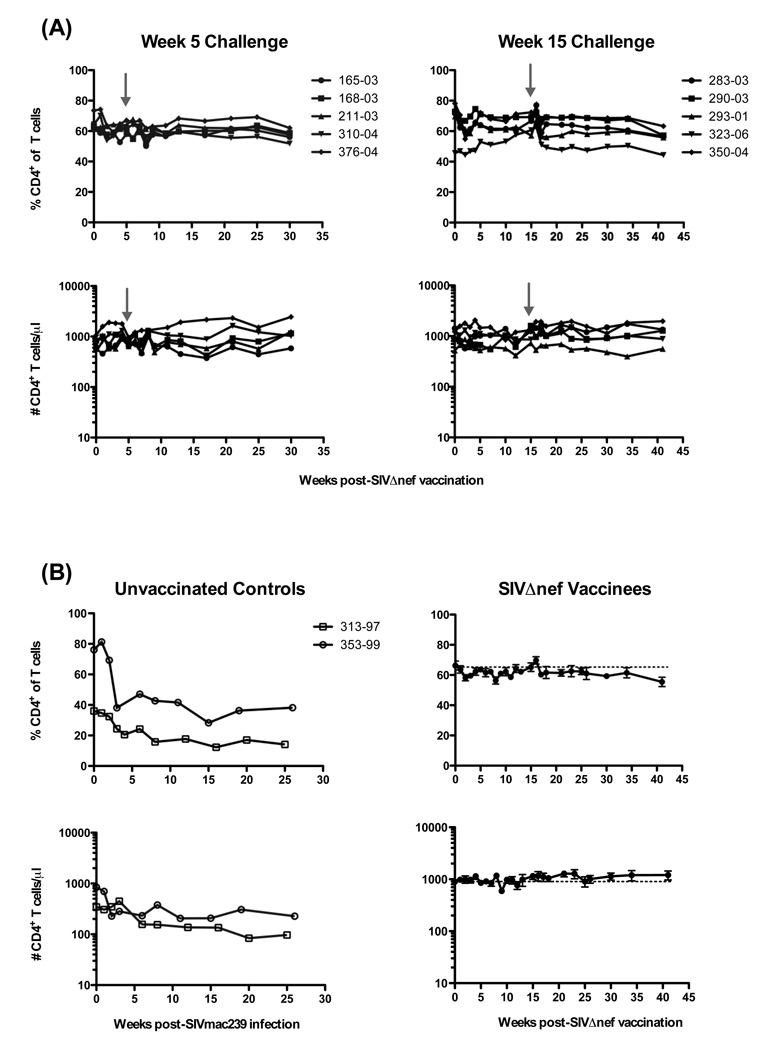
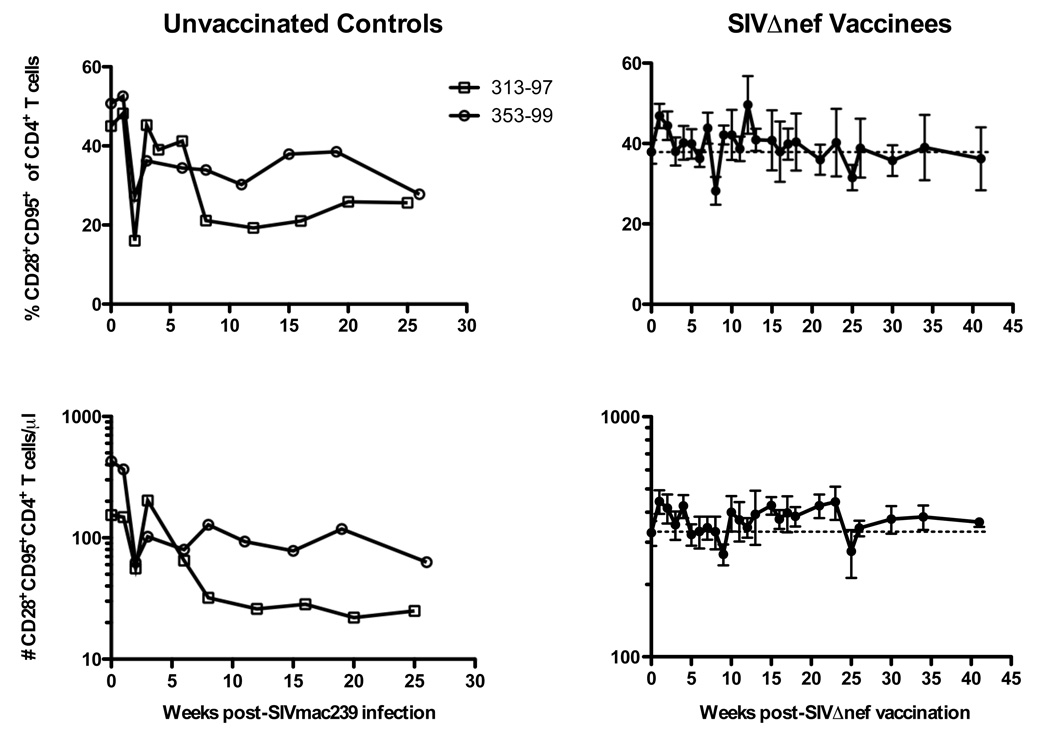
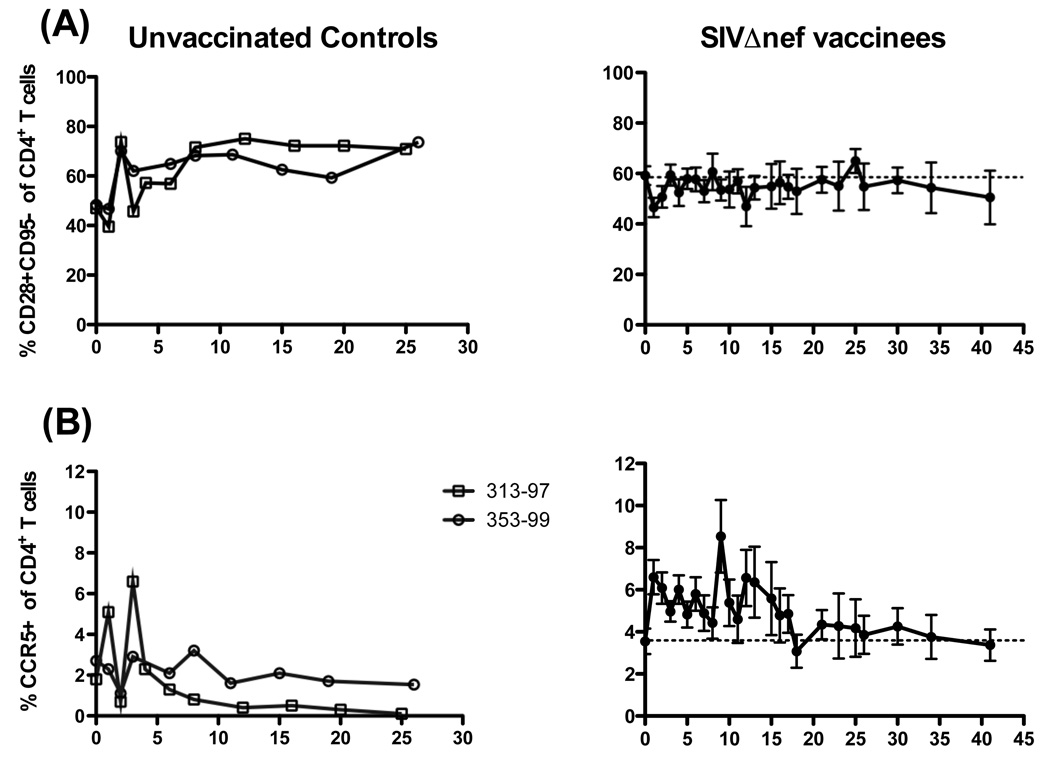
Ten normal rhesus macaques were vaccinated with SIVΔnef. Subsequently, five macaques were challenged with SIVmac239 at 5 weeks and five at 15 weeks post-vaccination (Fig 3A). Neither animal group demonstrated any significant changes in CD4+ T cell numbers or frequencies after vaccination or after challenge (Fig. 3A). Therefore, these ten animals were grouped collectively for further analyses (Fig. 3B, right panels). In contrast to SIVmac239Δnef-vaccinated animals, percentages of CD4+ T cells declined in frequency by ~50% by week 5 post-challenge and remained reduced through 26 weeks post-challenge (Fig 3B, left panels). Similarly, the absolute numbers of CD4+ T cells declined to 97 and 227 at weeks 25 (Mm # 353-99) and 26 (Mm # 313-97), respectively. Interestingly, CD28+ memory CD4+ T cells increased transiently in both frequency and absolute number over baseline levels (Fig. 4, right panels), reaching statistical significance at week one post-vaccination (P = 0.002 and P = 0.0273, respectively, Wilcoxon test). As expected, CD28+CD95+ memory CD4+ T cells declined rapidly during acute SIVmac239 infection, followed by a rebound period, and then gradually declined longitudinally (Fig. 4, left panels). By comparison there was ~ 40% increase in the frequency of CD28+CD95− naïve CD4+ T cells two weeks after SIVmac239 infection of unvaccinated controls which was sustained throughout the observation period (Fig. 5A), likely due to decline in the frequency of central memory cells. This was not however observed after SIVmac239Δnef vaccination, where the naïve population was stable.
Figure 3.
Longitudinal analysis of circulating CD4+ T cells in SIVΔnef-vaccinated and SIVmac239-infected rhesus macaques. (A) Frequencies (upper panels) and absolute numbers (lower panels) of circulating CD4+CD3+ lymphocytes are shown for SIVΔnef-vaccinated macaques challenged intravenously with SIVmac239 at either 5 or 15 weeks post-vaccination; grey arrows indicate 5 and 15 week challenges, respectively. (B) Frequencies and absolute numbers of CD4+CD3+ lymphocytes in two unvaccinated controls challenged with SIVmac239 (left panels) and means ± SEM of SIVΔnef-vaccinated macaques (right panels) combined from (A). Dashed horizontal lines indicate means at day of vaccination (day 0).
Figure 4.
Longitudinal analysis of central memory CD4+ T cells. Frequencies (upper panels) and absolute numbers (lower panels) of CD28+CD95+ “central memory” CD4+ T cells in two SIVmac239-infected macaques (left panels) and means ± SEM of ten SIVΔnef-vaccinated animals as shown in Figure 3.
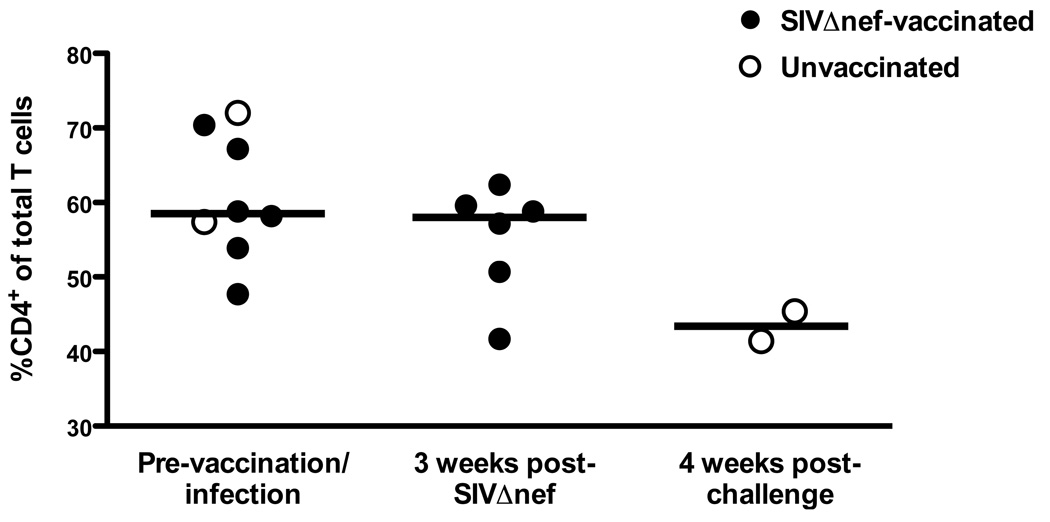
Figure 5.
CD4+ T cell frequencies in the rectal mucosa of SIV-naïve, SIVΔnef-vaccinated, and SIVmac239-infected rhesus macaques. Percentages of CD4+ T cells are shown as frequencies among total T cells in lymphocytes isolated from rectal biopsies performed at one week prior to and three weeks after SIVΔnef vaccination and four weeks after SIVmac239 challenge (weeks 5 and 15 challenge groups are combined). Bars indicate medians of two to eight animals.
Since CD4+ T cell depletion in HIV and SIV infections is most prominent in the gut mucosa, we also examined the frequency of CD4+ T cells among the total T cell population in rectal biopsies (Fig. 6). When comparing pre-vaccination/infection biopsies to those taken 3 weeks post-SIVΔnef-vaccination, the median values (Pre – 58.5%, week 3 – 58.0%) were equivalent, while the frequency of CD4+ T cells in rectal biopsies had declined in both unvaccinated controls.
Figure 6.
Longitudinal analysis of CD4+ T cell subsets. Frequencies of (A) CCR5+ and (B) CD28+CD95− naïve CD4+ T cells in SIVmac239-infected and SIVΔnef-vaccinated macaques.
Because the chemokine receptor CCR5 also delineates memory cells and is a necessary coreceptor for SIV infection, we also evaluated its expression on circulating CD4+ T cells (Fig. 2C). Interestingly, while the frequency of CCR5 expressing CD4+ T cells eventually declined in wild-type infection (Fig. 5B), after SIVmac239Δnef vaccination, CCR5 expression significantly increased at one week post-vaccination (P = 0.0039, Wilcoxon test; means, week 0 – 3.55%, week 1 – 6.6%). Although not statistically significant, CCR5 expression remained elevated until ~ 18 weeks post-vaccination (mean at 18 weeks, 3.08%).
SIVmac239Δnef activates CD4+ T cells after vaccination
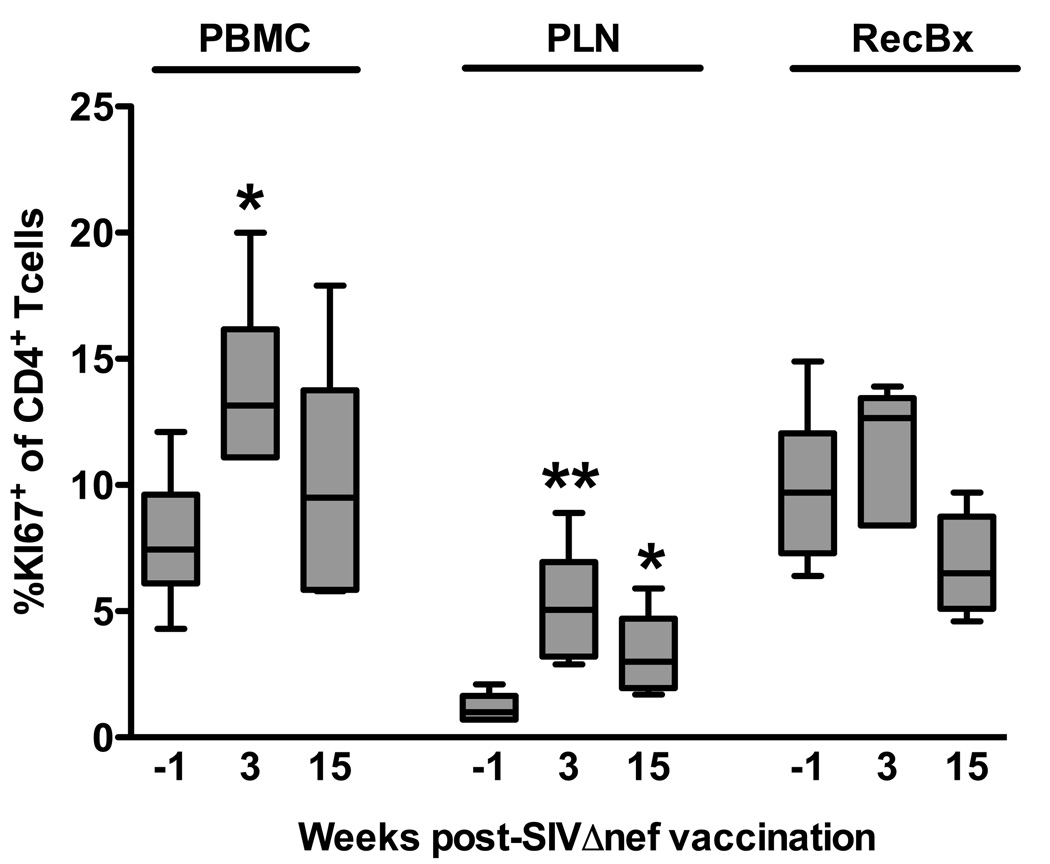
An acute increase in CCR5 expression on CD4+ T cells suggested SIVΔnef vaccination could be activating these cells. To further evaluate this possibility we analyzed intracellular Ki67 expression in bulk CD4+ T cells in PBMC, peripheral lymph nodes, and in rectal biopsies. Ki67 was upregulated acutely in all three tissues, but only significantly in PBMC and peripheral lymph nodes (Fig. 7). By 15 weeks post-vaccination, Ki67 expression had declined to near pre-vaccination levels, but in peripheral lymph nodes was still significantly higher than baseline. It is also interesting to note that basal levels of Ki67 expression were significantly lower in peripheral lymph nodes than in either PBMC (P = 0.0022, Mann Whitney test) or rectal biopsies (P = 0.0050).
Figure 7.
Ki67 expression in CD4+ T cells after SIVΔnef vaccination. Intracellular Ki67 expression was quantified in CD4+ T cells by flow cytometry in lymphocytes isolated from blood (PBMC), peripheral lymph node biopsies (PLN), and rectal biopsies (RecBx) taken one week prior to and three weeks after SIVΔnef vaccination. Bars indicate medians of six animals; *, P < 0.05; **, P < 0.01; Mann Whitney test.
Discussion
In this study we comprehensively examined the cellular dynamics of memory and bulk CD4+ T cells after vaccination with the live attenuated SIV strain, SIVmac239Δnef. Unlike pathogenic lentivirus infections, SIVmac239Δnef-vaccinated animals demonstrated no loss of CD4+ T cells either in the periphery or in gut mucosa. However, we did find a transient increase in CD4+ T cell activation that declined coinciding with control of viremia, in contrast to the sustained increases in CD4+ T cell activation observed in HIV-1 and pathogenic SIV infections [9–11, 31]. Although the transient induction of CD4+ T cell activation by SIVmac239Δnef did not appear to influence protection against wild-type virus challenge in this study, it could have implications for target cell availability, and ultimately vaccine efficacy.
Previous studies evaluating CD4+ T cell loss in macaques infected with live attenuated SIVs have yielded conflicting results. Similar to our findings, Picker et al. [22] reported no evidence for loss of bulk or memory CD4+ T cells in peripheral blood or bronchoalveolar lavage in macaques vaccinated with SIVmac239Δnef. In contrast, naïve macaques vaccinated as infants with high doses of SIVmac239Δ3 almost uniformly progressed to simian AIDS [24], indicating that the infant immune system can be vulnerable to even attenuated lentivirus strains. However, vaccination of adult macaques with SIVmac239Δ3 was much less likely to cause disease – AIDS-like symptoms occurred in less than 20% of vaccinated macaques and only after a prolonged observation greater than 3 years or after steroid-induced immunosuppression [23–25]. Another nef-defective SIV, SIVmacC8, was also found to cause CD4+ T cell loss as early as 12 weeks, but only in 1 animal [32, 33]. Although the number of macaques in these studies progressing to disease is small, we cannot exclude the possibility that some fraction of SIVmac239Δnef-vaccinated animals could develop some symptoms of disease, particularly since our original observation period was concluded at 40 weeks and Picker et al. [22] only evaluated animals up to 200 days post-vaccination.
Although SIVmac239Δnef was derived over 15 years ago [14], the specific protective correlates remain poorly understood. Many immune components have been proposed to be involved in mediating protection – humoral immunity, cytoxic T lymphocytes, innate immune responses, and CD4+ T cells [18, 19]. Since SIVmac239Δnef primarily replicates in CD4+ T cells in lymphoid tissue [21] a hypothesized mechanism of protection was that SIVmac239Δnef might actually deplete target cells, decreasing permissivity to superinfection with wild-type viruses. However, we demonstrate herein that in fact the opposite is true, at least acutely, – the frequency of memory CD4+ T cells transiently increases following SIVmac239Δnef vaccination. Therefore, we must consider the opposite hypothesis – that increased numbers of target cells could exacerbate infection rates after wild-type virus challenge. In this study, when vaccinated macaques were challenged at 5 weeks post-vaccination (when levels of Ki67 and CCR5 expression were still elevated) all animals were sterilely protected, indicating that the increased target cell availability and activation did not results in superinfection with the SIVΔnef parental virus, SIVmac239. However, in a previous kinetic study, when SIVmac239Δnef-vaccinated macaques were challenged with the SIVmac239-related virus, SIVmac251 animals became infected at 5, but not 15 weeks vaccination [15]. Therefore, increases in activation or target cell availability might contribute to superinfection with viruses other than the parental strain, although the relative contribution of this effect is difficult to assess, since changes in SIV-specific immune responses also occur during this time frame.
While the question of whether live attenuated SIVs can induce AIDS-like disease in rhesus macaques remains controversial, our data show no evidence for this in primary infection or up to ~ 40 week post-vaccination. Also, since no loss of CD4+ T cells occurred in peripheral blood or in the gut mucosa, this suggests that these macaques are less likely to progress to immunodeficiency, since early CD4+ T cell loss often dictates disease course [1, 2, 22, 34]. Furthermore, while our results indicate that acute increases in activated memory CD4+ T cells do not influence infection rates upon challenge with SIVmac239, we cannot exclude the possibility that the transient increase in CD4+ T cell activation may affect the susceptibility to challenge in other experimental settings. More detailed studies will be needed to assess the potential consequences of activation of the CD4+ T cell memory pool to protective immunity induced by live attenuated SIV.
Acknowledgements
We thank Michelle Connole and Yi Yu for expert technical assistance, Carolyn O'Toole for help with manuscript preparation, and Angela Carville and the staff of the Department of Primate Medicine for assistance with animal experiments. We also thank Louis Picker, Andrew Sylvester, Laurel Nomura, and Holden Maecker for the development of and provision of reagents for the nonhuman primate whole blood leukocyte counting tubes.
Funding
This study was funded by an International AIDS Vaccine Initiative (IAVI) grant and NIH grants R01 AI062412 and P01 AI071306 all to RPJ. Additional funding was provided by the NEPRC base grant P51 RR00168. RKR was also supported by a CHAVI/HVTN Early Career Investigator (ECI) award.
References
- 1.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattapallil JJ, Douek DC, Hill BJ, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 3.Veazey RS, DeMaria M, Chalifoux LV, Shvetz D, Pauley D, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. The gastrointestinal tract as a major site of CD4 T lymphocyte depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 4.Chun TW, Chadwick K, Margolick J, Siliciano RF. Differential susceptibility of naive and memory CD4+ T cells to the cytopathic effects of infection with human immunodeficiency virus type 1 strain LAI. J Virol. 1997;71:4436–4444. doi: 10.1128/jvi.71.6.4436-4444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elrefaei M, McElroy MD, Preas CP, Hoh R, Deeks S, Martin J, Cao H. Central memory CD4+ T cell responses in chronic HIV infection are not restored by antiretroviral therapy. J Immunol. 2004;173:2184–2189. doi: 10.4049/jimmunol.173.3.2184. [DOI] [PubMed] [Google Scholar]
- 6.Schnittman SM, Lane HC, Greenhouse J, Justement JS, Baseler M, Fauci AS. Preferential infection of CD4+ memory T cells by HIV-1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc Natl Acad Sci USA. 1990;87:6058–6062. doi: 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spina CA, Prince HE, Richman DD. Preferential replication of HIV-1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J Clin Invest. 1997;99:1774–1785. doi: 10.1172/JCI119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veazey RS, Mansfield KG, Tham IC, Carville AC, Shvetz DE, Forand AE, Lackner AA. Dynamics of CCR5 expression by CD4+ T cells in lymphoid tissues during simian immunodeficiency virus infection. J Virol. 2000;74:11001–11007. doi: 10.1128/jvi.74.23.11001-11007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, Shih R, Lewis J, Wiley DJ, Phair JP, Wolinsky SM, Detels R. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 10.Grossman Z, Meier-Schellersheim M, Sousa AE, Victorino RM, Paul WE, Gupta AK. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat Med. 2002;8:319–323. doi: 10.1038/nm0402-319. [DOI] [PubMed] [Google Scholar]
- 11.Hazenberg MD, Stuart JW, Otto SA, Borleffs JC, Boucher CA, de Boer RJ, Miedema F, Hamann D. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART) Blood. 2000;95:249–255. [PubMed] [Google Scholar]
- 12.Wyand MS, Manson K, Montefiori DC, Lifson JD, Johnson RP, Desrosiers RC. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J Virol. 1999;73:8356–8363. doi: 10.1128/jvi.73.10.8356-8363.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wyand MS, Manson KH, Garcia-Moll M, Montefiori D, Desrosiers RC. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniel MD, Kirchhoff F, Czajak SC, Sehgal PK, Desrosiers RC. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 15.Connor RI, Montefiori DC, Binley JM, Moore JP, Bonhoeffer S, Gettie A, Fenamore EA, Sheridan KE, Ho DD, Dailey PJ, Marx PA. Temporal analyses of virus replication, immune responses, and efficacy in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J Virol. 1998;72:7501–7509. doi: 10.1128/jvi.72.9.7501-7509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almond N, Kent K, Cranage M, Rud E, Clarke B, Stott EJ. Protection by attenuated simian immunodeficiency virus in macaques against challenge with virus-infected cells. Lancet. 1995;345:1342–1344. doi: 10.1016/s0140-6736(95)92540-6. [DOI] [PubMed] [Google Scholar]
- 17.Almond N, Rose J, Sangster R, Silvera P, Stebbings R, Walker B, Stott EJ. Mechanisms of protection induced by attenuated simian immunodeficiency virus. I. Protection cannot be transferred with immune serum. Journal of General Virology. 1997;78:1919–1922. doi: 10.1099/0022-1317-78-8-1919. [DOI] [PubMed] [Google Scholar]
- 18.Gauduin MC, Glickman RL, Ahmad S, Yilma T, Johnson RP. Immunization with live attenuated simian immunodeficiency virus induces strong type 1 T helper responses and beta-chemokine production. Proc Natl Acad Sci USA. 1999;96:14031–14036. doi: 10.1073/pnas.96.24.14031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gauduin MC, Yu Y, Barabasz A, Carville A, Piatak M, Lifson JD, Desrosiers RC, Johnson RP. Induction of a virus-specific effector-memory CD4+ T cell response by attenuated SIV infection. J Exp Med. 2006;203:2661–2672. doi: 10.1084/jem.20060134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson RP, Glickman RL, Yang JQ, Kaur A, Dion JT, Mulligan MJ, Desrosiers RC. Induction of vigorous cytotoxic T-lymphocyte responses by live attenuated simian immunodeficiency virus. J Virol. 1997;71:7711–7718. doi: 10.1128/jvi.71.10.7711-7718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stahl-Hennig C, Steinman RM, Ten Haaft P, Uberla K, Stolte N, Saeland S, Tenner-Racz K, Racz P. The simian immunodeficiency virus deltaNef vaccine, after application to the tonsils of Rhesus macaques, replicates primarily within CD4+ T cells and elicits a local perforin-positive CD8+ T-cell response. J Virol. 2002;76:688–696. doi: 10.1128/JVI.76.2.688-696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picker LJ, Hagen SI, Lum R, Reed-Inderbitzin EF, Daly LM, Sylwester AW, Walker JM, Siess DC, Piatak M, Jr, Wang C, Allison DB, Maino VC, Lifson JD, Kodama T, Axthelm MK. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J Exp Med. 2004;200:1299–1314. doi: 10.1084/jem.20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baba TW, Liska V, Khimani AH, Ray NB, Dailey PJ, Penninck D, Bronson R, Greene MF, McClure HM, Martin LN, Ruprecht RM. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat Med. 1999;5:194–203. doi: 10.1038/5557. [DOI] [PubMed] [Google Scholar]
- 24.Baba TW, Jeong YS, Pennick D, Bronson R, Greene MF, Ruprecht RM. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- 25.Hofmann-Lehmann R, Vlasak J, Williams AL, Chenine AL, McClure HM, Anderson DC, O'Neil S, Ruprecht RM. Live attenuated, nef-deleted SIV is pathogenic in most adult macaques after prolonged observation. AIDS. 2003;17:157–166. doi: 10.1097/00002030-200301240-00004. [DOI] [PubMed] [Google Scholar]
- 26.Mansfield K, Lang SM, Gauduin MC, Sanford HB, Lifson JD, Johnson RP, Desrosiers RC. Vaccine protection by live, attenuated simian immunodeficiency virus in the absence of high-titer antibody responses and high-frequency cellular immune responses measurable in the periphery. J Virol. 2008;82:4135–4148. doi: 10.1128/JVI.00015-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdel-Motal UM, Gillis J, Manson K, Wyand M, Montefiori D, Stefano-Cole K, Montelaro RC, Altman JD, Johnson RP. Kinetics of expansion of SIV Gag-specific CD8+ T lymphocytes following challenge of vaccinated macaques. Virol. 2005;333:226–238. doi: 10.1016/j.virol.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 28.Macchia I, Gauduin MC, Kaur A, Johnson RP. Expression of CD8alpha identifies a distinct subset of effector memory CD4+ T lymphocytes. Immunol. 2006;119:232–242. doi: 10.1111/j.1365-2567.2006.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montes M, Jaensson EA, Orozco AF, Lewis DE, Corry DB. A general method for bead-enhanced quantitation by flow cytometry. J Immunol Methods. 2006;317:45–55. doi: 10.1016/j.jim.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 31.Kaur A, Hale CL, Ramanujan S, Jain RK, Johnson RP. Differential dynamics of CD4+ and CD8+ T lymphocyte proliferation and activation in acute simian immunodeficiency virus infection. J Virol. 2000;74:8413–8424. doi: 10.1128/jvi.74.18.8413-8424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rud EW, Cranage M, Yon J, Quirk J, Ogilvie L, Cook N, Webster S, Dennis M, Clarke BE. Molecular and biological characterization of simian immunodeficiency virus macaque strain 32H proviral clones containing nef size variants. J Gen Virol. 1994;75:529–543. doi: 10.1099/0022-1317-75-3-529. [DOI] [PubMed] [Google Scholar]
- 33.Whatmore AM, Cook N, Hall GA, Sharpe S, Rud EW, Cranage MP. Repair and evolution of nef in vivo modulates simian immunodeficiency virus virulence. J Virol. 1995;69:5117–5123. doi: 10.1128/jvi.69.8.5117-5123.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]