Abstract
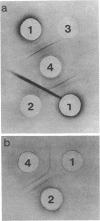
Treatment with urea-mercaptoethanol of purified spores of Bacillus thuringiensis, other Bacillus species, and Clostridium roseum solubilizes a protein fraction between 5 and 12% of the dry weight of the spores. This fraction behaves identically to the crystal protein of B. thuringiensis on acrylamide-gel electrophoresis. The protein from all of the Bacillus species shows partial homology with crystal protein, using the Ouchterlony immunodiffusion technique. A further fraction, similar in amount, can be removed from spores of B. thuringiensis by the addition of sodium lauryl sulfate to the urea-mercaptoethanol. Spores of B. thuringiensis extracted in these ways show no difference when compared to untreated spores with respect to viability or resistance to heat and ultraviolet-irradiation. The extracted spores do show differences in their germination requirements and their susceptibility to phase-darkening by lysozyme. It is concluded that an urea-mercaptoethanol-soluble protein or class of protein is a widespread component of bacterial spores, possibly located in the spore coat, and that this protein may be related to the crystal protein of B. thuringiensis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. I., Fitz-James P. C. Biosynthesis of bacterial spore coats. J Mol Biol. 1968 Apr 14;33(1):199–212. doi: 10.1016/0022-2836(68)90288-x. [DOI] [PubMed] [Google Scholar]
- BERGER J. A., MARR A. G. Sonic disruption of spores of Bacillus cereus. J Gen Microbiol. 1960 Feb;22:147–157. doi: 10.1099/00221287-22-1-147. [DOI] [PubMed] [Google Scholar]
- Delafield F. P., Somerville H. J., Rittenberg S. C. Immunological homology between crystal and spore protein of Bacillus thuringiensis. J Bacteriol. 1968 Sep;96(3):713–720. doi: 10.1128/jb.96.3.713-720.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOULD G. W., HITCHINS A. D. SENSITIZATION OF BACTERIAL SPORES TO LYSOZYME AND TO HYDROGEN PEROXIDE WITH AGENTS WHICH RUPTURE DISULPHIDE BONDS. J Gen Microbiol. 1963 Dec;33:413–423. doi: 10.1099/00221287-33-3-413. [DOI] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- SACKS L. E., PERCELL P. B., THOMAS R. S., BAILEY G. F. KINETICS OF DRY RUPTURE OF BACTERIAL SPORES IN THE PRESENCE OF SALT. J Bacteriol. 1964 Apr;87:952–960. doi: 10.1128/jb.87.4.952-960.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville H. J., Delafield F. P., Rittenberg S. C. Biochemical homology between crystal and spore protein of Bacillus thuringiensis. J Bacteriol. 1968 Sep;96(3):721–726. doi: 10.1128/jb.96.3.721-726.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]