Abstract
(+)-Pulegone is a central intermediate in the biosynthesis of (-)-menthol, the most significant component of peppermint essential oil. Depending on environmental conditions, this branch point metabolite may be reduced to (-)-menthone en route to menthol, by pulegone reductase (PR), or oxidized to (+)-menthofuran, by menthofuran synthase (MFS). To elucidate regulation of pulegone metabolism, we modified the expression of mfs under control of the CaMV 35S promoter in transformed peppermint plants. Overexpression and cosuppression of mfs resulted in the respective increase or decrease in the production of menthofuran, indicating that the control of MFS resides primarily at the level of transcription. Significantly, in both WT peppermint as well as in all transformed plants, the flux of (+)-pulegone through PR correlated negatively with the essential oil content of menthofuran, such that menthofuran, and pulegone increased, or decreased, in concert. These results suggested that menthofuran itself might influence the reduction of pulegone. Although (+)-menthofuran did not inhibit (+)-PR activity, stem feeding with menthofuran selectively decreased pr transcript levels in immature leaves, thereby accounting for decreased reductase activity and increased pulegone content. These data demonstrate that the metabolic fate of (+)-pulegone is controlled through transcriptional regulation of mfs and that menthofuran, either directly or indirectly, influences this process by down-regulating transcription from pr and/or decreasing pr message stability. The ability to reduce both menthofuran and pulegone levels is of commercial significance in improving essential oil quality; however, the physiological rationale for such complex regulation is presently unclear.
Keywords: monoterpene biosynthesis, menthol biosynthesis, menthofuran synthase, pulegone reductase, Mentha piperita
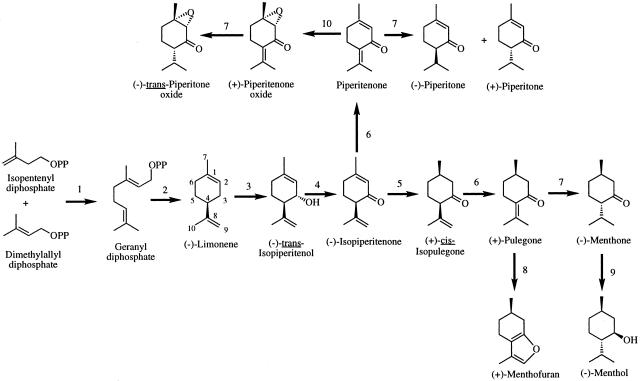
Monoterpenes are the major essential oil constituents of members of the mint (Lamiaceae) family, including peppermint (Mentha piperita), which has been developed as a model system for the study of monoterpene metabolism (1, 2). Monoterpene biosynthesis in mints is localized specifically to the glandular trichomes (1, 3, 4), and the pathway in peppermint leading to the principal oil component (-)-menthol is complex in involving multiple steps and a range of different reaction types (5). Thus, after the conversion of the primary metabolites isopentenyl diphosphate and dimethylallyl diphosphate to geranyl diphosphate, the cyclization of this universal monoterpene precursor to the committed intermediate (-)-limonene, and the cytochrome P450-mediated hydroxylation to (-)-trans-isopiperitenol, a sequence of five steps produce (-)-menthol (Fig. 1). Menthol is the most abundant (and characteristic) component of the essential oil of mature peppermint plants, but the overall quality of the oil, and thus its commercial value, is determined by the compositional balance of several oil constituents (6, 7). Typically, superior oils contain high quantities of menthol, moderate amounts of menthone, and low levels of pulegone and menthofuran. In this context, the monoterpene ketone (+)-pulegone assumes central importance because it is the precursor of (-)-menthone, (-)-menthol, and of the side-product (+)-menthofuran (8).
Fig. 1.
The principal pathways for monoterpene biosynthesis in peppermint. The responsible enzymes are as follows: geranyl diphosphate synthase (1); (-)-limonene synthase (2); cytochrome P450 (-)-limonene-3-hydroxylase (3); (-)-trans-isopiperitenol dehydrogenase (4); (-)-isopiperitenone reductase (5); (+)-cis-isopulegone isomerase (6); (+)-PR (7); cytochrome P450 (+)-MFS (8); (-)-menthone reductase (9); and the terpenoid epoxidase (10). OPP denotes the diphosphate moiety.
With the pathway for the biosynthesis of peppermint monoterpenes defined (9), attention has turned to the regulation of metabolism of these natural products. Developmental and environmental factors are known to greatly influence the yield and composition of peppermint oil. For example, oil yield and menthol content increase with leaf (and thus oil gland) maturity, and a range of stress conditions (related to light, temperature and moisture status) tend to promote the accumulation of pulegone and menthofuran (10–13). The means by which these factors influence pathway flux or the specific steps of monoterpene metabolism are not understood.
Studies with intact plants indicate that monoterpene production is restricted to developing oil glands of young leaves (14) and that metabolic turnover and evaporative losses of oil components play very minor roles in determining oil yield and composition (1). The correlation between in vitro activity for the nine enzymatic steps of menthol biosynthesis and the rate of biosynthesis measured in vivo suggests that monoterpene production is controlled by the coordinately regulated activity of the relevant biosynthetic enzymes, with the exception of menthone reductase, which appears notably late in development (2). These results, combined with the lack of evidence for the control of pathway enzyme activity by allosteric modulation or covalent modification (9), suggest that oil yield, as well as composition, reflect the simple kinetic consequences of the levels of biosynthetic enzymes present, as determined by transcriptional and translational production of these pathway catalysts and their subsequent turnover.
In a recent effort to improve the essential oil composition of peppermint (15), plants were transformed with a homologous antisense version of the menthofuran synthase (mfs) cDNA (8) driven by the CaMV 35S promoter. The resulting transgenic plants were of normal appearance, growth habit, and essential oil yield, yet contained less than half of the menthofuran content of WT mint grown under unstressed or stressed conditions. Curiously, although transgenic down-regulation of mfs by the antisense approach led to the anticipated decrease in oil content of (+)-menthofuran, it did not increase the content of (+)-pulegone as would be expected via the decreased conversion of this ketone intermediate to menthofuran coupled to a presumed fixed rate of pulegone reduction to menthone via pulegone reductase (PR). Rather, a decrease in the oil content of both menthofuran and pulegone was observed in the transgenic antisense mfs plants, implying another level of control beyond the predicted, simple kinetic redistribution of pathway intermediates. This unusual observation represents a quite favorable compositional change because both menthofuran and pulegone are considered undesirable monoterpene components when present in peppermint essential oil at levels exceeding a few percent.
To further examine this phenomenon, the consequences of overexpression and cosuppression of mfs on oil composition were evaluated, as were the influence of these genetic modifications, and of menthofuran itself, on PR. The results indicate that menthofuran down-regulates transcript level for the branch-point enzyme PR, and by this mechanism decreases reductase capacity to consequently increase pulegone content.
Materials and Methods
General Methods. Peppermint plants were grown under optimum conditions as described (1, 15). To induce stress, plants were transferred to a growth chamber at constant temperature of 26°C and 16 h light period at an intensity of 80–100 μmol·m-2·s-1; plants were analyzed after 4 weeks under this regime.
The source and derivation of the pGAdekG/Nib.L transformation vector (16) have been described (15). The cDNA encoding MFS was released from the original clone (8) by EcoRI/KpnI digestion and used to replace the GUS-Nib fusion in the parent vector by insertion between the CaMV tandem 35S promoter and the Agrobacterium nopaline synthase transcriptional terminator (15). The resulting construct, designated pGAMFSS, was electroporated into Agrobacterium tumefaciens strain EHA105 by using a MicroPulser (Bio-Rad) according to the manufacturer's instructions. A single transformant was isolated and used to infect peppermint leaf disks, followed by selection/regeneration employing established procedures (17, 18).
Protocols for RNA isolation and blot analysis have been described (15). 32P-Labeled DNA probes were prepared by random priming of the homologous cDNAs encoding MFS, PR, ubiquitin, and deoxyxylulose phosphate reductoisomerase (DXR) (1, 19–21). Digital images of gels and blots were obtained by using the GEL DOC 2000/CHEMI DOC gel documentation system (Bio-Rad), and signal intensities were quantified by densitometry by using the NIH SCION IMAGE 1.57 software for the Macintosh. To correct for loading variations, signals for mfs, pr, and dxr were standardized to those of ubiquitin, or to those of the ethidium bromide stained 18S ribosomal RNA, in corresponding samples. At least three replicates for each treatment group were compared with those of controls by performing the Student t test at the 95% confidence level by using Microsoft EXCEL software for the Macintosh.
Sampling procedures for essential oil isolation have been described (15). For evaluation of oil composition and yield, the essential oil was isolated from 5-g samples of leaves by simultaneous steam distillation–pentane extraction using (+)-camphor as internal standard, and aliquots of the distillate were analyzed by capillary GC (with GC-MS for component identification) using an established protocol (1). Monoterpene standards were from our own collection. Reported yield values represent the mean and SE of five independent measurements for each plant, with statistical analysis as described above. Between-sample variation for all monoterpene components was <5% of the indicated value in all cases.
Menthofuran Treatments. Monoterpene feeding experiments under greenhouse conditions were conducted by placing the cut stems of 10 peppermint shoots (≈10 cm in length) in water or in a saturated aqueous solution of menthofuran or menthone in a 250-ml Erlenmeyer flask. The shoots were transferred to fresh solution after 6 h and 12 h of treatment. After 18 h, immature leaves (<2 cm long) were excised for RNA isolation and enzyme extraction.
For foliar application, peppermint plants grown under optimal conditions were sprayed with aqueous solutions containing 0.1% Tween 20 and concentrations of (+)-menthofuran ranging from 0 to 50 μM. Plants were sprayed three times at 6-h intervals before immature leaves were harvested for RNA extraction and Northern blot analysis as above.
Enzyme Isolation and Assay. The preparation of operationally soluble enzymes and microsomal enzymes from peppermint leaves has been described (2). Assays for the microsomal epoxidation of (+)-piperitone and piperitenone, and for the reduction of (+)-piperitenone oxide in soluble enzyme extracts, have also been described, as have the preparation of the required substrates and authentic product standards (22). The PR assay was from an earlier protocol (23). For inhibition studies, 5-μM to 200-μM concentrations of (+)-menthofuran in pentane (the solvent was without effect) were added to the mixture before the substrate, with preincubation for 30 min before the addition of 5 μM, 50 μM, or 100 μM (+)-pulegone to initiate the reaction under the described assay conditions (23). Inhibition data were analyzed by Dixon plotting (24). The assay for neomycin phosphotransferase-II (NPT-II) activity was performed by using the soluble enzyme preparation and a published procedure (25).
Results and Discussion
Effects of mfs Expression on Pulegone Content. In prior work (15), it was shown that transgenic down-regulation of mfs, by the antisense approach, led to the anticipated decrease in peppermint oil content of (+)-menthofuran but surprisingly did not increase (+)-pulegone content as might be expected via the decreased conversion of this central ketone intermediate to (+)-menthofuran (see Fig. 1). Rather, a decrease in the oil content of both menthofuran and pulegone was observed in the transgenic antisense plants. Because the expected chemotype for an altered biosynthetic pathway was not observed (i.e., the absence of the subsequent product of the pathway accompanied by the accumulation of the intermediate(s) before the site of restriction), it was clear that additional regulatory influences were operating on monoterpene metabolism in these transgenic mint plants, most likely at the central branch-point step mediated by PR.
To examine this unusual phenomenon in greater detail, the influences of overexpression and cosuppression of mfs on pulegone content and PR were examined. For this purpose, peppermint was transformed with the homologous copy of the mfs cDNA (8) driven by the CaMV 35S promoter, resulting in the regeneration of 67 independent transformants. Gene transfer in these plants was confirmed by selection on kanamycin and by direct assay (25) for the selectable marker, neomycin phosphotransferase-II. Essential oil composition of WT and transgenic plants was determined by capillary GC and GC-MS (1). Both immature and fully expanded leaves were evaluated for oil composition because monoterpene biosynthesis is most rapid in developing oil glands of young leaves (14, 26) and because increased flux through PR in maturing oil glands results in depletion and dilution of earlier pathway intermediates via the derived products menthone and, ultimately, menthol (2).
The results of the analytical screen indicated that the oil of 16 mfs sense transformants (immature leaves) contained substantially more menthofuran than did the oil of WT control plants. These plants (now designated HMF for high menthofuran) also accumulated higher quantities of pulegone than did controls, which was accompanied by a decrease in menthone content (Table 1). The correlation of increased menthofuran content with increased pulegone content, although unusual, was anticipated from the prior results (15) and was consistent with the overexpression of mfs in these HMF plants while indicating that flux through PR was coincidentally compromised under these conditions. Consistent with prior observations on the oil content of mature leaves of unstressed plants (1), the developmentally regulated production of menthol in mature leaves of HMF transformants had depleted pulegone to below the limit of detection and had diluted the menthofuran content to near WT levels (Table 1).
Table 1. Essential oil composition of WT peppermint and representative transgenic plants that overexpress (HMF) or cosuppress (LMF) MFS.
| Immature leaves†
|
Mature leaves†
|
Pooled leaves‡
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Monoterpene component* | WT | HMF | LMF | WT | HMF | LMF | WT | HMF | LMF |
| Limonene | 5.5 | 3.5 | 8.7 | 0.9 | 0.6 | 1.6 | 1.1 | 1.4 | 2.2 |
| 1,8-Cineole | 1.7 | 3.5 | 1.6 | 9.5 | 9.7 | 7.7 | 7.2 | 3.7 | 6.5 |
| Menthone | 71.4 | 55.5 | 67.5 | 25.6 | 29.6 | 6.3 | 14.5 | 18.3 | 46.6 |
| Sabinene hydrate | 3.4 | 2.9 | 2.4 | 5.6 | 4.2 | 4.4 | 4.4 | 2.2 | 3.3 |
| Menthofuran | 6.4 | 15.4 | 0.3 | 2.3 | 2.4 | n.d. | 24.3 | 42.3 | 0.4 |
| Isomenthone | 5.3 | 4.3 | 1.6 | 4.3 | 4.4 | 0.2 | 1.8 | 2.2 | 0.7 |
| Neomenthol | 0.2 | 0.2 | 0.4 | 2.6 | 2.5 | 5.3 | 0.6 | 0.4 | 1.8 |
| Pulegone | 3.7 | 10.7 | 1.0 | n.d. | n.d. | n.d. | 35.0 | 22.1 | 9.9 |
| Menthol | 0.9 | 2.5 | 3.0 | 48.4 | 45.2 | 64.1 | 9.9 | 6.5 | 19.7 |
| Piperitone | 1.0 | 0.8 | 2.1 | 0.9 | 1.3 | 1.7 | 0.4 | 0.2 | 1.0 |
| trans-Piperitone oxide | n.d. | n.d. | 8.4 | n.d. | n.d. | 5.4 | n.d. | n.d. | 6.2 |
| Piperitenone oxide | n.d. | n.d. | 0.4 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.1 |
n.d., Not detected (<0.1%).
Concentration of each oil component is expressed as percentage of total oil
These plants were grown under optimal conditions
These plants were grown under stress conditions
The analytical screen of the original 67 independent transformants also produced four plants (designated LMF for low menthofuran) with exceptionally low (<0.5% of the oil) menthofuran content (Table 1). The essential oil of these plants (immature leaves) also contained a substantially lower content of pulegone than did controls. Interestingly, the monoterpene epoxyketones (-)-trans-piperitone oxide and (+)-piperitenone oxide were also observed; these compounds are not normally detected in peppermint oil. In the oil of mature leaves from these LMF plants, neither pulegone nor menthofuran was detectable, and, with increased flux through pulegone and menthone, the menthol content was very high; the trans-piperitone oxide content of the oil of fully mature leaves still exceeded 5%. Because of the exceptionally low content of menthofuran in these four LMF plants, they seemed to be cosuppressed (27, 28) for mfs and, as an apparent consequence, exhibited improved flux through PR to decrease the level of this intermediate and increase the content of the reduction products menthone (LMF immature leaves) and, ultimately, menthol (LMF mature leaves). Essential oil yields (in mg/g of frozen tissue) for LMF transformants ranged from 2.5 ± 0.46 to 3.3 ± 0.30 and was not significantly (P < 0.05) different from that of representative HMF (3.3 ± 0.48) or WT peppermint plants (2.9 ± 0.40), indicating that overall monoterpene flux was unaffected by these transformations.
In a final experiment of this type, the transgenic HMF and LMF plants and WT controls were grown under stress conditions (reduced photon flux density and increased night temperature) known to promote the accumulation of menthofuran and pulegone, with but modest influence on yield (10, 11, 15). Under these conditions, WT plants produce an oil (all leaves pooled) with 24% menthofuran and 35% pulegone whereas the LMF plants (pooled sample) produce very little menthofuran (<1% of the oil) and <10% pulegone (Table 1). In the HMF plants, the pooled oil content of menthofuran was exceptionally high (42%), and the pulegone content exceeded 22%. Thus, in all plants examined (WT and transgenic) under either growth condition, an increased menthofuran content was accompanied by a metabolically counterintuitive increase in the content of the precursor pulegone.
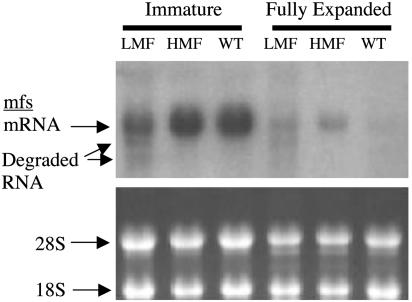
These analytical results suggested that mfs-overexpressed and mfs-cosuppressed transgenic mint had been obtained, and this supposition was confirmed by RNA blot analysis (Fig. 2), which showed that mfs was highly expressed in young leaves of WT and HMF plants [standardized signal intensities (SSI) of 0.83 ± 0.02 and 0.77 ± 0.01 for WT and HMF, respectively] whereas this transcript was much less abundant and underwent obvious degradation in young leaves of LMF plants (SSI of 0.37 ± 0.01), indicative of posttranscriptional gene silencing (28) in this case. In mature leaves, the mfs message (mfs driven by the CaMV 35S promoter) was measurable in HMF plants (SSI of 0.24 ± 0.01) and was detectable in LMF plants [barely visible (SSI of ≈0.08) and with degradation in this instance] but had disappeared entirely in the mature leaves of WT plants in which essential oil monoterpene biosynthesis had long since ceased (1, 2, 26). These data indicated that, in all cases in which the expression of mfs was transcriptionally manipulated, a positive correlation was observed between menthofuran content and the level of the mfs transcript, thus implying that menthofuran biosynthesis is controlled primarily by transcriptional regulation of mfs. The data also indicated that high level expression of mfs, possibly through the agency of menthofuran, adversely influenced the transcription and/or translation of the pr gene or inhibited the activity of PR itself to decrease flux through this central, downstream branch of the monoterpene biosynthetic pathway in peppermint and thereby promote the accumulation of pulegone (Fig. 1). In addition, the observation that stressed LMF plants accumulated considerable amounts of pulegone (≈10% of the oil, Table 1), although menthofuran levels in these plants were still low, indicates that additional factors regulate the flux of pulegone at this critical step. It should be noted here that expression from the CaMV 35S promoter is presumably constitutive but that metabolic consequences occur only within the glandular sites of essential oil biosynthesis. Because of the difficulties in obtaining comparable oil gland preparations from leaves of different stages of development (29), RNA blot analyses were conducted by using whole leaf extracts. All measurements were made relative to WT plants, which reflect only glandular content of the target message, and the consistency of blotting results with oil chemistry indicates that extrapolation to gland metabolism is appropriate and the conclusions justified.
Fig. 2.
mRNA levels for mfs in immature and fully expanded leaves of WT and transgenic peppermint plants that overexpress (HMF) or cosuppress (LMF) mfs. Total leaf RNA was resolved on an agarose-formaldehyde gel (5 μg per lane), blotted, hybridized to the radiolabeled mfs cDNA as probe, and exposed to x-ray film (Upper). (Lower) Ribosomal RNA bands visualized with ethidium bromide that were used to verify RNA loading before transfer.
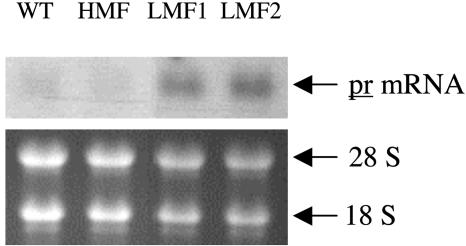
Effects of mfs Expression on PR. To examine the influence of mfs expression on the expression of the pr gene, the levels of the pr transcript were evaluated in immature leaves of HMF and LMF plants by RNA blot analysis using labeled PR cDNA (20, 21) as probe. These results (Fig. 3) indicated that pr transcript levels were significantly higher in LMF plants (SSI of 1.14 ± 0.03 and 1.92 ± 0.07 for LMF1 and LMF2, respectively) compared with WT (SSI of 0.35 ± 0.03) or HMF (SSI of 0.14 ± 0.01) plants, suggesting that expression of mfs (and the production of relatively high levels of menthofuran) results in the suppression of pr expression or the accelerated turnover of the pr message.
Fig. 3.
mRNA levels for pr in leaves of WT and transgenic peppermint plants that overexpress (HMF) or cosuppress (LMF) mfs. Total leaf RNA was resolved on an agarose-formaldehyde gel (5 μg per lane), blotted, hybridized to the radiolabeled pr cDNA as probe, and exposed to x-ray film (Upper). (Lower) Ribosomal RNA bands visualized with ethidium bromide that were used to verify RNA loading before transfer.
To examine the influence of mfs expression on PR itself, parallel assays were performed with partially purified enzyme preparations from immature leaves of WT, LMF, and HMF plants raised under optimum (unstressed) and stressed conditions. These results (Table 2) clearly indicated that reductase activity was substantially lower in HMF plants than in either WT or LMF plants grown under either environmental condition. These differences in activity levels provide a clear rationale for the elevation of pulegone production in HMF plants (and in WT plants under stress) by the diminished pathway flux through PR to menthone (Fig. 1). These results indicate that high-level expression of mfs (in HMF plants and in WT plants under stress) is responsible for the decrease in PR mRNA levels that correlates with a decrease in PR activity and consequent increase in pulegone level.
Table 2. PR activity in soluble protein extracts from immature leaves of WT peppermint plants and transgenic plants that overexpress (HMF) or cosuppress (LMF) mfs.
| Pulegone reductase activity, nmol·mg protein-1·h-1*
|
||
|---|---|---|
| Plant line | Stressed plants† | Unstressed plants† |
| WT | 1,133 ± 26 | 1,271 ± 110 |
| LMF | 3,406 ± 31 | 1,288 ± 13 |
| HMF | 433 ± 23 | 635 ± 26 |
Average rates ± SE are reported
Unstressed plants were grown under optimal conditions, and stressed plants were grown under reduced light and higher temperature
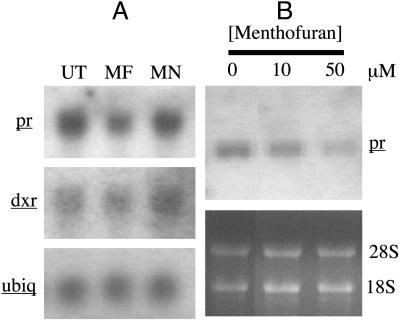
Effects of Menthofuran on PR. To determine whether the influences on PR resulting from mfs expression are mediated by menthofuran itself, the effects of exogenous (+)-menthofuran, and menthone as a control, on pr mRNA and activity levels were examined. RNA blot analysis, using labeled pr cDNA (20, 21) as probe, of young leaves of unstressed WT plants revealed that (+)-menthofuran, when stem fed at ≈5 mM, near saturation (30), notably reduced the content of pr message (SSI of 2.29 ± 0.04 and 1.41 ± 0.09 for untreated and menthofuran treated plants, respectively) (Fig. 4A). Parallel assays using cell-free preparations from the corresponding plants revealed that the decrease in pr mRNA levels resulting from application of menthofuran was accompanied by a similar decrease in PR activity (Table 3). Menthofuran had no effect on the transcript levels of dxr (SSI of 0.9 ± 0.06, and 1.03 ± 0.08, for untreated and menthofuran-treated plants, respectively), which is responsible for an early step in the supply of isoprenoid precursors (19) (Fig. 4A). Furthermore, application of menthone at 4.5 mM, near saturation (31), had no effect on the levels of transcripts for pr (SSI of 2.24 ± 0.10) or dxr (SSI of 1.03 ± 0.08) (Fig. 4A), or on the activity of PR (Table 3).
Fig. 4.
(A) mRNA levels for ubiquitin (ubiq), pr, and dxr in young leaves of untreated peppermint plants (UT) and plants treated with menthofuran (MF) or menthone (MN). Band intensities for dxr and pr were measured by densitometry and standardized to the signal for ubiquitin in each treatment group. Transcript levels for dxr were not affected by either treatment, whereas pr transcript levels were significantly reduced (P < 0.05) by menthofuran but not by menthone treatment. (B) pr transcript levels in immature leaves of unstressed peppermint plants treated with aqueous solutions containing 0, 10, or 50 μM menthofuran. Signal intensities for the pr mRNA bands were standardized to those for the 28S ribosomal RNA bands in corresponding samples.
Table 3. Pulegone reductase activity in soluble protein extracts from immature leaves of WT peppermint shoots fed with water alone (Control) or water containing saturating concentrations of menthone (MN) or menthofuran (MF).
Average rates ± SE are reported
Not significantly different (P > 0.06) from control
Significantly different (P < 0.05) from control
In a separate experiment, peppermint plants grown under optimal conditions were sprayed with aqueous solutions containing various concentrations of menthofuran. The results demonstrated that foliar application of menthofuran to intact plants also resulted in a decrease in pr mRNA abundance. Thus, pr transcript levels were significantly (P < 0.05) lower in plants treated with aqueous solutions containing 10 and 50 mM menthofuran (SSI of 3.2 ± 0.02 and 1.79 ± 0.22, respectively) than those treated with the vehicle alone (SSI of 4.41 ± 0.02), as evidenced by Northern blot analysis of extracted total RNA (Fig. 4B).
The possible inhibition of PR by (+)-menthofuran was also examined directly in partially purified preparations of the enzyme, at concentrations of menthofuran ranging from 5 to 200 μM and with substrate concentrations ranging from 5 μMto100 μM (data not shown). These results showed that menthofuran did not significantly influence the activity of PR in vitro.
Origin of Monoterpene Epoxyketones. As noted above, the LMF plants (cosuppressed for mfs) produced readily measurable amounts of the epoxyketones trans-piperitone oxide and piperitenone oxide (Table 1). These epoxyketones are reported infrequently in peppermint, and the total content very rarely exceeds a few percent of the distilled essential oil (32). The biosynthesis of these compounds in mint has been described (22) and has been shown to involve the isomerization of isopiperitenone to piperitenone by isopulegone isomerase, followed by cytochrome P450-dependent epoxidation of piperitenone to (+)-piperitenone oxide, then NADPH-dependent reduction of the Δ4,8-double bond to (-)-trans-piperitone oxide by PR (Fig. 1) (23, 33). The NADPH/O2-dependent epoxidase capable of converting piperitenone to (+)-piperitenone oxide was demonstrated in microsomal preparations from immature leaves of LMF transgenics. These microsomal preparations also catalyzed the epoxidation of (+)-piperitone to an oxide of undefined stereochemistry (which was not detected in the essential oil). Neither epoxidase activity was detected in microsomal preparations from WT plants, indicating that the expression of the epoxidase(s) is suppressed in WT peppermint in which mfs is expressed. The NADPH-dependent reductase that catalyzes the conversion of (+)-piperitenone oxide to (-)-trans-piperitone oxide was detected in soluble enzyme preparations from both WT and LMF plants. Therefore, it can be concluded that the absence of (-)-trans-piperitone oxide in WT plants is due to the absence of the relevant intermediate (+)-piperitenone oxide; the direct epoxidation of (+)-piperitone does not seem to be of biological significance. In addition to the epoxidase, this metabolic diversion may also relate to alteration in the isopiperitenone reductase that could limit conversion to isopulegone or to an influence on the isomerase that mediates conversion to piperitenone as the precursor of the oxides (Fig. 1). The cytochrome P450 epoxidase and the isomerase have not been well characterized, and the corresponding genes have not been identified; therefore, the available tools for defining the context for epoxidase and isomerase expression are limited. Thus, although the biochemistry of the epoxyketones is defined, the molecular origins of these compounds as a consequence of mfs cosuppression are uncertain.
Conclusions. In the absence of menthofuran, resulting from cosuppression of mfs, PR is readily synthesized, resulting in high throughput of this critical intermediate step to process pulegone and increase essential oil content of the derived products menthone and menthol. Conversely, expression (particularly overexpression) of mfs, which results in the production of high levels of menthofuran, leads to a notable decrease of pr message, accompanied by a decrease in reductase activity, with the consequence of increasing pulegone concentration in the oil. This effect is mediated by menthofuran itself, as demonstrated by feeding experiments with WT plants, and it seems to be specific to PR. DXR, which catalyzes the committed step of the DXP pathway of plastidial isoprenoid biosynthesis and is rate-limiting in monoterpene biosynthesis in mint (15), is unaffected by application of exogenous menthofuran.
These results provide an unusual example of a small molecule (which is regarded as a dead-end metabolite; see Fig. 1) acting to down-regulate (at the mRNA level) a downstream step of an extended biosynthetic pathway. The data are presently insufficient to determine whether the effects of menthofuran as a regulator of PR are direct or involve other intermediaries, or whether these effects are exerted at the levels of pr transcription or translation, or pr message stability. Although the mechanistic basis of this unusual “feed-forward inhibition” phenomenon and its possible physiological consequences are not understood, these findings have important application in the production of commercial mint oils of high quality, and they may have broad implications for the control of natural products biosynthetic pathways. More detailed studies on the control of PR are clearly warranted.
Acknowledgments
We thank Robert Long and Matthew Williams for technical assistance, Joyce Tamura for typing the manuscript, Julianna Gothard and Craig Whitney for raising the plants, and Gregory Biza (I.P. Callison & Sons, Chehalis, WA) and Norman W. Rowe (William Leman Co., Bremen, IN) for the gifts of monoterpene standards. The work was supported in part by the Department of Energy (Division of Energy Biosciences), the Mint Industry Research Council, and the Agricultural Research Center, Washington State University (Project No. 0268).
Abbreviations: DXR, deoxyxylulose phosphate reductoisomerase; PR, pulegone reductase; MFS, menthofuran synthase; HMF, high menthofuran; LMF, low menthofuran; SSI, standardized signal intensity.
References
- 1.Gershenzon, J., McConkey, M. & Croteau, R. (2000) Plant Physiol. 122, 205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McConkey, M., Gershenzon, J. & Croteau, R. (2000) Plant Physiol. 122, 215-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gershenzon, J., Maffei, M. & Croteau, R. (1989) Plant Physiol. 89, 1351-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCaskill, D., Gershenzon, J. & Croteau, R. (1992) Planta 187, 445-454. [DOI] [PubMed] [Google Scholar]
- 5.Croteau, R. & Gershenzon, J. (1994) Recent Adv. Phytochem. 28, 193-229. [Google Scholar]
- 6.Guenther, E. (1961) Perfum. Ess. Oil. Rec. 1961, 632-642. [Google Scholar]
- 7.Court, W. A., Roy, R. C. & Pocs, R. (1993) Can. J. Plant Sci. 73, 815-824. [Google Scholar]
- 8.Bertea, C. M., Schalk, M., Karp, F., Maffei, M. & Croteau, R. (2001) Arch. Biochem. Biophys. 390, 279-286. [DOI] [PubMed] [Google Scholar]
- 9.Wise, M. L. & Croteau, R. (1999) in Comprehensive Natural Products Chemistry: Isoprenoids Including Carotenoids and Steroids, ed. Cane, D. E. (Elsevier Science, London), Vol. 2, pp. 97-153. [Google Scholar]
- 10.Burbott, A. J. & Loomis, W. D. (1967) Plant Physiol. 42, 20-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark, R. J. & Menary, R. C. (1980) Aust. J. Plant Physiol. 7, 685-692. [Google Scholar]
- 12.Voirin, B., Brun, N. & Bayet, C. (1990) Phytochemistry 29, 749-755. [Google Scholar]
- 13.Brun, N., Colson, M., Perrin, A. & Voirin, B. (1991) Can. J. Bot. 69, 2271-2278. [Google Scholar]
- 14.Turner, G. W., Gershenzon, J. & Croteau, R. B. (2000) Plant Physiol. 124, 655-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahmoud, S. S. & Croteau, R. (2001) Proc. Natl. Acad. Sci. USA 98, 8915-8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.An, G. (1987) Methods Enzymol. 153, 293-305. [Google Scholar]
- 17.Niu, X., Lin, K., Hasegawa, P. M., Bressan, R. A. & Weller, S. C. (1998) Plant Cell Rep. 17, 165-171. [DOI] [PubMed] [Google Scholar]
- 18.Niu, X., Li, X., Veronese, P., Bressan, R. A., Weller, S. C. & Hasegawa, P. M. (2000) Plant Cell Rep. 19, 304-310. [DOI] [PubMed] [Google Scholar]
- 19.Lange, B. M. & Croteau, R. (1999) Arch. Biochem. Biophys. 365, 170-174. [DOI] [PubMed] [Google Scholar]
- 20.Lange, B. M., Wildung, M. R., Stauber, E. J., Sanchez, C., Pouchnik, D. & Croteau, R. (2000) Proc. Natl. Acad. Sci. USA 97, 2934-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McConkey, M. (2001) Ph.D. thesis (Washington State Univ.).
- 22.Croteau, R., Karp, F., Wagschal, K. C., Satterwhite, D. M., Hyatt, D. C. & Skotland, C. B. (1991) Plant Physiol. 96, 744-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Croteau, R. & Venkatachalam, K. V. (1986) Arch. Biochem. Biophys. 249, 306-315. [DOI] [PubMed] [Google Scholar]
- 24.Dixon, M. & Webb, E. C. (1964) Enzymes (Academic, New York), 2nd Ed., pp. 328-331.
- 25.Platt, S. G. & Yang, N. S. (1987) Anal. Biochem. 162, 529-535. [DOI] [PubMed] [Google Scholar]
- 26.Turner, G. W., Gershenzon, J. & Croteau, R. B. (2000) Plant Physiol. 124, 665-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smyth, D. R. (1997) Curr. Biol. 7, 793-795. [DOI] [PubMed] [Google Scholar]
- 28.Vaucheret, H., Ingelbrecht, I., VanMontagu, M. & Depicker, A. (1998) Plant J. 16, 651-659.10069073 [Google Scholar]
- 29.Gershenzon, J., McCaskill, D., Rajaonarivony, J. I. M., Mihaliak, C., Karp, F. & Croteau, R. (1992) Anal. Biochem. 200, 130-138. [DOI] [PubMed] [Google Scholar]
- 30.Smyrl, T. G. & Le Maguer, M. (1980) J. Chem. Eng. Data 25, 150-152. [Google Scholar]
- 31.Weidenhame, J. D., Macias, F. A., Fischer, N. H. & Williamson, G. B. (1993) J. Chem. Ecol. 19, 1799-1807. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence, B. M. (1978) Ph.D. thesis (Rijksuniversiteit Groningen).
- 33.Kjonaas, R. B., Venkatachalam, K. V. & Croteau, R. (1985) Arch. Biochem. Biophys. 238, 49-60. [DOI] [PubMed] [Google Scholar]