Abstract
Although waves in networks of astrocytes in vivo are well documented, propagation in vivo is much more complex than in culture, and there is no consensus concerning the dominant roles of intercellular and extracellular messengers [inositol 1,4,5–trisphosphate (IP3) and adenosine-5′-triphosphate (ATP)] that mediate waves. Moreover, to date only simplified models that take very little account of the geometrical struture of the networks have been studied. Our aim in this paper is to develop a mathematical model based on realistic cellular morphology and network connectivity, and a computational framework for simulating the model, in order to address these issues. In the model, wave propagation through a network of astrocytes is driven by IP3 diffusion between cells and ATP transport in the extracellular space. Numerical simulations of the model show that different kinetic and geometric assumptions give rise to differences in wave propagation patterns, as characterized by the velocity, propagation distance, time delay in propagation from one cell to another, and the evolution of Ca2+ response patterns. The temporal response patterns in cells are different from one cell to another, and the response patterns evolve from one type to another as a wave propagates. In addition, the spatial patterns of wave propagation depend on whether IP3, ATP, or both are mediating messengers. Finally, two different geometries that reflect the in vivo and in vitro configuration of astrocytic networks also yield distinct intracellular and extracellular kinetic patterns. The simulation results as well as the linear stability analysis of the model lead to the conclusion that waves in astrocyte networks are probably mediated by both intercellular IP3 transport and nonregenerative (only the glutamate-stimulated cell releases ATP) or partially regenerative extracellular ATP signaling.
Calcium (Ca2+) is one of the most versatile and widely used second-messenger molecules and plays a pivotal role in neurotransmission, muscle contraction, gene expression, and a variety of other intracellular processes.13, 37 Because high levels of intracellular calcium are toxic, and because it cannot be degraded as many other signaling molecules are, cells control the intracellular calcium level at around 100 nM (compared to millimolar extracellular levels) by buffering, sequestration in specialized compartments, and by expulsion to the extracellular space.37, 110, 115 In addition to intracellular homeostatic mechanisms to control , sophisticated intracellular signal transduction pathways that involve different proteins modulated by Ca2+ have evolved for communication between cells.7, 38, 39, 41, 42, 60, 61, 62, 70, 100, 119 In the central nervous system, glial cells (collectively, astrocytes, oligodendrocytes, and microglia), which are 10–15 times more numerous than neurons, make up about half of the total brain weight. Astrocytes, which are the dominant glial cell type, had been regarded as maintenance and support cells for neurons until recently, because they lack sodium channels and are electrically nonexcitable.117 It has been found experimentally that waves propagate through networks of astrocytes, and there is a great deal of interest in understanding their role in the brain. In this paper we develop mathematical models that shed light on what factors control the spread of such waves.
INTRODUCTION
Glutamate induced mobilization in astrocytes
A major metabotropic pathway from agonist to calcium changes is via receptor-activated G proteins that initiate production of inositol 1,4,5–trisphosphate (IP3), which then binds to IP3 receptors on calcium channels in the membrane of the endoplasmic reticulum (ER), an intracellular Ca2+ store. Calcium release from the ER is terminated by Ca2+ inhibition of channel opening at high concentrations14 and pumps restore to resting levels. Typically the reuptake makes the Ca2+ signal a transient “spike” and allows the cell to maintain very low levels of resting .
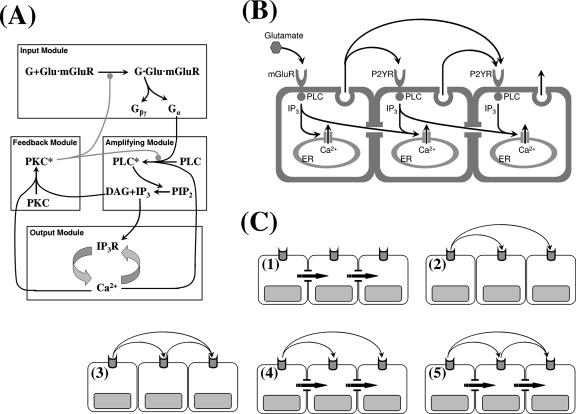
It was shown previously that the intracellular network that controls dynamics is comprised of four modules [cf. Fig. 1a] that can be summarized as follows:67 (1) the ligand and receptor kinetics at the plasma membrane (the input module), (2) a Gq-type G-protein-activated module in which activated phospholipase C (PLC) leads to the production of IP3 and diacylglycerol (DAG) from phosphatidylinositol-biphosphate (PIP2) (the amplifying module), (3) an IP3∕IP3-receptor system that controls the Ca2+ release from the ER by calcium-induced calcium release (CICR) (the output module), and (4) a feedback module involving DAG-Ca2+ activation of protein kincase C (PKC), which leads to downregulation of receptors and PLC (the feedback module). The input module receives stimulatory ligand and inhibitory PKC signals as inputs and produces Gα and Gβγ as outputs, the former of which serves as an input to the amplifying module. The amplifying module produces its outputs, IP3 and DAG, from the hydrolysis of PIP2 by Gα-activated PLC. While soluble IP3 diffuses into the cytoplasm and functions as an input to the output module, hydrophobic DAG stays at the inner leaflet of the plasma membrane. The output module comprises the Ca2+ handling mechanisms such as IP3-stimulated release from the ER and Sarco∕Endoplasmic Reticulum calcium ATPase (SERCA) uptake and outputs . Finally, the feedback module receives and DAG as inputs and produces the activated state of PKC, which downregulates the activity of input and amplifying modules. A detailed model for calcium dynamics in isolated cells based on this modular decomposition was derived and analyzed earlier.67
Figure 1.
(a) The modular representation of the glutamate-induced Ca2+ release pathway. (b) A schematic overview of the possible mechanism of wave propagation in an astrocyte network. (c) Case studies of wave propagation under all the possible combination of intracellular and extracellular messengers: (1) direct coupling and no extracellular signal, (2) nonregenerative extracellular signal and no direct coupling, (3) regenerative extracellular signal and no direct coupling, (4) direct coupling and nonregenerative extracellular signal, and (5) regenerative extracellular signal and direct coupling. Note that autocrine ATP signaling is neglected for the glutamate-stimulated cell.
Ca2+ wave propagation in astrocyte networks
It is now believed, after numerous reports of waves in astrocyte networks following various stimuli,89, 93, 97, 84, 86, 24, 45, 95 that astrocytes modulate neural network activities via astro-astro and astroneuronal cross-talk, although their physiological roles in vivo are still subject to debate.1, 37, 45, 43, 35, 83, 116, 78, 99 One example of the cross-talk is reflected in adenosine-5′-triphosphate (ATP)-mediated calcium waves, which demonstrate the coupling between intracellular calcium dynamics and cell-cell communication via the extracellular space.1, 37, 56 Such waves, which typically decay in time and in space as they propagate, have a maximal propagation range of 200–350 μm and a maximal velocity of 15–27 μm2∕s.15, 113, 120, 82, 18
There is substantial evidence that waves in astrocytes are mediated by direct coupling between astrocytes via transport through gap junctions49, 105, 114, 94 and∕or by paracrine ATP signaling via the extracellular space.26, 54, 18, 17, 65, 103 The mode of communication used depends on the astrocyte subtype,45 and there is a significant diversity with respect to interactions with surrounding cells.1 For example, gap junctional coupling appears to be important in astrocytes in the neocortex, while paracrine ATP signaling can induce waves independent of gap junctional coupling in astrocytes in the hippocampus.45 However, the findings that Ca2+ waves can propagate between physically separated astrocytes54, 58, 11 and in cultured astrocytes in which gap junctional coupling was pharmacologically impaired53, 63 suggest that extracellular ATP signaling plays a major role in in vitro, although it may not be the only mode. The overview of these possible mechanisms of wave propagation in an astrocyte network is described in Fig. 1b.
Just as is strictly regulated by cells, the level of ATP is also tightly controlled, but the relative levels are reversed. While cytosolic ATP is >5 mM in most cells,50, 51 extracellular [ATP] is kept around 1 nM (Ref. 64) by various enzymes. Thus large amounts of ATP can be released into the extracellular space under pathological conditions such as tissue injury, cell lysis, and cell ischemia.3, 108 Under physiological conditions, cytosolic ATP can be released via transmembrane transport in response to receptor activation in both vascular smooth muscle cells and endothelial cells.96, 98, 68, 123 In most neurons, ATP is stored in vesicles with neurotransmitters and coreleased.16, 40 Under experimental conditions various environmental stressors, such as a mechanical stress, have been used as stimuli to release ATP. It has also been reported that ATP may be released via spontaneous changes in cell volume via volume-regulated anion channels (VRACs).92 Similarly, there are multiple possible ATP release mechanisms in astrocytes. Hemichannel-mediated ATP release,25, 4, 103, 20, 65, 2 vesicular release,22, 18, 17, 122 and P2X7 ATP receptor mediated release104 have been postulated, but recent evidence suggests that vesicular release is the most probable mechanism.18, 17 At present there is no evidence of transporter-mediated cellular uptake of ATP, even though nucleotides and nucleobases are taken up by several transport systems.52
Extracellular ATP can reach biologically active levels from nanomolar to micromolar concentrations near a release site50, 73, 82, 121 and participates in various signaling processes,34, 32, 69 but the half-life of extracellular ATP is very short due to the presence of potent degrading enzymes. It was reported that ATP reaches a local peak as high as 10–75 μM (Refs. 109, 121, 82) and that the ATP “front” diffuses outward at about 41 μm∕s, which exceeds the speed of 28 μm∕s for the wave front.82 The maximal detectable ATP spread ranges from 84 to 120 μm depending on the stimulus source.82, 4, 58 The measured diffusion coefficient for the extracellular ATP ranges from 160 to 330 μm2∕s,91, 59, 81 which is much slower than in the cytosol. Estimated degradation rates for ATP in the extracellular space of astrocytes range from 3.466∕s to 4×10−4∕s.64
Extracellular ATP can bind to metabotropic ATP receptors (P2YRs) on cells, and at sufficiently high levels it can initiate signaling cascades, including release. The effective dosage of ATP for astrocyte response has been reported to be 0.74–3 μM.82, 64 There are two distinct ATP binding sites on P2YR—low affinity site and high affinity site—the former having a Kd=20±5 μM with a total concentration of Bmax=150 nM∕106 cells of P2YR, while the latter having a Kd=2.5±0.2 μM with Bmax=52 nM∕106 cells and a dissociation rate of 1.2×10−3∕s.76 [Concentrations are based on the volumes of rat and human astrocytes, which have been estimated as 66×103 and 18×105 μm3 (Refs. 85, 21).] In the model we fix the ATP release rate constant so that the peak ATP concentration is ∼30 μM. Since there are considerable differences in ATP degradation rates reported in the literature, the extracellular ATP decay rate is treated as a parameter to control the extracellular ATP level. For simplicity, we will not distinguish the two different ATP binding sites on P2YR and set Bmax=0.1 μM and Kd=10 μM.
The rationale for the model structure
increases are often found to be spatially synchronized in cultured astrocytes, which usually form an adherent cell monolayer that is well approximated as a rectangular tessellation of a two-dimensional (2D) domain.37, 45, 11 This has led to suggestions that long-range propagation of in cultured astrocytes is doubtful under physiological conditions.45, 1 However, in vivo astrocytes are interconnected by well-developed fine processes that constrain the waves to propagate along certain routes and provide weaker gap-junctional connectivity between cells. Even for the same cell type, differences exist between cultured astrocytes and astrocytes in vivo, and as a result, there are conflicting opinions concerning the roles played by intercellular and extracellular messengers in astrocytic waves. As we show later, using a realistic morphology has important implications for complex network dynamics, although other factors also have significant effects. Our results suggest that morphological differences in cultured and intact astrocytes can cause different , IP3, and ATP wave propagation patterns in terms of propagation velocity, propagation distance, amplitude, and delays between cells.
Many theoretical studies have been done to understand the phenotypical properties of waves reported for different cell types in various contexts.60, 10 For example, it is known how to predict the range of propagation in a highly simplified model of gap-junction-coupled cells.5 However, to our knowledge no studies have considered realistic morphological differences between cells in different experimental studies. We considered a realistic yet simple mathematical model of elevation and wave propagation in two different geometries mimicking astrocytic networks in vivo and in vitro and tested it under various scenarios of wave propagation [Fig. 1c] based on a number of simplifying assumptions. First, we assumed that sustained glutamate stimulus is locally restricted in one cell, yet the glutamate concentration is high enough so that ATP released from neighboring astrocytes does not influence the response kinetics in the glutamate-stimulated cell. Second, all the astrocytes share identical physiological properties, which means that the same system of partial differential equations (PDEs) is valid for all the cells. Third, the distribution of ER is homogeneous throughout the cell body and processes of an astrocyte, i.e., the shape of the ER network matches the shape of an astrocyte. Fourth, P2YRs are also uniformly distributed over the cell body and processes of an astrocyte, and gap junctions may exist between adjacent astrocytes. Finally, we assume that the only difference between astrocytes in vivo and in vitro is in their morphology.
THE MATHEMATICAL MODEL FOR WAVES
An overview of the spatial model
In the previous study,67 a model of ligand-induced intracellular Ca2+ oscillations was developed and analyzed to understand the bifurcation structure of the different types of oscillations, particularly sinusoidal oscillations and baseline spiking, in terms of the role of PKC and PLC in determining the intracellular level of IP3. Our objective here is to extend this to allow different modes of cell-cell communication, either directly via gap junctions or indirectly via the extracellular space. The goal is to develop a PDE model in order to understand wave propagation through an astrocytic network and ATP wave propagation in the extracellular space. As previously mentioned, substantial differences in waves were reported among different subtypes of astrocytes that result from the diversity of interactions with surrounding cells.45 The differences are even larger between astrocytes in vitro (cultured) and in vivo.45
To determine how the different configurations of astrocyte network geometries influence wave propagation, the PDE model must be solved numerically in both realistic (in vivo) and simplified (in vitro) geometries for astrocyte networks, assuming only morphological differences between cultures and intact astrocytes (Fig. 2). In both cases, simulations under all possible combinations of direct coupling using an intracellular messenger (IP3) and indirect communication using an extracellular messenger (ATP) were done to study the properties of waves. The cases considered are (1) direct coupling and no extracellular signal, (2) nonregenerative extracellular signal and no direct coupling, (3) regenerative extracellular signal and no direct coupling, (4) direct coupling and nonregenerative extracellular signal, and (5) regenerative extracellular signal and direct coupling [Fig. 1c].
Figure 2.
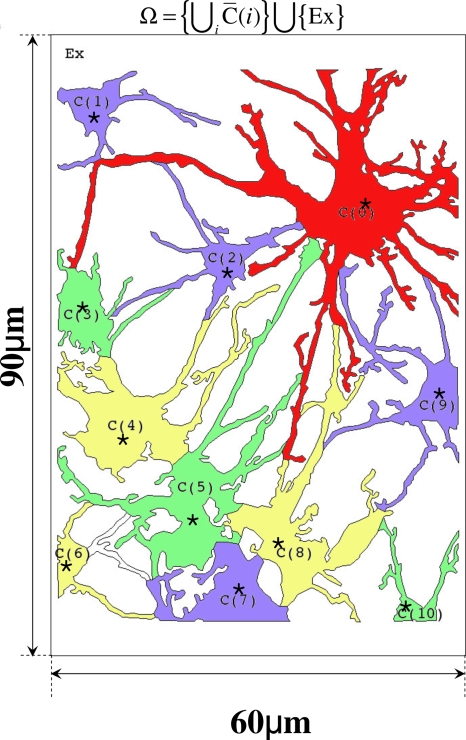
The astrocytic network geometry used in the in vivo model. Here and in the simplified model, C(0) is the cell stimulated by glutamate. The extracellular space is defined as , where is the extracellular domain above C(i) and Ex is the cell free space.
Simplification of the intracellular temporal model
It can be shown that the full temporal model studied earlier67 can be reduced to interactions between the key component in each of the modules, namely, IP3, IP3R, Ca2+, and PKC. Formally, this can be done by introducing a time scale , converting the ordinary differential equation (ODE) system into nondenominational form to identify fast and slow steps as measured by the sizes of nondimensional groups and applying the pseudosteady state hypothesis to reduce some ODEs to algebraic equations.47, 66 By simplifying the resulting algebraic-differential equations we obtain a system that captures the slow dynamics to leading order in the small parameter. This leads to the following four-dimensional system:
| (1) |
where P, K, R, and C represent the concentrations of IP3, Ca2+⋅PKC complex, Ca2+⋅Ca2+⋅IP3⋅IP3R, and . The parameters that appear in Eq. 1 are given in Table 1. Notice that C couples the first two equations to the last two, whereas P couples the last two to the first two. The reader can interpret the equations in terms of the interactions between the modules. For example, K, which arises from the feedback, affects the output via the first term in the first equation. The inhibitory feedback pathway of PKC dynamics is well documented in various studies.8, 30, 31, 29, 112, 6, 28, 46, 23, 80 Further discussion of the physical interpretation of Eq. 1 can be found in Appendix A and Ref. 66.
Table 1.
Parameters and their meaning. In the simplified geometry (in vitro model), kperm P=1 were used ( *). For IP3 mediated wave (without ATP binding kinetics), kin=0 was chosen ( †), while kperm P=0 was used to study ATP mediated wave without IP3 diffusion ( ‡).
| Parameter | Unit | Meaning | Value |
|---|---|---|---|
| DA | μm2 sec−1 | Diffusion coefficient of ATP in extracellular space | 330 |
| DP | μm2 sec−1 | Diffusion coefficient of IP3 | 300 |
| DK | μm2 sec−1 | Diffusion coefficient of Ca2+⋅PKC | 30 |
| DC | μm2 sec−1 | Effective diffusion coefficient of | 30 |
| k1 | sec−1 | Ca2+ dependence of IP3 production | 4.7994 |
| k2 | μM−1 | PKC dependence of IP3 production | 0.0943 |
| k3 | sec−1 | IP3 degradation rate | 2.5000 |
| k4 | μM−1 sec−1 | Rate constant for PKC and Ca2+ binding | 0.6000 |
| k5 | sec−1 | K degradation rate | 0.5000 |
| k6 | μM−2 sec−1 | Binding rate constant for IP3, 2Ca2+ | 139.09 |
| k7 | μM−1 | Affinity constants for IP3, IP3R | 8.5000 |
| k8 | μM−1 | Affinity constants for Ca2+, | 9.0909 |
| k9 | sec−1 | degradation rate | 0.2100 |
| k10 | sec−1 | Basal Ca2+ release rate from ER | 0.0185 |
| μM−2 | Affinity constant for C, P, and free IP3R | ⋅ | |
| μM−1 sec−1 | Ca2+ release rate from IP3R Ca2+ channels | ⋅ | |
| k11 | μM−3 | 15841 | |
| k12 | μM sec−1 | The maximal Ca2+ pumping rate | 7.5000 |
| kc | μM | Volume averaged Ca2+ concentration | 7.0000 |
| kp2 | μM | Ca2+ sensitivity of the SERCA pump | 0.1300 |
| KT | μM | Total K concentration | 1.0000 |
| RT | μM | Total R concentration | 0.8000 |
| AT | μM | ATP concentration in the cytosol | 5000 |
| kin | sec−1 | Rate of ATP induced IP3 production | 30∕0† |
| k−ATP | sec−1 | ATP decay rate in extracellular space | 1 |
| Bmax | μM | The total concentration of P2YR | 0.1 |
| Kd | μM | Dissociation constant of ATP and P2YR | 10 |
| kATP | sec−1 | Maximal ATP release rate | 0.184 |
| γ | μm−1 | Extracellular volume dependent parameter | 1.087 |
| ρ | μM | IP3 dependency parameter in ATP release | 10 |
| kperm P | μm∕s | Gap junctional permeability for IP3 | 2∕1*∕0‡ |
| L | μm | The height of extracellular space | 0.9 |
Geometry of the astrocyte networks
We later derive equations for waves in cultured astrocytes and for in vivo networks and here describe the geometry we use. Cultured astrocytes are often confluent, and to understand waves in this context we consider a finite line of cells wherein each is coupled to its nearest neighbors via gap junctions, as shown in Fig. 1b. This line of cells is covered by a thin extracellular space that extends above and to the end of the line, in which ATP can diffuse, and we homogenize this system in the vertical direction so as to reduce it to 2D system of coupled squares of 15×15 μm2 with fluid layer above it. We solve the equations both within the squares and in the exterior fluid layer using equations and boundary conditions given later. Similar simplified model geometry was also studied extensively in Refs. 11, 101, 102, 12.
The geometry of a realistic in vivo astrocyte network is very complicated, as shown in Fig. 2, and some simplifications are necessary. From the morphological point of view, astrocytes in vivo have well developed processes in both number and size covering most of the dendrites, axons, and synapses, as well as the larger soma.1, 21, 85 To capture these characteristics, a realistic astrocyte network in 60×90 μm2 rectangular domain was considered which was modified from original confocal immunofluorescence images of the vitreal surface of the rat retina.81 The locations at which Ci, Pi, and A in C(i) were measured were marked as ⋆ [Fig. 2b], where A represents extracellular ATP concentration. The distance between measuring point from C(0) in descending order are C(2), C(9), C(1), C(3), C(4), C(8), C(5), C(10), C(7), and C(6) with distances 20.3, 29.8, 40.4, 42.4, 46.4, 49.3, 50.3, 58.2, 59.3, and 64.76 (μm). This system is also treated as having been homogenized the vertical direction, and equations given later are solved in the domain defined by the cells and that defined by the extracellular space. Here there is an additional difficulty, in that to be entirely faithful to the in vivo geometry we should treat the system as a two phase system (cell and fluid) as above, but here the fraction of the phases varies from point to point. This extension significantly complicates the problem computationally, and this will be pursued elsewhere.
Several lines of evidence indicate that gap junctional hemichannels play an important role by providing a direct path to second messengers such as IP3.15, 49, 72, 105, 114 A portion of mobilized also diffuses through the gap junctional hemichannels. However, due to various of Ca2+ buffer proteins in the cytosol, the amount is negligible and we ignore diffusion of between cells.110, 9 We assume that astrocytic gap junctions are located at the end of astrocytic processes as well as on the part of the boundary where astrocyte bodies.79 Because it was hard to distinguish different cells from the image, the cell boundaries other than processes in the model were simply assigned. It was also assumed that the ATP receptors are present over the entire cell surface, although some studies indicate that a localization of ATP receptors either in an astrocyte cell body or processes could be specific to the astrocyte subtype.48, 90
The governing equations for the spatial model
Let C(0) be a cell stimulated by glutamate (parametrized by k1) and C(i), i≠0 be the surrounding cells (Fig. 2), and let Xi denote a quantity X in the ith cell. In the subdomain C(0) (Fig. 2), for t>0,
| (2) |
where R0 is assumed to be immobile (DR=0). The initial conditions on C(0)×{t=0} are given by
| (3) |
Wherever cell C(0) meets other cells we impose the boundary conditions
| (4) |
while all other boundaries are impermeable to all species. When we consider extracellular messenger-mediated waves without IP3 diffusion between cells, we simply set kperm P=0.
Since ATP affects Ca2+ wave propagation via binding to P2YR, the equation for IP3 involves an input that depends on ATP binding kinetics (Bmax=0.1 μM, Kd=10 μM) as well as on (Ci) and PKC (Ki). A similar term is absent from the glutamate-stimulated cell because we neglect the ATP stimulation relative to that by glutamate. Therefore,
| (5) |
with initial conditions and boundary conditions. Notice that the glutamate-dependent source term in P0(k1) is replaced by the ATP-dependent term kinBmaxA∕(Kd+A) in Pi for i≠0. When waves are mediated only by the intracellular messenger (IP3), we set kin=0 in C(i) for i≠0.
On the other hand, ATP kinetics are defined domainwise in the extracellular space, with the boundary ∂Ω (Fig. 2), where is a domain right above C(i) in extracellular space and Ex is cell-free extracellular space. Notice that ∂Ω consists of four sides of the rectangular Ω in the realistic geometry [Fig. 2b, while ∂Ω is found at both sides of the simplified geometry [Fig. 2a]. In , with an extracellular volume dependent parameter γ, the equation of ATP kinetics (see Appendix B for more details) is
| (6) |
where AT is the intracellular concentration of ATP with the initial condition
The function ϕ describes ATP release kinetics either by hemichannels or by vesicular release. Because ϕ is unknown, we choose ϕ(Pi)=Pi∕(ρ+Pi) for some constant ρ (Table 1). It should be noted that a recent study118 suggested that ϕ is a bell-shaped function of , but we assumed that ATP release is triggered by IP3.20, 22
On the other hand, for nonregenerative ATP release [Fig. 1(C2), (C4)], there is no source term in any but the glutamate-stimulated cell, and thus kATP=0 in Eq. 6 for , i≠0. This applies in the cell-free region as well. In all cases, we use the boundary condition on ∂Ω as D(∂A∕∂n)=−A to reflect leakage of ATP to the surroundings [Fig. 2c].
For the numerical computations, Eqs. 2, 3, 4, 5, 6 in the domains in Fig. 2 were solved by the finite element method implemented by FEMLAB® with a choice of linear Lagrangian interpolation for the shape functions. The system of PDEs described on geometries defined in Fig. 2 was incorporated into FEMLAB and UMFPACK (Ref. 33) was chosen to solve the resulting nonsymmetric, sparse linear systems.
RESULTS AND DISCUSSION
Ca2+ wave propagation and evolution of response patterns in a network of cells
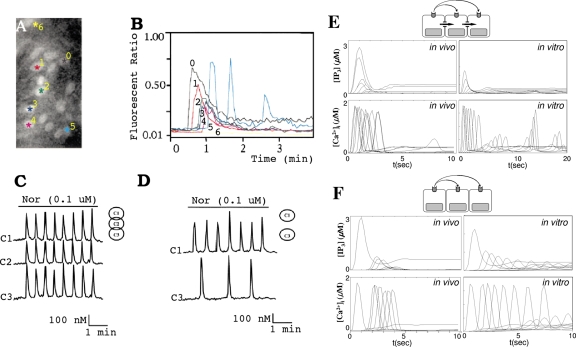
In a previous study it was demonstrated that the different response types are determined by [IP3], which, in turn, is controlled by the activities of PLC and PKC.67 The effect of [IP3] on the complexity of calcium oscillations in a single cell was also addressed by Pittà et al.88 in a simpler framework. Since IP3 levels are different from one cell to another in an astrocytic network, we may expect different types of response in different cells. Indeed Venance et al.113 reported an evolution of response patterns in rat astrocytes [Figs. 3a, 3b]. In Figs. 3a, 3b, cells 1–4 show a gradual change from a “transient with plateau-type” response pattern (cell 0) to a “transient without plateau-type” response pattern. Interestingly, the beginnings of a baseline spiking-type response pattern were observed in the fifth cell, whereas the sixth showed no response.
Figure 3.
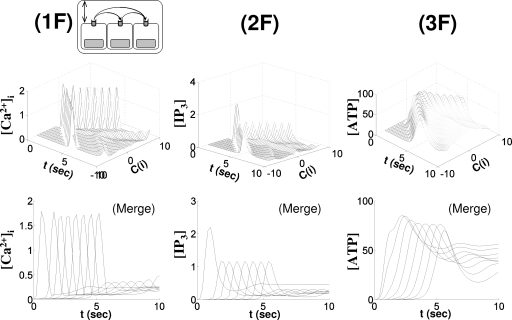
Evolution of the response types in cells. [(a) and (b)] Distribution and pattern of responses to focal application of receptor agonist in rat astrocytes in a 200×100 μm2 rectangle (Ref. 113). [(c) and (d)] Noradrenaline-induced oscillation in rat hepatocytes when three cells are connected and when the intermediate cell was excised (Ref. 111). [(e) and (f)] Simulation results from simplified geometry (in vitro) and realistic geometry (in vivo) corresponding to (a), (b) and (c), (d). The traces from left to right in (e) (in vivo) correspond to C(i), i=0, 1, 3, 2, 9, 4, 8, 5, 7, 6, and 10 in descending order. The traces from left to right in (f) (in vivo) correspond to C(i), i=0, 2, 9, 1, 5, 8, and 4 with subthreshold responses in C(3) and C(10). The traces from top to bottom in (e) [IP3] correspond to C(i), i=1, 0, 2, 3, 9, 4, 8, 5, 7, 6, and 10 in descending order. Also, in (f), [IP3], the traces from top to bottom correspond to in C(i), i=0, 2, 9, 1, 5, 8, 4, 6, and 7 in descending order and [IP3]≃0 in C(3) and C(10). In (e) (in vitro) and (f) (in vitro), the traces from top to bottom are C(i),i=0,±1,±2,…,±7.
Another interesting observation was made by Tordjmann et al.,111 who found that noradrenaline-induced oscillation patterns in three interconnected rat hepatocytes were similar, whereas different patterns were observed in the first and third cells when those cells were physically separated by excising the intermediate second cell, thereby presumably removing direct coupling through gap junctions. Also, longer delays in the initial transients were observed in the latter case [Figs. 3c, 3d]. (Here one must be careful when dynamics in hepatocytes and astrocytes are compared because waves in hepatocytes are predominantly carried by IP3 through intercellular gap junctions111 while both intracellular IP3 and extracellular messengers are believed equally importantly in waves in astrocytes.53, 25, 27, 26, 54, 44
To determine whether the model can reproduce the results in Figs. 3a, 3b, 3c, 3d, Eqs. 2, 3, 4, 5, 6 were solved numerically on the geometries (Fig. 2) under the assumption of either only an intracellular messenger and nonregenerative extracellular messenger or only nonregenerative extracellular messenger [Fig. 1(C4), (C2)]. A sustained glutamate input (k1) was used as a stimulus in both simplified and realistic geometries. For quantification, the values of and [IP3] in the center of cell (⋆ in Fig. 2) were calibrated in the realistic geometry, while the average values of and IP3 in cells were computed for the simplified geometry.
Figure 3ein vivo indicates that as the [IP3] level decreases from the stimulated cell [C(0)] to remote cells, the response patterns evolve from transient with plateau at C(0) to oscillations at C(9) and to baseline spiking at C(10), as reported in Ref. 67. Results for the model of an in vitro astrocytic network are similar, except that the amplitude of [IP3] is about half of that of in vivo model, because there are more gap junctions present in the in vivo model than in the in vitro model (Fig. 2).
Figures 3c, 3d can be understood in the same context in terms of [IP3]. In the interconnected rat hepatocytes [Fig. 3c], there was almost no transition in response patterns from one cell to another, implying that the [IP3] levels in each cell are in a similar range or at least over some effective dosage (∼0.1 μM) while quite different [IP3] levels are expected when the intermediate cell is excised and IP3 diffusion is absent. The decrease level of [IP3] available in the third cell implies the transition from high to low frequency baseline spiking.67 The longer delay in the initial spikes in Fig. 3d was also reproducible in both in vivo and in vitro model when gap junctional permeability (kperm P) was set to zero, although the delay was more evident in the latter model [Figs. 3e, 3f].
Gap junction mediated versus P2YR mediated waves
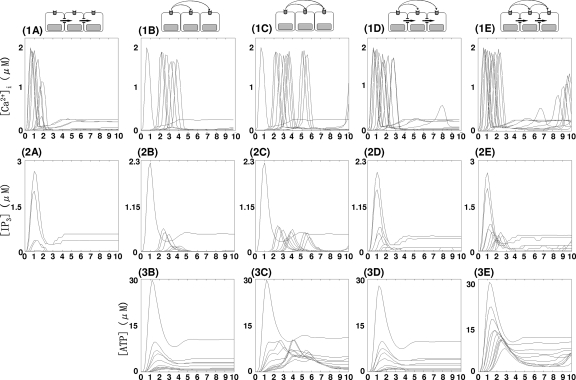
In the absence of extracellular signaling, the distance that an initial calcium spike spreads depends on IP3 diffusion. The threshold value of IP3 for initiating a spike is ∼0.1 μM (Figs. 456, Table 2) and [IP3] decreases rapidly from one cell to the next as IP3 diffuses through astrocytic gap junctions [Figs. 4(2A), 5(2A), and 6(2A), Table 2]. As a result, the amplitudes of spikes along the spreading wave decrease rapidly and the wave dies within ∼58.2 μm from C(0) [C(6), C(7), and C(10) in Fig. 4(1A), Table 2] in the realistic geometry, whereas it vanishes in the fourth cell in the simplified geometry [Fig. 6(1A)]. Also, the velocity of waves decreases as shown in Figs. 5(1A) and 6(1A) and Table 2.
Figure 4.
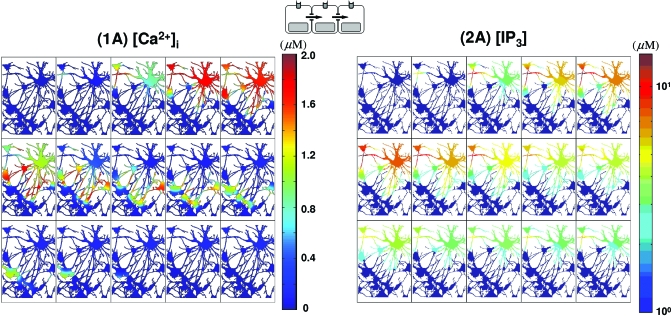
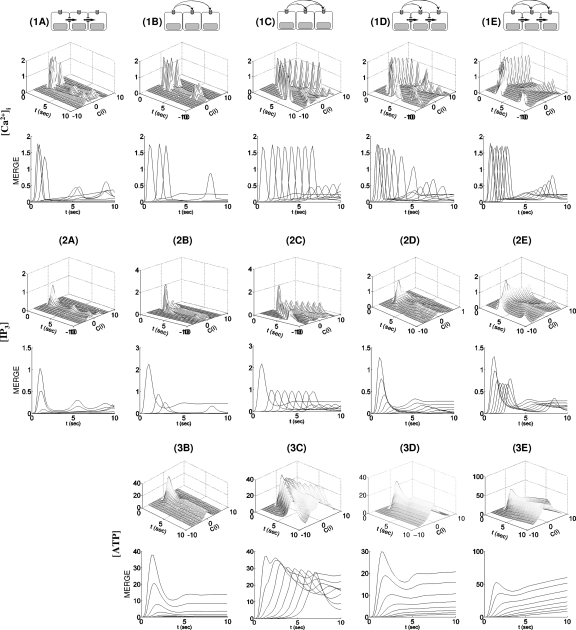
Intracellular-messenger-mediated waves in a realistic network. (1A) waves by a glutamate stimulus in C(0) and mediated only by IP3 diffusion are shown on a linear scale. (2A) The corresponding IP3 waves plotted on a log scale. The temporal sequence of images in (1A) and (2A) runs from left to right and top to bottom. The elapsed time between images in (1A) and (2A) is Δt=0.2 s with a total time of 3 s (t=0,0.2,0.4,…,2.8,3).
Figure 5.
A composite summary of the waves in the realistic cell network. , [IP3], and [ATP] in C(i) were measured at the locations marked as ⋆ in Fig. 2. The locations of the measuring point in descending order are C(2), C(9), C(1), C(3), C(4), C(8), C(5), C(10), C(7), and C(6) with distances from C(0) of 20.3, 29.8, 40.4, 42.4, 46.4, 49.3, 50.3, 58.2, 59.3, and 64.76 (μm). In (1A), the traces whose peaks are above 0.5 μM correspond to C(0), C(1), C(3), C(2), C(9), C(8), and C(4) from left to right, while the traces whose peaks are below 0.5 μM correspond to C(5) and C(7) from top to bottom. In (2A), the traces from top to bottom represent [IP3] in C(1), C(0), C(3), C(2), and C(10). In (1B), (1C), (2B), and (2C), the traces are of C(0), C(2), C(9), C(1), C(5), C(8), C(4), C(3), C(7), C(6), and C(10), while in distal order of C(0)-C(2)-C(9)-C(1)-C(5)-C(8)-C(4)-C(3)-C(7)-C(6)-C(10) from left top to bottom right traces in (3B) and (3C). In (1D) and (1E), the traces from left to right are from C(0), C(1), C(3), C(2), C(9), C(4), C(8), C(5), C(7), C(6) and C(10), whereas C(1)-C(0)-C(2)-C(3)-C(9)-C(4)-C(8)-C(7)-C(10) in (2D) and C(1)-C(0)-C(3)-C(2)-C(9)-C(4)-C(5)-C(8)-C(7)-C(6)-C(10) in (2E) from left top to bottom right. Finally, from left top to bottom right, C(0)-C(2)-C(1)-C(9)-C(3)-C(4)-C(8)-C(10)-C(7)-C(6) in (3D) and C(0)-C(1)-C(2)-C(3)-C(9)-C(4)-C(5)-C(8)-C(7)-C(6)-C(10) in (3E).
Figure 6.
waves in the simplified cell geometry. In the upper panels for , [IP3], and [ATP], the x- and y-axes represent time in seconds and the location of cells C(i) for i=1,…,10, respectively. On the z-axis is shown the average of the quantity over the cell (i.e., the integral of the quantity divided by the cell volume) plotted in increments of t=0.2. The lower panels (MERGE) showed the projected traces from the upper panels onto the y=0 plane.
Table 2.
The characteristics of waves mediated by IP3 and∕or ATP in vivo model.
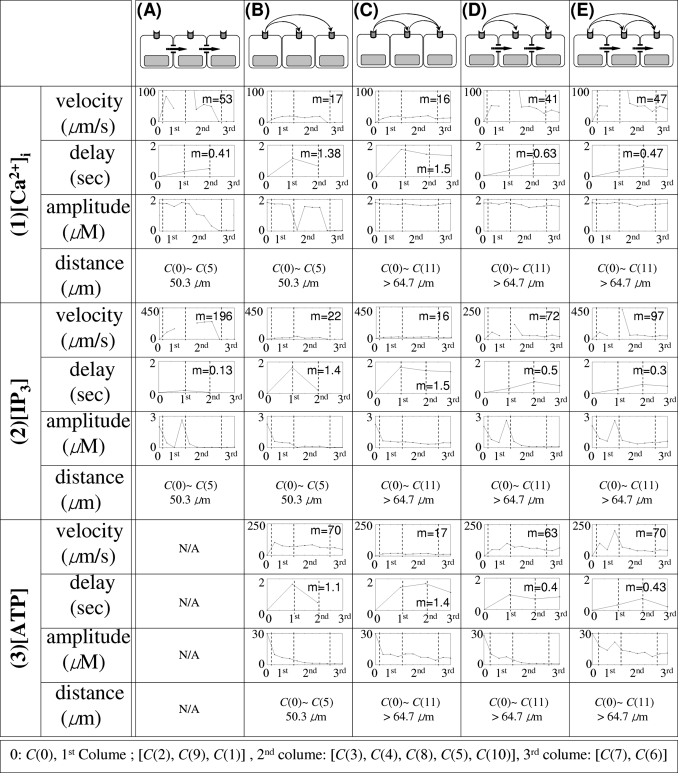 | |
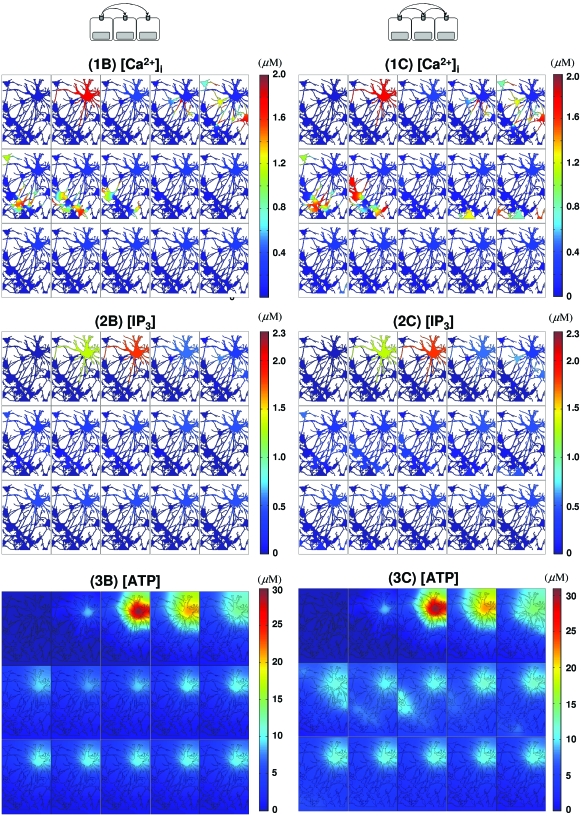
On the other hand, when waves are mediated by nonregenerative ATP diffusion, [IP3] decreases more slowly than when the waves are mediated by IP3 diffusion through gap junctions [Figs. 5(2B), 6(2B), and 7(2B), Table 2]. Although the waves died after a few cells [Figs. 5(1B) and 6(1B), Table 2] similar to the case of gap junction mediated waves, the amplitudes of initial spikes and the propagation velocity along wave remained constant [Figs. 5(1B) and 6(1B), Table 2].
Figure 7.
Extracellular messenger mediated waves. waves initiated by a glutamate stimulus in C(0) and mediated only by ATP are shown. In (1B)–(3B), excitation is mediated by nonregenerative ATP spread, while regenerative ATP release is present in (1C)–(3C). The images in each panel [(1B)–(3B) and (1C)–(3C)] run from left to right and top to bottom. In each panel, the time elapsed between images is Δt=0.6 s with total time of 8.4 s (t=0,0.6,1.2,…,7.8,8.4).
The effective dosage of ATP to trigger spike in the model was 3 μM [Figs. 5(3B) and 6(3B), Table 2], which is similar to the value reported in the literature.82, 64 The velocity as measured by the spread of the peak of nonregenerative ATP wave spread was ∼50 μm∕s in the realistic geometry [Fig. 5(3B), Table 2] and ∼40 μm∕s in the simplified geometry [4 cells in 1.5 s, Fig. 6(3B)], which is similar to reported values (41 μm∕s; Ref. 82).
Another noticeable feature of waves by mediated by IP3 diffusion is that there is very little delay in propagation between cells [Figs. 5(1A) and 6(1A), Table 2], while waves by ATP signal show a longer delay time for propagation from one cell to the next [Figs. 5(1B) and 6(1B), Table 2]. Although the diffusion coefficient of ATP is larger than that of IP3 (Table 1), the results in Figs. 5(1A), 5(2A), 6(1A), and 6(2A) illustrate the higher velocity of gap junction mediated waves compared to P2YR mediated waves. This difference stems from the fact that IP3 is the critical species for initiating release, and when it spreads via gap junction there is little delay in initiating release, whereas additional time for IP3 production is required for ATP signaling by P2YR. Because of this delay, P2YR mediated waves are expected to be slower than gap junction mediated waves, and this is borne out by the measured speeds: ∼15 μm∕s [Figs. 5(1B) and 6(1B), Table 2] versus 40 μm∕s [Figs. 5(1A) and 6(1A), Table 2] in both simplified and realistic geometries. These also compare favorably to the experimentally observed ranges (15–27 μm2∕s; Refs. 15, 18, 82, 113, 120). It has also been reported that in wave propagation from Muller cells into astrocytes, there was a considerable delay, 2.6±0.2 s, while there was a shorter delay (0.85 s) in the case of wave spread from astrocyte processes to an adjacent Muller cell endfoot.82 Other studies demonstrated a longer delay (5–10 s) in waves between different layers of cells in a hippocampal slice.57 In the model, the delay times range between 0.4 and 1.6 s [Figs. 5(1B) and 6(1B), Table 2].
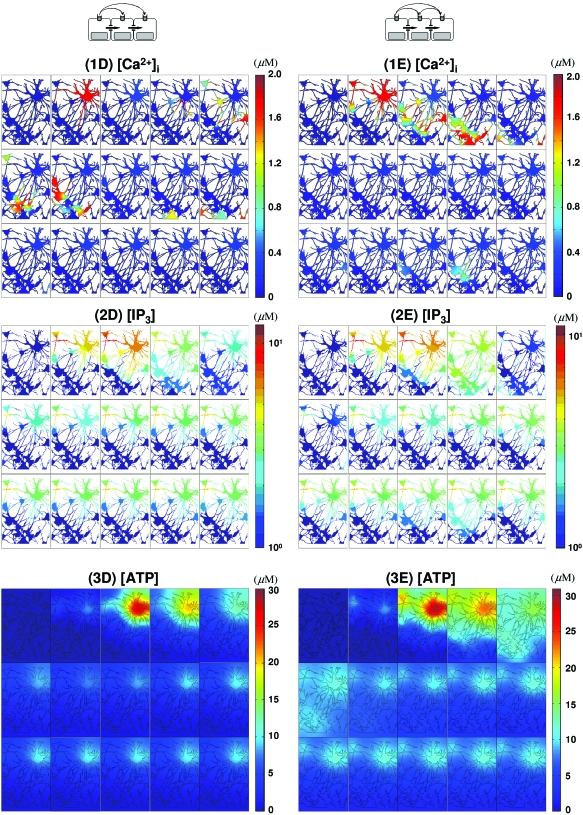
Another difference between P2YR and gap junction mediated waves in vivo is reflected in the local IP3 concentration. Local [IP3] can be larger than 10 μM [Figs. 4(2A), 8(2D), and 8(2E)] for the in vivo gap junction mediated waves due to more “focused” diffusion along fine astrocytic processes. In contrast, the maximum [IP3] remains less than 3 μM for P2YR mediated waves [Figs. 7(2B) and 7(2C)]. On the other hand, in the simplified model which has more open connectivity in gap junctions, IP3 concentrations are less than 3 μM in all cases, which is probably due to large gap junctional boundaries between cells (Fig. 6). A more detailed discussion on gap junctional connectivity and wave patterns can be found in Ref. 36.
Figure 8.
Extracellular volume and wave by regenerative extracellular messenger
The effects of regenerative ATP signaling
Another contentious issue in signaling is whether or not ATP signaling is regenerative.37 To see how the regenerative of ATP release affects the waves, Eqs. 2, 3, 4, 5, 6 were solved either for kATP=0 or 0.184∕s. The velocities of initial spikes under the two scenarios were not distinguishable, even though the distance of wave spread was larger for regenerative ATP [(1B) and (1C) in Figs. 567, Table 2]. Other differences can be seen from the ATP and IP3 profiles. When ATP release is assumed to be nonregenerative, peaks of [ATP] transients quickly decrease due to enzymatic degradation, whereas the summation of regenerative ATP release from each cell and diffusive spread from neighboring cells gives rise to steady amplitude of [ATP] transients in the regenerative case [Figs. 5(3B), 5(3C), 6(3B), and 6(3C)]. Although the waves travel further in the regenerative case, the ATP wave speed in both cases was ∼40–50 μm∕s [measured by the initial spikes in Figs. 5(3B), 5(3C), 6(3B), and 6(3C)].
Similar to what is observed for ATP wave propagation, in both cases the and IP3 wave velocities are similar at ∼40 μm∕s [(1B), (1C), (2B), and (2C) in Figs. 56]. The peak IP3 amplitude of the stimulated cell is unchanged at 2.3 μM but the peak IP3 amplitudes of other cells diminish as a function of distance for nonregenerative ATP signaling, while they remain constant from the second cell on in the regenerative case due to ATP-stimulated IP3 downstream of the stimulated cell [Figs. 5(2B), 5(2C), 6(2B), and 6(2C)]. Also, a slight decrease in the amplitude of transients was observed in the nonregenerative ATP mediated wave, but the amplitude of transients remains constant in the regenerative ATP mediated waves [Figs. 5(1B), 5(1C), 6(1B), and 6(1C)].
Given the variety of calcium responses including oscillations that exist in a single cell,67 it is worthwhile to understand why there is no sustained wave propagation in a network. To this end we tested the linear stability of the steady state solution of Eq. 1 assuming zero gap-junctional permeability, which is equivalent to assuming that waves are mediated solely by ATP. The analysis of Eq. 1 shows that below the effective ATP dosage for response, all the real parts of the eigenvalues of Eq. 1 linearized about the steady state solution are negative, which prevents initiation of the response. Therefore, without regenerative ATP release the level of ATP that reaches the neighboring cells eventually drops below the effective ATP dosage as a result of diffusive spreading and enzymatic degradation (see Appendix C for details), and sustained propagation of waves is precluded. Furthermore, we have not found conditions that lead to propagation even if regenerative release is included.
Synergy of intra- and extracellular messengers
Thus far we have investigated the properties of response patterns initiated by either IP3 or ATP alone. Now we consider cases when both intra- and nonregenerative extracellular messengers carry release signals. Because we assume nonregenerative ATP signaling, the intracellular dynamics other than in C(0) do not influence ATP evolution [Figs. 7(3B) and 8(3D)].
Recall that the time scale of direct IP3 diffusion is faster than IP3 generation via ATP signaling [Figs. 5(2A), 5(2B), 6(2A), and 6(2B)]. When both IP3 and ATP are used for mobilization, IP3 diffusion dominates wave initiation as far as the IP3 concentration is above the effective threshold (∼0.1 μM). Beyond that distance the generation of IP3 via the ATP pathway serves to elevate IP3 above the threshold. Therefore, in short range wave propagation by pure IP3 diffusion is dominant, while wave propagation by ATP signaling is dominant in cells distant from the stimulation point [(1A), (1B), and (1D) in Figs. 56]. As the result, longer delay in wave propagation is observed for remote cell locations, in contrast with rapid continuous wave propagation near the stimulated cell [Figs. 5(1D) and 6(1D)].
Another consequence of synergy between intra- and extracellular messengers is the propagation distance. While either an ATP signal without IP3 diffusion or IP3 diffusion without an ATP signal results in decaying waves, the synergistic activity of the two pathways gives rise to permanent waves that propagate through the entire computational domain, as shown in Figs. 479. More quantitatively, (1A) and (2A) in Figs. 56 indicate that no responses were found in the distant cells [C(i), i=6, 7, 10 in the realistic geometry and i≥4 in the simplified geometry] when either intra- or extracellular messenger was applied independently. However, when both intra- and extracellular messengers are present, transients are observed in all cells [Figs. 5(1D) and 6(1D), Table 2]. This indicates that when both IP3 and ATP are used for mobilization, the fast IP3 diffusion can either mobilize or sensitize cells by eliciting a subthreshold responses. After additional IP3 is received via the slower process of IP3 production by ATP, the IP3 level in sensitized cells reaches the threshold level (∼0.1 μM) and transients are induced. As shown in (1B) and (2D) in Figs. 56, a slight decrease in the amplitude of transients was observed in the nonregenerative ATP mediated wave, while a constant amplitude of transients was observed in the regenerative ATP-mediated waves [Figs. 5(1C) and 6(1C)]. A similar observation can be made concerning ATP-mediated waves even with IP3 diffusion through gap junctions. Moreover, regenerative ATP-mediated waves propagate like true traveling wave patterns, retaining the initial spike profile along the wave [Figs. 5(1C), 5(1E), 6(1C), and 6(1E)].
Figure 9.
The synergy between intra- and extracellular messengers in waves. waves initiated by glutamate in C(0) and mediated by both ATP and IP3 are shown. In (1D)–(3D) are shown waves mediated by nonregenerative ATP spread, while regenerative ATP release is present in (1E)–(3E). The color maps for and ATP are on a linear scale [(1D), (3D), (1E), and (3E)], while IP3 [(2D) and (2E)] is scaled logarithmically. The images in each panel [(1D)–(3D), (1E)–(3E)] are read from left to right and top to bottom and the time elapsed between images in each panel is Δt=0.6 s with total time of 8.4 s (t=0,0.6,1.2,…,7.8,8.4).
Another interesting result is that when IP3 diffusion is included in the realistic geometry, a second wave was initiated from C(4) to C(9) which died out beyond C(9) [Figs. 5(1E) and 8(1E)]. In this case, C(2) shows a transient with plateau type of response pattern similar to that C(0) and C(1), while the cells C(4)⋯C(9) show an oscillatory response pattern. Apparently the signal transferred from C(9) to its downstream neighbors was not strong enough to elevate IP3 above threshold for wave initiation, but even so, the signal from C(9) integrated the signal relayed from C(4) and could, in living cells, influence the future response of these downstream cells [Figs. 5(1E) and 8(1E)].
In both the simplified and realistic geometries, the ATP wave propagation pattern indicates that for regenerative ATP without IP3 diffusion, the peak amplitude of ATP was lower than for regenerative ATP with IP3 diffusion [Figs. 5(3C), 5(3E), 6(3C), and 6(3E)]. Also, the maximum point of ATP spread from C(0) can be easily identified in Figs. 5(3C) and 6(3C), while when there is regenerative ATP with IP3 diffusion, the extracellular effect of ATP released from a cell cannot be clearly identified [Figs. 5(3E) and 6(3E)]. Also, accumulation of ATP was observed in the simplified geometry due to the ATP released into the restricted extracellular space, something that is not observed in the sparsely distributed cells in the realistic geometry [Figs. 5(3E) and 6(3E)]. This may also explain why extracellular ATP is believed to be a major contributor to waves in cultured astrocytes.54, 58, 11, 53, 63
The role of the extracellular volume
In reality the extracellular space in the brain provides a tortuous path for molecular diffusion, and it is important to understand how the extracellular volume influences ATP signaling. For this purpose, the volume of the extracellular space was modified via an extracellular volume dependent parameter γ=1∕L, where L is the thickness of the extracellular space (Appendix B). Here γ was chosen as 1.087 μm−1, but because the ATP concentration in a cell is high (30 μM), any change in L could result in a large change in ATP dynamics. To take this factor into account, we redid some computations in the case of regenerative ATP signaling using , which in effect doubles the source term γkATPϕ(P)(AT−A) in Eq. 6.
In comparison with Fig. 6(3C), the peak amplitude of ATP was doubled (from 38 to 85 μM), and the propagation of ATP waves showed similar traveling wavelike patterns (Fig. 8). However, the average ATP and IP3 wave velocity increased from 24.49 [Fig. 6(3C)] to 34.29 μm∕s [Fig. 8(3F)]. The IP3 profiles were not distinguishable between the two cases having similar amplitudes [Fig. 6(3C) and Fig. 8(3F)]. Similarly, wave velocity increased from 24.00 to 26.09 μm∕s with constant amplitudes and the delay between cells was shortened [Fig. 6(3C) and Fig. 8(3F)]. Although the extracellular volume strongly influences the ATP dynamics, the effects on intracellular amplitudes of and IP3 were minimal [Figs. 6(1C), 6(2C), 8(1F), and 8(2F)]. Thus the primary effect of the higher ATP signal is to speed up wave initiation and propagation.
In light of the nonuniform and complex spatial distribution of cells and extracellular space in the brain, these results imply that wave patterns can be very complex with a wide range of speeds and response times. Especially, when regenerative ATP is involved, the local maximal ATP that defines local wave velocity and delay time between cells was proportional to γ, and amplification and dilution of the strength of extracellular signaling could be accomplished by modifying the extracellular geometry. There is evidence that astrocytes have the ability to control the extracellular volume by gating VRACs and swelling in K+ ion concentration and ATP dependent manner, even though the underlying mechanism has not been fully understood.106, 87, 75, 71, 77 Therefore, ATP may have more complex, indirect, self-regulatory roles in diffusion by modulating volume-sensitive anion channels, which in turn affects ATP diffusion.
CONCLUSIONS
It is widely believed that both direct coupling via the intracellular messenger IP3 and indirect coupling via the extracellular messenger ATP are involved in cell-cell signaling in astrocyte networks, but the relative importance of each mode has not been established in general. The model developed herein, which utilizes a detailed model of signal transduction and intracellular calcium dynamics for single cells developed earlier,67 allows for both modes of transport in both simplified and realistic network topologies. Simulations of the model in simplified and realistic geometries demonstrated that waves induced by individual messengers have distinct characteristics of propagation speed, propagation distance, delay between cells, and transient profiles. It was also found that synergistic effects of intracellular IP3 and extracellular ATP on waves can be very complex, but the model developed here can be used to explore these effects.
While the IP3-mediated waves propagate rapidly with at most a short delay between cells, they only propagate for a few cells and the corresponding amplitude of transients decreases significantly from cell to cell. Similar effects are observed for ATP-mediated waves, but the delay time in waves between cells is much longer for reasons adduced earlier, which leads to slower wave propagation and slower decay of transients.
waves mediated by both IP3 and ATP display a mix of all the characteristics of the separate cases. While there is little or no delay in the wave close to stimulated cell, longer delays were observed in the remote cells. Overall decay of wave front transients was similar to that of ATP mediated wave, and wave propagation reached all the cells in the domain of consideration.
When regenerative ATP release was considered, the waves display a more permanent form and propagate at a constant speed, regardless of whether or not IP3 served as a messenger. However, the wave speed was much larger when both IP3 and regenerative ATP were involved [Fig. 7(1C), (1E)]. The characteristics of waves in this case are summarized in Table 2. One clear conclusion is that regenerative release of ATP can lead to long distance propagation in networks.
While the qualitative behaviors of responses are independent of the geometries considered, IP3 kinetics strongly depend on the geometries especially on the gap junctional connectivity among the astrocytes. When higher gap junctional connectivity was established through an astrocytic network, IP3 easily diffuses out to neighboring cells, thereby controlling the [IP3] in cells. In contrast, the lower gap junctional connectivity observed in the realistic geometry of an astrocyte network leads to local [IP3] greater than 10 μM.
Regenerative ATP-driven waves also show geometry dependence. When cell density in the astrocytic network is high as in the case of the simplified geometry, regenerative ATP along can exceed the ATP decay rate and lead to local elevated concentrations for the parameters chosen. However, in the realistic geometry where there is a large area of cell free domains, the ATP released decays rapidly and the concentration remains close to the steady state level. Of course no geometry dependency in observed ATP is non-regenerative because the only ATP release is from the stimulated cell.
Experimentally observed waves in astrocyte networks exhibit decaying speeds (from the site of initiation) in the range of 200 μm,15, 113 a maximal propagation range of 200–350 μm in radius, and a maximal speed of 15–27 μm∕s.15, 113, 120, 82, 18 Our results replicate the decaying amplitudes when waves are mediated by either IP3 or IP3 and nonregenerative ATP, and the decrease in the velocity is observed in both cases. The maximal velocity in both cases is over 40 μm∕s, which is larger than the values reported in the literature. However, when IP3 was the only messenger, the effective range of waves was much lower than 200–350 μm, while waves propagate over 100 μm. In contrast, when regenerative ATP release is involved the waves display a more permanent form, which has not been reported. From this we conclude that waves in an astrocyte network are probably mediated by both intracellular IP3 and nonregenerative extracellular ATP (or partially regenerative ATP as suggested in Ref. 74).
ACKNOWLEDGMENTS
This work was supported by NIH Grant GM 29123 (Hans G. Othmer) and NIH RO1 GM073846 (Anne K. Kenworthy).
APPENDIX A: PHYSICAL INTERPRETATION OF SIMPLIFIED TEMPORAL MODEL
Examination of Eq. 1 indicates that each equation has a source term and a decay term. For example,
where Jsource=k1C∕(1+k2K) and Jdecay=k3P. Therefore IP3 production by PLC (Jsource) is a function of cytosolic free and PKC with property Jsource∝C, 1∕K with dependence on some parameters k1(s−1) and k2(μM−1). Since K is positive and the denominator (1+k2K)≥1, the expression shows explicit inhibition of PKC in IP3 production. Likewise, the activation of PKC shows the relationships and free PKC (K0−K) and
where Jactivate=k4C(K0−K) and Jdecay=−k5K. Here, k4(μM−1 s−1) is the rate constant for K and C binding and k5 is decay rate for K.
Although in R-kinetics, the source term is quite complicated comparing with previous two cases, we can apply similar argument. Previously, the Tang and Othmer107 Ca2+ model was implemented for IP3 induced Ca2+ release from the ER,
where R (Ca2+⋅Ca2+⋅IP3⋅IP3R)∝P, C2, and free IP3R. This leads us to
for activated state (Jactivate∝PC2IP3R) and decay (Jdecay∝R) of R. Because we want an ODE system of P, K, R, and C, we followed the computation described in Refs. 107, 66 to remove the dependency on free IP3R of Jactivate. This step leads us to and as desired, where k6(μM−2 s−1) is the binding rate constant for P, C2, and IP3R, k9(s−1) is the offrate constant of R, k7(μM−1), and k8(μM−1) are the affinity constant for (IP3, IP3R) and (, IP3⋅IP3R).
Finally, the cytosolic dynamics is governed by following equation:
where is the basal release from ER, is the IP3 induced release, and is the clearance of by SERCA pump on ER. If we let kc (micromolar) be volume averaged concentration in cytosol (i.e., the equilibrium of levels the cytosolic concentration approach when the whole ER network is ruptured), then
where k10(s−1) is the basal release rate. The IP3 induced Ca2+ release is a function of (kc−C) and Ca2+⋅IP3⋅IP3R (i.e., JIP3∝(kc−C)[Ca2+⋅IP3⋅IP3R]), but if we use the similar argument to express Ca2+⋅IP3⋅IP3R in term of R as we used in free IP3R (Refs. 107, 66) to get
where denotes affinity constant for C, P, and free IP3R binding, and denotes Ca2+ release rate from IP3R Ca2+Ca2+ channels. If we define , then we can combine and as ,
Finally, we assume that JSERCA follows the Hill-type kinetics with Hill coefficient two67 so that
where k12(μM s−1) and kp2(μM) denote the maximal Ca2+ pumping rate and Ca2+ sensitivity of the SERCA pump, respectively.
APPENDIX B: ATP KINETICS
Let the height of extracellular space at the location of ATP release be L and define M as total mass of ATP (A) in the infinitesimal volume at the ATP release site (i.e., M=ALδxδy). From the conservation of mass and ATP decay by enzyme, the change in M over time is given by
| (B1) |
where and denote mass fluxes while represents loss of mass due to decay.
Assuming Fick’s law diffusion for the fluxes, applying a Taylor series expansion, and truncating, we obtain
| (B2) |
which does not involve z.
If the extracellular space is assumed to be uniform in height L, the value of L is computed from the observation that body fluid is composed of 28.0 l of intracellular fluid and 14.0 l of extracellular fluid, which is again composed of 3.0 l plasma fluid and 11.0 l of interstitial fluid. From the ratio of interstitial and intracellular fluids of 11/28,55 we have L=0.4l(μm), where l is the thickness of cells. With a choice of l=2.33, the cell thickness, we estimated L=0.92 μm (Table 1, Fig. 2).
Because the ATP release mechanism is unknown, we assume that ATP release is proportional to the ATP concentration gradient between intra- and extracellular spaces in IP3 dependent manner,
where kATP(s−1) is the ATP release rate, ϕ(P) is the IP3 dependence of ATP release, and AT is the intracellular concentration of ATP. The dependence of ATP release on IP3 has been reported from some studies,19, 20 but the function ϕ is unknown and we will choose ϕ(P1)=P1∕(ρ+P1) in the current model. We will approximate kATP(s−1) so that the peak amplitude of ATP release is 30 μM.
Putting this and Eq. B2 together, we get
| (B3) |
In summary, the ATP kinetics can be described domainwise as the initial boundary value problem
where i=0,…,10 and γ=1∕L. For nonregenerative ATP release, we set kATP=0.
APPENDIX C: WAVE PROPAGATION
Since we are interested in P2YR mediated waves, we assume that kperm P=0 in this section. A necessary condition for a wave initiated in one astrocyte to propagate to neighboring astrocytes is that [IP3] in the neighboring astrocytes should be above the effective dosage for response. Our previous study67 indicates that various responses may occur for [IP3] above the effective dosage via a change in the linear stability of the steady state solution. If [IP3] is not high enough then the steady state solution remains stable and there exists no response in the cell, i.e., a wave stops. In this sense, wave propagation in an astrocytic network is completely determined by the linear stability of the steady state solution to Eq. 1,
| (C1) |
which was studied extensively in Ref. 107 for K=0. Let , which is above the effective dosage such that linearized Eq. C1 at the steady state solution has a pair of complex conjugate eigenvalues. By a continuity argument applied to the eigenvalues, we can prove that there exists an open set around where the linearized equation C1 at the steady state solution has complex conjugate eigenvalues.
Next, If we write Eq. 1 as dX∕dt=Φ(X), where X=(P,K,R,C), the steady state solutions of Eq. 1 are given by . Linearizing about , we get
| (C2) |
and the linear stability of is determined by the eigenvalues Λ=(μ1,μ2,λ1,λ2) of the matrix . Because there exists an open set around where linearized Eq. C1 at the steady state solution has complex conjugate eigenvalues, if P and K dynamics are confined in the open set around , Eq. C2 also has complex conjugate eigenvalues. Indeed, we can find an invariant rectangular domain near bounded by
To see this, we explicitly compute by solving the following algebraic equations:
| (C3) |
| (C4) |
| (C5) |
| (C6) |
Beginning with Eq. C4, by solving for ,
| (C7) |
This implies that the steady state of PKC follows sigmoidal or hyperbolic dose-response curve in in which the binding of a ligand to a single binding site is completely defined by the concentration of the binding site (Bmax=KT) and the concentration of unbound ligand at which the binding site is 50% occupied [the equilibrium dissociation constant (Kd=k5∕k4)]. Equation C7 further indicates that (dK∕dt)<0 on and (dK∕dt)>0 on . Substituting Eq. C7 into Eq. C3, we also get
| (C8) |
which implies that (dP∕dt)<0 on and (dP∕dt)>0 on . Note that Eqs. C7, C8 indicate that (even for some k5∕k4) and for , or as a function of k1∕k3 (i.e., for some k1∕k3). Because ϵ is an arbitrarily small positive number, we may assume that the invariant domain is a proper subset of the open set in which Eq. C1 is oscillatory. This result further indicates that the steady state is stable (the real parts of associated eigenvalues are negative), ruling out the existence of any periodic orbit.
We further represent nullclines of C and R explicitly. By using Eq. C8, from Eq. C5,
| (C9) |
which is a sigmoidal curve in C. Note that can be regarded as a function of k1∕k2 if we divide both denominator and numerator by k1. Finally to get , if we rewrite Eq. C6, as
| (C10) |
Equations C9, C10 provide explicit expressions for versus so that we can plot the nullclines, dC∕dt=0 and dR∕dt=0. For the C nullcline, if we solve Eq. C10 for , then
| (C11) |
So far, it was shown that R−C system in Eq. 1 can have complex conjugate eigenvalues. Also, the nullclines of Eq. 1 can be reduced into manifolds in space. By studying the local behavior of the R−C nullclines f(C,R)=0 and g(C,R)=0 at a steady state , we provide a condition for wave propagations in terms of k1(A).
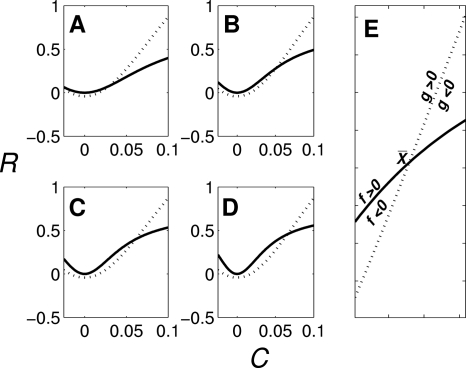
Since the right-hand side of Eq. C11 is independent of k1 as we can see from Figs. 10a, 10b, 10c, 10d, C nullcline (g(C,R)=0) does not change as k1 varies. Only R nullcline [f(C,R)=0] changes, i.e., nullcline moves upward changing its curvature (and eigenvalues) as k1 (PLC activity) increases. Also, for , both and increase as k1 increases.
Figure 10.
Nullclines of C and R as k1 varies C nullcline [g(C,R)=0: dotted line] and R nullcline [f(C,R)=0: solid line]. [(a)–(d)] C−R nullclines for k1=0.5, 1, 1.5, and 2. (e) C−R nullclines near the steady state point .
Consider now Fig. 10e. If the complex conjugate eigenvalues λ1 and λ2 are associated with , λi, i=1,2 satisfy
At the steady state , ∂f∕∂C<0, ∂f∕∂R>0, and ∂g∕∂C<0, ∂g∕∂R>0, which indicates that the sign of tr A=λ1+λ2=∂f∕∂C+∂g∕∂R cannot be determined. Also, from [dR∕dC]g(C)=0>[dR∕dC]f(C)=0,
as expected from any complex conjugate eigenvalues.
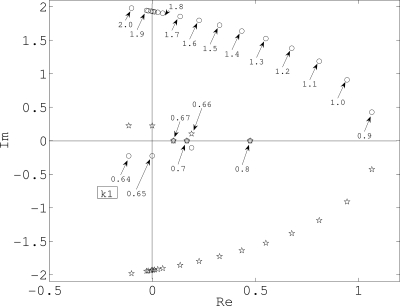
To investigate how the sign of the real parts of the complex eigenvalues varies with respect to k1(A), the characteristic equation for was solved numerically. The numerical solution shows that there are two negative eigenvalues and a pair of conjugate complex eigenvalues. Figure 11 shows the location of conjugate eigenvalues in the complex plane. The stable steady state loses stability via a Hopf bifurcation as a pair of conjugate eigenvalues crosses the imaginary axis at k1=0.65. At about 0.7 the pair merges to become real and remains so for k1=0.7–0.9. Beyond k1∼0.9, these eigenvalues become complex and the steady state regains stability near k1=1.85. This corresponds to the transient with plateau-type response.67
Figure 11.
Conjugate complex eigenvalues.
This result suggests that for waves to be propagated to the neighboring cells, k1,kin(BmaxA∕Kd+A)>0.6 is required in each cells. However, under nonregenerative ATP release assumption, extracellular ATP is attenuated due to diffusion and enzymes as it propagates, and eventually kin(BmaxA∕Kd+A) becomes less than 0.6 (A<2.5 μM; recall that the effective ATP dosage was 3 μM), at which the real part of complex conjugate eigenvalues becomes negative.
References
- Agulhon, C., Petravicz, J., McMullen, A. B., Sweger, E. J., Minton, S. K., Taves, S. R., Casper, K. B., Fiacco, T. A., and McCarthy, K. D., “What is the role of astrocyte calcium in neurophysiology?,” Neuron 59, 932–946 (2008). 10.1016/j.neuron.2008.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmi, F., Hernandez, V. H., Crispino, G., Seydel, A., Ortolano, S., Roper, S. D., Kessaris, N., Richardson, W., Rickheit, G., Filippov, M. A., Monyer, H., and Mammano, F., ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear,” Proc. Natl. Acad. Sci. U.S.A. 105, 18770–18775 (2008). 10.1073/pnas.0800793105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apolloni, S., Montilli, C., Finocchi, P., and Amadio, S., “Membrane compartments and purinergic signalling: P2x receptors in neurodegenerative and neuroinflammatory events,” FEBS J. 276, 354–364 (2009). 10.1111/j.1742-4658.2008.06796.x [DOI] [PubMed] [Google Scholar]
- Arcuino, G., Lin, J. H.-C., Takano, T., Liu, C., Jiang, L., Gao, Q., Kang, J., and Nedergaard, M., “Intercellular calcium signaling mediated by point-source burst release of ATP,” Proc. Natl. Acad. Sci. U.S.A. 99, 9840–9845 (2002). 10.1073/pnas.152588599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson, D. G., Mantzaris, N. V., and Othmer, H. G., “Wave propagation and blocking in inhomogeneous media,” Discrete Contin. Dyn. Syst. 13, 843–876 (2005). 10.3934/dcds.2005.13.843 [DOI] [Google Scholar]
- Ashida, N., Ueyama, T., Rikitake, K., Shirai, Y., Eto, M., Kondoh, T., Kohmura, E., and Saito, N., “Ca2+ oscillation induced by P2Y2 receptor activation and its regulation by a neuron-specific subtype of PKC (gammaPKC),” Neurosci. Lett. 446, 123–128 (2008). 10.1016/j.neulet.2008.09.047 [DOI] [PubMed] [Google Scholar]
- Atri, A., Amundson, J., Clapham, D., and Sneyd, J., “A single-pool model for intracellular calcium oscillations and waves in the xenopus laevis oocyte,” Biophys. J. 65, 1727–1739(1993). 10.1016/S0006-3495(93)81191-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babwah, A. V., Dale, L. B., and Ferguson, S. S. G., “Protein kinase C isoform-specific differences in the spatial-temporal regulation and decoding of metabotropic glutamate receptor1a-stimulated second messenger responses,” J. Biol. Chem. 278, 5419–5426 (2003). 10.1074/jbc.M211053200 [DOI] [PubMed] [Google Scholar]
- Barrow, S. L., Sherwood, M. W., Dolman, N. J., Gerasimenko, O. V., Voronina, S. G., and Tepikin, A. V., “Movement of calcium signals and calcium-binding proteins: Firewalls, traps and tunnels,” Biochem. Soc. Trans. 34, 381–384 (2006). 10.1042/BST0340381 [DOI] [PubMed] [Google Scholar]
- Bartlett, P. J., Young, K. W., Nahorski, S. R., and Challiss, R. A. J., “Single cell analysis and temporal proling of agonist-mediated inositol 1,4,5-trisphosphate, Ca2+, diacylglycerol, and protein kinase C signaling using fluorescent biosensors,” J. Biol. Chem. 280, 21837–21846 (2005). 10.1074/jbc.M411843200 [DOI] [PubMed] [Google Scholar]
- Bennett, M. R., Buljan, V., Farnell, L., and Gibson, W. G., “Purinergic junctional transmission and propagation of calcium waves in spinal cord astrocyte networks,” Biophys. J. 91, 3560–3571 (2006). 10.1529/biophysj.106.082073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, M. R., Farnell, L., and Gibson, W. G., “A quantitative model of cortical spreading depression due to purinergic and gap-junction transmission in astrocyte networks,” Biophys. J. 95, 5648–5660 (2008). 10.1529/biophysj.108.137190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge, M. J., Bootman, M. D., and Lipp, P., “Calcium—A life and death signal,” Nature (London) 395, 645–648 (1998). 10.1038/27094 [DOI] [PubMed] [Google Scholar]
- Bezprozvanny, I., Watras, J., and Ehrlich, B. E., “Bell-shaped calcium-response curves of ins(1,4,5)p3- and calcium-gated channels from endoplasmic reticulum of cerebellum,” Nature (London) 351, 751–754 (1991). 10.1038/351751a0 [DOI] [PubMed] [Google Scholar]
- Blomstrand, F., Aberg, N. D., Eriksson, P. S., Hansson, E., and Rönnbäck, L., “Extent of intercellular calcium wave propagation is related to gap junction permeability and level of connexin-43 expression in astrocytes in primary cultures from four brain regions,” Neuroscience 92, 255–265 (1999). 10.1016/S0306-4522(98)00738-6 [DOI] [PubMed] [Google Scholar]
- Bobalova, J. and Mutafova-Yambolieva, V. N., “Co-release of endogenous ATP and noradrenaline from guinea-pig mesenteric veins exceeds co-release from mesenteric arteries,” Clin. Exp. Pharmacol. Physiol. 28, 397–401 (2001). 10.1046/j.1440-1681.2001.03460.x [DOI] [PubMed] [Google Scholar]
- Bowser, D. N. and Khakh, B. S., “Two forms of single-vesicle astrocyte exocytosis imaged with total internal reflection fluorescence microscopy,” Proc. Natl. Acad. Sci. U.S.A. 104, 4212–4217 (2007). 10.1073/pnas.0607625104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser, D. N. and Khakh, B. S., “Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes,” J. Gen. Physiol. 129, 485–491 (2007). 10.1085/jgp.200709780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braet, K., Aspeslagh, S., Vandamme, W., Willecke, K., Martin, P. E. M., Evans, W. H., and Leybaert, L., “Pharmacological sensitivity of ATP release triggered by photoliberation of inositol-1,4,5-trisphosphate and zero extracellular calcium in brain endothelial cells,” J. Cell Physiol. 197, 205–213 (2003). 10.1002/jcp.10365 [DOI] [PubMed] [Google Scholar]
- Braet, K., Vandamme, W., Martin, P. E. M., Evans, W. H., and Leybaert, L., “Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26,” Cell Calcium 33, 37–48 (2003). 10.1016/S0143-4160(02)00180-X [DOI] [PubMed] [Google Scholar]
- Bushong, E. A., Martone, M. E., Jones, Y. Z., and Ellisman, M. H., “Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains,” J. Neurosci. 22, 183–192 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco, S., Calegari, F., Pravettoni, E., Pozzi, D., Taverna, E., Rosa, P., Matteoli, M., and Verderio, C., “Storage and release of ATP from astrocytes in culture,” J. Biol. Chem. 278, 1354–1362 (2003). 10.1074/jbc.M209454200 [DOI] [PubMed] [Google Scholar]
- Codazzi, F., Teruel, M. N., and Meyer, T., “Control of astrocyte Ca2+ oscillations and waves by oscillating translocation and activation of protein kinase C,” Curr. Biol. 11, 1089–1097 (2001). 10.1016/S0960-9822(01)00326-8 [DOI] [PubMed] [Google Scholar]
- Cornell-Bell, A. H., Finkbeiner, S. M., Cooper, M. S., and Smith, S. J., “Glutamate induces calcium waves in cultured astrocytes: Long-range glial signaling,” Science 247, 470–473 (1990). 10.1126/science.1967852 [DOI] [PubMed] [Google Scholar]
- Cotrina, M. L., Lin, J. H., Alves-Rodrigues, A., Liu, S., Li, J., Azmi-Ghadimi, H., Kang, J., Naus, C. C., and Nedergaard, M., “Connexins regulate calcium signaling by controlling ATP release,” Proc. Natl. Acad. Sci. U.S.A. 95, 15735–15740 (1998). 10.1073/pnas.95.26.15735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina, M. L., Lin, J. H.-C., López-García, J. C., Naus, C. C. G., and Nedergaard, M., “ATP-mediated glia signaling,” J. Neurosci. 20, 2835–2844 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina, M. L., Lin, J. H.-C., and Nedergaard, M., “Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling,” J. Neurosci. 18, 8794–8804 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, M. L., Filtz, T. M., and Harden, T. K., “Protein kinase C-promoted inhibition of galpha(11)-stimulated phospholipase C-beta activity,” Mol. Pharmacol. 56, 265–271 (1999). [DOI] [PubMed] [Google Scholar]
- Dale, L. B., Babwah, A. V., Bhattacharya, M., Kelvin, D. J., and Ferguson, S. S., “Spatial-temporal patterning of metabotropic glutamate receptor-mediated inositol 1,4,5-triphosphate, calcium, and protein kinase C oscillations: Protein kinase C-dependent receptor phosphorylation is not required,” J. Biol. Chem. 276, 35900–35908 (2001). 10.1074/jbc.M103847200 [DOI] [PubMed] [Google Scholar]
- Dale, L. B., Babwah, A. V., and Ferguson, S. S. G., “Mechanisms of metabotropic glutamate receptor desensitization: Role in the patterning of eector enzyme activation,” Neurochem. Int. 41, 319–326 (2002). 10.1016/S0197-0186(02)00073-6 [DOI] [PubMed] [Google Scholar]
- Dale, L. B., Bhattacharya, M., Anborgh, P. H., Murdoch, B., Bhatia, M., Nakanishi, S., and Ferguson, S. S., “G protein-coupled receptor kinase-mediated desensitization of metabotropic glutamate receptor 1a protects against cell death,” J. Biol. Chem. 275, 38213–38220 (2000). 10.1074/jbc.M006075200 [DOI] [PubMed] [Google Scholar]
- Davalos, D., Grutzendler, J., Yang, G., Kim, J. V., Zuo, Y., Jung, S., Littman, D. R., Dustin, M. L., and Gan, W. B., “ATP mediates rapid microglial response to local brain injury in vivo,” Nat. Neurosci. 8, 752–758 (2005). 10.1038/nn1472 [DOI] [PubMed] [Google Scholar]
- Davis, T. and Duff, I., “An unsymmetric-pattern multifrontal method for sparse LU factorization,” SIAM J. Matrix Anal. Appl. 18, 140–158 (1997). 10.1137/S0895479894246905 [DOI] [Google Scholar]
- Delicado, E., Jimenez, A., Carrasquero, L., Castro, E., and Miras-Portugal, M., “Cross-talk among epidermal growth factor, ap (5) a, and nucleotide receptors causing enhanced ATP Ca2+ signaling involves extracellular kinase activation in cerebellar astrocytes,” J. Neurosci. Res. 81, 789–796 (2005). 10.1002/jnr.20609 [DOI] [PubMed] [Google Scholar]
- Ding, S., Fellin, T., Zhu, Y., Lee, S. Y., Auberson, Y. P., Meaney, D. F., Coulter, D. A., Carmignoto, G., and Haydon, P. G., “Enhanced astrocytic Ca2+ signals contribute to neuronal excitotoxicity after status epilepticus,” J. Neurosci. 27, 10674–10684 (2007). 10.1523/JNEUROSCI.2001-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokukina, I., Gracheva, M., Grachev, E., and Gunton, J., “Role of network connectivity in intercellular calcium signaling,” Physica D 237, 745–754 (2008). 10.1016/j.physd.2007.10.010 [DOI] [Google Scholar]
- Dupont, G., Combettes, L., and Leybaert, L., “Calcium dynamics: Spatio-temporal organization from the subcellular to the organ level,” Int. Rev. Cytol. 261, 193–245 (2007). 10.1016/S0074-7696(07)61005-5 [DOI] [PubMed] [Google Scholar]
- Dupont, G. and Goldbeter, A., “Oscillations and waves of cytosolic calcium: Insights from theoretical models,” BioEssays 14, 485–493 (1992). 10.1002/bies.950140711 [DOI] [PubMed] [Google Scholar]
- Dupont, G. and Goldbeter, A., “Properties of intracellular Ca2+ waves generated by a model based on Ca2+-induced Ca2+ release,” Biophys. J. 67, 2191–2204 (1994). 10.1016/S0006-3495(94)80705-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espallergues, J., Solovieva, O., Técher, V., Bauer, K., Alonso, G., Vincent, A., and Hussy, N., “Synergistic activation of astrocytes by ATP and norepinephrine in the rat supraoptic nucleus,” Neuroscience 148, 712–723 (2007). 10.1016/j.neuroscience.2007.03.043 [DOI] [PubMed] [Google Scholar]
- Falcke, M., Li, Y., Lechleiter, J. D., and Camacho, P., “Modeling the dependence of the period of intracellular Ca2+ waves on serca expression,” Biophys. J. 85, 1474–1481 (2003). 10.1016/S0006-3495(03)74580-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall, C. P., Wagner, J. M., Loew, L. M., and Nuccitelli, R., “Cortically restricted production 1 of IP3 leads to propagation of the fertilization Ca2+ wave along the cell surface in a model of the xenopus egg,” J. Theor. Biol. 231, 487–496 (2004). 10.1016/j.jtbi.2004.06.019 [DOI] [PubMed] [Google Scholar]
- Fiacco, T. A., Agulhon, C., Taves, S. R., Petravicz, J., Casper, K. B., Dong, X., Chen, J., and McCarthy, K. D., “Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity,” Neuron 54, 611–626 (2007). 10.1016/j.neuron.2007.04.032 [DOI] [PubMed] [Google Scholar]
- Fiacco, T. A. and McCarthy, K. D., “Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous ampa receptor currents in CA1 pyramidal neurons,” J. Neurosci. 24, 722–732 (2004). 10.1523/JNEUROSCI.2859-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiacco, T. A. and McCarthy, K. D., “Astrocyte calcium elevations: Properties, propagation, and effects on brain signaling,” Glia 54, 676–690 (2006). 10.1002/glia.20396 [DOI] [PubMed] [Google Scholar]
- Filtz, T. M., Cunningham, M. L., Stanig, K. J., Paterson, A., and Harden, T. K., “Phosphorylation by protein kinase C decreases catalytic activity of avian phospholipase C-beta,” Biochem. J. 338, 257–264 (1999). 10.1042/0264-6021:3380257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, A. C., Mathematical Models in the Applied Sciences (Cambridge University Press, Cambridge, UK, 1997). [Google Scholar]
- Franke, H., Krügel, U., Grosche, J., Heine, C., Härtig, W., Allgaier, C., and Illes, P., “P2Y receptor expression on astrocytes in the nucleus accumbens of rats,” Neuroscience 127, 431–441 (2004). 10.1016/j.neuroscience.2004.05.003 [DOI] [PubMed] [Google Scholar]
- Giaume, C. and Venance, L., “Intercellular calcium signaling and gap junctional communication in astrocytes,” Glia 24, 50–64 (1998). [DOI] [PubMed] [Google Scholar]
- Gordon, J. L., “Extracellular ATP: effects, sources and fate,” Biochem. J. 233, 309–319 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble, F. M., Loussouarn, G., Tucker, S. J., Zhao, C., Nichols, C. G., and Ashcroft, F. M., “A novel method for measurement of submembrane ATP concentration,” J. Biol. Chem. 275, 30046–30049 (2000). 10.1074/jbc.M001010200 [DOI] [PubMed] [Google Scholar]
- Griffith, D. A. and Jarvis, S. M., “Nucleoside and nucleobase transport systems of mammalian cells,” Biochim. Biophys. Acta 1286, 153–181 (1996). [DOI] [PubMed] [Google Scholar]
- Guan, X., Cravatt, B. F., Ehring, G. R., Hall, J. E., Boger, D. L., Lerner, R. A., and Gilula, N. B., “The sleep-inducing lipid oleamide deconvolutes gap junction communication and calcium wave transmission in glial cells,” J. Cell Biol. 139, 1785–1792 (1997). 10.1083/jcb.139.7.1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie, P. B., Knappenberger, J., Segal, M., Bennett, M. V. L., Charles, A. C., and Kater, S. B., “ATP released from astrocytes mediates glial calcium waves,” J. Neurosci. 19, 520–528 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, J., Guyton Physiology Review (Saunders, 2005). [Google Scholar]
- Hamilton, N., Vayro, S., Kirchhoff, F., Verkhratsky, A., Robbins, J., Gorecki, D., and Butt, A., “Mechanisms of ATP-and glutamate-mediated calcium signaling in white matter astrocytes,” Glia 56, 734–749 (2008). 10.1002/glia.20649 [DOI] [PubMed] [Google Scholar]
- Harris-White, M. E., Zanotti, S. A., Frautschy, S. A., and Charles, A. C., “Spiral intercellular calcium waves in hippocampal slice cultures,” J. Neurophysiol. 79, 1045–1052 (1998). [DOI] [PubMed] [Google Scholar]
- Hassinger, T. D., Guthrie, P. B., Atkinson, P. B., Bennett, M. V., and Kater, S. B., “An extracellular signaling component in propagation of astrocytic calcium waves,” Proc. Natl. Acad. Sci. U.S.A. 93, 13268–13273 (1996). 10.1073/pnas.93.23.13268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubley, M. J., Locke, B. R., and Moerland, T. S., “The effects of temperature, pH, and magnesium on the diffusion coefficient of ATP in solutions of physiological ionic strength,” Biochim. Biophys. Acta 1291, 115–121 (1996). [DOI] [PubMed] [Google Scholar]
- Höfer, T., Venance, L., and Giaume, C., “Control and plasticity of intercellular calcium waves in astrocytes: A modeling approach,” J. Neurosci. 22, 4850–4859 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri, M. S. and Keizer, J., “On the roles of Ca2+ diffusion, Ca2+ buffers, and the endoplasmic reticulum in IP3-induced Ca2+ waves,” Biophys. J. 69, 2139–2153 (1995). 10.1016/S0006-3495(95)80088-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri, M. S. and Keizer, J., “Agonist-induced calcium waves in oscillatory cells: A biological example of Burgers’ equation,” Bull. Math. Biol. 59, 1125–1144 (1997). 10.1007/BF02460104 [DOI] [PubMed] [Google Scholar]
- John, G. R., Scemes, E., Suadicani, S. O., Liu, J. S., Charles, P. C., Lee, S. C., Spray, D. C., and Brosnan, C. F., “Il-1beta differentially regulates calcium wave propagation between primary human fetal astrocytes via pathways involving P2 receptors and gap junction channels,” Proc. Natl. Acad. Sci. U.S.A. 96, 11613–11618 (1999). 10.1073/pnas.96.20.11613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph, S. M., Buchakjian, M. R., and Dubyak, G. R., “Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes,” J. Biol. Chem. 278, 23331–23342 (2003). 10.1074/jbc.M302680200 [DOI] [PubMed] [Google Scholar]
- Kang, J., Kang, N., Lovatt, D., Torres, A., Zhao, Z., Lin, J., and Nedergaard, M., “Connexin 43 hemichannels are permeable to ATP,” J. Neurosci. 28, 4702–4711 (2008). 10.1523/JNEUROSCI.5048-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, M., “Temporal and spatial aspects of calcium dynamics in astrocytes,” Ph.D. thesis, University of Minnesota, 2004. [Google Scholar]
- Kang, M. and Othmer, H. G., “The variety of cytosolic calcium responses and possible roles of PLC and PKC,” Phys. Biol. 4, 325–343 (2007). 10.1088/1478-3975/4/4/009 [DOI] [PubMed] [Google Scholar]
- Katsuragi, T., Sato, C., Usune, S., Ueno, S., Segawa, M., and Migita, K., “Caffeine-inducible ATP release is mediated by Ca2+-signal transducing system from the endoplasmic reticulum to mitochondria,” Naunyn-Schmiedeberg’s Arch. Pharmacol. 378, 93–101 (2008). 10.1007/s00210-008-0292-9 [DOI] [PubMed] [Google Scholar]
- Kawano, S., Otsu, K., Kuruma, A., Shoji, S., Yanagida, E., Muto, Y., Yoshikawa, F., Hirayama, Y., Mikoshiba, K., and Furuichi, T., “ATP autocrine/paracrine signaling induces calcium oscillations and NFAT activation in human mesenchymal stem cells,” Cell Calcium 39, 313–324 (2006). 10.1016/j.ceca.2005.11.008 [DOI] [PubMed] [Google Scholar]
- Keener, J. and Sneyd, J., Mathematical Physiology (Springer, Berlin, 2001). [Google Scholar]
- Kimelberg, H. K., “Increased release of excitatory amino acids by the actions of ATP and peroxynitrite on volume-regulated anion channels (VRACs) in astrocytes,” Neurochem. Int. 45, 511–519 (2004). 10.1016/j.neuint.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Konietzko, U. and Müller, C. M., “Astrocytic dye coupling in rat hippocampus: Topography, developmental onset, and modulation by protein kinase C,” Hippocampus 4, 297–306 (1994). 10.1002/hipo.450040313 [DOI] [PubMed] [Google Scholar]
- Lazarowski, E. R., Watt, W. C., Stutts, M. J., Boucher, R. C., and Harden, T. K., “Pharmacological selectivity of the cloned human P2U-purinoceptor: potent activation by diadenosine tetraphosphate,” Br. J. Pharmacol. 116, 1619–1627 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald, C. L., Yu, D., Buibas, M., and Silva, G. A., “Diffusion modeling of ATP signaling suggests a partially regenerative mechanism underlies astrocyte intercellular calcium waves,” Front. Neuroeng. 1, 1–13 (2008). 10.3389/neuro.16.001.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazel, T., Richter, F., Vargová, L., and Syková, E., “Changes in extracellular space volume and geometry induced by cortical spreading depression in immature and adult rats,” Physiol. Res. 51, S85–S93 (2002). [PubMed] [Google Scholar]
- Merten, M. D., Saleh, A., Kammouni, W., Marchand, S., and Figarella, C., “Characterization of two distinct P2Y receptors in human tracheal gland cells,” Eur. J. Biochem. 251, 19–24 (1998). 10.1046/j.1432-1327.1998.2510019.x [DOI] [PubMed] [Google Scholar]
- Mongin, A. A. and Kimelberg, H. K., “ATP potently modulates anion channel-mediated excitatory amino acid release from cultured astrocytes,” Am. J. Physiol.: Cell Physiol. 283, C569–C578 (2002). [DOI] [PubMed] [Google Scholar]
- Nadkarni S., Jung P., and Levine H., “Astrocytes optimize the synaptic transmission of information,” PLOS Comput. Biol. 4, e1000088 (2008). 10.1371/journal.pcbi.1000088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy, J. I. and Rash, J. E., “Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS,” Brain Res. Rev. 32, 29–44 (2000). 10.1016/S0165-0173(99)00066-1 [DOI] [PubMed] [Google Scholar]
- Nash, M. S., Schell, M. J., Atkinson, P. J., Johnston, N. R., Nahorski, S. R., and Challiss, R. A. J., “Determinants of metabotropic glutamate receptor-5-mediated Ca2+ and inositol 1,4,5-trisphosphate oscillation frequency. Receptor density versus agonist concentration,” J. Biol. Chem. 277, 35947–35960 (2002). 10.1074/jbc.M205622200 [DOI] [PubMed] [Google Scholar]
- Newman, E. A., Glial cells of the rat retina (http://www.manticmoo.com/articles/jeff/programming/latex/bibtextypes. php).
- Newman, E. A., “Propagation of intercellular calcium waves in retinal astrocytes and Müller cells,” J. Neurosci. 21, 2215–2223 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni, Y., Malarkey, E. B., and Parpura, V., “Vesicular release of glutamate mediates bidirectional signaling between astrocytes and neurons,” J. Neurochem. 103, 1273–1284 (2007). 10.1111/j.1471-4159.2007.04864.x [DOI] [PubMed] [Google Scholar]
- Zur Nieden, R. Z. and Deitmer, J. W., “The role of metabotropic glutamate receptors for the generation of calcium oscillations in rat hippocampal astrocytes in situ,” Cereb. Cortex 16, 676–687 (2006). 10.1093/cercor/bhj013 [DOI] [PubMed] [Google Scholar]
- Oberheim, N. A., Wang, X., Goldman, S., and Nedergaard, M., “Astrocytic complexity distinguishes the human brain,” Trends Neurosci. 29, 547–553 (2006). 10.1016/j.tins.2006.08.004 [DOI] [PubMed] [Google Scholar]
- Piet, R. and Jahr, C. E., “Glutamatergic and purinergic receptor-mediated calcium transients in Bergmann glial cells,” J. Neurosci. 27, 4027–4035 (2007). 10.1523/JNEUROSCI.0462-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piet, R., Vargová, L., Syková, E., Poulain, D. A., and Oliet, S. H. R., “Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk,” Proc. Natl. Acad. Sci. U.S.A. 101, 2151–2155 (2004). 10.1073/pnas.0308408100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pittà, M. D., Volman, V., Levine, H., Pioggia, G., Rossi, D. D., and Ben-Jacob, E., “Coexistence of amplitude and frequency modulations in intracellular calcium dynamics,” Phys. Rev. E 77, 030903(R) (2008). 10.1103/PhysRevE.77.030903 [DOI] [PubMed] [Google Scholar]
- Porter, J. T. and McCarthy, K. D., “Astrocytic neurotransmitter receptors in situ and in vivo,” Prog. Neurobiol. 51, 439–455 (1997). 10.1016/S0301-0082(96)00068-8 [DOI] [PubMed] [Google Scholar]
- Ralevic, V. and Burnstock, G., “Receptors for purines and pyrimidines,” Pharmacol. Rev. 50, 413–492 (1998). [PubMed] [Google Scholar]
- Rostovtseva, T. K. and Bezrukov, S. M., “ATP transport through a single mitochondrial channel, VDAC, studied by current fluctuation analysis,” Biophys. J. 74, 2365–2373 (1998). 10.1016/S0006-3495(98)77945-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabirov, R. Z., Dutta, A. K., and Okada, Y., “Volume-dependent ATP-conductive large-conductance anion channel as a pathway for swelling-induced ATP release,” J. Gen. Physiol. 118, 251–266 (2001). 10.1085/jgp.118.3.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson, M. J., Charles, A. C., Boitano, S., and Dirksen, E. R., “Mechanisms and function of intercellular calcium signaling,” Mol. Cell Endocrinol. 98, 173–187 (1994). 10.1016/0303-7207(94)90136-8 [DOI] [PubMed] [Google Scholar]
- Scemes, E., “Components of astrocytic intercellular calcium signaling,” Mol. Neurobiol. 22, 167–179 (2000). 10.1385/MN:22:1-3:167 [DOI] [PubMed] [Google Scholar]
- Scemes, E. and Giaume, C., “Astrocyte calcium waves: What they are and what they do,” Glia 54, 716–725 (2006). 10.1002/glia.20374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedaa, K. O., Bjur, R. A., Shinozuka, K., and Westfall, D. P., “Nerve and drug-induced release of adenine nucleosides and nucleotides from rabbit aorta,” J. Pharmacol. Exp. Ther. 252, 1060–1067 (1990). [PubMed] [Google Scholar]
- Shelton, M. K. and McCarthy, K. D., “Hippocampal astrocytes exhibit Ca2+-elevating muscarinic cholinergic and histaminergic receptors in situ,” J. Neurochem. 74, 555–563 (2000). 10.1046/j.1471-4159.2000.740555.x [DOI] [PubMed] [Google Scholar]
- Shinozuka, K., Hashimoto, M., Masumura, S., Bjur, R. A., Westfall, D. P., and Hattori, K., “In vitro studies of release of adenine nucleotides and adenosine from rat vascular endothelium in response to alpha 1-adrenoceptor stimulation,” Br. J. Pharmacol. 113, 1203–1208 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silchenko, A. N. and Tass, P. A., “Computational modeling of paroxysmal depolarization shifts in neurons induced by the glutamate release from astrocytes,” Biol. Cybern. 98, 61–74 (2008). 10.1007/s00422-007-0196-7 [DOI] [PubMed] [Google Scholar]
- Sneyd, J., Girard, S., and Clapham, D., “Calcium wave propagation by calcium-induced calcium release: An unusual excitable system,” Bull. Math. Biol. 55, 315–344 (1993). [DOI] [PubMed] [Google Scholar]
- Stamatakis, M. and Mantzaris, N. V., “Modeling of ATP-mediated signal transduction and wave propagation in astrocytic cellular networks,” J. Theor. Biol. 241, 649–668 (2006). 10.1016/j.jtbi.2006.01.002 [DOI] [PubMed] [Google Scholar]
- Stamatakis, M. and Mantzaris, N. V., “Astrocyte signaling in the presence of spatial inhomogeneities,” Chaos 17, 033123-1–033123-12 (2007). 10.1063/1.2767409 [DOI] [PubMed] [Google Scholar]
- Stout, C. E., Costantin, J. L., Naus, C. C. G., and Charles, A. C., “Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels,” J. Biol. Chem. 277, 10482–10488 (2002). 10.1074/jbc.M109902200 [DOI] [PubMed] [Google Scholar]
- Suadicani, S. O., Brosnan, C. F., and Scemes, E., “P2x7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling,” J. Neurosci. 26, 1378–1385 (2006). 10.1523/JNEUROSCI.3902-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suadicani, S. O., Flores, C. E., Urban-Maldonado, M., Beelitz, M., and Scemes, E., “Gap junction channels coordinate the propagation of intercellular Ca2+ signals generated by P2y receptor activation,” Glia 48, 217–229 (2004). 10.1002/glia.20071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykova, E., “The extracellular space in the CNS: Its regulation, volume and geometry in normal and pathological neuronal function,” Neuroscientist 3, 28–41 (1997). [Google Scholar]
- Tang, Y. and Othmer, H. G., “Frequency encoding in excitable systems with applications to calcium oscillations,” Proc. Natl. Acad. Sci. U.S.A. 92, 7869–7873 (1995). 10.1073/pnas.92.17.7869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoufik, E. and Probert, L., “Ischemic neuronal damage,” Curr. Pharm. Des. 14, 3565–3573 (2008). 10.2174/138161208786848748 [DOI] [PubMed] [Google Scholar]
- Taylor, A. L., Kudlow, B. A., Marrs, K. L., Gruenert, D. C., Guggino, W. B., and Schwiebert, E. M., “Bioluminescence detection of ATP release mechanisms in epithelia,” Am. J. Physiol. 275, C1391–C1406 (1998). [DOI] [PubMed] [Google Scholar]
- Thul, R., Bellamy, T. C., Roderick, H. L., Bootman, M. D., and Coombes, S., “Calcium oscillations,” Adv. Exp. Med. Biol. 641, 1–27 (2008). 10.1007/978-0-387-09794-7_1 [DOI] [PubMed] [Google Scholar]
- Tordjmann, T., Berthon, B., Claret, M., and Combettes, L., “Coordinated intercellular calcium waves induced by noradrenaline in rat hepatocytes: Dual control by gap junction permeability and agonist,” EMBO J. 16, 5398–5407 (1997). 10.1093/emboj/16.17.5398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino, M., Sakai, N., Kashiwagi, K., Shirai, Y., Shinohara, Y., Hirose, K., Iino, M., Yamamura, T., and Saito, N., “Isoform-specic phosphorylation of metabotropic glutamate receptor 5 by protein kinase C (PKC) blocks Ca2+ oscillation and oscillatory translocation of Ca2+-dependent PKC,” J. Biol. Chem. 279, 2254–2261 (2004). 10.1074/jbc.M309894200 [DOI] [PubMed] [Google Scholar]
- Venance, L., Stella, N., Glowinski, J., and Giaume, C., “Mechanism involved in initiation and propagation of receptor-induced intercellular calcium signaling in cultured rat astrocytes,” J. Neurosci. 17, 1981–1992 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky, A. and Kettenmann, H., “Calcium signalling in glial cells,” Trends Neurosci. 19, 346–352 (1996). 10.1016/0166-2236(96)10048-5 [DOI] [PubMed] [Google Scholar]
- Verkhratsky, A., Orkand, R. K., and Kettenmann, H., “Glial calcium: homeostasis and signaling function,” Physiol. Rev. 78, 99–141 (1998). [DOI] [PubMed] [Google Scholar]
- Volman, V., Ben-Jacob, E., and Levine, H., “The astrocyte as a gatekeeper of synaptic information transfer,” Neural Comput. 19, 303–326 (2007). 10.1162/neco.2007.19.2.303 [DOI] [PubMed] [Google Scholar]
- Volterra, A. and Meldolesi, J., “Astrocytes, from brain glue to communication elements: The revolution continues,” Nat. Rev. Neurosci. 6, 626–640 (2005). 10.1038/nrn1722 [DOI] [PubMed] [Google Scholar]
- De Vuyst, E. D., Decrock, E., Cabooter, L., Dubyak, G. R., Naus, C. C., Evans, W. H., and Leybaert, L., “Intracellular calcium changes trigger connexin hemichannel opening,” EMBO J. 25, 34–44 (2006). 10.1038/sj.emboj.7600908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, J., Li, Y. X., Pearson, J., and Keizer, J., “Simulation of the fertilization Ca2+ wave in xenopus laevis eggs,” Biophys. J. 75, 2088–2097 (1998). 10.1016/S0006-3495(98)77651-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z., Haydon, P. G., and Yeung, E. S., “Direct observation of calcium-independent intercellular ATP signaling in astrocytes,” Anal. Chem. 72, 2001–2007 (2000). 10.1021/ac9912146 [DOI] [PubMed] [Google Scholar]
- Wilson, P. D., Hovater, J. S., Casey, C. C., Fortenberry, J. A., and Schwiebert, E. M., “ATP release mechanisms in primary cultures of epithelia derived from the cysts of polycystic kidneys,” J. Am. Soc. Nephrol. 10, 218–229 (1999). [DOI] [PubMed] [Google Scholar]
- Zhang, Z., Chen, G., Zhou, W., Song, A., Xu, T., Luo, Q., Wang, W., Gu, X., and Duan, S., “Regulated ATP release from astrocytes through lysosome exocytosis,” Nat. Cell Biol. 9, 945–953 (2007). 10.1038/ncb1620 [DOI] [PubMed] [Google Scholar]
- Zhao, Y., Migita, K., Sato, C., Usune, S., Iwamoto, T., and Katsuragi, T., “Endoplasmic reticulum is a key organella in bradykinin-triggered ATP release from cultured smooth muscle cells,” J. Pharmacol. Sci. 105, 57–65 (2007). 10.1254/jphs.FP0070865 [DOI] [PubMed] [Google Scholar]