Summary
Since the recognition that costimulatory signals are critical for optimal T cell activation, proliferation, and differentiation, there has been an explosion in the study of costimulatory molecules and their roles in enhancing anti-donor T cell responses following transplantation. Here, we focus on the bench to beside translation of blocking agents designed to target three critical costimulatory pathways: the CD28/CD80/CD86 pathway, the CD154/CD40 pathway, and the LFA-1/ICAM pathway. While blockade of each of these pathways proved promising in inhibiting donor-reactive T cell responses and promoting long-term graft survival in murine models of transplantation, the progression of development of therapeutic agents to block these pathways has each taken a slightly different course. Both logistical and biological pitfalls have accompanied the translation of blockers of all three pathways into clinically applicable therapies, and the development of costimulatory blockade as a substitute for current standard-of-care calcineurin inhibitors has by no means reached completion. Collaboration between both the basic and clinical arenas will further propel the development of costimulation blockers currently in the pipeline, as well as of novel methods to target these critical pathways during transplantation.
Keywords: transplantation, tolerance, costimulation blockade, CD28 pathway, CD154 pathway, LFA-1 pathway
Introduction
T cells play a central role in mediating transplant rejection, orchestrating the generation of inflammatory events that ultimately lead to graft destruction. Thus, the focus of immunosuppressive therapies in transplantation is to block T cell activation, thereby attenuating these processes. Defining the requirements for T cell activation has been the focus of much research over the past several decades (1). In order to become activated, T cells must encounter their cognate ligand, composed of a peptide epitope presented by a self MHC molecule, on the surface of an antigen-presenting cell (APC) (1). In addition, as first demonstrated by Jenkins and Schwartz in 1987, naïve T cells are exquisitely dependent on signaling through costimulatory molecules for efficient activation (2). Since the recognition that costimulatory signals are critical for optimal T cell activation, proliferation, and differentiation, there has been an explosion in the study of costimulatory molecules and their roles in enhancing anti-donor T cell responses following transplantation. Several families of costimulatory molecules have been identified as being important in the generation of donor-specific T cell responses, including the immunoglobulin superfamily, the tumor necrosis factor superfamily, and the integrin family. In general, costimulatory molecules may function either to augment or attenuate antigen-specific T cell responses, may differ as to whether they are constitutively or inducibly expressed, and also may diverge with respect to their kinetics of expression over the course of an immune response. Understanding which, where, and when costimulatory molecules are most critical for augmenting anti-donor T cell responses is essential to being able to design therapies to interrupt these pathways.
CD28:CD80/CD86 blockade—progression from bench to the bedside
One promising therapy designed to regulate the immune response to donor tissue and thereby prevent rejection involves the use of reagents designed to block the costimulatory molecules that are required for T cell activation (3). The most well-studied and perhaps the most essential of these costimulatory molecules is CD28, which is ligated by B7.1 (CD80) or B7.2 (CD86) on the surface of an APC (4). Signaling through CD28 increases transcription and mRNA stability of IL-2 (5, 6), elevates the expression of anti-apoptotic molecules such as Bcl-xL (7), and decreases the threshold level of T cell receptors (TCRs) required for activation (8). In addition to CD28, activated T cells express the negative regulatory molecule CTLA-4, which binds the B7 proteins with a 10-20-fold higher affinity than CD28, thereby preventing costimulation of T cells through CD28 engagement (9). Throughout the 1990s, the dramatic impact of the inhibition of CD28 signaling on graft survival began to be appreciated (10, 11). For example, CD80/CD86 double knockout mice exhibit an inability to reject cardiac allografts, although they can reject skin and islet transplants (12, 13). Therefore, attempts were made to inhibit CD28-mediated signaling in the hopes of attenuating donor-specific T cell responses. As is very often the case in biology, there turned out to be several ways to skin the proverbial cat, and it is often impossible to guess a priori which method will prove to be the most efficacious.
Therapeutics designed to inhibit CD28-mediated costimulation
One method of blocking CD28-mediated signals that was brought to bear was the development of anti-CD80/CD86 monoclonal antibodies. These monoclonal antibodies proved efficacious in delaying renal allograft rejection in non-human primate models even in the absence of additional therapies such as calcineurin inhibitors (CNIs) or steroids (14-16). Development of this modality progressed as far as Phase I clinical trials, where these monoclonal antibodies (h1F1 and h3D1) proved to be safe when tested as part of a combination maintenance immunosuppressive regimen containing CNIs, anti-proliferative agents (mycophenolate mofeteil, or MMF), and steroids (17). However, phase II clinical trials to evaluate efficacy of these therapeutics were never pursued.
A second strategy to inhibit CD28-mediated signals was the development of monoclonal antibodies specific for CD28 itself. Initial reports showed that short-term administration of anti-CD28 monoclonal antibodies prevented rejection in rodent models of renal allotransplantation (18, 19). Paradoxically, it was recently also demonstrated that agonism of the CD28 pathway might also result in attenuation of the alloimmune response to transplanted tissue (20-23). In a full MHC-mismatch murine graft-versus-host disease (GVHD) model, Anasetti and colleagues showed that agonizing the CD28 pathway could induce activation-induced cell death (AICD) of the recipient-specific T cells, thereby protecting the hosts from developing GVHD (20, 21). Furthermore, agonistic anti-CD28 mAb has been shown to synergize with rapamycin (20), which blocks the IL-2 dependent activation of the mammalian target of rapamycin (mTOR) (24), and was shown to further enhance survival and prevent GVHD in a full MHC-mismatch model. (20). However, agonism of the CD28 pathway as a means to induce activation induced cell death failed in a minor antigen mismatch model (25). These results are particularly important given the tragic results of a Phase I clinical trial in humans earlier this year (26), where six healthy volunteers experienced a systemic inflammatory response following administration of an agonistic anti-CD28 mAb (26). While both murine and Cynomolgus macaque recipients in previous studies failed to develop this severe systemic inflammatory response syndrome after anti-CD28 administration (20, 22, 23), further testing revealed that human T cells that receive CD28 agonism could become hyper-activated and secrete inflammatory cytokines (27). This situation highlight the fact that even non-human primate preclinical studies cannot entirely predict the effects of new candidate therapeutics in humans.
A third alternate strategy designed to block CD28 signaling made use of this property through the creation of a CTLA-4 immunoglobulin (Ig) fusion protein, which combines the extracellular binding domain of CTLA-4 with the Fc portion of human IgG1. This biologic binds CD80 and CD86 with a high avidity, thereby preventing ligation of CD28 on potentially alloreactive T cells (4). In the absence of CD28-mediated signaling, T cells fail to be optimally stimulated, resulting in abortive activation, decreased IL-2 production, down-regulation of the IL-2 receptor, and increased donor-reactive T cell death. Importantly, treatment with CTLA-4 Ig prevented the development of anti-donor antibody and T cell responses, and resulted in long-term survival of islet, cardiac, and renal allografts in murine models (28-32). While treatment with CTLA-4 Ig in rodent models proved highly efficacious, experiments in non-human primates demonstrated much more modest prolongation in allograft survival (33, 34).
Rational development of LEA29Y: A partnership of pharma and fundamental immunology
Despite these initially underwhelming results, interest in the CTLA-4 Ig fusion protein as a potential therapeutic in transplantation remained. In vivo studies suggested that CTLA-4 Ig did not completely block T cell activation and proliferation (35-38), and further studies revealed that CTLA-4 Ig is 100-fold less potent in inhibiting CD86-mediated costimulation as compared to CD80-mediated costimulation (39). Because the contact residues between CTLA-4 and CD86 had been completely elucidated (40), modification of these residues with the intent of increasing the affinity of CTLA-4Ig for CD86 was theoretically possible. For these reasons, further development of CTLA-4 Ig derivatives as a therapy for allograft rejection was pursued by Bristol Myers Squibb company, in collaboration with our laboratory. The result was the rational development of LEA29Y, a second-generative derivative of CTLA-4 Ig in which two amino acids within the CD86 binding domain had been substituted, resulting in a molecule possessing an approximately four-fold increase in affinity for CD86, two-fold increase in affinity for CD80, and 10-fold increase in its ability to inhibit T cell activation in vitro when compared to CTLA-4 Ig (41). Translation of LEA29Y into non-human primate models of renal transplantation showed superior prolongation in graft survival as compared to CTLA-4 Ig as monotherapy, and dramatically improved survival when used as part of a combined immunosuppressive regimen containing either MMF and steroids or anti-IL-2R (basliximab) (41). LEA29Y also showed efficacy as the mainstay maintenance immunosuppressive agent in a non-human primate model of neonatal porcine islet xenotransplantation (42). The development of non-CNI-based regimens for islet transplantation is critical, as the risk of nephrotoxicity associated with CNI-based regimens, is, for most diabetic patients, too great to warrant transplantation. Based on these encouraging results in non-human primate models, LEA29Y (belatacept) moved into Phase II clinical trials to evaluate the efficacy of this drug in kidney transplant recipients as the principal component of an immunosuppressive regimen consisting of basiliximab, steroids, and MMF in preventing biopsy-proven rejection as compared to cyclosporine. This study revealed similar rates of acute rejection between the belatacept and cyclosporine treated groups (43). Importantly, recipients treated with belatacept showed reduced CNI-related renal toxicities, including chronic allograft nephropathy, and showed improvement in renal function as compared to cyclosporine-treated recipients (43). Furthermore, data suggested reduced incidence of hyperlipidemia, hypertension, and post-transplant diabetes in belatacept-treated as compared to cyclosporin-treated patients. Analysis of two multi-center Phase III clinical trials of belatacept should be completed this year.
The story of the development of CD28 blockers for therapeutic use in transplantation demonstrates critical aspects of the translational process that we believe aided in the successful development of this therapeutic. First, at all stages of the process there was collaboration and dialogue between academic scientists and the biotechnology and pharmaceutical industries. Second, this partnership allowed a biologic that conceivably could have moved into clinical trials in transplantation (CTLA-4 Ig) to be brought back to basic scientists who used knowledge gleaned from fundamental immunology studies to modify and improve the reagent, which then moved forward in clinical trials.
Blockade of the CD40/CD154 Pathway: The Road Not Taken (So Far)
A second major pathway of intense interest in the transplantation community is the CD40/CD154 pathway. CD40 is a member of the TNF receptor superfamily, is constitutively expressed on B cells, macrophages, and dendritic cells, and can be inducibly expressed by endothelial cells in the presence of inflammation. Ligation of CD40 by CD154 is critical for B cell activation and class-switching, as well as for DC activation and maturation. Signaling downstream of the CD40 molecules is mediated by TRAFs and leads to the activation of NFκB, which in turn results in the upregulation of MHC molecules, upregulation of the costimulatory molecules CD80 and CD86, enhanced DC survival, and increased production of inflammatory cytokines such as TNF and IL-12. The ligand that binds to CD40, CD154, is rapidly induced on CD4+ and some CD8+ T cells following antigen encounter.
Initial efforts aimed at blocking the CD40/CD154 pathway involved the use of monoclonal antibodies specific for the CD154 ligand, and blockade of the pathway via this means has shown great promise in both rodent and non-human primate models (44-49). The development of reagents designed to block the CD40 pathway, as with CTLA-4 Ig, was aided by close collaboration between academic laboratories and biotechnology/pharmaceutical companies, including Biogen Idec and BMS. However, clinical trials using anti-CD154 monoclonal antibodies (mAb) were halted following evidence of unanticipated thromboembolic side effects (50-52). It is now appreciated that CD154 expressed by platelets as well as present in soluble form is involved in the formation and stabilization of thrombi (53, 54). While thromboembolic complications were not observed with all anti-CD154 reagents and dosing strategies (51, 52, 55), clinical development of traditional anti-CD154 mAbs has been largely suspended. Nonetheless, interrupting the CD40/CD154 signaling pathway remains one of the most potent means for inhibiting rejection in experimental models and thus an attractive albeit elusive therapeutic target. Encouragingly, however, this effect of the stabilization of arterial thrombi is thought not to involve the CD40 molecule, as CD154-/- mice exhibit unstable thrombi, whereas CD40-/- mice do not exhibit this phenotype. These findings therefore suggest that targeting CD40 rather than CD154 may allow interruption of the CD40/CD154 interaction between T cells and APCs, while not precipitating dysregulated thrombostasis in vivo.
Studies using anti-CD40 mAb conducted outside the field of transplantation have, for the most part, focused on agonistic properties of these antibodies, with the intent of augmenting immune responses to T-independent antigens or boosting anti-tumor and anti-virus activity (56-60). However, several groups, including our own, have pursued the use of anti-CD40 monoclonal antibodies as a means of attenuating anti-donor T cell responses during transplantation in both murine and non-human primate allograft models (61-63). In contemplating whether targeting the CD40 pathway would be a useful and effective target, much debate arose regarding the mechanism of exactly how anti-CD154 monoclonal antibodies mediated their effects. Our favored hypothesis is that the critical effect of CD154 blockade is to inhibit ligation of the CD40 molecule, resulting in diminished DC activation, maturation, and longevity. This concept is well supported in the literature, in that interrupting CD40-mediated signals delivered to APC during T cell priming has been shown to profoundly impact the dynamics of the T cell:APC interaction. Alternatively, it has been postulated that antibodies bound to CD154 serve to deplete activated T cells (64) or may induce reciprocal negative signaling upon the T cell itself (65).
Anti-CD40 Monoclonal Antibodies: Another Alternative Strategy
Because we believed that the effect of blocking CD154 was ultimately to inhibit CD40 signaling, we pursued the generation and testing of anti-CD40 monoclonal antibodies to interrupt the CD154/CD40 pathway without perturbing hemostasis. Again partnering academia with pharma, two novel murine anti-CD40 binding antibodies, 7E1-G1, a rat IgG1 isotype, and its natural switch variant 7E1-G2b, a rat IgG2b isotype, were developed. Standard hybridoma selection and purification techniques were used to identify and isolate the clone 7E1-G1, which was chosen as the most promising candidate to function as a CD40 antagonist based on its specificity for murine CD40, ability to inhibit ligand binding, and paucity of agonism (66). This decision again highlights one of the biggest challenges in translation of therapeutics from the bench to the bedside: where to place one's bets. In this case, it was hypothesized that an antibody which strongly induced both complement fixation and fragment crystallizable (Fc) receptor interactions would be the most favorable depleting antigen-bearing, CD40-expressing APC and therefore most effective at promoting graft survival. However, rat IgG1 antibody isotypes have previously been shown to weakly fix complement and interact poorly with Fc receptors (67-69). Thus, an antibody which possessed the same specificity as 7E1-G1, but which was of the IgG2b isotype, was sought. With this aim, using the sib-selection technique, the isotype switch variant 7E1-G2b was generated (66, 70).
While in vitro proliferation assays to measure the agonist properties of the two anti-CD40 antibodies revealed similar weak responses when plate-bound, 7E1-G1 but not 7E1-G2b led to strong proliferation when present as a soluble stimulus. Importantly, 7E1-G2b was as effective as anti-CD154 in synergizing with CTLA4-Ig to promote long-term graft survival in murine models of both allogeneic skin and bone marrow transplantation, while 7E1-G1 was not (Larsen, unpublished observations). These data suggest that an appropriately designed anti-CD40 antibody can promote graft survival as well as anti-CD154, making 7E1-G2b an attractive substitute in murine models of costimulation blockade-based tolerance regimens.
Based on these encouraging results from murine studies, a humanized mouse-anti-human CD40 mAbs were developed and tested for efficacy in prolonging renal and islet allografts in Rhesus macaque models. In one study, this monoclonal was shown to block CD154 binding and inhibit CD154-induced B cell proliferation in vitro, but also exhibited weak agonistic properties (71). Importantly, humanized anti-CD40 mAbs promoted moderate prolongation of graft survival when used as a monotherapy but effectively synergized with LEA29Y in prolonging graft survival in non-human primate models of renal and islet transplantation (61, 62, 71), and also syngerized with chimeric anti-CD86 mAbs in prolonging renal allograft survival in the rhesus (61). These results do not formally exclude alternate possibilities, but they strongly suggest that the inhibition of positive CD40-mediated signaling events is an important mechanism of action of both anti-CD40 and anti-CD154 antibodies. The mechanisms behind anti-CD40 mediated suppression and how it compares to anti-CD154 mediated suppression remain unclear, and warrant further study.
LFA-1/ICAM in Transplantation Tolerance: An Old Pathway Revisited
Thus far we have discussed the development of CD28 blocker belatacept, in which continuous progress has more or less steadily been made, from its inception in murine models, through trials in non-human primates, revision in vitro, and progression back through pre-clinical models and finally into clinical trials in renal transplantation. The progression of CD154 blockers seemed at one time to be equally promising, but are now stalled as an alternative approach to block CD154/CD40 interactions is sought and more mechanistic information about the precise effects of anti-CD154 in vivo are amassed. The road towards therapeutically targeting a third costimulatory pathway, the LFA-1/ICAM pathway, during transplantation has been slightly more circuitous. Interest into the potential utility of blocking of LFA-1/ICAM interactions for the attenuation of potent donor-reactive T cell response and protection from graft rejection was intense in the early 1990s, but investigation over the last decade had focused more on its role in the treatment of autoimmunity. Quite recently, however, there has been renewed interest in this pathway as a therapeutic target for the prevention of graft rejection following transplantation (72).
Leukocyte function-associated antigen (LFA-1) is a β2 integrin which is composed of two chains: a unique alpha chain (CD11a) and a beta chain (CD18) which is also used by other β2 integrin proteins. Through its interaction with intracellular adhesion molecules (ICAMs), LFA-1 plays an important role in both T cell trafficking and costimulation of T cells through its involvement in the immunological synapse. During the initial phases of T cell activation, LFA-1 is localized at the center of the T cell:APC contact, whereas the TCR:pMHC molecules are found mainly around the periphery. At later timepoints, however, TCRs move to the center of the synapse and are downregulated, leaving a ring of LFA-1 surrounding the center of the synapse, and this ring persists for many hours. As such, LFA-1 plays an integral role in the stabilization of T cell:APC contacts, thus ensuring optimum activation of T cells. In addition to its adhesive functions, LFA-1 delivers costimulatory signals that are important for T cell activation and cytotoxic T cell function (73-75). LFA-1 also is expressed on B cells and the blockade of LFA-1, when combined with other immunosuppressive agents, inhibited the formation of anti-donor antibodies (76-78). Lastly, LFA-1 expression is increased on memory T cells relative to their naïve counterparts (79, 80), suggesting a potential therapeutic role for LFA-1 blockade in the setting of existing anti-donor T cell memory.
Targeting the LFA-1/ICAM Pathway in Rodent Models of Allograft Rejection
Over the last two decades, therapeutic targeting of LFA-1 has shown great promise in experimental transplant models. Murine studies of anti-LFA-1 antibodies alone or in combination with other agents have shown dramatic prolongation of islet, heart and skin allograft survival and, in some models, transplantation tolerance (77, 78, 81-88). Specifically, anti-LFA-1 has shown utility in murine models when combined with CTLA-4 Ig, anti-CD154, anti-ICAM-1, anti-CD45RB, calcineurin inhibitors, and everolimus (89).
The mechanisms underlying the ability of LFA-1 blockade prolong graft survival, either alone or in combination with other therapeutics, are at present unclear. What is clear from numerous reports is that anti-LFA-1 treatment does not result in pan T cell depletion, and while it does induce a lymphocytosis for the duration of treatment (90), T cells are eventually able to infiltrate the graft (82). Furthermore, several studies have demonstrated that the tolerance or long-term graft survival induced via LFA-1 blockade post-transplant is resistant to subsequent immunizations with donor splenocytes (81, 82). Taken together, these results suggest that the mechanism of prolongation in graft survival following LFA-1 blockade is more likely to be due to interruption of the costimulatory and adhesive role of this pathway in facilitating optimum T cell priming in the immunological synapse, as opposed to the blocking of lymphocyte trafficking into the graft.
Translation of LFA-1 Blockade in Transplantation and Autoimmunity
Given the wide variety of regimens and protocols using LFA-1 blockade that were developed in murine models, one challenge that presented itself was the decision of which of these regimens to begin to translate clinically. Early attempts to further evaluate purely murine anti-LFA-1 mAbs in human clinical kidney and bone marrow transplantation during the 1990s similarly showed some promise: combined anti-LFA-1 and anti-CD2 mAb proved to have efficacy in bone marrow transplantation in pediatric populations with acute lymphoblastic leukemia (91). However, this success in children was unfortunately not mirrored in adult recipients of bone marrow transplants (92). Experience in solid-organ transplantation saw similarly mediocre results: a French pilot study of murine anti-LFA-1 mAbs in preventing rejection of renal allografts failed to demonstrate efficacy. Despite a 1996 multi-center trial which demonstrated that anti-LFA-1 monoclonal antibodies were as effective as the pan-T cell depleting agent thymoglobulin in induction therapy in renal transplantation (93), these early clinical trials led to a dead end and these therapeutics were ultimately not developed. It is likely that their efficacy may have been limited by the suboptimal properties of murine mAbs as therapeutics. Unfortunately, no further collaboration between industry and academia were undertaken at that point to guide the further development of anti-LFA-1 mAbs.
In the later 1990s, the development of therapeutic anti-LFA-1 mAbs shifted focus to autoimmunity, leading to the development of efalizumab, which has now received FDA-approval for the treatment of severe plaque psoriasis (12, 13). Efalizumab, a humanized monoclonal antibody that binds to CD11a, reversibly blocks the binding of LFA-1 to its ligand ICAM-1, and does not result in T cell depletion in vivo. This monoclonal antibody therefore represents a potentially new class of therapeutic agents that both attenuate T cell activation and inhibit cell migration during undesired donor-reactive immune responses in vivo (14). In patients with moderate to severe plaque psoriasis, efalizumab resulted in significant improvements when tested as a monotherapy, demonstrating outcomes that were comparable to those reported with cyclosporine in previous trials (94). Overall, the safety profile of efalizumab is tolerable, with little evidence of nephrotoxicity, diabetes, hypertension, opportunistic infections, or neurological disorders (95-100). However, two cases of progressive multifocal leukoencephalopathy (PML) in patients both > 70 years of age have recently been reported from post-marketing surveillance. This rare but potentially fatal JC polyoma-associated disease has thus occurred in only two of 46,000 treated subjects. While this is clearly a devastating complication, the major challenge for the field of transplantation will be to assess the relative risk of common and familiar toxicities such as cardiovascular morbidities, post-transplant diabetes, dyslipidemia, hypertension, and accelerated allograft failure from calcineurin inhibitor-derived nephrotoxicities, with the risk of unfamiliar but rare complications such as PML. Overall, the large number of treated psoriatic patients combined with relatively rare incidence of side effects suggests that blockade of the LFA-1 costimulatory pathway may be an efficacious and safe method of preventing transplant rejection, thus bypassing the nephrotoxicity and other side effects associated with current calcineurin inhibitor-based immunosuppressive regimens.
Given the encouraging results of humanized anti-LFA-1 monoclonal antibodies in attenuating unwanted immune responses in psoriasis, renewed interest in this pathway as a potential target in transplantation has surfaced. One promising study involved the use of anti-LFA-1 monotherapy in prolonging cardiac allograft survival in a robust fully allogeneic rhesus macaque model (101). Ongoing studies in non-human primates have demonstrated that LFA-1 blockade is efficacious in preventing islet allograft rejection in non-human primates when combined with rapamycin (Larsen, unpublished data). In addition, efalizumab has been evaluated in a pilot trial in human renal transplant recipients. In this study, it was evaluated in multi-drug regimens in combination with cyclosporine. A very low incidence of rejection was observed in all of the groups receiving efalizumab (7.8%). In a pilot study of human alloislet transplants, LFA-1 blockade has shown efficacy in promoting graft survival when combined with mycophenolate mofeteil. With the advent of humanized monoclonal antibodies for blockade of the LFA-1 pathway, this target has once again engaged the interest of the transplant community, and as more clinical trials are in the pipeline, both the efficacy and safety of this reagent as a useful tool in the anti-rejection armamentarium remains to be discovered.
From the Bench to the Bedside and Back: Current Challenges in Costimulation Blockade Based Immunosuppression
In this article we have discussed the development of blocking agents for three pathways: CD28, CD40, and LFA-1, as they progressed from pre-clinical through translational models and finally into clinical trials. While the future of all of these therapeutic targets remains to be played out through further modifications and future clinical trials, one concept that is becoming increasingly clear with all types of immunosuppressive regimens is that it may not be feasible, or even desirable, to develop a one-size-fits-all approach to immunotherapy for transplantation. Rather, the mechanistic studies that have accompanied these translational studies, and that are currently ongoing, have suggested that several important factors may determine the relative likelihood of success of costimulation-based therapies in a given patient. Here, we revisit three such parameters that mechanistic studies in murine models have identified as being critical for the induction of long-term graft survival. Understanding these parameters may help us refine costimulation blockade based therapies and tailor them to individual patients.
Precursor Frequency of Donor-reactive T cell responses: A barrier to long-term graft survival induced by costimulation blockade
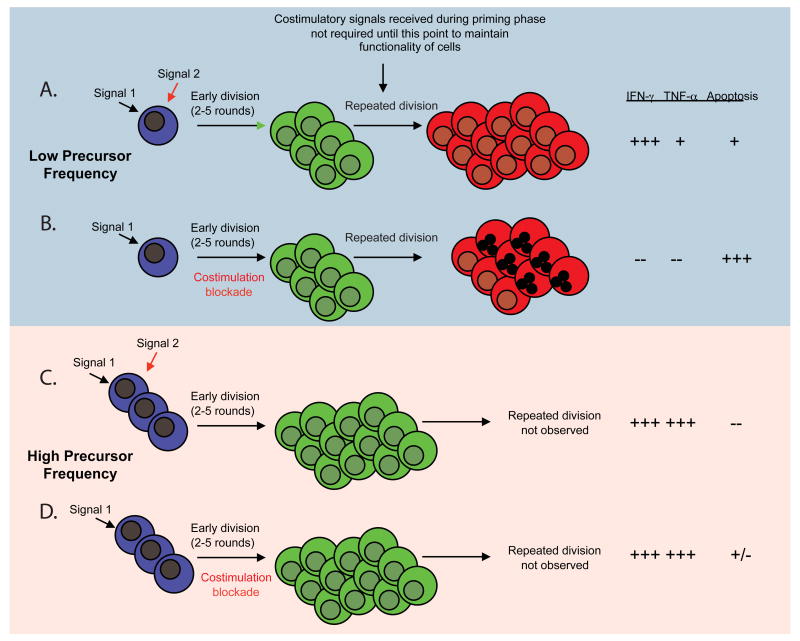
While we have highlighted the relatively large degree of success of costimulatory blockade in promoting long-term graft survival in rodent models of transplantation, this success is by no means uniform. Rather, several models in which “breakthrough” rejection occurs despite costimulation blockade have been identified. As a result, we have gone back to the bench to investigate those factors that are most critical in inducing “costimulation blockade resistant rejection.” First, we recently demonstrated a critical role for both naïve CD4+ and CD8+ T cell precursor frequency in determining their abilities to mediate costimulation blockade-resistant rejection following transplantation (102, 103). Specifically, when present at high frequency approximating those encountered across full MHC disparities, naive graft-specific CD8+ T cells proliferated, differentiated, and accumulated at high numbers even in the presence of CTLA-4 Ig and anti-CD154 costimulatory blockade, resulting in more “high-quality,” multicytokine producing effector cells and the rapid precipitation of graft rejection (102, 104). In marked contrast, naïve graft-specific CD8+ T cells stimulated at lower frequency failed to accumulate and did not differentiate into high quality effectors that were capable of rejecting a skin graft in the presence of CD28 and CD40 pathway blockade (Figure 1). Thus, naïve CD8+ T cells present at high frequency appear to obviate the need for costimulation during priming. In subsequent studies, we observed that a critical CD4+ T cell threshold precursor frequency was necessary for the provision of help following blockade of the CD28 and CD154 costimulatory pathways, as measured by increased B cell and CD8+ T cell responses and precipitation of graft rejection (105). In contrast to high-frequency CD8+ T cell responses, this effect was observed even though the proliferative and cytokine responses of Ag-specific CD4+ T cells were inhibited. Thus, we conclude that an initial high frequency of donor-reactive CD4+ T cells uncouples T cell proliferative and effector cytokine production from the provision of T cell help. The findings that donor-reactive T cell precursor frequency may potently impact graft survival under treatment with costimulation blockade may have important potential clinical implications. As new costimulation blockade-based modalities enter clinical trials in transplantation, it will be important to fully comprehend and evaluate the circumstances that predict a greater likelihood of success or portend break-through rejection, such as high initial CD8+ or CD4+ donor-reactive T cell frequency. If initial donor-reactive T cell precursor frequency also proves to have a potent effect in human allo-specific T cell responses, this could prompt consideration of alternative approaches to organ allocation. For example, this knowledge might re-emphasize class I and/or class II MHC matching as a means to effectively lower the graft-specific CD8+ and CD4+ T cell frequencies, respectively. More likely, however, identification of low CD4+ or CD8+ donor-reactive precursor frequency in a particular patient could be used to direct the reduction of immunosuppression or prompt attempts at tolerance induction in that individual, whereas detection of high donor-reactive T cell frequencies might direct the intensification of immunosuppression for the control of break-through CD8+ T cell responses. Furthermore, these results might suggest that costimulatory blockade may potently synergize with other therapeutics designed to globally reduce the initial frequency of all T cells, such as anti-CD52 (106, 107) or anti-CD3 mAbs (108), in that these agents would effectively lower the initial frequency of graft-specific T cells and thereby increase the efficacy of treatment with costimulatory blockade.
Figure 1.
Impact of antigen-specific T cell precursor frequency on susceptibility to costimulation blockade. A, Cells stimulated at low precursor frequency are required to undergo multiple rounds of division in order to generate a threshold number of effector cells needed to mediate graft rejection. B, Cells that undergo multiple rounds of division in the absence of costimulation fail to differentiate into competent effectors and undergo increased cell death at later rounds of division. C, In contrast, cells that are stimulated at high naïve T cell precursor frequency must undergo many fewer rounds of division in order to a sufficient number of effector cells to mediate graft rejection. D, Populations that had undergone fewer rounds of division in the absence of costimulation had better effector function and reduced death as compared to those that underwent more rounds of division in the absence of costimulation.
Heterologous Immunity and The Memory Barrier to Transplantation Tolerance
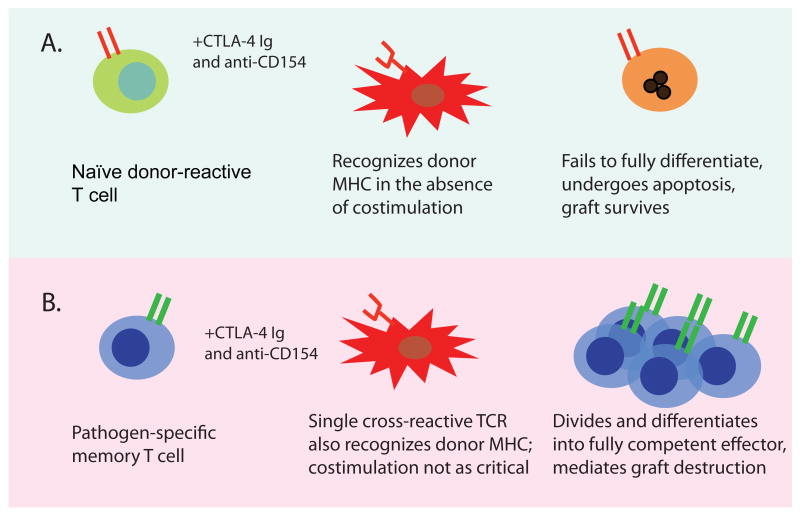
Thus far we have highlighted the ability of blockade of T cell costimulatory pathways to potently and specifically attenuate naïve anti-donor T cell responses following transplantation in mouse, monkey, and man. However, numerous studies have shown that the presence of donor-reactive memory T cells in the recipient poses a sometimes insurmountable barrier to long-term graft survival and tolerance induction. Donor-reactive memory T cells may arise from prior transplantation, blood transfusion, pregnancy, or environmental exposure to pathogens. Pathogen-specific memory T cells, by virtue of the inherent degeneracy of TCR recognition of peptide:MHC ligands, may exhibit cross-reactivity with allogeneic peptide:MHC complexes and thereby mediate graft rejection (Figure 2). Thus, a proportion of the alloreactive T cell repertoire may reside within the memory T cell compartment in socially-housed non-human primates as well as humans. The problem as it relates to effective post-transplant immunosuppression is that memory T cells are phenotypically and functionally distinct from their naïve counterparts, in that they possess a lower activation threshold and are poised to respond rapidly upon restimulation (109, 110). For instance, memory cells exhibit reduced requirements for both signal one (the strength and/or duration of TCR stimulation) and importantly, for signal two (costimulation) (110, 111). In addition, memory T cells have been shown to express higher levels of adhesion molecules (LFA-1, CD44), cytokine receptors (CD122) and anti-apoptotic molecules of the bcl-2 family relative to naïve T cells (112-114). Numerous studies have shown that memory T cells seem to be refractory to control via a wide variety of therapeutic interventions, perhaps due to these reduced activation requirements and increased expression of anti-apoptotic molecules. Furthermore, CD40 and CD28 blockade-based protocols that effectively tolerize naïve donor-specific T cells may not be effective against donor-specific memory T cells elicited either by exposure to donor antigens or viral pathogens (115-118). For example, while a costimulation blockade-based tolerance regimen effectively prevented skin graft rejection in naïve mice, mice that had been previously infected with one, two, or three different viruses demonstrated increasing resistance to the induction of tolerance by costimulation blockade (116). Costimulation blockade-induced tolerance can be broken by both CD4+ and CD8+ donor-specific memory cells, as evidenced by a variety of murine model systems (116, 119). In addition, we have demonstrated that a critical frequency of memory T cells is required to generate costimulation blockade-resistant rejection, in that adoptive transfer of 105 but not 104 alloreactive memory cells into naïve recipients resulted in graft loss despite treatment with costimulation blockade (116). Therefore, in addition to their clearly reduced activation threshold, a critical component involved in the costimulation blockade resistance of memory T cells may be their heightened precursor frequency. Taken together, these studies demonstrate that high-frequency T cell responses, present either as naïve alloreactive populations or as memory T cell populations cross-reactive for an alloantigen, may obviate the need for costimulation and play a large role in mediating costimulation blockade-resistant allograft rejection. Moreover, the immune history of a transplant recipient and frequencies of donor-cross-reactive memory T cells may predict the likelihood of success or failure of long-term graft survival induced by costimulation blockade-based therapies.
Figure 2.
Heterologous immunity to pathogens is a potent barrier to transplantation tolerance/long-term graft survival induced by costimulation blockade. A, Naïve donor-reactive T cells encounter donor antigen under the cover of costimulation blockade, fail to fully differentiate, undergo apoptosis, and therefore fail to mediate destruction of the graft. B, Memory T cells specific for pathogen-derived epitopes presented by self MHC may cross-react with donor-derived MHC and become activated. By virtue of their reduced requirement for CD28 and CD40 mediated costimulation during reactivation, these pathogen-derived memory T cells may then expand, differentiate, and mediate graft destruction.
What's good for the T cell response is bad for the graft: the opposing roles of IFN-γ in costimulation blockade-induced transplant survival
A third parameter that impacts the effectiveness of costimulation blockade appears to be the presence of interferon-gamma (IFN-γ) following transplantation. Early studies had suggested that IFN-γ deficient animals were resistant to the effects of costimulation blockade (120-123), but many studies have suggested that IFN-γ augments, instead of inhibits, CD8+ T cell responses (124-126). We assessed the expansion of donor-specific CD8 T cell populations during the course of the immune response to fully MHC disparate skin allografts and found that although costimulation blockade delayed the emergence of a population of donor-specific CD8+ effector T cells, a small “breakthrough” population was detectable around the time of graft loss in costimulation blockade-treated recipients (127). Notably, the presence of IFN-γ was critical for the expansion of this donor-specific CD8+ T cell population under costimulation blockade, because this population failed to expand in IFN-γR deficient animals or when IFN-γ was neutralized in vivo (127). Strikingly, however, the absence of a detectable population of donor-specific effector CD8 T cells allografts did not confer protection of the allografts, as grafts were rapidly rejected when IFNγ was neutralized following treatment with costimulation blockade. This result is explained by the finding that the grafted skin itself required IFN-γ in order to survive after allotransplantation under costimulation blockade (127). Numerous reports have also confirmed the critical importance of graft-directed action of IFN-γ on the survival of murine liver allografts as well as on the prevention of early necrosis of heart or kidney allografts in untreated recipients (128-132), and we have shown the necessity of IFN-γ for graft survival in a model in which the donor-reactive T cell response is attenuated via costimulation blockade (127). In summary, these studies imply that accelerated graft loss in IFN-γ-/-recipients is due not to uncontrolled expansion of costimulation blockade-resistant alloreactive T cells, but rather to the lack of direct IFN-γ signals to the graft to support tissue survival even at times when the recipient T cell response is undetectable.
These mechanistic studies, while defining a critical role for IFN-γ in the “break-through” CD8+ T cell response following treatment with costimulation blockade, also highlight the important role of this cytokine in maintaining tissue integrity of the graft following transplantation. Thus, translation of IFN-γ neutralization into a clinically therapeutic may be hampered by its pleiotropic effects on the anti-donor T cell response and the graft. The story of IFN-γ in transplantation is an excellent example of how basic mechanistic studies will allow us to evaluate which targets are good candidates for clinical translation, and which, due to properties such as those described for IFN-γ, may be unsuitable.
Conclusions
Attempts at translating blockade of three key costimulatory pathways for the prolongation of graft survival from the bench to the clinic over the last two decades have provided us with good lessons to come away with. First, the pace and outcome of translational science is hard to predict—sometimes it progresses nicely (but not with out road blocks, as in the case of CTLA-4 Ig), sometimes it takes a long and tortuous route to come to fruition (as in the case of anti-LFA-1), and sometimes it just gets stalled in the mud (as in the case of anti-CD154). Second, while there has been a recent healthy focus on the potential negative impact of unmanaged conflicts on interest in biomedical research, translation of these therapeutics was greatly aided, not surprisingly, by the early engagement and support of pharmaceutical institutions to drive translational studies. Third, these tales highlight the critical importance of the non-human primate as an intermediate step between murine screening and mechanistic models and clinical trials. Lastly, as demonstrated by the mechanistic studies that shed light on specific factors that may enhance or mitigate the efficacy of costimulation blockade-based therapy in transplantation, collaboration between basic and clinical scientists is crucial for the successful translation of new therapeutics. These interactions will propel the development of both novel methods to target currently-appreciated pathways and the discovery of new molecules and pathways critical in tipping the balance between transplant rejection and tolerance.
References
- 1.Germain RN, Stefanova I. The dynamics of T cell receptor signaling: complex orchestration and the key roles of tempo and cooperation. Annu Rev Immunol. 1999;17:467–522. doi: 10.1146/annurev.immunol.17.1.467. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khoury S, Sayegh MH, Turka LA. Blocking costimulatory signals to induce transplantation tolerance and prevent autoimmune disease. International reviews of immunology. 1999;18:185–199. doi: 10.3109/08830189909043024. [DOI] [PubMed] [Google Scholar]
- 4.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 5.Lindstein T, June CH, Ledbetter JA, Stella G, Thompson CB. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989;244:339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- 6.Fraser JD, Irving BA, Crabtree GR, Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991;251:313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- 7.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 8.Howland KC, Ausubel LJ, London CA, Abbas AK. The roles of CD28 and CD40 ligand in T cell activation and tolerance. J Immunol. 2000;164:4465–4470. doi: 10.4049/jimmunol.164.9.4465. [DOI] [PubMed] [Google Scholar]
- 9.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 10.Lenschow D, Zeng Y, Thistlethwaite J, Montag A, Brady W, Gibson M, Linsley P, Bluestone J. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4Ig. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 11.Turka LA, Linsley PS, Lin H, Brady W, Leiden JM, Wei R, Gibson ML, Zheng X, Myrdal S, Gordon D, Bailey T, Boiling SF, Thompson CB. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc Natl Acad Sci USA. 1992;89:11102–11105. doi: 10.1073/pnas.89.22.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szot GL, Zhou P, Sharpe AH, He G, Kim O, Newell KA, Bluestone JA, Thistlethwaite JR., Jr Absence of host B7 expression is sufficient for long-term murine vascularized heart allograft survival. Transplantation. 2000;69:904–909. doi: 10.1097/00007890-200003150-00040. [DOI] [PubMed] [Google Scholar]
- 13.Mandelbrot DA, Furukawa Y, McAdam AJ, Alexander SI, Libby P, Mitchell RN, Sharpe AH. Expression of B7 molecules in recipient, not donor, mice determines the survival of cardiac allografts. J Immunol. 1999;163:3753–3757. [PubMed] [Google Scholar]
- 14.Birsan T, Hausen B, Higgins JP, Hubble RW, Klupp J, Stalder M, Celniker A, Friedrich S, O'Hara RM, Morris RE. Treatment with humanized monoclonal antibodies against CD80 and CD86 combined with sirolimus prolongs renal allograft survival in cynomolgus monkeys. Transplantation. 2003;75:2106–2113. doi: 10.1097/01.TP.0000066806.10029.7A. [DOI] [PubMed] [Google Scholar]
- 15.Kirk AD, Tadaki DK, Celniker A, Batty DS, Berning JD, Colonna JO, Cruzata F, Elster EA, Gray GS, Kampen RL, Patterson NB, Szklut P, Swanson J, Xu H, Harlan DM. Induction therapy with monoclonal antibodies specific for CD80 and CD86 delays the onset of acute renal allograft rejection in non-human primates. Transplantation. 2001;72:377–384. doi: 10.1097/00007890-200108150-00005. [DOI] [PubMed] [Google Scholar]
- 16.Hausen B, Klupp J, Christians U, Higgins JP, Baumgartner RE, Hook LE, Friedrich S, Celnicker A, Morris RE. Coadministration of either cyclosporine or steroids with humanized monoclonal antibodies against CD80 and CD86 successfully prolong allograft survival after life supporting renal transplantation in cynomolgus monkeys. Transplantation. 2001;72:1128–1137. doi: 10.1097/00007890-200109270-00025. [DOI] [PubMed] [Google Scholar]
- 17.Vincenti F. What's in the Pipeline? New immunosuppressive Drugs in Transplantation. American Journal of Transplantation. 2002;2:898–903. doi: 10.1034/j.1600-6143.2002.21005.x. [DOI] [PubMed] [Google Scholar]
- 18.Haspot F, Seveno C, Dugast AS, Coulon F, Renaudin K, Usal C, Hill M, Anegon I, Heslan M, Josien R, Brouard S, Soulillou JP, Vanhove B. Anti-CD28 antibody-induced kidney allograft tolerance related to tryptophan degradation and TCR class II B7 regulatory cells. Am J Transplant. 2005;5:2339–2348. doi: 10.1111/j.1600-6143.2005.01018.x. [DOI] [PubMed] [Google Scholar]
- 19.Laskowski IA, Pratschke J, Wilhelm MJ, Dong VM, Beato F, Taal M, Gasser M, Hancock WW, Sayegh MH, Tilney NL. Anti-CD28 monoclonal antibody therapy prevents chronic rejection of renal allografts in rats. J Am Soc Nephrol. 2002;13:519–527. doi: 10.1681/ASN.V132519. [DOI] [PubMed] [Google Scholar]
- 20.Albert MH, Yu XZ, Martin PJ, Anasetti C. Prevention of lethal acute GVHD with an agonistic CD28 antibody and rapamycin. Blood. 2005;105:1355–1361. doi: 10.1182/blood-2004-08-3305. [DOI] [PubMed] [Google Scholar]
- 21.Yu XZ, Albert MH, Martin PJ, Anasetti C. CD28 ligation induces transplantation tolerance by IFN-gamma-dependent depletion of T cells that recognize alloantigens. J Clin Invest. 2004;113:1624–1630. doi: 10.1172/JCI20940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu XZ, Martin PJ, Anasetti C. CD28 signal enhances apoptosis of CD8 T cells after strong TCR ligation. J Immunol. 2003;170:3002–3006. doi: 10.4049/jimmunol.170.6.3002. [DOI] [PubMed] [Google Scholar]
- 23.Yu XZ, Bidwell SJ, Martin PJ, Anasetti C. CD28-specific antibody prevents graft-versus-host disease in mice. J Immunol. 2000;164:4564–4568. doi: 10.4049/jimmunol.164.9.4564. [DOI] [PubMed] [Google Scholar]
- 24.Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplantation proceedings. 2003;35:7S–14S. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 25.Ford ML, Wagener ME, Gangappa S, Pearson TC, Larsen CP. Antigenic disparity impacts outcome of agonism but not blockade of costimulatory pathways in experimental transplant models. Am J Transplant. 2007;7:1471–1481. doi: 10.1111/j.1600-6143.2007.01826.x. [DOI] [PubMed] [Google Scholar]
- 26.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 27.Stebbings R, Findlay L, Edwards C, Eastwood D, Bird C, North D, Mistry Y, Dilger P, Liefooghe E, Cludts I, Fox B, Tarrant G, Robinson J, Meager T, Dolman C, Thorpe SJ, Bristow A, Wadhwa M, Thorpe R, Poole S. “Cytokine storm” in the phase I trial of monoclonal antibody TGN1412: better understanding the causes to improve preclinical testing of immunotherapeutics. J Immunol. 2007;179:3325–3331. doi: 10.4049/jimmunol.179.5.3325. [DOI] [PubMed] [Google Scholar]
- 28.Baliga P, Chavin KD, Qin L, Woodward J, Lin J, Linsley PS, Bromberg JS. CTLA4Ig prolongs allograft survival while suppressing cell-mediated immunity. Transplantation. 1994;58:1082–1090. [PubMed] [Google Scholar]
- 29.Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4Ig. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 30.Judge TA, Tang A, Turka LA. Immunosuppression through blockade of CD28:B7-mediated costimulatory signals. Immunol Res. 1996;15:38–49. doi: 10.1007/BF02918283. [DOI] [PubMed] [Google Scholar]
- 31.Azuma H, Chandraker A, Nadeau K, Hancock WW, Carpenter CB, Tilney NL, Sayegh MH. Blockade of T-cell costimulation prevents development of experimental chronic renal allograft rejection [see comments] Proc Natl Acad Sci USA. 1996;93:12439–12444. doi: 10.1073/pnas.93.22.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandraker A, Azuma H, Nadeau K, Carpenter CB, Tilney NL, Hancock WW, Sayegh MH. Late blockade of T cell costimulation interrupts progression of experimental chronic allograft rejection. J Clin Invest. 1998;101:2309–2318. doi: 10.1172/JCI2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, Hong X, Thomas D, Fechner JH, Jr, Knechtle SJ. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A. 1997;94:8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levisetti MG, Padrid PA, Szot GL, Mittal N, Meehan SM, Wardrip CL, Gray GS, Bruce DS, Thistlethwaite JR, Jr, Bluestone JA. Immunosuppressive effects of human CTLA4Ig in a non-human primate model of allogeneic pancreatic islet transplantation. Journal of Immunology. 1997;159:5187–5191. [PubMed] [Google Scholar]
- 35.Ronchese F, Hausmann B, Hubele S, Lane P. Mice transgenic for a soluble form of murine CTLA-4 show enhanced expansion of antigen-specific CD4+ T cells and defective antibody production in vivo. J Exp Med. 1994;179:809–817. doi: 10.1084/jem.179.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sayegh MH, Akalin E, Hancock WW, Russell ME, Carpenter CB, Linsley PS, Turka LA. CD28-B7 blockade after alloantigenic challenge in vivo inhibits Th1 cytokines but spares Th2. J Exp Med. 1995;181:1869–1874. doi: 10.1084/jem.181.5.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khoury SJ, Akalin E, Chandraker A, Turka L, Linsley PS, Sayegh MH, Hancock WW. CD28-B7 costimulatory blockade by CTLA4-Ig prevents actively induced experiemental autoimmune encephalomyelitis and inhibits Th1 but spares Th2 cytokines in the central nervous system. J Immunol. 1995;155:4521–4524. [PubMed] [Google Scholar]
- 38.Griggs ND, Agersborg SS, Noelle RJ, Ledbetter JA, Linsley PS, Tung KS. The relative contribution of the CD28 and gp39 costimulatory pathways in the clonal expansion and pathogenic acquisition of self-reactive T cells. J Exp Med. 1996;183:801–810. doi: 10.1084/jem.183.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors [published erratum appears in Immunity 1995 Feb;2(2):following 203] Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 40.Peach RJ, Bajorath J, Brady W, Leytze G, Greene J, Naemura J, Linsley PS. Complementarity determining region 1 (CDR1)- and CDR3-analogous regions in CTLA-4 and CD28 determine the binding to B7-1. J Exp Med. 1994;180:2049–2058. doi: 10.1084/jem.180.6.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobertm E, Anderson D, Cowan S, Price K, Naemura J, Emswiler J, Greene J, Turk LA, Bajorath J, Townsend R, Hagerty D, Linsley PS, Peach RJ. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5:443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 42.Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, Bello-Laborn H, Hacquoil B, Strobert E, Gangappa S, Weber CJ, Pearson TC, Rajotte RV, Larsen CP. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12:304–306. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 43.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Lang P, Grinyo J, Halloran PF, Solez K, Hagerty D, Levy E, Zhou W, Natarajan K, Charpentier B. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 44.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Cho HR, Aruffo A, Hollenbaugh D, Linsley PS, Winn KJ, Pearson TC. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 45.Kirk AD, Burkly LC, Batty DS, Baumgartner RE, Berning JD, Buchana K, Fechner JH, Jr, Germond RL, Kampen RL, Patterson NB, Swanson SJ, Tadaki DK, TenHoor CN, White L, Knechtle SJ, Harlan DM. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5:686–693. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- 46.Kenyon NS, Fernandez LA, Lehmann R, Masetti M, Ranuncoli A, Chatzipetrou M, Iaria G, Han D, Wagner JL, Ruiz P, Berho M, Inverardi L, Alejandro R, Mintz DH, Kirk AD, Harlan DM, Burkly LC, Ricordi C. Long-term survival and function of intrahepatic islet allografts in baboons treated with humanized anti-CD154. Diabetes. 1999;48:1473–1481. doi: 10.2337/diabetes.48.7.1473. [DOI] [PubMed] [Google Scholar]
- 47.Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, Hong X, Thomas D, Fechner JH, Knechtle SJ. CTLA4Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA. 1997;94:8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parker DC, Greiner DL, Phillips NE, Appel MC, Steele AW, Durie FH, Noelle RJ, Mordes JP, Rossini AA. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc Natl Acad Sci USA. 1995;92:9560–9564. doi: 10.1073/pnas.92.21.9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hancock WW, Sayegh MH, Zheng XG, Peach R, Linsley PS, Turka LA. Costimulatory function and expression of CD40 ligand, CD80, and CD86 in vascularized murine cardiac allograft rejection. Proc Natl Acad Sci USA. 1996;93:13967–13972. doi: 10.1073/pnas.93.24.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanmaz T, Fechner JJ, Jr, Torrealba J, Kim HT, Dong Y, Oberley TD, Schultz JM, Bloom DD, Katayama M, Dar W, Markovits J, Schuler W, Hu H, Hamawy MM, Knechtle SJ. Monotherapy with the novel human anti-CD154 monoclonal antibody ABI793 in rhesus monkey renal transplantation model. Transplantation. 2004;77:914–920. doi: 10.1097/01.tp.0000116392.72152.75. [DOI] [PubMed] [Google Scholar]
- 51.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 52.Weaver TA, Charafeddine AH, Kirk AD. Costimulation blockade: towards clinical application. Front Biosci. 2008;13:2120–2139. doi: 10.2741/2829. [DOI] [PubMed] [Google Scholar]
- 53.Inwald DP, McDowall A, Peters MJ, Callard RE, Klein NJ. CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ Res. 2003;92:1041–1048. doi: 10.1161/01.RES.0000070111.98158.6C. [DOI] [PubMed] [Google Scholar]
- 54.Andre P, Prasad KS, Denis CV, He M, Papalia JM, Hynes RO, Phillips DR, Wagner DD. CD40L stabilizes arterial thrombi by a beta3 integral--dependent mechanism. Nat Med. 2002;8:247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 55.Kean LS, Gangappa S, Pearson TC, Larsen CP. Transplant tolerance in non-human primates: progress, current challenges and unmet needs. Am J Transplant. 2006;6:884–893. doi: 10.1111/j.1600-6143.2006.01260.x. [DOI] [PubMed] [Google Scholar]
- 56.Schoenberger SP, Toes REM, van der Voort EIH, Offringa R, Melief CJM. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 57.Staveley-O'Carroll K, Schell TD, Jimenez M, Mylin LM, Tevethia MJ, Schoenberger SP, Tevethia SS. In vivo ligation of CD40 enhances priming against the endogenous tumor antigen and promotes CD8+ T cell effector function in SV40 T antigen transgenic mice. J Immunol. 2003;171:697–707. doi: 10.4049/jimmunol.171.2.697. [DOI] [PubMed] [Google Scholar]
- 58.Maxwell JR, Campbell JD, Kim CH, Vella AT. CD40 activation boosts T cell immunity in vivo by enhancing T cell clonal expansion and delaying peripheral T cell deletion. Journal of Immunology. 1999;162:2024–2034. [PubMed] [Google Scholar]
- 59.Bennett SRM, Carbone FR, Karamalis F, Flavell RA, Miller JFAP, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 60.Dullforce P, Sutton DC, Heath AW. Enhancement of T cell-independent immune responses in vivo by CD40 antibodies. Nat Med. 1998;4:88–91. doi: 10.1038/nm0198-088. [DOI] [PubMed] [Google Scholar]
- 61.Haanstra KG, Ringers J, Sick EA, Ramdien-Murli S, Kuhn EM, Boon L, Jonker M. Prevention of kidney allograft rejection using anti-CD40 and anti-CD86 in primates. Transplantation. 2003;75:637–643. doi: 10.1097/01.TP.0000054835.58014.C2. [DOI] [PubMed] [Google Scholar]
- 62.Haanstra KG, Sick EA, Ringers J, Wubben JA, Kuhn EM, Boon L, Jonker M. Costimulation blockade followed by a 12-week period of cyclosporine A facilitates prolonged drug-free survival of rhesus monkey kidney allografts. Transplantation. 2005;79:1623–1626. doi: 10.1097/01.tp.0000158426.64631.ed. [DOI] [PubMed] [Google Scholar]
- 63.Masunaga T, Yamashita K, Sakihama H, Hashimoto T, Hua N, Imai A, Inobe M, Miyazaki T, Todo S, Uede T. Dimeric but not monomeric soluble CD40 prolongs allograft survival and generates regulatory T cells that inhibit CTL function. Transplantation. 2005;80:1614–1622. doi: 10.1097/01.tp.0000181093.50141.6c. [DOI] [PubMed] [Google Scholar]
- 64.Monk NJ, Hargreaves RE, Marsh JE, Farrar CA, Sacks SH, Millrain M, Simpson E, Dyson J, Jurcevic S. Fc-dependent depletion of activated T cells occurs through CD40L-specific antibody rather than costimulation blockade. Nat Med. 2003;9:1275–1280. doi: 10.1038/nm931. [DOI] [PubMed] [Google Scholar]
- 65.Blair PJ, Riley JL, Harlan DM, Abe R, Tadaki DK, Hoffmann SC, White L, Francomano T, Perfetto SJ, Kirk AD, June CH. CD40 ligand (CD 154) triggers a short-term CD4(+) T cell activation response that results in secretion of immunomodulatory cytokines and apoptosis. J Exp Med. 2000;191:651–660. doi: 10.1084/jem.191.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aruffo AA, Hollenbaugh D, Siadak AW, Berry KK, Harris L, Thorne BA, Bajorath J. In: Methods of using antibodies against human CD40. U. S. P. Office, editor. Bristol-Myers Squibb Company; United States: 1999. [Google Scholar]
- 67.Hale G, Clark M, Waldmann H. Therapeutic potential of rat monoclonal antibodies: isotype specificity of antibody-dependent cell-mediated cytotoxicity with human lymphocytes. J Immunol. 1985;134:3056–3061. [PubMed] [Google Scholar]
- 68.Bruggemann M, Teale C, Clark M, Bindon C, Waldmann H. A matched set of rat/mouse chimeric antibodies. J Immunol. 1989;142:3145–3150. [PubMed] [Google Scholar]
- 69.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 70.Hale G, Cobbold SP, Waldmann H, Easter G, Matejtschuk P, Coombs RR. Isolation of low-frequency class-switch variants from rat hybrid myelomas. J Immunol Methods. 1987;103:59–67. doi: 10.1016/0022-1759(87)90242-0. [DOI] [PubMed] [Google Scholar]
- 71.Adams AB, Shirasugi N, Jones TR, Durham MM, Strobert EA, Cowan S, Rees P, Hendrix R, Price K, Kenyon NS, Hagerty D, Townsend R, Hollenbaugh D, Pearson TC, Larsen CP. Development of a chimeric anti-CD40 monoclonal antibody that synergizes with LEA29Y to prolong islet allograft survival. J Immunol. 2005;174:542–550. doi: 10.4049/jimmunol.174.1.542. [DOI] [PubMed] [Google Scholar]
- 72.Vincenti F, Kirk AD. What's next in the pipeline. Am J Transplant. 2008;8:1972–1981. doi: 10.1111/j.1600-6143.2008.02403.x. [DOI] [PubMed] [Google Scholar]
- 73.Van Seventer GA, Shimizu Y, Horgan KJ, Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990;144:4579–4586. [PubMed] [Google Scholar]
- 74.Zuckerman LA, Pullen L, Miller J. Functional consequences of costimulation by ICAM-1 on IL-2 gene expression and T cell activation. J Immunol. 1998;160:3259–3268. [PubMed] [Google Scholar]
- 75.Cai Z, Brunmark A, Jackson MR, Loh D, Peterson PA, Sprent J. Transfected Drosophila cells as a probe for defining the minimal requirements for stimulating unprimed CD8+ T cells. Proc Natl Acad Sci U S A. 1996;93:14736–14741. doi: 10.1073/pnas.93.25.14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Owens T. A role for adhesion molecules in contact-dependent T help for B cells. European journal of immunology. 1991;21:979–983. doi: 10.1002/eji.1830210418. [DOI] [PubMed] [Google Scholar]
- 77.Metzler B, Gfeller P, Bigaud M, Li J, Wieczorek G, Heusser C, Lake P, Katopodis A. Combinations of anti-LFA-1, everolimus, anti-CD40 ligand, and allogeneic bone marrow induce central transplantation tolerance through hemopoietic chimerism, including protection from chronic heart allograft rejection. J Immunol. 2004;173:7025–7036. doi: 10.4049/jimmunol.173.11.7025. [DOI] [PubMed] [Google Scholar]
- 78.Rayat GR, Gill RG. Indefinite survival of neonatal porcine islet xenografts by simultaneous targeting of LFA-1 and CD154 or CD45RB. Diabetes. 2005;54:443–451. doi: 10.2337/diabetes.54.2.443. [DOI] [PubMed] [Google Scholar]
- 79.Sanders ME, Makgoba MW, Sharrow SO, Stephany D, Springer TA, Young HA, Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988;140:1401–1407. [PubMed] [Google Scholar]
- 80.Okumura M, Fujii Y, Takeuchi Y, Inada K, Nakahara K, Matsuda H. Age-related accumulation of LFA-1 high cells in a CD8+CD45RAhigh T cell population. Eur J Immunol. 1993;23:1057–1063. doi: 10.1002/eji.1830230512. [DOI] [PubMed] [Google Scholar]
- 81.Nicolls MR, Coulombe M, Beilke J, Gelhaus HC, Gill RG. CD4-dependent generation of dominant transplantation tolerance induced by simultaneous perturbation of CD154 and LFA-1 pathways. J Immunol. 2002;169:4831–4839. doi: 10.4049/jimmunol.169.9.4831. [DOI] [PubMed] [Google Scholar]
- 82.Nicolls MR, Coulombe M, Yang H, Bolwerk A, Gill RG. Anti-LFA-1 Therapy Induces Long-Term Islet Allograft Acceptance in the Absence of IFN-gamma or IL-4. J Immunol. 2000;164:3627–3634. doi: 10.4049/jimmunol.164.7.3627. [DOI] [PubMed] [Google Scholar]
- 83.Isobe M, Suzuki J, Yamazaki S, Sekiguchi M. Acceptance of primary skin graft after treatment with anti-intercellular adhesion molecule-1 and anti-leukocyte function-associated antigen-1 monoclonal antibodies in mice. Transplantation. 1996;62:411–413. doi: 10.1097/00007890-199608150-00019. [DOI] [PubMed] [Google Scholar]
- 84.Isobe M, Yagita H, Okumura K, Ihara A. Specific acceptance of cardiac allograft after treatment with antibodies to ICAM-1 and LFA-1. Science. 1992;255:1125–1127. doi: 10.1126/science.1347662. [DOI] [PubMed] [Google Scholar]
- 85.Corbascio M, Ekstrand H, Osterholm C, Qi Z, Simanaitis M, Larsen CP, Pearson TC, Riesbeck K, Ekberg H. CTLA4Ig combined with anti-LFA-1 prolongs cardiac allograft survival indefinitely. Transpl Immunol. 2002;10:55–61. doi: 10.1016/s0966-3274(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 86.Corbascio M, Mahanty H, Osterholm C, Qi Z, Pearson TC, Larsen CP, Freise CE, Ekberg H. Anti-lymphocyte function-associated antigen-1 monoclonal antibody inhibits CD40 ligand-independent immune responses and prevents chronic vasculopathy in CD40 ligand-deficient mice. Transplantation. 2002;74:35–41. doi: 10.1097/00007890-200207150-00007. [DOI] [PubMed] [Google Scholar]
- 87.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Gray GS, Vallera DA. Coblockade of the LFA1:ICAM and CD28/CTLA4:B7 pathways is a highly effective means of preventing acute lethal graft-versus-host disease induced by fully major histocompatibility complex-disparate donor grafts. Blood. 1995;85:2607–2618. [PubMed] [Google Scholar]
- 88.Wang Y, Gao D, Lunsford KE, Frankel WL, Bumgardner GL. Targeting LFA-1 synergizes with CD40/CD40L blockade for suppression of both CD4-dependent and CD8-dependent rejection. Am J Transplant. 2003;3:1251–1258. doi: 10.1046/j.1600-6143.2003.00201.x. [DOI] [PubMed] [Google Scholar]
- 89.Nicolls MR, Gill RG. LFA-1 (CD11a) as a therapeutic target. Am J Transplant. 2006;6:27–36. doi: 10.1111/j.1600-6143.2005.01158.x. [DOI] [PubMed] [Google Scholar]
- 90.Hamann A, Jablonski-Westrich D, Duijvestijn A, Butcher EC, Baisch H, Harder R, Thiele HG. Evidence for an accessory role of LFA-1 in lymphocyte-high endothelium interaction during homing. J Immunol. 1988;140:693–699. [PubMed] [Google Scholar]
- 91.Cavazzana-Calvo M, Bordigoni P, Michel G, Esperou H, Souillet G, Leblanc T, Stephan JL, Vannier JP, Mechinaud F, Reiffers J, Vilmer E, Landman-Parker J, Benkerrou M, Baruchel A, Pico J, Bernaudin F, Bergeron C, Plouvier E, Thomas C, Wijdenes J, Lacour B, Blanche S, Fischer A. A phase II trial of partially incompatible bone marrow transplantation for high-risk acute lymphoblastic leukaemia in children: prevention of graft rejection with anti-LFA-1 and anti-CD2 antibodies. Societe Francaise de Greffe de Moelle Osseuse. British journal of haematology. 1996;93:131–138. doi: 10.1046/j.1365-2141.1996.4831024.x. [DOI] [PubMed] [Google Scholar]
- 92.Maraninchi D, Mawas C, Stoppa AM, Gaspard MH, Marit G, Van Ekthoven A, Reiffers J, Olive D, Hirn M, Delaage M, et al. Anti LFA1 monoclonal antibody for the prevention of graft rejection after T cell-depleted HLA-matched bone marrow transplantation for leukemia in adults. Bone marrow transplantation. 1989;4:147–150. [PubMed] [Google Scholar]
- 93.Hourmant M, Bedrossian J, Durand D, Lebranchu Y, Renoult E, Caudrelier P, Buffet R, Soulillou JP. A randomized multicenter trial comparing leukocyte function-associated antigen-1 monoclonal antibody with rabbit antithymocyte globulin as induction treatment in first kidney transplantations. Transplantation. 1996;62:1565–1570. doi: 10.1097/00007890-199612150-00006. [DOI] [PubMed] [Google Scholar]
- 94.Stern RS. A promising step forward in psoriasis therapy. Jama. 2003;290:3133–3135. doi: 10.1001/jama.290.23.3133. [DOI] [PubMed] [Google Scholar]
- 95.Leonardi C, Menter A, Hamilton T, Caro I, Xing B, Gottlieb AB. Efalizumab: results of a 3-year continuous dosing study for the long-term control of psoriasis. Br J Dermatol. 2008;158:1107–1116. doi: 10.1111/j.1365-2133.2008.08548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lebwohl M, Tyring SK, Hamilton TK, Toth D, Glazer S, Tawfik NH, Walicke P, Dummer W, Wang X, Garovoy MR, Pariser D. A Novel Targeted T-Cell Modulator, Efalizumab, for Plaque Psoriasis. New England Journal of Medicine. 2003;349:2004–2013. doi: 10.1056/NEJMoa030002. [DOI] [PubMed] [Google Scholar]
- 97.Gordon KB, Papp KA, Hamilton TK, Walicke PA, Dummer W, Li N, Bresnahan BW, Menter A. Efalizumab for patients with moderate to severe plaque psoriasis: a randomized controlled trial. Jama. 2003;290:3073–3080. doi: 10.1001/jama.290.23.3073. [DOI] [PubMed] [Google Scholar]
- 98.Gottlieb AB, Hamilton T, Caro I, Kwon P, Compton PG, Leonardi CL. Long-term continuous efalizumab therapy in patients with moderate to severe chronic plaque psoriasis: updated results from an ongoing trial. Journal of the American Academy of Dermatology. 2006;54:S154–163. doi: 10.1016/j.jaad.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 99.Langley RG, Carey WP, Rafal ES, Tyring SK, Caro I, Wang X, Wetherill G, Gordon KB. Incidence of infection during efalizumab therapy for psoriasis: analysis of the clinical trial experience. Clinical therapeutics. 2005;27:1317–1328. doi: 10.1016/j.clinthera.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 100.Leonardi CL, Papp KA, Gordon KB, Menter A, Feldman SR, Caro I, Walicke PA, Compton PG, Gottlieb AB. Extended efalizumab therapy improves chronic plaque psoriasis: results from a randomized phase III trial. Journal of the American Academy of Dermatology. 2005;52:425–433. doi: 10.1016/j.jaad.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 101.Poston RS, Robbins RC, Chan B, Simms P, Presta L, Jardieu P, Morris RE. Effects of humanized monoclonal antibody to rhesus CD11a in rhesus monkey cardiac allograft recipients. Transplantation. 2000;69:2005–2013. doi: 10.1097/00007890-200005270-00006. [DOI] [PubMed] [Google Scholar]
- 102.Ford ML, Koehn BH, Wagener ME, Jiang W, Gangappa S, Pearson TC, Larsen CP. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med. 2007 doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ford ML, Wagener ME, Hanna SS, Pearson TC, Kirk AD, Larsen CP. A Critical Precursor Frequency of Donor-Reactive CD4+ T Cell Help is Required for CD8+ T Cell-Mediated CD28/CD154-Independent Rejection. J Immunol. 2008;180:7207–7211. doi: 10.4049/jimmunol.180.11.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koehn BH, Ford ML, Ferrer IR, Borom K, Gangappa S, Kirk AD, Larsen CP. PD-1-dependent mechanisms maintain peripheral tolerance of donor-reactive CD8+ T cells to transplanted tissue. J Immunol. 2008;181:5313–5322. doi: 10.4049/jimmunol.181.8.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ford ML, Wagener ME, Hanna SS, Pearson TC, Kirk AD, Larsen CP. A critical precursor frequency of donor-reactive CD4+ T cell help is required for CD8+ T cell-mediated CD28/CD154-independent rejection. J Immunol. 2008;180:7203–7211. doi: 10.4049/jimmunol.180.11.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bloom DD, Hu H, Fechner JH, Knechtle SJ. T-lymphocyte alloresponses of Campath-1H-treated kidney transplant patients. Transplantation. 2006;81:81–87. doi: 10.1097/01.tp.0000191940.13473.59. [DOI] [PubMed] [Google Scholar]
- 107.Knechtle SJ, Pirsch JD, Fechner JHJ, Becker BN, Friedl AA, Colvin RB, Lebeck LK, Chin LT, Becker YT, Odorico JS, D'Alessandro AM, Kalayoglu AM, Hamawy MM, Hu AH, Bloom DD, Sollinger HW. Campath-1H induction plus rapamycin monotherapy for renal transplantation: results of a pilot study. Am J Transplant. 2003;3:722–730. doi: 10.1034/j.1600-6143.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- 108.Knechtle SJ. Treatment with immunotoxin. Philos Trans R Soc Lond B Biol Sci. 2001;356:681–689. doi: 10.1098/rstb.2001.0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lanzavecchia A, Sallusto F. From synapses to immunological memory: the role of sustained T cell stimulation. Curr Opin Immunol. 2000;12:92–98. doi: 10.1016/s0952-7915(99)00056-4. [DOI] [PubMed] [Google Scholar]
- 110.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290:92–97. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 111.London CA, Lodge MP, Abbas AK. Functional responses and costimulator dependence of memory CD4+ T cells. J Immunol. 2000;164:265–272. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- 112.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 113.Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol. 2000;164:3950–3954. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 114.Grayson JM, Murali-Krishna K, Altman JD, Ahmed R. Gene expression in antigen-specific CD8+ T cells during viral infection. J Immunol. 2001;166:795–799. doi: 10.4049/jimmunol.166.2.795. [DOI] [PubMed] [Google Scholar]
- 115.Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J Immunol. 2002;169:3686–3693. doi: 10.4049/jimmunol.169.7.3686. [DOI] [PubMed] [Google Scholar]
- 116.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, Pearson TC, Ahmed R, Larsen CP. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2:501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 118.Zhai Y, Meng L, Gao F, Busuttil RW, Kupiec-Weglinski JW. Allograft rejection by primed/memory CD8+ T cells is CD154 blockade resistant: therapeutic implications for sensitized transplant recipients. J Immunol. 2002;169:4667–4673. doi: 10.4049/jimmunol.169.8.4667. [DOI] [PubMed] [Google Scholar]
- 119.Chen Y, Heeger PS, Valujskikh A. In Vivo Helper Functions of Alloreactive Memory CD4+ T Cells Remain Intact Despite Donor-Specific Transfusion and Anti-CD40 Ligand Therapy. Journal of Immunology. 2004;172:5456–5466. doi: 10.4049/jimmunol.172.9.5456. [DOI] [PubMed] [Google Scholar]
- 120.Bishop DK, Chan Wood S, Eichwald EJ, Orosz CG. Immunobiology of allograft rejection in the absence of IFN-gamma: CD8+ effector cells develop independently of CD4+ cells and CD40-CD40 ligand interactions. J Immunol. 2001;166:3248–3255. doi: 10.4049/jimmunol.166.5.3248. [DOI] [PubMed] [Google Scholar]
- 121.Hassan AT, Dai Z, Konieczny BT, Ring GH, Baddoura FK, Abou-Dahab LH, El-Sayed AA, Lakkis FG. Regulation of alloantigen-mediated T-cell proliferation by endogenous interferon-gamma: implications for long-term allograft acceptance. Transplantation. 1999;68:124–129. doi: 10.1097/00007890-199907150-00023. [DOI] [PubMed] [Google Scholar]
- 122.Kishimoto K, Sandner S, Imitola J, Sho M, Li Y, Langmuir PB, Rothstein DM, Strom TB, Turka LA, Sayegh MH. Th1 cytokines, programmed cell death, and alloreactive T cell clone size in transplant tolerance. J Clin Invest. 2002;109:1471–1479. doi: 10.1172/JCI14947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Konieczny BT, Dai Z, Elwood ET, Saleem S, Linsley PS, Baddoura FK, Larsen CP, Pearson TC, Lakkis FG. IFN-gamma is critical for long-term allograft survival induced by blocking the CD28 and CD40 ligand T cell costimulation pathways. J Immunol. 1998;160:2059–2064. [PubMed] [Google Scholar]
- 124.Turner SJ, Olivas E, Gutierrez A, Diaz G, Doherty PC. Disregulated influenza A virus-specific CD8+ T cell homeostasis in the absence of IFN-gamma signaling. J Immunol. 2007;178:7616–7622. doi: 10.4049/jimmunol.178.12.7616. [DOI] [PubMed] [Google Scholar]
- 125.Whitmire JK, Earn B, Benning N, Whitton JL. Direct interferon-gamma signaling dramatically enhances CD4+ and CD8+ T cell memory. J Immunol. 2007;179:1190–1197. doi: 10.4049/jimmunol.179.2.1190. [DOI] [PubMed] [Google Scholar]
- 126.Whitmire JK, Tan JT, Whitton JL. Interferon-gamma acts directly on CD8+ T cells to increase their abundance during virus infection. J Exp Med. 2005;201:1053–1059. doi: 10.1084/jem.20041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Coley SM, Ford ML, Hanna SC, Wagener ME, Kirk AD, Larsen CP. IFN-gamma dictates allograft fate via opposing effects on the graft and on recipient CD8 T cell responses. J Immunol. 2009;182:225–233. doi: 10.4049/jimmunol.182.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Famulski KS, Sis B, Billesberger L, Halloran PF. Interferon-gamma and donor MHC class I control alternative macrophage activation and activin expression in rejecting kidney allografts: a shift in the Th1-Th2 paradigm. Am J Transplant. 2008;8:547–556. doi: 10.1111/j.1600-6143.2007.02118.x. [DOI] [PubMed] [Google Scholar]
- 129.Halloran PF, Afrouzian M, Ramassar V, Urmson J, Zhu LF, Helms LM, Solez K, Kneteman NM. Interferon-gamma acts directly on rejecting renal allografts to prevent graft necrosis. Am J Pathol. 2001;158:215–226. doi: 10.1016/s0002-9440(10)63960-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Halloran PF, Miller LW, Urmson J, Ramassar V, Zhu LF, Kneteman NM, Solez K, Afrouzian M. IFN-gamma alters the pathology of graft rejection: protection from early necrosis. J Immunol. 2001;166:7072–7081. doi: 10.4049/jimmunol.166.12.7072. [DOI] [PubMed] [Google Scholar]
- 131.Mele TS, Kneteman NM, Zhu LF, Ramassar V, Urmson J, Halloran B, Churchill TA, Jewell L, Kane K, Halloran PF. IFN-gamma is an absolute requirement for spontaneous acceptance of liver allografts. Am J Transplant. 2003;3:942–951. doi: 10.1034/j.1600-6143.2003.00153.x. [DOI] [PubMed] [Google Scholar]
- 132.Miura M, El-Sawy T, Fairchild RL. Neutrophils mediate parenchymal tissue necrosis and accelerate the rejection of complete major histocompatibility complex-disparate cardiac allografts in the absence of interferon-gamma. Am J Patthol. 2003;162:509–519. doi: 10.1016/s0002-9440(10)63845-x. [DOI] [PMC free article] [PubMed] [Google Scholar]