Abstract
Obstructive sleep apnea may cause vascular inflammation and atherosclerosis, which has been attributed to intermittent hypoxia (IH). Recent data suggest that IH, but not sustained hypoxia (SH), activates proinflammatory genes in HeLa cells. Effects of IH and SH on the gene expression profile in human aortic endothelial cells (HAEC) have not been compared. We perfused media with alternating flow of 16% and 0% O2 (IH) or constant flow of 4% O2 (SH-4%), 8% O2 (SH-8%), or 16% O2 (control) for 8 h. Illumina gene microarrays were performed, with subsequent verification by real-time PCR. Proinflammatory cytokines in the media were measured by ELISA. Both IH and SH-4% upregulated proinflammatory genes, including heat shock protein 90-kDa B1, tumor necrosis factor superfamily member 4, and thrombospondin 1. Among all proinflammatory genes, only IL-8 mRNA showed significantly higher levels of expression (1.78-fold) during IH, compared with SH-4%, but both types of hypoxic exposure elicited striking three- to eightfold increases in IL-8 and IL-6 protein levels in the media. IH and SH-4% also upregulated antioxidant genes, including heme oxygenase-1 and nuclear factor (erythroid-derived 2)-like 2 (NRF2), whereas classical genes regulated by hypoxia-inducible factor 1 (HIF-1), such as endothelin and glucose transporter GLUT1, were not induced. SH-8% induced changes in gene expression and cytokine secretion that were similar to those of IH and SH-4%. In conclusion, short exposures to IH and SH upregulate proinflammatory and antioxidant genes in HAEC and increase secretion of proinflammatory cytokines IL-8 and IL-6 into media in similar fashions.
Keywords: gene microarrays, interleukin-8, interleukin-6, heme oxygenase-1, nuclear factor (erythroid-derived 2)-like 2
obstructive sleep apnea (OSA) is associated with high cardiovascular mortality and morbidity (38, 49, 71). High cardiovascular risk in OSA can be attributed to accelerated atherosclerosis (10). Treatment with continuous positive airway pressure (CPAP) for 3 mo inhibited the progression of atherosclerosis in patients with OSA, which suggests that OSA plays a causal role in atherogenesis (9). OSA induces chronic intermittent hypoxia (IH) during sleep, and the atherogenic effects of OSA have been linked to IH (10, 32). Furthermore, we previously developed a mouse model of chronic IH, which mimics the oxygen profile in patients with OSA, and have shown that exposure to chronic IH in conjunction with a high-cholesterol diet for 12 wk causes atherosclerosis in C57BL/6J mice (59) and accelerates progression of atherosclerosis in apolipoprotein E (ApoE)-deficient mice (21).
In addition to OSA, evidence for a role of IH in the pathogenesis of atherosclerosis can be found in daily commuters from low to high altitude, who develop an atherogenic lipid profile (5a). In contrast, the impact of sustained hypoxia (SH) on atherosclerosis is equivocal. For example, high altitude dwellers show low incidence of atherosclerotic heart disease (12), whereas rabbits and ApoE−/− mice exposed to sustained hypoxia show rapid progression of atherosclerosis (28, 43). Along these lines, patients with chronic hypoxemia due to chronic obstructive pulmonary disease and interstitial lung disease showed high cardiovascular morbidity, independent of their smoking history, which has been attributed to the chronic inflammatory state (18, 63).
Mechanisms involved in the development of atherosclerosis in OSA or IH are not clear, but multiple factors are likely to contribute, including dyslipidemia (39, 53, 58), hypertension (46), and systemic oxidative stress (29). OSA is also characterized by systemic inflammation with increased circulating levels of the proinflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin (IL)-8, and IL-6 (56, 57, 69), which can accelerate atherosclerosis (7), and decreased levels of anti-inflammatory and antiatherogenic IL-10 (15, 65).
IH may lead to atherosclerosis by inducing direct vascular injury (8, 32). Interestingly, IH upregulated redox-sensitive proinflammatory nuclear factor-κB (NF-κB) in HeLa cells and bovine aortic endothelial cells (55, 56), whereas SH activated the transcription factor hypoxia-inducible factor 1 (HIF-1) but not NF-κB, suggesting differential cellular responses to IH and SH (56). The in vitro findings are clinically relevant, because NF-κB is tightly linked to oxidative stress and hypercytokinemia observed in OSA (7, 57). Despite the fact that endothelial cells are likely to be a major site of injury during the development of atherosclerosis, the expression profiles in human endothelial cells exposed to IH and SH have not been compared previously. We chose human aortic endothelial cells (HAEC) for our study because of their relevance for atherosclerosis and commercial availability. We hypothesized that IH will induce genes of inflammation and oxidative stress in HAEC, which will not occur after SH. We exposed HAEC to IH or SH for 8 h and 1) performed gene arrays with total RNA isolated from HAEC with subsequent verification of differentially expressed genes of inflammation and oxidative stress in real-time PCR and 2) measured protein levels of the proinflammatory and proatherogenic cytokines TNF-α, IL-8, and IL-6 (56, 57, 69) and anti-inflammatory, antiatherogenic IL-10 (15, 65) in media.
METHODS
Cell culture.
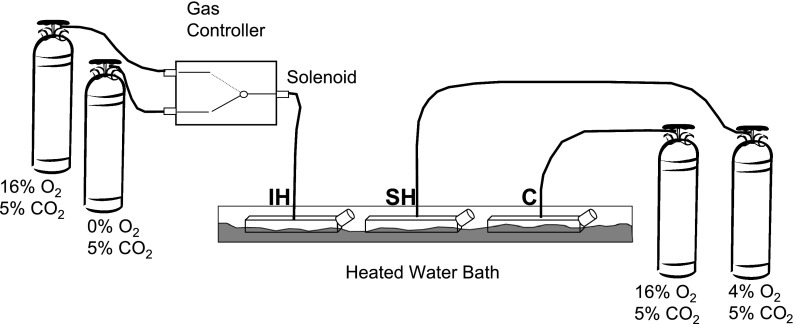
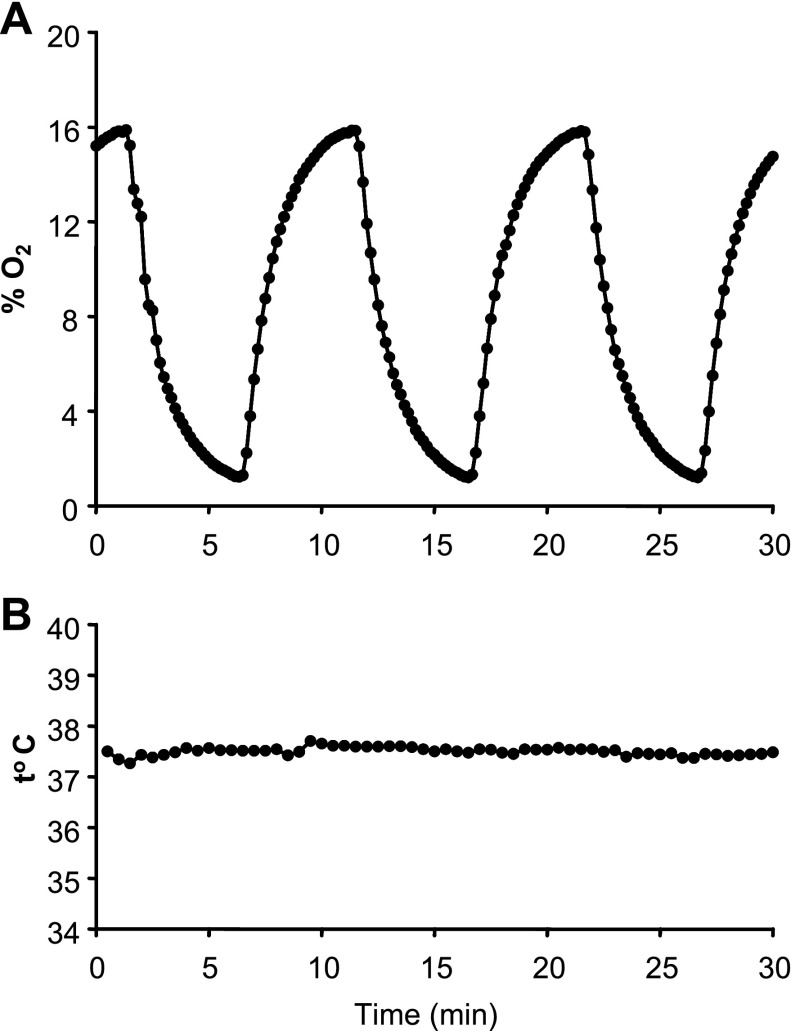
HAEC from three different donors were obtained commercially from Cambrex (East Rutherford, NJ). Cells at passage 9 were grown to 100% confluence in T-75 tissue culture flasks in EGM-2 complete medium (Clonetics) and placed in basal medium 24 h before exposures began. Immediately before exposure, 40 ml of fresh medium was added to each flask and small holes for gas inlets and outlets were created in the tops of the flasks with a heated awl. Flasks were placed in a heated water bath, which maintained medium temperature at 37°C, and were gassed with 1) 16% O2, 5% CO2, balance N2 (control); 2) 5 min of 16% O2, 5% CO2, balance N2 alternating with 5 min of 0% O2, 5% CO2, balance N2 (IH); or 3) 4% O2, 5% CO2, balance N2 (SH-4%) as shown in Fig. 1. Preliminary experiments had shown that six cycles per hour was the highest frequency of IH that could reach the desired zenith and nadir of O2 in the medium (Fig. 2). Initial experiments were performed to record temperature and % O2 in the medium simultaneously during the protocol with an OM-4 oxygen meter (Microelectrodes, Bedford, NH) and an electronic temperature probe (4600; YSI, Dayton, OH), and data were captured with a Powerlab 8Sp Data Acquisition system (AD Instruments, Colorado Springs, CO). The % O2 in the medium cycled according to the desired profile, and the temperature remained stable throughout the 8-h exposure. After our model was validated, HAEC were exposed to IH, SH, or control conditions for 8 h. The exposure was repeated five times (n = 5, including 3 times with HAEC from donor 1 and once each with HAEC from donors 2 and 3). Upon cessation of the exposure, flasks were immediately placed on ice, and cells and medium were collected, snap-frozen in liquid nitrogen, and stored at −80°C.
Fig. 1.
Intermittent hypoxia was induced in human aortic endothelial cells (HAEC). T-75 flasks with HAEC were placed in a 37°C water bath and gassed with 1) 16% O2, 5% CO2, balance N2 [control (C)]; 2) 5 min of 16% O2, 5% CO2, balance N2 alternating with 5 min of 0% O2, 5% CO2, balanced N2 [intermittent hypoxia (IH)]; 3) 4% O2, 5% CO2, balance N2 [sustained hypoxia (SH-4%)]; or 4) 8% O2, 5% CO2, balance N2 [sustained hypoxia (SH-8%)] for 8 h.
Fig. 2.
Representative O2 profile (A) and temperature (B) during IH were recorded simultaneously in the T-75 flask. An OM-4 oxygen meter (Microelectrodes, Bedford, NH) and an electronic temperature probe (4600; YSI, Dayton, OH) were utilized, and data were captured with a Powerlab 8Sp Data Acquisition system (AD Instruments, Colorado Springs, CO).
The regimen of hypoxia was selected on the basis of the following considerations: 1) control conditions corresponded to partial pressure of O2 (Po2) ∼114 mmHg slightly exceeding the Po2 of arterial blood in a healthy human and ensuring that neither hypoxia nor hyperoxia was present; 2) SH corresponded to a Po2 of 28.5 mmHg, which was consistent with severe hypoxia in vivo; 3) IH was comparable in severity to SH, given that although the mean O2 concentration during IH was ∼7.5%, Po2 of 53.5 mmHg, the nadir of O2 was 1%, Po2 of 7 mmHg. Nevertheless, given that the mean O2 during IH was higher than during SH-4%, we performed a second set of experiments, which included identical IH and control exposure but SH induced with 8% O2 (SH-8%) corresponding to Po2 of 57 mmHg.
Immunocytochemistry.
HAEC at passage 9 were fixed with 10% buffered formalin and then stained with 1) mouse monoclonal anti-human thrombomodulin antibody (R&D Systems, Minneapolis, MN) and Alexa Fluor 594 goat anti-mouse IgG (Invitrogen, Carlsbad, CA) and counterstained with 4′,6-diamidino-2-phenylindole (DAPI) or 2) mouse monoclonal anti-smooth muscle actin from Sigma and Alexa Fluor 488 goat anti-mouse IgG (Invitrogen) and counterstained with DAPI. Staining was detected with filters U-N31000 [exciter 360 nm/emitter 460 nm (DAPI)], U-N31001 [exciter 480 nm/emitter 535 nm (actin/Alexa Fluor 488)], and U-N31002 [exciter 540 nm/emitter 605 nm (thrombomodulin/Alexa Fluor 594)] from Chroma Technology (Rockingham, VT) on an Olympus microscope.
Illumina array studies.
Microarray studies were performed in the experiments with IH and SH-4% with standard protocols. Total RNA was isolated from HAEC with the TRIzol reagent method (Invitrogen; catalog no. 15596-026) and subsequent RNEasy clean up (Qiagen, Valencia, CA; catalog no. 74104). Total RNA (0.5 μg from each sample) was labeled with the Illumina TotalPrep RNA Amplification Kit (Ambion, Austin, TX; catalog no. IL1791). mRNA was converted into double-stranded cDNA with an oligo(dT) primer containing the T7 RNA polymerase promoter. Single-stranded RNA (cRNA) was synthesized from double-stranded cDNA in an in vitro transcription reaction. cRNA was labeled by incorporating biotin-16-UTP. Biotin-labeled cRNA (0.85 μg) was hybridized (16 h) to Illumina's Sentrix HumanRef-8 Expression BeadChips (Illumina, San Diego, CA; catalog no. 11201828). The hybridized biotinylated cRNA was detected with streptavidin-Cy3 and quantified with Illumina's BeadStation 500GX Genetic Analysis Systems scanners. Preliminary analysis of the scanned data was performed with Illumina BeadStudio software. The resulting digitized matrix was processed by a platform approach modified for Illumina described previously (16).
Real-time PCR.
cDNA was synthesized from total RNA with the Advantage RT for PCR kit from Clontech (Palo Alto, CA). Real-time reverse transcriptase-PCR (RT-PCR) was performed with primers from Invitrogen and Taqman probes from Applied Biosystems (Foster City, CA). The sequences of primers and probes for 18S were described previously (33–35, 58, 59). The sequences of primers and probes for heat shock protein 90 kDa β1 (HSP90-B1) were designed based on GenBank sequence NM_003299: forward primer 5′-CCAGTTTGGTGTCGGTTTCTATTC-3′, reverse primer 5′-TCTTGGGTCAGCAATTACAGAAAA-3′, and probe 5′-AGGTTATTGTCACTTCAAAAC-3′. The sequences of primers for thrombospondin 1 (THBS1) were designed based on GenBank sequence NM_003246.2: forward primer 5′-CGGTTAAGAAGCTCTCCTGGCAAC-3′ and reverse primer 5′-GCCCTGTCTTCCTGCACAAAC-3′. The sequences of primers for tumor necrosis factor superfamily member 4 (TNFSF4) were designed based on GenBank sequence NM_003326.2: forward primer 5′-CTCCTTGATGGTGGCCTCTCTG-3′ and reverse primer 5′-GACAATAAAGTCATCCAGGGAGGTATTG-3′. The sequences of primers for heme oxygenase-1 (HO-1) were designed based on GenBank sequence NM_002133.1: forward primer 5′-ACATTGCCAGTGCCACCAAG-3′ and reverse primer 5′-CGCATATCTCCAGGGAGTTCATG[FAM]G-3′. The sequences of primers and probes for nuclear factor (erythroid-derived 2)-like 2 (NRF2) were designed based on GenBank sequence NM_006164: forward primer 5′-GAAGTGTCAAACAGAATGGTCCTAAA-3′, reverse primer 5′-GCCCCTGAGATGGTGACAA-3′, and probe 5′-CACCAGTACATTCTTC-3′. The efficiency of all RT-PCRs was 85–110%. The mRNA expression levels were normalized to 18S rRNA concentrations with the following formula: gene of interest/18S = 2Ct(18S) − Ct(gene of interest), where Ct is threshold cycle, and then expressed as a ratio of hypoxic conditions (IH or SH) to control.
ELISA.
Immediately after exposures, the medium was collected and stored at −80°C. ELISA was performed with kits for TNF-α, IL-6, IL-8, and IL-10 from R&D Systems. Samples were run in duplicate according to the manufacturer's instructions. Average optical densities were used to calculate concentrations based on the standard curves generated with control peptides provided by the manufacturer.
Statistical analysis.
The new concept for sample size determination in microarray experiments for class comparisons was employed as described previously (67). This sample size prediction concept is based on variability of the population, the desired detectable differences in gene expression, the power to detect expression differences, and an acceptable error rate. We generated four gene expression profiles for each experimental condition and used control values from untreated HAEC cells to calculate hybridization signal variability. Identified standard deviation (σ = 0.219) was submitted to the microarray sample size identifying formula accepting a 5% false discovery rate (q, significance level α = 0.05) and imposing 90% of detecting power (1 − β). The power.t.test function of R2.3.1 program (www.r-project.org) was implemented: >power.t.test(n=4,delta=NULL,sd=0.219,sig.level=0.05,power=0.90,type=“two.sample”, alternative =“two.sided”). These calculations identified fold change log2 (Δ = 0.606) that corresponded to numerical 1.52-fold change. Significance Analysis of Microarrays (SAM 2.20) was conducted with 1,000 permutations of 5 control and 5 treated HAEC samples without application of arbitrary restrictions. Genes with ≥1.52-fold change and q < 5% were considered significantly affected by IH or SH. Finally, the Medline database (www.pubmed.com; National Library of Medicine, Bethesda, MD) was searched for all differentially expressed genes through December 2009. The search strategy was as follows: [gene of interest(MeSH)] AND [inflammation(MeSH)] OR [atherosclerosis(MeSH)] OR [oxidative stress(MeSH)] OR [mitochondrial electron transport(MeSH)] OR [hypoxia (MeSH)].
All values obtained in PCR and ELISA are reported as means ± SE. Statistical comparisons between groups were performed by ANOVA with Tukey's post hoc test. A P value of <0.05 was considered significant.
RESULTS
HAEC at passage 9 preserved the endothelial cell phenotype, which was evident from positive thrombomodulin staining and negative smooth muscle actin staining (Fig. 3).
Fig. 3.
HAEC at passage 9 were stained for thrombomodulin (A) and smooth muscle cell actin (B) (immunofluorescence; original magnification ×200). In A, thrombomodulin stained in red is present in the cell membrane and, to a lesser extent, in the cytoplasm; nuclei are counterstained with 4′,6-diamidino-2-phenylindole (DAPI) in blue. In B, main photograph depicts the absence of smooth muscle cell actin with positive DAPI staining for nuclei; inset shows smooth muscle cells, cytoplasm of which is positive for actin stained in green.
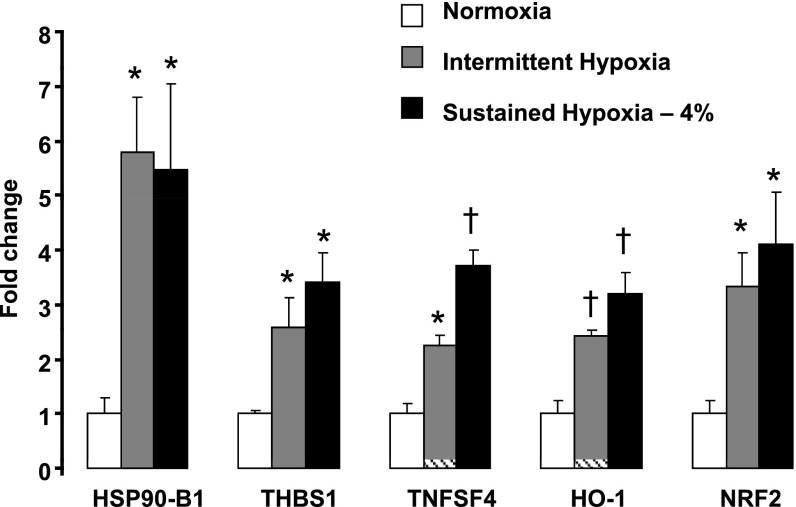
In HAECs, IH upregulated 407 genes and downregulated 151 genes, whereas SH-4% upregulated 213 genes and downregulated 221 genes (Supplemental Tables S1 and S2).1 The Medline analysis of the differentially expressed genes revealed that IH and SH-4% activated a number of the proinflammatory and anti-inflammatory genes (Tables 1 and 2). Gene microarrays showed that both stimuli upregulated the following proinflammatory genes: heat shock proteins 70 kDa and 90 kDa (HSP70, HSP90-A1, and HSP90-B1); histone 2, H2be; histone 1, H3d; and histone deacetylase 5; and proatherogenic chemokine (C-X-C motif) receptor 4 (CXCR4) (40). Robust induction of HSP90-B1 by both IH and SH-4% was verified by real-time PCR (Fig. 4). Gene microarrays also showed a 1.5- to 1.9-fold increase in expression of several proinflammatory genes by IH but not by SH-4% (Table 1). These genes included THBS1 (64), TNFSF4 (also known as OX40 ligand) (68), proatherogenic cytokine IL-8 (5), metalloproteinase 10 (54), integrin β1 (70), and apoptotic protein caspase 3 (27). However, real-time PCR revealed that expression of THBS1 and TNFSF4 was increased by both IH and SH-4% (Fig. 4). Several anti-inflammatory genes were also upregulated by both IH and SH-4%, including annexin A1, which prevents adhesion of neutrophils to endothelium (17), adenosine A2a receptor, agonists of which decrease TNF-α expression (60), antiapoptotic heat shock protein 27 (30), and NF-κB inhibitor α (IκBα) (Table 2). Interestingly, none of the pro- or anti-inflammatory genes was significantly downregulated by either condition.
Table 1.
Proinflammatory genes differentially regulated by intermittent hypoxia and sustained hypoxia (4% O2) compared with normoxia
| IH |
SH-4% |
||||
|---|---|---|---|---|---|
| Probe ID | Gene Name | Fold Change | q, % | Fold Change | q, % |
| Genes upregulated by intermittent and sustained hypoxia | |||||
| GI_4507676-S | Heat shock protein 90 kDa β1 (HSP90-B1) | 3.70 | <0.10 | 2.28 | <0.10 |
| GI_40254815-S | Heat shock protein 90 kDa, α1 (HSP90-A1) | 3.40 | <0.10 | 1.71 | <0.10 |
| GI_22027639-S | Histone 2, H2be (HIST2H2BE) | 1.89 | <0.10 | 2.07 | <0.10 |
| GI_4504252-S | H2A histone family, member X (H2AFX) | 1.81 | 0.289 | 1.84 | <0.10 |
| GI_39812261-S | Histone 1, H3d (HIST1H3D) | 1.80 | <0.10 | 1.76 | <0.10 |
| GI_4503174-S | Chemokine (C-X-C motif) receptor 4 (CXCR4) | 1.70 | <0.10 | 1.94 | <0.10 |
| GI_21237796-A | Histone deacetylase 5 (HDAC5), transcript variant 1 | 1.69 | 0.162 | 1.64 | 0.178 |
| Genes upregulated by intermittent hypoxia only | |||||
| GI_40317625-S | Thrombospondin 1 (THBS1) | 1.86 | <0.10 | NS | |
| GI_4505564-S | Procollagen-proline, 2-oxoglutarate 4-dioxygenase (proline 4-hydroxylase), α polypeptide I (P4HA1) | 1.82 | 0.162 | NS | |
| GI_23510446-S | Tumor necrosis factor (ligand) superfamily, member 4 (tax-transcriptionally activated glycoprotein 1, 34 kDa) (TNFSF4) | 1.78 | <0.10 | NS | |
| GI_19743812-I | Integrin, β1 (fibronectin receptor, β polypeptide, antigen CD29 includes MDF2, MSK12) (ITGB1), transcript variant 1A | 1.66 | 1.656 | NS | |
| GI_4505204-S | Matrix metalloproteinase 10 (stromelysin 2) (MMP10) | 1.66 | 0.386 | NS | |
| GI_22027623-A | TNF receptor-associated factor 4 (TRAF4), transcript variant 2 | 1.65 | <0.10 | NS | |
| GI_14790114-A | Caspase 3, apoptosis-related cysteine protease (CASP3), transcript variant β | 1.64 | 0.162 | NS | |
| GI_18379365-S | Zinc metalloproteinase (STE24 homolog, yeast) (ZMPSTE24) | 1.61 | 0.162 | NS | |
| GI_19743818-A | Integrin, β1 (fibronectin receptor, β polypeptide, antigen CD29 includes MDF2, MSK12) (ITGB1), transcript variant 1D | 1.60 | 2.136 | NS | |
| GI_28610153-S | Interleukin 8 (IL8) | 1.57 | 2.931 | NS | |
IH, intermittent hypoxia; SH-4%, sustained hypoxia (4% O2); q, false discovery rate; NS, not significant.
Table 2.
Anti-inflammatory genes differentially regulated by intermittent hypoxia and sustained hypoxia (4% O2) compared with normoxia
| IH |
SH-4% |
||||
|---|---|---|---|---|---|
| Probe ID | Gene Name | Fold Change | q, % | Fold Change | q, % |
| GI_4502100-S | Annexin A1 (ANXA1) | 2.97 | <0.10 | 1.76 | 0.178 |
| GI_17136146-S | Adenosine A2a receptor (ADORA2A) | 2.63 | <0.10 | 2.93 | <0.10 |
| GI_21361242-S | Heat shock 70 kDa protein 5 (glucose-regulated protein, 78 kDa) (HSPA5) | 2.08 | <0.10 | 1.83 | <0.10 |
| GI_29743323-S | Similar to Heat shock 27 kDa protein (HSP 27) (Stress-responsive protein 27) (SRP27) (Estrogen-regulated 24 kDa protein) (28 kDa heat shock protein) (LOC347348) | 1.89 | <0.10 | 1.94 | <0.10 |
| GI_10092618-S | Nuclear factor of κ light polypeptide gene enhancer in B-cells inhibitor, α (NFKBIA) | 1.87 | <0.10 | 2.01 | <0.10 |
Fig. 4.
Expression of heat shock protein 90 kDa β1 (HSP90-B1), thrombospondin 1 (THBS1), tumor necrosis factor (ligand) superfamily, member 4 (TNFSF4), heme oxygenase-1 (HO-1), and nuclear factor (erythroid-derived 2)-like 2 (NRF2) mRNA in HAEC after exposure to control condition, IH, or SH (4% O2) for 8 h measured by real-time PCR (n = 5 for each exposure). Results were normalized as ratios to 18S and then expressed as fold change compared with control condition. *P < 0.05, †P < 0.01 for difference between hypoxia and control.
The Medline analysis of the differentially expressed genes revealed that both IH and SH-4% upregulated a number of antioxidant genes, including HO-1, selenoproteins, thioredoxin, and an important transcription factor of antioxidant defense, NRF2 (24). Induction of HO-1 and NRF2 by IH and SH-4% was confirmed by real-time PCR (Fig. 4). Both conditions resulted in upregulation of genes encoding such critical proteins of the mitochondrial electron transport chain as cytochrome-c oxidases, NAD(P)H quinone oxidoreductases (NQO), and ATP synthase (11) (Table 3). IH, but not SH-4%, induced expression of antioxidant thioredoxin reductase 1, and von Hippel-Lindau (VHL) binding protein 1. In contrast, SH-4%, but not IH, upregulated VEGF.
Table 3.
Genes of oxidative stress, mitochondrial electron transport and hypoxia differentially regulated by intermittent hypoxia and sustained hypoxia (4% O2) compared with normoxia
| IH |
SH-4% |
||||
|---|---|---|---|---|---|
| Probe ID | Gene Name | Fold Change | q, % | Fold Change | q, % |
| Genes upregulated by intermittent and sustained hypoxia | |||||
| GI_4504436-S | Heme oxygenase (decycling) 1 (HO1) | 3.18 | <0.10 | 2.76 | <0.10 |
| GI_27754195-S | NAD(P)H quinone oxidoreductase 3 (NQO3) | 3.16 | <0.10 | 3.27 | <0.10 |
| GI_27754205-S | Cytochrome-c oxidase II (MTCO2) | 3.01 | <0.10 | 3.00 | <0.10 |
| GI_27754207-S | ATP synthase 6 (MTATP6) | 2.55 | <0.10 | 2.49 | <0.10 |
| GI_20149575-S | Nuclear factor (erythroid-derived 2)-like 2 (NRF2) | 2.31 | <0.10 | 1.65 | <0.10 |
| GI_27754203-S | Cytochrome-c oxidase I (MTCO1) | 2.20 | 0.289 | 2.61 | <0.10 |
| GI_25014098-S | Selenoprotein K (SELK) | 2.16 | <0.10 | 2.24 | <0.10 |
| GI_27754201-S | NAD(P)H quinone oxidoreductase 2 (NQO2) | 2.02 | 0.693 | 2.7 | 0.178 |
| GI_27754199-S | NAD(P)H quinone oxidoreductase 1 (NQO1) | 1.98 | 0.162 | 2.11 | <0.10 |
| Genes upregulated by intermittent hypoxia only | |||||
| GI_33519429-A | Thioredoxin reductase 1 (TXNRD1), transcript variant 4 | 1.96 | 1.656 | NS | |
| GI_42741647-S | 15 kDa selenoprotein (SEP15), transcript variant 1 | 1.77 | <0.10 | NS | |
| GI_34222132-S | Thioredoxin domain containing (TXNDC) | 1.76 | 0.693 | NS | |
| GI_21614497-S | von Hippel-Lindau binding protein 1 (VBP1) | 1.61 | 1.656 | NS | |
| Genes upregulated by sustained hypoxia only | |||||
| GI_19924300-S | Vascular endothelial growth factor C (VEGFC) | NS | 1.57 | 0.384 | |
Only five genes demonstrated statistical differences in the levels of expression between IH and SH-4% (Table 4). Compared with SH-4%, IH resulted in higher levels of expression of proinflammatory IL-8, anti-inflammatory annexin A1, and general transcription factor IIF, which is important for RNA polymerization (44). Compared with IH, SH-4% induced higher levels of expression of proinflammatory HSP40 (51), whereas antiapoptotic BCL2-associated athanogene 3 (BAG3) (4) was decreased. Thus IH and SH-4% induced similar genomic profiles in HAEC.
Table 4.
Genes differentially regulated by intermittent hypoxia compared with sustained hypoxia (4% O2)
| Probe ID | Gene Name | Fold Change | q, % |
|---|---|---|---|
| GI_28610153-S | Interleukin 8 (IL8) | 1.78 | <0.1 |
| GI_4502100-S | Annexin A1 (ANXA1) | 1.69 | <0.1 |
| GI_4758487-S | General transcription factor IIF, polypeptide 2 (30 kDa subunit) (GTF2F2) | 1.66 | <0.1 |
| GI_33354248-S | DnaJ (Hsp40) homolog, subfamily A, member 4 (DNAJA4) | −1.56 | <0.1 |
| GI_14043023-S | BCL2-associated athanogene 3 (BAG3) | −1.58 | <0.1 |
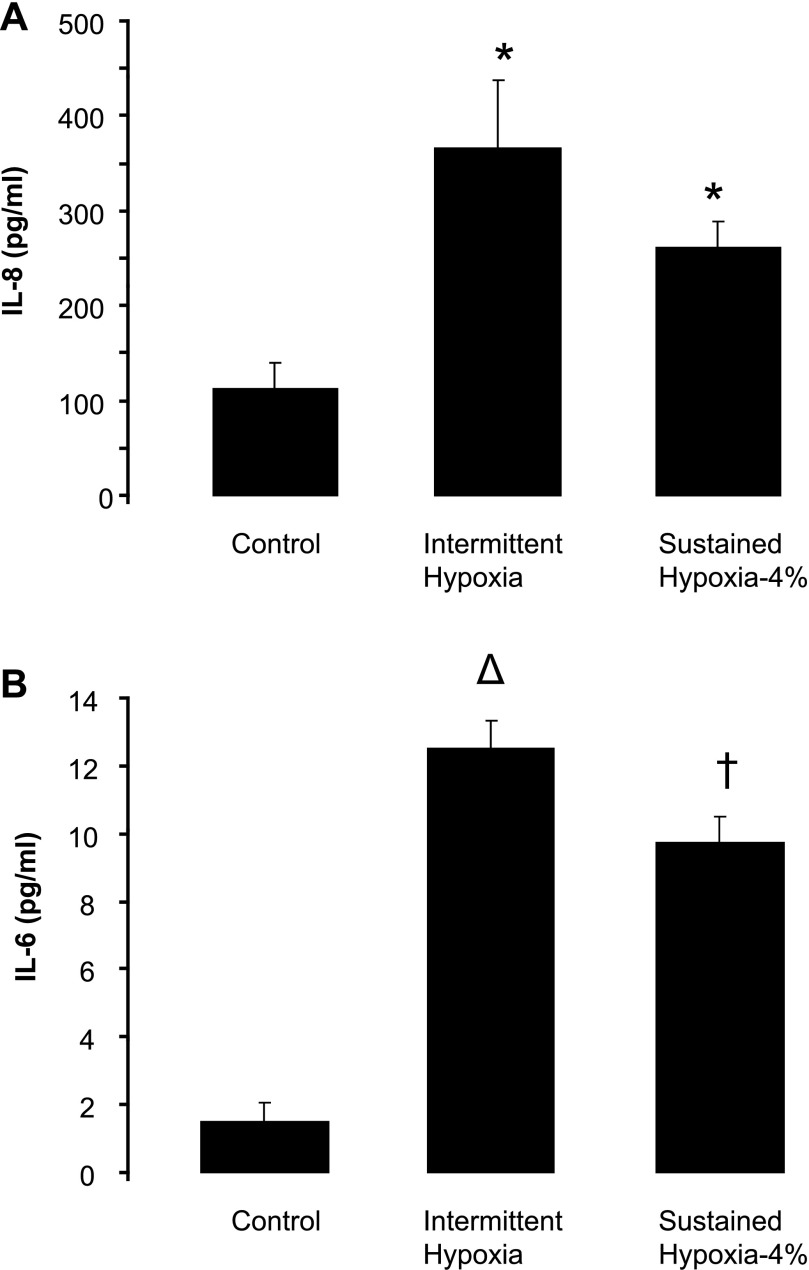
Secretion of proinflammatory cytokines into supernatant was examined by ELISA. In contrast to the microarray data, both IH and SH-4% resulted in 3.5- to 4.5-fold increases in IL-8 protein levels (Fig. 5A). Moreover, IH and SH-4% induced striking 6- to 8-fold increases in IL-6 protein levels, despite unchanged mRNA levels (Fig. 5B). TNF-α and IL-10 protein levels were undetectable in both control and hypoxic conditions.
Fig. 5.
Levels of proinflammatory cytokines interleukin (IL)-8 (A) and IL-6 (B) in the medium of HAEC after exposure to normoxia, IH, or SH (4% O2) for 8 h measured by ELISA (n = 5 for each exposure). *P < 0.05, †P < 0.01, ΔP < 0.001 for difference between hypoxia and control.
In a separate series of experiments, we explored whether similarities between IH and SH persist when the same regimen of IH is compared with SH-8%. Compared with control conditions, IH induced a 2.61 ± 0.84-fold increase in HSP90-B1 mRNA (P < 0.05), whereas SH-8% induced a 2.12 ± 0.91-fold increase P = 0.05); there was no difference in HSP90-B1 mRNA between IH and SH-8% (P = 0.67). IH induced a 2.84 ± 0.62-fold increase in THBS1 mRNA (P < 0.05), and SH-8% induced a 3.36 ± 0.73-fold increase (P < 0.05); there was no difference in THBS1 mRNA between IH and SH-8% (P = 0.71). IH induced a 2.41 ± 0.38-fold increase in TNFSF4 mRNA (P < 0.05), and SH-8% induced a 3.19 ± 0.48-fold increase (P < 0.05); there was no difference in TNFSF4 mRNA between IH and SH-8% (P = 0.46). IH induced a 3.25 ± 0.62-fold increase in NRF2 mRNA (P < 0.01), and SH-8% induced a 3.17 ± 0.82-fold increase (P < 0.05); there was no difference in NRF2 mRNA between IH and SH-8% (P = 0.93). Levels of IL-8 in the medium were identical after IH and SH-8%, 1,148 ± 141 and 1,145 ± 88 pg/ml, respectively, which was significantly higher than under control conditions (429 ± 48 pg/ml, P < 0.001 with IH and SH-8%). Levels of IL-6 in the medium were also similar in both hypoxic conditions. Thus IH and SH-8% comparisons yielded the same results as IH and SH-4% comparisons: IH and SH-8% induce similar changes in expression of proinflammatory and anti-oxidant genes and secretion of proinflammatory cytokines.
DISCUSSION
There is a growing body of literature suggesting that IH of OSA induces systemic and vascular inflammation leading to atherosclerosis that is uniquely different from SH. However, genomic responses in human endothelial cells exposed to IH and SH have not been compared. The purpose of this work was to examine changes in gene expression in HAEC induced by IH and SH. Although we hypothesized that IH and SH would induce distinct genetic profiles, the main finding of our study was that IH and SH exhibited similar expression profiles, upregulating proinflammatory and antioxidant genes. The only proinflammatory atherogenic cytokine differentially expressed in the two conditions was IL-8, but the differences between IH and SH did not persist at the level of secreted protein. Finally, similarities between gene expression and proinflammatory cytokine secretion were present when IH was compared with two levels of SH, 4% and 8% O2. In the discussion below, we explore a potential role of IH and SH in atherosclerosis and putative pathways involved in cellular responses to IH and SH in the human endothelium.
Overall, our data suggest that IH and SH induce similar inflammatory changes in the human endothelium at the transcriptional and posttranscriptional levels. There are several possible interpretations of our data in relationship to atherosclerosis in humans. First, SH may be as atherogenic as IH, as could also be inferred from previous work in animal models of SH and atherosclerosis (28, 43). Human observational studies in denizens of high altitude lack appropriate controls (12) and cannot dispel this hypothesis. In addition, these subjects can be genetically adapted to residence at high altitude. Second, we have identified early changes in the expression profile during IH and SH; it is conceivable that proinflammatory changes will persist in IH because of the lack of adaptation, which will occur at in SH conditions. Late changes in gene expression may differ from acute changes, and our findings may not be relevant for a chronic disease such as atherosclerosis. Third, IH may have a more profound proinflammatory effect on other cellular populations important for the development of atherosclerosis, such as macrophages and lymphocytes (1, 31), which were not examined in our study. Finally, proatherogenic effects of IH of OSA may be systemic rather than local and related to dyslipidemia (58, 59), insulin resistance (47, 48, 50), and hypertension (46), which do not develop in SH.
We were surprised by the finding that both IH and SH induced proinflammatory genes regulated by NF-κB. Our results stand in stark contrast to those of Ryan et al. (56), where IH, but not SH, induced NF-κB-regulated genes and increased NF-κB DNA binding activity in HeLa cells. In our study, both stimuli induced the NF-κB pathway, including NF-κB-regulated HSP90 (3), TNFSF4 (22), and CXCR4 (25) and upregulated NF-κB activator histone deacetylase (36). IL-8 was the only NF-κB-regulated gene, which was differentially expressed in the two conditions, but the difference was no longer present at the protein level. The dissimilarities between our study and that of Ryan et al. could be attributed to differences in cell types and exposure regimens. Ryan et al. (56) maintained HeLa cells at constant levels of O2 in the SH and control protocols and alternated preconditioned hypoxic and normoxic media in the IH protocol, whereas we used HAEC and perfused the media with gas in all types of exposure. In addition, levels of O2 at control and SH conditions differ in our study and that of Ryan et al., which could also contribute to the differences in outcomes. Our study has also shown that, while IH led to a modest 1.57-fold increase in IL-8 mRNA, both IH and SH induced a robust 2.5- to 4-fold increase in IL-8 protein in the medium. These findings suggest that both hypoxic stimuli acted posttranscriptionally, at the level of either protein synthesis or secretion. A similar phenomenon occurred with IL-6, which was unchanged by hypoxia at the mRNA level but exhibited a striking 6- to 8-fold increase in the protein level in both IH and SH conditions. An increase in secretion of IL-6 and IL-8 by human endothelium in response to hypoxia has been reported previously and was attributed to the effect of reactive oxygen species, but molecular mechanisms remain obscure (2). Thus our work demonstrates that IH and SH induce similar proinflammatory changes in HAEC at transcriptional and posttranscriptional levels.
In our study, neither IH nor SH upregulated classical HIF-1-dependent genes, such as endothelin and glucose transporter GLUT1 (20, 61). Several potential HIF-1 targets, HO-1, cytochrome-c oxidase, and adenosine A2a receptor (13, 37, 45), were induced by IH and SH, while VEGFC was induced only by SH. However, these genes are also NF-κB targets (6, 41, 42) and therefore, could be upregulated by NF-κB. In addition, HO-1 is regulated by NRF2, as discussed further below. Our findings differ from previous reports showing HIF-1 induction in cell culture by SH (20, 56, 62). Such a discrepancy may be a result of differences in cell type or more severe sustained hypoxia used by other investigators (20, 56, 62). A lack of HIF-1 induction by IH was previously reported by Ryan et al. (56), who exposed HeLa cells to 16 cycles of IH with inspired O2 fraction (FiO2) alternating between 12% and 1%. We have exposed HAEC to 48 cycles of IH (6 cycles/h for 8 h). Yuan et al. (72) exposed PC-12 rat pheochromocytoma cells to IH with FiO2 changing between 20% and 1% and demonstrated that HIF-1 activity depends on the number of IH cycles, with a marked increase in HIF-1 activity noted only after 60 cycles. Thus the absence of HIF-1 activation in our study and that of Ryan et al. could be related to a low number of cycles and/or differences in cell type.
IH uniquely predisposes to oxidative stress compared with SH (26). Therefore, we anticipated that IH and SH will have a distinct effect on genes of oxidative stress and antioxidant defense. Surprisingly, our study has shown that IH and SH induce similar antioxidant responses, which are mediated via a key transcription factor of the antioxidant defense, NRF2. NRF2 is regulated predominantly at the posttranscriptional level. In unstressed basal conditions NRF2 is bound to Keap1 and localized in the cytoplasm. Upon the addition of electrophiles or oxidants, NRF2 is released from Keap1 and translocates into nucleus to bind to an antioxidant/electrophile response element (ARE) and activate transcription of antioxidant genes (23, 24, 52). NRF2 upregulates a variety of antioxidant genes and cytoprotective enzymes. We found that both SH and IH increased expression of NRF2 mRNA. Although this finding does not necessarily suggest that NRF2 is activated, increased expression of such NRF2-regulated genes as NQO1, HO-1, and thioredoxin reductase indicates that both hypoxic stimuli may indeed induce NRF2 (14, 23, 24, 66, 73).
In conclusion, we demonstrated that short exposures to IH and SH upregulate proinflammatory and antioxidant genes in HAEC and increase secretion of proinflammatory cytokines IL-6 and IL-8 into the medium in similar fashions. Our study had several limitations. First, proatherogenic and proinflammatory effects of IH may occur systemically as a result of IH-induced hypertension, dyslipidemia, and lipid peroxidation, and therefore in vitro models may have inherent limitations for studying IH. Second, our protocol of exposure to IH and SH-4% could be considered too severe compared with OSA or IH in vivo. Other investigators used comparable or even more severe regimens of in vitro hypoxia (56, 72). In addition, moderate levels of sustained hypoxia (SH-8%) alter gene expression and cytokine secretion in a similar manner, suggesting that our findings in IH and SH-4% may have clinical relevance. Third, our study describes the gene expression profile but does not dissect mechanisms of endothelial cell response to hypoxic stimuli. Nevertheless, an important implication of the study is that perturbation of NF-κB and NRF2 pathways and excessive secretion of proinflammatory cytokines by endothelial cells may contribute to the pathogenesis of atherosclerosis in hypoxic conditions, including OSA.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-68715, HL-80105, and P50-HL-084945, American Heart Association Grant 0765293U, American Heart Association Mid-Atlantic Affiliate Postdoctoral Fellowship 0625514U, and Research Fellowship Grant RE 2842/1-1 from the German Research Foundation (DFG).
DISCLOSURES
The authors have no conflicts of interest to report.
Supplementary Material
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med 12: 178–180, 2006. [DOI] [PubMed] [Google Scholar]
- 2. Ali MH, Schlidt SA, Chandel NS, Hynes KL, Schumacker PT, Gewertz BL. Endothelial permeability and IL-6 production during hypoxia: role of ROS in signal transduction. Am J Physiol Lung Cell Mol Physiol 277: L1057–L1065, 1999. [DOI] [PubMed] [Google Scholar]
- 3. Ammirante M, Rosati A, Gentilella A, Festa M, Petrella A, Marzullo L, Pascale M, Belisario MA, Leone A, Turco MC. The activity of hsp90 alpha promoter is regulated by NF-kappaB transcription factors. Oncogene 27: 1175–1178, 2008. [DOI] [PubMed] [Google Scholar]
- 4. Antoku K, Maser RS, Scully WJ, Jr, Delach SM, Johnson DE. Isolation of Bcl-2 binding proteins that exhibit homology with BAG-1 and suppressor of death domains protein. Biochem Biophys Res Commun 286: 1003–1010, 2001. [DOI] [PubMed] [Google Scholar]
- 5. Boisvert WA, Santiago R, Curtiss LK, Terkeltaub RA. A leukocyte homologue of the IL-8 receptor CXCR-2 mediates the accumulation of macrophages in atherosclerotic lesions of LDL receptor-deficient mice. J Clin Invest 101: 353–363, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a. Brito J, Siques P, Leon-Velarde F, De La Cruz JJ, Lopez V, Herruzo R. Chronic intermittent hypoxia at high altitude exposure for over 12 years: assessment of hematological, cardiovascular, and renal effects. High Alt Med Biol 8: 236–244, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Cogswell PC, Kashatus DF, Keifer JA, Guttridge DC, Reuther JY, Bristow C, Roy S, Nicholson DW, Baldwin AS., Jr NF-kappaB and IkappaBalpha are found in the mitochondria. Evidence for regulation of mitochondrial gene expression by NF-kappaB. J Biol Chem 278: 2963–2968, 2003. [DOI] [PubMed] [Google Scholar]
- 7. de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol 25: 904–914, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Dematteis M, Julien C, Guillermet C, Sturm N, Lantuejoul S, Mallaret M, Levy P, Gozal E. Intermittent hypoxia induces early functional cardiovascular remodeling in mice. Am J Respir Crit Care Med 177: 227–235, 2008. [DOI] [PubMed] [Google Scholar]
- 9. Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Effects of CPAP on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med 176: 706–712, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med 172: 613–618, 2005. [DOI] [PubMed] [Google Scholar]
- 11. Dudkina NV, Eubel H, Keegstra W, Boekema EJ, Braun HP. Structure of a mitochondrial supercomplex formed by respiratory-chain complexes I and III. Proc Natl Acad Sci USA 102: 3225–3229, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fujimoto N, Matsubayashi K, Miyahara T, Murai A, Matsuda M, Shio H, Suzuki H, Kameyama M, Saito A, Shuping L. The risk factors for ischemic heart disease in Tibetan highlanders. Jpn Heart J 30: 27–34, 1989. [DOI] [PubMed] [Google Scholar]
- 13. Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 129: 111–122, 2007. [DOI] [PubMed] [Google Scholar]
- 14. Gong P, Stewart D, Hu B, Li N, Cook J, Nel A, Alam J. Activation of the mouse heme oxygenase-1 gene by 15-deoxy-Delta(12,14)-prostaglandin J2 is mediated by the stress response elements and transcription factor Nrf2. Antioxid Redox Signal 4: 249–257, 2002. [DOI] [PubMed] [Google Scholar]
- 15. Gozal D, Serpero LD, Sans CO, Kheirandish-Gozal L. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep Med 9: 254–259, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grigoryev DN, Mathai SC, Fisher MR, Girgis RE, Zaiman AL, Housten-Harris T, Cheadle C, Gao L, Hummers LK, Champion HC, Garcia JG, Wigley FM, Tuder RM, Barnes KC, Hassoun PM. Identification of candidate genes in scleroderma-related pulmonary arterial hypertension. Transl Res 151: 197–207, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayhoe RP, Kamal AM, Solito E, Flower RJ, Cooper D, Perretti M. Annexin 1 and its bioactive peptide inhibit neutrophil-endothelium interactions under flow: indication of distinct receptor involvement. Blood 107: 2123–2130, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Hubbard RB, Smith C, Le Jeune I, Gribbin J, Fogarty AW. The association between idiopathic pulmonary fibrosis and vascular disease: a population-based study. Am J Respir Crit Care Med 178: 1257–1261, 2008. [DOI] [PubMed] [Google Scholar]
- 20. Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1alpha. Genes Dev 12: 149–162, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jun J, Reinke C, Bedja D, Berkowitz D, Bevans-Fonti S, Li J, Barouch LA, Gabrielson K, Polotsky VY. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis (October 17, 2009). doi:10.1016/j.atherosclerosis.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kawamata S, Hori T, Imura A, Takaori-Kondo A, Uchiyama T. Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF-kappaB activation. J Biol Chem 273: 5808–5814, 1998. [DOI] [PubMed] [Google Scholar]
- 23. Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47: 89–116, 2007. [DOI] [PubMed] [Google Scholar]
- 24. Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal 7: 385–394, 2005. [DOI] [PubMed] [Google Scholar]
- 25. Kukreja P, Abdel-Mageed AB, Mondal D, Liu K, Agrawal KC. Up-regulation of CXCR4 expression in PC-3 cells by stromal-derived factor-1alpha (CXCL12) increases endothelial adhesion and transendothelial migration: role of MEK/ERK signaling pathway-dependent NF-kappaB activation. Cancer Res 65: 9891–9898, 2005. [DOI] [PubMed] [Google Scholar]
- 26. Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, Prabhakar NR. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol 575: 229–239, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lakhani SA, Masud A, Kuida K, Porter GA, Jr, Booth CJ, Mehal WZ, Inayat I, Flavell RA. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 311: 847–851, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lau AK, Chaufour X, McLachlan C, Leichtweis SB, Celermajer DS, Sullivan C, Stocker R. Intimal thickening after arterial balloon injury is increased by intermittent repetitive hypoxia, but intermittent repetitive hyperoxia is not protective. Atherosclerosis 185: 254–263, 2006. [DOI] [PubMed] [Google Scholar]
- 29. Lavie L. Obstructive sleep apnoea syndrome—an oxidative stress disorder. Sleep Med Rev 7: 35–51, 2003. [DOI] [PubMed] [Google Scholar]
- 30. Lepedda AJ, Cigliano A, Cherchi GM, Spirito R, Maggioni M, Carta F, Turrini F, Edelstein C, Scanu AM, Formato M. A proteomic approach to differentiate histologically classified stable and unstable plaques from human carotid arteries. Atherosclerosis 203: 112–118, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lessner SM, Prado HL, Waller EK, Galis ZS. Atherosclerotic lesions grow through recruitment and proliferation of circulating monocytes in a murine model. Am J Pathol 160: 2145–2155, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Levy P, Pepin JL, Arnaud C, Baguet JP, Dematteis M, Mach F. Obstructive sleep apnea and atherosclerosis. Prog Cardiovasc Dis 51: 400–410, 2009. [DOI] [PubMed] [Google Scholar]
- 33. Li J, Grigoryev DN, Ye SQ, Thorne L, Schwartz AR, Smith PL, O'Donnell CP, Polotsky VY. Chronic intermittent hypoxia upregulates genes of lipid biosynthesis in obese mice. J Appl Physiol 99: 1643–1648, 2005. [DOI] [PubMed] [Google Scholar]
- 34. Li J, Savransky V, Nanayakkara A, Smith PL, O'Donnell CP, Polotsky VY. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J Appl Physiol 102: 557–563, 2007. [DOI] [PubMed] [Google Scholar]
- 35. Li J, Thorne LN, Punjabi NM, Sun CK, Schwartz AR, Smith PL, Marino RL, Rodriguez A, Hubbard WC, O'Donnell CP, Polotsky VY. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res 97: 698–706, 2005. [DOI] [PubMed] [Google Scholar]
- 36. Lindstrom TM, Mohan AR, Johnson MR, Bennett PR. Histone deacetylase inhibitors exert time-dependent effects on nuclear factor-kappaB but consistently suppress the expression of proinflammatory genes in human myometrial cells. Mol Pharmacol 74: 109–121, 2008. [DOI] [PubMed] [Google Scholar]
- 37. Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105: 659–669, 2005. [DOI] [PubMed] [Google Scholar]
- 38. Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep 31: 1079–1085, 2008. [PMC free article] [PubMed] [Google Scholar]
- 39. McArdle N, Hillman D, Beilin L, Watts G. Metabolic risk factors for vascular disease in obstructive sleep apnea: a matched controlled study. Am J Respir Crit Care Med 175: 190–195, 2007. [DOI] [PubMed] [Google Scholar]
- 40. Melchionna R, Porcelli D, Mangoni A, Carlini D, Liuzzo G, Spinetti G, Antonini A, Capogrossi MC, Napolitano M. Laminar shear stress inhibits CXCR4 expression on endothelial cells: functional consequences for atherogenesis. FASEB J 19: 629–631, 2005. [DOI] [PubMed] [Google Scholar]
- 41. Morello S, Ito K, Yamamura S, Lee KY, Jazrawi E, Desouza P, Barnes P, Cicala C, Adcock IM. IL-1beta and TNF-alpha regulation of the adenosine receptor (A2A) expression: differential requirement for NF-kappaB binding to the proximal promoter. J Immunol 177: 7173–7183, 2006. [DOI] [PubMed] [Google Scholar]
- 42. Naidu S, Wijayanti N, Santoso S, Kietzmann T, Immenschuh S. An atypical NF-kappaB-regulated pathway mediates phorbol ester-dependent heme oxygenase-1 gene activation in monocytes. J Immunol 181: 4113–4123, 2008. [DOI] [PubMed] [Google Scholar]
- 43. Nakano D, Hayashi T, Tazawa N, Yamashita C, Inamoto S, Okuda N, Mori T, Sohmiya K, Kitaura Y, Okada Y, Matsumura Y. Chronic hypoxia accelerates the progression of atherosclerosis in apolipoprotein E-knockout mice. Hypertens Res 28: 837–845, 2005. [DOI] [PubMed] [Google Scholar]
- 44. Nikolov DB, Burley SK. RNA polymerase II transcription initiation: a structural view. Proc Natl Acad Sci USA 94: 15–22, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ockaili R, Natarajan R, Salloum F, Fisher BJ, Jones D, Fowler AA, III, Kukreja RC. HIF-1 activation attenuates postischemic myocardial injury: role for heme oxygenase-1 in modulating microvascular chemokine generation. Am J Physiol Heart Circ Physiol 289: H542–H548, 2005. [DOI] [PubMed] [Google Scholar]
- 46. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342: 1378–1384, 2000. [DOI] [PubMed] [Google Scholar]
- 47. Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol 552: 253–264, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Polotsky VY, Patil SP, Savransky V, Laffan A, Fonti S, Frame LA, Steele KE, Schweizter MA, Clark JM, Torbenson MS, Schwartz AR. Obstructive sleep apnea, insulin resistance, and steatohepatitis in severe obesity. Am J Respir Crit Care Med 179: 228–234, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, Shahar E, Unruh ML, Samet JM. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 6: e1000132, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol 99: 1998–2007, 2005. [DOI] [PubMed] [Google Scholar]
- 51. Qiu XB, Shao YM, Miao S, Wang L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell Mol Life Sci 63: 2560–2570, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reddy NM, Kleeberger SR, Yamamoto M, Kensler TW, Scollick C, Biswal S, Reddy SP. Genetic dissection of the Nrf2-dependent redox signaling-regulated transcriptional programs of cell proliferation and cytoprotection. Physiol Genomics 32: 74–81, 2007. [DOI] [PubMed] [Google Scholar]
- 53. Robinson GV, Pepperell JC, Segal HC, Davies RJ, Stradling JR. Circulating cardiovascular risk factors in obstructive sleep apnoea: data from randomised controlled trials. Thorax 59: 777–782, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rodriguez JA, Orbe J, Martinez de LS, Calvayrac O, Rodriguez C, Martinez-Gonzalez J, Paramo JA. Metalloproteinases and atherothrombosis: MMP-10 mediates vascular remodeling promoted by inflammatory stimuli. Front Biosci 13: 2916–2921, 2008. [DOI] [PubMed] [Google Scholar]
- 55. Ryan S, McNicholas WT, Taylor CT. A critical role for p38 map kinase in NF-kappaB signaling during intermittent hypoxia/reoxygenation. Biochem Biophys Res Commun 355: 728–733, 2007. [DOI] [PubMed] [Google Scholar]
- 56. Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 112: 2660–2667, 2005. [DOI] [PubMed] [Google Scholar]
- 57. Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med 174: 824–830, 2006. [DOI] [PubMed] [Google Scholar]
- 58. Savransky V, Jun J, Li J, Nanayakkara A, Fonti S, Moser AB, Steele KE, Schweitzer MA, Patil SP, Bhanot S, Schwartz AR, Polotsky VY. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ Res 103: 1173–1180, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med 175: 1290–1297, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Scheibner KA, Boodoo S, Collins S, Black KE, Chan-Li Y, Zarek P, Powell JD, Horton MR. The adenosine a2a receptor inhibits matrix-induced inflammation in a novel fashion. Am J Respir Cell Mol Biol 40: 251–259, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Semenza GL. O2-regulated gene expression: transcriptional control of cardiorespiratory physiology by HIF-1. J Appl Physiol 96: 1173–1177, 2004. [DOI] [PubMed] [Google Scholar]
- 62. Shimoda LA, Fallon M, Pisarcik S, Wang J, Semenza GL. HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 291: L941–L949, 2006. [DOI] [PubMed] [Google Scholar]
- 63. Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest 127: 1952–1959, 2005. [DOI] [PubMed] [Google Scholar]
- 64. Stenina OI, Plow EF. Counterbalancing forces: what is thrombospondin-1 doing in atherosclerotic lesions? Circ Res 103: 1053–1055, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev 86: 515–581, 2006. [DOI] [PubMed] [Google Scholar]
- 66. Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res 62: 5196–5203, 2002. [PubMed] [Google Scholar]
- 67. Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98: 5116–5121, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. van Wanrooij EJ, van Puijvelde GH, de VP, Yagita H, van Berkel TJ, Kuiper J. Interruption of the Tnfrsf4/Tnfsf4 (OX40/OX40L) pathway attenuates atherogenesis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol 27: 204–210, 2007. [DOI] [PubMed] [Google Scholar]
- 69. Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, Kales A, Chrousos GP. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab 85: 1151–1158, 2000. [DOI] [PubMed] [Google Scholar]
- 70. Wang X, Feuerstein GZ, Gu JL, Lysko PG, Yue TL. Interleukin-1beta induces expression of adhesion molecules in human vascular smooth muscle cells and enhances adhesion of leukocytes to smooth muscle cells. Atherosclerosis 115: 89–98, 1995. [DOI] [PubMed] [Google Scholar]
- 71. Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 31: 1071–1078, 2008. [PMC free article] [PubMed] [Google Scholar]
- 72. Yuan G, Nanduri J, Bhasker CR, Semenza GL, Prabhakar NR. Ca2+/calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem 280: 4321–4328, 2005. [DOI] [PubMed] [Google Scholar]
- 73. Zhang X, Chen X, Song H, Chen HZ, Rovin BH. Activation of the Nrf2/antioxidant response pathway increases IL-8 expression. Eur J Immunol 35: 3258–3267, 2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.