Abstract
Histones play a crucial role in the organization of DNA in the nucleus, but their presence can prevent interactions with DNA binding proteins responsible for repair of DNA damage. Uracil is an abundant mutagenic lesion recognized by uracil DNA glycosylase (UDG) in the first step of base excision repair (BER). In nucleosome core particles (NCPs), we find substantial differences in UDG-directed cleavage at uracils rotationally positioned toward (U-In) or away from (U-Out) the histone core, or midway between these orientations (U-Mid). Whereas U-Out NCPs show a cleavage rate just below that of naked DNA, U-In and U-Mid NCPs have markedly slower rates of cleavage. Crosslinking of U-In DNA to histones in NCPs yields a greater reduction in cleavage rate but, surprisingly, yields a higher rate of cleavage in U-Out NCPs compared with uncrosslinked NCPs. Moreover, the next enzyme in BER, APE1, stimulates the activity of human UDG in U-Out NCPs, suggesting these enzymes interact on the surface of histones in orientations accessible to UDG. These data indicate that the activity of UDG likely requires “trapping” transiently exposed states arising from the rotational dynamics of DNA on histones.
Keywords: chromatin, DNA damage, glycosylase, histones, APE1 endonuclease
DNA repair is essential for cell survival and prevention of mutagenesis (1). Base excision repair (BER) is a key member of the cellular DNA repair mechanisms and is responsible for detection of a wide range of prevalent DNA lesions, including alkylated nucleobases and uracil (2, 3). The first step of BER is recognition of the modified base by a glycosylase, which cleaves the N-glycosidic bond and excises the base from its deoxyribose sugar, leaving an abasic (AP) site. Because the AP site repair intermediate is a liability to the cell, some glycosylases are retained at the site with high affinity, released only when the next factor in BER, the AP endonuclease (APE1), is available (4). APE1 specifically recognizes AP sites in the DNA and incises the phosphodiester bond 5′ of the abasic residue. This single strand break is typically the substrate for DNA polymerase β (Pol β), which exerts DNA-deoxyribophosphodiesterase activity to cleave off the abasic sugar and uses the intact strand as template to synthesize the missing base (5). Repair of the lesion is complete after ligation of the newly synthesized strand, usually by DNA ligase III (6). Although these enzymatic steps can be accomplished by purified enzymes on naked DNA in vitro, there are accessory factors in cells, including XRCC1, that ensure efficiency and coordination of the repair process (6, 7). Importantly, BER in the cell must take place on DNA in the context of chromatin.
Chromatin is a dynamic structure, consisting of DNA and numerous proteins that fold and pack the genetic material at many levels to maintain organization and to regulate gene expression (8 –10). The primary level of chromatin packaging is the nucleosome core particle (NCP), consisting of 147 bp of DNA wrapped around an octamer of the four core histones H2A, H2B, H3, and H4 (11). These nucleosome cores are located in close proximity along the chromosomes, separated by short, variable lengths of “linker” DNA (∼20–90 bp), which are bound by either of the linker histones H1 or H5.
Although the density of chromatin is an essential factor for its role in organizing DNA, its presence can be problematic for normal DNA metabolic processes, including DNA repair and transcription (8, 10, 12 –15). DNA associated with nucleosomes is constrained, and the general helical mobility necessary for access to binding factors is impeded (12). In addition, the DNA in direct contact with histones is occluded from interaction with other proteins, as intervals of the helix along the histone core surface are buried away from the solvent and thus virtually inaccessible (13, 16).
There are two primary “active” mechanisms used by the cell, acting independently or in combination, to overcome the nucleosome-associated barriers to DNA metabolism. Chromatin structure can be rearranged by ATP-dependant chromatin remodeling complexes which can remove, replace, and reposition nucleosomes along the DNA (17). In addition, histones are targeted for a wide range of posttranslational modifications, including methylation and acetylation, which can alter DNA–histone interactions and/or “mark” NCPs, for recognition, to aid additional activities (10).
Importantly, intrinsic nucleosome dynamics may serve a “passive” role for contributing to exposure of otherwise inaccessible sites on DNA. For example, nucleosomes exhibit spontaneous transient (partial) unwrapping in vitro (18 –20). Thus, even without active nucleosomal displacement and modification, DNA repair proteins may use NCP dynamics for surveillance of DNA to detect damaged bases within nucleosomes. This is particularly relevant for the initiation of BER, in which a glycosylase could have difficulty accessing certain bases along the helix because of their orientation relative to the histones surface.
The effect of lesion orientation and the impact of spontaneous molecular motions promoting BER have been examined in previous studies attempting to understand glycosylase accessibility to their substrates in chromatin. Two studies assessing uracil DNA glycosylase (UDG) activity on uracil-containing DNA assembled in NCPs showed reduced rates of UDG activity, ranging from 3- to 10-fold (21, 22). However, these studies differ in their conclusions regarding the orientation effects of uracil relative to the histones, as only the latter study showed a difference in UDG activity between “outward-” and “inward-” oriented uracil residues (22).
The bifunctional glycosylase hNTH1 also shows orientation-driven DNA cleavage activity in NCPs assembled with thymine glycol–containing DNA (23). Increased cleavage was measured at lesions facing away from the histone surface versus those facing in, and differences between orientations were less pronounced at positions closer to the end of the DNA (23). Among these glycosylase studies it is evident that increasing the glycosylase concentration reduces the histone-associated inhibition of activity (21 –23). Together, the hNTH1 and UDG data support the idea that glycosylases are sterically occluded from access to their respective substrates by the presence of histones. In addition, they suggest that glycosylases may gain accessibility to lesions based on molecular motion within the nucleosome core, as measured previously using restriction enzyme accessibility to monitor the unwrapping of DNA near the NCP edges (18, 19). Curiously, measurements of the time in which regions of the DNA along the nucleosome are unwrapped vary greatly, from the ends to the center of the histone octamer (dyad axis), by a factor of ∼10,000-fold (20). With such a great difference in exposure based on unwrapping, it is surprising that the glycosylase studies on NPCs show only modest differences in activity between inward and outward orientations, especially near the dyad center of the DNA.
In this study, we determined the impact of histones on the recognition step of BER by measuring UDG-directed DNA cleavage (with or without APE1) at uracil residues carefully positioned near the dyad axis of NPCs in three distinct orientations. Using a strong NCP positioning sequence and hydroxyl radical footprinting, we verified that uracils in the U-In, U-Out, and U-Mid NCPs were oriented toward the histone core, toward the solvent, and midway between these orientations, respectively. To determine the impact of DNA rotational dynamics in the NCPs on UDG activity, we used formaldehyde crosslinking to suppress DNA movement on the histone octamer surface. Finally, we assessed the potential role of AP endonuclease in enhancing glycosylase activity in the context of histones by comparison of human UDG (hUNG2) activity at each uracil orientation in the presence or absence of APE1.
Results
Reconstitution and Verification of Nucleosome Core Particles with Uracil Residues in Specific Rotational Orientations.
To create stable mononucleosome substrates with particular orientations of uracil bases relative to the histone surface, we used synthesized 150 base oligonucleotides containing the 15 base canonical glucocorticoid receptor response element (GRE) sequence flanked by the well-characterized nucleosome positioning TG motif sequences (TG-GRE-TG) (16, 24, 25). Three different cytosine bases within the GRE were replaced with uracil, labeled U-In, U-Mid, and U-Out (Materials and Methods), in reference to their predicted orientations relative to the histone octamer determined by the TG positioning motifs. Cytosine residues were chosen because of the biological relevance of replacement by uracil from naturally occurring spontaneous cytosine deamination, leading to G:U mispairs in DNA (26). We also created a strand that encoded the native GRE sequence without uracil (UND). The oligos with uracil were labeled with 32P at the 5′ end and annealed with the complementary strand to create the double strand DNA substrates used in this study. The structure of the histone octamer at the dyad axis and the predicted orientations of the uracil residues after pairing with their complementary strand are shown in Fig. 1.
Fig. 1.
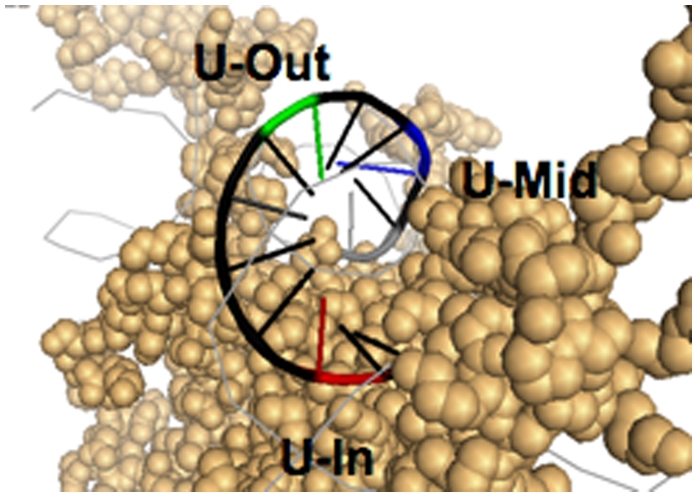
Uracil orientation in NCP substrates. Positioning of uracil residues in DNA on the surface of the histone octamer [figure created using PyMOL; structure taken from Davey et al. (54), Protein Data Bank Code 1KX5].
Uracil-containing NCPs were reconstituted from purified chicken erythrocyte mononucleosomes using salt dialysis, as described (25). As expected, the labeled TG-GRE-TG DNA assembled efficiently with the histone octamers, independent of the presence or location of uracil in the sequence (Fig. S1). To verify the rotational orientation of the uracil residues, we performed hydroxyl radical footprinting on reconstituted NCPs (27). When this method is performed on NCPs, the reaction products separated on a sequencing gel reveal the regions of the DNA backbone that are exposed to solvent from those associated with the histones, yielding a periodic pattern reflecting the helical rotation of a DNA strand (Fig. S2). To determine the orientation of uracil in the reconstituted NCPs, the three different constructs were digested with UDG and APE1, which cleaves the DNA backbone specifically at the uracil residues, and compared the location of the digestion products with the NCP footprint (Fig. S2). Peak analysis of the scan provides a clear visualization of the comparison between the NCP pattern and the location of the uracil residues within the digested DNA substrates (Fig. 2). The data indicate that the relative orientations of the uracils in the U-In, U-Mid, and U-Out NCPs accurately represent their respective orientations on the histone surface.
Fig. 2.
Hydroxyl radical footprint of reconstituted nucleosome core particles and relative uracil positions in the DNA substrate. Figure shows scans of a sequencing gel (Fig. S2) with the hydroxyl radical footprint of the undamaged DNA substrate associated with the histone octamer and the relative uracil positions of the U-In, U-Mid, and U-Out DNA constructs, after treatment with UDG and APE1. A labeled 10-bp DNA marker is also scanned for relative positioning, and the inverted triangle marks the dyad center of the NCP. Note that the actual position of the uracil residues of each substrate, in comparison with the NCP footprint, will be one nucleotide longer (shifted one peak to the left), as the APE1 endonuclease cleaves on the 5′ side of the lesion, between the uracil residue and the 5′ radiolabeled end.
Unbiased UDG/APE1 Activity at Uracils in Different Sequence Contexts.
It has been reported that the rate of glycosylase activity associated with repair of a modified nucleobase can be greatly affected by DNA sequence context (28, 29). Therefore, we treated the naked uracil-containing DNAs with limiting amounts (0.2 nM) of UDG and APE1 to determine their relative rates of cleavage. After treatment over a course of 60 min, it was clear that the uracils in the U-In and U-Out DNA were digested at very similar rates, whereas the U-Mid DNA construct showed a slightly lower rate of initial cleavage (Fig. S3). Importantly, there appears to be little difference in the relative rates of cleavage among the DNAs, eliminating the possibility that the sequence context of the uracil residues accounts for any measured differences in the enzymatic cleavage of these DNAs associated with NCPs.
Extreme Range of UDG/APE1 Activity Among Different Uracil Orientations in NCPs.
To determine the effect of different uracil orientations on the activity of UDG/APE1, we examined the time courses of cleavage of the three DNA substrates in NCPs. For each of the NCP species, two UDG concentrations were used (with APE1 in equimolar concentrations to UDG), and cleavage of the DNA was assessed over the course of 1 h on denaturing polyacrylamide gels (Fig. S4). Quantification and plotting of the percent cleavage as a function of time revealed that the difference in UDG/APE1 activity was so large between constructs that they could not be compared directly at a common UDG concentration (Fig. 3). For example, at 2 nM UDG, there is measurable cleavage activity among the NCPs and their respective naked DNAs, but cleavage of the U-Out naked DNA and NCP is virtually saturated (Fig. 3). By reducing the UDG/APE1 concentrations 10-fold, a difference between the U-Out naked DNA and the U-Out NCP is seen (Fig. 3, Lower). Thus, the outward oriented uracil shows a histone-associated decrease in UDG activity when the amount of UDG is reduced to a reaction-limiting concentration.
Fig. 3.
Assessment of UDG and APE1 activity on NCP substrates with different uracil orientations. Plots of relative rates of cleavage of each DNA/NCP substrate (U-In, U-Mid, and U-Out in Upper, Middle, and Lower, respectively) over a 1-h time course. Dashed lines represent naked DNA of each construct; solid lines represent NCPs. UDG concentrations are listed on the Right Insets. For each construct, the data for naked DNA and NCPs incubated with 2 nM UDG is denoted by open boxes and open diamonds, respectively. Data for NCPs incubated with 20 nM UDG (For U-In and U-Mid only) are denoted by open triangles; data for naked DNA and NCPs incubated with 0.2 nM UDG (U-Out only) are denoted by open and closed circles, respectively. Each data point represents the mean ± 1 SD of at least three independent experiments.
The U-In and U-Mid NCPs showed extensive reduction in the formation of cleavage products relative to their naked DNA controls, with U-In showing the greatest reduction (Fig. 3, Upper). Some of this cleavage inhibition was overcome by increasing the UDG/APE1 concentration by 10-fold (20 nM), but still did not show the formation of cleavage products close to that of the naked DNAs at 2 nM (Fig. 3, Upper and Middle, respectively). Importantly, although APE1 is used in the reaction to create the strand breaks necessary for formation of the cleavage products, UDG is the rate limiting factor in these measurements, as we found that reducing the APE1 concentration in the reaction to as low as 1% of the UDG concentration did not effect the rates of substrate cleavage. To get a sense of the relative differences in UDG activity among the NCPs, formation of cleavage products in the U-Out NCPs at 0.2 nM UDG is roughly equivalent to that seen for U-Mid NCPs at 100-fold UDG concentration (20 nM) (Fig. 3; compare Middle and Lower). These data highlight the vast differences in the impact of histone binding on activity of the glycosylase due to the orientation of the lesion.
Restricting Movement of DNA in NCPs Changes UDG Activity.
The DNA in NCPs is dynamic on the surface of histone octamers leading to transient, variable states of unwrapping that may perpetuate along the entire length of a DNA strand (18 –20), and it is likely that some movement of the DNA is present throughout the nucleosome core (30). To explore the impact of DNA rotational dynamics on the activity of UDG/APE1 cleavage in our NCPs, we restricted movement of the DNA on the surface of the histone octamers using formaldehyde crosslinking (31, 32). NCPs were treated with 1% formaldehyde for 1 h, followed by subsequent crosslink quenching with glycerol and dialysis. The formation of covalent bonds between the DNA and histones was verified on denaturing gels, in which no evidence of free DNA was found in samples after the crosslinking procedure (Fig. S5).
Formaldehyde crosslinking did not affect the activity of UDG/APE1 on naked DNA but, surprisingly, did lead to increased cleavage of the U-Out NCP (Fig. 4, Upper), bringing the rate of cleavage closer to that of naked DNA. This indicates that in the orientation associated with U-Out, UDG/APE1 activity is optimal, and any movement of the DNA on the histone surface during the course of the reaction presents less favorable orientations. Conversely, crosslinking caused a dramatic decrease in the cleavage rate of the U-In NCPs, virtually eliminating digestion after the first 5 min (Fig. 4, Lower). The early appearance of U-In cleavage products may be due to “locking” of some of the uracils in the histone-crosslinked DNA into more accessible orientations in a small fraction of the NCPs. Because formaldehyde treatment occurs over 1 h, the DNA dynamics in NCPs allow some crosslinks to “lock in” less frequent topological states. This may be affecting U-Out NCPs as well, in which some of these NCPs may be crosslinked in less favorable conformations (flattening the cleavage curve in the crosslinked U-Out sample, as in Fig. 4, Upper). Finally, the U-Mid NCPs did not appear to be significantly affected by formaldehyde treatment (Fig. 4, Lower). This may reflect the movement of uracil into both favorable and unfavorable transient states with roughly equal but opposite impacts on UDG accessibility. Thus, preventing U-Mid from vacillating into these different orientations would have a minimal effect on the rate of cleavage.
Fig. 4.
UDG and APE1 cleavage of formaldehyde crosslinked NCPs. (Upper) Time course after Incubation with 0.2 nM UDG of Naked DNA (squares) and U-Out NCPs (triangles) with and without 1 h preincubation with formaldehyde (open symbols/dashed lines and closed symbols/solid lines, respectively). (Lower) Time course after incubation with 20 nM UDG of U-In (circles) and U-Mid NCPs (diamonds) with and without 1 h preincubation with formaldehyde (open symbols/dashed lines and closed symbols/solid lines, respectively). Each data point represents the mean ± 1 SD of at least three independent experiments.
APE1 Stimulates hUDG Activity on NCPs.
Some glycosylases have very high affinity for AP sites (their reaction product), and their rate of activity is reduced over time by the removal of free glycosylase enzymes from the reaction, a process known as “product inhibition” (33, 34). APE1 has been shown to enhance efficiency of these glycosylases by releasing the enzyme from its product or preventing rebinding to the AP site, and thereby recycling the enzyme for binding to other lesions. APE1 has also been shown to enhance the efficiency of human UDG (hUDG, commonly known as hUNG2) (35), presumably by the same mechanism. Although it is not known whether APE1 stimulation of hUDG occurs in the context of NCPs, the presence (or absence) of APE1 in previous assessments of UDG activity on NCPs has been suggested to be a factor in discrepancies associated with the impact of uracil orientation (36). Whereas UDG activity appeared to be unaffected by uracil orientation when measuring uracil base excision (done in the absence of APE1) (21), measuring cleavage of the DNA as an indicator of UDG activity (with APE1) has shown orientation dependence in this study and elsewhere (22). We wished to determine whether APE1 stimulates hUDG activity in the context of NCPs, in addition to determining whether the effect of histones on UDG activity is independent of APE1.
We first verified that hUDG shows the broad range of activity among the uracil orientations seen in the recombinant E.coli UDG used in the previous experiments. We measured the relative rates of cleavage among the different NCP and naked DNA substrates in the absence of APE1 to prevent the potential for an APE1 effect on the activity of hUDG. To reveal hUDG activity, the DNA was purified after incubations with the catalytic domain of hUDG and treated with APE1 to allow complete cleavage at all abasic sites in the DNA. As with E. coli UDG, we found a large difference in formation of cleavage products between the U-In and U-Out NCPs, with U-Mid NCPs closely mimicking the U-In orientation (Fig. 5). Also, like the E. coli enzyme, a 10-fold increase in hUDG concentration (20 nM) was necessary for the U-In and U-Mid NCPs for reliable quantification over the time course (at 20 nM UDG, both naked DNA and U-Out NCP samples are completely cleaved within 15 min). Thus, it is clear that human UDG is also greatly affected by the orientation of uracil residues in NCPs, and that the absence of APE1 does not abrogate the effect.
Fig. 5.
Relative rates of hUDG and APE1-directed cleavage DNA and NCP constructs. Naked DNA (open triangles with dashed line) and NCP substrates U-In (small filled squares), U-mid (filled triangles), and U-Out (large filled squares) are represented. The hUDG concentration for the naked and U-Out NCPs is 2 nM, and for the U-In and U-Mid NCPs is 20 nM. Each data point represents the mean ± 1 SD of at least three independent experiments.
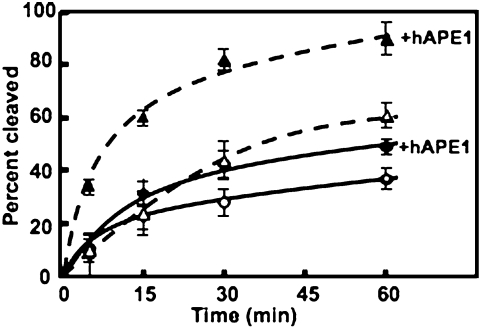
To examine the possibility that the AP endonuclease may promote glycosylase activity in the context of histones, we repeated hUDG activity experiments in the presence of equimolar concentrations of APE1. APE1-enhancement of hUDG activity is clearly measurable on naked DNA substrates, enhancing the rate of cleavage by ∼2- to 3-fold (Fig. 6, dashed lines). It also appears that hUDG activity is enhanced by the presence of APE1 on U-Out NCPs, although not to the same extent as naked DNA (∼1.3- to 1.5-fold). However, unlike the naked DNA and U-Out NCPs, the enhancement of hUDG activity in the presence of equimolar concentrations of APE1 was not detected in the U-In and U-Mid NCPs (Fig. S6). Thus, the association of DNA with histones does not eliminate APE1-dependent enhancement of hUDG activity in the solvent-exposed uracil orientation.
Fig. 6.
Influence of APE1 on hUDG activity in naked DNA and U-Out NCPs. Naked DNA (triangles and dashed lines) or U-Out NCPs (circles and solid lines) incubated with hUDG in the presence (filled symbols) or absence (open symbols) of APE1. Each data point represents the mean ± 1 SD of at least three independent experiments.
Discussion
Glycosylase recognition of a damaged substrate initiates the BER process by direct lesion binding. Bulky chemical adducts and UV light–induced pyrimidine dimers, repaired by nucleotide excision repair (NER), lead to distortions in the topology of the DNA helix. Some of these lesions can alter nucleosome structure (25, 37) and many disrupt transcription elongation complexes (8, 38 –40). However, most chemical modifications recognized by glycosylases cause comparatively small perturbations in DNA, with little evidence for preferential nucleosome perturbations or, in most cases, blockage of transcription (41 –43). Thus, in the cell, there appears to be a heavy reliance on intrinsic accessibility to damaged chromatin-associated DNA bases by glycosylases.
In this study, we found extreme differences in the rates of UDG activity dependent on the orientation of the uracil relative to the histone surface near the dyad center of NCPs, pointing to a clear effect of lesion proximity to histones. The effect of orientation in our study was substantially greater than that measured in previous studies on UDG and hNTH1 (21 –23). However, after submission of this manuscript, another report appeared showing a reduction of ∼3,000- to ∼10,000-fold in UDG activity at uracils oriented toward histones in NCPs (44), in close agreement with our results.
Variation in DNA sequence may account for some of the differences among the previous studies, including the use of Lytechinus variegatus 5S rDNA as the NCP positioning sequence (21, 23). This sequence has reduced histone binding energy and multiple translational settings compared to the sequence used in this study (45). To improve homogeneity in our NCP preparations, we used the strongly binding TG nucleosome positioning sequence. The minimal impact of uracil orientation in a previous study that also used the TG positioning motifs (22) is explained in a subsequent study from our laboratory, in which the actual orientations of the uracil positions used were assessed by hydroxyl radical footprinting (46). We found that the two orientations used by Beard et al. (22) may not have represented truly “in” and “out” orientations but, rather, shifted one or two bases from the vertical axis (46). Considering the wide range of orientations possible on the surface of histones for different sequences, assessment of the NCPs via hydroxyl radical footprinting was critical for our choice of base orientation in this study.
The crosslinking experiments proved to be particularly revealing of the importance of DNA dynamics on histones in exposing unfavorable base orientations to the solvent. As expected, the crosslinked U-In NCP showed very little UDG activity, at least after the first 5 min, highlighting the necessity for DNA movement on the histone surface for transient exposure of unfavorable helical orientations.
Surprisingly, the U-Out NCPs showed an increase in the rate of DNA cleavage after crosslinking, suggesting that crosslinking restricts movement of the DNA that restrains some bases to favorable positions. Indeed, results with the U-Mid NCP, which showed little difference between the crosslinked and noncrosslinked states, point to a similar conclusion. Our data showing increased DNA cleavage in U-Mid NCPs with a higher UDG concentration suggest transient states of increased glycosylase accessibility. However, because immobilization of the DNA did not affect the measured UDG activity on U-Mid NCPs, uracil residues in the U-Mid orientation in noncrosslinked NCPs must be vacillating between both more accessible and less accessible orientations. Thus, our crosslinking results suggest that, at least near the dyad center of NCP DNA, access to uracils by UDG is driven more by rotational dynamics of DNA on the histone surface than by transient unwrapping.
The decrease in glycosylase activity that we found for the outward oriented lesion, relative to naked DNA, despite the otherwise “optimal” positioning, was also seen in the previous glycosylase studies using NCPs (21 –23). One explanation is that this may be due to the interference by the histones of one-dimensional glycosylase “scanning” along the DNA, a mechanism proposed for detection of damaged substrates (35, 47, 48). Our crosslinking results, showing increased UDG activity in the immobilized U-Out NCPs, approaching the rates measured for naked DNA, suggest that at least some of the histone-associated glycosylase inhibition is due to transient rotation of uracil into less accessible orientations. However, we cannot eliminate the possibility that a decrease in glycosylase scanning may account for some of the reduced UDG activity on NCPs, as the rate of cleavage in the U-Out NCPs did not equal that measured with naked DNA, even after crosslinking.
The high affinity of glycosylases for AP sites is thought to be an important factor in protecting these sites, and potentially recruiting the next enzyme in the BER pathway (APE1). This process of presenting the product of each reaction as a viable substrate for the next repair step is referred to as “passing the baton” (4). This accounts for the product inhibition associated with UDG activity, which is overcome by the “hand-off” of the abasic site to the AP endonuclease. We saw clear evidence of the APE1 stimulation of hUDG activity in the naked DNA and U-Out NCP, indicating that the association of DNA with histones does not greatly affect the ability of AP endonuclease to remove the glycosylase from the abasic repair intermediate, at least at an AP site facing away from the histone core. Because we did not find an APE1-associated increase in cleavage of the U-In and U-Mid NCPs (Fig. S5), it is tempting to speculate that, at unfavorable orientations, there is a histone-associated impairment of the endonuclease to recycle the glycosylase from its product, or that APE1 is prevented from accessing buried abasic sites. However, the 20-nM concentration of hUDG in the U-In and U-Mid NCP cleavage assays (necessary for quantifiable detection of cleavage) is in vast excess (10-fold) relative to the uracil-containing NCP substrates. Thus, whereas turnover of the limited amount of hUDG is important for maintaining high rates of cleavage in the naked DNA and U-Out NCPs (where the enzyme to substrate ratio is close to unity), any APE1-associated effect on hUDG recycling during the U-In and U-Mid NCP reactions would provide a negligible increase in the available pool of hUDG in the reaction. This punctuates the conclusion that, in the U-In and U-Mid NCPs, it is the inability of the glycosylase to access the lesion that is responsible for the reduction of hUDG activity between the unfavorable uracil orientations.
There are undoubtedly many modes of motion among the protein and DNA molecules that make up chromatin. In the more open nucleosome-loaded regions, the intrinsic, spontaneous dynamics of nucleosomes are likely contributing to the control of DNA binding factors and enzyme access to DNA, including proteins responsible for recognizing DNA lesions. Our results suggest that at least some regions of DNA in chromatin are likely afforded little access to DNA-binding proteins, and that rotational motion of the DNA may be the primary mechanism by which glycosylases can access these buried sites. Furthermore, the marked difference in rates of glycosylase activity among the uracil orientations indicate a potential persistence of lesions facing toward the histones and thereby influencing mutagenesis. Indeed, recent studies have found the highest occurrence of single nucleotide polymorphisms (SNPs) among human CpG sequences in regions of closed chromatin (49), and a 146-nucleotide periodicity of SNPs in transcription start sites among humans (50). These results may reflect the conserved placement of nucleosomes in these regions, and point to the direct impact of histones on mutation rate.
Materials and Methods
Detailed methods are provided in SI Text.
Oligonucleotide Synthesis, 32P Labeling, and NCP Reconstitution.
Double-strand DNA substrates for study contained a central 15 bp GRE sequence flanked by the TG nucleosomal positioning sequences (13, 24, 25), and were created by annealing of 150 base oligonucleotides (synthesized by solid phase synthesis by Midland Certified Reagent Company). Three of the oligos (U-IN, U-MID, and U-OUT) each contained uracils at a different position within the GRE; one oligo (UND) had the same GRE sequence with no uracils; and one oligo (PARTNER) served as the partner strand for annealing with the rest. Sequences of the GRE at the center of each of the four oligos are as follows: GRE: TGTACAGCATGTTCT; U-In: TGTACAGCATGTTUT; U-Mid: TGTAUAGCATGTTCT; U-Out: TGTACAGUATGTTCT. Before annealing with the partner strand, oligos were radiolabeled with 32P at the 5′ ends with T4 polynucleotide kinase (Invitrogen) and γ-[32P]ATP (PerkinElmer) for 30 min at 37° C. Mononucleosomes were prepared by histone octamer transfer, combining the radiolabeled 150-bp DNA substrates with chicken erythrocyte core particles prepared from chicken erythrocytes (51) at high ionic strength, and subsequent incremental diaylsis as described previously (25, 46).
UDG/hUDG and APE1 Digestion.
Treatment of DNAs and NCPs with recombinant E.coli UDG (Ung; New England Biolabs) or the catalytic domain of hUDG (hUNG2 with N-terminal deletion of 84-amino acids entailing single-strand DNA and RPA binding domains (52); kindly provided by Samuel Wilson (National Institute on Environmental Health Sciences) and human APE1 (New England Biolabs) were initiated by adding UDG or hUDG and APE to final concentrations of 0.2, 2, or 20 nM each, with APE1 concentrations always equal to UDG concentrations unless otherwise specified. Incubations were at 37°C for 5, 15, 30, and 60 min. Reactions were terminated with addition phenol:chloroform:isopropanol (PCI; 20:19:1) at equal volume to the reaction, and DNA recovered by ethanol precipitation. For reactions with hUDG in the absence of APE1, the reaction conditions were run as described above without APE1. After DNA isolation, a second digestion reaction was run on the naked DNA, with only APE1 at 2 nM for 30 min at 37°C to reveal all of the abasic sites. All naked DNA samples had chicken erythrocyte core particles added to a concentration of 300 pM to adjust for the excess core particles present in reconstituted nucleosome samples. Digested DNAs were separated on 10% polyacrylamide (0.5% bisacrylamide) 7M Urea denaturing gels in 1× TBE buffer, exposed to PhosphorImager screens (Molecular Dynamics), visualized on a STORM 840 PhosphorImager (Amersham), and images were analyzed with IMAGEQUANT software (Molecular Dynamics).
Formaldehyde Crosslinking and Hydroxyl Radical Footprinting.
To covalently crosslink DNA to associated histones, NCPs were treated with 1% formaldehyde (from 37% stock) on ice for 1 h, after which crosslinking activity was quenched by addition of glycerol to a final concentration of 1%. Formaldehyde- and mock-treated samples were dialyzed in NCP reconstitution buffer (10 mM Tris, pH7.5, 1 mM EDTA, 50 mM NaCl) for 1 h at 4°C to remove glycerol and formaldehyde. UDG/APE1 digestions were performed as described above, but reactions were terminated with addition of EDTA (to 40 mM) and boiled for 1 min to eradicate repair enzyme activity. Samples were then treated with high NaCl (to 1 M) for 5 h at 65°C to remove crosslinks. DNA was subsequently isolated by PCI and ethanol precipitation and separated and analyzed as described above. Hydroxyl radical footprinting of the NCPs was performed as described previously (53). For uracil location control samples, DNAs containing uracil were each treated with 20 nM UDG and 10 nM APE1 and incubated at 37°C for 60 min.
Supplementary Material
Acknowledgments
The authors thank Dr. Samuel Wilson, National Institute on Environmental Health Sciences (NIEHS), for providing hUDG and Dr. Raymond Reeves, Washington State University, for providing chicken erythrocyte NCPs. This study was supported by National Institutes of Health (NIH) Grants ES004106 and ES002614 from the NIEHS.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914443107/DCSupplemental.
References
- 1.Friedberg EC, et al. DNA Repair and Mutagenesis. Washington, DC: ASM Press; 2006. [Google Scholar]
- 2.Seeberg E, Eide L, Bjørås M. The base excision repair pathway. Trends Biochem Sci. 1995;20:391–397. doi: 10.1016/s0968-0004(00)89086-6. [DOI] [PubMed] [Google Scholar]
- 3.Memisoglu A, Samson L. Base excision repair in yeast and mammals. Mutat Res. 2000;451:39–51. doi: 10.1016/s0027-5107(00)00039-7. [DOI] [PubMed] [Google Scholar]
- 4.Dianov GL, Parsons JL. Co-ordination of DNA single strand break repair. DNA Repair (Amst) 2007;6:454–460. doi: 10.1016/j.dnarep.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto Y, Kim K. Excision of deoxyribose phosphate residues by DNA polymerase beta during DNA repair. Science. 1995;269:699–702. doi: 10.1126/science.7624801. [DOI] [PubMed] [Google Scholar]
- 6.Cappelli E, et al. Involvement of XRCC1 and DNA ligase III gene products in DNA base excision repair. J Biol Chem. 1997;272:23970–23975. doi: 10.1074/jbc.272.38.23970. [DOI] [PubMed] [Google Scholar]
- 7.Dianova II, et al. XRCC1-DNA polymerase beta interaction is required for efficient base excision repair. Nucleic Acids Res. 2004;32:2550–2555. doi: 10.1093/nar/gkh567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Allis CD, Jenuwein T, Reinberg D, Caparros ML. Epigenetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2007. [Google Scholar]
- 10.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 12.Golding A, Chandler S, Ballestar E, Wolffe AP, Schlissel MS. Nucleosome structure completely inhibits in vitro cleavage by the V(D)J recombinase. EMBO J. 1999;18:3712–3723. doi: 10.1093/emboj/18.13.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Q, Wrange O. Accessibility of a glucocorticoid response element in a nucleosome depends on its rotational positioning. Mol Cell Biol. 1995;15:4375–4384. doi: 10.1128/mcb.15.8.4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolffe AP, Kurumizaka H. The nucleosome: A powerful regulator of transcription. Prog Nucleic Acid Res Mol Biol. 1998;61:379–422. doi: 10.1016/s0079-6603(08)60832-6. [DOI] [PubMed] [Google Scholar]
- 15.Gong F, Kwon Y, Smerdon MJ. Nucleotide excision repair in chromatin and the right of entry. DNA Repair (Amst) 2005;4:884–896. doi: 10.1016/j.dnarep.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Wrange O. Translational positioning of a nucleosomal glucocorticoid response element modulates glucocorticoid receptor affinity. Genes Dev. 1993;7(12A):2471–2482. doi: 10.1101/gad.7.12a.2471. [DOI] [PubMed] [Google Scholar]
- 17.Clapier CR, Cairns BR. The biology of chromatin remodeling complexes. Annu Rev Biochem. 2009;78:273–304. doi: 10.1146/annurev.biochem.77.062706.153223. [DOI] [PubMed] [Google Scholar]
- 18.Anderson JD, Widom J. Sequence and position-dependence of the equilibrium accessibility of nucleosomal DNA target sites. J Mol Biol. 2000;296:979–987. doi: 10.1006/jmbi.2000.3531. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JD, Thåström A, Widom J. Spontaneous access of proteins to buried nucleosomal DNA target sites occurs via a mechanism that is distinct from nucleosome translocation. Mol Cell Biol. 2002;22:7147–7157. doi: 10.1128/MCB.22.20.7147-7157.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li G, Levitus M, Bustamante C, Widom J. Rapid spontaneous accessibility of nucleosomal DNA. Nat Struct Mol Biol. 2005;12:46–53. doi: 10.1038/nsmb869. [DOI] [PubMed] [Google Scholar]
- 21.Nilsen H, Lindahl T, Verreault A. DNA base excision repair of uracil residues in reconstituted nucleosome core particles. EMBO J. 2002;21:5943–5952. doi: 10.1093/emboj/cdf581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beard BC, Wilson SH, Smerdon MJ. Suppressed catalytic activity of base excision repair enzymes on rotationally positioned uracil in nucleosomes. Proc Natl Acad Sci USA. 2003;100:7465–7470. doi: 10.1073/pnas.1330328100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prasad A, Wallace SS, Pederson DS. Initiation of base excision repair of oxidative lesions in nucleosomes by the human, bifunctional DNA glycosylase NTH1. Mol Cell Biol. 2007;27:8442–8453. doi: 10.1128/MCB.00791-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrader TE, Crothers DM. Effects of DNA sequence and histone-histone interactions on nucleosome placement. J Mol Biol. 1990;216:69–84. doi: 10.1016/S0022-2836(05)80061-0. [DOI] [PubMed] [Google Scholar]
- 25.Kosmoski JV, Smerdon MJ. Synthesis and nucleosome structure of DNA containing a UV photoproduct at a specific site. Biochemistry. 1999;38:9485–9494. doi: 10.1021/bi990297h. [DOI] [PubMed] [Google Scholar]
- 26.Frederico LA, Kunkel TA, Shaw BR. Cytosine deamination in mismatched base pairs. Biochemistry. 1993;32:6523–6530. doi: 10.1021/bi00077a005. [DOI] [PubMed] [Google Scholar]
- 27.Tullius TD, Dombroski BA, Churchill ME, Kam L. Hydroxyl radical footprinting: A high-resolution method for mapping protein-DNA contacts. Methods Enzymol. 1987;155:537–558. doi: 10.1016/0076-6879(87)55035-2. [DOI] [PubMed] [Google Scholar]
- 28.Nilsen H, Yazdankhah SP, Eftedal I, Krokan HE. Sequence specificity for removal of uracil from U.A pairs and U.G mismatches by uracil-DNA glycosylase from Escherichia coli, and correlation with mutational hotspots. FEBS Lett. 1995;362:205–209. doi: 10.1016/0014-5793(95)00244-4. [DOI] [PubMed] [Google Scholar]
- 29.Xia L, et al. Human 3-methyladenine-DNA glycosylase: Effect of sequence context on excision, association with PCNA, and stimulation by AP endonuclease. J Mol Biol. 2005;346:1259–1274. doi: 10.1016/j.jmb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Zlatanova J, Bishop TC, Victor JM, Jackson V, van Holde K. The nucleosome family: Dynamic and growing. Structure. 2009;17:160–171. doi: 10.1016/j.str.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Solomon MJ, Varshavsky A. Formaldehyde-mediated DNA-protein crosslinking: A probe for in vivo chromatin structures. Proc Natl Acad Sci USA. 1985;82:6470–6474. doi: 10.1073/pnas.82.19.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGhee JD, von Hippel PH. Formaldehyde as a probe of DNA structure. II. Reaction with endocyclic imino groups of DNA bases. Biochemistry. 1975;14:1297–1303. doi: 10.1021/bi00677a030. [DOI] [PubMed] [Google Scholar]
- 33.Waters TR, Gallinari P, Jiricny J, Swann PF. Human thymine DNA glycosylase binds to apurinic sites in DNA but is displaced by human apurinic endonuclease 1. J Biol Chem. 1999;274:67–74. doi: 10.1074/jbc.274.1.67. [DOI] [PubMed] [Google Scholar]
- 34.Hill JW, Hazra TK, Izumi T, Mitra S. Stimulation of human 8-oxoguanine-DNA glycosylase by AP-endonuclease: Potential coordination of the initial steps in base excision repair. Nucleic Acids Res. 2001;29:430–438. doi: 10.1093/nar/29.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parikh SS, et al. Base excision repair initiation revealed by crystal structures and binding kinetics of human uracil-DNA glycosylase with DNA. EMBO J. 1998;17:5214–5226. doi: 10.1093/emboj/17.17.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jagannathan I, Cole HA, Hayes JJ. Base excision repair in nucleosome substrates. Chromosome Res. 2006;14:27–37. doi: 10.1007/s10577-005-1020-7. [DOI] [PubMed] [Google Scholar]
- 37.Mann DB, Springer DL, Smerdon MJ. DNA damage can alter the stability of nucleosomes: Effects are dependent on damage type. Proc Natl Acad Sci USA. 1997;94:2215–2220. doi: 10.1073/pnas.94.6.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Studitsky VM, Walter W, Kireeva M, Kashlev M, Felsenfeld G. Chromatin remodeling by RNA polymerases. Trends Biochem Sci. 2004;29:127–135. doi: 10.1016/j.tibs.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 39.van Hoffen A, Balajee AS, van Zeeland AA, Mullenders LH. Nucleotide excision repair and its interplay with transcription. Toxicology. 2003;193:79–90. doi: 10.1016/j.tox.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Perlow RA, et al. DNA adducts from a tumorigenic metabolite of benzo[a]pyrene block human RNA polymerase II elongation in a sequence- and stereochemistry-dependent manner. J Mol Biol. 2002;321:29–47. doi: 10.1016/s0022-2836(02)00593-4. [DOI] [PubMed] [Google Scholar]
- 41.Tornaletti S, Maeda LS, Lloyd DR, Reines D, Hanawalt PC. Effect of thymine glycol on transcription elongation by T7 RNA polymerase and mammalian RNA polymerase II. J Biol Chem. 2001;276:45367–45371. doi: 10.1074/jbc.M105282200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tornaletti S, Maeda LS, Kolodner RD, Hanawalt PC. Effect of 8-oxoguanine on transcription elongation by T7 RNA polymerase and mammalian RNA polymerase II. DNA Repair (Amst) 2004;3:483–494. doi: 10.1016/j.dnarep.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Wang W, Sitaram A, Scicchitano DA. 3-Methyladenine and 7-methylguanine exhibit no preferential removal from the transcribed strand of the dihydrofolate reductase gene in Chinese hamster ovary B11 cells. Biochemistry. 1995;34:1798–1804. doi: 10.1021/bi00005a037. [DOI] [PubMed] [Google Scholar]
- 44.Cole HA, Tabor-Godwin JM, Hayes JJ. Uracil DNA glycosylase activity on nucleosomal DNA depends on rotational orientation of targets. J Biol Chem. 2010;285:2876–2885. doi: 10.1074/jbc.M109.073544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flaus A, Luger K, Tan S, Richmond TJ. Mapping nucleosome position at single base-pair resolution by using site-directed hydroxyl radicals. Proc Natl Acad Sci USA. 1996;93:1370–1375. doi: 10.1073/pnas.93.4.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Svedruzić ZM, Wang C, Kosmoski JV, Smerdon MJ. Accommodation and repair of a UV photoproduct in DNA at different rotational settings on the nucleosome surface. J Biol Chem. 2005;280:40051–40057. doi: 10.1074/jbc.M509478200. [DOI] [PubMed] [Google Scholar]
- 47.Higley M, Lloyd RS. Processivity of uracil DNA glycosylase. Mutat Res. 1993;294:109–116. doi: 10.1016/0921-8777(93)90019-d. [DOI] [PubMed] [Google Scholar]
- 48.Bennett SE, Sanderson RJ, Mosbaugh DW. Processivity of Escherichia coli and rat liver mitochondrial uracil-DNA glycosylase is affected by NaCl concentration. Biochemistry. 1995;34:6109–6119. doi: 10.1021/bi00018a014. [DOI] [PubMed] [Google Scholar]
- 49.Prendergast JG, et al. Chromatin structure and evolution in the human genome. BMC Evol Biol. 2007;7:72. doi: 10.1186/1471-2148-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Higasa K, Hayashi K. Periodicity of SNP distribution around transcription start sites. BMC Genomics. 2006;7:66. doi: 10.1186/1471-2164-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Libertini LJ, Small EW. Salt induced transitions of chromatin core particles studied by tyrosine fluorescence anisotropy. Nucleic Acids Res. 1980;8:3517–3534. doi: 10.1093/nar/8.16.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kavli B, et al. hUNG2 is the major repair enzyme for removal of uracil from U:A matches, U:G mismatches, and U in single-stranded DNA, with hSMUG1 as a broad specificity backup. J Biol Chem. 2002;277:39926–39936. doi: 10.1074/jbc.M207107200. [DOI] [PubMed] [Google Scholar]
- 53.Bashkin J, Hayes JJ, Tullius TD, Wolffe AP. Structure of DNA in a nucleosome core at high salt concentration and at high temperature. Biochemistry. 1993;32:1895–1898. doi: 10.1021/bi00059a002. [DOI] [PubMed] [Google Scholar]
- 54.Davey CA, Sargent DF, Luger K, Maeder AW, Richmond TJ. Solvent mediated interactions in the structure of the nucleosome core particle at 1.9 a resolution. J Mol Biol. 2002;319:1097–1113. doi: 10.1016/S0022-2836(02)00386-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.