Abstract
Nkx2.2 is an essential regulator of pancreatic endocrine differentiation. Nkx2.2-null mice are completely devoid of β-ells and have a large reduction of α- and PP cells. In the place of these islet populations, there is a corresponding increase in the ghrelin-positive ε-cells. Molecular studies have indicated that Nkx2.2 functions as an activator and repressor to regulate islet cell fate decisions. To determine whether Nkx2.2 is solely important for islet cell fate decisions or also has the capability to control ghrelin at the promoter level, we studied the transcriptional regulation of the ghrelin promoter within the pancreas, in vitro and in vivo. These studies demonstrate that both of the previously identified transcriptional start sites in the ghrelin promoter are active within the embryonic pancreas; however, the long transcript is preferentially up-regulated in the Nkx2.2-null pancreas. We also show that the promoter region between −619 and −488 bp upstream of the translational start site is necessary for repression of ghrelin in αTC1 and βTC6 cells. Surprisingly, we also show that Nkx2.2 is able to bind to and activate the ghrelin promoter in several cell lines that do or do not express endogenous ghrelin. Together, these results suggest that the up-regulation of ghrelin expression in the Nkx2.2-null mice is not due to loss of repression of the ghrelin promoter in the nonghrelin islet populations. Furthermore, Nkx2.2 may contribute to the activation of ghrelin in mature islet ε-cells.
Characterization of ghrelin promoter activity in the pancreas demonstrates that Nkx2.2 binds to and activates ghrelin transcription.
Ghrelin was first identified as an endogenous ligand for the GH secretagogue receptor GHSR1a and is a potent stimulator of GH secretion (1). It has also been shown to act as an orexigenic factor. Cerebral administration of ghrelin in rats acutely stimulated food intake and increased long-term weight gain (2,3,4,5). Intravenous injection of ghrelin into human patients also resulted in increased food intake (6). However, ghrelin-null mice show no significant differences with respect to bone mineral density, fat content, body weight, food intake, and serum leptin or glucose concentration before or after fasting (7,8), suggesting that ghrelin is sufficient but not necessary to stimulate appetite. Ghrelin has been shown to be a paracrine signal in the testes where it controls the replication of immature Leydig cell steroidogenesis and stem cell factor expression in Sertoli cells (9,10). Ghrelin and GHSR1a are also expressed in the embryonic pancreas; however, ghrelin does not appear to influence the pancreatic developmental program during embryogenesis (11,12).
In the adult rodent, plasma ghrelin levels depend almost entirely on ghrelin production by X/A-like cells in the stomach (13,14). Several studies have demonstrated that ghrelin expression in the stomach is regulated by the energy status of the organism; ghrelin production in the stomach and ghrelin levels in the bloodstream are elevated during fasting conditions and in response to glucagon administration (15). Conversely, ghrelin expression is reduced in response to feeding, hyperglycemia, and obesity. During embryogenesis, ghrelin production is undetectable in the stomach and rises to significant levels only after birth (16,17). On the other hand, ghrelin is detected as early as embryonic d 10.5 (e10.5) in the mouse embryonic pancreas, remaining high through late gestation and decreasing shortly after birth. Several other hormones, including gastrin, cocaine- and amphetamine-regulated transcript (CART), and IGF-II, are expressed transiently in the embryonic and/or perinatal islet; however their expression is limited to subpopulations of the major endocrine cell types and for the most part do not overlap with the ghrelin-producing ε-cell population (18,19,20,21). Furthermore, ghrelin expression is evident at a much earlier stage of pancreas development than gastrin, CART, and IGF-II (22,23). Interestingly, the decline of ghrelin expression in the pancreas corresponds with the increase in ghrelin production in the stomach (24). Similar developmental expression patterns were seen in human fetuses where ghrelin cell numbers in the pancreas were highest during midgestation, preceding ghrelin expression in the stomach, and decreased in neonates. However, unlike the rodent, a few ghrelin-positive cells remain in the adult human pancreas (23). Furthermore, unlike the stomach, fasting does not affect ghrelin levels in the pancreas (24). The differential spatial and temporal expression patterns of ghrelin between stomach and pancreas suggest that there may be key differences in their transcriptional regulation within these two tissues.
In light of the variety of metabolic effects ghrelin exerts in response to energy balance, several studies have explored the regulation of ghrelin gene transcription. Characterization of the ghrelin promoter has identified the presence of two transcription initiation sites. The short transcript is present in almost all mouse, rat, and human cell lines tested, and the long transcript is most abundant in the human medullary TT cells and in rat stomach and placental tissue (15). Interestingly, this study also identified several structural differences between the rat and human core promoters; however, rat and human ghrelin regulation both depended on activation by the upstream stimulatory factor (USF) and were similarly regulated by glucagon induction (15). More recently, it was shown that Krueppel-like factor 4 also positively regulates human ghrelin expression in the human gastric cancer AGS cells (25).
In the mouse and human embryonic pancreas, ghrelin expression is restricted to the ε-cell population and a small number of the glucagon-producing α-cell population (21,22,26). Little is known about ghrelin regulation in the pancreas, although it was recently demonstrated that the essential islet transcriptional regulator factor Pax4 directly represses mouse ghrelin promoter activity in islet β-cell lines (27). Consistent with this observation, ghrelin expression becomes de-repressed in a population of cells that coexpress glucagon, IAPP, and low levels of Pdx1 in the Pax4-null mice. Nkx2.2 is another essential pancreatic transcription factor that affects the expression of ghrelin during pancreatic development. Nkx2.2-null mice lose all insulin-producing β-cells and the majority of the glucagon-producing α-cell population; instead, the islet is predominantly populated by ghrelin-expressing cells (26). We recently demonstrated that the increase in ghrelin cell numbers is not caused by the loss of insulin (11). Furthermore, in contrast to the Pax4-null mice, the ghrelin-producing population in the Nkx2.2-null pancreas does not coexpress glucagon or other islet hormones. Based on additional marker analysis, the ghrelin-positive cells present in the Nkx2.2-null islet do not appear to represent misexpression of ghrelin in defined endocrine populations. However, unlike the wild-type ghrelin-producing population, these mutant ghrelin cells erroneously express low levels of several α- and β-cell factors (26,28) (Hill, J. T., unpublished). Because we have previously demonstrated that Nkx2.2 functions as a repressor and an activator of transcription (29), we wished to determine whether the increased ghrelin expression observed in the Nkx2.2-null mice was due to a loss of Nkx2.2-mediated repression of ghrelin transcription in a non-ε-cell islet endocrine population.
To more carefully characterize ghrelin promoter regulation by Nkx2.2, we first clarified several aspects of ghrelin promoter regulation in the mouse pancreas. In this study, we show that the embryonic pancreas expresses a mixture of the short and long transcripts; however, the long transcript is preferentially up-regulated in the absence of Nkx2.2. In addition, we characterized ghrelin promoter activity in αTC1 and βTC6 cell lines. From this analysis, we determined that the promoter region upstream of the TATA box is not necessary for ghrelin transcription in the embryonic mouse pancreas, but is required for repression of the ghrelin promoter in αTC1 and βTC6 cells. We also show that Nkx2.2 is able to bind this region but, surprisingly, activates the ghrelin gene in all cell lines tested. This finding supports the idea that the increase of ghrelin-expressing cells in the Nkx2.2-null pancreas is not simply caused by the ectopic activation of the ghrelin promoter in a non-ε-cell endocrine population. Further studies are underway to elucidate the role of Nkx2.2 in regulating islet-cell fate choices during embryonic development.
Results
Identification of the ghrelin transcript produced in the embryonic pancreas
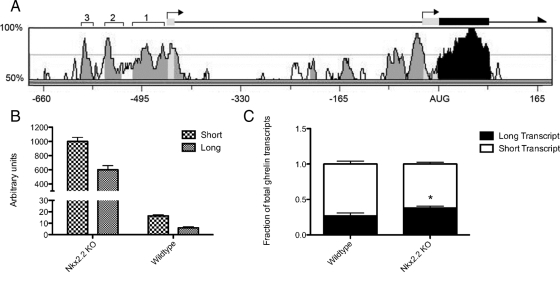
The ghrelin gene contains two transcriptional start sites (Fig. 1A, bent arrows). The upstream site lies 451 bases upstream of the translational start site and includes a small (20 bp) noncoding first exon, whereas the downstream start site lies at the start of the second exon, 24 bases upstream of the AUG (30). Transcripts that are made from each of the start sites produce identical proteins; however, use of each start site appears to be controlled in a tissue-specific manner. For example, in adult mice, the short transcript is found primarily in the pituitary and testes, whereas the long transcript is produced primarily in the stomach (15). Although adult tissues tested generally produced either the long or the short transcript, the placenta was shown to produce both transcripts (15). The tissue-specific distribution of the long and short transcripts indicates that there may be unique transcriptional programs that are necessary for ghrelin activation in each of the ghrelin-producing cell types.
Figure 1.
Activity of both ghrelin transcription initiation sites in the embryonic pancreas. A, Schematic representation of the ghrelin gene including rVista alignment of the mouse and human promoters. Transcription initiation sites are shown as bent arrows. Conserved (>70% homology) intronic and promoter regions, noncoding exonic regions, and coding regions are shaded in light gray, dark gray, and black, respectively. Three conserved regions upstream of exon 1 are labeled with square brackets. Numbers along the bottom of the chart represent the number of base pairs upstream and downstream of the AUG initiation codon. B, Real-time quantification of the long and short transcripts made in the mouse embryonic pancreas in both wild-type and Nkx2.2 knockout (KO) mice at e18.5. Both the long and short transcripts were greatly increased (long transcript 99.0-fold; short transcript 61.6-fold) in the Nkx2.2-null mice. C, Comparison of the proportion of long and short transcripts. The long transcript represented 26.8% of all ghrelin transcripts in the wild-type mice and 37.9% of the transcripts in the Nkx2.2 knockout mice. The increased proportion of the long transcript in the Nkx2.2 knockout mice was statistically significant using the unpaired Student's t test (*, P = 0.0426; n =3). Error bars represent the sem.
To determine which transcriptional start site is used within the embryonic pancreas, we performed qRT-PCR on RNA extracts from e18.5 wild-type and Nkx2.2-null pancreata. Twenty-eight percent of pancreatic ghrelin transcripts include the first exon in wild-type pancreata. Both transcripts were increased in the Nkx2.2-null pancreas (long transcript 99.0-fold; short transcript 61.6-fold; Fig. 1B). Interestingly, the proportion of total transcripts that included the first exon was significantly increased to 37% in the Nkx2.2-null pancreas (Fig. 1C). Therefore, loss of Nkx2.2 results in an increase of both transcripts but has a more potent effect on the long transcript in embryonic mouse islets.
Characterization of the ghrelin promoter in pancreatic islet cells
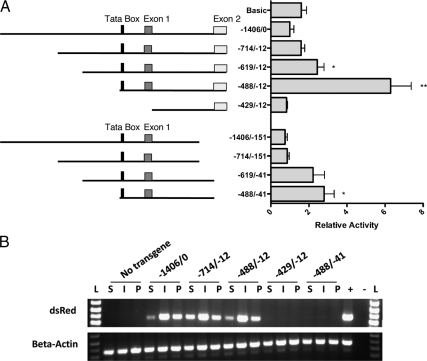
A previous study identified the minimal human and rat promoters necessary for ghrelin expression in AGS cells, a gastric carcinoma cell line (15). In both rat and human, the region upstream of the TATA box was not necessary for promoter activation. However, elements downstream of the TATA box had differing effects in the human and rat ghrelin genes. The presence of the region spanning the first intron was necessary for activity in the human promoter but repressed activity in the rat promoter. To test the importance of the promoter regions upstream and downstream of the TATA box in the embryonic mouse pancreas, we performed deletion analysis of the full-length 1406-bp mouse promoter region corresponding to the full-length human promoter (15,27). Promoter fragments containing the 1406-bp sequence and subsequently shorter 5′ upstream sequences were fused to a luciferase reporter in the pGL3-Basic plasmid and transiently transfected into αTC1 cells (Fig. 2A). A pancreatic ghrelin cell line does not exist; however, the αTC1 cell line expresses endogenous ghrelin at low levels, consistent with the presence of a small population of glucagon/ghrelin coexpressing cells exist in the mouse and human embryonic islet (22,26). Analogous to previous studies of the mouse ghrelin promoter, and similar to what was found with the rat promoter (15,27), the 1406-bp full-length ghrelin promoter and a shorter 714-bp promoter showed little activation over the pGL3-Basic vector control in both αTC1 cells and βTC6 cell lines (Fig. 2A and supplemental Fig. 1, published as supplemental data on The Endocrine Society's Journals Online web site at http://mend. endojournals.org). However, deletion of the region upstream of −619 resulted in a significant activation of the promoter (P = 0.016). Further deletion of the 5′ promoter region to just upstream of the TATA Box (−488/−12) showed the highest amount of activation with a 6.3-fold increase over the full promoter (−1406/−12) (P = 0.003). Deletion of the downstream initiation sites (−151/−13 or −41/−13) resulted in similar expression patterns but with lower overall activity (P = 0.02).
Figure 2.
Identification of the minimum active promoter in vitro and in vivo. A, Relative expression of mouse ghrelin promoter deletion constructs. Constructs were cloned into the pGL3 luciferase reporter vector (Promega) and electroporated into αTC1 cells. The graph on the right shows the relative luciferase activity of each construct. Significance was determined by the unpaired Student's t test (*, P < 0.05; **, P < 0.01). B, RT-PCR amplification of dsRED-E5 mRNA from stomach (S), intestine (I), and pancreas (P) of P0 mice. Several transgenic lines were tested for each promoter construct, and representative samples are shown here (see supplemental Table 1). β-Actin was used as a loading control. L, Ladder.
To understand ghrelin promoter activity in vivo, we generated transgenic reporter mice with specific regions of the ghrelin promoter driving the fluorescent protein, dsRed-E5 (pTimer1; Clontech, Mountain View, CA). DsRed-E5 was chosen for its ability to change from green to red fluorescence over time, allowing for relative detection of early to late born cells (31). Based on the in vitro luciferase results, we chose to generate pTimer1:pGhr[−1406/0], pTimer1:pGhr[−714/−12], pTimer1:pGhr[−488/−12], pTimer1:pGhr[−429/ −12], and pTimer1:pGhr[−488/−41]. Unfortunately, we were unable to detect dsRed expression by direct fluorescence or by indirect immunofluorescence with the anti-dsRed antibody (Clontech), presumably because expression levels were below the level of detection. We were able to detect dsRED mRNA expression using RT-PCR, which was sufficient for the assessment of transgene transcriptional expression within the tissues but did not allow us to distinguish ghrelin expression in the individual cell populations. Ghrelin promoter activity was assayed by expression of dsRed-E5 mRNA in perinatal stomach, intestine, and pancreas by RT-PCR (Fig. 2B and supplemental Table 1). Consistent with in vitro luciferase data, the minimal mouse ghrelin promoter necessary for transcription was (−488/−12). In addition, the transgenic mouse studies demonstrate that although the (−41/−12) ghrelin promoter region was not critical for activity in αTC1 cells, it is required for transcription of Ghrelin:dsRed in vivo, although this activity may represent expression of ghrelin in the pancreatic ε-cell population.
Nkx2.2 binds and activates the ghrelin promoter
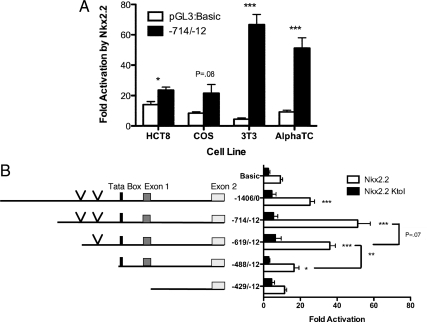
A ghrelin-producing cell population replaces all of the β-cells and of the majority of α-cells in the Nkx2.2-null pancreas (26). To determine whether this phenotype can be explained by loss of repression of the ghrelin promoter in the absence of Nkx2.2, we examined the effects of Nkx2.2 on ghrelin transcription in a number of cell lines that have medium to high endogenous ghrelin promoter activity, including intestinal HCT8 cells, COS7 cells, and NIH3T3 cells (15,27). In addition, we assessed the effect of Nkx2.2 on ghrelin expression in αTC1 cells, which express lower levels of ghrelin but should contain relevant Nkx2.2 coregulatory proteins. Each cell line was transiently transfected with the pcDNA3:Nkx2.2 expression plasmid and either the pGL3:Basic vector or the pGL3:Ghrelin (−714/−12) promoter element. Surprisingly, Nkx2.2 significantly activated the ghrelin promoter in each cell line when compared with the pGL3 vector alone controls, with the exception of COS7 cells where only moderate activation was achieved. There was no evidence of repression by Nkx2.2 (Fig. 3A). Previous studies have demonstrated that Nkx2.2 functions as a repressor of transcription through its interaction with members of the Grg corepressor family (29,32). In the brain, Nkx2.2 interacts with Grg4, and in the pancreatic islet Nkx2.2 interacts with Grg3. We confirmed that Grg3 is expressed in αTC1 cells (data not shown). Subsequent cotransfection of Grg3 with Nkx2.2 into either the NIH3T3 cells or HCT8 cells did not alter the ability of Nkx2.2 to activate the ghrelin promoter (data not shown). These results suggest that the failure of Nkx2.2 to repress the ghrelin promoter is not due to the absence of its endogenous corepressor protein.
Figure 3.
Nkx2.2 is able to activate the ghrelin promoter. A, A luciferase construct containing the −714/−12 ghrelin promoter region was transfected into HCT8, COS7 (COS), NIH3T3 (3T3), and αTC1 cell lines with PCDNA3:Nkx2.2 or pCDNA3 backbone. Graph shows fold activation of the pGL3:−714/−12 or pGL3:basic by Nkx2.2 (luciferase activity in pCDNA3:Nkx2.2 transfected cells/activity of PCDNA3 backbone transfected cells). B, Luciferase constructs were transfected with pCDNA3:Nkx2.2, pCDNA3:Nkx2.2 K→I, or pCDNA3 backbone into NIH3T3 cells. Graph on the right shows fold activation of each ghrelin promoter construct by Nkx2.2. Asterisks indicate significant change over activation of PGL3-Basic control. Significance was determined by the unpaired Student's t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Vertical arrows indicate the location of Nkx2.2-binding consensus core sequences.
To better understand the mechanism by which Nkx2.2 was able to activate rather than repress the ghrelin promoter, we assessed the ability of Nkx2.2 to regulate truncated versions of the ghrelin promoter. Luciferase constructs containing the ghrelin promoter regions (−1406/0), (−714/−12), (−619/−12), (−488/−12), and (−429/12) were transiently transfected into the NIH3T3 cell line with pCDNA3.1:Nkx2.2, pCDNA3:Nkx2.2K→I or the pCDNA3.1 vector control. The pCDNA3.1:Nkx2.2K→I construct contains a point mutation in the DNA-binding region of Nkx2.2 that prevents direct binding of the protein to DNA (33). These promoter activity assays revealed that the ghrelin promoter constructs (−1406/0), (−714/−12), and (−619/−12) are significantly activated by Nkx2.2 (Fig. 3B). There was only a small reduction in activation when the (−714/−619) region was deleted; however, activation of the ghrelin promoter by Nkx2.2 decreased significantly when the region within (−619/−488) was deleted. Taken together, it appears that Nkx2.2 functions on the promoter within the (−714/−488) region. Interestingly, this area also corresponds to two of three highly conserved sequences in the ghrelin promoter, which we refer to as region 2 and 3 (Fig. 1A). Cotransfection with Nkx2.2K→I did not result in activation of any promoters relative to the vector-only controls, which also confirms that Nkx2.2 DNA-binding activity is required for ghrelin activation (Fig. 3B).
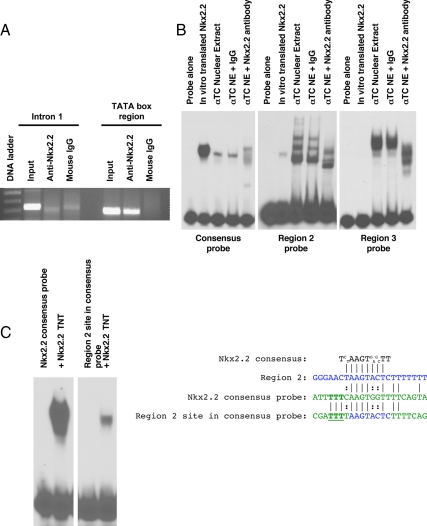
We used in silico sequence analysis to examine the ghrelin promoter region between −714 and −488 bp for the presence of the Nkx2.2 consensus core site. Within this region, we were able to identify two occurrences of the Nkx2.2 core recognition sequence, AAGT (34) (vertical arrows in Fig. 3B). One core sequence was identified on the negative strand at (−629/−625) within the conserved promoter region 3; the other was on the positive strand at (−574/−570) within the conserved promoter region 2. These sites also correspond to the area of the ghrelin promoter that is necessary for highest activation by Nkx2.2 (−714/−619) and the minimal region necessary for Nkx2.2 activation (−619/−488), respectively. To determine whether Nkx2.2 occupied the endogenous ghrelin promoter within this region in vivo, we performed chromatin immunoprecipitation (ChIP) (Fig. 4A). PCR amplification of the TATA box region showed that Nkx2.2 is able to bind the endogenous ghrelin promoter. As expected, primers designed to amplify a region near the downstream initiation site, which does not contain Nkx2.2 consensus sites, failed to amplify this promoter region (Fig. 4A).
Figure 4.
Nkx2.2 binds the ghrelin promoter as part of a heteromeric complex. A, ChIP analysis of Nkx2.2 binding to the ghrelin promoter. Two primer sets were used to show binding near the TATA box and in intron 1. B, EMSA analysis of Nkx2.2 binding. Probes were generated for conserved regions 2 and 3 (see Fig. 1A) of the ghrelin promoter, corresponding with the two predicted Nkx2.2-binding sites. EMSA were performed with Nkx2.2 protein synthesized with rabbit reticulolysate (labeled Nkx2.2 TNT) or αTC1 nuclear extract. Supershifts were performed using monoclonal anti-Nkx2.2 antibody or mouse IgG as a control. C, EMSA of a hybrid probe containing the Nkx2.2 binding site from region 2 of the ghrelin promoter flanked by the 5′- and 3′-arms of the Nkx2.2 consensus probe using in vitro-synthesized Nkx2.2 protein. Alignment of the Nkx2.2 consensus site with region 2 of the ghrelin promoter (blue), the Nkx2.2 consensus probe (green), and the hybrid probe (core sequence from region 2 in blue and flanking regions from Nkx2.2 consensus probe in green) used in EMSA analysis. Aligned bases (│) and use of alternative bases in the consensus sequence (:) are indicated. From the alignment, three bases were identified adjacent to the core Nkx2.2 consensus sequence that were essential to allow Nkx2.2 binding (bold and underlined).
To determine whether Nkx2.2 binds directly to the two predicted consensus sites within the ghrelin promoter (−714/−488) region, we performed EMSA analysis (Fig. 4B). Probes for each of the conserved regions containing the predicted Nkx2.2-binding sites were incubated with in vitro-translated Nkx2.2 or αTC1 cells nuclear extract. In vitro-synthesized Nkx2.2 bound each site only very weakly. Incubation with αTC1 cells nuclear extract resulted in two distinct DNA-binding complexes that were disrupted with the addition of anti-Nkx2.2 antibody, indicating that Nkx2.2 is part of these complexes. Furthermore, the complex bound the DNA with greater affinity than Nkx2.2 protein alone. These experiments suggest that Nkx2.2 is able to bind the predicted binding sites, and formation of a protein complex is required to strengthen and/or stabilize binding.
Because this was the first time we observed increased Nkx2.2-binding affinity due to the presence of other protein factors, we wished to determine whether the increased affinity was due to variations within the core Nkx2.2-binding site, which was slightly different from the published consensus site, or due to the DNA flanking sequences present in the ghrelin promoter. To test this, we generated a hybrid EMSA probe that contained the region 2 8-bp core Nkx2.2-binding site (AAGTACTC) and surrounded it with the flanking regions of the Nkx2.2 consensus probe (Fig. 4C). Probes were incubated with in vitro-synthesized Nkx2.2 to test for binding without the formation of a complex (Fig. 4C). In vitro-synthesized Nkx2.2 was able to bind the hybrid probe with much higher affinity than the region 2 probe, indicating that the surrounding regions in the ghrelin promoter normally interfere with Nkx2.2 binding. Alignment of the region 2 site with the Nkx2.2 consensus probe and the hybrid probe showed that only three bases in the 5′-flanking region (AAG vs. TTT) differed between the region 2 probe and the hybrid probe to allow binding (Fig. 4C, underlined). Therefore, it appears that Nkx2.2 DNA binding is affected by regions outside of the canonical Nkx2.2 binding site, but binding to sites without the appropriate flanking sequences can be stabilized by the presence of cellular cofactors.
Discussion
Ghrelin is an orexigenic hormone that is widely expressed in many tissues. Discovery of ghrelin expression in the pancreas led to the identification of a novel endocrine cell population in the islet: the ε-cells (21,26,35). ε-Cells are unique among the islet cell types in that they are present only during embryonic pancreas development (24). Furthermore, ghrelin cells appear to be reciprocally regulated with the other islet cells by several essential transcription factors (26,35,36). The purpose of the current study is to understand the transcriptional regulation of ghrelin within the islets and to determine whether the increase of ghrelin in the Nkx2.2-null mice is due to direct de-repression of the ghrelin promoter in the islet. Surprisingly, our studies suggest a more complex role for Nkx2.2 in the regulation of the ghrelin cell fate vs. regulation of the ghrelin promoter in mature islet cells.
Null mutations in the essential pancreatic transcription factors Nkx2.2, Pax4, and Pax6 all result in increased ghrelin expression within the endocrine cells of the embryonic pancreas (26,35,36,37). Pax4 has recently been shown to directly bind and repress the ghrelin promoter (27). Our results show that Nkx2.2 is also able to bind the ghrelin promoter but functions to activate ghrelin transcription in all cell lines tested. These results appear contradictory to the Nkx2.2-null phenotype; however, the opposing results likely reflect the different functions for Nkx2.2 in regulating islet cell fate decisions during the process of cell specification and hormone promoter regulation in the mature islet cell. The activation (rather than repression) of the ghrelin promoter is also consistent with the presence of Nkx2.2 in the mouse and human islet ghrelin cells (Leclerc, K., and L. Sussel, unpublished data) (38). Taken together, our studies indicate that the up-regulation of ghrelin in the Nkx2.2-null pancreas does not appear to be due to direct loss of repression of the ghrelin promoter in nonghrelin islet cell types. Consistently, the small percentage of glucagon-positive α-cells that persist within the Nkx2.2-null islet do not express ghrelin; the Nkx2.2-null islet is completely devoid of the glucagon/ghrelin double-positive cell population.
An interesting observation regarding Nkx2.2 regulation of the ghrelin promoter also emerged from this study. Nkx2.2 has been previously shown to bind the MafA, NeuroD/β2, and insulin II promoters, and in each case, in vitro-translated Nkx2.2 was sufficient for DNA binding (33,39,40). Because Nkx2.2 appears to require a protein complex to bind the ghrelin promoter with high affinity, these studies appear to have uncovered an additional and novel mechanism for Nkx2.2 binding. Changes in the sequences immediately upstream of the Nkx2.2-binding site in region 2 restored Nkx2.2-binding ability, suggesting that the presence of Nkx2.2 within a protein complex allows Nkx2.2 to bind certain DNA sequence contexts by stabilizing Nkx2.2/DNA interaction, facilitating Nkx2.2 competition for DNA with other protein complexes, or by changing the DNA structure. Interestingly, the study by Watada and colleagues (34) that originally characterized the Nkx2.2-binding consensus site demonstrated that DNA sequence substitutions in the 5′- or 3′-flanking regions of the Nkx2.2 consensus binding site negatively affected the binding affinity of Nkx2.2. Further studies are ongoing to determine the nature of the Nkx2.2 protein complexes that form on the ghrelin promoter. Neither of the characterized ghrelin regulators, the upstream stimulatory factors USF1/2 or Pax4, bind to the ghrelin promoter within the vicinity of the Nkx2.2-binding sites (15,27). Furthermore, cotransfection of Pax4 or Pax6 with Nkx2.2 did not change Nkx2.2 ability to activate the ghrelin promoter (data not shown). Consistent with Nkx2.2's activation function on the ghrelin promoter, the Grg3 corepressor protein is not part of the α-cell protein complex bound to the ghrelin promoter, although it is present within the cell (data not shown). It is likely that the combination of protein cofactors and promoter context will influence the transcriptional activity of Nkx2.2 within a cell.
Understanding the regulation of ghrelin by Nkx2.2 was facilitated by the characterization of the ghrelin promoter in the mouse islet. These studies demonstrated that both ghrelin transcriptional start sites are actively used within the mouse embryonic pancreas, but the long transcript is preferentially up-regulated in the absence of Nkx2.2. This may reflect the induction of an Nkx2.2-independent mechanism of transcriptional regulation that is occurring in the mutant islet cell populations. The promoter analysis also provided a basic understanding of how ghrelin is regulated in general. Deletion of the downstream initiator sequence reduced activity of the promoter by approximately 50%, consistent with a loss of the short but not long transcript. However, removal of the TATA box-containing region (−488/−429) abolished all promoter activity, suggesting that the short transcript is not regulated by a distinct TATA-less promoter. We also showed that the promoter region (−488/−429) is necessary for ghrelin activity in the pancreas both in vitro and in vivo, whereas the downstream initiation site (−41/−12) is necessary only for transcription in vivo. Therefore, we conclude that the (−488/−12) region represents the minimal active promoter for ghrelin expression within the embryonic islet. Interestingly, the TATA box lies at the immediate 5′-end of the (−488/−429) region that is necessary for basal transcription, suggesting that the promoter regions upstream of the TATA box are not necessary for ghrelin activation. This result corresponds with the minimal active promoters identified in the human and rat stomach (15). However, unlike the promoter studies in stomach cells, removal of the (−619/−488) region in αTC1 cell lines resulted in activation of the gene. Therefore, it is possible that the (−619/−488) region is necessary for active repression, and Nkx2.2 may compete with repressors for binding to this promoter region to activate ghrelin in certain cellular contexts.
Materials and Methods
GenomeVista alignment of human and mouse ghrelin genes
Alignment of the mouse and human ghrelin genes was done using the GenomeVista program (http://pipeline.lbl.gov/cgi-bin/GenomeVista) with the following parameters: calculation window, 20 bp; Min Cons Width, 20 bp; Cons Identity, 70%. Mouse was used as the base genome.
Reverse-transcription quantitative PCR
To determine the transcripts made in the embryonic pancreas, whole RNA extracts were prepared from e18.5 pancreata of wild-type and Nkx2.2-null mice using the RNeasy micro kit (QIAGEN, Valencia, CA). cDNA was then prepared using the SuperScript III kit (Invitrogen, Carlsbad, CA) and oligo-deoxythymidine primers. cDNA (200 ng) was amplified by real-time PCR using the following primers: forward for long transcript, 5′-ACATCCCCAGGCATTCCAG-3′; forward for short transcript, 5′-TGCTGTCTTCAGGCACCATCT-3′; reverse primer for both, 5′-TTACTTGTCAGCTGGCGCCT-3′. Standard curves were generated using a plasmid containing a ghrelin cDNA clone covering the same region as the long PCR. Cyclophilin B was used as a loading control. For expression analysis of Nkx2.2, Grg2, and Grg3 in αTC1 cell lines, whole RNA extracts were made from cell cultures of αTC1 cells at approximately 80% confluency using the RNeasy mini kit (QIAGEN). cDNA was prepared using the SuperScript III kit (Invitrogen) and random hexamer primers. cDNA (200 ng for ghrelin PCR or 40 ng for Nkx2.2, Grg2, and Grg3) was amplified by real-time PCR. Quantification was measured by Sybr Green 1 fluorescence.
Transfection constructs
The plasmid, pGL3:pGhr[−1406/−12] was generated by inserting 1.4 kb of the 5′-flanking region of the ghrelin gene was inserted into the MluI/BglII sites of pGL3-Basic plasmid (Clontech; B. Sosa-Pineda). pGL3:pGhr[−714/−12] was generated by removal of 712 bp (KpnI/PvuII) from pGL3:pGhr[−1406/−12] and religated. pGL3:pGhr[−1406/−151] was constructed by removal of 128 bp (HindIII/HindIII) from pGL3:pGhr[−1406/−12] and religated. The remaining promoter deletion constructs were generated with forward and reverse primers, subcloned into TOPObluntII, and then cloned into pGL3 in KpnI/BglII sites for pGL3:pGhr[−619/−12], pGL3:pGhr[−488/−12], and pGL3:pGhr [−429/−12] or KpnI/XhoI sites for pGL3:pGhr[−619/−41]. The forward primers were as follows: [−619/−597] 5′-CTGTCCTCCACTGTTTGTTCTT-3′, [−488/−464] 5′-CCTATATAAGGAGAAGCCGGTGAG-3′, [−429/−404] 5′-CTCTCTTAGCTGTCTGTATATGTGTG-3′, and [−41/−64] 5′-CTTCTGGTAGCCTACTTCATCTCC-3′. All of the above constructs used the common reverse primer [−12/−33] 5′-AGATCTGAGGACAGATGACCTG-3′.
Transient transfections
A ratio of 50:1 (16 μg/0.32 μg) of pGL3:pGhr/pRL-null plasmids were electroporated into cells or transfected using Fugene transfection reagent (Roche, Indianapolis, IN). After 48 h, cells were harvested and assayed for luciferase activity using the dual luciferase assay kit (Promega, Madison, WI). For cotransfections of PCDNA3:Nkx2.2 expression plasmids with the PGL3 constructs, equal amounts of the constructs were used. Expression of Nkx2.2 and Nkx2.2KtoI was confirmed by Western blot analysis. At least three independent experiments were performed in duplicate, and the unpaired Student's t test was used to measure significance of changes between sample conditions.
Transgenic mice
Transgenic constructs were created using fragments of the ghrelin promoter and the β-globin intron cloned upstream of dsRedE5 of the pTimer-1 vector (Clontech). Plasmids were created by a triple ligation into the pBluescriptKS+ vector (KpnI/EcoRI). For pKS+:pGhr[−1406/−12], [−488/−12], [−429/ −12], and [−619/−41], their respective promoter regions from the plasmids previously described for transfection pGL3:pGhr (KpnI/BglII) and β-globin intron from pdx1β-globin (BamHI/EcoRI) (41) were cloned into the pTimer1 vector. Transgenic constructs were then digested with XhoI/SfiI for all constructs except for pTimer1:pGhr[−619/−41], which was digested with SacI/SfiI to create linearized DNA for injection. Transgenic constructs containing the ghrelin promoter, β-intron, and dsRedE5 were sent to the University of Colorado at Denver and Health Sciences Center Cancer Core Transgenic Facility for microinjection. Founders were identified by PCR of genomic tail DNA with the forward primer in the ghrelin promoter (5′-CTCTCTTAGCTGTCTGTATATGTGTG-3′) and the reverse primer in the pTimer1 vector (5′-ATCTCGAACTCGTGGCCGTT-3′).
DsRedE5 mRNA detection
Total RNA was isolated from pancreas, stomach, and intestine of newly born (P0) pups or e17.5 embryos of founder lines using the RNeasy micro kit (QIAGEN). cDNA was prepared using SuperScript III kit (Invitrogen) oligo-deoxythymidine primers. mRNA for dsRed (345-bp fragment) was detected by PCR using 5′-TCAAGGAGTTCATGCGCTTCAAGG-3′ and 5′-TCACCTTGTAGATGAAGCAGCCGT-3′ primers. β-Actin (5′-AATCGTGCGTGACATCAAAG-3′ and 5′-AAGCACTTGCGGTGCACGAT-3′ for a 550-bp fragment) mRNA was detected as a control.
EMSA
Nuclear extracts were prepared from αTC1 cells using the nuclear extract kit (Active Motif, Carlsbad, CA). In vitro-synthesized Nkx2.2 protein was made using the TNT-coupled reticulolysate system (Promega). Supershifts were performed with 2 μl mouse monoclonal antibody (Developmental Studies Hybridoma Bank, Iowa City, IA). The following oligos were purchased and annealed to create double-stranded probes with 5′ overhangs: Nkx2.2 consensus, 5′-TCGACGCGATTTTTCAAGTGGTTTTCAGTAGC-3′ and 5′-AGCTGCTACTGAAAACCACTTGAAAAATCGCG-3′; region 2, 5′-GTGAGGGGAAAAAAAGAGTACTTAGTTCCCAAGGAATGCAATA-3′ and 5′-GCAGTATTGCATTCCTTGGGAACTAAGTACTCTTTTTTTCCCC-3′; region 3, 5′-ACTTTGGCACCTGTCCTCCACTGTTTGTTCTTCAA-3′ and 5′-TCACTGTTGAAGAACAAACAGTGGAGGACAGGTG-3′; and the hybrid region 2/consensus probe, 5′-TCGACGCGATTTTAAGTACTCTTTTCAGTAGC-3′ and 5′-AGCTGCTACTGAAAAGAGTACTTAAAATCGCG-3′. Probes were labeled by filling in 5′ overhangs with [32P]dCTP. The binding buffer included 100 mm Tris HCl (pH 7.5), 500 mm NaCl, 5 mm EDTA, 10 mm MgCl2, 40% glycerol, 5 mm dithiothreitol, 10× BSA, and 0.1 μg/μl of poly-deoxyinosine-deoxycytosine. Binding reactions were incubated on ice for 30 min with 5 μg nuclear protein or 5 μl in vitro-synthesized protein and 25,000 cpm of labeled probe. Samples were run on 5% nondenaturing polyacrylamide gels at 180 V for 1.5 h in 1× TGE buffer (250 mm Tris base, 1.9 m glycine, and 10 mm EDTA).
ChIP
Pancreata were dissected from e13.5 pancreata one litter at a time. Each litter was formaldehyde cross-linked (1% in PBS) for 10 min while continuing to dissect. Cross-linking reaction was stopped with fresh 2.5 m glycine for 5 min. Tissue was dounce homogenized. Cells were spun down and lysed on ice for 15 min. Cross-linked chromatin complexes were fragmented by sonication with a Diagnode BioRuptor (8 min to 30 sec on/off), resulting in chromatin fragments from 200–600 bp long. Fragmented tissue chromatin (250 μl) used in ChIP reactions was brought up to 1 ml with dilution buffer, and 2 μg mouse monoclonal antibody (Developmental Studies Hybridoma Bank) or mouse IgG was added. Antibody complexes were pulled down using blocked protein-A/G beads and washed through a series of buffers. Chromatin complexes were eluted off of beads by increasing pH (NaHCO3) and cross-links reversed at 65 C followed by protease and ribonuclease treatment. Chromatin fragments from the pull-down were phenol/chloroform extracted (for tissue chromatin, two rounds of ligation-mediated PCR amplification were used) and amplified by PCR with the following primers: −234/−12 region, 5′-CCTCTATCACATCAGGCTCTGTCT-3′ and 5′-AGATCTGAGGACAGATGACCTG-3′; −486/−283 region, 5′CCTATATAAGGAGAAGCCGGTGAG3′ and 5′-CTTCTGGTAGCCTACTTCATCTCC-3′.
Supplementary Material
Acknowledgments
We thank Lynda Elghazi and Beatriz Sosa-Pineda for providing us with the mouse ghrelin promoter construct. We thank members of the Sussel lab for critical reading of the manuscript.
Footnotes
This work was supported by American Diabetes Association Regular Research Grant 1-05-RA-131, National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (U01 DK072504), and the Naomi Berrie Diabetes Center (to L.S.); NIH Predoctoral Training Awards in Molecular Biology (T32-GM08730) and Endocrinology (T32-DK07328) (to J.H.); and National Institute of General Medical Sciences (F31-GM75456-01) (to C.C.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 4, 2009
Abbreviations: ChIP, Chromatin immunoprecipitation; e10.5, embryonic d 10.5.
References
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K 1999 Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660 [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S 2001 A role for ghrelin in the central regulation of feeding. Nature 409:194–198 [DOI] [PubMed] [Google Scholar]
- Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K, Hayashi T, Inoue G, Hosoda K, Kojima M, Kangawa K, Nakao K 2001 Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes 50:227–232 [DOI] [PubMed] [Google Scholar]
- Tschöp M, Smiley DL, Heiman ML 2000 Ghrelin induces adiposity in rodents. Nature 407:908–913 [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR 2000 The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141:4325– 4328 [DOI] [PubMed] [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR 2001 Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 86:5992 [DOI] [PubMed] [Google Scholar]
- Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R, Moncrieffe M, Thabet K, Cox HJ, Yancopoulos GD, Wiegand SJ, Sleeman MW 2004 Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci USA 101:8227–8232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Ahmed S, Smith RG 2003 Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol 23:7973–7981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro ML, Gaytán F, Caminos JE, Pinilla L, Casanueva FF, Aguilar E, Diéguez C, Tena-Sempere M 2002 Cellular location and hormonal regulation of ghrelin expression in rat testis. Biol Reprod 67:1768–1776 [DOI] [PubMed] [Google Scholar]
- Barreiro ML, Gaytan F, Castellano JM, Suominen JS, Roa J, Gaytan M, Aguilar E, Dieguez C, Toppari J, Tena-Sempere M 2004 Ghrelin inhibits the proliferative activity of immature Leydig cells in vivo and regulates stem cell factor messenger ribonucleic acid expression in rat testis. Endocrinology 145:4825–4834 [DOI] [PubMed] [Google Scholar]
- Hill JT, Mastracci TL, Vinton C, Doyle ML, Anderson KR, Loomis ZL, Schrunk JM, Minic AD, Prabakar KR, Pugliese A, Sun Y, Smith RG, Sussel L 2009 Ghrelin is dispensable for embryonic pancreatic islet development and differentiation. Regul Pept 157:51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama H, Funahashi H, Hirayama M, Takenoya F, Kita T, Kato S, Sakurai J, Lee EY, Inoue S, Date Y, Nakazato M, Kangawa K, Shioda S 2005 Morphological analysis of ghrelin and its receptor distribution in the rat pancreas. Regul Pept 126:67–71 [DOI] [PubMed] [Google Scholar]
- Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M 2000 Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141:4255–4261 [DOI] [PubMed] [Google Scholar]
- Gomez G, Englander EW, Greeley Jr GH 2004 Nutrient inhibition of ghrelin secretion in the fasted rat. Regul Pept 117:33–36 [DOI] [PubMed] [Google Scholar]
- Wei W, Wang G, Qi X, Englander EW, Greeley Jr GH 2005 Characterization and regulation of the rat and human ghrelin promoters. Endocrinology 146:1611–1625 [DOI] [PubMed] [Google Scholar]
- Hayashida T, Nakahara K, Mondal MS, Date Y, Nakazato M, Kojima M, Kangawa K, Murakami N 2002 Ghrelin in neonatal rats: distribution in stomach and its possible role. J Endocrinol 173:239–245 [DOI] [PubMed] [Google Scholar]
- Lee HM, Wang G, Englander EW, Kojima M, Greeley Jr GH 2002 Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology 143:185–190 [DOI] [PubMed] [Google Scholar]
- Bardram L, Hilsted L, Rehfeld JF 1990 Progastrin expression in mammalian pancreas. Proc Natl Acad Sci USA 87:298–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DJ, Hogg J, Petrik J, Arany E, Han VK 1999 Cellular distribution and ontogeny of insulin-like growth factors (IGFs) and IGF binding protein messenger RNAs and peptides in developing rat pancreas. J Endocrinol 160:305–317 [DOI] [PubMed] [Google Scholar]
- Wierup N, Kuhar M, Nilsson BO, Mulder H, Ekblad E, Sundler F 2004 Cocaine- and amphetamine-regulated transcript (CART) is expressed in several islet cell types during rat development. J Histochem Cytochem 52:169–177 [DOI] [PubMed] [Google Scholar]
- Wierup N, Yang S, McEvilly RJ, Mulder H, Sundler F 2004 Ghrelin is expressed in a novel endocrine cell type in developing rat islets and inhibits insulin secretion from INS-1 (832/13) cells. J Histochem Cytochem 52:301–310 [DOI] [PubMed] [Google Scholar]
- Jørgensen MC, Ahnfelt-Rønne J, Hald J, Madsen OD, Serup P, Hecksher-Sørensen J 2007 An illustrated review of early pancreas development in the mouse. Endocr Rev 28:685–705 [DOI] [PubMed] [Google Scholar]
- Wierup N, Svensson H, Mulder H, Sundler F 2002 The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Pept 107:63–69 [DOI] [PubMed] [Google Scholar]
- Chanoine JP, Wong AC 2004 Ghrelin gene expression is markedly higher in fetal pancreas compared with fetal stomach: effect of maternal fasting. Endocrinology 145:3813–3820 [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kang YM, Moon CS, Joe MK, Lim JH, Suh YH, Song J, Jung MH 2009 KLF4 positively regulates human ghrelin expression. Biochem J 420:403–411 [DOI] [PubMed] [Google Scholar]
- Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L 2004 Ghrelin cells replace insulin-producing β-cells in two mouse models of pancreas development. Proc Natl Acad Sci USA 101:2924–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Elghazi L, Martin S, Martins I, Srinivasan RS, Geng X, Sleeman M, Collombat P, Houghton J, Sosa-Pineda B 2008 Ghrelin is a novel target of Pax4 in endocrine progenitors of the pancreas and duodenum. Dev Dyn 237:51–61 [DOI] [PubMed] [Google Scholar]
- Chao CS, Loomis ZL, Lee JE, Sussel L 2007 Genetic identification of a novel NeuroD1 function in the early differentiation of islet α, PP and ε-cells. Dev Biol 312:523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle MJ, Loomis ZL, Sussel L 2007 Nkx2.2-repressor activity is sufficient to specify α-cells and a small number of β-cells in the pancreatic islet. Development 134:515–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Hayashida Y, Iguchi T, Nakao N, Nakai N, Nakashima K 2001 Organization of the mouse ghrelin gene and promoter: occurrence of a short noncoding first exon. Endocrinology 142:3697–3700 [DOI] [PubMed] [Google Scholar]
- Bertera S, Geng X, Tawadrous Z, Bottino R, Balamurugan AN, Rudert WA, Drain P, Watkins SC, Trucco M 2003 Body window-enabled in vivo multicolor imaging of transplanted mouse islets expressing an insulin-Timer fusion protein. Biotechniques 35:718–722 [DOI] [PubMed] [Google Scholar]
- Muhr J, Andersson E, Persson M, Jessell TM, Ericson J 2001 Groucho-mediated transcriptional repression establishes progenitor cell pattern and neuronal fate in the ventral neural tube. Cell 104:861–873 [DOI] [PubMed] [Google Scholar]
- Anderson KR, Torres CA, Solomon K, Becker TC, Newgard CB, Wright CV, Hagman J, Sussel L 2009 Cooperative transcriptional regulation of the essential pancreatic islet gene NeuroD1 (β2) by Nkx2.2 and neurogenin 3. J Biol Chem 284:31236–31248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watada H, Mirmira RG, Kalamaras J, German MS 2000 Intramolecular control of transcriptional activity by the NK2-specific domain in NK-2 homeodomain proteins. Proc Natl Acad Sci USA 97:9443–9448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller RS, Jenny M, Collombat P, Mansouri A, Tomasetto C, Madsen OD, Mellitzer G, Gradwohl G, Serup P 2005 Genetic determinants of pancreatic ε-cell development. Dev Biol 286:217–224 [DOI] [PubMed] [Google Scholar]
- Wang J, Elghazi L, Parker SE, Kizilocak H, Asano M, Sussel L, Sosa-Pineda B 2004 The concerted activities of Pax4 and Nkx2.2 are essential to initiate pancreatic β-cell differentiation. Dev Biol 266:178–189 [DOI] [PubMed] [Google Scholar]
- Sosa-Pineda B 2004 The gene Pax4 is an essential regulator of pancreatic β-cell development. Mol Cells 18:289–294 [PubMed] [Google Scholar]
- Andralojc KM, Mercalli A, Nowak KW, Albarello L, Calcagno R, Luzi L, Bonifacio E, Doglioni C, Piemonti L 2009 Ghrelin-producing epsilon cells in the developing and adult human pancreas. Diabetologia 52:486–493 [DOI] [PubMed] [Google Scholar]
- Cissell MA, Zhao L, Sussel L, Henderson E, Stein R 2003 Transcription factor occupancy of the insulin gene in vivo. Evidence for direct regulation by Nkx2.2. J Biol Chem 278:751–756 [DOI] [PubMed] [Google Scholar]
- Raum JC, Gerrish K, Artner I, Henderson E, Guo M, Sussel L, Schisler JC, Newgard CB, Stein R 2006 FoxA2, Nkx2.2, and PDX-1 regulate islet β-cell-specific mafA expression through conserved sequences located between base pairs −8118 and −7750 upstream from the transcription start site. Mol Cell Biol 26:5735–5743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgaard GA, Jensen JN, Jensen J 2003 FGF10 signaling maintains the pancreatic progenitor cell state revealing a novel role of Notch in organ development. Dev Biol 264:323–338 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.