Summary
Plant cell signaling triggers the abscission of entire organs, such as fruit, leaves and flowers. Previously, we characterized an ADP-ribosylation factor GTPase-activating protein, NEVERSHED (NEV), that regulates membrane trafficking and is essential for floral organ shedding in Arabidopsis. Through a screen for mutations that restore organ separation in nev flowers, we have identified a leucine-rich repeat receptor-like kinase, EVERSHED (EVR), that functions as an inhibitor of abscission. Defects in the Golgi structure and location of the trans-Golgi network in nev abscission zone cells are rescued by a mutation in EVR, suggesting that EVR might regulate membrane trafficking during abscission. In addition to shedding their floral organs prematurely, nev evr flowers show enlarged abscission zones. A similar phenotype was reported for plants ectopically expressing INFLORESCENCE DEFICIENT IN ABSCISSION, a predicted signaling ligand for the HAESA/HAESA-LIKE2 receptor-like kinases, indicating that this signaling pathway may be constitutively active in nev evr flowers. We present a model in which EVR modulates the timing and region of abscission by promoting the internalization of other receptor-like kinases from the plasma membrane.
Keywords: SOBIR1, Receptor-like kinase, Membrane trafficking, Abscission, ARF-GAP, NEVERSHED, Arabidopsis
INTRODUCTION
Plants have evolved expansive groups of transmembrane receptor-like kinases (RLKs) that allow complex sampling of environmental conditions and developmental status. With over 600 RLKs annotated in the Arabidopsis genome, signaling complexity is enhanced by genetic redundancy (Shiu and Bleecker, 2001). Allowing for even further complexity, RLKs containing extracellular leucine-rich repeats (LRRs) can oligomerize and participate in multiple receptor complexes (Dievárt and Clark, 2003). For example, by interacting with the ligand-binding LRR-RLKs BRASSINOSTEROID INSENSITIVE1 (BRI1) and FLAGELLIN-SENSITIVE2 (FLS2), BRI1-ASSOCIATED RECEPTOR KINASE1 (BAK1) functions in two discrete signaling complexes that regulate cell growth and pathogen defense, respectively (Li et al., 2002; Chinchilla et al., 2007).
Although RLKs are expected to play crucial roles in plant development, deduction of these roles has often required the phenotypical analysis of multiple loss-of-function mutants (Shpak et al., 2004; DeYoung et al., 2006; Morillo and Tax, 2006; Xu et al., 2008). A recent breakthrough is the discovery that a pair of LRR-RLKs, HAESA (HAE) and HAESA-LIKE2 (HSL2), are redundantly required for floral organ abscission (Jinn et al., 2000; Cho et al., 2008; Stenvik et al., 2008). Whereas the outer whorls of the Arabidopsis flower, containing the sepals, petals and stamens, are shed following fertilization in wild type, floral organs remain attached indefinitely in hae hsl2 flowers. As shown for other LRR-RLKs (Li et al., 2002; Kinoshita et al., 2005; Karlova et al., 2006; Chinchilla et al., 2007), HAE and HSL2 probably function as members of larger receptor complexes that are activated through interactions with an extracellular ligand. A small, secreted peptide, INFLORESCENCE DEFICIENT IN ABSCISSION (IDA), is required for abscission and is likely to be the ligand for HAE/HSL2 (Butenko et al., 2003; Cho et al., 2008; Stenvik et al., 2008; Butenko et al., 2009).
With the identification of these and other mutations that block or delay floral organ shedding (reviewed by Leslie et al., 2007; González-Carranza et al., 2007; McKim et al., 2008; Ogawa et al., 2009; Liljegren et al., 2009), the Arabidopsis flower has become a model for studying abscission. In plants, abscission events allow the shedding of leaves, flowers, fruit and seeds, and can facilitate growth, reproduction, and defense against pathogens. As with most developmental events, proper timing and spacing are crucial during organ separation (Roberts and González-Carranza, 2007). In order for shedding to occur at the correct time, cell wall modifying enzymes must be secreted from cells within abscission zones (AZs) at the base of each organ to be shed (Roberts et al., 2002). If these enzymes are released too soon, premature organ loss could have an irreparable effect on reproduction (e.g. shedding of stamens before pollination). Conversely, a delay or block in abscission can also have an adverse effect on plant growth and reproduction (e.g. lack of seed dispersal) (Pinyopich et al., 2003). Of equal importance is the spatial regulation of abscission; the release of cell wall modifying enzymes must be restricted to discrete AZs to prevent general loss of tissue integrity or the shedding of neighboring organs. For example, misexpression of IDA leads to the deregulated expansion of floral AZ cells and can cause premature organ shedding, as well as ectopic loss of developing fruit (Stenvik et al., 2006; Cho et al., 2008).
Membrane trafficking is emerging as an additional means of regulating plant transmembrane RLKs and is likely to modulate signaling during complex developmental events (Geldner and Robatzek, 2008). We recently reported that NEVERSHED (NEV), an ADP-ribosylation factor GTPase-activating protein, is required for proper membrane trafficking and abscission (Liljegren et al., 2009). Mutations in NEV lead to altered organization of the Golgi and the associated trans-Golgi network, the accumulation of vesicles in paramural bodies, impaired fruit growth, and a block in organ separation. We hypothesized that NEV, which is localized to the trans-Golgi network and other distinct endosomal compartments, is required for the proper trafficking of key abscission factors.
Here, we report that mutations in EVERSHED (EVR), which encodes an LRR-RLK, rescue organ shedding in nev flowers. The sepals, petals and stamens of nev evr flowers are shed prematurely, and ectopic AZ cell expansion occurs. Loss of EVR kinase activity also restores normal Golgi morphology in nev AZ cells. Our results suggest that the EVR RLK functions as an inhibitor of abscission.
MATERIALS AND METHODS
Plants
The evr-1 and evr-2 alleles were obtained through ethyl methanesulfonate screens of nev-3 (Landsberg erecta, Ler) plants as described (Liljegren et al., 2000). The evr-3 (sobir1-12; SALK_050715) and evr-4 (srrlk-2; SALK_009453) alleles (Col) contain T-DNA insertions within codons 516 and 600, respectively (Alonso et al., 2003; Gao et al., 2009; Katiyar-Agarwal et al., 2007). The At1g17750 (SALK_098161) allele contains an insertion within codon 137. Genotyping primers and restriction enzymes are listed in Table S1 in the supplementary material. Mutant alleles described previously include nev-2, nev-3, nev-6, ida-2, hae-1, hsl2-1 and pepr1-1 (SALK_014538) (Yamaguchi et al., 2006; Cho et al., 2008; Stenvik et al., 2008; Liljegren et al., 2009).
A 759-bp region of EVR, from 750 nucleotides upstream of the predicted translational start site into the third codon, was PCR amplified from Col DNA with 5′-GATCAAGCTTTCACTATGGAACCCACAG-3′ and 5′-GATCTCTAGAACAGCCATTTTAATTAGAG-3. This fragment was cloned into pCR2.1 (Life Technologies, Carlsbad, CA, USA), excised with HindIII/XbaI, and cloned into pDW137 (Blázquez et al., 1997) to create a translational fusion of the EVR promoter to β-glucuronidase (GUS). A 1689-bp region of HAE (Jinn et al., 2000) was amplified from Col DNA with 5′-GTTTCTGTTGCATGTCAGGATTAGC-3′ and 5′-GGATCCAGCATTTTTTTGGAAAAGGAATCG-3′, cloned into pCR2.1, excised with XbaI/BamHI, and cloned into pDW137. Eleven out of 12 HAE::GUS T1 lines showed AZ expression. GUS expression was analyzed as described (Blázquez et al., 1997).
A 2670-bp region of EVR, including the predicted promoter and open reading frame, was amplified from Col DNA with 5′-CACCTCACTATGGAACCCACAGCG-3′ and 5′-GTGCTTGATCTGGGACAACATGGTC-3′, cloned into pENTR/D-TOPO (Life Technologies) and recombined into pGWB40 to add a YFP C-terminal tag (Nakagawa et al., 2007). Sixty-four EVR::EVR-YFP T1 lines were generated.
To generate 35S::IDA-GFP/GUS plants, a tandem repeat of the 35S promoter was excised as an EcoRI/HindIII fragment from pBIN-JIT (Ferrandiz et al., 2000) and cloned in pBluescriptIISK(+) (Stratagene, La Jolla, CA, USA). The IDA cDNA was amplified with 5′-AAGCTTGACCCTTCATTCATTTACTC-3′ and 5′-GTCGACTGAGGAAGAGAGTTAACAAAAGAG-3′ from Col DNA, cloned into pCR2.1, excised with HindIII/SalI, and cloned into the 35S/pBluescript construct. The 35S::IDA fragment was excised with SacI/SalI, and replaced the 35S::CLV3 fragment in the pBGF-0 vector (Chytilova et al., 1999; Rojo et al., 2002) to create a translational fusion of IDA to GFP/GUS. Twelve out of 68 T1 lines showed the described phenotype.
Mapping
To determine the identity of EVR, the nev-3 evr-1 mutant was crossed to nev-6 (Col). Using 631 F2 plants and PCR-based markers, including several designed from Ler polymorphisms (http://www.Arabidopsis.org/Cereon/), the evr-1 mutation was mapped to a 122-kb interval on chromosome 2, between the CER100665 and CER103194 markers.
Kinase activity
EVR kinase domains were amplified with 5′-CACCAGAGGATCAGAAAAACCACCAGG-3′ and 5′-CTAGTGCTTGATCTGGGACAACATG-3′ from Col and evr-2 DNA, to create KDWT and KDE407K, respectively. Fragments were cloned into pENTR/D-TOPO. Site-directed mutagenesis (Stratagene) with 5′-GGAAGATCATAGCTGTGGAGAAAGTGATCCAACCG-3′ and 5′-CGGTTGGATCACTTTCTCCACAGCTATGATCTTCC-3′ was used to generate EVR-KDK377E. Recombination with pDEST17 (Life Technologies) generated N-terminal 6×His-tagged EVR kinase domains for expression in Escherichia coli. Recombinant proteins were purified by Co2+ affinity chromatography (Clontech Laboratories, Mountain View, CA, USA).
To generate EVR-specific antiserum, a C-terminal peptide, CTLDDPKQRPNSKDVRTMLSQIKH, was synthesized and used to immunize chickens (Aves Labs, Tigard, Oregon, USA). Antisera were used at the following dilutions: anti-EVR (1:10,000), anti-phosphoserine (Sigma-Aldrich; 1:2000), anti-phosphotyrosine (Sigma-Aldrich; 1:2000), anti-phosphothreonine (Zymed/Invitrogen; 1:800). HRP-conjugated goat anti-chicken (Abcam, Cambridge, MA, USA) and chicken anti-mouse (Santa Cruz Biotechnology, Santa Cruz, CA, USA) secondary antibodies were used at a 1:10,000 dilution.
Microscopy
For scanning electron microscopy, flowers were fixed in 2% glutaraldehyde in 0.05 M sodium phosphate buffer, treated with 2% osmium tetroxide and dehydrated through an ethanol series. Samples were dried using a Samdri-795 critical point dryer (Tousimis Research Corporation, Rockville, MD, USA) and coated in gold-palladium using a Hummer X sputtering system (Anatech, Alexandria, VA, USA). Images were captured using a Zeiss Supra 25 scanning electron microscope with SmartSEM acquisition and imaging software. For transmission electron microscopy, flowers were analyzed as described (Liljegren et al., 2009). Confocal laser scanning microscopy of leaf petiole (stem), root, and cotyledon epidermal cells was performed with an LSM-510 (Carl Zeiss, Thornwood, NY, USA). Image brightness and contrast were adjusted with Adobe Photoshop CS4.
RESULTS
Mutations in EVR restore floral organ shedding in nev plants
Wild-type floral organs are shed after fertilization, whereas floral organs senesce and remain attached indefinitely in nevershed (nev) flowers (Fig. 1A,B). To identify novel regulators of abscission, we carried out a suppressor screen for mutations that restore floral organ shedding in nev-3 plants (M.W.L., E. Fulcher and S.J.L., unpublished). Two recessive mutations identified in this screen form a complementation group that we have named evershed (evr) (Fig. 1C; see also Fig. S1A in the supplementary material). The interaction between NEV and EVR was subsequently observed in multiple allele combinations (see Fig. S1C-F in the supplementary material). Because the phenotypes of nev evr-1 and nev evr-2 flowers are indistinguishable, analysis was primarily performed using the evr-2 allele.
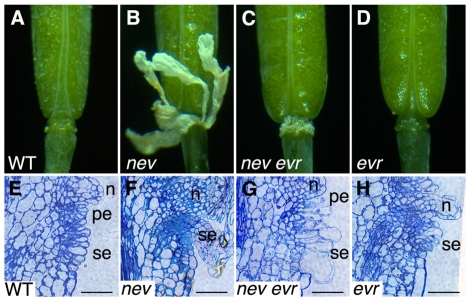
Fig. 1.
Mutations in EVR rescue organ separation in nev flowers. (A) Wild-type flower after abscission (stage 17). (B) nev mutant flowers (stage 17) retain their floral organs indefinitely. (C) Organ shedding is rescued in nev evr flowers (stage 17). (D) evr flower after abscission (stage 17). (E-H) Longitudinal sections of wild-type (E), nev (F), nev evr (G) and evr (H) flowers (stage 16) stained with Toluidine Blue. In nev evr flowers (G), the remaining abscission zone (AZ) cells expand to a greater extent than do those of wild type (E). The sepal (se) and petal (pe) AZ regions are indicated, as are the nectaries (n). Scale bars: 50 μm.
Mutations in NEV block abscission during the separation phase; the early patterning and differentiation of nev abscission zone (AZ) cells are unaffected (Liljegren et al., 2009). The secondary loss of EVR restored cell separation within nev AZs, such that all of the outer floral organs were shed from nev evr flowers (Fig. 1C; see also Fig. S1A in the supplementary material). As the densely cytoplasmic cells of nev sepal and petal AZs fail to become vacuolated and expand as they do in wild type (Fig. 1E,F) (Liljegren et al., 2009), we examined longitudinal sections of nev evr flowers at the time of shedding to see whether these defects were rescued by mutations in EVR. We found that cell vacuolation and expansion are not only rescued, but that nev evr AZ cells expand to a greater extent than do those of wild type (Fig. 1E-G).
To determine whether mutations in EVR affect organ separation in otherwise wild-type flowers, we isolated evr-1 and evr-2 single mutants. Floral organ shedding and AZ expansion appeared to be unaffected by loss of EVR alone compared with wild type (Fig. 1A,D,E,H; see also Fig. S1B in the supplementary material).
Organ abscission occurs prematurely in nev evr flowers
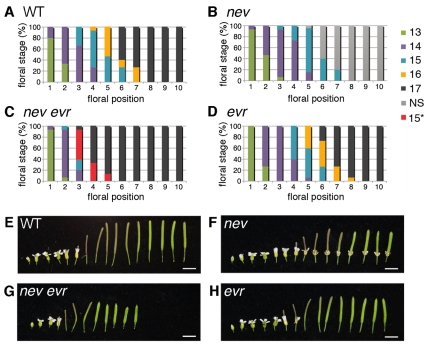
By examining the progression through flower development, we discovered that nev evr floral organs are shed prematurely (Fig. 2A,C,E,G). In wild-type flowers, buds open and pollen grains are released by the anthers (stage 13); fertilization of the ovules occurs shortly thereafter (stage 14) (Müller, 1961; Smyth et al., 1990). After the fruit begins to elongate (stage 15), the outer floral organs start to senesce and undergo abscission (stage 16) (Fang and Fernandez, 2002). To compare these developmental stages in wild-type and mutant flowers, the youngest open bud (stage 13) at the inflorescence apex was defined as the first floral position, the next older flower produced as the second position, and so on (Patterson, 2001). The percentage of flowers at each floral stage (13-17) was assessed for positions one to ten. Whereas all floral organs were shed (stage 17) from wild-type inflorescences by position 8, shedding was complete in nev evr inflorescences by position 6 (Fig. 2A,C). In nev evr flowers, abscission occurred before the petals wither, at a developmental timepoint resembling stage 15 (Fig. 2C, stage 15*) rather than stage 16. Similar results were obtained with petal breakstrength assays (Lease et al., 2006) of wild-type, nev and nev evr flowers. Whereas a similar force was required to remove petals from nev flowers at each position, the force required to remove nev evr petals dropped to zero by position 4, one position earlier than for wild type (see Fig. S2 in the supplementary material). These results suggest that EVR prevents premature organ abscission during the transition between fertilization and early fruit development.
Fig. 2.
Abscission occurs prematurely in nev evr flowers. Progression of flower development in wild-type, nev, nev evr and evr plants from the first open flower to maturing fruit. (A-D) From the first open flower (position 1), stages were assessed for up to 10 flowers per inflorescence (n=15 inflorescences per genotype). For each position, the percentage of flowers at each stage is shown. In wild-type flowers (A), organ separation (stage 16) is first observed at position 5.7±1.0. By position 8, all flowers have shed their organs (stage 17). nev flowers (B) retain their floral organs and are labeled as NS (non-shedding, stage 16 on). In nev evr flowers (C), organ separation (stage 15*) is first observed at position 3.4±0.5 and is complete by position 6. In evr flowers (D), organ separation (stage 16) is first observed at position 5.9±0.8 and is complete by position 9. (E-H) evr-2 fruit (stage 17; 8.7±0.3 mm; n=11) are 79% the length of wild type (11.0±0.5 mm; n=11). nev-3 evr-2 fruit (6.4±0.3 mm; n=13) are 88% the length of nev-3 fruit (7.3±0.5 mm; n=15). Scale bars: 5 mm.
nev and nev evr mutants show other growth defects, including reduced stature (see Fig. S3A in the supplementary material) and decreased fruit growth (Fig. 2F,G) (Liljegren et al., 2009). To confirm that premature shedding of nev evr floral organs was not due to a general defect in the timing of floral meristem formation, we also tracked individual wild-type and mutant flowers over the course of 5 days, from bud opening (day 0; stage 13) to the completion of abscission (stage 17). Whereas wild-type floral organs began shedding 3 days post-bud opening, nev evr floral organs began shedding one day earlier (see Fig. S3B in the supplementary material). These results suggest that nev evr floral organs abscise prematurely with respect to both floral age and stage of development.
Mutations in EVR alone did not substantially affect the timing of abscission (Fig. 2D,H; see also Fig. S3B in the supplementary material). However, evr fruit were significantly shorter (79%) than wild type (Fig. 2E,H), and nev evr fruit were shorter (88%) than nev fruit (Fig. 2F,G).
AZ regions in nev evr flowers are larger and show increased cell expansion
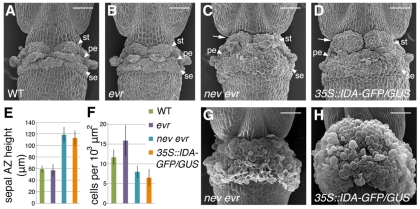
We found that expansion of nev evr AZ cells is increased in comparison to wild type at the time of shedding (Fig. 1E,G). To further characterize this phenotype, we took scanning electron micrographs (SEMs) of wild-type, evr and nev evr flowers after organ separation was complete (youngest stage 17). In wild-type flowers, discrete AZs were found at the base of the developing fruit (Fig. 3A). Loss of EVR alone did not appear to affect AZ development in comparison to wild type (Fig. 3B). By contrast, nev evr AZs were less defined and appeared to expand into the neighboring fruit and stem tissues (Fig. 3C). In a comparison of sepal AZ height in stage 17 SEMs, we found that nev evr sepal AZs were 2-fold taller than either wild-type or evr sepal AZs (Fig. 3E). In addition, the stem-like base of the fruit known as the gynophore was hidden owing to expansion of the stamen AZs in mature nev evr flowers (Fig. 3C, arrow). Cells within nev evr sepal AZs were also significantly larger in comparison to wild-type and evr cells (cells/103 μm2 measurement, Fig. 3F), indicating that cell expansion contributes in part to the increase in AZ size. In older flowers, the cell expansion was even more striking — discrete AZs were less recognizable and the floral nectaries became enveloped in the expanding tissue (Fig. 3G). Later on, the expanded nev evr AZ cells burst, leaving behind a visible collar of rough tissue around the base of the fruit (Fig. 1C).
Fig. 3.
Ectopic AZ cell expansion occurs in nev evr flowers. (A-D) Scanning electron micrographs (SEMs) of flowers immediately after organ separation (first stage 17 flower). Owing to increased cell expansion and the presence of additional cells, nev evr flowers (C), like flowers constitutively expressing IDA (D), develop larger AZs than do wild-type (A) and evr (B) flowers, which cover the stem-like gynophore of the fruit (arrows). (E,F) Quantification of AZ size (E) and cell expansion (F) in wild-type and mutant flowers (n≥4, second stage 17 flower). In nev evr and 35S::IDA-GFP/GUS flowers, the average heights of the sepal AZs are 2-fold greater than those of wild-type or evr flowers (E). A decrease in the number of sepal AZ cells in a defined area was observed for nev evr and 35S::IDA-GFP/GUS flowers compared with wild-type and evr flowers, suggesting that cell expansion contributes in part to the increase in AZ size (F). (G,H) The expanding AZs of older nev evr (G) and 35S::IDA-GFP/GUS (Col) (H) flowers (stage 17) envelop the nectaries and form visible collars of tissue at the fruit bases. The sepal (se), petal (pe) and stamen (st) AZs cannot be distinguished. Scale bars: 100 μm.
A similar phenotype was reported for flowers misexpressing the putative signaling ligand INFLORESCENCE DEFICIENT IN ABSCISSION (IDA) (Stenvik et al., 2006; Cho et al., 2008). We generated plants that constitutively express an IDA-GFP/GUS translational fusion under the control of the viral 35S promoter. We also observed enlargement of the floral AZs and overexpansion of individual AZ cells (Fig. 3D-F,H). Similar to what we observed in nev evr flowers, premature abscission of the sepals, petals and stamens has been reported for 35S::IDA flowers (Stenvik et al., 2006). These results suggest that the regulation of AZ size and cell expansion by NEV, EVR and IDA is important for the proper timing of organ shedding in Arabidopsis.
EVR encodes a membrane-localized LRR-RLK with dual specificity
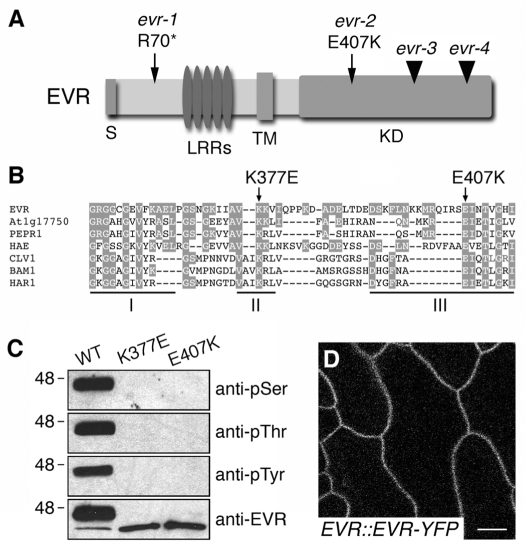
The nev evr phenotype suggests an important role for EVR in abscission. To clarify its mechanism of action, we identified single nucleotide changes for the evr-1 and evr-2 alleles within At2g31880, one of the 34 predicted genes in the mapping interval. This intronless gene encodes a 641 amino acid protein of the leucine-rich repeat receptor-like kinase (LRR-RLK) family (Fig. 4A). Eleven additional alleles of EVR were identified in a screen for mutations that rescue seedling lethality in bak1-interacting receptor-like kinase1 (bir1) mutants (Gao et al., 2009). Loss of BIR1 is predicted to cause constitutive activation of a SUPPRESSOR OF BIR1 (SOBIR1)/EVR-mediated signaling pathway for disease resistance (Gao et al., 2009). Also encoded within the EVR open-reading frame is a heterogeneous cluster of small RNAs that are expressed upon pathogen infection, and for which EVR has also been named small RNA-generating RLK (Katiyar-Agarwal et al., 2007) (see Fig. S4A in the supplementary material).
Fig. 4.
EVR encodes a membrane-localized LRR-RLK. (A) Diagram of the EVR protein, with the sites of the identified evr mutations indicated. The regions corresponding to the signal peptide (S), leucine-rich repeats (LRRs), transmembrane domain (TM) and kinase domain (KD) are indicated. Point mutations are marked by arrows, and T-DNA insertions by arrowheads. (B) Sequence alignment of kinase subdomains I-III from EVR and related LRR-RLKs from Arabidopsis and Lotus japonicus. Amino acids conserved between EVR and other proteins are shaded. The sites of the conserved glutamic acid in subdomain III affected by the evr-2 mutation and an invariant lysine in subdomain II required for kinase activity are marked by arrows above the alignment. (C) The recombinant EVR KD autophosphorylates at serine, threonine and tyrosine residues in vitro. Mutations in subdomains II (K377E) and III (E407K) of the kinase domain block the kinase activity of EVR. EVR antiserum recognizes ~46 and ~40 kDa phosphorylated and unphosphorylated proteins, respectively. (D) EVR localizes to the plasma membrane. Fluorescent localization of the EVR-YFP marker in epidermal cells of wild-type leaf petioles (stems). Scale bar: 10 μm.
Based on the sequence of its kinase domain, EVR has been assigned to the 28-member LRR-RLK subfamily XI (Shiu and Bleecker, 2001). This subfamily includes the HAESA (HAE) and HAESA-LIKE2 (HSL2) RLKs known to redundantly promote organ shedding (Jinn et al., 2000; Cho et al., 2008; Stenvik et al., 2008), the CLATAVA1 and BARELY ANY MERISTEM RLKs that control stem cell differentiation in shoot and flower meristems (Clark et al., 1997; DeYoung et al., 2006; DeYoung and Clark, 2008), the HAIKU RLK that regulates seed size (Luo et al., 2005) and the PEP1 RECEPTOR1 (PEPR1) that is involved in pathogen response (Yamaguchi et al., 2006). Although most members of subfamily XI have extensive sets of 19-32 LRRs in their extracellular domains (Shiu and Bleecker, 2001), EVR has only five predicted LRRs (Fig. 4A). However, in the context of the entire LRR-RLK family, EVR is not unique; the majority of Arabidopsis LRR-RLKs have fewer than ten LRRs (Shiu and Bleecker, 2001).
The evr-1 and evr-2 mutations are both predicted to be loss-of-function alleles that are unlikely to affect transcriptional regulation of EVR or the alternatively encoded small RNAs. The evr-1 allele introduces a stop codon upstream of the first LRR, suggesting that it is a null allele (Fig. 4A). The evr-2 allele changes a conserved glutamic acid to a lysine within subdomain III of the kinase domain (Fig. 4A,B). This subdomain is involved in ATP binding and catalysis; a loss-of-function mutation in the HAR1 LRR-RLK gene of Lotus japonicus introduces an identical missense mutation (Nishimura et al., 2002; Diévart and Clark, 2003). Two additional mutant alleles (Alonso et al., 2003), evr-3 and evr-4, contain T-DNA insertions within subdomains VII and X of the kinase domain, respectively (Fig. 4A; see also Fig. S1H,I in the supplementary material). These mutations also restore organ separation and cause premature abscission in nev flowers (see Fig. S1G,J-L, Fig. S2 in the supplementary material; data not shown).
Although up to 20% of Arabidopsis RLKs are predicted to be kinase dead (Castells and Casacuberta, 2007), isolation of the evr-2 mutation suggests that EVR kinase activity is required to regulate abscission (Fig. 4A,B). To facilitate the analysis of EVR kinase activity, we generated EVR-specific antiserum using a 24-amino acid peptide corresponding to its unique C terminus. The EVR antibody recognizes the EVR-KDs of wild type (WT), a kinase-dead mutant (K377E) (Horn and Walker, 1994) and the evr-2 mutant (E407K) expressed as N-terminal 6×His-tagged fusion proteins in E. coli (Fig. 4B,C). Whereas the purified KDK377E and KDE407K proteins migrate as single bands of ~40 kDa near the predicted size of 39 kDa, the purified KDWT migrates as two distinct bands of ~40 and 46 kDa, suggesting that the wild-type protein is phosphorylated (Fig. 4C).
To test whether EVR is a functional serine/threonine kinase, we used phosphoserine and phosphothreonine antisera to detect phosphorylated residues on the recombinant KDs. Both antisera recognized the upper KDWT band but not the lower, presumably unphosphorylated, KDWT band. Neither KDK377E nor KDE407K appear to have kinase activity, as the antisera did not recognize these mutant proteins (Fig. 4C). As some LRR-RLKs have also been demonstrated to be dual-specificity kinases (Mu et al., 1994; Oh et al., 2009), we also tested the ability of the recombinant EVR-KDs to undergo autophosphorylation at tyrosine residues. We found that phosphotyrosine antiserum exhibited the same recognition pattern as that of the phosphoserine and phosphothreonine antisera (Fig. 4C), and that treatment with calf intestinal alkaline phosphatase was able to completely dephosphorylate the tyrosine residues of EVR-KDWT (see Fig. S4B in the supplementary material). These results suggest that EVR is a dual-specificity kinase that autophophorylates serine, threonine and tyrosine residues in vitro.
EVR, like other transmembrane receptor-like kinases, is predicted to localize to the plasma membrane (Alexandersson et al., 2004). To visualize EVR localization in vivo, we generated transgenic plants expressing EVR-YFP under control of the EVR promoter (see Fig. S4A in the supplementary material). Using confocal laser scanning microscopy, six out of six independent transgenic lines showed localization of EVR-YFP chimeric protein to the periphery of epidermal cells (Fig. 4D), and early colocalization of EVR-YFP with the lipophilic dye FM4-64 was observed (Fig. S4C in the supplementary material). These results suggest that EVR is localized to the plasma membrane and/or internal membrane compartments (Vida and Emr, 1995).
EVR is expressed in floral organ AZs prior to cell separation
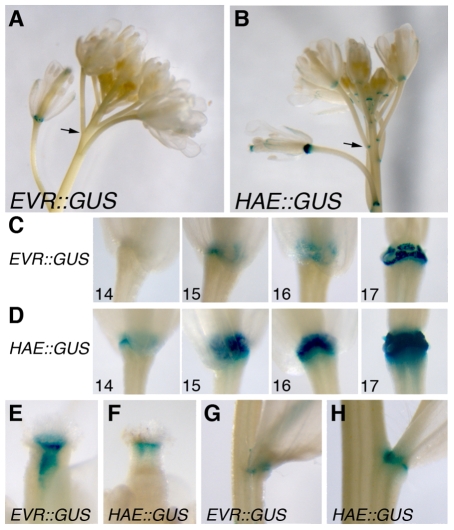
RT-PCR and global expression experiments indicate that EVR transcripts are present in multiple tissues, with increasing expression in older flowers (see Fig. S5A,B in the supplementary material) (Schmid et al., 2005). To determine whether EVR is expressed at the right time and place to modulate organ separation, we generated transgenic plants that express β-glucuronidase (GUS) under the control of the predicted EVR promoter (see Fig. S4A in the supplementary material). Of 83 T1 plants examined, 58 showed GUS expression in floral organ AZs, typically just prior to organ shedding (stage 15) with strengthened expression in older flowers (stages 16 and early 17; Fig. 5A,C). This profile is similar to the reported expression patterns of HAE::GUS and HSL2::GUS (Fig. 5A-D) (Jinn et al., 2000; Cho et al., 2008), and is consistent with a role for EVR in modulating the timing of organ shedding.
Fig. 5.
EVR is expressed in organ AZs. (A-H) The regulatory regions of EVR and HAE direct expression of β-glucuronidase (GUS) in AZs (A-D), in internal tissues of the floral style (E,F), and at the junction between the cauline leaves and the inflorescence stem (G,H). The HAE::GUS marker is also expressed at the bases of the floral pedicels (B, arrow). Within floral AZs (C), the EVR promoter directs GUS expression prior to organ separation (stage 15), during abscission (stage 16), and as the remaining cells form protective scar tissue (stage 17). GUS expression directed by the HAE promoter shows a similar, yet stronger profile in AZs, with an earlier stage of initiation (Jinn et al., 2000).
Two independent EVR::GUS lines with single transgene insertions were characterized in the T2 generation. In addition to expression in floral organ AZs, each line showed GUS expression within the style of the developing fruit (stage 15; Fig. 5E), at the bases of the cauline leaves (Fig. 5G), and in the stems of the first rosette leaves (see Fig. S5C in the supplementary material). Because evr mutants showed defects in fruit growth (Fig. 2H), and global transcriptional analysis suggests that EVR has a broader expression profile than our EVR::GUS markers (see Fig. S5B in the supplementary material) (Schmid et al., 2005); these might not reflect the complete expression profile of EVR. The HAE promoter was also observed to direct GUS expression in the style (Fig. 5F), at the cauline leaf bases (Fig. 5H), and at the junction between the floral and inflorescence stems (Fig. 5B, arrow). These observations suggest that EVR and HAE may co-regulate the development of a set of plant tissues.
Mutations in EVR restore the structure of the Golgi and the location of the TGN in nev AZ cells
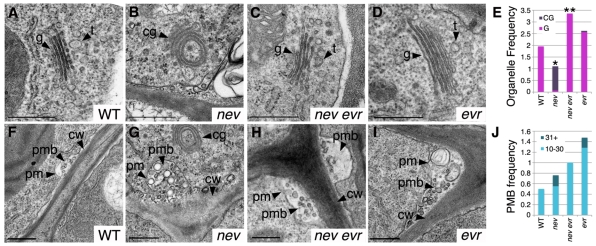
As mutations in EVR rescue abscission in nev flowers, we examined AZ cells at the time of organ shedding to determine whether the membrane trafficking defects observed in nev flowers were also rescued. Loss of NEV leads to a distinctive bending of the Golgi cisternae, disruption of the trans-Golgi network (TGN) and the accumulation of clusters of vesicles known as paramural bodies (PMBs) between the plasma membrane and cell wall of AZ cells (Liljegren et al., 2009). Unlike nev cells, nev evr cells contain flat stacks of Golgi cisternae with a wild-type appearance and closely associated TGN (Fig. 6A-C,E). The Golgi stacks and TGN of evr cells resemble those of wild type (Fig. 6D-E). As with nev cells, PMBs with more than 30 vesicles were observed in evr cells, but not in wild-type or nev evr cells (Fig. 6F-J; see also Fig. S6 in the supplementary material). As the defects in Golgi morphology and TGN location correlate with non-shedding floral organs, these might represent the primary cellular changes associated with and potentially responsible for the block of organ shedding in nev flowers. Furthermore, the restoration of Golgi/TGN structure in nev evr flowers and the defects in PMB formation in evr flowers suggest the possibility that, like NEV, EVR might regulate membrane trafficking at the transition to floral organ shedding.
Fig. 6.
Mutations in EVR restore Golgi structure and location of the TGN in nev flowers. Transmission electron micrographs and analysis of cells in sepal AZ regions at the time of organ separation for wild-type (stage 16), nev (stage 16 non-shedding), nev evr (stage 15*) and evr (stage 16) flowers. (A-D) Instead of the flat stacks of Golgi cisternae characteristic of wild type (A), circularized multilamellar structures are observed in nev cells (B). Golgi with a wild-type appearance are found in nev evr (C) and evr cells (D). We frequently observed vesicular-tubular structures characteristic of the trans-Golgi network (TGN) (81%, n=16) closely associated with the Golgi cisternae (34±21 nm, n=13) in wild-type cells (A), whereas the TGN (15%, n=13) was less often observed near the circularized multilamellar structures (40±25 nm, n=2) of nev cells (B). The location of the TGN was restored in nev evr cells (83% associated with Golgi, n=24; 37±12 nm, n=20) and was unaffected by loss of EVR alone (76% associated with Golgi, n=21; 38±25 nm, n=15). (E) Frequency of flat Golgi cisternae (G, pink) and circularized multilamellar structures (CG, purple) per cell in sections of wild-type and mutant sepal AZ regions. For each genotype, n (cells)≥11. Statistical differences between nev and wild type, and between nev evr and nev tissues are indicated by single and double asterisks, respectively (Fisher's exact test, P<0.0001). A statistical difference was not detected between evr and wild-type tissues. (F-I) Paramural bodies (PMBs) were observed in the cells of wild-type (F), nev (G), nev evr (H) and evr (I) flowers. Whereas PMBs were observed in cells from each genotype, PMBs with greater than 30 vesicles were only observed in nev and evr cells. (J) Frequency of PMBs (10-30 and 31+ vesicles) per cell in sections of wild-type and mutant sepal AZ regions. For each genotype, n (cells)≥11. Statistical differences in PMB accumulation were not detected. cg, circularized multilamellar structures; cw, cell wall; g, Golgi cisternae; pm, plasma membrane; pmb, paramural body; t, trans-Golgi network. Scale bars: 0.5 μm.
Mutations in EVR-like genes do not restore abscission in nev flowers
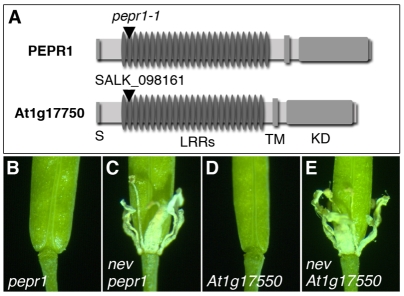
To potentially identify additional regulators of abscission, we evaluated whether mutations in two EVR-related genes could also rescue nev-mediated defects. EVR shares 42 and 45% amino acid identity within the kinase domains of the PEPR1 and At1g17750 LRR-RLKs, respectively (Fig. 4B). PEPR1 was previously found to be a receptor for the PEP1 peptide involved in the innate immune response of plants to pathogen attack (Yamaguchi et al., 2006), and At1g17750 has been shown to be transcriptionally induced by the fungal protein, Nep1 (Necrosis and ethylene-inducing peptide1) (Qutob et al., 2006). EVR is also expressed in response to viral and bacterial infection (Whitham et al., 2003; Katiyar-Agarwal et al., 2007; Ascencio-Ibanez et al., 2008). Despite the similarities within the kinase domain and the overlapping expression patterns (see Fig. S5B in the supplementary material), predicted loss-of-function mutations in PEPR1 and At1g17750 did not rescue organ shedding in nev flowers (Fig. 7). These results suggest that these closely related kinases do not regulate organ separation, or that their roles are hidden by additional genetic redundancy.
Fig. 7.
Mutations in PEPR1 or At1g17750 do not rescue shedding in nev flowers. (A) Diagrams of the predicted PEPR1 and At1g17750 proteins, with the sites of the T-DNA mutations indicated by arrowheads. The regions corresponding to the signal peptide (S), leucine-rich repeats (LRRs), transmembrane domain (TM, and kinase domain (KD) are indicated. (B-E) Floral organ shedding occurs normally in pepr1 (B) and At1g17750 (D) mutant flowers (stage 17), but is blocked in nev pepr1 (C) and nev At1g17750 (E) flowers (stage 17).
Mutations in EVR do not rescue shedding in ida or hae hsl2 mutant flowers
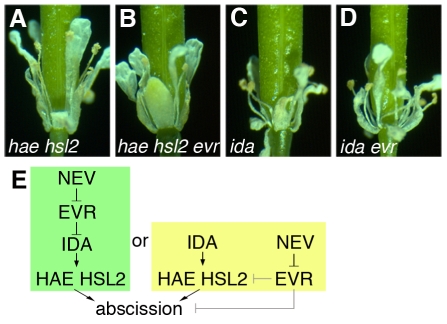
As mutations in NEV, IDA, and the redundant genes HAE and HSL2 all appear to block floral organ shedding at a similar point during the cell separation stage (Butenko et al., 2003; Cho et al., 2008; Stenvik et al., 2008; Liljegren et al., 2009), we sought to use mutations in EVR to test the genetic relationship between these genes. We found that loss of EVR does not rescue shedding in either ida or hae hsl2 flowers (Fig. 8A-D). These results suggest that NEV and EVR could act upstream of IDA and HAE/HSL2, or in a parallel pathway that converges at the point of HAE/HSL2 function or further downstream (Fig. 8E).
Fig. 8.
Mutations in EVR do not restore abscission in ida or hae hsl2 mutant flowers. (A-D) Organ separation is blocked in ida (C) and hae hsl2 (A) mutant flowers (stage 17), and is not rescued in ida evr (D) or hae hsl2 evr (B) flowers (stage 17). (E) Alternative pathways for the relationships between NEV, EVR, IDA and HAE/HSL2. In both, EVR is predicted to act as a negative regulator of abscission and to function downstream of NEV. In the simplest model, NEV and EVR act upstream of both IDA and HAE/HSL2. Alternatively, NEV and EVR could function in a converging pathway, in which EVR acts upon HAE/HSL2, or further downstream.
DISCUSSION
Here, we report the characterization of EVR, an Arabidopsis LRR-RLK that modulates floral organ abscission and promotes fruit development. Our studies suggest that EVR functions to inhibit organ separation by regulating signaling that affects the timing of AZ activity, AZ size and cell expansion.
We were able to detect a role for EVR in organ separation through a sensitized genetic screen of nev flowers. NEV encodes an ADP-ribosylation factor GTPase-activating protein that is likely to regulate membrane trafficking during multiple aspects of plant development (Liljegren et al., 2009). At the time of abscission, loss of NEV activity could disrupt the movement of signaling molecules crucial for activating this process, thereby blocking release of the hydrolytic enzymes essential for the AZ cell wall modifications associated with cell separation. As described below, we propose that secondary loss of the EVR LRR-RLK might bypass the requirement for NEV-mediated trafficking during abscission by affecting the localization and/or activity of such a signaling complex.
We have discovered that EVR acts as both a temporal and spatial regulator of abscission. EVR regulatory regions first direct GUS expression in floral AZs during the transition from fertilization to fruit growth (stage 15; Fig. 5A,C). In wild-type flowers of the Ler ecotype, organ separation (stage 16) occurs about three days after the buds open and the stamens release their pollen (see Fig. S3B in the supplementary material). Loss of EVR causes premature abscission of floral organs in nev flowers (stage 15*) two positions earlier than in wild-type inflorescences (Fig. 2C), and about two days after bud opening (see Fig. S3B in the supplementary material). These results indicate that younger flowers are competent to respond to signals to shed their organs, as was previously observed for flowers treated with ethylene or misexpressing IDA (Butenko et al., 2003; Stenvik et al., 2006). More importantly, our results suggest that EVR is one of the factors that acts to inhibit this response. Although mutations in EVR alone do not affect the timing of abscission, multiple levels of genetic redundancy probably exist to ensure that organ separation does not occur prematurely.
Mutations in EVR appear to alter the spatial restriction of AZ signaling. Whereas smooth, scar-like surfaces form at the sites of organ detachment in wild-type flowers (Fig. 3A), increased expansion of individual AZ cells leads to visible collars of rough, broken tissue at the bases of nev evr fruit (Fig. 1C, Fig. 3F,G). AZ regions are also enlarged in nev evr flowers compared with wild type (Fig. 3E). These AZ phenotypes strongly resemble those of plants constitutively expressing IDA (Fig. 3E-H) (Stenvik et al., 2006; Cho et al., 2008), suggesting that excess levels of the IDA ligand or an activated HAE/HSL2 receptor complex might be present in nev evr flowers. An intriguing possibility is that ectopic AZ signaling causes cell wall loosening and expansion of cells both within and neighboring the original AZs of nev evr flowers. Although the relationship between AZ cell expansion and organ separation in wild-type Arabidopsis flowers is unclear (Patterson, 2001), cell expansion might physically enable the shedding of floral organs during the cell wall loosening process. In this capacity, the contribution of AZ cell expansion to organ abscission might be analogous to that of lignified fruit cells to Arabidopsis pod dehiscence (Liljegren et al., 2004).
With five LRRs in its extracellular domain, EVR may inhibit organ separation by regulating the behavior of ligand-binding LRR-RLKs. One of the best characterized interactions between non-ligand and ligand-binding LRR-RLKs is that of BAK1 (SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE3) and FLAGELLIN-SENSITIVE2 (FLS2) during pathogen-triggered immunity (Geldner and Robatzek, 2008; Boller and Felix, 2009). Recognition of the flagellin-derived flg22 peptide by FLS2 triggers interaction with BAK1 (Chinchilla et al., 2007; Heese et al., 2007). As a co-receptor for FLS2, BAK1 promotes both flg22-induced signaling and internalization of FLS2 from the plasma membrane (Chinchilla et al., 2007). Although EVR may function similarly to BAK1 — by mediating the membrane trafficking of a ligand-binding receptor — we predict that it plays a unique role by acting as an inhibitor of its LRR-RLK partner prior to ligand binding, rather than as a co-receptor after ligand binding. Further studies will be necessary to pinpoint the location(s) of EVR activity; our initial results suggest that EVR functions at the plasma membrane and in closely associated vesicles (Fig. 4D; see also Fig. S4C in the supplementary material).
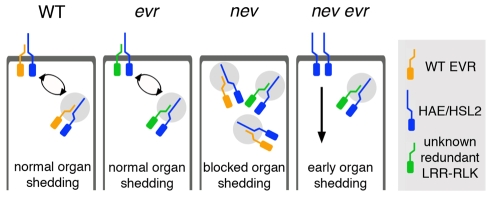
A model for EVR function during the transition from fertilization to floral organ shedding is shown in Fig. 9. In wild-type AZ cells, plasma membrane-localized EVR may interact with and inhibit the activity of ligand-binding LRR-RLK(s), such as HAE and HSL2. In contrast to BAK1, for which FLS2 binding is enhanced by the presence of ligand, EVR may promote the internalization of an inactive receptor complex, thereby limiting the pool of available receptors and delaying abscission signaling. Increased availability of the HAE/HSL2 receptors, or of the putative ligand IDA, may trigger cell separation in older flowers. Loss of EVR function alone would not alter the timing of floral organ abscission owing to the activity of a redundant LRR-RLK(s). This RLK would also be predicted to promote internalization of inactive receptor complexes in wild-type cells. Loss of NEV function in the TGN and other endosomal compartments could alter the trafficking of receptor complexes containing EVR and other inhibitory RLKs, such that the components required to activate cell separation are not recycled back to the plasma membrane. Secondary loss of EVR function could bypass the requirement for NEV in floral organ shedding, potentially resulting in a constitutive signal for cell separation that is no longer internalized or targeted for degradation. Increased signaling might be responsible for the premature organ shedding, enlarged AZs and deregulated cell expansion that we observe in nev evr flowers.
Fig. 9.
A model for EVR function during the transition to floral organ separation. In wild-type AZ cells before organ shedding, EVR might inhibit cell separation by interacting with ligand-binding LRR-RLKs, such as HAE/HSL2, that trigger cell wall loosening and separation. This interaction could promote the internalization and recycling of inactive receptor complexes through the endosomal system. Continued signaling from a ligand-activated HAE/HSL2 complex would lead to organ shedding in older flowers. The EVR RLK may act redundantly with another LRR-RLK(s), such that loss of EVR alone would not alter the timing of abscission. Disrupting NEV activity might alter the trafficking of receptor complexes containing EVR or EVR-like RLKs, thereby blocking a signal required for cell separation. Mutations in EVR could bypass the requirement for NEV in floral organ shedding, resulting in constitutive signaling of the HAE/HSL2 LRR-RLKs, premature activation of organ separation, the enlargement of AZ regions, and deregulated AZ cell expansion.
In addition to restoring abscission, we found that a mutation in EVR rescues the structural defects in the Golgi and TGN that are present in nev flowers. Defects in NEV-mediated membrane trafficking might leave the integrity of the Golgi/TGN vulnerable to organizational stress due to the high volume of traffic blocked during organ abscission. If mutations in EVR restore abscission signaling and thereby alleviate the backlog of traffic in nev AZ cells, they might be sufficient to indirectly restore Golgi/TGN structure. Alternatively, EVR/SOBIR1 might function in a pathway(s) that directly regulates Golgi dynamics during periods of cellular stress, such as abscission and pathogen attack. Perturbations in Golgi structure have been associated with cell death-associated kinase signaling and GTPase activity (Landry et al., 2009), and AZ tissues that undergo periodically high volumes of membrane trafficking might require specific signaling pathways to maintain the integrity of membrane-bound organelles. Future experiments investigating the role of the EVR RLK in regulating membrane trafficking during abscission are likely to advance our understanding of the signaling complexities that control plant growth and development.
Supplementary Material
Acknowledgements
We thank M. Duncan, M. Peifer, J. Reed, J. Kieber, B. Duronio, J. Dangl, C. Argueso, C. Burr and M. Simon for helpful discussions; V. Madden, S. Ray and T. Perdue for assistance with TEM, SEM and confocal microscopy; I. Chen, A. Larberg and R. Mulamba for technical assistance; J. Fletcher for the 35S::CLV3-GFP/GUS construct; T. Nakagawa for the pGWB destination vectors; ABRC for seed stocks; and Monsanto for access to Ler polymorphisms. This research was supported by an NSF grant (IOB-0517550) and an UNC URC grant to S.J.L. M.E.L. was supported by William R. Kenan, William C. Coker and Alma Holland Beers fellowships, and the UNC Curriculum in Genetics and Molecular Biology; M.W.L. was supported by the NIH-funded UNC Developmental Biology training program, and by William C. Coker and Alma Holland Beers fellowships. Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.041335/-/DC1
References
- Alexandersson E., Saalbach, G., Larsson, C. and Kjellbom, P. (2004). Arabidopsis plasma membrane proteomics identifies components of transport, signal transduction and membrane trafficking. Plant Cell Physiol. 45, 1543-1556. [DOI] [PubMed] [Google Scholar]
- Alonso J. M., Stepanova, A. N., Leisse, T. J., Kim, C. J., Chen, H., Shinn, P., Stevenson, D. K., Zimmerman, J., Barajas, P., Cheuk, R., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301, 653-657. [DOI] [PubMed] [Google Scholar]
- Ascencio-Ibáñez J. T., Sozzani, R., Lee, T.-J., Chu, T.-M., Wolfinger, R. D., Cella, R. and Hanley-Bowdoin, L. (2008). Global analysis of Arabidopsis gene expression uncovers a complex array of changes impacting pathogen response and cell cycle during geminivirus infection. Plant Physiol. 148, 436-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez M. A., Soowal, L. N., Lee, I. and Weigel, D. (1997). LEAFY expression and flower initiation in Arabidopsis. Development 124, 3835-3844. [DOI] [PubMed] [Google Scholar]
- Boller T. and Felix, G. (2009). A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60, 379-406. [DOI] [PubMed] [Google Scholar]
- Butenko M. A., Patterson, S. E., Grini, P. E., Stenvik, G. E., Amundsen, S. S., Mandal, A. and Aalen, R. B. (2003). INFLORESCENCE DEFICIENT IN ABSCISSION controls floral organ abscission in Arabidopsis and identifies a novel family of putative ligands in plants. Plant Cell 15, 2296-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenko M. A., Vie, A. K., Brembu, T., Aalen, R. B. and Bones, A. M. (2009). Plant peptides in signaling: looking for new partners. Trends Plant Sci. 14, 255-263. [DOI] [PubMed] [Google Scholar]
- Castells E. and Casacuberta, J. M. (2007). Signalling through kinase-defective domains: the prevalence of atypical receptor-like kinases in plants. J. Exp. Bot. 58, 3503-3511. [DOI] [PubMed] [Google Scholar]
- Chinchilla D., Zipfel, C., Robatzek, S., Kemmerling, B., Nürnberger, T., Jones, J. D., Felix, G. and Boller, T. (2007). A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448, 497-500. [DOI] [PubMed] [Google Scholar]
- Cho S. K., Larue, C. T., Chevalier, D., Wang, H., Jinn, T.-L., Zhang, S. and Walker, J. C. (2008). Regulation of floral organ abscission in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 105, 15629-15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chytilova E., Macas, J. and Galbraith, D. W. (1999). Green fluorescent protein targeted to the nucleus, a transgenic phenotype useful for studies in plant biology. Ann. Bot. 83, 645-654. [Google Scholar]
- Clark S. E., Williams, R. W. and Meyerowitz, E. M. (1997). The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89, 575-585. [DOI] [PubMed] [Google Scholar]
- DeYoung B. J. and Clark, S. E. (2008). BAM receptors regulate stem cell specification and organ development through complex interactions with CLAVATA signaling. Genetics 180, 895-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung B. J., Bickle, K. L., Schrage, K. J., Muskett, P., Patel, K. and Clark, S. E. (2006). The CLAVATA1-related BAM1, BAM2 and BAM3 receptor kinase-like proteins are required for meristem function in Arabidopsis. Plant J. 45, 1-16. [DOI] [PubMed] [Google Scholar]
- Diévart A. and Clark, S. E. (2003). Using mutant alleles to determine the structure and function of leucine-rich repeat receptor-like kinases. Curr. Opin. Plant Biol. 6, 507-516. [DOI] [PubMed] [Google Scholar]
- Fang S.-C. and Fernandez, D. E. (2002). Effect of regulated overexpression of the MADS domain factor AGL15 on flower senescence and fruit maturation. Plant Physiol. 130, 78-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrándiz C., Liljegren, S. J. and Yanofsky, M. F. (2000). Negative regulation of the SHATTERPROOF genes by FRUITFULL during Arabidopsis fruit development. Science 289, 436-438. [DOI] [PubMed] [Google Scholar]
- Gao M., Wang, X., Wang, D., Xu, F., Ding, X., Zhang, Z., Bi, D., Chen, Y. T., Chen, S., Li, X. and Zhang, Y. (2009). Regulation of cell death and innate immunity by two receptor-like kinases in Arabidopsis. Cell Host Microbe 6, 34-44. [DOI] [PubMed] [Google Scholar]
- Geldner N. and Robatzek, S. (2008). Plant receptors go endosomal: a moving view on signal transduction. Plant Physiol. 147, 1565-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N., Hyman, D. L., Wang, X., Schumacher, K. and Chory, J. (2007). Endosomal signaling of plant steroid receptor kinase BRI1. Genes Dev. 21, 1598-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Carranza Z. H., Rompa, U., Peters, J. L., Bhatt, A. M., Wagstaff, C., Stead, A. D. and Roberts, J. A. (2007). Hawaiian skirt: an F-box gene that regulates organ fusion and growth in Arabidopsis. Plant Physiol. 144, 1370-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heese A., Hann, D. R., Gimenez-Ibanez, S., Jones, A. M., He, K., Li, J., Schroeder, J. I., Peck, S. C. and Rathjen, J. P. (2007). The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc. Natl. Acad. Sci. USA 104, 12217-12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn M. A. and Walker, J. C. (1994). Biochemical properties of the auto-phosphorylation of RLK5, a receptor-like protein kinase from Arabidopsis thaliana. Biochim. Biophys. Acta 1208, 65-74. [DOI] [PubMed] [Google Scholar]
- Jinn T. L., Stone, J. M. and Walker, J. C. (2000). HAESA, an Arabidopsis leucine-rich repeat receptor kinase, controls floral organ abscission. Genes Dev. 14, 108-117. [PMC free article] [PubMed] [Google Scholar]
- Karlova R., Boeren, S., Russinova, E., Aker, J., Vervoort, J. and de Vries, S. C. (2006). The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 protein complex includes BRASSINOSTEROID-INSENSITIVE 1. Plant Cell 18, 626-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar-Agarwal S., Gao, S., Vivian-Smith, A. and Jin, H. (2007). A novel class of bacteria-induced small RNAs in Arabidopsis. Genes Dev. 21, 3123-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Caño-Delgado, A., Seto, H., Hiranuma, S., Fujioka, S., Yoshida, S. and Chory, J. (2005). Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature 433,167-171. [DOI] [PubMed] [Google Scholar]
- Landry M., Sicotte, A., Champagne, C. and Lavoie, J. N. (2009). Regulation of cell death by recycling endosomes and Golgi membrane dynamics via a pathway involving Src-family kinases, Cdc42 and Rab11a. Mol. Biol. Cell 20, 4091-4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lease K. A., Cho, S. K. and Walker, J. C. (2006). A petal breakstrength meter for Arabidopsis abscission studies. Plant Methods 2, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie M. E., Lewis, M. L. and Liljegren, S. J. (2007). Organ Abscission. In Plant Cell Separation and Adhesion (ed. J. Roberts and Z. Gonzalez-Carranza), p. 106-136. Oxford: Blackwell Publishing.
- Li J., Wen, J., Lease, K. A., Doke, J. T., Tax, F. E. and Walker, J. C. (2002). BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110, 213-222. [DOI] [PubMed] [Google Scholar]
- Liljegren S. J., Ditta, G. S., Eshed, Y., Savidge, B., Bowman, J. L. and Yanofsky, M. F. (2000). SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 404, 766-770. [DOI] [PubMed] [Google Scholar]
- Liljegren S. J., Roeder, A. H., Kempin, S. A., Gremski, K., Østergaard, L., Guimil, S., Reyes, D. K. and Yanofsky, M. F. (2004). Control of fruit patterning in Arabidopsis by INDEHISCENT. Cell 161, 843-853. [DOI] [PubMed] [Google Scholar]
- Liljegren S. J., Leslie, M. E., Darnielle, L., Lewis, M. W., Taylor, S. M., Luo, R., Geldner, N., Chory, J., Randazzo, P. A., Yanofsky, M. F., et al. (2009). Regulation of membrane trafficking and organ separation by the NEVERSHED ARF-GAP protein. Development 136, 1909-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M., Dennis, E. S., Berger, F., Peacock, W. J. and Chaudhury, A. (2005). MINISEED3 (MIN3), a WRKY family gene, and HAIKU2 (IKU2), a leucine-rich repeat (LRR) KINASE gene, are regulators of seed size in Arabidopsis. Proc. Natl. Acad. Sci. USA 102, 17531-17536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim S. M., Stenvik, G.-E., Butenko, M. A., Kristiansen, W., Cho, S. K., Hepworth, S. R., Aalen, R. B. and Haughn, G. W. (2008). The BLADE-ON-PETIOLE genes are essential for abscission zone formation in Arabidopsis. Development 135, 1537-1546. [DOI] [PubMed] [Google Scholar]
- Morillo S. A. and Tax, F. E. (2006). Functional analysis of receptor-like kinases in monocots and dicots. Curr. Opin. Plant Biol. 9, 460-469. [DOI] [PubMed] [Google Scholar]
- Mu J.-H., Lee, H.-S. and Kao, T.-H. (1994). Characterization of a pollen-expressed receptor-like kinase gene of Petunia inflata and the activity of its encoded kinase. Plant Cell 6, 709-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A. (1961). Zur Charakterisierung der Blüten und Infloreszenzen von Arabidopsis thaliana (L.) Heynh. Kulturpflanze 9, 364-393. [Google Scholar]
- Nakagawa T., Kurose, T., Hino, T., Tanaka, K, Kawamukai, M., Niwa, Y., Toyooka, K., Matsuoka, K., Jinbo, T. and Kimura, T. (2007). Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104, 34-41. [DOI] [PubMed] [Google Scholar]
- Nishimura R., Hayashi, M., Wu, G.-J., Kouchi, H., Imaizumi-Anraku, H., Murakami, Y., Kawasaki, S., Akao, S., Ohmori, M., Nagasawa, M., et al. (2002). HAR1 mediates systemic regulation of symbiotic organ development. Nature 420, 426-429. [DOI] [PubMed] [Google Scholar]
- Ogawa M., Kay, P., Wilson, S. and Swain, S. M. (2009). ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE1 (ADPG1), ADPG2, and QUARTET2 are polygalacturonases required for cell separation during reproductive development in Arabidopsis. Plant Cell 21, 216-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M.-H., Wang, X., Kota, U., Goshe, M. B., Clouse, S. D. and Huber, S. C. (2009). Tyrosine phosphorylation of the BRI1 receptor kinase emerges as a component of brassinosteroid signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 106, 658-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson S. E. (2001). Cutting loose. Abscission and dehiscence in Arabidopsis. Plant Physiol. 126, 494-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinyopich A., Ditta, G. S., Savidge, B., Liljegren, S. J., Baumann, E., Wisman, E. and Yanofsky, M. F. (2003). Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature 424, 85-88. [DOI] [PubMed] [Google Scholar]
- Qutob D., Kemmerling, B., Brunner, F., Kufner, I., Engelhardt, S., Gust, A. A., Luberacki, B., Seitz, H. U., Stahl, D., Rauhut, T., et al. (2006). Phytotoxicity and innate immune responses induced by Nep1-like proteins. Plant Cell 18, 3721-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. A. and González-Carranza, Z. H. (2007). Abscission. In Handbook of Plant Science, Volume 1 (ed. K. Roberts), p. 512-519. Chichester: John Wiley & Sons. [Google Scholar]
- Roberts J. A., Elliott, K. A. and González, Z. H. (2002). Abscission, dehiscence, and other cell separation processes. Annu. Rev. Plant Biol. 53, 131-158. [DOI] [PubMed] [Google Scholar]
- Rojo E., Sharma, V. K., Kovaleva, V., Raikhel, N. V. and Fletcher, J. C. (2002). CLV3 is localized to the extracellular space, where it activates the Arabidopsis CLAVATA stem cell signaling pathway. Plant Cell 14, 969-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M., Davison, T. S., Henz, S. R., Pape, U. J., Demar, M., Vingron, M., Schölkopf, B., Weigel, D. and Lohmann, J. U. (2005). A gene expression map of Arabidopsis thaliana development. Nat. Genet. 37, 501-506. [DOI] [PubMed] [Google Scholar]
- Shiu S. H. and Bleecker, A. B. (2001). Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Natl. Acad. Sci. USA 98, 10763-10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpak E. D., Berthiaume, C. T., Hill, E. J. and Torii, K. U. (2004). Synergistic interaction of three ERECTA-family receptor-like kinases controls Arabidopsis organ growth and flower development by promoting cell proliferation. Development 131, 1491-1501. [DOI] [PubMed] [Google Scholar]
- Smyth D. R., Bowman, J. L. and Meyerowitz, E. M. (1990). Early flower development in Arabidopsis. Plant Cell 2, 755-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenvik G.-E., Butenko, M. A., Urbanowicz, B. R., Rose, J. K. and Aalen, R. B. (2006). Overexpression of INFLORESCENCE DEFICIENT IN ABSCISSION activates cell separation in vestigial abscission zones in Arabidopsis. Plant Cell 18, 1467-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenvik G.-E., Tandstad, N. M., Guo, Y., Shi, C.-L., Kristiansen, W., Holmgren, A., Clark, S. E., Aalen, R. B. and Butenko, M. A. (2008). The EPIP peptide of INFLORESCENCE DEFICIENT IN ABSCISSION is sufficient to induce abscission in Arabidopsis through the receptor-like kinases HAESA and HAESA-LIKE2. Plant Cell 20, 1805-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup G., Cohen, S. and Garbers, D. L. (1981). Selective dephosphorylation of proteins containing phosphotyrosine by alkaline phosphatases. J. Biol. Chem. 15, 8197-8201. [PubMed] [Google Scholar]
- Vida T. A. and Emr, S. D. (1995). A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 128, 779-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham S. A., Quan, S., Chang, H.-S., Cooper, B., Estes, B., Zhu, T., Wang, X. and Hou, Y.-M. (2003). Diverse RNA viruses elicit the expression of common sets of genes in susceptible Arabidopsis thaliana plants. Plant J. 33, 271-283. [DOI] [PubMed] [Google Scholar]
- Xu S.-L., Rahman, A., Baskin, T. I. and Kieber, J. J. (2008). Two leucine-rich repeat receptor kinases mediate signaling, linking cell wall biosynthesis and ACC synthase in Arabidopsis. Plant Cell 20, 3065-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Pearce, G. and Ryan, C. A. (2006). The cell surface leucine-rich repeat receptor for AtPep1, an endogenous peptide elicitor in Arabidopsis, is functional in transgenic tobacco cells. Proc. Natl. Acad. Sci. USA 103, 10104-10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.