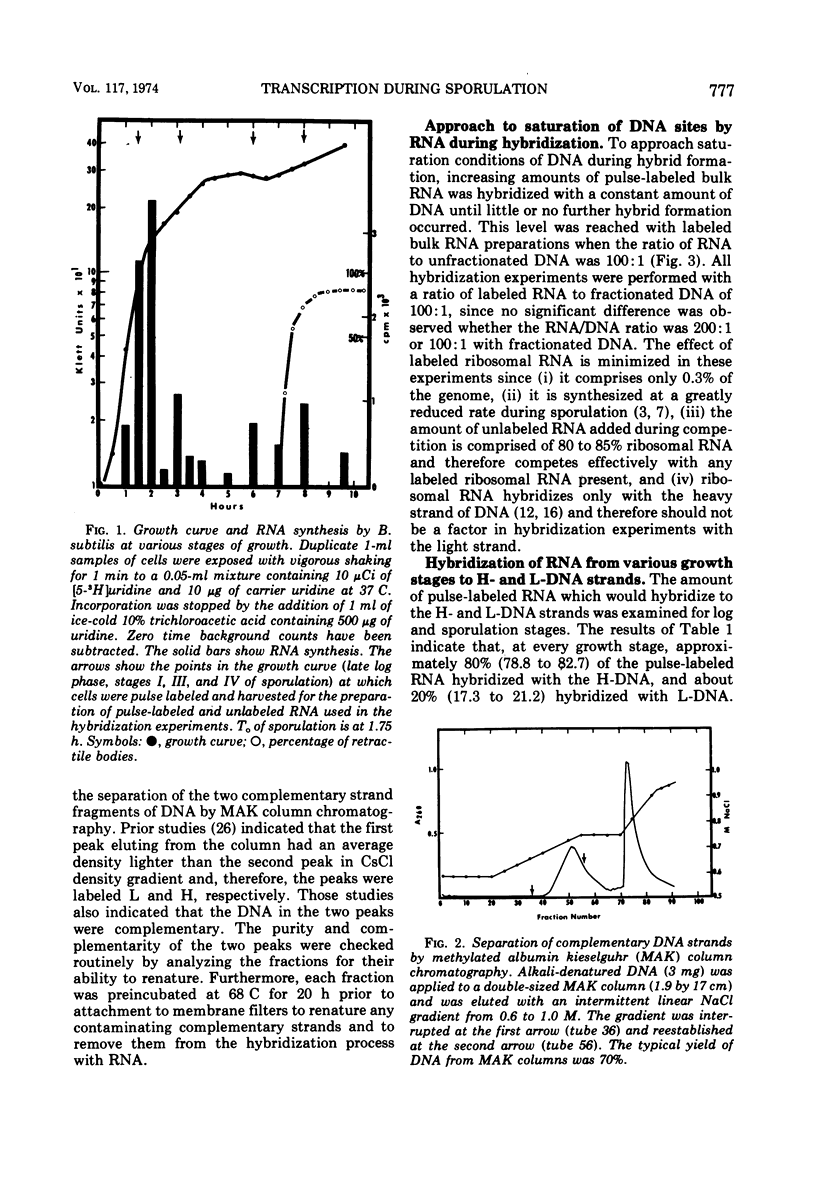
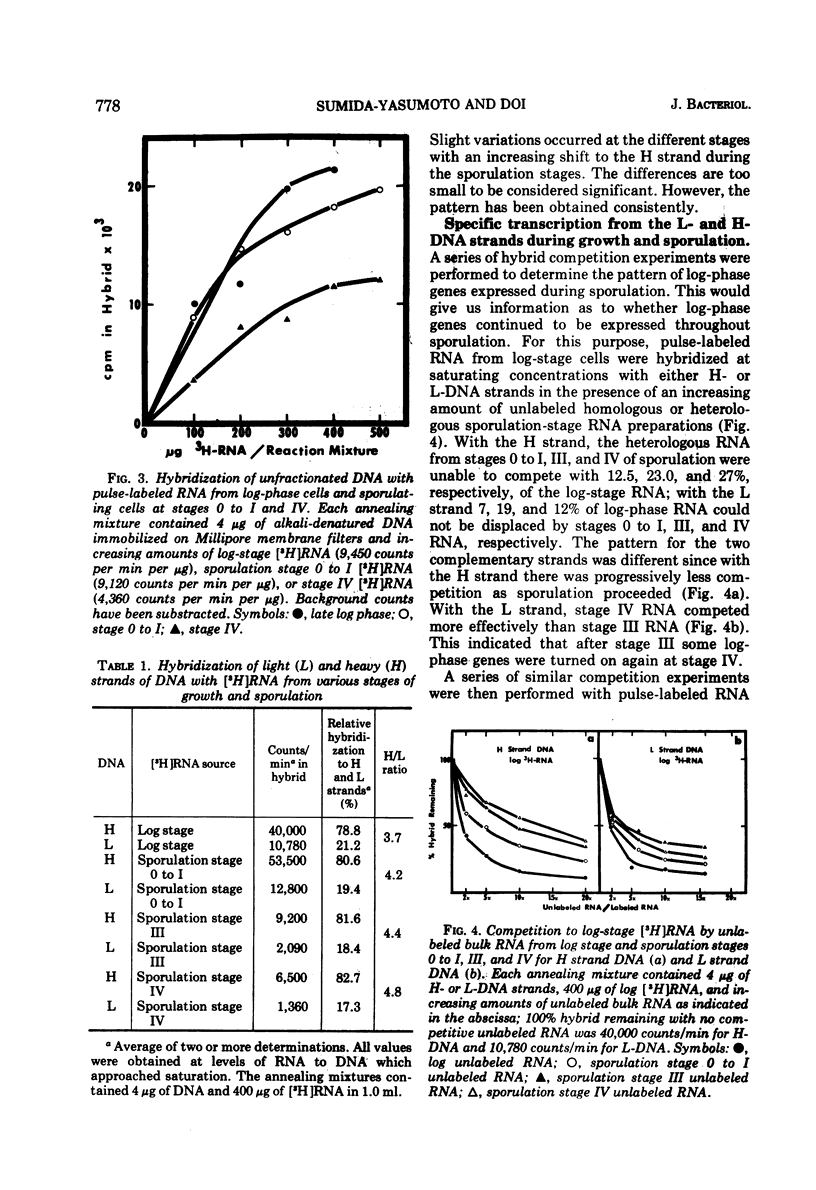
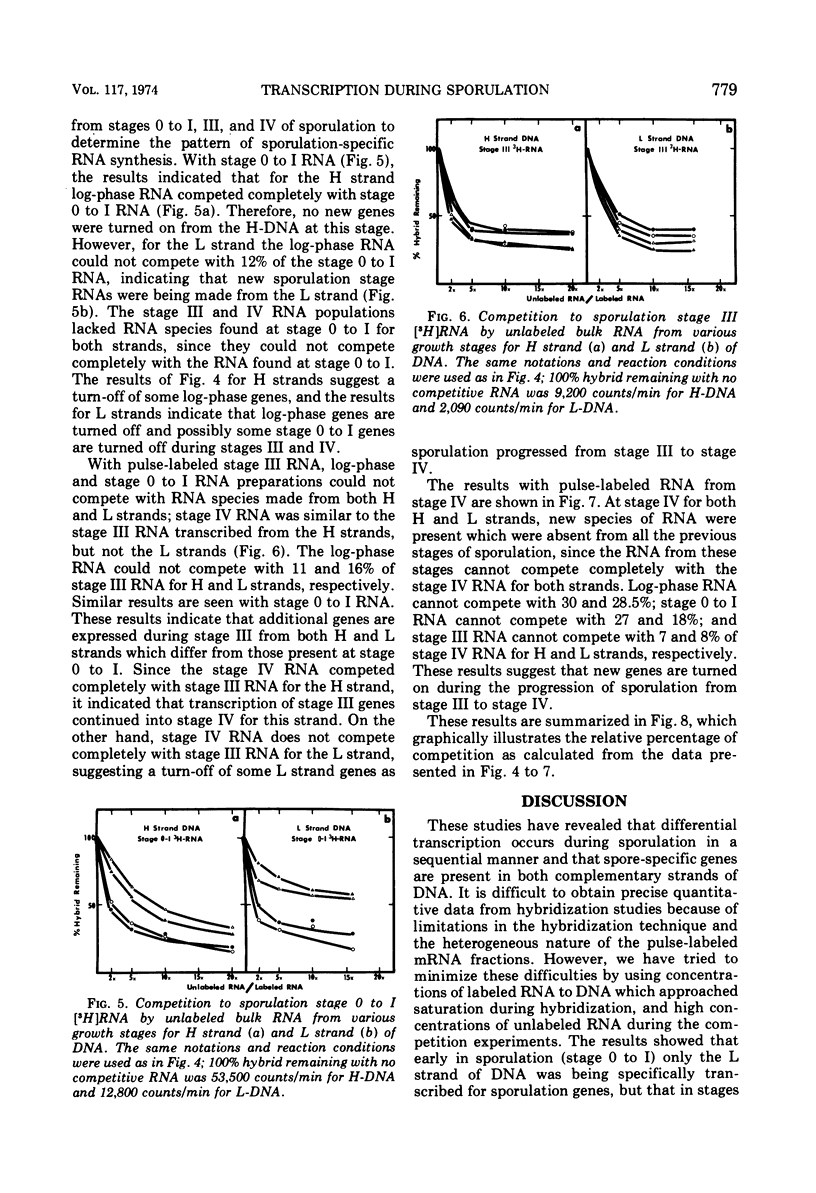
Abstract
The messenger ribonucleic acid (mRNA) pattern of log-phase and sporulating cells of Bacillus subtilis has been analyzed by deoxyribonucleic acid (DNA)-RNA hybrid studies with the complementary-strand fragments of DNA. Approximately 80% of the mRNA of log-phase and sporulating cells from stages I, III, and IV hybridizes with the heavy DNA fragments, and 20% hybridizes with the light DNA fragments. Hybrid competition studies indicated that there was either a greatly reduced rate of transcription or a turn-off of some log-phase genes during the sporulation stages. However, a significant amount of log-phase gene transcription occurred even at late stages of sporulation. Similar studies indicate a significantly increased rate of transcription or a turn-on of sporulation phase genes during the latter stages of sproulation. There is a sequential increase in the amount of sporulation-specific transcription from both complementary-strand fragments of DNA. These results indicate that the RNA polymerase population in sporulating cells can transcribe both log-phase and sporulation-phase genes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARONSON A. I. CHARACTERIZATION OF MESSENGER RNA IN SPORULATING BACILLUS CEREUS. J Mol Biol. 1965 Mar;11:576–588. doi: 10.1016/s0022-2836(65)80012-2. [DOI] [PubMed] [Google Scholar]
- BALASSA G. RENOUVELLEMENT DE L'ACIDE RIBONUCL'EIQUE AU COURS DE LA SPORULATION DE BACILLUS SUBTILIS. Biochim Biophys Acta. 1963 Nov 22;76:410–416. doi: 10.1016/0006-3002(63)90060-x. [DOI] [PubMed] [Google Scholar]
- Bonamy C., Hirschbein L., Szulmajster J. Synthesis of ribosomal ribonucleic acid during sporulation of Bacillus subtilis. J Bacteriol. 1973 Mar;113(3):1296–1306. doi: 10.1128/jb.113.3.1296-1306.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOI R. H., IGARASHI R. T. GENETIC TRANSCRIPTION DURING MORPHOGENESIS. Proc Natl Acad Sci U S A. 1964 Sep;52:755–762. doi: 10.1073/pnas.52.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCioccio R. A., Strauss N. Patterns of transcription in Bacillus subtilis during sporulation. J Mol Biol. 1973 Jun 25;77(2):325–336. doi: 10.1016/0022-2836(73)90338-0. [DOI] [PubMed] [Google Scholar]
- Doi R. H., Brown L. R., Rodgers G., Hsu Y. Bacillus subtilis mutant altered in spore morphology and in RNA polymerase activity. Proc Natl Acad Sci U S A. 1970 Jun;66(2):404–410. doi: 10.1073/pnas.66.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey C., Losick R., Sonenshein A. L. Ribosomal RNA synthesis is turned off during sporulation of Bacillus subtilis. J Mol Biol. 1971 Apr 14;57(1):59–70. doi: 10.1016/0022-2836(71)90119-7. [DOI] [PubMed] [Google Scholar]
- Korch C. T., Doi R. H. Electron microscopy of the altered spore morphology of a ribonucleic acid polymerase mutant of Bacillus subtilis. J Bacteriol. 1971 Mar;105(3):1110–1118. doi: 10.1128/jb.105.3.1110-1118.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton T. J. An RNA polymerase mutation causing temperature-sensitive sporulation in Bacillus subtilis. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1179–1183. doi: 10.1073/pnas.70.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton T. J., Doi R. H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971 May 25;246(10):3189–3195. [PubMed] [Google Scholar]
- Leighton T. J., Dor R. H., Warren R. A., Kelln R. A. The relationship of serine protease activity to RNA polymerase modification and sporulation in Bacillus subtilis. J Mol Biol. 1973 May 5;76(1):103–122. doi: 10.1016/0022-2836(73)90083-1. [DOI] [PubMed] [Google Scholar]
- Linn T. G., Greenleaf A. L., Shorenstein R. G., Losick R. Loss of the sigma activity of RNA polymerase of Bacillus subtilis during sporulation. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1865–1869. doi: 10.1073/pnas.70.6.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Shorenstein R. G., Sonenshein A. L. Structural alteration of RNA polymerase during sporulation. Nature. 1970 Aug 29;227(5261):910–913. doi: 10.1038/227910a0. [DOI] [PubMed] [Google Scholar]
- Margulies L., Remeza V., Rudner R. Asymmetric template function of microbial deoxyribonucleic acids: transcription of messenger ribonucleic acid. J Bacteriol. 1971 Sep;107(3):610–617. doi: 10.1128/jb.107.3.610-617.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies L., Remeza V., Rudner R. Asymmetric template function of microbial deoxyribonucleic acids: transcription of ribosomal and soluble ribonucleic acids. J Bacteriol. 1970 Sep;103(3):560–568. doi: 10.1128/jb.103.3.560-568.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConaughy B. L., Laird C. D., McCarthy B. J. Nucleic acid reassociation in formamide. Biochemistry. 1969 Aug;8(8):3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- Oishi M. The transcribing strands of bacillus subtilis DNA for ribosomal and transfer RNA. Proc Natl Acad Sci U S A. 1969 Jan;62(1):256–262. doi: 10.1073/pnas.62.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner R., Ledoux M., Mazelis A. Distribution of pyrimidine oligonucleotides in strands L and H of Bacillus subtilis DNA. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2745–2749. doi: 10.1073/pnas.69.9.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZULMAJSTER J., CANFIELD R. E., BLICHARSKA J. [Action of actinomycin D on the sporulation of Bacillus subtilis]. C R Hebd Seances Acad Sci. 1963 Feb 25;256:2057–2060. [PubMed] [Google Scholar]
- Santo L., Leighton T. J., Doi R. H. Ultrastructural analysis of sporulation in a conditional serine protease mutant of Bacillus subtilis. J Bacteriol. 1972 Jul;111(1):248–253. doi: 10.1128/jb.111.1.248-253.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshein A. L., Losick R. RNA polymerase mutants blocked in sporulation. Nature. 1970 Aug 29;227(5261):906–909. doi: 10.1038/227906a0. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Pratt I. Cell wall polymers of Bacillus sphaericus 9602. II. Synthesis of the first enzyme unique to cortex synthesis during sporulation. J Bacteriol. 1970 Aug;103(2):305–317. doi: 10.1128/jb.103.2.305-317.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. B., Hsu W. T., Foft J. W., Scherberg N. H. Transfer RNA coded by the T4 bacteriophage genome. Proc Natl Acad Sci U S A. 1968 Sep;61(1):114–121. doi: 10.1073/pnas.61.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa T., Doi R. H. Preferential transcription of Bacillus subtilis light deoxyribonucleic acid strands during sporulation. J Bacteriol. 1971 May;106(2):305–310. doi: 10.1128/jb.106.2.305-310.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


