Abstract
The genetic polymorphisms of Echinococcus spp. in the eastern Tibetan Plateau and the Xinjiang Uyghur Autonomous Region were evaluated by DNA sequencing analyses of genes for mitochondrial cytochrome c oxidase subunit 1 (cox1) and nuclear elongation factor-1 alpha (ef1a). We collected 68 isolates of Echinococcus granulosus sensu stricto (s.s.) from Xinjiang and 113 isolates of E. granulosus s. s., 49 isolates of Echinococcus multilocularis and 34 isolates of Echinococcus shiquicus from the Tibetan Plateau. The results of molecular identification by mitochondrial and nuclear markers were identical, suggesting the infrequency of introgressive hybridization. A considerable intraspecific variation was detected in mitochondrial cox1 sequences. The parsimonious network of cox1 haplotypes showed star-like features in E. granulosus s. s. and E. multilocularis, but a divergent feature in E. shiquicus. The cox1 neutrality indexes computed by Tajima's D and Fu's Fs tests showed high negative values in E. granulosus s. s. and E. multilocularis, indicating significant deviations from neutrality. In contrast, the low positive values of both tests were obtained in E. shiquicus. These results suggest the following hypotheses: (i) recent founder effects arose in E. granulosus and E. multilocularis after introducing particular individuals into the endemic areas by anthropogenic movement or natural migration of host mammals, and (ii) the ancestor of E. shiquicus was segregated into the Tibetan Plateau by colonizing alpine mammals and its mitochondrial locus has evolved without bottleneck effects.
Keywords: Echinococcus, Mitochondrial DNA, Genetic diversity, Population genetic structure, China
1. Introduction
Metacestodes of the dog tapeworm Echinococcus granulosus sensu stricto (s.s.) and the fox tapeworm Echinococcus multilocularis are highly pathogenic to humans, and cause cystic and alveolar echinococcoses, respectively. Humans become infected through oral ingestion of eggs derived from faeces of canine definitive hosts. Sheep are a main intermediate host for E. granulosus s. s., whereas arvicoline rodents serve as intermediate hosts for E. multilocularis (Eckert and Deplazes, 2004). Besides the main two species, Echinococcus equinus, Echinococcus ortleppi, Echinococcus canadensis, Echinococcus felidis, Echinococcus shiquicus, Echinococcus vogeli and Echinococcus oligarthrus have been regarded as valid by recent phylogenetic studies (Xiao et al., 2005; Nakao et al., 2007; Hüttner et al., 2008). However, the species status of E. canadensis is still debatable (Thompson, 2008).
In China, E. granulosus s. s. and E. multilocularis are widespread in western, northern and central parts of the country (Wang et al., 2008), and hyperendemic foci exist within pastoral areas of the eastern Tibetan Plateau (Schantz et al., 2003; Li et al., 2008) and the Xinjiang Uyghur Autonomous Region (Wang et al., 2001). Both endemic regions are geographically separated by the Kunlum mountains and the Taklamakan Desert. The alpine steppe of the Tibetan Plateau supports human pastoral activity for the raising of yak and sheep. In Xinjiang, nomads and semi-nomads keep livestock on low-altitude grassland. This type of sheep husbandry system, including the use of pastoral dogs, is essential to maintain the synanthropic cycle of E. granulosus s. s. In contrast, the rodent fauna of grassland and the migration of foxes are key factors in establishing the endemic foci of E. multilocularis (Giraudoux et al., 2006). Human infections with E. multilocularis are extremely frequent in the Tibetan communities of Sichuan province (Craig, 2006), and the role of dogs in the communities is important to infections (Wang et al., 2006). Thus, the life cycle of E. multilocularis is altered to be synanthropic in hyperendemic areas.
Species of Echinococcus prevailing in China have been clarified by molecular taxonomic studies using mtDNA markers (McManus et al., 1994; Zhang et al., 1998; Yang et al., 2005; Bart et al., 2006; Xiao et al., 2004, 2005, 2006; Ma et al., 2008; Li et al., 2008). The molecular identification of Echinococcus isolates from various origins showed the following host-parasite relationships in China: (i) domestic mammals (sheep, cattle, goats, yaks and dogs) for E. granulosus s. s., (ii) domestic mammals (camels, cattle and dogs) for E. canadensis (G6 genotype), (iii) wildlife (voles, hares, pikas, red foxes and Tibetan foxes) and domestic mammals (dogs) for E. multilocularis and (iv) wildlife (pikas and Tibetan foxes) for E. shiquicus. Human infections have been confirmed for all species except E. shiquicus. Interestingly, E. canadensis G6 has been found only in Xinjiang (Zhang et al., 1998) and E. shiquicus seems to be restricted in the Tibetan Plateau (Xiao et al., 2005). All of the epidemiological information provides a basis to consider the natural history of Echinococcus in China. Previous studies demonstrated that intraspecific mtDNA variations occurred in E. granulosus s. s. (Yang et al., 2005; Bart et al., 2006; Ma et al., 2008), E. multilocularis (Yang et al., 2005) and E. shiquicus (Xiao et al., 2005). However, the genetic populations of Echinococcus spp. in China have never been characterized in an evolutionary context.
In this study, the genetic diversities of E. granulosus s. s., E. multilocularis and E. shiquicus were explored by using mtDNA and nDNA markers. We sampled the isolates of E. granulosus s. s. from Xinjiang and the isolates of all three species from the eastern Tibetan Plateau. The main purpose of this study was to evaluate the population genetic structures of the three species. The resultant data and epidemiological information enabled us to suggest evolutionary hypotheses on how the parasites have spread in China.
2. Materials and methods
2.1. Isolates collected
An Echinococcus isolate was defined as a unilocular cyst or a separated alveolar cyst from an intermediate host. During the period from 2002 to 2007, the larval isolates of Echinococcus spp. from various hosts were collected in the eastern Tibetan Plateau (Qinghai and Sichuan provinces) and the Xinjiang Uyghur Autonomous Region. Table 1 summarizes the number and origin of isolates examined in the two localities. For E. granulosus s. s., 113 isolates in the highlands and 68 isolates in the lowlands were treated as two separate populations. On the other hand, 49 isolates of E. multilocularis and 34 isolates of E. shiquicus were obtained only from the highlands. One isolate in the lowlands was identified as E. canadensis G6 genotype. In addition, 57 isolates of E. granulosus s. s. in Peru (Moro et al., 2009) served as a foreign control for population genetic analyses. The species identification of those isolates was validated by DNA sequencing described below.
Table 1.
Number of Echinococcus isolates used for this study.
| Species and localities |
Origins of larval isolates |
||||||
|---|---|---|---|---|---|---|---|
| Human | Sheep | Yak | Rodent a | Hare b | Pika c | Total | |
| Echinococcus granulosus | |||||||
| Qinghai & Sichuan | 37 | 57 | 19 | 0 | 0 | 0 | 113 |
| Xinjiang | 54 | 14 | 0 | 0 | 0 | 0 | 68 |
| Total | 91 | 71 | 19 | 0 | 0 | 0 | 181 |
| Echinococcus multilocularis | |||||||
| Qinghai & Sichuan | 20 | 0 | 0 | 26 | 1 | 2 | 49 |
| Echinococcus shiquicus | |||||||
| Qinghai & Sichuan | 0 | 0 | 0 | 0 | 0 | 34 | 34 |
| Echinococcus canadensis (G6) | |||||||
| Xinjiang | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
Microtus fuscus, Microtus limnophilus and Cricetulus kamensis.
Lepus oiostolus.
Ochotona curzoniae.
2.2. PCR amplification and sequencing
The genomic DNA of each isolate was prepared from ethanol-preserved larval cysts by using a DNeasy blood and tissue kit (Qiagen), and used as a template for PCR. Partial fragments of a mitochondrial gene for cytochrome c oxidase subunit 1 (cox1) and a nuclear gene for elongation factor-1 alpha (ef1a) were amplified by PCR using specific primers reported previously (Nakao et al., 2000; Moro et al., 2009). The PCR mixture was prepared in a 25 μl final volume containing 1 μl template DNA, 200 μM of each dNTP, 0.2 μM of each primer, 0.5 U of Ex-Taq polymerase (Takara) and the manufacturer-supplied reaction buffer. Thermal reactions were performed for 35 cycles of 94°C for 30 s, 55°C for 30 s and 72°C for 60 s. Amplified DNA fragments were purified with QIAquick spin columns (Qiagen), and sequenced directly with a BigDye terminator cycle sequencing kit (Applied Biosystems). The resultant sequence ladders were read by an ABI PRISM 377 genetic analyzer (Applied Biosystems).
2.3. Data analysis
In each species of Echinococcus, multiple alignments in NEXUS format were made manually by editing the plain text files of nucleotide sequences. Amino acid sequences were inferred from the nucleotide sequences by echinoderm mitochondrial genetic code (Nakao et al., 2000; Telford et al., 2000) or standard genetic code. Percentage divergence values of nucleotide sequences were determined using the MEGA4 package (Tamura et al., 2007) using Kimura's two parameter model (Kimura, 1980) with a γ-shaped parameter (a=0.5). The identification of haplotypes and the drawing of their networks were executed by TCS 1.2 software (Clement et al., 2000) using statistical parsimony (Templeton et al. 1992). The network estimation was run at a 95% connection limit.
Population diversity indexes (number of haplotypes, haplotype diversity and nucleotide diversity) were calculated using DnaSP 4.5 software (Rozas et al., 2003). The population genetics package Arlequin 3.1 (Excoffier et al., 2005) was employed to calculate the neutrality indexes of Tajima's D (Tajima, 1989) and Fu's Fs (Fu, 1997). The degree of gene flow between two populations was estimated using a pairwise fixation index (Fst) as determined by the Arlequin package. The three geographic populations of E. granulosus s. s. from Xinjiang, the eastern Tibetan Plateau and Peru (out of China) were used to compute the Fst values.
3. Results
3.1. Variations in nucleotide sequences
In our targeted regions of Echinococcus DNA, deletion or insertion mutations were not observed, even in different species. The total numbers of nucleotides examined were therefore stable in mitochondrial cox1 (789 sites) and nuclear ef1a (656 sites). The cox1 sequences could be amplified in all of the isolates of E. granulosus s. s. (n = 181), E. multilocularis (n = 49), E. shiquicus (n = 34) and E. canadensis G6 (n = 1). However, the PCR positive rate of ef1a was relatively lower than that of cox1, probably due to the low copy number of the nuclear gene. Each Echinococcus sp. retained the species-specific nucleotide sequences of cox1 and ef1a.
Considerable intraspecific variations were detected only in the mtDNA sequences of cox1 (Table 1), indicating its primary use for population genetic analyses. Synonymous substitutions exceeded non-synonymous substitutions in the cox1 sequences of E. granulosus s. s. and E. shiquicus. Out of all of the point mutations, 24 sites (49.0%) of E. granulosus s. s., one site (25.0%) of E.multilocularis and 11 sites (64.7%) of E. shiquicus were parsimony informative. Relatively to E. shiquicus, singleton substitutions were abundant in E.granulosus s. s. and E. multilocularis. The pairwise divergence of the cox1 sequences was computed among individual isolates at an intraspecific level. The maximum values of the divergence were 0.9% in E. granulosus s. s., 0.3% in E. multilocularis and 1.5% in E. shiquicus. The autochthonous species E.shiquicus appeared to have the most variable mtDNA.
3.2. Haplotype networks
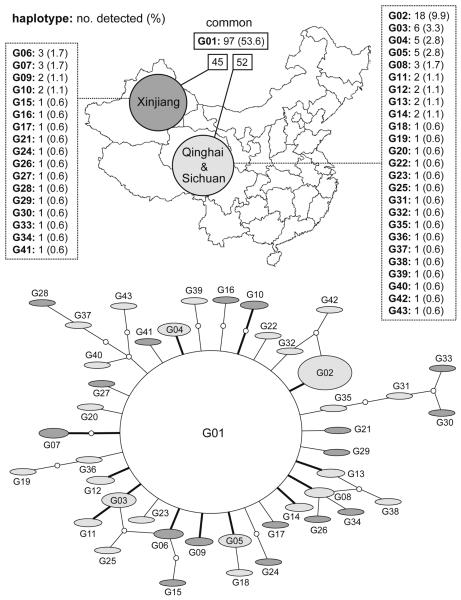
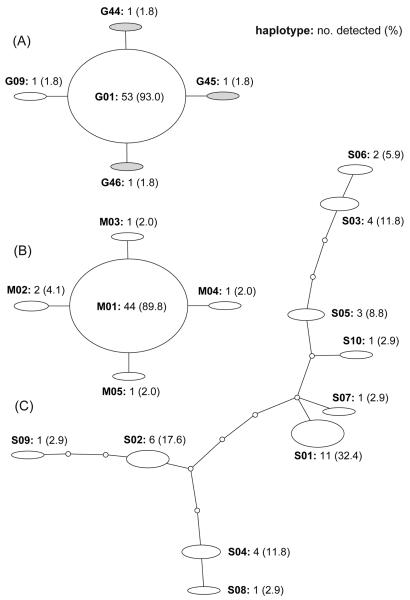
In E. granulosus s. s., 43 mtDNA haplotypes were found in 181 isolates from Xinjiang and the eastern Tibetan Plateau (Qinghai and Sichuan). To discern a genealogical relationship among the haplotypes, we constructed a statistical parsimony network. As shown in Fig. 1, each of the two regions possessed geographically specific haplotypes. The network, however, showed a star-like expansion, and one common haplotype (G01) occupied the centre of the network. The numbers of mutational steps between the common haplotype and the others ranged from one to five, and the frequency of the common haplotype was 53.6% in the population. A similar star-like network was observed in the Peruvian population of E. granulosus s. s. (Fig. 2A). Five mtDNA haplotypes were detected in the 57 Peruvian isolates (Moro et al., 2009), but the majority of the isolates (93.0%) belonged to the haplotype G01, which was the most common in the Chinese populations. A single-nucleotide variation was identified between members of the five haplotypes, three of which were geographically specific to Peru.
Fig. 1.
Frequencies of mitochondrial cytochrome c oxidase subunit 1 (cox1) haplotypes in Chinese Echinococcus granulosus and their network based on statistical parsimony. In the network, the size of ovals indicates the frequency of the haplotypes. Small circles show hypothetical haplotypes. The haplotypes whose frequencies are more than 1% are connected with bold lines. Dark ovals represent the haplotypes found in Xinjiang, whereas the localities of gray ovals are Qinghai and Sichuan. A white oval shows the common haplotype.
Fig. 2.
The statistical parsimony networks of mitochondrial cytochrome c oxidase subunit 1 (cox1) haplotypes in Echinococcus spp. The size of ovals indicates the frequency of the haplotypes. Small circles show hypothetical haplotypes. (A) The Peruvian population of Echinococcous granulosus sensu stricto. Gray ovals represent the haplotypes specific to Peru. (B) The Tibetan population of Echinococcus multilocularis. (C) The Tibetan population of Echinococcus shiquicus.
We detected five mtDNA haplotypes in 49 isolates of E. multilocularis from the eastern Tibetan Plateau. These were illustrated as a star-like network with one major haplotype (M01), which comprised 89.8% of the isolates examined (Fig. 2B). All variations found in the five haplotypes were single-nucleotide polymorphisms. As opposed to the convergent networks of E. granulosus s. s. and E. multilocularis, a divergent network was found in E. shiquicus (Fig. 2C). Although 10 mtDNA haplotypes were detected in 34 isolates of E. shiquicus, major haplotypes were absent in the population. The maximum number of mutational steps was 13 in the network of E. shiquicus.
3.3. Diversity and neutrality indexes
Diversity indexes for Echinococcus populations in each locality were calculated using the data set of cox1 (Table 3). In the Chinese populations of Echinococcus spp., both values of haplotype and nucleotide diversities were the highest in E. shiquicus but the lowest in E. multilocularis. In the case of E. granulosus s. s., the Peruvian population showed the lowest values compared with the Chinese populations. The high levels of haplotype diversity were kept in the Chinese populations of E. granulosus s. s., but their nucleotide diversity was relatively low because of the richness of single nucleotide substitutions.
Table 3.
Diversity and neutrality indexes for Echinococcus populations calculated from the nucleotide data set of mitochondrial cytochrome c oxidase subunit 1 (cox1) gene.
| Species and localities |
Diversity Neutrality |
|||||
|---|---|---|---|---|---|---|
| n | Hn | Hd ± S.D. | π ± S.D. | D | Fs | |
| Echinococcus granulosus | ||||||
| Qinghai & Sichuan | 113 | 26 | 0.760 ± 0.038 | 0.0017 ± 0.0002 | −2.323a | −26.023a |
| Xinjiang | 68 | 18 | 0.562 ± 0.073 | 0.0015 ± 0.0003 | −2.456a | −15.762a |
| Total (China) | 181 | 43 | 0.702 ± 0.038 | 0.0017 ± 0.0002 | −2.536a | −61.569a |
| Out of China (Peru) | 57 | 5 | 0.137 ± 0.062 | 0.0002 ± 0.0001 | −1.849a | −5.889a |
| Echinococcus multilocularis | ||||||
| Qinghai & Sichuan | 49 | 5 | 0.195 ± 0.075 | 0.0003 ± 0.0001 | −1.765a | −4.788a |
| Echinococcus shiquicus | ||||||
| Qinghai & Sichuan | 34 | 10 | 0.847± 0.040 | 0.0055 ± 0.0004 | 0.164 | 0.258 |
Abbreviations are number of isolates examined (n), number of haplotypes (Hn), haplotype diversity (Hd), nucleotide diversity (π), Tajima's D (D) and Fu's Fs (Fs).
Significant P values (P < 0.01).
Neutrality indexes calculated by Tajima's D and Fu's Fs tests are also shown in Table 3. The highly negative values were recorded in the Chinese populations of E. granulosus s. s. and E. multilocularis, indicating a significant deviation from neutrality. The Peruvian population of E. granulosus s. s. also showed significant negative values. By contrast, relatively low positive values were obtained in the population of E. shiquicus, which kept highly polymorphic mtDNA.
3.4. Fixation index for the populations of E. granulosus s. s
Using the data set of mtDNA, the values of the pairwise fixation index (Fst) were computed to estimate the degree of gene flow among three geographic populations of E. granulosus s. s in China and Peru (Table 4). Since one common haplotype existed predominantly in the three localities, the Fst values between the populations were very small, ranging from 0.036 to 0.009. These low values implied that the populations were not genetically differentiated from one another.
Table 4.
Pairwise fixation index (Fst values) between Echinococcus granulosus sub-populations calculated from the nucleotide data set of mitochondrial cytochrome c oxidase subunit 1 (cox1) gene.
Significant P values (P < 0.01).
4. Discussion
Echinococcoses caused by E. granulosus s. s. and E. multilocularis lead to considerable social and economic losses in the endemic communities of the eastern Tibetan Plateau (Budke et al., 2005). Furthermore, E. shiquicus has been recently discovered in the plateau (Xiao et al., 2005). Information on the population genetic structures of these sympatric species is necessary to better understand the process of intra- and interspecific gene flows, and may provide a foundation for future epidemiological studies on the transmission dynamics of the parasites. In the present study, mtDNA revealed the basic structures of Echinococcus populations in the eastern Tibetan Plateau.
We chose the protein-coding gene cox1 as a marker for Echinococcus mtDNA. In other organisms, the mitochondrial control region has been generally used to infer genealogical relationships. However, the corresponding mtDNA regions of Echinococcus spp. contain highly repetitive sequences, which are unsuitable for phylogenetic studies (Nakao et al., 2007). The cox1 gene of Echinococcus spp. has already been shown to be a promising candidate for the classification of intra- and interspecific variants even in the short sequence (366 nucleotide sites) (Bowles et al., 1992). In this study, we determined the relatively long sequence of cox1 (789 nucleotide sites), and detected a sufficient number of haplotypes to analyze the population genetic structures of each species. The haplotypes are only loosely correlated with the intraspecific genotypes of E. granulosus s. s. (G1, G2 and G3) and E. multilocularis (M1 and M2) defined by Bowles et al. (1992). The negative selection of the cox1 for the purging of deleterious mutations has been demonstrated by the codon-based Z-test (Tamura et al., 2007) using the corresponding sequences of E. granulosus s. s., E. multilocularis, E. equinus, E. ortleppi, E. canadensis, E. felidis, E. vogeli and E. oligarthrus (M. Nakao, unpublished data). The fragments of the nuclear gene ef1a were also sequenced in this study, but their species-specific sequences were not polymorphic at an intraspecific level. The results of species identification by the mitochondrial and nuclear markers were identical, suggesting the infrequency of introgressive hybridization among the sympatric species.
The cox1 haplotypes of E. granulosus s. s. found in this study did not show an apparent phylogeographic structuring in China. The parsimony network analysis revealed that the haplotypes exhibit a star-like expansion from a main founder haplotype, suggesting that the populations of eastern Tibet and Xinjiang are not fully differentiated from each other. It is noteworthy that the same founder was predominant in the Peruvian population. It seems unlikely that mutations of the founder haplotype are advantageous because the amino acid sequence deduced from the founder is the same as those from other minor haplotypes. The common genetic structure between geographically unrelated populations enables us to speculate that one particular lineage of E. granulosus s. s. is widespread globally. The genetic non-differentiation between the local populations is also demonstrated by extreme low values of the fixation index Fst. Furthermore, the significant negative values of the neutrality indexes Tajima's D and Fu's Fs suggest that bottleneck events might occur in the recent past. It is most likely that demographic expansions of the parasite occurred after introducing particular individuals into the endemic areas by anthropogenic movements of host mammals (sheep and dogs).
It is assumed that E. granulosus s. s. was introduced into South America from Europe through livestock importation after the colonial period. The higher values of haplotype and nucleotide diversities in the Chinese populations of E. granulosus s. s. suggest that China historically preceded Peru in the time of initial founder introduction. One could speculate that bottleneck events might also occur in the ancestral population of E. granulosus s. s. during the colonization of domestic sheep as an intermediate host. Archaeological and genetic evidence suggest that sheep were domesticated in the Ancient Near East (Pedrosa et al., 2005), but the genealogical survey of Chinese domestic sheep showed the possibility that additional domestication events occurred independently in other regions (Chen et al., 2006). The lack of archaeoparasitological data does not permit us to infer how and when the parasite invaded China. However, the Ancient Near East is one possible candidate for the cradle of E. granulosus s. s. The population genetic structures of E. granulosus s. s. should be compared in various endemic areas to clarify its ancestral origin and the process of its worldwide dispersal.
Our previous study has already indicated the rarity of mtDNA polymorphism in the Tibetan population of E. multilocularis (Xiao et al., 2005). The present study furthermore revealed that a particular cox1 sequence was positioned as a basal haplotype, suggesting that a founder effect arose in the population. Our recent phylogeographic study on E. multilocularis showed that the worldwide isolates were classified into European, Asian and North American clades except the Inner Mongolia isolates from the corsac fox Vulpes corsac (Nakao et al., 2009). The geographic clustering indicates a possibility that genetic changes occurred in E. multilocularis after the fragmentation of the population during the Pleistocene ice ages. The red fox Vulpes vulpes, which has a flexible ability to adapt to various environments, extended its distributional range in the Holarctic region, and might play an essential role in introducing E. multilocularis into new areas. It seems likely that an epidemic of the parasite in the eastern Tibetan Plateau was initiated by natural migration of red foxes in the recent past.
The Tibetan indigenous species E. shiquicus showed a quite different pattern of population genetic structure when compared with E. granulosus s. s. and E. multilocularis. Statistical neutrality tests and haplotype network analyses suggest a possibility that the mitochondrial locus of E. shiquicus has evolved without bottleneck effects. Our previous report clarified that E. shiquicus utilizes the Tibetan fox Vulpes ferrilata as a definitive host and the plateau pika Ochotona curzoniae as an intermediate host (Xiao et al., 2005). Both the autochthonous mammals are adapted to the high altitude steppe but do not survive in lowlands. We can therefore consider that E. shiquicus has been segregated in the plateau since the parasite's ancestor colonized the alpine mammals. The lasting geographic segregation seems to be a cause for extraordinary richness of polymorphism in Tibetan E. shiquicus.
Diploid organisms having a mixed sexual and asexual reproduction system show different patterns from theoretical population genetic models (Prugnolle et al., 2005). The adult tapeworms of Echinococcus are hermaphroditic, and self-fertilization mainly occurs in the small intestine of canine definitive hosts (Haag et al., 1999). The larvae furthermore proliferate asexually in the viscera of intermediate hosts, and the clonal offspring develop into adults in a definitive host. The biphasic reproduction of Echinococcus spp. may strongly affect their population genetic structures and promote a very low genetic variability of nuclear loci. In this study we used a haploid maternally inherited mtDNA marker to examine the population genetic structure of Echinococcus because of the lack of appropriate nuclear markers. A panel of single-locus nuclear markers is required for further population genetic studies to elucidate the evolutionary backgrounds of Echinococcus worldwide.
Table 2.
Number of nucleotide substitutions in mitochondrial cytochrome c oxidase subunit 1 (cox1) and nuclear elongation factor-1 alpha (ef1a) genes amplified from Echinococcus spp. in China.
|
cox1 (789) |
ef1a (656) |
|||||
|---|---|---|---|---|---|---|
| Species | n | S | NS | n | S | NS |
| Echinococcus granulosus | 181 | 30 | 19 | 122 | 0 | 0 |
| Echinococcus multilocularis | 49 | 1 | 3 | 38 | 0 | 0 |
| Echinococcus shiquicus | 34 | 16 | 1 | 30 | 3 | 0 |
Number of the total nucleotide sites examined is shown in parentheses. Abbreviations are number of isolates examined (n), synonymous substitutions (S) and non-synonymous substitutions (NS).
Acknowledgements
This study was supported by the US National Institutes of Health Funds (the NIH/NSF Ecology and Infectious Diseases Program, # TWO 1565, Principal Investigator: P. S. Craig), the Japan Society for the Promotion of Science (JSPS) (17256002, 21256003) and JSPS-Asia/Africa Science Platform Fund (2007-2011) to A. Ito. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Center or the National Institutes of Health. The authors thank many colleagues and field workers who collected the isolates of E. granulosus s. s., E. multilocularis and E. shiquicus in China.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Nucleotide sequence data reported in this paper are available in DDBJ/EMBL/GenBank databases under the accession nos. AB491414-AB491471.
References
- Bart JM, Abdukader M, Zhang YL, Lin RY, Wang YH, Nakao M, Ito A, Craig PS, Piarroux R, Vuitton DA, Wen H. Genotyping of human cystic echinococcosis in Xinjiang, PR China. Parasitology. 2006;133:571–579. doi: 10.1017/S0031182006000734. [DOI] [PubMed] [Google Scholar]
- Bowles J, Blair D, McManus DP. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992;54:165–173. doi: 10.1016/0166-6851(92)90109-w. [DOI] [PubMed] [Google Scholar]
- Budke CM, Jiamin Q, Qian W, Torgerson PR. Economic effects of echinococcosis in a disease-endemic region of the Tibetan Plateau. Am. J. Trop. Med. Hyg. 2005;73:2–10. [PubMed] [Google Scholar]
- Chen SY, Duan ZY, Sha T, Xiangyu J, Wu SF, Zhang YP. Origin, genetic diversity, and population structure of Chinese domestic sheep. Gene. 2006;376:216–223. doi: 10.1016/j.gene.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Clement M, Posada D, Crandall K. TCS: a computer program to estimate gene genealogies. Mol. Ecol. 2000;9:1657–1660. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- Craig PS. Epidemiology of human alveolar echinococcosis in China. Parasitol. Int. 2006;55:S221–S225. doi: 10.1016/j.parint.2005.11.034. [DOI] [PubMed] [Google Scholar]
- Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffier L, Laval G, Schneider S. Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol. Bioinform. Online. 2005;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- Fu YX. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics. 1997;147:915–925. doi: 10.1093/genetics/147.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudoux P, Pleydell D, Raoul F, Quéré JP, Wang Q, Yang Y, Vuitton DA, Qiu J, Yang W, Craig PS. Transmission ecology of Echinococcus multilocularis: what are the ranges of parasite stability among various host communities in China? Parasitol. Int. 2006;55:S237–S246. doi: 10.1016/j.parint.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Haag KL, Araújo AM, Gottstein B, Siles-Lucas M, Thompson RC, Zaha A. Breeding systems in Echinococcus granulosus (Cestoda; Taeniidae): selfing or outcrossing? Parasitology. 1999;118:63–71. doi: 10.1017/s0031182098003485. [DOI] [PubMed] [Google Scholar]
- Hüttner M, Nakao M, Wassermann T, Siefert L, Boomker JD, Dinkel A, Sako Y, Mackenstedt U, Romig T, Ito A. Genetic characterization and phylogenetic position of Echinococcus felidis (Cestoda: Taeniidae) from the African lion. Int. J. Parasitol. 2008;38:861–868. doi: 10.1016/j.ijpara.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Li T, Ito A, Nakaya K, Qiu J, Nakao M, Zhen R, Xiao N, Chen X, Giraudoux P, Craig PS. Species identification of human echinococcosis using histopathology and genotyping in northwestern China. Trans. R. Soc. Trop. Med. Hyg. 2008;102:585–590. doi: 10.1016/j.trstmh.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SM, Maillard S, Zhao HL, Huang X, Wang H, Geng PL, Bart JM, Piarroux R. Assessment of Echinococcus granulosus polymorphism in Qinghai province, People's Republic of China. Parasitol. Res. 2008;102:1201–1206. doi: 10.1007/s00436-008-0894-7. [DOI] [PubMed] [Google Scholar]
- McManus DP, Ding Z, Bowles J. A molecular genetic survey indicates the presence of a single, homogeneous strain of Echinococcus granulosus in north-western China. Acta Trop. 1994;56:7–14. doi: 10.1016/0001-706x(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Moro P, Nakao M, Ito A, Schantz PM, Cavero C, Cabrera L. Molecular identification of Echinococcus isolates from Peru. Parasitol. Int. 2009;58:184–186. doi: 10.1016/j.parint.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Nakao M, McManus DP, Schantz PM, Craig PS, Ito A. A molecular phylogeny of the genus Echinococcus inferred from complete mitochondrial genomes. Parasitology. 2007;134:713–722. doi: 10.1017/S0031182006001934. [DOI] [PubMed] [Google Scholar]
- Nakao M, Sako Y, Yokoyama N, Fukunaga M, Ito A. Mitochondrial genetic code in cestodes. Mol. Biochem. Parasitol. 2000;111:415–424. doi: 10.1016/s0166-6851(00)00334-0. [DOI] [PubMed] [Google Scholar]
- Nakao M, Xiao N, Okamoto M, Yanagida T, Sako Y, Ito A. Geographic pattern of genetic variation in the fox tapeworm Echinococcus multilocularis. Parasitol. Int. 2009;58 doi: 10.1016/j.parint.2009.07.010. in press. [DOI] [PubMed] [Google Scholar]
- Pedrosa S, Uzun M, Arranz JJ, Gutiérrez-Gil B, San Primitivo F, Bayón Y. Evidence of three maternal lineages in Near Eastern sheep supporting multiple domestication events. Proc. Biol. Sci. 2005;272:2211–2217. doi: 10.1098/rspb.2005.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prugnolle F, Liu H, Meeûs T, Balloux F. Population genetics of complex life-cycle parasites: an illustration with trematodes. Int. J. Parasitol. 2005;35:255–263. doi: 10.1016/j.ijpara.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Rozas J, Sánchez-DelBarrio JC, Messeguer X, Rozas R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics. 2003;19:2496–2497. doi: 10.1093/bioinformatics/btg359. [DOI] [PubMed] [Google Scholar]
- Schantz PM, Wang H, Qiu J, Liu FJ, Saito E, Emshoff A, Ito A, Roberts JM, Delker C. Echinococcosis on the Tibetan Plateau: prevalence and risk factors for cystic and alveolar echinococcosis in Tibetan populations in Qinghai Province, China. Parasitology. 2003;127:S109–S120. [PubMed] [Google Scholar]
- Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123:585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Telford MJ, Herniou EA, Russell RB, Littlewood DT. Changes in mitochondrial genetic codes as phylogenetic characters: two examples from the flatworms. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11359–11364. doi: 10.1073/pnas.97.21.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton AR, Crandall KA, Sing CF. A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. III. Cladogram estimation. Genetics. 1992;132:619–633. doi: 10.1093/genetics/132.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RCA. The taxonomy, phylogeny and transmission of Echinococcus. Exp. Parasitol. 2008;119:439–446. doi: 10.1016/j.exppara.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Wang Q, Qiu J, Yang W, Schantz PM, Raoul F, Craig PS, Giraudoux P, Vuitton DA. Socioeconomic and behavior risk factors of human alveolar echinococcosis in Tibetan communities in Sichuan, People's Republic of China. Am. J. Trop. Med. Hyg. 2006;74:856–862. [PubMed] [Google Scholar]
- Wang YH, Rogan MT, Vuitton DA, Wen H, Bartholomot B, Macpherson CN, Zou PF, Ding ZX, Zhou HX, Zhang XF, Luo J, Xiong HB, Fu Y, McVie A, Giraudoux P, Yang WG, Craig PS. Cystic echinococcosis in semi-nomadic pastoral communities in north-west China. Trans. R. Soc. Trop. Med. Hyg. 2001;95:153–158. doi: 10.1016/s0035-9203(01)90142-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang X, Liu X. Echinococcosis in China, a review of the epidemiology of Echinococcus spp. Ecohealth. 2008;5:115–126. doi: 10.1007/s10393-008-0174-0. [DOI] [PubMed] [Google Scholar]
- Yang YR, Rosenzvit MC, Zhang LH, Zhang JZ, McManus DP. Molecular study of Echinococcus in west-central China. Parasitology. 2005;131:547–555. doi: 10.1017/S0031182005007973. [DOI] [PubMed] [Google Scholar]
- Xiao N, Li TY, Qiu JM, Nakao M, Chen XW, Nakaya K, Yamasaki H, Schantz PM, Craig PS, Ito A. The Tibetan hare Lepus oiostolus: a novel intermediate host for Echinococcus multilocularis. Parasitol. Res. 2004;92:352–353. doi: 10.1007/s00436-003-1048-6. [DOI] [PubMed] [Google Scholar]
- Xiao N, Nakao M, Qiu J, Budke CM, Giraudoux P, Craig PS, Ito A. Dual infection of animal hosts with different Echinococcus species in the eastern Qinghai-Tibet plateau region of China. Am. J. Trop. Med. Hyg. 2006;75:292–294. [PubMed] [Google Scholar]
- Xiao N, Qiu J, Nakao M, Li T, Yang W, Chen X, Schantz PM, Craig PS, Ito A. Echinococcus shiquicus n. sp., a taeniid cestode from Tibetan fox and plateau pika in China. Int. J. Parasitol. 2005;35:693–701. doi: 10.1016/j.ijpara.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Zhang LH, Chai JJ, Jiao W, Osman Y, McManus DP. Mitochondrial genomic markers confirm the presence of the camel strain (G6 genotype) of Echinococcus granulosus in north-western China. Parasitology. 1998;116:29–33. doi: 10.1017/s0031182097001881. [DOI] [PubMed] [Google Scholar]