Abstract
Enhanced redox-stress caused by neuroinflammation, mitochondria, and NADPH oxidases has been hypothesized to play critical roles in disease progression of amyotrophic lateral sclerosis (ALS). However, distinguishing whether the redox-stress observed in ALS is due to a primary defect in cellular reactive oxygen species metabolism/catabolism, or is a secondary consequence of neuroinflammation, has been difficult and the issue remains a matter of debate. Emerging evidence suggests that defects in genes that regulate NADPH oxidases may account for at least some forms of ALS. NADPH oxidases are key signaling complexes that influence cellular responses to growth factors and cytokines. In this context, NADPH oxidase-derived reactive oxygen species exert spatial control over the redox-dependent activation of certain pro-inflammatory receptors. Understanding the biology of how NADPH oxidases control cell signaling may help to clarify how genetic determinants of ALS lead to dysregulated pro-inflammatory signaling. This review provides a framework for understanding endosomal signaling through NADPH oxidases and potential mechanisms whereby gene defects in various forms of ALS may influence this cellular process and lead to motor neuron degeneration. Lastly, this review discusses past and current efforts to treat ALS using antioxidant therapies, as well as the limitations and advantages of each of these approaches. Antioxid. Redox Signal. 11, 1569–1586.
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive and lethal degenerative disorder of upper and lower motor neurons in the brain and spinal cord. ALS disease onset is insidious and progresses linearly from time of diagnosis, with death occurring in most of the patients within 3–5 years. Most cases begin after age 40 and the mean age at onset is 58 years. About 50% of patients die within 3 years, and ∼90% die within 5 years, while only a small proportion survive beyond 8 years. About 10% of ALS cases are clearly genetic or familial (FALS), with the rest being classified as sporadic (SALS).
Despite a great deal of academic, societal, and corporate interest in determining the root cause of the disease and developing adequate drugs and treatment regimens, to date only one drug—riluzole (Rilutektm)—has been approved for the treatment of ALS (28). However, the mechanism of action of riluzole is unclear and it has been estimated to prolong life by only ∼3 months. Otherwise, treatments for ALS are largely palliative, and developing a new therapy for ALS remains a high priority. Thus, like many other progressive neurodegenerative diseases such as Parkinson's, Alzheimer's, and Huntington's disease, ALS represents a largely unmet medical need (28, 46, 125). Studies on rare familial forms of several of these diseases have provided insights into the molecular basis of their pathogenesis (20, 52, 109), and are beginning to reveal a general unifying set of factors in these slowly progressing diseases. These include protein aggregation, mitochondrial injury, inflammatory response, and nitrosative and oxidative stress.
ALS is a multigenic disease with a complex etiology (20, 46). Sporadic ALS cases may result from interactions between genetic and environmental components, each of which may contribute to the pathophysiology of the disease. Most, but not all, FALS exhibits an autosomal dominant pattern of inheritance, and several FALS-associated mutant genes have been identified (109). Most notable among these is the gene encoding superoxide dismutase type 1 (SOD1) (117). Recently, analysis of one such mutant gene, SOD1G93A, in animal models has provided important insights into the mechanism underlying the dominant, or toxic gain-of-function, phenotype and its potential to lead to oxidative damage (55, 90). Similarly, research on a rare juvenile form of ALS caused by mutations in the ALS2 gene has uncovered potential new paradigms for pathogenesis (26, 54, 144). Interestingly, as discussed in this review, the mechanisms involved in ALS pathogenesis in the context of ALS2 and SOD1 mutations overlap more extensively than previously thought.
Much of the difficulty in developing effective therapies for ALS reflects our relatively poor understanding of ALS pathogenesis (20, 46, 112). For instance, an extremely diverse set of biochemical processes has been implicated in the pathogenesis of ALS disease:
glutamate-mediated excitotoxicity
mitochondrial dysfunction
neuroinflammation (microglial proliferation)
apoptosis
protein aggregation
autophagy
abnormal axonal transport
oxidative stress
Several of these events—including glutamate excitotoxicity, mitochondrial dysfunction, and neuroinflammation—are discussed in detail in other reviews in this Forum issue.
One key hypothesis for the genesis of ALS is that motor neuron damage initially results as a consequence of oxidative stress (7). However, there is significant uncertainty as to whether the toxicity is mediated only by events that occur primarily in neurons, or if non-neuronal cells contribute as well. Recent evidence suggests that the neurodegeneration is non-cell autonomous (i.e., not driven by events solely within the neuron), and that both neuronal cells and non-neuronal microglia are involved in ALS pathogenesis (13, 29, 86), even though degeneration is manifested only in the neurons. This review attempts to place in context potential pathologic mechanisms and disease-causing genes that may regulate oxidative stress in ALS.
Oxidative damage is known to increase with age, and the main risk factors for ALS—those that are consistently identified in epidemiology studies—are increasing age, male gender, and smoking (5, 10, 38). Moreover, biochemical markers of oxidative injury are abnormally high in postmortem samples from ALS patients (126). Oxidative stress results when the cell's ability to generate reactive oxygen species (ROS) exceeds its antioxidant capacity; when ROS or other radicals are not detoxified by antioxidants, cellular macromolecules (phospholipids, DNA, proteins) become targets for oxidative modifications that may compromise their function (46). In addition, other mechanisms that have been proposed to play a role in ALS disease, such as excitotoxcity and defective axonal transport, may be consequences of oxidative stress (21, 46). Excitatory neurotransmission and the metabolism of these neurotransmitters are sources of oxidative stress in cells such as motor neurons, which receive high levels of excitatory input. Mechanisms that lead to glutamate excitotoxicity include the disruption of intracellular calcium homeostasis and increased ROS production due to perturbed mitochondrial function and activation of calcium-dependent enzymes. The latter include neuronal nitric oxide synthase (nNOS), which catalyzes the production of nitric oxide (57). Both superoxide and nitric oxide are produced in abundant quantity by phagocytes, including microglia of the nervous system (21, 141). There is also evidence for increased oxidative damage in both sporadic ALS and a familial form that is associated with mutations in SOD1 (42, 141). Although the cause of disease initiation and progression in human patients is not known, and it also remains unclear whether oxidative damage occurs as a secondary consequence of the initiating toxic events, sequelae involving both motor neurons and surrounding glia are likely to contribute to neuronal death. Thus, mitigating ROS overproduction would be expected to prevent or retard the progression of this neurodegenerative disease. However, in clinical trials with antioxidants, ALS patients have not demonstrated a survival benefit (105). Hence, it appears that our understanding of the mechanisms that underlie the disease processes in ALS is not yet sufficiently advanced for us to rationally design antioxidant therapies (32, 81).
Genes known to cause juvenile (ALS2) and familial (SOD1) forms of ALS, as well as those closely linked to sporadic forms, are known to influence the activity of the small GTPase Rac1, which regulates several NADPH oxidase (Nox) complexes. Additionally, Nox1 and Nox2 appear to influence disease progression in the SOD1G93A mouse model, whereas Nox4 appears to be closely linked to sporadic ALS. Together, these finding suggest the intriguing possibility that multiple distinct defects in the regulation of Rac/Nox pathways may lead to enhanced redox-stress in ALS. This review will discuss the field's current understanding of how NADPH oxidases can control cell signaling and the relevance of these pathways to the pathoprogression and treatment of ALS.
NADPH Oxidases Influence Disease Progression in a SOD1G93A Transgenic Mouse Model of Familial ALS
Redox-stress is thought to be an important component of disease progression in ALS (7, 109). For example, as was discovered more than 10 years ago, protein carbonyl and nitrotyrosine modifications (both markers of oxidative protein damage) occur at unusually high levels in the spinal cords of ALS patients (1, 2, 8, 122). What remains significantly less clear is whether this increased redox-stress is a primary defect or a secondary consequence of disease. Recently, two studies have shed some light on the involvement of NADPH oxidases in the disease progression observed in a familial mouse model of ALS that overexpresses a human SOD1G93A transgene. NADPH oxidases are multi-subunit enzyme complexes that generate superoxide (O2•−) by transferring an electron from NADPH to molecular oxygen. Seven known NADPH oxidase catalytic subunits exist (Nox1, Nox2gp91phox, Nox3, Nox4, Nox5, Duox1, and Duox2) (60, 80). The most widely characterized of these is phagocytic gp91phox (also known as Nox2), which is also expressed in microglia (141) and a variety of other nonphagocytic cell types. Rac, a small GTPase, is an essential activator of Nox2 and Nox1, along with several other subunits (p40phox, p47phox, p67phox, NoxO1, and NoxA1) that can act to promote Nox complex activation in a cell type-specific fashion (60, 80). Since NADPH oxidases (Nox) generate the O2•− substrate of the SOD1 dismutation reaction (2O2•− + 2H+ → H2O2 + O2) (94), this class of Nox enzymes has recently generated considerable interest in studies of ALS.
A recent study has demonstrated that SOD1G93A ALS transgenic mice produce elevated levels of Nox2 and O2•− in spinal cord microglia (141). This same study demonstrated that Nox2-deficient SOD1G93A congenic C57BL/6J mice live, on average, 13 days longer than Nox2-WT SOD1G93A mice on the same background (increase from 122 to 135 days). Increased survival was accompanied by reduced ROS in the spinal cord (141). A subsequent study demonstrated that both Nox1 and Nox2 influence the survival of SOD1G93A ALS transgenic mice on a B6SJL hybrid background (90). In this study, homozygous deletion of either Nox1 or Nox2 significantly delayed disease onset, and enhanced the survival of hemizygous SOD1G93A ALS mice. Deletion of the Nox2 gene had the greatest impact on survival (97 days difference; 132–229 days), and also led to a four-fold increase in the survival index (time to death after onset of disease). Nox1 deficiency gave rise to a much smaller, but still significant, increase in survival (33 days difference; 129–162 days), but did not influence the survival index. Additional results from this study demonstrated reduced ROS production and microglial activation in the spinal cords, as well as increased rotarod performance and stride length, in Nox2-deficient SOD1G93A ALS mice, consistent with changes in disease onset (90). Although these studies differed with respect to the magnitude of their findings, collectively they led to the conclusion that both Nox1 and Nox2 are NADPH oxidase isoforms that influence disease progression in the SOD1G93A ALS mouse model, but that Nox2 plays a more major role.
The reason for differences in outcomes between the above-described studies (90, 141) remains unclear, but likely has to do with background variation in the mice used to study Nox2 involvement. Indeed, differences in genetic background have been shown to influence the survival of hemizygous SOD1G93A transgenic mice (58, 139), with a 14–28 day increase in survival on the C57BL/6 congenic, as compared to the B6SJL hybrid, background. Currently, it is hypothesized that multiple SJL-derived modifier genes act in concert with Nox2-deficiency to significantly enhance the survival of SOD1G93A mice, and pedigree analysis of survival from the F1 and F2 generations in the Marden et al. study is consistent with this hypothesis (90). The lack of a high degree of variability in survival among siblings with Nox2-deficient SOD1G93A genotypes in the F1 and F2 generations suggests that a single modifier gene likely does not account for the increased survival associated with the Nox2 mutant allele on the SOD1G93A B6SJL mixed genetic background.
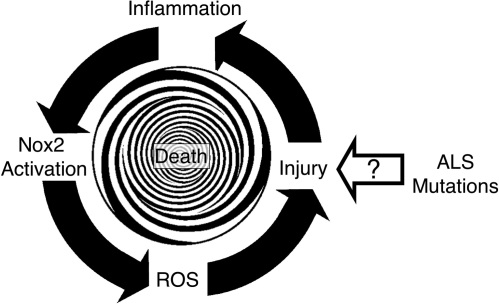
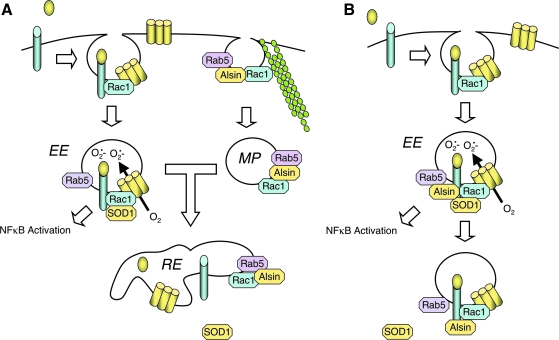
Despite the discovery that Nox2 contributes to inflammatory components of ALS (90, 141), the mechanism(s) whereby mutations in SOD1 lead to dysregulation of O2•− production by Nox2 remains poorly understood. The two studies described above suggest that Nox2 hyperactivation is likely the result of the enhanced inflammation and microglial activation that are typically associated with disease progression in ALS (109). In this context, one hypothesis to explain increased Nox2 expression and enhanced redox-stress in ALS is shown in Fig. 1. In this working model, Nox2 hyperactivation is not the primary event that leads to disease, but rather a consequence of some other cellular defect caused by a mutation in either SOD1 or another gene(s) that leads to ALS (see other Forum Review articles for elaboration). However, as disease progresses, inflammation and enhanced Nox2 activation in microglia lead to redox-stress and neuronal death. Neuronal death, in turn, leads to more inflammation, recruitment of more microglia to the damaged tissues, and further Nox2 activation. Thus, Nox2-mediated inflammation amplifies disease progression by increasing the redox-burden. This repetitive injury may lead to a spiraling decline in motor neuron function, and eventually to death. However, this model cannot explain how Nox1 might also be involved in disease progression, since Nox2 is thought to be the primary isoform that controls inflammatory responses in phagocytes and microglia.
FIG. 1.
Inflammation model for Nox2 involvement in ALS disease progression. According to this model, primary cellular defects in ALS lead to cellular injury by unknown mechanisms. This injury leads to inflammation and Nox2 activation in microglia. Activated microglia in turn produce ROS via Nox2, and this leads to further injury. This cycle repeats until death occurs.
Endosomal Signaling through Rac-Regulated NADPH Oxidases: Relevance to Pro-Inflammatory Signaling in ALS
Over the last two decades, superoxide and hydrogen peroxide produced by NADPH oxidases have been linked to a number of highly spatially- and temporally-regulated signaling mechanisms. In the context of the pathogenesis model presented in Fig. 1, understanding how NADPH oxidases control pro-inflammatory signaling pathways becomes extremely relevant to ALS. Two highly relevant pro-inflammatory cytokines linked to the progression of disease in ALS include interleukin-1-beta (IL-1β) and tumor necrosis factor alpha (TNFα). Levels of these two pro-inflammatory cytokines are elevated in the blood and/or cerebrospinal fluid (CSF) of humans with ALS (33, 111). Elevation of TNFα protein and mRNA are also observed in spinal cords from SOD1G93A (59) and SOD1G37R (101) ALS mice, and this correlates with enhanced NFκB activation (101). IL-1β is also induced in the spinal cords of ALS mice; however, gene disruption leading to the elimination of this cytokine does not delay disease onset (101). Similarly, SOD1G93A and SOD1G37R ALS mice bred onto the TNFα knockout background also do not exhibit increased survival (49). Hence, the current thinking is that it is the cumulative effect of multiple pro-inflammatory cytokines that influences the course of neuroinflammation and disease in ALS mice.
The activation of the pro-inflammatory transcription factor NFκB by IL-1β and TNFα has been long recognized to be redox-dependent (68, 129). More recently, the redox-dependent processes that control NFκB activation by these cytokines have been shown to also involve NADPH oxidases (specifically Nox1 and Nox2). In this context, endosomal activation of Nox1 and/or Nox2 has been shown to control redox-dependent activation of both the IL-1β and TNFα receptors (82, 83, 97, 100). Although these receptors have unique effector pathways responsible for activating NFκB, they share a similar dependence on redox-active signaling endosomes for their activation cascades. For these reasons, redox-active signaling endosomes have been recently named redoxosomes (103). The mechanisms of redoxosome activation may be particular relevant to redox-signaling in ALS. In this section we review the features of redoxosome biology that are the foundation of later discussion, which places this subject in the context of new paradigms for thinking about disease mechanisms relevant to ALS.
Redoxosomes are signaling endosomes that generate O2•− in their lumen in response to stimuli such IL-1β (82, 97) and TNFα (83, 97, 138) (Fig. 2). ROS generated by redoxosomes act as second messengers to facilitate the redox-dependent activation of the IL-1β receptor (IL-1R) and TNFα receptor (TNFR1). An important feature of redoxosomal biology is its dependence on endocytosis of the ligand-activated receptor, together with NADPH oxidases from the plasma membrane; inhibiting receptor endocytosis prevents the NADPH-dependent ROS production in redoxosomes that is required for activation of the proinflammatory transcription factor NFκB (82, 83). In this context, Rac1 appears to play a critical role, as it is required for bringing both IL-1R and Nox2 from the plasma membrane into the endosome in a mammary epithelial cell line (82). Similar processes likely control TNFR1 recruitment into redoxosomes in this cell type (83). In smooth muscle cells, Nox1 appears to be capable of replacing Nox2 function in redoxosomes following both IL-1β and TNFα stimulation (97), suggesting that Rac1-dependent NADPH oxidases (i.e., Nox1 and Nox2) perform cell type-specific redoxosomal signaling. ROS generated by redoxosomes play an important role in the redox-activation of IL-1 and TNF receptor complexes at the endosome surface. It is hypothesized that the conversion of O2•− to H2O2 is required for redox-signaling, since the loading of endosomes with both purified SOD1 and catalase proteins is needed to inhibit redox-dependent activation of NFκB by these receptors (82, 83).
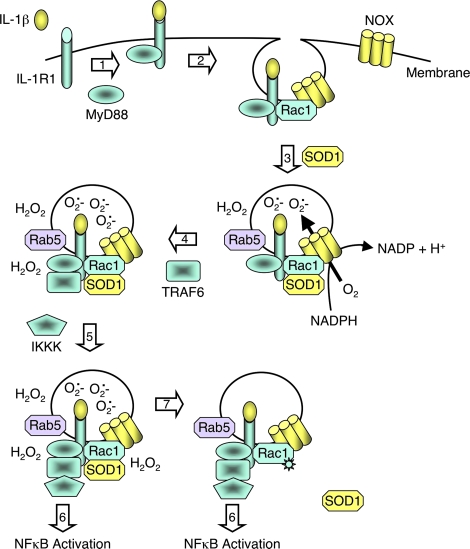
FIG. 2.
IL-1β mediates NFκB activation through redoxosomal activation. Schematically drawn are steps involved in the redox-activation of NFκB by IL-1β (82, 97, 100). Step 1: IL-1β binding to the IL-1 receptor (IL-1R1) facilitates recruitment of the effector MyD88 to the plasma membrane. Step 2: MyD88 binding to the ligand/receptor complex facilitates dynamin-dependent endocytosis and recruitment of Rac1 to the receptor. Rac1 is responsible for co-endocytosis of the Nox complex with the ligand-activated receptor. Step 3: Endocytosis of the IL-1R1/Nox complex leads to NADPH-dependent O2•− production in the endosomal lumen. In the case of Rab5 recruitment, is currently unclear whether it occurs at the endosome or the plasma membrane. However, superoxide production initiates in the Rab5-positive, early endosomal compartment. Additionally, SOD1 is recruited to the cytoplasmic face of the redoxosome, where it binds to Rac1 and stabilizes the GTP-bound active form of Rac1. Step 4: The localized production of ROS by newly formed redoxosomes facilitates H2O2-mediated recruitment of TRAF6 to IL-1R1 on the redoxosome surface. Extra-redoxosomal H2O2 may be generated either in the cytoplasm, following exit of O2•− from the lumen via chloride channels, or within the redoxosome lumen by spontaneous dismutation of O2•− within the lumen, followed by diffusion across the redoxosomal membrane. Step 5: The IL-1R1/MyD88/TRAF6 complex is now competent to recruit IKK kinases (IKKKs), and the recruited IKKKs can then phosphorylate cytoplasmically localized IKK complexes to activate NFκB. Step 6: As ROS levels outside the redoxosome rise, Rac1 is oxidized and SOD1 disengages. The uncoupling of SOD1 from Rac1 leads to: enhanced GTP hydrolysis by Rac1, inactivation of the Nox complex, and the termination of ROS production. This redox-dependent uncoupling of SOD1 from Rac1 appears to be defective in certain ALS-associated SOD1 mutants, and may lead to increases redox stress in the context of stimulating ligands that utilize redoxosomal pathways. Similar redoxosomal mechanisms appear to control TNFα-mediated activation of NFκB (83, 97). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
The production of localized O2•− and H2O2 by redoxosomes is thought to restrict ROS production spatially to sites of receptor activation, and hence to control downstream signaling events. The redox-dependent event required for the activation of both IL-1R and TNFR1 on redoxosomes appears to involve H2O2-mediated recruitment of TRAFs to these receptor complexes (Fig. 2). TRAF recruitment to each of these receptors (TRAF6 → IL-1R1 and TRAF2 → TNFR1) is a key event that is required for the formation of an active IKK kinase (IKKK) complex on these receptors, and this in turn facilitates phosphorylation and activation of the IKK complex. Activation of the IKK complex then leads to phosphorylation of IκB (inhibitor of NFκB) and subsequent mobilization of NFκB to the nucleus, where it can transcriptionally activate pro-inflammatory pathways. For a more comprehensive review on the effectors of both the IL-1R and TNFR1 pathways that lead to redoxosomal activation of NFκB, see the review by Oakley (103).
Why are redoxosomal pathways potentially important in ALS? The answer to this question stems from the finding that SOD1 actively recruits to both IL-1β- and TNFα-activated redoxosomes (Fig. 2) (82, 83, 100). Additionally, SOD1 appears to be functionally important for the activation of NFκB; SOD1-deficient fibroblasts fail to effectively activate NFκB following IL-1β stimulation (100). Furthermore, the expression of ALS mutant forms of SOD1 (L8Q, G93A, and G93C) in both glial and neuronal cell lines enhances NADPH-dependent O2•− production and Rac1 activation in endomembrane fractions (55). This enhanced redox-stress also led to decreased cell viability in both cell types. Given the link between Nox1/Nox2 and disease progression in SOD1G93A transgenic mice (90, 141), these in vitro findings are of considerable interest, because they demonstrate that mutant SOD1 expression can lead to enhanced Nox-activation in the absence of inflammation. The placement of SOD1 on redoxosomes also suggested the potential for a direct functional involvement of SOD1 in regulating Nox-mediated signaling by redoxosomes.
The finding that SOD1 is recruited to the redoxosome surface might suggest that it functions to locally dismutate O2•− to H2O2, and in this manner facilitates redox-dependent receptor activation. However, unlike H2O2, which freely diffuses across membranes (25), ·O2– is relatively impermeable to cellular membranes (88, 119). Hence, if indeed SOD1 dismutation activity plays a role in this process, a pathway that allows O2•− to pass through endosomal membranes must exist. Recently, studies in isolated endosomes and reconstituted endomembrane proteoliposomes have demonstrated that indeed O2•− can permeate redoxosomal membranes, and that it does so through an unknown chloride channel(s) (100). Cumulatively, these findings suggest that SOD1 is potentially important for redoxosomal ROS metabolism and signaling.
The SOD1/Rac1 Interaction Serves as a Redox-Sensor for Redoxosomal Nox Regulation: Functional Abnormalities in ALS SOD1 Mutants
The importance of SOD1 in regulating redoxosomal ROS was elucidated by the finding that SOD1 can directly associate with Rac1 (Fig. 2) (55). Immunoprecipitation studies have demonstrated that Rac1 is associated with SOD1 in multiple organs including the kidney, liver, and brain. Additionally, in vitro studies with purified His-tagged Rac1 deletion mutants and bovine SOD1 have demonstrated that these two proteins directly associate via a region of Rac1 contained between amino acids 35 and 70. This region of Rac1 spans several domains that are important for nucleotide binding (i.e., switch I, G2, switch II, and G3 domains) (140). Reconstitution assays using His-tagged Rac1 have demonstrated that the interaction between Rac1 and SOD1 depends on the redox-state and nucleotide-bound state of Rac1 (55). When Rac1 is in a reduced state and bound to GTP, it most efficiently binds to SOD1. Oxidation of Rac1 with as little as 50–100 pM of hydrogen peroxide prevents SOD1 association with Rac1. This process is reversible when Rac1 is reduced by the addition of 100–300 μM DTT. The association of SOD1 with Rac1 leads to an inhibition in the intrinsic GTPase activity of Rac1 (55), and hence would be expected to activate Rac1-regulated NADPH oxidases under reducing conditions. Indeed, the addition of SOD1 to isolated fibroblast endosomes in vitro leads to enhanced NADPH-dependent superoxide production by Nox2 (55); however, this enhancement is transient, since the accumulation of ROS leads to the dissociation of SOD1 from Rac1, and to inactivation of Rac1 through the hydrolysis of GTP to GDP. Thus, SOD1 appears to act as a redox-sensor that controls Rac1-mediated NADPH oxidase activation in the redoxosome (Fig. 3). Superoxides also appear to be capable of dissociating SOD1 from Rac1-GTP in vitro, leading to enhanced GTP hydrolysis by Rac1 (55). Although it is clear that the oxidation of Rac1 by very low levels of hydrogen peroxide plays an important role in dissociating SOD1 from Rac1, it remains to be determined if SOD1 locally provides this source of hydrogen peroxide for Rac1 oxidation on the redoxosome surface.
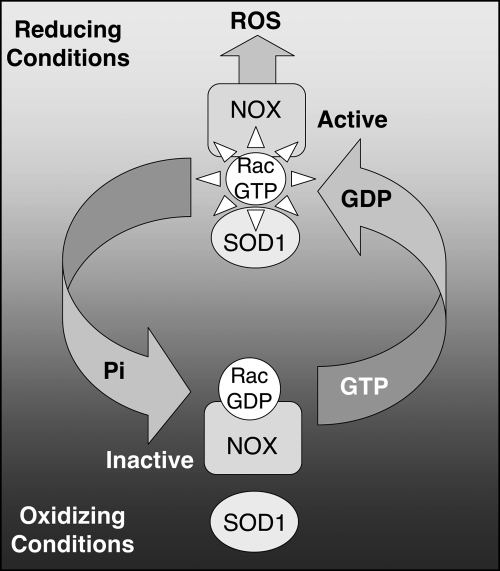
FIG. 3.
Redox-dependent association of SOD1 with Rac1 serves as a redox-sensing mechanism that controls NADPH oxidase activation. Under reducing conditions, SOD1 associates with Rac1-GTP, and inhibits intrinsic and GAP-mediated GTP hydrolysis, maintaining Rac1 in an active state. This active form of Rac1 stimulates Nox activation and ROS production. When NADPH oxidase produces sufficient ROS to establish an oxidizing local environment, SOD1 disengages from Rac1 and Rac1-GTP is hydrolyzes to Rac1-GDP. This leads to shut down of the Nox complex.
Of importance to ALS, studies have demonstrated that certain mutant forms of SOD1 (L8Q and G10V) have increased affinity for Rac1 (55). In this context, these SOD1 mutants more effectively stabilized the Rac1-GTP complex by decreasing intrinsic GTPase activity. Most interestingly, the association of mutant SOD1 with Rac1 was redox-insensitive, and the two proteins failed to dissociate in the presence of xanthine/xanthine oxidase derived O2•−, a finding distinctly different from that of wild-type SOD1. These findings suggested that ALS-causing mutants of SOD1 can alter Rac1 activation, and hence may also influence the activity of Rac1-regulated Nox complexes. In vivo support for this hypothesis comes from findings in SOD1G93A transgenic mice, in which both Rac1 activation and Nox-dependent ROS production are elevated in the spinal cord (55, 90, 141). However, conclusive evidence that an ALS-associated SOD1 mutant can alter redoxosomal Nox2 activation came from reconstitution studies in isolated endosomes from primary fibroblasts that had been isolated from wild-type and Nox2-deficient mice (55). In this study, the addition of purified SOD1L8Q protein to isolated endosomes gave rise to prolonged NADPH-dependent O2•− production in comparison to that stimulated by the addition of SOD1WT protein. However, Nox2-deficient endosomes exhibited significantly reduced NADPH-dependent O2•− production following the addition of SOD1WT protein, suggesting that, at least in this cell type, SOD1 activated Nox2. These findings suggest that SOD1 can directly regulate endosomal Nox activation by acting as a redox-sensor for Rac1 activation and inactivation. Furthermore, redox-sensitive uncoupling of SOD1 from Rac1 appears to be dysfunctional in certain ALS-associated mutants of SOD1, and this defect leads to hyperactivation of Nox-derived O2•− by endomembranes. Such findings may help to explain the gain-of-function phenotype caused by familial ALS SOD1 mutations associated with enhanced redox-stress.
The ability of H2O2 in pico-molar quantities to liberate SOD1 from Rac-GTP, and to allow for GTP hydrolysis to occur, suggests that the mechanism underlying in vivo Nox regulation may be exquisitely sensitive to small changes in cellular ROS. This mechanism may allow Rac1 to sense and regulate changes in cellular O2•− through SOD1 enzymatic conversion of O2•− to H2O2. Such spatial regulation may be a key aspect of SOD1 function as a redox-sensor of inflammatory signals that utilize the redoxosome. Hence, we propose an alternative model for pathogenesis in certain familial forms of ALS involving SOD1 mutations (Fig. 4). According to this model, SOD1 mutations directly dysregulate redoxosomal Nox2 (and potentially also Nox1) activation, leading to heightened and prolonged ROS production in response to pro-inflammatory signals. In the context of TNFα and IL-1β receptor activation, elevated endosomal Nox activation would be expected to enhance downstream pro-inflammatory signaling pathways such as NFκB (82, 83). Very recent support for the model shown in Fig. 4 has emerged from mixed cultures of human embryonic stem cell-derived astrocytes and motor neurons (89). This study demonstrated that expression of the SOD1G37R ALS mutant protein in astrocytes leads to increased Nox2-dependent ROS production and hyperactivation of pro-inflammatory pathways that can selectively kill motor neurons in a non-cell-autonomous fashion.
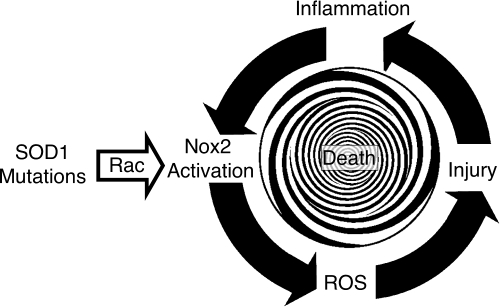
FIG. 4.
Model for disease progression in familial forms of ALS that are caused by defects in SOD1-mediated regulation of the Rac/Nox2 complex. In this model, mutations in SOD1 leads to enhanced redox-insensitive binding to Rac-GTP that stabilizes the GTP-bound active form of Rac. This enhanced activation elevates Nox2 activity, increasing ROS production by microglia. This inciting event begins the cascade of injury and enhanced inflammation that in turn lead to microgliosis, and thus to more Nox2-expressing cells. The expanded population of Nox2-expressing microglia further increases ROS production via a dysregulated interaction between mutant-SOD1 and Rac, and this cycle repeats until death occurs.
Whether SOD1-mediated regulation of Nox2 is the event responsible for initiating disease progression in ALS, or only enhances ROS-mediated injury, remains to be determined. In this context, it is also important to point out that the regulation of ROS production by Rac1 is important for many cellular processes that are involved in signal transduction (129), actin cytoskeletal rearrangements (71, 87, 102), cell migration (143), cell proliferation (66, 99, 108, 143), and cell differentiation (113). Given that many of these pathways also involve activation and recruitment of Rac1 to specific membrane domains, it is also possible that SOD1/Rac1 interactions may influence other cellular aspects of disease progression in ALS.
ALS2 and SOD1 Inherited Forms of ALS Exhibit Defects in Rac1 GTPase Regulation: A Potential Unifying Theme for Molecular Pathophysiology in ALS
The identification of SOD1 as an effector of Rac1, and the finding that ALS mutations in SOD1 enhance Rac1 activity, becomes even more interesting when one considers that mutations in the ALS2 gene lead to a juvenile form of inherited ALS (26, 54, 144). The ALS2 gene encodes two alternatively spliced products that encode a long (1659 amino acid) and a short (396 amino acid) form of a protein called Alsin. The full-length Alsin protein has three independent guanine nucleotide exchange factor (GEF)-like domains. These include: an N-terminal regulator of chromatin condensation (RCC1)-like domain, which resembles the GEF for the Ran GTPase; a middle Dbl homology (DH)- and pleckstrin homology (PH)-like domain similar to the GEF for Rho GTPase; and a C-terminal vacuolar protein sorting 9 (VPS9)-like domain that is homologous to the Rab GTPase GEF (26, 54). The exact mechanism by which mutations in ALS2 lead to ALS-like disease remains unknown, and this is confounded by the fact that motor neuron degeneration is not observed in ASL2 knockout mice (23). Interestingly, however, ALS2 knockout mice do have increased susceptibility to paraquat-induced neuronal oxidative stress (23), lymphopenia and hematopoiesis abnormalities (40), and endosome trafficking defects (34).
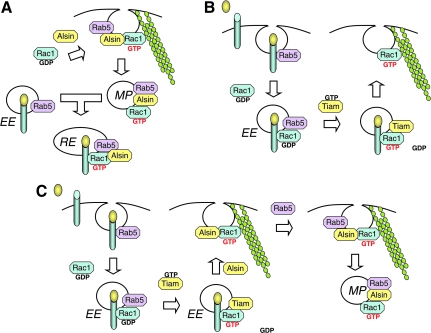
Several molecular findings suggest that Alsin may be an important determinant of endosomal dynamics, and hence relevant to SOD1-mediated redoxosomal signaling defects in ALS. Although a link between Alsin activity and the regulation of endosomal NADPH oxidases remains highly speculative, we review aspects of Alsin biology that are potentially important to this line of thinking. Alsin was originally thought to serve as a GEF for both Rab5 and Rac1 GTPases, and hence is considered a candidate activator of each of these proteins (106, 131). Although the GEF activity of Alsin with respect to Rab5 has been reproduced by several research laboratories, a relatively recent study suggests that Alsin functions as an effector, rather than a GEF, of Rac1 (76). This finding is of considerable interest since SOD1 also acts as an effector for Rac1 (rather than as a traditional GEF or GTPase-activating protein (GAP)) (Fig. 3) (55). Both the Rab5-GEF and Rac1-effector functions of Alsin appear to influence endocytic mechanisms and endosomal dynamics; activated Rac1 appears to recruit Alsin to the plasma membrane at sites of Rab5-dependent macropinocytosis, and Alsin appears to also promote the fusion of macropinosomes with EEA1/Rab5-positive early endosomes (Fig. 5A) (76).
FIG. 5.
Models for Alsin function in endosomal dynamics. (A) According to a model proposed by Kunita et al., Alsin plays an important role in macropinocytosis following its recruitment by Rac1 to plasma membrane ruffles at sites of organized actin filaments (76). In this context, Alsin may stabilize Rac1-GTP and hence act as a Rac1-effector. In addition, the Rab5-GEF function of Alsin appears to control maturation of the macropinosome (MP) and later fusion of macropinosome with the early endosomal compartment (EE). This process may be important for the movement of endosomal components into the recycling endosome (RE). (B) In a model proposed by Palamidessi et al., Rab5-mediated endocytosis into the early endosomal compartment is required for Rac1 activation by Tiam1, a Rac1-GEF that exchanges GDP for GTP. Rac1-GTP is then recruited to the plasma membrane where it facilitates the formation of actin-based migratory protrusions (107). (C) In a combined model that takes into account findings from both the Kunita and Palamidessi studies, Tiam1 activates Rac1 prior to recruitment of Alsin to the plasma membrane. As in model B, this process may be initiated by a ligand stimulus that leads to the formation of early endosomes. Once Rab5/Alsin/Rac1-GTP complexes are formed at the plasma membrane, spatially-restricted association with organized actin filaments leads to the formation of macropinosomes and downstream endosomal dynamics controlled by this compartment. The potential involvement of Tiam1 in this process is particularly interesting, since its homolog, Tiam2, has been associated with sporadic ALS (37). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
These functions of Alsin in endosome formation and trafficking are intriguing when compared to the biology of receptor-mediated redoxosome formation in the context of IL-1β and TNFα signaling pathways. For example, IL-1β-activated redoxosomes have been shown to initiate Nox2- and/or Nox1-dependent O2•− production in the early endosomal compartment, as determined by affinity isolation of Rab5 endosomes and the cellular co-localization of early endosomal antigen (EEA1) with O2•−-sensitive fluorochromes (82, 97) (Fig. 2). Furthermore, the recruitment of Nox2 from the plasma membrane into IL-1R1-stimulated redoxosomes is dependent on Rac1 (82). Lastly, SOD1 is recruited to Rab5-positive IL-1β/IL-1R-containing redoxosomes (100), where it binds to Rac1 and regulates Nox2 activity in a redox-dependent fashion (55) (Fig. 3). However, in contrast to Rac1/Alsin-dependent macropinocytosis, which is most likely dynamin-independent (14, 31, 76), endocytosis of ligand-activated IL-1R1 is dependent on dynamin rather than on Rac1 (82). Interestingly, Alsin has been shown to selectively bind ALS mutants of SOD1 via its DH/PH domain (69), a finding reminiscent of enhanced binding of ALS SOD1 mutants to Rac1 (55).
Cumulatively, these findings place four early endosomal proteins (SOD1, Alsin, Rab5, and Rac1) in intersecting endosomal compartments where both Alsin and SOD1 have been shown to play roles as effectors of Rac1 (55, 76). Interestingly, it was also recently demonstrated that Rab5-mediated endocytosis is required for Rac1 activation in the endosomal compartment following mitogenic stimulation, and that this process plays a role in localizing activated Rac1 to the plasma membrane where it facilitates the formation of actin-based migratory protrusions (107) (Fig. 5B). In this context, Tiam1 (a Rac1-GEF) activates Rac1 in the early endosomal compartment prior to its association with actin at the plasma membrane. Given that actin-based rearrangements are also important for Alsin/Rac1-dependent macropinocytosis (76) (Fig. 5A), it is possible that this Tiam-mediated activation of Rac1 also plays a role in macropinocytic mechanisms involving Alsin (Fig. 5C). Hence, Rac1/SOD1/Alsin/Rab5 signaling networks may act in concert to regulate cell signaling at the endosomal level, and dysfunction of these pathways in ALS (through mutations in either Alsin or SOD1) may lead to disease. As discussed later in this review, the potential involvement of TIAM proteins in Alsin-mediated endosomal dynamics is of special interest since the TIAM2 gene was identified as associated with sporadic forms of ALS (37).
Given that both Alsin and SOD1 are effectors of Rac1, it is tempting to speculate that defects in either protein could influence redox-signaling at the endosomal level. This would be consistent with two sets of findings. First, Alsin overexpression can mitigate neurotoxicity caused by the expression of a mutant form of SOD1 (69), suggesting that Alsin counterbalances a gain of function in SOD1. In glial and neuronal cell lines, the expression of SOD1 mutants leads to enhanced ROS production in the endomembrane compartment, and ultimately to cell death (55). Inhibiting ROS production under these conditions—by applying the Nox2 inhibitor apocynin—protects glial cells from this SOD1 mutant toxicity (55). Whether Alsin's association with mutant SOD1 and/or Rac1 also mitigates hyperactivation of Nox2 in the endosomal compartment remains to be determined. Second, ALS2 knockout mice demonstrate an increased susceptibility to paraquat-induced neuronal oxidative stress (23) as well as defects in endosome trafficking (34). Hence, Alsin appears to protect cells from redox-stress while also influencing endosomal dynamics. Cumulatively, these findings suggest the possibility that Alsin may protect cells from redox-stress by associating with mutant SOD1 and/or Rac1 on Nox-active endosomes.
Two models that could potentially explain how Alsin and SOD1 might influence Nox activation in the endosomal compartment are shown in Fig. 6. According to the first model, Alsin might play a role in the processing of receptor-activated redoxosomes to an inactive state (Fig. 6A). In this model, fusion of macropinosomes with receptor-activated redoxosomes leads to inactivation of the Nox complex by recycling or degradation of its components in the endocytic pathway. Loss of either Alsin or SOD1 function would in this case dysregulate the pathway by reducing the rate of fusion between these compartments, due to altered Rac1 or Rab5 activation. In contrast, Alsin overexpression might accelerate the fusion process, leading to a more rapid disposal of redoxosomes. According to the second model, Alsin may play an unknown role in receptor-mediated endocytosis and the regulation of Rab5, Rac1, and/or SOD1 in the redoxosome (Fig. 6B). In this context, Alsin binding to Rac1 and/or SOD1 could influence activation of the Nox complex in redoxosomes by facilitating conformational changes in the Rac1/SOD1 complex. In this model, Alsin could be part of a redox-sensor required to shut down the Nox complex, potentially by competing for SOD1 binding to Rac1 on the redoxosome. Although these models are currently both highly speculative and other scenarios could be envisions, both are consistent with Alsin protecting against redox-stress.
FIG. 6.
Two models that position Alsin as a protective modulator of redox-signaling at the endosomal level. (A) This model proposes that Alsin facilitates endosomal processing of redoxosomes. Accordingly, ligand-triggered redoxosome formation mobilizes Nox to the early endosomal (EE) compartment, where Rac1/SOD1 complex formation leads to Nox activation and ROS production (55, 82). Macropinosomes (MP) form through the previously described process involving Alsin, Rac1, and Rab5 (76), and this compartment may be important for directing redoxosomes to the recycling endosomal (RE) compartment. This Alsin/Rab5-facilitated fusion event could lead to the processing of redoxosomal components and inactivation of the Nox complex. (B) In a second model, ligand stimulation leads to direct recruitment of Alsin to the redoxosome, through its binding to a component(s) of the Rab5, Rac1, SOD1 complex. This large molecular complex may facilitate the redox-dependent uncoupling of SOD1 from Rac1 to shut down the Nox complex following signal transmission. Such a scenario might involve competitive binding of Alsin to Rac1 in a manner that excludes SOD1 from the complex (as shown), or changes to the complex that enhance Rac1-GTP hydrolysis. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Genome Analysis of Sporadic ALS Uncovers Other Potential Nox/Rac Regulators
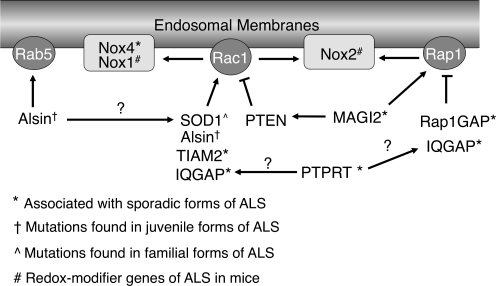
A recent study described a genome-wide search for single-nucleotide polymorphisms (SNPs) that associate with sporadic ALS (37). This search of over 750,000 SNPs in over 1,200 ALS patients and 1,500 normal subjects identified 10 genetic loci with a significant association with sporadic ALS in three independent population series, and 41 additional loci that were significant in two of the three series. Interestingly, six of the associated genes encode proteins known to either directly or indirectly influence Rac1 and/or NADPH oxidases activation (Fig. 7). This section places in context the known functions of these genes—in regulating Rac1, NADPH oxidases, and/or endosomal dynamics—that may influence redoxosome function. With speculative models, we will attempt to lay a foundation for further thinking about how diverse mutations in sporadic and inherited forms of ALS may have overlapping mechanisms of pathogenesis.
FIG. 7.
Potential relationships between gene products that have been linked to ALS disease progression in mouse models, sporadic ALS, and various inherited forms of ALS. Arrows denote an activating relationship, and flat-headed lines denote an inhibitory relationship. Question marks denote a previously described relationship that would have unknown consequence in the current scheme (i.e., SOD1/Alsin interaction), or a potential relationship based on a findings from a close homolog (i.e., PTPRT/PTPRM). See text for details.
Nox4
The Nox4 gene was identified as significantly linked to sporadic ALS in two of three series of patient studies (37). Given the finding that deletion of Nox1 or Nox2 genes in SOD1G93A transgenic mice significantly prolongs survival (90, 141), the finding that the Nox4 isoform of NADPH oxidase may also be linked to certain sporadic forms of ALS is quite intriguing. Unlike Nox1 and Nox2, Nox4 appears to be significantly regulated at the transcriptional level in response to disease processes and certain stimuli (9, 60). Nox4 is expressed in neurons, and its mRNA increases following ischemia (133). The mechanisms that control Nox4 activation appear to be quite different than those for Nox1 and Nox2 activation (60). For example, Rac1 and other co-activator cytosolic subunits appear to be dispensable for Nox4 activation in certain cell systems (9, 91). However, others have also demonstrated that Rac1 is required for Nox4 activation by certain cell stimuli (48). It is interesting that mutant SOD1 overexpression enhances Nox-dependent ROS in endomembranes from both glial and neuronal cell lines. However, only ROS production by glial cells is inhibited by apocynin (a known Nox2 inhibitor that likely prevents p47phox recruitment to the Nox2 complex) (55). Hence, it is possible that p47phox-independent NADPH oxidases (such as Nox4 or Nox1) are responsible for neuronal ROS production stimulated by mutant SOD1.
TIAM2 (STEF)
The TIAM2 gene was identified as significantly linked to sporadic ALS in two of three series of patient studies (37). Tiam1 and Tiam2 (also known as STEF) are two closely related Rac1 guanine nucleotide exchange factors (GEFs) (27, 53, 61, 95, 96). Both are expressed in the brain and other neuronal tissues, and activate Rac1 by exchanging bound GDP for GTP. Tiam GEFs are generally thought to regulate the actin cytoskeletal at the membrane through the activation of Rac1 (96), and have been shown to be involved in neuronal developmental, growth, and migration (70, 93, 146). Vav1 is another Rac-specific GEF (22), and is generally thought to be responsible for activating Rac-dependent NADPH oxidases such as Nox1 and Nox2 (45, 98, 116, 137). Although Tiam2 has not been reported to directly influence the activation NADPH oxidase, Tiam2 association with the small GTPase Rap1 can control activation of Rac1 (147). Interestingly, Rap1 (also known as Krev-1), which associates with the phagocytic Nox2 complex (39, 114), was recently demonstrated to positively influence Nox2-dependent superoxide production in neutrophils of Rap1a knockout mice (84). Hence, Tiam2 may indirectly influence Nox activation through its control of Rap1/Rac1/2 dynamics at the membrane. The recent characterization of Alsin as a Rac1 effector that influences endocytosis from actin cytoskeletal contacts at the plasma membrane (76), provides yet another potential commonality between ALS2 and TIAM2-associated sporadic forms of ALS (i.e., both proteins regulate Rac1 and actin cytoskeletal dynamics). Additionally, SOD1 has also been identified as a Rac1 effector that, when mutated in ALS, leads to excessive activation of both Rac1 and NADPH oxidase (55). Hence, Tiam2, Alsin, and SOD1 are all Rac1 effectors/activators that are associated with both inherited and sporadic forms of ALS (Fig. 8).
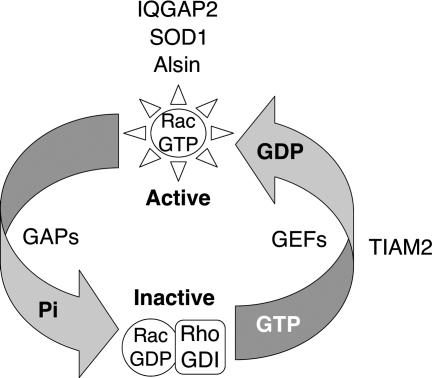
FIG. 8.
Rac1 effectors and activators identified or associated with various forms of ALS in humans. Rac1 activity is controlled by nucleotide binding. The inactive form, Rac1-GDP, is held in the cytoplasm through its association with RhoGDI. Rac1 is activated by guanine nucleotide exchange factors (GEFs) and inactivated by GTPase activating proteins (GAPs). GEFs facilitate the exchange of GDP for GTP, while GAPs enhance GTP hydrolysis. IQGAP2, SOD1, and Alsin are known Rac1 effectors that bind to and stabilize the GTP-bound form of Rac1. These three molecules do not appear to act as either traditional Rac1-GEFs or GAPs (19, 55, 76). In contrast, TIAM2 serves as a GEF for Rac1 (27). TIAM2 and IQGAP2 genes were recently associated with sporadic ALS (37). SOD1 and Alsin have also been shown to influence the dynamics of Rac1 activation, to recruit to the endosomal compartment, and to also be mutated in certain forms of ALS (55, 76, 130, 131). See text for more detailed descriptions of these factors and their potential relationships to ALS.
IQGAP2
The IQGAP2 gene was identified as significantly linked to sporadic ALS in two of three series of patient studies (37). IQGAP-1, -2, and -3 are related calmodulin-binding IQ-motif proteins with significant homology to GAPs (17, 78). These proteins regulate actin cytoskeletal dynamics at the plasma membrane and cell migration, by controlling activation of the Cdc42 and Rac1 GTPases (17, 18, 92). Typically, guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) are thought to either activate or inactivate small GTPases by exchanging GDP for GTP (GEFs), or by enhancing GTPase activity (GAPs), respectively (15). Although the name implies that IQGAPs might inhibit small GTPases through GAP-associated activity, this is actually not the case. IQGAP1 associates with the GTP-bound forms of Cdc41 and Rac1 through a GAP-related domain (77), and suppresses intrinsic GTPase activity by stabilizing the GTP-bound form, which in turn increases the activity of both Cdc41 and Rac1 (17, 56). Unlike IQGAP1, IQGAP2 appears to associate equally with both GDP- and GTP-bound forms of Rac1 and Cdc42. However, similar to IQGAP1, IQGAP2 retains the ability to inhibit both RhoGAP-mediated and intrinsic GTP hydrolysis by Rac1 and Cdc42 (19, 120). Additionally, IQGAP3, which is highly expressed in the brain, has been shown to be required for the activation of Cdc42- and Rac1-dependent neurite outgrowth during neuronal morphogenesis (136). Hence, much like Alsin (76) and SOD1 (55), IQGAPs appears to function as effectors of Rac1. It is also interesting to note that IQGAP2 and SOD1 can both associate with GDP- and GTP-bound forms of Rac1 (19, 55, 120), although in the case of SOD1 there is a nucleotide preference that is dependent on the redox-state of Rac1 (55). IQGAP1 has been shown to increase ROS production by activating Nox2, and in this manner facilitates migratory wound healing in vascular endothelial cells (65). However, it is currently unclear if IQGAP2 also influences Nox activation. In summary, the studies by Dunckley et al. that have identified the TIAM2 and IQGAP2 genes as tightly linked to sporadic forms of ALS (37) add two additional Rac1 effectors to the existing list of Alsin and SOD1. Interestingly, TIAM2, IQGAP2, Alsin, and SOD1 all appear to influence the abundance/stability of the GTP-bound form of Rac1, and hence the mechanism by which defects in these proteins lead to ALS may overlap (Fig. 8).
PTPRT (PTPrho)
The protein tyrosine phosphatase receptor type-T (called PTPRT or PTPrho) gene was shown to be significantly linked to sporadic ALS in three of three series of patient studies (37). This gene encodes one of four related transmembrane proteins that fall into a family of receptor protein tyrosine phosphatases (RPTPs) (12). This family of proteins is characterized by the presence of a novel extracellular N-terminal MAM domain (meprin/A5/micro), which includes an immunoglobulin-like domain and four fibronectin type III (FN) repeats, and plays a role in homophilic cell adhesion (3, 4). PTPRT (PTPrho) is most closely related to PTPRM (PTPmu), another member of the family of RPTPs. Both of these proteins are highly expressed in the brain base on NCBI Unigene EST expression profiles. Interestingly, PTPRM directly interacts with IQGAP1 to promote neurite outgrowth, and peptide competition of IQGAP1 binding to Cdc42 and Rac1 blocks this PTPRM-mediated process (110). These findings are similar to those demonstrating that IQGAP3 is required for the induction of Cdc42- and Rac1-dependent neurite outgrowth during neuronal morphogenesis (136). Hence, PTPRM appears to activate Cdc42 and Rac1-dependent neurite outgrowth through IQGAP1. It is currently unclear if PTPRT, which has been linked to sporadic ALS (37), also possesses the ability to influence the activation of Cdc42 and Rac1. However, if PTPRT functions similarly to PTPRM in this context, PTPRT would join Alsin, SOD1, IQGAP2, and Tiam2 as activators/effectors of Rac1. More research in this area is obviously needed to support this hypothesis.
RAP1GAP (GARNL4)
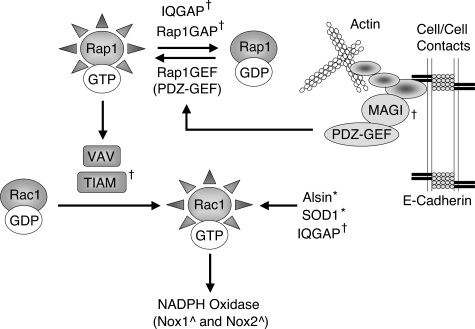
The RAP1GAP gene (also known as GARNL4) was shown to be significantly linked to sporadic ALS in two of three series of patient studies (37). Rap1GAP (GTPase activating Rap/RanGAP domain-like 4) is a Rap1 GTPase activating protein (GAP) and hence inactivates the small GTPase Rap1 by promoting the hydrolysis of bound GTP (i.e., Rap1-GTP is active and Rap1-GDP is inactive). Rap1 is a member of the Ras family of small GTPases and participates in numerous cell signaling events at the plasma membrane, where it controls integrin-mediated cell adhesion and the formation of cadherin-based cell-cell junctions (74). Interestingly, Rap1 physically associates with and activates VAV, TIAM2, and TIAM1 (all GEFs for Rac1) (6, 43, 147). As discussed above, the TIAM2 gene was also associated with sporadic ALS (37). It is currently thought that Rap1 may coordinate the recruitment of Rac1-GEFs to sites of cell–cell contact, and provide a link to the actin cytoskeleton (74) (Fig. 9). Interestingly, IQGAP also binds to both the GDP- and GTP-bound forms of Rap1, and appears to negatively regulate Rap1 activation, either by promoting Rap1 association with Rap1GAPs or by inhibiting its association with Rap1GEFs (67). The IQGAP gene was also associated with sporadic ALS in genetic studies (37). Activation of Rap1 is mediated by Rap1GEFs, including PDZ-GEF1, which is of particular interest since it associates with the MAGI scaffold proteins at β/α-catenin/cadherin-based cell–cell contacts to control Rap1 activation (74, 118) (Fig. 9). Overexpression of Rap1GAP also impairs Rap1-mediated VE-cadherin-dependent cell adhesion (118). In this context, MAGI controls the ability of PDZ-GEF1 to activate Rap1 following cell–cell contact, and hence counterbalances Rap1GAP activity. As discussed below, MAGI2 is yet another gene identified in genetic association studies of sporadic ALS (37).
FIG. 9.
Mechanistic relationships between actin cytoskeletal dynamics and the control of small GTPases Rap1 and Rac1. Shown is a schematic diagram outlining known relationships between genes associated with sporadic ALS (IQGAP, Rap1GAP, MAGI, and TIAM) (37), and their potential influence on Rac1 activation and function. Arrows denote an activating relationship between connected effectors. †denotes genes identified in genomic screens as strongly associated with sporadic ALS (37). *denotes genes known to be mutated in inherited forms of ALS. ^denotes mouse redox-modifiers genes known to influence disease progression in the SOD1G93A transgenic mouse model. See text for details.
Rap1GAP is also potentially of interest from the standpoint of redox-stress, since it was first shown to be highly expressed in neutrophils and to be associated with the NADPH oxidase complex (39, 114). For many years, Rap1 was thought to serve as the small GTPase required for Nox2 activation. Although Rac1/2 was later shown to have this role, recent studies have begun to indicate that Rap1 can also influence Nox2 activation. For example, Rap1a knockout mice have reduced respiratory bursts in isolated neutrophils (84), suggesting that Rap1 does indeed influence Nox2 activation (perhaps indirectly through the recruitment and activation of Vav, a Rac1-GEF). However, another report suggests that Rap1 suppresses Ras-mediated production of ROS in T-cells that is thought to be derived from NADPH oxidase (115).
Many of the emerging features of Rap1 paint a picture of protein/protein interactions and connected pathways suggestive of signaling networks for ALS-causing mutations and ALS-associated genes. At face value, many of these known or potential ALS-causing genes—such as IQGAP, Rap1GAP, MAGI2, TIAM2, ALS2, and SOD1—appear unrelated. However, as shown in Fig. 9, these genes and their protein products may indeed be connected—by cellular processes that regulate the cytoskeleton, Rac1, and/or Rac1-regulated NADPH oxidases. For example, mutations in Rap1Gap and IQGAP that inhibit their association with Rap1 might lead to higher levels of activated Rap1-GTP, which would in turn lead to enhanced Rac1 activation. Similarly, defects in MAGI2 could potentially influence PDZ-GEF activation, leading to reduced or enhanced Rap1 → Rac1 activation. Lastly, mutations in TIAM, IQGAP, Alsin, and/or SOD1 also have a proven ability, or the potential, to alter Rac1 activation. It remains to be determined whether the connections across these pathways are a conserved feature of the pathophysiologic mechanisms in ALS, or simply coincidence.
MAGI2
The MAGI2 gene (also known as membrane-associated guanylate kinase inverted-2) was shown to be significantly linked to sporadic ALS in three of three series of patient studies (37). MAGI1 and MAGI2 are scaffold proteins that connect cadherin cell-cell contacts and actin cytoskeleton to signaling domains at the plasma membrane. As discussed above (Fig. 9), MAGI proteins are required for Rap1 activation initiated following cell–cell contact (74). Interestingly, MAGI2 directly binds to and activates PTEN, a membrane-associated lipid phosphatase (64, 135, 142). The PTEN protein is also stabilized by interactions with MAGI2 at the membrane (128), and negatively regulates Rac1 and Cdc42, and inhibits cell migration facilitated by these two small GTPases (85). In this context, Liliental et al. demonstrated enhanced Rac1 and Cdc4 activation in PTEN knockout cells that demonstrated a significantly increases migratory phenotype. This phenotype was also reversed by expression of dominant-negative forms of Rac1 and Cdc42. Hence, MAGI2 may also have the ability to regulate Rac1 through its control of PTEN (Fig. 7). In this context, null mutations in MAGI2 would be expected to lead to enhanced Rac1 activation by suppressing PTEN-mediated inhibition of Rac1.
Therapeutic Approaches to ALS
Clinical trials aimed at identifying drugs for the treatment of ALS have been conducted for over 50 years. Although over 20 drugs have been evaluated in clinical trails over the past 10 years, only riluzole (Rilutek) has emerged as a product (28, 81). Moreover, although the clinical trials for riluzole demonstrated a statistically enhanced survival of 60 days for patients being treated with the drug (11, 79), new therapeutic candidates are still being sought due to its limited efficacy. New sets of hypotheses have been generated based on the pathogenesis of ALS and on findings from genetic, pathological, and biochemical postmortem evaluations. Drugs candidates have been chosen for their potential to reduce excitotoxicity, apoptosis, motor neuron degeneration, bioenergetics, or oxidative damage (24, 62, 81, 132). As discussed throughout this review, oxidative stress has been implicated in the pathogenesis of ALS. Therefore, a relatively straightforward approach to development of therapies has been to evaluate anti-oxidants as a means of controlling this process. However, results from clinical trials for ALS that are based on anti-oxidant strategies have so far yielded unimpressive results (105), as highlighted below.
Many natural biological agents such as vitamins C and E have antioxidant effects and are readily available; thus physicians commonly advise ALS patients to take these (105). However, although randomized clinical studies of high-dose vitamin E (5,000 mg) intake for 18 months in patients who were also taking riluzole found this regimen to be safe, they also indicated that the patients derived no survival benefit (44, 105).
Creatine improves motor function in transgenic SOD1-based animal models of ALS, however, results in human patients have not been successful. In ALS transgenic mice, oral administration of creatine has a positive impact on abnormal mitochondrial function (72, 73). However, in clinical studies in which creatine was supplemented in the diet, patients were found not to derive any benefit (51, 123, 124).
Coenzyme Q10, a cofactor in the electron transfer chain, is also a scavenger of free radicals in lipid and mitochondrial membranes, and is thought to improve energy production by the mitochondrial membrane and the cell. Although the administration of high doses (up to 3,000 mg) of CoQ10 plus vitamin E (1,500 mg) to ALS patients was found to be generally safe, no clinical benefit was detected (41).
Edaravone, a potent scavenger of free radicals that is approved in Japan for the treatment of ALS, was tested in 20 Japanese patients in a small double-blind study (145). The study showed only limited improvement in the ALSFRS-R score.
Two compounds with antioxidant properties remain in clinical studies. The manganese porphyrin AEOL 10150 is a potent catalytic antioxidant (16), and the injection of this compound into ALS mice when they became symptomatic markedly prolonged survival (30). Two Phase I studies testing the safety and feasibility of AEOL 10150 have been completed, and they indicate that the administration of this drug at doses up to 2 mg/kg/day was safe and well tolerated, with an excellent pharmacokinetic profile in ALS subjects (104, 132). A follow-on, phase I open-label study of AEOL 10150 was recently initiated in a patient diagnosed with progressive and debilitating ALS, as a compassionate-use, multiple-dose study; this trial is designed to evaluate the safety and efficacy of AEOL 10150 in an ALS patient over an extended period of time.
The second compound currently under study is KNS-760704, which provides protection from oxidative stress-related neurotoxic cascades and has neuroprotective properties (50). A double-blind, randomized, placebo-controlled study evaluating the safety and efficacy of KNS-760704 compared to placebo is ongoing. The study will be conducted in two parts. In Part 1, ∼80 eligible patients will receive one of the following treatments for 12 weeks: placebo, low-dose, mid-dose, or high-dose KNS-760704. Participants who complete all 12 weeks of the Part 1 phase will be eligible for randomization in the Part 2 phase of the study. The duration of Part 2 of the study will be 28 weeks. Subjects of the Part 2 study will divided randomly into one of two active treatment groups for 24 weeks (low-dose or high-dose KNS-760704), and will receive placebo for the remaining 4-week period (63).
Several therapies developed based on other current hypotheses for ALS pathogenesis have also been tested in double-blind, placebo-controlled clinical trials in ALS patients, but have failed (35, 81). Specifically, these trials have tested therapies based on anti-glutamate, antioxidant, neuroprotection, anti-inflammatory and anti-apoptotic strategies (35, 46, 81).
The antiexcitotoxic drugs are intended to have a therapeutic impact on the harmful effects of repetitive firing or on the elevation of intracellular calcium by calcium-permeable glutamate receptors (21, 46). Repetitive firing in response to excessive glutamate release is a well-known cause of neuronal cell death. Glutamate transporters normally provide rapid uptake of glutamate, and ALS patients often have increased glutamate in the cerebrospinal fluid, due to reduced glutamate transport—most often because of loss of the excitatory amino acid transporter 2 (EAAT2) (46, 57). Therefore, drugs that can affect this process have been pursued as potential therapeutics. To date, however, riluzole is the only one of these drugs for which test results have been sufficiently compelling to lead to its approval for the treatment of ALS. Notably, the exact mode of action of riluzole, a benzothiazole compound, is unknown and may actually be related to inactivation of voltage-dependent sodium channels or interference with intracellular signaling events, rather than to inhibition of glutamate release (11, 36, 79).
Intuitively, and from data derived from rodent studies, it was reasonable to evaluate the activity of neurotrophic factors for their ability to rescue or protect motor neurons from degeneration. However, pharmacokinetics, bioavailability, dose-limiting toxicities, and antibody-based inactivation of the neurotrophic factor have all contributed to a lack of success to date in clinical trials that have evaluated such factors (132). Also, minocycline, a tetracycline antibiotic with neuroprotective and anti-inflammatory properties, appeared to be effective in delaying disease onset and survival in ALS transgenic mice (75, 134, 148, 149). However, in a Phase III clinical trial of ALS subjects, patients treated with this drug unexpectedly exhibited substantially more rapid disease progression than those treated with the placebo (47).
Other modalities such as protease inhibitors, stem cell therapy, and gene therapy are either being considered or tested in clinical trials (46). Also, novel approaches to shut down the production of mutant SOD1 are under development for the subset of patients with these mutations (127). However, effective drug treatments for patients have remained elusive despite advances in our understanding of ALS pathology. Although the ALS field has gained valuable experience in the design and conduct of clinical trials from the clinical studies completed to date, it is unclear why none of these therapies except riluzole, which has only a modest effect on survival, has had a positive impact in patients. Whether the reason the experimental treatment did not show efficacy in any of these cases was because the therapy was truly not effective, the biological targets were misguided, or the trial design was inadequate, is difficult to assess. For instance, in spite of a strong scientific rationale for developing an antioxidant-based therapy, antioxidants—including vitamins C, E, selegiline, selenium, methionine, and—no significant effect on the primary outcome measure was observed in a meta-analysis of all antioxidant trials combined (105). Suggested limitations of these trials include insufficient power (Vitamin E, creatine) and low dosage (creatine) (81).
Although some of the potential therapeutics described above were efficacious in animal models, such as the mutant SOD1 transgenic mouse, these preclinical results obviously have been unreliable in predicting the results of human studies (35). A recently published study suggests that most of the published results on the mutant SOD1 transgenic mouse models may actually reflect noise in survival distribution, and the authors recommend a stringent minimum study design (121). Whether the mutant SOD1 transgenic mouse accurately represents human sporadic ALS is also uncertain.
Many of the antioxidant therapies tested thus far may not have been effective because they are not based on inhibiting a validated therapeutic target or a specific pathway. As discussed earlier in this review, an interaction between SOD1 and Rac1 serves to regulate ROS production by Nox2. Additionally, several other genes that are either known to cause ALS or are associated with the disease appear to converge on Rac1 regulation. As in the case of mutant SOD1, these mutations may lead to alterations in the regulation of NADPH oxidases and in the overproduction of ROS. These events may precipitate a pathogenic cascade that impairs mitochondrial function and inhibits EAAT2 (the main glial glutamate transporter protein), which results in the presence of an excess of glutamate and a consequent increase in intracellular calcium, and ultimately in enhanced oxidative stress (46). Microglial activation in patients with either sporadic ALS or familial ALS results in the release of neuroinflammatory proteins, amplifying oxidative stress that ultimately promote neuronal death via programmed cell death. Thus, inhibiting the key biological target Nox2 using a potent inhibitor should suppress the pathogenic cascade. The administration of apocynin, an inhibitor of Nox2 activity, has been shown to improve the survival of SOD1G93A transgenic mice and also to protect a glial cell line from ROS-induced cell death caused by overexpression of SOD1L8Q or SOD1G93A (55). More recently, apocynin has also been show to protect human motor neurons from non-cell-autonomous pro-inflammatory toxicity derived from co-cultured human astrocytes expressing SOD1G37R (89). Thus, based on its mechanism of action, apocynin may be considered a disease-modifying therapy for ALS. Safety and toxicology studies are required to test its therapeutic activity in humans. Initial clinical studies of apocynin will include an evaluation of the dose-response relationship, and a demonstration of biological activity using relevant markers. Once these are obtained, efficacy studies will be considered.
Concluding Remarks and Future Directions
Despite a tremendous amount of research on ALS (over 10,800 publications to date), a unifying pathophysiologic mechanism for this devastating disease has eluded the field for decades. Additionally, apparent differences in the mechanism(s) that underlie disease pathology in the mouse models of ALS and human ALS patients have made it challenging to use rational design in developing therapies with clinical efficacy. One might argue that a unifying pathophysiologic mechanism does not exist because multiple independent pathways can lead to the motor neuron degeneration that is observed in ALS. This may indeed be the case. However, it is equally possible that ALS-causing genes and gene modifiers will fall into functionally-related groups—and that unifying pathways for disease causation will emerge from shared, overlapping, and interconnected cellular functions. The recent success in genome-wide genetic association studies in sporadic ALS patients has begun to support this concept, and has identified a wide array of candidate genes that may either cause ALS or influence the progression of this disease. Interestingly, many of these genes are known to influence endosomal and cytoskeletal dynamics, and the regulation of both Rac1 and NADPH oxidases. It is currently unclear which subsets of these genes directly dysregulate ROS production and/or the inflammatory responses observed in ALS. However, regulation of Rac1 appears to be central to both the inherited forms of ALS caused by defects in the ALS2 and SOD1 genes and a subset of genes that have been associated with sporadic ALS. Given the finding that ROS is elevated in ALS-affected tissues, genes that affect ROS production, either directly or indirectly through inflammatory processes, may provide a unifying mechanism for disease causation and disease severity. The challenge over the next decade will be to place the abundance of genes that are associated with sporadic ALS into the biologic context of the simpler monogenetic causes of ALS. Such studies will hopefully lead to a clearer understanding of pathophysiologic mechanisms, and also to more effective methods with which to approach disease treatment.
Acknowledgments
This work was supported by NIDDK (RO1 DK051315 to JFE) and the Roy J. Carver Chair in Molecular Medicine (JFE). We also gratefully acknowledge Dr. Christine Blaumueller for editorial assistance.
Abbreviations
ALS, amyotrophic lateral sclerosis; ALS2, amyotrophic lateral sclerosis 2 gene; Alsin, amyotrophic lateral sclerosis protein 2; CSF, cerebrospinal fluid; Duox, dual oxidase; EEA1, early endosomal antigen 1; FALS, familial amyotrophic lateral sclerosis; GDP, guanosine diphosphate; GFP, green fluorescent protein; GTP, guanosine triphosphate; H2O2, hydrogen peroxide; IL-1β, interleukin-1 β; IL-1R1, interleukin-1 receptor 1; IKK, IκB kinase; IKKK, IκB kinase kinase; MyD88, myeloid differentiation primary response gene; NADPH, nicotinamide–adenine dinucleotide phosphate (reduced); NFκB, nuclear factor κB; Nox, NADPH oxidase; Rac1, Ras related C3 botulinum toxin substrate 1; redoxosome, redox-active endosome; RhoGDI, Rho GDP-dissociation inhibitor; ROS, reactive oxygen species; SOD1, superoxide dismutase 1; SALS; sporadic amyotrophic lateral sclerosis; TNFα, tumor necrosis factor α; TNFR1, tumor necrosis factor receptor 1; TRAF2, tumor necrosis factor receptor associated factor 2; TRAF6, tumor necrosis factor receptor-associated factor 6; TRADD, tumor necrosis factor receptor-associated death domain.
References
- 1.Abe K. Pan LH. Watanabe M. Kato T. Itoyama Y. Induction of nitrotyrosine-like immunoreactivity in the lower motor neuron of amyotrophic lateral sclerosis. Neurosci Lett. 1995;199:152–154. doi: 10.1016/0304-3940(95)12039-7. [DOI] [PubMed] [Google Scholar]
- 2.Abe K. Pan LH. Watanabe M. Konno H. Kato T. Itoyama Y. Upregulation of protein-tyrosine nitration in the anterior horn cells of amyotrophic lateral sclerosis. Neurol Res. 1997;19:124–128. doi: 10.1080/01616412.1997.11740784. [DOI] [PubMed] [Google Scholar]
- 3.Aricescu AR. Hon WC. Siebold C. Lu W. van der Merwe PA. Jones EY. Molecular analysis of receptor protein tyrosine phosphatase mu-mediated cell adhesion. EMBO J. 2006;25:701–712. doi: 10.1038/sj.emboj.7600974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aricescu AR. Siebold C. Jones EY. Receptor protein tyrosine phosphatase micro: Measuring where to stick. Biochem Soc Trans. 2008;36:167–172. doi: 10.1042/BST0360167. [DOI] [PubMed] [Google Scholar]
- 5.Armon C. An evidence-based medicine approach to the evaluation of the role of exogenous risk factors in sporadic amyotrophic lateral sclerosis. Neuroepidemiology. 2003;22:217–228. doi: 10.1159/000070562. [DOI] [PubMed] [Google Scholar]
- 6.Arthur WT. Quilliam LA. Cooper JA. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J Cell Biol. 2004;167:111–122. doi: 10.1083/jcb.200404068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barber SC. Mead RJ. Shaw PJ. Oxidative stress in ALS: A mechanism of neurodegeneration and a therapeutic target. Biochim Biophys Acta. 2006;1762:1051–1067. doi: 10.1016/j.bbadis.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Beal MF. Ferrante RJ. Browne SE. Matthews RT. Kowall NW. Brown RH., Jr Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann Neurol. 1997;42:644–654. doi: 10.1002/ana.410420416. [DOI] [PubMed] [Google Scholar]
- 9.Bedard K. Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 10.Beghi E. Logroscino G. Chio A. Hardiman O. Mitchell D. Swingler R. Traynor BJ. The epidemiology of ALS and the role of population-based registries. Biochim Biophys Acta. 2006;1762:1150–1157. doi: 10.1016/j.bbadis.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Bensimon G. Lacomblez L. Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 12.Besco J. Popesco MC. Davuluri RV. Frostholm A. Rotter A. Genomic structure and alternative splicing of murine R2B receptor protein tyrosine phosphatases (PTPkappa, mu, rho and PCP-2) BMC Genomics. 2004;5:14. doi: 10.1186/1471-2164-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boillee S. Yamanaka K. Lobsiger CS. Copeland NG. Jenkins NA. Kassiotis G. Kollias G. Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 14.Bonazzi M. Spano S. Turacchio G. Cericola C. Valente C. Colanzi A. Kweon HS. Hsu VW. Polishchuck EV. Polishchuck RS. Sallese M. Pulvirenti T. Corda D. Luini A. CtBP3/BARS drives membrane fission in dynamin-independent transport pathways. Nat Cell Biol. 2005;7:570–580. doi: 10.1038/ncb1260. [DOI] [PubMed] [Google Scholar]
- 15.Bos JL. Rehmann H. Wittinghofer A. GEFs and GAPs: Critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Bowler RP. Sheng H. Enghild JJ. Pearlstein RD. Warner DS. Crapo JD. A catalytic antioxidant (AEOL 10150) attenuates expression of inflammatory genes in stroke. Free Radic Biol Med. 2002;33:1141–1152. doi: 10.1016/s0891-5849(02)01008-0. [DOI] [PubMed] [Google Scholar]
- 17.Brandt DT. Grosse R. Get to grips: Steering local actin dynamics with IQGAPs. EMBO Rep. 2007;8:1019–1023. doi: 10.1038/sj.embor.7401089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briggs MW. Sacks DB. IQGAP proteins are integral components of cytoskeletal regulation. EMBO Rep. 2003;4:571–574. doi: 10.1038/sj.embor.embor867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brill S. Li S. Lyman CW. Church DM. Wasmuth JJ. Weissbach L. Bernards A. Snijders AJ. The Ras GTPase-activating-protein-related human protein IQGAP2 harbors a potential actin binding domain and interacts with calmodulin and Rho family GTPases. Mol Cell Biol. 1996;16:4869–4878. doi: 10.1128/mcb.16.9.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown RH., Jr Robberecht W. Amyotrophic lateral sclerosis: Pathogenesis. Semin Neurol. 2001;21:131–139. doi: 10.1055/s-2001-15260. [DOI] [PubMed] [Google Scholar]
- 21.Bruijn LI. Miller TM. Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- 22.Bustelo XR. Vav proteins, adaptors and cell signaling. Oncogene. 2001;20:6372–6381. doi: 10.1038/sj.onc.1204780. [DOI] [PubMed] [Google Scholar]
- 23.Cai H. Lin X. Xie C. Laird FM. Lai C. Wen H. Chiang HC. Shim H. Farah MH. Hoke A. Price DL. Wong PC. Loss of ALS2 function is insufficient to trigger motor neuron degeneration in knock-out mice but predisposes neurons to oxidative stress. J Neurosci. 2005;25:7567–7574. doi: 10.1523/JNEUROSCI.1645-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carri MT. Grignaschi G. Bendotti C. Targets in ALS: Designing multidrug therapies. Trends Pharmacol Sci. 2006;27:267–273. doi: 10.1016/j.tips.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Chance B. Sies H. Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 26.Chandran J. Ding J. Cai H. Alsin and the molecular pathways of amyotrophic lateral sclerosis. Mol Neurobiol. 2007;36:224–231. doi: 10.1007/s12035-007-0034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu CY. Leng S. Martin KA. Kim E. Gorman S. Duhl DM. Cloning and characterization of T-cell lymphoma invasion and metastasis 2 (TIAM2), a novel guanine nucleotide exchange factor related to TIAM1. Genomics. 1999;61:66–73. doi: 10.1006/geno.1999.5936. [DOI] [PubMed] [Google Scholar]
- 28.Choudry RB. Cudkowicz ME. Clinical trials in amyotrophic lateral sclerosis: The tenuous past and the promising future. J Clin Pharmacol. 2005;45:1334–1344. doi: 10.1177/0091270005282631. [DOI] [PubMed] [Google Scholar]
- 29.Clement AM. Nguyen MD. Roberts EA. Garcia ML. Boillee S. Rule M. McMahon AP. Doucette W. Siwek D. Ferrante RJ. Brown RH., Jr. Julien JP. Goldstein LS. Cleveland DW. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- 30.Crow JP. Calingasan NY. Chen J. Hill JL. Beal MF. Manganese porphyrin given at symptom onset markedly extends survival of ALS mice. Ann Neurol. 2005;58:258–265. doi: 10.1002/ana.20552. [DOI] [PubMed] [Google Scholar]
- 31.Damke H. Baba T. van der Bliek AM. Schmid SL. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J Cell Biol. 1995;131:69–80. doi: 10.1083/jcb.131.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delanty N. Dichter MA. Antioxidant therapy in neurologic disease. Arch Neurol. 2000;57:1265–1270. doi: 10.1001/archneur.57.9.1265. [DOI] [PubMed] [Google Scholar]
- 33.Dengler R. von Neuhoff N. Bufler J. Krampfl K. Peschel T. Grosskreutz J. Amyotrophic lateral sclerosis: New developments in diagnostic markers. Neurodegener Dis. 2005;2:177–184. doi: 10.1159/000089623. [DOI] [PubMed] [Google Scholar]
- 34.Devon RS. Orban PC. Gerrow K. Barbieri MA. Schwab C. Cao LP. Helm JR. Bissada N. Cruz–Aguado R. Davidson TL. Witmer J. Metzler M. Lam CK. Tetzlaff W. Simpson EM. McCaffery JM. El–Husseini AE. Leavitt BR. Hayden MR. Als2-deficient mice exhibit disturbances in endosome trafficking associated with motor behavioral abnormalities. Proc Natl Acad Sci USA. 2006;103:9595–9600. doi: 10.1073/pnas.0510197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dib M. Amyotrophic lateral sclerosis: progress and prospects for treatment. Drugs. 2003;63:289–310. doi: 10.2165/00003495-200363030-00004. [DOI] [PubMed] [Google Scholar]
- 36.Doble A. The pharmacology and mechanism of action of riluzole. Neurology. 1996;47:S233–241. doi: 10.1212/wnl.47.6_suppl_4.233s. [DOI] [PubMed] [Google Scholar]
- 37.Dunckley T. Huentelman MJ. Craig DW. Pearson JV. Szelinger S. Joshipura K. Halperin RF. Stamper C. Jensen KR. Letizia D. Hesterlee SE. Pestronk A. Levine T. Bertorini T. Graves MC. Mozaffar T. Jackson CE. Bosch P. McVey A. Dick A. Barohn R. Lomen–Hoerth C. Rosenfeld J. O'Connor D T. Zhang K. Crook R. Ryberg H. Hutton M. Katz J. Simpson EP. Mitsumoto H. Bowser R. Miller RG. Appel SH. Stephan DA. Whole-genome analysis of sporadic amyotrophic lateral sclerosis. N Engl J Med. 2007;357:775–788. doi: 10.1056/NEJMoa070174. [DOI] [PubMed] [Google Scholar]
- 38.Eisen A. Krieger C. Amyotrophic Lateral Sclerosis: A Synthesis of Research and Clinical Practice. New York, NY: Cambridge University Press; 1998. [Google Scholar]
- 39.el Benna J. Ruedi JM. Babior BM. Cytosolic guanine nucleotide-binding protein Rac2 operates in vivo as a component of the neutrophil respiratory burst oxidase. Transfer of Rac2 and the cytosolic oxidase components p47phox and p67phox to the submembranous actin cytoskeleton during oxidase activation. J Biol Chem. 1994;269:6729–6734. [PubMed] [Google Scholar]
- 40.Erie EA. Shim H. Smith AL. Lin X. Keyvanfar K. Xie C. Chen J. Cai H. Mice deficient in the ALS2 gene exhibit lymphopenia and abnormal hematopietic function. J Neuroimmunol. 2007;182:226–231. doi: 10.1016/j.jneuroim.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferrante KL. Shefner J. Zhang H. Betensky R. O'Brien M. Yu H. Fantasia M. Taft J. Beal MF. Traynor B. Newhall K. Donofrio P. Caress J. Ashburn C. Freiberg B. O'Neill C. Paladenech C. Walker T. Pestronk A. Abrams B. Florence J. Renna R. Schierbecker J. Malkus B. Cudkowicz M. Tolerance of high-dose (3,000 mg/day) coenzyme Q10 in ALS. Neurology. 2005;65:1834–1836. doi: 10.1212/01.wnl.0000187070.35365.d7. [DOI] [PubMed] [Google Scholar]
- 42.Ferrante RJ. Browne SE. Shinobu LA. Bowling AC. Baik MJ. MacGarvey U. Kowall NW. Brown RH., Jr. Beal MF. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J Neurochem. 1997;69:2064–2074. doi: 10.1046/j.1471-4159.1997.69052064.x. [DOI] [PubMed] [Google Scholar]
- 43.Fukuyama T. Ogita H. Kawakatsu T. Inagaki M. Takai Y. Activation of Rac by cadherin through the c-Src-Rap1-phosphatidylinositol 3-kinase-Vav2 pathway. Oncogene. 2006;25:8–19. doi: 10.1038/sj.onc.1209010. [DOI] [PubMed] [Google Scholar]
- 44.Galbussera A. Tremolizzo L. Brighina L. Testa D. Lovati R. Ferrarese C. Cavaletti G. Filippini G. Vitamin E intake and quality of life in amyotrophic lateral sclerosis patients: A follow-up case series study. Neurol Sci. 2006;27:190–193. doi: 10.1007/s10072-006-0668-x. [DOI] [PubMed] [Google Scholar]
- 45.Gianni D. Bohl B. Courtneidge SA. Bokoch GM. The involvement of the tyrosine kinase c-src in the regulation of reactive oxygen species generation mediated by NADPH oxidase-1. Mol Biol Cell. 2008;19:2984–2994. doi: 10.1091/mbc.E08-02-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goodall EF. Morrison KE. Amyotrophic lateral sclerosis (motor neuron disease): Proposed mechanisms and pathways to treatment. Expert Rev Mol Med. 2006;8:1–22. doi: 10.1017/S1462399406010854. [DOI] [PubMed] [Google Scholar]
- 47.Gordon PH. Moore DH. Miller RG. Florence JM. Verheijde JL. Doorish C. Hilton JF. Spitalny GM. MacArthur RB. Mitsumoto H. Neville HE. Boylan K. Mozaffar T. Belsh JM. Ravits J. Bedlack RS. Graves MC. McCluskey LF. Barohn RJ. Tandan R. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: A phase III randomised trial. Lancet Neurol. 2007;6:1045–1053. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- 48.Gorin Y. Ricono JM. Kim NH. Bhandari B. Choudhury GG. Abboud HE. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol Renal Physiol. 2003;285:F219–229. doi: 10.1152/ajprenal.00414.2002. [DOI] [PubMed] [Google Scholar]
- 49.Gowing G. Dequen F. Soucy G. Julien JP. Absence of tumor necrosis factor-alpha does not affect motor neuron disease caused by superoxide dismutase 1 mutations. J Neurosci. 2006;26:11397–11402. doi: 10.1523/JNEUROSCI.0602-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gribkoff VK. Bozik ME. KNS-760704 [(6R)-4,5,6,7-tetrahydro-N6-propyl-2, 6-benzothiazole-diamine dihydrochloride monohydrate] for the treatment of amyotrophic lateral sclerosis. CNS Neurosci Ther. 2008;14:215–226. doi: 10.1111/j.1755-5949.2008.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Groeneveld GJ. Veldink JH. van der Tweel I. Kalmijn S. Beijer C. de Visser M. Wokke JH. Franssen H. van den Berg LH. A randomized sequential trial of creatine in amyotrophic lateral sclerosis. Ann Neurol. 2003;53:437–445. doi: 10.1002/ana.10554. [DOI] [PubMed] [Google Scholar]
- 52.Gros–Louis F. Gaspar C. Rouleau GA. Genetics of familial and sporadic amyotrophic lateral sclerosis. Biochim Biophys Acta. 2006;1762:956–972. doi: 10.1016/j.bbadis.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Habets GG. van der Kammen RA. Stam JC. Michiels F. Collard JG. Sequence of the human invasion-inducing TIAM1 gene, its conservation in evolution and its expression in tumor cell lines of different tissue origin. Oncogene. 1995;10:1371–1376. [PubMed] [Google Scholar]
- 54.Hadano S. Kunita R. Otomo A. Suzuki–Utsunomiya K. Ikeda JE. Molecular and cellular function of ALS2/alsin: Implication of membrane dynamics in neuronal development and degeneration. Neurochem Int. 2007;51:74–84. doi: 10.1016/j.neuint.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 55.Harraz MM. Marden JJ. Zhou W. Zhang Y. Williams A. Sharov VS. Nelson K. Luo M. Paulson H. Schoneich C. Engelhardt JF. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest. 2008;118:659–670. doi: 10.1172/JCI34060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hart MJ. Callow MG. Souza B. Polakis P. IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. EMBO J. 1996;15:2997–3005. [PMC free article] [PubMed] [Google Scholar]
- 57.Heath PR. Shaw PJ. Update on the glutamatergic neurotransmitter system and the role of excitotoxicity in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:438–458. doi: 10.1002/mus.10186. [DOI] [PubMed] [Google Scholar]
- 58.Heiman–Patterson TD. Deitch JS. Blankenhorn EP. Erwin KL. Perreault MJ. Alexander BK. Byers N. Toman I. Alexander GM. Background and gender effects on survival in the TgN(SOD1-G93A)1Gur mouse model of ALS. J Neurol Sci. 2005;236:1–7. doi: 10.1016/j.jns.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Hensley K. Fedynyshyn J. Ferrell S. Floyd RA. Gordon B. Grammas P. Hamdheydari L. Mhatre M. Mou S. Pye QN. Stewart C. West M. West S. Williamson KS. Message and protein-level elevation of tumor necrosis factor alpha (TNF alpha) and TNF alpha-modulating cytokines in spinal cords of the G93A-SOD1 mouse model for amyotrophic lateral sclerosis. Neurobiol Dis. 2003;14:74–80. doi: 10.1016/s0969-9961(03)00087-1. [DOI] [PubMed] [Google Scholar]
- 60.Hordijk PL. Regulation of NADPH oxidases: The role of Rac proteins. Circ Res. 2006;98:453–462. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- 61.Hoshino M. Sone M. Fukata M. Kuroda S. Kaibuchi K. Nabeshima Y. Hama C. Identification of the stef gene that encodes a novel guanine nucleotide exchange factor specific for Rac1. J Biol Chem. 1999;274:17837–17844. doi: 10.1074/jbc.274.25.17837. [DOI] [PubMed] [Google Scholar]
- 62.ALS Association. Calabasas Hills, CA: [Google Scholar]
- 63.Knopp Neuroscience Inc. Pittsburgh, PA: [Google Scholar]
- 64.Hu Y. Li Z. Guo L. Wang L. Zhang L. Cai X. Zhao H. Zha X. MAGI-2 inhibits cell migration and proliferation via PTEN in human hepatocarcinoma cells. Arch Biochem Biophys. 2007;467:1–9. doi: 10.1016/j.abb.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 65.Ikeda S. Yamaoka–Tojo M. Hilenski L. Patrushev NA. Anwar GM. Quinn MT. Ushio–Fukai M. IQGAP1 regulates reactive oxygen species-dependent endothelial cell migration through interacting with Nox2. Arterioscler Thromb Vasc Biol. 2005;25:2295–2300. doi: 10.1161/01.ATV.0000187472.55437.af. [DOI] [PubMed] [Google Scholar]
- 66.Irani K. Xia Y. Zweier JL. Sollott SJ. Der CJ. Fearon ER. Sundaresan M. Finkel T. Goldschmidt–Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 67.Jeong HW. Li Z. Brown MD. Sacks DB. IQGAP1 binds Rap1 and modulates its activity. J Biol Chem. 2007;282:20752–20762. doi: 10.1074/jbc.M700487200. [DOI] [PubMed] [Google Scholar]
- 68.Kabe Y. Ando K. Hirao S. Yoshida M. Handa H. Redox regulation of NF-kappaB activation: Distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal. 2005;7:395–403. doi: 10.1089/ars.2005.7.395. [DOI] [PubMed] [Google Scholar]
- 69.Kanekura K. Hashimoto Y. Niikura T. Aiso S. Matsuoka M. Nishimoto I. Alsin, the product of ALS2 gene, suppresses SOD1 mutant neurotoxicity through RhoGEF domain by interacting with SOD1 mutants. J Biol Chem. 2004;279:19247–19256. doi: 10.1074/jbc.M313236200. [DOI] [PubMed] [Google Scholar]
- 70.Kawauchi T. Chihama K. Nabeshima Y. Hoshino M. The in vivo roles of STEF/Tiam1, Rac1 and JNK in cortical neuronal migration. EMBO J. 2003;22:4190–4201. doi: 10.1093/emboj/cdg413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kheradmand F. Werner E. Tremble P. Symons M. Werb Z. Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science. 1998;280:898–902. doi: 10.1126/science.280.5365.898. [DOI] [PubMed] [Google Scholar]
- 72.Klivenyi P. Ferrante RJ. Matthews RT. Bogdanov MB. Klein AM. Andreassen OA. Mueller G. Wermer M. Kaddurah–Daouk R. Beal MF. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat Med. 1999;5:347–350. doi: 10.1038/6568. [DOI] [PubMed] [Google Scholar]
- 73.Klivenyi P. Kiaei M. Gardian G. Calingasan NY. Beal MF. Additive neuroprotective effects of creatine and cyclooxygenase 2 inhibitors in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurochem. 2004;88:576–582. doi: 10.1046/j.1471-4159.2003.02160.x. [DOI] [PubMed] [Google Scholar]
- 74.Kooistra MR. Dube N. Bos JL. Rap1: A key regulator in cell-cell junction formation. J Cell Sci. 2007;120:17–22. doi: 10.1242/jcs.03306. [DOI] [PubMed] [Google Scholar]
- 75.Kriz J. Nguyen MD. Julien JP. Minocycline slows disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2002;10:268–278. doi: 10.1006/nbdi.2002.0487. [DOI] [PubMed] [Google Scholar]
- 76.Kunita R. Otomo A. Mizumura H. Suzuki–Utsunomiya K. Hadano S. Ikeda JE. The Rab5 activator ALS2/alsin acts as a novel Rac1 effector through Rac1-activated endocytosis. J Biol Chem. 2007;282:16599–16611. doi: 10.1074/jbc.M610682200. [DOI] [PubMed] [Google Scholar]
- 77.Kuroda S. Fukata M. Kobayashi K. Nakafuku M. Nomura N. Iwamatsu A. Kaibuchi K. Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. J Biol Chem. 1996;271:23363–23367. doi: 10.1074/jbc.271.38.23363. [DOI] [PubMed] [Google Scholar]
- 78.Kuroda S. Fukata M. Nakagawa M. Fujii K. Nakamura T. Ookubo T. Izawa I. Nagase T. Nomura N. Tani H. Shoji I. Matsuura Y. Yonehara S. Kaibuchi K. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin- mediated cell-cell adhesion. Science. 1998;281:832–835. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- 79.Lacomblez L. Bensimon G. Leigh PN. Guillet P. Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347:1425–1431. doi: 10.1016/s0140-6736(96)91680-3. [DOI] [PubMed] [Google Scholar]
- 80.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 81.Lanka V. Cudkowicz M. Therapy development for ALS: Lessons learned and path forward. Amyotroph Lateral Scler. 2008;9:131–140. doi: 10.1080/17482960802112819. [DOI] [PubMed] [Google Scholar]
- 82.Li Q. Harraz MM. Zhou W. Zhang LN. Ding W. Zhang Y. Eggleston T. Yeaman C. Banfi B. Engelhardt JF. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol. 2006;26:140–154. doi: 10.1128/MCB.26.1.140-154.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Q. Spencer NY. Oakley FD. Buettner GR. Engelhardt JF. Endosomal Nox2 facilitates redox-dependent induction of NF-κB by TNF-α. Antioxid Redox Signal. 2009;11:1249–1263. doi: 10.1089/ars.2008.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Y. Yan J. De P. Chang HC. Yamauchi A. Christopherson KW., 2nd Paranavitana NC. Peng X. Kim C. Munugalavadla V. Kapur R. Chen H. Shou W. Stone JC. Kaplan MH. Dinauer MC. Durden DL. Quilliam LA. Rap1a null mice have altered myeloid cell functions suggesting distinct roles for the closely related Rap1a and 1b proteins. J Immunol. 2007;179:8322–8331. doi: 10.4049/jimmunol.179.12.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liliental J. Moon SY. Lesche R. Mamillapalli R. Li D. Zheng Y. Sun H. Wu H. Genetic deletion of the Pten tumor suppressor gene promotes cell motility by activation of Rac1 and Cdc42 GTPases. Curr Biol. 2000;10:401–404. doi: 10.1016/s0960-9822(00)00417-6. [DOI] [PubMed] [Google Scholar]
- 86.Lobsiger CS. Cleveland DW. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci. 2007;10:1355–1360. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lopes N. Gregg D. Vasudevan S. Hassanain H. Goldschmidt–Clermont P. Kovacic H. Thrombospondin 2 regulates cell proliferation induced by Rac1 redox-dependent signaling. Mol Cell Biol. 2003;23:5401–5408. doi: 10.1128/MCB.23.15.5401-5408.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lynch RE. Fridovich I. Permeation of the erythrocyte stroma by superoxide radical. J Biol Chem. 1978;253:4697–4699. [PubMed] [Google Scholar]
- 89.Marchetto MC. Muotri AR. Mu Y. Smith AM. Cezar GG. Gage FH. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 90.Marden JJ. Harraz MM. Williams AJ. Nelson K. Luo M. Paulson H. Engelhardt JF. Redox modifier genes in amyotrophic lateral sclerosis in mice. J Clin Invest. 2007;117:2913–2919. doi: 10.1172/JCI31265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martyn KD. Frederick LM. von Loehneysen K. Dinauer MC. Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 92.Mataraza JM. Briggs MW. Li Z. Entwistle A. Ridley AJ. Sacks DB. IQGAP1 promotes cell motility and invasion. J Biol Chem. 2003;278:41237–41245. doi: 10.1074/jbc.M304838200. [DOI] [PubMed] [Google Scholar]
- 93.Matsuo N. Hoshino M. Yoshizawa M. Nabeshima Y. Characterization of STEF, a guanine nucleotide exchange factor for Rac1, required for neurite growth. J Biol Chem. 2002;277:2860–2868. doi: 10.1074/jbc.M106186200. [DOI] [PubMed] [Google Scholar]
- 94.McCord JM. Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 95.Mertens AE. Roovers RC. Collard JG. Regulation of Tiam1-Rac signalling. FEBS Lett. 2003;546:11–16. doi: 10.1016/s0014-5793(03)00435-6. [DOI] [PubMed] [Google Scholar]
- 96.Michiels F. Habets GG. Stam JC. van der Kammen RA. Collard JG. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature. 1995;375:338–340. doi: 10.1038/375338a0. [DOI] [PubMed] [Google Scholar]
- 97.Miller FJ., Jr. Filali M. Huss GJ. Stanic B. Chamseddine A. Barna TJ. Lamb FS. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 98.Ming W. Li S. Billadeau DD. Quilliam LA. Dinauer MC. The Rac effector p67phox regulates phagocyte NADPH oxidase by stimulating Vav1 guanine nucleotide exchange activity. Mol Cell Biol. 2007;27:312–323. doi: 10.1128/MCB.00985-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moore KA. Sethi R. Doanes AM. Johnson TM. Pracyk JB. Kirby M. Irani K. Goldschmidt–Clermont PJ. Finkel T. Rac1 is required for cell proliferation and G2/M progression. Biochem J. 1997;326:17–20. doi: 10.1042/bj3260017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mumbengegwi DR. Li Q. Li C. Bear CE. Engelhardt JF. Evidence for a superoxide permeability pathway in endosomal membranes. Mol Cell Biol. 2008;28:3700–3712. doi: 10.1128/MCB.02038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nguyen MD. Julien JP. Rivest S. Induction of proinflammatory molecules in mice with amyotrophic lateral sclerosis: No requirement for proapoptotic interleukin-1beta in neurodegeneration. Ann Neurol. 2001;50:630–639. doi: 10.1002/ana.1256. [DOI] [PubMed] [Google Scholar]
- 102.Nimnual AS. Taylor LJ. Bar-Sagi D. Redox-dependent downregulation of Rho by Rac. Nat Cell Biol. 2003;5:236–241. doi: 10.1038/ncb938. [DOI] [PubMed] [Google Scholar]
- 103.Oakley FD. Abbott D. Li Q. Engelhardt JF. Signaling components of redox active endosomes: The redoxosomes. Antioxid Redox Signal. 2009;11:1313–1333. doi: 10.1089/ars.2008.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Orrell RW. AEOL-10150 (Aeolus) Curr Opin Investig Drugs. 2006;7:70–80. [PubMed] [Google Scholar]
- 105.Orrell RW. Lane RJ. Ross M. A systematic review of antioxidant treatment for amyotrophic lateral sclerosis/motor neuron disease. Amyotroph Lat Scler. 2008;9:195–211. doi: 10.1080/17482960801900032. [DOI] [PubMed] [Google Scholar]
- 106.Otomo A. Hadano S. Okada T. Mizumura H. Kunita R. Nishijima H. Showguchi–Miyata J. Yanagisawa Y. Kohiki E. Suga E. Yasuda M. Osuga H. Nishimoto T. Narumiya S. Ikeda JE. ALS2, a novel guanine nucleotide exchange factor for the small GTPase Rab5, is implicated in endosomal dynamics. Hum Mol Genet. 2003;12:1671–1687. doi: 10.1093/hmg/ddg184. [DOI] [PubMed] [Google Scholar]
- 107.Palamidessi A. Frittoli E. Garre M. Faretta M. Mione M. Testa I. Diaspro A. Lanzetti L. Scita G. Di Fiore PP. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–147. doi: 10.1016/j.cell.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 108.Pani G. Colavitti R. Bedogni B. Anzevino R. Borrello S. Galeotti T. A redox signaling mechanism for density-dependent inhibition of cell growth. J Biol Chem. 2000;275:38891–38899. doi: 10.1074/jbc.M007319200. [DOI] [PubMed] [Google Scholar]
- 109.Pasinelli P. Brown RH. Molecular biology of amyotrophic lateral sclerosis: Insights from genetics. Nat Rev Neurosci. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- 110.Phillips–Mason PJ. Gates TJ. Major DL. Sacks DB. Brady–Kalnay SM. The receptor protein-tyrosine phosphatase PTPmu interacts with IQGAP1. J Biol Chem. 2006;281:4903–4910. doi: 10.1074/jbc.M506414200. [DOI] [PubMed] [Google Scholar]
- 111.Poloni M. Facchetti D. Mai R. Micheli A. Agnoletti L. Francolini G. Mora G. Camana C. Mazzini L. Bachetti T. Circulating levels of tumour necrosis factor-alpha and its soluble receptors are increased in the blood of patients with amyotrophic lateral sclerosis. Neurosci Lett. 2000;287:211–214. doi: 10.1016/s0304-3940(00)01177-0. [DOI] [PubMed] [Google Scholar]
- 112.Polymenidou M. Cleveland DW. Motor neuron disease: The curious ways of ALS. Nature. 2008;454:284–285. doi: 10.1038/454284a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Puceat M. Travo P. Quinn MT. Fort P. A dual role of the GTPase Rac in cardiac differentiation of stem cells. Mol Biol Cell. 2003;14:2781–2792. doi: 10.1091/mbc.E02-09-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Quinn MT. Parkos CA. Walker L. Orkin SH. Dinauer MC. Jesaitis AJ. Association of a Ras-related protein with cytochrome b of human neutrophils. Nature. 1989;342:198–200. doi: 10.1038/342198a0. [DOI] [PubMed] [Google Scholar]
- 115.Remans PH. Gringhuis SI. van Laar JM. Sanders ME. Papendrecht–van der Voort EA. Zwartkruis FJ. Levarht EW. Rosas M. Coffer PJ. Breedveld FC. Bos JL. Tak PP. Verweij CL. Reedquist KA. Rap1 signaling is required for suppression of Ras-generated reactive oxygen species and protection against oxidative stress in T lymphocytes. J Immunol. 2004;173:920–931. doi: 10.4049/jimmunol.173.2.920. [DOI] [PubMed] [Google Scholar]
- 116.Roepstorff K. Rasmussen I. Sawada M. Cudre–Maroux C. Salmon P. Bokoch G. van Deurs B. Vilhardt F. Stimulus-dependent regulation of the phagocyte NADPH oxidase by a VAV1, Rac1, and PAK1 signaling axis. J Biol Chem. 2008;283:7983–7993. doi: 10.1074/jbc.M708281200. [DOI] [PubMed] [Google Scholar]
- 117.Rosen DR. Siddique T. Patterson D. Figlewicz DA. Sapp P. Hentati A. Donaldson D. Goto J. O'Regan JP. Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 118.Sakurai A. Fukuhara S. Yamagishi A. Sako K. Kamioka Y. Masuda M. Nakaoka Y. Mochizuki N. MAGI-1 is required for Rap1 activation upon cell–cell contact and for enhancement of vascular endothelial cadherin-mediated cell adhesion. Mol Biol Cell. 2006;17:966–976. doi: 10.1091/mbc.E05-07-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Salvador A. Sousa J. Pinto RE. Hydroperoxyl, superoxide and pH gradients in the mitochondrial matrix: a theoretical assessment. Free Radic Biol Med. 2001;31:1208–1215. doi: 10.1016/s0891-5849(01)00707-9. [DOI] [PubMed] [Google Scholar]
- 120.Schmidt VA. Scudder L. Devoe CE. Bernards A. Cupit LD. Bahou WF. IQGAP2 functions as a GTP-dependent effector protein in thrombin-induced platelet cytoskeletal reorganization. Blood. 2003;101:3021–3028. doi: 10.1182/blood-2002-09-2807. [DOI] [PubMed] [Google Scholar]
- 121.Scott S. Kranz JE. Cole J. Lincecum JM. Thompson K. Kelly N. Bostrom A. Theodoss J. Al–Nakhala BM. Vieira FG. Ramasubbu J. Heywood JA. Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph Lateral Scler. 2008;9:4–15. doi: 10.1080/17482960701856300. [DOI] [PubMed] [Google Scholar]
- 122.Shaw PJ. Ince PG. Falkous G. Mantle D. Oxidative damage to protein in sporadic motor neuron disease spinal cord. Ann Neurol. 1995;38:691–695. doi: 10.1002/ana.410380424. [DOI] [PubMed] [Google Scholar]
- 123.Shefner JM. Cudkowicz ME. Schoenfeld D. Conrad T. Taft J. Chilton M. Urbinelli L. Qureshi M. Zhang H. Pestronk A. Caress J. Donofrio P. Sorenson E. Bradley W. Lomen–Hoerth C. Pioro E. Rezania K. Ross M. Pascuzzi R. Heiman–Patterson T. Tandan R. Mitsumoto H. Rothstein J. Smith–Palmer T. MacDonald D. Burke D. A clinical trial of creatine in ALS. Neurology. 2004;63:1656–1661. doi: 10.1212/01.wnl.0000142992.81995.f0. [DOI] [PubMed] [Google Scholar]
- 124.Shefner JM. Cudkowicz ME. Zhang H. Schoenfeld D. Jillapalli D. The use of statistical MUNE in a multicenter clinical trial. Muscle Nerve. 2004;30:463–469. doi: 10.1002/mus.20120. [DOI] [PubMed] [Google Scholar]
- 125.Shoulson I. Experimental therapeutics of neurodegenerative disorders: Unmet needs. Science. 1998;282:1072–1074. doi: 10.1126/science.282.5391.1072. [DOI] [PubMed] [Google Scholar]
- 126.Simpson EP. Yen AA. Appel SH. Oxidative stress: A common denominator in the pathogenesis of amyotrophic lateral sclerosis. Curr Opin Rheumatol. 2003;15:730–736. doi: 10.1097/00002281-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 127.Smith RA. Miller TM. Yamanaka K. Monia BP. Condon TP. Hung G. Lobsiger CS. Ward CM. McAlonis–Downes M. Wei H. Wancewicz EV. Bennett CF. Cleveland DW. Antisense oligonucleotide therapy for neurodegenerative disease. J Clin Invest. 2006;116:2290–2296. doi: 10.1172/JCI25424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Subauste MC. Nalbant P. Adamson ED. Hahn KM. Vinculin controls PTEN protein level by maintaining the interaction of the adherens junction protein beta-catenin with the scaffolding protein MAGI-2. J Biol Chem. 2005;280:5676–5681. doi: 10.1074/jbc.M405561200. [DOI] [PubMed] [Google Scholar]
- 129.Sulciner DJ. Irani K. Yu ZX. Ferrans VJ. Goldschmidt–Clermont P. Finkel T. rac1 regulates a cytokine-stimulated, redox-dependent pathway necessary for NF-kappaB activation. Mol Cell Biol. 1996;16:7115–7121. doi: 10.1128/mcb.16.12.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Topp JD. Carney DS. Horazdovsky BF. Biochemical characterization of Alsin, a Rab5 and Rac1 guanine nucleotide exchange factor. Methods Enzymol. 2005;403:261–276. doi: 10.1016/S0076-6879(05)03022-3. [DOI] [PubMed] [Google Scholar]
- 131.Topp JD. Gray NW. Gerard RD. Horazdovsky BF. Alsin is a Rab5 and Rac1 guanine nucleotide exchange factor. J Biol Chem. 2004;279:24612–24623. doi: 10.1074/jbc.M313504200. [DOI] [PubMed] [Google Scholar]
- 132.Traynor BJ. Bruijn L. Conwit R. Beal F. O'Neill G. Fagan SC. Cudkowicz ME. Neuroprotective agents for clinical trials in ALS: A systematic assessment. Neurology. 2006;67:20–27. doi: 10.1212/01.wnl.0000223353.34006.54. [DOI] [PubMed] [Google Scholar]
- 133.Vallet P. Charnay Y. Steger K. Ogier–Denis E. Kovari E. Herrmann F. Michel JP. Szanto I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 134.Van Den Bosch L. Tilkin P. Lemmens G. Robberecht W. Minocycline delays disease onset and mortality in a transgenic model of ALS. Neuroreport. 2002;13:1067–1070. doi: 10.1097/00001756-200206120-00018. [DOI] [PubMed] [Google Scholar]
- 135.Vazquez F. Grossman SR. Takahashi Y. Rokas MV. Nakamura N. Sellers WR. Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J Biol Chem. 2001;276:48627–48630. doi: 10.1074/jbc.C100556200. [DOI] [PubMed] [Google Scholar]
- 136.Wang S. Watanabe T. Noritake J. Fukata M. Yoshimura T. Itoh N. Harada T. Nakagawa M. Matsuura Y. Arimura N. Kaibuchi K. IQGAP3, a novel effector of Rac1 and Cdc42, regulates neurite outgrowth. J Cell Sci. 2007;120:567–577. doi: 10.1242/jcs.03356. [DOI] [PubMed] [Google Scholar]
- 137.Wilkinson B. Koenigsknecht–Talboo J. Grommes C. Lee CY. Landreth G. Fibrillar beta-amyloid-stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem. 2006;281:20842–20850. doi: 10.1074/jbc.M600627200. [DOI] [PubMed] [Google Scholar]
- 138.Woo CH. Kim TH. Choi JA. Ryu HC. Lee JE. You HJ. Bae YS. Kim JH. Inhibition of receptor internalization attenuates the TNFalpha-induced ROS generation in non-phagocytic cells. Biochem Biophys Res Commun. 2006;351:972–978. doi: 10.1016/j.bbrc.2006.10.154. [DOI] [PubMed] [Google Scholar]
- 139.Wooley CM. Sher RB. Kale A. Frankel WN. Cox GA. Seburn KL. Gait analysis detects early changes in transgenic SOD1(G93A) mice. Muscle Nerve. 2005;32:43–50. doi: 10.1002/mus.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Worthylake DK. Rossman KL. Sondek J. Crystal structure of Rac1 in complex with the guanine nucleotide exchange region of Tiam1. Nature. 2000;408:682–688. doi: 10.1038/35047014. [DOI] [PubMed] [Google Scholar]
- 141.Wu DC. Re DB. Nagai M. Ischiropoulos H. Przedborski S. The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc Natl Acad Sci USA. 2006;103:12132–12137. doi: 10.1073/pnas.0603670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wu X. Hepner K. Castelino–Prabhu S. Do D. Kaye MB. Yuan XJ. Wood J. Ross C. Sawyers CL. Whang YE. Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2. Proc Natl Acad Sci USA. 2000;97:4233–4238. doi: 10.1073/pnas.97.8.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yamaoka-Tojo M. Ushio–Fukai M. Hilenski L. Dikalov SI. Chen YE. Tojo T. Fukai T. Fujimoto M. Patrushev NA. Wang N. Kontos CD. Bloom GS. Alexander RW. IQGAP1, a novel vascular endothelial growth factor receptor binding protein, is involved in reactive oxygen species–dependent endothelial migration and proliferation. Circ Res. 2004;95:276–283. doi: 10.1161/01.RES.0000136522.58649.60. [DOI] [PubMed] [Google Scholar]
- 144.Yang Y. Hentati A. Deng HX. Dabbagh O. Sasaki T. Hirano M. Hung WY. Ouahchi K. Yan J. Azim AC. Cole N. Gascon G. Yagmour A. Ben–Hamida M. Pericak–Vance M. Hentati F. Siddique T. The gene encoding alsin, a protein with three guanine-nucleotide exchange factor domains, is mutated in a form of recessive amyotrophic lateral sclerosis. Nat Genet. 2001;29:160–165. doi: 10.1038/ng1001-160. [DOI] [PubMed] [Google Scholar]
- 145.Yoshino H. Kimura A. Investigation of the therapeutic effects of edaravone, a free radical scavenger, on amyotrophic lateral sclerosis (Phase II study) Amyotroph Lateral Scler. 2006;7:241–245. doi: 10.1080/17482960600881870. [DOI] [PubMed] [Google Scholar]
- 146.Yoshizawa M. Hoshino M. Sone M. Nabeshima Y. Expression of stef, an activator of Rac1, correlates with the stages of neuronal morphological development in the mouse brain. Mech Dev. 2002;113:65–68. doi: 10.1016/s0925-4773(01)00650-5. [DOI] [PubMed] [Google Scholar]
- 147.Zaldua N. Gastineau M. Hoshino M. Lezoualc'h F. Zugaza JL. Epac signaling pathway involves STEF, a guanine nucleotide exchange factor for Rac, to regulate APP processing. FEBS Lett. 2007;581:5814–5818. doi: 10.1016/j.febslet.2007.11.053. [DOI] [PubMed] [Google Scholar]
- 148.Zhang W. Narayanan M. Friedlander RM. Additive neuroprotective effects of minocycline with creatine in a mouse model of ALS. Ann Neurol. 2003;53:267–270. doi: 10.1002/ana.10476. [DOI] [PubMed] [Google Scholar]
- 149.Zhu S. Stavrovskaya IG. Drozda M. Kim BY. Ona V. Li M. Sarang S. Liu AS. Hartley DM. Wu DC. Gullans S. Ferrante RJ. Przedborski S. Kristal BS. Friedlander RM. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]