Abstract
In Drosophila, germ cell survival and directionality of migration are controlled by two lipid phosphate phosphatases (LPP), wunen (wun) and wunen-2 (wun2). wun wun2 double mutant analysis reveals that the two genes, hereafter collectively called wunens, act redundantly in primordial germ cells. We find that wunens mediate germ cell-germ cell repulsion and that this repulsion is necessary for germ cell dispersal and proper transepithelial migration at the onset of migration and for the equal sorting of the germ cells between the two embryonic gonads during their migration. We propose that this dispersal function optimizes adult fecundity by assuring maximal germ cell occupancy of both gonads. Furthermore, we find that the requirement for wunens in germ cell survival can be eliminated by blocking germ cell migration. We suggest that this essential function of Wunen is needed to maintain cell integrity in actively migrating germ cells.
Keywords: Drosophila, Germ cell, Migration, Lipid phosphate phosphatase, Cell repulsion
INTRODUCTION
Germ cells are those cells that will eventually give rise to the gametes and therefore the next generation. In Drosophila, germ cells form at the posterior of the syncytial blastoderm. During gastrulation they are carried into the posterior midgut pocket (stage 9) from where they migrate through the midgut epithelium. From the basal side of the midgut (stage 10), they migrate into the mesoderm where they associate with the somatic gonadal precursors (SGPs; stage 11). SGPs are specified in bilateral clusters within parasegments 10, 11 and 12. During gonad formation at stage 13, the three clusters of SGPs come together to form a line of cells. Germ cells and SGPs then undergo a coalescence event to form a compact rounded embryonic gonad.
Genetic screens have identified a number of genes that regulate germ cell migration (for a review, see Kunwar et al., 2006). Subsequent to crossing the gut, germ cell migration is highly dependent on the activity of two redundant genes, wun and wun2 (hereafter collectively called wunens). These genes are zygotically expressed in somatic cells in regions of the embryo that germ cells avoid (Starz-Gaiano et al., 2001; Zhang et al., 1997). For example, they are expressed in regions of the midgut and in the CNS, the later being responsible for repelling germ cells from the midline of the embryo, causing the germ cells to split into two bisymmetrical groups (Sano et al., 2005). In embryos lacking either wun or wun2, there are only minor defects in germ cell migration. However, in embryos lacking both wun and wun2 in somatic tissues, most germ cells fail to reach the SGPs, instead scattering throughout the posterior of the embryo (Sano et al., 2005; Starz-Gaiano et al., 2001; Zhang et al., 1997).
Overexpression of either wun or wun2 causes germ cells to avoid the region of ectopic expression and induces germ cell death (Starz-Gaiano et al., 2001). Thus, wun and wun2 act redundantly in somatic tissues and are necessary and sufficient to repel germ cells. Wun and Wun2 are lipid phosphate phosphatases (LPPs), transmembrane enzymes that dephosphorylate extracellular lipid phosphates (for a review, see Sigal et al., 2005). These data suggest that an extracellular lipid phosphate is regulating both germ cell migration directionality and survival.
We and others found that, in addition to its expression in somatic cells, wun2 is also maternally provided and functions in germ cells (Hanyu-Nakamura et al., 2004; Renault et al., 2004). Intriguingly, Wun2 in germ cells is necessary to protect them from death: lack of wun2 function in germ cells results in germ cell death immediately once they have crossed the midgut at stage 10. This death can be suppressed by reducing the levels of somatic wunens. wun2 is therefore part of the mechanism in germ cells required to perceive the wunen signal from the soma. We have proposed that germ cell survival is controlled through competition between somatic wunens and germ cell Wun2 for a common extracellular lipid phosphate substrate (Renault et al., 2004).
In this paper, we explore genetic redundancy and temporal and spatial requirements of wunen signalling. We determine that, similar to the situation in somatic cells, Wun2 function in germ cells is partially redundant with Wun. We find that, in embryos lacking both maternal and zygotic wunens, the germ cells fail to migrate across the midgut epithelium. Thus, wunens act earlier than previously appreciated in the process of transepithelial migration. We also find that germ cell wunens mediate germ cell-germ cell repulsion, providing a mechanism to ensure that germ cells partition evenly to the two embryonic gonads, which is important for maximal adult fecundity.
MATERIALS AND METHODS
wun wun2 double mutant manufacture
wun and wun2 lie adjacent to each other on chromosome 2L, making it exceedingly difficult to recombine existing wun and wun2 single alleles to create a double-mutant line. Therefore, we performed ethyl methanesulfonate (EMS) mutagenesis to induce a mutation in wun in the background of a line that is already a null mutant for wun2 (wun2EP2650ex34), as described in Renault et al. (Renault et al., 2004) (see Fig. S1 in the supplementary material). Null alleles of wun are semi-lethal and female-sterile; therefore, we scored for flies that were lethal or semi-lethal and sterile over a deficiency for the region, Df(2R)wunGL. We recovered a single double-mutant allele, wun49 wun2EP2650ex34. The EMS mutation in wun49 was sequenced and corresponds to a C-to-T change, which would result in a proline-to-histidine amino acid change at position 189 or 268 of splice forms Wun-PA and Wun-PB, respectively (FlyBase). Although this residue is conserved in all fly LPPs, it is substituted for a tyrosine or phenylalanine in mammalian LPPs (see Fig. S1 in the supplementary material). Like a previously identified wun null allele, wun9, the new allele, is semi-lethal and female-sterile in trans to itself, the wun9 allele, or a deletion for the region; therefore, we believe it represents a null allele or at least a very strong hypomorph. wun49 wun2EP2650ex34 was recombined onto a p{w+ FRT}42B-containing chromosome and used to make germline clones.
Drosophila lines
The following Drosophila alleles were used: shgA9-49 [a hypomorphic allele resulting from an E336K change in the extracellular domain, described in Kunwar et al. (Kunwar et al., 2008), causing weaker cell-cell adhesion], srp6g (a null allele courtesy of Rolf Reuter, University of Tübingen), UAS moeGFP (courtesy of Tom Millard, University of Manchester), wun2N14 (courtesy of Akira Nakamura, RIKEN Center for Developmental Biology), nos>moeGFP [described in Sano et al. (Sano et al., 2005)], nos>moeRFP (kindly provided by Matthew DeGennaro, New York University) and wun2EP2208ex60 [described in Renault et al. (Renault et al., 2004)]. UAS Rac, UAS RacV12, UAS RacN17 and UAS Rho1N19 flies were provided by Denise Montell (John Hopkins University). UAS Rho, UAS Rho1V14 flies were provided by Marek Mlodzik (Mount Sinai School of Medicine). To produce embryos with few germ cells, gclA26-10 (courtesy of Alexy Arkov, Murray State University) in trans to Df(2R)PurP133 mothers were mated to wild-type males.
Embryo staining and scoring
Egg layings were carried out at room temperature. For bright field microscopy, embryos were fixed in 4% formaldehyde and stained using the following antibodies: rabbit anti-Vasa antibody (1:10,000, a gift from A. Williamson and H. Zinszner, New York University) and anti-rabbit biotin (1:500, Jackson ImmunoResearch), mounted in Epon and viewed on a Zeiss Axioskop microscope. For immunofluorescent images, embryos were heat-fixed, stained using chicken anti-Vasa (1:10,000) and anti-alpha Spectrin (1:10, 3A9 from DSHB), mounted in Aqua-Poly/Mount (Polysciences) and viewed using a Zeiss LSM 510 Meta confocal microscope.
Live imaging
For wild type, embryos laid by females carrying nos>moeGFP on the X chromosome were used. For wun wun2 M–Z–, embryos laid by nos>moeGFP; wun49 wun2EP2650ex34 germline clone females mated to Df(2R)wunGL males were used. Dechorionated embryos were mounted in Halocarbon Oil 200 on an oxygen-permeable membrane (YSL Inc.). Images were acquired at room temperature, and maximum intensity projections and movies were made using Image J (NIH). The wild-type embryo was imaged at 2-minute intervals with 7 sections, spaced 4 μm apart, using a modified Olympus BX51 microscope with a 40× objective (Olympus U Plan Fluorite, oil, 1.3 NA), 80 MHz Ti:Saph laser (Coherent Chameleon Ultra 1) and PrairieView software (Prairie Technologies). The mutant was imaged at 1-minute intervals with either 12 or 13 sections, spaced 2 μm apart, using a Nikon Eclipse E600FN microscope with a 60× objective (Nikon Plan Apo, water, 1.2 NA), the Radiance System (Bio-Rad Laboratories), a 10 W pumped laser (model Tsunami; Spectra-Physics) and LaserSharp 2000 software (Bio-Rad Laboratories).
Germ cell transplants
Embryos were collected from flies laying at room temperature. Germ cells from stage-5 nos>moeRFP;;nos>moeRFP donor embryos were used. Recipient embryos were either wild type (containing nos>moeGFP to label the germ cells) or those laid by wun49 wun2EP2650ex34 germline clone females carrying nosGAL4VP16 mated to UAS moeGFP males (wun wun2 M–Z+ embryos).
Transplants were carried out at 18°C. Recipient and donor embryos were dechorionated in 50% bleach for 3 minutes, rinsed in water, lined up on apple juice agar slices then transferred to glue-coated coverslips. Recipient embryos were desiccated for 15 minutes to obtain mildly flaccid embryos. Embryos were covered with 10S-halocarbon oil. Germ cells were removed from donor embryos at stage 5 by injection through the anterior pole using an 8 μm spiked pipette (BioMedical Instruments). Germ cells were transplanted into recipient embryos by injection into the posterior. Embryos were aged overnight at 18°C to stage 14 and each embryo was scored live for germ cell survival using a Zeiss AxioImager with a 20× objective with GFP and RFP filters. Germ cells that failed to migrate and remained inside the gut were not scored.
The auto-fluorescence of the gut might cause some germ cells to not be visible by fluorescence microscopy, possibly leading to an underestimate of the surviving germ cells. We fixed and used the anti-Vasa antibody to stain uninjected host embryos to accurately score the number of surviving germ cells at stage 14. We found no significant difference in the counting of surviving germ cells in the soma or gonad per embryo between stage-14 fixed embryos (average of 1.9 germ cells per embryo, s.d.=2.7, n=27) compared with living embryos (2.3 germ cells, s.d.=2.7, n=52).
RESULTS
Wun2 acts with Wun in germ cells
Lack of wun2 in germ cells results in germ cell death (Hanyu-Nakamura et al., 2004; Renault et al., 2004). However, not all wun2-null germ cells die, and the surviving germ cells are able to migrate to the gonad and give rise to fertile adults (Renault et al., 2004). Given that wun2 is redundant with wun in the soma (Starz-Gaiano et al., 2001), we tested whether removal of both wun and wun2 from germ cells resulted in more germ cell death than wun2 alone.
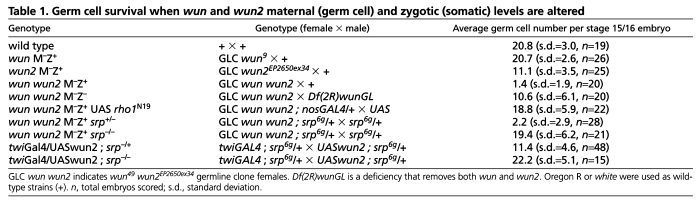
Germ cells are transcriptionally quiescent during early development and therefore rely on maternally supplied mRNA and protein (Van Doren et al., 1998). We created a wun and wun2 double-mutant allele (see Materials and methods) and used this allele to generate wun and wun2 double maternal-mutant embryos (hereafter referred to as wun wun2 M–Z+ embryos; `M' denotes the maternal genotype and `Z' denotes the zygotic genotype) using the germline clone technique (Chou and Perrimon, 1996). In such embryos, the germ cells are mutant for both wun and wun2 but the soma is wild type owing to zygotic expression supplied by a wild-type chromosome from the male. In wun wun2 M–Z+ embryos, germ cells are formed with wild-type numbers but die with, on average, 1.4 germ cells remaining by the end of embryogenesis (Fig. 1D-F; Table 1). This phenotype is much more severe than in wun2 M–Z+ embryos in which 11.1 germ cells typically remain by the end of embryogenesis (Table 1) (Renault et al., 2004). Removal of germ cell wun alone does not affect germ cell survival (Table 1). Taken together, we conclude that wun2 acts redundantly with wun in germ cells.
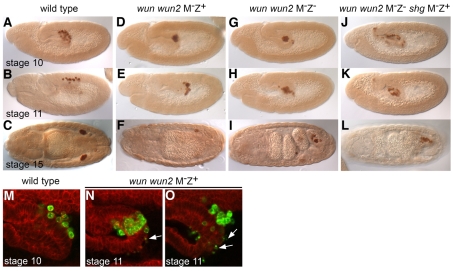
Fig. 1.
Wunens are required for germ cell dispersal and proper transepithelial migration. (A-L) Germ cells stained with an anti-Vasa antibody in lateral stage 10, lateral stage 11 or dorsal stage 15 embryos of wild type (A-C), wun wun2 M–Z+ (D-F), wun wun2 M–Z– (G-I), wun wun2 M–Z– shgA9-49 M–Z+ (J-L). (M-O) Lateral view of the midgut of stage 10-11 embryos. Germ cells are visualized with anti-Vasa (green) and cell membranes with anti-α-Spectrin (red). Germ cells individualize and cross the gut in wild-type embryos (M), but fail to individualize whilst still managing to cross the gut as a cluster in wun wun2 M–Z+ embryos (N,O). Note the large number of Vasa-positive cell fragments (arrows) in wun wun2 M–Z+ embryos (N,O). The remaining germ cells will also eventually die as embryogenesis proceeds, leading to, on average, only 1.4 germ cells surviving by stage 15/16.
Table 1.
Germ cell survival when wun and wun2 maternal (germ cell) and zygotic (somatic) levels are altered
The amount of germ cell death in our wun2 RNA-null alleles, wun2EP2650ex34 and wun2EP2208ex60, is less than that reported for an EMS-induced allele, wun2N14, described in Hanyu-Nakamura et al. (Hanyu-Nakamura et al., 2004). This later allele causes a premature stop codon at residue Trp111, and the truncated protein would include the first transmembrane domain but no catalytic domains. Embryos laid by mothers mutant for the RNA-null alleles wun2EP2650ex34 and wun2EP2208ex60 in trans to a deficiency contain, on average, 10.3 and 9.3 germ cells at late stages, respectively, whereas in embryos laid by females containing wun2N14 in trans to the same deficiency, the number of germ cells surviving was 2.3 (see Table S1 in the supplementary material). Similar differences in germ cell survival were obtained in embryos derived from wun2N14 and wun2EP2650ex34 germ-line clone females (see Table S1 in the supplementary material). Therefore, our results further suggest that the wun2N14 allele acts as a dominant-negative allele and interferes with both Wun and Wun2 function, possibly by heterodimerization (Burnett et al., 2004; Long et al., 2008), thereby creating a more severe germ cell phenotype than loss of wun2 alone.
To determine that the maternal requirement for both wun and wun2 reflects a requirement in the germ cells, we used the nanosGAL4VP16 driver to express wun or wun2 specifically in germ cells in wun wun2 M–Z+ embryos. Expression of either Wun or Wun2 rescues germ cell death with equal efficiency (see Table S1 in the supplementary material), indicating that these two proteins are functionally redundant in germ cells. Phosphatase activity is crucial for germ cell survival because expression of a catalytic dead form of Wun2 (Wun2 H326K) (Starz-Gaiano et al., 2001) could not rescue the death of wun wun2 double-mutant germ cells (see Table S1 in the supplementary material).
Wunens are required for transepithelial migration
We examined further the behaviour of germ cells in wun wun2 M–Z+ embryos during their migration. In the wild type, about 30 germ cells form at the posterior pole of the embryo. During gastrulation, they are carried inside the embryo in close apposition to each other and the posterior midgut primordium. During the process of transepithelial migration, germ cells polarize, individualize and rapidly migrate through the posterior midgut epithelium (Kunwar et al., 2008). Once germ cells have passed through the midgut, they sort bilaterally into two groups to reach the embryonic gonads which are specified on either side within the lateral mesoderm. Subsequently, germ cells associate with the somatic gonads and coalesce into the embryonic gonad. In wun wun2 M–Z+ embryos, a normal number of germ cells was formed and the germ cells were correctly positioned in the midgut pocket at stage 9, prior to active migration. The mutant germ cells, however, failed to individualize and did not cross the midgut epithelium at stage 10 (compare Fig. 1A with D). Eventually, the mutant germ cells did cross the midgut at stage 11. They did not cross as individual cells, as in wild type, but instead moved as a cluster of cells (Fig. 1N). This delayed migration occurred concurrent with the epithelial-to-mesenchymal transition of the midgut cells, suggesting that cellular changes within the midgut might be necessary to permit this type of migration. The mutant germ cells remained in close association, failed to migrate further into the overlying mesoderm (Fig. 1E) and eventually underwent cell death (Fig. 1O).
The death of wun2-null germ cells can be suppressed by reducing the levels of somatic wunens (Hanyu-Nakamura et al., 2004; Renault et al., 2004). We asked whether the death of wun wun2 double-mutant germ cells could be also suppressed by reducing somatic wun and wun2 levels. Removing somatic wunens (wun wun2 M–Z– embryos) could suppress the death of wun wun2 double-mutant germ cells with, on average, 10.6 germ cells surviving at the end of embryogenesis (Table 1; Fig. 1G-I). This number is less than in wild type but we conclude that wun wun2 double-mutant germ cells remain sensitive to somatic wunens.
Intriguingly, the germ cells surviving in wun wun2 M–Z– embryos were exclusively located inside the midgut (Fig. 1I). We found that the germ cells failed to individualize at stage 10, just as in wun wun2 M–Z+ embryos, but in this case the germ cells did not cross the midgut epithelium, but remained in a clump inside the midgut pocket (Fig. 1G,H). These data demonstrate an earlier role for wunens than had previously been shown, namely in the correct dispersal and subsequent transepithelial migration of germ cells.
Lack of transepithelial migration is not due to inappropriate germ cell-germ cell adhesion or a failure to be motile.
The failure of germ cells to cross the midgut in wun wun2 M–Z– embryos might be because germ cells are unable to correctly downregulate germ cell-germ cell adhesion. We recently identified DE-cadherin, encoded by the shg gene, as being important for maintaining the adhesion between germ cells whilst they are inside the midgut (Kunwar et al., 2008). To determine whether wunens regulate migration primarily by downregulating germ cell-germ cell adhesion via DE-cadherin, we generated wun wun2 M–Z– shg M–Z+ embryos. We found that, in such embryos, the germ cells dispersed inside the midgut (Fig. 1J), as they do in shg M–Z+ embryos (data not shown), but this was not sufficient to allow the germ cells to migrate across the midgut at any stage (Fig. 1K,L). Therefore, the failure of germ cells to migrate in wun wun2 M–Z– embryos is not due to an inability to downregulate germ cell-germ cell adhesion.
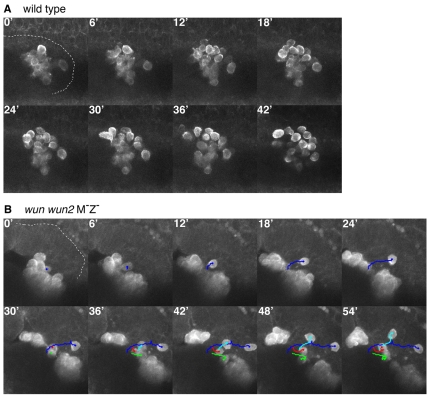
To test if germ cells in wun wun2 M–Z– embryos are still motile we used two-photon microscopy and a germ cell-specific expression system, which translates the actin binding domain of Moesin fused to GFP under the control of nanos regulatory sequences (Sano et al., 2005). In wun wun2 M–Z– embryos, the germ cells appeared as a tight clump. Although the vast majority of germ cells remained in a cluster and did not appear to be motile, occasionally germ cells could be visualized detaching from the clump and migrating within the midgut with normal amoeboid-like motion (Fig. 2). We conclude that wunens are not required for germ cell motility per se. Given the repulsion of germ cells from wunen-expressing somatic cells (Starz-Gaiano et al., 2001; Zhang et al., 1997), we suggest that germ cell wunens mediate germ cell-germ cell repulsion.
Fig. 2.
Germ cells retain motility in embryos lacking wunens. (A,B) Movie stills at 6-minute intervals from a lateral view of a stage 10 wild-type (A) and stage 11 wun wun2 M–Z– (B) embryo (anterior left, dorsal uppermost, germ cells labelled with a moeGFP construct). The extent of the posterior midgut is outlined for the first time-point in each movie series. In the wild type, the germ cells quickly disperse. In the mutant, although most germ cells remain clumped and do not migrate (for example, red and green tracking), single germ cells (blue and cyan tracking) can be seen to break away and migrate with typical amoeboid motion. For full movies, see Movies 1 and 2 in the supplementary material.
Germ cell-germ cell repulsion ensures equal distribution of germ cells between the embryonic gonads
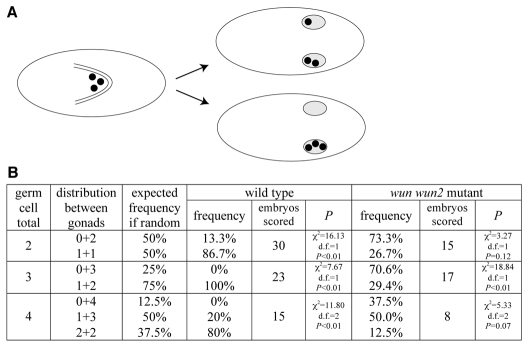
Our data suggest that wunen-mediated germ cell-germ cell repulsion mediates germ cell dispersal and is required for transepithelial migration at the onset of migration. We next wanted to know whether germ cell-germ cell repulsion had additional functional consequences for later stages of germ cell migration. Once germ cells have passed through the posterior midgut, they split and sort into the two embryonic gonads. This process is important for the reproductive success of the organism. If germ cells reach only one embryonic gonad, then, in the adult female for example, only one ovary would be functional, severely reducing fecundity. To test whether germ cell sorting into two gonads was a regulated process, we first analyzed the process in wild-type embryos with a genetic background where the number of germ cells was reduced. We scored the sorting of the germ cells between the embryonic gonads in embryos laid by mothers mutant for a weak germ cell-less (gcl) mutation. Such embryos formed between 2 and 4 germ cells (Fig. 3). In principle, we could imagine three scenarios: (1) random segregation if the process was unregulated, (2) germ cells ending up predominantly in one gonad if germ cells had a tendency to follow a `pioneer' leading them to the gonad, or (3) equal germ cell sorting if germ cells would repel each other to maximize reproductive fitness. We found that the distribution of germ cells was significantly different from the expected frequency if the process was considered random, and was in favour of an equal distribution between the gonads (Fig. 3). We conclude that mechanisms exist to ensure that germ cells split between the gonads.
Fig. 3.
Germ cells split evenly between the embryonic gonads. (A) Cartoons representing the dorsal view of a stage 9 embryo (left) with germ cells (black circles) in the gut and stage 16 embryos (right) with germ cells in the embryonic gonads (grey ovals). In embryos containing few germ cells, the germ cells can split evenly between the two embryonic gonads (upper embryo) or all migrate into the same gonad (lower embryo), which would leave one ovary or testis devoid of germ cells in the adult. (B) Distribution of germ cells between embryonic gonads in embryos with few, but otherwise wild-type, germ cells [embryos laid by gclA26-10/Df(2R)PurP133 mothers] and germ cells lacking wunens (wun wun2 M–Z+ embryos). P value shows the probability that the observed result is significantly different from the expected distribution of germ cells if the process was random (given in the third column) using a χ2 test.
To determine whether Wunen function in germ cells is involved in this process, we asked whether the few germ cells that survive in the absence of germ cell wunens (in wun wun2 M–Z+ embryos) still distribute evenly between the gonads. We found that, in embryos containing 3 germ cells, there was a significant bias toward germ cells ending up in one gonad versus being split between the gonads (Fig. 3). In the case of embryos with 2 or 4 germ cells, there was no significant difference between the distribution of germ cells and expected frequency if the process was considered random (Fig. 3). We propose that wunen-mediated germ cell-germ cell repulsion that occurs at the initiation of migration provides a mechanism to distribute the germ cells evenly between the embryonic gonads and, in the absence of germ cell wunens, the sorting becomes random or rather favours the accumulation of germ cells in just one gonad.
Wunens are required for germ cell survival only during their active migration
As germ cells in wun wun2 M–Z+ embryos die during their attempted trans-midgut migration, we observed a large amount of small Vasa-positive fragments (Fig. 1N,O). Previously, we and others showed that wunen-mediated germ cell death is non-apoptotic (Hanyu-Nakamura et al., 2004; Renault et al., 2004; Sano et al., 2005). These new observations lead us to speculate that these germ cell fragments represent cell remnants and that this germ cell death is caused by germ cell disintegration.
We further noted that germ cells in wun wun2 M–Z+ embryos only begin to die at stage 10 when they initiate migration and not before. One possibility is that Wunen function for survival is only required during the migration of germ cells. To test this hypothesis, we blocked germ cell migration using a number of strategies to see if this would rescue the death of germ cells in wun wun2 M–Z+ embryos. The Rho family of small GTPases are crucial for reorganization of the actin cytoskeleton during cell movement (Burridge and Wennerberg, 2004). We previously found that, in a wild-type background, germ cell expression of dominant-negative Rho1 (Rho1N19) was able to block the transepithelial migration of germ cells leading the majority of germ cells to adhere to each other inside the midgut at late stages (Fig. 4B) (Kunwar et al., 2003). This is similarly true for dominant-active Rac (RacV12; Fig. 4D). However, in both cases, a few germ cells were able to cross the midgut but ended up scattered individually in the posterior of the embryo at stage 16 (Fig. 4B,D, arrowheads). We found that germ cell expression of Rho1N19 or RacV12 in wun wun2 M–Z+ embryos rescued germ cell death (Fig. 4C,E; Table 1). Furthermore, the surviving germ cells were localized exclusively inside the gut and we did not detect individual germ cells outside of the gut at late stages (Fig. 4C,E).
Fig. 4.
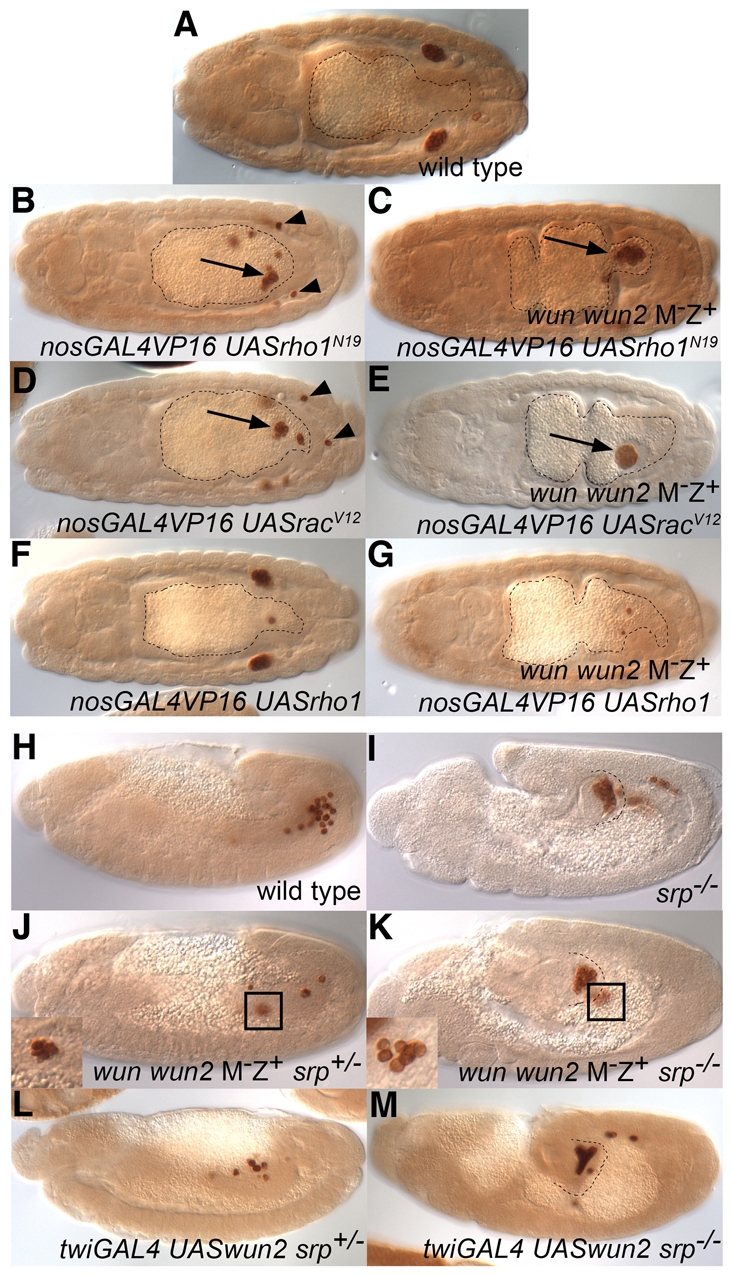
Blocking germ cell migration suppresses the death of wun and wun2 double-mutant germ cells. (A-G) Dorsal view of stage 15/16 Vasa-stained embryos with midguts outlined. (A) Wild-type embryo. (B-G) Embryos laid by nanosGAL4VP16/+ (B,D,F) or wun wun2 germline clone; nanosGAL4VP16/+ (C,E,G) females crossed to UASrho1N19 (B,C), UASracV12 (D,E) or UASrho1 (F,G) males. Germ cell expression of dominant-active Rac (racV12) and dominant-negative Rho1 (rho1N19), but not wild-type Rho1, in wild-type embryos results in most germ cells remaining inside of the midgut (arrows; B,D). The few germ cells that do mange to cross the gut epithelium are scattered in the soma (arrowheads). Germ cell expression of dominant-active Rac and dominant-negative Rho1, but not wild-type Rho1, in germ cells lacking wunens suppresses the germ cell death (C,E,G). The surviving germ cells are found in a clump in the gut (arrows; C,E), with no germ cells in the soma. (H-M) Lateral view of stage 12 or 13 Vasa-stained embryos with the extent of the posterior midgut outlined for srp mutant embryos. (H) Wild-type embryo. (I) srp6g/srp6g embryo. (J,K) wun wun2 M–Z+ embryos that are also zygotically srp6g heterozygous (J) or homozygous (K). At stage 12, many small, irregularly shaped dying germ cells can be seen in a clump in wun wun2 M–Z+ embryos (J, inset). In wun wun2 M–Z+ srp sibling embryos, germ cells can be found in a tight clump in the gut but many are found outside with wild-type morphology and size (K, inset). (L,M) Embryos overexpressing wun2 in the mesoderm using twiGAL4 and UASwun2 have reduced germ cell numbers owing to germ cell death (L), which can be suppressed in srp6g homozygous embryos (M).
Expression of wild-type or dominant-negative Rac (RacN17), and wild-type or constitutively active Rho1 (Rho1V14), did not rescue the death of germ cells lacking wun and wun2 (Fig. 4G; data not shown) and expression of these forms in a wild-type background did not block germ cell transepithelial migration or affect later germ cell migration (Fig. 4F; data not shown). Therefore, suppression of germ cell death correlates with the ability of these constructs to block migration.
In the second strategy, we used a cell-non-autonomous method to block transepithelial migration: serpent (srp) encodes a GATA transcription factor necessary for proper specification of the midgut. In srp mutant embryos, the midgut through which the germ cells migrate is transformed into hindgut, preventing the germ cells from crossing (Jaglarz and Howard, 1994; Reuter, 1994). In late srp mutant embryos, most of the germ cells are tightly clumped in a ball inside the posterior midgut pocket; however, a few germ cells move through and are found in a loose cluster on the other side of the gut (Fig. 4I). We found that srp was able to rescue the death of germ cells in wun wun2 M–Z+ embryos (Fig. 4J,K; Table 1). The surviving germ cells in wun wun2 M–Z+ srp– embryos were found both in a tight clump inside the gut but also frequently in loosely associated groups outside of the gut (Fig. 4K, inset). These later germ cells are normal in size and morphology, indicating that it is not the germ cell aggregation or localization within the midgut that causes the germ cells to survive in wun wun2 M–Z+ srp– embryos.
Wunens are also able to affect germ cell survival through their expression in the soma. In particular, overexpression of either wun or wun2 in somatic cells is able to induce germ cell death (Starz-Gaiano et al., 2001). We therefore tested whether the germ cell death resulting from wun2 overexpression can be suppressed by blocking their migration. We found that srp was able to rescue the death of germ cells in embryos overexpressing wun2 in the mesoderm using the GAL4 driver twiGAL4 (Fig. 4L,M; Table 1). Thus, the death resulting from wun2 overexpression in the soma and lack of wunens in germ cells both require that germ cells actively migrate.
Germ cell requirement for wunens is cell-autonomous
Wunens act to dephosphorylate a cell-surface lipid phosphate, which thereby modulates germ cell survival. Thus, it is in principle possible that wunens expressed on the surface of one germ cell could provide phosphatase activity, and perhaps the dephosphorylated lipid product, to neighbouring cells and thereby enable these germ cells to survive and migrate properly. To test this idea, we examined whether transplanted wild-type germ cells could rescue the survival of wun wun2 mutant germ cells, which would normally die during embryogenesis. We transplanted stage-5 wild-type germ cells, labelled with the actin binding domain of Moesin fused to RFP, into the posterior pole of stage-5 wun wun2 M–Z+ embryos, whose germ cells were labelled with Moesin actin binding domain fused to GFP (Fig. 5A,C,D). We scored the numbers of wunen mutant host and wild-type transplanted germ cells visible at stage 14 (Fig. 5B). We found no significant difference (Mann-Whitney U test, U=1068, P=0.38) between the number of surviving host germ cells in embryos in which no transplanted germ cells were visible (average=2.3, n=52, s.d.=2.7) and in embryos in which one or more transplanted germ cells were visible (average=2.7, n=37, s.d.=2.7). In addition, we attempted to correlate the numbers of surviving wun wun2-null host germ cells with the number of transplanted wild-type germ cells using a Spearman rank-order correlation test but no significant correlation was observed (n=66, rs=–0.0236, P=0.85). This argues that the cell surface phosphatase reaction is cell-autonomous.
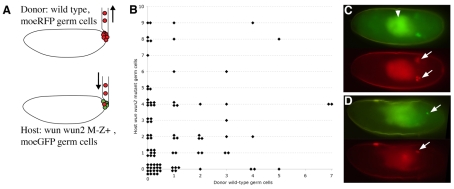
Fig. 5.
Transplantation of wild-type germ cells does not rescue wun wun2-null germ cell survival. (A) Transplantation scheme. Germ cells from stage-5 nos>moeRFP embryos (donor) were injected into the posterior pole of stage-5 embryos laid by wun wun2 germ line clone females (wun wun2 M–Z+) carrying nosGAL4VP16 mated to UAS moeGFP males (host). (B) Scattergram showing the number of host mutant germ cells in soma or gonad versus the number of donor wild-type germ cells in soma or gonad. (C,D) Fluorescent images of living embryos, anterior to the left, showing host moeGFP germ cells (green) and transplanted moeRFP germ cells (red). (C) Stage-15 wild-type host embryo. Transplanted germ cells are able to survive and migrate to the gonads (arrows). The gut autofluorescence is marked with an arrowhead. (D) Stage-14 wun wun2 M–Z+ host embryo showing a single surviving host germ cell (upper arrow) and two transplanted germ cells (lower arrow).
DISCUSSION
Our data show that Wun and Wun2 act redundantly, not only in the soma, but also in germ cells. Analysis of loss-of-function double mutants reveals that wunens are required earlier than previously described, during transepithlelial migration, the first active migratory step of germ cells. Wunens act specifically and cell-autonomously in germ cells and this function is needed to maintain cellular integrity necessary for germ cell survival and migration. Wunen-mediated germ cell-germ cell repulsion provides a mechanism to disperse and distribute the germ cells evenly between the embryonic gonads, thereby maximizing adult fecundity.
The early transcriptional quiescence of Drosophila germ cells requires that many germ cell components be maternally supplied. Loss of maternal wun2 leads to germ cell death (Hanyu-Nakamura et al., 2004; Renault et al., 2004). Mutations in wun, although having no maternal phenotype by themselves, substantially enhance the phenotype caused by loss of maternal wun2. Therefore, similar to the situation that occurs in somatic cells, wun2 is redundant with wun in germ cells. Expression of Wun or Wun2 individually is able to fully substitute for the loss of both genes in germ cells (see Table S1 in the supplementary material). Overexpression of either gene in somatic cells causes identical amounts of germ cell death (our unpublished results). We conclude that Wun and Wun2 are fully functionally redundant, which supports the notion there is a single in vivo substrate that both proteins can dephosphorylate with equal efficiency. The germ cell death caused by lack of Wun2 in germ cells (Renault et al., 2004), which does not occur upon removal of just Wun, is probably caused by differences in the expression levels of the two genes in germ cells.
We have found that the requirement of germ cells for wunens for their survival can be abrogated if the migration of the germ cells is blocked. Although we cannot rule out the possibility that germ cells become reliant on wunens owing to a signalling event that occurs concomitant with migration or that the midgut provides a protective environment, we favour the idea that the migration process itself creates physical stress that, without wunens, leads to cell disintegration and death.
Transepithelial migration involves the rapid dispersal of the germ cells, which move as individually migrating cells across the midgut epithelium (Fig. 2) (Kunwar et al., 2008). We find that wunens play a role in this process based upon germ cell behaviour in two genetic backgrounds. First, in embryos lacking germ cell and somatic wunens (wun wun2 M–Z– embryos), the majority of germ cells remain inside of the midgut. Second, in embryos lacking just germ cell wunens (wun wun2 M–Z+ embryos), the germ cells fail to individualize but do cross the midgut epithelium. The germ cells never appear as individual cells but remain in a tight cluster. Their migration is delayed compared with wild type and is concurrent with the epithelial-to-mesenchymal transition of the midgut cells, which might be necessary to permit this movement of clustered cells.
Two interpretations of these data are possible. First, that germ cell wunens act to reduce adhesion between the germ cells, allowing them to disperse. However, reducing DE-cadherin in germ cells, sufficient to get germ cell dispersal, is not sufficient to cause the germ cells to migrate through the midgut in wun wun2 M–Z– embryos (Fig. 1J-L). The alternative explanation is that wunen-expressing germ cells also repel each other, given that wunen-expressing somatic cells repel germ cells. Wunen-mediated germ cell-germ cell repulsion would thereby provide a mechanism to explain the rapid movement of these cells away from each other that occurs during transepithelial migration (Kunwar et al., 2008).
We wondered if germ cell-germ cell repulsion might confer any advantages to the organism. We find that germ cell-germ cell repulsion serves to distribute the germ cells evenly between the two embryonic gonads, which are located on either side of the midline (Fig. 3). Full fertility of the adult can be achieved even if only a few germ cells reach the embryonic gonad. This is because germ cell division during larval stages can compensate for deficits in the number of germ cells reaching the gonad (Gilboa and Lehmann, 2006). If, however, no germ cells reach a gonad, compensatory proliferation is not possible and the adult ovary or testis will be devoid of eggs or sperm, respectively. Therefore, distribution of germ cells between the gonads becomes crucial when there are few germ cells. For germ cells to reach both gonads, they must sort bilaterally to either side of the midline. Wunens are expressed in the central nervous system in the middle of the embryo and this tissue repels germ cells from the midline toward the lateral sides (Sano et al., 2005). This mechanism might be sufficient to get germ cells to the lateral sides but could actually hinder the equal distribution of germ cells because it also prevents germ cells from crossing the midline from one side of the embryo to the other (Sano et al., 2005). Therefore, it is important that germ cells are already evenly spread laterally when they exit the midgut. Wunen-mediated germ cell-germ cell repulsion inside the midgut is well placed to ensure that germ cells exit the midgut in all directions and therefore are likely to sort equally to lateral sides and hence to both gonads.
If germ cells repel each other, then how do they come together to cluster at the embryonic gonad? Although it is possible that attractive cues from the gonad act to overcome repulsive effects of the germ cells, we note that the coalescence of the germ cells into the embryonic gonad is driven by the somatic cells of the gonad and not by the germ cells themselves (Clark et al., 2007; Jenkins et al., 2003). Wun2 expression in germ cells remains constant throughout embryogenesis (our unpublished results); therefore, we do not believe that regulation of Wun2 levels plays a role in germ cell coalescence.
These findings have provided new insight into how wunens act at the molecular level in transepithelial migration, and germ cell migration in general, and extend our previous model (Fig. 6) (Renault et al., 2004). Wunens dephosphorylate a lipid phosphate, which is required for germ cell survival and attraction. Expression of wunens on somatic cells leads to spatial differences in lipid phosphate levels. Germ cells move out of the gut owing to expression of wunens on midgut cells, which depletes lipid phosphate levels. Expression of wunens on germ cells causes local depletions in lipid phosphate levels and hence germ cells migrate away to avoid contact with each other (Fig. 6).
Fig. 6.
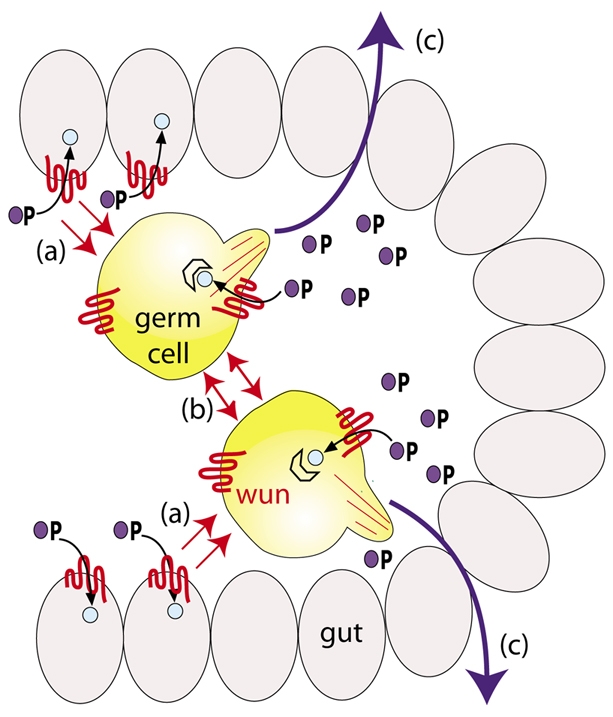
Model showing how wunen activity leads to germ cell-germ cell repulsion. Model depicts germ cells inside the midgut pocket in a stage-10 embryo. Wunen in somatic cells depletes a lipid phosphate attractant (blue circle with P) required for survival, leading to repulsion of germ cells from wunen-expressing somatic cells (a). Germ cells also deplete the lipid phosphate attractant leading to germ cell-germ cell repulsion (b). This leads germ cells to cross the midgut in all directions (c), ensuring that the germ cells become partitioned equally to the embryonic gonads. The extent to which competition between soma and germ cells occurs inside the midgut pocket is uncertain. Germ cells do cross the midgut in wun wun2 zygotic mutants in which the midgut cells would lack wunens, therefore germ cell-germ cell repulsion is sufficient for crossing the midgut.
Given that wunens act to produce a dephosphorylated product that might be accessible to neighbouring cells, we examined whether the requirement for germ cell wunens is completely cell-autonomous. We found that transplanted wild-type germ cells were unable to suppress the death of wun wun2 mutant germ cells (Fig. 5). We therefore find no evidence that germ cell wunens can supply the dephosphorylated product to neighbouring germ cells. If this were the case, then we might expect wun wun2 mutant germ cells to be attracted and remain associated with wunen-positive somatic cells. In fact, the opposite is true and wunen-positive somatic cells repel germ cells.
The splitting of germ cells from an initial cluster between two gonads occurs in a number of species outside of Drosophila. In the mouse, the germ cells are specified in the primitive streak as a single group. Although germ cells do not immediately sort laterally at this stage, exit from their site of origin is regulated by a repulsive factor, Ifitm1, that is expressed on germ cells and surrounding cells (Tanaka et al., 2005). The germ cells invade the endoderm and migrate inside the hindgut. The germ cells exit the hindgut dorsally and it is at this point that the germ cells split into two groups as they migrate towards the bilateral genital ridges (Kunwar et al., 2006). Whether germ cell-germ cell repulsion mechanisms operate at this stage remains to be evaluated. In other species, zebrafish for example, the situation is different. The germ cells form at four different locations randomly located with respect to the body axis. The migration of the germ cells to the gonads therefore involves the convergence of pairs of germ cell clusters rather than the splitting of a single cluster (Weidinger et al., 1999).
The dispersal of cells is a common occurrence in development. For example, the migration and scattering of hemocytes in the Drosophila embryo or dispersal of the neural crest cells in vertebrates. Wunens, and thereby the action of LPPs, present a novel mechanism that mediates repulsion. Examining to what extent cell-to-cell repulsion is used, rather than attractive cues, will provide new insights into the strategies used to disperse cells.
Supplementary Material
Acknowledgments
We thank members of the fly community for fly stocks and the Developmental Studies Hybridoma Bank (University of Iowa, Department of Biology, Iowa City, IA, USA) for antibodies. We thank Kaspar Feldmeier for statistical help and members of the Lehmann laboratory and Gáspár Jékely for advice on the manuscript. A.D.R. was a Charles H. Revson Senior Fellow in Biomedical Science and R.L. is an HHMI investigator and a member of the Kimmel Center for Biology and Medicine. This work was supported by NIH grant R01 HD041900. Deposited in PMC for release after 6 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.046110/-/DC1
References
- Burnett C., Makridou P., Hewlett L., Howard K. (2004). Lipid phosphate phosphatases dimerise, but this interaction is not required for in vivo activity. BMC Biochem. 5, 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Wennerberg K. (2004). Rho and Rac take center stage. Cell 116, 167-179 [DOI] [PubMed] [Google Scholar]
- Chou T. B., Perrimon N. (1996). The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics 144, 1673-1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. B., Jarman A. P., Finnegan D. J. (2007). Live imaging of Drosophila gonad formation reveals roles for Six4 in regulating germline and somatic cell migration. BMC Dev. Biol. 7, 52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa L., Lehmann R. (2006). Soma-germline interactions coordinate homeostasis and growth in the Drosophila gonad. Nature 443, 97-100 [DOI] [PubMed] [Google Scholar]
- Hanyu-Nakamura K., Kobayashi S., Nakamura A. (2004). Germ cell-autonomous Wunen2 is required for germline development in Drosophila embryos. Development 131, 4545-4553 [DOI] [PubMed] [Google Scholar]
- Jaglarz M. K., Howard K. R. (1994). Primordial germ cell migration in Drosophila melanogaster is controlled by somatic tissue. Development 120, 83-89 [DOI] [PubMed] [Google Scholar]
- Jenkins A. B., McCaffery J. M., Van Doren M. (2003). Drosophila E-cadherin is essential for proper germ cell-soma interaction during gonad morphogenesis. Development 130, 4417-4426 [DOI] [PubMed] [Google Scholar]
- Kunwar P. S., Starz-Gaiano M., Bainton R. J., Heberlein U., Lehmann R. (2003). Tre1, a G protein-coupled receptor, directs transepithelial migration of Drosophila germ cells. PLoS Biol. 1, 372-384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunwar P. S., Siekhaus D. E., Lehmann R. (2006). In vivo migration: a germ cell perspective. Annu. Rev. Cell Dev. Biol. 22, 237-265 [DOI] [PubMed] [Google Scholar]
- Kunwar P. S., Sano H., Renault A. D., Barbosa V., Fuse N., Lehmann R. (2008). Tre1 GPCR initiates germ cell transepithelial migration by regulating Drosophila E-cadherin. J. Cell Biol. 183, 157-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J. S., Pyne N. J., Pyne S. (2008). Lipid phosphate phosphatases form homo- and hetero-oligomers: catalytic competency, subcellular distribution and function. Biochem. J. 411, 371-377 [DOI] [PubMed] [Google Scholar]
- Renault A. D., Sigal Y. J., Morris A. J., Lehmann R. (2004). Soma-germ line competition for lipid phosphate uptake regulates germ cell migration and survival. Science 305, 1963-1966 [DOI] [PubMed] [Google Scholar]
- Reuter R. (1994). The gene serpent has homeotic properties and specifies endoderm versus ectoderm within the Drosophila gut. Development 120, 1123-1135 [DOI] [PubMed] [Google Scholar]
- Sano H., Renault A. D., Lehmann R. (2005). Control of lateral migration and germ cell elimination by the Drosophila melanogaster lipid phosphate phosphatases Wunen and Wunen 2. J. Cell Biol. 171, 675-683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal Y. J., McDermott M. I., Morris A. J. (2005). Integral membrane lipid phosphatases/phosphotransferases: common structure and diverse functions. Biochem. J. 387, 281-293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starz-Gaiano M., Cho N. K., Forbes A., Lehmann R. (2001). Spatially restricted activity of a Drosophila lipid phosphatase guides migrating germ cells. Development 128, 983-991 [DOI] [PubMed] [Google Scholar]
- Tanaka S. S., Yamaguchi Y. L., Tsoi B., Lickert H., Tam P. P. (2005). IFITM/Mil/fragilis family proteins IFITM1 and IFITM3 play distinct roles in mouse primordial germ cell homing and repulsion. Dev. Cell 9, 745-756 [DOI] [PubMed] [Google Scholar]
- Van Doren M., Williamson A. L., Lehmann R. (1998). Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr. Biol. 8, 243-246 [DOI] [PubMed] [Google Scholar]
- Weidinger G., Wolke U., Koprunner M., Klinger M., Raz E. (1999). Identification of tissues and patterning events required for distinct steps in early migration of zebrafish primordial germ cells. Development 126, 5295-5307 [DOI] [PubMed] [Google Scholar]
- Zhang N., Zhang J., Purcell K. J., Cheng Y., Howard K. (1997). The Drosophila protein Wunen repels migrating germ cells. Nature 385, 64-67 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.