Abstract
Apoptosis is an ancient form of regulated cell death that functions under pathological and non-pathological contexts in all metazoans. More than a decade of intense research has led to extensive characterization of the core molecular mechanisms for apoptotic cell death. This includes the identification of a family of cysteine proteases, caspases, which are critical for the execution of apoptosis. Whereas completion of the proteolytic caspase cascade leads to elimination of a cell by apoptosis, caspase activation, when finely tuned, directs alternative cellular functions independent of cell death. Exciting recent developments have focused on uncovering non-apoptotic roles of caspases in highly specialized cellular frameworks, ranging from immune regulation to spermatogenesis.
INTRODUCTION
Apoptosis is a highly regulated form of cell death that is known to sculpt tissues during development and maintain tissue homeostasis by eliminating unnecessary or harmful cells. Genetic analysis of the nematode C. elegans provided the first insights into the molecular mechanism of apoptosis (Yuan et al., 1993). The canonical apoptotic pathways are well conserved in all metazoans from invertebrates to vertebrates (Figure 1) (Degterev et al., 2003; Miura et al., 1993). Extensive research in the past 15 years established the critical role of caspases, a family of cysteine proteases, in mediating the signal transduction and execution of apoptosis. While CED-3 is the sole caspase required for programmed cell death in C. elegans, multiple caspases are required for apoptosis in more complex organisms, such as flies (4 caspases) and mammals (10 caspases in mouse; 11 caspases in human). Such expansion of the caspase family during evolution might have arisen for dual purposes: the function of caspases in the execution of apoptotic cell death and the ability of caspases to carry out multiple cellular processes that do not necessarily involve cell death.
Figure 1. The canonical apoptotic pathway is well conserved in C. elegans, Drosophila, and mammals.
Functional orthologs are color-coded. In C. elegans inhibition of the anti-apoptotic Bcl-2 family member, CED-9, by the pro-apoptotic BH3-only protein, EGL-1, releases CED-4 to promote activation of the caspase, CED-3. In Drosophila, the pro-apoptotic multi-domain Bcl-2 family members, Debcl and Buffy, promote Dark-mediated activation of Dronc followed by induction of the caspase cascade. Similarly, mammalian pro-apoptotic BH3-only proteins inhibit the anti-apoptotic Bcl-2 family members, Bcl-2 and Bcl-xL, to promote oligomerization of the multi-domain Bcl-2 family members, Bax and Bak. Adaptor proteins, such as Apaf-1, promote caspase activation, leading to apoptotic cell death. Note that in mammals, the caspase family has expanded substantially (see text). Bcl, B-cell lymphoma; CED, cell death abnormal; BH3, Bcl-2 homology 3; EGL, egg-laying defective; Dark, Drosophila Apaf-1 related killer.
Members of the caspase family are subdivided into upstream ‘initiator’ caspases (in fly, Dredd, Dronc; in mammals, caspase-1, -2, -4, -5, -8, -9, -10, -11, -12), which respond to pro-apoptotic signals, and downstream ‘executioner’ caspases (in fly, Drice, Dcp-1; in mammals, caspase-3, -6, -7, -14). Initiator caspases are molecularly defined by the presence of a long N-terminal prodomain, which mediates the formation of protein complexes that provide the molecular platform for caspase activation and inhibition. Caspases are synthesized as zymogens and their activation requires allosteric conformational changes and/or specific cleavage after a selective aspartate residue. The initial processing of the inactive caspase separates the large and small subunits, followed by removal of the N-terminal domain to form the catalytically active protease (Degterev et al., 2003). Apoptosis ensues when the active caspases cleave their respective substrates, leading to characteristic morphological features of apoptotic cell death, including membrane blebbing, pyknotic nuclei, cell rounding, and formation of apoptotic vesicles (Clarke, 1990).
In order to assemble a large multimeric platform for caspase activation, adaptor proteins recruit specific initiator caspases via homotypic interaction with the long prodomain of caspases, either a caspase-recruitment domain (CARD; in C. elegans, CED-3; in D. melanogaster, Dronc; in mammals, caspase-1, -2, -4, -5, -9, -11, -12) or death-effector domain (DED; in D. melanogaster, Dredd; in mammals, caspase-8, -10) (Degterev et al., 2003). In response to specific developmental cues, the adaptor protein, CED-4, which is composed of an N-terminal CARD and a nucleotide-binding domain (NBD, also known as nucleotide-oligomerization domain (NOD)), oligomerizes to promote the activation of CED-3 (Yang et al., 1998). Drosophila Apaf-1 (also called Dark, Hac-1, or Dapaf-1), a CED-4 like molecule, is responsible for the activation of the major fly initiator caspase, Dronc, in response to cellular stresses such as DNA damage (Kanuka et al., 1999; Rodriguez et al., 1999; Zhou et al., 1999). Drosophila FADD (dFADD, Fas associated protein with death domain) is an additional adaptor protein that promotes DREDD activation during the innate immune response to microbial infection (Hu and Yang, 2000; Kanuka et al., 1999; Rodriguez et al., 1999; Zhou et al., 1999). Whereas a single adaptor protein, CED-4, is required for the activation of CED-3 in C. elegans, multiple adaptor proteins are required for caspase activation in mammals by promoting the formation of unique protein complexes under different conditions.
In mammals, four specific caspase activating complexes have been characterized. These complexes include the apoptosome, which mediates the activation of caspase-9 via interaction with the adaptor APAF-1 (apoptotic protease-activating factor-1) in the presence of cytochrome c; the death inducing signaling complex (DISC), which mediates the activation of caspase-8 via interaction with the adaptor FADD; the inflammasomes, which mediate the activation of caspase-1 and caspase-5 via interaction with the adaptors, ASC (apoptosis-associated speck-like protein containing a CARD) or the family of NLRs (nucleotide-binding and oligomerization domain (NOD)-like receptors); and the PIDDosome, which mediates the activation of caspase-2 via interaction with the adaptors RAIDD (RIP (receptor-interacting protein)-associated ICH-1/CED-3 homologous protein with a death domain; also called CRADD) (Duan and Dixit, 1997) and PIDD (p53-induced protein with a death domain) (Festjens et al., 2006). Importantly, the functions of these complexes are highly specialized such that they are activated in response to distinct pro-apoptotic and pro-inflammatory signals. The apoptosome is formed in response to cytochrome c release resulting from the loss of mitochondrial integrity, while the DISCs assemble after the stimulation of the death receptors by their respective ligands such as Fas ligand (FasL) or tumor necrosis factor-α (TNFα). In addition, the inflammasomes are involved in the activation of innate immune response to intracellular pathogens, whereas the PIDDosome responds to DNA damage (Tinel and Tschopp, 2004) as well as other cellular stresses. The complexity of environmental stresses at both cellular and organismal levels faced by mammals may have been a strong selective force that led to the specialization of these caspase complexes.
Although the critical function of caspases in apoptosis is firmly established, an increasing number of studies have surprisingly revealed various non-apoptotic functions of capsases (Table 1). The first indication of a non-apoptotic role for caspases was the initial characterization of caspase-1 in the processing of the proinflammatory cytokine, proIL-1β, into mature IL-1β in response to bacterial infection in mammals (Cerretti et al., 1992; Thornberry and Molineaux, 1995). It has become increasingly clear that caspases and their respective adaptor proteins mediate multiple cellular processes far beyond apoptotic cell death per se. In this review, we would like to discuss the emerging evidence for non-apoptotic functions of caspases in mediating immunity, cell fate specification, cell survival, cell cycle regulation, cell proliferation, and cell migration.
Table 1.
The function of caspases in non-apoptotic processes.
| Organism | Caspase | Non-Apoptotic Function |
|---|---|---|
| C. elegans | CED-3 | Immunity |
| Drosophila | Dronc | Compensatory proliferation, Dendritic pruning, Spermatogenesis, Neuronal differentiation, Border cell migration |
| Dredd | Immunity | |
| Drice | Compensatory proliferation, Spermatogenesis, Neuronal differentiation | |
| Dcp-1 | Compensatory proliferation | |
| Mammals | Caspase-1 | Innate immunity, Protein secretion |
| Caspase-2 | Not determined | |
| Caspase-3 | Adaptive immunity, Lymphocyte proliferation, Erythroblast, Macrophage, Megakaryocyte, Lens epithelial cell, Skeletal muscle cell, Osteoblast, and Placental trophoblast differentiation, Stem cell maintenance | |
| Caspase-4 | Not determined | |
| Caspase-5 | Innate immunity | |
| Caspase-6 | Not determined | |
| Caspase-7 | Not determined | |
| Caspase-8 | Innate and adaptive immunity, Lymphocyte proliferation, Macrophage differentiation | |
| Caspase-9 | Not determined | |
| Caspase-10 | Not determined | |
| Caspase-11 | Innate immunity, Macrophage cell migration | |
| Caspase-12 | Not determined | |
| Caspase-14 | Keratinocyte differentiation |
CASPASE FUNCTION IN IMMUNITY
Caspases in invertebrate immunity: Regulation of anti-microbial peptides
The function of caspases in mediating the innate immune response is well conserved from worm and fly to mammals. Feeding C. elegans on a lawn of pathogenic bacteria, such as S. typhimurium, leads to substantial gonadal cell death and eventually death of the organism. Interestingly, ced-3 and ced-4 may play an important role in defending worms against pathogenic bacteria as ced-3 and ced-4 mutants are hypersensitive to the killing induced by S. typhimurium infection (Aballay and Ausubel, 2001). The host defense role of CED-3 and CED-4 may not be restricted to pathogenic bacteria as replication of vaccinia virus is also enhanced in ced-3 and ced-4 mutants (Liu et al., 2006). Thus, ced-3 and ced-4 may play an important role in restricting both bacterial and viral pathogens in C. elegans. However, it is still not clear whether CED-3/CED-4 function in the host response by directly promoting cell death in a cell autonomous manner or by indirectly regulating the immune response in a cell non-autonomous manner.
In Drosophila, two signaling networks define the innate immune system: the Toll pathway, which responds to Gram-positive bacteria and pathogenic fungi, and the immune deficiency pathway (IMD), which primarily defends against infection by Gram-negative bacteria (Ferrandon et al., 2007). Drosophila can discriminate between Gram-positive and Gram-negative bacteria by their unique composition of peptidoglycan, which forms a polymeric layer of glycan molecules in the outer coat for Gram-positive bacteria or the inner coat for Gram-negative bacteria. Two members of the family of peptidoglycan-recognition proteins (PGRPs), PGRP-LC and –LE, specifically detect Gram-negative bacteria and activate the IMD signaling pathway. Engagement of the transmembrane receptor, PGRP-LC (Gottar et al., 2002) and the intracellular receptor, PGRP-LE, recruits IMD, which is similar to the mammalian RIPs, most likely by an unknown intermediate (Kaneko et al., 2006). IMD then initiates the formation of molecular complexes that facilitate the transcriptional activation of a major immune regulator, the NF-κB factor-like molecule, Relish (Georgel et al., 2001).
DREDD, the Drosophila caspase-8 ortholog, functions in the IMD pathway downstream of the innate immune receptors by regulating the expression of anti-microbial peptides, an important host defense mechanism (Leulier et al., 2000). dredd mutant flies are highly sensitive to E. coli infection and fail to produce anti-microbial peptides, a phenotype similar to flies deficient in dFADD, the fly ortholog of the caspase-8 adaptor protein, FADD (Leulier et al., 2002). These data suggest the following model whereby IMD recruitment to PGRP-LC provides a scaffold for FADD and DREDD complex formation, inducing the activation of Relish and the expression of anti-microbial peptides (Stoven et al., 2003). Thus, DREDD performs an important host defense response by promoting the expression of anti-microbial peptides upon bacterial challenge. Interestingly, this function of DREDD may be highly similar to that of its mammalian ortholog, caspase-8, as described below.
Caspases in mammalian immunity
Mammalian caspases and innate immunity: more than IL-1β
Compared to their counterparts in Drosophila and C. elegans, mammalian caspases play a more dominant and intricate role in regulating the host inflammatory response. In mammals, two major types of caspase complexes function in regulating host immunity: DISC, which is the intracellular signaling complex formed upon stimulation of the death receptors, and the inflammasomes, which are the protein complexes responsible for the activation of caspase-1.
Once an appropriate pro-inflammatory cytokine, such as TNFα, engages a death receptor in the TNF receptor (TNF-R) superfamily, DISC formation is initiated. This leads to aggregation and internalization of the TNF-R complex, including TRADD, FADD, and caspase-8 or -10. Although this protein complex is primarily described in mediating caspase-dependent cell death, non-apoptotic functions of caspase-8 have been recently elucidated recently, including NF-κB activation in immunity, monocyte cell differentiation, and T and B cell proliferation. In this section we will focus on the role of caspase-8 in regulating the pro-inflammatory response following the activation of NF-κB, which is strikingly reminiscent of the IMD pathway in Drosophila innate immunity.
One indication that caspase-8 regulates the immune response is the observation that caspase-8 deficient mice are unable to clear a viral infection compared to control mice (Salmena et al., 2003). Furthermore, Listeria monocytogenes was found to colonize the liver of liver-specific caspase-8 deficient mice more efficiently than that of wild-type mice, suggesting that caspase-8 limits the growth of this intracellular pathogen in vivo (Ben Moshe et al., 2007). This may be due to ablated NF-κB activation as caspase-8 has been implicated in mediating the transcriptional response to antigen stimulation of antigen receptors, Fc receptors, or Toll-like receptors, in mammalian lymphocytes (Hu and Yang, 2000; Lemmers et al., 2007). Caspase-8 was also found to be associated with the IκB kinase (IKK) complex inducing the transcriptional activation of NF-κB. Thus, mammalian caspase-8 has a role in regulating innate immunity similar to that of DREDD in the fly IMD pathway. In addition to promoting NF-κB activation, caspase-8 might directly regulate pro-inflammatory cytokines. A role of caspase-8 in regulating proinflammatory cytokines could be considered as stimulation of Fas ligand was found to induce the processing and secretion of IL1-β in peritoneal exudate cells in a caspase-1 independent manner (Miwa et al., 1998). However, the mechanism by which caspase-8 mediates cytokine secretion has not been explored.
The signaling events regulated by the family of pro-inflammatory caspases define an additional branch of caspase-mediated immune regulation, which includes possibly five CARD-containing members (human caspase-1, -4, and -5; mouse caspase-1, -11, -12). A large body of work focuses on understanding the molecular regulation of caspase-1 during inflammation. The first insight into the regulation of caspase-1 activation was the observation that caspase-11 deficient mice show a remarkably similar phenotype as caspase-1 deficient mice (Wang et al., 1998). These mice are highly resistant to LPS-induced septic shock and defective in IL-1 production, indicating that caspase-11 is required for caspase-1 activation. Interestingly endogenous caspase-11 and caspase-1 form heterodimers, suggesting that a caspase complex may be necessary for proper IL-1 processing. Indeed a subsequent study describes a pro-inflammatory caspase complex containing human caspase-1 and caspase-5, which is closely related to caspase-11, and additional adaptor proteins (Martinon et al., 2002). Consistent with the previous observations in mice, the presence of both human caspases leads to efficient production of IL-1β. The authors of the study coined the term, ‘inflammasome,’ to describe this caspase-1 containing complex, which will be discussed below.
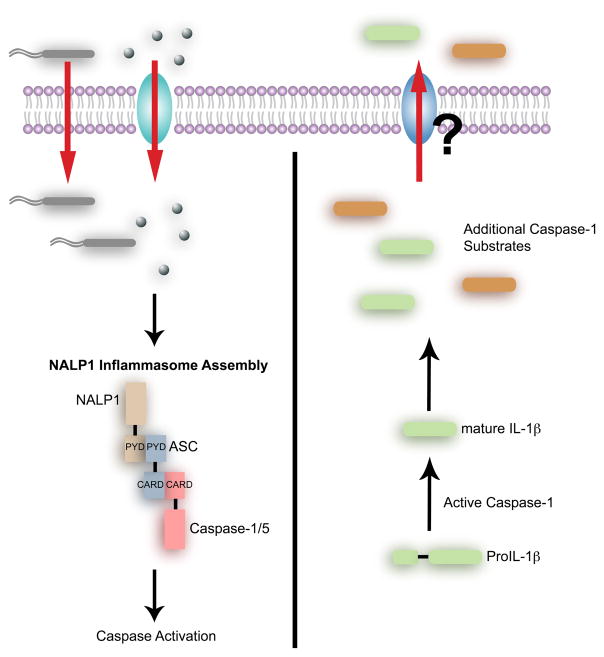
Currently, four caspase-1 activating complexes have been characterized in promoting a pro-inflammatory response in response to infection. Despite their highly specialized capacity to recognize specific classes of invasive microbes, these complexes share a similar molecular composition of an NLR family protein, ASC, and caspase-1. Once an invasive pathogen is sensed in the cytosol, oligomerization of these protein complexes is induced, leading to caspase-1 mediated processing of proIL-1β into mature IL-1β. These complexes include the NALP1 (NACHT- (nucleotide-binding and oligomerization domain), LRR- (leucine rich repeat domain), PYD (pyrin domain)) inflammasome, the (Cyropyrin (NALP3) inflammasome, the IPAF (Ice protease-activating factor) inflammasome, and the Francisella-sensing inflammasome.
The characterization of the NALP1 inflammasome in a cell-free system was defined the first caspase-1 activating complex (Martinon et al., 2002). In response to LPS treatment, caspase-1 and caspase-5 are recruited to NALP1 through a CARD-CARD interaction with the adaptor protein, ASC (Figure 2). Importantly, the formation of this multimeric complex provides the platform for caspase activation and IL-1β maturation. The physiological significance of this caspase-1 complex was indicated by the identification of nalp1b as a Bacillus anthracis lethal toxin (LT) susceptibility gene in mice (Boyden and Dietrich, 2006). Loss of NALP1b or caspase-1 provided protection against LT-induced cell death in LT-sensitive macrophages, suggesting that LT induces caspase-1 dependent cell death. However, the mechanism by which NALP1b promotes caspase-1 activation needs further clarification. Unlike human NALP1, mouse NALP1b lacks the PYD domain, which is required for recruiting ASC, suggesting that mechanism of NALP1-caspase-1 complex formation is species-dependent. Nevertheless, it is clear that these bacterial products directly or indirectly induce the formation of the NALP1 inflammasome. One study provides strong evidence that muramyl dipeptide (MDP), a component of peptidoglycan, induces assembly of the NALP1 inflammasome in vitro, indicating that NALP1 directly senses MDP (Faustin et al., 2007). It seems unlikely that NALP1 is also a direct microbial sensor for LPS and LT, which are structurally distinct. Two possibilities can explain LPS and LT-dependent caspase-1 activation by NALP1— these bacterial products may be recognized by unknown host factors that in turn, activate the inflammasome, or alternatively, MDP may be present in the commercial preparations of LPS or LT. Indeed, contamination of MDP in LPS preparations has been previously reported (Faustin et al., 2007; Girardin et al., 2003; Inohara et al., 2003). It will be important to reconcile these two possibilities.
Figure 2. Inflammatory caspases mediate an innate immune response to microbial invasion.
Cellular recognition of foreign invaders leads to formation of inflammatory caspase-containing protein complexes, called the inflammasomes. The best characterized is the NALP1 inflammasome, which consists of a NLR family member, an adaptor protein called ASC, and caspase-1/5. Inflammasome assembly leads to activation of caspase-1 and maturation of IL-1β. A recent study has implicated caspase-1 in regulating non-conventional protein secretion, which may be intimately involved in the inflammatory response (Keller et al., 2008). However the exact mechanism for caspase-1 in mediating protein secretion remains to be determined.
Interestingly, the NALP3 inflammasome promotes caspase-1 activation in response to a plethora of microbial products and host products secreted by dying cells. Such bacterial products include LPS (Mariathasan et al., 2006; Sutterwala et al., 2006), bacterial RNA (Kanneganti et al., 2006b), viral RNA (Kanneganti et al., 2006a), bacterial lipopeptide (Mariathasan et al., 2006), as well as exposure to the Gram-positive bacteria, Staphylococcus aureus and Listeria monocytogenes (Mariathasan et al., 2006). The NALP3 inflammasome may be specific for Gram-positive bacteria infection as NALP3 deficient macrophages infected with Gram-negative bacteria normally secrete IL-1. Furthermore, NALP3-dependent IL-1 production is implicated in the inflammatory response to the cellular deposition of uric acid crystals in the autoinflammatory disease, gout (Martinon et al., 2006). A recent study proposes that the NALP3 inflammasome mediates the immunostimulatory properties of aluminium adjuvants, which can form insoluble particles, in a similar manner as described for uric acid crystals (Eisenbarth et al., 2008). Because NALP3 responds to a multitude of foreign and cellular signals, it is most likely that additional host factors, which function as direct microbial sensors, promote complex formation. This seems to be a likely possibility as there is currently no evidence for the aforementioned NALP3 stimulating agents in direct assembly of the NALP3-caspase-1 complex (Kanneganti et al., 2007).
Unlike the NALP3 inflammasome, the IPAF inflammasome promotes caspase-1 activation in response to both Gram-negative and Gram-positive bacteria, including Salmonella typhimurium (Mariathasan et al., 2004), Legionella pneumophila (Amer et al., 2006), Pseudomonas aeruginosa (Franchi et al., 2007; Miao et al., 2008; Sutterwala et al., 2007), and Listeria monocytogenes (Warren et al., 2008). Subsequent studies have identified flagellin, the proteinaceous component of flagella, as the bacterial factor that promotes NALP3 induced caspase-1 activation (Amer et al., 2006; Franchi et al., 2007; Miao et al., 2008; Ren et al., 2006). In particular, bacterial pathogens deficient in flagellin are unable to induce IL-1β processing in macrophages. Concordantly, direct delivery of purified flagellin into the host cytosol induces caspase-1 activation in an IPAF-dependent manner, demonstrating the sufficiency of flagellin to elicit an innate immune response. These findings indicate that the IPAF inflammasome may function as a microbial sensor or responds to a host factor that specifically recognizes bacterial flagellin.
The inflammasome that responds to the intracellular Gram-negative pathogen, Francisella tularensis, is the least understood caspase-1 activating complex. Although ASC is required for caspase-1 activation induced by Francisella infection, the NLR family member that senses the pathogen is currently unknown as NALP3 and IPAF seem to play negligible roles (Mariathasan et al., 2005; Mariathasan et al., 2006). Cytosolic Francisella may be necessary for host recognition because vacuolar escape mutants cannot stimulate caspase-1 activation (Gavrilin et al., 2006). This suggests that the microbial product that triggers an immune response may not easily bypass the vacuolar membrane or may not be actively secreted by the pathogen. Moreover, caspase-1 activation is abrogated in macrophages deficient in the type I interferon-receptor, which is known to respond to cytosolic pathogens, suggesting that a component of the type I IFN signaling network may promote inflammasome assembly in response to Francisella infection (Henry and Monack, 2007).
One conundrum in the field is the observation that caspase-1 influences the secretion of IL-1α and IL-1β, but only proIL-1β is a direct caspase-1 substrate (Howard et al., 1991; Li et al., 1995). Although the cleavage sites for proIL-1α and proIL-1β are unique (cleavage of proIL-1β is Asp dependent), production of both cytokines is curiously reduced in caspase-1 deficient mice upon stimulation. Furthermore, the secretion of proIL-1α and proIL-1β does not require the classical secretory pathway, which pertains to the export of proteins tagged with a signal peptide in an ER/Golgi-dependent manner. This suggests that the maturation of IL-1 depends on a completely independent mechanism. More than a decade later, one group has set out to unravel this mystery. Using a proteomic approach, these authors identified almost 1,000 extracellular proteins, whose secretion depends on caspase-1 activity rather than the classic protein export system (Figure 2) (Keller et al., 2008). Such leaderless proteins include fibroblast growth factor-2 (FGF-2), caspase-1, proIL-1α, and proIL-1β. Although FGF-2 and proIL-1α are not direct substrates of caspase-1, both proteins can interact with the protease, suggesting that caspase-1 can directly and indirectly promote protein secretion. It remains to be seen, however, how caspase-1 regulates the secretion of such substrates. The ability of caspase-1 to regulate the secretion of this staggering number of proteins underscores that the importance of the non-apoptotic functions of this caspase are yet to be fully understood. It also raises the question whether other caspases might also regulate protein secretion.
Mammalian caspases in adaptive immunity: the role of secretion?
Specific to vertebrates, adaptive immunity is characterized by the exquisite specificity for foreign molecules and the ability to remember certain microbes in anticipation for future infections, such that a highly potent defense could be mounted. Negative regulation of adaptive immunity by eliminating activated or auto-reactive immune cells is a well-established apoptotic role for caspases. Surprisingly, a number of caspases have also been found to play positive roles in the adaptive immune response by promoting proliferation and activation of lymphocytes.
In addition to defects in lymphocyte apoptosis, individuals with an inherited genetic deficiency in caspase-8 exhibit reduced activation of T cells, B cells, and natural killer cells after stimulation (Chun et al., 2002). These defects lead to immunodeficiency characterized by increased susceptibility to viral infections in affected individuals. Consistent with a positive role of caspase-8 in mediating T cell activation, T cell-specific caspase-8 deficient mice show a decrease in the number of peripheral T cells and impaired T cell response to stimulation (Salmena et al., 2003). The effect of caspase-8 in mediating T cell proliferation may be coordinated with FADD, as FADD−/− T cells also exhibit a similar proliferation defect (Zhang et al., 1998). On the other hand, caspase-8 may not be required for activation-induced B cell proliferation as mice with a deletion of casapse-8 specifically in B cells manifest normal lymphocyte development (Lemmers et al., 2007). However, attenuated antibody production is observed after viral infection, suggesting that caspase-8 is still important for a proper humoral response. Surprisingly, B cell development is impaired in FADD deficient mice in striking contrast to that of caspase-8 deficient B cells, suggesting that FADD might have a caspase-8-independent effect on B cell proliferation (Zhang et al., 1998). Thus, caspase-8 may regulate activation-induced lymphocyte proliferation in both FADD-dependent and FADD-independent manner.
Recently two studies have proposed a new hypothesis for the requirement of caspase-8 in the activation and clonal expansion of T cells (Bell et al., 2008; Ch’en et al., 2008). Surprisingly, the authors found that although caspase-8 or FADD deficient T cells are able to undergo apparently normal proliferation in response to antigen stimulation, these cells subsequently die through a RIP1 kinase-dependent but caspase-independent necrotic cell death pathway. This type of necrotic cell death, which has been termed necroptosis (Degterev et al., 2005), can be effectively inhibited by a RIP1 kinase inhibitor, necrostatin-1 (Nec-1) (Bell et al., 2008; Ch’en et al., 2008). Interestingly, caspase-8 deficiency in T cells may lead to increased RIP1 kinase activity (Ch’en et al., 2008). Thus, caspase-8 may be required to promote the survival of activated T cells by suppressing RIP1 kinase activity. It remains to be seen, however, if defects in the activation and expansion of T cells, B cells, and natural killer cells in individuals with an inherited genetic deficiency in caspase-8 (Chun et al., 2002) is due to activation of necroptosis. Since inhibition of caspases by a pan caspase inhibitor zVAD.fmk leads to activation of necroptosis by autocrine secretion of TNFα (Hitomi et al. 2008), we speculate that caspase-8 deficiency may lead to activation of necroptosis in immune cells by increased secretion of TNFα (Figure 3).
Figure 3. The role of caspase-8 in maintaining the survival of activated T cells.
Caspase-8 deficient T cells undergo necroptosis in response to antigen stimulation, which can be inhibited by necrostatin-1 (Nec-1). Autocrine secretion of TNFα is hypothesized to mediate necroptosis in activated caspase-8 deficient T cells.
In addition to caspase-8, caspase-3 may also influence the adaptive immune response by regulating B cell proliferation. Loss of caspase-3 in mice results in lymphadenopathy and splenomegaly due to hyperproliferation of both B and T lymphocytes (Woo et al., 2003). The increased proliferation of peripheral T cells in caspase-3 deficient mice has been found to be due to defects in apoptosis (Woo et al., 1998). However, hyperproliferation of peripheral B cells in caspase-3 deficient mice may result from altered cell cycle regulation rather than apoptosis resistance (Woo et al., 2003). Interestingly, caspase-3 deficient B cells display an increase in the levels of PCNA, a processivity factor for DNA polymerase δ and ε, and elevated cyclin dependent kinase activity, which is associated with increased cell cycle progression. Surprisingly, p21 protein levels are also increased in caspase-3 deficient B cells, which may be due to the lack of cleavage as p21 is a caspase-3 substrate (Levkau et al., 1998). Although it may seem counterintuitive that an increase in p21—a cyclin dependent kinase inhibitor—is observed in hyperproliferating cells, a deficiency of p21 inhibits the proliferation of caspase-3 deficient B cells in vivo, suggesting that p21 may have an alternative role in promoting cell cycle progression (Woo et al., 2003). Taken together, the findings described above elucidated the complex roles of caspases in influencing multiple aspects of the mammalian immune response.
We still have much to learn as to how caspases function in regulating cell proliferation. We speculate that the roles of caspase-3 and caspase-8 could mediate an extracellular mechanism, such as secretion of paracrine or autocrine factors, and contribute to the intracellular signaling events described above, leading to increased cell proliferation. Since caspase-1 has a role in regulating FGF-2 secretion (Keller et al., 2008), it is not too far fetched that caspase-3 and caspase-8 might also influence the secretion of certain growth factors that can promote cell proliferation in a cell non-autonomous manner. In fact, there are suggestions from studies on compensatory proliferation during Drosophila development that caspases may regulate the expression of non-cell autonomous mitogenic pathways.
CASPASES IN OTHER PHYSIOLOGICAL PROCESSES
In the remaining sections, we will discuss the growing body of work that reveals the physiological functions of caspases during fly and mammalian development, namely tissue regeneration after injury, cell differentiation, and cell migration and motility.
Caspases regulate compensatory proliferation: regulation of mitogenic pathways
Maintaining tissue homeostasis after injury is a critical survival response for metazoans. We are most likely familiar with the ability of our skin epithelium to regenerate after tissue damage caused by ultraviolet radiation from the sun and other injuries (Fuchs, 2007). Cell growth and proliferation has been the primary focus in the study of tissue regeneration; however, it is the integration of cell death (or cell loss) and cell proliferation that shapes the course of tissue maintenance. Fascinatingly, dying cells in Drosophila stimulate proliferation of neighboring cells in a cell non-autonomous manner, termed compensatory proliferation. In particular the larval imaginal disc, which develops into the adult wing, has a remarkable capacity to recover from extensive tissue damage: even destroying 40%–60% of cell by lethal irradiation is not enough to prevent normal wing size development (Haynie and Bryant, 1977; James and Bryant, 1981; Milan et al., 1997). Surprisingly, this staggering cellular feat may be under the control of caspases.
The utilization of an effector caspase inhibitor from baculovirus, p35, revealed that the activation of upstream caspase Dronc, but not downstream caspases Dcp-1 or Drice, is a prerequisite for compensatory proliferation in the developing wing (Huh et al., 2004a; Kondo et al., 2006; Ryoo et al., 2004; Wells et al., 2006). Because phagocytes rapidly remove dying cells in vivo, expression of p35 allows the execution of certain upstream apoptotic events without cell death. Interestingly, the activation of upstream apoptotic signaling leads to the upregulation of Wingless (Wg; fly ortholog of Wnt) and Decapentaplegic (Dpp; fly ortholog of TGF-β (Transforming Growth Factor-β)) (Huh et al., 2004a; Perez-Garijo et al., 2004; Perez-Garijo et al., 2005; Ryoo et al., 2004), secreted mitogens that are known to play a role during development and tissue regeneration (Stoick-Cooper et al., 2007). Activation of the Wg pathway by dying cells in turn triggers the proliferation of surrounding tissue in a non-cell autonomous manner. Thus, this might provide another example of caspases in mediating the secretion of extracellular factors. However the mechanism by which Dronc mediates the secretion of Wg and Dpp remains to be understood.
In developing Drosophila larval imaginal eye disc, caspases also play an important role in mediating apoptosis-induced compensatory proliferation. Apoptosis-induced compensatory proliferation in proliferating and differentiating tissues within the developing fly eye requires independent pathways mediated by upstream or downstream caspases. In the anterior proliferating eye disc, Dronc plays a dominant role in regulating compensatory proliferation; whereas in the posterior differentiating eye disc, Dcp-1 and Drice activity are required to promote proliferation of nearby undifferentiated cells (Fan and Bergmann, 2008). As described for the wing disc, Dronc-dependent induction of compensatory proliferation in the anterior eye disc corresponds to an increased expression of Wg and Dpp in the apoptotic cells. In the differentiating posterior eye imaginal disc, however, Hedgehog is induced in the apoptotic photoreceptor neurons, stimulating a mitogenic pathway distinct from that of Wg and Dpp. Thus, the mechanism by which compensatory proliferation is induced (Wg/Wnt or Hh pathways) may depend on the differentiated state of the tissue.
Taken together, these findings indicate that caspase activation drives apoptosis-induced compensatory proliferation; however, many questions remain to be answered. What is the regulatory mechanism that controls the induction of growth promoting signals by dying cells? More importantly, how does caspase activity regulate different mitogenic pathways to mediate compensatory proliferation? Caspases may process proteins that influence transcriptional activation of Dmp53 and in turn promote compensatory proliferation. A more parsimonious explanation is that caspases may directly or indirectly influence the secretion of mitogenic factors. Such mitogenic factors may require cleavage by caspases for their bioactivity.
The ability of apoptotic cells to regulate the expression of secreted mitogenic factors as a mechanism mediating compensatory proliferation is very interesting. If similar phenomenon can be observed in mammalian cells, one may raise the question as to if inducing apoptosis of cancer cells might lead to proliferation of remaining cancer cells. Given that caspase-1 regulates the unconventional protein secretion of a large number of extracellular proteins involved in tissue repair and inflammation (Keller et al., 2008), it may not be surprising that multiple caspases have similar capacity in regulating protein secretions under different conditions.
Apoptosis-induced compensatory proliferation in developing tissues has an uncanny resemblance to tissue regeneration of adult tissues, a process that utilizes adult stem cells to replace lost tissue. The developing fly eye tissue provides an interesting system to study regeneration of differentiated tissue as apoptotic neurons are implicated in inducing proliferation of the neighboring undifferentiated cells (Fan and Bergmann, 2008). It would be exciting if an analogous mechanism also regulates regeneration of adult tissue. An emerging system to study adult tissue maintenance is the freshwater planarian Schmidtea mediterranea, which demonstrate a remarkable regenerative capacity. However it is unclear whether cell death pathways control the regenerative capacity of planarians as elucidation of these pathways is currently underway (Pellettieri and Sanchez Alvarado, 2007). An enticing area of research could be the crosstalk between cell death and cell proliferation in the potential regulation of adult tissue regeneration in planarians. Identification of such signaling effectors could help to rebuild damaged tissue for biomedical applications.
Caspases in “subcellular” apoptosis
Whereas full caspase activation mediates total cellular destruction during apoptotic cell death, caspase activation in a temporally and spatially restricted manner mediates localized cellular remodeling. Such caspase-mediated remodeling of organelles and cellular structures may be considered as a highly specialized form of “subcellular” apoptosis in that caspases carry out strictly defined functions independent of cell death. In this regard, it is possible that the ability of caspases to regulate the protein secretion may also be an example of subcellular compartmentalization of caspase activation.
Caspases in the enucleation process
Terminal differentiation of certain cell types, such as lens epithelial cells, erythroblasts, megakaryocytes, and keratinocytes, requires elimination of nuclei. Interestingly, caspases have been found to play a role in this “nuclear” apoptosis process. For example, the enucleation process of lens epithelial cells is associated with extensive nuclear DNA fragmentation, a typical marker for caspase activation (Bassnett and Mataic, 1997; Ishizaki et al., 1998). Inhibition of caspase activity by the pan caspase inhibitor, z-VAD.fmk, prevents the encucleating process, suggesting that caspases are involved in the specific elimination of nuclei as opposed to elimination of the whole cell (Ishizaki et al., 1998). From these studies, Raff and colleagues proposed that caspases might be necessary in general for the removal of nuclei or other organelles/structures in differentiating cells.
The terminal maturation of erythroblasts into anucleate red blood cells also requires caspase activity. Although erythroblasts are sensitive to apoptotic cell death upon stimulation of death receptors (De Maria et al., 1999), transient activation of effector caspases, such as caspase-3, is detected during normal erythroid maturation (Carlile et al., 2004; Zermati et al., 2001). Moreover, the loss of caspase-3 blocks the enucleation process in a human erythroid culture system (Carlile et al., 2004). Caspase activation during enucleation is associated with the cleavage of lamin B and acinus, which are necessary for the maintenance of nuclear integrity and condensed chromatin, respectively (Zermati et al., 2001). It is still not clear how differentiating cells restrain activated caspases to selectively remove the nucleus without compromising cellular integrity. In this regard, one recent study suggests that the molecular chaperone, Hsp70, may protect GATA-1, a master regulator of erythroid maturation, from caspase-3 mediated cleavage during the enucleation process (Ribeil et al., 2007). It is unclear, however, whether the selective masking of caspase substrates by Hsp70 provides a universal control mechanism for substrate specificity. Localized caspase activation may offer an alternative mechanism for mediating cleavage of distinct substrates during enucleation. Because a staggering number caspase substrates have been systematically identified (Mahrus et al., 2008; Van Damme et al., 2005), the question regarding the mode for selective caspase activity in non-apoptotic processes in general is still ripe for discovery. It remains to be seen whether such activation involves one of the caspase adaptor complexes discussed above.
Enucleation is important during the differentiation of megakaryocytes into mature platelets. Unlike that of lens epithelial cells and erythroblasts, this process is considered more akin to cytokinesis, where cytoplasmic fragmentation of megakaryocytes leads to the formation of anucleate cells rather than removal of the nucleus per se (Radley and Scurfield, 1980). Interestingly this fragmentation process may be under the control of caspase-3 and caspase-9 (De Botton et al., 2002). Activated caspase-3 was detected in maturing megakaryocytes with punctate cytoplasmic distribution and outer mitochondrial membrane permeabilization as assessed by the release of cytochrome c but no detectable DNA fragmentation. Thus, caspase activation in the enucleation process may involve the destruction of nucleus, such as during the differentiation of lens epithelial cells, or without nuclear destruction, such as during the differentiation of megakaryocytes into platelets. In either case, the mechanism by which ”nuclear apoptosis” is orchestrated without destruction of the cytoplasmic compartment remains to be determined.
Caspase activation is also involved in the terminal differentiation of keratinocytes into anucleate cells that form the outer layer of the skin. The implicated caspase, caspase-14, was first identified for its unusual expression pattern in embryonic tissue and adult skin (Van de Craen et al., 1998). Further characterization revealed that caspase-14 expression is highly specific for keratinocytes during the enucleation process (Lippens et al., 2000). A recent study sheds some light into the mechanism by which caspase-14 controls skin development. Caspase-14 deficient mice present a unique phenotype of shiny and lichenified skin (Denecker et al., 2007). A key event in keratinocyte differentiation is the cleavage and subsequent aggregation of filaggrin, an abundant protein in the epidermis, leading to the collapse of the anucleate cells and formation of the outer skin layer (Smith et al., 2006). Although it was previously thought that caspase-14 lacked protease activity (Van de Craen et al., 1998), caspase-14 was found to directly cleave filaggrin in vitro (Denecker et al., 2007). These findings indicate that caspase-14 plays a surprising role in maintaining the integrity of skin, though it is unclear whether caspase-14 directly regulates the enucleation process of keratinocytes.
Caspases in dendritic pruning
An emerging area of interest is the possible role of caspases in influencing neural connectivity. Selective removal of neuronal dendrites, or dendritic pruning, is essential for normal brain function. This multistep process involves cytoskeletal destabilization, leading to severing of dendrites from the soma and subsequent engulfment by glia; thus, pruning of dendritic branches might also be considered as ‘dendritic apoptosis’. Interestingly, two recent studies indicate that localized caspase activity is important for the selective removal of sensory neuron dendrites during fly metamorphosis (Figure 4) (Kuo et al., 2006; Williams et al., 2006). Dronc activity is restricted to the dendrites that are eventually removed by glia, suggesting that Dronc is involved in initiating dendritic pruning during fly larval development. However, it is unclear whether Dronc activation is required for severing of dendrites prior to engulfment (Kuo et al., 2006) or required for targeting dendrites to glia after dendrite severing (Williams et al., 2006). Further characterization regarding the regulation of caspase activation revealed that the ubiquitin-proteasome system (UPS) promotes the degradation of DIAP1 (Drosophila Inhibitor of Apoptosis Protein 1), a potent inhibitor of caspases, and releases caspases from inhibition (Kuo et al., 2006). Thus, localized degradation of DIAP1 might spatially control caspase activation, though it remains to be determined how ubiquitin-dependent degradation of DIAP1 is initiated and restricted to dendrites.
Figure 4. Local caspase activation influences dendritic pruning during Drosophila metamorphosis.
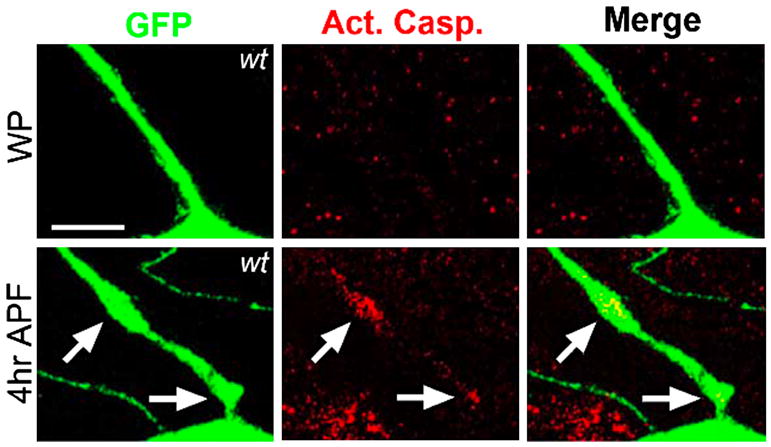
Extensive severing of neuronal dendrites occurs in the class IV dendritic arborization (C4da) neurons within the fly peripheral nervous system during metamorphosis (Kuo et al., 2006). Local activation of caspases is restricted to dendrites targeted for degradation at 4hr after pupae formation (APF) as indicated by antibody staining with anti-activated caspase-3. Activated caspase-3 is not observed at early metamorphosis or white pupae stage (WP). Images courtesy of Yuh-Nung Jan.
The ability of caspases in regulating dendritic pruning may have direct consequence on brain function. For example, caspase-3 has been proposed to play a role in the habituation of birdsong in zebra finch, which offers an in vivo setting to understand learning and memory (Huesmann and Clayton, 2006). Reminiscent to that of fly, caspase-3 activity is confined to dendritic spines within the auditory forebrain of zebra finch and may be necessary for proper memory consolidation of a song stimulus. Thus, caspase activity may influence postsynaptic remodeling in response to certain environmental stimuli. Elucidating a possible mechanism for caspase-mediated dendritic pruning during learning and memory could be an interesting avenue of study.
Caspases and spermatogenesis
Localized caspase activity has also been found to play an important role during sperm development. Dramatic removal of bulk cytoplasm occurs in the terminal differentiation of spermatid into individual sperm, which could be considered the ‘apoptotic cytoplasm’. Surprisingly multiple fly caspases have been implicated in this highly specialized cellular event, known as spermatid individualization. Drice activity is observed at the onset of spermatid individualization, though this caspase may be more important for driving cytoplasmic elimination (Arama et al., 2003). drice mutants, however, show partial defects in sperm maturation (Muro et al., 2006) similar to that of dFadd and dredd mutants (Huh et al., 2004b). On the other hand, RNAi against dark or overexpression of dominant-negative Dronc results in more severe phenotypes, where the cytoplasm is mostly unaffected and individualization is blocked (Arama et al., 2003; Huh et al., 2004a; Huh et al., 2004b). Thus, cytoplasmic removal during spermatogenesis may be dependent on Dronc and Dark activity, though other caspases may contribute to the nearly complete removal of cytoplasmic material. Importantly, a recent study points to the UPS in the regulation of caspase activation by mediating degradation of the IAP-like protein, dBruce, leading to the subsequent release of caspases from inhibition (Arama et al., 2007). Coincidently, this mechanism is similar to the one proposed for the regulation of caspase activation during dendritic pruning (see above). It will be interesting to determine how dBruce destabilization leads to local caspase activity.
Caspase function in cell differentiation
Cell differentiation also involves dramatic cellular re-organization without subcellular remodeling, such as cell fusion events. Emerging evidence suggests that this type of cell differentiation is also regulated by caspases.
Caspases and neuronal differentiation
A well-known observation in the apoptosis field is that differentiating cells are more refractory to apoptosis compared to less differentiated cells. As differentiating neurons are largely apoptosis resistant, the finding that caspase activity, when finely tuned, can influence the differentiation of neuronal precursor cells into neurons is quite intriguing. Curiously, one additional sensory organ precursor (SOP) cell, equivalent to the mammalian sensory neuron, is found in dark fly mutants (Kanuka et al., 2005). In addition, overexpression of dominant-negative Dronc or silencing of Dronc by RNAi leads to a similar phenotype. The survival of an extra neuron in animals deficient in caspase activity was found to be more complex than a simple failure in apoptosis. Miura and colleagues discovered that cleavage-dependent activation of a glycogen synthase kinase-3β-like protein, Sgg46, by Dronc or Drice negatively controls SOP cell development (Kanuka et al., 2005). Activation of Sgg46 leads to down-regulation of the Wingless pathway, which is essential for the generation of a sensory bristle from an SOP cell. Thus, Dronc or Drice may have an instructive role in the regulation of neuronal development by antagonizing the Wingless pathway.
If caspases can have instructive roles in regulating neuronal development, one might expect that there should be regulatory mechanisms that restrict caspase activity in neuronal development independent of cell death. The identification of DmIKKε (also called Ik2), the fly ortholog of the noncanonical IκB kinase family (IKKε or NAK), in a dominant-modifier screen in Drosophila for suppressors of caspase activation begins to address this question (Figure 5) (Kuranaga et al., 2006). DmIKKε was found to regulate Dronc activity by promoting phosphorylation and subsequent degradation of DIAP1. Importantly, RNAi against DmIKKε leads to the development of extra SOP cells, which phenocopies dronc and dark mutants. Thus, DmIKKε may control the levels of DIAP1 to induce a certain threshold of caspase activity that is still compatible with cell survival. This regulation of caspase activation may be conserved in mammalian cells as NAK controls XIAP, a mammalian ortholog of DIAP1, in a similar fashion (Kuranaga et al., 2006). This mechanism could apply to mammalian neuronal development as increased caspase-3 activity has been observed during the differentiation of neural progenitor cells derived from mouse embryos (Fernando et al., 2005).
Figure 5. Non-apoptotic function of caspases in Drosophila neural development.
Low-level caspase activity, which would otherwise fall below the threshold of detection in some assays, was measured using a fluorescence resonance energy transfer (FRET)-based probe containing enhanced cyan fluorescence protein (ECFP) as the FRET donor and a variant of enhanced yellow fluorescence protein, Venus, as the FRET acceptor. A caspase cleavage site was designed within the peptide linker that connects ECFP and Venus (Takemoto et al., 2003). In situ confocal imaging of the FRET emission ratio (Venus/ECFP) reveals caspase activity in wing imaginal discs (A), including the specific subset of cells that have the potential to give rise to scutellar sensory organ precursors (B, upper panel). This level of caspase activation is dependent on DmIKKε-activated degradation of DIAP1 and is insufficient to induce apoptosis. Rather, it is required for proper regulation of sensory organ precursor cell number (Kuranaga et al., 2006). Suppression of caspase activation via loss of DmIKKεresults in the eventual formation of an extra sensory bristle, which co-stains with a neuronal marker, anti-senseless (B, lower panels). Images courtesy of Masayuki Miura.
Caspases and other cell types
Caspases have been implicated in the differentiation process for a number of other cell types; however, the regulatory mechanism is largely unknown for these cases. For example, caspase activation is observed during the differentiation of monocytes into macrophages but not into dendritic cells (Netea et al., 2008; Sordet et al., 2002). Concordantly, loss of caspase-8 leads to more severe defects in the development of macrophages than that of dendritic cells (Kang et al., 2004). Identification of possible caspase substrates that are cleaved during macrophage differentiation has been initiated (Cathelin et al., 2006). Caspase-8 has also been associated with specialized cell fusion events that are necessary for the differentiation of human placental villous trophoblast, an epithelial layer important for transporting nutrients directly from mother to fetus (Black et al., 2004). Other cell differentiation processes may require caspase-3 activation. Caspase-3 activation is observed during neuronal differentiation (Fernando et al., 2005) and in glial cells within the developing cerebellum (Oomman et al., 2006). Appropriate skeletal myogenesis characterized by a series of myoblast fusion events may also depend on caspase-3 activity (Fernando et al., 2002). Cleavage-dependent activation of MST1 kinase (Mammalian Sterile Twenty-like 1) by caspase-3 may drive skeletal muscle differentiation as expression of the truncated kinase rescues the differentiation defect in caspase-3 deficient myoblasts. It is intriguing that caspase-3 has been linked to skeletal muscle differentiation as well as osteogenic differentiation (Miura et al., 2004) because both are necessary for a functional skeletal system.
Caspases and stem cells
The function of caspases in cell differentiation may extend beyond the terminal differentiation to include stem cell pluropotency and hematopoiesis. A defining characteristic of stem cells is their dual capacity to self-renew and to differentiate into specific somatic cells. Surprisingly, two recent studies implicate caspase-3 in controlling the fate of both embryonic stem cells (ESCs) and adult hematopoietic stem cells (HSCs).
The self-renewing state of ESCs is controlled by a set of transcription factors, Oct4, Sox2, and Nanog, which constitutes a robust auto-regulatory signaling circuit (Boyer et al., 2005). Increased caspase activity is observed in differentiating ESCs and is associated with caspase-3 mediated cleavage of human Nanog (Fujita et al., 2008). ESCs expressing a caspase cleavage-resistant Nanog mutant are refractory to differentiation induction similar to that of cells deficient in caspase-3, suggesting that Nanog may be the key caspase substrate for limiting the self-renewing capacity of ESCs. Moreover, loss of caspase-3 leads to increased proliferation of long-term repopulating HSCs with a concordant defect in differentiation due to altered ERK (extracellular signal-related kinase) signaling (Janzen et al., 2008). This finding suggests that caspase-3 may help to maintain the quiescent state of HSCs. In light of these studies, the role of caspase-3 in stem cell maintenance may go hand in hand with the role of this caspase in promoting the differentiation of stem cells. Determining whether caspases function at the molecular bifurcation of the signal transduction pathways that direct cell lineage specification and simultaneously block stem cell self-renewal properties is a key unanswered question.
Caspases in cell migration and motility
A tractable system to study cell migration in vivo is the migratory behavior of border cells, which are the follicle cells within the egg chamber, during fly oogenesis. Surprisingly, a screen for suppressors of the migration defect caused by overexpression of dominant-negative Rac GTPase led to the identification of DIAP1 (Geisbrecht and Montell, 2004). Further characterization revealed that overexpression of dominant-negative Dronc and Dark also rescued this migration defect. However expression of p35 was unable to promote border cell migration. These findings suggest that the upstream caspase Dronc may inhibit Rac-dependent cell motility. One hint as to how cell migration might be affected by Dronc is the observation of a disorganized actin cytoskeleton in the oocytes of flies expressing a hypomorphic allele of DIAP1 (Geisbrecht and Montell, 2004). In addition, regulation of DIAP1 levels by DmIKKε has also been implicated in the organization of actin cytoskeleton at the cell edge (Oshima et al., 2006). DmIKKε mutants show increased branching of the actin cytoskeleton, leading to enhanced membrane ruffling, whereas hyperactivation of DmIKKε results in decreased actin assembly. Though it is unclear how Dronc might regulate actin dynamics, one possibility is that caspases have a conserved role in actin depolymerization.
An important aspect of a proper host response is appropriate immune cell migration and motility. In this paradigm, our laboratory discovered that caspase-11 has a surprising role in regulating actin dynamics during inflammation in murine macrophages. In particular, caspase-11 interacts with actin interacting protein 1 (Aip1) to promote cofilin-mediated actin depolymerization (Li et al., 2007). Conversely Flightless-I, a member of the gelsolin superfamily of actin remodeling proteins, inhibits caspase-11 and caspase-1 mediated IL-1β maturation by controlling the localization and activation of these proinflammatory caspases (Li et al., 2008). The link between caspases and actin dynamics might provide an important regulatory venue for organization of the actin cytoskeleton as well as localized caspase activity.
Concluding remarks
It is remarkable how the identification of a simple cell death machinery in C. elegans paved the path for elucidating the complex apoptotic machinery in mammals. Though extensive research has characterized caspases as key executioners of apoptotic cell death, recent advances implicate caspases in diverse non-apoptotic processes. While full activation of the caspase cascades associated with cell death is rare in long-lived cell types, such as neurons, localized or limited activation of caspases may be more common and persistent in order to carry out their non-apoptotic functions.
Much remains to be learned as to how caspases are regulated in these highly specialized cellular processes. Such regulatory mechanisms will be intricate in nature in order to provide a suitable balance between sufficient caspase activation to execute a certain non-apoptotic function and maintenance of cellular integrity. In Drosophila, it is clear that protein levels of DIAP1 influences caspase activation by regulating either localization of caspase activity or extent of caspase activation. [this sentence was unclear]. To date, a similar mechanism has yet to be implicated in regulating the activation of mammalian caspases in non-apoptotic processes, such as “subcellular” apoptosis. Interestingly post-translational modifications, including phosphorylation and S-nitrosylation, can directly regulate caspase activity and influence apoptotic cell death (Allan and Clarke, 2007; Allan et al., 2003; Cardone et al., 1998; Hess et al., 2005; Nutt et al., 2005). Furthermore these modifications are influenced by specific pro-apoptotic stimuli—for example, dephosphorylation of caspase-2 is induced upon metabolic stress (Nutt et al., 2005). This mechanism could provide the specificity required for appropriate caspase activation in highly specialized cellular contexts. A more speculative possibility is that activated caspases might be unstable in living cells. After executing their non-apoptotic function in a localized manner, such short-lived active caspases could be eliminated quickly by cellular degradative mechanisms. It will be of great interest to test the temporal regulation of caspase activation as well as the threshold of caspase activity under non-apoptotic conditions. Refinement of the currently available fluorescent caspase reporters will be useful in teasing apart such mechanisms (Bardet et al., 2008; Takemoto et al., 2003). With our current technology and knowledge, the time is ripe for further exploration of the non-apoptotic roles of caspases and for the systematic identification of caspase substrates that might function as critical effectors for these cellular processes (Dix et al., 2008; Keller et al., 2008).
Acknowledgments
We apologize to our colleagues whose work could not be cited due to space limitations. We thank Andrew D. Steele for critical reading of the manuscript. This work was supported in part by a NIH Director’s Pioneer Award (DP1OD000580) and a grant from the National Institute of Aging (R37-012859) to J. Yuan, and a Kirchstein National Research Service Award from the National Institute of Neurological Disorders and Stroke (grant 1F31NS057872-01) to C.H. Yi.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aballay A, Ausubel FM. Programmed cell death mediated by ced-3 and ced-4 protects Caenorhabditis elegans from Salmonella typhimurium-mediated killing. Proc Natl Acad Sci U S A. 2001;98:2735–2739. doi: 10.1073/pnas.041613098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan LA, Clarke PR. Phosphorylation of caspase-9 by CDK1/cyclin B1 protects mitotic cells against apoptosis. Mol Cell. 2007;26:301–310. doi: 10.1016/j.molcel.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR. Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol. 2003;5:647–654. doi: 10.1038/ncb1005. [DOI] [PubMed] [Google Scholar]
- Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, Nunez G. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- Arama E, Agapite J, Steller H. Caspase activity and a specific cytochrome C are required for sperm differentiation in Drosophila. Dev Cell. 2003;4:687–697. doi: 10.1016/s1534-5807(03)00120-5. [DOI] [PubMed] [Google Scholar]
- Arama E, Bader M, Rieckhof GE, Steller H. A ubiquitin ligase complex regulates caspase activation during sperm differentiation in Drosophila. PLoS Biol. 2007;5:e251. doi: 10.1371/journal.pbio.0050251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardet PL, Kolahgar G, Mynett A, Miguel-Aliaga I, Briscoe J, Meier P, Vincent JP. A fluorescent reporter of caspase activity for live imaging. Proc Natl Acad Sci U S A. 2008;105:13901–13905. doi: 10.1073/pnas.0806983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassnett S, Mataic D. Chromatin degradation in differentiating fiber cells of the eye lens. J Cell Biol. 1997;137:37–49. doi: 10.1083/jcb.137.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell BD, Leverrier S, Weist BM, Newton RH, Arechiga AF, Luhrs KA, Morrissette NS, Walsh CM. FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proc Natl Acad Sci U S A. 2008;105:16677–16682. doi: 10.1073/pnas.0808597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Moshe T, Barash H, Kang TB, Kim JC, Kovalenko A, Gross E, Schuchmann M, Abramovitch R, Galun E, Wallach D. Role of caspase-8 in hepatocyte response to infection and injury in mice. Hepatology. 2007;45:1014–1024. doi: 10.1002/hep.21495. [DOI] [PubMed] [Google Scholar]
- Black S, Kadyrov M, Kaufmann P, Ugele B, Emans N, Huppertz B. Syncytial fusion of human trophoblast depends on caspase 8. Cell Death Differ. 2004;11:90–98. doi: 10.1038/sj.cdd.4401307. [DOI] [PubMed] [Google Scholar]
- Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Carlile GW, Smith DH, Wiedmann M. Caspase-3 has a nonapoptotic function in erythroid maturation. Blood. 2004;103:4310–4316. doi: 10.1182/blood-2003-09-3362. [DOI] [PubMed] [Google Scholar]
- Cathelin S, Rebe C, Haddaoui L, Simioni N, Verdier F, Fontenay M, Launay S, Mayeux P, Solary E. Identification of proteins cleaved downstream of caspase activation in monocytes undergoing macrophage differentiation. J Biol Chem. 2006;281:17779–17788. doi: 10.1074/jbc.M600537200. [DOI] [PubMed] [Google Scholar]
- Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA, et al. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992;256:97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- Ch’en IL, Beisner DR, Degterev A, Lynch C, Yuan J, Hoffmann A, Hedrick SM. Antigen-mediated T cell expansion regulated by parallel pathways of death. Proc Natl Acad Sci U S A. 2008;105:17463–17468. doi: 10.1073/pnas.0808043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, Dale JK, Puck J, Davis J, Hall CG, et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419:395–399. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- Clarke PG. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol (Berl) 1990;181:195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- De Botton S, Sabri S, Daugas E, Zermati Y, Guidotti JE, Hermine O, Kroemer G, Vainchenker W, Debili N. Platelet formation is the consequence of caspase activation within megakaryocytes. Blood. 2002;100:1310–1317. doi: 10.1182/blood-2002-03-0686. [DOI] [PubMed] [Google Scholar]
- De Maria R, Zeuner A, Eramo A, Domenichelli C, Bonci D, Grignani F, Srinivasula SM, Alnemri ES, Testa U, Peschle C. Negative regulation of erythropoiesis by caspase-mediated cleavage of GATA-1. Nature. 1999;401:489–493. doi: 10.1038/46809. [DOI] [PubMed] [Google Scholar]
- Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22:8543–8567. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- Denecker G, Hoste E, Gilbert B, Hochepied T, Ovaere P, Lippens S, Van den Broecke C, Van Damme P, D’Herde K, Hachem JP, et al. Caspase-14 protects against epidermal UVB photodamage and water loss. Nat Cell Biol. 2007;9:666–674. doi: 10.1038/ncb1597. [DOI] [PubMed] [Google Scholar]
- Dix MM, Simon GM, Cravatt BF. Global mapping of the topography and magnitude of proteolytic events in apoptosis. Cell. 2008;134:679–691. doi: 10.1016/j.cell.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Dixit VM. RAIDD is a new ‘death’ adaptor molecule. Nature. 1997;385:86–89. doi: 10.1038/385086a0. [DOI] [PubMed] [Google Scholar]
- Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Bergmann A. Distinct mechanisms of apoptosis-induced compensatory proliferation in proliferating and differentiating tissues in the Drosophila eye. Dev Cell. 2008;14:399–410. doi: 10.1016/j.devcel.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, Reed JC. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Fernando P, Brunette S, Megeney LA. Neural stem cell differentiation is dependent upon endogenous caspase 3 activity. Faseb J. 2005;19:1671–1673. doi: 10.1096/fj.04-2981fje. [DOI] [PubMed] [Google Scholar]
- Fernando P, Kelly JF, Balazsi K, Slack RS, Megeney LA. Caspase 3 activity is required for skeletal muscle differentiation. Proc Natl Acad Sci U S A. 2002;99:11025–11030. doi: 10.1073/pnas.162172899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol. 2007;7:862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- Festjens N, Cornelis S, Lamkanfi M, Vandenabeele P. Caspase-containing complexes in the regulation of cell death and inflammation. Biol Chem. 2006;387:1005–1016. doi: 10.1515/BC.2006.124. [DOI] [PubMed] [Google Scholar]
- Franchi L, Stoolman J, Kanneganti TD, Verma A, Ramphal R, Nunez G. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol. 2007;37:3030–3039. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita J, Crane AM, Souza MK, Dejosez M, Kyba M, Flavell RA, Thomson JA, Zwaka TP. Caspase activity mediates the differentiation of embryonic stem cells. Cell Stem Cell. 2008;2:595–601. doi: 10.1016/j.stem.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilin MA, Bouakl IJ, Knatz NL, Duncan MD, Hall MW, Gunn JS, Wewers MD. Internalization and phagosome escape required for Francisella to induce human monocyte IL-1beta processing and release. Proc Natl Acad Sci U S A. 2006;103:141–146. doi: 10.1073/pnas.0504271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbrecht ER, Montell DJ. A role for Drosophila IAP1-mediated caspase inhibition in Rac-dependent cell migration. Cell. 2004;118:111–125. doi: 10.1016/j.cell.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Georgel P, Naitza S, Kappler C, Ferrandon D, Zachary D, Swimmer C, Kopczynski C, Duyk G, Reichhart JM, Hoffmann JA. Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev Cell. 2001;1:503–514. doi: 10.1016/s1534-5807(01)00059-4. [DOI] [PubMed] [Google Scholar]
- Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- Gottar M, Gobert V, Michel T, Belvin M, Duyk G, Hoffmann JA, Ferrandon D, Royet J. The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature. 2002;416:640–644. doi: 10.1038/nature734. [DOI] [PubMed] [Google Scholar]
- Haynie J, Bryant P. The effects of X-rays on the proliferation dynamics of cells in the imaginal wing disc of Drosophila melanogaster. Development Genes and Evolution. 1977;183:85–199. doi: 10.1007/BF00848779. [DOI] [PubMed] [Google Scholar]
- Henry T, Monack DM. Activation of the inflammasome upon Francisella tularensis infection: interplay of innate immune pathways and virulence factors. Cell Microbiol. 2007;9:2543–2551. doi: 10.1111/j.1462-5822.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- Howard AD, Chartrain N, Ding GF, Kostura MJ, Limjuco G, Schmidt JA, Tocci MJ. Probing the role of interleukin-1 beta convertase in interleukin-1 beta secretion. Agents Actions Suppl. 1991;35:77–83. [PubMed] [Google Scholar]
- Hu S, Yang X. dFADD, a novel death domain-containing adapter protein for the Drosophila caspase DREDD. J Biol Chem. 2000;275:30761–30764. doi: 10.1074/jbc.C000341200. [DOI] [PubMed] [Google Scholar]
- Huesmann GR, Clayton DF. Dynamic role of postsynaptic caspase-3 and BIRC4 in zebra finch song-response habituation. Neuron. 2006;52:1061–1072. doi: 10.1016/j.neuron.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JR, Guo M, Hay BA. Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase Dronc in a nonapoptotic role. Curr Biol. 2004a;14:1262–1266. doi: 10.1016/j.cub.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Huh JR, Vernooy SY, Yu H, Yan N, Shi Y, Guo M, Hay BA. Multiple apoptotic caspase cascades are required in nonapoptotic roles for Drosophila spermatid individualization. PLoS Biol. 2004b;2:E15. doi: 10.1371/journal.pbio.0020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- Ishizaki Y, Jacobson MD, Raff MC. A role for caspases in lens fiber differentiation. J Cell Biol. 1998;140:153–158. doi: 10.1083/jcb.140.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AA, Bryant PJ. A quantitative study of cell death and mitotic inhibition in gamma-irradiated imaginal wing discs of Drosophila melanogaster. Radiat Res. 1981;87:552–564. [PubMed] [Google Scholar]
- Janzen V, Fleming HE, Riedt T, Karlsson G, Riese MJ, Lo Celso C, Reynolds G, Milne CD, Paige CJ, Karlsson S, et al. Hematopoietic stem cell responsiveness to exogenous signals is limited by caspase-3. Cell Stem Cell. 2008;2:584–594. doi: 10.1016/j.stem.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Yano T, Aggarwal K, Lim JH, Ueda K, Oshima Y, Peach C, Erturk-Hasdemir D, Goldman WE, Oh BH, et al. PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan. Nat Immunol. 2006;7:715–723. doi: 10.1038/ni1356. [DOI] [PubMed] [Google Scholar]
- Kang TB, Ben-Moshe T, Varfolomeev EE, Pewzner-Jung Y, Yogev N, Jurewicz A, Waisman A, Brenner O, Haffner R, Gustafsson E, et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol. 2004;173:2976–2984. doi: 10.4049/jimmunol.173.5.2976. [DOI] [PubMed] [Google Scholar]
- Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, Nunez G. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006a;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006b;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- Kanuka H, Kuranaga E, Takemoto K, Hiratou T, Okano H, Miura M. Drosophila caspase transduces Shaggy/GSK-3beta kinase activity in neural precursor development. Embo J. 2005;24:3793–3806. doi: 10.1038/sj.emboj.7600822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanuka H, Sawamoto K, Inohara N, Matsuno K, Okano H, Miura M. Control of the cell death pathway by Dapaf-1, a Drosophila Apaf-1/CED-4-related caspase activator. Mol Cell. 1999;4:757–769. doi: 10.1016/s1097-2765(00)80386-x. [DOI] [PubMed] [Google Scholar]
- Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- Kondo S, Senoo-Matsuda N, Hiromi Y, Miura M. DRONC coordinates cell death and compensatory proliferation. Mol Cell Biol. 2006;26:7258–7268. doi: 10.1128/MCB.00183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CT, Zhu S, Younger S, Jan LY, Jan YN. Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating Drosophila sensory neuron dendrite pruning. Neuron. 2006;51:283–290. doi: 10.1016/j.neuron.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Kuranaga E, Kanuka H, Tonoki A, Takemoto K, Tomioka T, Kobayashi M, Hayashi S, Miura M. Drosophila IKK-related kinase regulates nonapoptotic function of caspases via degradation of IAPs. Cell. 2006;126:583–596. doi: 10.1016/j.cell.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Lemmers B, Salmena L, Bidere N, Su H, Matysiak-Zablocki E, Murakami K, Ohashi PS, Jurisicova A, Lenardo M, Hakem R, Hakem A. Essential role for caspase-8 in Toll-like receptors and NFkappaB signaling. J Biol Chem. 2007;282:7416–7423. doi: 10.1074/jbc.M606721200. [DOI] [PubMed] [Google Scholar]
- Leulier F, Rodriguez A, Khush RS, Abrams JM, Lemaitre B. The Drosophila caspase Dredd is required to resist gram-negative bacterial infection. EMBO Rep. 2000;1:353–358. doi: 10.1093/embo-reports/kvd073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leulier F, Vidal S, Saigo K, Ueda R, Lemaitre B. Inducible expression of double-stranded RNA reveals a role for dFADD in the regulation of the antibacterial response in Drosophila adults. Curr Biol. 2002;12:996–1000. doi: 10.1016/s0960-9822(02)00873-4. [DOI] [PubMed] [Google Scholar]
- Levkau B, Koyama H, Raines EW, Clurman BE, Herren B, Orth K, Roberts JM, Ross R. Cleavage of p21Cip1/Waf1 and p27Kip1 mediates apoptosis in endothelial cells through activation of Cdk2: role of a caspase cascade. Mol Cell. 1998;1:553–563. doi: 10.1016/s1097-2765(00)80055-6. [DOI] [PubMed] [Google Scholar]
- Li J, Brieher WM, Scimone ML, Kang SJ, Zhu H, Yin H, von Andrian UH, Mitchison T, Yuan J. Caspase-11 regulates cell migration by promoting Aip1-Cofilin-mediated actin depolymerization. Nat Cell Biol. 2007;9:276–286. doi: 10.1038/ncb1541. [DOI] [PubMed] [Google Scholar]
- Li J, Yin HL, Yuan J. Flightless-I regulates proinflammatory caspases by selectively modulating intracellular localization and caspase activity. J Cell Biol. 2008;181:321–333. doi: 10.1083/jcb.200711082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- Lippens S, Kockx M, Knaapen M, Mortier L, Polakowska R, Verheyen A, Garmyn M, Zwijsen A, Formstecher P, Huylebroeck D, et al. Epidermal differentiation does not involve the pro-apoptotic executioner caspases, but is associated with caspase-14 induction and processing. Cell Death Differ. 2000;7:1218–1224. doi: 10.1038/sj.cdd.4400785. [DOI] [PubMed] [Google Scholar]
- Liu WH, Lin YL, Wang JP, Liou W, Hou RF, Wu YC, Liao CL. Restriction of vaccinia virus replication by a ced-3 and ced-4-dependent pathway in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2006;103:4174–4179. doi: 10.1073/pnas.0506442103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahrus S, Trinidad JC, Barkan DT, Sali A, Burlingame AL, Wells JA. Global sequencing of proteolytic cleavage sites in apoptosis by specific labeling of protein N termini. Cell. 2008;134:866–876. doi: 10.1016/j.cell.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- Miao EA, Ernst RK, Dors M, Mao DP, Aderem A. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc Natl Acad Sci U S A. 2008;105:2562–2567. doi: 10.1073/pnas.0712183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milan M, Campuzano S, Garcia-Bellido A. Developmental parameters of cell death in the wing disc of Drosophila. Proc Natl Acad Sci U S A. 1997;94:5691–5696. doi: 10.1073/pnas.94.11.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Chen XD, Allen MR, Bi Y, Gronthos S, Seo BM, Lakhani S, Flavell RA, Feng XH, Robey PG, et al. A crucial role of caspase-3 in osteogenic differentiation of bone marrow stromal stem cells. J Clin Invest. 2004;114:1704–1713. doi: 10.1172/JCI20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Zhu H, Rotello R, Hartwieg EA, Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993;75:653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- Miwa K, Asano M, Horai R, Iwakura Y, Nagata S, Suda T. Caspase 1-independent IL-1beta release and inflammation induced by the apoptosis inducer Fas ligand. Nat Med. 1998;4:1287–1292. doi: 10.1038/3276. [DOI] [PubMed] [Google Scholar]
- Muro I, Berry DL, Huh JR, Chen CH, Huang H, Yoo SJ, Guo M, Baehrecke EH, Hay BA. The Drosophila caspase Ice is important for many apoptotic cell deaths and for spermatid individualization, a nonapoptotic process. Development. 2006;133:3305–3315. doi: 10.1242/dev.02495. [DOI] [PubMed] [Google Scholar]
- Netea MG, Lewis EC, Azam T, Joosten LA, Jaekal J, Bae SY, Dinarello CA, Kim SH. Interleukin-32 induces the differentiation of monocytes into macrophage-like cells. Proc Natl Acad Sci U S A. 2008;105:3515–3520. doi: 10.1073/pnas.0712381105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt LK, Margolis SS, Jensen M, Herman CE, Dunphy WG, Rathmell JC, Kornbluth S. Metabolic regulation of oocyte cell death through the CaMKII-mediated phosphorylation of caspase-2. Cell. 2005;123:89–103. doi: 10.1016/j.cell.2005.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomman S, Strahlendorf H, Dertien J, Strahlendorf J. Bergmann glia utilize active caspase-3 for differentiation. Brain Res. 2006;1078:19–34. doi: 10.1016/j.brainres.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Oshima K, Takeda M, Kuranaga E, Ueda R, Aigaki T, Miura M, Hayashi S. IKK epsilon regulates F actin assembly and interacts with Drosophila IAP1 in cellular morphogenesis. Curr Biol. 2006;16:1531–1537. doi: 10.1016/j.cub.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Pellettieri J, Sanchez Alvarado A. Cell turnover and adult tissue homeostasis: from humans to planarians. Annu Rev Genet. 2007;41:83–105. doi: 10.1146/annurev.genet.41.110306.130244. [DOI] [PubMed] [Google Scholar]
- Perez-Garijo A, Martin FA, Morata G. Caspase inhibition during apoptosis causes abnormal signalling and developmental aberrations in Drosophila. Development. 2004;131:5591–5598. doi: 10.1242/dev.01432. [DOI] [PubMed] [Google Scholar]
- Perez-Garijo A, Martin FA, Struhl G, Morata G. Dpp signaling and the induction of neoplastic tumors by caspase-inhibited apoptotic cells in Drosophila. Proc Natl Acad Sci U S A. 2005;102:17664–17669. doi: 10.1073/pnas.0508966102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JM, Scurfield G. The mechanism of platelet release. Blood. 1980;56:996–999. [PubMed] [Google Scholar]
- Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2006;2:e18. doi: 10.1371/journal.ppat.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeil JA, Zermati Y, Vandekerckhove J, Cathelin S, Kersual J, Dussiot M, Coulon S, Moura IC, Zeuner A, Kirkegaard-Sorensen T, et al. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature. 2007;445:102–105. doi: 10.1038/nature05378. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Oliver H, Zou H, Chen P, Wang X, Abrams JM. Dark is a Drosophila homologue of Apaf-1/CED-4 and functions in an evolutionarily conserved death pathway. Nat Cell Biol. 1999;1:272–279. doi: 10.1038/12984. [DOI] [PubMed] [Google Scholar]
- Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell. 2004;7:491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au PY, Berry DM, Tamblyn L, Shehabeldin A, Migon E, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17:883–895. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FJ, Irvine AD, Terron-Kwiatkowski A, Sandilands A, Campbell LE, Zhao Y, Liao H, Evans AT, Goudie DR, Lewis-Jones S, et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet. 2006;38:337–342. doi: 10.1038/ng1743. [DOI] [PubMed] [Google Scholar]
- Sordet O, Rebe C, Plenchette S, Zermati Y, Hermine O, Vainchenker W, Garrido C, Solary E, Dubrez-Daloz L. Specific involvement of caspases in the differentiation of monocytes into macrophages. Blood. 2002;100:4446–4453. doi: 10.1182/blood-2002-06-1778. [DOI] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Moon RT, Weidinger G. Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes Dev. 2007;21:1292–1315. doi: 10.1101/gad.1540507. [DOI] [PubMed] [Google Scholar]
- Stoven S, Silverman N, Junell A, Hedengren-Olcott M, Erturk D, Engstrom Y, Maniatis T, Hultmark D. Caspase-mediated processing of the Drosophila NF-kappaB factor Relish. Proc Natl Acad Sci U S A. 2003;100:5991–5996. doi: 10.1073/pnas.1035902100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galan JE, Askenase PW, Flavell RA. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Takemoto K, Nagai T, Miyawaki A, Miura M. Spatio-temporal activation of caspase revealed by indicator that is insensitive to environmental effects. J Cell Biol. 2003;160:235–243. doi: 10.1083/jcb.200207111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornberry NA, Molineaux SM. Interleukin-1 beta converting enzyme: a novel cysteine protease required for IL-1 beta production and implicated in programmed cell death. Protein Sci. 1995;4:3–12. doi: 10.1002/pro.5560040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinel A, Tschopp J. The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science. 2004;304:843–846. doi: 10.1126/science.1095432. [DOI] [PubMed] [Google Scholar]
- Van Damme P, Martens L, Van Damme J, Hugelier K, Staes A, Vandekerckhove J, Gevaert K. Caspase-specific and nonspecific in vivo protein processing during Fas-induced apoptosis. Nat Methods. 2005;2:771–777. doi: 10.1038/nmeth792. [DOI] [PubMed] [Google Scholar]
- Van de Craen M, Van Loo G, Pype S, Van Criekinge W, Van den brande I, Molemans F, Fiers W, Declercq W, Vandenabeele P. Identification of a new caspase homologue: caspase-14. Cell Death Differ. 1998;5:838–846. doi: 10.1038/sj.cdd.4400444. [DOI] [PubMed] [Google Scholar]
- Wang S, Miura M, Jung YK, Zhu H, Li E, Yuan J. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell. 1998;92:501–509. doi: 10.1016/s0092-8674(00)80943-5. [DOI] [PubMed] [Google Scholar]
- Warren SE, Mao DP, Rodriguez AE, Miao EA, Aderem A. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J Immunol. 2008;180:7558–7564. doi: 10.4049/jimmunol.180.11.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells BS, Yoshida E, Johnston LA. Compensatory proliferation in Drosophila imaginal discs requires Dronc-dependent p53 activity. Curr Biol. 2006;16:1606–1615. doi: 10.1016/j.cub2006.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DW, Kondo S, Krzyzanowska A, Hiromi Y, Truman JW. Local caspase activity directs engulfment of dendrites during pruning. Nat Neurosci. 2006;9:1234–1236. doi: 10.1038/nn1774. [DOI] [PubMed] [Google Scholar]
- Woo M, Hakem R, Furlonger C, Hakem A, Duncan GS, Sasaki T, Bouchard D, Lu L, Wu GE, Paige CJ, Mak TW. Caspase-3 regulates cell cycle in B cells: a consequence of substrate specificity. Nat Immunol. 2003;4:1016–1022. doi: 10.1038/ni976. [DOI] [PubMed] [Google Scholar]
- Woo M, Hakem R, Soengas MS, Duncan GS, Shahinian A, Kagi D, Hakem A, McCurrach M, Khoo W, Kaufman SA, et al. Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 1998;12:806–819. doi: 10.1101/gad.12.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Chang HY, Baltimore D. Essential role of CED-4 oligomerization in CED-3 activation and apoptosis. Science. 1998;281:1355–1357. doi: 10.1126/science.281.5381.1355. [DOI] [PubMed] [Google Scholar]
- Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- Zermati Y, Garrido C, Amsellem S, Fishelson S, Bouscary D, Valensi F, Varet B, Solary E, Hermine O. Caspase activation is required for terminal erythroid differentiation. J Exp Med. 2001;193:247–254. doi: 10.1084/jem.193.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- Zhou L, Song Z, Tittel J, Steller H. HAC-1, a Drosophila homolog of APAF-1 and CED-4 functions in developmental and radiation-induced apoptosis. Mol Cell. 1999;4:745–755. doi: 10.1016/s1097-2765(00)80385-8. [DOI] [PubMed] [Google Scholar]