Abstract
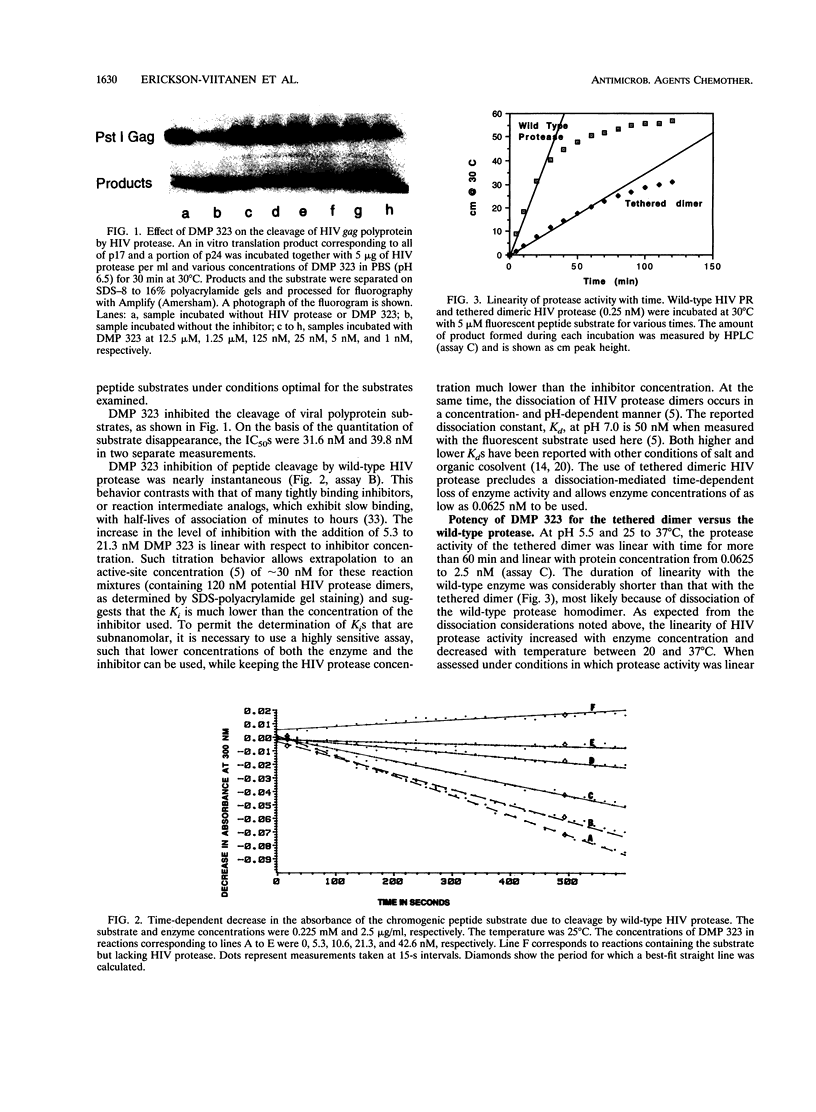
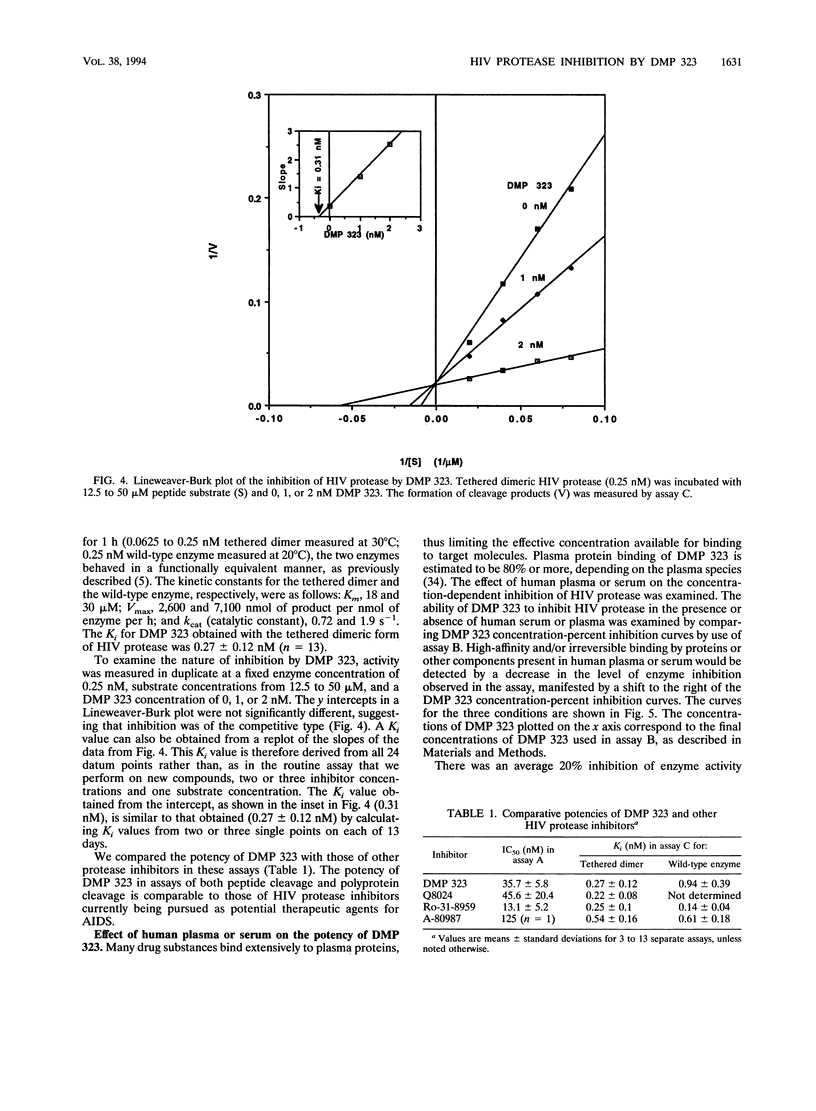
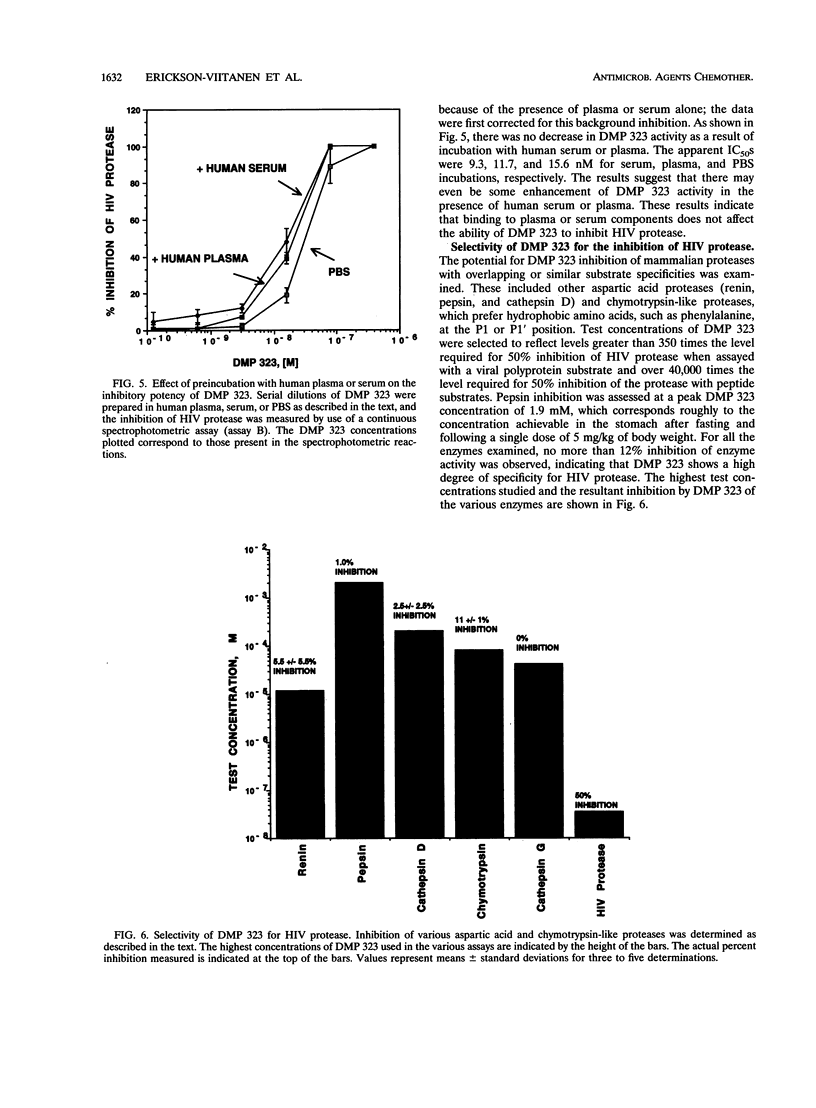
DMP 323 is a potent inhibitor of the protease of human immunodeficiency virus (HIV), with antiviral activity against both HIV type 1 and HIV type 2. This compound is representative of a class of small, novel, nonpeptide cyclic urea inhibitors of HIV protease that were designed on the basis of three-dimensional structural information and three-dimensional database searching. We report here studies of the kinetics of DMP 323 inhibition of the cleavage of peptide and HIV-1 gag polyprotein substrates. DMP 323 acts as a rapidly binding, competitive inhibitor of HIV protease. DMP 323 is as potent against both peptide and viral polyprotein substrates as A-80987, Q8024, and Ro-31-8959, which are among the most potent inhibitors of HIV protease described in the literature to date. Incubation with human plasma or serum did not decrease the effective potency of DMP 323 for HIV protease, suggesting that plasma protein binding is of a low affinity relative to that of HIV protease. DMP 323 was also assessed for its ability to inhibit the mammalian proteases renin, pepsin, cathepsin D, cathepsin G, and chymotrypsin. No inhibition of greater than 12% was observed for any of these enzymes at concentrations of DMP 323 that were 350 to 40,000 times higher than that required to inhibit the viral protease 50%.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J. Cathepsin G. Methods Enzymol. 1981;80(Pt 100):561–565. doi: 10.1016/s0076-6879(81)80044-4. [DOI] [PubMed] [Google Scholar]
- Burton J., Cody R. J., Jr, Herd J. A., Haber E. Specific inhibition of renin by an angiotensinogen analog: studies in sodium depletion and renin-dependent hypertension. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5476–5479. doi: 10.1073/pnas.77.9.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., McGowan M. H., Kettner C. A., Schloss J. V., Erickson-Viitanen S., Yin F. H. High-level synthesis of recombinant HIV-1 protease and the recovery of active enzyme from inclusion bodies. Gene. 1990 Mar 15;87(2):243–248. doi: 10.1016/0378-1119(90)90308-e. [DOI] [PubMed] [Google Scholar]
- Cheng Y. S., Yin F. H., Foundling S., Blomstrom D., Kettner C. A. Stability and activity of human immunodeficiency virus protease: comparison of the natural dimer with a homologous, single-chain tethered dimer. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9660–9664. doi: 10.1073/pnas.87.24.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody R. J., Burton J., Evin G., Poulsen K., Herd J. A., Haber E. A substrate analog inhibitor of renin that is effective in vivo. Biochem Biophys Res Commun. 1980 Nov 17;97(1):230–235. doi: 10.1016/s0006-291x(80)80158-6. [DOI] [PubMed] [Google Scholar]
- Debouck C. The HIV-1 protease as a therapeutic target for AIDS. AIDS Res Hum Retroviruses. 1992 Feb;8(2):153–164. doi: 10.1089/aid.1992.8.153. [DOI] [PubMed] [Google Scholar]
- DelMar E. G., Largman C., Brodrick J. W., Geokas M. C. A sensitive new substrate for chymotrypsin. Anal Biochem. 1979 Nov 1;99(2):316–320. doi: 10.1016/s0003-2697(79)80013-5. [DOI] [PubMed] [Google Scholar]
- Erickson-Viitanen S., Manfredi J., Viitanen P., Tribe D. E., Tritch R., Hutchison C. A., 3rd, Loeb D. D., Swanstrom R. Cleavage of HIV-1 gag polyprotein synthesized in vitro: sequential cleavage by the viral protease. AIDS Res Hum Retroviruses. 1989 Dec;5(6):577–591. doi: 10.1089/aid.1989.5.577. [DOI] [PubMed] [Google Scholar]
- Erickson J., Neidhart D. J., VanDrie J., Kempf D. J., Wang X. C., Norbeck D. W., Plattner J. J., Rittenhouse J. W., Turon M., Wideburg N. Design, activity, and 2.8 A crystal structure of a C2 symmetric inhibitor complexed to HIV-1 protease. Science. 1990 Aug 3;249(4968):527–533. doi: 10.1126/science.2200122. [DOI] [PubMed] [Google Scholar]
- Jordan S. P., Zugay J., Darke P. L., Kuo L. C. Activity and dimerization of human immunodeficiency virus protease as a function of solvent composition and enzyme concentration. J Biol Chem. 1992 Oct 5;267(28):20028–20032. [PubMed] [Google Scholar]
- Kempf D. J., Codacovi L., Wang X. C., Kohlbrenner W. E., Wideburg N. E., Saldivar A., Vasavanonda S., Marsh K. C., Bryant P., Sham H. L. Symmetry-based inhibitors of HIV protease. Structure-activity studies of acylated 2,4-diamino-1,5-diphenyl-3-hydroxypentane and 2,5-diamino-1,6-diphenylhexane-3,4-diol. J Med Chem. 1993 Feb 5;36(3):320–330. doi: 10.1021/jm00055a003. [DOI] [PubMed] [Google Scholar]
- Kleinert H. D., Rosenberg S. H., Baker W. R., Stein H. H., Klinghofer V., Barlow J., Spina K., Polakowski J., Kovar P., Cohen J. Discovery of a peptide-based renin inhibitor with oral bioavailability and efficacy. Science. 1992 Sep 25;257(5078):1940–1943. doi: 10.1126/science.1411510. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Emini E. A., Schleif W. A., Davis L. J., Heimbach J. C., Dixon R. A., Scolnick E. M., Sigal I. S. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4686–4690. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmic P. Kinetic assay for HIV proteinase subunit dissociation. Biochem Biophys Res Commun. 1993 Mar 31;191(3):998–1003. doi: 10.1006/bbrc.1993.1316. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam P. Y., Jadhav P. K., Eyermann C. J., Hodge C. N., Ru Y., Bacheler L. T., Meek J. L., Otto M. J., Rayner M. M., Wong Y. N. Rational design of potent, bioavailable, nonpeptide cyclic ureas as HIV protease inhibitors. Science. 1994 Jan 21;263(5145):380–384. doi: 10.1126/science.8278812. [DOI] [PubMed] [Google Scholar]
- Martin J. A. Recent advances in the design of HIV proteinase inhibitors. Antiviral Res. 1992 Apr;17(4):265–278. doi: 10.1016/0166-3542(92)90022-w. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Powers J. C., Ashe B. M., Zimmerman M. Mapping the extended substrate binding site of cathepsin G and human leukocyte elastase. Studies with peptide substrates related to the alpha 1-protease inhibitor reactive site. J Biol Chem. 1979 May 25;254(10):4027–4032. [PubMed] [Google Scholar]
- Otto M. J., Reid C. D., Garber S., Lam P. Y., Scarnati H., Bacheler L. T., Rayner M. M., Winslow D. L. In vitro anti-human immunodeficiency virus (HIV) activity of XM323, a novel HIV protease inhibitor. Antimicrob Agents Chemother. 1993 Dec;37(12):2606–2611. doi: 10.1128/aac.37.12.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl L. H., Taylor W. R. A structural model for the retroviral proteases. Nature. 1987 Sep 24;329(6137):351–354. doi: 10.1038/329351a0. [DOI] [PubMed] [Google Scholar]
- Peng C., Ho B. K., Chang T. W., Chang N. T. Role of human immunodeficiency virus type 1-specific protease in core protein maturation and viral infectivity. J Virol. 1989 Jun;63(6):2550–2556. doi: 10.1128/jvi.63.6.2550-2556.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe M., Wu J. K., Lin T. Y., Hoogsteen K., Bull H. G., Slater E. E. Renin cleavage of a human kidney renin substrate analogous to human angiotensinogen, H-Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Ser-OH, that is human renin specific and is resistant to cathepsin D. Anal Biochem. 1984 Aug 1;140(2):459–467. doi: 10.1016/0003-2697(84)90194-5. [DOI] [PubMed] [Google Scholar]
- Roberts N. A., Martin J. A., Kinchington D., Broadhurst A. V., Craig J. C., Duncan I. B., Galpin S. A., Handa B. K., Kay J., Kröhn A. Rational design of peptide-based HIV proteinase inhibitors. Science. 1990 Apr 20;248(4953):358–361. doi: 10.1126/science.2183354. [DOI] [PubMed] [Google Scholar]
- Royer W. E., Jr, Love W. E., Fenderson F. F. Cooperative dimeric and tetrameric clam haemoglobins are novel assemblages of myoglobin folds. Nature. 1985 Jul 18;316(6025):277–280. doi: 10.1038/316277a0. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tang J. Cathepsin D from porcine and bovine spleen. Methods Enzymol. 1981;80(Pt 100):565–581. doi: 10.1016/s0076-6879(81)80045-6. [DOI] [PubMed] [Google Scholar]
- Wlodawer A., Erickson J. W. Structure-based inhibitors of HIV-1 protease. Annu Rev Biochem. 1993;62:543–585. doi: 10.1146/annurev.bi.62.070193.002551. [DOI] [PubMed] [Google Scholar]
- Wondrak E. M., Louis J. M., Oroszlan S. The effect of salt on the Michaelis Menten constant of the HIV-1 protease correlates with the Hofmeister series. FEBS Lett. 1991 Mar 25;280(2):344–346. doi: 10.1016/0014-5793(91)80327-y. [DOI] [PubMed] [Google Scholar]



