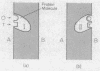
Abstract
It is suggested that active transport, muscle contraction, and ribosomal translocation may all make use of a common allosteric mechanism in which ATP or GTP serves as both the effector and substrate and in which a conformational change in a protein (enzyme) moves or exerts a force on a second ligand. The enzymatic splitting of ATP or GTP provides the driving force for the process and allows repetition of the steady-state cycle.
Full text
PDF
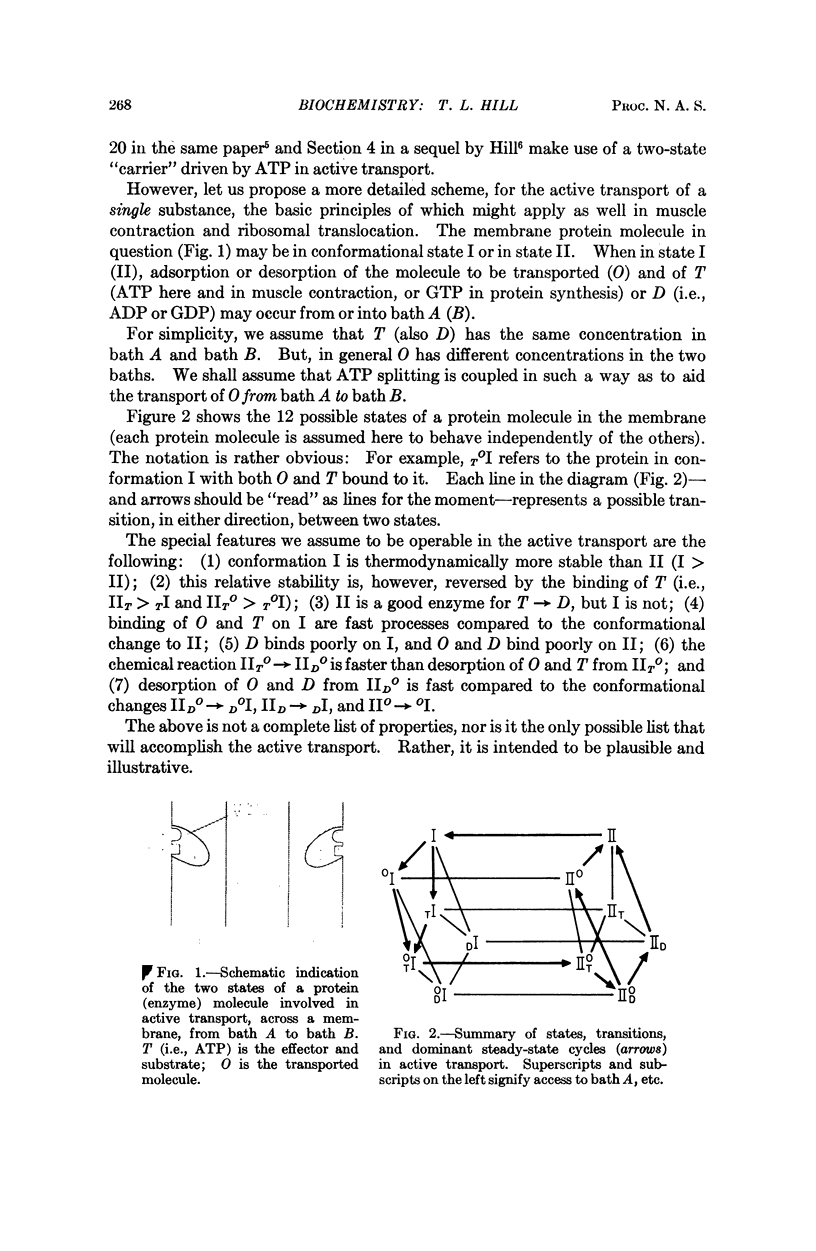

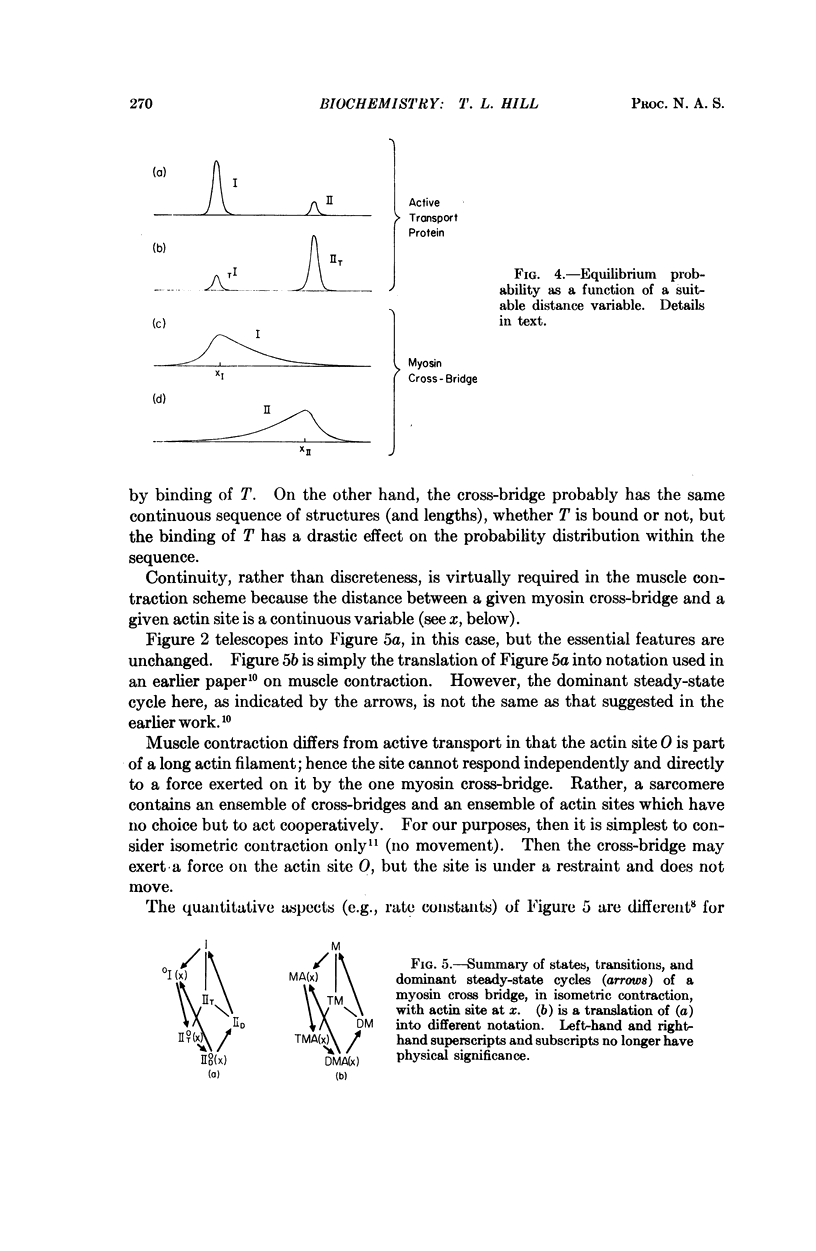




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Civan M. M., Podolsky R. J. Contraction kinetics of striated muscle fibres following quick changes in load. J Physiol. 1966 Jun;184(3):511–534. doi: 10.1113/jphysiol.1966.sp007929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller W., Hodgson A. Conformation of the anticodon loop intRNA. Nature. 1967 Aug 19;215(5103):817–821. doi: 10.1038/215817a0. [DOI] [PubMed] [Google Scholar]
- Hill T. L., Kedem O. Studies in irreversible thermodynamics. 3. Models for steady state and active transport across membranes. J Theor Biol. 1966 Apr;10(3):399–441. doi: 10.1016/0022-5193(66)90136-6. [DOI] [PubMed] [Google Scholar]
- Hill T. L. Studies in irreversible thermodynamics. IV. Diagrammatic representation of steady state fluxes for unimolecular systems. J Theor Biol. 1966 Apr;10(3):442–459. doi: 10.1016/0022-5193(66)90137-8. [DOI] [PubMed] [Google Scholar]
- Hill T. L., White G. M. On the sliding-filament model of muscular contraction, IV. Calculation of force-velocity curves. Proc Natl Acad Sci U S A. 1968 Nov;61(3):889–896. doi: 10.1073/pnas.61.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipmann F. Polypeptide chain elongation in protein biosynthesis. Science. 1969 May 30;164(3883):1024–1031. doi: 10.1126/science.164.3883.1024. [DOI] [PubMed] [Google Scholar]
- MONOD J., JACOB F. Teleonomic mechanisms in cellular metabolism, growth, and differentiation. Cold Spring Harb Symp Quant Biol. 1961;26:389–401. doi: 10.1101/sqb.1961.026.01.048. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y., Lipmann F. The interrelationship between guanosine triphosphatase and amino acid polymerization. Arch Biochem Biophys. 1966 Sep 26;116(1):344–351. doi: 10.1016/0003-9861(66)90040-3. [DOI] [PubMed] [Google Scholar]