Abstract
We have performed deuterium exchange mass spectrometry (DXMS) to probe the conformational changes that the bacterial MutS homodimer and the homologous eukaryotic heterodimer Msh2-Msh6 undergo when binding to ATP or DNA. The DXMS data support the view that high affinity binding to mispair-containing DNA and low affinity binding to fully base-paired DNA both involve forming rings by MutS protein family dimers around the DNA; however, mispair binding protects additional regions from deuterium exchange. DXMS also reveals two distinct conformations upon binding one or two ATP molecules and that binding of two ATP molecules propagates conformational changes to other regions of the protein complexes. The regions showing major changes in deuterium exchange upon ATP binding tend to occur in regions distinct from those involved in DNA binding, suggesting that although communication occurs between DNA and nucleotide binding, sliding clamps formed by binding both ATP and mispairs could result from the simultaneous action of two independent conformational changes.
Keywords: DNA Damage, DNA Repair, DNA Replication, Mass Spectrometry (MS), Protein-DNA Interaction
Introduction
DNA mismatch repair (MMR)3 recognizes and repairs mispaired nucleotides that arise in DNA as a result of errors during DNA replication, chemical damage to DNA and DNA precursors, and during the formation of heteroduplex recombination intermediates (1–4). The MutS homodimer detects mispairs in DNA in bacteria (2, 5–8), whereas mispaired bases in eukaryotes are recognized by two different partially redundant heterodimers of MutS homologs, Msh2-Msh6 (MutSα) and Msh2-Msh3 (MutSβ), that have different mispair binding specificities (3, 9–12). The ability of MutS, as well as Msh2-Msh6 and Msh2-Msh3, to recruit other MMR proteins and trigger downstream events after recognizing mispairs is mediated by dynamic interactions with DNA and ATP (13–20).
Extensive biochemical studies have revealed that the function of MutS and its homologs depends upon extensive communication between the two ATP-binding sites and on communication between the ATP-binding sites and the DNA-binding site, likely mediated by conformational changes that are induced in the protein. Details of these interactions appear to be generally conserved between MutS and its homologs (21, 22), but they are probably best understood in the context of Msh2-Msh6, in which the two ATP-binding sites can be distinguished. In solution, Msh2-Msh6 containing two bound ATP molecules does not form stable complexes with DNA (13); however, under conditions where ATP can be hydrolyzed, the most stable form of the protein contains ADP bound in the Msh2 nucleotide-binding site (23), and this state can bind both base-paired and mispaired DNA, albeit with a higher affinity for mispaired DNA (13, 23). Binding of Msh2-Msh6 to mispaired DNA suppresses ATP hydrolysis (24), primarily at the Msh6 site (23). Thus, mispaired DNA promotes increased steady state levels of ATP-bound Msh6, which facilitates binding of MutL homologs (23, 25) and the dissociation of ADP from the Msh2 site (23). Binding of ATP or ATPγS at the Msh2 site converts the mispair-bound form of Msh2-Msh6 into a form that slides along the DNA (23, 26), which causes rapid dissociation from free DNA ends but slow dissociation from substrates with blocked ends (13, 26). In contrast, Msh2-Msh6 bound to fully base-paired DNA does not suppress ATP hydrolysis (24), and Msh2-Msh6 directly dissociates from fully base-paired DNA when challenged with ATP or ATPγS, rather than forming a sliding clamp (13–20, 23).
Structures of mispair-bound bacterial MutS and human Msh2-Msh6 have revealed important features of how mispairs are recognized (5, 6, 27). Both complexes form asymmetric rings around DNA, with only one subunit (Msh6 in Msh2-Msh6) directly contacting the mispair by bending the DNA and stacking the mispaired base with a conserved phenylalanine of the mispair recognition domain. The ring itself consists of the core domain (domain III), the clamp domain (domain IV), and the nucleotide-binding domain (domain V). The mispair-binding domain (domain I) is linked to the connector domain (domain II), and the connector domain is linked to the core domain by what appear to be flexible linkers. Unfortunately, attempts to use x-ray crystallography to understand the induced conformational changes in MutS that have been predicted by biochemical studies by soaking crystals of MutS bound to mispaired DNA with ATP and ATP analogs have yielded only limited insight (22, 28). Thus, the nature of the dynamic conformational changes that underlie the communication between the ATP-binding sites and the DNA-binding site remains poorly understood.
In this study, we have used deuterium exchange mass spectrometry (DXMS) (29, 30) to probe the structural changes that occur when MutS and Msh2-Msh6 interact with base-paired DNA, mispaired DNA, and ATP by monitoring the solvent accessibility of main-chain amides in the protein. Our results are consistent with the MutS or Msh2-Msh6 complexes forming a ring around the DNA in the absence of a mispair but that mispair binding can be distinguished from base pair binding by additional protection of two regions of the mispair-binding domain. In addition, our results demonstrate that binding of one or two ATP molecules by Msh2-Msh6 involves two conformational changes that protect part or all of the dimeric interface between the nucleotide-binding domain, respectively. The binding of two ATPs to Msh2-Msh6 is also associated with additional changes in deuteration in Msh2-Msh6, but the inability of the dual ATP-bound form to bind DNA does not appear to be due to rigid clamp formation. Both DNA and ATP binding do, however, appear to modulate the interaction between the connector domain, which contains the MutL homolog-binding interface (31), and the core domain. Most changes in deuteration in the mispair-bound and ATP-bound MutS and Msh2-Msh6 affect different regions of the protein, suggesting that the DNA and ATP requirements for forming the ternary complex and the sliding clamp involve the sum of two independent sets of conformational changes, one driven by mispaired DNA and the other by nucleotide binding.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
His6-MutS, Msh2-Msh6, and Msh2-Msh6-S1036P were expressed in Escherichia coli and purified as described previously (23, 24, 32–34). A mutant derivative of the dual expression plasmids pET11a-MSH2-MSH6 expressing the Msh2-Msh6-S1036P complex has been described elsewhere (35).
DXMS
DXMS analysis was performed essentially as described previously (31). MutS or Msh2-Msh6 with or without GT mispair-containing DNA (71 bp), GC base pair-containing DNA of the same sequence (71 bp), ATP, and/or ATPγS in 10 μl was mixed with 30 μl of D2O-containing buffer. Final concentrations were 3.6 μm MutS and 4.0 μm DNA or 250 μm ATPγS, as indicated, in buffer containing 10 mm Tris, pH 8, 4 mm MgCl2, and 95 mm NaCl or 11 μm Msh2-Msh6 and 60 μm DNA or 250 μm ATP, as indicated, in buffer containing 10 mm Tris, pH 8, 2 mm EDTA, and 110 mm NaCl. For the low ATP samples, 4 μm Msh2-Msh6 and 4 μm ATP were used. Samples were incubated for 30, 100, 300, 1000, or 3000 s, or a subset of these times in selected experiments, at 4 °C. At the indicated times, the sample was added to vials containing 60 μl of quench solution (0.8% formic acid and 0.8 m guanidinium HCl for MutS or 0.8% formic acid and 3.2 m guanidinium HCl) and immediately frozen at −80 °C. In addition, a nondeuterated sample (incubated in H2O buffer) and a fully deuterated sample (incubated in D2O buffer containing 0.5% formic acid for 16 h at 25 °C) were prepared. The 100-μl samples were manually thawed and immediately passed through an immobilized protease column (66-μl bed volume) of porcine pepsin (Sigma) coupled to 20AL support (PerSeptive Biosystems, Foster City, CA) at a flow rate of 100 μl/min. Proteolytic fragments were collected contemporaneously on a C18 high pressure liquid chromatography reverse phase column (Vydac) and eluted by a linear gradient (5–45% solvent B in 30 min, 50 μl/min: solvent A, 0.05% trifluoroacetic acid; solvent B, 80% acetonitrile, 0.01% trifluoroacetic acid). Mass spectrometric analysis was performed using a Thermo Finnigan LCQ Classic mass spectrometer operated with capillary temperature at 200 °C and spray voltage of 5000 V. Deuterium quantification data were collected in MS1 profile mode, and peptide identification data were collected in MS2 mode. The SEQUEST software program (Thermo Finnigan Inc.) was used to identify the likely sequence of the parent peptide ions. Identified peptides were examined to determine whether the quality of the measured isotopic envelope of peptides was sufficient to allow accurate measurement of the geometric centroid of isotopic envelopes on deuterated samples. Only peptides that were unambiguously detected in all conditions for a given experiment are reported here. A smaller number of peptides, ∼5–10%, were variably detected between the differing conditions and were not reported. Specialized software was used to determine deuterium content in functionally deuterated samples as described previously (36–38). The standard deviation of deuterium incorporation measured in replicate determinations is typically less than 5% of the mean (39). Examples of data where peptide-to-peptide variation of deuteration can be seen (in either the same peptides found with different charges or different but largely overlapping peptides) are in supplemental Tables S1–S7. Molecular images in which changes in deuteration were mapped to the E. coli MutS and human Msh2-Msh6 structures were generated with PyMOL (40).
RESULTS
DXMS Analysis of MutS
We first used DXMS to examine the solvent accessibility of E. coli MutS alone as MutS has a much lower sequence complexity than Saccharomyces cerevisiae Msh2-Msh6, 91 versus 249 kDa. The extent of deuteration of MutS was first determined at six different times of exposure to deuterium ranging from 10 to 3000 s (Fig. 1A). 114 partially overlapping high quality MutS peptides were recovered and identified by mass spectrometry from the six sets of reactions, resulting in 67% coverage of MutS sequence. Three classes of peptides were observed. The first class of peptides corresponded to five regions of MutS, which were found to be completely deuterated at the earliest time point examined, 10 s (Fig. 1B). These included the N terminus, regions of the clamp domain, the nucleotide-binding domain, and the flexible linker (residues 801–820) between the nucleotide-binding domain and the C-terminal dimerization/tetramerization domain (Fig. 1, C and D) (32), indicative of contiguous stretches of unstructured regions whose amides are exposed to solvent water (41, 42). The second class of peptides corresponded to five regions of MutS and showed little or no deuterium exchange, even at the longest time point examined, 3000 s. These peptides were found in the core domain of MutS (Fig. 1, C and D) and reflect the much slower exchange rates (that can be up to 109-fold reduced) in rigid protein regions that likely only exchange during transient unfolding fluctuations. Finally, the third and largest class of peptides showed intermediate levels of deuteration where the level of deuteration observed generally increased with increasing time of incubation with deuterium and likely represent structured regions that are less ordered than the rigid regions.
FIGURE 1.
DXMS reveals regions of disorder in MutS. A, percentage deuterium incorporation for individual peptides of MutS after 30 (black) or 300 s (green) are shown as bars spanning over the indicated sequence on the x axis. Regions containing peptides that fully exchange rapidly or do not exchange at all are boxed. B, kinetics of deuteron incorporation for a representative peptide from each region boxed in A. C, regions containing extremely rapidly (red) or slowly (blue) exchanging peptides mapped onto the six domains of the MutS monomer are distinguished by color on a ribbon diagram constructed from the x-ray structures of residues 1–800 (Protein Data Bank code 1e3m (5)) and 820–853 (Protein Data Bank code 2ok2 (32)) with a linker of arbitrary conformation. D, regions from above are mapped onto a MutS dimer (right), and regions 8–10 are mapped onto a ribbon diagram of the ATPase domain (left).
To determine the effect of binding to a GT mispair on the structure of MutS, the extent of deuteration of MutS bound to a 71-bp DNA substrate containing a central GT mispair was determined at different time points ranging from 10 to 3000 s and compared with the data generated for MutS alone (Fig. 2A). For peptides with substantial differences in deuteration, the extent of deuteration was always highly visible and usually maximal at the 300-s time point (Fig. 2B); as a consequence, we have compared the extent of deuteration of peptides at the 300-s time point (Fig. 2A; supplemental Table S1). Fourteen regions of MutS showed clearly decreased deuteration (increased protection from solvent) upon GT mispair binding, whereas the extent of deuteration of the rest of the protein was within 10% of the MutS-only sample. These 14 protected regions of MutS were primarily located in the DNA-contacting domains, i.e. the mispair-binding and the clamp domains (Fig. 2C). The rate of deuterium exchange in most of the unstructured and rigid regions in the MutS-only dataset was not affected by the presence of the mispair (supplemental Fig. 1A), except for one unstructured region in the clamp domain that showed reduced deuterium exchange in the MutS-GT sample (residues 436–447; Fig. 1, region 5; Fig. 2, region 6; and supplemental Fig. 1). Intermediate exchanging regions in the MutS-alone samples with fairly high deuterium exchange (above 75% at 300 s) showed reduced deuterium exchange in the MutS-GT sample (supplemental Fig. 1B), except for the flexible linker between the nucleotide-binding domain and the connector domain, two regions of the nucleotide-binding domain, including the Walker A motif, and the flexible linker between the nucleotide-binding and dimerization/tetramerization domains. Decreased deuteration of residues 301–322 in the core domain mapped to an interface with residues 260–270 in the connector domain that also showed a slight decrease in deuteration (Fig. 2A), which suggests that mispair binding may stabilize the interaction between the connector and core domains. Decreased deuteration in the nucleotide-binding domain occurred in a region implicated in ATP hydrolysis (see “Discussion”). Additional regions showing protection also included the folded portion of the C-terminal dimerization/tetramerization domain (32).
FIGURE 2.
DNA alters solvent accessibility for regions of MutS. A, change in percent deuteration of individual peptides from MutS bound to a 71-bp DNA duplex containing a central GT mispair and MutS at 300 s. Green bars that span the indicated sequence on the x axis indicate peptides measured in both samples. Positive and negative y axis values indicate enhancement and protection from deuterium exchange, respectively, caused by addition of the DNA substrate. Regions of substantial protection are boxed. B, kinetics of deuteron incorporation for a representative peptide of MutS in the presence (red) and absence (black) of DNA for each region boxed in A. C, regions of protection upon DNA addition (blue) mapped onto the ribbon diagram of MutS.
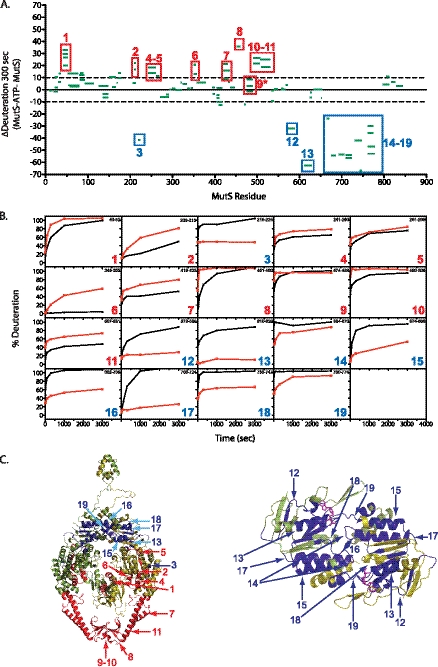
To determine the effect of ATP binding on the structure of MutS, the extent of deuteration of MutS in the presence of 250 μm ATPγS and 4 mm Mg2+ was determined at different time points ranging from 10 to 3000 s and compared with the data generated for MutS alone (Fig. 3, A and B). Unlike the changes in deuteration observed in the presence of mispairs, we observed regions of both decreased and increased deuteration in the presence of ATPγS (supplemental Table S2). The 10 regions of increased deuteration mapped primarily to the clamp domain and also included the mispair-binding, connector, and core domains. Of particular note were amino acids 349–355 at the interface between the connector and core domains, which is a very slowly exchanging region in the MutS-only samples (supplemental Fig. 2) and is consistent with ATPγS-dependent reorganization of the connector domain packing, and amino acids 209–213 in the connector domain, which showed increased deuteration in the presence of ATPγS and is part of the interface that mediates the ATP-dependent interaction of MutS with MutL (31). Three other regions of increased deuteration in the mispair-binding and clamp domains corresponded to regions of decreased deuteration in the presence of mispaired DNA (supplemental Fig. 3). Of the nine regions of MutS that had decreased deuteration in the presence of ATPγS, eight mapped to the MutS nucleotide-binding domain and were located within the series of α-helices that are predicted to pack against each other during the ATP-induced conversion of the open to closed forms of these nucleotide-binding sites as predicted from structural studies of the ATP-bound form of Rad50 (43); two of these regions were almost completely deuterated at the very earliest time points in the MutS-only samples (supplemental Fig. 2), and only the region containing amino acids 573–589, which includes part of the nucleotide-binding domain that recognizes the adenine base, had decreased deuteration in both the MutS-ATPγS and MutS-GT datasets (supplemental Fig. 3).
FIGURE 3.
ATPγS alters solvent accessibility for regions of MutS. A, change in percent deuteration of individual peptides from MutS in the presence of 250 μm ATPγS and MutS at 300 s. Peptides measured in both samples are shown as green bars spanning over the indicated sequence on the x axis. Negative and positive y axis values indicate increased protection and increased solvent accessibility, respectively, caused by addition ATPγS. Regions of substantial change upon ATPγS addition are boxed. B, kinetics of deuteron incorporation for a representative peptide of MutS in the presence (red) and absence (black) of ATPγS for each region boxed in A. C, regions of solvent protection (blue) and increased solvent accessibility (red) upon ATPγS addition are mapped onto the ribbon diagram of MutS.
DXMS Analysis of Msh2-Msh6
Analysis of the E. coli MutS DXMS data is complicated by the fact that the MutS homodimer is functionally and conformationally asymmetric (5, 6, 21), but analysis of the deuterium exchange data superimposes the results from both subunits. We therefore performed DXMS on the S. cerevisiae Msh2-Msh6 complex, which has two subunits with distinct roles in the binding of both nucleotides and DNA; this proved possible despite the fact that the Msh2-Msh6 heterodimer contains 249 kDa of unique sequence. The extent of deuteration of Msh2 and Msh6 peptides in samples containing only Msh2-Msh6 was monitored at 30 and 300 s. We analyzed 123 and 128 partially overlapping high quality peptides from Msh2 and Msh6, which corresponded to 71 and 69% sequence coverage, respectively (Fig. 4). As with MutS, these peptides fell into groups that were fully deuterated at the early time point, those resistant to deuteration at the late time point, and those with intermediate levels of deuteration where the extent of deuteration increased with increasing times of incubation. The majority of the peptides from the Msh2 and Msh6 proteins consisted of those with intermediate levels of deuteration; however, a number of regions showed extensive deuteration even at the earliest time points (Fig. 4A), including the ∼300-amino acid N terminus of Msh6 that was previously shown to be unstructured (33).
FIGURE 4.
DXMS reveals regions of disorder in Msh2-Msh6. A, percentage deuterium incorporation (y axis) is displayed for individual peptides of Msh2-Msh6 after 30 s (black bars) or 300 s (green bars) that span the indicated sequence on the x axis. Regions containing peptides that fully exchange rapidly or do not exchange at all are boxed. B, regions containing extremely rapidly (red) and slowly (blue) exchanging peptides are mapped onto the structure of the Msh2-Msh6 heterodimer. C, regions 8–9 and 19–23 are mapped onto a ribbon diagram of the ATPase domain.
To analyze the effects of DNA binding to Msh2-Msh6, we analyzed Msh2-Msh6 by DXMS in the presence of a 71-bp DNA containing either a central GT mispair or a central GC base pair. In the presence of a GT mispair, three regions of Msh2 in the clamp domain contained peptides that showed reduced deuteration (Fig. 5, A and C, and supplemental Table S3). Remarkably, domain I of Msh2, which is equivalent to the mispair binding domains of MutS and Msh6, but only makes nonspecific DNA contacts, showed no alteration in the kinetics of deuteration. In contrast, the mispair-binding domain of Msh6 showed two regions of decreased deuteration in the presence of DNA (Fig. 5, A and C, and supplemental Table S4). The first included a loop (Fig. 5, region 4) containing Phe-337 that stacks on the mispaired base. The second included a helix-loop-helix motif (Fig. 5, region 5) that had very rapid deuterium exchange in the absence of DNA (supplemental Fig. 4). This motif is predicted to interact with the DNA backbone at Arg-412 based on the MutS crystal structure, and mutations that alter Arg-412 result in MMR defects (44, 45); however, this region does not contact the DNA in the human Msh2-Msh6 crystal structure (27) due to its involvement in a crystal packing contact. Like Msh2, Msh6 also showed decreased deuteration in the clamp domain at the Msh2-Msh6 interface. We only observed one large change in the levels of deuteration of Msh2-Msh6 in the presence of mispaired DNA outside of the regions involved in DNA recognition. This region displayed increased deuteration relative to the Msh2-Msh6-only sample and was located at the C-terminal end of the Msh6 nucleotide-binding domain. Most of the changes in deuteration of the clamp domain observed upon mispair binding were also observed in experiments with fully base paired DNA (Fig. 5B), except that the overall magnitude of the protection from deuteration and the size of the protected regions were substantially decreased. This change in the magnitude of protection from deuteration may be consistent with a similar mode of binding to both mispaired and base-paired DNAs combined with a lower affinity for fully base-paired DNA. In addition, the protected regions of the mispair and backbone contacting region of the mispair-binding domain of Msh6 were not protected in the presence of fully base-paired DNA (Fig. 5B).
FIGURE 5.
DNA alters solvent accessibility for regions of Msh2-Msh6. A, change in percent deuteration of individual peptides from Msh2-Msh6 bound to a 71-bp DNA duplex containing a central GT mispair and Msh2-Msh6 at 30 s (black) and 300 s (green). Peptides measured in both samples are shown as black and green bars spanning over the indicated sequence on the x axis. Positive and negative y axis values indicate enhancement and protection from deuterium exchange, respectively, caused by addition of the DNA substrate. Regions of substantial protection are boxed. B, change in percent deuteration of individual peptides from Msh2-Msh6 bound to a 71-bp DNA duplex containing a central GC base pair and Msh2-Msh6 at 30 and 300 s as shown in A. C, regions of solvent protection (blue) and increased solvent accessibility (red) upon DNA addition are mapped onto the ribbon diagram of Msh2-Msh6.
To understand the effects of ATP binding on Msh2-Msh6, DXMS was used to analyze the solvent accessibility of Msh2-Msh6 in the presence of either 4 or 250 μm ATP in the absence of magnesium. The 4 μm ATP concentration was chosen as it should result in saturation of the high affinity nucleotide-binding site in Msh6 without significant binding to the lower affinity Msh2 nucleotide-binding site, whereas the 250 μm ATP concentration was chosen as it should result in saturation of both the Msh2 and Msh6 nucleotide-binding sites (23). Similar to MutS, most of the changes in solvent accessibility in both Msh2 and Msh6 seen at high concentrations of ATP (250 μm ATP) were increased protection from deuterium exchange (Fig. 6A). These protected regions were primarily in the nucleotide-binding domain (Fig. 6C), which contains regions that very rapidly exchange in the absence of nucleotide (supplemental Fig. S5). The nucleotide-induced reduction of deuterium exchange of these rapidly exchanging regions (Fig. 6, regions 18–21) is consistent with the protection of a proteolysis site in this region by the binding of ATP to the Msh6 nucleotide-binding site (25), and deletion of this region in Thermus aquaticus MutS causes in vivo MMR defects (46). In contrast to MutS, there were no extensive increases in deuterium exchange and hence increases in solvent accessibility in the clamp domain of either Msh2 or Msh6. However, there were a few regions of moderately increased deuteration, which mapped to the mispair-binding and core domains of Msh6, and a few regions of moderately reduced deuteration in the mispair-binding domain and the clamp domain of Msh6 (Fig. 6C). At 4 μm ATP, there were substantially fewer regions with altered solvent accessibility (Fig. 6B). These regions all showed decreased deuteration and mapped to the region immediately around the high affinity Msh6 nucleotide-binding site (Fig. 6D). The additional changes in solvent accessibility observed in other regions of Msh2 and Msh6 at 250 μm ATP (Fig. 6A) were not observed at 4 μm ATP (Fig. 6B).
FIGURE 6.
ATP(−Mg2+) alters solvent accessibility for regions of Msh2-Msh6. A, change in percent deuteration of individual peptides from Msh2-Msh6 at 250 μm ATP(−Mg2+) predicted to fill both nucleotide binding sites and Msh2-Msh6 at 30s (black) and 300s (green). Peptides measured in both samples are shown as black and green bars spanning over the indicated sequence on the x axis. Positive and negative y axis values indicate enhancement and protection from deuterium exchange, respectively, caused by addition of the ATP(−Mg2+). Regions of substantial change are boxed. B, change in percent deuteration of individual peptides from Msh2-Msh6 at 4 μm ATP(−Mg2+) predicted to only fill the Msh6 binding site and Msh2-Msh6 at 30s and 300s as shown in “A”. C, regions of solvent protection (blue) and increased solvent accessibility (red) upon 250 μm ATP(−Mg2+) addition are mapped onto the ribbon diagram of Msh2-Msh6 (left) and the Msh2-Msh6 dimeric nucleotide binding domains (right). D, regions of the nucleotide binding domains protected at the lower 4 μm ATP(−Mg2+) concentration are depicted at the bottom.
DXMS Analysis of Msh2-Msh6-S1036P
To further explore the nucleotide-triggered conformational changes of Msh2-Msh6, we also performed DXMS on an Msh2-Msh6 complex containing the Msh6-S1036P amino acid substitution. The S1036P amino acid substitution alters the ABC ATPase “signature motif” and affects an amino acid that contacts ATP in the Msh2 nucleotide-binding site. The msh6-S1036P mutation causes a dominant negative phenotype (45) and results in a mutant Msh2-Msh6 complex that binds mispaired bases but is defective for binding of ATP to the Msh2 nucleotide-binding site (47), formation of the sliding clamp on mispaired DNA, and Msh2-Msh6-Mlh1-Pms1 ternary complex formation (25, 47).
DXMS of Msh2-Msh6-S1036P in the presence of 250 μm ATPγS and 4 mm Mg2+ revealed substantially fewer changes in deuteration than seen in the wild-type Msh2-Msh6 complex (Fig. 7). A number of peptides of the Msh2-Msh6-S1036P complex near the Msh6 nucleotide-binding site were protected from deuterium exchange in the presence of ATPγS. In contrast, much of the protection from solvent accessibility of peptides from near the Msh2 nucleotide-binding site observed in wild-type Msh2-Msh6 at high nucleotide concentrations was absent; however, the observed protection at the Msh2 site was somewhat increased compared with the wild-type Msh2-Msh6 complex at 4 μm ATP. Importantly, many of the changes in solvent accessibility observed outside of the nucleotide-binding domain in the wild-type complex at the higher ATP concentration were missing in the Msh2-Msh6-S1036P complex, which is also similar to the wild-type Msh2-Msh6 complex at 4 μm ATP. Taken together, the similarity of the DXMS data for the Msh2-Msh6-S1036P at 250 μm ATPγS and the Msh2-Msh6 complex at 4 μm ATP is consistent with a model in which the defect caused by Msh6-S1036P is due to substantially reduced ATP binding to the Msh2-binding site.
FIGURE 7.
Altered deuteration of the Msh2-Msh6-S1036P complex. A, change in percent deuteration of individual peptides from Msh2-Msh6-S1036P at 250 μm ATPγS at 30s (black) and 300s (green). Peptides measured in both samples are shown as black and green bars spanning over the indicated sequence on the x axis. Positive and negative y axis values indicate enhancement and protection from deuterium exchange, respectively, caused by addition of ATPγS. Regions of substantial protection are boxed. B, regions of solvent protection (blue) upon 250 μm ATPγS addition are mapped onto the ribbon diagram of Msh2-Msh6 (left) and the Msh2-Msh6 dimeric nucleotide binding domains (right).
DISCUSSION
Biochemical and genetic studies of MutS and Msh2-Msh6 have provided extensive evidence for dynamic interactions between these proteins and both base-paired and mispaired DNA, whereas structural studies have provided a well characterized view of the static structure of these proteins bound to mispaired DNA in the presence of ADP. However, attempts to use crystallography to address the dynamic conformational changes predicted to occur in these complexes on the basis of biochemical data have been problematic, which is likely due to the constraints placed on the conformations of these complexes by the crystal lattice, such as by the effective concentrations of mispaired DNA and by crystal packing forces. Here, we have used DXMS as an indirect probe of the conformational changes in MutS or Msh2-Msh6 upon binding base-paired DNA, mispaired DNA, or ATP in solution to address various hypotheses about the dynamic structures of the MutS family of proteins as they interact with their substrates.
Binding of ATP to both MutS and Msh2-Msh6 caused extensive solvent protection along the dimer interface of the nucleotide-binding domains, consistent with an open to closed transition predicted by the increased affinities between the nucleotide-binding domains in the presence of ATP (22) and by comparison with the conformation of the ATP-bound state of related ATPases such as Rad50 (43). Comparison of ATP concentrations predicted to fill only the Msh6 nucleotide-binding site or both the Msh2 and Msh6 nucleotide-binding sites provides evidence for two conformational changes. When only the Msh6 nucleotide-binding site was filled, changes in deuteration were only seen in the region immediately around the Msh6 nucleotide-binding site, consistent with the observation of a conformational change at the Msh6 nucleotide-binding site detected using a proteolysis assay (25). Nucleotide binding in both sites appears to mediate a second conformational change that protects the entire dimeric interface between the nucleotide-binding domains. Furthermore, additional changes in deuterium exchange were present in both the Msh2-Msh6 and MutS data sets that appear to reflect conformational changes that are propagated to other regions of the complexes, including moderately increased deuterium exchange of the connector domain, which is involved in ATP- and mispair-dependent MutL homolog binding (31). It is possible that this ATP-induced conformational change is a component of the ATP- and mispaired DNA-binding induced conformational changes required for the formation of MutS-MutL and Msh2-Msh6-Mlh1-Pms1 ternary complexes. The two-stage nature of this ATP-mediated conformational change is also consistent with the pattern of changes in deuterium exchange observed with the Msh2-Msh6-S1036P, which has defects in binding ATP in the low affinity Msh2-binding site and whose defects are consistent with a Msh2-Msh6 complex with impaired ATP binding at the Msh2 site.
The ATP-bound, DNA-free forms of MutS and Msh2-Msh6 are incapable of forming stable complexes with either fully base-paired or mispair-containing DNAs. One attractive hypothesis is that the dual ATP-bound forms of MutS and Msh2-Msh6 form rigid rings that are incapable of binding DNA, similar to the sliding clamp state formed on mispaired DNA (26). Analysis of the effects of ATP binding on deuterium exchange suggests that this hypothesis is incorrect. We did not observe the same types of extensive protection of the clamp domain in MutS, Msh2, or Msh6 in the presence of ATP (Figs. 2C and 5C) that was observed upon DNA binding (Figs. 3C and 6C) or would have been anticipated from the increased ordering of clamp domain when comparing the DNA-bound and DNA-free crystal form of T. aquaticus MutS (6). On the other hand, sliding clamp formation must involve disruption of mispair recognition, and the observed increased deuteration observed in the mispair-binding and connector domains in the presence of ATP (Fig. 3C and Fig. 6C) could be consistent with conformational changes that may prevent mispair recognition in the dual ATP-bound state and thus allow the clamp structure to slide along the DNA.
There are significant differences in the binding affinity of MutS and Msh2-Msh6 for fully base-paired or mispair-containing DNA (9) and how these protein-DNA complexes respond to challenge with ATP; however, it has not been clear whether these differences reflect different modes of binding DNA (e.g. nonspecific binding to base-paired DNA versus binding as a ring on mispaired DNA) or similar DNA-binding modes that respond differently to challenge with ATP. Importantly, we observed that when both MutS and Msh2-Msh6 are bound to base-paired and mispaired DNA, the clamp domains of MutS, Msh2, and Msh6 that wrap around the DNA showed similar patterns of reduced deuterium exchange. These data are consistent with the view that rings are formed around DNA independently of whether a mispair is present in the DNA and that the differences between binding mispaired DNAs versus base-paired DNAs are more subtle, likely reflecting the additional interaction of the mispair binding domain with the mispair.
Detailed examination of differences in solvent accessibility gives some clues for how ATP binding induces conversion of the mispair-bound form of these proteins to sliding clamps while inducing direct dissociation of the base pair-bound forms of these proteins. Decreased deuteration of two regions of the mispair-binding domain was observed in the presence of a mispair that was not observed when a base pair was present. These two regions mapped to the mispair-contacting region of Msh6 and contained the mispair-contacting residue Phe-337 and the DNA backbone-contacting residue Arg-412 (5, 6, 27). Genetic studies have shown that these two residues are required for MMR in contrast to most of the other nonspecific contacts seen in crystal structures in MutS and Msh2-Msh6 that are generally not required for MMR (44, 45, 48, 49). Overall, these results support three hypotheses about the interactions between these two regions of Msh6 and MutS and mispaired DNA. First, these interactions are responsible for the structural changes in MutS and Msh6 induced by mispair binding that result in decreased deuteration of Msh6. Second, these interactions and the structural changes they induce are likely responsible for the increased affinity of MutS and Msh2-Msh6 for mispaired DNA versus base-paired DNA. Finally, these interactions likely underlie the differences in response to ATP binding by the mispair-bound versus the base pair-bound forms of MutS and Msh2-Msh6, possibly by altering the relative stabilities of different conformations and/or the relative kinetics by which a particular conformation is formed.
Mispair binding inhibits pre-steady state ATP hydrolysis by MutS and Msh2-Msh6 (24), and in Msh2-Msh6 this inhibition appears to occur at the Msh6 nucleotide-binding site (23). Analysis of the regions of reduced deuteration in MutS in the presence of mispaired DNA could be consistent with a conformational change being propagated from the DNA-binding site through the core domain into the nucleotide-binding domain (Fig. 2C). Intriguingly, the regions of increased protection in the nucleotide-binding domain corresponded to the part of the protein that recognizes the adenine base in ATP (amino acids 573–609) and a region adjacent to Walker B (amino acids 725–729) that includes His-728. His-728 is conserved and is at the correct location to coordinate and/or activate a water molecule for ATP hydrolysis, consistent with the hypothesis that conformational changes in this region affect ATP hydrolysis. Consistent with this, a mutation causing an amino acid substitution (H1096A) in S. cerevisiae MSH6 causes MMR defects (45) and reduces the catalytic efficiency of ATP hydrolysis (50). However, we did not observe a similar alteration of deuteration of Msh2-Msh6 in the presence of mispaired DNA (Fig. 5C). It is possible that our data cannot distinguish between the possibilities that MutS and Msh2-Msh6 might differ in their mechanisms for mispair suppression of ATPase activity or that they share a conserved mechanism that is not detectable in Msh2-Msh6 by changes in protein backbone deuteration, consistent with the extensive protection of the Msh6 His-1096 region from deuteration even in the absence of mispaired DNA.
In conclusion, DXMS has provided a sensitive method that has allowed us to begin to probe the structures of some of the less stable, dynamic forms of MutS and Msh2-Msh6. Remarkably, the regions undergoing changes in deuteration in Msh2-Msh6 under DNA and ATP binding conditions tend to be distinct, with the exception of the Msh6 region containing amino acids 1149–1172 (supplemental Table 4), which suggests that ternary complex and sliding clamp formation may occur through the action of two independent conformational changes occurring simultaneously. A similar pattern was also observed with MutS (supplemental Fig. 3). Taken together, these results provide evidence for conformational changes in the MutS family of proteins that have yet to be observed by crystallography, many of which should allow the development of hypotheses about how MMR is coordinated in vivo and what specific defects in these conformational changes are caused by mutations in genes encoding MutS family proteins that cause biochemical defects in MMR.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants GM50006 and CA92584 (to R. D. K.), AI076961, AI081982, AI2008031, AI072106, AI068730, GM037684, GM020501, and GM066170 (to V. L. W.), and Grants CA099835 and CA118595 from Innovative Technologies for the Molecular Analysis of Cancer Program (to V. L. W.). This work was also supported by Discovery Grant UC10591 from the University of California Industry-University Cooperative Research Program, BiogenIDEC corporate sponsor (to V. L. W.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–5 and Tables S1–S7.
- MMR
- mismatch repair
- ATPγS
- adenosine 5′-O-(thiotriphosphate)
- DXMS
- deuterium exchange mass spectrometry.
REFERENCES
- 1.Schofield M. J., Hsieh P. (2003) Annu. Rev. Microbiol. 57, 579–608 [DOI] [PubMed] [Google Scholar]
- 2.Marti T. M., Kunz C., Fleck O. (2002) J. Cell. Physiol. 191, 28–41 [DOI] [PubMed] [Google Scholar]
- 3.Iyer R. R., Pluciennik A., Burdett V., Modrich P. L. (2006) Chem. Rev. 106, 302–323 [DOI] [PubMed] [Google Scholar]
- 4.Li G. M. (2008) Cell Res. 18, 85–98 [DOI] [PubMed] [Google Scholar]
- 5.Lamers M. H., Perrakis A., Enzlin J. H., Winterwerp H. H., de Wind N., Sixma T. K. (2000) Nature 407, 711–717 [DOI] [PubMed] [Google Scholar]
- 6.Obmolova G., Ban C., Hsieh P., Yang W. (2000) Nature 407, 703–710 [DOI] [PubMed] [Google Scholar]
- 7.Su S. S., Modrich P. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 5057–5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiricny J. (2006) Nat. Rev. Mol. Cell Biol. 7, 335–346 [DOI] [PubMed] [Google Scholar]
- 9.Marsischky G. T., Kolodner R. D. (1999) J. Biol. Chem. 274, 26668–26682 [DOI] [PubMed] [Google Scholar]
- 10.Marsischky G. T., Filosi N., Kane M. F., Kolodner R. (1996) Genes Dev. 10, 407–420 [DOI] [PubMed] [Google Scholar]
- 11.Acharya S., Wilson T., Gradia S., Kane M. F., Guerrette S., Marsischky G. T., Kolodner R., Fishel R. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 13629–13634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genschel J., Littman S. J., Drummond J. T., Modrich P. (1998) J. Biol. Chem. 273, 19895–19901 [DOI] [PubMed] [Google Scholar]
- 13.Mendillo M. L., Mazur D. J., Kolodner R. D. (2005) J. Biol. Chem. 280, 22245–22257 [DOI] [PubMed] [Google Scholar]
- 14.Selmane T., Schofield M. J., Nayak S., Du C., Hsieh P. (2003) J. Mol. Biol. 334, 949–965 [DOI] [PubMed] [Google Scholar]
- 15.Acharya S., Foster P. L., Brooks P., Fishel R. (2003) Mol. Cell 12, 233–246 [DOI] [PubMed] [Google Scholar]
- 16.Blackwell L. J., Bjornson K. P., Allen D. J., Modrich P. (2001) J. Biol. Chem. 276, 34339–34347 [DOI] [PubMed] [Google Scholar]
- 17.Flores-Rozas H., Kolodner R. D. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 12404–12409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grilley M., Welsh K. M., Su S. S., Modrich P. (1989) J. Biol. Chem. 264, 1000–1004 [PubMed] [Google Scholar]
- 19.Hall M. C., Matson S. W. (1999) J. Biol. Chem. 274, 1306–1312 [DOI] [PubMed] [Google Scholar]
- 20.Räschle M., Marra G., Nyström-Lahti M., Schär P., Jiricny J. (1999) J. Biol. Chem. 274, 32368–32375 [DOI] [PubMed] [Google Scholar]
- 21.Lamers M. H., Winterwerp H. H., Sixma T. K. (2003) EMBO J. 22, 746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamers M. H., Georgijevic D., Lebbink J. H., Winterwerp H. H., Agianian B., de Wind N., Sixma T. K. (2004) J. Biol. Chem. 279, 43879–43885 [DOI] [PubMed] [Google Scholar]
- 23.Mazur D. J., Mendillo M. L., Kolodner R. D. (2006) Mol. Cell 22, 39–49 [DOI] [PubMed] [Google Scholar]
- 24.Antony E., Hingorani M. M. (2003) Biochemistry 42, 7682–7693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hargreaves V. V., Shell S. S., Mazur D. J., Hess H. T., Kolodner R. D. (2010) J. Biol. Chem. 285, 9301–9310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gradia S., Subramanian D., Wilson T., Acharya S., Makhov A., Griffith J., Fishel R. (1999) Mol. Cell 3, 255–261 [DOI] [PubMed] [Google Scholar]
- 27.Warren J. J., Pohlhaus T. J., Changela A., Iyer R. R., Modrich P. L., Beese L. S. (2007) Mol. Cell 26, 579–592 [DOI] [PubMed] [Google Scholar]
- 28.Alani E., Lee J. Y., Schofield M. J., Kijas A. W., Hsieh P., Yang W. (2003) J. Biol. Chem. 278, 16088–16094 [DOI] [PubMed] [Google Scholar]
- 29.Wales T. E., Engen J. R. (2006) Mass Spectrom. Rev. 25, 158–170 [DOI] [PubMed] [Google Scholar]
- 30.Hoofnagle A. N., Resing K. A., Ahn N. G. (2003) Annu. Rev. Biophys. Biomol. Struct. 32, 1–25 [DOI] [PubMed] [Google Scholar]
- 31.Mendillo M. L., Hargreaves V. V., Jamison J. W., Mo A. O., Li S., Putnam C. D., Woods V. L., Jr., Kolodner R. D. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 22223–22228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendillo M. L., Putnam C. D., Kolodner R. D. (2007) J. Biol. Chem. 282, 16345–16354 [DOI] [PubMed] [Google Scholar]
- 33.Shell S. S., Putnam C. D., Kolodner R. D. (2007) Mol. Cell 26, 565–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng G., Winkler M. E. (1995) BioTechniques 19, 956–965 [PubMed] [Google Scholar]
- 35.Shell S. S., Putnam C. D., Kolodner R. D. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 10956–10961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Black B. E., Foltz D. R., Chakravarthy S., Luger K., Woods V. L., Jr., Cleveland D. W. (2004) Nature 430, 578–582 [DOI] [PubMed] [Google Scholar]
- 37.Englander J. J., Del Mar C., Li W., Englander S. W., Kim J. S., Stranz D. D., Hamuro Y., Woods V. L., Jr. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 7057–7062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pantazatos D., Kim J. S., Klock H. E., Stevens R. C., Wilson I. A., Lesley S. A., Woods V. L., Jr. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 751–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu S., Kim Y., Li S., Durrant E. S., Pace R. M., Woods V. L., Jr., Gentry M. S. (2009) Biochemistry 48, 9891–9902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeLano W. L. (2002) The PyMOL User's Manual, DeLano Scientific, San Carlos, CA [Google Scholar]
- 41.Englander S. W., Englander J. J. (1994) Methods Enzymol. 232, 26–42 [DOI] [PubMed] [Google Scholar]
- 42.Bai Y., Englander J. J., Mayne L., Milne J. S., Englander S. W. (1995) Methods Enzymol. 259, 344–356 [DOI] [PubMed] [Google Scholar]
- 43.Hopfner K. P., Karcher A., Shin D. S., Craig L., Arthur L. M., Carney J. P., Tainer J. A. (2000) Cell 101, 789–800 [DOI] [PubMed] [Google Scholar]
- 44.Drotschmann K., Yang W., Brownewell F. E., Kool E. T., Kunkel T. A. (2001) J. Biol. Chem. 276, 46225–46229 [DOI] [PubMed] [Google Scholar]
- 45.Das Gupta R., Kolodner R. D. (2000) Nat. Genet. 24, 53–56 [DOI] [PubMed] [Google Scholar]
- 46.Biswas I., Obmolova G., Takahashi M., Herr A., Newman M. A., Yang W., Hsieh P. (2001) J. Mol. Biol. 305, 805–816 [DOI] [PubMed] [Google Scholar]
- 47.Hess M. T., Mendillo M. L., Mazur D. J., Kolodner R. D. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 558–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamamoto A., Schofield M. J., Biswas I., Hsieh P. (2000) Nucleic Acids Res. 28, 3564–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bowers J., Sokolsky T., Quach T., Alani E. (1999) J. Biol. Chem. 274, 16115–16125 [DOI] [PubMed] [Google Scholar]
- 50.Hess M. T., Gupta R. D., Kolodner R. D. (2002) J. Biol. Chem. 277, 25545–25553 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.