Abstract
Oxidative modification of LDL is known to elicit an array of pro-atherogenic responses, but it is generally underappreciated that oxidized LDL (OxLDL) exists in multiple forms, characterized by different degrees of oxidation and different mixtures of bioactive components. The variable effects of OxLDL reported in the literature can be attributed in large part to the heterogeneous nature of the preparations employed. In this review, we first describe the various subclasses and molecular composition of OxLDL, including the variety of minimally modified LDL preparations. We then describe multiple receptors that recognize various species of OxLDL and discuss the mechanisms responsible for the recognition by specific receptors. Furthermore, we discuss the contentious issues such as the nature of OxLDL in vivo and the physiological oxidizing agents, whether oxidation of LDL is a prerequisite for atherogenesis, whether OxLDL is the major source of lipids in foam cells, whether in some cases it actually induces cholesterol depletion, and finally the Janus-like nature of OxLDL in having both pro- and anti-inflammatory effects. Lastly, we extend our review to discuss the role of LDL oxidation in diseases other than atherosclerosis, including diabetes mellitus, and several autoimmune diseases, such as lupus erythematosus, anti-phospholipid syndrome, and rheumatoid arthritis. Antioxid. Redox Signal. 13, 39–75.
I. Introduction
There is overwhelming evidence that LDL is oxidatively modified in vivo, and that this modification results in an increase in its proinflammatory and proatherogenic properties. However, despite extensive studies over the last 3 decades from numerous laboratories, the sites of LDL oxidation in vivo, the nature of the physiological oxidizing agents, the nature and composition of oxidized LDL in circulation, and the pathophysiological relevance of LDL oxidation for atherosclerosis and other diseases are all matters of controversy. Because of the heterogeneity of the oxidized LDL preparations, whether prepared in vitro or isolated from the natural sources, there is no consensus on the exact definition or composition of oxidized LDL. In this review, we will briefly summarize the biochemistry and composition of the various preparations of oxidized LDL described in the literature, and discuss their pathophysiological properties and potential therapeutic implications. Special attention will be paid to the relationship between the extent of LDL modification and its biological effects, the specific actions of the bioactive components of oxidized LDL, and the controversial aspects of the role of oxidatively modified LDL in cholesterol loading and atherogenesis. The reader is referred to several excellent articles on the historical aspects of LDL oxidation hypothesis (269, 302, 303), mechanisms of oxidation, composition of oxidized LDL preparations, immunoassays for oxidized LDL (38, 284), clinical trials of antioxidant drugs, and studies with experimental models of atherosclerosis (33, 146, 164, 191, 240, 263, 280).
II. Definitions, Biochemistry, and Composition
The term “oxidized LDL” is used to describe a wide variety of LDL preparations that have been oxidatively modified ex vivo under defined conditions, or isolated from biological sources. The major problem in comparing the results of oxidized LDL studies from various laboratories is the heterogeneity of the preparations employed. There is no accepted ‘gold standard’ for preparing oxidized LDL ex vivo, and the preparations isolated from the tissues differ greatly from lab to lab, both in the composition and biological effects. The oxidized LDL preparations described in literature are broadly (and somewhat arbitrarily) divided into two main categories: “minimally modified LDL” (MM-LDL) and “(fully or extensively) oxidized” LDL (OxLDL). The major difference between the two groups is that the MM-LDL, while chemically different from unmodified LDL, is still recognized by the LDL receptor, but not by most of the known scavenger receptors. On the other hand, the OxLDL preparations are all recognized by a variety of scavenger receptors but not by the LDL receptor. Each of the two categories of oxidized LDL is composed of an array of preparations that differ widely from each other in composition and biological effects. As to be expected, the type of oxidizing agent used and the conditions of oxidation of LDL determine the chemical and biological properties of OxLDL. Unfortunately, most studies do not report the detailed composition of OxLDL used, or even the exact conditions of LDL oxidation, which complicates the comparison of their biological effects. Even when identical conditions are used to oxidize the LDL ex vivo, the products could differ significantly, depending upon the fatty acid composition and antioxidant status of the starting LDL preparation.
A. Minimally modified LDL
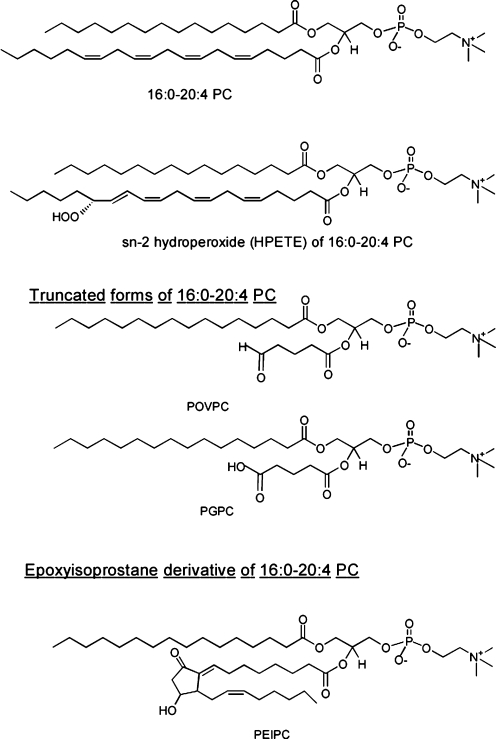
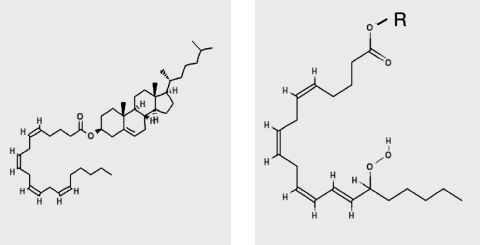
Minimally modified LDL (MM-LDL) is a general term used to describe a variety of LDL preparations that are sufficiently modified to be chemically distinguished from unmodified LDL, but retain the ability to bind to LDL receptor, are not recognized by most scavenger receptors, and have distinct biological activity not shown by unmodified LDL, such as the induction of chemotactic or pro-inflammatory proteins by endothelial cells and macrophages. Since the MM-LDL have been prepared by a wide range of methods, they also differ significantly from each other in their chemical and biological properties (Table 1). Furthermore, since LDL itself is composed of several distinct subfractions that differ in density, size, composition, and antioxidant levels, the oxidation of total LDL gives rise to a mixture of OxLDL species even under controlled conditions. The ‘average’ LDL particle has been calculated to contain 600 molecules of free cholesterol, 1600 molecules of cholesteryl ester, 700 molecules of phospholipid (64% PC, 1.5% PE, 26% SM, and 11% LPC), 180 molecules of TG, and 1 molecule of ApoB (124). In addition, varying amounts of antioxidants (α tocopherol, γ tocopherol, ubiquinol, lycopene, β carotene) are present in the LDL particles (257). Although there are several oxidizable components in LDL, the polyunsaturated fatty acids (mostly arachidonic acid and linoleic acid) of LDL lipids are the major targets of oxidizing agents. The first detectable product of lipid oxidation is the hydroperoxy derivative of a phospholipid (Fig. 1). This also results in the rearrangement of double bonds to form conjugated dienes that are conveniently detected by an increase in absorbance at 235 nm (A235). Further oxidation results in the truncation of sn-2 acyl chain, forming short-chain aldehyde or carboxy derivatives. The aldehydes may form adducts with the lysine residues of apo B, either before or after hydrolysis from the phospholipids by phospholipase A2. HNE (4-hydroxynonenal) is one of the most abundant aldehydes in oxidized LDL, which derivatizes thiols and free amino groups of LDL Apo B and cellular proteins.
Table 1.
Minimally Modified LDL Preparations and their Properties
| Method | References | Composition reported | Receptor binding | Comments |
|---|---|---|---|---|
| Storage of LDL at 4°C in dark for 3–6 Months | (7, 20) | 3 nmol TBARS 6 nmol Chol expoxide and 2 nmol peroxide/ mg Chol | LDL receptor | No increase in conjugated diene; no change in electrophoretic mobility |
| Treat LDL with 1 μM FeSO4 for 96 h or 0.5 μM FeSO4 at RT for 48 h | (20, 119) | 5–10 nmol TBARS/ mg chol; POVPC and PGPC formation | LDL receptor | Increase in conjugated dienes; Reacts with DLH3 antibody |
| Treat LDL with 15-LPO expressing cells | (30, 260) | 12.6 nmol TBARS/mg prot; 7% loss of 18:2; mild loss of protein | LDL receptor, CD-14 | |
| Lipoxygenase treatment | (93) | Oxygenated phospholipids and cholesteryl esters | Macrophage activation | |
| Subject LDL to hemoglobin treatment under hypoxia | (16) | Negative charge; stimulates cell proliferation | ||
| Limited Cu2+oxidation of LDL | (21) | 2.3 nmol TBARS/mg? | LDL receptor | Inhibits LCAT |
| LDL isolated from plasma | (252) | 4.6 nmol TBARS/ mg Chol; enriched in oxysterols and lipid hydroperoxides | Negatively charged | |
| HOCl modification of LDL (myeloperoxidase) | (185, 304) | Increased lipid hydroperoxide, no increase in TBARS; no loss of vitamin E; | Negatively charged |
FIG. 1.
Structures of PAPC (16:0-20:4 PC) and selected oxidation products. See text for abbreviations.
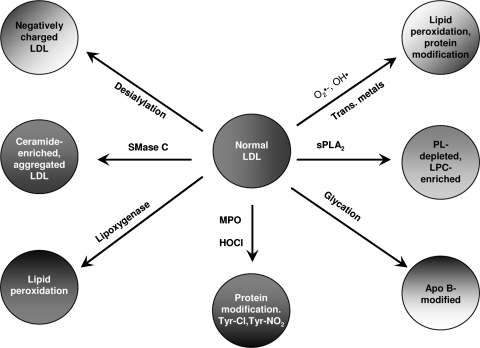
Malondialdehyde (MDA), another prominent aldehyde product of lipid peroxidation, as well as of eicosanoid metabolism, can also form adducts with the lysine residues of Apo B. MDA-modified LDL has also been isolated and characterized from the plasma of patients with coronary heart disease (105). The modification of the protein results in alteration of the electrophoretic mobility, as well as the biological properties of LDL. Apo B can also be oxidized directly by the oxidizing agents such as HOCl generated by myeloperoxidase (96) without the need for the aldehydes produced from lipid peroxidation. In this case, Apo B is predominantly modified at the tyrosine residues. LDL can also be directly modified by various enzymes such as phospholipases, sphingomyelinase, and lipoxygenase to give rise to products that are atherogenic. The various types of MM-LDL that may be formed in vivo are shown in Figure 2.
FIG. 2.
Potential pathways of MM-LDL formation in vivo. The physiological modification of LDL takes place by a variety of reactions, both enzymatic and nonenzymatic. The products of all these reactions can be rightfully designated as MM-LDL, although the oxidation may not be the primary event in many of these modifications. Lipid peroxidation is the primary reaction only in the lipoxygenase and free radical mediated pathways. Hydrolysis of SM by secretory SMase C may occur in acute phase response when the SMase level is increased in plasma (305) or by the putative SMase intrinsic to LDL (103). The hydrolysis of LDL SM to ceramide increases the oxidative susceptibility of LDL (271), and also results in the formation of aggregated LDL (249) that is superior to OxLDL in loading of macrophages with cholesterol. The action of sPLA2 on LDL produces an LPC-enriched LDL that should have strong chemotactic and pro-inflammatory effects. PAF acetylhydrolase (Lp-PLA2) may be responsible for the hydrolysis of oxidatively truncated PC in LDL, releasing the cytotoxic aldehydes in addition to LPC. Desialylated LDL has been shown to be present in circulation (279), and is formed either by the action of sialidase or by free radical mediated reactions. This LDL was shown to promote foam cell formation. Glycation of LDL, which is more prevalent in diabetes (80), also increases foam cell formation, and increases the susceptibility of LDL to oxidation. The myeloperoxidase (MPO)-mediated oxidation of LDL results primarily in the modification of tyrosine residues of Apo B (97).
B. Extensively oxidized LDL
When LDL is oxidatively modified to a level where it becomes unrecognizable by the LDL receptor, but instead becomes a ligand for various scavenger receptors, it is categorized as maximally oxidized, fully oxidized, or extensively oxidized LDL, and is referred to as OxLDL in this review. While the MM-LDL contains typically 3–12 nmol of TBARS/ mg Apo B (Table 1), the extensively oxidized LDL may contain over 30 nmol of TBARS/ mg Apo B. The OxLDL preparations described in the literature also encompass a wide range of particles that differ in the lipid composition, protein modification, and degradation, and biological activities. Therefore, in this review we tried to include the extent of LDL oxidation, and the major bioactive molecules responsible for each activity, where such information is available. It should be pointed out that although the extent of LDL oxidation is most often expressed as nmol of TBARS in the sample, the assay of TBARS has many drawbacks (87), and one has to be cautious in comparing the OxLDL samples from different laboratories solely on the basis of TBARS values.
III. What Is the Nature of Oxidized LDL That Occurs In Vivo?
Although there is compelling evidence that oxidation of LDL takes place in vivo, the detailed characterization of naturally occurring OxLDL is technically challenging, and largely remains elusive. Because of the possible artifactual generation of OxLDL during the isolation from tissues or plasma, the true nature of physiologically occurring OxLDL has been difficult to assess. There is convincing evidence that OxLDL is present in the atherosclerotic lesions of both humans and experimental animals (302, 310), although its role in the initiation and development of the lesion is still a matter of contention. It is generally accepted that the oxidation of LDL occurs mostly in the subendothelial space of the arteries, not in the circulation. It should be pointed out that the extensively oxidized LDL may have a very short half life in the plasma because it is likely to be cleared rapidly from the circulation by the reticuloendothelial system. However, small but significant amounts of oxidized LDL (predominantly the MM-LDL) are immunologically detectable in normal plasma, and are increased significantly in several disease states, including coronary heart disease, diabetes, and renal disease (119, 284). The detection of oxidized LDL in the plasma has been facilitated by the development of monoclonal antibodies (mAbs) specific for the epitopes of oxidized Apo B or oxidized lipids bound to Apo B. The three well-established mAbs used for the immunoassays of oxidized LDL are: a) FOH1a/DLH3, which was generated by immunizing mice against human coronary atheroma, and which recognizes the phosphorylcholine moiety of oxidized PC, but not of normal, PC (120); b) 4E6, which was generated by immunizing mice with Cu2+-oxidized LDL, and which recognizes the MDA-modified lysine epitopes of Apo B (104); and c) E06, which was established from the B cell clones of nonimmunized Apo E-deficient mice, and also recognizes the phosphorylcholine moiety of oxidized but not normal PC (213). Another line of evidence for the existence of OxLDL in the plasma is the presence of autoantibodies for oxidized LDL and their correlation with heart disease (181).
While the immunological methods are useful analytical tools for screening large populations, and for distinguishing between normal and disease states, they do not provide structural and compositional information of the whole oxidized LDL particles present in vivo. Although oxidized LDL isolated from atherosclerotic lesions has been studied in several laboratories, it may represent a more highly oxidized form that has been further modified by other components of the lesion, and is therefore not the oxidized LDL involved in lesion formation. Ideally, one needs to isolate oxidized LDL from the plasma under mild conditions that do not generate artifacts, and characterize the particles by physicochemical and biochemical methods and by proteomic analysis. An electronegative LDL (also termed LDL- or L5) has been isolated from the plasma by ion exchange chromatography and ultracentrifugation by several laboratories, and appears to have several features of oxidized LDL, including low vitamin E content, reduced affinity to LDL receptor, binding to LOX-1, and pro-inflammatory, pro-apoptotic, and cytotoxic effects on cells (174, 245). This sub-fraction of LDL, which comprises about 1% of total LDL in normal plasma, is significantly increased in hyperlipidemia and diabetes. The negative charge of this LDL fraction (L5) could be due to nonoxidative modifications, perhaps increased free fatty acid content (72), and this preparation may represent a mixture of LDL species modified by different pathways, rather than by oxidation alone (245). Nevertheless, a recent study by Lu et al. (2009) showed that both L5 and OxLDL (generated by Cu2+oxidation of LDL) induced LOX-1 in endothelial cells and competed for uptake by this receptor (175). Holvoet et al. (105) isolated and characterized a modified form of LDL from the plasma of patients with acute myocardial infarction using gel filtration and ion exchange chromatography. This form of LDL (which was increased by 7-fold in the patients, compared to controls) had a higher cholesterol/protein ratio, a 50% decrease in arachidonate content, and 192 blocked lysines (compared to 7 in normal LDL). These characteristics, coupled with its ability to generate foam cells in vitro, and its high immunoreactivity with MDA-antibody suggest that it is equivalent to the in vitro-generated MDA-LDL, although no LPC was detected in the sample. Its possible overlap with the electronegative LDL described above has not been investigated.
IV. What Are the Physiological Oxidizing Agents In Vivo?
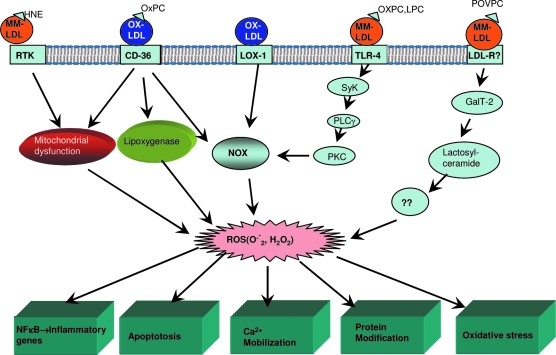
It is generally accepted that very little oxidation of LDL takes place in the circulation because of the abundance of antioxidants, such as tocopherol, ascorbate, urate, apolipoproteins, and serum albumin. Instead, the bulk of LDL oxidation takes place in the subendothelial space of arterial wall, where LDL may be sequestered by the proteoglycans, and where the relative concentration of antioxidants is much lower than in plasma. Wen and Leake (299) demonstrated that LDL can also be oxidized intracellularly, most probably in the lysosomal compartment of macrophages. LDL oxidation also could take place at the sites of inflammation because of the infiltration of neutrophils and monocytes/macrophages, and because of the increased vascular permeability and consequent increase in LDL concentration in the tissues at the sites of inflammation. The arterial wall cells generate both free radicals and nonradical oxidants through various enzymatic mechanisms. The free radicals produced by the cells include superoxide, hydroxyl radicals, carbon-center radicals, nitric oxide, and thiyl and perthiyl radicals. Although free transition metals are commonly used for the in vitro oxidation of LDL, their role in the physiological oxidation of LDL is controversial because significant amounts of free iron or copper are not found in vivo. However, the iron-containing proteins (e.g., ferritin, transferrin, hemoglobin, myoglobin) and copper-containing proteins (e.g., ceruloplasmin) have been shown to oxidize LDL in vitro and therefore may be physiologically relevant in the generation of OxLDL in vivo (64). Furthermore, free iron can be released from ferritin following its reduction to ferrous state by SOD (51) and one of the 7 copper atoms bound to ceruloplasmin is exchangeable with chelators (64). The free radicals oxidize preferentially the polyunsaturated fatty acids, whose breakdown products would ultimately derivatize Apo B and alter its receptor recognition. The nonradical oxidants that tend to modify the proteins directly (especially the cysteine, methionine and tyrosine) include H2O2, hypochlorite, and peroxynitrite. The oxidants in the vessel wall are generated by the actions of NADPH oxidase (NOX), xanthine oxidase, NO synthase, myeloperoxidase, and lipoxygenase, all of which have been shown to be present in the atherosclerotic lesions (268). It should be pointed out that the various oxidizing agents do not act in isolation, but in fact a consecutive action of several agents and enzymes is more likely to be involved in the generation of fully oxidized LDL in vivo.
V. Bioactive Compounds in OxLDL
The biological activities of the MM-LDL and OxLDL are due to the numerous new compounds generated by the oxidative modification of LDL. Some of the biological effects of OxLDL can be attributed to individual components while others are due to the combined effects of various compounds of the OxLDL particle. The various constituents of OxLDL known to exert biological effects are shown in Table 2.
Table 2.
Bioactive Components of OxLDL
| Compound | Biological effects | References |
|---|---|---|
| Phospholipid products | ||
| sn-2 short chain PAPC products (POVPC, PGPC, | Monocyte adhesion, induction of IL-8, activation of SREBP CD-36 ligands | (309) |
| sn-2 epoxy products (PEIPC, PECPC) | Monocyte binding, induction of MCP-1, IL-8 | (228) |
| sn-2 acyl hydroxy and hydroperoxy products | ?? | (273, 297) |
| LPC | Chemotactic to monocytes, upregulation of cytokines, adhesion molecules | (180) |
| LPA | Platelet activation, mitogenic effect, PPAR activation | (258) |
| PAF-like (sn-1 ether) products | Platelet aggregation, monocyte activation | (136) |
| Sphingolipid products | ||
| Ceramide, sphingosine, sphingosine phosphate | LDL aggregation | (249) |
| Mitogenesis of SMC | (11) | |
| Free fatty acid products | ||
| HODE, HPODE, HETE, HPETE Isoprostanes | PPAR activation; G2A ligands; monocytes adhesion; Inhibition of superoxide production (15 HETE) | (210, 301) |
| Free aldehydes MDA, HNE | Induction of COX-2, MCP-1, TGFβ1 | (131, 160) |
| Oxysterols | ||
| 7-keto, 7αOH, 24OH, 25OH, 27OH chol. Cholesteryl ester hydroperoxides | Inhibition of sterol synthesis and sterol efflux | (33) |
| Macrophage activation | (93) | |
| Apo B modification | ||
| Lysine adducts, tyrosine adducts, cysteine adducts | Antigenicity, scavenger receptor recognition, loss of LDL receptor recognition | (115) |
A. Phospholipid products
1. Lysophospholipid products
One of the first components of OxLDL to be shown to have specific biological effects on cells is lysophosphatidylcholine (LPC), which is also present in normal LDL, but at a lower concentration. LPC is generated by three different pathways in the plasma. Large amounts (about 120 nmol/h/ml) of LPC are generated continuously in the plasma by the action of LCAT, the enzyme responsible for esterification of cholesterol in plasma (74). This LPC is mostly carried by serum albumin and is delivered to liver and other tissues for further metabolism. A small but significant percentage of it is, however, found in the LDL fraction. The majority of LPC present in OxLDL, on the other hand, is formed from the actions of Ca2+-independent lipoprotein-associated PLA2 (Lp-PLA2, also called the PAF acetyl hydrolase), which efficiently hydrolyzes the oxidized acyl groups from the sn-2 position of phospholipids, in addition to PAF. Interestingly, the electronegative LDL fraction (LDL-) described above is enriched with this enzyme, and may be responsible for the generation of LPC in OxLDL in vivo. Another source of LPC in the lipoproteins is the action of Ca2+-dependent group IIa sPLA2, which hydrolyzes long-chain PCs. However, the activity of this enzyme in normal plasma is very low, and its contribution to plasma LPC is unknown. It has been shown, nevertheless, that transgenic mice overexpressing sPLA2 IIa develop more atherosclerosis (281), although the noncatalytic effects of the enzyme could be involved in atherosclerosis. The sPLA2-treated LDL is also more susceptible to oxidation by endothelial cells (158). It is also possible that the enzyme hydrolyzes oxidized PC better than normal PC. In addition to sPLA2 IIa, plasma and several tissues contain group V and group X sPLA2, which are more efficient in the hydrolysis of PC to LPC (73, 195, 261). LPC is a chemotactic agent for monocytes, and therefore helps recruit more circulating monocytes into the arterial wall (229). Other pro-inflammatory effects of LPC include the stimulation of superoxide generation, stimulation of inflammatory cytokines including IL-1β and IL-8 by monocyte/macrophages, inhibition of endothelium-dependent arterial relaxation, upregulation of adhesion molecule synthesis by endothelial cells, upregulation of IL-2 and interferon γ synthesis by the lymphocytes, and stimulation of smooth muscle proliferation (180). More recently, Hara et al. (91) showed that LPC upregulates the OxLDL receptor LOX-1, chemokine receptors, and several activation related transcription factors in human T-lymphocyte cell lines. Thus LPC appears to affect all the cells involved in inflammation and atherosclerosis, and contributes to all stages of atherosclerosis. Interestingly LPC has also been reported to have some anti-atherogenic effects such as promotion of cholesterol efflux and Apo E secretion from the macrophage foam cells (90).
Another bioactive lysophospholipid that is present in OxLDL is lysophosphatidic acid (LPA). This compound is generated from LPC by the action lysophospholipase D (autotaxin) (290), and is a well-known mitogen that acts through specific G-protein coupled receptors. Seiss et al. (257, 259) demonstrated the accumulation of LPA during the oxidation of LDL, as well as in atherosclerotic lesions, and identified it as the factor responsible for platelet activation by OxLDL, although the receptor responsible for this effect remains elusive. The sn-1 alkyl analogs of LPA were shown to be more prevalent in the mildly oxidized LDL, and are 20 times more potent than the acyl analogs in platelet activation (257). In addition to promoting chemokine expression by endothelial cells, LPA stimulates the uptake of OxLDL itself through upregulation of scavenger receptor A in macrophages (41), and increases monocytes migration at low concentrations (83). It was also shown to stimulate SMC proliferation through the activation of the transcription factor Egr-1 (48).
2. sn-2 short chain PCs
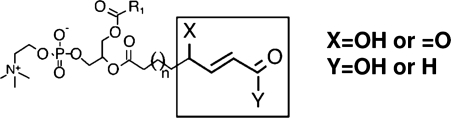
Several breakdown products of 16:0-20:4 PC and 16:0-18:2 PC with truncated sn-2 acyl chain (POVPC, PGPC, PONPC, etc, see Fig. 1) have been identified in OxLDL, and numerous biological activities have been attributed to them (272). It should, however, be noted that because of the propensity of these compounds to react with the functional groups of Apo B and the cellular proteins, some of their effects could be indirect, through the loss or modification of function of the corresponding protein. The ligand-binding properties of OxLDL for scavenger receptors such as CD36 can be attributed largely to the truncated OxPC products, with part of the effect due to their protein adducts, and the rest due to the free compounds (94). The structure-function studies with synthetic phospholipids revealed that CD36 recognition requires a phospholipid with a truncated sn-2 chain that contains a terminal γ-hydroxy, α,β-unsaturated carbonyl (94) (also see Fig. 5). Some of the OxPC products also exhibit anti-inflammatory activities. For example, KOdiAPC and POVPC were shown to inhibit TLR-4 and TLR-2 binding of LPS (296). Specific oxidation products of PAPC also inhibit the SR-B1-mediated selective uptake of CE from HDL, and thus inhibit the reverse cholesterol transport pathway (9).
FIG. 5.
The core structural motif of oxidized phospholipids responsible for OxLDL recognition by CD36 receptors [from (228)]. The structural requirements for OxPC-CD36 interaction were identified by OxLDL lipid extraction, fractionation by reverse phase HPLC, and then testing the ability of different lipids to inhibit the binding of NO2-LDL to HEK293 cells transfected with CD36. Molecular structures for the major biologically active constituents were determined by tandem mass spectrometry [for more detail, see (228)]. The figure shows the core structural motif conserved among different various oxidized PC species that support their binding to CD36.
3. PAF-like products
Phospholipids with sn-1 ether linkage and a short chain acyl group at sn-2 position resemble PAF structurally, and may act through the PAF receptor, or conversely, compete with PAF for the receptor binding and inhibit its action (179). Several PAF-like compounds are formed during the oxidation of LDL, the predominant ones having a 4:0 or 4:1 acyl group at sn-2 (179). They are about 10-fold less potent than PAF in their cellular effects, but because of their relatively high concentration in OxLDL, may contribute significantly to the pro-inflammatory properties of OxLDL. Another PAF analog found in OxLDL, namely the 1-hexadecyl 2-azelaoyl PC (which contains a 9-carbon dicarboxylic acid at sn-2) is known to disrupt mitochondrial function, and was recently shown to be a ligand for the PAF receptor (212).
4. sn-2 epoxy PCs
Isoprostanes are commonly accepted as one of the most reliable markers of oxidative stress in vivo. Most of the isoprostanes in the plasma are, however, not in the free form, but esterified to phospholipids. The phospholipids containing the epoxyisoprostane groups have been shown to exhibit several biological activities. Berliner and colleagues (297) first identified a pro- inflammatory epoxyisoprostane derivative of PAPC (sn-1-16:0-2 (5,6 epoxyisoprostane E2) glycero3 phosphorylcholine or PEIPC) (Fig. 1) which stimulated the adhesion of monocytes to endothelial cells. They also later showed the presence of five PEIPC species and four PECPC species in OxLDL, all of which induced synthesis of IL-8 and MCP-1 by endothelial cells (273). Interestingly, the epoxy isoprostane PCs were shown to be protective of endothelial barrier function (through Rac and Cdc42 signaling), whereas the truncated products of PAPC oxidation were disruptive to the barrier function (301). PEIPC was also shown to activate the prostaglandin receptor subtype EP2 (163), which in turn could increase the cAMP levels in monocytes and consequently downregulate TNFα expression.
B. Sphingolipid products
1. Ceramide
Although the presence of ceramide in OxLDL has not been unequivocally demonstrated, oxidation of LDL is correlated with plasma ceramide levels (115), and the SM of OxLDL is more susceptible to hydrolysis by SMase (81). Conversely, SMase treatment of LDL increases its susceptibility to oxidation (271), and increases the aggregation of LDL and subsequent foam cell formation (249). The reported presence of SMase C activity intrinsic to Apo B (138) is intriguing and could give rise to ceramide. However, this activity appears to be inhibited by LDL oxidation and therefore its role in the generation of ceramide in oxidized LDL is unclear. The ceramide in LDL has also been shown to induce apoptosis in endothelial cells (31).
2. Sphingosine 1-phosphate
Sphingosine 1-phosphate (S1P) is present in the plasma at low concentrations, but is mainly associated with HDL, and its levels actually appear to decrease in LDL during oxidation (212). However, Auge et al. (11) have demonstrated that OxLDL induces the formation of S1P in the cells through the combined activation of SMase C, ceramidase, and sphingosine kinase. Similarly, Hammad et al. (88) reported the extracellular generation of S1P in monocytes incubated with OxLDL-immune complex (IC). Furthermore, platelet activation, which is induced by OxLDL, also results in the release of S1P. Thus OxLDL stimulates the formation of S1P through several pathways, and this could be one mechanism by which OxLDL stimulates smooth muscle cell proliferation. In addition, S1P is known to increase platelet aggregation, and expression of adhesion molecules. However, there is also some evidence for the anti-atherogenic effects of S1P. For example, it attenuates the TLR-2 signaling, specifically the NFκB-driven pathways, resulting in an anti-inflammatory response (58). It also appears to inhibit SMC migration, and stimulates NO production in endothelial cells (212).
C. Free fatty acid products
Fatty acids from sn-2 position of OxPL are released by the action of sPLA2, or Lp-PLA2 (PAF- AH). Furthermore, the various lipoxygenases (LOs) oxidize the free fatty acids (arachidonic and linoleic acids) as well as esterified fatty acids to the hydroperoxy derivatives (69). At least three types of LO, namely 12/15 LO, platelet 12-LO, and 5-LO are implicated in LDL oxidation and vascular remodeling (151). These modified free fatty acids have been shown to be ligands for PPARα and PPARγ. The isoprostane derivatives (free and phospholipid-bound) are reliable markers of oxidative stress in vivo (190). They were also reported to have biological functions such as renal vasoconstrictor activity, through the activation of prostanoid TP receptors (47, 194).
The hydroperoxy derivatives of 18:2 and 20:4 (13(S) HODE, 15(S) HETE) were shown to be 100 times more potent than H2O2 in oxidizing PAPC and producing more pro-inflammatory products (205). On the other hand, some oxidized fatty acids have anti-inflammatory activities. For example, 15 HPETE and 15-HETE inhibit TNFα-induced upregulation of ICAM-1, E-selectin, and VCAM-1 in endothelial cells (109). In addition, 8-HETE stimulates PPARα, while 15-HETE, 9-HODE, and 13-HODE activate PPARγ (199), (108), all of which would be potentially anti-inflammatory. Furthermore, 15-HETE has been shown to inhibit superoxide production and migration of PMN across the endothelium (301). In general, the products of 12/15 LO tend to be pro-inflammatory, while the products of 15 LO exhibit anti-inflammatory properties (301).
D. Oxysterols
The major oxysterols found OxLDL prepared in vitro are 7 keto-cholesterol, 7α-OH and 7β-OH cholesterols, and cholesterol epoxides, while the side-chain oxidation products are minor components (33). The oxysterols identified in the electronegative LDL (LDL-) isolated from plasma include 7α-OH, 7β-OH, 7-keto, and 5,6 epoxy cholesterols (251). On the other hand, the oxysterols that accumulate in atherosclerotic lesions are predominantly side-chain oxidized compounds (mostly 27- OH, generated from mitochondrial oxidation) (33). This does not appear to support a role for oxysterols in the initiation of atherosclerosis. However the oxysterols could exert several biological activities in the arterial cells, including apoptosis, cytotoxicity, and regulation of gene expression (33, 170). Several oxysterols appear to upregulate the ROS levels by stimulating the NOX pathway, and to upregulate the synthesis of TGFβ (160). Both 7β-OH cholesterol and 25-OH cholesterol have recently been shown to induce interleukin-8 secretion through the activation of ERK ½ signaling pathway in monocytes (159).
E. Cholesteryl ester products
The majority of the di- and polyunsaturated fatty acids in LDL are present as cholesteryl esters, and therefore, quantitatively most of the oxidized fatty acids in the fully oxidized LDL are esterified to cholesterol, especially since unlike the phospholipids, cholesteryl esters are not hydrolyzed in the plasma. The hydroperoxides and hydroxides of cholesteryl esters are the major lipid oxidation products found in human atherosclerotic lesions (124) (see Fig. 7). Some of the biological effects of MM-LDL and OxLDL have been attributed to the cholesteryl ester hydroperoxides. These effects include upregulation of CD36 through PPARα activation (122), monocyte adhesion, and phosphorylation of ERK ½, and Akt (93, 110). The core aldehyde, 9-oxononanoyl cholesterol was shown to upregulate the expression of TGFβ-1 and TGFβ receptor, apparently through the stimulation of ERK ½ (70). Although cholesteryl esters are normally present in the interior of the LDL particle, it is suggested that the oxygenation of the acyl groups results in their appearance on the surface and thus become accessible as ligands for cell surface receptors (93). The covalent binding of the oxidized cholesteryl esters to Apo B was also demonstrated to occur in vivo, and such adducts were detected in atherosclerotic lesions (135).
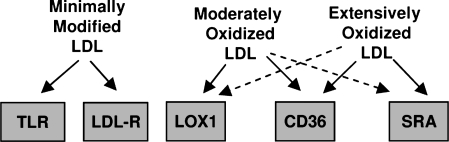
FIG. 7.
Summary for the relationship between different scavenger receptors and degree of LDL oxidation.
F. Hydroxynonenal and malondialdehyde
There are several short-chain aldehydes formed from the oxidative cleavage of unsaturated fatty acids, the most extensively studied ones being hydroxynonenal (HNE) and malondialdehyde (MDA). HNE is formed from the degradation of n-6 fatty acids, while MDA is formed from the peroxidation of all unsaturated fatty acids containing two or more methylene interrupted double bonds, and is also generated during thromboxane formation. The estimation of MDA by the TBARS assay is the most widely used measure of lipid peroxidation, and the extent of LDL oxidation is often expressed as MDA equivalents per mg of Apo B. Both MDA and HNE are highly reactive and form adducts with the thiol and amino groups of Apo B and other proteins with which they come in contact. The change in the electrophoretic mobility of LDL following oxidation is due to this reaction, and most of the biological activity of HNE can be attributed to its high reactivity towards the functional groups (cysteine, lysine, histidine) of the cellular proteins. For example, it reacts with several tyrosine kinase receptors, such as EGFR and PDGFR, and regulates their activities (206), and also generates ROS intracellularly through the interruption of mitochondrial function (157). It inhibits IκB kinase by derivatization and thus inhibits the NFκB-mediated transcription of inflammatory genes. Gene expression of several important proteins, including TGFβ1, PDGF, MCP-1 is regulated through the modulation of nuclear binding of the transcription factor AP-1 (160). HNE also induces COX-2 expression through a CD36-dependent pathway (131).
G. Products of Apo B modification
Apo B is modified by derivatization of various functional groups of amino acids such as lysine, cysteine, histidine, tryptophan, and tyrosine (118). In addition, the myeloperoxidase oxidation of LDL results in the formation of chloro- and nitro-tyrosine derivatives. Modification of about 16% of the lysine residues of Apo B by MDA has been shown to result in the loss of recognition by LDL receptor and the appearance of epitopes for recognition by the scavenger receptors (86), as described in more detail in the next section of the review. In addition to the derivatization of side chains, some cleavage of peptide chains, and cross-linking of polypeptides could occur during LDL oxidation. Interestingly, the activation of a latent SMase activity intrinsic to Apo B was reported to be due to a conformational change that occurs during lipolysis (138). This SMase activity could be responsible for the generation of ceramide and aggregation of LDL that occurs following LDL oxidation, although it was reported to be inhibited by oxidation (138). Bancells et al. (17) recently reported that the electronegative LDL (LDL-) isolated from the plasma is enriched in the SMase/lysophospholipase activity, while the ‘normal’ LDL (LDL+) is not. It is unclear, however, whether this lipolytic activity is intrinsic to Apo B or a separate protein that associates preferentially with electronegative LDL.
In summary, oxidation of LDL leads to the generation of several bioactive lipids, as well as modification of the functional groups of the Apo B that lead to its recognition and uptake by various scavenger receptors, as described in detail in the next section.
VI. OxLDL–Cellular Interactions: Patterns of OxLDL Recognition
Goldstein et al. were the first to demonstrate that the uptake of modified LDL is mediated by a receptor distinct from the LDL receptor (75). Specifically, the uptake and lysosomal degradation of 125I-acetyl-LDL (acLDL) by mouse peritoneal macrophages was shown to be 20-fold higher than the uptake of 125I-LDL, indicating that a high-affinity surface binding site is responsible for the recognition of acLDL but not native LDL. Since the macrophage binding site also recognized malylated LDL and several other negatively charged ligands, it was suggested that negative charges are important for binding of acLDL to this site. A similar binding activity was found in macrophages and monocytes but not in lymphocytes or fibroblasts. Goldstein et al. (1979) suggested, therefore, that this receptor may mediate the degradation of denatured LDL. Indeed, Henriksen et al. (98, 99) have shown that when LDL was pre-incubated with endothelial cells, its degradation by macrophages was 3–5 times more rapid than degradation of control LDL.
Furthermore, degradation of endothelial cells-modified LDL was inhibited by acLDL indicating that the two types of LDL compete for the same pathway. Comparison of endothelial-modified LDL and LDL oxidized by exposure to Cu2+suggested that all the changes associated with endothelial-mediated modification of LDL can be attributed to oxidation (267). Further studies have shown that acLDL receptors are present not only in macrophages but also in other cell types, including endothelial cells (222), smooth muscle cells (221), and fibroblasts (221). It is important to note, however, that numerous studies have shown that there are significant differences in binding and internalization of acLDL and OxLDL by different scavenger receptors, as well as in the ability of the two types of lipoproteins to load cells with cholesterol. Today, multiple receptors belonging to several different classes have been identified to recognize OxLDL and mediate OxLDL-cellular interactions (Fig. 3). The biochemistry, classification, and various biological functions of the scavenger receptors, including their endocytotic activity, signaling and the roles of host-defense mechanisms have been described in several excellent reviews (191, 197, 224). The goal of this part of our review is to discuss the differences in the recognition patterns of the major OxLDL receptors to the different forms of OxLDL.
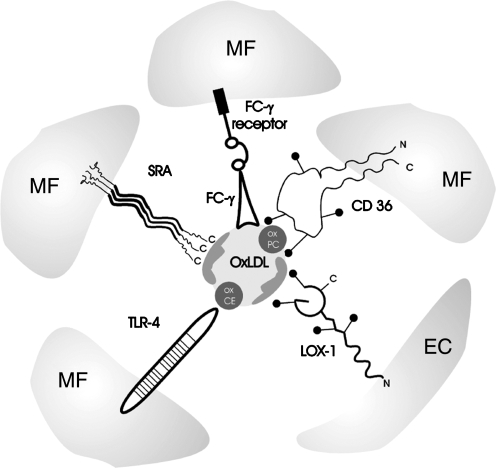
FIG. 3.
Mechanisms of OxLDL recognition by different scavenger receptors. Multiple types of scavenger receptors have been identified to recognize and interact with different forms of OxLDL. The major scavenger receptors responsible for OxLDL uptake by macrophages (MF) are: class A scavenger receptors SRAI/II and class B scavenger receptor CD36. OxLDL immune complexes OxLDL are recognized and metabolized via Fcγ receptors. OxLDL, particularly MM-LDL may also be recognized by TLR-4 receptors. Each of these receptors recognizes a different component of the OxLDL particle with SRAI/II receptors recognizing modification of the Apo B protein, CD36 recognizing oxidized phospholipids, and TLR-4 recognizing oxidized cholesteryl esters. The major OxLDL uptake pathway in endothelial cells (ECs) is LOX-1 receptor that also recognizes Apo B modifications. ECs also express CD36 and other types of scavenger receptors. Scavenger receptors are also expressed in other cell types, including smooth muscle cells and platelets. Receptor structures represent the basic domain architecture of the different receptors [receptor structures are adapted from (224)].
A. Class A scavenger receptors: Extensively oxidized LDL
The first scavenger receptors that to be identified on the molecular level were class A type I and II (SR-AI and II), two isoforms that are derived from alternative splicing of a single gene product (66, 141, 236). The first receptor was identified as a 220 kDa protein that exhibits acLDL binding activity but does not bind native LDL and is highly expressed in liver, spleen, adrenal gland, and in the lung, the latter presumably because of resident alveolar macrophages (141). Both scavenger receptors AI and AII were shown to be trimeric membrane glycoproteins with the only difference in the cysteine-rich C-terminal domain where a 110-amino acid sequence in SR-AI is replaced by a 6-amino acid sequence in SR-AII (140, 141, 236). In spite of the truncated C- terminus in SR-AII receptor, the affinities of the two receptors to acLDL are similar (236). Both subtypes of the class A receptors can be endogenously expressed in the same cells (66, 200). SRAI/II receptors are predominantly expressed in macrophages of various organs and induced during monocyte–macrophage differentiation with most prominent expression in macrophage-derived foam cells in fatty streaks and atherosclerotic lesions (79, 102, 200). In addition to macrophages, SRA receptors were also shown to be expressed in smooth muscle cells (55, 79, 162) and sinusoidal endothelial cells in the liver (111). A third splice variant of SRA receptors, SR-AIII, found in human macrophages was shown to be nonfunctional, being trapped in the endoplasmic reticulum (78). Expression of SR-AIII, however, has a dominant negative effect on SR-AI and ST-AII, suggesting that it may play a role in the regulation of SR-AI/II function (78).
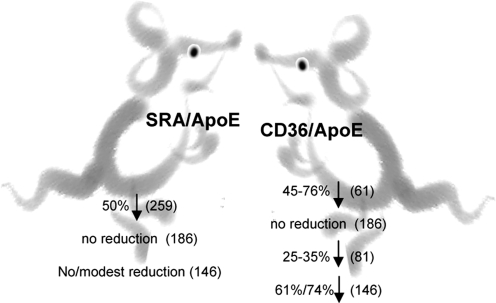
A major feature of the SR-AI/II receptors is their broad specificity to a variety of ligands, such as different types of modified lipoproteins, an array of negatively charged nonlipoprotein ligands, as well as different types of bacteria (145, 223). Multiple studies have shown that SR-AI/II receptors bind both acLDL and OxLDL, but there are significant differences in the binding affinities of the two modified LDLs and the mechanisms responsible for the binding appear to be complex. Specifically, Freeman et al. (67) showed that the affinity of oxidized LDL was lower than that of acLDL for both type I and type II scavenger receptors when the two proteins were expressed in Chinese Hamster Ovary cells, which normally have little scavenger receptor activity. Competition studies showed that while acLDL is an efficient competitor for OxLDL binding, OxLDL does not compete efficiently with acLDL. It was proposed that OxLDL and acLDL bind to nonidentical but partially interacting sites of the receptors. Consistent with these studies, Dejager et al. (55) also showed that acLDL is a more efficient competitor for OxLDL binding than OxLDL for acLDL-binding both in smooth muscle cells and in macrophages, further suggesting that not all of the acLDL binding sites can bind OxLDL. In contrast to acLDL, however, OxLDL did not result in massive lipid accumulation in SRAI/II-expressing CHO cells. However, as described below in more detail, the role of SRAI/II receptors in the uptake and degradation of OxLDL was further confirmed by demonstrating that targeted disruption of SRAI/II receptors in mice results in ∼30%–50% decrease in the uptake and degradation of Cu2+-oxidated LDL by peritoneal macrophages (the uptake of acLDL was reduced by more than 80%) (153, 171, 275). Furthermore, disruption of the SRAI/II receptors significantly inhibited the uptake and degradation of circulating LDL fraction isolated from apo E -deficient (30% inhibition) and LDL receptor-deficient mice (25% inhibition), demonstrating that these receptors constitute major pathways for the uptake of LDL modified in vivo (313).
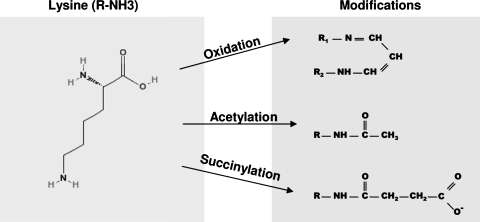
In terms of the degree of LDL oxidation, SRA receptors are generally considered to be most specific for extensively oxidized LDL. The initial clue came from the ability of the SRA receptors to recognize different modifications of LDL including acetylation, acetoacetylation, succinylation, or malondialdehyde treatment, all of which modify the lysine residues of the LDL protein (35). Furthermore, chemical modifications that lead to the recognition of LDL by the SRA receptors involve neutralization of the positively charged residues and it was suggested that oxidation may have a similar effect (35). More specifically, neutralization of at least 16% of the lysine residues of apo-B protein was required for the receptor recognition, and progressive modifications resulted in an increase of binding and degradation (85). Modifications of >60% of the lysine residues either by acetylation or by succinylation was required for the maximal uptake (85). Figure 4 shows schematically the structural modifications that result in lysine charge neutralization and recognition by the SRA receptors (from Ref. 85). Indeed, LDL oxidation was also shown to modify the lysine residues of the LDL protein with 32% of lysines being modified in extensively oxidized LDL (20 hours oxidation with 5 μM Cu2+(265). In contrast, methylation of the lysines, which does not alter the charge of these residues (no significant change in the electrophoretic mobility of LDL) was not sufficient to induce recognition of LDL by the macrophage scavenger receptors (265). Moreover, methylation of LDL prior to oxidation prevented its recognition by the receptors, suggesting that methylation protected lysine residues from oxidative changes, possibly by inhibiting the binding of lipid peroxidation products to lysine residues (265, 311). Similar to the requirement for lysine modifications for the recognition of acLDL, recognition of OxLDL was also induced by the modification of 15%–20% of lysine residues with further increase as the degree of lysine derivatization was increased to over 30% (311). Consistent with the critical role of the protein modifications, Parthasarathy et al. (216) showed that delipidated apoproteins isolated from OxLDL are effectively taken up by the macrophages and that the uptake of OxLDL-derived apoprotein is competitively inhibited by acLDL and by MDA-conjugated LDL (216). In a later study, Terpstra et al. (278) showed that binding and uptake of OxLDL by peritoneal macrophages can also be effectively blocked by a lipid emulsion extracted from OxLDL (but not from native LDL), indicating that OxLDL lipids are also recognized by the macrophage scavenger receptors. The inhibitory effect depended on the degree of LDL oxidation: microemulsions prepared from LDL oxidized for 2 hours (with 10 μ M Cu2+) corresponding to minimally-oxidized LDL had no effect, but 6 hours oxidation which corresponds to moderately-oxidized LDL was sufficient to have the inhibitory effect. Furthermore, uptake of OxLDL was also significantly inhibited by oxidized phospholipids (1-palmitoyl-2-arachidonoyl-PC), suggesting that oxidized phospholipids play a significant role in OxLDL binding to macrophage scavenger receptors (278). Consistent with these observations, OxLDL uptake by macrophages was also inhibited by monoclonal antibodies that show specific binding to oxidized phospholipids (106). Interestingly, most of the autoantibodies that were isolated from apo E-deficient mice on the basis of their binding to OxLDL were shown to bind oxidized phospholipids (106). These studies clearly show that lipid moieties are also important for the recognition of OxLDL by macrophage scavenger receptors but in light of the later studies (e.g., Refs. 29, 225–228), it appears that these effects may be attributed to CD36 receptors, a class B of scavenger receptors described below, rather than to SRAI/II receptors.
FIG. 4.
Structural modification of Apo B lysine residues critical for OxLDL recognition by SRAI/II receptors [adapted from (85)]. The major modifications of the Apo B protein leading to the recognition by the SRA receptors include oxidation by lipid oxidation products, such as malondialdehyde, hydroxynoneanl, or trancated phospholipids, as well as acetylation and succinylation. All the modifications occur on the lysine residues with 15%–60% of lysines being required to be modified for the interaction with SRA receptors. The products of these modifications are malondialdehyde, acetic anhydride, and succinic anhydride for oxidation, acetylation, and succinylation, respectively. All three modifications result in lysine charge change with the net change per lysine being Δ = −1 for oxidation and acetylation and Δ = −2 for succinylation.
The mode of oxidation also plays a major role in OxLDL recognition by the SRA receptors. Babiy and Gebecki (14) showed that, in contrast to Cu2+oxidation, oxidation of LDL by ionizing radiation does not produce an OxLDL species that is efficiently recognized by the macrophage receptors. Furthermore, they showed that the major difference between LDL oxidized by the two methods is the degree of LDL hydroperoxide decomposition: while Cu2+oxidation resulted in significant hydroperoxide degradation, ionizing radiation did not. The uptake and accumulation of cholesterol were highly dependent on the degree of hydroperoxide degradation (14), suggesting that OxLDL is not recognized by the macrophage scavenger receptors unless the lipid hydroperoxide groups are decomposed, which in turn derivatize LDL Apo B. Further insights into the structural requirements of the recognition of OxLDL by the SRA receptors were obtained by comparing two chemical modifications of the lysine residues in the LDL apolipoprotein: formation of a lysine pyrrole that neutralizes lysine charges or formation of pyridinium ring that retains the charges (227). Both forms of the modified LDL were taken up by the macrophages, resulting in accumulation of cholesteryl ester and foam formation; but “neutral” LDL was taken up more efficiently than the “charged” LDL. However, only the pyrrole modification of LDL that neutralizes the lysine charges induced its recognition by the SRA receptors heterologously expressed in CHO cells whereas the non-neutralizing form of LDL was recognized by CD36 receptor, a class B scavenger receptor described in detail below (227). Taken together, these multiple lines of evidence indicate that oxidative modifications of the LDL protein, specifically the neutralization of lysine residues are critical for the recognition of OxLDL by macrophage class A scavenger receptors.
In addition to SRAI/II receptors, several other members have been identified as class A scavenger receptors family: MARCO, SCARA5, and SRCL (also known as CL-P1) but these receptors have not been shown to constitute major pathways for OxLDL uptake (191, 197, 224, 250). Macrophage Receptor with a Collagenous Structure (MARCO) that has been identified in a subset of macrophages residing in spleen and medullary cord lymph nodes (59, 60) is well established to bind Gram-negative and Gram-positive bacteria including E. coli and S. aureus (59), but its role in binding and internalization of OxLDL is somewhat controversial. Initially, Elomaa et al. (59) showed that MARCO expressed in COS cells can bind acLDL, as assayed by the uptake of DiI-acLDL, but a later study of Elshourbagy et al. (60) showed that while MARCO binds E. coli and S. aureus, it does not bind either acLDL or OxLDL. They also showed that neither acLDL nor OxLDL could compete with E. coli binding to MARCO. More recently, another member of the SRA family, scavenger receptor with C-type lectin (SRCL) or collectin placenta 1 (CL-P1), was identified (202, 203, 211). Similarly to SRAI/II, CL-P1 recognizes extensively oxidized LDL but in contrast to previously identified members of the SRA family, CL-P1 receptor is not expressed in monocyte-macrophage lineage cells and binds OxLDL but not acLDL (211). CL-P1 also binds E. coli and S. aureus, as well as yeast (203, 211). Finally, another member of the SRA family of the scavenger receptors SCAPA5 was recently identified in epithelial cells but this receptor does not bind or internalize either acLDL or OxLDL (128).
B. Class B scavenger receptors: Extensively and moderately oxidized LDL
The first member of the SRB family to be identified as a receptor for OxLDL was CD36, an 88 kDa glycoprotein expressed in macrophages, platelets, and endothelial cells (61). Similarly to SRA receptors, CD36 was shown to bind and internalize OxLDL but not unmodified LDL, but in contrast to the SRA receptors, OxLDL binding to CD36 was not inhibited by acLDL (61). Consistent with these observations, a stable expression of CD36 in NIH-3T3 fibroblasts resulted in OxLDL but not LDL or acLDL binding (208). Other studies, however, reported that CD36 can also bind acLDL (2, 63). More importantly, multiple studies have shown that CD36 differs from SRA receptors in its affinity to moderately-oxidized LDL. Specifically, Endemann et al. (61) showed that 4 hours of oxidation (with 5 μ M Cu2+) was sufficient to induce uptake of OxLDL by CD36, with the maximal uptake observed after 10 hours of oxidation. In contrast, no uptake by SRA receptors was observed after 4 hours and more than 20 hours of oxidation were required for the maximal uptake. The uptake of extensively oxidized LDL, however, by CD36 and by SRA receptors were similar.
In contrast to SRA receptors, binding of OxLDL to CD36 was abrogated by delipidation of the lipoprotein, indicating that these receptors do not recognize LDL protein alone (208). Consistent with these observations, OxLDL uptake by CD36 receptors is competitively inhibited by oleic and linoleic fatty acids, suggesting that it is the lipid moiety that is critical for binding of OxLDL to CD36 receptors (208). These receptors were also shown to specifically bind anionic phospholipids, phosphatidylserine (PS), and phosphatidylinositol (PI) with both PS and PI liposomes competing with acLDL (232). However, Boullier et al. (29) showed that CD36 receptors can be inhibited both by Apo B and by OxLDL-derived lipid microemulsions. In addition, they showed that Apo B and the lipids reciprocally compete with each other for CD36 binding, suggesting that they compete for the same binding site. As pointed out by the authors, it appears paradoxical that the apoprotein and the lipid moieties would bind to the same site of the receptor and they suggested that the most probable explanation of these observations is that some fraction of oxidized phospholipids remains associated with the protein during the extraction procedure. Thus, the discrepancy between this study and the studies of Nicholson et al. (208) described above, might be due to the differences in the extraction protocols. Partial inhibition of OxLDL apoprotein binding to CD36 by a monoclonal antibody that recognizes oxidized phospholipids is also consistent with this conclusion (29, 106). Further studies provided compelling evidence for the critical role of oxidized phospholipids in OxLDL recognition by CD36 receptors. Specifically, in contrast to SRAI/II receptors that require neutralization of the lysine residues to recognize OxLDL, CD36 receptors recognize both the neutralizing and the non-neutralizing modifications of OxLDL (227). Significantly, CD36 receptors were also shown to recognize LDL modified by a myeloperoxidase–hydrogen–peroxide–nitrite (MPO–H2O2–NO-2) system that is abundant in monocytes and neutrophils (225). Exposure of LDL to the MPO system results in nitration of Apo B tyrosyl residues and lipid peroxidation. Only a brief exposure (2 hours) to MPO was sufficient to convert LDL to NO2-LDL, a ligand for CD36 supporting the notion that CD36 recognizes mildly-oxidized LDL. A significantly longer exposure (>8 hours) was required for the recognition by SR-AI receptors and the latter correlated with an increase in relative electrophoretic mobility, as expected from the earlier studies. Most importantly, lipid extracts of NO2-LDL were shown to be potent competitors for NO2-LDL binding to CD36 receptors (225). More specifically, lipid oxidation products of 1-palmitoyl-2-arachidonyl- glycero-3-phosphocholine (PAPC) were identified as the lipid moieties critical for the recognition of OxLDL by CD36 receptors (225). Furthermore, systematic structural analysis revealed a group of specific molecular species of choline glycerophospholipids that are responsible for the recognition by CD36 receptors (228). Figure 5 shows the key structures of oxidized PC that have been identified to be responsible for the binding of OxLDL to CD36 receptors (from 185). Modifications of the LDL protein had no effect. Thus, conversion of LDL into a ligand for CD36 appears to be a very early event during LDL oxidation, occurring before substantial modification of ApoB, as monitored by loss of free lysine residues and alteration in relative electrophoretic mobility (225). To test further whether modification of the LDL apoprotein and/or lipid–protein adducts are also important for the recognition of OxLDL by CD36, Podrez et al. (226) measured CD36-mediated OxLDL uptake when the particle was first methylated to protect the lysine residues of the apoprotein and then oxidized by Cu2+. As was shown earlier (265, 311), methylation prevented OxLDL uptake by SRA receptors but it had no effect on the uptake by CD36 receptors, supporting the conclusion that recognition of OxLDL by CD36 receptors critically depends on the lipid modifications of the particle (226).
Relative contributions of the CD36 and SRAI/II pathways to OxLDL uptake by macrophages are comparable for the extensively oxidized LDL, with CD36 playing a more major role in the uptake of mildly oxidized and MPO-modified LDL. First, Endemann et al. (61) showed that blocking CD36 with an antibody decreases the uptake of extensively oxidized LDL by 50% in macrophage-like THP cells and platelets. Moreover, targeted disruption of CD36 receptors in mice resulted in ∼50–60% decrease in the uptake extensively oxidized LDL (63, 153), as compared to ∼30%–50% decrease in SRAI/II deficient mice (153, 171, 275). The uptake of mildly-oxidized LDL (8 hours Cu2+oxidation) was decreased by ∼70% by CD36 disruption and ∼40% by SRAI/II disruption (153). The uptake of acLDL was decreased only by 13% by CD36 disruption (153) vs. 80% in SRAI/II knockout macrophages (275). CD36 also appears to be the principal pathway for the internalization of LDL modified by a myeloperoxidase–hydrogen–peroxide–nitrite system, a more physiological method of oxidation described above (225) with 60%–77% inhibition of MPO-modified LDL in CD36-deficient macrophages and 30% inhibition in SRAI/II-deficient cells (63, 153). Importantly, disruption of CD36 also significantly inhibited the uptake of circulating LDL fraction isolated from apo E and LDL receptor-deficient mice (50% and 25% inhibition, respectively). Taken together, these studies demonstrate that CD36 constitutes a major pathway for the uptake of modified LDL by macrophages.
The second receptor in this class to be identified was SR-BI/II (2, 298). Similar to CD36 receptor, SR-B1 binds both OxLDL and acLDL, but in contrast to all other scavenger receptors identified earlier SR-B1 also recognizes native LDL (2). Most importantly, SR-B1 was identified as the principal receptor for high-density lipoproteins (HDL)(1, 233, 283, 291) that is highly expressed in liver and steroidogenic tissues, the principal sites of selective uptake of CE in vivo (40, 155, 189) and plays a major role in the reverse cholesterol transport and cholesterol clearance (289, 312). Overexpression of SR-BI in LDL receptor knockout mice resulted in a protective effect, decreasing mean atherosclerotic lesion area by 80% (8) while targeted disruption of SR-BI had a proatherogenic effect (114). SR-B1 was also shown to internalize oxidized forms of HDL (287), but this topic is beyond the scope of this review.
C. Class E scavenger receptors: Mildly oxidized LDL
Lectin-like oxidized LDL receptor-1 (LOX-1, GenBank designation OLR1) is a major receptor for OxLDL in endothelial cells (224). It was first identified by expression cloning of the cDNA library of bovine aortic endothelial cells (247). Expression of LOX-1 in CHO cells resulted in binding and degradation of OxLDL comparable to that in cells expressing SRA receptors. The binding was effectively inhibited by OxLDL but not by native LDL or acLDL (193, 247). The human homolog isolated in the same study from human lungs was shown to have similar properties. In vivo, LOX-1 receptor was shown to be most abundant in vascular-rich organs, such as lungs, placenta, and brain (247). It was also identified in thoracic and carotid arteries, including atheromatous regions (247). Furthermore, while in early lesions LOX-1 was found mainly in endothelial cells, in more advanced lesions it was also highly expressed in macrophages and smooth muscle cells that accumulated in the intima (133).
Similarly to SRAI/II receptors, LOX-1 also binds the delipidated form of OxLDL with the same efficiency as untreated OxLDL, suggesting that LOX-1 recognizes the modified Apo B (193). In terms of the degree of LDL oxidation, however, LOX-1 was shown to have a higher affinity to mildly-oxidized form (3–6 hours oxidation) rather than extensively-oxidized LDL (12–24 hours oxidation), suggesting that LOX-1 may also recognize oxidized lipids (130). This pattern of OxLDL recognition is different from the patterns that were reported for both SRAI/II and CD36 receptors. As described above, SRAI/II receptors recognize mainly extensively-oxidized LDL with maximal uptake observed after 20 hours of oxidation, which is required for sufficient modification of lysine residues of the apoprotein. Also, the fact that in contrast to SRAI/II receptors, LOX-1 does not bind acLDL, indicates that the mechanisms by which SRAI/II and LOX-1 recognize OxLDL are significantly different. On the other hand, while LOX-1and CD36 are similar in terms of their overall affinity to mildly/moderately oxidized LDL, CD36 is sensitive only to oxidized phospholipids, while LOX-1 is sensitive to the modifications of the protein. One explanation to reconcile these observations is to suggest that LOX-1 may recognize oxidized lipids that are covalently bound to the apolipoprotein and are not removed during the delipidation process. Alternatively, it is possible that some modification of the protein occurs relatively early in the oxidation process and that these modifications are recognized by LOX-1 receptors. In addition, it was shown recently that LOX-1 can also bind phosphadidylserine, one of the major cellular phospholipids that flips from the inner to the outer leaflet of the plasma membrane in apoptotic cells (196). It was also shown that phosphatidylserine may be involved in LOX-1 recognition of platelets (129). Furthermore, since OxLDL can compete with apoptotic cells for binding and internalization by macrophages, it was suggested that oxidation of LDL may lead to a structure that is in some way homologous to phosphatidylserine-rich domains on apoptotic cells (244). It is possible, therefore, that phosphatidylserine may not only be responsible for LOX-1 recognition of apoptotic cells but also may be important for LOX-1 recognition of OxLDL (196). Most importantly, Mehta et al. have shown recently that targeted disruption of the LOX-1 gene resulted in a significant decrease in OxLDL binding to aortic endothelium and preservation of endothelial function in LDL-R deficient mice (186). The impact of OxLDL on endothelial function is described further in a later section of the review.
D. Fcγ receptor: OxLDL immune complexes
High affinity OxLDL binding was also found for FcγRII-R2 (Fc receptor), a member of the family of receptors that mediate the uptake of immune complexes via recognition of the Fc region of IgG (231, 262). This receptor was initially identified by the expression cloning of mouse peritoneal macrophages and when expressed in a null cell line resulted in specific internalization of OxLDL. The uptake of OxLDL by the FcγRII-R2 receptor was blocked by a monoclonal antibody to the receptor but not by native LDL or acLDL (262). Fcγ receptors were also shown to uptake LDL antigen–antibody complexes with LDL particles forming immune complexes with autoantibodies, predominantly of the IgG isotype, subclasses IgG1 and IgG3 which react with MDA-modified and myeloperoxidase-modified LDL (166, 241). Indeed, once LDL is oxidized it becomes highly immunogenic. Multiple studies have shown that humoral response to OxLDL is marked by the presence of high titers of IgG and IgM antibodies against oxidation-specific epitopes of OxLDL and immune complexes with OxLDL in plasma and athersoclerotic lesions in animals and humans (22, 23). However, the exact epitopes are yet to be identified. Interestingly, natural antibodies, a specific type of autoantibodies produced by B1 cells that are considered to be innate-like part of the adaptive immune system providing the first line of defense against viral and bacterial pathogens, recognize similar epitopes on OxLDL, apoptotic cells and some bacteria such as pneumococci and salmonella (254). More specifically, these antibodies bind to oxidized phosphorylcholine (PC)-containing phospholipids, such as 1-palmitoyl-2-(5-oxovaleroyl)-sn- glycero-3-phosphocholine (POVPC), but not to native low density lipoprotein (LDL) and nonoxidized phosphatidylcholine such as in 1-palmitoyl-2-arachidonyl-sn-glyceroyl-3 phosphorylcholine (PAPC) (254). These interactions of natural antibodies and oxidation epitopes from phospholipids link host responses in infection, autoimmunity, and atherosclerosis (22, 23). Immunizing LDL receptor knockout mice with pneumococci lead to formation of high levels of OxLDL-specific IgM and a modest reduction in atherosclerosis. However, pneumococcal vaccination in humans failed to induce production of these atheroprotective antibodies (209). However, the titers of OxLDL-specific antibodies were also shown to correlate with the extent (23, 293) or progression of atherosclerosis as well as constitute the risk for development of myocardial infarction (243, 293). These findings are still somewhat controversial and the discrepancies could be due to differences in individual responses (types of antibodies produced, levels of the antibodies, and their avidity) (293) or OxLDL preparations (112). Additionally, several groups have reported a significant correlation between soluble LDL-IC and the presence of coronary artery disease (CAD) (293).
Furthermore, expression of the FcγRII-R2 receptors is regulated by OxLDL exposure and the effect depends on the degree of LDL oxidation (234). A recent study by Nagarajan et al. (198) has shown that FcγRII-R2 receptors are involved in the adhesion of circulating monocytes to endothelial cells and that this effect depends on the formation of OxLDL immune complexes. The authors propose that FcγRII-R2 expressed on monocytes binds OxLDL immune complexes on the surface of endothelial cells, which results in enhanced secretion of pro-inflammatory chemokines. Thus, while Fcγ receptors may not play a major role in the uptake of OxLDL alone, these receptors play a major role in the uptake of OxLDL immune complexes, as well as contribute to the development of athero-sclerosis by inducing an inflammatory response. The relation between OxLDL and immune responses is discussed in the later sections of this review.
E. Other scavenger receptors
Several other types of scavenger receptors were shown to bind OxLDL and/or acLDL but their roles in OxLDL uptake are less clear. For example, DSR-CI, a class C scavenger receptor Drosophila melanogaster was shown to bind acLDL but so far there are no known homologues in mammalian cells (218). Another example is class D scavenger receptors, CD68 and macrosialin, that can bind OxLDL, but no OxLDL uptake was observed when macrosialin was expressed in COS or CHO cells (52). Downregulation of macrosialin expression in macrophages also had no effect on OxLDL uptake (52). It was also shown that CD68 is predominantly expressed intracellular in late endosomes and lysosomes and it was suggested, therefore, that while it is unlikely to play a major role in OxLDL internalization, it may contribute to the processing of OxLDL in lysosomes (191, 224). Finally, it is important to note that in addition to lipoprotein uptake, scavenger receptors play multiple roles in regulation of host defense, phagocytosis, antigen presentation, and other functions, but discussion of these functions are beyond the scope of this review. More information about the properties and functions of different scavenger receptors can be found in many excellent reviews (3, 28, 42, 45, 152, 207, 223, 224, 266).
F. Alternative pathways for minimally-oxidized LDL
Multiple studies have shown that a very mildly oxidized form of LDL that contains only early lipid peroxidized products is not modified sufficiently to be recognized by the scavenger receptors described above. However, this minimal modification is still sufficient for inducing an array of pro-atherogenic responses, including an increase in endothelial-monocyte adhesion (20) and activation of macrophages (188). Furthermore, while minimally-modified LDL (MM-LDL) that was obtained by storage of LDL at 4°C for 3–6 months was indistinguishable from native LDL in terms of the recognition by the LDL receptor, it was found to induce significant inflammatory response (20). The active component responsible for the proatherogenic effects on endothelial cells was found in the charged lipid phase, suggesting that it is the polar lipids that are responsible for the pro-atherogenic activity (20). As described in the beginning of this review, a more physiological way to obtain minimal modification of LDL is exposure to 12/15 lipoxygenase (12/15-LO), an enzyme that is responsible for oxygenation of fatty acids. Indeed, genetic disruption of 12/15-LO was shown to have an atheroprotective effect (49). Miller et al. (188) were the first to show that MM-LDL modified by the exposure to 12/15LO over expressing fibroblasts is specifically recognized by a Toll-Like Receptor-4 (TLR-4,CD14), resulting in activation of TLR-4-dependent signaling pathways, which in turn lead to actin polymerization and cell spreading. The binding of MM-LDL to TLR-4 was specific and distinct from the LPS binding. It is also important to note that exposure of peritoneal macrophages to MM-LDL significantly upregulated the uptake and degradation of extensively-oxidized LDL, suggesting that MM-LDL upregulates the expression of the scavenger receptors. More recently, Harkewicz et al. (93) have identified cholesteryl ester (CE) hydroperoxides as the active component of MM-LDL responsible for their biological activity in macrophages (Fig. 6). Indeed, 12/15-LO is known to oxidize arachidonic acid at positions 12 and/or 15 and it may oxidize the esterified fatty acids of phospholipids and cholesteryl esters. Furthermore, MM-LDL-induced activation of TLR4 receptor results in initiation of macropinocytosis, which in turn results in increased uptake of small molecules present in the fluid phase including both native and oxidized LDL (44). This pathway is also shown to induce significant lipid accumulation both in vitro and in vivo (44).
FIG. 6.
Structures of cholesteryl ester hydroperoxides responsible for OxLDL–TLR-4 interaction [from (93)]. Cholesteryl ester hydroperoxides have been identified as the biological components of MM-LDL responsible for its interaction with TLR-4 receptors by comparing the lipid profiles of MM-LDL with unmodified LDL and then testing the biological activities of the different components. The figure shows the structure of cholesteryl arachidonate, one of the most common cholesteryl esters found in LDL and the fatty acid portion of cholesteryl arachidonate hydroperoxide (15-HpETE) (R stands for cholesterol) that is responsible for the biological activity of MM-LDL. Similar observations were made for cholesteryl linoleate, another common cholesteryl ester of LDL.
In summary, the recognition of OxLDL by the different receptors depends on specific components of the particle with modifications of the protein being critical for SRAI/II and LOX1 receptors and modifications of the lipids being critical for CD36 and TLR receptors (Fig. 3). There is also a clear preference of the different receptors to different stages of LDL oxidation, although there is also a significant overlap (Fig. 7). The specificity of the different receptors to different oxidation states of OxLDL may underlie the variety of OxLDL-induced biological responses. In the next section of the review, we will describe the complex relationship between OxLDL and pro- and anti-inflammatory responses, as well as discuss the signaling pathways that may be responsible for these effects.
VII. OxLDL, the Janus-Faced Particle: Pro- and Anti-Inflammatory Properties
OxLDL is composed of a complex mixture of several bioactive compounds, each of which has independent and sometimes opposing cellular effects. Therefore, it is not surprising that OxLDL has been reported to have both pro- and anti-inflammatory effects, as well as pro-and anti-apoptotic effects, and pro-and anti-angiogenic effects. The mode and extent of oxidation of LDL plays an important role in determining the overall pro- or anti-inflammatory result, as does the cell system being studied, and the receptors being engaged. In some cases the pro-inflammatory effects predominate at the early stages of OxLDL exposure, while the anti-inflammatory effects at a later stage (235). The pro-inflammatory effects are primarily manifested through the transcription factors NFκB, AP-1, STAT 1/3, NFAT, SP-1, and HF-1 in various cells (Table 3), while the anti-inflammatory effects are expressed through the activation of PPARs, Nrf2, and HO-1 (Table 4). The effect on the pro-inflammatory NFκB, interestingly appears to be biphasic, with a stimulatory effect at low concentration of OxLDL, and an inhibitory effect at high concentration (235). The individual components of OxLDL responsible for the pro- and anti-inflammatory effects have been identified at least in a few cases. For example LPC has been shown to be responsible for the stimulation of the pro-inflammatory AP-1 (184) while 7-keto cholesterol stimulated the production of fibronectin through ROS-dependent SP-1 activation (4). Cholesteryl ester hydroperoxides stimulate ERK1 phosphorylation and cytokine secretion (93), while HNE, the aldehyde degradation product of linoleic acid, activates the receptor tyrosine kinases (RTK) in the membranes by adduct formation and triggering ROS generation (206). OxPAPC products have been shown to be ligands for CD36 (245), and agents that directly bind CD36 may be anti-inflammatory because of the induction of IL-10 (215). Similarly the hydroxyl and hydroperoxy free fatty acids are ligands for the PPARγ, which is known to have anti-inflammatory function because of its suppression of induction of pro-inflammatory cytokines (276). Although the individual lipids exert their cellular effects when added as pure compounds, it is not clear whether in vivo they enter the cells through (lipid) specific receptor-mediated pathways, or as part of the whole OxLDL particles. Specific receptors for LPC have been proposed, although not yet clearly identified as in the case of PAF-receptor. Since OxPAPC and other OxPCs are also found in the MM-LDL, which can enter the cells through LDL receptor, the effects of these molecules may not require the expression of specific scavenger receptors.
Table 3.
Pro-Inflammatory Transcription Factors Induced by OxLDL
| Transcription Factor | Active Component of OxLDL | Target Genes and Cellular Effects (Selected) |
|---|---|---|
| NFκB | LPC, 13-HODE | Inflammatory cytokines, Immune receptors Adhesion molecules Impaired Glut4 function |
| AP-1 | LPC Oxidized PC | TNFα, Osteopontin Endothelin-1, ABCA1 TGFβ, MMP-9 |
| STAT 1/3 | ?? | Cytokines Apoptotic pathway sPLA2, cPLA2 |
| NFAT | Oxidized PC ROS | Cytokines Angiogenesis Tumorigenicity |
| Sp-1 | 7-keto cholesterol | VCAM1, ICAM1, TGFβ PDGFβ, Osteopontin Tissue factor |
| (HF-1) | ?? | VEGF-1, PAI-1 COX-2, VCAM1, IL1β |
Table 4.
Anti-inflammatory Transcription Factors Induced by OxLDL
| Transcription Factor | Active Component of OxLDL | Target Genes and Cellular Effects |
|---|---|---|
| PPARα | 9-HODE, 13-HODE HETE, Free fatty acids | ↑ Lipolysis, ↑ Fatty acid oxidation ↓ NFκB activity ↓ VCAM-1, ↓ Tissue factor |
| PPARγ | Prostaglandin PGJ2 9-HODE, 13-HODE, Arachidonate PAF-like products | SR-B1, CD-36, LPL, ↑ Insulin sensitivity Adipocyte differentiation |
| Nuclear factor erythroid-related factor2 (Nrf2) | 4-HNE Lipid hydroperoxides | ↓ VCAM-1, ↑ Heme oxygenase-1 |
| Smad3 | ?? | PAI-1 |
In addition to stimulating the expression of the transcription factors shown in Table 4, the components of OxLDL exert anti-inflammatory effects at various levels. For example, extracellularly, oxidized phospholipids competitively inhibit the binding of bacterial LPS to TLR-4, thus inhibiting the LPS-mediated inflammatory reactions. Oxidized fatty acids inhibit the TNFα-induced upregulation of adhesion molecules and activate PPARs (245). In the nucleus, oxidized lipids have been shown to inhibit DNA binding and transactivation of NFκB. Oxidized PC in MM-LDL was shown to induce heme oxygenase-1 (HO-1) in EC and SMC (117). HO-1 has both antioxidant and anti-inflammatory properties (27). The oxidized phospholipids in MM-LDL were also shown to elevate cAMP levels in endothelial cells, which is known to be anti-inflammatory because of its inhibition of adhesion molecule synthesis and secretion of inflammatory cytokines, possibly through an inhibition of the NFκB pathway (27). In another example of the Janus-like nature of oxidized LDL, Boullier et al. (30) reported that while OxLDL is pro-apoptotic to macrophages, MM-LDL (prepared by the lipoxygenase method) showed anti-apoptotic effects, and in fact counteracted the effects of OxLDL by activating the pro-survival PI3K/Akt signaling pathway. In contrast, the effects on SMC appear exactly opposite to those on macrophages because Loidl et al. (165) reported that MM-LDL stimulated ceramide generation in these cells through the activation of acid SMase, and thus promoted apoptosis. It should, however, be noted that the “MM-LDL” in the latter study was prepared by dialysis of LDL against 5 μ M FeSO4 for 48 h, and that this preparation contained 30–60 nmol of MDA/mg Apo B. By way of comparison, Auge et al. (10) oxidized LDL by UV irradiation (resulting in the formation of only 4 nmol of TBARS/mg Apo B, and no loss of amino groups), and found that this preparation activated SMC proliferation through the generation of S1P and activation of EGFR/PI3kinase/Akt pathways. The degree of oxidation obviously affects the composition of the active components of OxLDL, and the balance of pro- and anti-inflammatory or apoptotic effects. As mentioned previously, the long-chain oxygenated forms of PAPC (PEIPC, PECPC) exhibit protective effects on endothelial barrier, while their degradation products (POVPC, PGPC, LPC) show exactly the opposite effects (301). The conflicting results obtained from different studies not only illustrate the multipotential nature of oxidized LDL, but also the pitfalls associated with comparing the data obtained with different MM-LDL preparations.
VIII. Reactive Oxygen Species and OXLDL Effects
Although reactive oxygen species (ROS) are involved in the formation of OxLDL, many of the biological effects of OxLDL appear to require the generation of more ROS intracellularly. In fact, the activation of various transcription factors as well as the calcium mobilization that occurs following OxLDL treatment can be inhibited by cell permeable free radical scavengers and antioxidants, indicating the importance of ROS as a proximal event in its cellular effects. Intracellular ROS are generated by several pathways, the most important being the NADPH oxidase (NOX) system. ROS are also generated by the lipoxygenase/ cyclooxygenase system, and by the mitochondrial dysfunction caused by OxLDL. Once formed, the ROS induce a multitude of cellular effects, depending upon the cellular systems (Fig. 8). At least two of the known OxLDL receptors (LOX-1 and TLR-4) have been linked to the generation of superoxide and H2O2 through the NOX pathway. Cominacini et al. (46) first reported that the formation of superoxide and the consequent inactivation of intracellular NO occurs in endothelial cells within 1 min of the engagement of LOX-1 receptor by OxLDL. The NFκB activation by OxLDL in endothelial cells also involves ROS generation through the LOX-1-mediated pathway (161). The involvement of TLR-4 in the generation of ROS was reported by Bae et al. (15) who showed that the interaction of MM-LDL with TLR-4 in the macrophages activated the spleen tyrosine kinase (SyK), which phosphorylates PLCγ1, and sequentially leads to the activation of protein kinase C, and activation of NOX-2, and production of superoxide (Fig. 8). A CD36-dependent ROS generation was also recently proposed by Park et al (214) in macrophages. Although the intermediate steps between CD36 and NOX activation have not been determined, the generation of ROS was found to be essential for the modulation of macrophage spreading and migration. The involvement of lipoxygenase reaction as well as mitochondrial dysfunction in the generation of ROS and consequent apoptosis of smooth muscle cells (SMC) was suggested by the results of Hsieh et al. (107). While the OxLDL receptor involved in this process has not been investigated, the likely candidate is CD36, since it was shown to be implicated in the role of lipoxygenase-mediated ROS generation that down regulates insulin-like growth factor receptor-1 in SMC in response to OxLDL (101). POVPC, the oxidation product of PAPC was shown to mimic the effect of MM-LDL in activating lactosylceramide production, leading to ROS generation (245). Since MM-LDL can enter the cells through the LDL receptor pathway, the effects of POVPC may not require the signaling through the scavenger receptor pathway. Another pathway for ROS generation is the mitochondrial damage induced directly by HNE (157), which appears to be independent of OxLDL receptors. In summary, the intracellular generation of ROS may represent a key common event in the action of OxLDL taken up by different pathways. Therefore, therapeutic strategies for the treatment of inflammatory diseases and atherosclerosis should not only include extracellular antioxidants that prevent the oxidation of LDL, but also membrane-permeable antioxidants that could inhibit ROS generation inside the cells. Part of the failure of the antioxidants in the clinical trials reported so far could be due to their inability to influence the intracellular generation of ROS in response to OxLDL. The hydroperoxy fatty acids produced by the intracellular lipoxygenases have been shown to regulate the expression of several redox-sensitive genes by augmenting the cellular oxidizing potential (151).
FIG. 8.
Importance of intracellular reactive oxygen species (ROS) in the effects of oxidized LDL. Evidence indicates that a common cellular event following the binding of OxLDL to various membrane receptors is the generation of intracellular ROS. The stimulation of pro-inflammatory genes, apoptotic events, and calcium mobilization in the cells are all preceded by ROS generation through the various mechanisms shown. It should, however, be pointed out that not all reactions shown here take place in every cell type. An important consequence of ROS generation is the ‘feed forward’ effect of further oxidation of LDL by the ROS secreted from the cells. The active molecules in OxLDL responsible for the individual pathways have been identified at least in some cases. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Since it is well recognized today that chronic inflammation underlies the development of atherosclerosis, the pro-inflammatory signaling pathways described in this section may constitute the basis for the initiation of lesion formation. In the next section, we will discuss the role of OxLDL in the lipid accumulation and foam cell formation, a hallmark of the pronounced atherosclerosis.
IX. OxLDL Uptake in Foam Cell Formation and Lesion Development
The hallmark of atherosclerosis is accumulation of cholesterol-laden macrophages (foam cells) in the vascular wall, as described in many previous reviews (147, 164, 240, 263). The goal of this part of our review is to discuss the evidence for OxLDL as a critical factor in cholesterol accumulation by macrophages, foam cell formation, and lesion development. In general, once taken up by the cell, LDL particles enter the lysosomal compartment where it is expected to be degraded by an array of hydrolytic acid lipases and proteases, leading to the degradation of a lipoprotein particle to its components including cholesterol (35, 36). The resulting excess of free cholesterol is then transported to the endoplasmic reticulum where it is esterified by the enzyme, acyl-CoA:cholesterol acyltransferase (ACAT), resulting in formation of cholesteryl ester that is stored in cytoplasmic inclusions. However, since multiple studies demonstrated that exposure of cultured macrophages to native LDL is not sufficient to result in significant cholesterol accumulation, it was proposed that modification of native LDL, particularly its oxidation, is a prerequisite for cholesterol accumulation in macrophages and foam cell formation (12, 264, 303). Two general lines of evidence support this hypothesis: (1) ability of macrophages to internalize and degrade modified LDL resulting in cholesterol accumulation and (2) inhibition of foam cell formation by the downregulation of the scavenger receptors. However, while numerous studies have shown that exposure to acetylated LDL indeed induces massive accumulation of cholesterol in macrophages, as was first demonstrated by Goldstein et al. (75), the ability of oxidized LDL to load cells with cholesterol is more controversial. In this review, we will discuss how different forms of OxLDL and different OxLDL uptake pathways affect macrophage cholesterol accumulation and foam cell formation.
A. OxLDL-induced cholesterol loading of macrophages in vitro
Extensively oxidized LDL. Several studies have shown that exposing different types of macrophages to extensively oxidized LDL results in significant accumulation of free cellular cholesterol but only a small increase in cholesteryl ester. Specifically, Roma et al. (238) showed that incubation of J774 macrophage cell line with extensively oxidized LDL (24 hours oxidation by 20 μ M Cu2+) resulted in a significant (2.4 fold) accumulation of total cellular cholesterol with the bulk of accumulated cholesterol (85%) being in the form of free cholesterol and only a small increase in cellular cholesteryl ester. Furthermore, there was only a small increase in synthesis of cholesteryl oleate, indicating further that the uptake of OxLDL failed to induce ACAT activation. In contrast, exposure to acLDL in the same series of experiments resulted in significant accumulation of both free cellular cholesterol and cholesteryl ester (237, 238). A lack of cholesterol esterification could not be attributed to OxLDL-induced cytotoxicity or to direct inhibition of ACAT (237), an effect that was previously reported in endothelial cells (127). To verify whether Cu2+-oxidized LDL used in this study is a relevant model for biologically oxidized LDL, cholesterol accumulation was compared in J774 macrophages exposed to Cu2+-oxidized LDL (24 hours) or to LDL oxidized by the incubation with endothelial-like EAhy-926 cells (237). The two effects were very similar: in both cases, there was a significant increase in free cholesterol but little increase of esterified cholesterol, the latter not exceeding 21% of total cholesterol (237).
Consistent with the studies of Roma et al. (92), Brown et al. (32, 34) showed that incubation of mouse peritoneal macrophages with extensively oxidized LDL (24 hours oxidation with 20 μM Cu2+) also resulted in strong (5-fold) increase in total cellular cholesterol with free cholesterol constituting ∼40%–50%, and cholesteryl ester about 5%–10%. Oxysterols, predominantly 7-ketocholesterol, were reported to comprise up to 50% of total sterol content of OxLDL-loaded cells (32, 34). Furthermore, macrophages accumulated free and esterified sterols in very similar proportions to those found in OxLDL itself and the pools were not significantly affected by the ACAT inhibition suggesting that the esters of sterols found in the cells are not generated by the ACAT activity but instead derive directly from the donor OxLDL (32). Macrophages derived from human peripheral blood monocytes appear to be even more resistant to OxLDL-induced accumulation of cellular cholesterol (288). In this case, accumulation of cellular cholesterol (∼30% increase) was observed only after a prolonged exposure (7 days) with no effect after shorter periods of time. At the same time, incubation with acLDL resulted in significant cholesterol accumulation with more than 3.5-fold increase after 7 days. Consistent with these observations, only acLDL-treated cells were stained strongly with Oil Red O stain, indicating significant accumulation of cellular lipids whereas cells incubated with strongly-oxidized LDL showed only mild staining (288).
To explain the lack of cholesterol esterification, it was proposed that deficient degradation of OxLDL which results in trapping of the LDL particle in lysosomes prevents the delivery of free cholesterol to ACAT-sensitive pool. Indeed, several studies have shown that oxidized LDL is resistant to acid proteolysis and is not efficiently degraded (126, 172, 237). Specifically, Lougheed et al. (172) showed that in contrast to acLDL that is degraded by more than 90%, OxLDL is resistant to cathepsins, the enzymes responsible for the lysosomal degradation of LDL and only ∼50% of OxLDL is efficiently degraded. The same effect was observed for mildly oxidized and extensively oxidized LDL (172). Similarly, Jessup et al. (126) showed that OxLDL is more resistant to proteolysis by lysosomal enzymes than native LDL while acLDL is more sensitive to degradation. It was also shown that defective hydrolysis is accompanied by the accumulation of OxLDL in the lysosomal compartment (176, 237). Thus, resistance of OxLDL to cathepsins and retention in the lysosomal compartment may explain the poor cholesterol esterification in the cytoplasm by ACAT and depositions of OxLDL components in the lysosomes (178), which by itself may have significant pathological consequences, as reviewed by Jerome (123). However, Brown et al. (34) found that there was no selective retention of free cholesterol in the lysosomes (34). Instead, it was shown that a limited supply of fatty acid co-substrates may be responsible for the poor esterification rate (34). Other studies, however, showed that exposure to extensively oxidized LDL does lead to a significant increase in cholesteryl ester [e.g., (44)]. Taken together, these studies suggest that it is still controversial whether exposure of macrophages to extensively oxidized LDL in vitro may fully simulate cholesterol accumulation in foam cells in vivo. Furthermore, it was proposed that extensively oxidized LDL may be ineffective in loading cells with cholesterol because heavy oxidation results in the formation of an array of oxysterols and oxidized cholesteryl esters while depleting the LDL particle of unoxidized cholesterol and cholesteryl ester (32, 82). Further studies, therefore, focused on mildly oxidized LDL.
Mildly oxidized LDL was shown to be significantly more efficient in loading macrophages with both free cholesterol and cholesteryl ester, however this effect was strongly species dependent (308). In mouse peritoneal macrophages, exposure to mildly oxidized (2 hours oxidation) resulted in significant accumulation of free cholesterol within the first 24 hours of the exposure, after which most of free cholesterol (∼75%) was esterified and the excess was stored as cytoplasmic cholesteryl ester droplets (308), as is observed in foam cells. However, in human THP1-derived macrophages, while exposure to OxLDL also resulted in a significant increase in both free cholesterol and cholesteryl ester, the lipids accumulated in the lysosomal compartment. Moreover, while inhibition of ACAT activity resulted in a significant decrease in cholesteryl ester in mouse macrophages, there was only little effect on the level of cholesteryl ester in human macrophages. The latter observations suggest that the major source of cholesteryl ester in OxLDL-loaded human macrophages is not esterification of cholesterol by ACAT but direct uptake from the OxLDL particle. In this respect, the pattern of degradation of mildly oxidized LDL observed in this study was similar to that described for extensively oxidized LDL described above. However, the type of esters accumulated in the lysosomes appear to depend on the degree of LDL oxidation. For extensively oxidized LDL, ester accumulation is mainly comprised of oxidized esters derived from OxLDL, whereas for mildly oxidized LDL, the majority of cholesteryl esters accumulating in the lysosomes are degradable unoxidized esters (125, 308). In this case, accumulation of the esters in the lysosomal compartment was proposed to be due to the inactivation of lysosomal hydrolases (125). Importantly, LDL modified by the myeloperoxidase–peroxinitrite system (NO2-LDL), which is considered to be closer to the physiological form of OxLDL, also appears to be more efficient than Cu2+-oxidized LDL in promoting cholesterol accumulation and esterification in vitro (225). More specifically, exposure of mouse macrophages to NO2-LDL resulted in significant increase in cholesteryl ester synthesis and accumulation of the lipid in the cytosol in the CD36-dependent way (225). However, in contrast to these studies, no cholesterol accumulation was observed in human macrophages derived from human peripheral blood monocytes exposed to mildly oxidized LDL (288). Thus, while the preponderance of evidence suggests that mildly oxidized forms of OxLDL are more efficient than extensively-oxidized LDL in loading macrophages with both free cholesterol and cholesteryl esters, there are still significant discrepancies between different studies. These discrepancies may arise from the differences between human and mouse macrophages, suggesting that more studies are needed to determine the ability of different types of OxLDL to induce cholesterol loading in human macrophages.
B. OxLDL-induced cholesterol loading in vivo
The evidence for the critical role of OxLDL in cholesterol loading and formation of foam cells in vivo comes primarily from the studies of targeted disruption of different scavenger receptors. As described above, macrophages isolated from mice that lack SRAI/II or CD36 receptors are significantly impaired in their ability to internalize OxLDL in vitro (153, 171, 275). It is also well established that disruption of SRAI/II and CD36 scavenger receptors result in a significant decrease in the appearance of foam cells and lipid depositions in vivo [e.g. (13, 192, 242, 275)]. Furthermore, analysis of free cholesterol and cholesteryl ester levels in macrophages isolated from male SRAI/II and CD36 knockout mice reveals that disruption of each of the pathways results in 40%–60% decrease reduction in free cellular cholesterol with a corresponding decrease of 70%–80% in cholesteryl ester (192). The impacts of disruption of the SRAI/II and CD36 pathways were comparable. Surprisingly, while both pathways were efficient in male mice, neither pathway had a significant impact on cholesterol accumulation in macrophages isolated from female SRAI/II and CD36 knockout mice (192). The gender specificity of this effect is not clear. In any case, there is an important difference between OxLDL-induced cholesterol accumulation in vitro and an apparent OxLDL-induced cholesterol accumulation in vivo, as determined from the disruption of the major OxLDL uptake pathways. As described above, incubation of macrophages with OxLDL, particularly when it is extensively oxidized in vitro, results in accumulation of free cholesterol but not of cholesteryl ester. In contrast, a significant reduction in the level of cholesteryl ester in macrophages lacking SRAI/II or CD36 receptors reported in the study of Moore et al. (192) suggests that OxLDL contributes significantly to the accumulation of cholesteryl ester. It is also important to note that disruption of SRAI/II receptors which recognize extensively oxidized LDL rather than mildly-oxidized LDL had an even stronger negative effect on the accumulation of cholesteryl ester than disruption of the CD36 pathway (192).
What are the possible explanations for these discrepancies? One possibility that has been proposed in the literature is that OxLDL–immune complexes may be significantly more efficient in inducing macrophage activation and foam cell formation than OxLDL alone (89). The predominant OxLDL antibody isotype is IgG (293) that, as described above, is recognized by Fcγ receptors (FcγR), which is well known to induce macrophage activation and therefore are pro-inflammatory. Specifically, anti-OxLDL IgG antibodies, subclasses 1 and 3, can activate the complement system by the classical pathway and interact with FcγR in phagocytic cells (293). Furthermore, OxLDL induces overexpression of FcγR on monocytes as they differentiate to macrophages further increasing the inflammatory response (234). Cross-linking of FcγR by OxLDL-IC activate macrophages and may trigger signal transductions pathways that are not induced when OxLDL uptake is mediated by scavenger receptors (89). They also have higher levels of cholesteryl esters and increased release of cytokines when compared to the ones exposed to OxLDL only. Finally, foam cells generated by IC uptake have prolonged survival compared to the ones induced by OxLDL alone (89).
However, in addition to the IgG antibodies, OxLDL may also induce IgM antibodies, which may have a protective role. IgM antibodies do not interact with FcγR of phagocytic cells (293). Moreover, interaction with FcγR does not induce the inflammatory response. In contrast, OxLDL IgM antibodies may be atheroprotective because they inhibit the uptake of OxLDL by macrophages. However, while anti-OxLDL IgM are predominant antibodies in different mouse models of atherosclerosis, their role in humans is debatable. While some studies showed a negative correlation between IgM MDA–LDL antibody levels and carotid intima media thickness (IMT) (132) and found that IgM anti-OxLDL antibodies correlated with the reduction in the development of carotid atherosclerosis in hypertensive patients (270). Other studies showed that these antibodies actually correlate with a more rapid progression of carotid disease based on IMT measurement (293).
It is also possible that MM-LDL may induce LDL and OxLDL uptake via macropinocytosis (44) and since both MM-LDL and OxLDL are likely to co-exist in the environment of an atherosclerotic lesion in vivo, it is possible that it is the simultaneous exposure to different types of OxLDL that is important for lipid accumulation.
C. Disruption of OxLDL uptake pathways in lesion formation
Multiple studies investigated the impact of targeted disruption of the major OxLDL uptake pathways on the extent of lesion formation in different models of atherosclerosis-susceptible mice. Most but not all of the studies have found that deletion of both SRAI/II and CD36 receptors results in marked decrease in the areas of atherosclerotic lesions (Fig. 9). More specifically, in the earlier studies, Suzuki et al. (275) have shown that disruption of SRAI/II receptors resulted in more than 50% decrease in the area of atherosclerotic plaques in the double SRA/apoE knockout mice fed a high cholesterol diet. Furthermore, a decrease in the area of the plaques was observed in spite of an increase in plasma cholesterol in the double knockout mice, as compared with apoE knockout, suggesting that modification of LDL within the arterial wall and their uptake by macrophages is more critical for the plaque formation than the plasma cholesterol level. Surprisingly, however, no significant differences were observed in the levels of the plasma cholesterol and triglycerides between SRA-deficient and normal mice, and the clearance rate of acLDL injected into the circulation was also not affected. Consistent with these studies, targeted disruption of SRA receptors also resulted in a significant decrease in atherosclerotic lesions in LDL receptor-deficient mice, with the effects ranging between 30% to more than 90% depending on the duration of the diet with a stronger effect observed after a more prolonged period (13, 242).
FIG. 9.
Decrease in the area of atherosclerotic lesions in SRAI/II/Apo E and CD36/Apo E double KO mice. Targeted disruption of SRAI/II receptors in ApoE KO mice was shown to decrease the area of atherosclerotic lesions in some but not in all studies. Similar observations were made on the other pro-atherogenic genetic backgrounds. Disruption of CD36 on the Apo E-deficient background resulted in the decrease in atherosclerotic lesions in the majority but also not in all studies. Several factors, such as severity of the disease, duration of the diet, and the specific site of the vascular tree that was analyzed in these studies were suggested to be responsible for the variability in the responses. Numbers in parenthesis are references.
Similarly, targeted disruption of CD36 in apoE knockout mice resulted in 45%–75% decrease in lesion formation (63). Furthermore, a lack of CD36 in macrophages alone was sufficient to dramatically decrease the area of atherosclerotic lesions (88%), whereas re-introduction of macrophage CD36 resulted in a significant increase in lesion area (62). In more advanced atherosclerosis, the effect was more modest with 25%–35% decrease in CD36/apoE double knockout mice (84). There was also significant variation in lesions at different areas of the vascular tree. In case of CD36 deficiency, the effect was observed mainly in the areas that are less prone to the development of atherosclerosis. Specifically, there was no decrease in lesion formation in sinus and arch of the aortas, the regions that are most prone to the development of atherosclerosis, and it was suggested that these regions may accumulate mostly extensively oxidized LDL that can be taken up more efficiently by the SRA receptors (63). Deletion of LOX-1 also resulted in a significant (∼50%) decrease in the area of atherosclerotic lesions and intima thickness in aortas of LDL-receptor deficient mice (186).
However, SRA deficiency on the background of ApoE3Leiden mice with dysfunctional Apo E variant had no beneficial effect on atherosclerosis development, instead leading to more complex lesions without any decrease in the lesion area (53). More surprisingly, Moore et al. (192) found no decrease in atherosclerosis in apoE knockout mice lacking either SRAI/II or CD36 receptors. Quite to the opposite, in spite of a decrease in foam cell formation, histological analysis revealed that the cross-sectional lesion areas in aortic sinus region were 20%–40% percent greater both in mice lacking SRAI/II receptors and mice lacking CD36 receptors. An increase in lesion formation correlated with an increase in serum cholesterol, which was also observed in the earlier studies (275). The authors pointed out that while elimination of SRAI/II and CD36 pathways decreases lipid accumulation leading to decreased lesion areas as detected by en face measurements, this decrease is not reflected in the intimal area measurements. Moore et al. (192) also found that some of the lesions in CD36/apoE double knockout mice contain large acellular regions characteristic of a necrotic core and it was suggested that the loss of OxLDL uptake may lead to increased extracellular accumulation of the lipids which may contribute to the development of the disease. Moore et al. (192) proposed, therefore, that in contrast to previous studies that supported a pro-atherogenic role of the macrophage scavenger receptors, these pathways may be anti-atherogenic, providing a protective effect. This hypothesis was further addressed in a recent study of Kuchibhotla et al. (150) by comparing both the en face and the histological analysis of the lesion in Apo E knockout mice lacking SRAI/II and CD36 receptors. In this study, deletion of CD36 pathway resulted in a strong protective effect, decreasing lesion areas by 60%–70%, but no additional decrease in lesion formation was observed after deletion of both CD36 and SRAI/II receptors. Deletion of SRAI/II alone had no effect on lesion area in male mice and a modest effect in female mice. Thus, while the effect of SRAI/II was somewhat comparable with the results of Moore et al. (192), effect of CD36 deletion was dramatically different. One of the proposed explanations was a difference in the duration of the diet, with the earlier study looking at early lesions and the later study looking at more advanced lesions (150, 192). Variability between the different areas of the vascular tree may also contribute to the conflicting results. Clearly, it is necessary to understand in detail the signaling pathways that are activated by OxLDL uptake via different receptors and how these signaling pathways may lead to the development of the inflammatory process that underlies atherosclerosis development.
X. OXLDL in Endothelial Cells: Cholesterol, Caveolae, and Lesion Formation
Numerous studies have shown that endothelial dysfunction develops in the early stages of cardiovascular disease (CVD) and is a strong predictor of CVD development [reviewed by (77, 240, 256)]. It is also well known that exposure to OxLDL induces an array of endothelial responses, including inhibition of endothelial-induced release of nitric oxide, a vasorelaxation factor that also has anti-inflammatory and antithrombotic properties, regulation of endothelial permeability and inflammation, and altered angiogenesis [reviewed by (24, 100, 253)]. However, it is also being increasingly recognized that the impact of OxLDL on endothelial cholesterol may be complex. The goal of this part of our review is to analyze the studies addressing this issue.
A. OxLDL-induced impact on endothelial cholesterol in vitro: Loading or depletion?
In spite of the fact that endothelial cells express multiple scavenger receptors that bind and internalize OxLDL (3), exposure of endothelial cells to OxLDL in vitro was reported to result in cholesterol depletion rather than cholesterol loading. Specifically, Jialal et al. (127) reported that while OxLDL was efficiently degraded by human endothelial cells isolated from umbilical vein, there was no significant increase either in free or esterified forms of cholesterol after 24 hours of exposure. Furthermore, exposure to OxLDL resulted in significant inhibition of ACAT activity, resulting in more than 30% decrease in cholesterol esterification rate. Surprisingly, Blair et al. (25) showed that a short (30 min) exposure to OxLDL not only does not induce cholesterol accumulation in endothelial cells but actually results in dramatic depletion of cholesterol from the caveolae, a specific membrane domain that constitutes a scaffold for multiple signaling events and is cholesterol rich. As expected, cholesterol depletion of caveolae was accompanied with the loss of caveolin from the membrane. Moreover, since caveolae cover only a relatively minor part of the whole surface of the plasma membrane (less than 10%) there is no real contradiction between cholesterol depletion of caveolae observed by Blair et al. (25) and a lack of any effect on endothelial cholesterol level observed in the earlier study (127). While it is definitely counterintuitive that exposure to OxLDL may result in cholesterol depletion, this surprising effect has been supported by both direct and indirect observations by several other studies. First, a later study by the same group (285) reported that exposing the cells to HDL that serves as cholesterol donor reverses OxLDL-induced cholesterol depletion of endothelial caveolae. In terms of the mechanism, Uittenbogaard et al. (285) demonstrated that OxLDL-induced internalization of endothelial nitric oxide synthase (eNOS) that accompanied cholesterol depletion was blocked by CD36-blocking antibodies, implicating CD36 receptors in OxLDL- induced cholesterol depletion. Moreover, consistent with these observations, Yeh et al. (309) showed that cholesterol depletion can also be induced by exposing the cells to oxidized phospholipid oxPAPC, an active component of oxidized LDL that is a ligand for CD36 receptors. In this study, however, there was only a moderate (∼30%) decrease in cholesterol level of caveolin-rich membrane fractions, suggesting that activation of CD36 by oxPAPC is not sufficient to induce the full effect of OxLDL. Alternatively, it is also possible that caveolin-rich fractions isolated in the two studies contained different subpopulations of lipid rafts.
Consistent with these studies, we have also shown that a short exposure of endothelial cells to OxLDL induces internalization of another lipid raft marker GM1 (39). Furthermore, we have shown that depletion of endothelial cholesterol with a cholesterol acceptor methyl-β-cyclodextrin had a similar effect. However, in contrast to the study of Blair et al but similarly to the earlier study of Jialal et al. (127), we did not detect any OxLDL-induced changes in the level of cholesterol either in caveolin-rich or in caveolin-poor domains. It is most likely that the reason for this discrepancy was the nature of caveolin-rich fractions isolated in the two studies. In our study, caveolin-rich fractions contained ∼75% of total membrane cholesterol, while in the study of Blair et al., caveolin-rich fractions were isolated by a different procedure contained only ∼4% cellular cholesterol, indicating that it contains only a subpopulation of lipid rafts. These observations suggest that OxLDL may specifically affect a subpopulation of caveolin-rich membrane domains. Importantly, in spite of the discrepancy in detecting OxLDL-induced decrease in the level of endothelial cholesterol, our studies provide several lines of evidence supporting the notion that exposure to OxLDL results in cholesterol depletion rather than cholesterol enrichment of endothelial cells. First, we have shown that exposure to OxLDL induces an increase in endothelial stiffness, force generation and the ability of the cells to form endothelial networks and that all of these effects could be simulated by cholesterol depletion (39). More recently, we have shown that OxLDL facilitates the ability of endothelial cells to realign in the direction of the flow and that this effect was also simulated by cholesterol depletion and rescued by loading the cells with cholesterol (144). Taken together, these studies demonstrate that acute exposure of endothelial cells to OxLDL disrupts cholesterol-rich membrane domains. The key question, however, is whether acute short exposure to OxLDL has the same effect as OxLDL in vivo.
B. Dyslipidemia-induced disruption of endothelial caveolae in vivo
Two lines of evidence suggest that dyslipidemia in vivo is also associated with the disruption of caveolae in vascular tissues. First, Darblade et al. (50) have shown that endothelial cells covering fatty streaks in the vessels of cholesterol-fed rabbits have fewer caveolae than endothelial cells in control tissues, as estimated by electron microscopy. Treating endothelial cells in culture with methyl-β-cyclodextrin that depletes cellular cholesterol had a very similar effect on the abundance of endothelial caveolae as dyslipidemic conditions in vivo. Furthermore, a decrease in endothelial caveolae both in vitro and in vivo was associated with impairment of endothelial NO production. Consistent with these observations, Kincer et al. (137) showed that cholesterol content of caveolae isolated from vascular tissues of apoE-deficient mice is significantly lower than cholesterol content of caveolae in control mice. It is also important to note that since endothelial cells constitute only a single layer on the inner surface of the blood vessels, the majority of the vascular tissue is contributed by smooth muscle cells. Therefore, cholesterol depletion of caveolae in the vascular tissues of apoE-deficient mice indicates that this effect occurs also in smooth muscle cells. Our observations showing that endothelial cells isolated from aortas of hypercholesterolemic pigs are stiffer than cells isolated from control animals, an effect that we observed in OxLDL-treated and cholesterol depleted but not in cholesterol enriched cells (39) are also consistent with the notion that plasma dyslipidemia results in disruption of endothelial caveolae. The question is whether cholesterol depletion from caveolae is pro- or anti-atherogenic. On one hand, a loss of caveolin-1 (Cav-1) in apoE-/- mice results in a decreased lesion formation, suggesting that disruption of membrane rafts may be anti-atherogenic (65). This observation is also consistent with enhanced activation of eNOS in Cav-1-deficient mice, an effect attributed to the loss of inhibitory Cav-1/eNOS interaction (37, 57). However, exposure of ECs to OxLDL inhibits NO release, an effect attributed to cholesterol depletion of caveolae (25). These observations suggest that OxLDL-induced cholesterol depletion and internalization of caveolae have pro-atherogenic rather than anti-atherogenic effect. Taken together, these studies suggest that it is the disruption of caveolae rather than cholesterol loading that may be responsible for OxLDL-induced impairment of endothelial function.
XI. Is Oxidation of LDL Necessary for the Development of Atherosclerosis?
The OxLDL hypothesis of atherosclerosis was first proposed by Steinberg and colleagues (264) based on the fact that normal LDL uptake does not cause foam cell formation, whereas the modification of LDL, such as acetylation, results in massive uptake of LDL by the scavenger receptors and foam cell formation. The essentials of this hypothesis are as follows. The polyunsaturated fatty acids in the LDL are oxidized by enzymatic and nonenzymatic pathways in the arterial tissue, specifically by the endothelial cells and macrophages. The lipid degradation products derivatize functional groups on Apo B, rendering the latter unrecognizable by the normal LDL receptor, but making it a ligand for the various scavenger receptors on the macrophages that take up OxLDL in unregulated manner and become foam cells. The OxLDL also contains factors that have chemotactic effects on monocytes, cytotoxic effects on endothelial cells, and growth promoting effects on smooth muscle cells, further promoting atherosclerosis.
A. Evidence supporting the hypothesis
Ever since the original hypothesis proposed by Steinberg and colleagues (264), supporting evidence for the role of oxidized LDL in atherogenesis has come from several laboratories, through the study of experimental models as well as from clinical studies. The most compelling arguments for the role of LDL oxidation can be summarized as follows: 1). OxLDL has been shown to be present in atherosclerotic lesions, but not in normal artery (239). 2). Autoantibodies generated against epitopes of OxLDL are present in the circulation, and, moreover, the titers of these antibodies correlate positively with the severity of the atherosclerosis and are predictive of the disease (119, 284, 292). 3). Studies with various animal models (rabbit, mouse, hamster, guinea pig, and monkey) and with numerous types of antioxidants (including vitamin E, probucol, analogues of probucol, and coenzyme Q) have all demonstrated a protective effect of the antioxidants against atherosclerosis (302), although the clinical trials in humans with vitamin E were not positive. 4). Genetic ablation of various scavenger receptors in mice results in a significant reduction in atherosclerosis (54, 150, 177, 186), showing the importance of these receptors, as well as their ligands (OxLDL) in the development of the lesions. 5). Deletion of 12/15 lipoxygenase, which is a physiological oxidizing agent for LDL, similarly ameliorates atherosclerosis (113), whereas its overexpression accelerates atherosclerosis (92), supporting the importance of oxidative modification of LDL. 6). Recent studies of Kato et al.(134) in Apo E-deficient mice show that the increase in plasma levels of OxLDL occurs before the development of the lesions. 7). Specific reduction of plasma OxLDL by overexpression of hepatic LOX-1 receptor was shown to prevent progression of atherosclerotic lesions in Apo E-deficient mice, even in the presence of severe hypercholesterolemia (116). 8). The negative results with the antioxidant trials in humans may have been due to the use of wrong type of antioxidant at wrong doses and for too short a period of time (302). Furthermore, vitamin E does not prevent the peroxidase-mediated oxidation, and in fact, addition of vitamin E, as well as some other antioxidants, paradoxically increased the rate of LDL oxidation (217, 246). These observations led to the hypothesis that small amounts of antioxidants may actually enhance peroxidase- mediated LDL oxidation (246) (217). It was also proposed, however, that the presence of high concentrations of antioxidants may block the propagation of oxidation (217, 246). In addition, part of the reason for the lack of the protective effect of antioxidants appears to be the inability of α-tocopherol to prevent decomposition of preformed lipid peroxides (230).
B. Evidence inconsistent with the hypothesis
Although there is a general consensus that the oxidative modification of LDL results in its increased uptake by macrophages, resulting in the formation of foam cells in vitro, there are several observations that suggest that OxLDL generation may not be a prerequisite for the lesion formation in vivo. The evidence for this view may be summarized as follows: 1). Foam cell formation can occur in the presence of native LDL, when the macrophages are activated by PMA (148). It is also well known that the selective modification of Apo B by acetylation or cationization can result in massive accumulation of lipids in macrophages, in the absence of significant oxidation of LDL lipids (19, 75). Treatment of LDL with physiologically relevant enzymes such as sPLA2, SMase C, and phospholipase C increases its retention by proteoglycans in the arterial tissue, leading to internalization by the nonscavenger receptor pathways (148). Therefore, it is not essential for the LDL lipids to be oxidized for the unregulated uptake of LDL and generation of foam cells. 2). While there is strong evidence that OxLDL is present in the arteries, it has not been conclusively shown that the lipids present in foam cells are indeed derived from OxLDL, as opposed to from the native, aggregated, or nonoxidatively modified LDL. As discussed above, the accumulation of cholesteryl esters in the cells after incubation with OxLDL is much lower than observed with other forms of modified LDL. The time course of accumulation of lipids in human atherosclerotic lesions showed that the accumulation of oxidized lipids occurred later than the accumulation of unoxidized lipids (286), suggesting that the initiation of the lesion formation occurred before the oxidation of LDL lipids. 3). Advanced human lesions contain significant concentrations of antioxidants such as vitamin E and vitamin C, which is inconsistent with the fact that the oxidation of LDL requires a complete depletion of these antioxidants before lipid oxidation can occur (286). Furthermore, the anti-atherosclerotic effects of probucol and its analogs do not correlate with their antioxidant effects, but with their ability to induce hemoxygenase-1, an anti-inflammatory enzyme (306) . 4). Perhaps the most troubling findings against the OxLDL hypothesis are the clinical trials with antioxidants such as vitamin E. The majority of the randomized clinical trials on the effect of vitamin E supplementation on cardiovascular disease showed no protective effect in large population studies (95, 302), casting doubts on the hypothesis that prevention of LDL oxidation would be protective against atherosclerosis. It has been pointed out, however, that the design of these studies may be flawed, and that they do not necessarily disprove the hypothesis (217, 302). Compounds that promote the reduction of hydroperoxides to hydroxides have been suggested to be more effective in treating atherosclerosis than vitamin E, which is more effective in preventing the initiation of lipid peroxidation and lesion formation (230).
In summary, while all of the above arguments cast some doubt on the absolute requirement of LDL oxidation for the initiation of atherosclerosis, there is no doubt that OxLDL promotes the lesion development in many ways. Furthermore, it is also increasingly recognized that OxLDL may not only be important for the development of atherosclerosis but also contribute to other diseases, particularly to diabetes mellitus, which is a major risk factor for cardiovascular disease. There is also emerging evidence that OxLDL may play important role in several autoimmune diseases independently of atherosclerosis. These studies are described in the next two sections of the review.
XII. OxLDL in Diabetes Mellitus
Diabetes mellitus (DM) is a chronic disease characterized by pathologic glucose metabolism and is associated with significant morbidity and mortality. Multiple studies have demonstrated that there is a clear connection between oxidative stress and development of type 2 diabetes mellitus (T2DM) that is related to insulin resistance. First, several reports showed increased plasma OxLDL levels in the metabolic syndrome as well as in T2DM and obesity (248). It is also known that hyperglycemia induces nonenzymatic oxidation of a variety of lipids and proteins. Furthermore, several studies have shown that increased levels of ROS in peripheral blood contribute to the development of insulin resistance in muscle and adipose tissues with worsening beta-cell insulin secretion. One of the mechanisms proposed to be involved in this process is OxLDL-induced impairment of glucose transporter 4 (GLUT4) (248). This transporter is stored in intracellular vesicles in insulin-sensitive cells like adipocytes and myocytes and translocates to the plasma membrane upon stimulation by insulin. Exposure of adipocytes to OxLDL was shown to induce a marked insulin-desensitization characterized by a decrease in glucose uptake that was due to the impairment of GLUT4 translocation.
Another mechanism by which OxLDL may contribute to the development of diabetes is vascular damage that is due to the generation of glucose-oxidized LDL (g-OxLDL). Indeed, it is well known that hyperglycemia induces glycation of proteins and lipids, resulting in formation of advanced glycation end products (AGEs) (274). Accumulation of AGEs in the vessel wall has been shown to promote vascular disease in DM. The receptor for AGE (RAGE) is expressed on the surface of vascular endothelial cells, smooth muscle cells, and macrophages, and is involved in endothelial activation in diabetic patients. There is an enhanced expression of RAGE and its ligands in the atherosclerotic lesion in diabetes. Since OxLDL contains AGE epitopes, it can bind RAGE in macrophages, especially in hyperlipidemic state and enhance macrophage proliferation and oxidative stress (274).
OxLDL may also be involved in the development of renal disease in DM, since it participates in the development of glomerulosclerosis and interstitial fibrosis in patients with chronic kidney diseases (156). LDL trapped in the mesangial matrix or tubulointerstitium, may be oxidized due to depletion of antioxidants found in plasma and extracellular fluid. The inflamed glomeruli or interstitium are infiltrated by neutrophils and monocyte/macrophages making condition for LDL oxidation highly favorable, which is confirmed by demonstration of OxLDL in renal biopsies. OxLDL induces foam cell formation in glomeruli and further increases production of inflammatory cytokines, chemokines, and growth factors. This induces injury and death to surrounding cells, resulting in renal fibrosis (156). More specifically, it appears that glomerulosclerosis and interstitial fibrosis are mediated by activation of TGFβ. Nakhjavani et al. showed that ox-LDL and TGF-ß are significantly elevated in diabetic patients (204). Ox-LDL correlated with serum TGF-ß in T2DM patients and remained significant after adjustment for age, sex, and BMI (204).
Ox-LDL stimulates the expression of TGFβ-1 in cultured mesangial and glomerular epithelial cell and TGFβ1 protein itself can induce ROS generation in mesangial cells. TGF-β/Smad signaling in mesangial cells affects TGF-β-inducible promoters in nucleus including α2(I) collagen, TGF-β1, and PAI-1 genes whose products are responsible for the fibrotic effect (156). OxLDL also induces Ras/ERK activation, leading again to Smad activation. This was also noted to be the pathway by which OxLDL activation of AngII/R leads to Smad activation. TGF-β, angiotensin II (Ang II), and ischemia can upregulate tubulointerstitial LOX-1 expression, further enhancing deleterious effects of OxLDL on tubulointerstitum ultimately leading to fibrosis (307).
Importantly, LDL like any plasma protein, is susceptible to AGE modification and when glycated it becomes more prone to oxidation and more atherogenic (294). It was also shown that glucose-oxidized LDL (g-OxLDL) enhances the proliferative response of SMC to insulin-like growth factor I and since migration and proliferation of SMC are important for the formation of atherosclerotic lesions, g-OxLDL-induced SMC proliferation contributes to lesion formation in DM (5). Also, AGE-modified proteins are immunogenic and may induce inflammation. The AGE-LDL autoantibodies isolated from the sera of patients with diabetes mellitus are mostly IgG1 and IgG3 subtypes, the very same subsets of IgG to OxLDL that are known to promote atherosclerosis (294).
However, interestingly, Garrido–Sanchez et al. found that there is an inverse correlation between the levels of anti-OxLDL antibodies and development of carbohydrate disorder: Surprisingly, patients that initially had no carbohydrate disorder and presented with low levels of antioxidized LDL antibodies (below 50th percentile) had a 1.5-fold greater relative risk of developing some carbohydrate metabolism disorder after 6 years than patients with high levels of the antibodies (above 50th percentile) (71). Furthermore, patients with some form of carbohydrate metabolism disorder and low antibodies had even higher risk (9.79-fold) of developing T2DM than those whose antibody levels were high. This association was independent of the presence of other variables that have a known risk for T2DM. It is possible that anti-OxLDL antibodies are actually protective because the OxLDL immune complexes may be blocking the pathological effect of OxLDL on glucose metabolism (71).
Thus, multiple lines of evidence suggest that OxLDL is involved in DM disease development, particularly in DM-associated kidney disease, as well as in DM-associated cardiovascular disease. It is also important to note that OxLDL may be an important factor in other kidney diseases that lead to glomerulosclerosis (156), but this topic is beyond the scope of the current review. In the next part of the review, we will discuss the role of OxLDL in several autoimmune diseases. Indeed, as described above, OxLDL immune complexes were shown to play important roles in both atherosclerosis and diabetes. However, it may be of even greater importance in autoimmune diseases that are characterized by increased production of autoantibodies.
XIII. OxLDL in Autoimmune Diseases
It is well known that OxLDL induces a variety of autoimmune responses through both adaptive and innate mechanisms.
A. Systemic lupus erythematosus and antiphospholipid syndrome
Systemic lupus erythematosus (SLE) is a chronic inflammatory disease of unknown cause which can affect many organs and is characterized by the production of a number of antinuclear and other autoantibodies and immune complex (IC) formation. Antiphospholipid syndrome (APS) is an autoimmune disease characterized by the presence of a heterogeneous group of antiphospholipid antibodies (aPL), that clinically presents with thromboembolic complications, and/or pregnancy morbidity. APS can exist independently of lupus (primary APS) but is often found in lupus patients (secondary APS), suggesting that the two diseases are associated. There is emerging evidence that OxLDL may contribute to clinical manifestations of both diseases.
Several lines of evidence suggest that SLE is associated with increased oxidative stress. One, it is well known that increased oxidative damage is present in SLE patients compared to normal controls (181). Two, oxidative modification of 60 kD Ro ribonucleoprotein was shown to be pathogenic. Specifically, antibodies to this ribonucleoprotein are one of the markers of lupus, and oxidative modifications of this antigen were shown to induce lupus-like disease when introduced to rabbits (154). However, only few studies focused on evaluation of significance of OxLDL or oxidation in general with the disease activity and the results are mostly conflicting. One study found that 30% of the patients with SLE have marked changes in titers of anti-OxLDL antibodies over time, which correlated significantly with disease activity markers, particularly with complement levels (76). However, it is difficult to exclude the possibility that the correlation between OxLDL levels and SLE severity could be attributed to SLE-associated increase in atherosclerosis and decrease in complement levels. Another study showed that renal manifestations of lupus were associated with OxLDL/E06 and also with anti-OxLDL of the IgM subclass (68). Lastly, circulating complexes of OxLDL with β2 glycoprotein (β2GPI) were associated with renal involvement in SLE but not with disease activity (18, 182). It is important to note that appearance of antibodies to β2 glycoprotein is associated with both lupus and APS. However, no association between anti-OxLDL antibodies (either IgG or IgM) with nephritis, hemolytic anemia, or thrombocytopenia was found in another study that focused predominantly on Hispanic lupus population who often present with more severe disease (282).
In APS, an increase in OxLDL is associated with the disease activity (168). As described above, one of the major criteria for APS is the presence of aPL antibodies. These antibodies actually recognize phospholipid-binding plasma proteins, such as β2GPI in the complex with negatively charged phospholipids or OxLDL(168). β2GPI, a 50 kDa single-chain polypeptide, by itself is an anticoagulant. Hence, anti-OxLDL/β2GPI antibodies promote thrombosis. IgG and IgM antibodies to OxLDL/B2GP complexes in APS patients were found to have strong correlation with arterial and venous thrombosis but not with pregnancy morbidity (139, 168). The positive predictive value of IgG anti-OxLDL/β2GPI antibodies for arterial and venous thrombosis in patients with secondary APS was 92%, for arterial thrombosis was 88.9%, and for venous thrombosis was not statistically significant (167). However, Pengo et al. failed to prove correlation of these antibodies with arterial and venous thrombosis in patients with primary and secondary APS (219). Thus, whereas there are still conflicting reports, the preponderance of evidence suggests a significant correlation.
Importantly, it is well known that lupus is associated with increased atherosclerosis. There is also emerging evidence suggesting that atherosclerosis is also exaggerated in APS patients. Here, we will discuss the evidence suggesting that OxLDL is an important factor in the acceleration of atherosclerosis in lupus and APS patients. The reason that it may be an accelerating factor is that while OxLDL is quickly removed from the circulation, OxLDL/β2GPI immune complexes that form in these diseases due to the increased production of the antibodies persist for prolonged period of time, significantly increasing macrophage activation and foam cell formation (169). As described above, IgG anti-β2GPI antibodies are considered to act as proatherogenic factors by increasing the macrophage uptake of OxLDL/β2GPI complexes by binding to Fcγ receptors (139, 181). Indeed, immunostaining studies showed co-localization of OxLDL and β2GPI in atherosclerotic lesions (169, 183), supporting the notion that these complexes are deposited in the lesions and are atherogenic. Furthermore, circulating OxLDL/β2GPI immune complexes and corresponding antibodies are found in sera of patients with systemic autoimmune diseases, such as SLE and APS (154). More specifically, several studies have shown that IgG anti-OxLDL/β2GPI antibody levels are higher in SLE patients (18, 182) than in healthy controls, and even higher in SLE patients with APS (139), as compared to the ones without APS. Their correlation with atherosclerotic disease in patients with autoimmune diseases is still under investigation. One group showed that IgG anti-β2GPI-OxLDL independently predicted intima–media thickness of carotid artery and was inversely related to decrease in paraoxonase (PON) activity, in patients with primary APS (6) However, Bassi et al. did not find any correlation between IgG or IGM antibodies specific to these complexes and subclinical atherosclerosis in SLE patients (18)
Another connection between the autoimmune diseases and atherosclerosis is increased levels of C-reactive protein (CRP). CRP is an acute-phase protein produced mainly in liver, as well as by endothelial cells, macrophages, and smooth muscle cells in atherosclerotic lesions (181). It is also well known to be increased in autoimmune diseases, particularly during the exacerbation periods. Several studies have shown that high sensitivity CRP is a significant predictor of future cardiac events, and a reduction in its serum levels by statins correlates with a reduction in cardiovascular morbidity and mortality (142). CRP binds to OxLDL but not to native LDL via oxidized phosphatidylcholines and enhances binding of OxLDL to macrophages via FcγR (181). . The generation of CRP/OxLDL/β2GPI complexes seems to be associated with arterial inflammation, hyperglycemia, and hypercholesterolemia (277). Immunohistochemistry staining co-localized OxLDL, β2GPI, and CRP in carotid artery plaques (277). CRP can also directly interact with LOX-1, and the binding mechanism appears to be distinct from that for OxLDL (255). The treatment of human endothelial cells with CRP resulted in the induction of LOX-1 and its downstream genes IL-8, ICAM-1, and VCAM-1. CRP can induce endothelial LOX-1 expression through the FcγR, resulting in the induction of endothelial cell–monocyte adhesion and increased OxLDL uptake (255). CRP most likely induces LOX-1 expression through the activation of FcγR early in the inflammatory process, but once LOX-1 is expressed, it may function synergistically with the FcγR and lead to endothelial dysfunction and inflammation. These data imply that CRP may not be just a marker of atherosclerosis, but may be involved in lesion formation. Similarly, autoimmune diseases may cause exacerbation of atherosclerosis via an increase in another inflammatory marker serum amyloid A (SAA) that also complexes with LDL. SAA–LDL complex is produced at the sites of vascular injury from oxidatively denaturated LDL, and several reports have shown that SAA-LDL may be a marker of arterial plaque activity in patients with stable coronary artery disease (143). Thus, an increase in CRP and/or SAA levels in autoimmune diseases may have a significant impact on atherosclerosis development.
B. Rheumatoid arthritis
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic inflammation and joint destruction. Several studies have shown occurrence of oxidative damage in the joints in inflammatory arthritis. Two decades ago, Blake et al. (26) proposed that exercise-induced hypoxic-reperfusion injury in the joint is mediated by an increase in ROS generation. Hypoxic-reperfusion injury in the joint develops because during the exercise, intra-articular pressure (the pressure within the joint fluid) in the inflamed joints rises above synovial capillary perfusion pressure (pressure within the blood vessels of the joint tissue), disrupting the blood flow and inducing intra-articular hypoxia. Then, once exercise seized, the intra-articular pressure drops, resulting in an increased blood flow and reperfusion injury. This is not present in noninflamed joints where there is only small reduction of capillary perfusion. Blake et al. (26) found that hypoxic-reperfusion injury during the exercise induced a small but significant increase in lipid peroxidation products in the synovial fluid (fluid within the joint). Similar peroxidation products were found in the synovial fluid and in sera, as estimated by fluorescent IgG in these patients. The IgG antibodies and their subclasses have a high reactivity with ROS, which results in free radical alteration of IgG, rendering them immunogenic and reactive with rheumatoid factor (RF). The immune complexes of these antibodies and the rheumatoid factor are deposited in the joint tissue causing further tissue damage. These findings have been confirmed by other studies (187).
Overall, oxygen free radicals have been identified in synovial fluid of 90% of patients with RA (154). It was also found that ROS correlate with the levels of TNFα in the blood, which is a major marker of RA activity. Oxidative stress is also associated with sequential oxidation processes that generate advanced glycation end (AGE) products that are damaging to proteins. The circulating IgM anti-IgG AGE products have been identified in RA patients (mainly in RF-positive patients). Pentosidine, an AGE modification product, was elevated in 50% of patients with RA and its levels correlated with clinical disease. RA treatment decreased concentrations of MDA and induced statistically significant increase in the concentrations of antioxidants. It was suggested, therefore, that therapeutic co-administration of antioxidants may be beneficial in RA (154).
The first description of the OxLDL in the synovium (joint tissue) came from Winyard's group who detected both intracellular and extracellular OxLDL in the rheumatoid synovium using polyclonal rabbit antibody specific for human OxLDL (300). Intracellular staining was largely confined to foamy macrophages, which were found in the vessel proximity, and it was hypothesized that macrophages engulfed OxLDL shortly after diapedesis. These foamy cells were arranged linearly similar to the ‘fatty streak’ of atherosclerotic lesions. Consistent with this study, James et al. in 1998 found that LDL in inflamed synovial fluid was slightly more electronegative than LDL from matched plasma samples (121). They concluded that LDL in synovial fluid is mildly oxidized. It is known that synovium and synovial fluid in inflammatory joint disease have cellular content similar to that in atheroma, namely macrophages, lymphocytes, leukocytes, endothelial and SMC (121). Most of these cells can potentially oxidatively modify LDL.
Furthermore, generation of OxLDL in the inflamed joints may initiate the vicious cycle of further inflammation by inducing expression of LOX1 on endothelial cells (201), which increases OxLDL-induced endothelial damage, disrupting the endothelial barrier and increasing vascular permeability within the synovial vessels. The accumulated LDL and OxLDL in joint fluid might further permeate joint cartilage and induce further inflammation. Moreover, Nakagawa et al. showed that in zymosan-induced arthritis, one of the RA animal models, the expression of LOX-1 was not restricted to blood vessels, but was also detectable in other cell types within the joint, such as chondrocytes and synoviocytes, and was accompanied by the accumulation of OxLDL. Importantly, blocking LOX-1 with specific antibodies resulted in a decrease in joint swelling, cartilage degradation and serum TNFα levels, indicating that LOX-1 plays a major role in articular inflammation (201).
In the previous section, it was discussed that anti-OxLDL antibodies generated in SLE and APS may contribute to the disease activity. Similarly, Lourida et al. found higher antibody titers against mildly OxLDL in patients with early RA and it was independently associated with early disease development, providing evidence that mildly OxLDL may be implicated in the pathophysiology of RA (173). RA-specific treatment for one year resulted in a significant decrease of autoantibody titers against OxLDL, suggesting that treatment of the baseline disease may be also beneficial for decreasing the incidence of atherosclerosis.
Also, similarly to SLE, RA is strongly associated with increased cardiovascular morbidity and mortality (149). The majority of cardiovascular deaths in active RA occur due to accelerated atheroscleorsis. IgG OxLDL antibodies were associated with carotid atherosclerosis in patients with RA based on IMT measurements, and were independent of traditional risk factors for atherosclerotic diseases (295). In addition, OxLDL antibodies in RA are strongly related with the degree of inflammation, and their presence is positively correlated with CRP and negatively with HDL (220). In contrast to SLE, while RA patients showed higher anti-OxLDL/β2GPI antibody levels than the controls, this difference was not statistically significant (181). However, OxLDL, β2GPI, and CRP were found to co-localize in carotid lesions of RA patients, suggesting that these immune complexes may contribute to the development of atherosclerosis (277).
The question is, as with other inflammatory diseases, whether the treatment of the disease will decrease the development of the atherosclerosis and cardiovascular events. The results so far are conflicting. It was noted that RA patients treated with methotrexate, one of the most efficient anti-inflammatory drugs at present, had a 70% reduced cardiovascular mortality compared to those treated with other traditional antirheumatic drugs after adjusting for potential confounders (43). However, TNFα blockade, a newer anti-RA treatment strategy that is at least as efficient as methotrexate in treating inflammation appears not to be as beneficial as methotrexate for cardiovascular mortality. It is still too early though to reach firm conclusions about the efficacy of this therapy for atheroslerosis. Some studies showed transient improvement in lipid profile; others showed improvement in flow-mediated dilation or aortic pulse-wave velocity, while others found no change in the augmentation index (a composite measure of systemic arterial stiffness and wave-reflection amplitude or intensity) (56). Currently there are no available data regarding RA treatment-induced changes in the OxLDL level or titers of different antibodies to OxLDL and its complexes and their effect in atherosclerosis.
In summary, since inflammation induces lipid oxidation, any chronic infection or inflammatory disease such as RA, SLE, Sjogren's syndrome, vasculitidies (Behcet's disease or giant cell arthritis) may induce OxLDL production. Indeed, OxLDL and its antibodies to OxLDL or its complexes were found in several immune diseases. OxLDL is also found in the diseases where ischemia or hypoxia play major roles in initiation and disease manifestation, such as in obstructive sleep apnea. Yet, the role of this oxidized lipoprotein in pathogenesis of these diseases is still very much unknown or controversial.
XIV. Concluding Remarks
Oxidized LDL represents not only a heterogeneous mixture of LDL particles of varying degrees of oxidation, but each particle contains scores of bioactive compounds, including oxidized phospholipids, lysophospholipids, oxysterols, oxidized fatty acids, and variably modified Apo B. The complexity of the OxLDL studies is reflected in the plethora of cellular events and pathologies attributed to it and its components. In this review, we have explored the relationship between the mode of LDL oxidation and its biological effects, including recognition by various scavenger receptors, activation of different signaling pathways and cholesterol accumulation, the primary mechanisms of OxLDL-induced vascular injury. It is clear that there is a significant degree of specificity to different forms of OxLDL in terms of their recognition by the scavenger receptors and subsequent signaling. It is less clear, however, which of these forms is predominant in vivo and has the most pronounced effect on the development of atherosclerotic lesions and other pathophysiological consequences. Considerable progress has been made in recent years in identifying the bioactive products of phospholipids, especially those derived from PAPC and PLPC, as well as in the identification of the major cellular receptors for OxLDL uptake. However, there are still unresolved issues, including the identity of the physiological oxidizing agents, the characteristics of naturally occurring OxLDL species, and the importance of lipid oxidation for the formation of foam cells and atherogenesis. Furthermore, the use of poorly defined OxLDL preparations in various studies led to irreproducible, and at times conflicting data on the biological activities and proposed mechanisms. There is thus a need for the standardization of OxLDL preparations and development of criteria to define and classify the various preparations based on a more robust analytical methodology than TBARS assay, which is fraught with numerous pitfalls. Some of the potential candidates include the analysis of one or more oxidized PAPC products by LC/MS, measuring the epitopes of modified Apo B by specific monoclonal antibodies, and the quantitation of modified lysine residues of Apo B.
The role of OxLDL in nonatherosclerotic diseases has been attracting more attention recently, especially the immunological diseases such as lupus, APS, and RA, as well as diabetes and renal disease. Although OxLDL has been primarily assumed to be a pro-inflammatory and pro-atherogenic particle, recent evidence clearly indicates the anti-inflammatory properties of some of the OxLDL components, and the investigation of the mechanisms involved may provide novel therapeutic opportunities in the prevention and treatment of atherosclerosis and other inflammatory diseases. The apparently critical role of intracellular ROS in mediating the multiple effects of OxLDL provides another therapeutic opportunity to target the intracellular ROS generation rather than focusing on the inhibition of OxLDL generation with traditional antioxidants that act extracellularly, and had disappointing outcomes to date in large-scale clinical trials.
Abbreviations Used
- ACAT
acyl CoA:cholesterol acyltransferase
- acLDL
acetylated LDL
- AGE
advanced glycation end products
- Ang
angiotensin
- AP-1
activator protein-1
- aPL
antiphospholipid antibodies
- Apo
apolipoprotein
- APS
antiphospholipid syndrome
- β2GPI
β2 glycoprotein
- CAD
coronary artery disease
- Cav
caveolin
- CD
cluster differentiation
- CE
cholesteryl ester
- CHD
coronary heart disease
- CHO
Chinese hamster ovary
- CL-P1
collectin placenta 1
- COX-2
cyclooxygenase 2
- cPLA2
cytosolic phospholipase A2
- CRP
C-reactive protein
- DSR-C
Drosophila scavenger receptor class C
- EC
endothelial cell
- EGR
early growth response factor
- eNOS
endothelial nitric oxide synthase
- ERK
extracellular signal regulated kinase
- FcγR:
Fcγ receptor
- FcμR:
Fcμ receptor
- GLUT
glucose transporter
- g-OxLDL
glucose-OxLDL
- HDL
high density lipoprotein
- HETE
hydroxyl-eicosatetraenoic acid
- HF-1
hypoxia inducible factor
- HNE
4-hydroxy-2-nonenal
- HO-1
hemeoxygenase-1
- HODE
hydroxy-octadecadienoic acid
- HPETE
hydroperoxy-eicosatetraenoic acid
- HPODE
hydroperoxy-octadecadienoic acid
- IC
immune complex
- ICAM
intercellular adhesion molecule
- Ig
immunoglobulins
- IL
interleukin
- IMT
intima media thickness
- KOdiAPC
keto-hydroxy dicarboxylic acid PC
- LCAT
lecithin:cholesterol acyltransferase
- LDL
low density lipoprotein
- LO
lipoxygenase
- LOX-1
leptin-like OxLDL receptor-1
- LPA
lysophosphatidic acid
- LPC
lysophosphatidylcholine
- LPS
lipopolysaccharide
- MARCO
Macrophage Receptor with a Collagenous Structure
- MCP-1
monocyte chemotactic protein-1
- MM-LDL
minimally modified LDL
- MPO
myeloperoxidase
- NFAT
nuclear factor of activated T-cells
- NFκB
nuclear factor κB
- NO
nitric oxide
- NOX
NADPH-oxidase
- OxLDL
(Fully or extensively) oxidized LDL
- OxPC
oxidized phosphatidylcholine
- PAF
platelet activating factor
- PAI-1
plasminogen activator inhibitor-1
- PAPC
1-palmitoyl 2-arachidonoyl PC
- PC
phosphatidyl choline
- PC PGPC
1-palmitoyl 2-glutaroyl PC
- PC PMA
phorbol 12-myristate 13-acetate
- PDGF
platelet derived growth factor
- PE
phosphatidyl ethanolamine
- PEIPC
1-palmitoyl 2-(5.6)-epoxy isoprostane E2
- PI3K
phosphoinositide 3 kinase
- PKC
protein kinase C
- PLC
phospholipase C
- PLPC
1-palmitoyl 2-linoleoyl
- PONPC
1-palmitoyl 2-(9-oxononanoyl) PC
- POVPC
1-palmitoyl 2-(5-oxovaleroyl) PC
- PPAR
peroxisome proliferator activated receptor
- RAGE
receptor for AGE
- RF
rhematoid factor
- ROS
reactive oxygen species
- RTK
receptor tyrosine kinase
- SAA
serum amyloid A
- SCARA-5
scavenger receptor class A-5
- SLE
systemic lupus erythematosus
- SM
sphingomyelin
- Smad3
mothers against decapentaplegic homolog 3
- SMase
sphingomyelinase
- SMC
smooth muscle cell
- SOD
superoxide dismutase
- SP-1
specific protein-1
- sPLA2
secretory phospholipase A2
- SR-A
scavenger receptor-class A
- SR-B1
scavenger receptor-class B1
- SRCL
scavenger receptor with C-type lectin
- STAT
signal transducer and activator of transcription
- T2DM
type 2 diabetes milletus
- TBARS
thiobarbituric acid reactive substances
- TG
triacylglycerol
- TGF β
transforming growth factor β
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- VCAM
vascular cell adhesion molecule
- VEGF
vascular endothelial growth factor
Footnotes
Reviewing Editors: Eiji Matsuura, Cecile Maziere, Maureen McMahon, Sampath Parthasarathy, and Sreenivasan Ponnambalam
Acknowledgments
This work was supported by NIH Grants HL073965 and HL083298 for IL and HL 68585, and DK 078165 for PVS. We also thank Mr. Gregory Kowalsky for his help in formatting the figures and designing figure cartoons.
References
- 1.Acton S. Rigotti A. Landschulz KT. Xu S. Hobbs HH. Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 2.Acton SL. Scherer PE. Lodish HF. Krieger M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J Biol Chem. 1994;269:21003–21009. [PubMed] [Google Scholar]
- 3.Adachi H. Tsujimoto M. Endothelial scavenger receptors. Prog Lipid Res. 2006;45:379–404. doi: 10.1016/j.plipres.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Akiba S. Chiba M. Mukaida Y. Sato T. Involvement of reactive oxygen species and SP-1 in fibronectin production by oxidized LDL. Biochem Biophys Res Commun. 2003;310:491–497. doi: 10.1016/j.bbrc.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 5.Allen LB. Capps BE. Miller EC. Clemmons DR. Maile LA. Glucose-oxidized low-density lipoproteins enhance insulin-like growth factor I-stimulated smooth muscle cell proliferation by inhibiting integrin-associated protein cleavage. Endocrinology. 2009;150:1321–1329. doi: 10.1210/en.2008-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ames PRJ. Delgado Alves J. Lopez LR. Gentile F. Margarita A. Pizzella L. Batuca J. Scenna G. Brancaccio V. Matsuura E. Antibodies against β 2 -glycoprotein I complexed with an oxidised lipoprotein relate to intima thickening of carotid arteries in primary antiphospholipid syndrome. Clin Devel Immunol. 2006;13:1–9. doi: 10.1080/17402520600554930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson JW. Gowri MS. Turner J. Nichols L. Diwadkar VA. Chow CK. Oeltgen PR. Antioxidant supplementation effects on low-density lipoprotein oxidation for individuals with type 2 diabetes mellitus. J Am Coll Nut. 1999;18:451–461. doi: 10.1080/07315724.1999.10718883. [DOI] [PubMed] [Google Scholar]
- 8.Arai T. Wang N. Bezouevski M. Welch C. Tall AR. Decreased atherosclerosis in heterozygous low density lipoprotein receptor-deficient mice expressing the scavenger receptor BI transgene. J Biol Chem. 1999;274:2366–2371. doi: 10.1074/jbc.274.4.2366. [DOI] [PubMed] [Google Scholar]
- 9.Ashraf MZ. Kar NS. Chen X. Choi J. Salomon RG. Febbraio M. Podrez EA. Specific oxidized phospholipids inhibit scavenger receptor BI-mediated selective uptake of cholesteryl esters. J Biol Chem. 2008;283:10408–10414. doi: 10.1074/jbc.M710474200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auge N. Garcia V. Maupas-Schwalm F. Levade T. Salvayre R. Negre-Salvayre A. Oxidized LDL-induced smooth muscle cell proliferation involves the EGF receptor/PI-3 kinase/Akt and the sphingolipid signaling pathways. Arterioscler Thromb Vasc Biol. 2002;22:1990–1995. doi: 10.1161/01.atv.0000043453.21629.3b. [DOI] [PubMed] [Google Scholar]
- 11.Auge N. Nikolova–Karakashian M. Carpentier S. Parthasarathy S. Negre–Salvayre A. Salvayre R. Merrill AH., Jr. Levade T. Role of sphingosine 1-phosphate in the mitogenesis induced by oxidized low density lipoprotein in smooth muscle cells via activation of sphingomyelinase, ceramidase, and sphingosine kinase. J Biol Chem. 1999;274:21533–21538. doi: 10.1074/jbc.274.31.21533. [DOI] [PubMed] [Google Scholar]
- 12.Aviram M. Modified forms of low density lipoprotein in atherosclerosis. Atherosclerosis. 1993;98:1–9. doi: 10.1016/0021-9150(93)90217-i. [DOI] [PubMed] [Google Scholar]
- 13.Babaev VR. Gleaves LA. Carter KJ. Suzuki H. Kodama T. Fazio S. Linton MF. Reduced atherosclerotic lesions in mice deficient for total or macrophage-specific expression of scavenger receptor-A. Arterioscler Thromb Vasc Biol. 2000;20:2593–2599. doi: 10.1161/01.atv.20.12.2593. [DOI] [PubMed] [Google Scholar]
- 14.Babiy AV. Gebicki JM. Decomposition of lipid hydroperoxides enhances the uptake of low density lipoprotein by macrophages. Acta Biochim Pol. 1999;46:31–42. [PubMed] [Google Scholar]
- 15.Bae YS. Lee JH. Choi SH. Kim S. Almazan F. Witztum JL. Miller YI. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: Toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res. 2009;104:210–218. doi: 10.1161/CIRCRESAHA.108.181040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balagopalakrishna C. Bhunia AK. Rifkind JM. Chatterjee S. Minimally modified low density lipoproteins induce aortic smooth muscle cell proliferation via the activation of mitogen activated protein kinase. Mol Cell Biochem. 1997;170:85–89. doi: 10.1023/a:1006840927835. [DOI] [PubMed] [Google Scholar]
- 17.Bancells C. Benitez Sn. Villegas S. Jorba O. Ordonez–Llanos J. Sachez–Quesada JL. Novel phospholipolytic activities associated with electronegative low-density lipoprotein are involved in increased self-aggregation. Biochemistry. 2008;47:8186–8194. doi: 10.1021/bi800537h. [DOI] [PubMed] [Google Scholar]
- 18.Bassi N. Ghirardello A. Iaccarino L. Zampieri S. Rampudda ME. Atzeni F. Sarzi- -Puttini P. Shoenfeld Y. Doria A. OxLDL/[beta]2GPI-anti-OxLDL/[beta]2GPI complex and atherosclerosis in SLE patients. Autoimmun Rev. 2007;7:52–58. doi: 10.1016/j.autrev.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Basu SK. Goldstein JL. Anderson RGW. Brown MS. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci USA. 1976;73:3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berliner JA. Territo MC. Sevanian A. Ramin S. Kim JA. Bamshad B. Esterson M. Fogelman AM. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest. 1990;85:1260–1266. doi: 10.1172/JCI114562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bielicki JK. Forte TM. McCall MR. Minimally oxidized LDL is a potent inhibitor of lecithin: cholesterol acyltransferase activity. J Lipid Res. 1996;37:1012–1021. [PubMed] [Google Scholar]
- 22.Binder CJ. Chang MK. Shaw PX. Miller YI. Hartvigsen K. Dewan A. Witztum JL. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 23.Binder CJ. Silverman GJ. Natural antibodies and the autoimmunity of atherosclerosis. Springer Semin Immunopathol. 2005;26:385–404. doi: 10.1007/s00281-004-0185-z. [DOI] [PubMed] [Google Scholar]
- 24.Birukov KG. Oxidized lipids: The two faces of vascular inflammation. Curr Atheroscler Rep. 2006;8:223–231. doi: 10.1007/s11883-006-0077-x. [DOI] [PubMed] [Google Scholar]
- 25.Blair A. Shaul PW. Yuhanna IS. Conrad PA. Smart EJ. Oxidized low-density lipoprotein displaces endothelial Nitric-oxide synthase from plasmalemmal caveolae and impairs eNOS activation. J Biol Chem. 1999;274:32512–32519. doi: 10.1074/jbc.274.45.32512. [DOI] [PubMed] [Google Scholar]
- 26.Blake DR. Unsworth J. Outhwaite JM. Morris CJ. Merry P. Kidd BL. Ballard R. Gray L. Lunec J. Hypoxic-reperfusion injury in the inflamed human joint. Lancet. 1989;333:289–293. doi: 10.1016/s0140-6736(89)91305-6. [DOI] [PubMed] [Google Scholar]
- 27.Bochkov V. Leitinger N. Anti-inflammatory properties of lipid oxidation products. J Mol Med. 2003;81:613–626. doi: 10.1007/s00109-003-0467-2. [DOI] [PubMed] [Google Scholar]
- 28.Boullier A. Bird DA. Chang IK. Dennis EA. Friedman P. Gillotte–Taylor K. Horkko S. Palinski W. Quehenberger O. Shaw P. Steinberg D. Terpstra V. Witztum JL. Scavenger receptors, oOxidized LDL, and atherosclerosis. Ann NY Acad Sci. 2001;947:214–223. doi: 10.1111/j.1749-6632.2001.tb03943.x. [DOI] [PubMed] [Google Scholar]
- 29.Boullier A. Gillotte KL. Horkko S. Green SR. Friedman P. Dennis EA. Witztum JL. Steinberg D. Quehenberger O. The binding of oxidized low density lipoprotein to mouse CD36 is mediated in part by oxidized phospholipids that are associated with both the lipid and protein moieties of the lipoprotein. J Biol Chem. 2000;275:9163–9169. doi: 10.1074/jbc.275.13.9163. [DOI] [PubMed] [Google Scholar]
- 30.Boullier A. Li Y. Quehenberger O. Palinski W. Tabas I. Witztum JL. Miller YI. Minimally oxidized LDL offsets the apoptotic effects of extensively oxidized LDL and free cholesterol in macrophages. Arterioscler Thromb Vasc Biol. 2006;26:1169–1176. doi: 10.1161/01.ATV.0000210279.97308.9a. [DOI] [PubMed] [Google Scholar]
- 31.Boyanovsky B. Karakashian A. King K. Giltiay N. Nikolova–Karakashian M. Uptake and metabolism of low density lipoproteins with elevated ceramide content by human microvascular endothelial cells. Implications for the regulation of apoptosis. J Biol Chem. 2003;278:26992–26999. doi: 10.1074/jbc.M301536200. [DOI] [PubMed] [Google Scholar]
- 32.Brown AJ. Dean RT. Jessup W. Free and esterified oxysterol: formation during copper-oxidation of low density lipoprotein and uptake by macrophages. J Lipid Res. 1996;37:320–335. [PubMed] [Google Scholar]
- 33.Brown AJ. Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. 1999;142:1–28. doi: 10.1016/s0021-9150(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 34.Brown AJ. Mander EL. Gelissen IC. Kritharides L. Dean RT. Jessup W. Cholesterol and oxysterol metabolism and subcellular distribution in macrophage foam cells: Accumulation of oxidized esters in lysosomes. J Lipid Res. 2000;41:226–237. [PubMed] [Google Scholar]
- 35.Brown MS. Goldstein JL. Lipoprotein metabolism in the macrophage. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- 36.Brown MS. Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 37.Bucci M. Gratton JP. Rudic RD. Acevedo L. Roviezzo F. Cirino G. Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med. 2000;6:1362–1367. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- 38.Burkitt MJ. A critical overview of the chemistry of copper-dependent low density lipoprotein oxidation: Roles of lipid hydroperoxides, [alpha]-tocopherol, tThiols, and ceruloplasmin. Arch Biochem Biophys. 2001;394:117–135. doi: 10.1006/abbi.2001.2509. [DOI] [PubMed] [Google Scholar]
- 39.Byfield FJ. Tikku S. Rothblat GH. Gooch KJ. Levitan I. OxLDL increases endothelial stiffness, force generation and network formation. J Lipid Res. 2006;47:715–723. doi: 10.1194/jlr.M500439-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Cao G. Garcia CK. Wyne KL. Schultz RA. Parker KL. Hobbs HH. Structure and localization of the human gene encoding SR-BI/CLA-1. Evidence for transcriptional control by steroidogenic factor 1. J Biol Chem. 1997;272:33068–33076. doi: 10.1074/jbc.272.52.33068. [DOI] [PubMed] [Google Scholar]
- 41.Chang CL. Hsu HY. Lin HY. Chiang W. Lee H. Lysophosphatidic acid-induced oxidized low-density lipoprotein uptake is class A scavenger receptor-dependent in macrophages. Prostagland Other Lipid Med. 2008;87:20–25. doi: 10.1016/j.prostaglandins.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Chen M. Masaki T. Sawamura T. LOX-1, the receptor for oxidized low-density lipoprotein identified from endothelial cells: Implications in endothelial dysfunction and atherosclerosis. Pharmacol Therapeut. 2002;95:89–100. doi: 10.1016/s0163-7258(02)00236-x. [DOI] [PubMed] [Google Scholar]
- 43.Choi HK. Hernán MA. Seeger JD. Robins JM. Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: A prospective study. Lancet. 2002;359:1173–1177. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- 44.Choi S–H. Harkewicz R. Lee JH. Boullier A. Almazan F. Li AC. Witztum JL. Bae YS. Miller YI. Lipoprotein accumulation in macrophages via Toll-like receptor-4-dependent fluid phase uptake. Circ Res. 2009;104:1355–1363. doi: 10.1161/CIRCRESAHA.108.192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collot–Teixeira S. Martin J. McDermott–Roe C. Poston R. McGregor JL. CD36 and macrophages in atherosclerosis. Cardiovasc Res. 2007;75:468–477. doi: 10.1016/j.cardiores.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 46.Cominacini L. Pasini AF. Garbin U. Davoli A. Tosetti ML. Campagnola M. Rigoni A. Pastorino AM. Lo Cascio V. Sawamura T. Oxidized low fensity lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappa B through an increased production of intracellular reactive oxygen species. J Biol Chem. 2000;275:12633–12638. doi: 10.1074/jbc.275.17.12633. [DOI] [PubMed] [Google Scholar]
- 47.Cracowski JL. Durand T. Bessard G. Isoprostanes as a biomarker of lipid peroxidation in humans: Physiology, pharmacology and clinical implications. Trends Pharmacol Sci. 2002;23:360–366. doi: 10.1016/s0165-6147(02)02053-9. [DOI] [PubMed] [Google Scholar]
- 48.Cui MZ. Laag E. Sun L. Tan M. Zhao G. Xu X. Lysophosphatidic acid induces early growth response gene 1 expression in vascular smooth muscle cells: CRE and SRE mediate the transcription. Arterioscler Thromb Vasc Biol. 2006;26:1029–1035. doi: 10.1161/01.ATV.0000214980.90567.b5. [DOI] [PubMed] [Google Scholar]
- 49.Cyrus T. Pratico D. Zhao L. Witztum JL. Rader DJ. Rokach J. FitzGerald GA. Funk CD. Absence of 12/15-lipoxygenase expression decreases lipid peroxidation and atherogenesis in apolipoprotein E-deficient mice. Circulation. 2001;103:2277–2282. doi: 10.1161/01.cir.103.18.2277. [DOI] [PubMed] [Google Scholar]
- 50.Darblade B. Caillaud D. Poirot M. Fouque M. Thiers JC. Rami J. Bayard F. Arnal JF. Alteration of plasmalemmal caveolae mimics endothelial dysfunction observed in atheromatous rabbit aorta. Cardiovasc Res. 2001;50:566–576. doi: 10.1016/s0008-6363(01)00251-6. [DOI] [PubMed] [Google Scholar]
- 51.David GM. The iron hypothesis. Does iron cause atherosclerosis? Clin Cardiol. 1996;19:925–929. doi: 10.1002/clc.4960191205. [DOI] [PubMed] [Google Scholar]
- 52.de Beer MC. Zhao Z. Webb NR. van der Westhuyzen DR. de Villiers WJS. Lack of a direct role for macrosialin in oxidized LDL metabolism. J Lipid Res. 2003;44:674–685. doi: 10.1194/jlr.M200444-JLR200. [DOI] [PubMed] [Google Scholar]
- 53.de Winther MPJ. Gijbels MJJ. van Dijk KW. van Gorp PJJ. Suzuki H. Kodama T. Frants RR. Havekes LM. Hofker MH. Scavenger receptor deficiency leads to more complex atherosclerotic lesions in APOE3Leiden transgenic mice. Atherosclerosis. 1999;144:315–321. doi: 10.1016/s0021-9150(98)00332-3. [DOI] [PubMed] [Google Scholar]
- 54.de Winther MPJ. van Dijk KW. Havekes LM. Hofker MH. Macrophage scavenger receptor Class A: A multifunctional receptor in aAtherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:290–297. doi: 10.1161/01.atv.20.2.290. [DOI] [PubMed] [Google Scholar]
- 55.Dejager S. Mietus–Synder M. Pitas RE. Oxidized low density lipoproteins bind to the scavenger receptor expressed by rabbit smooth muscle cells and acrophages. Arterioscler Thromb. 1993;13:371–378. doi: 10.1161/01.atv.13.3.371. [DOI] [PubMed] [Google Scholar]
- 56.Dixon WG. Symmons DPM. What effects might anti-TNF[alpha] treatment be expected to have on cardiovascular morbidity and mortality in rheumatoid arthritis? A review of the role of TNF[alpha] in cardiovascular pathophysiology. Annals Rheum Dis. 2007;66:1132–1136. doi: 10.1136/ard.2006.063867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drab M. Verkade P. Elger M. Kasper M. Lohn M. Lauterbach B. Menne J. Lindschau C. Mende F. Luft FC. Schedl A. Haller H. Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 58.Duenas AI. Aceves M. Fernandez–Pisonero I. Gomez C. Orduna A. Crespo MS. Garcia–Rodriguez C. Selective attenuation of Toll-like receptor 2 signalling may explain the atheroprotective effect of sphingosine 1-phosphate. Cardiovasc Res. 2008;79:537–544. doi: 10.1093/cvr/cvn087. [DOI] [PubMed] [Google Scholar]
- 59.Elomaa O. Kangas M. Sahlberg C. Tuukkanen J. Sormunen R. Liakka A. Thesleff I. Kraal G. Tryggvason K. Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of acrophages. Cell. 1995;80:603–609. doi: 10.1016/0092-8674(95)90514-6. [DOI] [PubMed] [Google Scholar]
- 60.Elshourbagy NA. Li X. Terrett J. VanHorn S. Gross MS. Adamou JE. Anderson KM. Webb CL. Lysko PG. Molecular characterization of a human scavenger receptor, human MARCO. Eur J Biochem. 2000;267:919–926. doi: 10.1046/j.1432-1327.2000.01077.x. [DOI] [PubMed] [Google Scholar]
- 61.Endemann G. Stanton LW. Madden KS. Bryant CM. White RT. Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- 62.Febbraio M. Guy E. Silverstein RL. Stem cell transplantation reveals that absence of macrophage CD36 is protective against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:2333–2338. doi: 10.1161/01.ATV.0000148007.06370.68. [DOI] [PubMed] [Google Scholar]
- 63.Febbraio M. Podrez EA. Smith JD. Hajjar DP. Hazen SL. Hoff HF. Sharma K. Silverstein RL. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fox PL. Mazumder B. Ehrenwald E. Mukhopadhyay CK. Ceruloplasmin and cardiovascular disease. Free Radical Biol Med. 2000;28:1735–1744. doi: 10.1016/s0891-5849(00)00231-8. [DOI] [PubMed] [Google Scholar]
- 65.Frank PG. Lee H. Park DS. Tandon NN. Scherer PE. Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- 66.Freeman M. Ashkenas J. Rees DJG. Kingsley DM. Copeland NG. Jenkins NA. Krieger M. An ancient, highly conserved family of cysteine-rich protein domains revealed by cloning type I and type II murine macrophage scavenger receptors. Proc Natl Acad Sci U A. 1990;87:8810–8814. doi: 10.1073/pnas.87.22.8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freeman M. Ekkel Y. Rohrer L. Penman M. Freedman NJ. Chisolm GM. Krieger M. Expression of type I and type II bovine scavenger receptors in Chinese hamster ovary cells: Lipid droplet accumulation and nonreciprocal cross competition by acetylated and oxidized low density lipoprotein. Proc Natl Acad Sci USA. 1991;88:4931–4935. doi: 10.1073/pnas.88.11.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frostegård J. Svenungsson E. Wu R. Gunnarsson I. Lundberg IE. Klareskog L. Hörkkö S. Witztum JL. Lipid peroxidation is enhanced in patients with systemic lupus erythematosus and is associated with arterial and renal disease manifestations. Arthritis Rheum. 2005;52:192–200. doi: 10.1002/art.20780. [DOI] [PubMed] [Google Scholar]
- 69.Funk CD. Cyrus T. 12/15-Lipoxygenase, oxidative modification of LDL and atherogenesis. Trends Cardiovasc Med. 2004;11:116–124. doi: 10.1016/s1050-1738(01)00096-2. [DOI] [PubMed] [Google Scholar]
- 70.Gargiulo S. Gamba P. Sottero B. Biasi F. Chiarpotto E. Serviddio G. Vendemiale G. Poli Leonarduzzi G. The core-aldehyde 9-oxononanoyl cholesterol increases the level of transforming growth factor beta1-specific receptors on promonocytic U937 cell membranes. Aging Cell. 2009;8:77–87. doi: 10.1111/j.1474-9726.2009.00454.x. [DOI] [PubMed] [Google Scholar]
- 71.Garrido–Sánchez L. Cardona F. García–Fuentes E. Rojo–Martínez G. Gómez- -Zumaquero JM. Picón MJ. Soriguer FJ. Tinahones FJ. Anti-oxidized low-density lipoprotein antibody levels are associated with the development of type 2 diabetes mellitus. Eur J Clin Invest. 2008;38:615–621. doi: 10.1111/j.1365-2362.2008.01988.x. [DOI] [PubMed] [Google Scholar]
- 72.Gaubatz JW. Gillard BK. Massey JB. Hoogeveen RC. Huang M. Lloyd EE. Raya JL. Yang CY. Pownall HJ. Dynamics of dense electronegative low density lipoproteins and their preferential association with lipoprotein phospholipase A2. JLipid Res. 2007;48:348–357. doi: 10.1194/jlr.M600249-JLR200. [DOI] [PubMed] [Google Scholar]
- 73.Gesquiere L. Cho W. Subbaiah PV. Role of group IIa and group V secretory phospholipases A 2 in the metabolism of lipoproteins. Substrate specificities of the enzymes and the regulation of their activities by sphingomyelin. Biochemistry. 2002;41:4911–4920. doi: 10.1021/bi015757x. [DOI] [PubMed] [Google Scholar]
- 74.Glomset JA. The plasma lecithin:cholesterol acyltransferase reaction. J Lipid Res. 1968;9:155–167. [PubMed] [Google Scholar]
- 75.Goldstein JL. Ho YK. Basu SK. Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci USA. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gomez–Zumaquero JM. Tinahones FJ. De Ramon E. Camps M. Garrido L. Soriguer FJ. Association of biological markers of activity of systemic lupus erythematosus with levels of anti-oxidized low-density lipoprotein antibodies. Rheumatology. 2004;43:510–513. doi: 10.1093/rheumatology/keh109. [DOI] [PubMed] [Google Scholar]
- 77.Gonzalez MA. Selwyn AP. Endothelial function, inflammation, and prognosis in cardiovascular disease. Am J Med. 2003;115:99S–106S. doi: 10.1016/j.amjmed.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 78.Gough PJ. Greaves DR. Gordon S. A naturally occurring isoform of the human macrophage scavenger receptor (SR-A) gene generated by alternative splicing blocks modified LDL uptake. J Lipid Res. 1998;39:531–543. [PubMed] [Google Scholar]
- 79.Gough PJ. Greaves DR. Suzuki H. Hakkinen T. Hiltunen MO. Turunen M. Herttuala SY. Kodama T. Gordon S. Analysis of macrophage scavenger receptor (SR-A) expression in human aortic atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1999;19:461–471. doi: 10.1161/01.atv.19.3.461. [DOI] [PubMed] [Google Scholar]
- 80.Graier WF. Kostner GM. Glycated low-density lipoprotein and atherogenesis: the missing link between diabetes mellitus and hypercholesterolaemia? Eur J Clin Invest. 1997;27:457–459. doi: 10.1046/j.1365-2362.1997.1470696.x. [DOI] [PubMed] [Google Scholar]
- 81.Grandl M. Bared SM. Liebisch G. Werner T. Barlage S. Schmitz G. E-LDL and Ox-LDL differentially regulate ceramide and cholesterol raft microdomains in human Macrophages. Cytometry A. 2006;69:189–191. doi: 10.1002/cyto.a.20232. [DOI] [PubMed] [Google Scholar]
- 82.Greenspan P. Yu H. Mao F. Gutman RL. Cholesterol deposition in macrophages: foam cell formation mediated by cholesterol-enriched oxidized low density lipoprotein. J Lipid Res. 1997;38:101–109. [PubMed] [Google Scholar]
- 83.Gustin C. Van Steenbrugge M. Raes M. LPA modulates monocyte migration directly and via LPA-stimulated endothelial cells. Am J Physiol Cell Physiol. 2008;295:C905–C914. doi: 10.1152/ajpcell.00544.2007. [DOI] [PubMed] [Google Scholar]
- 84.Guy E. Kuchibhotla S. Silverstein R. Febbraio M. Continued inhibition of atherosclerotic lesion development in long-term Western diet fed CD36°/apoE° mice. Atherosclerosis. 2007;192:123–130. doi: 10.1016/j.atherosclerosis.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 85.Haberland ME. Olch CL. Folgelman AM. Role of lysines in mediating interaction of modified low density lipoproteins with the scavenger receptor of human monocyte macrophages. J Biol Chem. 1984;259:11305–11311. [PubMed] [Google Scholar]
- 86.Haberland ME. Steinbrecher UP. Modified low-density lipoproteins: Diversity and biological relevance in atherogenesis. Monogr Hum Genet. 1992;14:35. [Google Scholar]
- 87.Halliwell B. Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hammad SM. Taha TA. Nareika A. Johnson KR. Lopes–Virella MF. Obeid LM. Oxidized LDL immune complexes induce release of sphingosine kinase in human U937 monocytic cells. Prostagland Other Lipid Med. 2006;79:126–140. doi: 10.1016/j.prostaglandins.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 89.Hammad SM. Twal WO. Barth JL. Smith KJ. Saad AF. Virella G. Argraves WS. Lopes–Virella MF. Oxidized LDL immune complexes and oxidized LDL differentially affect the expression of genes involved with inflammation and survival in human U937 monocytic cells. Atherosclerosis. 2009;202:394–404. doi: 10.1016/j.atherosclerosis.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hara S. Shike T. Takasu N. Mizui T. Lysophosphatidylcholine promotes cholesterol efflux from mouse macrophage foam cells. Arterioscler Thromb Vasc Biol. 1997;17:1258–1266. doi: 10.1161/01.atv.17.7.1258. [DOI] [PubMed] [Google Scholar]
- 91.Hara Y. Kusumi Y. Mitsumata M. Li XK. Fujino M. Lysophosphatidylcholine upregulates LOX-1, chemokine receptors, and activation-related transcription factors in human T-cell line Jurkat. J Thromb Thrombolysis. 2008;26:113–118. doi: 10.1007/s11239-007-0158-x. [DOI] [PubMed] [Google Scholar]
- 92.Harats D. Shaish A. George J. Mulkins M. Kurihara H. Levkovitz H. Sigal E. Overexpression of 15-lipoxygenase in vascular endothelium accelerates early atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:2100–2105. doi: 10.1161/01.atv.20.9.2100. [DOI] [PubMed] [Google Scholar]
- 93.Harkewicz R. Hartvigsen K. Almazan F. Dennis EA. Witztum JL. Miller YI. Cholesteryl ester hydroperoxides are biologically active components of minimally oxidized low density lipoprotein. J Biol Chem. 2008;283:10241–10251. doi: 10.1074/jbc.M709006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hazen SL. Oxidized phospholipids as endogenous pattern recognition ligands in Innate Immunity. J Biol Chem. 2008;283:15527–15531. doi: 10.1074/jbc.R700054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heinecke JW. Lipoprotein oxidation in cardiovascular disease: Chief culprit or innocent bystander? J Exp Med. 2006;203:813–816. doi: 10.1084/jem.20060218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heinecke JW. Mechanisms of oxidative damage of low density lipoprotein in human atherosclerosis. Curr Opin Lipidol. 1997;8:268–274. doi: 10.1097/00041433-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 97.Heinecke JW. Oxidized amino acids: Culprits in human atherosclerosis and indicators of oxidative stress. Free Radical Biol Med. 2002;32:1090–1101. doi: 10.1016/s0891-5849(02)00792-x. [DOI] [PubMed] [Google Scholar]
- 98.Henriksen T. Mahoney EM. Steinberg D. Enhanced macrophage degradation of biologically modified low density lipoprotein. Arteriosclerosis. 1983:149–159. doi: 10.1161/01.atv.3.2.149. [DOI] [PubMed] [Google Scholar]
- 99.Henriksen T. Mahoney EM. Steinberg D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoproteins. Proc Natl Acad Sci USA. 1981;78:6499–6503. doi: 10.1073/pnas.78.10.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Henry PD. Hyperlipidemic endothelial injury and angiogenesis. Basic Res Cardiol. 1994;89:107–114. doi: 10.1007/978-3-642-85660-0_10. [DOI] [PubMed] [Google Scholar]
- 101.Higashi Y. Peng T. Du J. Sukhanov S. Li Y. Itabe H. Parthasarathy S. Delafontaine P. A redox-sensitive pathway mediates oxidized LDL-induced downregulation of insulin-like growth factor-1 receptor. J Lipid Res. 2005;46:1266–1277. doi: 10.1194/jlr.M400478-JLR200. [DOI] [PubMed] [Google Scholar]
- 102.Hiltunen TP. Luoma JS. Nikkari T. Yla–Herttuala S. Expression of LDL receptor, VLDL receptor, LDL receptor–related protein, and scavenger receptor in rabbit atherosclerotic lesions: Marked induction of scavenger receptor and VLDL receptor expression during lesion development. Circulation. 1998;97:1079–1086. doi: 10.1161/01.cir.97.11.1079. [DOI] [PubMed] [Google Scholar]
- 103.Holopainen JM. Medina OP. Metso AJ. Kinnunen PKJ. Sphingomyelinase activity associated with human plasma low density lipoprotein. Possible functional implications. J Biol Chem. 2000;275:16484–16489. doi: 10.1074/jbc.275.22.16484. [DOI] [PubMed] [Google Scholar]
- 104.Holvoet P. Donck J. Landeloos M. Brouwers E. Luijtens K. Arnout J. Lesaffre E. Vanrenterghem Y. Collen D. Correlation between oxidized low density lipoproteins and von Willebrand factor in chronic renal failure. Thrombosis Haemost. 1996;76:663–669. [PubMed] [Google Scholar]
- 105.Holvoet P. Perez G. Zhao Z. Brouwers E. Bernar H. Collen D. Malondialdehyde-modified low density lipoproteins in patients with atherosclerotic disease. J Clin Invest. 1995;95:2611–2619. doi: 10.1172/JCI117963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hörkkö S. Bird DA. Miller E. Itabe H. Leitinger N. Subbanagounder G. Berliner JA. Friedman P. Dennis EA. Curtiss LK. Palinski W. Witztum JL. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest. 1999;103:117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hsieh CC. Yen MH. Yen CH. Lau YT. Oxidized low density lipoprotein induces apoptosis via generation of reactive oxygen species in vascular smooth muscle cells. Cardiovasc Res. 2001;49:135–145. doi: 10.1016/s0008-6363(00)00218-2. [DOI] [PubMed] [Google Scholar]
- 108.Huang JT. Welch JS. Ricote M. Binder CJ. Willson TM. Kelly C. Witztum JL. Funk CD. Conrad D. Glass CK. Interleukin-4-dependent production of PPAR-[gamma] ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 109.Huang ZH. Bates EJ. Ferrante JV. Hii CST. Poulos A. Robinson BS. Ferrante A. Inhibition of stimulus-induced endothelial cell intercellular adhesion molecule-1, E-selectin, and vascular cellular adhesion molecule-1 expression by arachidonic acid and its hydroxy and hydroperoxy derivatives. Circ Res. 1997;80:149–158. doi: 10.1161/01.res.80.2.149. [DOI] [PubMed] [Google Scholar]
- 110.Huber J. Boechzelt H. Karten B. Surboeck M. Bochkov VN. Binder BR. Sattler W. Leitinger N. Oxidized cholesteryl linoleates stimulate endothelial cells to bind monocytes via the extracellular signal-regulated kinase ½ pathway. Arterioscler Thromb Vasc Biol. 2002;22:581–586. doi: 10.1161/01.atv.0000012782.59850.41. [DOI] [PubMed] [Google Scholar]
- 111.Hughes DA. Fraser IP. Gordon S. Murine macrophage scavenger receptor: In vivo expression and function as receptor for macrophage adhesion in lymphoid and non-lymphoid organs. Eur J Immunol. 1995;25:466–473. doi: 10.1002/eji.1830250224. [DOI] [PubMed] [Google Scholar]
- 112.Hulthe J. Antibodies to oxidized LDL in atherosclerosis development. Clinical and animal studies. Clinica Chimica Acta. 2004;348:1–8. doi: 10.1016/j.cccn.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 113.Huo Y. Zhao L. Hyman MC. Shashkin P. Harry BL. Burcin T. Forlow SB. Stark MA. Smith DF. Clarke S. Srinivasan S. Hedrick CC. Pratico D. Witztum JL. Nadler JL. Funk CD. Ley K. Critical role of macrophage 12/15-lipoxygenase for atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;110:2024–2031. doi: 10.1161/01.CIR.0000143628.37680.F6. [DOI] [PubMed] [Google Scholar]
- 114.Huszar D. Varban ML. Rinninger F. Feeley R. Arai T. Fairchild–Huntress V. Donovan MJ. Tall AR. Increased LDL cholesterol and atherosclerosis in LDL receptor-deficient mice with attenuated expression of scavenger receptor B1. Arterioscler Thromb Vasc Biol. 2000;20:1068–1073. doi: 10.1161/01.atv.20.4.1068. [DOI] [PubMed] [Google Scholar]
- 115.Ichi I. Nakahara K. Miyashita Y. Hidaka A. Kutsukake S. Inoue K. Maruyama T. Miwa Y. Harada M. Tsushima M. Kojo S Kisei Cohort Study G. Association of ceramides in human plasma with risk factors of atherosclerosis. Lipids. 2006;41:859. doi: 10.1007/s11745-006-5041-6. [DOI] [PubMed] [Google Scholar]
- 116.Ishigaki Y. Katagiri H. Gao J. Yamada T. Imai J. Uno K. Hasegawa Y. Kaneko K. Ogihara T. Ishihara H. Sato Y. Takikawa K. Nishimichi N. Matsuda H. Sawamura T. Oka Y. Impact of plasma oxidized low-density lipoprotein removal on atherosclerosis. Circulation. 2008;118:75–83. doi: 10.1161/CIRCULATIONAHA.107.745174. [DOI] [PubMed] [Google Scholar]
- 117.Ishikawa K. Navab M. Leitinger N. Fogelman AM. Lusis AJ. Induction of heme oxygenase-1 inhibits the monocyte transmigration induced by mildly oxidized LDL. J Clin Invest. 1997;100:1209–1216. doi: 10.1172/JCI119634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Itabe H. Oxidative Modification of LDL: Its Pathological Role in Atherosclerosis. Clin Rev Allergy Immunol. 2009;37:4–11. doi: 10.1007/s12016-008-8095-9. [DOI] [PubMed] [Google Scholar]
- 119.Itabe H. Mori M. Fujimoto Y. Higashi Y. Takano T. Minimally modified LDL is an oxidized LDL enriched with oxidized phosphatidylcholines. J Biochem (Tokyo) 2003;134:459–465. doi: 10.1093/jb/mvg164. [DOI] [PubMed] [Google Scholar]
- 120.Itabe H. Yamamoto H. Imanaka T. Shimamura K. Uchiyama H. Kimura J. Sanaka T. Hata Y. Takano T. Sensitive detection of oxidatively modified low density lipoprotein using a monoclonal antibody. J Lipid Res. 1996;37:45–53. [PubMed] [Google Scholar]
- 121.James MJ. van Reyk D. Rye KA. Dean RT. Cleland LG. Barter PJ. Jessup W. Low density lipoprotein of synovial fluid in inflammatory joint disease is mildly oxidized. Lipids. 1998;33:1115–1121. doi: 10.1007/s11745-998-0313-8. [DOI] [PubMed] [Google Scholar]
- 122.Jedidi I. Couturier M. Thqrond P. GardFs–Albert M. Legrand A. Barouki R. Bonnefont–Rousselot D. Aggerbeck M. Cholesteryl ester hydroperoxides increase macrophage CD36 gene expression via PPAR[alpha] Biochem Biophys Res Commun. 2006;351:733–738. doi: 10.1016/j.bbrc.2006.10.122. [DOI] [PubMed] [Google Scholar]
- 123.Jerome W. Advanced atherosclerotic foam cell formation has features of an acquired lysosomal storage disorder. Rejuvenation Res. 2006;9:245–255. doi: 10.1089/rej.2006.9.245. [DOI] [PubMed] [Google Scholar]
- 124.Jessup W. Krithairides L. Stocker R. Lipid oxidation in atherogenesis: An overview. Biochem Soc Trans. 2004;32:134–138. doi: 10.1042/bst0320134. [DOI] [PubMed] [Google Scholar]
- 125.Jessup W. Kritharides L. Metabolism of oxidized LDL by macrophages. Curr Opin Lipidol. 2000;11:473–481. doi: 10.1097/00041433-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 126.Jessup W. Mander EL. Dean RT. The intracellular storage and turnover of apolipoprotein B of oxidized LDL in macrophages. Biochim Biophys Acta. 1992;1126:167–177. doi: 10.1016/0005-2760(92)90287-6. [DOI] [PubMed] [Google Scholar]
- 127.Jialal I. Chait A. Differences in the metabolism of oxidatively modified low density lipoprotein and acetylated low density lipoprotein by human endothelial cells: Inhibition of cholesterol esterification by oxidatively modified low density lipoprotein. J Lipid Res. 1989;30:1561–1568. [PubMed] [Google Scholar]
- 128.Jiang Y. Oliver P. Davies KE. Platt N. Identification and characterization of murine SCARA5, a novel class A scavenger receptor that is expressed by populations of epithelial cells. J Biol Chem. 2006;281:11834–11845. doi: 10.1074/jbc.M507599200. [DOI] [PubMed] [Google Scholar]
- 129.Kakutani M. Masaki T. Sawamura T. A platelet–endothelium interaction mediated by lectin-like oxidized low-density lipoprotein receptor-1. Proc Natl Acad Sci USA. 2000;97:360–364. doi: 10.1073/pnas.97.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kakutani M. Ueda M. Naruko T. Masaki T. Sawamura T. Accumulation of LOX-1 ligand in plasma and atherosclerotic lesions of Watanabe heritable hyperlipidemic rabbits: Identification by a novel enzyme immunoassay. Biochem Biophys Res Commun. 2001;282:180–185. doi: 10.1006/bbrc.2001.4508. [DOI] [PubMed] [Google Scholar]
- 131.Kanayama M. Yamaguchi S. Shibata T. Shibata N. Kobayashi M. Nagai R. Arai H. Takahashi K. Uchida K. Identification of a serum component that regulates cyclooxygenase-2 gene expression in cooperation with 4-hydroxy-2-nonenal. J Biol Chem. 2007;282:24166–24174. doi: 10.1074/jbc.M703212200. [DOI] [PubMed] [Google Scholar]
- 132.Karvonen J. Paivansalo M. Kesaniemi YA. Horkko S. Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation. 2003;108:2107–2112. doi: 10.1161/01.CIR.0000092891.55157.A7. [DOI] [PubMed] [Google Scholar]
- 133.Kataoka H. Kume N. Miyamoto S. Minami M. Moriwaki H. Murase T. Sawamura T. Masaki T. Hashimoto N. Kita T. Expression of lectin-like oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation. 1999;99:3110–3117. doi: 10.1161/01.cir.99.24.3110. [DOI] [PubMed] [Google Scholar]
- 134.Kato R. Mori C. Kitazato K. Arata S. Obama T. Mori M. Takahashi K. Aiuchi T. Takano T. Itabe H. Transient increase in plasma oxidized LDL during the progression of atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2009;29:33–39. doi: 10.1161/ATVBAHA.108.164723. [DOI] [PubMed] [Google Scholar]
- 135.Kawai Y. Saito A. Shibata N. Kobayashi M. Yamada S. Osawa T. Uchida K. Covalent binding of oxidized cholesteryl esters to protein: Implications for oxidative modification of low density lipoprotein and atherosclerosis. J Biol Chem. 2003;278:21040–21049. doi: 10.1074/jbc.M212426200. [DOI] [PubMed] [Google Scholar]
- 136.Kern H. Volk T. Knauerschiefer S. Mieth T. Rustow B. Kox WJ. Schlame M. Stimulation of monocytes and platelets by short-chain phosphatidylcholines with and without terminal carboxyl group. Biochim Biophys Acta. 1998;1394:33–42. doi: 10.1016/s0167-4889(98)00093-7. [DOI] [PubMed] [Google Scholar]
- 137.Kincer JF. Uittenbogaard A. Dressman J. Guerin TM. Febbraio M. Guo L. Smart EJ. Hypercholesterolemia promotes a CD36-dependent and endothelial nitric-oxide synthase-mediated vascular dysfunction. J Biol Chem. 2002;277:23525–23533. doi: 10.1074/jbc.M202465200. [DOI] [PubMed] [Google Scholar]
- 138.Kinnunen PKJ. Holopainen JM. Sphingomyelinase activity of LDL: A link between atherosclerosis, ceramide, and apoptosis? Trend Cardiovasc Med. 2002;12:37–42. doi: 10.1016/s1050-1738(01)00143-8. [DOI] [PubMed] [Google Scholar]
- 139.Kobayashi K. Kishi M. Atsumi T. Bertolaccini ML. Makino H. Sakairi N. Yamamoto I. Yasuda T. Khamashta MA. Hughes GRV. Koike T. Voelker DR. Matsuura E. Circulating oxidized LDL forms complexes with {beta}2-glycoprotein I: Implication as an atherogenic autoantigen. J Lipid Res. 2003;44:716–726. doi: 10.1194/jlr.M200329-JLR200. [DOI] [PubMed] [Google Scholar]
- 140.Kodama T. Freeman M. Rohrer L. Zabrecky J. Matsudaira P. Krieger M. Type I macrophage scavenger receptor contains α-helical and collagen-like coiled coils. Nature. 1990;343:531–535. doi: 10.1038/343531a0. [DOI] [PubMed] [Google Scholar]
- 141.Kodama T. Reddy P. Kishimoto C. Krieger M. Purification and characterization of a bovine acetyl low density lipoprotein receptor. Proc Natl Acad Sci USA. 1988;85:9238–9242. doi: 10.1073/pnas.85.23.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kones R. The Jupiter study, CRP screening, and aggressive statin therapy. Implications for the primary prevention of cardiovascular disease. Ther Adv Cardiovasc Dis. 2009;3:309–315. doi: 10.1177/1753944709337056. [DOI] [PubMed] [Google Scholar]
- 143.Kotani K. Satoh N. Kato Y. Araki R. Koyama K. Okajima T. Tanabe M. Oishi M. Yamakage H. Yamada K. Hattori M. Shimatsu A. A novel oxidized low-density lipoprotein marker, serum amyloid A-LDL, is associated with obesity and the metabolic syndrome. Atherosclerosis. 2009;204:526–531. doi: 10.1016/j.atherosclerosis.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 144.Kowalsky GB. Byfield FJ. Levitan I. OxLDL facilitates flow-induced realignment of aortic endothelial cells. Am J Physiol Cell Physiol. 2008;295:C332–C340. doi: 10.1152/ajpcell.00335.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Krieger M. Herz J. Structures and functions: macrophage scavenger receptors and LDL receptor-related protein (LRP) Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- 146.Kris–Etherton PM. Lichtenstein AH. Howard BV. Steinberg D. Witztum JL for the Nutrition Committee of the American Heart Association Council on Nutrition PAaM. Antioxidant vitamin supplements and cardiovascular disease. Circulation. 2004;110:637–641. doi: 10.1161/01.CIR.0000137822.39831.F1. [DOI] [PubMed] [Google Scholar]
- 147.Kruth HS. Lipoprotein cholesterol and atherosclerosis. Curr Mol Med. 2001;1:633–653. doi: 10.2174/1566524013363212. [DOI] [PubMed] [Google Scholar]
- 148.Kruth HS. Huang W. Ishii I. Zhang WY. Macrophage foam cell formation with native low density lipoprotein. J Biol Chem. 2002;277:34573–34580. doi: 10.1074/jbc.M205059200. [DOI] [PubMed] [Google Scholar]
- 149.Ku IA. Imboden JB. Hsue PY. Ganz P. Rheumatoid arthritis: A model of systemic inflammation driving atherosclerosis. Circul J. 2009;73:977–985. doi: 10.1253/circj.cj-09-0274. [DOI] [PubMed] [Google Scholar]
- 150.Kuchibhotla S. Vanegas D. Kennedy DJ. Guy E. Nimako G. Morton RE. Febbraio M. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc Res. 2008;78:185–196. doi: 10.1093/cvr/cvm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kuhn H. Chaitidis P. Roffeis J. Walther M. Arachidonic acid metabolites in the cardiovascular system: The role of lipoxygenase isoforms in atherogenesis with particular emphasis on vascular remodeling. J Cardiovasc Pharmacol. 2007;50:609–620. doi: 10.1097/FJC.0b013e318159f177. [DOI] [PubMed] [Google Scholar]
- 152.Kume N. Kita T. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) in atherogenesis. Trends Cardiovasc Med. 2001;11:22–25. doi: 10.1016/s1050-1738(01)00079-2. [DOI] [PubMed] [Google Scholar]
- 153.Kunjathoor VV. Febbraio M. Podrez EA. Moore KJ. Andersson L. Koehn S. Rhee JS. Silverstein R. Hoff HF. Freeman MW. Scavenger receptors Class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 154.Kurien BT. Scofield RH. Autoimmunity and oxidatively modified autoantigens. Autoimmun Rev. 2008;7:567–573. doi: 10.1016/j.autrev.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Landschulz KT. Pathak RK. Rigotti A. Krieger M. Hobbs HH. Regulation of scavenger receptor, class B, type I, a high density lipoprotein receptor, in liver and steroidogenic tissues of the rat. J Clin Invest. 1996;98:984–995. doi: 10.1172/JCI118883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee HS. Song CY. Oxidized low-density lipoprotein and oxidative stress in the development of glomerulosclerosis. Am J Nephrol. 2009;29:62–70. doi: 10.1159/000151277. [DOI] [PubMed] [Google Scholar]
- 157.Lee JY. Jung GY. Heo HJ. Yun MR. Park JY. Bae SS. Hong KW. Lee WS. Kim CD. 4-Hydroxynonenal induces vascular smooth muscle cell apoptosis through mitochondrial generation of reactive oxygen species. Toxicol Lett. 2006;166:212–221. doi: 10.1016/j.toxlet.2006.07.305. [DOI] [PubMed] [Google Scholar]
- 158.Leitinger N. Watson AD. Hama SY. Ivandic B. Qiao JH. Huber J. Faull KF. Grass DS. Navab M. Fogelman AM. de Beer FC. Lusis Aj. Berliner JA. Role of group II secretory phospholipase A 2 in atherosclerosis: 2. Potential involvement of biologically active oxidized phospholipids. Arterioscler Thromb Vasc Biol. 1999;19:1291–1298. doi: 10.1161/01.atv.19.5.1291. [DOI] [PubMed] [Google Scholar]
- 159.Lemaire–Ewing Sp. Berthier A. Royer M. Logette E. Corcos L. Bouchot A. Monier S. Prunet C. Raveneau M. Rebe C. Desrumaux C. Lizard G. Neel D. 7b- Hydroxycholesterol and 25-hydroxycholesterol-induced interleukin-8 secretion involves a calcium-dependent activation of c-fos via the ERK1/2 signaling pathway in THP-1 cells. Cell Biol Toxicol. 2009;25:127–139. doi: 10.1007/s10565-008-9063-0. [DOI] [PubMed] [Google Scholar]
- 160.Leonarduzzi G. Chiarpotto E. Biasi F. Poli G. 4-Hydroxynonenal and cholesterol oxidation products in atherosclerosis. Mol Nut Food Res. 2005;49:1044–1049. doi: 10.1002/mnfr.200500090. [DOI] [PubMed] [Google Scholar]
- 161.Li D. Mehta JL. Intracellular signaling of LOX-1 in endothelial cell apoptosis. Circ Res. 2009;104:566–568. doi: 10.1161/CIRCRESAHA.109.194209. [DOI] [PubMed] [Google Scholar]
- 162.Li H. Freeman MW. Libby P. Regulation of smooth muscle cell scavenger receptor expression in vivo by atherogenic diets and in vitro by cytokines. J Clin Invest. 1995;95:122–133. doi: 10.1172/JCI117628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li R. Mouillesseaux KP. Montoya D. Cruz D. Gharavi N. Dun M. Koroniak L. Berliner JA. Identification of prostaglandin E2 receptor subtype 2 as a receptor activated by OxPAPC. Circ Res. 2006;98:642–650. doi: 10.1161/01.RES.0000207394.39249.fc. [DOI] [PubMed] [Google Scholar]
- 164.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 165.Loidl A. Claus R. Ingolic E. Deigner HP. Hermetter A. Role of ceramide in activation of stress-associated MAP kinases by minimally modified LDL in vascular smooth muscle cells. Biochim Biophys Acta Mol Basis Dis. 2004;1690:150–158. doi: 10.1016/j.bbadis.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 166.Lopes–Virella MF. Binzafar N. Rackley S. Akira T. La Via M. Virella G. The uptake of LDL-IC by human macrophages: Predominant involvement of the Fc[gamma]RI receptor. Atherosclerosis. 1997;135:161. doi: 10.1016/s0021-9150(97)00157-3. [DOI] [PubMed] [Google Scholar]
- 167.Lopez D. Garcia–Valladares I. Palafox–Sanchez CA. De La Torre IG. Kobayashi K. Matsuura E. Lopez LR. Oxidized low-density lipoprotein/β2-glycoprotein I complexes and autoantibodies to oxLig-1/β2-glycoprotein I in patients with systemic lupus erythematosus and antiphospholipid syndrome. Am J Clin Pathol. 2004;121:426–436. doi: 10.1309/2AUE-6HD4-W6TL-EUU5. [DOI] [PubMed] [Google Scholar]
- 168.Lopez D. Kobayashi K. Merrill JT. Matsuura E. Lopez LR. IgG autoantibodies against β2-glycoprotein I complexed with a lipid ligand derived from oxidized low-density lipoprotein are associated with arterial thrombosis in antiphospholipid syndrome. Clin Devel Immunol. 2003;10:203–211. doi: 10.1080/10446670310001642113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lopez LR. Buckner TR. Hurkey BL. Kobayashi BL. Matsuura E. Determination of oxidized low-density lipoproteins (ox-LDL) versus ox-LDL/β2GPI complexes for the assessment of autoimmune-mediated atherosclerosis. Ann NY Acad Sci. 2007;1109:303–310. doi: 10.1196/annals.1398.036. [DOI] [PubMed] [Google Scholar]
- 170.Lordan S. Mackrill JJ. O'Brien NM. Oxysterols and mechanisms of apoptotic signaling: Implications in the pathology of degenerative diseases. J Nut Biochem. 2009;20:321–336. doi: 10.1016/j.jnutbio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 171.Lougheed M. Lum CM. Ling W. Suzuki H. Kodama T. Steinbrecher U. High affinity saturable uptake of oxidized low density lipoprotein by macrophages from mice lacking the scavenger receptor class A Type I/II. J Biol Chem. 1997;272:12938–12944. doi: 10.1074/jbc.272.20.12938. [DOI] [PubMed] [Google Scholar]
- 172.Lougheed M. Zhang HF. Steinbrecher UP. Oxidized low density lipoprotein is resistant to cathepsins and accumulates within macrophages. J Biol Chem. 1991;266:14519–14525. [PubMed] [Google Scholar]
- 173.Lourida E. Georgiadis A. Papavasiliou E. Papathanasiou A. Drosos A. Tselepis A. Patients with early rheumatoid arthritis exhibit elevated autoantibody titers against mildly oxidized low-density lipoprotein and exhibit decreased activity of the lipoprotein-associated phospholipase A2. Arthritis Res Ther. 2007;9:R19. doi: 10.1186/ar2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lu J. Jiang W. Yang JH. Chang PY. Walterscheid JP. Chen HH. Marcelli M. Tang D. Lee YT. Liao WSL. Yang CY. Chen CH. Electronegative LDL impairs vascular endothelial cell integrity in diabetes by disrupting fibroblast growth factor 2 (FGF2) autoregulation. Diabetes. 2008;57:158. doi: 10.2337/db07-1287. [DOI] [PubMed] [Google Scholar]
- 175.Lu J. Yang JH. Burns AR. Chen HH. Tang D. Walterscheid JP. Suzuki SS. Yang CY. Sawamura T. Chen CH. Mediation of electronegative low-density lipoprotein signaling by LOX-1: A possible mechanism of endothelial apoptosis. Circ Res. 2009;104:619–627. doi: 10.1161/CIRCRESAHA.108.190116. [DOI] [PubMed] [Google Scholar]
- 176.Mander EL. Dean RT. Stanley KK. Jessup W. Apolipoprotein B of oxidized LDL accumulates in the lysosomes of macrophages. Biochim Biophys Acta (BBA)–Lipids Lipid Metab. 1994;1212:80–92. doi: 10.1016/0005-2760(94)90192-9. [DOI] [PubMed] [Google Scholar]
- 177.Manning–Tobin JJ. Moore KJ. Seimon TA. Bell SA. Sharuk M. Varez–Leite JI. de Winther MPJ. Tabas I. Freeman MW. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 2009;29:19–26. doi: 10.1161/ATVBAHA.108.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Maor I. Aviram M. Oxidized low density lipoprotein leads to macrophage accumulation of unesterified cholesterol as a result of lysosomal trapping of the lipoprotein hydrolyzed cholesteryl ester. J Lipid Res. 1994;35:803–819. [PubMed] [Google Scholar]
- 179.Marathe GK. Prescott SM. Zimmerman GA. McIntyre TM. Oxidized LDL contains inflammatory PAF-like phospholipids. Trend Cardiovasc Med. 2001;11:139. doi: 10.1016/s1050-1738(01)00100-1. [DOI] [PubMed] [Google Scholar]
- 180.Matsumoto T. Kobayashi T. Kamata K. Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr Med Chem. 2007;14:3209–3220. doi: 10.2174/092986707782793899. [DOI] [PubMed] [Google Scholar]
- 181.Matsuura E. Hughes GRV. Khamashta MA. Oxidation of LDL and its clinical implication. Autoimmun Rev. 2008;7:558–566. doi: 10.1016/j.autrev.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 182.Matsuura E. Kobayashi K. Inoue K. Lopez LR. Shoenfeld Y. Oxidized LDL/β2 glycoprotein I complexes: New aspects in atherosclerosis. Lupus. 2005;14:736–741. doi: 10.1191/0961203305lu2211oa. [DOI] [PubMed] [Google Scholar]
- 183.Matsuura E. Lopez L. Autoimmune-mediated atherothrombosis. Lupus. 2008;17:879–888. doi: 10.1177/0961203308093553. [DOI] [PubMed] [Google Scholar]
- 184.Maziere C. Maziere JC. Activation of transcription factors and gene expression by oxidized low-density lipoprotein. Free Radical Biol Med. 2009;46:127–137. doi: 10.1016/j.freeradbiomed.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 185.McCall MR. Carr AC. Forte TM. Frei B. LDL modified by hypochlorous scid is a potent inhibitor of lecithin-cholesterol scyltransferase sctivity. Arterioscler Thromb Vasc Biol. 2001;21:1040–1045. doi: 10.1161/01.atv.21.6.1040. [DOI] [PubMed] [Google Scholar]
- 186.Mehta JL. Sanada N. Hu CP. Chen J. Dandapat A. Sugawara F. Satoh H. Inoue K. Kawase Y. Jishage K–I. Suzuki H. Takeya M. Schnackenberg L. Beger R. Hermonat PL. Thomas M. Sawamura T. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res. 2007;100:1634–1642. doi: 10.1161/CIRCRESAHA.107.149724. [DOI] [PubMed] [Google Scholar]
- 187.Merry P. Grootveld M. Lunec J. Blake D. Oxidative damage to lipids within the inflamed human joint provides evidence of radical-mediated hypoxic-reperfusion injury. Am J Clin Nutr. 1991;53:362S–369S. doi: 10.1093/ajcn/53.1.362S. [DOI] [PubMed] [Google Scholar]
- 188.Miller YI. Viriyakosol S. Binder CJ. Feramisco JR. Kirkland TN. Witztum JL. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem. 2003;278:1561–1568. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- 189.Mizutani T. Sonoda Y. Minegishi T. Wakabayashi K. Miyamoto K. Cloning, characterization, and cellular distribution of rat scavenger receptor class B Type I (SRBI) in the ovary. Biochem Biophys Res Commun. 1997;234:499–505. doi: 10.1006/bbrc.1997.6646. [DOI] [PubMed] [Google Scholar]
- 190.Montuschi P. Barnes PJ. Roberts LJ. Isoprostanes: Markers and mediators of oxidative stress. FASEB J. 2004;18:1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 191.Moore KJ. Freeman MW. Scavenger receptors in atherosclerosis: Beyond lipid uptake. Arterioscler Thromb Vasc Biol. 2006;26:1702–1711. doi: 10.1161/01.ATV.0000229218.97976.43. [DOI] [PubMed] [Google Scholar]
- 192.Moore KJ. Kunjathoor VV. Koehn SL. Manning JJ. Tseng AA. Silver JM. McKee M. Freeman MW. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Moriwaki H. Kume N. Sawamura T. Aoyama T. Hoshikawa H. Ochi H. Nishi E. Masaki T. Kita T. Ligand specificity of LOX-1, a novel receptor for oxidized low-density lipoprotein. Arterioscler Thromb Vasc Biol. 1998;18:1541–1547. doi: 10.1161/01.atv.18.10.1541. [DOI] [PubMed] [Google Scholar]
- 194.Morrow JD. The Isoprostanes—Unique products of arachidonate peroxidation: Their role as mediators of oxidant stress. Curr Pharm Des. 2006;12:895–902. doi: 10.2174/138161206776055985. [DOI] [PubMed] [Google Scholar]
- 195.Murakami M. Kudo I. New phospholipase A2 isozymes with a potential role in atherosclerosis. Curr Opin Lipidol. 2003;14:431–436. doi: 10.1097/00041433-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 196.Murphy JE. Tacon D. Tedbury PR. Hadden JM. Knowling S. Sawamura T. Peckham M. Phillips SEV. Walker JH. Ponnambalam S. LOX-1 scavenger receptor mediates calcium-dependent recognition of phosphatidylserine and apoptotic cells. Biochem J. 2006;393:107–115. doi: 10.1042/BJ20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Murphy JE. Tedbury PR. Homer–Vanniasinkam S. Walker JH. Ponnambalam S. Biochemistry and cell biology of mammalian scavenger receptors. Atherosclerosis. 2005;182:1. doi: 10.1016/j.atherosclerosis.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 198.Nagarajan S. Anti-OxLDL IgG blocks OxLDL interaction with CD36, but promotes Fc[gamma]R, CD32A-dependent inflammatory cell adhesion. Immunol Lett. 2007;108:52–61. doi: 10.1016/j.imlet.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 199.Nagy L. Tontonoz P. Alvarez JGA. Chen H. Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPAR gamma. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 200.Naito M. Suzuki H. Mori T. Matsumoto A. Kodama T. Takahashi K. Coexpression of type I and type II human macrophage scavenger receptors in macrophages of various organs and foam cells in atherosclerotic lesions. Am J Pathol. 1992;141:591–599. [PMC free article] [PubMed] [Google Scholar]
- 201.Nakagawa T. Akagi M. Hoshikawa H. Chen M. Yasuda T. Mukai S. Ohsawa K. Masaki T. Nakamura T. Sawamura T. Lectin-like oxidized low-density lipoprotein receptor 1 mediates leukocyte infiltration and articular cartilage destruction in rat zymosan–induced arthritis. Arthritis Rheum. 2002;46:2486–2494. doi: 10.1002/art.10504. [DOI] [PubMed] [Google Scholar]
- 202.Nakamura K. Funakoshi H. Miyamoto K. Tokunaga F. Nakamura T. Molecular cloning and functional characterization of a human scavenger receptor with C-type lectin (SRCL), a novel member of a scavenger receptor family. Biochem Biophys Res Commun. 2001;280:1028–1035. doi: 10.1006/bbrc.2000.4210. [DOI] [PubMed] [Google Scholar]
- 203.Nakamura K. Funakoshi H. Tokunaga F. Nakamura T. Molecular cloning of a mouse scavenger receptor with C-type lectin (SRCL)(1), a novel member of the scavenger receptor family. Biochim Biophys Acta. 2001;1522:53–58. doi: 10.1016/s0167-4781(01)00284-6. [DOI] [PubMed] [Google Scholar]
- 204.Nakhjavani M. Esteghamati A. Asgarani F. Khalilzadeh O. Nikzamir A. Safari R. Association of oxidized low-density lipoprotein and transforming growth factor-beta in type 2 diabetic patients: A cross-sectional study. Trans Res. 2009;153:86–90. doi: 10.1016/j.trsl.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 205.Navab M. Hama SY. Anantharamaiah GM. Hassan K. Hough GP. Watson AD. Reddy ST. Sevanian A. Fonarow GC. Fogelman AM. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: Steps 2 and 3. J Lipid Res. 2000;41:1495–1508. [PubMed] [Google Scholar]
- 206.Negre–Salvayre A. Vieira O. Escargueil–Blanc I. Salvayre R. Oxidized LDL and 4-hydroxynonenal modulate tyrosine kinase receptor activity. Mol Aspects Med. 2003;24:251–261. doi: 10.1016/s0098-2997(03)00020-7. [DOI] [PubMed] [Google Scholar]
- 207.Nicholson AC. Expression of CD36 in macrophages and atherosclerosis. The role of lipid regulation of PPAR[gamma] signaling. Trends Cardiovasc Med. 2004;14:8–12. doi: 10.1016/j.tcm.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 208.Nicholson AC. Frieda S. Pearce A. Silverstein RL. Oxidized LDL binds to CD36 on human monocyte-derived macrophages and transfected cell lines: Evidence implicating the lipid moiety of the lipoprotein as the binding site. Arterioscler Thromb Vasc Biol. 1995;15:269–275. doi: 10.1161/01.atv.15.2.269. [DOI] [PubMed] [Google Scholar]
- 209.Nilsson J. Hansson GK. Autoimmunity in atherosclerosis: A protective response losing control? J Intern Med. 2008;263:464–478. doi: 10.1111/j.1365-2796.2008.01945.x. [DOI] [PubMed] [Google Scholar]
- 210.Obinata H. Hattori T. Nakane S. Tatei K. Izumi T. Identification of 9- hydroxyoctadecadienoic acid and other oxidized free fatty acids as ligands of the G protein-coupled receptor G2A. J Biol Chem. 2005;280:40676–40683. doi: 10.1074/jbc.M507787200. [DOI] [PubMed] [Google Scholar]
- 211.Ohtani K. Suzuki Y. Eda S. Kawai T. Kase T. Keshi H. Sakai Y. Fukuoh A. Sakamoto T. Itabe H. Suzutani T. Ogasawara M. Yoshida I. Wakamiya N. The membrane-type collectin CL-P1 is a scavenger eeceptor on vascular endothelial cells. J Biol Chem. 2001;276:44222–44228. doi: 10.1074/jbc.M103942200. [DOI] [PubMed] [Google Scholar]
- 212.Okajima F. Plasma lipoproteins behave as carriers of extracellular sphingosine 1- phosphate: Is this an atherogenic mediator or an anti-atherogenic mediator? Biochim Biophys Acta. 2002;1582:132–137. doi: 10.1016/s1388-1981(02)00147-6. [DOI] [PubMed] [Google Scholar]
- 213.Palinski W. Horkko S. Miller E. Steinbrecher UP. Powell HC. Curtiss LK. Witztum JL. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein e-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest. 1996;98:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Park YM. Febbraio M. Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest. 2009;119:136–145. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Parsons MS. Barrett L. Little C. Grant MD. Harnessing CD36 to rein in inflammation, Endocr Metab Immune. Disord Drug Targets. 2008;8:184–191. doi: 10.2174/187153008785700073. [DOI] [PubMed] [Google Scholar]
- 216.Parthasarathy S. Fong LG. Otero D. Steinberg D. Recognition of solubilized apoproteins from delipidated, oxidized low density lipoprotein (LDL) by the acetyl-LDL receptor. Proc Natl Acad Sci USA. 1987;84:537–540. doi: 10.1073/pnas.84.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Parthasarathy S. Litvinov D. Selvarajan K. Garelnabi M. Lipid peroxidation and decomposition. Conflicting roles in plaque vulnerability and stability. Biochim Biophys Acta. 2008;1781:221–231. doi: 10.1016/j.bbalip.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Pearson A. Lux A. Krieger M. Expression cloning of dSR-CI, a class C macrophage-specific scavenger receptor from Drosophila melanogaster. Proc Natl Acad Sci USA. 1995;92:4056–4060. doi: 10.1073/pnas.92.9.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pengo V. Bison E. Ruffatti A. Iliceto S. Antibodies to oxidized LDL/[beta]2- glycoprotein I in antiphospholipid syndrome patients with venous and arterial thromboembolism. Thromb Res. 2008;122:556–559. doi: 10.1016/j.thromres.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 220.Peters MJL. van Halm VP. Nurmohamed MT. Damoiseaux J. Tervaert JWC. Twisk JWR. Dijkmans BAC. Voskuyl AE. Relations between autoantibodies against oxidized low-density lipoprotein, inflammation, subclinical atherosclerosis, and cardiovascular disease in rheumatoid arthritis. J Rheumatol. 2008;35:1495–1499. [PubMed] [Google Scholar]
- 221.Pitas RE. Expression of the acetyl low density lipoprotein receptor by rabbit fibroblasts and smooth muscle cells. Up-regulation by phorbol esters. J Biol Chem. 1990;265:12722–12727. [PubMed] [Google Scholar]
- 222.Pitas RE. Boyles J. Mahley RW. Bissell DM. Uptake of chemically modified low density lipoproteins in vivo is mediated by specific endothelial cells. J Cell Biol. 1985;100:103–117. doi: 10.1083/jcb.100.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Plüddemann A. Mukhopadhyay S. Gordon S. The interaction of macrophage receptors with bacterial ligands. Expert Rev Mol Med. 2006;22:1–25. doi: 10.1017/S1462399406000159. [DOI] [PubMed] [Google Scholar]
- 224.Plüddemann A. Neyen C. Gordon S. Macrophage scavenger receptors and host-derived ligands. Methods. 2007;43:207–217. doi: 10.1016/j.ymeth.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 225.Podrez EA. Febbraio M. Sheibani N. Schmitt D. Silverstein RL. Hajjar DP. Cohen PA. Frazier WA. Hoff HF. Hazen SL. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J Clin Invest. 2000;105:1095–1108. doi: 10.1172/JCI8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Podrez EA. Hoppe G. O'Neil J. Hoff HF. Phospholipids in oxidized LDL not adducted to apoB are recognized by the CD36 scavenger receptor. Free Rad Biol Med. 2003;34:356–364. doi: 10.1016/s0891-5849(02)01294-7. [DOI] [PubMed] [Google Scholar]
- 227.Podrez EA. Hoppe G. O'Neil J. Sayre LM. Sheibani N. Hoff HF. Macrophage receptors responsible for distinct recognition of low density lipoprotein containing pyrrole or pyridinium adducts: Models of oxidized low density lipoprotein. J Lipid Res. 2000;41:1455–1463. [PubMed] [Google Scholar]
- 228.Podrez EA. Poliakov E. Shen Z. Zhang R. Deng Y. Sun M. Finton PJ. Shan L. Gugiu B. Fox PL. Hoff HF. Salomon RG. Hazen SL. Identification of a novel fFamily of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J Biol Chem. 2002;277:38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 229.Quinn MT. Parthasarathy S. Steinberg D. Lysophosphatidylcholine: A chemotactic factor for human monocytes and its potential role in atherogenesis. Proc NatlAcad Sci USA. 1988;85:2805–2809. doi: 10.1073/pnas.85.8.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Raghavamenon A. Garelnabi M. Babu S. Aldrich A. Litvinov D. Parthasarathy S. Tocopherol is ineffective in preventing the decomposition of preformed lipid peroxides and may promote the accumulation of toxic aldehydes: A potential explanation for the failure of antioxidants to affect human atherosclerosis. Antioxid Redox Signal. 2009;11:1237–1248. doi: 10.1089/ars.2008.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ravetch JV. Kinet JP. Fc Receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 232.Rigotti A. Acton SL. Krieger M. The Class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. J Biol Chem. 1995;270:16221–16224. doi: 10.1074/jbc.270.27.16221. [DOI] [PubMed] [Google Scholar]
- 233.Rigotti A. Trigatti BL. Penman M. Rayburn H. Herz J. Krieger M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci USA. 1997;94:12610–12615. doi: 10.1073/pnas.94.23.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rios FJ. Jancar S. Melo IB. Ketelhuth DF. Gidlund M. Role of PPAR-gamma in the modulation of CD36 and FcgammaRII induced by LDL with low and high degrees of oxidation during the differentiation of the monocytic THP-1 cell line. Cell Physiol Biochem. 2008;22:549–556. doi: 10.1159/000185539. [DOI] [PubMed] [Google Scholar]
- 235.Robbesyn F. Salvayre R. Negre–Salvayre A. Dual role of oxidized LDL on the NF-KappaB signaling pathway. Free Rad Res. 2004;38:541–551. doi: 10.1080/10715760410001665244. [DOI] [PubMed] [Google Scholar]
- 236.Rohrer L. Freeman M. Kodama T. Penman M. Krieger M. Coiled-coil fibrous domains mediate ligand binding by macrophage scavenger receptor type II. Nature. 1990;343:570–572. doi: 10.1038/343570a0. [DOI] [PubMed] [Google Scholar]
- 237.Roma P. Bernini F. Fogliatto R. Bertulli SM. Negri S. Fumagalli R. Catapano AL. Defective catabolism of oxidized LDL by J774 murine macrophages. J Lipid Res. 1992;33:819–829. [PubMed] [Google Scholar]
- 238.Roma P. Catapano AL. Bertulli SM. Varesi L. Fumagalli R. Bernini F. Oxidized LDL increase free cholesterol and fail to stimulate cholesterol esterification in murine macrophages. Biochem Biophys Res Commun. 1990;171:123–131. doi: 10.1016/0006-291x(90)91365-y. [DOI] [PubMed] [Google Scholar]
- 239.Rosenfeld ME. Palinski W. Yla–Herttuala S. Butler S. Witztum JL. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990;10:336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- 240.Ross R. Atherosclerosis—An inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 241.Saad AF. Virella G. Chassereau C. Boackle RJ. Lopes–Virella MF. OxLDL immune complexes activate complement and induce cytokine production by MonoMac 6 cells and human macrophages. J Lipid Res. 2006;47:1975–1983. doi: 10.1194/jlr.M600064-JLR200. [DOI] [PubMed] [Google Scholar]
- 242.Sakaguchi H. Takeya M. Suzuki H. Hakamata H. Kodama T. Horiuchi S. Gordon S. van der Laan LJ. Kraal G. Ishibashi S. Kitamura N. Takahashi K. Role of macrophage scavenger receptors in diet-induced atherosclerosis in mice. Lab Invest. 1998;78:423–434. [PubMed] [Google Scholar]
- 243.Salonen JT. Korpela H. Salonen R. Nyyssonen K. Yla–Herttuala S. Yamamoto R. Butler S. Palinski W. Witztum JL. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. The Lancet. 1992;339:883–887. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- 244.Sambrano GR. Terpstra V. Steinberg D. Independent mechanisms for macrophage binding and macrophage phagocytosis of damaged erythrocytes: Evidence of receptor cooperativity. Arterioscler Thromb Vasc Biol. 1997;17:3442–3448. doi: 10.1161/01.atv.17.12.3442. [DOI] [PubMed] [Google Scholar]
- 245.Sanchez–Quesada JL. Benitez S. Ordonez–Llanos J. Electronegative low-density lipoprotein. Curr Opin Lipidol. 2004;15:329–335. doi: 10.1097/00041433-200406000-00014. [DOI] [PubMed] [Google Scholar]
- 246.Santanam N. Parthasarathy S. Paradoxical actions of antioxidants in the oxidation of low density lipoprotein by peroxidases. J Clin Invest. 1995;95:2594–2600. doi: 10.1172/JCI117961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Sawamura T. Kume N. Aoyama T. Moriwaki H. Hoshikawa H. Aiba Y. Tanaka T. Miwa S. Katsura Y. Kita T. Masaki T. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;6:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 248.Scazzocchio B. Vari R. D'Archivio M. Santangelo C. Filesi C. Giovannini C. Masella R. Oxidized LDL impair adipocyte response to insulin by activating serine/threonine kinases. J Lipid Res. 2009;50:832–845. doi: 10.1194/jlr.M800402-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Schissel SL. Tweedie–Hardman J. Rapp JH. Graham G. Williams KJ. Tabas I. Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J Clin Invest. 1996;98:1455–1464. doi: 10.1172/JCI118934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Selman L. Skjodt K. Nielsen O. Floridon C. Holmskov U. Hansen S. Expression and tissue localization of collectin placenta 1 (CL-P1, SRCL) in human tissues. Mol Immunol. 2008;45:3278. doi: 10.1016/j.molimm.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 251.Sevanian A. Bittolobon G. Cazzolato G. Hodis H. Hwang J. Zamburlini A. Maiorino M. Ursini F. Ldl(-) is a lipid hydroperoxide-enriched circulating lipoprotein. J Lipid Res. 1997;38:419–428. [PubMed] [Google Scholar]
- 252.Sevanian A. Hodis HN. Hwang J. McLeod LL. Peterson H. Characterization of endothelial cell injury by cholesterol oxidation products found in oxidized LDL. J Lipid Res. 1995;36:1971–1986. [PubMed] [Google Scholar]
- 253.Shaul PW. Endothelial nitric oxide synthase, caveolae, and the development of atherosclerosis. J Physiol. 2003;15:21–33. doi: 10.1113/jphysiol.2002.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Shaw PX. Goodyear CS. Chang MK. Witztum JL. Silverman GJ. The autoreactivity of anti-phosphorylcholine antibodies for atherosclerosis-associated neo-antigens and apoptotic cells. J Immunol. 2003;15:6151–6157. doi: 10.4049/jimmunol.170.12.6151. [DOI] [PubMed] [Google Scholar]
- 255.Shih HH. Zhang S. Cao W. Hahn A. Wang J. Paulsen JE. Harnish DC. CRP is a novel ligand for the oxidized LDL receptor LOX-1. Am J Physiol Heart Circ Physiol. 2009;296:H1643–H1650. doi: 10.1152/ajpheart.00938.2008. [DOI] [PubMed] [Google Scholar]
- 256.Shimokawa H. Primary endothelial dysfunction: Atherosclerosis. J Mol Cell Cardiol. 1999;31:23–37. doi: 10.1006/jmcc.1998.0841. [DOI] [PubMed] [Google Scholar]
- 257.Siess W. Platelet interaction with bioactive lipids formed by mild oxidation of low- density lipoprotein. Pathophysiol Haemost Thromb. 2006;35:292–304. doi: 10.1159/000093222. [DOI] [PubMed] [Google Scholar]
- 258.Siess W. Tigyi G. Thrombogenic and atherogenic activities of lysophosphatidic acid. J Cell Biochem. 2004;92:1086–1094. doi: 10.1002/jcb.20108. [DOI] [PubMed] [Google Scholar]
- 259.Siess W. Zangl KJ. Essler M. Bauer M. Brandl R. Corrinth C. Bittman R. Tigyi G. Aepfelbacher M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci USA. 1999;96:6931–6936. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sigari F. Lee C. Witztum JL. Reaven PD. Fibroblasts that overexpress 15- lipoxygenase generate bioactive and minimally modified LDL. Arterioscler Thromb Vasc Biol. 1997;17:3639–3645. doi: 10.1161/01.atv.17.12.3639. [DOI] [PubMed] [Google Scholar]
- 261.Singh DK. Subbaiah PV. Modulation of the activity and arachidonic acid selectivity of group X secretory phospholipase A 2 by sphingolipids. J Lipid Res. 2007;48:683–692. doi: 10.1194/jlr.M600421-JLR200. [DOI] [PubMed] [Google Scholar]
- 262.Stanton LW. White RT. Bryant CM. Protter AA. Endemann G. A macrophage Fc receptor for IgG is also a receptor for oxidized low density lipoprotein. J Biol Chem. 1992;267:22446–22451. [PubMed] [Google Scholar]
- 263.Stary HC. Chandler AB. Glagov S. Guyton JR. Insull WJ. E. RM. Schaffer SA. J. SC. Wagner WD. Wissler RW. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994;89:2462–2478. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- 264.Steinberg D. Parthasarathy S. Carew TE. Khoo JC. Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 265.Steinbrecher UP. Oxidation of human low density lipoprotein results in derivatization of lysine residues of apolipoprotein B by lipid peroxide decomposition products. J Biol Chem. 1987;262:3603–3608. [PubMed] [Google Scholar]
- 266.Steinbrecher UP. Receptors for oxidized low density lipoprotein. Biochim Biophys Acta. 1999;1436:279–298. doi: 10.1016/s0005-2760(98)00127-1. [DOI] [PubMed] [Google Scholar]
- 267.Steinbrecher UP. Witztum JL. Parthasarathy S. Steinberg D. Decrease in reactive amino groups during oxidation or endothelial cell modification of LDL. Correlation with changes in receptor-mediated catabolism. Arteriosclerosis. 1987;7:135–143. doi: 10.1161/01.atv.7.2.135. [DOI] [PubMed] [Google Scholar]
- 268.Stocker R. Keaney KJ., Jr New insights on oxidative stress in the artery wall. J Throm Haem. 2005;3:1825–1834. doi: 10.1111/j.1538-7836.2005.01370.x. [DOI] [PubMed] [Google Scholar]
- 269.Stocker R. Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 270.Su J. Georgiades A. Wu R. Thulin T. de Faire U. Frostegård J. Antibodies of IgM subclass to phosphorylcholine and oxidized LDL are protective factors for atherosclerosis in patients with hypertension. Atherosclerosis. 2006;188:160–166. doi: 10.1016/j.atherosclerosis.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 271.Subbaiah PV. Subramanian VS. Wang K. Novel physiological function of sphingomyelin in plasma. Inhibition of lipid peroxidation in low density lipoproteins. J Biol Chem. 1999;274:36409–36414. doi: 10.1074/jbc.274.51.36409. [DOI] [PubMed] [Google Scholar]
- 272.Subbanagounder G. Watson AD. Berliner JA. Bioactive products of phospholipid oxidation: Isolation, identification, measurement and activities. Free Radical Biol Med. 2000;28:1751–1761. doi: 10.1016/s0891-5849(00)00233-1. [DOI] [PubMed] [Google Scholar]
- 273.Subbanagounder G. Wong JW. Lee H. Faull KF. Miller E. Witztum JL. Berliner JA. Epoxyisoprostane and epoxycyclopentenone phospholipids regulate monocyte chemotactic protein-1 and interleukin-8 synthesis. Formation of these oxidized phospholipids in response to interleukin-1 beta. J Biol Chem. 2002;277:7271–7281. doi: 10.1074/jbc.M107602200. [DOI] [PubMed] [Google Scholar]
- 274.Sun L. Ishida T. Yasuda T. Kojima Y. Honjo T. Yamamoto Y. Yamamoto H. Ishibashi S. Hirata K-i. Hayashi Y. RAGE mediates oxidized LDL-induced pro- inflammatory effects and atherosclerosis in non-diabetic LDL receptor-deficient mice. Cardiovasc Res. 2009;82:371–381. doi: 10.1093/cvr/cvp036. [DOI] [PubMed] [Google Scholar]
- 275.Suzuki H. Kurihara Y. Takeya M. Kamada N. Kataoka M. Jishage K. Ueda O. Sakaguchi H. Higashi T. Suzuki T. Takashima Y. Kawabe Y. Cynshi O. Wada Y. Honda M. Kurihara H. Aburatani H. Doi T. Matsumoto A. Azuma S. Noda T. Toyoda Y. Itakura H. Yazaki Y. Kodama T. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 276.Szanto A. Nagy L. The many faces of PPAR[gamma]: Anti-inflammatory by any means? Immunobiology. 2008;213:789–803. doi: 10.1016/j.imbio.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 277.Tabuchi M. Inoue K. Usui–Kataoka H. Kobayashi K. Teramoto M. Takasugi K. Shikata K. Yamamura M. Ando K. Nishida K. Kasahara J. Kume N. Lopez LR. Mitsudo K. Nobuyoshi M. Yasuda T. Kita T. Makino H. Matsuura E. The association of C-reactive protein with an oxidative metabolite of LDL and its implication in atherosclerosis. J Lipid Res. 2007;48:768–781. doi: 10.1194/jlr.M600414-JLR200. [DOI] [PubMed] [Google Scholar]
- 278.Terpstra V. Bird DA. Steinberg D. Evidence that the lipid moiety of oxidized low density lipoprotein plays a role in its interaction with macrophage receptors. Proc Natl Acad Sci USA. 1998;95:1806–1811. doi: 10.1073/pnas.95.4.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Tertov VV. Sobenin IA. Orekhov AN. Similarity between naturally occurring modified desialylated, electronegative and aortic low density lipoprotein. 1996;25:313–319. doi: 10.3109/10715769609149054. [DOI] [PubMed] [Google Scholar]
- 280.Thomson MJ. Puntmann V. Kaski JC. Atherosclerosis and oxidant stress: The end of the road for antioxidant vitamin treatment? Cardiovasc Drugs Ther. 2007;21:195–210. doi: 10.1007/s10557-007-6027-1. [DOI] [PubMed] [Google Scholar]
- 281.Tietge UJF. Pratico D. Ding T. Funk CD. Hildebrand RB. Van Berkel T. Van Eck M. Macrophage-specific expression of group IIA sPLA2 results in accelerated atherogenesis by increasing oxidative stress. J Lipid Res. 2005;46:1604–1614. doi: 10.1194/jlr.M400469-JLR200. [DOI] [PubMed] [Google Scholar]
- 282.Toloza SMA. Uribe AG. McGwin GJ. Alarcón GS. Fessler BJ. Bastian HM. Vilá LM. Wu R. Shoenfeld Y. Roseman JM. Reveille JD. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA): XXIII. Baseline predictors of vascular events. Arthritis Rheum. 2004;50:3947–3957. doi: 10.1002/art.20622. [DOI] [PubMed] [Google Scholar]
- 283.Trigatti B. Rigotti A. Scavenger receptor class B type I (SR-BI) and high-density lipoprotein metabolism: Recent lessons from genetically manipulated mice. Int J Tissue React. 2000;22:29–37. [PubMed] [Google Scholar]
- 284.Tsimikas S. Oxidative biomarkers in the diagnosis and prognosis of cardiovascular disease. Am J Cardiol. 2006;98:9P–17P. doi: 10.1016/j.amjcard.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 285.Uittenbogaard A. Shaul PW. Yuhanna IS. Blair A. Smart EJ. High density lipoprotein prevents oxidized low density lipoprotein-induced inhibition of endothelial nitric-oxide synthase localization and activation in caveolae. J Biol Chem. 2000;275:11278–11283. doi: 10.1074/jbc.275.15.11278. [DOI] [PubMed] [Google Scholar]
- 286.Upston JM. Niu X. Brown AJ. Mashima R. Wang H. Senthilmohan R. Kettle AJ. Dean RT. Stocker R. Disease stage-dependent accumulation of lipid and protein oxidation products in human atherosclerosis. Am J Pathol. 2002;160:701–710. doi: 10.1016/S0002-9440(10)64890-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Valiyaveettil M. Kar N. Ashraf MZ. Byzova TV. Febbraio M. Podrez EA. Oxidized high-density lipoprotein inhibits platelet activation and aggregation via scavenger receptor B1. Blood. 2008;111:1962–1971. doi: 10.1182/blood-2007-08-107813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.van den Eijnden MMED. van Noort JT. Hollaar L. van der Laarse A. Bertina RM. Cholesterol or triglyceride loading of human monocyte-derived macrophages by incubation with modified lipoproteins does not induce tissue factor expression. Arterioscler Thromb Vasc Biol. 1999;19:384–392. doi: 10.1161/01.atv.19.2.384. [DOI] [PubMed] [Google Scholar]
- 289.van der Velde AE. Groen AK. Shifting gears: Liver SR-BI drives reverse cholesterol transport in macrophages. J Clin Invest. 2005;115:2699–2701. doi: 10.1172/JCI26241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.van Meeteren LA. Moolenaar WH. Regulation and biological activities of the autotaxin-LPA axis. Prog Lipid Res. 2007;46:145–160. doi: 10.1016/j.plipres.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 291.Varban ML. Rinninger F. Wang N. Fairchild–Huntress V. Dunmore JH. Fang Q. Gosselin ML. Dixon KL. Deeds JD. Acton SL. Tall AR. Huszar D. Targeted mutation reveals a central role for SR-BI in hepatic selective uptake of high density lipoprotein cholesterol. Proc Natl Acad Sci USA. 1998;95:4619–4624. doi: 10.1073/pnas.95.8.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Verhoye E. Langlois MR. Circulating oxidized low-density lipoprotein: A biomarker of atherosclerosis and cardiovascular risk? Clin Chem Lab Med. 2009;47:128. doi: 10.1515/CCLM.2009.037. [DOI] [PubMed] [Google Scholar]
- 293.Virella G. Lopes–Virella MF. Atherogenesis and the humoral immune response to modified lipoproteins. Atherosclerosis. 2008;200:239–246. doi: 10.1016/j.atherosclerosis.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Virella G. Thorpe SR. Alderson NL. Stephan EM. Atchley D. Wagner F. Lopes- -Virella MF. Autoimmune response to advanced glycosylation end-products of human LDL. J Lipid Res. 2003;44:487–493. doi: 10.1194/jlr.M200370-JLR200. [DOI] [PubMed] [Google Scholar]
- 295.Wada Y. Kuroda T. Murasawa A. Tanabe N. Nakano M. Gejyo F. Autoantibodies against oxidized low-density lipoprotein (LDL) and carotid atherosclerosis in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2005;23:482–486. [PubMed] [Google Scholar]
- 296.Walton KA. Cole AL. Yeh M. Subbanagounder G. Krutzik SR. Modlin RL. Lucas RM. Nakai J. Smart EJ. Vora DK. Berliner JA. Specific phospholipid oxidation products inhibit ligand activation of toll-like receptors 4 and 2. Arterioscler Thromb Vasc Biol. 2003;23:1197–1203. doi: 10.1161/01.ATV.0000079340.80744.B8. [DOI] [PubMed] [Google Scholar]
- 297.Watson AD. Subbanagounder G. Welsbie DS. Faull KF. Navab M. Jung ME. Fogelman AM. Berliner JA. Structural identification of a novel pro-inflammatory epoxyisoprostane phospholipid in mildly oxidized low density lipoprotein. J Biol Chem. 1999;274:24787–24798. doi: 10.1074/jbc.274.35.24787. [DOI] [PubMed] [Google Scholar]
- 298.Webb NR. de Villiers WJ. Connell PM. de Beer FC. van der Westhuyzen DR. Alternative forms of the scavenger receptor BI (SR-BI) J Lipid Res. 1997;38:1490–1495. [PubMed] [Google Scholar]
- 299.Wen Y. Leake DS. Low density lipoprotein undergoes oxidation within lysosomes in cells. Circ Res. 2007;100:1337–1343. doi: 10.1161/CIRCRESAHA.107.151704. [DOI] [PubMed] [Google Scholar]
- 300.Winyard PGTF. Esterbauer H. Kus ML. Blake DR. Morris CJ. Presence of foam cells containing oxidised low density lipoprotein in the synovial membrane from patients with rheumatoid arthritis. Ann Rheum Dis. 1993;52:677–680. doi: 10.1136/ard.52.9.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wittwer J. Hersberger M. The two faces of the 15-lipoxygenase in atherosclerosis. Prostagland Leukotrienes Essent Fatty Acids. 2007;77:67–77. doi: 10.1016/j.plefa.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 302.Witztum JL. Steinberg D. The oxidative modification hypothesis of atherosclerosis: Does it hold for humans? Trends Cardiovasc Med. 2001;11:93. doi: 10.1016/s1050-1738(01)00111-6. [DOI] [PubMed] [Google Scholar]
- 303.Witztum JL. Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Woenckhaus C. Kaufmann A. Bufeld D. Gemsa D. Sprenger H. Gröne HJ. Hypochlorite-modified LDL: Chemotactic potential and chemokine induction in human monocytes. Clin Immunol Immunopathol. 1998;86:27–33. doi: 10.1006/clin.1997.4453. [DOI] [PubMed] [Google Scholar]
- 305.Wong M–L. Xie B. Beatini N. Phu P. Marathe S. Johns A. Gold PW. Hirsch E. Williams KJ. Licinio J. Tabas I. Acute systemic inflammation up-regulates secretory sphingomyelinase in vivo: A possible link between inflammatory cytokines and atherogenesis. Proc Natl Acad Sci USA. 2000;97:8681–8686. doi: 10.1073/pnas.150098097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wu BJ. Kathir K. Witting PK. Beck K. Choy K. Li C. Croft KD. Mori TA. Tanous D. Adams MR. Lau AK. Stocker R. Antioxidants protect from atherosclerosis by a heme oxygenase-1 pathway that is independent of free radical scavenging. J Exp Med. 2006;203:1117–1127. doi: 10.1084/jem.20052321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Yamamoto N. Toyoda M. Abe M. Kobayashi T. Kobayashi K. Kato M. Miyauchi M. Kimura M. Umezono T. Suzuki D. Lectin-like oxidized LDL receptor-1 (LOX-1) expression in the tubulointerstitial area likely plays an important role in human diabetic nephropathy. Int Med. 2009;48:189–194. doi: 10.2169/internalmedicine.48.1251. [DOI] [PubMed] [Google Scholar]
- 308.Yancey PG. Jerome WG. Lysosomal sequestration of free and esterified cholesterol from oxidized low density lipoprotein in macrophages of different species. J Lipid Res. 1998;39:1349–1361. [PubMed] [Google Scholar]
- 309.Yeh M. Cole AL. Choi J. Liu Y. Tulchinsky D. Qiao J–H. Fishbein MC. Dooley AN. Hovnanian T. Mouilleseaux K. Vora DK. Yang W–P. Gargalovic P. Kirchgessner T. Shyy JYJ. Berliner JA. Role for sterol regulatory element-binding protein in activation of endothelial cells by phospholipid oxidation products. Circ Res. 2004;95:780–788. doi: 10.1161/01.RES.0000146030.53089.18. [DOI] [PubMed] [Google Scholar]
- 310.Yla–Herttuala S. Is oxidized low-density lipoprotein present in vivo? Curr Opin Lipidol. 1998;9:337–344. doi: 10.1097/00041433-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 311.Zhang H. Yang Y. Steinbrecher UP. Structural requirements for the binding of modified proteins to the scavenger receptor of macrophages. J Biol Chem. 1993;268:5535–5542. [PubMed] [Google Scholar]
- 312.Zhang Y. Da Silva JR. Reilly M. Billheimer JT. Rothblat GH. Rader DJ. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest. 2005;115:2870–2874. doi: 10.1172/JCI25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Zhao Z. de Beer MC. Cai L. Asmis R. de Beer FC. de Villiers WJS. van der Westhuyzen DR. Low-density lipoprotein from apolipoprotein E-deficient mice induces macrophage lipid accumulation in a CD36 and scavenger receptor class A-dependent manner. Arterioscler Thromb Vasc Biol. 2005;25:168–173. doi: 10.1161/01.ATV.0000149145.00865.d9. [DOI] [PubMed] [Google Scholar]