Abstract
Background
Obesity and type 2 diabetes are associated with renal dysfunction which improves after roux-en-Y gastric bypass (RYGB) surgery. We prospectively studied, during a 12-month follow-up period, changes in glomerular and tubular functions that occurred in excessively obese diabetic and non-diabetic subjects after RYGB.
Methods
The cohort included 35 patients, 54% of them with type 2 diabetes. Glomerular filtration rate (GFR) was estimated using creatinine clearance. Tubular function was studied by measuring the ratio of urinary cystatin C to urinary creatinine (UCC ratio).
Results
Baseline renal parameters, anthropometric characteristics as well as changes in body mass index following the surgical procedures were similar between the two cohorts. Creatinine clearance decreased 15% in diabetics (p = 0.02) and 21% in non-diabetics (p = 0.03), 12 months after RYGB. A significant change in GFR was seen earlier in the non-diabetics (−29% after 6 months, p = 0.003). UCC ratio underwent a significant increase at both 6 and 12 months follow-ups (p = 0.03 and 0.003, respectively) only in the diabetic group.
Conclusion
RYGB improved GFR 12 months after surgery with non-diabetics showing a higher propensity. Tubular function remained unchanged in the non-diabetic but worsening occurred in diabetic subjects. These results underscore the importance of reversal of excessive obesity prior to the onset of frank diabetes.
INTRODUCTION
Obesity, defined as a body mass index (BMI) equal to or more than 30 kg/m2,1 is becoming a major health concern in the United States of America (USA) and worldwide. Excess weight carries several health risks and decreases life expectancy.2 Obesity related co-morbidities, mainly type 2 diabetes (T2D), cardiovascular disease, dyslipidemia and hypertension are well documented.3–7
An equally important but less investigated adverse effect of obesity is impaired renal function. Obesity is associated with worsening albuminuria,8 proteinuria9,10 and end-stage renal disease.11 Additionally, autopsy and biopsy studies have associated obesity with structural changes in the kidney and increased kidney weight, which are indicative of hyperfiltration.12,13 Chagnac et al. showed that obese non-diabetic subjects (BMI >38 kg/m2) have significantly elevated (+51%) glomerular filtration rates (GFR), elevated (+31%) renal plasma flow (RPF), and increased (+13%) filtration fraction (GFR/RPF) when compared to lean controls.14 Similar observations were obtained in overweight subjects with BMI between 25 and 30 kg/m2.15 Reduction in BMI, whether induced by surgical approaches15,17 or caloric restriction16,18, results in improved kidney function.
Obesity and diabetes are two closely associated morbidities. Subjects with a BMI ≥ 30kg/m2 have an adjusted relative risk of 3.44 for diagnosed diabetes16 and more than 50% lifetime risk of developing diabetes.17 Also, 48% of patients with diabetes have a BMI ≥ 30 kg/m2.18 Moreover, diabetic nephropathy is the single most common cause of end-stage renal disease in the USA, Europe and Japan.19
Significant weight loss and reversal of T2D are common occurrences following bariatric surgery. Roux-en-Y gastric bypass (RYGB), the most commonly performed surgical approach for obesity in the USA,20 is associated with 83% reversal of diabetes through 14 years of follow-up.21
No published reports have yet evaluated prospectively the effects of diabetes on renal changes after bariatric surgery. In the current study, we tested whether the presence of diabetes preoperatively would affect the changes in renal function differently than non-diabetic obese after RYGB in two groups of matched obese subjects with and without diabetes. We assessed glomerular filtration using creatinine clearance, and renal tubular function using plasma and urinary measurements of creatinine22,23 and cystatin C. Cystatin C is a low molecular weight (13.3 kD) protein that is produced at constant rates by all nucleated cells. It is freely filtered across the glomerular membrane and is completely reabsorbed and rapidly broken down by proximal tubular cells.24 Together, they increase the accuracy for estimating renal function tests25 especially for patients with early kidney disease and T2D.26,27
METHODS AND PROCEDURES
Study Subjects
Study subjects were recruited from the Surgical Weight Loss Clinic at Vanderbilt University Medical Center (VUMC) and all had been approved for RYGB (BMI range: 38.5–69 kg/m2) and were divided into two groups that were matched for BMI and gender: (i) One group consisted of 19 subjects with established T2D and (ii) another 16 subjects were non-diabetic. The diagnosis of T2D was based on fasting blood sugar (FBS) levels of ≥ 126 mg/dl28 or already established diagnoses by the primary physician, where the patients were taking anti-diabetic medications. Hypertension was defined as either having two separate recorded blood pressure measurements ≥ 140/90 mm Hg and/or subjects receiving antihypertensive therapy. All subjects included were non-smokers. The Health Science Committee of the Institutional Review Board at VUMC approved all procedures and protocols. Written informed consent was signed by all subjects prior to participation. All studies were performed at the Vanderbilt Clinical Research Center.
Study Protocol
Renal function tests were performed in the preoperative period (within 2–3 weeks prior to the surgical procedure) and at 6 and 12 months postoperatively. Demographic and clinical (including medication list) information for all patients were updated at each follow-up visit. Simultaneous 24-h urine collections were obtained for all patients. The RYGB surgery (100–150 cm Roux limb) was performed at VUMC by one of three surgeons. All vitamin and nutritional deficiencies were corrected through strict follow-up in the Surgical Weight Loss Clinic before the patients underwent surgery. Diabetes was considered resolved if FBS levels were below 126 mg/dl with no current medical therapy for diabetes.
Measurements
(i) GFR (ml/min) was estimated using creatinine clearance that was calculated according to:29
Creatinine Clearance (ml/min) = [Urine creatinine (mg/dl) × Urine volume (ml/24h)]/[Plasma creatinine (mg/dl) × 1440 (min/24h)]
(ii) Renal tubular function was assessed using the urinary cystatin C to urinary creatinine (UCC) ratio. (iii) Waist circumference was measured at the level of the anterior superior iliac crests; hip measurement was recorded as the widest part around the buttocks region.
Samples analyses
Levels of plasma and urine creatinine were determined by colorimetric assays (VITROS® 250, Ortho-Clinical Diagnostics, Rochester, NY, USA). We assayed urine cystatin C using the Quantikine Human cystatin C immunoassay Enzyme Linked Immunosorbant Assay kit (R&D Systems, Inc., Minneapolis, MN, USA). Urine samples for albumin were measured using a turbidometric immunoassay (Beckman Coulter Unicel® DxC 800, Brea, CA, USA) and total protein with a colorimetric assay (Roche COBAS® Integra 800, Indianapolis, IN, USA). Serum glucose was measured in triplicate via the glucose oxidase method using a Beckman glucose analyzer (Beckman Instruments, Fullerton, CA, USA). Glycosylated hemoglobin (HgbA1c) was assayed with high pressure liquid chromatography (BIO-RAD®, Hercules, CA, USA).
Statistical Analyses
We analyzed the data using non-parametric statistical methods. We used the (i) Wilcoxon signed-rank test for related samples to test for differences between time points within the diabetic and non-diabetic groups, and (ii) the Mann-Whitney U test for between-group differences. All analyses were conducted using the SPSS (version 15.0, Chicago, IL, USA) software program. Summary data are reported as the mean ± standard deviation (SD). Statistical significance was determined if p values were < 0.05.
RESULTS
Anthropometric and baseline characteristics (table 1)
Table 1.
Baseline Demographics.
| Diabetes | Yes | No* |
|---|---|---|
| Number of subjects | 19 | 16 |
| Females (%) | 90 | 94 |
| Age (years) | 45 ± 9 | 42 ± 10 |
| BMI (kg/m2) | 47 ± 8 | 48 ± 8 |
| Waist circumference (cm) | 132 ± 13 | 126 ± 18 |
| Waist to hip ratio | 0.92 ± 0.13 | 0.88 ± 0.12 |
| Hypertension (%) | 63 | 44 |
| Systolic blood pressure (mm Hg) | 137 ± 19 | 126 ± 14 |
| Diastolic blood pressure (mm Hg) | 76 ± 12 | 73 ± 7 |
| Plasma creatinine (mg/dl) | 0.64 ± 0.11 | 0.72 ± 0.12 |
| Creatinine clearance (ml/min) | 155 ± 57 | 148 ± 37 |
| Urinary cystatin (µg/day) | 81 ± 58 | 71 ± 33 |
| Urinary creatinine (mg/day) | 14.7± 5.9 | 14.4 ± 4.4 |
| UCC ratio (µg/mmole) | 5.8 ± 3.1 | 5.7 ± 2.2 |
| Urinary albumin (mg/day) | 26 ± 50 | 10 ± 6 |
| Urinary protein (mg/day) | 181 ± 165 | 122 ± 53 |
BMI, body mass index. UCC, urinary cystatin C to urinary creatinine
Data expressed as mean ± SD.
Comparison of data obtained in diabetic vs. non-diabetic subjects using the non-parametric Mann-Whitney U test demonstrated no significance in any of the parameters tested.
We studied 35 morbidly obese subjects with 19 (54%) were diabetic and 16 (46%) were matched non-diabetics. The average BMI was 47 ± 8 kg/m2 for diabetics and 48 ± 8 kg/m2 for non-diabetics, respectively (p = 0.4). The majority of the patients were females (90% and 94% for diabetics and non-diabetics, respectively) reflective of the general population of obese subjects requiring bariatric surgery.20 Most (77%) of the patients were Caucasians.
The baseline measurements for BMI, waist circumference, waist-to-hip ratio, systolic and diastolic blood pressures, plasma creatinine, creatinine clearance, plasma cystatin C, urinary cystatin C, urinary creatinine, the UCC ratio, urinary albumin and protein were comparable between diabetic and non-diabetic subjects; no significant difference was found for any of the parameters studied (table 1). Nearly 63% of the diabetic subjects and 44% of non-diabetic subjects were considered hypertensive. Hypertension had no effect on the increase in UCC ratios in the diabetics.
Changes in BMI and diabetes after RYGB
As shown in table 2, BMI decreased significantly at each follow-up visit in both groups. After 12 months, BMI diminished 33% in the diabetics (p < 0.001) and 35% in the non-diabetics (p < 0.001). No noticeable difference was observed between diabetics and non-diabetics at baseline, 6 months and 12 months, respectively.
Table 2.
BMI and renal parameters after RYGB.
| Diabetes | Baseline | 6 months | 12 months | |
|---|---|---|---|---|
| BMI (kg/m2) | YES | 47 ± 8 | 35 ± 6*† | 32 ± 6‡ |
| NO | 48 ± 8 | 35 ± 7*† | 31 ± 6‡ | |
| Plasma creatinine (mg/dl) |
YES | 0.64 ± 0.11 | 0.63 ± 0.09 | 0.63 ± 0.09 |
| NO | 0.72 ± 0.12 | 0.68 ± 0.15 | 0.71 ± 0.11 | |
| Urinary cystatin (µg/day) |
YES | 81 ± 58 | 90 ± 35 | 83 ± 40 |
| NO | 71 ± 33 | 55 ± 26 | 75 ± 31 | |
| Urinary creatinine (mg/day) |
YES | 14.7± 5.9 | 10.9 ± 4.9* | 12 ± 3.4‡ |
| NO | 14.4 ± 4.4 | 9.6 ± 4.2*† | 12.4 ± 3.6 | |
| Urinary albumin (mg/day) |
YES | 26 ± 50 | 18 ± 33 | 15 ± 29 |
| NO | 10 ± 6 | 5 ± 2*† | 14 ± 20 | |
| Urinary protein (mg/day) |
YES | 181 ± 165 | 109 ± 68 | 133 ± 67 |
| NO | 122 ± 53 | 80 ± 39† | 125 ± 58 |
BMI, body mass index. RYGB, Roux-en-Y gastric bypass.
Data expressed as mean ± SD.
p<0.05, baseline vs. 6 months;
p<0.05, 6 months vs. 12 months;
p<0.05, baseline vs. 12 months. (nonparametric, Wilcoxon signed-rank test)
Diabetes resolved in 16/19 patients, resulting in 84% resolution rate 12 months after RYGB. Of the 19 patients with diabetes at baseline, 16 were taking oral medications, one patient was on insulin and 2 patients were diet-controlled. The mean FBS in the diabetics decreased from 149 ± 48 mg/dl in the preoperative period to 102 ± 33 mg/dl at 12 months (p < 0.001). Likewise, plasma HgbA1c levels decreased from preoperative values of 6.7 ± 0.8% to 5.9 ± 0.9% at 12-months postoperatively (p = 0.002).
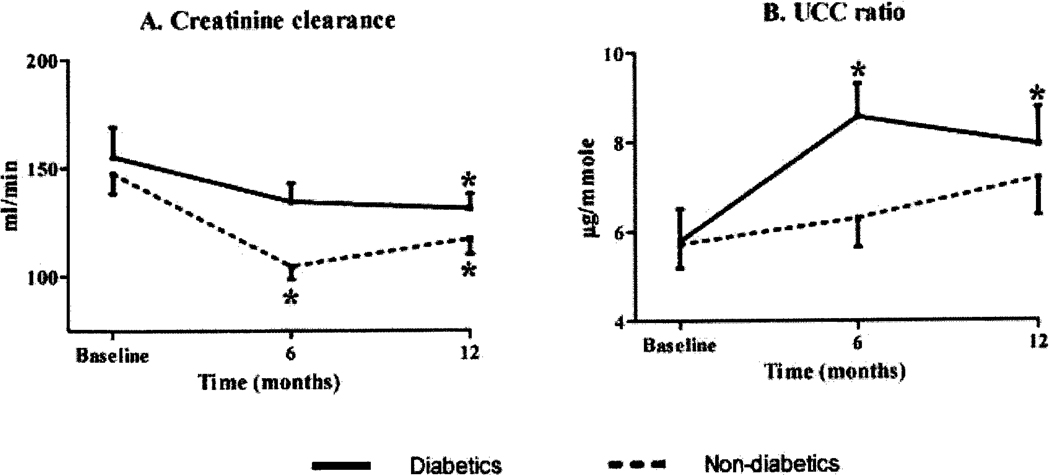
Changes in renal function after RYGB (figure 1 and table 2)
Figure 1.
Comparison of the changes in creatinine clearance (panel A) and UCC ratio (panel B) seen 6 and 12 months after RYGB between diabetic (straight line) and non-diabetic obese subjects (dotted line). *, p<0.05 compared to baseline within each group.
GFR was estimated using creatinine clearance. We noted a significant drop in creatinine clearance in both groups after surgery. In the diabetics, there was a 15% drop in creatinine clearance from baseline to 12 months (p = 0.02), while there was a 21% decrease in the non-diabetics for the same time period (p = 0.03); parenthetically, the drop in the diabetics was more prominent at 6-months postoperatively (−29%, p = 0.003), and this accounted for the significant differences between the two groups (p = 0.02) seen at this time point.
Estimates of renal tubular function were obtained from measurements of the UCC ratios. As shown in table 2 and figure 1B, the UCC ratio increased significantly only in the diabetic group (p < 0.05). There was no significant change in the urinary levels of cystatin C in both groups.
The averages of baseline albuminuria were normal for diabetics and non-diabetics (< 30mg/day), indicating minimal nephropathy. For urinary protein the diabetic group had a baseline value of 181 ± 165 mg/day considered higher than the upper limit for normal individuals (<150 mg/day). RYGB resulted in significant drop in both urinary albumin (p = 0.009) and protein (p < 0.05) excretions in the non-diabetic group within the first six months postoperatively, but these rebounded such that the levels at 12 months were not significantly different from baseline, nor were they different from those obtained in the diabetic group. Though a biologically/clinically relevant decrease in urinary protein was seen for diabetics (from 181 ± 165 mg/day at baseline to 133 ± 67 mg/day after 12 months), this did not reach statistical significance (p=0.2).
DISCUSSION
This longitudinal study confirms some of the previous findings of altered kidney function in the obese population. In particular, our obese subjects with minimal apparent nephropathy (as evidenced by minimal albuminuria and proteinuria) have glomerular and tubular abnormalities. RYGB resulted in significant improvements in renal hyperfiltration in both diabetics and non-diabetics. Non-diabetics manifested significant improvements in GFR within 6 months, while the improvements in the diabetics did not occur until 12 months after RYGB. RYGB was associated with no change of the renal tubular function in the non-diabetic subject, while renal tubular dysfunction in the diabetics continued to worsen after RYGB.
The nature of kidney damage in patients with T2D remains unclear. Histopathological lesions are known to appear long before any disturbance in clinical parameters occur.30 Nephropathy lesions for T2D have been described to be more heterogeneous and consist of three types: (a) typical glomerular lesions as seen in type 1 diabetes; (b) less glomerular lesions but significant tubule-interstitial fibrosis and atrophy; and (c) glomerular disease on top of type 1 diabetic glomerulosclerosis.31 Patients with associated microalbuminuria (30 mg/dl <24-h urinary albumin<300 mg/dl) tend to have higher proportion (nearly 41%) of severe tubule-interstitial disease.32 Findings from the current study supported by observations in the literature, suggest that RYGB results in significant improvements in renal hyperfiltration. In a cross-sectional study, Navarro-Diaz et al. demonstrated significant improvements in creatinine clearance mostly during the first year after RYGB, with little additional improvement by 24 months;33 their cohort, however, did not include diabetic patients. Serra et al. reported a significant improvement in hyperfiltration 12 months after RYGB; diabetes was present in only 17% of their study group.34 Additionally, the findings from the current study indicate that the improvements in GFR, though seen in both groups, occurred faster in the non-diabetics (within 6 months) and normalized after 12 months (117 ± 29 ml/min). After one year, creatinine clearance levels in the diabetics remained higher (131 ± 29 ml/min) than the normal limit for GFR (≤120 ml/min)
In our study, we estimated changes in tubular function using the UCC ratio, which requires measurements of Cystatin C. Uchida et al. studied 1,887 individuals including healthy adults and patients with chronic renal failure and found the UCC ratio to be a good marker of renal tubular dysfunction.35 A high UCC ratio was found to correlate with tubule-interstitial disease and with heavy proteinuria.36 In our study the diabetic subjects had significantly higher increases in the UCC ratio suggesting that the presence of tubular dysfunction in the diabetics persists and/or continues to worsen following RYGB. More basic research and longer follow-up needs to be undertaken to better define the level of tubular dysfunction associated with obesity and T2D.
In summary, obese subjects manifest significant alteration in glomerular and tubular functions and these occur with minimal evidence of nephropathy. RYGB leads to significant improvement in renal function in morbidly obese subjects. In non-diabetics renal defects, primarily glomerular, normalize with RYGB. Contrastingly, in diabetics, the renal defects are both glomerular and tubular in nature; RYGB improves but does not normalize the glomerular defects while the renal tubular defects continue to worsen. Therefore, the regular assessment of renal function in morbidly obese subjects may prompt early surgical intervention.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (NIH) grant NIDDK 5R01DK70860. Support was provided in part by the Vanderbilt Clinical Research Center grant (M01 RR 00095 NCRR/NIH, CTSA grant 1 UL1 RR024975), the Diabetes Center Grant (DK20593) and the Digestive Diseases Research Center grant (DK058404).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. Geneva: World Health Organization; Obesity : preventing and managing the global epidemic : report of a WHO consultation. 2000 [PubMed]
- 2.Lubetkin EI, Jia H. Health-Related Quality of Life, Quality-Adjusted Life Years, and Quality-Adjusted Life Expectancy in New York City from 1995 to 2006. J Urban Health. 2009 doi: 10.1007/s11524-009-9344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sowers JR. Obesity as a cardiovascular risk factor. Am J Med. 2003;115 Suppl 8A:37S–41S. doi: 10.1016/j.amjmed.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Hall JE, Louis K. Dahl Memorial Lecture. Renal and cardiovascular mechanisms of hypertension in obesity. Hypertension. 1994;23:381–394. doi: 10.1161/01.hyp.23.3.381. [DOI] [PubMed] [Google Scholar]
- 5.Herrara MF, Lozano-Salazar RR, Gonzalez-Barranco J, Rull JA. Diseases and problems secondary to massive obesity. Eur J Gastroenterol Hepatol. 1999;11:63–67. doi: 10.1097/00042737-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. Jama. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 7.Garrison RJ, Kannel WB, Stokes J, 3rd, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med. 1987;16:235–251. doi: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 8.Ferris M, Hogan SL, Chin H, Shoham DA, Gipson DS, Gibson K, et al. Obesity, albuminuria, and urinalysis findings in US young adults from the Add Health Wave III study. Clin J Am Soc Nephrol. 2007;2:1207–1214. doi: 10.2215/CJN.00540107. [DOI] [PubMed] [Google Scholar]
- 9.Weisinger JR, Kempson RL, Eldridge FL, Swenson RS. The nephrotic syndrome: a complication of massive obesity. Ann Intern Med. 1974;81:440–447. doi: 10.7326/0003-4819-81-4-440. [DOI] [PubMed] [Google Scholar]
- 10.Praga M, Hernandez E, Herrero JC, Morales E, Revilla Y, Diaz-Gonzalez R, et al. Influence of obesity on the appearance of proteinuria and renal insufficiency after unilateral nephrectomy. Kidney Int. 2000;58:2111–2118. doi: 10.1111/j.1523-1755.2000.00384.x. [DOI] [PubMed] [Google Scholar]
- 11.Stengel B, Tarver-Carr ME, Powe NR, Eberhardt MS, Brancati FL. Lifestyle factors, obesity and the risk of chronic kidney disease. Epidemiology. 2003;14:479–487. doi: 10.1097/01.EDE.0000071413.55296.c4. [DOI] [PubMed] [Google Scholar]
- 12.Kasiske BL, Napier J. Glomerular sclerosis in patients with massive obesity. Am J Nephrol. 1985;5:45–50. doi: 10.1159/000166902. [DOI] [PubMed] [Google Scholar]
- 13.Kasiske BL, Crosson JT. Renal disease in patients with massive obesity. Arch Intern Med. 1986;146:1105–1109. [PubMed] [Google Scholar]
- 14.Chagnac A, Weinstein T, Korzets A, Ramadan E, Hirsch J, Gafter U. Glomerular hemodynamics in severe obesity. Am J Physiol Renal Physiol. 2000;278:F817–F822. doi: 10.1152/ajprenal.2000.278.5.F817. [DOI] [PubMed] [Google Scholar]
- 15.Bosma RJ, van der Heide JJ, Oosterop EJ, de Jong PE, Navis G. Body mass index is associated with altered renal hemodynamics in non-obese healthy subjects. Kidney Int. 2004;65:259–265. doi: 10.1111/j.1523-1755.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- 16.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. Jama. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 17.Narayan KM, Boyle JP, Thompson TJ, Gregg EW, Williamson DF. Effect of BMI on lifetime risk for diabetes in the U.S. Diabetes Care. 2007;30:1562–1566. doi: 10.2337/dc06-2544. [DOI] [PubMed] [Google Scholar]
- 18.Jia H, Lubetkin EI. The impact of obesity on health-related quality-of-life in the general adult US population. J Public Health (Oxf) 2005;27:156–164. doi: 10.1093/pubmed/fdi025. [DOI] [PubMed] [Google Scholar]
- 19.Parving H, Mauer M, Ritz E. Diabetic nephropathy. In: Brenner B, editor. Brenner & Rector's THE KIDNEY. Philadelphia: SAUNDERS ELSEVIER; 2008. pp. 1265–1290. [Google Scholar]
- 20.Samuel I, Mason EE, Renquist KE, Huang YH, Zimmerman MB, Jamal M. Bariatric surgery trends: an 18-year report from the International Bariatric Surgery Registry. Am J Surg. 2006;192:657–662. doi: 10.1016/j.amjsurg.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Pories WJ, Albrecht RJ. Etiology of type II diabetes mellitus: role of the foregut. World J Surg. 2001;25:527–531. doi: 10.1007/s002680020348. [DOI] [PubMed] [Google Scholar]
- 22.Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van Lente F, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39:920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 23.Mahajan S, Mukhiya GK, Singh R, Tiwari SC, Kalra V, Bhowmik DM, et al. Assessing glomerular filtration rate in healthy Indian adults: a comparison of various prediction equations. J Nephrol. 2005;18:257–261. [PubMed] [Google Scholar]
- 24.Tenstad O, Roald AB, Grubb A, Aukland K. Renal handling of radiolabelled human cystatin C in the rat. Scand J Clin Lab Invest. 1996;56:409–414. doi: 10.3109/00365519609088795. [DOI] [PubMed] [Google Scholar]
- 25.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia LH, Bing XG, An XT. Serum cystatin C assay for the detection of early renal impairment in diabetic patients. J Clin Lab Anal. 2004;18:31–35. doi: 10.1002/jcla.20005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller TF, Raeder J, Oettl K, Zitta S, Klausmann G, Estelberger W, et al. Cystatin C does not detect acute changes in glomerular filtration rate in early diabetic nephropathy. Ren Fail. 2008;30:21–29. doi: 10.1080/08860220701741916. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Geneva: World Health Organization; International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia : report of a WHO/IDF consultation.
- 29.Aydin F, Gungor F, Cengiz AK, Tuncer M, Mahsereci E, Ozdem S, et al. Comparison of glomerular filtration rate measurements with the two plasma sample and single plasma sample, gamma camera Gates, creatinine clearance, and prediction equation methods in potential kidney donors with normal renal function. Nucl Med Commun. 2008;29:157–165. doi: 10.1097/MNM.0b013e3282f1bbde. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki D, Takano H, Toyoda M, Umezono T, Uehara G, Sakai T, et al. Evaluation of renal biopsy samples of patients with diabetic nephropathy. Intern Med. 2001;40:1077–1084. doi: 10.2169/internalmedicine.40.1077. [DOI] [PubMed] [Google Scholar]
- 31.Gambara V, Mecca G, Remuzzi G, Bertani T. Heterogeneous nature of renal lesions in type II diabetes. J Am Soc Nephrol. 1993;3:1458–1466. doi: 10.1681/ASN.V381458. [DOI] [PubMed] [Google Scholar]
- 32.Fioretto P, Mauer M, Brocco E, Velussi M, Frigato F, Muollo B, et al. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia. 1996;39:1569–1576. doi: 10.1007/s001250050616. [DOI] [PubMed] [Google Scholar]
- 33.Navarro-Diaz M, Serra A, Romero R, Bonet J, Bayes B, Homs M, et al. Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J Am Soc Nephrol. 2006;17:S213–S217. doi: 10.1681/ASN.2006080917. [DOI] [PubMed] [Google Scholar]
- 34.Serra A, Granada ML, Romero R, Bayes B, Canton A, Bonet J, et al. The effect of bariatric surgery on adipocytokines, renal parameters and other cardiovascular risk factors in severe and very severe obesity: 1-year follow-up. Clin Nutr. 2006;25:400–408. doi: 10.1016/j.clnu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Uchida K, Gotoh A. Measurement of cystatin-C and creatinine in urine. Clin Chim Acta. 2002;323:121–128. doi: 10.1016/s0009-8981(02)00177-8. [DOI] [PubMed] [Google Scholar]
- 36.Herget-Rosenthal S, van Wijk JA, Brocker-Preuss M, Bokenkamp A. Increased urinary cystatin C reflects structural and functional renal tubular impairment independent of glomerular filtration rate. Clin Biochem. 2007;40:946–951. doi: 10.1016/j.clinbiochem.2007.04.013. [DOI] [PubMed] [Google Scholar]