Abstract
Background
Human phosphodiesterase (PDE) type 8B (PDE8B) is located at 5q14.1 and is known as the PDE with the highest affinity to cAMP. We recently described a family with bilateral micronodular adrenocortical disease that was apparently caused by an inactivating PDE8B mutation (H305P). As a result of a genome-wide study, a strong association between six polymorphic variants in the PDE8B promoter and serum levels of the thyroid-stimulating hormone (TSH) has been recently reported. Despite an extended analysis of the regions surrounding 5q14.1, no other potential genetic variants that could be responsible for the associated TSH levels were found.
Methods
In this study, we genotyped by polymerase chain reaction the described six polymorphic variants in the PDE8B promoter in the family with micronodular adrenocortical disease and inactivating PDE8B mutation and analyzed their correlation with individual TSH values in the family members.
Results
We observed complete segregation between the reported association and individual TSH values in the family we studied. Haplotype analysis showed that the haplotype associated with the high TSH levels is different from the one that segregated with H305P, suggesting that the mutation most probably has arisen on an allele independent of the high TSH-associated allele.
Conclusions
The proposed mechanism by which PDE8B may influence TSH levels is through control of cAMP signaling. Our analysis revealed separate segregation of an inactivating PDE8B allele from the high-TSH-allele and showed low TSH levels in persons who carry an inactivating PDE8B allele. These data suggest that, indeed, PDE8B may be involved in regulation of TSH levels.
Introduction
Human phosphodiesterase (PDE) type 8B (PDE8B) is located at 5q14.1, regulates cAMP signaling, and has the highest affinity for cAMP compared with all other known PDEs (1). We recently described a family with bilateral micronodular adrenocortical disease (MAD); the latter was caused by an inactivating PDE8B mutation (H305P) (2). MAD is a distinct form of corticotrophin (ACTH)-independent adrenal hyperplasia characterized by multiple nodules of up to 1 cm in diameter that are surrounded by hyperplastic cortical tissue (3–5). This form of adrenocortical hyperplasia is one of the rare genetic causes of Cushing syndrome that affects primarily children and young adults and is linked to molecular defects of the cAMP signaling pathway (6,7).
As a result of a genome-wide study, a strong association between six polymorphic variants in the PDE8B promoter (rs461497, rs6453293, rs13158164, rs4704397, rs6885099, and rs2046045) and serum levels of the thyroid-stimulating hormone (TSH) has been recently reported (8). Despite an extended analysis of regions surrounding 5q14.1, no other potential sequence variants were found that could be linked to the regulation of the TSH levels (4,8). TSH regulates the thyroid function through the cAMP pathway, and its serum level is a sensitive predictor for thyroid activity, even within the normal range (9). Although TSH levels show certain heritability (at least in some ethnic groups), the genetic mechanism by which specific gene variants influence TSH levels remains unknown (9,10).
In this study, we genotyped the described six polymorphic variants in the PDE8B promoter (8) in a family with MAD that we have described before (2); its members carry an inactivating PDE8B mutation that leads to Cushing syndrome. We then analyzed segregation of these PDE8B alleles with individual TSH values in family members.
Materials and Methods
Subjects and their samples
The institutional review boards of National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), approved the genetic investigation of patients with adrenocortical tumors under NICHD protocols 95-CH-0059 and 00-CH-0160 after the patients had given informed consent. The clinical data for the herein studied family are presented in Table 1. The proband (CAR 559.03) was a girl found to have Cushing syndrome due to isolated MAD at age 2 years (2,7). In addition to characteristic Cushing syndrome signs (hypercholesterolemia and other metabolic abnormalities, obesity, and growth retardation) she presented also with elevated TSH that was unusual for her degree of hypercortisolemia. She continued to run relatively high TSH levels with negative antibodies, after her cure with surgery. She inherited the PDE8B inactivating mutation (H305P) from her father (CAR 559.01), who had mild hyperplasia of the adrenal glands on computed tomography, and moderate hypercholesterolemia and hypertension (2,7). In contrast to the proband, his TSH levels were in the low normal range. Similarly, the sister of the proband (CAR 559.04), who was also carrier of the H305P mutation in PDE8B, had normal-to-low TSH serum values; she was clinically normal and did not undergo extensive testing. The mother (CAR 559.02) was negative for the PDE8B H305H mutation and presented with intermediate TSH levels; she was positive for microsomal antibodies, but had no history of thyroid disease and no goiter.
Table 1.
Clinical Data of the Family Positive for Inactivating Mutation in Phosphodiesterase Type 8B (H305P)
| |
|
Thyroid hormones |
|
|||
|---|---|---|---|---|---|---|
| Patient code | Family relation | TSH (normal range, 0.4–4 mIU/L) | FT4 (μg/dL) | T3 (ng/mL) | Microsomal antibodies (normal range, <35.0 IU/mL) | PDE8B genotype |
| CAR 559.03 | Proband | 5.45 mIU/L | 1.5 | 129 | NA | wt/H305P |
| CAR 559.01 | Father | 1.13 mIU/L | 1.3 | NA | NA | wt/H305P |
| CAR 559.02 | Mother | 2.3 mIU/L | 6.9a | NA | 510 IU/mL | wt/wt |
| CAR 559.04 | Sister | 0.59 mIU/L | 1.3 | 1.4 | NA | wt/H305P |
Value in bold: outside the normal range.
For CAR 559.02, the total T4 has been measured.
FT4, free thyroxine; PDE8B, phosphodiesterase (PDE) type 8B; T3, triiodothyronine; TSH, thyroid-stimulating hormone; N/A, non-available.
DNA extraction and sequence analysis
Blood samples were collected; DNA was extracted from whole blood using standard procedures (7). All the primers used for the polymerase chain reaction amplification of the PDE8B promoter regions containing the single-nucleotide polymorphism (SNPs) of interest are listed in Table 2. After the amplification, the polymerase chain reaction products were agarose gel purified (Minelute; Qiagen, Valencia, CA) and bidirectionally sequenced on a 3130 × l Genetic Analyzer (Applied Biosystems, Foster City, CA). Sequences were analyzed using Vector NTI 10 software (Invitrogen, Carlsbad, CA).
Table 2.
Primers Used to Genotype the Six Polymorphic Variants in the Phosphodiesterase Type 8B Promoter Region
| Poly | Amplicon | Forward primer sequence (5′–3′) | Reverse primer sequence (5′–3′) | PCR product size (bp) |
|---|---|---|---|---|
| rs4361497 | PDE8B poly1 | gaagccagctgtggcattt | tggccttagataggcgtcat | 485 |
| rs6453293 | PDE8B poly2 | tgaggggcagtggctataaa | taatggaaaatggccaaagg | 600 |
| rs13158164 and rs4704327 | PDE8B poly3&4 | ccttgcagctcctttgctat | gctttgagctccaggcttag | 844 |
| rs6885099 | PDE8B poly5 | gggaatgtcacctttgttgg | gaacgagaaaacaggccaaa | 592 |
| rs2046045 | PDE8B poly6 | ggcttgtctctccagaatgc | cagctactcggaaggctgag | 472 |
PCR, polymerase chain reaction.
In silico analysis
The promoter region of PDE8B was analyzed for potential functional or expressed variants using online available tools: for detection of potential initiation exons we applied First exon finder (11); for the detection of possible splice sequences we applied Splice port (12) and Genescan (13).
Results
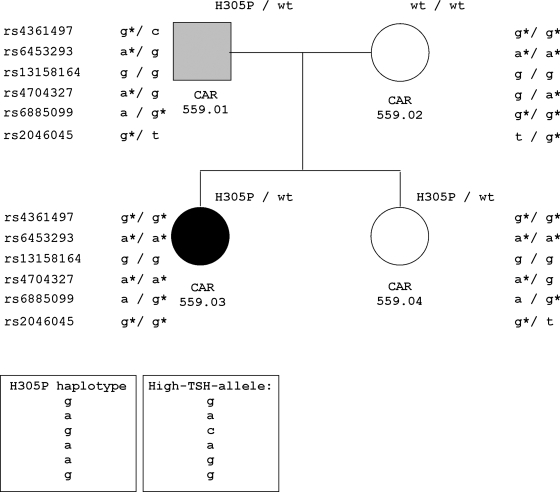
We first genotyped all four family members for the six PDE8B promoter variants; the results are presented in Figure 1. A schematic representation of the six polymorphic sites in relation to the PDE8B locus is shown on Figure 2. We determined the haplotype linked to the H305P mutation: rs461497 [g], rs6453293 [a], rs13158164 [g], rs4704397 [a], rs6885099 [a], rs2046045 [g], or [g-a-g-a-a-g], which was different from the one associated with increased TSH [g-a-c-a-g-g] (8), and thus, rejected our initial hypothesis of linkage between the high-TSH-associated haplotype and the inactivating mutation H305P in PDE8B. Next, we analyzed the segregation between the reported high-TSH-associated alleles and the individual TSH values in the members of the family we studied. Our results were completely consistent with the reported association: the proband (CAR 559.03), who carried both the H305P-linked and high-TSH-associated alleles (see Fig. 1), had elevated TSH levels (5.45 mIU/L, normal range 0.4–4). In contrast, her father (CAR 559.01) and sister (CAR 559.04), both positive for the H305P mutation, did not carry the high-TSH-linked allele and presented with TSH levels in the lower interval of the normal range (1.13 and 0.59 mIU/L, respectively). The mother (CAR 559.02), who shared the high-TSH-linked allele with the proband, had intermediate TSH levels (2.3 mIU/L).
FIG. 1.
PDE8B polymorphic variants that were associated with TSH levels in a family with inactivating PDE8B mutation (H305P). The asterisks indicate association with TSH levels. The haplotypes associated with the H305P mutation and with the high TSH levels are shown in boxes. PDE8B, phosphodiesterase (PDE) type 8B; TSH, thyroid-stimulating hormone.
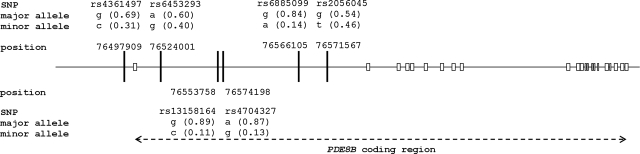
FIG. 2.
Schematic representation of the region between nucleotides 764,490,000 and 767,500,000 of chromosome 5. The positions of the six polymorphic sites in the PDE8B promoter are indicated. PDE8B exons are represented by white rectangles.
The correlation between the TSH levels and the high-TSH-linked allele was also supported by the cumulative number of high-TSH-associated alleles—9 (out of maximum possible 12) in the proband versus 5 and 7 in the father and the sister, respectively. When only the three SNPs showing the genome-wide significant association with TSH levels were taken into account (rs4704397, rs6885099, and rs2046045) (8), their cumulative number in the proband was 5 (out of a maximum of 6) compared to 3 in both the father and the sister, and 4 in the mother (see Fig. 1).
To search for closely positioned coding or functional PDE8B regions, we performed an in silico analysis on 75,000 bp from the 3′ region of the gene that contained the promoter, the previously recognized exon 1 and intron 1, the novel alternative exon 1 (7), and all six high-TSH-associated SNPs (11–13). Our analysis predicted with the highest confidence the two already detected in vivo initiation exons: the longest exon of the gene (isoforms 1–4), and the novel exon encoding 12 alternative AA (isoform 6) that was recently described (7,14,15); no other sequences from the region were scored reasonably high to initiate an analysis of their expression in vivo.
Discussion
The inactivating PDE8B mutation H305P was found on a novel isoform of the enzyme PDE8B (PDE8B6) that expresses a previously unknown initiating exon from the 3′ region of the promoter of the gene (7). In the present study, we determined the phase of the alleles for an inactivating PDE8B sequence alteration and six PDE8B promoter polymorphic variants that are reported to be associated with high TSH serum levels (8). The observed individual TSH values in the family we studied support the association of high TSH with the reported alleles. The haplotype associated with the high TSH levels was different from the one that segregated with H305P, suggesting that the mutation has arisen on an allele independent of the high TSH-associated allele.
In contrast to the proband, the mother, who was also harboring the high-TSH-associated allele, presented with intermediate TSH levels. Unfortunately, the mother was not available for further clinical investigation. Nevertheless, the observed genotype–phenotype correlations in the described family represent an informative example of the combined action of the known genetic factors that influence thyroid function.
The proposed mechanism by which PDE8B may influence TSH levels is through control of cAMP signaling. In this study, an inactivating PDE8B allele was present in association with both the high-TSH-allele (in the proband and her mother) and the low-TSH-level-associated alleles (in the father and the sister). Thus, PDE8B inactivation by this mutation that causes increased cAMP signaling in the adrenal cortex does not seem to lead to increased TSH in all subjects. Although tissue-specific effects need to be considered, it appears that the identified genetic variants that are associated with TSH levels in the population have a different effect that, at least in the case of this mutation, exceeds the increase of cAMP signaling mediated by PDE8B partial inactivation.
TSH production by the pituitary gland is regulated by the hypothalamic factors TSH-releasing hormone (TRH) and somatostatin (16,17). In addition, TRH and TSH secretion are regulated through a negative feedback mechanism by thyroid hormones (17). Since PDE8B is expressed primarily in the thyroid and significantly less in the pituitary and the hypothalamus, it is likely that it affects TSH responsiveness at the level of the thyroid cells more than at the level of pituitary production (16,17): if the identified genetic variants affect PDE8B activity in the thyroid, a decrease in cAMP levels there would lead to lower T3 and T4 production, which in turn would increase TSH levels.
Other molecular factors that may influence TSH levels in the population include the loci encoding the TSH and the TSH receptor, the latter harboring also the genes for thyroid transcription factor 1 (NKX2-1) and iodothyronine deiodinase type 2 (DIO2) (18,19). These genes are on different chromosomes and were not studied in the present investigation.
In conclusion, we report separate segregation of high TSH levels from a PDE8B inactivating allele in a family with bilateral adrenocortical hyperplasia. Our results support the suggested association (8) between the reported PDE8B haplotype and elevated TSH levels.
Acknowledgments
This work was supported by NIH intramural project Z01-HD-000642-04 to Dr. C.A. Stratakis (PDEGEN, NICHD, NIH). We are grateful to our patient and her family.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Bender AT. Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 2.Horvath A. Mericq V. Stratakis CA. Mutation in PDE8B, a cyclic AMP-specific phosphodiesterase in adrenal hyperplasia. N Engl J Med. 2008;358:750–752. doi: 10.1056/NEJMc0706182. [DOI] [PubMed] [Google Scholar]
- 3.Disorders of the human adrenal cortex. In: Flück CE, editor; Miller WL, editor. Endocrine Development. Vol. 13. Karger; Basel: 2008. pp. 117–132. [Google Scholar]
- 4.Stratakis CA. Boikos SA. Genetics of adrenal tumors associated with Cushing's syndrome: a new classification for bilateral adrenocortical hyperplasias. Nat Clin Pract Endocrinol Metab. 2007;3:748–757. doi: 10.1038/ncpendmet0648. [DOI] [PubMed] [Google Scholar]
- 5.Stratakis CA. Adrenocortical tumors, primary pigmented adrenocortical disease (PPNAD)/Carney complex, and other bilateral hyperplasias: the NIH studies. Horm Metab Res. 2007;39:467–473. doi: 10.1055/s-2007-981477. [DOI] [PubMed] [Google Scholar]
- 6.Horvath A. Boikos S. Giatzakis C. Robinson-White A. Groussin L. Griffin KJ. Stein E. Levine E. Delimpasi G. Hsiao HP. Keil M. Heyerdahl S. Matyakhina L. Libè R. Fratticci A. Kirschner LS. Cramer K. Gaillard RC. Bertagna X. Carney JA. Bertherat J. Bossis I. Stratakis CA. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet. 2006;38:794–800. doi: 10.1038/ng1809. [DOI] [PubMed] [Google Scholar]
- 7.Horvath A. Giatzakis C. Tsang K. Greene E. Osorio P. Boikos S. Libè R. Patronas Y. Robinson-White A. Remmers E. Bertherat J. Nesterova M. Stratakis CA. A cAMP-specific phosphodiesterase (PDE8B) that is mutated in adrenal hyperplasia is expressed widely in human and mouse tissues: a novel PDE8B isoform in human adrenal cortex. Eur J Hum Genet. 2008;16:1245–1253. doi: 10.1038/ejhg.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnaud-Lopez L. Usala G. Ceresini G. Mitchell BD. Pilia MG. Piras MG. Sestu N. Maschio A. Busonero F. Albai G. Dei M. Lai S. Mulas A. Crisponi L. Tanaka T. Bandinelli S. Guralnik JM. Loi A. Balaci L. Sole G. Prinzis A. Mariotti S. Shuldiner AR. Cao A. Schlessinger D. Uda M. Abecasis GR. Nagaraja R. Sanna S. Naitza S. Phosphodiesterase 8B gene variants are associated with serum TSH levels and thyroid function. Am J Hum Genet. 2008;82:1270–1280. doi: 10.1016/j.ajhg.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vassart G. Dumont JE. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev. 1992;13:596–611. doi: 10.1210/edrv-13-3-596. [DOI] [PubMed] [Google Scholar]
- 10.Pilia G. Chen WM. Scuteri A. Orrú M. Albai G. Dei M. Lai S. Usala G. Lai M. Loi P. Mameli C. Vacca L. Deiana M. Olla N. Masala M. Cao A. Najjar SS. Terracciano A. Nedorezov T. Sharov A. Zonderman AB. Abecasis GR. Costa P. Lakatta E. Schlessinger D. Heritability of cardiovascular and personality traits in 6,148 Sardinians. PLoS Genet. 2006;2:e132. doi: 10.1371/journal.pgen.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davuluri RV. Grosse I. Zhang MQ. Computational identification of promoters and first exons in the human genome. Nat Genet. 2001;29:412–417. doi: 10.1038/ng780. [DOI] [PubMed] [Google Scholar]
- 12.Dogan RI. Getoor L. Wilbur WJ. Mount SM. SplicePort—an interactive splice-site analysis tool. Nucleic Acids Res. 2007;35:W285–W291. doi: 10.1093/nar/gkm407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burge CB. Modeling dependencies in pre-mRNA splicing signals. In: Salzberg S, editor; Searls D, editor; Kasif S, editor. Computational Methods in Molecular Biology. Elsevier Science; Amsterdam: 1998. pp. 127–163. [Google Scholar]
- 14.Soderling SH. Bayuga SJ. Beavo JA. Cloning and characterization of a cAMP-specific cyclic nucleotide phosphodiesterase. Proc Natl Acad Sci U S A. 1998;95:8991–8996. doi: 10.1073/pnas.95.15.8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi M. Shimada Y. Nishimura Y. Hama T. Tanaka T. Genomic organization, chromosomal localization, and alternative splicing of the human phosphodiesterase 8B gene. Biochem Biophys Res Commun. 2002;297:1253–1258. doi: 10.1016/s0006-291x(02)02371-9. [DOI] [PubMed] [Google Scholar]
- 16.Barnett P. Somatostatin and somatostatin receptor physiology. Endocrine. 2003;20:255–264. doi: 10.1385/ENDO:20:3:255. [DOI] [PubMed] [Google Scholar]
- 17.Larsen PR. Thyroid-pituitary interaction: feedback regulation of thyrotropin secretion by thyroid hormones. N Engl J Med. 1982;306:23–32. doi: 10.1056/NEJM198201073060107. [DOI] [PubMed] [Google Scholar]
- 18.Baum AE. Solberg LC. Kopp P. Ahmadiyeh N. Churchill G. Takahashi JS. Jameson JL. Redei EE. Quantitative trait loci associated with elevated thyroid-stimulating hormone in the Wistar-Kyoto rat. Endocrinology. 2005;146:870–878. doi: 10.1210/en.2004-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moeller LC. Alonso M. Liao X. Broach V. Dumitrescu A. Van Sande J. Montanelli L. Skjei S. Goodwin C. Grasberger H. Refetoff S. Weiss RE. Pituitary-thyroid setpoint and thyrotropin receptor expression in consomic rats. Endocrinology. 2007;148:4727–4733. doi: 10.1210/en.2007-0236. [DOI] [PubMed] [Google Scholar]