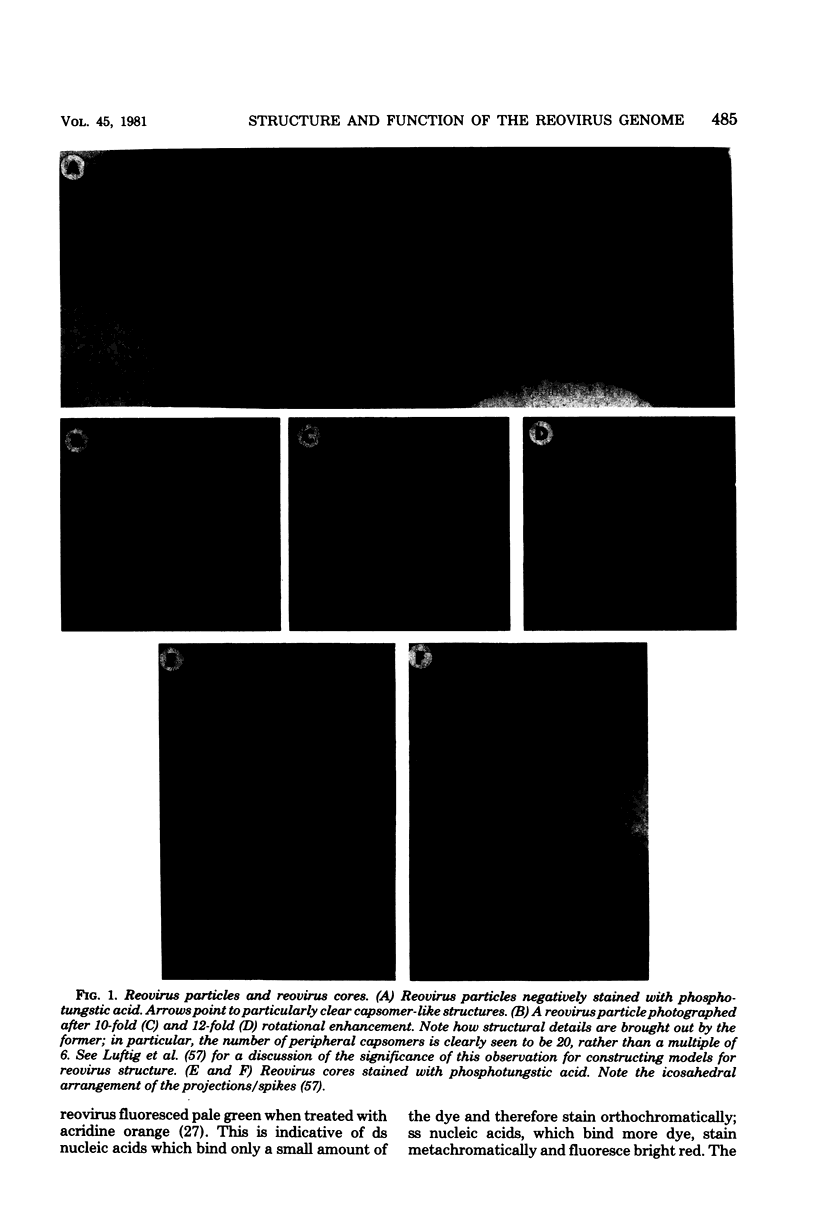
Full text
PDF




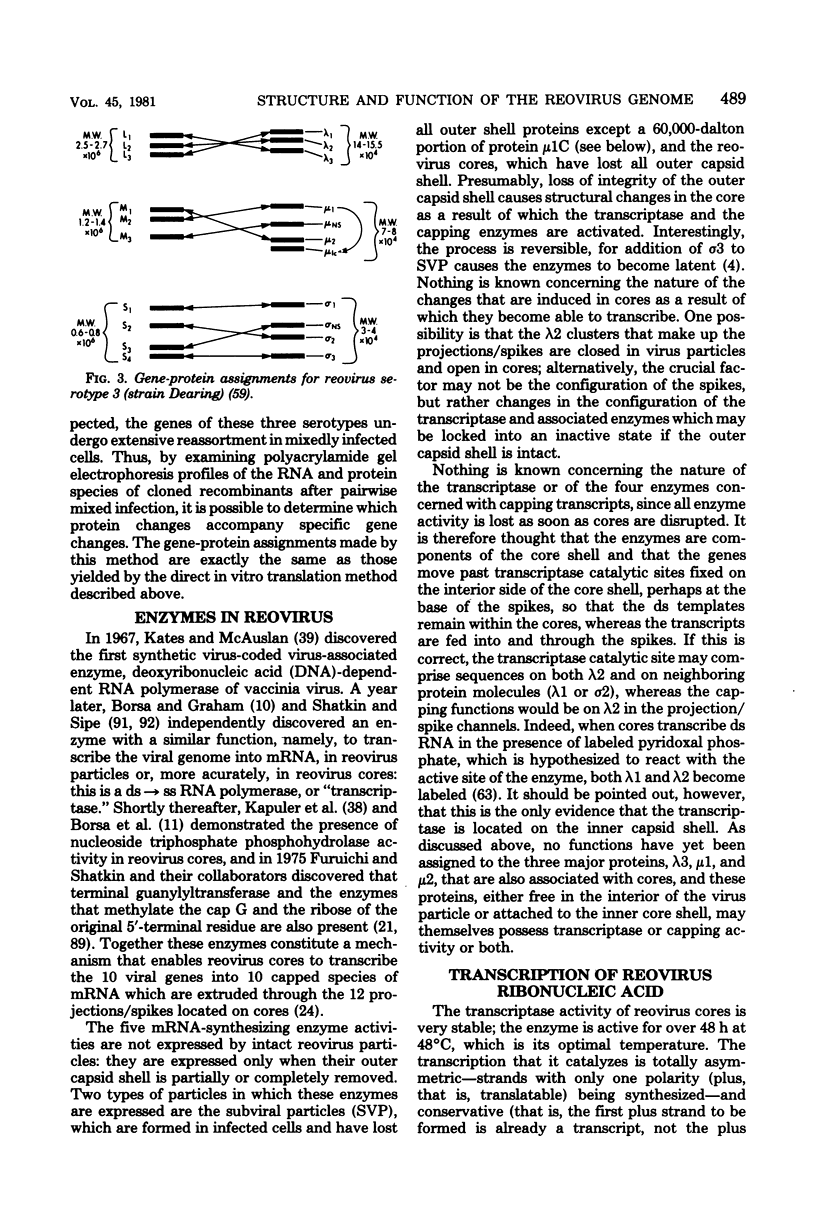

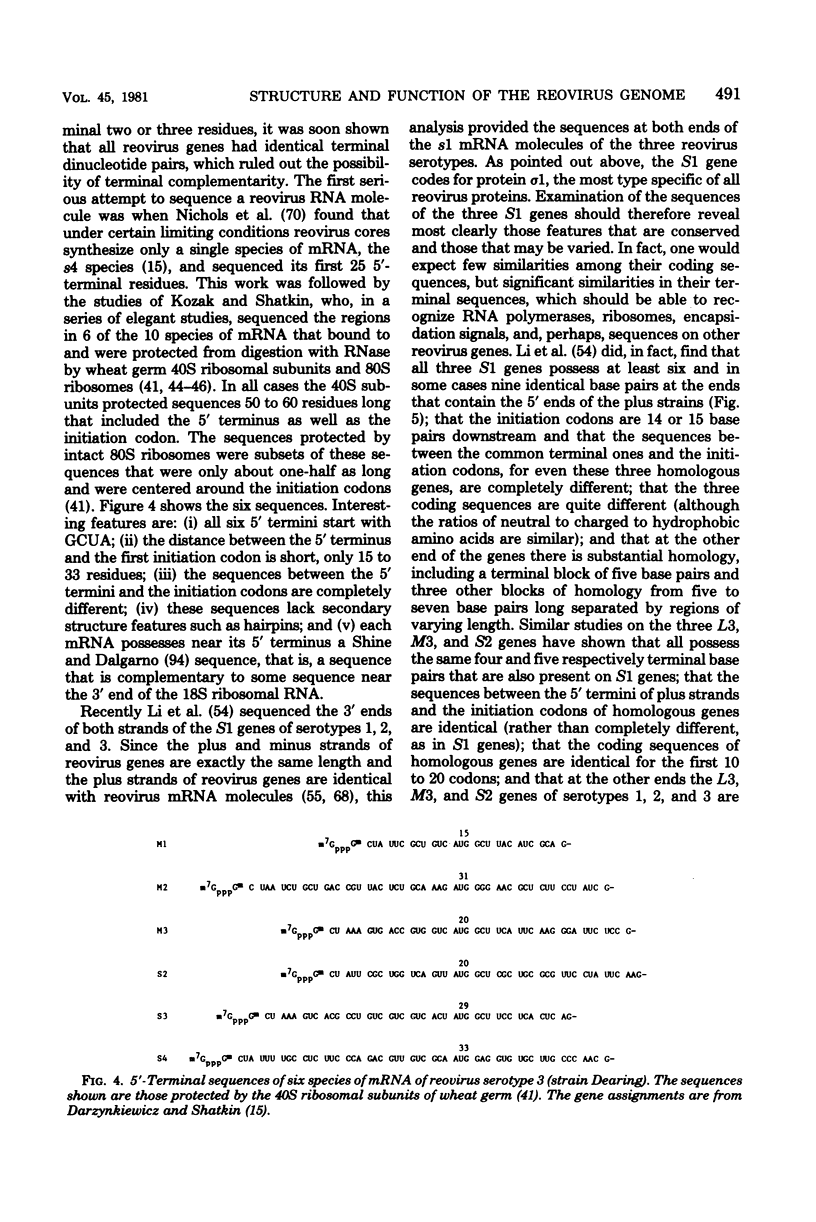
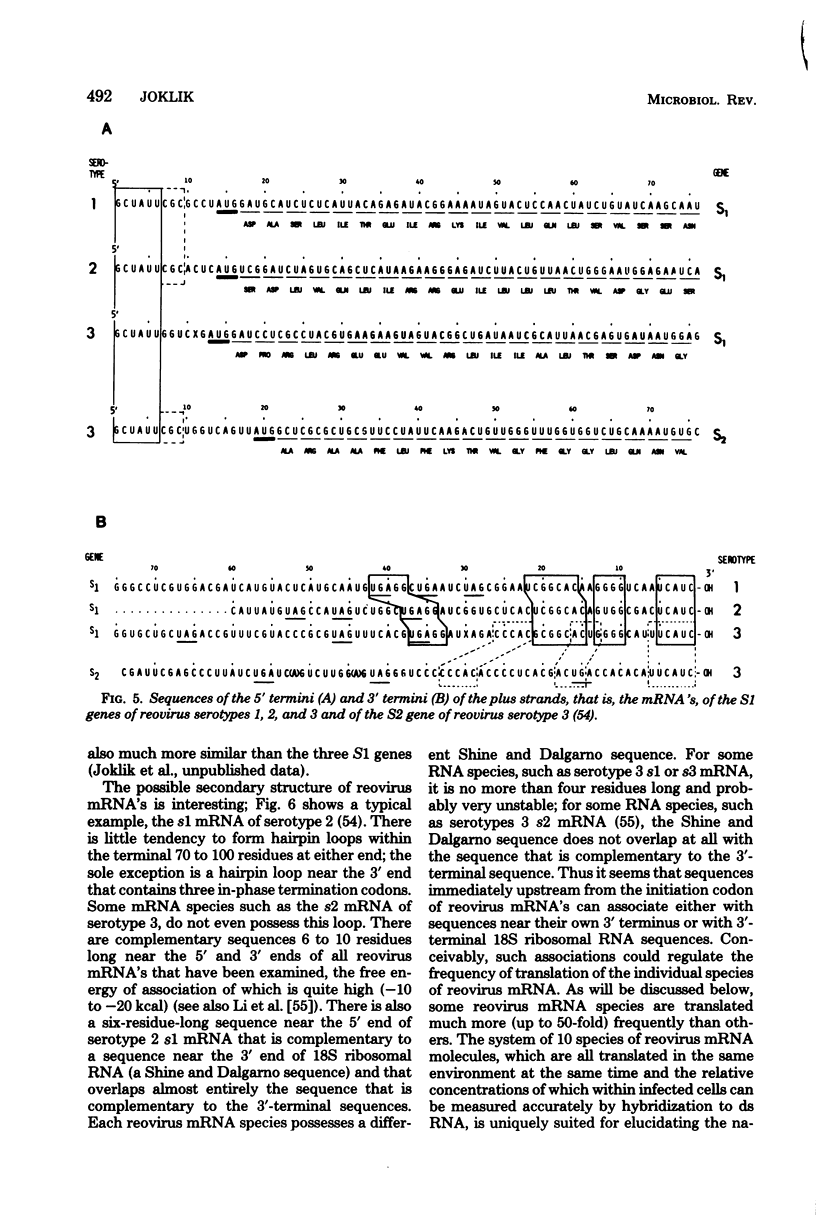
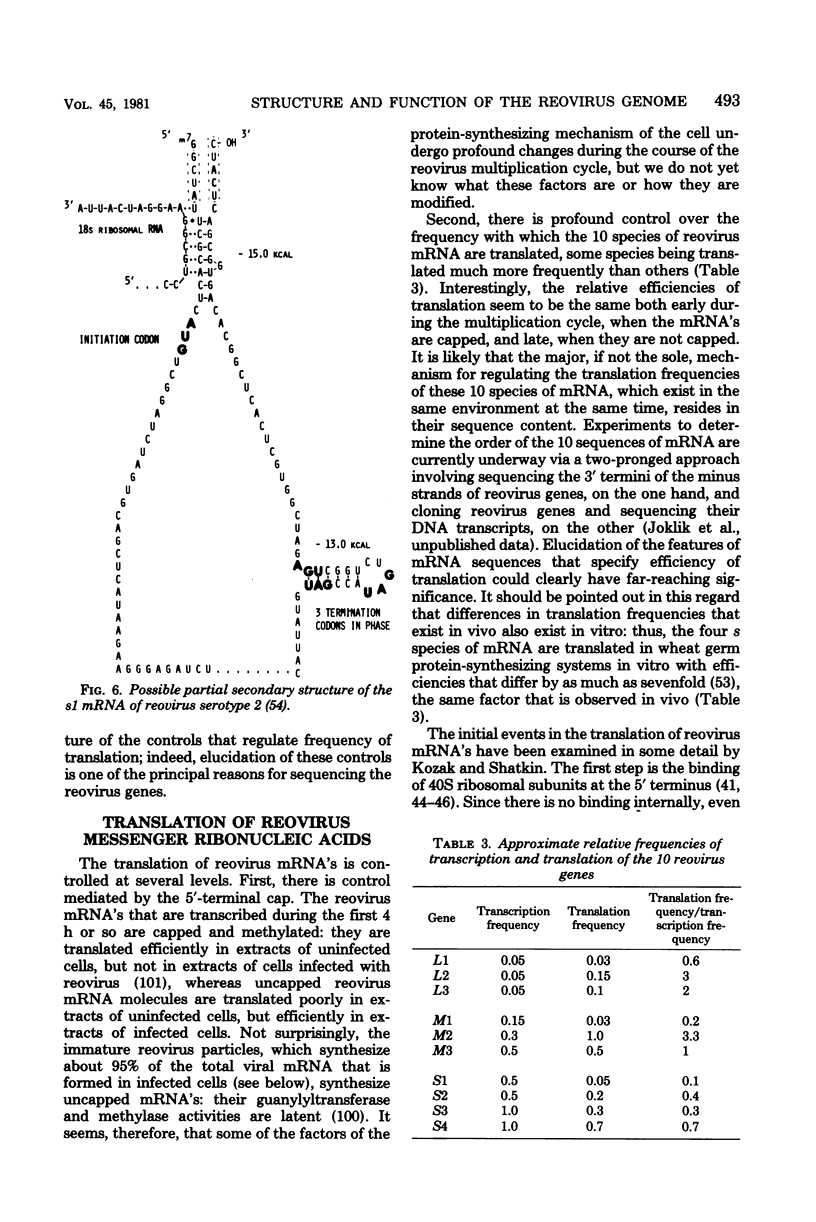
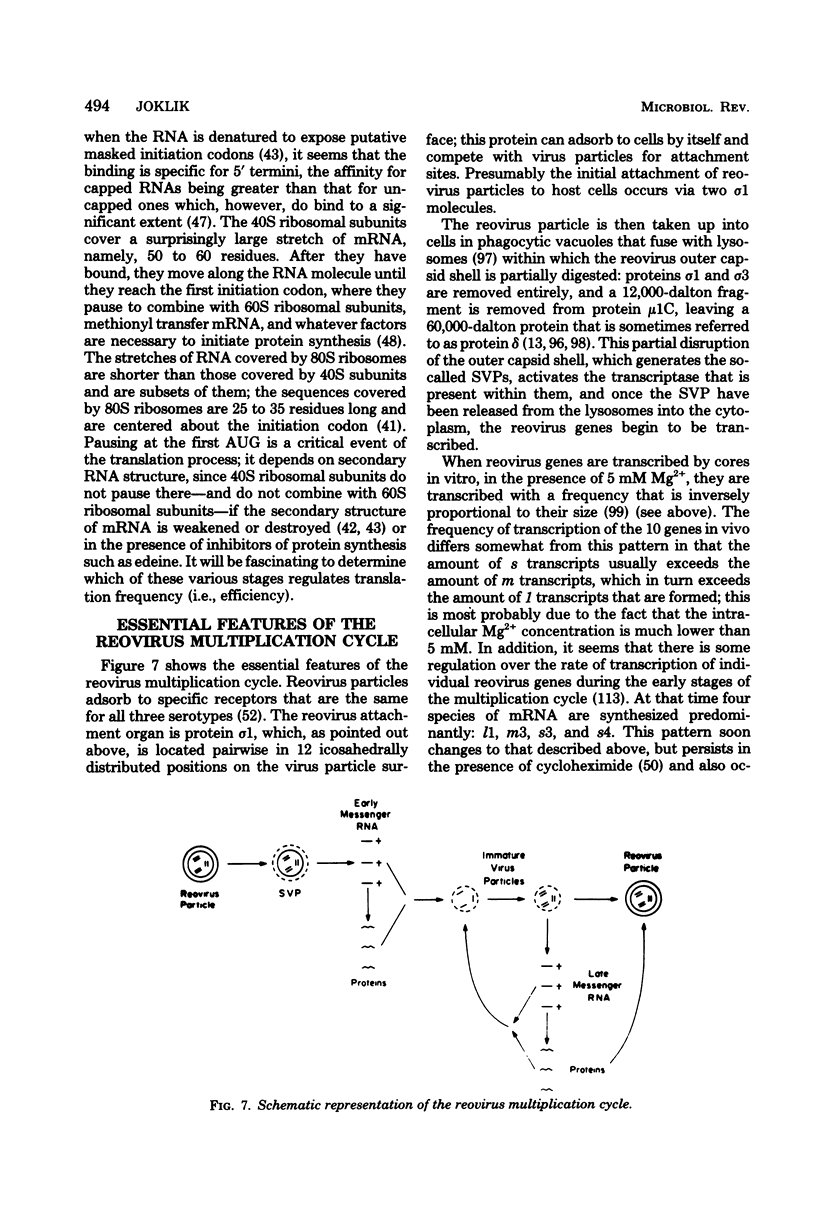







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acs G., Klett H., Schonberg M., Christman J., Levin D. H., Silverstein S. C. Mechanism of reovirus double-stranded ribonucleic acid synthesis in vivo and in vitro. J Virol. 1971 Nov;8(5):684–689. doi: 10.1128/jvi.8.5.684-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Graham A. F. Persistent infections in L cells with temperature-sensitive mutants of reovirus. J Virol. 1977 Aug;23(2):250–262. doi: 10.1128/jvi.23.2.250-262.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott S., Hutchinson F., Spencer M., Wilkins M. H., Fuller W., Langridge R. X-ray diffraction studies of double helical ribonucleic acid. Nature. 1966 Jul 16;211(5046):227–232. doi: 10.1038/211227a0. [DOI] [PubMed] [Google Scholar]
- Astell C., Silverstein S. C., Levin D. H., Acs G. Regulation of the reovirus RNA transcriptase by a viral capsomere protein. Virology. 1972 Jun;48(3):648–654. doi: 10.1016/0042-6822(72)90149-3. [DOI] [PubMed] [Google Scholar]
- Babiss L. E., Luftig R. B., Weatherbee J. A., Weihing R. R., Ray U. R., Fields B. N. Reovirus serotypes 1 and 3 differ in their in vitro association with microtubules. J Virol. 1979 Jun;30(3):863–874. doi: 10.1128/jvi.30.3.863-874.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy A. R., Joklik W. K. Studies on reovirus RNA. II. Characterization of reovirus messenger RNA and of the genome RNA segments from which it is transcribed. J Mol Biol. 1967 Oct 14;29(1):19–26. doi: 10.1016/0022-2836(67)90178-7. [DOI] [PubMed] [Google Scholar]
- Bellamy A. R., Joklik W. K. Studies on the A-rich RNA of reovirus. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1389–1395. doi: 10.1073/pnas.58.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy A. R., Nichols J. L., Joklik W. K. Nucleotide sequences of reovirus oligonucleotides: evidence for abortive RNA synthesis during virus maturation. Nat New Biol. 1972 Jul 12;238(80):49–51. doi: 10.1038/newbio238049a0. [DOI] [PubMed] [Google Scholar]
- Bellamy A. R., Shapiro L., August J. T., Joklik W. K. Studies on reovirus RNA. I. Characterization of reovirus genome RNA. J Mol Biol. 1967 Oct 14;29(1):1–17. doi: 10.1016/0022-2836(67)90177-5. [DOI] [PubMed] [Google Scholar]
- Borsa J., Graham A. F. Reovirus: RNA polymerase activity in purified virions. Biochem Biophys Res Commun. 1968 Dec 30;33(6):895–901. doi: 10.1016/0006-291x(68)90396-3. [DOI] [PubMed] [Google Scholar]
- Borsa J., Grover J., Chapman J. D. Presence of nucleoside triphosphate phosphohydrolase activity in purified virions of reovirus. J Virol. 1970 Sep;6(3):295–302. doi: 10.1128/jvi.6.3.295-302.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Lavi S., Shatkin A. J. Synthesis of all the gene products of the reovirus genome in vivo and in vitro. Cell. 1975 Feb;4(2):173–180. doi: 10.1016/0092-8674(75)90124-5. [DOI] [PubMed] [Google Scholar]
- Chang C. T., Zweerink H. J. Fate of parental reovirus in infected cell. Virology. 1971 Dec;46(3):544–555. doi: 10.1016/0042-6822(71)90058-4. [DOI] [PubMed] [Google Scholar]
- Cross R. K., Fields B. N. Temperature-sensitive mutants of reovirus type 3: studies on the synthesis of viral RNA. Virology. 1972 Dec;50(3):799–809. doi: 10.1016/0042-6822(72)90434-5. [DOI] [PubMed] [Google Scholar]
- DIENER T. O. Use of snake venom phosphodiesterase in place of pancreatic ribonuclease for the testing of nucleic acid preparations from tobacco mosaic virus. Nature. 1961 Feb 25;189:687–688. doi: 10.1038/189687b0. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz E., Shatkin A. J. Assignment of reovirus mRNA ribosome binding sites to virion genome segments by nucleotide sequence analyses. Nucleic Acids Res. 1980 Jan 25;8(2):337–350. doi: 10.1093/nar/8.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B. N., Joklik W. K. Isolation and preliminary genetic and biochemical characterization of temperature-sensitive mutants of reovirus. Virology. 1969 Mar;37(3):335–342. doi: 10.1016/0042-6822(69)90217-7. [DOI] [PubMed] [Google Scholar]
- Finberg R., Weiner H. L., Fields B. N., Benacerraf B., Burakoff S. J. Generation of cytolytic T lymphocytes after reovirus infection: role of S1 gene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):442–446. doi: 10.1073/pnas.76.1.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A., Weiner H. L. Interaction of reovirus with cell surface receptors. II. Generation of suppressor T cells by the hemagglutinin of reovirus type 3. J Immunol. 1980 Dec;125(6):2660–2664. [PubMed] [Google Scholar]
- Furuichi Y. Allosteric stimulatory effect of S-adenosylmethionine on the RNA polymerase in cytoplasmic polyhedrosis virus. A model for the positive control of eukaryotic transcription. J Biol Chem. 1981 Jan 10;256(1):483–493. [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Muthukrishnan S., Shatkin A. J. Reovirus messenger RNA contains a methylated, blocked 5'-terminal structure: m-7G(5')ppp(5')G-MpCp-. Proc Natl Acad Sci U S A. 1975 Jan;72(1):362–366. doi: 10.1073/pnas.72.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Shatkin A. J. 5'-termini of reovirus mRNA: ability of viral cores to form caps post-transcriptionally. Virology. 1977 Apr;77(2):566–578. doi: 10.1016/0042-6822(77)90482-2. [DOI] [PubMed] [Google Scholar]
- GOMATOS P. J., TAMM I., DALES S., FRANKLIN R. M. Reovirus type 3: physical characteristics and interaction with L cells. Virology. 1962 Jul;17:441–454. doi: 10.1016/0042-6822(62)90139-3. [DOI] [PubMed] [Google Scholar]
- Gaillard R. K., Joklik W. K. The antigenic determinants of most of the proteins coded by the three serotypes of reovirus are highly conserved during evolution. Virology. 1980 Dec;107(2):533–536. doi: 10.1016/0042-6822(80)90321-9. [DOI] [PubMed] [Google Scholar]
- Gillies S., Bullivant S., Bellamy A. R. Viral RNA polymerases: electron microscopy of reovirus reaction cores. Science. 1971 Nov 12;174(4010):694–696. doi: 10.1126/science.174.4010.694. [DOI] [PubMed] [Google Scholar]
- Gomatos P. J. RNA synthesis in reovirus-infected L929 mouse fibroblasts. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1798–1805. doi: 10.1073/pnas.58.4.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomatos P. J., Tamm I. THE SECONDARY STRUCTURE OF REOVIRUS RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):707–714. doi: 10.1073/pnas.49.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene M. I., Weiner H. L. Delayed hypersensitivity in mice infected with reovirus. II. Induction of tolerance and suppressor T cells to viral specific gene products. J Immunol. 1980 Jul;125(1):283–287. [PubMed] [Google Scholar]
- Harvey J. D., Bellamy A. R., Earnshaw W. C., Schutt C. Biophysical studies of reovirus type 3. IV. Low-angle x-ray diffraction studies. Virology. 1981 Jul 15;112(1):240–249. doi: 10.1016/0042-6822(81)90629-2. [DOI] [PubMed] [Google Scholar]
- Hayes E. C., Lee P. W., Miller S. E., Joklik W. K. The interaction of a series of hybridoma IgGs with reovirus particles. Demonstration that the core protein lambda 2 is exposed on the particle surface. Virology. 1981 Jan 15;108(1):147–155. doi: 10.1016/0042-6822(81)90534-1. [DOI] [PubMed] [Google Scholar]
- Huismans H., Joklik W. K. Reovirus-coded polypeptides in infected cells: isolation of two native monomeric polypeptides with affinity for single-stranded and double-stranded RNA, respectively. Virology. 1976 Apr;70(2):411–424. doi: 10.1016/0042-6822(76)90282-8. [DOI] [PubMed] [Google Scholar]
- Iglewski W. J., Franklin R. M. Purification and properties of reovirus ribonucleic acid. J Virol. 1967 Apr;1(2):302–307. doi: 10.1128/jvi.1.2.302-307.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Joklik W. K. Temperature-sensitive mutants of reovirus. 3. Evidence that mutants of group D ("RNA-negative") are structural polypeptide mutants. Virology. 1972 Oct;50(1):282–286. doi: 10.1016/0042-6822(72)90373-x. [DOI] [PubMed] [Google Scholar]
- Ito Y., Joklik W. K. Temperature-sensitive mutants of reovirus. II. Anomalous electrophoretic migration of certain hybrid RNA molecules composed of mutant plus strands and wild-type minus strands. Virology. 1972 Oct;50(1):202–208. doi: 10.1016/0042-6822(72)90360-1. [DOI] [PubMed] [Google Scholar]
- Johnson R. B., Jr, Soeiro R., Fields B. N. The synthesis of A-rich RNA by temperature-sensitive mutants of reovirus. Virology. 1976 Aug;73(1):173–180. doi: 10.1016/0042-6822(76)90071-4. [DOI] [PubMed] [Google Scholar]
- Joklik W. K. Studies on the effect of chymotrypsin on reovirions. Virology. 1972 Sep;49(3):700–715. doi: 10.1016/0042-6822(72)90527-2. [DOI] [PubMed] [Google Scholar]
- Kapuler A. M., Mendelsohn N., Klett H., Acs G. Four base-specific nucleoside 5'-triphosphatases in the subviral core of reovirus. Nature. 1970 Mar 28;225(5239):1209–1213. doi: 10.1038/2251209a0. [DOI] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Messenger RNA synthesis by a "coated" viral genome. Proc Natl Acad Sci U S A. 1967 Feb;57(2):314–320. doi: 10.1073/pnas.57.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavenoff R., Talcove D., Mudd J. A. Genome-sized RNA from reovirus particles. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4317–4321. doi: 10.1073/pnas.72.11.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Binding of wheat germ ribosomes to bisulfite-modified reovirus messenger RNA: evidence for a scanning mechanism. J Mol Biol. 1980 Dec 15;144(3):291–304. doi: 10.1016/0022-2836(80)90092-3. [DOI] [PubMed] [Google Scholar]
- Kozak M. Influence of mRNA secondary structure on binding and migration of 40S ribosomal subunits. Cell. 1980 Jan;19(1):79–90. doi: 10.1016/0092-8674(80)90390-6. [DOI] [PubMed] [Google Scholar]
- Kozak M. Nucleotide sequences of 5'-terminal ribosome-protected initiation regions from two reovirus messages. Nature. 1977 Sep 29;269(5627):391–394. doi: 10.1038/269390a0. [DOI] [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Characterization of ribosome-protected fragments from reovirus messenger RNA. J Biol Chem. 1976 Jul 25;251(14):4259–4266. [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Identification of features in 5' terminal fragments from reovirus mRNA which are important for ribosome binding. Cell. 1978 Jan;13(1):201–212. doi: 10.1016/0092-8674(78)90150-2. [DOI] [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Migration of 40 S ribosomal subunits on messenger RNA in the presence of edeine. J Biol Chem. 1978 Sep 25;253(18):6568–6577. [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Sequences and properties of two ribosome binding sites from the small size class of reovirus messenger RNA. J Biol Chem. 1977 Oct 10;252(19):6895–6908. [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Sequences of two 5'-terminal ribosome-protected fragments from reovirus messenger RNAs. J Mol Biol. 1977 May 5;112(1):75–96. doi: 10.1016/s0022-2836(77)80157-5. [DOI] [PubMed] [Google Scholar]
- LANGRIDGE R., GOMATOS P. J. The structure of RNA. Reovirus RNA and transfer RNA have similar three-dimensional structures, which differ from DNA. Science. 1963 Aug 23;141(3582):694–698. doi: 10.1126/science.141.3582.694. [DOI] [PubMed] [Google Scholar]
- Lau R. Y., Van Alstyne D., Berckmans R., Graham A. F. Synthesis of reovirus-specific polypeptides in cells pretreated with cycloheximide. J Virol. 1975 Sep;16(3):470–478. doi: 10.1128/jvi.16.3.470-478.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. W., Hayes E. C., Joklik W. K. Characterization of anti-reovirus immunoglobulins secreted by cloned hybridoma cell lines. Virology. 1981 Jan 15;108(1):134–146. doi: 10.1016/0042-6822(81)90533-x. [DOI] [PubMed] [Google Scholar]
- Lee P. W., Hayes E. C., Joklik W. K. Protein sigma 1 is the reovirus cell attachment protein. Virology. 1981 Jan 15;108(1):156–163. doi: 10.1016/0042-6822(81)90535-3. [DOI] [PubMed] [Google Scholar]
- Levin K. H., Samuel C. E. Biosynthesis of reovirus-specified polypeptides. purification and characterization of the small-sized class mRNAs of reovirus type 3: coding assignments and translational efficiencies. Virology. 1980 Oct 15;106(1):1–13. doi: 10.1016/0042-6822(80)90216-0. [DOI] [PubMed] [Google Scholar]
- Li J. K., Keene J. D., Scheible P. P., Joklik W. K. Nature of the 3'-terminal sequences of the plus and minus strands of the S1 gene of reovirus serotypes 1, 2 and 3. Virology. 1980 Aug;105(1):41–51. doi: 10.1016/0042-6822(80)90154-3. [DOI] [PubMed] [Google Scholar]
- Li J. K., Scheible P. P., Keene J. D., Joklik W. K. The plus strand of reovirus gene S2 is identical with its in vitro transcript. Virology. 1980 Aug;105(1):282–286. doi: 10.1016/0042-6822(80)90181-6. [DOI] [PubMed] [Google Scholar]
- Loh P. C., Shatkin A. J. Structural proteins of reoviruses. J Virol. 1968 Nov;2(11):1353–1359. doi: 10.1128/jvi.2.11.1353-1359.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Kilham S. S., Hay A. J., Zweerink H. J., Joklik W. K. An ultrastructural study of virions and cores of reovirus type 3. Virology. 1972 Apr;48(1):170–181. doi: 10.1016/0042-6822(72)90124-9. [DOI] [PubMed] [Google Scholar]
- Matsuhisa T., Joklik W. K. Temperature-sensitive mutants of reovirus. V. Studies on the nature of the temperature-sensitive lesion of the group C mutant ts447. Virology. 1974 Aug;60(2):380–389. doi: 10.1016/0042-6822(74)90333-x. [DOI] [PubMed] [Google Scholar]
- McCrae M. A., Joklik W. K. The nature of the polypeptide encoded by each of the 10 double-stranded RNA segments of reovirus type 3. Virology. 1978 Sep;89(2):578–593. doi: 10.1016/0042-6822(78)90199-x. [DOI] [PubMed] [Google Scholar]
- McDowell M. J., Joklik W. K. An in vitro protein synthesizing system from mouse L fibroblasts infected with reovirus. Virology. 1971 Sep;45(3):724–733. doi: 10.1016/0042-6822(71)90186-3. [DOI] [PubMed] [Google Scholar]
- McDowell M. J., Joklik W. K., Villa-Komaroff L., Lodish H. F. Translation of reovirus messenger RNAs synthetesized in vitro into reovirus polypeptides by several mammalian cell-free extracts. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2649–2653. doi: 10.1073/pnas.69.9.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward S., Graham A. F. Structural studies on reovirus: discontinuities in the genome. Proc Natl Acad Sci U S A. 1970 Feb;65(2):422–429. doi: 10.1073/pnas.65.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E. M., Kingsbury D. W. Pyridoxal phosphate as a probe of reovirus transcriptase. Biochemistry. 1980 Feb 5;19(3):484–489. doi: 10.1021/bi00544a014. [DOI] [PubMed] [Google Scholar]
- Morgan E. M., Zweerink H. J. Characterization of transcriptase and replicase particles isolated from reovirus-infected cells. Virology. 1975 Dec;68(2):455–466. doi: 10.1016/0042-6822(75)90286-x. [DOI] [PubMed] [Google Scholar]
- Morgan E. M., Zweerink H. J. Reovirus morphogenesis. Corelike particles in cells infected at 39 degrees with wild-type reovirus and temperature-sensitive mutants of groups B and G. Virology. 1974 Jun;59(2):556–565. doi: 10.1016/0042-6822(74)90465-6. [DOI] [PubMed] [Google Scholar]
- Mustoe T. A., Ramig R. F., Sharpe A. H., Fields B. N. A genetic map of reovirus. III. Assignment of the double-stranded RNA-positive mutant groups A, B, and G to genome segments. Virology. 1978 Apr;85(2):545–556. doi: 10.1016/0042-6822(78)90460-9. [DOI] [PubMed] [Google Scholar]
- Mustoe T. A., Ramig R. F., Sharpe A. H., Fields B. N. Genetics of reovirus: identification of the ds RNA segments encoding the polypeptides of the mu and sigma size classes. Virology. 1978 Sep;89(2):594–604. doi: 10.1016/0042-6822(78)90200-3. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan S., Shatkin A. J. Reovirus genome RNA segments: resistance to S-1 nuclease. Virology. 1975 Mar;64(1):96–105. doi: 10.1016/0042-6822(75)90082-3. [DOI] [PubMed] [Google Scholar]
- Nichols J. L., Bellamy A. R., Joklik W. K. Identification of the nucleotide sequences of the oligonucleotides present in reovirions. Virology. 1972 Aug;49(2):562–572. doi: 10.1016/0042-6822(72)90507-7. [DOI] [PubMed] [Google Scholar]
- Nichols J. L., Hay A. J., Joklik W. K. 5'-terminal nucleotide sequence of reovirus mRNA synthesized in vitro. Nat New Biol. 1972 Jan 26;235(56):105–107. doi: 10.1038/newbio235105a0. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Graham A. F. Appearance of defective virions in clones of reovirus. J Virol. 1970 Nov;6(5):693–694. doi: 10.1128/jvi.6.5.693-694.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer E. L., Martin M. L. The fine structure of the capsid of reovirus type 3. Virology. 1977 Jan;76(1):109–113. doi: 10.1016/0042-6822(77)90287-2. [DOI] [PubMed] [Google Scholar]
- RAMOS-ALVAREZ M., SABIN A. B. Characteristics of poliomyelitis and other enteric viruses recovered in tissue culture from healthy American children. Proc Soc Exp Biol Med. 1954 Dec;87(3):655–661. doi: 10.3181/00379727-87-21474. [DOI] [PubMed] [Google Scholar]
- RHIM J. S., SMITH K. O., MELNICK J. L. Complete and coreless forms of reovirus (ECHO 10). Ratio of number of virus particles to infective units in the one-step growth cycle. Virology. 1961 Dec;15:428–435. doi: 10.1016/0042-6822(61)90110-6. [DOI] [PubMed] [Google Scholar]
- ROSEN L. Serologic grouping of reoviruses by hemagglutination-inhibition. Am J Hyg. 1960 Mar;71:242–249. doi: 10.1093/oxfordjournals.aje.a120107. [DOI] [PubMed] [Google Scholar]
- Ralph S. J., Harvey J. D., Bellamy A. R. Subunit structure of the reovirus spike. J Virol. 1980 Dec;36(3):894–896. doi: 10.1128/jvi.36.3.894-896.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig R. F., Fields B. N. Revertants of temperature-sensitive mutants of reovirus: evidence for frequent extragenic suppression. Virology. 1979 Jan 15;92(1):155–167. doi: 10.1016/0042-6822(79)90221-6. [DOI] [PubMed] [Google Scholar]
- Ramig R. F., Mustoe T. A., Sharpe A. H., Fields B. N. A genetic map of reovirus. II. Assignment of the double-stranded RNA-negative mutant groups C, D, and E to genome segments. Virology. 1978 Apr;85(2):531–534. doi: 10.1016/0042-6822(78)90459-2. [DOI] [PubMed] [Google Scholar]
- Ramig R. F., White R. M., Fields B. N. Suppression of the temperature-sensitive phenotype of a mutant of reovirus type 3. Science. 1977 Jan 28;195(4276):406–407. doi: 10.1126/science.831284. [DOI] [PubMed] [Google Scholar]
- Rubin D. H., Fields B. N. Molecular basis of reovirus virulence. Role of the M2 gene. J Exp Med. 1980 Oct 1;152(4):853–868. doi: 10.1084/jem.152.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABIN A. B. Reoviruses. A new group of respiratory and enteric viruses formerly classified as ECHO type 10 is described. Science. 1959 Nov 20;130(3386):1387–1389. doi: 10.1126/science.130.3386.1387. [DOI] [PubMed] [Google Scholar]
- STANLEY N. F., DORMAN D. C., PONSFORD J. Studies on the pathogenesis of a hitherto undescribed virus (hepato-encephalomyelitis) producing unusual symptoms in suckling mice. Aust J Exp Biol Med Sci. 1953 Apr;31(2):147–159. doi: 10.1038/icb.1953.18. [DOI] [PubMed] [Google Scholar]
- Sakuma S., Watanabe Y. Incorporation of in vitro synthesized reovirus double-stranded ribonucleic acid into virus corelike particles. J Virol. 1972 Nov;10(5):943–950. doi: 10.1128/jvi.10.5.943-950.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma S., Watanabe Y. Reovirus replicase-directed synthesis of double-stranded ribonucleic acid. J Virol. 1972 Oct;10(4):628–638. doi: 10.1128/jvi.10.4.628-638.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma S., Watanabe Y. Unilateral synthesis of reovirus double-stranded ribonucleic acid by a cell-free replicase system. J Virol. 1971 Aug;8(2):190–196. doi: 10.1128/jvi.8.2.190-196.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuerch A. R., Joklik W. K. Temperature-sensitive mutants of reovirus. IV. Evidence that anomalous electrophoretic migration behavior of certain double-stranded RNA hybrid species is mutant group-specific. Virology. 1973 Nov;56(1):218–229. doi: 10.1016/0042-6822(73)90301-2. [DOI] [PubMed] [Google Scholar]
- Schuerch A. R., Matsuhisa T., Joklik W. K. Temperature-sensitive mutants of reovirus. VI. Mutant ts 447 and ts 556 particles that lack either one or two genome RNA segments. Intervirology. 1974;3(1-2):36–46. doi: 10.1159/000149740. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Inactivity of purified reovirus RNA as a template for E. coli polymerases in vitro. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1721–1728. doi: 10.1073/pnas.54.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J., LaFiandra A. J. Transcription by infectious subviral particles of reovirus. J Virol. 1972 Oct;10(4):698–706. doi: 10.1128/jvi.10.4.698-706.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J. Methylated messenger RNA synthesis in vitro by purified reovirus. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3204–3207. doi: 10.1073/pnas.71.8.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D., Loh P. Separation of ten reovirus genome segments by polyacrylamide gel electrophoresis. J Virol. 1968 Oct;2(10):986–991. doi: 10.1128/jvi.2.10.986-991.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D. RNA polymerase activity in purified reoviruses. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1462–1469. doi: 10.1073/pnas.61.4.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D. Single-stranded, adenine-rich RNA from purified reoviruses. Proc Natl Acad Sci U S A. 1968 Jan;59(1):246–253. doi: 10.1073/pnas.59.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Astell C., Christman J., Klett H., Acs G. Shythesis of reovirus oligo adenylic acid in vivo and in vitro. J Virol. 1974 Mar;13(3):740–752. doi: 10.1128/jvi.13.3.740-752.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Astell C., Levin D. H., Schonberg M., Acs G. The mechanisms of reovirus uncoating and gene activation in vivo. Virology. 1972 Mar;47(3):797–806. doi: 10.1016/0042-6822(72)90571-5. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Dales S. The penetration of reovirus RNA and initiation of its genetic function in L-strain fibroblasts. J Cell Biol. 1968 Jan;36(1):197–230. [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Schonberg M., Levin D. H., Acs G. The reovirus replicative cycle: conservation of parental RNA and protein. Proc Natl Acad Sci U S A. 1970 Sep;67(1):275–281. doi: 10.1073/pnas.67.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Joklik W. K. Studies on the in vitro transcription of reovirus RNA catalyzed by reovirus cores. Virology. 1969 Dec;39(4):822–831. doi: 10.1016/0042-6822(69)90019-1. [DOI] [PubMed] [Google Scholar]
- Skup D., Millward S. Reovirus-induced modification of cap-dependent translation in infected L cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):152–156. doi: 10.1073/pnas.77.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skup D., Millward S. mRNA capping enzymes are masked in reovirus progeny subviral particles. J Virol. 1980 May;34(2):490–496. doi: 10.1128/jvi.34.2.490-496.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Graham A. F. Generation of defective virus after infection of newborn rats with reovirus. J Virol. 1976 Oct;20(1):234–247. doi: 10.1128/jvi.20.1.234-247.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandidos D. A., Graham A. F. Nonpermissive infection of L cells by an avian reovirus: restricted transcription of the viral genome. J Virol. 1976 Sep;19(3):977–984. doi: 10.1128/jvi.19.3.977-984.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spendlove R. S., McClain M. E., Lennette E. H. Enhancement of reovirus infectivity by extracellular removal or alteration of the virus capsid by proteolytic enzymes. J Gen Virol. 1970 Aug;8(2):83–94. doi: 10.1099/0022-1317-8-2-83. [DOI] [PubMed] [Google Scholar]
- Stoltzfus C. M., Banerjee A. K. Two oligonucleotide classes of single-stranded ribopolymers in reovirus A-rich RNA. Arch Biochem Biophys. 1972 Oct;152(2):733–743. doi: 10.1016/0003-9861(72)90269-x. [DOI] [PubMed] [Google Scholar]
- VASQUEZ C., TOURNIER P. The morphology of reovirus. Virology. 1962 Aug;17:503–510. doi: 10.1016/0042-6822(62)90149-6. [DOI] [PubMed] [Google Scholar]
- Van Dijk A. A., Huismans H. The in vitro activation and further characterization of the bluetongue virus-associated transcriptase. Virology. 1980 Jul 30;104(2):347–356. doi: 10.1016/0042-6822(80)90339-6. [DOI] [PubMed] [Google Scholar]
- Vasquez C., Kleinschmidt A. K. Electron microscopy of RNA strands released from individual Reovirus particles. J Mol Biol. 1968 May 28;34(1):137–147. doi: 10.1016/0022-2836(68)90240-4. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Graham A. F. Structural units of reovirus ribonucleic acid and their possible functional significance. J Virol. 1967 Aug;1(4):665–677. doi: 10.1128/jvi.1.4.665-677.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Millward S., Graham A. F. Regulation of transcription of the Reovirus genome. J Mol Biol. 1968 Aug 28;36(1):107–123. doi: 10.1016/0022-2836(68)90223-4. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Prevec L., Graham A. F. Specificity in transcription of the reovirus genome. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1040–1046. doi: 10.1073/pnas.58.3.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Drayna D., Averill D. R., Jr, Fields B. N. Molecular basis of reovirus virulence: role of the S1 gene. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5744–5748. doi: 10.1073/pnas.74.12.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Fields B. N. Neutralization of reovirus: the gene responsible for the neutralization antigen. J Exp Med. 1977 Nov 1;146(5):1305–1310. doi: 10.1084/jem.146.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Greene M. I., Fields B. N. Delayed hypersensitivity in mice infected with reovirus. I. Identification of host and viral gene products responsible for the immune response. J Immunol. 1980 Jul;125(1):278–282. [PubMed] [Google Scholar]
- Weiner H. L., Powers M. L., Fields B. N. Absolute linkage of virulence and central nervous system cell tropism of reoviruses to viral hemagglutinin. J Infect Dis. 1980 May;141(5):609–616. doi: 10.1093/infdis/141.5.609. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Ramig R. F., Mustoe T. A., Fields B. N. Identification of the gene coding for the hemagglutinin of reovirus. Virology. 1978 May 15;86(2):581–584. doi: 10.1016/0042-6822(78)90099-5. [DOI] [PubMed] [Google Scholar]
- White C. K., Zweerink H. J. Studies on the structure of reovirus cores: selective removal of polypeptide lambda 2. Virology. 1976 Mar;70(1):171–180. doi: 10.1016/0042-6822(76)90247-6. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Ito Y., Matsuhisa T. Synthesis of reovirus double-stranded RNA within virionlike particles. Virology. 1972 Nov;50(2):349–358. doi: 10.1016/0042-6822(72)90386-8. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Joklik W. K. Studies on the intracellular synthesis of reovirus-specified proteins. Virology. 1970 Jul;41(3):501–518. doi: 10.1016/0042-6822(70)90171-6. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Morgan E. M., Skyler J. S. Reovirus morphogenesis: characterization of subviral particles in infected cells. Virology. 1976 Sep;73(2):442–453. doi: 10.1016/0042-6822(76)90405-0. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J. Multiple forms of SS leads to DS RNA polymerase activity in reovirus-infected cells. Nature. 1974 Feb 1;247(5439):313–315. doi: 10.1038/247313a0. [DOI] [PubMed] [Google Scholar]