Abstract
The NADPH oxidase (Nox) family of enzymes is comprised of seven members, Noxes 1–5 and the Duoxes 1 and 2. Nox5 was the last of the conventional Nox enzymes to be identified, and in comparison to its siblings, much less is known about its molecular regulation and even less regarding its functional significance. The loss of Nox5 from rodent genomes has contributed significantly to this deficit in knowledge, but recent discoveries have narrowed the gap. There are many differences between Nox5 and the other Nox isoforms including alternative splicing, transcriptional regulation, enzymatic control mechanisms, tissue distribution, and intracellular trafficking. The goal of this review is to outline recent advances in our knowledge of the genetic regulation, the molecular mechanisms governing its activity, and the functional significance of Nox5 in human physiology and pathophysiology. Antioxid. Redox Signal. 11, 2443–2452.
Introduction
It is well established that gp91phox (NADPH oxidase2 or Nox2) plays an important role in the function of phagocytic immune cells by generating large concentrations of superoxide and derivative reactive oxygen species (ROS) that participate in the killing of invading pathogens (4, 10). The discovery of distinct, yet closely related, Nox isoforms (Noxes 1, 3, and 4) and their expression in cells outside of the immune system has led to the pursuit of novel functional roles for these proteins (6–8, 18, 45, 68). Indeed, Noxes1, 2, and 4 have been shown to have important roles in both the physiological and pathophysiological function of the cardiovascular, pulmonary, and renal systems, and may also regulate the neoplastic potential of cells (10, 44). Nox3 plays a key role in the morphogenesis of inner ear otoconia and is vital for proper functioning of the vestibular system (53, 54). The molecular steps controlling the activity of Noxes1–4 have been intensively investigated over the past decade and have recently been summarized in a number of comprehensive review articles (10, 46, 60, 69). In comparison, the functional significance of Nox5 is poorly understood. However, this is changing rapidly, and recent studies have provided exciting new data that the regulation of Nox5 goes beyond the simple elevation of intracellular calcium and that in human cells, Nox5 is important for physiological and pathophysiological processes. This review will address the molecular regulation of Nox5 and highlight its participation in the regulation of cellular function.
Discovery and Genetic Regulation
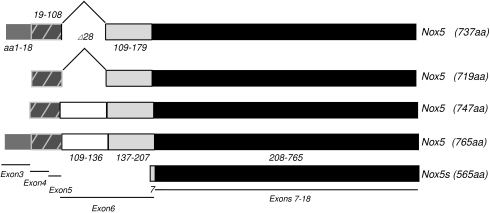
NADPH oxidase 5 or Nox5 was the fifth member of the Nox family of enzymes (excluding the Duoxes) to be identified and is genetically the most distinct. It was first reported in 2001 by two independent groups who discovered mRNA transcripts of a novel protein in human spleen, testis, and kidney that had significant homology to Nox2 and Nox1(8, 18). Currently there are five known splice variants of Nox5, Nox5α, β, δ, and γ, and a truncated variant (Nox5-S or ɛ). The Nox5 gene is located on chromosome 15 and is comprised of at least 18 exons with alternative use of exon 3 to generate the novel N-terminus of Nox5α and γ. Exons 4 and 5 are common to all isoforms, except for the short variant that does not possess the regulatory N-terminal EF-hand regions. Exon 6 is subject to intraexon splicing that yields three distinct protein regions, Nox5α/β, Nox5δ/γ, and the truncated Nox5-S. The remaining exons 7–18 are common to all isoforms (Fig. 1). Alternative splicing also leads to differences in the length of the 5′ untranslated region (8) and may be indicative of the presence of multiple promoter regions. In general, Nox5α and β are the most abundant isoforms expressed in cells. In some cell types, such as endothelial and vascular smooth muscle cells, all 5 isoforms are expressed together in varying proportions (11, 34). Alternatively, expression of individual isoforms can show a restricted pattern of distribution with Nox5α being the predominant Nox5 isoform in the spleen and Nox5β, the predominant isoform in the testis (8). In esophageal cancer cells, Nox5-S is the dominant isoform (24).
FIG. 1.
Representation of the genomic organization of Nox5 isoforms (based on homo sapiens chromosome 15, reference assembly NC_000015 region: 67009918.67136127). Numbers represent amino acids in each isoform. Accession numbers for Nox5α AF353088, Nox5β AF325189, Nox5δ AF325190, Nox5γ AF353089, Nox5-S AF317889.
Orthologs of Nox5 are present in the genomes of a number of other species ranging from mammals such as monkey, bovine, and canine, to fish such as zebrafish, and invertebrates such as sea urchins (39). However, Nox5 is absent from the rodent (rat, mouse) genomes. The functional significance of this is not yet clear, and it remains to be determined if rodents have developed mechanisms to compensate for the loss of Nox5 or whether the presence of Nox5 in the human genome is purposeful or simply vestigial. Given the high level of expression of Nox5 in the testis, particularly in pachytene spermatocytes, it might simply be a very good example of what has been described as antagonistic pleiotropy (44). This hypothesis posits that genes providing an early reproductive advantage, which may very well be the case with Nox5 and spermatogenesis, also contribute to chronic disease later in life. A major caveat to this hypothesis is that the functional significance of Nox5 is poorly understood. While we can assume, based on the literature, that increased expression of Nox5 and ROS production in cardiovascular disease (30) and cancer (15, 24, 35, 66) is detrimental, the possibility remains that Nox5 may be have important physiological roles that are yet be discovered. Regardless of the reasons, our dependence on rodents as models of human disease and the absence of Nox5 in rodent genomes have both contributed to our relatively poor understanding of the functional significance of Nox5 in humans.
Molecular Regulation
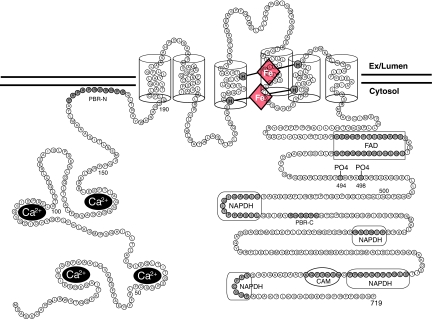
With the exception of Nox5-S, little is known about the functional significance of the various Nox5 splice variants. There is considerable controversy over whether Nox5-S, which lacks the N-terminal EF hand, can even produce superoxide. A number of studies have shown that Nox5-S is active basally in cells but does not respond to ionomycin (11, 24, 67), whereas others have reported that it is devoid of activity (8). It is possible that there are other cytosolic factors that could enable Nox5-S to function in select cell types, but these have not been reported. Although Nox5 is genetically the most distinct Nox isoform (27% identity versus Nox2), it retains significant structural similarity when compared to the other isoforms and is predicted to be an integral membrane protein with six membrane spanning α-helices (Fig. 2). The transmembrane domains 3 and 5 contain four highly conserved histidine residues (H268, H282, H356, and H369 of Nox5β) that coordinate the binding of two distinct heme molecules (Fig. 2). Equivalent histidines residues are highly conserved in other Nox isoforms. While these residues on Nox5 have not yet been proven to bind heme, it is very likely, given the conservation between isoforms. The C-terminus of Nox5 contains highly conserved binding sites for FAD and NADPH, which is also in agreement with that reported for other Nox isoforms (8, 38) (Fig. 2). The core function of Nox5 is based on a conformational change induced by calcium or other stimuli that enables electron flow from NADPH through the flavin FAD to the di-hemes for insertion into molecular oxygen and ultimately the release of superoxide.
FIG. 2.
Predicted transmembrane topology of Nox5 and functional motifs. The N-terminus contains four calcium-binding EF hands and a polybasic domain PBR-N. Six well-conserved regions span the membrane six times and coordinate the binding of two heme moieties via four histidine residues present on transmembrane domains III and V. The C-terminus contains a polybasic domain (PBR-C), conserved binding sites for NADPH and FAD, phosphorylation sites Thr494 and Ser498, and a calmodulin (CAM) binding site. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
The activity level of Noxes 1–3 is tightly regulated by the coordinated assembly of a number of distinct cytoplasmic proteins that coalesce into a functional enzymatic unit (10, 69). The regulation of Nox5 activity diverges from this theme and is not at all dependent on the presence of cytosolic factors that are known to activate Noxes1–3 (8). In addition, Noxes 1–4 have an absolute requirement for the transmembrane protein p22phox which they bind tightly. The p22phox:Nox heterodimer improves protein stability and coordinates the assembly of the activation complex (21). Nox5 has also been shown to bind p22phox, but the significance of this is unclear, as changes in the expression level of p22phox or dominant negative constructs of p22phox do not affect Nox5 activity (11, 40). Instead, the activity of Nox5 is governed by a unique intramolecular mode of regulation that is encoded within a single gene. As a result, the mRNA for Nox5 encodes a larger protein which is primarily distinguished from the other Nox isoforms by an N-terminal extension that contains four calcium binding EF hands.
The elevation of intracellular calcium promotes the occupation of the calcium-binding EF hand domains of Nox5. This triggers a conformational change between them to expose a hydrophobic motif that binds to a yet to be identified region in the C-terminus that enables electron flow to the heme moieties and consequently drives superoxide production (8). Therefore it is expected that extracellular stimuli that mobilize intracellular calcium would robustly increase superoxide in cells expressing Nox5. However, in a cell-free activity assay, the concentration of calcium required to maximally activate Nox5 was shown to be unusually high and unlikely to be achieved inside most cells (8). This raised the important question of how do cells adequately activate Nox5 in order to coordinate the appropriate response to extracellular stimuli. One possible explanation for this was shown recently by the ability of the phorbol ester/DAG mimetic (PMA) to robustly activate Nox5 without elevating intracellular calcium (33, 62). This indicated a new mode of regulation that was later determined to be due to the PKC-dependent phosphorylation of Nox5β on threonine 494 and serine 498 (Fig. 2). These modifications increase the calcium sensitivity of the enzyme and permit a higher level of enzyme activity at resting levels of intracellular calcium. In addition to being able to activate Nox5 without elevating calcium, PMA was shown to greatly potentiate the ability of Nox5 to produce superoxide in response to low concentrations of ionomycin, an effect dependent on the phosphorylation of these residues (33). Thus, simultaneous activation of both PMA-dependent and calcium-dependent pathways produces a much greater increase in superoxide production at lower levels of calcium. The ability of phosphorylation to modulate the response to calcium-dependent signals adds an additional level of control and thus diversifies the ability of Nox5 to produce ROS in response to appropriate stimuli.
Another mode of calcium sensitization that has been reported is via the direct binding of calcium-activated calmodulin. The C-terminus of Nox5 contains a calmodulin binding site that is situated between the two terminal NADPH binding sites (Fig. 2) and is well conserved among Nox isoforms. This binding of calcium-bound calmodulin to Nox5, but not to other Nox isoforms, was shown to increase its sensitivity to calcium (74). Given the location of the calmodulin-binding motif, it is reasonable to speculate that calmodulin binding promotes electron transfer, but the precise mechanism by which this occurs in Nox5 is not yet known. Adding to the intrigue, other Nox isoforms such as Nox4 also posses a calmodulin-binding site in the C-terminal end of the enzyme, but calmodulin does not increase ROS production (74). Recently, another element of regulatory control over Nox5 activity has been reported. The proto-oncogenic tyrosine kinase, c-abl, can bind to and increase Nox5 activity via a calcium-sensitive, hydrogen peroxide-dependent signaling pathway (22). The functional significance of this with regard to cancer, in particularly chronic myelogenous leukemia, is not well understood.
Subcellular Location
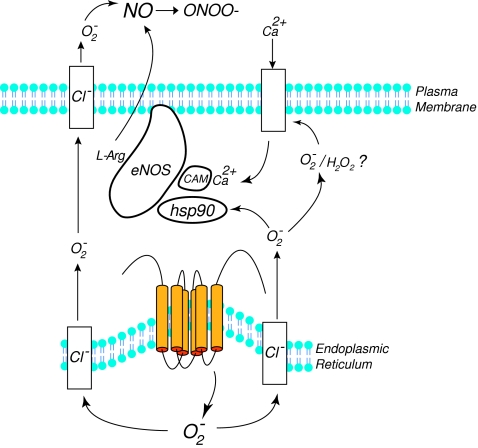
The intracellular location of NADPH oxidases can have a profound impact on the range of target molecules that can be modified by superoxide or secondary ROS (17, 73, 75). Indeed, spatial restriction is an important strategy used by other enzymes that generate highly reactive free radical gases such as the endothelial nitric oxide synthase (eNOS) (32, 79). Most studies to date have reported that Nox5 is expressed primarily within intracellular membranes. In transfected COS-7 and HEK293 cells and also in cultured human endothelial cells and prostate cancer cells, Nox5 can be found predominantly in perinuclear organelles that overlap with markers for the endoplasmic reticulum (11, 15, 33, 38, 62). Equivalent findings were achieved using the distinct approaches of both GFP fusion proteins in live cells and immunofluorescence of native proteins in fixed cells. The intracellular location of Nox5 also closely resembles that reported for other Nox isoforms in a variety of cell types (2, 9, 17, 77). Consistent with a location on intracellular membranes, the ability of Nox5 to produce superoxide inside the cell has also been reported (8, 15, 61). However, as depicted in Fig. 3, the location of Nox5 on intracellular membranes presents a logistical problem, as the superoxide generated inside the cell would be expected to be accumulate within these intracellular organelles. Superoxide is a charged molecule that is very reactive and it is widely assumed that it cannot traverse biological membranes unassisted (49, 52). Nox5 has been reported to produce both superoxide and hydrogen peroxide (8, 62) and hydrogen peroxide can readily pass through lipid membranes and be measured in the extracellular space. However, SOD-sensitive superoxide generated by Nox5 can be readily detected outside of the cell (8, 33). How this occurs remains poorly understood. It is known that a reduction in pH can promote the formation of hydroperoxyl radicals which can then traverse biological membranes. However, the pH of the endoplasmic reticulum is near neutral and is effectively connected to pH changes in the cytoplasmic environment (41), and thus the vast majority of superoxide formed is likely to remain in the charged water- soluble and membrane-impermeant state. An alternative pathway has recently been revealed by studies showing that endosomal and plasma membrane chloride channels rapidly conduct superoxide from intracellular organelles to the extracellular space (31, 52) (Fig. 3). It is suggested that superoxide rapidly traverses these channels to gain access to the cytosol or extracellular space.
FIG. 3.
Schematic depicting the pathways by which Nox5 activates eNOS. Co-expression of eNOS and Nox5 increases the activity of eNOS by promoting hsp90 binding. The release of superoxide from within intracellular organelles such as the ER is proposed to occur through chloride channels (Cl−) which also facilitate the release of superoxide to the extracellular space. Formation of hydrogen peroxide (H2O2) within the cell may also stimulate calcium entry which activates eNOS. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
In addition to these pathways, the possibility remains that extracellular superoxide may be generated by a comparatively smaller pool of highly active Nox5 that is present at the plasma membrane (38, 62). Indeed, it has been recently demonstrated that polybasic domains within the N-terminus of Nox5 (PBR-N, Fig. 2), bind phosphatidylinositol 4,5-bisphosphate, a phospholipid enriched at the plasma membrane, which promotes the trafficking and cell surface expression of Nox5 and accordingly enhances the release of superoxide into the extracellular space (38). The PBR-N is a highly conserved domain that is present in most orthologs of Nox5 and may account for the presence of Nox5 at the plasma membrane that has been reported in a number of cell types (38, 62). The C-terminus of Nox5, between the first two predicted NADPH binding sites (Fig. 2), contains another conserved polybasic domain that has been called the PBR-C. Mutation of this region reduces catalytic activity but does not affect intracellular location. The significance of this region is pending further investigation (38).
The functional relevance of intra- versus extracellular superoxide derived from Nox5 remains to be established. Based on studies investigating the interaction between Nox5 and eNOS, the production of intracellular superoxide is much more important for modifying eNOS activity compared to the extracellular release (80). Presumably this is due to the inability of extracellular superoxide to gain access the same intracellular environment at effective concentrations. It is also likely that other signaling events such as inhibition of protein tyrosine phosphatases are dependent on the intracellular release of superoxide and local formation of hydrogen peroxide (17, 73, 75).
The next section will focus on the relative expression of Nox5 in various tissues and highlight its potentials roles in cellular function.
Tissue Distribution of Nox5
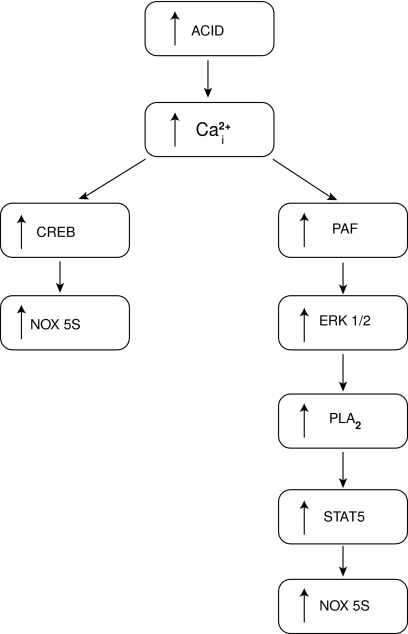
Nox5 is highly abundant in the spleen and testis (8) and to a lesser extent it is expressed in vascular tissue, cells of the gastrointestinal tract, reproductive systems, fetal organs, and various cancers (8, 18, 44). The transcriptional regulation of Nox5, which dictates its tissue- or cell-specific expression, is poorly understood. Acid treatment of esophageal adenocarcinoma cells and thrombin-treatment of endothelial cells have both been shown to increase Nox5 expression (11, 24). Both of these interventions elevate intracellular calcium, and Fu et al. reported that the calcium-sensitive transcription factor CREB can modulate expression levels of Nox5-S (24). Knockdown of CREB prevents acid-induced upregulation of Nox5, and chromatin immunoprecipitation (ChIP) assays in lysates of esophageal adenocarcinoma cells using CREB antibodies confirm the binding of CREB to a putative Nox5 promoter region (24). Two cAMP response elements are found in this promoter region (−6 to −1396 of ATG for Nox5-S). Another study by the same group reported that STAT5 could also modulate Nox5 expression, and one STAT5 binding site was found in the promoter for Nox5-S (−2249 to −2240) (66) (Fig. 4). It is important to note that this promoter region is significantly downstream to the transcriptional start site for the other isoforms of Nox5 and indicates the strong likelihood that other regions of the Nox5 gene and possibly other transcription factors are important for transcriptional regulation of Nox5. The promoter region(s) and transcription factors that govern the expression of the EF-hand containing Nox5 isoforms (α, β, δ, γ) are not yet described.
FIG. 4.
Mechanisms by which acid exposure increases the expression of Nox5-S in SEG1-EA cells.
The Cardiovascular System
NADPH oxidases have been shown to participate in virtually all aspects of cardiovascular function, including cell signaling, proliferation, apoptosis, permeability, migration, angiogenesis, hypertension, diabetes, atherosclerosis, and vasomotion. The primary Noxes described in blood vessels are Nox1, Nox2, and Nox4 (14, 28, 60). More recently, the mRNA and protein for Nox5 have been detected in human blood vessels (8, 11, 30, 34). To date, expression of Nox5 has been detected within blood vessels of the spleen and lung (11) and also in coronary blood vessels that perfuse the myocardium (30). Within these blood vessels, Nox5 has been detected in both the endothelial cell layer and in the underlying smooth muscle cells of the tunica media (11, 30). In cultured cells, expression of Nox5 has been documented in both endothelial and vascular smooth muscle cells (8, 11, 34). Fibroblasts do not appear to express Nox5 (Jagnandan and Fulton, unpublished observation).
The expression level of Nox5 is altered in cardiovascular disease. In particular, coronary artery disease is associated with increased expression of Nox5. These findings suggest that it may participate in the chronic dysfunction of vascular cells that is intimately connected with development of atherosclerosis. In blood vessels from individuals without coronary artery disease, Nox5 expression is very low, but is substantially increased in blood vessels with disease. At the cellular level, endothelial staining was evident in healthy vessels but was lost in those with more advanced lesions. In blood vessels with moderate lesions, robust staining was observed in the proliferating cells of the neointima. Staining was most intense in cells underlying advanced plaques. The cell types expressing Nox5 co-stained with smooth muscle markers and did not overlap with T-cell or macrophage specific markers (30), indicating that expression of Nox5 was confined to the vascular cell types.
Endothelial Cells
All five isoforms of Nox5 are expressed in endothelial cells and generate ROS in response to thrombin and ionomycin (11). The increased expression of Nox5 in endothelial cells encourages cellular proliferation and promotes the organization of endothelial cells into three-dimensional structures that resemble capillary networks. Knockdown of endogenous Nox5 expression with siRNA attenuates these effects and suggests that Nox5 is necessary for the normal function of endothelial cells. Increased expression of Nox5 transgenes in endothelial cells directly inhibits the extracellular actions of nitric oxide and prevents both cGMP accumulation and endothelium-dependent relaxation. Presumably these effects occur through the extremely avid interaction between nitric oxide and superoxide that yields peroxynitrite, as they are readily reversed by scavenging superoxide with extracellular superoxide dismutase (80). The direct scavenging of NO is unlikely to account for the increased proliferation and pro-angiogenic properties of Nox5 in the endothelium as NO itself is pro-proliferative and pro-angiogenic (57). Therefore, it is likely that ROS derived from Nox5 have other signaling actions within the cell.
Investigation into the effects of Nox5-derived ROS on endothelial function also revealed a paradoxical relationship. Instead of inhibiting eNOS activity as would have been predicted from the many studies showing that elevated ROS compromises BH4 levels and promotes the degradation of the active NOS dimer into inactive monomeric subunits (1, 16, 47), eNOS enzymatic activity was robustly increased. This ability of Nox5 to activate eNOS was shown in a heterologous expression system in COS cells, in cultured endothelial cells, and also in intact blood vessels. This effect was independent of changes in the phosphorylation of key sites on eNOS and was associated with an increase in eNOS:hsp90 binding (80). Hsp90 is a molecular chaperone that is important for overall eNOS enzymatic activity and also for the fidelity of NO synthesis (25, 55). Decreased eNOS:hsp90 binding is associated with the loss of NO production and a corresponding increase in superoxide production, a term coined NOS uncoupling (55, 56). In contrast, the increased hsp90 binding induced by Nox5 may actually prevent eNOS uncoupling and provide increased NO output to compensate for the reduced ability to activate NO-sensitive pathways. Another possible mechanism for the increase in eNOS activity could be due to the ROS-stimulated elevation of intracellular calcium (58), and calcium-activated calmodulin is the primary mechanism for eNOS activation (25) (Fig. 3). The depletion of tetrahydrobiopterin (BH4), a key NOS co-factor is widely reported to be a consequence of increased peroxynitrite production in the endothelium (42). Thus, another surprising observation was that the relatively short-term overproduction of ROS in endothelial cells via Nox5 was not sufficient to reduce BH4 levels to amounts that would constrain NO production. One possibility is that the ratio of hydrogen peroxide produced by Nox5, which stimulates BH4 synthesis (65), versus peroxynitrite which oxidizes BH4 (42) favors hydrogen peroxide, or alternatively that peroxynitrite is formed outside the cell.
Smooth Muscle
It is well established that platelet-derived growth factor (PDGF) is a potent mitogen for vascular smooth muscle cells (VSMC) and that its ability to increase cellular proliferation is sensitive to modulation of ROS levels (13, 64, 70). The importance of Nox5 to these events in human VSMC was unappreciated until recently. Nox5 expression has been documented in VSMC from human coronary arteries, aorta, and blood vessels of the spleen and lung (8, 11, 30, 34).The first indication that endogenously expressed Nox5 could contribute to VSMC function was the observation that PDGF-stimulated ROS production is dependent on changes in the level of intracellular calcium. This stimulus-driven elevation in ROS is primarily derived from Nox5 as it was reduced in VSMC in which Nox expression levels were suppressed by siRNA to Nox5, but not to Nox4. Furthermore, the ability of PDGF to phosphorylate Jak2 and activate the Jak2/stat3 signaling pathway and increase VSMC proliferation was blunted by depletion of Nox5 with siRNA (34). The precise mechanism by which Nox5 increases Jak2 phosphorylation and cellular proliferation is not yet known, but given the sensitivity of protein tyrosine phosphatases to ROS it seems likely that Nox5 inactivates specific PTPs (17, 36, 73, 75) (Fig. 5). In diseased coronary arteries, increased expression of Nox5 is seen in the proliferating smooth muscle cells of the neointima and staining is strongest in advanced lesions (30). While currently there is no evidence to suggest that Nox5 can modify the contractile responses of vascular smooth muscle, there is substantial indirect evidence to suggest that this is possible. Endothelial expression of Nox5 can potentiate contractile responses to phenylephrine (80), but this is more likely to be due to the direct scavenging of nitric oxide that is released from the endothelium to counterbalance changes in vascular tone (27, 29). A prominent cardiovascular effect of Nox1, which is primarily expressed in vascular smooth muscle, is to potentiate the pressor response to angiotensin II (20, 26). Orthologs of Nox5 in Drosophila have been shown to increase smooth muscle contraction through the formation of hydrogen peroxide (58). Collectively these findings suggest that Nox5 is expressed in vascular tissue and may contribute to a number of physiological functions, as well as diseases of the cardiovascular system.
FIG. 5.
Nox5-dependent signaling pathways that stimulate cellular proliferation. EA, esophageal adenocarcinoma; HCL, hairy cell leukemia; VSMC, vascular smooth muscle cells.
The Immune System
Nox5 was initially characterized as a gene that is highly expressed in the spleen and other lymphoid tissue such as the lymph nodes (8). While the expression of Nox5 is abundant in areas rich in mature B- and T-lymphocytes, it is not expressed in circulating lymphocytes. The significance of this is not well understood and it has been speculated that Nox5 in lymphocytes might participate in calcium signaling, proliferation, differentiation, and apoptosis (8). In rodents, Nox1 and 2 are the primary isoforms expressed in the spleen (50), whereas in humans it is Nox2 and Nox5 (18). ROS may contribute to aspects of lymphocyte signaling that are dependent on tyrosine phosphorylation by modifying the activity of protein tyrosine phosphatases such as SHP-2 (43). In addition, the absence of Nox5 in immune cells that are present in advanced atherosclerotic lesions (30) also suggests that Nox5 may be important in the development of the immune system but does not play an active role in cells in the periphery.
The Reproduction System
The presence or increased expression of genes that are detrimental to normal physiology represent an evolutionary enigma as they make little sense in the survival of the fittest. However, some of these genes may confer an early survival advantage by being expressed and having important functions in reproductive tissues. Indeed, this may be the case for Nox5. The mRNA for Nox5 is highly expressed in the testes and in particular is found in pachytene spermatocytes and to a lesser extent in round spermatids (8). Immunolocalization shows abundant staining near the lumen of the seminiferous tubules, associated with maturing spermatids, and in ejaculated spermatozoa Nox5 was detected in the rostral sperm head (59). Given the lack of direct functional studies, it is not yet clear whether Nox5 has a meaningful role in male reproductive function. Despite that limitation, considerable evidence exists to support the importance of ROS generation to a number of vital male reproductive functions including sperm maturation, capacitation, regulation of intracellular pH, hyperactivation, and acrosomal exocytosis (5, 19, 23). These events are associated with increased tyrosine phosphorylation and nitrosative modification of various signaling proteins. Collectively, the high level of expression of Nox5 and the importance of ROS in general to testicular function makes it difficult to envisage that Nox5 does not contribute significantly to reproductive function
Nox5 is also expressed in female reproductive organs, including the uterus, ovaries, and placenta (8, 18). As with male reproductive function, a lack of direct evidence limits our understanding of what specifically Nox5 contributes to the function of the female reproductive system. This is further complicated by a lack of general knowledge of what ROS contribute to these functions in general. Orthologs of Nox5 in Drosophila (dNox) are important for muscular contractions of the ovaries and egg laying. It is proposed that in response to a calcium stimulus, dNox5 generates hydrogen peroxide which promotes additional calcium entry and this positive feedback loop is responsible for a significantly greater muscular contraction. Loss of dNox reduces intracellular calcium levels, weakens ovarian contraction, and renders the insects sterile. The mechanism by which dNox promotes calcium influx is not known, but hydrogen peroxide is known to significantly elevate calcium in smooth muscle cells (3, 72). By analogy, it is possible that in humans Nox5 contributes to smooth muscle contraction in female reproductive organs. Other possible functions include angiogenesis during the menstrual cycle, NF-κB activation, apoptosis, and cellular proliferation (10).
Cancer
For many years ROS have been promoted as causative factors in the uncontrolled growth of neoplastic cells. Cancerous cells generate large quantities of ROS and have been shown to overexpress Nox enzymes and underexpress antioxidant defense enzyme (44, 71). However, much like cardiovascular disease, the ineffectiveness of antioxidants in vivo to prevent or treat cancer has been a major obstacle to the broad acceptance of this hypothesis (48, 63). There may be several reasons for this, as ROS can participate in opposing intracellular signaling pathways that not only promote growth but also stimulate apoptosis (10, 12, 60). In vitro, where variables can be more rigorously controlled, increased expression of Nox enzymes (Nox1) can in itself promote the transformation and uncontrolled growth of cells (68). This observation provided some of the first evidence that Nox-derived ROS in itself could directly modulate cellular growth and metastatic potential. Depending on the cell type of origin, cancer cells can differentially express individual Nox isoforms. However, the genomes of these cells are highly plastic and the level of expression of specific isoforms can vary significantly. The expression of Nox5 has been documented in a number of cancers or cancer cell lines including prostate (15), pancreatic (51), hairy cell leukemia (35), and esophageal cancer (24).
Consumption of antioxidants such as lycopene has been associated with reduced prostate cancer risk (76), and scavenging of ROS with antioxidants increases prostate cancer survival in mice (78). However, the role of ROS and lycopene in prostate cancer is not without controversy (37). The source of ROS in prostate cancer is poorly understood, but Nox5 protein and mRNA have been detected in human prostate cancer and the prostate cancer cell lines LNCaP and DU 145 (15). In DU145 cells, ROS production is calcium dependent, and antisense knockdown of Nox5, but not p22phox or Nox2, inhibits both ROS production and cellular proliferation. Inhibition of Nox activity with diphenyleneiodonium (DPI) or antioxidants increased apoptosis of DU145 cells. The proposed mechanism by which Nox5-derived ROS promotes the proliferation of prostate cells is through the reduced ability of the CREB/ATF transcription factors to bind DNA and decreased apoptosis(15). MAPK and NF-κB pathways were not involved. Of note, Nox5 was also strongly expressed in normal prostate tissue and this indicates that ROS production as an independent variable in these cells is unlikely to be sufficient to induce transformation. Thus, it is more likely that Nox5 acts in concert with other factors or cellular events to promote the proliferation of prostate cancer cells.
Repeated exposure of the esophagus to stomach acids, as in the case of gastroesophageal reflux disease (GERD), damages the squamous epithelium lining and promotes intestinal metaplasia. This condition, called Barrett's esophagus (BE), can lead to esophageal adenocarcinoma (EA) that has a particularly poor prognosis. The mechanism by which acid drives this process was unknown until recently when it was discovered that acid regulates the expression of Nox5 in esophageal adenocarcinoma cells (24). In this study, the authors found that acid treatment specifically increased the expression of the Nox5-S isoform and that this occurred in a calcium-dependent manner and was mediated by the calcium-sensitive transcription factor, CREB. Knockdown of Nox5-S with siRNA decreased ROS production in esophageal adenocarcinoma cells, dramatically inhibited cellular proliferation, and promoted apoptosis. Nox5-derived ROS also increases the expression of cyclooxygenase 2 which increases the proliferation of adenocarcinoma cells via the synthesis of prostaglandin E2 (67). Further studies by the same group have shown that PAF participates in the acid-induced upregulation of Nox5 and increases Nox5 expression via activation of the transcription factor STAT5 (66) (Fig. 4).
Another type of cancer expressing Nox5 is hairy cell leukemia (HCL). This cancer is characterized by the chronic overproduction of malignant B lymphocytes that contain thin membranous projections (hairy). ROS production in hairy cell (HCL) clones is sensitive to the nonselective flavoprotein inhibitor DPI and calcium modulation. While DPI, which inhibits all of the Nox enzymes, does not provide direct evidence for the involvement of Nox5, ROS production correlates linearly with the expression level of Nox5 protein. Inhibition of Nox5 with DPI also increases the activity of Src homology region 2 domain-containing phosphatase 1 (SHP-1). This effect was specific for SHP-1 as other tyrosine phosphatases, PTP-1B, and SHP-2 were not affected. The loss of SHP-1 increased total protein tyrosine phosphorylation(35), but the functional effects of this are not yet known (Fig. 5). The ability of the proto-oncogenic kinase C-able to increase Nox5 activity also suggests that it may play an important role in cancers such as leukemia (22). From these findings, it is clear that Nox5 is expressed in certain types of cancers and can contribute to the increased proliferation seen in these cells. However, given our comparatively poor understanding of Nox5, it is likely that the number of cancer cells expressing Nox5 will continue to grow.
Concluding Remarks
In the 8 years following the initial reports that described a new member of the NADPH oxidase family, we have gained considerable insights into the molecular regulation of Nox5. We now know where Nox5 resides within the cell and that calcium is far from the only mechanism governing its activity. We also know that Nox5 is expressed in a variety of cells outside the testis and lymphoid tissue, and that it participates in both physiological and pathophysiological functions. The lack of Nox5 in rodent genomes has hindered research into the functional significance of Nox5 and has also brought forth the proposition that it is not an essential gene and is the most dispensable of the Nox isoforms. The challenge ahead will be to identify whether Nox5 is necessary and sufficient for cellular function (or dysfunction) in the cell types in which it is expressed. To date, there are no selective inhibitors of Nox5 and the development of pharmacological tools to suppress Nox5 activity would be an invaluable resource to aid in this endeavor.
Abbreviations Used
- BH4
(6R)-5,6,7,8-tetrahydrobiopterin
- Duoxes
dual oxidases
- eNOS
endothelial nitric oxide synthase
- NO
nitric oxide
- Nox
NADPH oxidase
- phox
phagocyte oxidase
- PMA
phorbol 12-myristate 13-acetate
- ROS
reactive oxygen species
References
- 1.Alp NJ. Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:413–420. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- 2.Ambasta RK. Kumar P. Griendling KK. Schmidt HH. Busse R. Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 3.Ardanaz N. Beierwaltes WH. Pagano PJ. Distinct hydrogen peroxide-induced constriction in multiple mouse arteries: Potential influence of vascular polarization. Pharmacol Rep. 2008;60:61–67. [PubMed] [Google Scholar]
- 4.Babior BM. Lambeth JD. Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 5.Baker MA. Aitken RJ. The importance of redox regulated pathways in sperm cell biology. Mol Cell Endocrinol. 2004;216:47–54. doi: 10.1016/j.mce.2003.10.068. [DOI] [PubMed] [Google Scholar]
- 6.Banfi B. Malgrange B. Knisz J. Steger K. Dubois-Dauphin M. Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- 7.Banfi B. Maturana A. Jaconi S. Arnaudeau S. Laforge T. Sinha B. Ligeti E. Demaurex N. Krause KH. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science. 2000;287:138–142. doi: 10.1126/science.287.5450.138. [DOI] [PubMed] [Google Scholar]
- 8.Banfi B. Molnar G. Maturana A. Steger K. Hegedus B. Demaurex N. Krause KH. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem. 2001;276:37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 9.Bayraktutan U. Blayney L. Shah AM. Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:1903–1911. doi: 10.1161/01.atv.20.8.1903. [DOI] [PubMed] [Google Scholar]
- 10.Bedard K. Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 11.BelAiba RS. Djordjevic T. Petry A. Diemer K. Bonello S. Banfi B. Hess J. Pogrebniak A. Bickel C. Gorlach A. NOX5 variants are functionally active in endothelial cells. Free Radic Biol Med. 2007;42:446–459. doi: 10.1016/j.freeradbiomed.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 12.Benhar M. Engelberg D. Levitzki A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002;3:420–425. doi: 10.1093/embo-reports/kvf094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowen–Pope DF. Ross R. Platelet-derived growth factor. II. Specific binding to cultured cells. J Biol Chem. 1982;257:5161–5171. [PubMed] [Google Scholar]
- 14.Brandes RP. Schroder K. Composition and functions of vascular nicotinamide adenine dinucleotide phosphate oxidases. Trends Cardiovasc Med. 2008;18:15–19. doi: 10.1016/j.tcm.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Brar SS. Corbin Z. Kennedy TP. Hemendinger R. Thornton L. Bommarius B. Arnold RS. Whorton AR. Sturrock AB. Huecksteadt TP. Quinn MT. Krenitsky K. Ardie KG. Lambeth JD. Hoidal JR. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol. 2003;285:C353–369. doi: 10.1152/ajpcell.00525.2002. [DOI] [PubMed] [Google Scholar]
- 16.Cai S. Khoo J. Mussa S. Alp NJ. Channon KM. Endothelial nitric oxide synthase dysfunction in diabetic mice: Importance of tetrahydrobiopterin in eNOS dimerisation. Diabetologia. 2005;48:1933–1940. doi: 10.1007/s00125-005-1857-5. [DOI] [PubMed] [Google Scholar]
- 17.Chen K. Kirber MT. Xiao H. Yang Y. Keaney JF., Jr Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng G. Cao Z. Xu X. van Meir EG. Lambeth JD. Homologs of gp91phox: Cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 19.de Lamirande E. O'Flaherty C. Sperm activation: Role of reactive oxygen species and kinases. Biochim Biophys Acta. 2008;1784:106–115. doi: 10.1016/j.bbapap.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 20.Dikalova A. Clempus R. Lassegue B. Cheng G. McCoy J. Dikalov S. San Martin A. Lyle A. Weber DS. Weiss D. Taylor WR. Schmidt HH. Owens GK. Lambeth JD. Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 21.Dinauer MC. Pierce EA. Bruns GA. Curnutte JT. Orkin SH. Human neutrophil cytochrome b light chain (p22-phox). Gene structure, chromosomal location, and mutations in cytochrome-negative autosomal recessive chronic granulomatous disease. J Clin Invest. 1990;86:1729–1737. doi: 10.1172/JCI114898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Jamali A. Valente AJ. Lechleiter JD. Gamez MJ. Pearson DW. Nauseef WM. Clark RA. Novel redox-dependent regulation of NOX5 by the tyrosine kinase c-Abl. Free Radic Biol Med. 2008;44:868–881. doi: 10.1016/j.freeradbiomed.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ford WC. Regulation of sperm function by reactive oxygen species. Hum Reprod Update. 2004;10:387–399. doi: 10.1093/humupd/dmh034. [DOI] [PubMed] [Google Scholar]
- 24.Fu X. Beer DG. Behar J. Wands J. Lambeth D. Cao W. cAMP-response element-binding protein mediates acid-induced NADPH oxidase NOX5-S expression in Barrett esophageal adenocarcinoma cells. J Biol Chem. 2006;281:20368–20382. doi: 10.1074/jbc.M603353200. [DOI] [PubMed] [Google Scholar]
- 25.Fulton D. Gratton JP. Sessa WC. Post-translational control of endothelial nitric oxide synthase: Why isn't calcium/calmodulin enough? J Pharmacol Exp Ther. 2001;299:818–824. [PubMed] [Google Scholar]
- 26.Gavazzi G. Banfi B. Deffert C. Fiette L. Schappi M. Herrmann F. Krause KH. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006;580:497–504. doi: 10.1016/j.febslet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 27.Gold ME. Wood KS. Byrns RE. Fukuto J. Ignarro LJ. NG-methyl-L-arginine causes endothelium-dependent contraction and inhibition of cyclic GMP formation in artery and vein. Proc Natl Acad Sci USA. 1990;87:4430–4434. doi: 10.1073/pnas.87.12.4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griendling KK. Novel NAD(P)H oxidases in the cardiovascular system. Heart. 2004;90:491–493. doi: 10.1136/hrt.2003.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gryglewski RJ. Palmer RM. Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 30.Guzik TJ. Chen W. Gongora MC. Guzik B. Lob HE. Mangalat D. Hoch N. Dikalov S. Rudzinski P. Kapelak B. Sadowski J. Harrison DG. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol. 2008;52:1803–1809. doi: 10.1016/j.jacc.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawkins BJ. Madesh M. Kirkpatrick CJ. Fisher AB. Superoxide flux in endothelial cells via the chloride channel-3 mediates intracellular signaling. Mol Biol Cell. 2007;18:2002–2012. doi: 10.1091/mbc.E06-09-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwakiri Y. Satoh A. Chatterjee S. Toomre DK. Chalouni CM. Fulton D. Groszmann RJ. Shah VH. Sessa WC. Nitric oxide synthase generates nitric oxide locally to regulate compartmentalized protein S-nitrosylation and protein trafficking. Proc Natl Acad Sci USA. 2006;103:19777–19782. doi: 10.1073/pnas.0605907103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jagnandan D. Church JE. Banfi B. Stuehr DJ. Marrero MB. Fulton DJ. Novel mechanism of activation of NADPH oxidase 5: Calcium sensitization via phosphorylation. J Biol Chem. 2007;282:6494–6507. doi: 10.1074/jbc.M608966200. [DOI] [PubMed] [Google Scholar]
- 34.Jay DB. Papaharalambus CA. Seidel-Rogol B. Dikalova AE. Lassegue B. Griendling KK. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic Biol Med. 2008;45:329–335. doi: 10.1016/j.freeradbiomed.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamiguti AS. Serrander L. Lin K. Harris RJ. Cawley JC. Allsup DJ. Slupsky JR. Krause KH. Zuzel M. Expression and activity of NOX5 in the circulating malignant B cells of hairy cell leukemia. J Immunol. 2005;175:8424–8430. doi: 10.4049/jimmunol.175.12.8424. [DOI] [PubMed] [Google Scholar]
- 36.Kappert K. Sparwel J. Sandin A. Seiler A. Siebolts U. Leppanen O. Rosenkranz S. Ostman A. Antioxidants relieve phosphatase inhibition and reduce PDGF signaling in cultured VSMCs and in restenosis. Arterioscler Thromb Vasc Biol. 2006;26:2644–2651. doi: 10.1161/01.ATV.0000246777.30819.85. [DOI] [PubMed] [Google Scholar]
- 37.Kavanaugh CJ. Trumbo PR. Ellwood KC. The U.S. Food and Drug Administration's evidence-based review for qualified health claims: Tomatoes, lycopene, and cancer. J Natl Cancer Inst. 2007;99:1074–1085. doi: 10.1093/jnci/djm037. [DOI] [PubMed] [Google Scholar]
- 38.Kawahara T. Lambeth JD. Phosphatidylinositol (4,5)-bisphosphate modulates Nox5 localization via an N-terminal polybasic region. Mol Biol Cell. 2008;19:4020–4031. doi: 10.1091/mbc.E07-12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawahara T. Quinn MT. Lambeth JD. Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol. 2007;7:109. doi: 10.1186/1471-2148-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawahara T. Ritsick D. Cheng G. Lambeth JD. Point mutations in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J Biol Chem. 2005;280:31859–31869. doi: 10.1074/jbc.M501882200. [DOI] [PubMed] [Google Scholar]
- 41.Kim JH. Johannes L. Goud B. Antony C. Lingwood CA. Daneman R. Grinstein S. Noninvasive measurement of the pH of the endoplasmic reticulum at rest and during calcium release. Proc Natl Acad Sci USA. 1998;95:2997–3002. doi: 10.1073/pnas.95.6.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuzkaya N. Weissmann N. Harrison DG. Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: Implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 43.Kwon J. Qu CK. Maeng JS. Falahati R. Lee C. Williams MS. Receptor-stimulated oxidation of SHP-2 promotes T-cell adhesion through SLP-76-ADAP. EMBO J. 2005;24:2331–2341. doi: 10.1038/sj.emboj.7600706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lambeth JD. Nox enzymes, ROS, and chronic disease: An example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lambeth JD. Cheng G. Arnold RS. Edens WA. Novel homologs of gp91phox. Trends Biochem Sci. 2000;25:459–461. doi: 10.1016/s0968-0004(00)01658-3. [DOI] [PubMed] [Google Scholar]
- 46.Lambeth JD. Kawahara T. Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laursen JB. Somers M. Kurz S. McCann L. Warnholtz A. Freeman BA. Tarpey M. Fukai T. Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: Implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 48.Lonn E. Bosch J. Yusuf S. Sheridan P. Pogue J. Arnold JM. Ross C. Arnold A. Sleight P. Probstfield J. Dagenais GR. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: A randomized controlled trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 49.Lynch RE. Fridovich I. Permeation of the erythrocyte stroma by superoxide radical. J Biol Chem. 1978;253:4697–4699. [PubMed] [Google Scholar]
- 50.Maru Y. Nishino T. Kakinuma K. Expression of Nox genes in rat organs, mouse oocytes, and sea urchin eggs. DNA Seq. 2005;16:83–88. doi: 10.1080/10425170500069734. [DOI] [PubMed] [Google Scholar]
- 51.Mochizuki T. Furuta S. Mitsushita J. Shang WH. Ito M. Yokoo Y. Yamaura M. Ishizone S. Nakayama J. Konagai A. Hirose K. Kiyosawa K. Kamata T. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene. 2006;25:3699–3707. doi: 10.1038/sj.onc.1209406. [DOI] [PubMed] [Google Scholar]
- 52.Mumbengegwi DR. Li Q. Li C. Bear CE. Engelhardt JF. Evidence for a superoxide permeability pathway in endosomal membranes. Mol Cell Biol. 2008;28:3700–3712. doi: 10.1128/MCB.02038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakano Y. Longo-Guess CM. Bergstrom DE. Nauseef WM. Jones SM. Banfi B. Mutation of the Cyba gene encoding p22phox causes vestibular and immune defects in mice. J Clin Invest. 2008;118:1176–1185. doi: 10.1172/JCI33835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paffenholz R. Bergstrom RA. Pasutto F. Wabnitz P. Munroe RJ. Jagla W. Heinzmann U. Marquardt A. Bareiss A. Laufs J. Russ A. Stumm G. Schimenti JC. Bergstrom DE. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev. 2004;18:486–491. doi: 10.1101/gad.1172504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pritchard KA., Jr. Ackerman AW. Gross ER. Stepp DW. Shi Y. Fontana JT. Baker JE. Sessa WC. Heat shock protein 90 mediates the balance of nitric oxide and superoxide anion from endothelial nitric-oxide synthase. J Biol Chem. 2001;276:17621–17624. doi: 10.1074/jbc.C100084200. [DOI] [PubMed] [Google Scholar]
- 56.Pritchard KA., Jr. Groszek L. Smalley DM. Sessa WC. Wu M. Villalon P. Wolin MS. Stemerman MB. Native low-density lipoprotein increases endothelial cell nitric oxide synthase generation of superoxide anion. Circ Res. 1995;77:510–518. doi: 10.1161/01.res.77.3.510. [DOI] [PubMed] [Google Scholar]
- 57.Pyriochou A. Vassilakopoulos T. Zhou Z. Papapetropoulos A. cGMP-dependent and -independent angiogenesis-related properties of nitric oxide. Life Sci. 2007;81:1549–1–554. doi: 10.1016/j.lfs.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 58.Ritsick DR. Edens WA. Finnerty V. Lambeth JD. Nox regulation of smooth muscle contraction. Free Radic Biol Med. 2007;43:31–38. doi: 10.1016/j.freeradbiomed.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sabeur K. Ball BA. Characterization of NADPH oxidase 5 in equine testis and spermatozoa. Reproduction. 2007;134:263–270. doi: 10.1530/REP-06-0120. [DOI] [PubMed] [Google Scholar]
- 60.Selemidis S. Sobey CG. Wingler K. Schmidt HH. Drummond GR. NADPH oxidases in the vasculature: Molecular features, roles in disease and pharmacological inhibition. Pharmacol Ther. 2008;120:254–291. doi: 10.1016/j.pharmthera.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 61.Serrander L. Cartier L. Bedard K. Banfi B. Lardy B. Plastre O. Sienkiewicz A. Forro L. Schlegel W. Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serrander L. Jaquet V. Bedard K. Plastre O. Hartley O. Arnaudeau S. Demaurex N. Schlegel W. Krause KH. NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie. 2007;89:1159–1167. doi: 10.1016/j.biochi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 63.Sesso HD. Buring JE. Christen WG. Kurth T. Belanger C. MacFadyen J. Bubes V. Manson JE. Glynn RJ. Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: The Physicians' Health Study II randomized controlled trial. JAMA. 2008;300:2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shibanuma M. Kuroki T. Nose K. Stimulation by hydrogen peroxide of DNA synthesis, competence family gene expression and phosphorylation of a specific protein in quiescent Balb/3T3 cells. Oncogene. 1990;5:1025–1032. [PubMed] [Google Scholar]
- 65.Shimizu S. Shiota K. Yamamoto S. Miyasaka Y. Ishii M. Watabe T. Nishida M. Mori Y. Yamamoto T. Kiuchi Y. Hydrogen peroxide stimulates tetrahydrobiopterin synthesis through the induction of GTP-cyclohydrolase I and increases nitric oxide synthase activity in vascular endothelial cells. Free Radic Biol Med. 2003;34:1343–1352. doi: 10.1016/s0891-5849(03)00172-2. [DOI] [PubMed] [Google Scholar]
- 66.Si J. Behar J. Wands J. Beer DG. Lambeth D. Chin YE. Cao W. STAT5 mediates PAF-induced NADPH oxidase NOX5-S expression in Barrett's esophageal adenocarcinoma cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G174–183. doi: 10.1152/ajpgi.00291.2007. [DOI] [PubMed] [Google Scholar]
- 67.Si J. Fu X. Behar J. Wands J. Beer DG. Souza RF. Spechler SJ. Lambeth D. Cao W. NADPH oxidase NOX5-S mediates acid-induced cyclooxygenase-2 expression via activation of NF-kappaB in Barrett's esophageal adenocarcinoma cells. J Biol Chem. 2007;282:16244–16255. doi: 10.1074/jbc.M700297200. [DOI] [PubMed] [Google Scholar]
- 68.Suh YA. Arnold RS. Lassegue B. Shi J. Xu X. Sorescu D. Chung AB. Griendling KK. Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 69.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008;275:3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 70.Sundaresan M. Yu ZX. Ferrans VJ. Irani K. Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 71.Szatrowski TP. Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 72.Tabet F. Savoia C. Schiffrin EL. Touyz RM. Differential calcium regulation by hydrogen peroxide and superoxide in vascular smooth muscle cells from spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2004;44:200–208. doi: 10.1097/00005344-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 73.Terada LS. Specificity in reactive oxidant signaling: Think globally, act locally. J Cell Biol. 2006;174:615–623. doi: 10.1083/jcb.200605036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tirone F. Cox JA. NADPH oxidase 5 (NOX5) interacts with and is regulated by calmodulin. FEBS Lett. 2007;581:1202–1208. doi: 10.1016/j.febslet.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 75.Ushio–Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2009;11:1289–1299. doi: 10.1089/ars.2008.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Breemen RB. Pajkovic N. Multitargeted therapy of cancer by lycopene. Cancer Lett. 2008;269:339–351. doi: 10.1016/j.canlet.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van Buul JD. Fernandez–Borja M. Anthony EC. Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal. 2005;7:308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 78.Venkateswaran V. Fleshner NE. Sugar LM. Klotz LH. Antioxidants block prostate cancer in lady transgenic mice. Cancer Res. 2004;64:5891–5896. doi: 10.1158/0008-5472.CAN-04-0690. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Q. Church JE. Jagnandan D. Catravas JD. Sessa WC. Fulton D. Functional relevance of Golgi- and plasma membrane-localized endothelial NO synthase in reconstituted endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:1015–1021. doi: 10.1161/01.ATV.0000216044.49494.c4. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Q. Malik P. Pandey D. Gupta S. Jagnandan D. Belin de Chantemele E. Banfi B. Marrero MB. Rudic RD. Stepp DW. Fulton DJ. Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidase. Arterioscler Thromb Vasc Biol. 2008;28:1627–1633. doi: 10.1161/ATVBAHA.108.168278. [DOI] [PMC free article] [PubMed] [Google Scholar]