Abstract
The two-subunit cytochrome c oxidase from Paracoccus denitrificans has been sequentially digested with chymotrypsin and Staphylococcus aureus V8 protease. The smaller subunit of the enzyme (apparent Mr 32,000) was split into numerous peptides that were removed by anion-exchange HPLC. The larger subunit was only digested to a limited extent (from an apparent Mr 45,000 to Mr 43,000), and the spectral properties were preserved relative to the native enzyme (a reduced minus oxidized difference spectrum with maxima at 447 and 607 nm in the Soret and alpha region, respectively). As judged from CO-reduced spectra this proteolytically digested, one-fragment oxidase was found to contain an equal amount of cytochromes a and a3. The enzymatic activity with reduced cytochrome c as substrate in the presence of Triton X-100 proceeded with equal affinity (apparent Km = 0.5-1.0 microM) and with a Vmax of approximately 20% (40 s-1) of that found with the native enzyme (200 s-1). When the assay system was supplemented with soybean phospholipids, the Km became 2 microM for both enzymes and the Vmax became 730 and 170 s-1 for the native and the digested enzyme, respectively. Thus subunit I of P. denitrificans oxidase, and most probably of the other cytochrome c oxidases as well, contains both hemes and at least one Cu atom and has significant enzymatic activity.
Full text
PDF
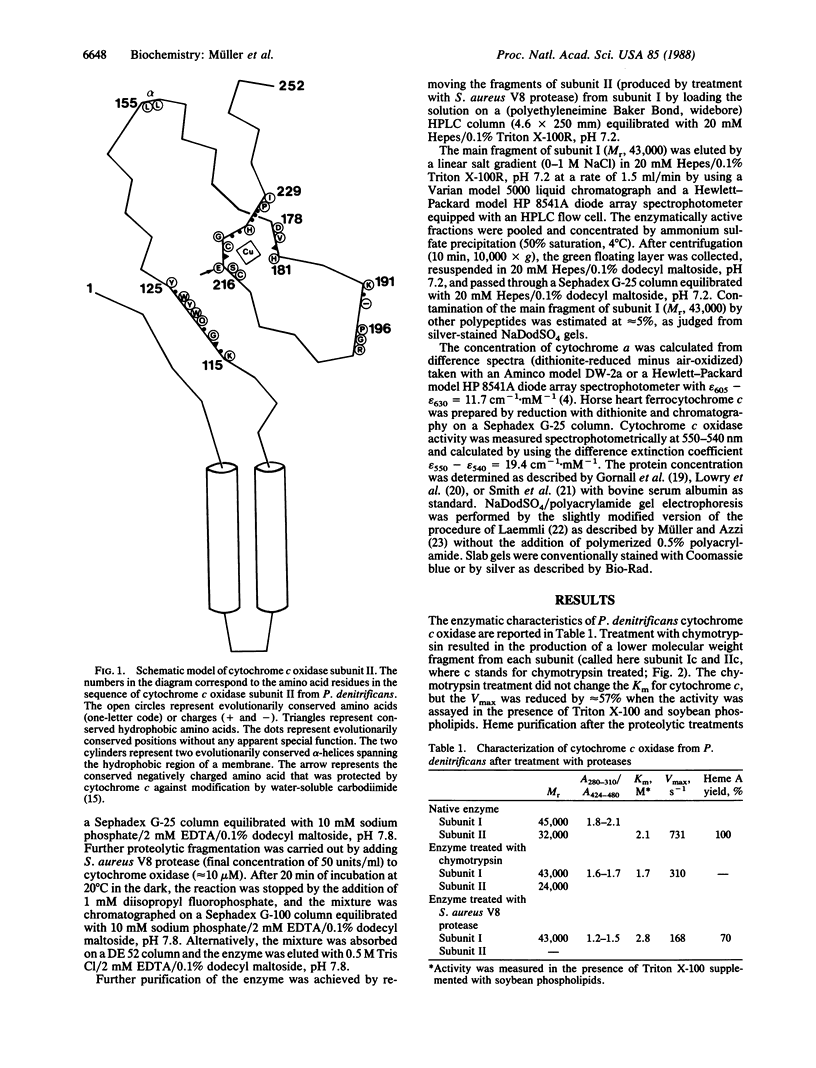
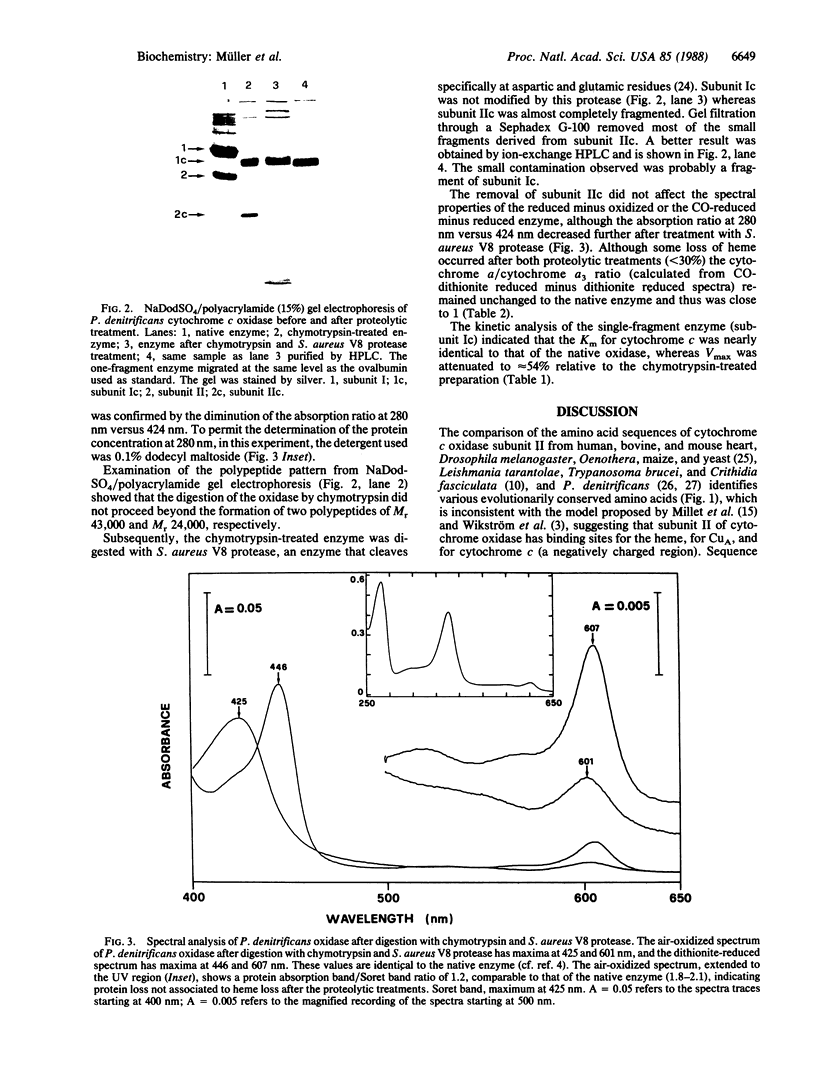


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benne R., Van den Burg J., Brakenhoff J. P., Sloof P., Van Boom J. H., Tromp M. C. Major transcript of the frameshifted coxII gene from trypanosome mitochondria contains four nucleotides that are not encoded in the DNA. Cell. 1986 Sep 12;46(6):819–826. doi: 10.1016/0092-8674(86)90063-2. [DOI] [PubMed] [Google Scholar]
- Bisson R., Gutweniger H., Montecucco C., Colonna R., Zanotti A., Azzi A. Covalent binding of arylazido derivatives of cytochrome c to cytochrome oxidase. FEBS Lett. 1977 Sep 1;81(1):147–150. doi: 10.1016/0014-5793(77)80948-4. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Eukaryotes with no mitochondria. 1987 Mar 26-Apr 1Nature. 326(6111):332–333. doi: 10.1038/326332a0. [DOI] [PubMed] [Google Scholar]
- Chance B., Kumar C., Powers L., Ching Y. C. "Peroxidatic" form of cytochrome oxidase as studied by X-ray absorption spectroscopy. Biophys J. 1983 Dec;44(3):353–363. doi: 10.1016/S0006-3495(83)84309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbley M. J., Azzi A. Resolution of bovine heart cytochrome c oxidase into smaller complexes by controlled subunit denaturation. Eur J Biochem. 1984 Mar 15;139(3):535–540. doi: 10.1111/j.1432-1033.1984.tb08038.x. [DOI] [PubMed] [Google Scholar]
- Drapeau G. R. Cleavage at glutamic acid with staphylococcal protease. Methods Enzymol. 1977;47:189–191. doi: 10.1016/0076-6879(77)47023-x. [DOI] [PubMed] [Google Scholar]
- Gelles J., Chan S. I. Chemical modification of the CuA center in cytochrome c oxidase by sodium p-(hydroxymercuri)benzoate. Biochemistry. 1985 Jul 16;24(15):3963–3972. doi: 10.1021/bi00336a025. [DOI] [PubMed] [Google Scholar]
- Gennis R. B., Casey R. P., Azzi A., Ludwig B. Purification and characterization of the cytochrome c oxidase from Rhodopseudomonas sphaeroides. Eur J Biochem. 1982 Jun 15;125(1):189–195. doi: 10.1111/j.1432-1033.1982.tb06667.x. [DOI] [PubMed] [Google Scholar]
- Hiesel R., Schobel W., Schuster W., Brennicke A. The cytochrome oxidase subunit I and subunit III genes in Oenothera mitochondria are transcribed from identical promoter sequences. EMBO J. 1987 Jan;6(1):29–34. doi: 10.1002/j.1460-2075.1987.tb04714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm L., Saraste M., Wikström M. Structural models of the redox centres in cytochrome oxidase. EMBO J. 1987 Sep;6(9):2819–2823. doi: 10.1002/j.1460-2075.1987.tb02578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac P. G., Jones V. P., Leaver C. J. The maize cytochrome c oxidase subunit I gene: sequence, expression and rearrangement in cytoplasmic male sterile plants. EMBO J. 1985 Jul;4(7):1617–1623. doi: 10.1002/j.1460-2075.1985.tb03828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadenbach B., Stroh A. Different reactivity of carboxylic groups of cytochrome c oxidase polypeptides from pig liver and heart. FEBS Lett. 1984 Aug 6;173(2):374–380. doi: 10.1016/0014-5793(84)80808-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li P. M., Gelles J., Chan S. I., Sullivan R. J., Scott R. A. Extended X-ray absorption fine structure of copper in CuA-depleted, p-(hydroxymercuri)benzoate-modified, and native cytochrome c oxidase. Biochemistry. 1987 Apr 21;26(8):2091–2095. doi: 10.1021/bi00382a005. [DOI] [PubMed] [Google Scholar]
- Ludwig B. Cytochrome c oxidase from Paracoccus denitrificans. Methods Enzymol. 1986;126:153–159. doi: 10.1016/s0076-6879(86)26017-6. [DOI] [PubMed] [Google Scholar]
- Ludwig B., Schatz G. A two-subunit cytochrome c oxidase (cytochrome aa3) from Paracoccus dentrificans. Proc Natl Acad Sci U S A. 1980 Jan;77(1):196–200. doi: 10.1073/pnas.77.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundeen M., Chance B., Powers L. The transmembrane helices of beef heart cytochrome oxidase. Biophys J. 1987 Apr;51(4):693-5, 697. doi: 10.1016/S0006-3495(87)83395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C. T., Scholes C. P., Chan S. I. The identification of histidine ligands to cytochrome a in cytochrome c oxidase. J Biol Chem. 1985 Mar 10;260(5):2857–2861. [PubMed] [Google Scholar]
- Millett F., de Jong C., Paulson L., Capaldi R. A. Identification of specific carboxylate groups on cytochrome c oxidase that are involved in binding cytochrome c. Biochemistry. 1983 Feb 1;22(3):546–552. doi: 10.1021/bi00272a004. [DOI] [PubMed] [Google Scholar]
- Mohana Rao J. K., Argos P. A conformational preference parameter to predict helices in integral membrane proteins. Biochim Biophys Acta. 1986 Jan 30;869(2):197–214. doi: 10.1016/0167-4838(86)90295-5. [DOI] [PubMed] [Google Scholar]
- Nicholls P., Chanady G. A. Titration and steady-state behaviour of the 830 nm chromophore in cytochrome c oxidase. Biochem J. 1982 Jun 1;203(3):541–549. doi: 10.1042/bj2030541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raitio M., Jalli T., Saraste M. Isolation and analysis of the genes for cytochrome c oxidase in Paracoccus denitrificans. EMBO J. 1987 Sep;6(9):2825–2833. doi: 10.1002/j.1460-2075.1987.tb02579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. A., Schwartz J. R., Cramer S. P. Structural aspects of the copper sites in cytochrome c oxidase. An X-ray absorption spectroscopic investigation of the resting-state enzyme. Biochemistry. 1986 Sep 23;25(19):5546–5555. doi: 10.1021/bi00367a030. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Steffens G. C., Biewald R., Buse G. Cytochrome c oxidase is a three-copper, two-heme-A protein. Eur J Biochem. 1987 Apr 15;164(2):295–300. doi: 10.1111/j.1432-1033.1987.tb11057.x. [DOI] [PubMed] [Google Scholar]
- Steffens G. J., Buse G. Studies on cytochrome c oxidase, IV[1--3]. Primary structure and function of subunit II. Hoppe Seylers Z Physiol Chem. 1979 Apr;360(4):613–619. [PubMed] [Google Scholar]
- Steinrücke P., Steffens G. C., Panskus G., Buse G., Ludwig B. Subunit II of cytochrome c oxidase from Paracoccus denitrificans. DNA sequence, gene expression and the protein. Eur J Biochem. 1987 Sep 15;167(3):431–439. doi: 10.1111/j.1432-1033.1987.tb13356.x. [DOI] [PubMed] [Google Scholar]
- Stevens T. H., Martin C. T., Wang H., Brudvig G. W., Scholes C. P., Chan S. I. The nature of CuA in cytochrome c oxidase. J Biol Chem. 1982 Oct 25;257(20):12106–12113. [PubMed] [Google Scholar]
- Vanneste W. H. The stoichiometry and absorption spectra of components a and a-3 in cytochrome c oxidase. Biochemistry. 1966 Mar;5(3):838–848. doi: 10.1021/bi00867a005. [DOI] [PubMed] [Google Scholar]
- Winter D. B., Bruyninckx W. J., Foulke F. G., Grinich N. P., Mason H. S. Location of heme a on subunits I and II and copper on subunit II of cytochrome c oxidase. J Biol Chem. 1980 Dec 10;255(23):11408–11414. [PubMed] [Google Scholar]
- Yoshida T., Lorence R. M., Choc M. G., Tarr G. E., Findling K. L., Fee J. A. Respiratory proteins from the extremely thermophilic aerobic bacterium, Thermus thermophilus. Purification procedures for cytochromes c552, c555,549, and c1aa3 and chemical evidence for a single subunit cytochrome aa3. J Biol Chem. 1984 Jan 10;259(1):112–123. [PubMed] [Google Scholar]