Abstract
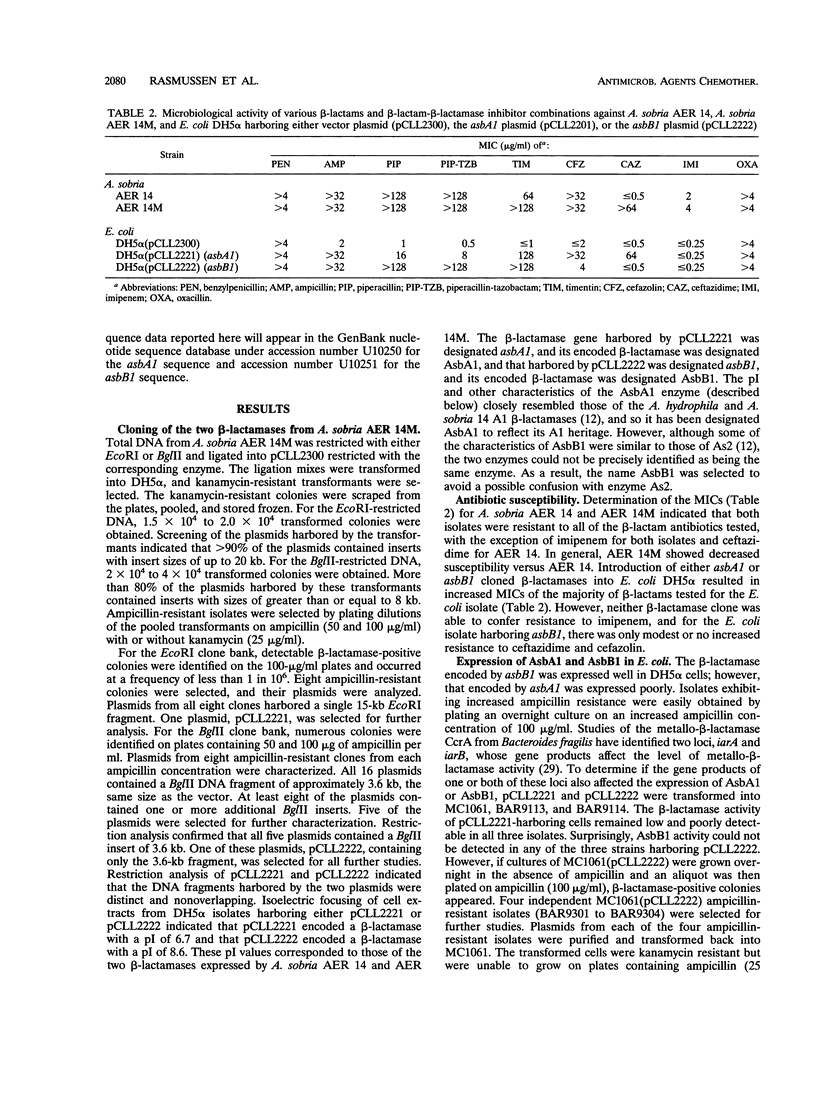
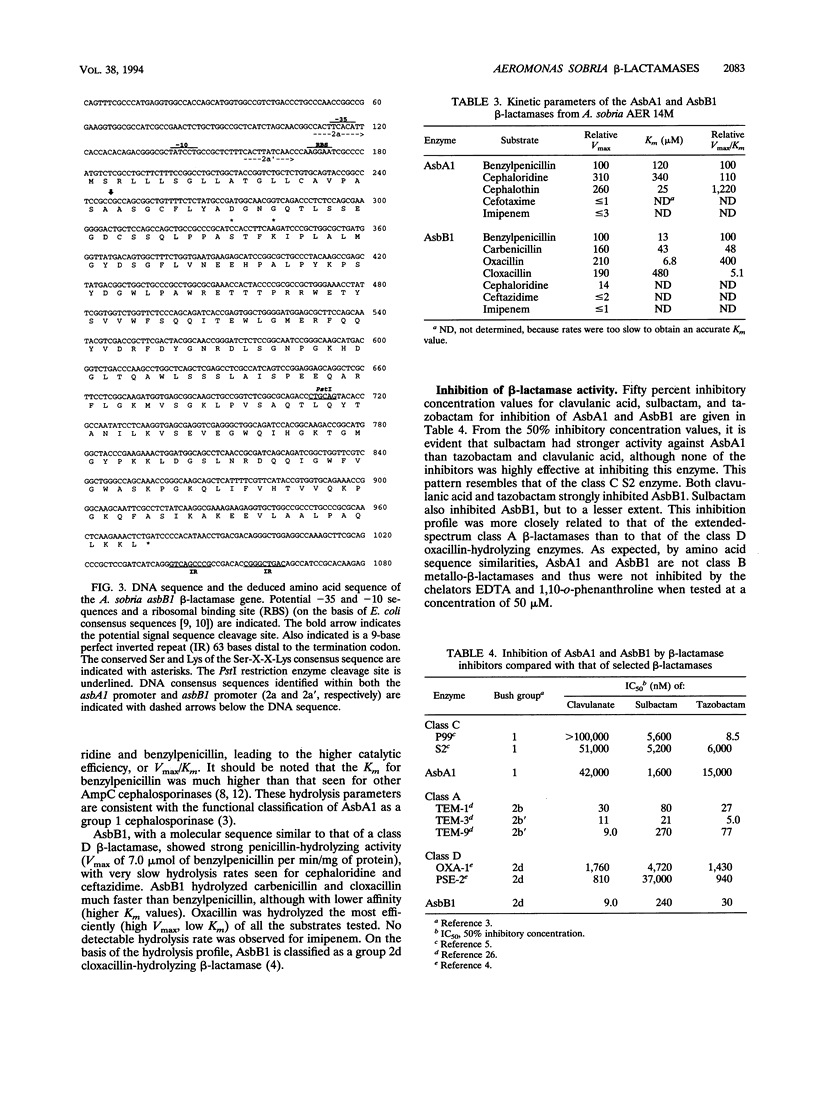
Two beta-lactamase genes, asbA1 and asbB1, encoding AsbA1 and AsbB1, respectively, have been cloned from Aeromonas sobria AER 14M into Escherichia coli. AsbA1 was expressed at low but detectable levels in all E. coli laboratory cloning strains tested. AsbB1 was expressed well in the E. coli cloning strain DH5 alpha. However, no enzyme activity could be detected from the same clone when placed in E. coli MC1061. Ampicillin-resistant mutants of E. coli MC1061 were obtained that expressed high levels of enzymatically active AsbB1. Four independent mutants were examined. All four mutations mapped to one locus, designated blpA (beta-lactamase permissive). The blpA locus was distinct from other known loci that play a role in beta-lactamase expression, i.e., the two loci that affect expression of the Bacteroides fragilis metallo-beta-lactamase and the ampC regulatory genes, ampD, ampE, and ampG. Sequence analysis of asbA1 and asbB1 revealed that AsbA1 was a class C beta-lactamase most closely related to the Pseudomonas aeruginosa chromosomal cephalosporinase and probably represents the common A. sobria cephalosporinase. AsbB1 was a class D enzyme most closely related to the oxacillin-hydrolyzing enzyme OXA-1, with 34% amino acid sequence identity. Purified AsbA1 was a typical cephalosporinase with a substrate profile that reflected high rates of hydrolysis of cephaloridine compared with benzylpenicillin. Purified AsbB1 showed strong penicillinase activity, with hydrolysis rates for carbenicillin and cloxacillin 2 to 2.5 times that for benzylpenicillin. Hydrolysis of imipenem was < or = 1% of that for benzylpenicillin. Both clavulanic acid and tazobactam strongly inhibited AsbB1, while sulbactam inhibited the AsbB1 enzyme less effectively. None of the inhibitors worked well against the AsbA1 enzyme. The chelators EDTA and 1,10-o-phenanthroline did not affect the activity of either enzyme. A. sobria AER 14M was found to produce both a group 1 cephalosporinase and a novel group 2d cloxacillin-hydrolyzing beta-lactamase that has been designated here OXA-12.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken J. S., Sanders C. C., Clark R. B., Hori M. Beta-lactam resistance in Aeromonas spp. caused by inducible beta-lactamases active against penicillins, cephalosporins, and carbapenems. Antimicrob Agents Chemother. 1988 Sep;32(9):1314–1319. doi: 10.1128/aac.32.9.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K. Characterization of beta-lactamases. Antimicrob Agents Chemother. 1989 Mar;33(3):259–263. doi: 10.1128/aac.33.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K. Classification of beta-lactamases: groups 2c, 2d, 2e, 3, and 4. Antimicrob Agents Chemother. 1989 Mar;33(3):271–276. doi: 10.1128/aac.33.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K., Macalintal C., Rasmussen B. A., Lee V. J., Yang Y. Kinetic interactions of tazobactam with beta-lactamases from all major structural classes. Antimicrob Agents Chemother. 1993 Apr;37(4):851–858. doi: 10.1128/aac.37.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Couture F., Lachapelle J., Levesque R. C. Phylogeny of LCR-1 and OXA-5 with class A and class D beta-lactamases. Mol Microbiol. 1992 Jun;6(12):1693–1705. doi: 10.1111/j.1365-2958.1992.tb00894.x. [DOI] [PubMed] [Google Scholar]
- Galleni M., Frère J. M. A survey of the kinetic parameters of class C beta-lactamases. Penicillins. Biochem J. 1988 Oct 1;255(1):119–122. doi: 10.1042/bj2550119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huovinen P., Huovinen S., Jacoby G. A. Sequence of PSE-2 beta-lactamase. Antimicrob Agents Chemother. 1988 Jan;32(1):134–136. doi: 10.1128/aac.32.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaconis J. P., Sanders C. C. Purification and characterization of inducible beta-lactamases in Aeromonas spp. Antimicrob Agents Chemother. 1990 Jan;34(1):44–51. doi: 10.1128/aac.34.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris B., Ghuysen J. M., Dive G., Renard A., Dideberg O., Charlier P., Frère J. M., Kelly J. A., Boyington J. C., Moews P. C. The active-site-serine penicillin-recognizing enzymes as members of the Streptomyces R61 DD-peptidase family. Biochem J. 1988 Mar 1;250(2):313–324. doi: 10.1042/bj2500313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F., Lindquist S., Normark S. Inactivation of the ampD gene causes semiconstitutive overproduction of the inducible Citrobacter freundii beta-lactamase. J Bacteriol. 1987 May;169(5):1923–1928. doi: 10.1128/jb.169.5.1923-1928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F., Normark S. Common mechanism of ampC beta-lactamase induction in enterobacteria: regulation of the cloned Enterobacter cloacae P99 beta-lactamase gene. J Bacteriol. 1987 Feb;169(2):758–763. doi: 10.1128/jb.169.2.758-763.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F., Westman L., Normark S. Regulatory components in Citrobacter freundii ampC beta-lactamase induction. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4620–4624. doi: 10.1073/pnas.82.14.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Lindberg F., Normark S. Binding of the Citrobacter freundii AmpR regulator to a single DNA site provides both autoregulation and activation of the inducible ampC beta-lactamase gene. J Bacteriol. 1989 Jul;171(7):3746–3753. doi: 10.1128/jb.171.7.3746-3753.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Weston-Hafer K., Schmidt H., Pul C., Korfmann G., Erickson J., Sanders C., Martin H. H., Normark S. AmpG, a signal transducer in chromosomal beta-lactamase induction. Mol Microbiol. 1993 Aug;9(4):703–715. doi: 10.1111/j.1365-2958.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Lodge J. M., Minchin S. D., Piddock L. J., Busby S. J. Cloning, sequencing and analysis of the structural gene and regulatory region of the Pseudomonas aeruginosa chromosomal ampC beta-lactamase. Biochem J. 1990 Dec 15;272(3):627–631. doi: 10.1042/bj2720627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massidda O., Rossolini G. M., Satta G. The Aeromonas hydrophila cphA gene: molecular heterogeneity among class B metallo-beta-lactamases. J Bacteriol. 1991 Aug;173(15):4611–4617. doi: 10.1128/jb.173.15.4611-4617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumovski L., Quinn J. P., Miyashiro D., Patel M., Bush K., Singer S. B., Graves D., Palzkill T., Arvin A. M. Outbreak of ceftazidime resistance due to a novel extended-spectrum beta-lactamase in isolates from cancer patients. Antimicrob Agents Chemother. 1992 Sep;36(9):1991–1996. doi: 10.1128/aac.36.9.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette M., Bissonnette L., Roy P. H. Precise insertion of antibiotic resistance determinants into Tn21-like transposons: nucleotide sequence of the OXA-1 beta-lactamase gene. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7378–7382. doi: 10.1073/pnas.84.21.7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen B. A., Gluzman Y., Tally F. P. Cloning and sequencing of the class B beta-lactamase gene (ccrA) from Bacteroides fragilis TAL3636. Antimicrob Agents Chemother. 1990 Aug;34(8):1590–1592. doi: 10.1128/aac.34.8.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen B. A., Gluzman Y., Tally F. P. Escherichia coli chromosomal mutations that permit direct cloning of the Bacteroides fragilis metallo-beta-lactamase gene, ccrA. Mol Microbiol. 1991 May;5(5):1211–1219. doi: 10.1111/j.1365-2958.1991.tb01895.x. [DOI] [PubMed] [Google Scholar]
- Singer M., Baker T. A., Schnitzler G., Deischel S. M., Goel M., Dove W., Jaacks K. J., Grossman A. D., Erickson J. W., Gross C. A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989 Mar;53(1):1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Yang Y., Rasmussen B. A., Bush K. Biochemical characterization of the metallo-beta-lactamase CcrA from Bacteroides fragilis TAL3636. Antimicrob Agents Chemother. 1992 May;36(5):1155–1157. doi: 10.1128/aac.36.5.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]


