Summary
The traditional view of hematopoiesis has been that all the cells of the peripheral blood are the progeny of a unitary homogeneous pool of hematopoietic stem cells (HSCs). Recent evidence suggests that the hematopoietic system is actually maintained by a consortium of HSC subtypes with distinct functional characteristics. We show here that myeloid-biased HSCs (My-HSCs) and lymphoid-biased (Ly-HSCs) can be purified according to their capacity for Hoechst dye efflux in combination with canonical HSC markers. These phenotypes are stable under natural (aging) or artificial (serial transplantation) stress and are exacerbated in the presence of competing HSCs. My- and Ly-HSCs respond differently to TGFβ1, presenting a possible mechanism for differential regulation of HSC subtype activation. This study demonstrates definitive isolation of lineage-biased HSC subtypes and contributes to the fundamental change in view that the hematopoietic system is maintained by a continuum of HSC subtypes, rather than a functionally uniform pool.
Introduction
Multipotent long-term HSCs (LT-HSCs) reside in the bone marrow and self-renew to sustain the stem cell pool and differentiate into short-term HSCs (ST-HSCs) or lineage-restricted progenitors that undergo extensive proliferation and differentiation to produce terminally-differentiated hematopoietic cells. Although various methods are used for HSC purification (Challen et al., 2009), ultimately, HSCs are defined not by phenotype, but by function in terms of hematopoietic reconstitution in bone marrow transplantation assays (Szilvassy et al., 1990; Spangrude et al., 1995). LT-HSCs can be operationally defined by the ability to contribute to greater than 1% of circulating white blood cells long-term (>16-weeks) after transplantation, with generation of myeloid and lymphoid progeny at levels >1% as an indicator of extensive self-renewal ability (Miller and Eaves, 1997; Ema et al., 2005; Dykstra et al., 2006). Since HSCs were first identified, the traditional view has been that the hematopoietic system is regenerated by a single pool of quiescent LT-HSCs that are recruited as needed. However, HSCs show heterogeneous behavior at the clonal level (Lemischka et al., 1986; Jordan and Lemischka, 1990; Smith et al., 1991), and recent studies suggest that the adult HSC compartment consists of a number of functionally distinct subsets with distinct self-renewal and differentiation potentials (Dykstra et al., 2007; Sieburg et al., 2006; Wilson et al., 2008).
By serial transplantation of single HSCs and their progeny, Dykstra et al demonstrated that HSC activity could be classified by four behaviors according to their lineage differentiation capacity as well as the length of time over which they could contribute to high levels of blood production (Dykstra et al., 2007). Another group proposed three classes of HSCs based on repopulation kinetics of mice transplanted with limiting dilutions of whole bone marrow – myeloid-biased (My-bi), lymphoid-biased (Ly-bi) and balanced HSCs (Bala) that generated myeloid and lymphoid cells in the same ratio as seen in the blood of unmanipulated mice (Muller-Sieburg et al., 2002; Muller-Sieburg et al., 2004; Sieburg et al., 2006). The behaviors of HSC subtypes are relatively stable over long periods in vivo, but are rapidly eroded in vitro (Wineman et al., 1996, Lemieux, 1996 #587). While these studies provide evidence for the existence of HSC subpopulations in terms of their functional properties, further understanding of the molecular mechanisms that empower each subset with their distinctive characteristics is impeded by a lack of approaches for their facile purification.
Our laboratory typically uses Hoechst 33342 staining to identify HSCs, which reside in the so called side population (SP) (Goodell et al., 1996). Although cells within the SP are very similar in terms of expression of canonical stem cell markers, it has been shown that cells from different regions of the SP possess different functional potentials, particularly over long periods of time (Goodell et al., 1997; Camargo et al., 2006). We recently reported heterogeneous expression of the signaling lymphocytic activation molecule (SLAM) family molecule CD150 within the SP, with CD150+ cells more prevalent in the lower-SP (Weksberg et al., 2008), suggesting this marker may help delineate HSC subtypes. This is consistent with the finding that CD150+ HSCs exhibit the highest long-term HSC activity correlating with persistent myelopoiesis (Kent et al., 2009).
Given these reports of functional diversity of phenotypically homogeneous HSCs, we sought to determine if Hoechst dye efflux could be used to discriminate different HSC subtypes. We demonstrate that lineage-biased HSCs can be prospectively isolated according to their capacity for dye efflux with further augmentation of this purification strategy using selection with CD150. By using the combination of SP and CD150, we show a very clear gradient of HSCs with distinct phenotypic, functional and molecular characteristics. We also identify the TGFβ signaling pathway as a potential mechanism of differential regulation between HSC subtypes and show that TGFβ1 exacerbates these functional differences in vitro and in vivo.
Results
Phenotypic Diversity of Cells From Different Regions of the Side Population
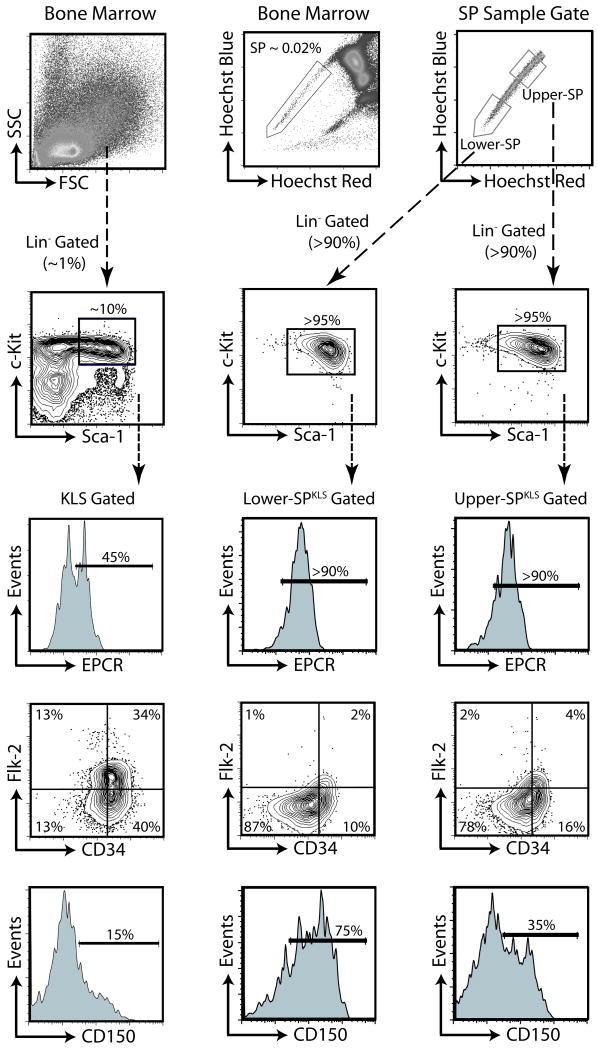
We typically purify HSCs by the phenotype of Side Population+ c-Kit+ Lineage- Sca-1+ (Figure S1A; henceforth called SPKLS). The SP contains virtually all of the long-term HSC activity (Goodell et al., 1996). Despite similar surface marker staining along the SP, there appears to be a gradient of functional activity along the SP (Goodell et al., 1997). Flow cytometric analysis of the regions designated as lower-SP and upper-SP (Figure 1) are extremely similar, and are essentially phenotypically indistinguishable from HSCs isolated using classical schemes (KLS Flk-2- CD34-) or newer markers (EPCR+ CD48-), although lower-SP cells have slightly brighter expression of Sca-1 and EPCR and slightly dimmer expression of Flk-2 and CD34 by mean fluorescent intensity (Figure S1B). In contrast, as previously reported (Weksberg et al., 2008), CD150 shows a bi-modal distribution on SPKLS cells, with the lower-SPKLS containing a higher frequency of CD150+ cells.
Figure 1.
Phenotypes of SP subtypes.
Left column shows surface marker HSC-identification strategy from whole bone marrow based on lineage-negative, c-kit, Scal-1 expression, as well as Flk-2 and CD34. Top middle panel demonstrates delineation of SP. Top right shows regions designated as lower-SP and upper-SP and below that, c-kit and Sca-1 cells expression of cells gated on these and through a lineage-negative gate. Surface markers on these cells, designated as SPKLS are shown below. CD150 expression is heterogeneous, with CD150+ cells more prevalent in the lower-SP (see also Figure S1).
Transplanted SP Subfractions Show Different Hematopoietic Lineage Outputs
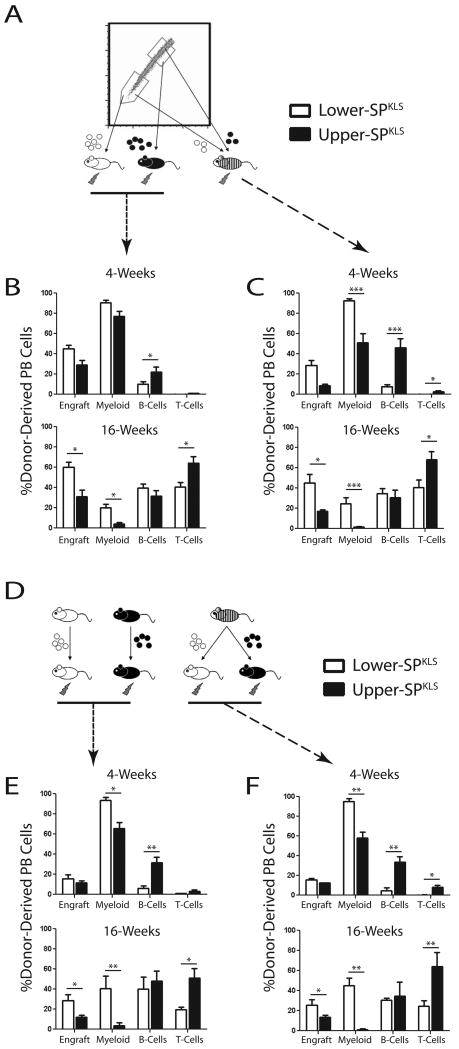
We investigated the functional characteristics of HSCs isolated from different regions of the SP by competitive transplantation assays, the gold standard for quantifying HSC activity. Competitive transplantations were performed such that test and recipient cell populations were distinguishable by combinations of CD45 alleles. Lethally irradiated recipients were transplanted with 200,000 competitor whole bone marrow cells along with either 200 lower-SPKLS cells or 200 upper-SPKLS cells (Figure 2A). SP sorting gates were set well apart to minimize cross-contamination. Peripheral blood was drawn every four weeks post-transplant to monitor donor cell engraftment and lineage distribution (Figure S2A).
Figure 2.
Repopulation kinetics of transplanted SP subtypes.
(A) Competitive transplant scheme for lower- and upper-SPKLS cells individually (200 per recipient) or in combination (100 of each subtype per recipient), with 200,000 whole bone marrow competitor cells in all transplants. (B) Overall peripheral blood contribution (Engraft) and proportional contribution to myeloid, B- and T-cells at 4- and 16-weeks after transplantation of SP fractions into separate mice. (C) Peripheral blood analysis after transplantation of both upper- and lower-SP into the same recipients. (D) Scheme for re-purification of primary HSCs and transplantation into secondary recipients. (E) Contribution to the blood of SP sub-populations after secondary transplantation at 4- and 16-weeks. (F) Reconstitution of blood in secondary recipients by SP originally co-transplanted in primary transplants (see also Figure S2A).
Multiple cohorts of bone marrow transplants experiments recapitulated previous work demonstrating higher long-term engraftment of lower-SPKLS cells compared to upper-SPKLS (approximately 2.5-fold higher peripheral blood chimerism 16-weeks post-transplant). However, we observed a striking previously unreported bias in terms of the hematopoietic lineage output of transplanted SP fractions (Figure 2B). While both lower- and upper-SPKLS cells were capable of forming all hematopoietic cell types, lower-SPKLS cells were biased towards myeloid differentiation while upper-SPKLS cells were biased towards lymphoid differentiation (B- and T-cells). At short-term timepoints (4-weeks post-transplant), lower-SPKLS cells generated significantly more myeloid cells (90.4 ± 2.9% vs 77.0 ± 5.2%) while upper-SPKLS cells produced significantly more B-cells (21.9 ± 5.3% vs 9.9 ± 2.8%). At long-term timepoints (16-weeks), lower-SPKLS cells retained a higher myeloid output (20.0 ± 3.3% vs 4.3 ± 1.4%), but upper-SPKLS cells generated significantly more T-cells (64.3 ± 6.4% vs 40.4 ± 4.5%) while the B-cell output of both populations was not significantly different. This showed that both populations have the potential to make all hematopoietic cell types, but they were inherently biased towards production of a particular hematopoietic branch.
Co-Transplantation of Competing SP Subfractions Enhances Lineage Biases
We next wondered whether the SP subfractions would behave differently in the presence of the other subtype. We repeated the transplantation experiments by co-transplanting 100 lower-SPKLS cells plus 100 upper-SPKLS cells in the presence of 200,000 whole bone marrow competitor cells (all distinguishable by CD45 alleles) into lethally irradiated mice. The lineage biases observed from transplantation of one SP subfraction alone held true when the two SP subfractions were co-transplanted into the same recipient animal, however the differences were magnified (Figure 2C). This suggests that while both populations are capable of forming all hematopoietic lineages, in the presence of the other HSC subtype (as would be the case in normal physiology), the majority of the output from each HSC subtype is strongly predisposed towards the differentiation program toward which they are more inherently inclined.
Lineage Biases of SP Subfractions are Retained Upon Serial Transplantation
In order to determine if the hematopoietic outputs of HSC subtypes was a stable phenotype and to establish both were true HSCs by definition of self-renewal, secondary transplantation of purified primary HSCs was performed. At >18-weeks post-transplant, donor HSCs from the bone marrow of primary recipients were re-purified (SPKLS + appropriate CD45 allele) and 200 were transplanted into secondary recipients along with 200,000 fresh whole bone marrow competitor cells. From primary recipients that had been co-transplanted with both lower- and upper-SPKLS cells, each donor HSC population was isolated and re-transplanted as individual populations into secondary recipients (Figure 2D). In all cases, all populations were still capable of generating all lineages in secondary recipients. Notably, the lineage biases initially observed were recapitulated upon secondary transfer, with lower-SPKLS cells generating more myeloid cells and upper-SPKLS cells producing more lymphoid cells (Figure 2E). In the primary transplants which received both HSC subtypes, the lineage output was comparable to secondary recipients receiving individually transplanted primary lower- or upper-SPKLS cells (Figure 2F). In other words, the differentiation biases remained the same, but were not as extreme as when they had been competed against purified HSCs of the other subtype.
These data indicate that both lower-SPKLS and upper-SPKLS are bona fide HSCs by definition of long-term, multi-lineage reconstitution (generating >1% white blood cells >16-weeks post-transplant and >1% contribution to both myeloid and lymphoid branches in primary and secondary recipients). However each population has an inherent bias towards generation of a particular hematopoietic branch that is maintained over multiple transplant generations. Statistical measures from all transplantation studies can be found in Table S1.
SP Subtypes Generate Different Proportions of Lineage-Specific Progenitors
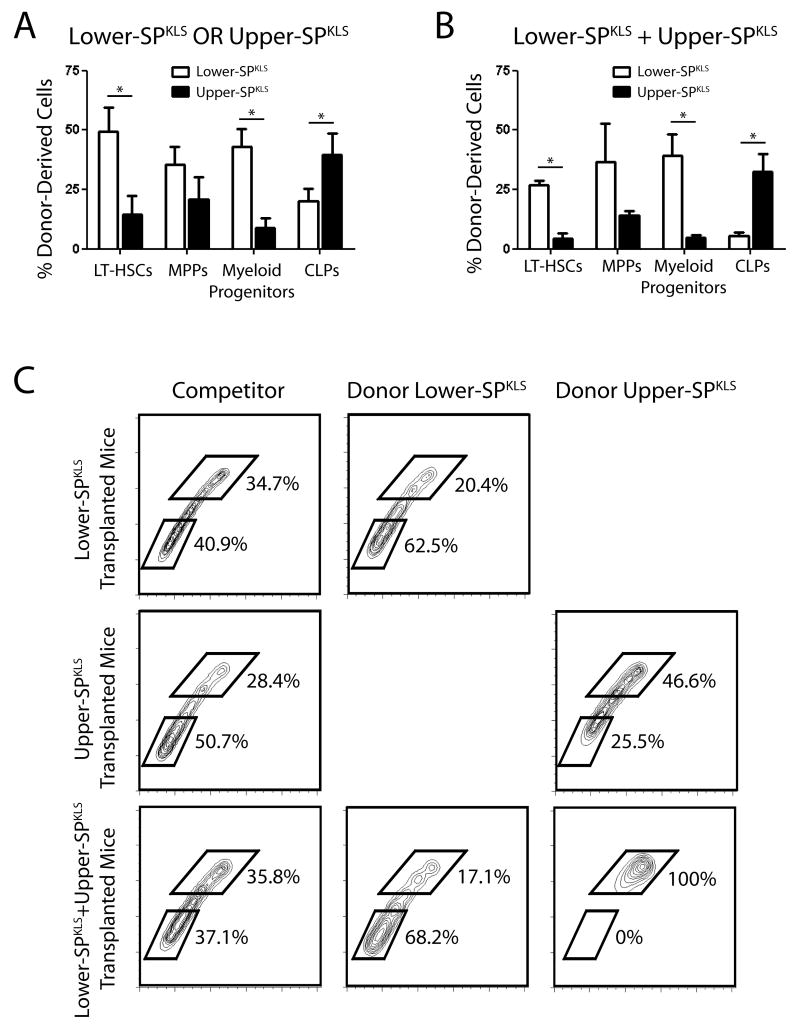
To determine if the lineage biases observed as mature hematopoietic cells in the peripheral blood resonated in hematopoietic progenitor populations, transplant recipients were sacrificed after long-term engraftment and donor cell contribution to stem and progenitor cell compartments was examined (Figure 3A). The higher levels of peripheral blood chimerism in mice transplanted with lower-SPKLS cells was reflected in a greater contribution to recipient LT-HSC (49.6 ± 9.7% vs 14.4 ± 8.1%) and MPP (35.4 ± 7.7% vs 20.9 ± 9.3%) compartments. The lineage biases in the peripheral blood were propagated from differences to contributions to the earliest committed progenitors with donor lower-SPKLS cells generating significantly more myeloid progenitors (MPs; 43.1 ± 7.6% vs 9.0 ± 4.1%) and upper-SPKLS producing more common lymphoid progenitors (CLPs; 39.5 ± 9.3% vs 20.2 ± 5.3%) in transplanted mice. In mice that were co-transplanted with both lower- and upper-SPKLS cells, the biases for lineage-specific progenitors were the same as for mice transplanted with individual HSC subtypes, although the differences were magnified.
Figure 3.
Donor-derived contribution to stem and progenitor cell compartments of transplant recipients.
Analysis of the bone marrow of recipient mice to determine donor cell contribution to stem and progenitor cell compartments 18-weeks post-transplant of SP subtypes transplanted separately (A) and into the same recipients (B). (C) Analysis of the donor cell contribution to SP of recipient mice. In reference to the whole bone marrow competitor cells which serve as an internal control, each SP subfraction tended to regenerate itself. Data presented are averages of three separate pooled experiments (see also Figure S2B).
Transplanted SP Subfractions Regenerate Themselves in Recipient Mice
Because the SP appears to allow HSC subtypes to be distinguished, we asked whether the HSC subtypes would preferentially repopulate the SP regions from which they were originally derived in the donor mice. Analysis of the SP profile of recipient mice, using the appropriate CD45 marker, showed striking differences in the regional contribution of donor cells to recipient SP fractions (Figure 3B). Donor lower-SPKLS cells tended to generate more lower-SP cells in recipient mice compared to internal control competitor (63.8% ± 3.0% vs 37.9 ± 3.0%) while the converse was true with donor upper-SPKLS cells generating more upper-SP compared to competitor (48.8 ± 2.3% vs 26.4 ± 1.2%). A much more dramatic effect was noticed in mice co-transplanted with both SP subfractions with each cell population strongly biased to regenerate the same population in the SP of the recipient animals. In co-transplanted mice, donor lower-SPKLS cells generated the majority of lower-SP cells (72.5 ± 5.3%) while donor upper-SPKLS cells almost exclusively generated upper-SP cells (95.8 ± 3.8%). In comparison, transplantation of non-fractionated SPKLS regenerates the SP in the same proportion as whole bone marrow competitor (Figure S2B). The determination that each transplanted SP subfraction tended to regenerate itself in the SP of recipient mice argues that these cell populations exist as distinct HSC subtypes in the bone marrow, with self-renewal to generate daughter HSCs that maintain the inherent properties of the parent.
CD150 Expression Augments the Discrimination of Lineage-Biased HSCs
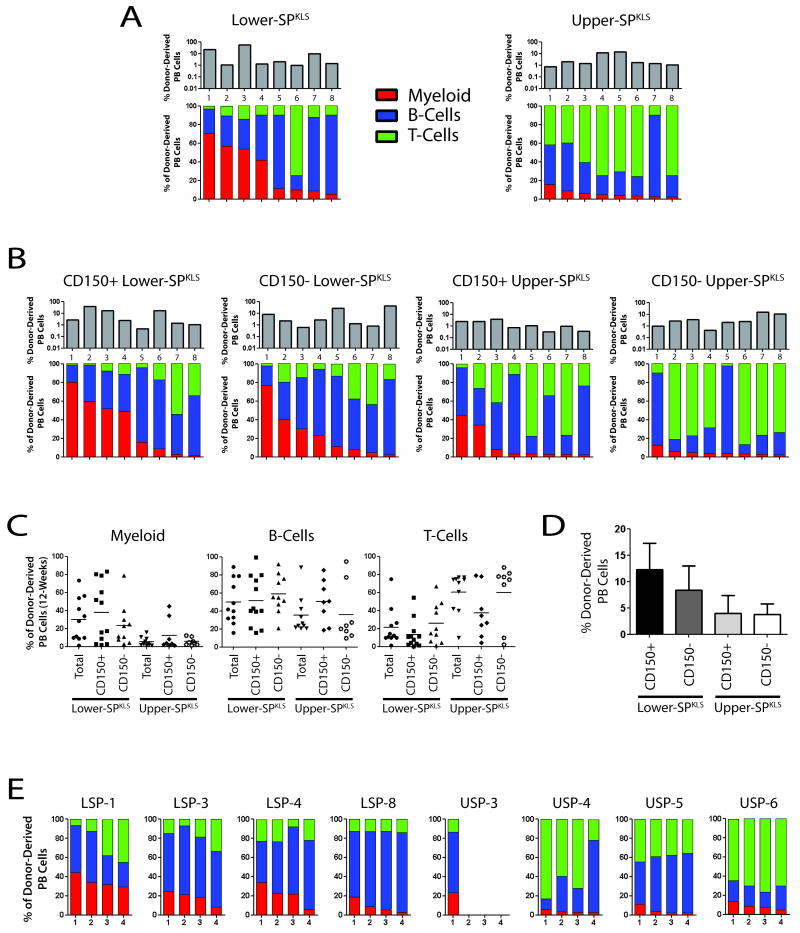
To ensure that the results described above were attributable to inherent differences in HSCs, and not simply impurities in the isolated populations, we repeated these experiments with single purified HSCs. Single lower- or upper-SPKLS HSCs were co-transplanted into lethally irradiated recipient mice along with 1 × 106 compromised (once-transplanted) whole bone marrow competitor cells; stable long-term engraftment was defined as at least 0.1% white blood cell contribution from test donor cells with tri-lineage contribution from donor-derived cells minimally at >1% myeloid, B- and T-cells >12-weeks post-transplant. In three separate cohorts of single-cell transplant experiments, the lineage biases observed with the bulk HSCs were evident and accentuated. Short times after transplant (4-weeks), single lower-SPKLS cells predominantly generated myeloid cells, whereas upper-SPKLS cells were much more effective at generating B-cells (Figure S3A) (T-cell development typically lags behind that of B- and myeloid cells). Twelve-weeks after transplantation, clones from both regions effectively generated all three lineages, but with pronounced myeloid and lymphoid biases for the lower-SPKLS and upper-SPKLS respectively (Figure 4A). The frequency of functional HSC, as measured by the proportion of mice successfully engrafted with a single HSC and showing tri-lineage contribution at 12-weeks after transplantation was 27.7% in the lower-SPKLS fraction (18 positive/65 transplanted mice) and 19.7% in the upper-SPKLS (15 positive/76 transplanted mice). This is comparable to that reported for other highly purified populations (Uchida et al., 2003; Matsuzaki et al., 2004; Takano et al., 2004; Camargo et al., 2006; Kiel et al., 2009).
Figure 4.
Clonal analysis of HSC subtypes using single-cell transplantation.
(A) Tracking of eight individual lower- and upper-SPKLS clones showing overall hematopoietic chimerism in recipient mice (engraftment; top panels in grey) and lineage distribution of test cells 12-weeks post-transplant. (B) Analysis of overall engraftment and lineage contribution of lower- and upper-SPKLS cells also fractionated with respect to CD150. (C) Distribution of lineage contribution 12-weeks after transplant from mice transplanted with single HSCs with the indicated phenotypes. (D) Average level of hematopoietic chimerism from single-cell transplantation experiments. (E) Lineage contribution measured 12 weeks after secondary transplantation of cells derived from primary mice reconstituted with a single HSC. Bone marrow from each clone was tested in four new recipients. All clones met the assigned threshold of at least 0.1% overall contribution to all 4 secondary recipients at 12-weeks post-transplant, with the exception of USP-3, in which 3/4 recipients fell just below the assigned threshold (see also Figure S3).
Given the recent evidence for CD150 expression correlating with HSC self-renewal (Kiel et al., 2005; Kent et al., 2009) and our report showing heterogeneous expression of CD150 in the SP (Weksberg et al., 2008), we repeated single-cell transplants with lower- or upper-SPKLS HSCs that were further subfractionated on the basis of CD150 expression. The results paralleled the above findings with some subtle differences. Short-term post-transplant, CD150- lower-SPKLS showed a marginal improvement in T-cell generation compared to their CD150+ counterparts, at the expense of myeloid cell production. Similarly, CD150+ upper-SPKLS showed, on average, enhanced myeloid cell production relative to CD150- upper-SPKLS (Figure S3B). Long-term post-transplant (>12-weeks), both CD150+ lower- and upper-SPKLS cells showed a greater propensity for myeloid cell production than their CD150− counterparts (Figure 4B). However, the effect of CD150 subfractionation was incremental compared to selection on the basis of Hoechst dye efflux, as both CD150+ and CD150− lower-SPKLS HSCs were more myeloid-biased than upper-SPKLS, irrespective of their CD150 status. Worth noting is the considerable degree of heterogeneity in lineage output on a clonal level even in these highly subfractionated bone marrow cell populations, particularly in long-term B-cell output (Figure 4C). Generally, higher B-cell development at early time-points is a harbinger of a lymphoid-biased clone, while high myeloid development heralds a myeloid-biased HSC; long-term B-cell development is generally relatively robust in both types.
Fractionation of SP subtypes by CD150 expression did not dramatically alter the HSC frequency in each test population. We could observe a clear gradient in HSC frequency with CD150+ lower-SPKLS having the highest HSC frequency (12 positive/45 transplanted mice; 26.7%), followed by CD150− lower-SPKLS (11/43; 25.6%), CD150+ upper-SPKLS (8/41; 19.5%) and CD150− upper-SPKLS (8/43; 18.6%). This is mirrored by a gradient in their average overall contribution to peripheral blood generation (Figure 4D). Single-cell transplantation is generally believed to underestimate the actual frequency of HSCs within a test population due to engraftment dynamics, and thus the true HSC frequency in these populations may be higher. It is not possible to determine whether these frequencies represent inherent limitations in the assay, or remaining heterogeneities in the population that additional markers will ultimately allow us to discern.
To conclusively examine the ability of single lower- and upper-SPKLS to regenerate HSC in the primary transplanted mice (self-renewal), we transplanted their progeny into secondary hosts and examined blood lineage regeneration (Figure 4E). From a cohort transplanted irrespective of CD150 status (shown in Figure 4A), we selected four lower- and four upper-SPKLS clones which showed >1% contribution to all hematopoietic lineages. Bone marrow from each of these recipients was harvested and transplanted into four secondary recipients. Of the lower-SPKLS donors, one was a highly myeloid-biased clone (LSP-1, presumptive CD150+), two were quasi-balanced clones (LSP-3 and-4) and one was a lymphoid-biased clone (LSP-8, presumptive CD150-). All secondary recipient mice showed engraftment with clone 1 retaining myeloid-bias, clones 3 and 4 showing a quasi-balanced lineage output and clone 8 retaining strong lymphoid-skewing. All of the upper-SPKLS donors were lymphoid-biased, and all secondary recipient mice showed tri-lineage engraftment except for those transplanted with bone marrow from USP clone 3, only one recipient of which reached our assigned threshold for calling successful engraftment of 0.1% (the other three being just below). Thus, the repopulation behavior measured in secondary recipients of clonally-derived upper-SPKLS cells replicated the lymphoid lineage-bias of their parent clone, and were bona fide HSC.
Aging Changes the Clonal Composition of the HSC Compartment due to a Relative Expansion of Myeloid-Biased Lower-SPKLS Cells
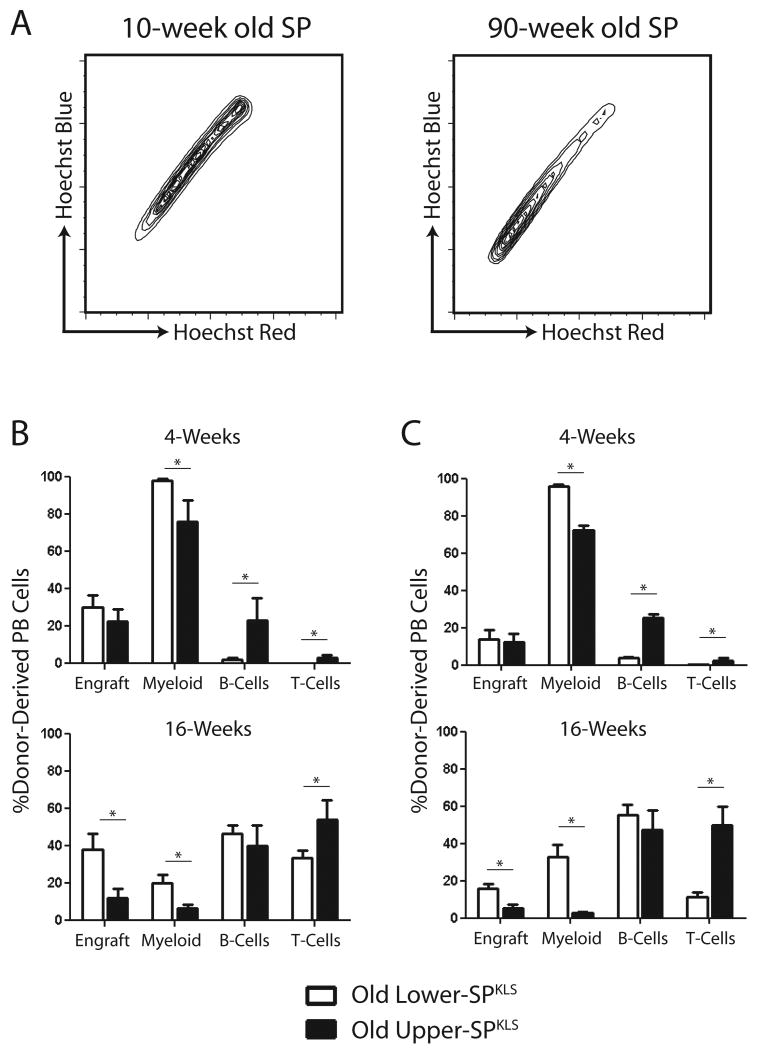
Our lab and others have shown that aging mouse HSCs show a decline long-term reconstituting potential (Chambers et al., 2007b; Rossi et al., 2007) and a deficit in lymphopoiesis (Sudo et al., 2000; Rossi et al., 2005). However, recent studies suggest that aging changes the clonal composition of the HSC compartment but does not alter the inherent properties of HSCs (Cho et al., 2008; Roeder et al., 2008). This implies that the age-related deficit in lymphopoiesis may not be due to a decline in lymphoid potential from HSCs, but rather due to a relative loss of lymphoid-biased HSCs over time. Strikingly, in the bone marrow of old mice there is an accumulation of lower-SP cells in the region we show to be enriched for myeloid-biased HSCs (Figure 5A). Competitive transplantation of bulk aged lower- and upper-SPKLS was performed to determine if the lineage potentials of HSC subtypes is altered with aging.
Figure 5.
Aging SP subtypes retain lineage-differentiation biases.
(A) Contour plot comparisons of young (10-week old) and old (90-week old) SPs showing an accumulation of lower-SP cells in aged mice. (B) Overall peripheral blood contribution (Engraft) and proportional contribution to myeloid, B- and T-cells at 4- and 16-weeks after transplantation of old and young SP fractions. (C) Peripheral blood contribution after secondary transplantation from recipients of aged lower- and upper-SPKLS cells.
In agreement with previous studies, aged HSCs were not as potent as their young counterparts in terms of long-term hematopoietic engraftment. However, analysis of the hematopoietic lineages formed from donor old lower- and upper-SPKLS cells mirrored that of young lower- and upper-SPKLS cells in both primary and secondary recipients (Figure 5B-C). Old lower-SPKLS had significantly greater myeloid potential (32.9% ± 9.8% vs 2.6 ± 0.9% 16-weeks post-transplant in secondary recipients) while upper-SPKLS showed greater propensity for lymphoid generation in terms of B-cells at short-term timepoints (32.1% ± 12.7% vs 2.0 ± 0.9% 4-weeks post-transplant in primary recipients) and T-cells at long-term timepoints (49.8% ± 9.9% vs 11.5 ± 2.1% 16-weeks post-transplant in secondary recipients). As with transplanted young SP fractions, old donor lower-SPKLS cells contributed more to LT-HSC, MPP and myeloid progenitor compartments in the bone marrow of primary recipients (Table S1) while old donor upper-SPKLS cells produced significantly more CLPs (22.1% ± 6.1% vs 3.3 ± 0.5%).
These data argue that aging does not alter the inherent lineage potential of individual HSCs, but rather the myeloid-bias seen in aging HSCs is due to a relative expansion of the myeloid-biased lower-SPKLS subpopulation. This shift in proportion can explain all of the observations of myeloid-lineage bias in aging HSCs from previous reports that were based on testing the entire HSC population. As the lineage outputs of SP subtypes are stable upon rounds of serial transplantation and in aging mice, lower-SPKLS cells will henceforth be called myeloid-biased HSCs (My-HSCs) and upper-SPKLS cells will be called lymphoid-biased HSCs (Ly-HSCs).
Ly-HSCs are more Proliferative HSCs
In order to gain insight into molecular mechanisms underlying the functional biases of HSC subtypes, we globally compared the gene expression profiles of My-HSCs and Ly-HSCs (Table S2). We identified 785 genes that were differentially expressed between My-HSCs and Ly-HSCs, with 434 higher in My-HSCs (Table S3) and 351 higher in Ly-HSCs (Table S4). The expression of a subset of the most significant differentially expressed genes were analyzed by real-time PCR on independently isolated samples (Figure S4A-B), demonstrating excellent corroboration of microarray data.
Ingenuity Pathway Analysis (IPA) revealed that Ly-HSCs expressed many genes reflective of highly activated cells; 9 of the 12 pathways significantly enriched in Ly-HSCs were related to cell cycle or metabolism (Figure S4C). A higher rate of turnover of Ly-HSCs compared to My-HSCs may lead to earlier exhaustion of this HSC subtype over the lifetime of the animal, perhaps consistent with the proportional change in HSC subtypes with age. Analysis of the cell cycle status of lower- and upper-SPKLS cells using PI staining, BrdU labeling, Hoechst / Pyronin Y staining and Ki67 staining (Figure S5) showed that Ly-HSCs are more proliferative while My-HSCs are more quiescent, both at any given point in time and over given time-frames.
TGFβ1 Elicits Different Responses from HSC Subtypes in vitro
Gene Ontology analysis (GO; Ogata et al., 1999) showed that one of the few molecular pathways significantly enriched in My-HSCs was the TGFβ signaling pathway. TGFβ signaling has been implicated in the regulation of HSC quiescence (Yamazaki et al., 2009), and TGFβ signaling-deficient HSCs have increased proliferative capacity in vitro, although in vivo HSC quiescence and maintenance of the HSC pool is not dependent on TGFβ signaling (Larsson et al., 2003; Larsson et al., 2005). HSCs have also been shown to have a biphasic response to TGFβ stimulation, with high concentrations being inhibitory and low concentrations stimulatory (Kale, 2004; Kale and Vaidya, 2004). These apparently contradictory responses of HSC to TGFβ signaling led us to hypothesize that TGFβ may exert different effects on My- and Ly-HSC subtypes.
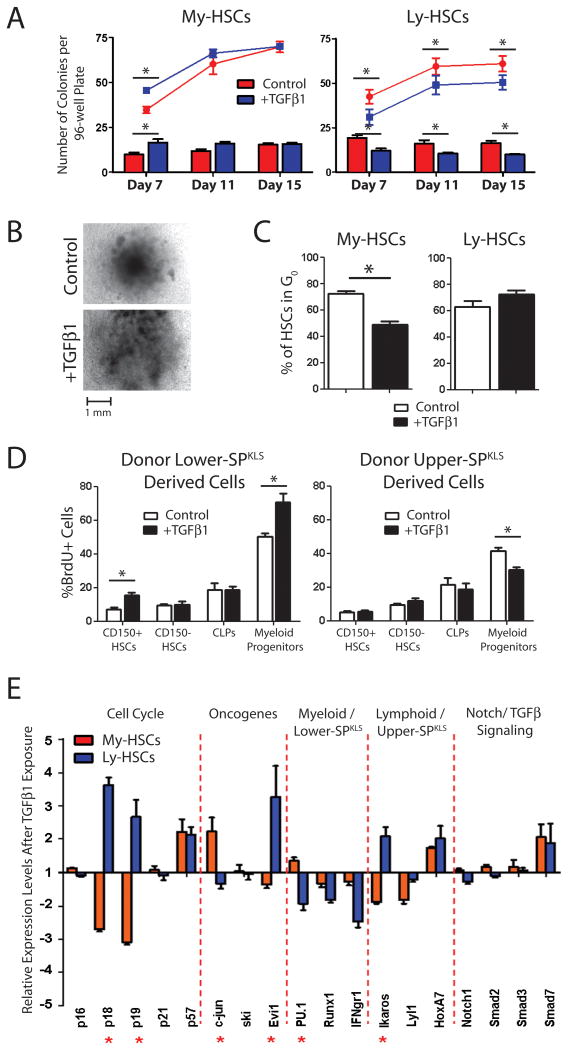
To test this, we subjected My- and Ly-HSCs to TGFβ1, -2 and -3 in a variety of assays to determine the effects of these growth factors on HSC subtypes. To summarize a number of experiments (Figure S6), we found high concentrations of all TGFβs (10 ng/μL) was inhibitory to for both My- and Ly-HSCs in vitro; however at lower concentrations of TGFβs (10 pg/μL) we found TGFβ1, -2 and -3 to be stimulatory for My-HSCs, while TGFβ1 and -2 were inhibitory to Ly-HSC (TGFβ3 was in general stimulatory for Ly-HSCs). Since the biggest distinctions in HSC subtype response were produced by exposure to TGFβ1, we focused on this factor for more detailed analysis.
We first tested the effect of TGFβ1 on HSC subtypes by colony formation assays in Methocult medium supplemented with TGFβ1 (Figure 6A). Exposure of My-HSCs to exogenous TGFβ1 accelerated initial colony formation (7-days), although the total number of colonies scored after 15-days was not different. This initial difference was due to the more rapid appearance of CFU-GM colonies relative to control My-HSCs. Conversely, exogenous TGFβ1 impeded colony formation of Ly-HSCs throughout the timecourse, due to the inhibition of CFU-GM colony differentiation. The size of multi-lineage CFU-GEMM colonies produced by My-HSCs in response to TGFβ1 was also greater compared to controls (Figure 6B), and flow cytometric analysis determined this was not due to over-representation of a specific cell type (Figure S6C). Thus TGFβ1 appears to be a general stimulatory factor for My-HSC proliferation.
Figure 6.
HSC subtypes show differential responses to TGFβ1.
(A) Low (10 pg/μL) concentrations of TGFβ1 accelerated colony formation of My-HSCs (more colonies at day 7), but did not change the total number of colonies formed (at day 15), while TGFβ1 proved inhibitory to Ly-HSC colony formation. The lines indicate the total number of colonies per plate, while the bar graphs represent the number of CFU-GM colonies at each timepoint. Data presented are cumulative of four individual experiments each comprising two replicate plates for each condition. (B) The CFU-GEMM colonies from My-HSCs in the presence of TGFβ1 were markedly larger in size than control colonies. (C) Analysis of Pyronin Y staining of My- and Ly-HSCs following 5-hour in vitro exposure to TGFβ1. Data are averages of three separate experiments. (D) BrdU uptake after in vivo TGFβ1 exposure by progeny of transplanted My- and Ly-HSCs. In analyzing the progeny of transplanted HSCs, CD150 expression was used as a surrogate marker for myeloid- (CD150+) and lymphoid-biased (CD150-) daughter HSCs. (E) Real-time PCR analysis of purified My- and Ly-HSCs following 5-hour in vitro TGFβ1 exposure. Data presented are averages for three separate experiments. Stars indicate the genes that exhibit statistically significant differences in response between My- and Ly-HSCs (see also Figure S5-7).
This differential effect of TGFβ1 could be due to enhancing proliferation, or differentiation, since both properties impact the outcome of methycellulose assays. To exclusively examine the effect on HSC proliferation over a shorter timeframe, My- and Ly-HSCs were purified and incubated with 10 pg/μL TGFβ1 for five hours and then subjected to Pyronin Y staining, which allows the proportion of HSC exiting G0 to be determined (Figure 6C). My-HSCs engaged the cell cycle following stimulation with TGFβ1, with a smaller proportion of cells, relative to controls, remaining in G0 following the incubation. In contrast, exit from G0 by Ly-HSCs was impeded by TGFβ1.
TGFβ1 Promotes Proliferation and Myeloid Differentiation Specifically in My-HSCs
To determine if exogenous TGFβ1 could also affect HSCs in vivo, groups of mice were injected with 0.1 μg TGFβ1 (Grzegorzewski et al., 1994) and then injected with BrdU to label HSCs that entered S-phase during the 12-hour BrdU exposure period. My- and Ly-HSCs were then purified and analyzed for BrdU incorporation (Figure S6E). Consistent with the in vitro studies, exposure to TGFβ1 produced a subtle yet significant stimulatory effect on My-HSC proliferation, while inhibiting turnover of Ly-HSCs.
In order to more accurately assess the in vivo response of HSC subtypes to TGFβ1, we transplanted a cohort of CD45.1/2 mice with limiting number of My- (25 CD45.1 lower-SPKLS) and Ly-HSCs (50 CD45.2 upper-SPKLS) and monitored the response of each transplanted cell population to exogenous TGFβ1 after stable hematopoietic reconstitution (12-weeks post-transplant). Test mice were administered three 0.1 μg TGFβ1 injections 24-hours apart and then labeled with BrdU 12-hours prior to sacrifice to determine the effect of TGFβ1 on turnover rate of donor cell-derived stem and progenitor cell compartments (Figure 6D; see Figure S7 for gating schemes). Transplanted My-HSCs responded to TGFβ1 by showing increased proliferation of CD150+ HSCs (as a surrogate marker for myeloid-biased HSCs; CD150+CD34-KLS) and myeloid progenitors, but no difference in proliferation of CD150− HSCs (surrogate marker for lymphoid-biased HSCs; CD150-CD34-KLS) or CLPs. The proliferation of transplanted Ly-HSC-derived myeloid progenitors was inhibited by exogenous TGFβ1 although no other significant changes were noted in any stem / progenitor cell compartment. Thus, TGFβ1 induces a proliferative response in My-HSCs, acting directly on their daughter HSCs and myeloid progenitors.
TGFβ1 Induces Opposing Transcriptional Responses in HSC Subtypes
In order to assess the molecular responses of HSC subtypes to TGFβs, real-time PCR analysis was conducted on My- and Ly-HSCs which were exposed to 10 pg/μL TGFβ in vitro for five-hours (Figure 6E). Genes for p18 and p19, which inhibit the G0 to G1 transition, were downregulated in My-HSCs exposed to TGFβ1, and upregulated in Ly-HSCs. No change was observed in p16 and p21 expression, and inhibitors of the later parts of the cell cycle such as p57 changed in parallel in My- and Ly-HSCs. Of the oncogenes which have been proposed to be regulated by TGFβ, Evi1 showed a strong up-regulation in Ly-HSCs in response to TGFβ1, and slight down-regulation in My-HSCs. Evi1 prevents terminal differentiation of bone marrow progenitors (Buonamici et al., 2003), thus its upregulation by Ly-HSCs in response to TGFβ may be one of the mechanisms behind TGFβ-induced proliferative suppression. Showing the opposite pattern was c-jun, induced during the G0 to G1 transition (Cosenza et al., 1994), which became up-regulated only in My-HSCs after TGFβ exposure Of genes known to be involved in myeloid or lymphoid differentiation, divergent responses in PU.1 and Ikaros expression were observed, regulators of myeloid and lymphoid differentiation respectively, with My-HSCs upregulating PU.1 and Ly-HSCs upregulating Ikaros. This suggests that TGFβ1 is biasing the stem cells even more towards their intrinsic differentiation fates. No significant differences were noted in the downstream regulators of TGFβ signaling Smad2, -3 and -7, although their activation is generally mediated by protein phosphorylation.
Discussion
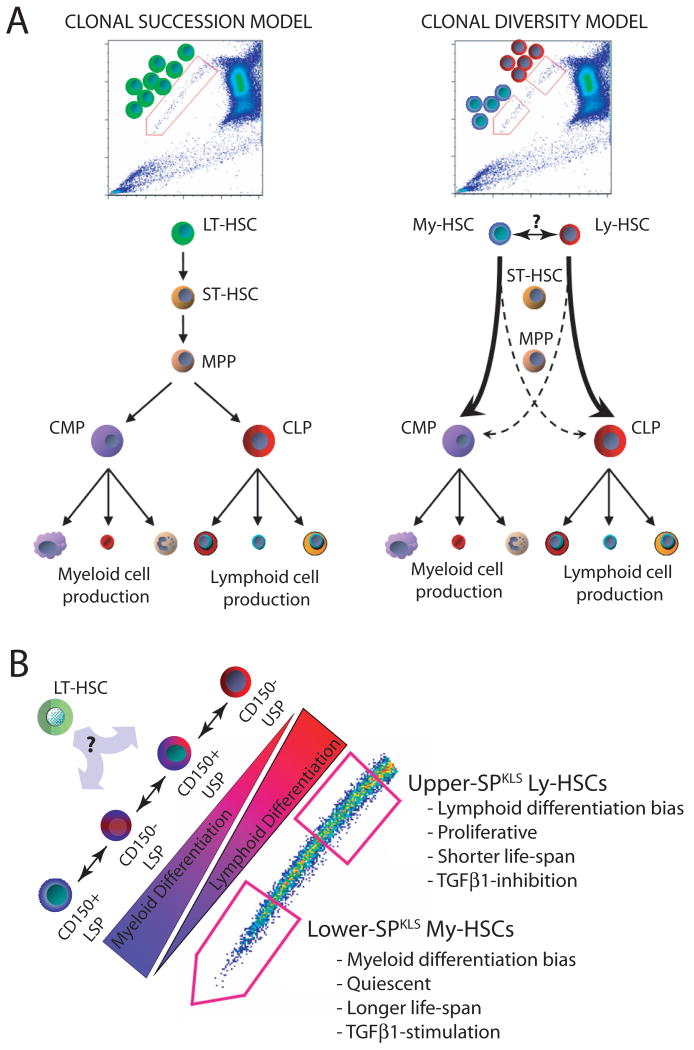
The classical model of hematopoietic hierarchy which proposes that all the mature cells of the peripheral blood are the progeny of a single LT-HSC, the so-called clonal succession model (Figure 7A), has come under scrutiny recently as more evidence accumulates that the HSC pool actually consists of multiple HSC subtypes with distinct functional potentials. The work presented here provides further evidence for this clonal diversity model (Figure 7B), and the combination of SP and CD150 shows a clear gradient in HSC potential suggesting a continuum of HSC is present in the marrow. By competing the subtypes against each other, we demonstrated that in the presence of the other subtypes, the HSC subtypes default to perform almost exclusively their primary function (i.e. their biases are extreme). This suggests that in vivo, most stem cells may be highly biased and behave not at all like classically-defined multi-lineage HSC.
Figure 7.
Model for HSC clonal diversity and its relation to the SP phenotype.
(A) The traditional clonal succession model (left) in which all mature blood cells are the progeny of a single uniform pool of LT-HSCs, and the clonal diversity model (right), supported by our data, in which distinct HSC subtypes are capable of contributing to all lineages, but are stably programmed to do so in a highly biased fashion (B) The SP allows visual representation of the continuum of HSC subtypes encompassing the spectrum from the most myeloid-biased CD150+ lower-SPKLS to the most lymphoid-biased CD150− upper-SPKLS. The HSC subtypes exhibit additional cellular, molecular, and functional distinctions. A parental unbiased HSC likely exists during development, and conceivably in the adult.
Initial studies that established this concept of biased HSC subtypes largely arose from analysis of the repopulation kinetics of mice transplanted with phenotypically identical populations (Muller-Sieburg et al., 2002; Muller-Sieburg et al., 2004; Dykstra et al., 2007). Further understanding of the mechanisms that regulate these HSC subtypes requires the ability to prospectively isolate them. The Eaves group recently showed that CD150 could be used to distinguish myeloid- and lymphoid-biased HSC purified on the basis of CD45+EPCR+CD48− expression (Kent et al., 2009), and were able to identify some differentially expressed genes.
Here, we show that Hoechst dye efflux and CD150 reveal a gradient of HSC self-renewal activity and myeloid bias correlating with the phenotypes (in order) CD150+lower-SPKLS ≫ CD150−lower-SPKLS ≫ CD150+upper-SPKLS ≫ CD150−upper-SPKLS with an opposing gradient of proliferative potential and lymphoid bias. However, while the average activities of the clones point to these correlations, there was substantial variation between individual HSCs. This implies that either the populations are still a mixture of more functionally distinct cells, or that the SP actually represents a continuum of HSC activities, which hampers the absolute segregation of distinct HSC phenotypes (Figure 7B). Because the peculiar Hoechst staining of the SP results from differential efflux of the dye by HSCs due to high multi-drug-resistance (MDR)–type transporter activity (Goodell et al., 1996; Zhou et al. 2001), it will be interesting to determine whether the discrete efflux properties will prove to have biological significance for the HSC subtypes.
Previous studies have shown that aging HSCs have reduced ability to reconstitute lethally irradiated recipients in transplantation assays (Morrison et al., 1996; Liang et al., 2005), a vastly different gene expression profile (Rossi et al., 2005; Chambers et al., 2007b), and a myeloid-differentiation bias compared to their young counterparts (Sudo et al., 2000; Kim et al., 2003). However, these studies compared the entire HSC pools from old versus young mice, not taking into account potential population dynamics. One study suggested that the age-related changes reflect changes in the clonal composition of the HSC pool of old mice (Cho et al., 2008). Indeed, we observed an accumulation of lower-SPKLS in the bone marrow of old mice, yet lower- and upper-SPKLS cells exhibit the same differentiation bias of their young counterparts. Thus, the apparent myeloid-bias of aging HSCs reflects a higher proportion of My-HSCs in the HSC pool, rather than alterations in the developmental potential of HSC with age. This could be due to differential responses to the aging cytokine milieu, the higher proliferative rate of Ly-HSCs which could lead to their exhaustion, and a greater self-renewal capacity of My-HSCs. Clearly, this dramatic proportional shift of these subpopulations with age has significant implications for the interpretation of studies that have described distinct molecular or biological properties of the aged HSC pool.
TGFβ-signaling has been implicated in maintaining HSC quiescence (Fortunel et al., 2000; Chabanon et al., 2008). Here, TGFβ1 stimulated My-HSCs to proliferate, whilst proving inhibitory to Ly-HSCs. This appears to be physiological relevant, as increased proliferation of My-HSCs was apparent in vitro and in vivo. Furthermore, when mice were co-transplanted with limited numbers of genetically distinguishable My- and Ly-HSCs, and then administered TGFβ1 after stable engraftment (Figure 6D), TGFβ1 acted directly on the transplanted My-HSC population, stimulating the proliferation of its daughter myeloid-biased HSCs and myeloid progenitors, while in the same animals simultaneously inhibiting the proliferation of transplanted Ly-HSC-derived myeloid progenitors. This highlights the unique responsiveness of distinct HSC subtypes, and their immediate progeny, to a growth factor and provides a potential mechanism for differential regulation of HSC subtypes.
One of the molecular mechanisms governing the proliferative response of My-HSCs to TGFβ1 appears to be downregulation of the G0 to G1 cell cycle inhibitors p18 and p19. Other differential responses were detected in HSC subtypes upon exposure to TGFβs, most notably the proto-oncogenes Evi1 (upregulated in Ly-HSCs) and c-jun (upregulated in My-HSCs). It is clear that My- and Ly-HSC subtypes have distinct molecular and cellular responses to TGFβ signaling, further enforcing their specific characteristics. It is possible that TGFβ signaling in the bone marrow niche functions as a “fine-tuning” mechanism for cross-talk between HSC subtypes to mediate the numbers of each actively engaged in hematopoiesis at any given point in time. We also speculate that the proportional increase of the My-HSC compartment with age could be due in part to differences in TGFβ ligand production, and the consequent response particularly of My-HSCs, in the inflammatory setting of an aging environment.
The My-HSCs exhibit the highest engraftment rate per mouse when single cells were transplanted, as well as the highest overall contribution to peripheral blood regeneration in each mouse. The secondary transplant data also suggest My-HSCs have higher self-renewal capacity. It is likely that if the contribution of the HSC subtypes were examined over a longer time-period (6 to 12 months), we would see further distinctions in the My- and Ly-HSCs with regard to their ability to sustain blood production. While clearly bona fide HSCs according to the rigorous criteria imposed here, blood production from Ly-HSCs may prove less durable, consistent with their lower potency and higher proliferative rate. The recent report of a class of HSCs with intermediate-term durability underscores this key property (Benveniste et al., 2010).
This visual continuum of HSCs with different dye efflux capacity, correlating with a functional gradient, makes it tempting to speculate that My-HSCs are the most primitive, generating the less-primitive, less quiescent Ly-HSC. While both HSC types clearly have the potential to generate each other (Figure 3C), each HSC subtype preferentially regenerated itself, arguing that these HSC pools operate largely independently of each other. This suggests a deterministic explanation for the data. Perhaps an omnipotent HSC present during development (conceivably still present in adult marrow) establishes a consortium of HSCs during seeding of the bone marrow. Their epigenetic state, or their different niches, may indelibly dictate their propensity to generate the downstream components of the blood in a biased fashion, as well as their self-renewal capacities. Thus, the properties of these HSC subtypes suggest a lineal relationship, but that may be a result of the limitations of the current assays and our historical interpretations.
Intriguingly, argument about the precursors of the hematopoietic systems can be traced back over 100 years when staining techniques enabled identification of different white blood cell lineages. “Unitarians” believed that erythrocytes, granulocytes and lymphocytes all came from a cell of common origin while “Dualists” argued that myeloid and lymphoid cells derived from committed precursors residing in distinct hematopoietic tissues (reviewed in Ramalho-Santos and Willenbring, 2007). The Unitarian concept may still hold true, but the mounting evidence for clonal diversity in the HSC pool with distinct subtypes dedicated to regenerating particular compartments argues that revision of the long-held view of a unipotent stem cell pool generating the entire branching hematopoietic differentiation tree must come under scrutiny. The clonal diversity model has important implications for experimental and clinical HSC biology including the selection of appropriate HSC subtypes for transplantation or cell / gene therapy applications, the incidence of myeloproliferative disorders in the elderly and the cellular and behavioral heterogeneity seen in genetically similar leukemias. Here we prospectively isolate functionally distinct HSC subtypes, and provide the first mechanistic insight into the molecular regulation of myeloid- and lymphoid-biased HSC function.
Experimental Procedures
Mice and Hematopoietic Stem Cell Purification
All animal procedures were IUCAC-approved and conducted in accordance institutional guidelines. Mice were housed in a specific pathogen-free facility and fed autoclaved acidified water and mouse chow ad libitum. All mice were C57Bl/6 background distinguished by CD45.1 or CD45.1 alleles. Whole bone marrow (WBM) was isolated from femurs and tibias, and SP cell staining was performed with Hoechst 33342 (Sigma-Aldrich) as previously reported (Goodell et al., 1996). WBM was resuspended in staining media at 106 cells/mL and incubated with 5 μg/mL Hoechst 33342 for 90 minutes at 37°C. For antibody staining, cells were suspended at a concentration of 108 cells/mL and incubated on ice for 20 minutes with various combinations antibodies (Supplemental Experimental Procedures). Cell sorting was performed on a MoFlo cell sorter (Coulter) and analysis on an LSRII (BD Biosciences).
Transplantation and Peripheral Blood Analysis
After a split dose of 10.5 Gy of irradiation, recipients were transplanted by intravenous injection. Donor HSCs were competed against 2 × 105 unfractionated WBM cells with the opposite CD45 allele (matched to the recipient). For peripheral blood analysis, mice were bled retro-orbitally, the red blood cells were lysed, and each sample was incubated with the following antibodies on ice for 20 minutes – CD45.1-FITC, CD45.2-APC, CD4-Pacific Blue, CD8-Pacific Blue, B220-Pacific Blue, B220-PeCy7, Mac1-PeCy7, and Gr-1-PeCy7 as previously described (Challen et al., 2009). Cells were then spun down, resuspended in a propidium iodide solution, and analysis was accomplished on live cells with an LSRII (Becton Dickinson). For secondary transplantation from mice transplanted with bulk lower- and upper-SPKLS cells, WBM was isolated >18 weeks after transplantation and SPKLS were purified, also sorting on the CD45 allele of the original donor SPKLS cells. 200 of these purified HSCs were competed against 2 × 105 fresh WBM cells.
Hematopoietic Progenitor Cell Analysis of Transplanted Mice
>18 weeks after transplantation, recipient mice were sacrificed, and bone marrow was isolated and analyzed using antibody schemes to identify the donor-derived multipotent progenitor (MPP; Lineage- Sca-1+ c-Kit+ Flk-2+), myeloid progenitor (MP; Lineage- Il7rα-Sca-1- c-Kit+), and common lymphoid progenitor (CLP; Lineage- Il7rα+ Sca-1+ c-Kit+) compartments as previously described (Akashi et al., 2000; Kondo et al., 1997).
Single Cell Transplantation
Compromised competitor WBM was derived by transplanting lethally irradiated CD45.1 mice with 1 × 106 CD45.1 WBM cells and harvesting the WBM 12-weeks post-transplant. 1 × 106 compromised WBM cells were seeded into wells of a 96-well plate, and single CD45.2 test HSCs were sorted directly into the wells. For secondary transplantation, WBM was harvested from each recipient and one femur equivalent was divided into four secondary CD45.1 hosts.
Methocult Assays
Single HSCs were sorted into 96-well plates containing Methocult 3434 medium (Stem Cell Technologies supplemented with penicillin / streptomycin and cultured in vitro at 37°C for two-weeks unless stated otherwise.
Microarray Analysis
Lower- and upper-SPKLS cells were sorted from three pools of 24 mice (10-week old male CD45.1 C57Bl/6) on separate days, yielding 12,000 – 16,000 HSCs for each of three biological replicates. Purity of each sort was >98%. RNA was isolated using the RNAqueous (Ambion), treated with DNase I, and precipitated with phenol:chloroform:isoamyl alcohol. The RNA was linearly amplified (Venezia et al., 2004), in two rounds of T7 in vitro transcription (MessageAmp, Ambion) and labeled with biotin-UTP and -CTP (Enzo Biotech) during the second amplification. Labeled RNA (20 μg) was diluted in fragmentation buffer, incubated at 94°C for 25 min and hybridized to Affymetrix MOE430.2 chips according to standard protocols. The raw image and intensity files were generated using GCOS 1.0 software (Affymetrix).
Microarray chips passed quality control tests, including a scale factor < 5, a 5′ to 3′ probe ratio < 20, a replicate correlation coefficient > 0.96, unbiased global clustering analysis, and limited RNA degradation analyzed using the 5′ to 3′ signal intensity ratios from chip probes. Normalization and model-based expression measurements were performed with GC-RMA (www.bioconductor.org; http://cran.r-project.org/). Determination of differentially expressed genes between samples was defined as fold-change > 2, adjusted p-value < 0.05, B-statistic > 0. Microarray data were further analyzed with Ingenuity Pathways Analysis (Ingenuity Systems). Data can be found in Gene Expression Omnibus (accession GSE16475).
Real-Time PCR Analysis
RNA was isolated using RNAqueous from FACS-sorted cells isolated independently from the samples used for microarray analysis. RNA was reverse transcribed with random hexamer primers using Super Script II (Invitrogen). cDNA input was standardized and RT-PCRs were performed with Taqman master Mix (Applied Biosystems), 18 s-rRNA probe (VIC-MGB; Applied Biosystems), and a gene-specific probe (FAM-MGB; Applied Biosystems) for 50 cycles using an AbiPrism 7900HT (Applied Biosystems). Samples were normalized to 18S and fold-change determined by the ΔΔCt method.
TGFβ
Recombinant TGFβ's (R&D Systems) were reconstituted according to the manufacturer's recommendations and assayed at the stated concentrations.
Cell Cycle Analysis of Transplanted HSC Progeny
Mice received one intraperitoneal injection of BrdU (Sigma-Aldrich; 1 mg/6 g of mouse weight) and sacrificed 12 hours later. WBM was stained with antibodies to identify stem and progenitor cell compartments (Figure S7) and then prepared for analysis of BrdU incorporation using the FITC-BrdU Flow Kit (BD Pharmingen).
Pyronin Y Staining
200,000 B220-FITC+ carrier splenocytes were pre-sorted into collection tubes. HSCs (more than 1000) were then sorted into the same tube. Following TGFβ1 incubation, cells were then incubated for 45 minutes with 20μg/mL Hoechst 33342 and 50 μg/mL Verapamil (Sigma-Aldrich) in phosphate buffered saline supplemented with 3% fetal bovine serum. Pyronin Y (Sigma-Aldrich) was then added at 1 μg/ml, and the cells were incubated for another 15 min at 37°C, washed, and immediately analyzed on a BD LSRII. During flow analysis, both Hoechst 33342 and Pyronin Y signal were displayed under linear mode. The carrier B-cells, were then a control to define the G0/G1 DNA content.
Statistics
Student t test and 1-way ANOVA's were used for statistical comparisons where appropriate. Significance is indicated on the figures using the following convention: *p<0.05, **p<0.01, ***p<0.001. Error bars on all graphs represent the SEM.
Supplementary Material
Acknowledgments
The authors have no financial conflicts of interest to disclose. The authors would like to thank all members of the Goodell lab for scientific advice, Jonathan Berg and Katherine King for manuscript review and Chris Threeton for flow cytometric sorting and analysis. G.A.C. was supported by an Australian National Health and Medical Research Council (NHMRC) CJ Martin Fellowship (384369). This work was also supported by NIH grants HL081007, EB005173, and DK58192, the Ellison foundation (AGSS178706), and the American Heart Association (0740020N).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolfsson J, Borge OJ, Bryder D, Theilgaard-Monch K, Astrand-Grundstrom I, Sitnicka E, Sasaki Y, Jacobsen SE. Upregulation of Flt3 expression within the bone marrow Lin(-)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- Balazs AB, Fabian AJ, Esmon CT, Mulligan RC. Endothelial protein C receptor (CD201) explicitly identifies hematopoietic stem cells in murine bone marrow. Blood. 2006;107:2317–2321. doi: 10.1182/blood-2005-06-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste P, Frelin C, Janmohamed S, Barbara M, Herrington R, Hyam D, Iscove NN. Intermediate-term hematopoietic stem cells with extended but time-limited reconstitution potential. Cell Stem Cell. 2010;6:48–58. doi: 10.1016/j.stem.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Buonamici S, Chakraborty S, Senyuk V, Nucifora G. The role of EVI1 in normal and leukemic cells. Blood Cells Mol Dis. 2003;31:206–212. doi: 10.1016/s1079-9796(03)00159-1. [DOI] [PubMed] [Google Scholar]
- Camargo FD, Chambers SM, Drew E, McNagny KM, Goodell MA. Hematopoietic stem cells do not engraft with absolute efficiencies. Blood. 2006;107:501–507. doi: 10.1182/blood-2005-02-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabanon A, Desterke C, Rodenburger E, Clay D, Guerton B, Boutin L, Bennaceur-Griscelli A, Pierre-Louis O, Uzan G, Abecassis L, et al. A crosstalk between stromal cell-derived factor-1 and transforming growth factor-beta controls the quiescence/cycling switch of CD34(+) progenitors through FoxO3 and mammalian target of rapamycin. Stem Cells. 2008;26:3150–3161. doi: 10.1634/stemcells.2008-0219. [DOI] [PubMed] [Google Scholar]
- Challen GA, Boles N, Lin KK, Goodell MA. Mouse hematopoietic stem cell identification and analysis. Cytometry A. 2009;75:14–24. doi: 10.1002/cyto.a.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SM, Boles NC, Lin KY, Tierney MP, Bowman TV, Bradfute SB, Chen AJ, Merchant AA, Sirin O, Weksberg DC, et al. Hematopoietic fingerprints: an expression database of stem cells and their progeny. Cell Stem Cell. 2007a;1:578–591. doi: 10.1016/j.stem.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007b;5:e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111:5553–5561. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza SC, Yumet G, Soprano DR, Soprano KJ. Induction of c-fos and c-jun mRNA at the M/G1 border is required for cell cycle progression. J Cell Biochem. 1994;55:503–512. doi: 10.1002/jcb.240550410. [DOI] [PubMed] [Google Scholar]
- Dykstra B, Kent D, Bowie M, McCaffrey L, Hamilton M, Lyons K, Lee SJ, Brinkman R, Eaves C. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell. 2007;1:218–229. doi: 10.1016/j.stem.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Dykstra B, Ramunas J, Kent D, McCaffrey L, Szumsky E, Kelly L, Farn K, Blaylock A, Eaves C, Jervis E. High-resolution video monitoring of hematopoietic stem cells cultured in single-cell arrays identifies new features of self-renewal. Proc Natl Acad Sci U S A. 2006;103:8185–8190. doi: 10.1073/pnas.0602548103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema H, Sudo K, Seita J, Matsubara A, Morita Y, Osawa M, Takatsu K, Takaki S, Nakauchi H. Quantification of self-renewal capacity in single hematopoietic stem cells from normal and Lnk-deficient mice. Dev Cell. 2005;8:907–914. doi: 10.1016/j.devcel.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Fortunel N, Hatzfeld J, Kisselev S, Monier MN, Ducos K, Cardoso A, Batard P, Hatzfeld A. Release from quiescence of primitive human hematopoietic stem/progenitor cells by blocking their cell-surface TGF-beta type II receptor in a short-term in vitro assay. Stem Cells. 2000;18:102–111. doi: 10.1634/stemcells.18-2-102. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, Paradis G, Grupp SA, Sieff CA, Mulligan RC, Johnson RP. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- Grzegorzewski K, Ruscetti FW, Usui N, Damia G, Longo DL, Carlino JA, Keller JR, Wiltrout RH. Recombinant transforming growth factor beta 1 and beta 2 protect mice from acutely lethal doses of 5-fluorouracil and doxorubicin. J Exp Med. 1994;180:1047–1057. doi: 10.1084/jem.180.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RJ, Wagner JE, Celano P, Zicha MS, Sharkis SJ. Separation of pluripotent haematopoietic stem cells from spleen colony-forming cells. Nature. 1990;347:188–189. doi: 10.1038/347188a0. [DOI] [PubMed] [Google Scholar]
- Jordan CT, Lemischka IR. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 1990;4:220–232. doi: 10.1101/gad.4.2.220. [DOI] [PubMed] [Google Scholar]
- Kale VP. Differential activation of MAPK signaling pathways by TGF-beta1 forms the molecular mechanism behind its dose-dependent bidirectional effects on hematopoiesis. Stem Cells Dev. 2004;13:27–38. doi: 10.1089/154732804773099236. [DOI] [PubMed] [Google Scholar]
- Kale VP, Vaidya AA. Molecular mechanisms behind the dose-dependent differential activation of MAPK pathways induced by transforming growth factor-beta1 in hematopoietic cells. Stem Cells Dev. 2004;13:536–547. doi: 10.1089/scd.2004.13.536. [DOI] [PubMed] [Google Scholar]
- Kent DG, Copley MR, Benz C, Wohrer S, Dykstra BJ, Ma E, Cheyne J, Zhao Y, Bowie MB, Zhao Y, et al. Prospective isolation and molecular characterization of hematopoietic stem cells with durable self-renewal potential. Blood. 2009;113:6342–6350. doi: 10.1182/blood-2008-12-192054. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Acar M, Radice GL, Morrison SJ. Hematopoietic stem cells do not depend on N-cadherin to regulate their maintenance. Cell Stem Cell. 2009;4:170–179. doi: 10.1016/j.stem.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kim M, Moon HB, Spangrude GJ. Major age-related changes of mouse hematopoietic stem/progenitor cells. Ann N Y Acad Sci. 2003;996:195–208. doi: 10.1111/j.1749-6632.2003.tb03247.x. [DOI] [PubMed] [Google Scholar]
- Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- Larsson J, Blank U, Helgadottir H, Bjornsson JM, Ehinger M, Goumans MJ, Fan X, Leveen P, Karlsson S. TGF-beta signaling-deficient hematopoietic stem cells have normal self-renewal and regenerative ability in vivo despite increased proliferative capacity in vitro. Blood. 2003;102:3129–3135. doi: 10.1182/blood-2003-04-1300. [DOI] [PubMed] [Google Scholar]
- Larsson J, Blank U, Klintman J, Magnusson M, Karlsson S. Quiescence of hematopoietic stem cells and maintenance of the stem cell pool is not dependent on TGF-beta signaling in vivo. Exp Hematol. 2005;33:592–596. doi: 10.1016/j.exphem.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Lemischka IR, Raulet DH, Mulligan RC. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986;45:917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106:1479–1487. doi: 10.1182/blood-2004-11-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki Y, Kinjo K, Mulligan RC, Okano H. Unexpectedly efficient homing capacity of purified murine hematopoietic stem cells. Immunity. 2004;20:87–93. doi: 10.1016/s1074-7613(03)00354-6. [DOI] [PubMed] [Google Scholar]
- Miller CL, Eaves CJ. Expansion in vitro of adult murine hematopoietic stem cells with transplantable lympho-myeloid reconstituting ability. Proc Natl Acad Sci U S A. 1997;94:13648–13653. doi: 10.1073/pnas.94.25.13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Muller-Sieburg CE, Cho RH, Karlsson L, Huang JF, Sieburg HB. Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired IL-7 responsiveness. Blood. 2004;103:4111–4118. doi: 10.1182/blood-2003-10-3448. [DOI] [PubMed] [Google Scholar]
- Muller-Sieburg CE, Cho RH, Thoman M, Adkins B, Sieburg HB. Deterministic regulation of hematopoietic stem cell self-renewal and differentiation. Blood. 2002;100:1302–1309. [PubMed] [Google Scholar]
- Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999;27:29–34. doi: 10.1093/nar/27.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passegue E, Wagers AJ, Giuriato S, Anderson WC, Weissman IL. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med. 2005;202:1599–1611. doi: 10.1084/jem.20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Santos M, Willenbring H. On the origin of the term “stem cell”. Cell Stem Cell. 2007;1:35–38. doi: 10.1016/j.stem.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Roeder I, Horn K, Sieburg HB, Cho R, Muller-Sieburg C, Loeffler M. Characterization and quantification of clonal heterogeneity among hematopoietic stem cells: a model-based approach. Blood. 2008;112:4874–4883. doi: 10.1182/blood-2008-05-155374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Weissman IL. Hematopoietic stem cell aging: mechanism and consequence. Exp Gerontol. 2007;42:385–390. doi: 10.1016/j.exger.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburg HB, Cho RH, Dykstra B, Uchida N, Eaves CJ, Muller-Sieburg CE. The hematopoietic stem compartment consists of a limited number of discrete stem cell subsets. Blood. 2006;107:2311–2316. doi: 10.1182/blood-2005-07-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LG, Weissman IL, Heimfeld S. Clonal analysis of hematopoietic stem-cell differentiation in vivo. Proc Natl Acad Sci U S A. 1991;88:2788–2792. doi: 10.1073/pnas.88.7.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude GJ. Characteristics of the hematopoietic stem cell compartment in adult mice. Int J Cell Cloning. 1992;10:277–285. doi: 10.1002/stem.5530100505. [DOI] [PubMed] [Google Scholar]
- Spangrude GJ, Brooks DM, Tumas DB. Long-term repopulation of irradiated mice with limiting numbers of purified hematopoietic stem cells: in vivo expansion of stem cell phenotype but not function. Blood. 1995;85:1006–1016. [PubMed] [Google Scholar]
- Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilvassy SJ, Humphries RK, Lansdorp PM, Eaves AC, Eaves CJ. Quantitative assay for totipotent reconstituting hematopoietic stem cells by a competitive repopulation strategy. Proc Natl Acad Sci U S A. 1990;87:8736–8740. doi: 10.1073/pnas.87.22.8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano H, Ema H, Sudo K, Nakauchi H. Asymmetric division and lineage commitment at the level of hematopoietic stem cells: inference from differentiation in daughter cell and granddaughter cell pairs. J Exp Med. 2004;199:295–302. doi: 10.1084/jem.20030929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Dykstra B, Lyons KJ, Leung FY, Eaves CJ. Different in vivo repopulating activities of purified hematopoietic stem cells before and after being stimulated to divide in vitro with the same kinetics. Exp Hematol. 2003;31:1338–1347. doi: 10.1016/j.exphem.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Venezia TA, Merchant AA, Ramos CA, Whitehouse NL, Young AS, Shaw CA, Goodell MA. Molecular signatures of proliferation and quiescence in hematopoietic stem cells. PLoS Biol. 2004;2:e301. doi: 10.1371/journal.pbio.0020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksberg DC, Chambers SM, Boles NC, Goodell MA. CD150-side population cells represent a functionally distinct population of long-term hematopoietic stem cells. Blood. 2008;111:2444–2451. doi: 10.1182/blood-2007-09-115006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Wineman J, Moore K, Lemischka I, Muller-Sieburg C. Functional heterogeneity of the hematopoietic microenvironment: rare stromal elements maintain long-term repopulating stem cells. Blood. 1996;87:4082–4090. [PubMed] [Google Scholar]
- Yamazaki S, Iwama A, Takayanagi S, Eto K, Ema H, Nakauchi H. TGF-beta as a candidate bone marrow niche signal to induce hematopoietic stem cell hibernation. Blood. 2009;113:1250–1256. doi: 10.1182/blood-2008-04-146480. [DOI] [PubMed] [Google Scholar]
- Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.