Abstract
Whole-plant carbohydrate partitioning involves the assimilation of carbon in leaves and its translocation to nonphotosynthetic tissues. This process is fundamental to plant growth and development, but its regulation is poorly understood. To identify genes controlling carbohydrate partitioning, we isolated mutants that are defective in exporting fixed carbon from leaves. Here we describe psychedelic (psc), a new mutant of maize (Zea mays) that is perturbed in carbohydrate partitioning. psc mutants exhibit stable, discrete chlorotic and green regions within their leaves. psc chlorotic tissues hyperaccumulate starch and soluble sugars, while psc green tissues appear comparable to wild-type leaves. The psc chlorotic and green tissue boundaries are usually delineated by larger veins, suggesting that translocation of a mobile compound through the veins may influence the tissue phenotype. psc mutants display altered biomass partitioning, which is consistent with reduced carbohydrate export from leaves to developing tissues. We determined that the psc mutation is unlinked to previously characterized maize leaf carbohydrate hyperaccumulation mutants. Additionally, we found that the psc mutant phenotype is inherited as a recessive, duplicate-factor trait in some inbred lines. Genetic analyses with other maize mutants with variegated leaves and impaired carbohydrate partitioning suggest that Psc defines an independent pathway. Therefore, investigations into the psc mutation have uncovered two previously unknown genes that redundantly function to regulate carbohydrate partitioning in maize.
CARBON fixation and utilization underpin all aspects of plant biology. Carbon fixed in the photosynthetic tissues must be properly sensed, translocated, allocated, and utilized to ensure proper growth, development, and reproduction. When any one aspect of carbohydrate partitioning is disrupted, yield is usually affected (Moore et al. 2003; Lu and Sharkey 2004; Niittyla et al. 2004; Srivastava et al. 2008). Selective breeding has led to crop domestication and yield improvements, but the pathways responsible for these modifications in the context of carbon partitioning have not been well characterized (Whitt et al. 2002). The regulation of carbon partitioning in plants is likely to be complex and encompass multiple steps, including allocation into soluble sugars vs. insoluble starch, use for cellular metabolism vs. sugar export, and local utilization for organ growth vs. long-distance partitioning to sustain nonphotosynthetic tissues (Wardlaw 1990; Cheng et al. 1996; Koch 1996; Hannah 2005; Zeeman et al. 2007; Braun and Slewinski 2009). However, little is known about the control of whole-plant carbohydrate partitioning or the signal transduction pathways regulating sucrose transport across cellular membranes (Chiou and Bush 1998; Lalonde et al. 2004; Turgeon 2006; Sauer 2007; Smith and Stitt 2007; Slewinski and Braun 2010).
Maize is a well-established model organism for studies of carbon physiology and sucrose transport in plants (Evert et al. 1978; Heyser et al. 1978; Evert 1980; Heyser 1980; Kalt-Torres et al. 1987; Nolte and Koch 1993; Slewinski et al. 2009). Although the pathway for carbon movement in maize leaves has been studied for more than 40 years, little is known about its regulation (Hofstra and Nelson 1969; Braun and Slewinski 2009). Maize leaves contain three orders of longitudinal veins: large, intermediate, and small. The different vein types have distinct functions in carbohydrate partitioning and can be distinguished anatomically (Russell and Evert 1985). Large veins contain metaxylem vessels and hypodermal sclerenchyma that connect the vein to both the adaxial and abaxial epidermis. Intermediate veins lack metaxylem vessels but contain hypodermal sclerenchyma subtending one or both epidermal surfaces. Small veins lack metaxylem vessels and hypodermal sclerenchyma. Small and intermediate veins, collectively referred to as minor veins, primarily function in sucrose uptake into the phloem from the photosynthetic cells (Fritz et al. 1983) and intergrade into the large veins toward the base of the leaf blade (Russell and Evert 1985). In contrast, the large veins are principally involved in long-distance transport (Fritz et al. 1989).
Maize utilizes C4 carbon assimilation and displays Kranz anatomy in its leaves: mesophyll cells encircle bundle sheath cells, which in turn surround the vein (Esau 1977; Edwards et al. 2001). Sucrose, the translocated form of carbohydrate, is synthesized in the mesophyll cells (Lunn and Furbank 1999) and diffuses symplastically through plasmodesmata, intercellular channels connecting adjacent plant cells, into the bundle sheath cells and then into the vascular parenchyma cells (Russin et al. 1996). By an unknown mechanism, sucrose is transported out of the vascular parenchyma cells into the apoplast (Evert et al. 1978; Heyser 1980; Slewinski et al. 2009). Sucrose transporters located in the plasma membrane of companion cells and/or sieve elements import sucrose from the apoplast, actively concentrating it within phloem tissue for long-distance transport to other parts of the plant (Lalonde et al. 2004; Sauer 2007).
Characterization of mutants that display abnormalities in carbohydrate partitioning represents one approach to identifying genes that function in this process. To date, only four maize mutants that hyperaccumulate carbohydrates within their leaves and that function independently of starch metabolism have been reported: sucrose export defective1 (sxd1), tie-dyed1 (tdy1), tie-dyed2 (tdy2), and sucrose transporter1 (sut1) (Russin et al. 1996; Blauth et al. 2001; Provencher et al. 2001; Dinges et al. 2003; Braun et al. 2006; Baker and Braun 2008; Slewinski et al. 2008, 2009). sut1 mutant leaves are uniformly chlorotic, whereas the other three mutants are unique in that they form both normal-appearing green and chlorotic leaf regions, the latter of which hyperaccumulate starch and soluble sugars. All four mutants hyperaccumulate starch in both the bundle sheath and mesophyll cells, whereas wild-type plants normally contain starch only in the bundle sheath cells (Rhoades and Carvalho 1944). Additionally, all four mutants show reduced plant stature, delayed reproductive maturity, and diminished reproductive tissues. Retention of carbohydrates in chlorotic leaf tissues is thought to cause these phenotypes.
Although variegated, the sxd1 mutant phenotype differs from the tdy mutants. sxd1 mutant phenotypic expression begins at the leaf tip and progressively spreads to the base as the leaf ages (Russin et al. 1996; Provencher et al. 2001). Sxd1 encodes tocopherol cyclase, which functions in vitamin E biosynthesis (Sattler et al. 2003). Vitamin E deficiency leads to ectopic callose deposition, which occludes plasmodesmata specifically at the bundle sheath-vascular parenchyma cell interface, thus blocking symplastic sucrose transport upstream of sucrose release into the apoplast (Russin et al. 1996; Botha et al. 2000).
In contrast to sxd1 mutants, the variegation pattern in tdy mutant leaves becomes visible upon leaf emergence from the whorl and remains fixed throughout the life of the leaf (Braun et al. 2006; Baker and Braun 2008). tdy mutant leaves develop large, nonclonal, chlorotic regions that violate cell lineage patterns of maize leaf development (Poethig and Szymkowiak 1995; Braun et al. 2006; Baker and Braun 2007, 2008). Hence, it was proposed that the Tdy genes may function as regulators of carbon flux rather than being directly involved in carbohydrate metabolism or transport (Braun et al. 2006; Baker and Braun 2008). A threshold model was hypothesized to explain the leaf variegation in tdy mutants: accumulation of a chloroplast byproduct, likely sucrose, above a threshold level induces the formation of a chlorotic tissue phenotype, while quantities below the threshold result in a normal-appearing green leaf region (Braun et al. 2006; Baker and Braun 2008).
Although in many ways the tdy mutant phenotypes are similar to those of sxd1, recent work determined that both Tdy1 and Tdy2 function independently of the Sxd1 pathway (Baker and Braun 2008; Ma et al. 2008). Furthermore, genetic analyses suggest that the two Tdy genes function in the same pathway (Baker and Braun 2008). Tdy1 encodes a novel, phloem-expressed, predicted transmembrane protein (Ma et al. 2009). The molecular identity of Tdy2 is not known.
To gain insight into additional genetic regulators of carbon flux and allocation, we have identified many new loci controlling carbohydrate partitioning in maize leaves from a large collection of variegated, chlorotic mutants. Here, we characterize the psychedelic (psc) mutant, named because of the unique pattern of chlorotic and green leaf variegation reminiscent of the fabrics and art associated with the “hippie” movement of the 1960s. psc mutants display stable, nonclonal, chlorotic, and green regions within leaf tissues. psc green tissue is comparable to wild-type green tissue, whereas psc chlorotic tissue hyperaccumulates starch and soluble sugars. To investigate the role Psc plays in controlling carbohydrate accumulation in maize leaves, we characterized the psc mutant phenotype and performed genetic analyses to determine whether Psc functions independently of the previously identified maize genes regulating carbohydrate partitioning.
MATERIALS AND METHODS
Growth conditions and genetic stocks:
Maize plants were grown under high-light conditions at the Pennsylvania State University Rock Springs Agronomy Farm during the summers of 2008 and 2009. Plants used for low-light experiments were grown in a shaded greenhouse in 400 μmol m−2 sec−1 light under a 12-hr day (30°) and 12-hr night (20°). The psc-Reference (hereafter psc) mutation arose spontaneously in a B73 inbred line. For phenotypic analyses, segregating 1:1 psc:wild-type families were generated by fertilizing heterozygous wild-type plants with pollen from homozygous psc mutant siblings. Complementation tests were performed by crossing psc mutant plants in the B73 background to homozygous tdy1-R, tdy2-R, and sxd1-1 mutant individuals introgressed six or more times into the B73 background. The F1 plants were self-fertilized to produce segregating F2 populations. At least two independent families were characterized for each of the double mutant analyses.
Starch staining and microscopy:
Starch was visualized by clearing leaf tissue in boiling 95% ethanol and staining with iodine potassium iodide (IKI) solution (Ruzin 1999). Free-hand leaf cross-sections were examined under bright-field and UV light using a Nikon Eclipse 80i fluorescent microscope with a 100-W mercury lamp as described (Baker and Braun 2007). Aniline blue staining of callose was performed according to Ma et al. (2008).
Carbohydrate quantification:
Carbohydrates were quantified according to Stitt et al. (1989). A total of 100 mg of tissue was collected between 5 and 6 pm on a sunny, cloudless day from leaves 15 and 16 on mature plants in mid-July 2008. Six biological replicates were conducted for each tissue type and the entire experiment was repeated three times with similar results. Data from a representative repeat are shown.
Photosynthetic, stomatal conductance and chlorophyll measurements:
Photosynthetic and stomatal conductance measurements were taken on fully expanded leaves 15 and 16 from mature plants grown in the field in midsummer between 2 and 4 pm using a LICOR 6400 photosynthesis system as described (Huang et al. 2009). Six biological replicates were performed for each tissue type and the entire experiment was repeated three times. Data from a representative repeat are shown. Total chlorophyll levels were measured with a SPAD 502 data logger on leaves 15 and 16 from mature plants. A total of 25 biological replicates were performed for each tissue type and the experiment was repeated three times. Data from a representative repeat are shown.
Morphometric analyses:
Morphometric analyses were done as described (Braun et al. 2006; Baker and Braun 2007; Ma et al. 2008). N = 12 for all parameters unless noted otherwise in figure legends and replicates were collected from two segregating families grown in separate field locations in the summer of 2008.
RESULTS
Identification of a new variegated maize mutant with starch hyperaccumulation in leaves:
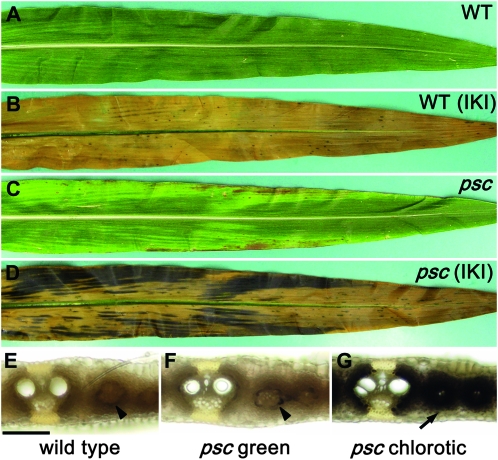
To identify additional genes controlling carbohydrate partitioning, we screened for mutants with phenotypes that resembled the tdy mutants. A spontaneous mutation arose in a B73 inbred line and was found to produce stable, nonclonal, variegated chlorotic leaf tissues (Figure 1, A and C). The stable, nonclonal phenotype indicated that the mutation is not caused by transposable element insertion or excision (Poethig and Szymkowiak 1995; Braun et al. 2006). The new mutant phenotype differed subtly from the tdy or sxd1 mutant phenotypes in that the chlorotic regions appeared to be more longitudinally streaked and more likely to be localized toward the leaf margins (Figure 1C). To assess whether the chlorotic regions contained excess carbohydrates, we stained mutant and wild-type leaves with IKI at the end of the night. Wild-type leaves contained little starch, as indicated by the pale yellow color of the tissue (Figure 1B). However, the mutant leaves contained abundant starch within the chlorotic regions, as evidenced by the dark brown coloration (Figure 1D). The green regions of mutant leaves did not appear to contain elevated starch contents. Therefore, the chlorotic leaf regions, like those in the tdy and sxd1 mutants, hyperaccumulated starch.
Figure 1.—
psc leaves display chlorotic regions that hyperaccumulate starch. (A) Wild-type (WT) leaves have uniform green coloration. (B) Wild-type leaves cleared and IKI stained showing that the leaf contains little starch after the dark period. (C) psc leaves display chlorotic and green tissues. (D) psc leaves cleared and IKI stained showing that chlorotic regions hyperaccumulate starch after the dark period, whereas green regions do not. Free-hand leaf cross-sections of (E) wild-type, (F) psc green, and (G) psc chlorotic tissues. psc chlorotic tissue hyperaccumulates starch in both the bundle sheath and mesophyll cells (arrow in G), whereas wild-type and psc green tissues contain trace amounts of starch in the bundle sheath cells (arrowheads in E and F) after the dark period. Bar, 100 μm for panels E–G.
To determine which photosynthetic cells accumulated excess starch in the mutant, histological analysis was performed. In wild-type leaves, at the end of the night, trace amounts of starch were present only in the bundle sheath cells (Figure 1E). Green tissue from mutant leaves showed a similar pattern and level of starch deposition (Figure 1F). However, in the mutant chlorotic leaf tissue, large quantities of starch were observed in both the bundle sheath and mesophyll cells (Figure 1G). Hence, the starch accumulation pattern within the chlorotic tissues of the new mutant was similar to the tdy and sxd1 mutants (Russin et al. 1996; Braun et al. 2006; Baker and Braun 2008).
To investigate whether the new mutation might be a new allele of tdy1, tdy2, or sxd1, we performed complementation crosses. In all cases, the F1 appeared normal, and the mutants complemented each other. These data, along with genetic analyses (see below), suggested that the new mutation represented a distinct locus, which we named psychedelic (psc) on the basis of its variegated appearance.
The psc phenotype displays duplicate-factor inheritance in some genetic backgrounds:
To characterize the inheritance of the psc trait, wild-type heterozygous siblings were self-pollinated. The psc phenotype segregated one mutant to three wild type in the progeny, suggesting a single recessive mutation in the B73 background (Table 1). However, this result differed in other genetic backgrounds. psc phenotypic individuals from the B73 line were crossed to the Mo17 inbred line and self-pollinated. Interestingly, the F2 generation segregated the psc mutant phenotype in a 1:15 ratio, suggesting duplicate-factor recessive inheritance (Table 2). To test this hypothesis, the F1 plants were backcrossed to the psc mutant in B73. The F2 progeny segregated psc mutants in a 1:3 ratio rather than a 1:1 ratio, supporting duplicate-factor inheritance (Table 2). Similar results were obtained when the same backcrossing scheme was performed with the W22 and A188 inbred lines (Table 2). These data indicate that the B73 inbred line is homozygous mutant for one of the unlinked psc duplicate loci, whereas the other inbred lines tested, each carry two functionally redundant genes.
TABLE 1.
psc displays single-factor recessive inheritance in the B73 inbred line
| Family no. | No. of mutants | No. of wild type | Total no. | % mutant |
|---|---|---|---|---|
| 1 | 6 | 21 | 27 | 22.2a |
| 2 | 8 | 30 | 38 | 21.1a |
| 3 | 5 | 23 | 28 | 17.9a |
| 4 | 16 | 39 | 55 | 29.1a |
| 5 | 13 | 36 | 49 | 26.5a |
| 6 | 11 | 41 | 52 | 21.2a |
| Total | 59 | 190 | 249 | 23.7a |
χ2 values fail to reject the null hypothesis of a 3:1 segregation ratio.
TABLE 2.
psc displays duplicate-factor recessive inheritance in the Mo17, A188, and W22 inbred lines
| Family no. | Female parent | Male parent | No. of mutants | No. of wild type | Total no. | % mutant |
|---|---|---|---|---|---|---|
| 1 | psc(B73) × Mo17 | Self-pollinated | 6 | 111 | 117 | 5.1a |
| 2 | psc(B73) × Mo17 | Self-pollinated | 12 | 158 | 170 | 7.1a |
| 3 | psc(B73) × Mo17 | Self-pollinated | 5 | 105 | 110 | 4.5a |
| 4 | psc(B73) × Mo17 | Self-pollinated | 7 | 116 | 123 | 5.7a |
| 5 | psc(B73) × Mo17 | Self-pollinated | 11 | 218 | 229 | 4.8a |
| 6 | psc(B73) × Mo17 | Self-pollinated | 17 | 227 | 244 | 7.0a |
| Total | 58 | 935 | 993 | 5.8a | ||
| 1 | psc(B73) × Mo17 | psc(B73) | 14 | 33 | 47 | 29.8b |
| 2 | psc(B73) × Mo17 | psc(B73) | 22 | 59 | 81 | 27.2b |
| 3 | psc(B73) × Mo17 | psc(B73) | 37 | 124 | 161 | 23.0b |
| 4 | psc(B73) × Mo17 | psc(B73) | 33 | 116 | 149 | 22.1b |
| 5 | psc(B73) × Mo17 | psc(B73) | 31 | 129 | 160 | 19.4b |
| 6 | psc(B73) × Mo17 | psc(B73) | 15 | 38 | 53 | 28.3b |
| 7 | psc(B73) × Mo17 | psc(B73) | 34 | 116 | 150 | 22.7b |
| 8 | psc(B73) × Mo17 | psc(B73) | 50 | 166 | 216 | 23.1b |
| Total | 236 | 781 | 1017 | 23.2b | ||
| 1 | psc(B73) × A188 | psc(B73) | 22 | 78 | 100 | 22.0b |
| 2 | psc(B73) × A188 | psc(B73) | 15 | 65 | 80 | 18.8b |
| Total | 37 | 143 | 180 | 20.5b | ||
| 1 | psc(B73) × W22 | psc(B73) | 27 | 84 | 111 | 24.3b |
| 2 | psc(B73) × W22 | psc(B73) | 32 | 73 | 105 | 30.5b |
| Total | 59 | 157 | 216 | 27.3b |
χ2 values fail to reject the null hypothesis of a 15:1 segregation ratio, but reject a 3:1 ratio.
χ2 values fail to reject the null hypothesis of a 3:1 segregation ratio, but reject a 1:1 ratio.
Soluble sugars hyperaccumulate in psc chlorotic regions and are associated with decreased photosynthesis:
To examine whether soluble sugars hyperaccumulated within psc leaves, we quantified their levels in leaves that were harvested at the end of the day, when carbohydrate contents peak. Green tissue in psc leaves had carbohydrate levels similar to wild type (Table 3). However, soluble sugars (sucrose, glucose, and fructose) and starch amounts were significantly increased in the psc chlorotic regions (Table 3). These data indicate that the carbohydrate partitioning defect in psc was not exclusive to starch, but also affected soluble sugars.
TABLE 3.
Carbohydrate levels, photosynthetic parameters, and chlorophyll content in wild-type and psc mutant plants
| Parameter | Wild type | psc green | psc chlorotic |
|---|---|---|---|
| Sucrosea | 18.54 ± 0.99 | 22.67 ± 2.13 | 43.54* ± 1.15 |
| Glucosea | 0.39 ± 0.05 | 0.43 ± 0.09 | 3.60* ± 0.16 |
| Fructosea | 0.80 ± 0.03 | 0.92 ± 0.14 | 2.18* ± 0.39 |
| Starcha | 23.65 ± 2.23 | 22.07 ± 2.45 | 41.33* ± 3.34 |
| Photosynthetic rateb | 41.78 ± 1.73 | 40.63 ± 1.30 | 1.70* ± 0.28 |
| Stomatal conductanceb | 0.196 ± 0.01 | 0.20 ± 0.01 | 0.017* ± 0.004 |
| Chlorophyll contentc | 51.3 ± 2.6 | 51.6 ± 2.6 | 16.1* ± 3.7 |
*Significantly different from wild type at P ≤ 0.05 using Student's t-test.
Data represent means of six samples ± the standard error. Units are mg per g fresh weight. Tissue was collected from summer nursery grown material at 8 pm.
Data represent means of six samples ± the standard error. Units for photosynthesis rate are μmol CO2 fixed m−2 s−1 and units for stomatal conductance measurements are μmol.
Chlorophyll content (N = 25) is expressed in relative units.
Sugars have been demonstrated to repress photosynthetic gene expression and chlorophyll abundance in maize and other plants (Sheen 1990; Goldschmidt and Huber 1992; Sheen 1994; Krapp and Stitt 1995; Koch 1996; Jeannette et al. 2000). Because of the high sugar levels in psc chlorotic leaf tissues, we investigated the photosynthetic capacity of mutant and wild-type leaves. Green tissues in psc leaves had chlorophyll levels, photosynthesis, and stomatal conductance rates similar to wild type (Table 3). However, the chlorotic regions displayed photosynthesis and gas exchange rates of only 4–9% of those observed in wild type (Table 3). Additionally, psc chlorotic tissues had ∼31% of the chlorophyll detected in the green tissues of psc or wild-type leaves. Hence, the excess accumulation of photoassimilates in psc chlorotic regions correlated with a downregulation of photosynthesis, decreased chlorophyll levels, and the closure of stomata.
Starch accumulation precedes chlorosis in developing psc leaves:
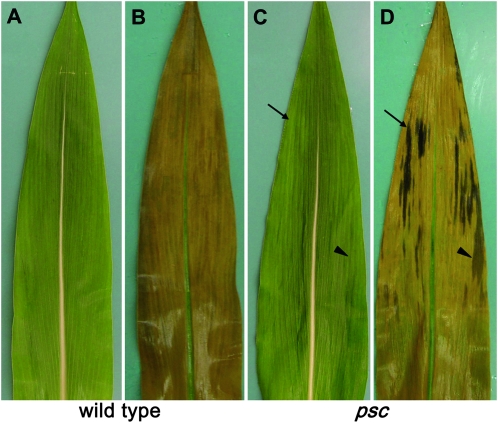
In the tdy1 and tdy2 mutants, starch hyperaccumulation precedes formation of chlorotic tissues (Braun et al. 2006; Baker and Braun 2008). To investigate whether carbohydrate accumulation also preceded chlorosis in psc leaves, we examined psc mutant and wild-type leaves in the process of emerging from the whorl. Developing leaves of psc mutants displayed chlorotic regions toward the mature tip of the leaves, but lacked chlorotic regions toward the immature leaf base (Figure 2, A and C). To inspect carbohydrate accumulation, we stained the leaves with IKI to monitor starch deposition. Wild-type leaves showed uniform, pale coloration and lacked any differential starch accumulation (Figure 2B). As expected, visible chlorotic regions within psc leaves showed strong starch staining (Figure 2D). However, the middle portion of psc leaves showed starch accumulation but did not display any observable difference in chlorophyll content (Figure 2C). These data imply that the visible chlorosis evident in psc mutant leaves is not directly due to defective chloroplasts, which accumulate excess carbohydrates. Instead, they suggest that the buildup of carbohydrates leads to the downregulation of chlorophyll accumulation in leaf tissues.
Figure 2.—
Starch accumulation precedes chlorosis in psc mutant leaves. (A and C) Leaf photographs. (B and D) Same leaves cleared and IKI stained. (A and B) Wild type. (C and D) psc mutant. Arrow points to a region in a psc mutant leaf that has hyperaccumulated starch and progressed to chlorosis, whereas the arrowhead shows a region that has begun to hyperaccumulate starch but has not yet progressed to chlorosis.
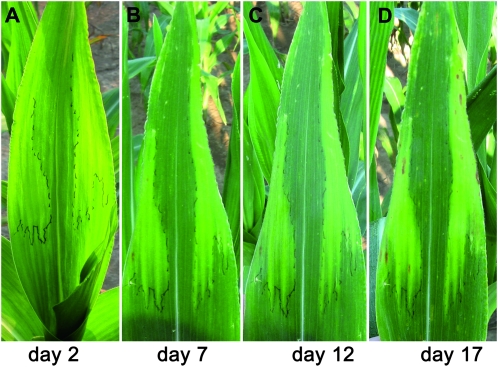
Chlorotic tissue boundaries are fixed and do not expand:
The chlorotic phenotype of psc mutant leaves appears soon after leaves emerge from the whorl and after carbohydrates have begun to accumulate in discrete regions. To determine whether increased accumulation of carbohydrates resulted in the progressive expansion of chlorotic tissue, as seen in sxd1 mutant leaves (Russin et al. 1996), we monitored the formation of a psc chlorotic region over time. One day after a leaf began emerging from the whorl it had a uniform, pale-green appearance and no chlorosis was visible (data not shown). Two days after leaf emergence commenced, chlorotic regions first appeared (Figure 3A). Visible chlorotic regions were outlined to mark their boundaries and thereby follow their development. Seven days after emergence, the leaf tip had fully matured. The chlorotic tissue had not increased in relative size as indicated by the black outline appearing coincident with the boundary between green and chlorotic tissues (Figure 3B). Continuing to monitor the leaf for 10 additional days showed that the boundary was stable (Figure 3, C and D). Hence, we propose that the formation of a psc chlorotic region occurs soon after leaf emergence, and that the tissue boundaries in mature leaves are permanent once formed, similar to tdy1 and tdy2 (Braun et al. 2006; Baker and Braun 2008).
Figure 3.—
The psc chlorotic leaf phenotype does not progressively expand. Photographs of the same psc mutant leaf over time. (A) Two days after leaf emergence. (B) Day 7. (C) Day 12. (D) Day 17.
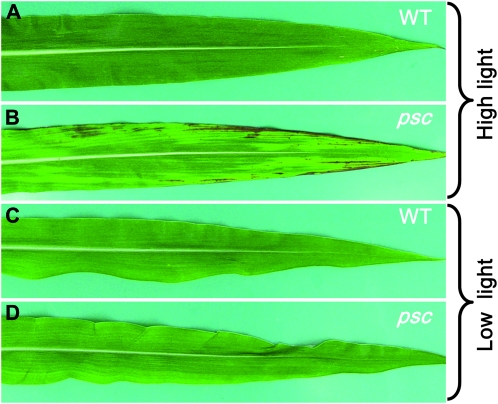
High light induces the psc chlorotic phenotype:
Expression of the tdy mutants chlorotic phenotype and the hyperaccumulation of carbohydrates require growth under high light (Braun et al. 2006; Baker and Braun 2008). To test whether light intensity influenced psc phenotypic expression, we grew wild-type and psc mutant plants in high- or low-light growth conditions. In high light, strong chlorotic regions were evident in psc leaves, whereas wild-type leaves appeared uniformly green (Figure 4, A and B). However, in low light, no chlorotic regions were visible in psc or wild-type leaves (Figure 4, C and D). These data suggest that high light is required to induce the psc phenotype, consistent with the idea that high rates of carbon assimilation underlie the formation of psc chlorotic leaf regions.
Figure 4.—
High light is required for psc chlorotic tissue formation. (A and C) Wild type (WT). (B and D) psc mutant. (A and B) Leaves from plants grown under 2100 μmol m−2 sec−1 light. (C and D) Leaves from plants grown under 400 μmol m−2 sec−1 light.
Veins are frequently found at psc tissue boundaries:
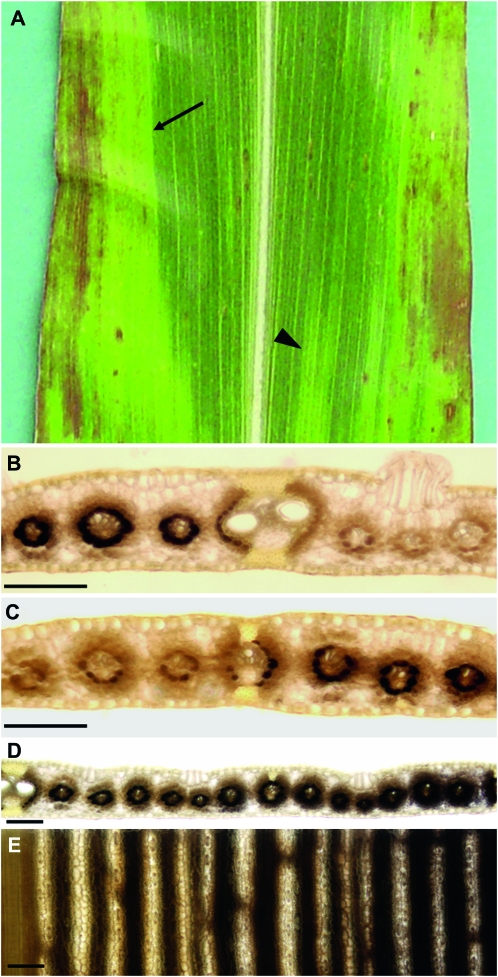
Similar to the tdy mutants (Baker and Braun 2007, 2008), the boundaries between the different psc leaf phenotypic regions are often sharp (Figure 5A). To ascertain the nature of these boundaries and what may limit the expansion of a chlorotic region, we histologically examined these tissues for morphological features. In the proximal–distal axis of the leaf, no obvious morphological landmarks were found at the tissue boundaries. However, along the midrib–margin axis, ∼70% of the distinct boundaries between psc green and chlorotic tissues occurred at large veins (Table 4). To determine whether the carbohydrate hyperaccumulation phenotype likewise showed a discrete boundary between chlorotic and green tissues, we analyzed leaf cross-sections from tissues harvested at the end of the night. Staining with IKI revealed that the boundary for both the tissue and starch accumulation phenotypes occurred at the large vein; psc chlorotic regions on one side of the vein contained abundant starch in the bundle sheath cells, whereas the green tissues on the other side of the vein had only trace amounts of starch (Figure 5B). In addition to large veins, 22% of the sharp boundaries between chlorotic and green tissue occurred at intermediate veins (Table 4). Starch staining revealed that the intermediate vein was also the boundary between greatly different levels of carbohydrates in the two tissues (Figure 5C). Intriguingly, for both the large and intermediate vein boundaries, chlorotic tissue adjacent to green tissue contained less starch than chlorotic tissue far from the tissue boundary (compare Figure 5, B and C with Figure 1G). Indeed, a gradual increase in starch accumulation in bundle sheath and mesophyll cells could be observed as the distance from the tissue boundary increased (Figure 5, D and E). Therefore, we infer that translocation of a mobile compound through the veins may influence the tissue phenotype.
Figure 5.—
Large and intermediate veins frequently delineate the boundary between psc chlorotic and green tissue. (A) psc mutant leaf showing a chlorotic and green tissue boundary at a large vein (arrow) as well as at an intermediate vein (arrowhead). (B) Cleared and IKI-stained cross-section of the boundary between chlorotic and green tissues marked with an arrow in A revealed it is located at a large vein. (C) Cleared and IKI-stained cross-section of the boundary between chlorotic and green tissues indicated with an arrowhead in A revealed it is located at an intermediate vein. (D) Lower magnification view of a cleared and IKI-stained cross-section of a chlorotic-green tissue boundary located at a large vein (left). (E) Abaxial view of D. In D and E, note the gradient of starch accumulation progressing into the chlorotic tissue (right). Bars, 100 μm.
TABLE 4.
psc chlorotic tissue boundary analysis
| Boundary location | % |
|---|---|
| Large vein | 72 |
| Intermediate vein | 22 |
| Other | 6 |
Twenty leaves were used for the analysis and 5 randomly selected, distinct tissue boundaries were analyzed per leaf.
Other refers to no distinguishable feature identified at tissue boundary.
Carbohydrate retention in psc leaves is correlated with decreased plant growth and yield:
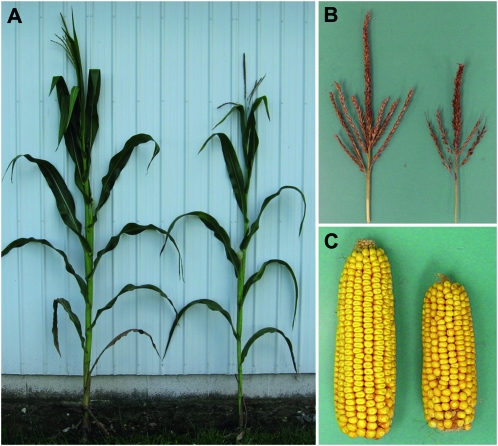
tdy and sxd1 mutant plants show decreased growth and yield (Russin et al. 1996; Braun et al. 2006; Baker and Braun 2008; Ma et al. 2008). Because psc mutant leaves retain large amounts of carbohydrates, we hypothesized that less sucrose would be delivered to developing tissues and result in reduced plant growth and development. To assess this idea, we quantified plant height, leaf production, time to flowering, and reproductive traits. At maturity, psc mutant plants were ∼12% shorter than wild type and produced about two fewer leaves (Figure 6A; Table 5). psc mutants also displayed a number of reproductive defects. The mutants were ∼4 days delayed in pollen shed and in producing silks relative to wild type (Table 5). The mutants also showed an ∼25% reduction in ear length, tassel length, and kernel number, and an ∼40% decrease in tassel branch number compared to wild type (Figure 6, B and C; Table 5). Additionally, the average kernel weight was reduced 18% in psc mutants. All of these phenotypes are consistent with carbohydrate retention within leaf tissues, which results in the failure to export enough sugars to sustain the growth of developing organs (Russin et al. 1996; Blauth et al. 2001; Niittyla et al. 2004; Braun et al. 2006; Baker and Braun 2008; Slewinski et al. 2009).
Figure 6.—
psc mutants have reduced growth of vegetative and reproductive tissues. (A) Photograph of mature psc (right) and wild-type sibling (left) field grown plants. (B) psc tassels (right) display diminished growth compared to wild-type tassels (left). (C) psc ears (right) display reduced size and yield compared to wild-type ears (left).
TABLE 5.
psc mutant plants have altered growth
| Growth parameters | Wild type | psc mutant | % WT |
|---|---|---|---|
| Plant height (cm) | 208.8 ± 2.4 | 184.3* ± 3.9 | 88.3 |
| Leaf number | 20.9 ± 0.2 | 19.1* ± 0.3 | 91.2 |
| Days to anthesis | 79.0 ± 0.3 | 82.8* ± 0.6 | 104.8 |
| Days to silking | 79.8 ± 0.4 | 84.1* ± 0.7 | 105.2 |
| Kernel weight (mg) | 18.1 ± 0.6 | 14.9* ± 0.6 | 82.3 |
| Kernel number | 589.3 ± 16.5 | 439.5* ± 14.5 | 74.6 |
| Ear length (cm) | 14.3 ± 0.3 | 10.8* ± 0.3 | 75.5 |
| Tassel length (cm) | 39.9 ± 1.0 | 28.5* ± 1.1 | 71.4 |
| Tassel branch number | 7.0 ± 0.1 | 4.1* ± 0.7 | 58.6 |
Values are the means ± the standard error. (N = 12) WT, wild type. Kernel weight is the average of 100 kernels. *Significantly different from wild type at P ≤ 0.05 using Student's t-test.
Psc functions independently of Tdy1, Tdy2 and Sxd1:
To determine whether Psc might function in the same genetic pathway as either the Tdy or Sxd1 genes, we generated double mutant families in the B73 background that segregated for psc and each of the other single mutants. In all F2 families, the psc mutation segregated independently from tdy1, tdy2, and sxd1, in each case exhibiting a segregation ratio that conformed to a 9:3:3:1 expectation (Table 6; supporting information, Table S1). These data support the findings from the complementation tests, indicating that the psc mutation defines different genes than those previously identified to function in carbohydrate partitioning in maize. Furthermore, these data indicate that Psc shows no epistatic relationships with Tdy1, Tdy2, or Sxd1.
TABLE 6.
Segregation data for psc and tdy1 F2 families
| Family no. | WT | psc | tdy1 | psc; tdy1 | Total | χ2 | Probability |
|---|---|---|---|---|---|---|---|
| 1 | 19 | 8 | 6 | 2 | 35 | 0.40 | P > 0.05 |
| 2 | 26 | 11 | 15 | 4 | 56 | 2.98 | P > 0.05 |
| 3 | 12 | 7 | 7 | 2 | 28 | 2.10 | P > 0.05 |
| 4 | 11 | 9 | 6 | 1 | 27 | 4.67 | P > 0.05 |
| 5 | 30 | 12 | 9 | 3 | 54 | 0.52 | P > 0.05 |
| 6 | 27 | 7 | 10 | 2 | 46 | 0.84 | P > 0.05 |
| Total | 125 | 54 | 53 | 14 | 246 | 3.79 | P > 0.05 |
χ2 analyses of psc and tdy1 segregating in six F2 families fail to reject a 9:3:3:1 expectation. WT, wild type.
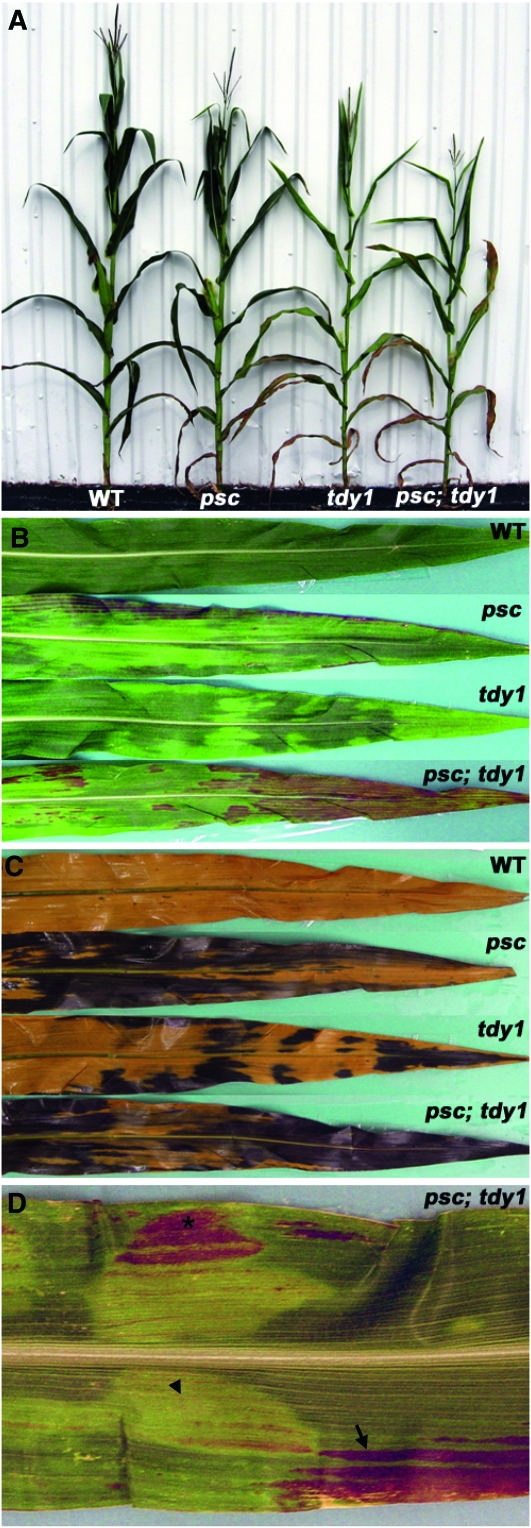
To investigate potential genetic interactions between psc and the tdy and sxd1 mutants, we characterized the F2 families for plant growth and time to reproductive maturity. At the whole-plant level, psc and tdy1 single mutants were 10–12% shorter than wild-type siblings (Figure 7A; Table 7), whereas the psc; tdy1 double mutant plants were 25% shorter than wild type. Similarly, both psc and tdy1 single mutants each flowered ∼5 days later than wild type, while the double mutant was twice as slow to shed pollen or extrude silks (Table 7). Hence, the psc; tdy1 double mutants displayed approximately double the phenotypic severity in plant height and time to flowering compared with the single mutants, suggesting an additive genetic interaction.
Figure 7.—
psc and tdy1 exhibit an additive genetic interaction. (A) Photograph of mature wild-type (WT), psc, tdy1, and psc; tdy1 double mutant plants. (B) Leaves from wild type, psc, tdy1, and psc; tdy1 double mutants. (C) Same leaves as shown in B, cleared and IKI stained. (D) Close-up of overlapping tdy1 and psc chlorotic tissues from a psc; tdy1 double mutant. Arrow indicates longitudinally streaked psc chlorotic tissue accumulating anthocyanins. Arrowhead indicates tdy1 chlorotic region. Asterisk indicates severely chlorotic tissue expressing both the psc and tdy1 chlorotic phenotypes and accumulating anthocyanins.
TABLE 7.
Growth parameters of psc; tdy1 double mutants
| Phenotype | Plant height (cm) | % WT | Days to anthesis | % WT | Days to silking | % WT |
|---|---|---|---|---|---|---|
| Wild type | 237.8 ± 0.2 | 100 | 85.2 ± 0.1 | 100 | 86.1 ± 0.1 | 100 |
| psc | 216.7* ± 0.4 | 91.1 | 89.6* ± 0.1 | 105.2 | 91.6** ± 0.1 | 106.4 |
| tdy1 | 209.7* ± 0.6 | 88.1 | 89.9* ± 0.1 | 105.4 | 91.5* ± 0.1 | 106.2 |
| psc; tdy1 | 177.4** ± 2.6 | 74.6 | 94.3** ± 0.3 | 110.7 | 96.3** ± 0.3 | 111.8 |
Values are the means ± standard error. N = 40 for wild type (WT), 25 psc, 26 tdy1, and 6 psc; tdy1. *Significantly different from wild type at P ≤ 0.05 using Student's t-test. **Significantly different from wild type, psc and tdy1 single mutants at P ≤ 0.05 using Student's t-test.
To test this hypothesis, we analyzed leaf chlorosis and starch accumulation in the F2 plants. Leaves of the psc; tdy1 double mutant exhibited a greater percentage of chlorotic tissues compared to either the psc or tdy1 single mutants, but still contained some distinct green regions (Figure 7B). Double mutant leaves also accumulated significantly more anthocyanins than the single mutants. Anthocyanins have been shown to accumulate in tissues containing high levels of carbohydrates that are experiencing osmotic stress (Russin et al. 1996; Chalker-Scott 1999; Baker and Braun 2007). IKI-staining the leaves confirmed that all of the chlorotic tissue contained excess starch, whereas the green regions of the single and double mutants appeared similar to wild type (Figure 7C). Within the double mutant leaves, the two single mutant variegation patterns appeared to independently overlay one another. Some leaf areas displayed the streaked, longitudinal chlorosis associated with the psc mutant phenotype (Figure 7D, arrow), while other regions showed the distinctive appearance of a tdy1 chlorotic region (Figure 7D, arrowhead). Moreover, there were regions in which the two distinct variegation patterns overlapped. Leaf regions expressing both mutant phenotypes exhibited more severe chlorosis than either single mutant (Figure 7D, asterisk). This strongly chlorotic tissue was visible before, accumulated anthocyanins earlier, and progressed to necrosis sooner than adjacent leaf tissue expressing only one of the chlorotic mutant phenotypes (data not shown). Hence, the leaf chlorosis and excess starch phenotypes in the double mutant leaves appeared to result from the independent expression of each single mutant phenotype, suggesting that Psc and Tdy1 function in separate genetic pathways.
Similar analyses with tdy2 and sxd1 suggest that Psc function is independent of previously defined pathways regulating carbohydrate partitioning in maize (Figure S1, Table S1, and Table S2). In support of this, psc mutants did not exhibit ectopic callose deposits at the bundle sheath–vascular parenchyma cell interface, which are present in sxd1 mutants, and did not influence this phenotype in the psc; sxd1 double mutant leaves (Figure S2) (Botha et al. 2000; Baker and Braun 2008; Ma et al. 2008).
DISCUSSION
We have taken a genetic approach to identify genes that promote carbon export from maize leaves. Previously, three variegated maize mutants, sxd1, tdy1, and tdy2 have been implicated as defective in carbon movement out of leaves. In this report, we describe a new mutant, psc, which hyperaccumulates carbohydrates within discrete chlorotic leaf regions. By characterizing the psc mutant phenotype and its genetic interaction with the other variegation mutants that accumulate excess carbohydrates, we determined that psc defines a new pair of redundantly acting genes regulating carbohydrate partitioning in maize.
Redundant genetic functions may be common in maize, as it is an ancient segmental allotetraploid with multiple duplicated, colinear chromosomal regions (Gaut and Doebley 1997). Previous studies found functional redundancy for maize genes that map to homeologous chromosomal positions (Rhoades 1951; Coe et al. 1981; Wright et al. 1992; Mena et al. 1996; Scanlon et al. 1996), indicating that the duplicated genes have identical or partially overlapping functions. For the later cases, these are likely caused by subfunctionalization, resulting in diverging tissue-specific expression patterns (Lynch and Force 2000; Langham et al. 2004). Indeed, recent analyses of the maize genome revealed hundreds of variants for gene copy number as well as thousands of present–absent variants between the B73 and Mo17 inbred lines (Schnable et al. 2009; Springer et al. 2009). These haplotype-specific sequences likely contribute to the tremendous phenotypic diversity present in maize and may explain differences in inheritance patterns of mutations observed in different maize inbred lines.
Psc promotes carbohydrate export from maize leaves:
Hyperaccumulation of starch in the mesophyll cells of C4 plants is only observed when carbon export is severely perturbed. Physiological disruptions leading to this phenotype include removing developing ears from adult plants (Allison and Weinmann 1970) or inhibiting phloem transport by techniques such as girdling (Goldschmidt and Huber 1992; Krapp and Stitt 1995; Jeannette et al. 2000; Slewinski et al. 2009). The psc, sxd1, tdy1, and tdy2 mutants all display carbohydrate hyperaccumulation in both bundle sheath and mesophyll cells within chlorotic leaf regions. Therefore, this indicates that they are defective in carbon export from leaves (Russin et al. 1996; Braun et al. 2006; Baker and Braun 2008; Slewinski et al. 2009).
Similar to the tdy mutants, high light is needed to generate the psc variegation phenotype. When grown under high-light conditions, psc mutant leaves exhibit stable, chlorotic regions that are first visible 2 days postemergence from the whorl. These chlorotic tissues form only during a limited developmental period as the leaf emerges and remain fixed throughout the life of the leaf. On the basis of these data, we hypothesize that the buildup of a photosynthetic byproduct in high light, for example sucrose, triggers psc chlorotic tissue formation during leaf development. Once leaf maturation progresses beyond a developmental time point, the psc mutant phenotype is irreversible. Thus, Psc is predicted to act at or prior to the time when the mutant phenotype is first detectable, potentially when the leaves are still immature sink tissues (Evert et al. 1996).
Larger order veins are implicated in psc chlorotic tissue formation. In the lateral dimension, 94% of the psc chlorotic region boundaries occurred at large or intermediate veins, suggesting that these veins limit the movement of the chlorotic-tissue-inducing compound. This may also account for the longitudinally streaked nature of the phenotype. In comparison, only 68–74% of the sharp tissue boundaries occurred at large and intermediate veins in tdy1 and tdy2 leaves (Baker and Braun 2007, 2008). Hypodermal sclerenchyma caps are present in both intermediate and large, but not small, veins (Russell and Evert 1985). Hence, these may function as barriers to restrict the diffusion of solutes in the apoplast and underlie the formation of a sharp boundary between chlorotic and green tissues. The sharp boundaries at these veins suggest that Psc may function to promote the long-distance translocation of sucrose within the phloem. Alternative possibilities are that Psc functions to induce sucrose export to the phloem or to sequester sugars in the vacuole and/or chloroplast, when excess levels of these substances are generated in high-light environments.
A threshold model to explain the variegation pattern of chlorosis as proposed for the tdy loci may also apply to psc (Braun et al. 2006; Baker and Braun 2008). In brief, the determination of leaf tissue to form a chlorotic region is based on a threshold of sucrose or other chloroplast byproduct which is experienced during a limited developmental window during leaf emergence. If the threshold is surpassed, a chlorotic region will form. If the concentration of this signal remains below the threshold, the tissue will mature to form normal green tissue. Vascular tissue may act to attenuate, export, or detoxify this signal, thus drastically reducing its concentration when large or intermediate veins are encountered. This may explain the observation that most chlorotic-green region boundaries occur at these veins.
Retention of carbohydrates and reduction in photosynthetic capacity are associated with a significant decrease in vegetative and reproductive growth in psc mutants. A decrease in vegetative growth is manifested as a reduction in plant height, whereas a decrease in reproductive growth is observed as reduced tassel size and branching, ear length, and time to flowering. Kernel number and mass are also significantly decreased in the psc mutant. These data suggest that Psc may play a role in maintaining a proper balance between carbohydrate assimilation, compartmentation, export, transport, or import within the developing maize plant.
The hyperaccumulation of carbohydrates within the chlorotic leaf regions may be caused by the import of carbohydrates from the surrounding green tissue. Alternatively, psc chlorotic tissue may result from a severe defect in carbon export. Support for the second possibility comes from the following observation: psc chlorotic tissue located directly adjacent to green tissue displays a reduced starch hyperaccumulation phenotype compared to psc chlorotic tissue further from the vein boundary. This starch accumulation pattern is the opposite of what would be predicted if psc chlorotic regions were importing carbohydrates from green tissues. The gradient in starch levels can be explained by a compensatory export affect from the green tissue, which presumably maintains normal export function and thereby siphons excess carbohydrates from the chlorotic tissue at the boundary. These data suggest that psc chlorotic tissues are impaired in carbohydrate transport, resulting in the overaccumulation of starch and sugars. Hence, we propose that Psc functions to promote carbohydrate export from maize leaves.
Although the psc mutant phenotype resembles those of the sxd1 and tdy mutants in displaying leaf variegation and carbohydrate retention, it is distinct from them in several ways. For instance, in sxd1 mutant leaves, the phenotype becomes progressively more chlorotic as the leaf ages (Russin et al. 1996), leading to premature leaf senescence, which is not observed in the psc mutants. Additionally, in sxd1 leaves, carbon export is blocked by ectopic callose deposition over the plasmodesmata at the bundle sheath–vascular parenchyma cell interface of minor veins (Botha et al. 2000), a phenotype not detected in psc mutants. An additive genetic interaction between psc and sxd1 suggests that Psc acts in a distinct pathway contributing to carbohydrate partitioning in maize leaves.
Both the psc and tdy mutants require high light to induce leaf chlorosis; however, the leaf variegation patterns are distinct. In contrast to the tdy mutants, psc mutants chlorotic regions appear longitudinally streaked and tend to be located near the leaf margins. These differences suggest that the psc and tdy mutant phenotypes are caused by divergent processes, such as different sensitivities to developmental timing, position within the leaf, light perception, signal transduction, and/or response. This hypothesis is supported by the variegation phenotype observed in psc; tdy double mutant leaves, wherein psc and tdy chlorotic tissues form independently of each other. Therefore, these data are consistent with the assertion that Psc and the Tdy genes function in separate genetic pathways.
Additive genetic interactions between psc and the tdy mutants in leaf variegation, plant growth, and reproductive traits suggest that Psc function is unrelated to the Tdy pathway. However, it cannot be excluded that psc is a hypomorphic rather than null mutation. If so, it could complicate the interpretation of the genetic interaction experiments. One method to determine the nature of mutant alleles in maize is to perform a dosage analysis (Muller 1932; Birchler 1994); however, the duplicate-factor recessive inheritance of the psc mutation hampers such an analysis. Nonetheless, our characterization of the psc mutant phenotype identifies a new genetic function that when disrupted leads to carbohydrate partitioning defects in maize.
In conclusion, characterization of the psc mutant phenotype and genetic analyses with similar carbohydrate accumulating mutants of maize suggest that the wild-type Psc genes act independently of previously identified genetic pathways. Future work to clone and characterize the Psc genes will elucidate how they redundantly function and contribute to the complex regulation of carbohydrate partitioning in higher plants.
Acknowledgments
We thank Tony Omeis and Scott Harkcom for excellent plant care and Sally Assmann for the use of the LICOR 6400. We thank two reviewers for improvements and the members of the Braun and McSteen labs for discussion of the data and comments on the manuscript. This work was supported by the National Research Initiative of the United States Department of Agriculture Cooperative State Research, Education and Extension Service, grant number 2008-35304-04597 (to D.M.B.).
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.113357/DC1.
References
- Allison, J. C. S., and H. Weinmann, 1970. Effect of absence of developing grain on carbohydrate content and senescence of maize leaves. Plant Physiol. 46 435–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, R. F., and D. M. Braun, 2007. tie-dyed1 functions non-cell autonomously to control carbohydrate accumulation in maize leaves. Plant Physiol. 144 867–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, R. F., and D. M. Braun, 2008. tie-dyed2 functions with tie-dyed1 to promote carbohydrate export from maize leaves. Plant Physiol. 146 1085–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler, J., 1994. Dosage analysis using B-A translocations, pp. 328–329 in The Maize Handbook, edited by M. Freeling and V. Walbot. Springer-Verlag, New York.
- Blauth, S., Y. Yao, J. Klucinec, J. Shannon, D. Thompson et al., 2001. Identification of Mutator insertional mutants of starch-branching enzyme 2a in corn. Plant Physiol. 125 1396–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha, C. E. J., R. H. M. Cross, A. J. E. van Bel and C. I. Peter, 2000. Phloem loading in the sucrose-export-defective (SXD-1) mutant maize is limited by callose deposition at plasmodesmata in bundle sheath-vascular parenchyma interface. Protoplasma 214 65–72. [Google Scholar]
- Braun, D. M., Y. Ma, N. Inada, M. G. Muszynski and R. F. Baker, 2006. tie-dyed1 regulates carbohydrate accumulation in maize leaves. Plant Physiol. 142 1511–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun, D. M., and T. L. Slewinski, 2009. Genetic control of carbon partitioning in grasses: roles of Sucrose Transporters and Tie-dyed loci in phloem loading. Plant Physiol. 149 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalker-Scott, L., 1999. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 70 1–9. [Google Scholar]
- Cheng, W. H., E. W. Taliercio and P. S. Chourey, 1996. The Miniature1 seed locus of maize encodes a cell wall invertase required for normal development of endosperm and maternal cells in the pedicel. Plant Cell 8 971–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou, T. J., and D. R. Bush, 1998. Sucrose is a signal molecule in assimilate partitioning. Proc. Natl. Acad. Sci. USA 95 4784–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe, E. H., S. M. McCormick and S. A. Modena, 1981. White pollen in maize. J. Hered. 72 318–320. [Google Scholar]
- Dinges, J. R., C. Colleoni, M. G. James and A. M. Myers, 2003. Mutational analysis of the pullulanase-type debranching enzyme of maize indicates multiple functions in starch metabolism. Plant Cell 15 666–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, G. E., V. R. Franceschi, M. S. B. Ku, E. V. Voznesenskaya, V. I. Pyankov et al., 2001. Compartmentation of photosynthesis in cells and tissues of C4 plants. J. Exp. Bot. 52 577–590. [PubMed] [Google Scholar]
- Esau, K., 1977. Anatomy of Seed Plants. John Wiley & Sons, New York.
- Evert, R. F., 1980. Vascular anatomy of angiospermous leaves, with special consideration of the maize leaf. Ber. Deutsch. Bot. Ges. 93 43–55. [Google Scholar]
- Evert, R. F., W. Eschrich and W. Heyser, 1978. Leaf structure in relation to solute transport and phloem loading in Zea mays L. Planta 138 279–294. [DOI] [PubMed] [Google Scholar]
- Evert, R. F., W. A. Russin and A. M. Bosabalidis, 1996. Anatomical and ultrastructural changes associated with sink-to-source transition in developing maize leaves. Int. J. Plant Sci. 157 247–261. [Google Scholar]
- Fritz, E., R. F. Evert and W. Heyser, 1983. Microautoradiographic studies of phloem loading and transport in the leaf of Zea mays L. Planta 159 193–206. [DOI] [PubMed] [Google Scholar]
- Fritz, E., R. F. Evert and H. Nasse, 1989. Loading and transport of assimilates in different maize leaf bundles: digital image analysis of 14C microautoradiographs. Planta 178 1–9. [DOI] [PubMed] [Google Scholar]
- Gaut, B. S., and J. F. Doebley, 1997. DNA sequence evidence for the segmental allotetraploid origin of maize. Proc. Natl. Acad. Sci. USA 94 6809–6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt, E. E., and S. C. Huber, 1992. Regulation of photosynthesis by end-product accumulation in leaves of plants storing starch, sucrose, and hexose sugars. Plant Physiol. 99 1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah, L. C., 2005. Starch synthesis in the maize endosperm. Maydica 50 497–506. [Google Scholar]
- Heyser, W., 1980. Phloem loading in the maize leaf. Ber. Deutsch. Bot. Ges. 93 221–228. [Google Scholar]
- Heyser, W., R. F. Evert, E. Fritz and W. Eschrich, 1978. Sucrose in the free space of translocating maize leaf bundles. Plant Physiol. 62 491–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstra, G., and C. Nelson, 1969. The translocation of photosynthetically assimilated 14C in corn. Can. J. Bot. 47 1435–1442. [Google Scholar]
- Huang, M., T. L. Slewinski, R. F. Baker, D. Janick-Buckner, B. Buckner et al., 2009. Camouflage patterning in maize leaves results from a defect in porphobilinogen deaminase. Mol. Plant 2 773–789. [DOI] [PubMed] [Google Scholar]
- Jeannette, E., A. Reyss, N. Gregory, P. Gantet and J. L. Prioul, 2000. Carbohydrate metabolism in a heat-girdled maize source leaf. Plant Cell Environ. 23 61–69. [Google Scholar]
- Kalt-Torres, W., P. S. Kerr, H. Usuda and S. C. Huber, 1987. Diurnal changes in maize leaf photosynthesis: I. Carbon exchange rate, assimilate export rate, and enzyme activities. Plant Physiol. 83 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, K. E., 1996. Carbohydrate-modulated gene expression in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47 509–540. [DOI] [PubMed] [Google Scholar]
- Krapp, A., and M. Stitt, 1995. An evaluation of direct and indirect mechanisms for the “sink-regulation” of photosynthesis in spinach: changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta 195 313–323. [Google Scholar]
- Lalonde, S., D. Wipf and W. B. Frommer, 2004. Transport mechanisms for organic forms of carbon and nitrogen between source and sink. Annu. Rev. Plant Biol. 55 341–372. [DOI] [PubMed] [Google Scholar]
- Langham, R. J., J. Walsh, M. Dunn, C. Ko, S. A. Goff et al., 2004. Genomic duplication, fractionation and the origin of regulatory novelty. Genetics 166 935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y., and T. D. Sharkey, 2004. The role of amylomaltase in maltose metabolism in the cytosol of photosynthetic cells. Planta 218 466–473. [DOI] [PubMed] [Google Scholar]
- Lunn, J. E., and R. T. Furbank, 1999. Tansley Review No. 105. Sucrose biosynthesis in C4 plants. New Phytol. 143 221–237. [Google Scholar]
- Lynch, M., and A. Force, 2000. The probability of duplicate gene preservation by subfunctionalization. Genetics 154 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y., R. F. Baker, M. Magallanes-Lundback, D. DellaPenna and D. M. Braun, 2008. Tie-dyed1 and Sucrose export defective1 act independently to promote carbohydrate export from maize leaves. Planta 227 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y., T. L. Slewinski, R. F. Baker and D. M. Braun, 2009. Tie-dyed1 encodes a novel, phloem-expressed transmembrane protein that functions in carbohydrate partitioning. Plant Physiol. 149 181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena, M., B. A. Ambrose, R. B. Meeley, S. P. Briggs, M. F. Yanofsky et al., 1996. Diversification of C-function activity in maize flower development. Science 274 1537–1540. [DOI] [PubMed] [Google Scholar]
- Moore, B., L. Zhou, F. Rolland, Q. Hall, W. H. Cheng et al., 2003. Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300 332–336. [DOI] [PubMed] [Google Scholar]
- Muller, H. J., 1932. Further studies on the nature and causes of gene mutations. Proceedings of the 6th International Congress of Genetics, Ithaca, NY, Vol. 1, pp. 213–255.
- Niittyla, T., G. Messerli, M. Trevisan, J. Chen, A. M. Smith et al., 2004. A previously unknown maltose transporter essential for starch degradation in leaves. Science 303 87–89. [DOI] [PubMed] [Google Scholar]
- Nolte, K. D., and K. E. Koch, 1993. Companion-cell specific localization of sucrose synthase in zones of phloem loading and unloading. Plant Physiol. 101 899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poethig, R. S., and E. J. Szymkowiak, 1995. Clonal analysis of leaf development in maize. Maydica 40 67–76. [Google Scholar]
- Provencher, L. M., L. Miao, N. Sinha and W. J. Lucas, 2001. Sucrose export defective1 encodes a novel protein implicated in chloroplast-to-nucleus signaling. Plant Cell 13 1127–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades, M., and A. Carvalho, 1944. The function and structure of the parenchyma sheath plastids of the maize leaf. Bull. Torrey Bot. Club 71 335–346. [Google Scholar]
- Rhoades, M. M., 1951. Duplicate genes in maize. Am. Nat. 85 105–110. [Google Scholar]
- Russell, S. H., and R. F. Evert, 1985. Leaf vasculature in Zea mays L. Planta 164 448–458. [DOI] [PubMed] [Google Scholar]
- Russin, W. A., R. F. Evert, P. J. Vanderveer, T. D. Sharkey and S. P. Briggs, 1996. Modification of a specific class of plasmodesmata and loss of sucrose export ability in the sucrose export defective1 maize mutant. Plant Cell 8 645–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzin, S., 1999. Plant Microtechnique and Microscopy. Oxford University Press, New York.
- Sattler, S. E., E. B. Cahoon, S. J. Coughlan and D. DellaPenna, 2003. Characterization of tocopherol cyclases from higher plants and cyanobacteria. Evolutionary implications for tocopherol synthesis and function. Plant Physiol. 132 2184–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer, N., 2007. Molecular physiology of higher plant sucrose transporters. FEBS Lett. 581 2309–2317. [DOI] [PubMed] [Google Scholar]
- Scanlon, M. J., R. G. Schneeberger and M. Freeling, 1996. The maize mutant narrow sheath fails to establish leaf margin identity in a meristematic domain. Development 122 1683–1691. [DOI] [PubMed] [Google Scholar]
- Schnable, P. S., D. Ware, R. S. Fulton, J. C. Stein, F. Wei et al., 2009. The B73 maize genome: complexity, diversity, and dynamics. Science 326 1112–1115. [DOI] [PubMed] [Google Scholar]
- Sheen, J., 1990. Metabolic repression of transcription in higher plants. Plant Cell 2 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen, J., 1994. Feedback-control of gene-expression. Photosynth. Res. 39 427–438. [DOI] [PubMed] [Google Scholar]
- Slewinski, T. L., and D. M. Braun, 2010. Current perspectives on the regulation of whole-plant carbohydrate partitioning. Plant Sci. 178 10.1016/j.plantsci.2010.1001.1010.
- Slewinski, T. L., Y. Ma, R. F. Baker, M. Huang, R. Meeley et al., 2008. Determining the role of Tie-dyed1 in starch metabolism: epistasis analysis with a maize ADP-glucose pyrophosphorylase mutant lacking leaf starch. J. Hered. 99 661–666. [DOI] [PubMed] [Google Scholar]
- Slewinski, T. L., R. Meeley and D. M. Braun, 2009. Sucrose transporter1 functions in phloem loading in maize leaves. J. Exp. Bot. 60 881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, A. M., and M. Stitt, 2007. Coordination of carbon supply and plant growth. Plant Cell Environ. 30 1126–1149. [DOI] [PubMed] [Google Scholar]
- Springer, N. M., K. Ying, Y. Fu, T. Ji, C.-T. Yeh et al., 2009. Maize inbreds exhibit high levels of copy number variation (CNV) and presence/absence variation (PAV) in genome content. PLoS Genet. 5 e1000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava, A. C., S. Ganesan, I. O. Ismail and B. G. Ayre, 2008. Functional characterization of the Arabidopsis thaliana AtSUC2 Suc/H+ symporter by tissue-specific complementation reveals an essential role in phloem loading but not in long-distance transport. Plant Physiol. 147 200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt, M., R. M. Lilley, R. Gerhardt and H. W. Heldt, 1989. Metabolite levels in specific cells and subcellular compartments of plant leaves. Methods Enzymol. 174 518–552. [Google Scholar]
- Turgeon, R., 2006. Phloem loading: how leaves gain their independence. BioScience 56 15–24. [Google Scholar]
- Wardlaw, I. F., 1990. Tansley Review No. 27. The control of carbon partitioning in plants. New Phytol. 116 341–381. [DOI] [PubMed] [Google Scholar]
- Whitt, S. R., L. M. Wilson, M. I. Tenaillon, B. S. Gaut and E. S. Buckler, 2002. Genetic diversity and selection in the maize starch pathway. Proc. Natl. Acad. Sci. USA 99 12959–12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, A. D., C. A. Moehlenkamp, G. H. Perrot, M. G. Neuffer and K. C. Cone, 1992. The maize auxotrophic mutant orange pericarp is defective in duplicate genes for tryptophan synthase beta. Plant Cell 4 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman, S., S. Smith and A. Smith, 2007. The diurnal metabolism of leaf starch. Biochem. J. 401 13–28. [DOI] [PubMed] [Google Scholar]