Abstract
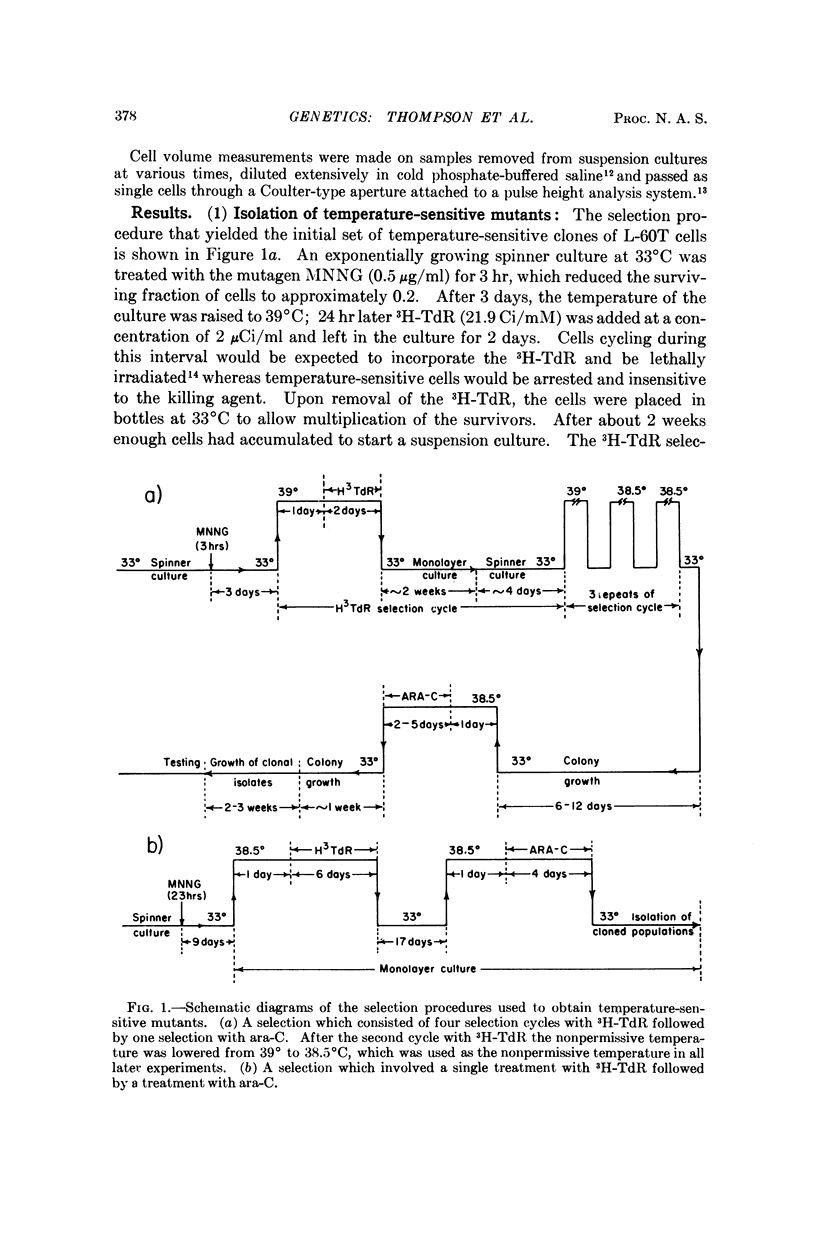
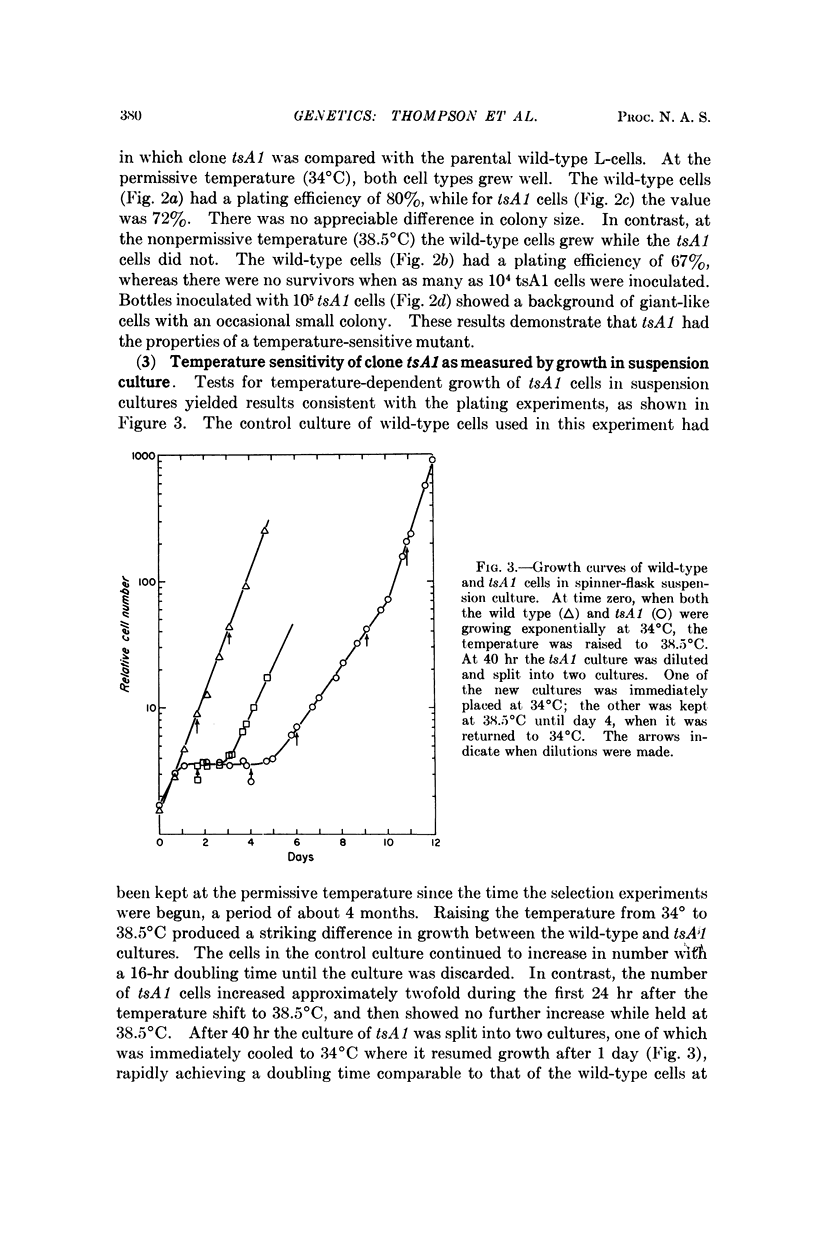
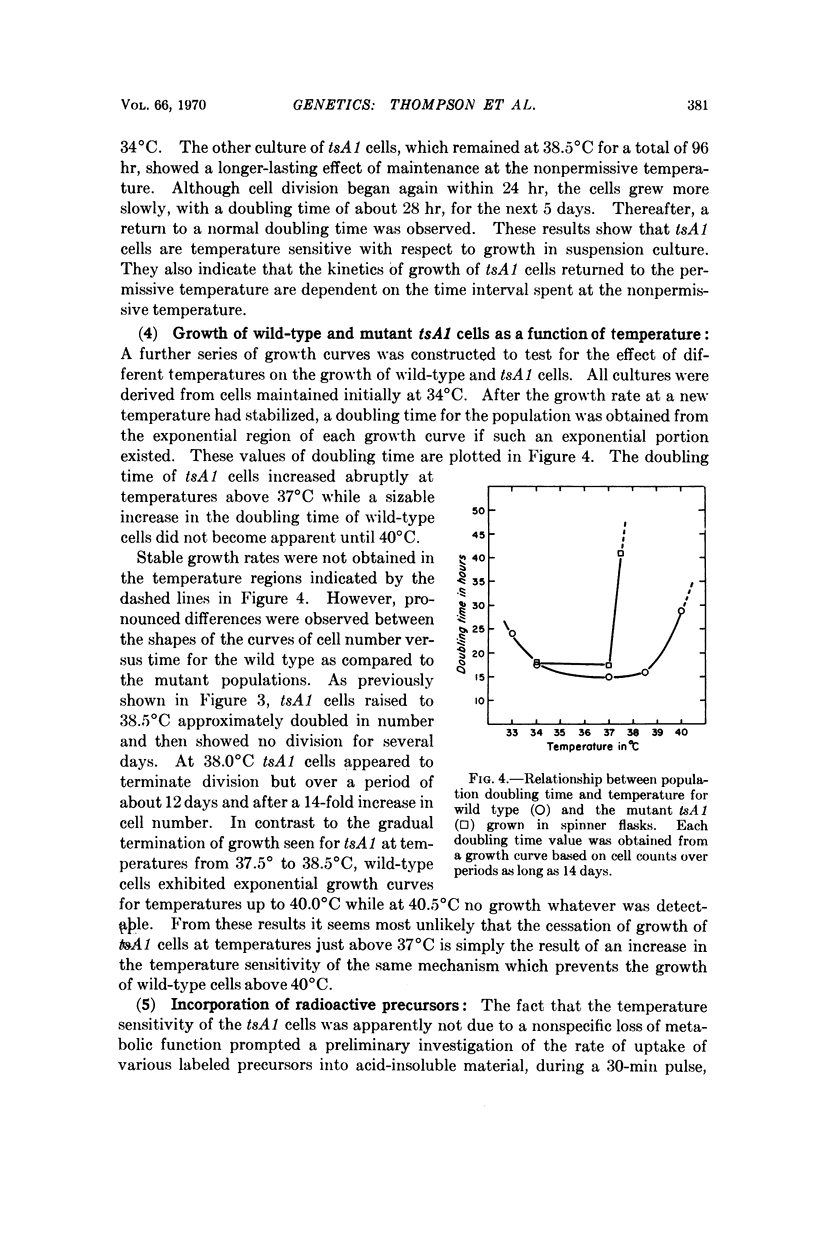
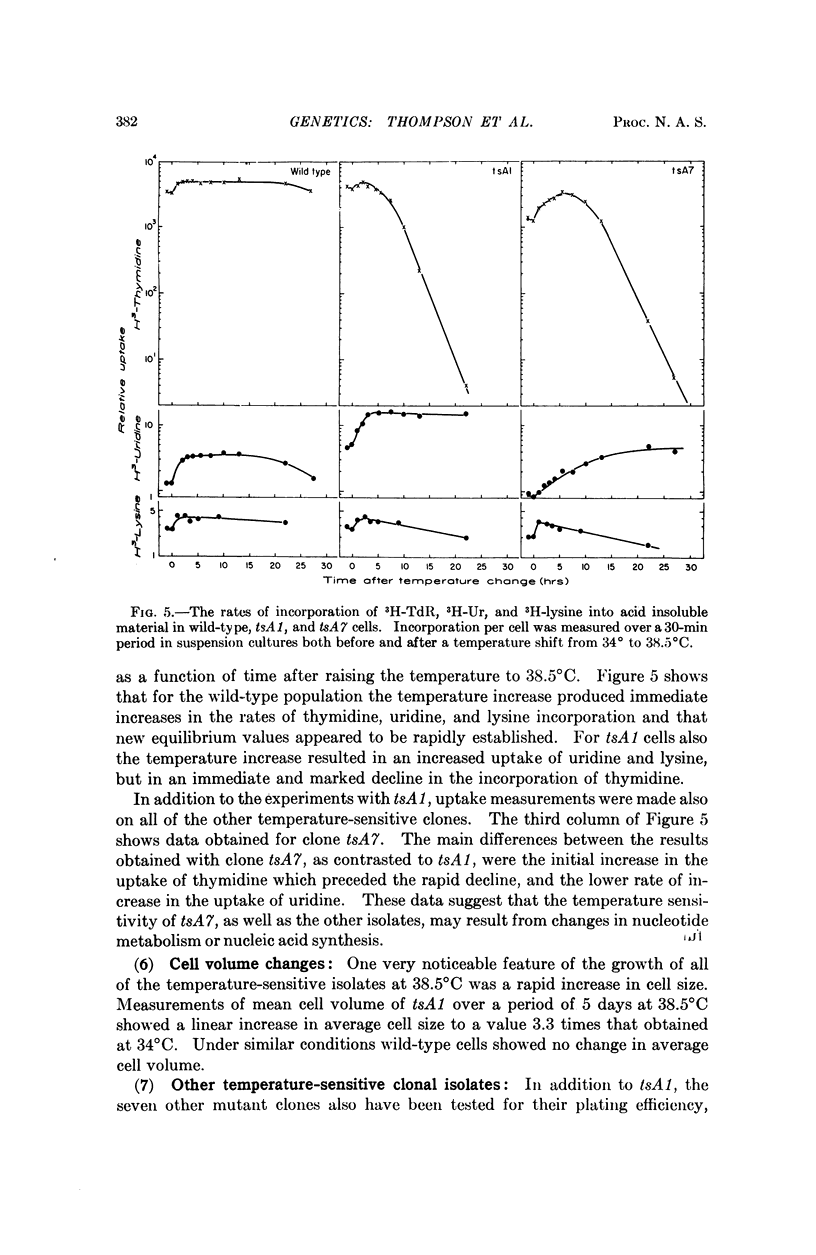
Procedures are described for the isolation of conditional lethal mutants of mouse L-60T cells. The mutant lines were temperature sensitive by the following criteria: (a) colony-forming ability, (b) growth in suspension culture, and (c) rate of uptake of tritiated-thymidine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacchetti S., Whitmore G. F. Actinomycin D: effects on mouse L-cells. Biophys J. 1969 Dec;9(12):1427–1445. doi: 10.1016/S0006-3495(69)86463-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu E. H., Brimer P., Jacobson K. B., Merriam E. V. Mammalian cell genetics. I. Selection and characterization of mutations auxotrophic for L-glutamine or resistant to 8-azaguanine in Chinese hamster cells in vitro. Genetics. 1969 Jun;62(2):359–377. doi: 10.1093/genetics/62.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu E. H., Malling H. V. Mammalian cell genetics. II. Chemical induction of specific locus mutations in Chinese hamster cells in vitro. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1306–1312. doi: 10.1073/pnas.61.4.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPHRUSSI B., SORIEUL S. [New observations on the in vitro hybridization of mouse cells]. C R Hebd Seances Acad Sci. 1962 Jan 3;254:181–182. [PubMed] [Google Scholar]
- Han A., Sinclair W. K. Sensitivity of synchronized Chinese hamster cells to ultraviolet light. Biophys J. 1969 Sep;9(9):1171–1192. doi: 10.1016/S0006-3495(69)86444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells, VII. Induction and isolation of nutritional mutants in Chinese hamster cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1275–1281. doi: 10.1073/pnas.60.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells. IX. Quantitation of mutagenesis by physical and chemical agents. J Cell Physiol. 1969 Dec;74(3):245–258. doi: 10.1002/jcp.1040740305. [DOI] [PubMed] [Google Scholar]
- Karon M., Shirakawa S. The locus of action of 1-beta-d-arabinofuranosylcytosine in the cell cycle. Cancer Res. 1969 Mar;29(3):687–696. [PubMed] [Google Scholar]
- Matsuya Y., Green H., Basilico C. Properties and uses of human-mouse hybrid cell lines. Nature. 1968 Dec 21;220(5173):1199–1202. doi: 10.1038/2201199a0. [DOI] [PubMed] [Google Scholar]
- Naha P. M. Temperature sensitive conditional mutants of monkey kidney cells. Nature. 1969 Sep 27;223(5213):1380–1381. doi: 10.1038/2231380a0. [DOI] [PubMed] [Google Scholar]
- TILL J. E., WHITMORE G. F., GULYAS S. Deoxyribonucleic acid synthesis in individual L-strain mouse cells. II. Effects of thymidine starvation. Biochim Biophys Acta. 1963 Jun 25;72:277–289. [PubMed] [Google Scholar]
- Whitmore G. F., Gulyas S. Synchronization of mammalian cells with tritiated thymidine. Science. 1966 Feb 11;151(3711):691–694. doi: 10.1126/science.151.3711.691. [DOI] [PubMed] [Google Scholar]



