This work shows that loss-of-function mutants of retromer large subunit genes ZIP3/VPS35a, VPS29, and VPS26a partially suppressed the defects in morphology and gravitropism of zig mutants. By contrast, mutations of the paralogous genes VPS35b, VPS35c, and VPS26b do not suppress zig. Functional differences among these paralogous genes suggest that VPS35A function differs from that of VPS35B or VPS35C.
Abstract
Arabidopsis thaliana zigzag (zig) is a loss-of-function mutant of Qb-SNARE VTI11, which is involved in membrane trafficking between the trans-Golgi network and the vacuole. zig-1 exhibits abnormalities in shoot gravitropism and morphology. Here, we report that loss-of-function mutants of the retromer large subunit partially suppress the zig-1 phenotype. Moreover, we demonstrate that three paralogous VPS35 genes of Arabidopsis have partially overlapping but distinct genetic functions with respect to zig-1 suppression. Tissue-specific complementation experiments using an endodermis-specific SCR promoter show that expression of VPS35B or VPS35C cannot complement the function of VPS35A. The data suggest the existence of functionally specialized paralogous VPS35 genes that nevertheless share common functions.
INTRODUCTION
Eukaryotic cells contain various endomembrane compartments that continuously communicate with each other and exchange materials through membrane trafficking. Membrane trafficking is essential for the transport of proteins and lipids to the appropriate sites of action in order to maintain the functions of organelles and to modulate higher-order cellular developmental processes. Analysis of the genomic sequence of Arabidopsis thaliana has revealed that several gene families involved in membrane trafficking are expanded in number relative to yeast or animal cells, including those that encode SNAREs (soluble NSF attachment protein receptors) and Rab GTPases, suggesting a more complex organization of endomembrane system than is found in other organisms (Rutherford and Moore, 2002; Sanderfoot, 2007).
Reverse genetics is a useful approach to study membrane trafficking in plant cells. However, the existence of paralogous genes often makes it difficult to study their physiological functions. Loss-of-function mutations of essential genes with or without redundancy tend to give rise to subtle phenotype or lethality, respectively. The functional significance of paralogous genes and the functional relationships between them are thus difficult to discern (Rojo and Denecke, 2008).
We have previously isolated the Arabidopsis zigzag (zig) mutant, a loss-of-function mutant of the gene encoding Qb-SNARE VPS10 interacting 11 (VTI11), and this mutant exhibits a defect in shoot gravitropism (Kato et al., 2002). The zig-1 mutant exhibits additional morphological abnormalities: its inflorescence stem elongates in a zigzag fashion, and its leaves are small and wrinkled (Yamauchi et al., 1997). VTI11 is localized to the trans-Golgi network (TGN), the prevacuolar compartment (PVC), and vacuoles (Zheng et al., 1999; Uemura et al., 2004; Niihama et al., 2005). SNAREs are classified to subgroups (Qa-, Qb-, Qc-, and R-SNARE) according to amino acid sequences of the SNARE motif (Fasshauer et al., 1998). A correct complex composed of four SNAREs from each subgroup can lead to membrane fusion. VTI11 forms a complex with Qa-SNARE SYP22/SGR3/VAM3, Qc-SNARE SYP5, and R-SNARE VAMP727, probably at the PVC and vacuoles (Sanderfoot et al., 2001; Yano et al., 2003; Ebine et al., 2008). Because the molecular function of SNAREs is to aid in vesicle targeting and docking, their intracellular localization should be closely linked to their site of action (Cai et al., 2007). This indicates that VTI11 functions in membrane trafficking between the TGN and the PVC/vacuoles. Consistent with this idea, abnormal vacuolar structures have been observed in zig-1 mutant cells (Morita et al., 2002).
Gravity is believed to be perceived by amyloplast sedimentation toward the direction of the gravitational force in the endodermal cell in Arabidopsis shoots (Fukaki et al., 1998; Morita and Tasaka, 2004). In zig-1 shoots, amyloplasts in the endodermal cells do not sediment in the direction of gravity (Morita et al., 2002). Live cell imaging enabled us to show that functions and membrane dynamics of vacuoles closely correlate with amyloplast movement (Saito et al., 2005). We also found that sgr3, an amino acid substitution mutant of SYP22, exhibits reduced shoot gravitropism without any obvious morphological defects except for horizontal elongation of lateral shoots (Fukaki et al., 1996; Yano et al., 2003). Abnormalities are also found both in amyloplast sedimentation and in vacuolar structure in the endodermal cells of sgr3 shoots. Furthermore, sgr3-type SYP22 exhibits a reduced ability to form a SNARE complex with VTI11 and SYP5 (Yano et al., 2003). These results indicate that disruption of membrane trafficking to vacuoles in endodermal cells affects shoot gravitropism in Arabidopsis.
To elucidate the genetic network of membrane trafficking to vacuoles, we have performed a screen to identify mutants that can suppress the phenotype of zig-1. A dominant mutation zig suppressor1 (zip1) could almost completely suppress the defects found in zig-1 (Niihama et al., 2005). The mutation was localized to the VTI12 gene, which is a homolog of VTI11 (Niihama et al., 2005). Although VTI11 and VTI12 share 60% amino acid sequence identity and exhibit some functional redundancy, their intracellular localization and their SNARE partners differ (Sanderfoot et al., 1999; Zheng et al., 1999; Bassham et al., 2000; Surpin et al., 2003). We found that the zip1 mutation likely imparts upon VTI12 the ability to substitute for the function of VTI11 by changing both the specificity of SNARE complex formation and its intracellular localization, suggesting a close functional relationship between these two VTI1 paralogs.
Here, we report a new zig suppressor mutant, zip3, which is a recessive mutant that partially suppresses both the gravitropism and morphological abnormalities of zig-1. A zip3 single homozygous mutant itself does not exhibit a remarkable phenotype. zip3 is a loss-of-function mutant of VPS35A, which is an ortholog of yeast VPS35, a component of the retromer involved in retrograde transport from the PVC/endosome to the TGN (Seaman et al., 1997). Recent studies in plants have demonstrated that the Arabidopsis genome contains three VPS35 paralogs referred to as A, B, and C (Shimada et al., 2006; Jaillais et al., 2007) that share redundant functions in plant viability (Yamazaki et al., 2008). We report here a distinct functional divergence between these three VPS35 paralogs with respect to their ability to suppress the zig-1 phenotype.
RESULTS
Phenotypic Characterization of the zip3 Mutant of zig-1
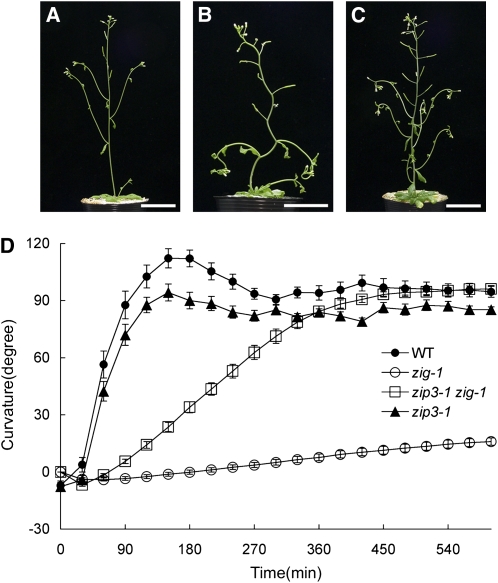
The zig-1 mutant exhibits abnormal morphology, including a zigzag-shaped inflorescence stem, small wrinkled leaves, and abnormal shoot gravitropism (Yamauchi et al., 1997; Kato et al., 2002). zip3-1 was isolated as a suppressor mutant of zig-1 from ethyl methanesulfonate–mutagenized zig-1 seeds (Niihama et al., 2005). The angles between adjacent internodes in the second metamer of zip3-1 zig-1 were smaller than those of zig-1 but larger than those of wild-type plants (Figures 1A to 1C; see Supplemental Figure 1A online). Consistently, the length of the internodes in the second metamer (internodes between the adjacent lateral branches and between the youngest leaf and the first lateral branch of the main shoot) and parameters of leaf shape in the zip3-1 zig-1 suppressor mutants were of intermediate value compared with those of the wild type and the zig-1 mutant (see Supplemental Figures 1B and 1C online). In a study of shoot gravitropism, when wild-type inflorescence stems were placed horizontally (gravistimulation), they bent upwards and became nearly perpendicular within ∼ 90 min (Figure 1D). As reported previously, zig-1 inflorescence stems showed little response (Figure 1D; Yamauchi et al., 1997). By contrast, stems of the zip3-1 zig-1 double mutant showed reduced but significant shoot gravitropism, with the curvature of the inflorescence stems reaching a perpendicular position within 6 h after gravistimulation (Figure 1D). The zip3-1 single mutant showed nearly normal shoot gravitropism. These results demonstrate that zip3-1 partially suppresses both the morphological and gravitropic phenotypes of zig-1.
Figure 1.
Phenotypes of the zip3-1 zig-1 Double Mutant.
(A) to (C) Six-week-old plants of wild type (A), zig-1 (B), and zip3-1 zig-1 (C). Bars = 3 cm.
(D) Gravitropic phenotypes of the wild type (closed circles), zig-1 (open circles), zip3-1 (closed triangles), and zip3-1 zig-1 (open squares). After 5-week-old plants were placed horizontally at 23 ° C under dim nondirectional light, inflorescence curvature was measured at 30-min intervals. Twenty individuals of each genotype were examined. Bars represent se.
[See online article for color version of this figure.]
ZIP3 Encodes VPS35A
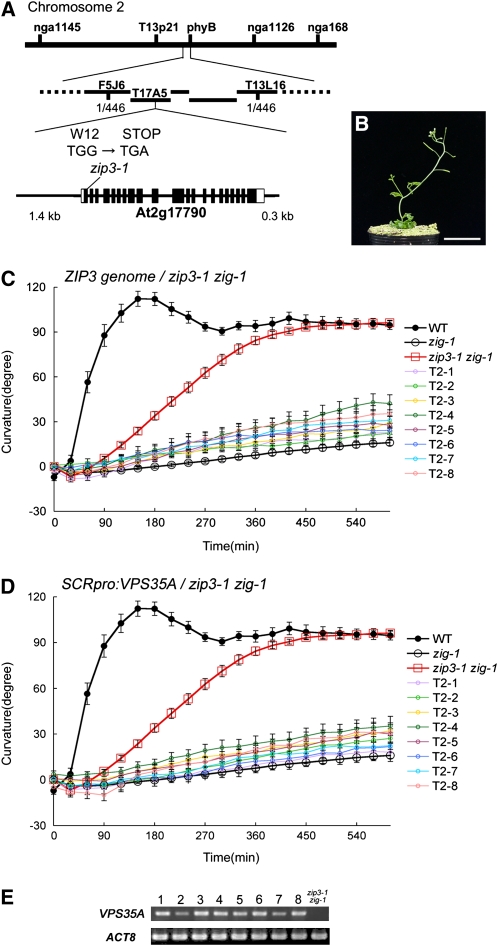
F2 progeny derived from a cross between zip3-1 zig-1 (Columbia [Col] background) and zig-3 (Wassilewskija background) were used to map ZIP3 to the middle of chromosome 2. We next mapped ZIP3 more finely using F2 progeny derived from a cross between zip3-1 zig-1 and a hybrid line zig-Ler (Landsberg erecta; see Methods). By analyzing 446 independent F2 progeny of the latter cross, the location of the ZIP3 locus was narrowed to a region between a polymorphic marker on BAC F5J6 and that on BAC T13L6. We found a single G-to-A substitution in one open reading frame (At2g17790), resulting in nonsense mutation at the twelfth Trp (W12; Figure 2A). By sequencing At2g17790 from an additional zip zig-1 mutant with a similar phenotype derived from the same screen for zig-1 suppressor mutants, we identified an allele, zip3-2, bearing a nonsense mutation at R200 (see Supplemental Figures 2A, 2C, and 2F online). Furthermore, two T-DNA insertion lines (SALK_125271 and SALK_039689) were crossed to zig-1, allowing us to confirm that both T-DNA insertion mutations are able to suppress the zig-1 phenotype (see Supplemental Figures 2A and 2D to 2F online).
Figure 2.
ZIP3 Is VPS35A.
(A) The ZIP3 locus was mapped between two cleaved-amplified polymorphic sequence markers, F5J6 and T13L16 on chromosome 2. A nonsense mutation was found at the twelfth Trp in the At2g17790 gene in the zip3-1 zig-1 mutant. The gene structure of At2g17790 is shown schematically; exons are indicated by boxes (open boxes, untranslated region; closed boxes, coding regions), and introns are indicated by lines between boxes.
(B) and (C) Complementation analysis.
(B) Aerial part of 5-week-old zip3-1 zig-1 T2 plant bearing the At2g17790 genomic fragment indicated in (A). Note that it shows a zig-1-like phenotype. Bar = 3 cm.
(C) Gravitropic response of wild-type (closed circles), zip3-1 zig-1 (red open squares), zig-1 (open circles), and eight independent T2 lines of zip3-1 zig-1 containing the At2g17790 genomic fragment (T2-1 to T2-8; open colored circles). Ten individuals of each transgenic line were examined. Bars represent se.
(D) and (E) Endodermis-specific expression of VPS35A extinguished the suppressive effect of zip3-1 on the zig-1 phenotype.
(D) Gravitropic response of wild-type (closed circles), zip3-1 zig-1 (red open squares), zig-1 (open circles), and independent eight T2 lines of zip3-1 zig-1 containing SCRpro:ZIP3/cVPS35A (T2-1 to T2-8; open colored circles). Ten individuals of each transgenic line were examined. Bars represent se.
(E) Expression of ZIP3/VPS35A derived from the transgene was confirmed by RT-PCR analyses of the eight transgenic lines. Analysis of the untransformed double mutant line is shown in the lane at the far right.
A 7.2-kb wild-type genomic fragment containing the At2g17790 open reading frame, predicted promoter region (1.4 kb), and 3 ′ downstream region (0.3 kb) was cloned and introduced into zip3-1 zig-1 plants for a complementation test (Figure 2A). Inflorescence stems of eight independent T2 lines of the resulting transgenic plants exhibited abnormal stem morphology and very reduced gravitropism with similar kinetics as the parental zig-1 single mutant (Figures 2B and 2C). Taken together, these results demonstrate that ZIP3 is At2g17790. At2g17790 was previously reported to encode VPS35A (Jaillais et al., 2007). In The Arabidopsis Information Resource, ZIP3 (At2g17790) is annotated as a protein similar to vacuolar protein sorting-associated protein 35 (Vps35) family proteins.
In Saccharomyces cerevisiae, Vps35p is a component of the large subunit of the retromer that is required for protein recycling from the PVC/endosomes to the TGN. Vps26p and Vps29p constitute the large subunit together with Vps35p. Components of the retromer large subunit are highly conserved in eukaryotes (Bonifacino and Rojas, 2006). Arabidopsis has three VPS35 genes (ZIP3/VPS35A, At2g17790; VPS35B, At1g75850; and VPS35C, At3g51310), two VPS26 genes (VPS26A, At5g53530; VPS26B, At4g27690; Jaillais et al., 2007), and one MAIGO1/VPS29 gene (At3g47810; Shimada et al., 2006).
Since zip3-1 is a nonsense mutation at Trp-12, it is most likely to be a null mutant allele. We confirmed by immunoblot analysis using anti-VPS35A (kindly provided by I. Hara-Nishimura) that VPS35A protein is not detected in zip3-1 or any of the other alleles (see Supplemental Figure 3 online). Considering the similar phenotype of the zip3 zig-1 alleles (see Supplemental Figure 2 online), they all appear to represent loss-of-function mutants of VPS35A. In addition, each single mutant allele showed no obvious phenotype for either gravitropism or stem morphology (see Supplemental Figure 4 online).
zip3-1 Also Suppresses the Cytological Phenotype of zig-1 in Endodermal Cells
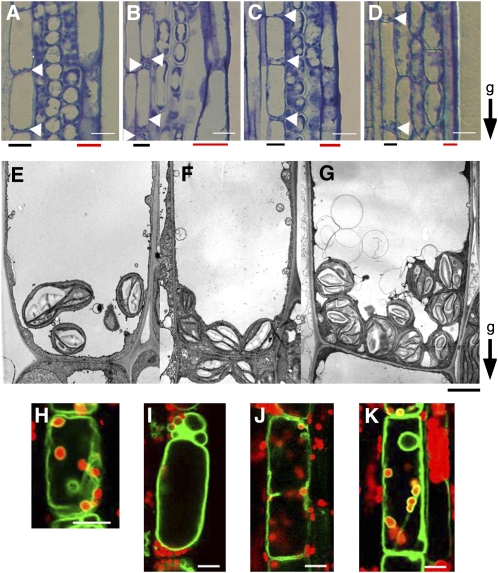
Plastids accumulating dense starch granules, called amyloplasts, are thought to act as statoliths in gravity sensing by higher plants (Sack, 1991). In wild-type Arabidopsis shoots, endodermal cells appear to serve as the gravity-sensing cells and contain amyloplasts that sediment in the direction of gravity (Figure 3A; Fukaki et al., 1998). By contrast, amyloplasts do not sediment in the endodermal cells of zig-1, sgr3, and sgr8/grv2/kam2 mutants (Figure 3B; Morita et al., 2002; Yano et al., 2003; Silady et al., 2004), suggesting that vacuolar functions mediated by TGN-PVC/vacuole membrane trafficking are important for amyloplast sedimentation. Interestingly, the majority of amyloplasts sedimented toward the direction of gravity in the zip3-1 zig-1 mutant (Figure 3C). To evaluate amyloplast sedimentation more quantitatively, the number of amyloplasts positioned at the upper, middle, or bottom region of endodermal cells was counted using embedded longitudinal sections, as shown in Figures 3A to 3D (see Supplemental Table 1 online). Although most amyloplasts were observed at the bottom region in the wild type and zip3-1 single mutants, amyloplasts were observed in all regions in zig-1 cells. By contrast, a majority of the amyloplasts was observed at the bottom region, with a smaller but significant number at the upper region in zip3-1 zig-1 cells (see Supplemental Table 1 online). Therefore, partial suppression of the gravitropic phenotype of zig-1 by zip3-1 mutation appears to correlate with the extent of amyloplasts sedimentation in endodermal cells.
Figure 3.
Endodermis of Inflorescence Stems.
(A) to (D) Longitudinal sections of inflorescence stems ( ∼ 2 to 3 cm below the apex) of the wild type (A), zig-1 (B), zip3-1 zig-1 (C), and zip3-1 (D). The growth orientation of stems was maintained during fixation. g, direction of gravity; red bars, epidermis; black bars, endodermis. Arrowheads indicate location of amyloplasts in endodermal cells. Bars = 20 μ m.
(E) to (G) Electron microscopy of endodermal cell of the wild type (E), zig-1(F), and zip3-1 zig-1 (G). g, direction of gravity. Bars = 2 μ m.
(H) to (K) Confocal images of amyloplasts and vacuolar membrane in living endodermal cells. The wild type (H), zig-1 (I), zip3-1 zig-1 (J), and zip3-1 (K). Amyloplasts and vacuolar membranes were observed using chlorophyll autofluorescence from plastids (red) and GFP fluorescence from GFP- γ -TIP (green), respectively. The transgenic line expressing GFP- γ -TIP under the control of the SCR promoter (Saito et al., 2005) was crossed with each mutant. The result of quantitative analysis based on this observation is shown in Supplemental Table 2 online.
Using electron microscopy, we previously observed that wild-type endodermal cell amyloplasts are enclosed by a vacuolar membrane with a thin cytoplasmic layer, whereas zig-1 amyloplasts are not enclosed by such a membrane and appear to be more localized toward the cell periphery (Figures 3E and 3F). By contrast, the amyloplasts in zip3 zig-1 cells are surrounded by a vacuolar membrane, although clusters of abnormal vesicles were occasionally observed (Figure 3G). To evaluate the extent of suppression of the zig-1 phenotype by zip3, we quantitatively analyzed cytological manifestations of the suppressed phenotype, including amyloplast movement and vacuolar membrane dynamics using live endodermal cells (Figures 3H to 3K). For these studies, we used the autofluorescence of the plastid and green fluorescent protein (GFP) fluorescence (following the expression of a GFP- γ -TIP fusion localized to vacuolar membrane) to visualize amyloplasts and the vacuolar membrane, respectively. We compared various physical characteristics of the amyloplasts and vacuolar membrane in wild-type and mutant endodermal cells (see Supplemental Table 2 online).
In all aspects, such as the number of amyloplasts enclosed by a vacuolar membrane, amyloplast and vacuolar dynamics, and the presence of an abnormal vesicular structure, zip3-1 zig-1 cells exhibited values that were intermediate between those of wild-type and zig-1 cells (see Supplemental Table 2 online). Thus, the zip3-1 suppressor mutation appears to partially restore the functional integrity of the vacuoles, suggesting that the zip3 mutation may partially restore a defect in membrane trafficking caused by loss-of-function of ZIG/VTI11. Moreover, the intermediate recovery of the cytological zig-1 phenotype by the zip3-1 mutation correlates with partial suppression of the gravitropic phenotype (Figure 1). When ZIP3/VPS35A was specifically expressed in the endodermis of zip3-1 zig-1 using the SCARECROW (SCR) promoter (Wysocka-Diller et al., 2000), the resulting transgenic plants (eight independent T2 lines) showed reduced gravitropism similar in level to the zig-1 single mutant (Figures 2D and 2E). This indicates that vacuolar integrity in the endodermal cells supported by membrane trafficking is closely linked to gravitropism of the inflorescence stems.
How Does the zip3 Mutation Suppress zig-1?
To address this question, we focused on VTI12, a paralog of ZIG/VTI11, since we have found that VTI12 is involved in suppression in the case of zip1 zig-1 (Niihama et al., 2005). VTI11 interacts with SYP22 on the PVC/vacuole, whereas VTI12 forms a complex with SYP4 on the TGN in wild-type cells (Sato et al., 1997; Zheng et al., 1999; Sanderfoot et al., 1999, 2001; Uemura et al., 2004). zip1, a gain-of-function mutation of VTI12, altered the intracellular localization and SNARE partner of VTI12, resulting in the substitution of zip1-type VTI12 for VTI11. In addition, overexpression of wild-type VTI12 can suppress the zig-1 phenotype, depending on its expression level (Surpin et al., 2003), probably due to the ability of wild-type VTI12 to substitute for VTI11.
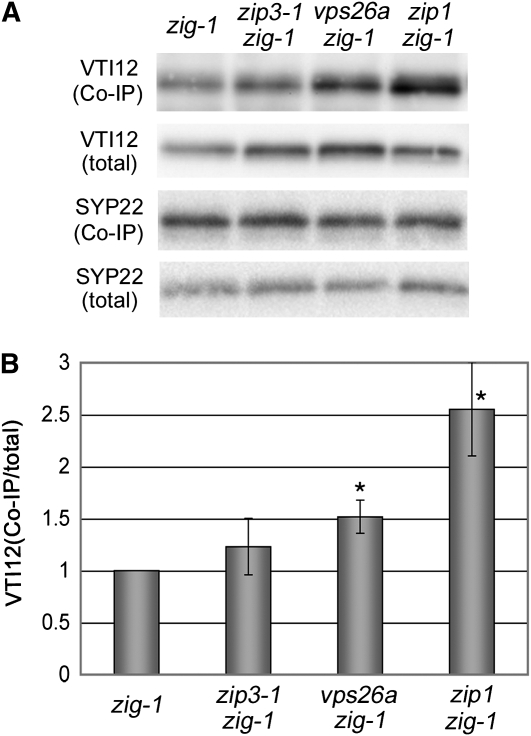
These results prompted us to test whether VTI12 is also involved in suppression of the zig-1 phenotype in zip3-1 zig-1 cells. We analyzed whether VTI12 interacts with SYP22 using anti-SYP22 antibody to immunoprecipitate protein from extracts prepared from the inflorescence stem from zig-1, zip3 zig-1, and zip1 zig-1 plants (Figure 4). In zig-1 plants, little VTI12 protein formed SNARE complexes with SYP22, indicating that wild-type VTI12 has some ability to interact with SYP22, but it was not sufficient to substitute for VTI11 function (Niihama et al., 2005). In addition, we confirmed that 2.5-fold more SYP22 was coimmunoprecipitated with VTI12 in zip1 zig-1 extracts compared with zig-1, as a positive experimental control (Niihama et al., 2005). In zip3-1 zig-1 extracts, a slightly increased amount of VTI12 was coimmunoprecipitated with SYP22, although the increase was not statistically significant (Figure 4).
Figure 4.
Interaction between SYP22 and VTI12.
(A) Immunoprecipitation analysis using anti-SYP22 antiserum with zig-1, zip3-1 zig-1, vps26a zig-1, and zip1 zig-1 plant extracts. Proteins were extracted from inflorescence stems of 6-week-old plants. VTI12 and SYP22 in immunoprecipitates were detected by immunoblotting using anti-VTI12 antiserum and anti-SYP22 antiserum, respectively (first and third panels from top). The total amounts of VTI12 and SYP22 are also indicated (second and fourth panels from top).
(B) The relative incorporation ratio of VTI12 into the SNARE complex containing SYP22 (immunoprecipitated VTI12/ total VTI12) was calculated based on results shown in (A). Average values of four independent experiments are presented with error bars showing sd. Values were normalized to that of zig-1 plants. Quantification of the band intensity was performed with the software Sion Image. The statistical significance against zig-1 control was tested using a Student t test (*P < 0.05).
Dysfunction of the Retromer Large Subunit Suppresses the zig-1 Phenotype
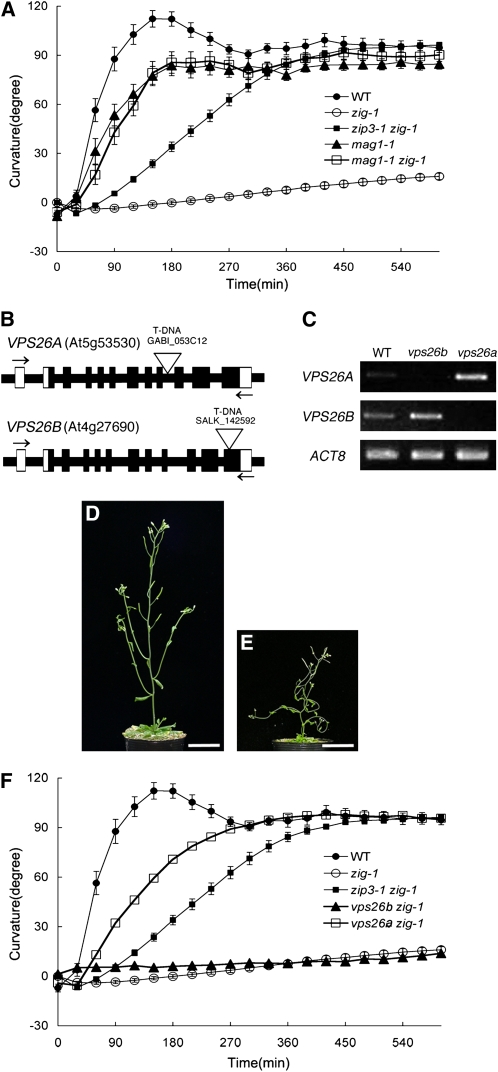
The molecular lesion found in zip3-1 demonstrates that a loss-of-function mutation of ZIP3/VPS35A, which probably causes dysfunction of the retromer containing VPS35A, suppressed the zig-1 phenotype. To test whether mutations in other large subunits can be suppressors of zig-1, we crossed zig-1 to mag1-1, a T-DNA insertion allele of VPS29, in which the expression level of VPS29 is much lower than in the wild type (Shimada et al., 2006). The mag1-1 single mutant showed reduced but significant gravitropism probably due to its thin stem and reduced growth rate (Figure 5A). mag1-1 zig-1 showed a significant gravitropic response comparable to that of the parental mag1-1 single mutant (Figure 5A). We note that mag1-1 suppressed the gravitropic phenotype of zig-1 to a greater extent than did zip3-1. The fact that the morphological phenotype of mag1-1 zig-1 was similar to that of the mag1-1 single mutant indicates that mag1-1 can suppress the morphological phenotype of zig-1 (see Supplemental Figure 5 online). Thus, the loss-of-function mutation in VPS29, a retromer component other than VPS35, can also suppress the zig-1 phenotype and the fact that its suppressive effect is greater than that of zip3 is likely due to the former being a single copy gene.
Figure 5.
Suppressive Effect of Loss-of-Function Mutations in Components of the Retromer Large Subunit.
(A) Gravitropic response of the wild type (closed circles), zip3-1 zig-1 (closed squares), zig-1 (open circles), mag1-1 (closed triangles), and mag1-1 zig-1 (open squares). At least 15 individuals of each genotype were examined. Bars represent se.
(B) Schematic structures of VPS26A and VPS26B genes and positions of T-DNA insertions. Boxes indicate exons, and white regions represent untranslated regions. Open triangles show positions of T-DNA insertions. Arrows indicate the position of primers used for RT-PCR analyses in (C).
(C) RT-PCR analysis to detect expression of VPS26A or VPS26B in wild-type and mutant plants using the primers indicated by arrows in (B)
(D) and (E) Morphological phenotypes of 6-week-old plants of vps26a zig-1 (D) and vps26b zig-1 (E). Bars = 3 cm.
(F) Gravitropic response of wild-type (closed circles), zip3-1 zig-1 (closed squares), zig-1 (open circles), vps26a zig-1 (open squares), and vps26b zig-1 (closed triangles). At least 15 individuals of each genotype were examined. Bars represent se.
[See online article for color version of this figure.]
In the Arabidopsis genome, there are two paralogs of the gene encoding the other large subunit of retromer, VPS26. Since mag1-1 can suppress zig, mutations in either VPS26A, VPS26B, or both are expected to suppress the zig-1 phenotype. We next crossed T-DNA insertion lines (vps26a, GABI_053C12; vps26b, SALK_142592) with zig-1 (Figure 5B). We could not detect full-length transcripts from either gene by RT-PCR analysis of total RNA from the corresponding insertion mutant (Figure 5C), indicating that these are loss-of-function mutants. Both single mutants were indistinguishable from the wild type both in stem morphology and in gravitropism (see Supplemental Figure 6 online). As expected, vps26a suppressed the zig-1 phenotype (Figures 5D and 5F). The gravitropic response of vps26a zig-1 inflorescence stems was greater than that of zip3-1 zig-1 stems but lesser than that of vps26a single mutant stems, suggesting that suppression by vps26a is partial. Interestingly, vps26b did not suppress the zig-1 phenotype at all (Figures 5E and 5F), suggesting that the genetic functions of vps26a and vps26b differ with respect to suppression of zig-1. These results demonstrate that loss-of-function mutations of components of the retromer large subunit, ZIP3/VPS35A, VPS29 and VPS26A, suppress the zig-1 phenotype. This suggests a close relationship between these three proteins, implying that VPS35A, VPS29, and VPS26A constitute a retromer large subunit. Furthermore, our results indicate that dysfunction of the retromer can suppress the zig-1 phenotype caused by loss-of-function mutation of Qb-SNARE VTI11.
Since the suppressive effect of vps26a was greater than that of zip3-1, we tested whether vps26a can affect VTI12-SYP22 complex formation. We performed immunoprecipitation analyses on extracts prepared from vps26a zig-1 plants (Figure 4). Interestingly, significantly increased amount of VTI12 coimmunoprecipitated with SYP22 from vps26a zig-1 extracts compared with that from zig-1 extracts. This result strongly suggests that VTI12-SYP22 complex formation is promoted by the vps26a mutation. We note that the amount of VTI12 coimmunoprecipitated with SYP22 correlates with the extent of suppression by each mutation, though the increase found in zip3-1 zig-1 was not statistically significant (zip3-1 < vps26a < zip1; Figures 1 and 5; Niihama et al., 2005). This result suggests that dysfunction of the retromer large subunits suppresses the zig-1 phenotype by promoting VTI12-SYP22 complex formation.
Functional Relationship among Paralogs of VPS35
The two VPS26 paralogs in the Arabidopsis genome may share redundant function for plant viability, since we were unable to generate a vps26a vps26b double mutant, at least for the allele combination we tested (data not shown). Interestingly, however, the roles of vps26a and vps26b with respect to suppression of the zig-1 phenotype appear to differ, as shown in Figures 5D to 5F. Thus, we postulate that our suppressor screen allowed us to discern subtle functional differences between redundant paralogous genes and the physiological significance of these differences.
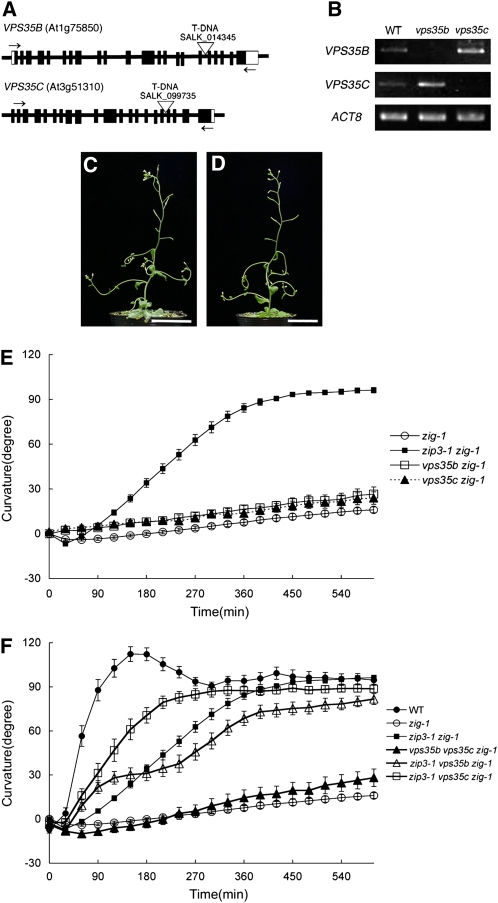
There are three VPS35 paralogs in Arabidopsis. We next investigated the functional relationship between the VPS35 paralogs by genetic analysis. T-DNA insertion lines (vps35b, SALK_014345; vps35c, SALK_099735) were crossed with zig-1 (Figure 6A). We were unable to detect full-length transcripts from each gene by RT-PCR performed on RNA isolated from the corresponding mutant (Figure 6B), indicating that these are loss-of-function mutants. As was the case for vps26, both vps35b and vps35c single mutants themselves showed no obvious phenotype in either stem morphology or gravitropism (see Supplemental Figure 7 online). Interestingly, neither vps35b nor vps35c was able to suppress the zig-1 phenotype (Figures 6C to 6E). This result indicates that the genetic functions of VPS35B and VPS35C differ from that of VPS35A with regard to the suppression of the zig-1 phenotype.
Figure 6.
Suppressive Effect of Loss-of-Function Mutations in VPS35 Paralogs.
(A) Schematic structures of VPS35B and VPS35C genes and positions of T-DNA insertions. Boxes indicate exons, and white regions represent untranslated regions. Open triangles show positions of T-DNA insertions. Arrows indicate the position of primers used for RT-PCR analyses to detect expression of VPS35B or VPS35C in (B).
(B) RT-PCR analysis to detect expression of VPS35B or VPS35C in wild-type and mutant plants using the primers indicated by arrows in (A).
(C) and (D) Morphological phenotypes of 6-week-old plants of vps35b zig-1 (C) and vps35c zig-1 (D). Bars = 3 cm.
(E) Gravitropic response of zip3-1 zig-1 (closed squares), zig-1 (open circles), vps35b zig-1 (open squares), and vps35c zig-1 (closed triangles). At least 15 individuals of each genotype were examined. Bars represent se.
(F) Gravitropic response of wild-type (closed circles), zip3-1 zig-1 (closed squares), zig-1 (open circles), zip3-1 vps35b zig-1 (open triangles), zip3-1 vps35c zig-1 (open squares), and vps35b vps35c zig-1 (closed triangles). At least 15 individuals of each genotype were examined. Bars represent se.
[See online article for color version of this figure.]
To further investigate redundancy between the three paralogs, we analyzed the ability of double mutations in VPS35 paralogs to suppress the gravitropic phenotype of zig-1. Inflorescence stems from vps35b vps35c zig-1 plants exhibited poor gravitropism, similar to that observed for the zig-1 single mutant (Figure 6F), consistent with what was observed for vps35b zig-1 or vps35c zig-1 double mutants (Figure 6E). By contrast, combination of either vps35b or vps35c mutation with the zip3-1 zig-1 genotype (zip3-1 vps35b zig-1 or zip3-1 vps35c zig-1) increased the gravitropic response. However, the response of zip3-1 vps35b zig-1 exhibited a biphasic curve with the first phase of the response (within 3 h) appearing faster than that of zip3-1 zig-1. Both vps35b and vps35c appear to enhance the suppressive effect of zip3-1, although the suppressive effect of vps35b is much weaker than that of vps35c. These results suggest that there is some genetic redundancy between the VPS35 paralogs with respect to suppression of the zig-1 phenotype.
Protein Functions of the VPS35 Paralogs Are Unlikely to Be Equivalent
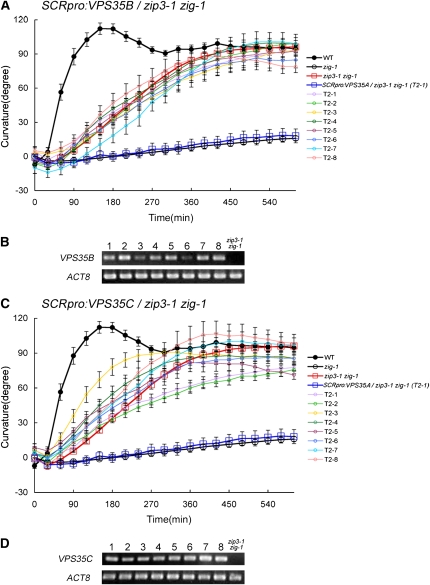
The genetic function of the VPS35 paralogs appeared to be distinct but somewhat overlapping. Although each paralog is ubiquitously expressed in all organs examined (Schmid et al., 2005), we do not know whether their level of expression is similar in all tissues or cells. Thus, we cannot determine whether the subtle differences in genetic function might be explained by differential patterns of expression of the paralogs or whether they might reflect differences in their molecular function. To address this question, VPS35B or VPS35C was expressed under the control of the endodermis-specific SCR promoter (SCRpro:VPS35B or SCRpro:VPS35C) in the zip3-1 zig-1 genetic background (Figure 7).
Figure 7.
Endodermis-Specific Expression of VPS35B or VPS35C in zip3-1 zig-1 Cells.
(A) and (C) Gravitropic response of wild-type (closed circles), zip3-1 zig-1 (red open squares), zig-1 (open circles), SCRpro:VPS35A/zip3 zig-1 (blue open squares), and eight independent T2 lines of zip3-1 zig-1 plants containing SCRpro:VPS35B (A) or SCRpro:VPS35C (C) (T2-1 to T2-8; open colored circles). Ten individuals of each transgenic line were examined. Bars represent se.
(B) and (D) Expression of VPS35B (B) or VPS35C (D) derived from the transgene was confirmed by RT-PCR analyses of the eight transgenic lines in each case.
As shown in Figure 2D, when ZIP3/VPS35A was expressed from the SCR promoter in zip3-1 zig-1 plants, the resulting transgenic plants exhibited a poor gravitropic response that was similar to the response observed in the zig-1 single mutant. If VPS35B and VPS35C have similar molecular functions as ZIP3/VPS35A, then the gravitropic response of the resulting transgenic plants would be predicted to be similar to that observed in zig-1 plants. However, SCRpro:VPS35B showed no effect on the gravitropic response of zip3-1 zig-1 plants, even though the transgene was clearly expressed in the plants, suggesting that molecular function of VPS35B differs at least partially from that of ZIP3/VPS35A (Figures 7A and 7B).
With respect to SCRpro:VPS35C, the transgenic lines exhibited a gravitropic response that was similar to that observed in zip3-1 zig-1 or SCRpro:VPS35B/zip3-1 zig-1 plants, although slight differences in the response were observed in independent lines (Figure 7C and 7D). Therefore, neither VPS35B nor VPS35C can substitute for ZIP3/VPS35A at least when expressed from the same promoter. These results suggest that the protein functions of VPS35B or VPS35C are distinct from those of ZIP3/VPS35A in the endodermis.
DISCUSSION
Functional Deficiency of the Retromer Large Subunit Suppresses the Loss-of-Function Mutant of Qb-SNARE VTI11, zig-1
Here, we demonstrate that zip3, which partially suppresses the morphological and gravitropic phenotype of zig-1, is a loss-of-function mutation of VPS35A (Figures 1 and 2; see Supplemental Figure 1 online). It is an ortholog of the yeast Vps35 gene, which is a component of the retromer large subunit functioning in the retrieval of the CPY (carboxypeptidase Y) sorting receptor (Vps10) to the Golgi from the PVC/endosome (Seaman et al., 1997). The retromer is composed of two subcomplexes: Vps26p-Vps29p-Vps35p, a heterotrimeric large subunit involved in cargo recognition, and the Vps5p-Vps17p complex promoting vesicle formation (Seaman et al., 1998; Nothwehr et al., 2000). All components, except for Vps17p, are highly conserved in higher eukaryotes. The human ortholog of Vps5p, termed sorting nexin 1 (SNX1) (Kurten et al., 1996; Horazdovsky et al., 1997; Nothwehr and Hindes, 1997), and other SNX family members are thought to function in a role similar to the Vps5p-Vps17p subcomplex (Bonifacino and Hurley, 2008).
In the Arabidopsis genome, retromer orthologs have been reported (Jaillais et al., 2006, 2007; Oliviusson et al., 2006; Shimada et al., 2006; Yamazaki et al., 2008). The presence of components of the large subunit (VPS26A, VPS29, and VPS35C) on the multivesicular body (MVB) has been demonstrated by immunoelectron microscopy (Oliviusson et al., 2006). Each loss-of-function yeast mutant lacking a retromer component exhibits a similar phenotype, such as mis-sorting of the CPY out of the cell (Horazdovsky et al., 1997; Nothwehr and Hindes, 1997; Seaman et al., 1997; Nothwehr et al., 1999; Reddy and Seaman, 2001). Similarly, all three Arabidopsis mutants, T-DNA insertion mutants of VPS26A or VPS35A and mag1-1 with reduced expression of VPS29, partially suppressed the zig-1 phenotype (Figure 5). The result suggests that VPS26A, VPS29, and VPS35A share the same molecular function, as part of the large subunit of the retromer in wild-type plants. Consistent with our genetic analysis, a recent study using the yeast two-hybrid analysis has shown that VPS35A can physically interact with VPS29 and VPS26A in yeast (Jaillais et al., 2007).
Possible Mechanism of zig-1 Suppression by Loss of Function of the Retromer
We previously reported that VTI12, a paralog of VTI11, can suppress the zig-1 phenotype. Genetic analysis has demonstrated that VTI11 and VTI12 have redundant functions, at least in part. Our previous data suggested that an excess amount of wild-type VTI12 or zip1-type VTI12 forms a SNARE complex with SYP22 leading to a functional substitution of the VTI11-SYP22 complex (Surpin et al., 2003; Niihama et al., 2005). We assumed that the mechanism of suppression in the case of loss-of-function mutants of the retromer could be explained by a similar scenario. However, amount of the VTI12-SYP22 SNARE complex was slightly increased but not significant in zip3-1 zig-1 compared with that detected in zig-1 plants used as a control (Figure 4).
By contrast, we detected a significant increase of VTI12-SYP22 complex formation in vps26a zig-1 (Figure 4). Unfortunately, we could not perform this experiment in mag1-1 zig-1 plants due to poor growth. VTI12-SYP22 complex formation was much more robust in zip1 zig-1 plants used as a positive control as reported previously (Niihama et al., 2005). The extent of suppression of the gravitropic phenotype of these zip zig-1 mutants positively correlated with the amount of the VTI12-SYP22 complex: zip1 zig-1 > vps26a zig-1 > zip3 zig-1. Our results suggest that VTI12-SYP22 complex formation is one of the mechanisms underlying suppression of the zig-1 phenotype through loss of function of the retromer, as is the case of zip1 zig-1. If so, how could dysfunction of the retromer promote VTI12-SYP22 complex formation? In wild-type cells, VTI12 is mainly localized to the TGN, and a fraction of VTI12 is localized to SYP22-positive PVC. Such dual localization of VTI12 suggests cycling of VTI12 between the TGN and the PVC (Uemura et al., 2004).
Retrieval trafficking from the PVC to the TGN is performed by the retromer. The retromer components VPS29, VPS26, and VPS35 are localized to the PVC/MVB (Oliviusson et al., 2006; Jaillais et al., 2007; Yamazaki et al., 2008). Phenotypes observed in retromer mutants, such as mis-sorting of vacuolar seed storage proteins to the extracellular space and increased accumulation of VSR receptors, indicate that the plant retromer is indeed involved in cargo retrieval from the PVC to the TGN in Arabidopsis (Shimada et al., 2006; Yamazaki et al., 2009). If we suppose that VTI12 reaching the PVC is immediately retrieved by the retromer, then we might expect that dysfunction of the retromer might lead to mis-sorting of VTI12 to the PVC and/or vacuole. This provides a possible explanation: in zip3 zig-1, vps26a zig-1, or mag1-1 zig-1 plants, VTI12 mis-sorted to the PVC and/or vacuoles due to dysfunction of the retromer could form a SNARE complex with SYP22 in the absence of VTI11, resulting in partial substitution of VTI11-SYP22 function.
We tested whether VTI12 is mislocalized to the PVC in zip3-1 zig-1 by analyzing colocalization between GFP-VTI12 and ARA6-RFP in endodermal cells (see Supplemental Figure 8 online). However, significant mislocalization of GFP-VTI12 to the ARA6-RFP-positive PVC could not be detected in zip3-1 zig-1 and zip3-1 compared with the wild type and zig-1. This could be due to the only partial suppressive ability of zip3-1. Alternatively, the result suggests that VTI12-SYP22 complex formation is promoted by a secondary effect of dysfunction of the retromer (e.g., disorganization of endomembrane system in the retromer mutants could result in increase of VTI12-SYP22 complex formation).
Possibly, the increase of the VTI12-SYP22 complex may not be the primary cause of the suppression. The loss-of-function of retromer may cause a detrimental effect not only on retrieval of specific cargos but also on retrieval of membrane lipids to the TGN, and this may result in accumulation of mis-sorted membrane to central vacuoles. Mis-sorting of membrane to vacuoles could recover vacuolar dynamics required for dynamic movement of amyloplasts in endodermal cells in zip3-1 zig-1 (Figure 3).
Functional Differences among Paralogs of VPS35
Arabidopsis has one VPS29, two VPS26, and three VPS35 genes. It has been reported that the vps29 null allele shows severe, pleiotropic effects on plant development and growth (Jaillais et al., 2007; Shimada et al., 2006). VPS35 function is probably essential because it has not been possible to generate a triple null mutant, vps35a vps35b vps35c (Yamazaki et al., 2008). We were unable to generate a vps26a vps26b double mutant from the F2 population (data not shown). By contrast, none of the vps35a, -b, or -c or vps26a or vps26b single mutants shows any obvious phenotype (see Supplemental Figures 6 and 7 online; Yamazaki et al., 2008). These results indicate that the paralogous genes of VPS35 and VPS26 have redundant functions regarding plant viability.
However, our genetic analysis revealed that VPS26A and VPS26B have distinct genetic functions with respect to suppression of the gravitropic phenotype of zig-1, since vps26a but not vps26b could suppress zig-1 (Figures 5D to 5F). Similarly, VPS35A also showed a distinct genetic function from its paralogs with regard to zig-1 suppression (Figures 6C to 6E). Analysis of the triple mutants (vps35b vps35c zig-1, zip3-1 vps35b zig-1, and zip3-1 vps35c zig-1) revealed a subtle functional relationship between the paralogs. The fact that vps35b and vps35c enhanced the suppressive effect of zip3 subtly and greatly, respectively, indicates that the genetic function of ZIP3/VPS35A and VPS35C are distinct but overlapping with respect to zig-1 suppression (Figure 6E).
Our genetic analysis implies partially overlapping but distinct functions of VPS35 paralogs. Such differences in genetic function, however, could result from differences in the expression pattern of each paralog. Meanwhile, all VPS35 genes are ubiquitously expressed in various organs (Schmid et al., 2005), although we have not analyzed the expression pattern of each gene at the tissue level. A tissue-specific complementation test using the SCR promoter demonstrated that expression of neither VPS35B nor VPS35C in the endodermis negates the suppressive effect of zip3 on the gravitropic phenotype (Figure 7). By contrast, expression of VPS35A by the same promoter did complement the effect of zip3, as expected (Figures 2D and 2E). We cannot exclude the possibility that all VPS35 proteins might share the same molecular function, but only VPS35A is stable in the endodermis of zip3-1 zig-1 background. However, these results indicate that VPS35B or VPS35C cannot substitute for the functions of ZIP3/VPS35A in mediating gravitropism in the endodermis, at least when expressed at similar levels of transcripts by the same promoter. Taken together, our results suggest that not only the genetic function but also the protein function of VPS35B or VPS35C is partially different from that of VPS35A.
How do the functions of the gene products of the VPS35 paralogs differ from each other? One possibility is a difference in recognition of cargo for retrograde trafficking, since VPS35 is the subunit responsible for binding to the cytoplasmic domains of cargo proteins (reviewed in Seaman, 2005; Collins, 2008). Analysis of the crystal structure of the C-terminal domain of human VPS35 (Hierro et al., 2007) in addition to secondary structure prediction strongly suggests that the entire VPS35 protein adopts an α -helical solenoid structure containing 17 HEAT-like helical repeats (Collins, 2008). It has been suggested that multiple regions of VPS35 are involved in binding to a cargo protein (Seaman, 2007; Canuel et al., 2008). In addition, the fact that distinct domains within yeast VPS35p mediate the retrieval of two different cargo proteins (Nothwehr et al., 1999) implies that the retromer may be able to associate with multiple cargo proteins at the same time (Collins, 2008).
In Arabidopsis, VPS35A shares 67 and 70% identity in amino acid sequence with VPS35B and VPS35C, respectively. Such a difference may give rise to variety in selectivity of cargo proteins. Another possibility is that the gene product of each VPS35 paralog may act as a retromer within distinct membrane compartments or subdomains. Although VPS35 has been shown to be localized to the PVC/MVB in plant cells (Oliviusson et al., 2006; Yamazaki et al., 2008), localization of each VPS35 paralog remains to be elucidated. It has been suggested that VPS26p recruits VPS35p to the membrane in yeast (Reddy and Seaman, 2001). Recent studies using mammalian cells suggest that formation of the entire heterotrimeric core is a prerequisite for membrane attachment (Collins et al., 2008). Attachment of the retromer core to the membrane is dependent upon the action of the membrane-associated SNX proteins (Rojas et al., 2007). Human VPS35 also binds to the SNX proteins, and other retromer components, in addition to VPS26 and VPS29 (Haft et al., 2000). These studies indicate that association with other retromer components including SNX proteins is important for recruitment of VPS35 to sites of action, implying that binding of each VPS35 paralog to other proteins or a combination of the components may affect the site of action of VPS35 in Arabidopsis. The above two possibilities do not seem to be mutually exclusive. VPS35A could differ from VPS35B with regard to cargo selectivity and/or intracellular localization. It might share some such functions with VPS35C, given the partial overlap of genetic function between VPS35A and VPS35C.
Recent phylogenetic studies suggest that several components of systems of membrane trafficking evolved through gene duplication and specialization (Dacks and Field, 2007; Dacks et al., 2008). Divergence of gene families encoding key factors of membrane trafficking, such as SNAREs and Rab GTPases, suggests a complexity of the endomembrane organization in plant cells. The functions of divergent gene family members have been suggested to be redundant, based on a subtle mutant phenotype or on the lethality of multiple mutants. Paralogous genes probably share ancestral functions but may have since developed distinct individual functions. Determining the precise individual functions of each paralog is a challenge because such functions are subtle, cryptic, and difficult to discern beyond being explained by simple genetic redundancy. The distinct and/or partially overlapping functions of the VPS35 paralogs reported here provide an example of the elucidation of such complexity. To elucidate more precisely the molecular mechanisms underlying functional differences between VPS35 paralogs, further investigations will be required. Variation in combinations of retromer complexes including SNX proteins, their intracellular localization, and cargo selectivity should be clarified in planta in the future. In addition, functional interaction between the retromer and its regulatory factors, such as Rab5 and Rab7, which has recently been suggested in animal cells (Rojas et al., 2008), during plant development is an important issue.
Shoot Gravitropism Is a Sensitive Indicator of the Status of Post-Golgi Trafficking in Endodermal Cells
The endodermis is the major site of gravity perception in the Arabidopsis shoot, and amyloplasts are thought to be statoliths that sediment toward the direction of gravity within the endodermal cell (Fukaki et al., 1998; Saito et al., 2005). In zig-1 cells, amyloplatsts do not sediment but are positioned at the cell periphery even within the upper portion of the cell (Morita et al., 2002). In addition, vacuole formation and function in the mutant are affected, and a dynamic vacuolar structure, including transvacuolar strands and the membrane surrounding the amyloplasts, are not observed in zig-1 cells (Saito et al., 2005). A similar vacuolar and amyloplast phenotype is observed in other sgr mutants, such as sgr2, sgr3, and sgr8/grv2. All of the genes responsible for these mutants have been suggested to be involved in post-Golgi membrane trafficking (Kato et al., 2002; Morita et al., 2002; Yano et al., 2003; Silady et al., 2004; Shimoi et al., 2005; Tamura et al., 2007; Kanamori et al., 2008; Silady et al., 2008).
ZIP3 also encodes a protein that is probably involved in retrograde transport from the PVC to the TGN. As we showed here, amyloplast sedimentation was almost normal (Figure 3), and vacuolar and amyloplasts dynamics in the endodermal cells were partially recovered in zip3 zig-1 plants (Figure 3). This partial suppression of the cytological phenotype in the endodermal cells correlates with the partial suppression of the physiological phenotype on gravitropism (Figure 1). Consistently, zip1 completely suppresses the zig-1 gravitropic phenotype as well as the cytological phenotype (Niihama et al., 2005). We have already demonstrated that the gravitropic defect in zig-1 plants can be attributed to the lack of VTI11 function in the endodermis (Morita et al., 2002). Expression of VPS35A under the control of the SCR promoter complemented the gravitropic suppression phenotype in zip3 zig-1 plants (Figures 2D and 2E), indicating that effect of the zip3 mutation on the endodermal cells is the cause of the suppression of the gravitropic phenotype. Taken together, we conclude that the gravitropic phenotype strongly correlates with aberrant post-Golgi membrane trafficking in endodermal cells.
Using this feature of endodermal cells, we found that the retromer, composed of VPS26A-VPS29-VPS35A, is genetically involved in the function of VTI1 family members. Moreover, this approach uncovered functional differences between paralogous genes, which are cryptic in the absence of the zig-1 mutation. Although the endodermis is nonessential for Arabidopsis viability, it is essential for gravity sensing. Therefore, we can use the gravitropic phenotype as a sensitive marker to monitor the status of membrane trafficking in endodermal cells. Our genetic strategy is useful to unravel the molecular network of membrane trafficking in plant cells that is thought to have complex endomembrane organization.
METHODS
Plant Materials and Growth Conditions
The Col-0 accession of Arabidopsis thaliana was used as the wild type. zig-1/ sgr4-1 is also a Col-0 accession, and both zip3-1 zig-1 and zip3-2 zig-1 were isolated from an M2 population of zig-1 seeds mutagenized with ethyl methanesulfonate (Niihama et al., 2005). zip3-3 (SALK_039689), zip3-4 (SALK_125271), vps26a (GABI_053C12), vps26b (SALK_142592), vps35b (SALK_014345), and vps35c (SALK_099735) were from the SALK T-DNA insertion collection (Col-0). mRNA expression of each gene in the corresponding mutant was examined by RT-PCR analysis using specific primer sets (cVPS35B-F2/-R2, cVPS35C-F2/-R2, cVPS26A-F1/-R2, and cVPS26B-F1/-R2; see Supplemental Table 3 online for their sequence). mag1-1 was kindly provided by I. Hara-Nishimura (Shimada et al., 2006). Seeds were surface sterilized with 5% sodium hypochlorite solution and then plated on Murashige and Skoog plates (1 × MS salt mixure, 1% [w/v] sucrose, 0.01% [w/v] myoinositole, 0.05% [w/v] gellan gum, and 0.05% MES-KOH, pH 5.7) followed by treatment at 4 ° C for 2 d. Seedlings were transplanted and grown on soil (mixture of vermiculite from Nittai and Metromix G550 from Sun Gro Horticulture covered with Ube Perlite Type I from Ube Kosan) under constant white light at 23 ° C.
Gravitropism Assay
To examine the gravitropic responses of inflorescence stems, intact 5-week-old plants with primary stems 4 to 8 cm in length were placed horizontally under nondirectional dim light at 23 ° C. Photographs were taken at indicated times, and then stem curvature was measured on the digital image with Image J (http://rsb.info.nih.gov/ij/) as the angle formed between the growing direction of the apex and the horizontal base line.
Mapping zip3
Based on the previous study, we had noticed Ler accessions contain natural variations that suppress the zig-1 phenotype to some extent. Since they behaved as quantitative trait loci, a mapping population generated by a cross between Ler and zip3-1 zig-1 should cause confusion. Thus, we first isolated F2 progeny from a cross between Ler and zig-1, which contained a homozygous zig-1 mutation and exhibited an obvious zig-1 phenotype. This renders suppressive factors in the Ler background negligible. Each inbred line was examined with polymorphic markers to select specific lines that contained a pair of or a region of chromosomes homozygously derived from Ler (zig-1Ler lines). Meanwhile, we roughly determined the ZIP3 locus on chromosome II using an F2 mapping population generated by cross between zig-3 derived from Wassilewskija accession and zip3-1 zig-1 because there is no suppressive factor in the Wassilewskija background. To map ZIP3 finely, we prepared a mapping population from a cross between zip3-1 zig-1 and a specific zig-1 Ler line containing a homozygous Ler region largely derived from the Ler ecotype on chromosome II. For fine-scale mapping, DNA was prepared from ∼ 220 F2 progeny. We made cleaved-amplified polymorphic sequence markers that can recognize polymorphisms between Col and Ler based on information provided by The Arabidopsis Information Resource.
Histological Analysis
For observation of endodermal cells, stem segments were fixed, embedded in Technovit7100, and sectioned as described previously (Morita et al., 2002). For electron microscopy, the samples of inflorescence stem were embedded in Spurr's resin (Nisshin EM) following the methods of Morita et al. (2002). Ultrathin sections were cut (70- to 90-nm thick), stained with uranyl acetate and lead nitrate, and examined with a transmission electron microscope (JEM 2000FXII; JEOL).
Confocal Microscopy
Inflorescence stems segments were excised from 5-week-old plants and sectioned longitudinally and manually. The sections were observed using a confocal laser scanning microscope (FV1000; Olympus). Then, GFP fluorescence and chlorophyll autofluorescence were detected with 500- to 510-nm and 640- to 700-nm spectral settings, respectively, following a 488-nm excitation.
Molecular Cloning and Plant Transformation
For the complementation analysis, a 7.2-kb genomic DNA fragment of ZIP3/VPS35A including the 1.4-kb putative promoter region and the 0.3-kb 3 ′ downstream region was amplified by PCR (primer set: gZIP3-F/gZIP3-R) using a BAC DNA T17A5 as a template and then cloned into the binary vector pBIN19 (GenBank accession number U09365). Total RNA was isolated from inflorescence stems with the RNeasy plant mini kit (Qiagen). cDNAs were synthesized using SuperScript II reverse transcriptase (Invitrogen). cDNA of ZIP3/VPS35A (primer set: cZIP3-F/cZIP3-R), VPS35B (primer set:; cVPS35B-F/cVPS35B-R), and VPS35C (primer set: cVPS35C-F/cVPS35C-R) were amplified by PCR using synthesized cDNAs as templates and then cloned into pCR-Blunt II–TOPO vector (ZERO Blunt TOPO PCR cloning kit; Invitrogen). The resulting cDNA was inserted into the region between the SCR promoter and NOS terminator in pBIN19. The DNA sequence of each construct was confirmed by sequencing. The resulting constructs were transformed into Agrobacterium tumefaciens strain GV3101 (pMP90) and then introduced into zip3-1 zig-1 plants by the floral dip method (Clough and Bent, 1998). T1 plants were selected for resistance against kanamycin (30 μ g/mL). Expression of transgenes (SCRpro:ZIP3/VPS35A, VPS35B, or VPS35C) was confirmed by RT-PCR analysis using primer sets that are able to specifically detect transcripts derived from the transgenes [primer sets: pSCR(-30)D/cZIP3-R2, pSCR(-30)D/cVPS35BR1, and pSCR(-30)D/cVPS35CR1]. The sequences of primers used are indicated in Supplemental Table 3 online.
Protein Extraction and Immunoprecipitation
Immunoprecipitation of detergent extracts from the shoots was performed as described previously (Yano et al., 2003), with minor modifications. Shoots (0.5 g) from 6-week-old plants without fruits were homogenized on ice in 10 mL of extraction buffer (50 mM HEPES-KOH, pH 6.5, 10 mM KOAc, 100 mM NaCl, 5 mM EDTA, and 0.4 M sucrose) with a protease inhibitor cocktail (Sigma-Aldrich). The homogenate was passed through Miracloth (Calbiochem) to remove debris. This extract was centrifuged at 15,000g for 30 min at 4 ° C. The pellet was resuspended in 1 mL of extraction buffer containing 1% (v/v) Triton X-100, protease inhibitor cocktail, and PreserveX-QML Plymeric Micelles (QBI Life Sciences) and then incubated at 4 ° C for 3 h with rotation. Insoluble material was removed by centrifugation at 10,000g for 10 min at 4 ° C. Protein concentration was determined with the BCA kit (Pierce) to use samples of equal protein amount for protein gel blot analysis or immunoprecipitation.
μ MACS ProteinA MicroBeads (Miltenyi Biotec) were preincubated with anti-SYP22 antibody for at least 1 h at 4 ° C on the rotator. The beads were then added to the extract and incubated for at least 4 h at 4 ° C with rotation. The samples were applied to μ -Columns (Miltenyi Biotec) attached to the magnetic field of the μ MACS separator (Miltenyi Biotec) and the flow-through was collected. Then, the column was washed five times with extraction buffer containing 1% (v/v) Triton X-100 and two times with PBS (130 mM NaCl, 7 mM Na2HPO4, and 3 mM NaH2PO4, pH 7.4). Protein (immunoprecipitate) was then eluted from the beads with 100 mL of SDS sample buffer. Equal volumes of total protein extract, flow-through, or immunoprecipitate were separated by SDS-PAGE followed by immunoblotting using anti-VTI12 antibody.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: ZIG/VTI11, At5g39510; ZIP3/VPS35A, At2g17790; VPS35B, At1g75850; VPS35C, At3g51310; VPS26A, At5g53530; VPS26B, At4g27690; and MAIGO1/VPS29, At3g47810. Accession numbers for T-DNA insertion lines are as follows: zip3-3, SALK_039689; zip3-4, SALK_125271, vps35b, SALK_014345; vps35c, SALK_099735; vps26a, GABI_053c12; and vps26b, SALK_142592.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Morphological Phenotype of zip3-1 zig-1.
Supplementa1 Figure 2. Phenotypes of Alleles of zip3 zig-1 Double Mutants..
Supplemental Figure 3. Immunoblot Analysis of ZIP3/VPS35A Protein..
Supplemental Figure 4. Phenotypes of Alleles of zip3 Single Mutants..
Supplemental Figure 5. Morphological Phenotypes of mag1-1 and mag1-1 zig-1 Mutants..
Supplemental Figure 6. Phenotypes of vps26 Single Mutants..
Supplemental Figure 7. Phenotypes of vps35b and vps35c Single Mutants..
Supplemental Figure 5. Localization of GFP-VTI12 and ARA6-mRFP in Endodermal Cells..
Supplemental Table 1. Localization of Amyloplasts in the Endodermal Cell..
Supplemental Table 2. Quantitative Analysis of the Cytological Phenotype of Living Endodermal Cells Based on the Observation as Shown in Figures 3H to 3K..
Supplemental Table 3. Primer Sets for Cloning and RT-PCR.
Supplementary Material
Acknowledgments
We thank Natasha V. Raikhel for providing the anti-VTI12 antibody, Ikuko Hara-Nishimura for the anti-VPS35A antibody, Takashi Ueda and Tomohiro Uemura for valuable discussions, and Nauko Inui and Kaori Kaminoyama for technical assistance. We also thank the SALK Institute and the Max Planck Institute for Plant Breeding Research for providing T-DNA insertion mutant lines. The financial support of a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan (16085205) and a grant from the Bioarchitect Project of RIKEN (to M.T.M.) is gratefully acknowledged.
References
- Bassham D.C., Sanderfoot A.A., Kovaleva V., Zheng H., Raikhel N.V. (2000). AtVPS45 complex formation at the trans-Golgi network. Mol. Biol. Cell 11: 2251–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J.S., Hurley J.H. (2008). Retromer. Curr. Opin. Cell Biol. 20: 427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J.S., Rojas R. (2006). Retrograde transport from endosomes to the trans-Golgi network. Nat. Rev. Mol. Cell Biol. 7: 568–579 [DOI] [PubMed] [Google Scholar]
- Cai H., Reinisch K., Ferro-Novick S. (2007). Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev. Cell 12: 671–682 [DOI] [PubMed] [Google Scholar]
- Canuel M., Lefrancois S., Zeng J., Morales C.R. (2008). AP-1 and retromer play opposite roles in the trafficking of sortilin between the Golgi apparatus and the lysosomes. Biochem. Biophys. Res. Commun. 366: 724–730 [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Collins B.M. (2008). The structure and function of the retromer protein complex. Traffic 9: 1811–1822 [DOI] [PubMed] [Google Scholar]
- Collins B.M., Norwood S.J., Kerr M.C., Mahony D., Seaman M.N., Teasdale R.D., Owen D.J. (2008). Structure of Vps26B and mapping of its interaction with the retromer protein complex. Traffic 9: 366–379 [DOI] [PubMed] [Google Scholar]
- Dacks J.B., Field M.C. (2007). Evolution of the eukaryotic membrane-trafficking system: Origin, tempo and mode. J. Cell Sci. 120: 2977–2985 [DOI] [PubMed] [Google Scholar]
- Dacks J.B., Poon P.P., Field M.C. (2008). Phylogeny of endocytic components yields insight into the process of nonendosymbiotic organelle evolution. Proc. Natl. Acad. Sci. USA 105: 588–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebine K., Okatani Y., Uemura T., Goh T., Shoda K., Niihama M., Morita M.T., Spitzer C., Otegui M.S., Nakano A., Ueda T. (2008). A SNARE complex unique to seed plants is required for protein storage vacuole biogenesis and seed development of Arabidopsis thaliana. Plant Cell 20: 3006–3021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer D., Sutton R.B., Brunger A.T., Jahn R. (1998). Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc. Natl. Acad. Sci. USA 95: 15781–15786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaki H., Fujisawa H., Tasaka M. (1996). SGR1, SGR2, SGR3: Novel genetic loci involved in shoot gravitropism in Arabidopsis thaliana. Plant Physiol. 110: 945–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaki H., Wysocka-Diller J., Kato T., Fujisawa H., Benfey P.N., Tasaka M. (1998). Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana. Plant J. 14: 425–430 [DOI] [PubMed] [Google Scholar]
- Haft C.R., Sierra M.L., Bafford R., Lesniak M.A., Barr V.A., Taylor S.I. (2000). Human orthologs of yeast vacuolar protein sorting proteins Vps26, Vps29, and Vps35: Assembly into multimeric complexes. Mol. Biol. Cell 11: 4105–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierro A., Rojas A.L., Rojas R., Murthy N., Effantin G., Kajava A.V., Steven A.C., Bonifacino J.S., Hurley J.H. (2007). Functional architecture of the retromer cargo-recognition complex. Nature 449: 1063–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horazdovsky B.F., Davies B.A., Seaman M.N., McLaughlin S.A., Yoon S., Emr S.D. (1997). A sorting nexin-1 homologue, Vps5p, forms a complex with Vps17p and is required for recycling the vacuolar protein-sorting receptor. Mol. Biol. Cell 8: 1529–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y., Fobis-Loisy I., Miège C., Rollin C., Gaude T. (2006). AtSNX1 defines an endosome for auxin-carrier trafficking in Arabidopsis. Nature 443: 106–109 [DOI] [PubMed] [Google Scholar]
- Jaillais Y., Santambrogio M., Rozier F., Fobis-Loisy I., Miège C., Gaude T. (2007). The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell 130: 1057–1070 [DOI] [PubMed] [Google Scholar]
- Kanamori T., Inoue T., Sakamoto T., Gengyo-Ando K., Tsujimoto M., Mitani S., Sawa H., Aoki J., Arai H. (2008). Beta-catenin asymmetry is regulated by PLA1 and retrograde traffic in C. elegans stem cell divisions. EMBO J. 27: 1647–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Morita M.T., Fukaki H., Yamauchi Y., Uehara M., Niihama M., Tasaka M. (2002). SGR2, a phospholipase-like protein, and ZIG/SGR4, a SNARE, are involved in the shoot gravitropism of Arabidopsis. Plant Cell 14: 33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurten R.C., Cadena D.L., Gill G.N. (1996). Enhanced degradation of EGF receptors by a sorting nexin, SNX1. Science 272: 1008–1010 [DOI] [PubMed] [Google Scholar]
- Morita M.T., Kato T., Nagafusa K., Saito C., Ueda T., Nakano A., Tasaka M. (2002). Involvement of the vacuoles of the endodermis in the early process of shoot gravitropism in Arabidopsis. Plant Cell 14: 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M.T., Tasaka M. (2004). Gravity sensing and signaling. Curr. Opin. Plant Biol. 7: 712–718 [DOI] [PubMed] [Google Scholar]
- Niihama M., Uemura T., Saito C., Nakano A., Sato M.H., Tasaka M., Morita M.T. (2005). Conversion of functional specificity in Qb-SNARE VTI1 homologues of Arabidopsis. Curr. Biol. 15: 555–560 [DOI] [PubMed] [Google Scholar]
- Nothwehr S.F., Bruinsma P., Strawn L.A. (1999). Distinct domains within Vps35p mediate the retrieval of two different cargo proteins from the yeast prevacuolar/endosomal compartment. Mol. Biol. Cell 10: 875–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr S.F., Ha S.A., Bruinsma P. (2000). Sorting of yeast membrane proteins into an endosome-to-Golgi pathway involves direct interaction of their cytosolic domains with Vps35p. J. Cell Biol. 151: 297–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr S.F., Hindes A.E. (1997). The yeast VPS5/GRD2 gene encodes a sorting nexin-1-like protein required for localizing membrane proteins to the late Golgi. J. Cell Sci. 110: 1063–1072 [DOI] [PubMed] [Google Scholar]
- Oliviusson P., Heinzerling O., Hillmer S., Hinz G., Tse Y.C., Jiang L., Robinson D.G. (2006). Plant retromer, localized to the prevacuolar compartment and microvesicles in Arabidopsis, may interact with vacuolar sorting receptors. Plant Cell 18: 1239–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy J.V., Seaman M.N. (2001). Vps26p, a component of retromer, directs the interactions of Vps35p in endosome-to-Golgi retrieval. Mol. Biol. Cell 12: 3242–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas R., Kametaka S., Haft C.R., Bonifacino J.S. (2007). Interchangeable but essential functions of SNX1 and SNX2 in the association of retromer with endosomes and the trafficking of mannose 6-phosphate receptors. Mol. Cell. Biol. 27: 1112–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas R., van Vlijmen T., Mardones G.A., Prabhu Y., Rojas A.L., Mohammed S., Heck A.J., Raposo G., van der Sluijs P., Bonifacino J.S. (2008). Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J. Cell Biol. 183: 513–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo E., Denecke J. (2008). What is moving in the secretory pathway of plants?. Plant Physiol. 147: 1493–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford S., Moore I. (2002). The Arabidopsis Rab GTPase family: Another enigma variation. Curr. Opin. Plant Biol. 5: 518–528 [DOI] [PubMed] [Google Scholar]
- Sack F.D. (1991). Plant gravity sensing. Int. Rev. Cytol. 127: 193–252 [DOI] [PubMed] [Google Scholar]
- Saito C., Morita M.T., Kato T., Tasaka M. (2005). Amyloplasts and vacuolar membrane dynamics in the living graviperceptive cell of the Arabidopsis inflorescence stem. Plant Cell 17: 548–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot A. (2007). Increases in the number of SNARE genes parallels the rise of multicellularity among the green plants. Plant Physiol. 144: 6–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot A.A., Kovaleva V., Bassham D.C., Raikhel N.V. (2001). Interactions between syntaxins identify at least five SNARE complexes within the Golgi/prevacuolar system of the Arabidopsis cell. Mol. Biol. Cell 12: 3733–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot A.A., Kovaleva V., Zheng H., Raikhel N.V. (1999). The t-SNARE AtVAM3p resides on the prevacuolar compartment in Arabidopsis root cells. Plant Physiol. 121: 929–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M.H., Nakamura N., Ohsumi Y., Kouchi H., Kondo M., Hara-Nishimura I., Nishimura M., Wada Y. (1997). The AtVAM3 encodes a syntaxin-related molecule implicated in the vacuolar assembly in Arabidopsis thaliana. J. Biol. Chem. 272: 24530–24535 [DOI] [PubMed] [Google Scholar]
- Schmid M., Davison T.S., Henz S.R., Pape U.J., Demar M., Vingron M., Schölkopf B., Weigel D., Lohmann J.U. (2005). A gene expression map of Arabidopsis thaliana development. Nat. Genet. 37: 501–506 [DOI] [PubMed] [Google Scholar]
- Seaman M.N. (2005). Recycle your receptors with retromer. Trends Cell Biol. 15: 68–75 [DOI] [PubMed] [Google Scholar]
- Seaman M.N. (2007). Identification of a novel conserved sorting motif required for retromer-mediated endosome-to-TGN retrieval. J. Cell Sci. 120: 2378–2389 [DOI] [PubMed] [Google Scholar]
- Seaman M.N., Marcusson E.G., Cereghino J.L., Emr S.D. (1997). Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J. Cell Biol. 137: 79–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman M.N., McCaffery J.M., Emr S.D. (1998). A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J. Cell Biol. 142: 665–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T., Koumoto Y., Li L., Yamazaki M., Kondo M., Nishimura M., Hara-Nishimura I. (2006). AtVPS29, a putative component of a retromer complex, is required for the efficient sorting of seed storage proteins. Plant Cell Physiol. 47: 1187–1194 [DOI] [PubMed] [Google Scholar]
- Shimoi W., Ezawa I., Nakamoto K., Uesaki S., Gabreski G., Aridor M., Yamamoto A., Nagahama M., Tagaya M., Tani K. (2005). p125 is localized in endoplasmic reticulum exit sites and involved in their organization. J. Biol. Chem. 280: 10141–10148 [DOI] [PubMed] [Google Scholar]
- Silady R.A., Ehrhardt D.W., Jackson K., Faulkner C., Oparka K., Somerville C.R. (2008). The GRV2/RME-8 protein of Arabidopsis functions in the late endocytic pathway and is required for vacuolar membrane flow. Plant J. 53: 29–41 [DOI] [PubMed] [Google Scholar]
- Silady R.A., Kato T., Lukowitz W., Sieber P., Tasaka M., Somerville C.R. (2004). The gravitropism defective 2 mutants of Arabidopsis are deficient in a protein implicated in endocytosis in Caenorhabditis elegans. Plant Physiol. 136: 3095–3103, discussion 3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surpin M., Zheng H., Morita M.T., Saito C., Avila E., Blakeslee J.J., Bandyopadhyay A., Kovaleva V., Carter D., Murphy A., Tasaka M., Raikhel N. (2003). The VTI family of SNARE proteins is necessary for plant viability and mediates different protein transport pathways. Plant Cell 15: 2885–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Takahashi H., Kunieda T., Fuji K., Shimada T., Hara-Nishimura I. (2007). Arabidopsis KAM2/GRV2 is required for proper endosome formation and functions in vacuolar sorting and determination of the embryo growth axis. Plant Cell 19: 320–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Ueda T., Ohniwa R.L., Nakano A., Takeyasu K., Sato M.H. (2004). Systematic analysis of SNARE molecules in Arabidopsis: Dissection of the post-Golgi network in plant cells. Cell Struct. Funct. 29: 49–65 [DOI] [PubMed] [Google Scholar]
- Wysocka-Diller J.W., Helariutta Y., Fukaki H., Malamy J.E., Benfey P.N. (2000). Molecular analysis of SCARECROW function reveals a radial patterning mechanism common to root and shoot. Development 127: 595–603 [DOI] [PubMed] [Google Scholar]
- Yamauchi Y., Fukaki H., Fujisawa H., Tasaka M. (1997). Mutations in the SGR4, SGR5 and SGR6 loci of Arabidopsis thaliana alter the shoot gravitropism. Plant Cell Physiol. 38: 530–535 [DOI] [PubMed] [Google Scholar]
- Yamazaki M., Shimada T., Takahashi H., Tamura K., Kondo M., Nishimura M., Hara-Nishimura I. (2008). Arabidopsis VPS35, a retromer component, is required for vacuolar protein sorting and involved in plant growth and leaf senescence. Plant Cell Physiol. 49: 142–156 [DOI] [PubMed] [Google Scholar]
- Yano D., Sato M., Saito C., Sato M.H., Morita M.T., Tasaka M. (2003). A SNARE complex containing SGR3/AtVAM3 and ZIG/VTI11 in gravity-sensing cells is important for Arabidopsis shoot gravitropism. Proc. Natl. Acad. Sci. USA 100: 8589–8594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., von Mollard G.F., Kovaleva V., Stevens T.H., Raikhel N.V. (1999). The plant vesicle-associated SNARE AtVTI1a likely mediates vesicle transport from the trans-Golgi network to the prevacuolar compartment. Mol. Biol. Cell 10: 2251–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.