Abstract
Plants can use ammonium (NH4+) as the sole nitrogen source, but at high NH4+ concentrations in the root medium, particularly in combination with a low availability of K+, plants suffer from NH4+ toxicity. To understand the role of K+ transporters and non-selective cation channels in K+/NH4+ interactions better, growth, NH4+ and K+ accumulation and the specific fluxes of NH4+, K+, and H+ were examined in roots of barley (Hordeum vulgare L.) and Arabidopsis seedlings. Net fluxes of K+ and NH4+ were negatively correlated, as were their tissue concentrations, suggesting that there is direct competition during uptake. Pharmacological treatments with the K+ transport inhibitors tetraethyl ammonium (TEA+) and gadolinium (Gd3+) reduced NH4+ influx, and the addition of TEA+ alleviated the NH4+-induced depression of root growth in germinating Arabidopsis plants. Screening of a barley root cDNA library in a yeast mutant lacking all NH4+ and K+ uptake proteins through the deletion of MEP1–3 and TRK1 and TRK2 resulted in the cloning of the barley K+ transporter HvHKT2;1. Further analysis in yeast suggested that HvHKT2;1, AtAKT1, and AtHAK5 transported NH4+, and that K+ supplied at increasing concentrations competed with this NH4+ transport. On the other hand, uptake of K+ by AtHAK5, and to a lesser extent via HvHKT2;1 and AtAKT1, was inhibited by increasing concentrations of NH4+. Together, the results of this study show that plant K+ transporters and channels are able to transport NH4+. Unregulated NH4+ uptake via these transporters may contribute to NH4+ toxicity at low K+ levels, and may explain the alleviation of NH4+ toxicity by K+.
Keywords: Ammonium toxicity, Arabidopsis, barley, competition, gadolinium, potassium nutrition, tetraethyl ammonium
Introduction
Ammonium (NH4+) is a central nitrogen compound in all organisms. In the autotrophic anabolism of plants, NH4+ is generated through the reduction of nitrate, or is directly taken up from the soil. In addition, NH4+ is generated via de-amination of organic compounds during protein turnover, photorespiration, and lignin biosynthesis (Joy, 1988).
It is well known that high concentrations of NH4+ can be toxic to plants leading to severe growth depression (Gerendás et al., 1997). Various hypotheses have been put forward aimed at identifying the cause of NH4+ toxicity. The majority of these hypotheses deal with the physiological changes associated with NH4+ assimilation and ion imbalances resulting from decreased uptake of essential cations such as K+, Mg2+, and Ca2+ (Barker et al., 1967; Gerendás et al., 1997; Roosta and Schjoerring, 2007). Rapid assimilation of NH4+ in roots is associated with a large requirement for carbohydrates and may, consequently, result in root carbon depletion (Kronzucker et al., 1998; Schjoerring et al., 2002). NH4+ assimilation also leads to a net release of H+ and a subsequent reduction in the concentration of dicarboxylic acids by decarboxylation, a cellular response to maintain cytoplasmic pH (Raven and Smith, 1976). Decarboxylation of dicarboxylic acids puts a further strain on the carbon budget of the root, and results in an increased uptake of anions for charge balance. In addition, NH4+ toxicity has been associated with hormonal imbalances (Barker, 1999; Walch-Liu et al., 2000) and a decrease in photosynthesis (Putrich and Barker, 1967; Gerendás et al., 1997).
Recently, an alternative hypothesis was put forward suggesting that growth depression of barley plants at high NH4+ was due to a futile cycling of NH4+ across the plasma membrane, where active extrusion of excess NH4+ by an as yet unknown transporter led to extensive energy consumption (Britto et al., 2001). The high respiration rate of roots in conjunction with the energy demand for active extrusion of NH4+ was suggested to be the fundamental cause of the damaging effects of high external NH4+. Thus, toxicity was explained by an excessive cycling at the plasma membrane as opposed to consequences of assimilation and accumulation in the cells. Several bacteria also show elevated respiration at high NH4+ and low K+ levels, caused by the energetically unfavourable cycling of NH4+ at the plasma membrane (Buurman et al., 1989, 1991). It was suggested that cycling of NH4+ across bacterial plasma membranes is caused by the disruption of the regulation of NH4+ uptake and non-specific influx through the kdp K+ transporter (Buurman et al., 1991).
It has long been known that toxicity of NH4+ is particularly pronounced during K+ deficiency (Wall, 1939). Similarly, addition of K+ can alleviate NH4+ toxicity (Barker et al., 1967; Cao et al., 1993). Toxicity of NH4+ and alleviation by K+ has also been shown in yeast, where high concentrations of NH4+ are toxic only when K+ levels are low (Hess et al., 2006).
NH4+ and K+ are highly similar regarding charge, size, and hydration energy, characteristics that are important for membrane transport (Wang et al., 1996; White, 1996). NH4+ has been shown to influence the uptake and accumulation of K+ (see, for example, Rufty et al., 1982; Allen and Raven, 1987; van Beusichem et al., 1988; Finnemann and Schjoerring, 1999; Angeles Martinéz-Cordero et al., 2005; Szczerba et al., 2008b, and references therein), and K+ influences the uptake and accumulation of NH4+ (Scherer et al., 1984; Nielsen and Schjoerring, 1998; Szczerba et al., 2008a). Reduced NH4+ uptake and accumulation through the provision of K+ may prevent competition with NH4+ in subsequent metabolic processes that require K+. It is therefore likely that the toxicity of NH4+ at high external concentrations is closely linked to its interference with K+ uptake and cellular homeostasis.
The genome of Arabidopsis thaliana contains around 20 genes encoding K+-selective transporters and 57 genes encoding cation channels (Mäser et al., 2001; Véry and Sentenac, 2002). The presence of the non-selective cation channel (NSCC) blocker lanthanum (La3+) reduced the uptake of NH4+ in leaves and roots (Nielsen and Schjoerring, 1998; Szczerba et al., 2008a) and several experiments have indicated that NSCCs can facilitate the transfer of NH4+ across membranes (Gassman and Schroeder, 1994; White, 1996; Davenport and Tester, 2000; Demidchik and Tester, 2002; Balleza et al., 2005). In addition, experiments using different heterologous expression systems have suggested that the Arabidopsis K+ channel AtKAT1 (Schachtman et al., 1992; Bertl et al., 1995; Cao et al., 1995; Uozumi et al., 1995; Moroni et al., 1998), K+ transporters AtKCO1 (Czempinski et al., 1997) and HvHKT2;1 (Santa-María et al., 2000) are also candidates for mediating NH4+ transport.
The aim of this work was to investigate the effect of different concentrations of NH4+ and K+ on the growth of barley (Hordeum vulgare L.) and Arabidopsis seedlings, and to demonstrate the interdependence of K+ and NH4+ fluxes during uptake. Pharmacological treatments with TEA+ and Gd3+ supported the indication from earlier studies that K+-selective channels as well as NSCCs transport NH4+ in the roots. Strikingly, treatment with TEA+ alleviated NH4+-induced growth depression in germinating Arabidopsis seedlings, indicating a role for K+-selective channels in mediating NH4+ uptake. A yeast complementation screen using a cDNA library from barley roots indicated that HvHKT2;1 transports NH4+, and that growth on NH4+ was inhibited by increasing K+ concentrations. Similar results were seen for AtAKT1 and AtHAK5. K+ transport through HvHKT2;1, AtAKT1, and AtHAK5 was inhibited by NH4+. Together these results demonstrate interference between K+ and NH4+ at uptake via HvHKT2;1, AtAKT1 and AtHAK5.
Materials and methods
Plant cultivation
Arabidopsis seeds (Col-0, Lehle seeds) were surface-sterilized by washing for 1 min with 50% ethanol, followed by incubation in 5% NaOCl and 0.02% SDS for 10 min. Thereafter, seeds were rinsed five times with sterile, double-deionized water. They were submerged in ±0.05% agarose and stratified for 2 d at 4 °C, either in the agarose-solution or on media in square 50 ml Petri dishes with the same composition as described by Cao et al. (1993). The medium was supplied with 0.4% agarose and 1% sucrose, and different combinations of 0.2 mM KCl, 6 mM NH4Cl, 1 mM GdCl3 or 10 mM TEACl. On the top of each plate two rows of seeds were sown, with 30 seeds in each row. Plates were organized horizontally, allowing seedlings to grow along the surface of the medium, in a controlled environment growth-chamber with a 85–110 μmol m−2 s−1 photon flux density for 8 h d−1, 75–80% air humidity, and 20 °C air temperature. Plants were cultured for 10 d prior to analysis.
Barley seeds (Hordeum vulgare L., cv. Antonia) were sown on vermiculite in a greenhouse (250–300 μmol m−2 s−1 photon flux density, 75–80% humidity, at 20 °C and 18 °C during 16/8 h light/dark, respectively). Seedlings were watered with (K+-free) double-deionized water. When the shoot was about 8 cm, four seedlings were transferred to 4.0 l buckets containing hydroponic medium without K+ or NH4+. The medium consisted of 0.3 mM MgSO4-7H2O, 0.1 mM NaCl, 0.2 mM NaH2PO4-H2O, 0.2 mM Na2SO4-10H2O, 0.15 mM Mg(NO3)2-6H2O, 0.6 mM Ca(NO3)2-4H2O, 1.5 mM Fe(III)-EDTA-Na, 150 μM MnSO4-H2O, 105 μM ZnSO4-7H2O, 120 μM CuSO4-5H2O, 300 μM H3BO3, and 120 μM Na2MoO4-2H2O. The pH was kept at 6.0 with 1.2 mM MES/NaOH. The solution was aerated with filtered air supplied through a syringe needle. After 3 d of growth in hydroponic medium, various concentrations of NH4+ and K+ were added using (NH4)2SO4 and/or K2SO4. The nutrient solution was changed daily. Five days after the initiation of the K+/NH4+ treatments, plants were harvested in a mixed sequence to reduce artefacts caused by circadian rhythms. For NH4+ and K+ determination, roots were washed in 2.5 g l−1 CaSO4, and three times in double-deionized water, then dried on paper tissue. Plants were fractionated into (i) oldest leaf, (ii) young leaves, (iii) stems, and (iv) roots. Each fraction was weighed, frozen in liquid nitrogen, and stored at –80 °C prior to extraction.
Root length measurements
Arabidopsis roots were magnified and measured using a scanner or a microscope (Leica Microsystems M500 or MZ FL III) connected to a camera (Leica Microsystems DC 300 F).
Measurement of soluble NH4+ content
Samples were homogenized with a steel pin, after which 200 mg was taken out and crushed with 100 μl acid-washed sand and 2 ml 10 mM ice-cold formic acid, in an agate mortar on ice. This suspension was centrifuged at 10 000 g and 4 °C. The supernatant was centrifuged again and the supernatant of this last centrifugation-step was diluted ten times with 10 mM formic acid. The NH4+ concentration was determined by measuring the fluorescence in 3 mM o-phthalaldehyde, 10 mM 2-mercaptoethanol, and 100 mM phosphate buffer adjusted to pH 6.8 as previously described (Husted et al., 2000).
Potassium determination with ICP-OES
Samples from the NH4+-extraction (described in the previous paragraph) were diluted ten times with 7% HNO3. The K+ concentration was determined by Induced Coupled Plasma-Optical Emission Spectroscopy (ICP-OES; Perkin-Elmer Optima 3000XL) at axial mode using a wavelength of 766.474 nm.
Flux measurements
Net fluxes of NH4+, K+, and H+ at the surface of Arabidopsis roots were measured non-invasively using the MIFE® technique (UTas Innovation, Hobart, Australia) essentially as described by Shabala et al. (1997, 2001) and Shabala (2000). Briefly, microelectrodes were pulled and salinized with tributylchlorosilane. After backfilling with an appropriate electrolyte, electrode tips were filled with commercially available ionophore cocktails (09882 for NH4+; 60031 for K+; 95297 for H+; all from Fluka) and mounted on a 3D-micromanipulator (MMT-5; Narishige), with their tips close together, 20 μm above the root surface. During measurements, a computer-controlled stepper motor moved the electrodes between two positions (20 μm and 40 μm, respectively) from the root surface in a 10 s square-wave manner. The CHART software (Shabala et al., 1997; Newman, 2001) recorded the potential difference between two positions and converted them into electrochemical potential differences using the calibrated Nernst slope of the electrodes. H+ fluxes were then calculated using the MIFEFLUX software for cylindrical diffusion geometry (Newman, 2001). NH4+ and K+ fluxes were calculated using the algorithm described in Knowles and Shabala (2004) taking into account the mutual interference between the two ions resulting from non-ideal selectivity of both the K+ and NH4+ ionophore cocktails.
Arabidopsis Col-0 plants were pre-grown on plates for 10 d as described. Eight to 10-mm-long apical root segments were excised and mounted horizontally in a Perspex holder using agar (see Babourina et al., 2000, for details). The holder was immediately placed in a 4 ml measuring chamber filled with 0.1 mM CaCl2, pH 5.7 (unbuffered), mounted on a computer-driven 3D-micromanipulator (MMT-5; Narishige) and left to equilibrate for 60 min before the addition of treatment. Steady-state ion fluxes were recorded over a period of 5 min, 15 min after the application of the appropriate treatment. Unless stated differently, 1 mM NH4+, 0.2 mM K+, 10 mM TEA, and 0.1 mM Gd3+ were used in the various treatments.
Yeast strains and expression of plant transporters
Three deletion mutants in the yeast Saccharomyces cerevisiae were generated in this study: a triple NH4+ transporter deletion mutant Δmep1-3, a K+ transporter double deletion mutant Δtrk1,2 and a 5-fold deletion mutant Δmep1-3 Δtrk1,2. All mutants were made in the BY background from the Euroscarf collection. To generate Δmep1-3, HIS3 was first generated by PCR using primers: fw 5′-CACATGTGCTAACCAAACATCAGTGGGTAGTAATCATTCGGCGTCAGCGGGTGTTGG-3′ and rev 5′-CGTTGGCATGCGATGAGGTCAGTTTCCTGCGCCATTTCAGTCTCGGTCTATTCTTTTGATTTAT-3′ to form a deletion cassette for MEP2. The PCR product was transformed into Δmep1 (Y04751, Mata his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 YGR121c::kanMX4), thus yielding a double mutant Δmep1,2. The double mutant was crossed with Δmep3 (Y15553, Matα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 YPR138c::kanMX4) to yield Δmep1-3 (Matα leu2Δ0 lys2Δ0 ura3Δ0 YGR121c::kanMX4 YNL142w::HIS3 YPR138c::kanMX4). Yeast strain Δtrk1,2 (Mata his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 YKR050w::kanMX4, YJL129c::kanMX4) was generated by crossing Δtrk1 (Y15121 Matα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 YKR050w::kanMX4) and Δtrk2 (Y01296 Mata his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 YJL129c::kanMX4). Likewise, the 5-fold deletion mutant strain Δmep1-3 trk1,2 (Mata leu2Δ0 ura3Δ0 YGR121c::kanMX4 YNL142w::HIS3 YPR138c::kanMX4, YKR050w::kanMX4, YJL129c::kanMX4) was generated by crossing the two aforementioned yeast strains Δmep1-3 and Δtrk1,2. All crossings (mating and sporulation) were done as described by Kassir and Simchen (1991). The deletion of genes was successfully verified by PCR, using a reverse primer for the flanking kanamycin gene (rev 5′-CTAGTAATCTTCAGGGGCC-3′) in combination with forward primers for the genes: MEP1 5′-ATGGCAACATCAGCTCG-3′, MEP3 5′-ATGGCTACATCTGCTAGAAGA-3′, TRK1 5′-TTTCACGAAAAGAGGGACAATGTAC-3′, and TRK2 5′-AGGATTCGTTGTGCTTGTTGAATCG-3′. The genotypes of the resulting yeast strains were characterized by cultivation on different amino acids and NH4+ and K+ conditions and their mating type was determined by mating with haploid strains with supplementing marker-genes and known mating type. To enable selection using two markers only, and to grow cells on media without supplementation by amino acids, yeast strains were transformed with single copy vectors from the pRS series containing appropriate marker genes.
Yeast strains were transformed with pYES2 containing cDNAs encoding Arabidopsis hak5L776H (Rubio et al., 2000), AtAKT1 in plasmid pFL61 (Sentenac et al., 1992) or a barley root cDNA library (Pedas et al., 2008). Transformation was done by electroporation, as described at http://www.agr.kuleuven.ac.be/dp/logt/protocol/yeastelectroporation.htm. After transformation, yeast cells were pre-grown on synthetic YNB medium without NH4+, supplemented with 100 mM K2SO4 and 0.1% proline, on 2% glucose and a pH of 5.5 (50 mM succinic acid/TRIS). Growth assays were carried out on synthetic medium containing 1% galactose, YNB without amino acids, K+ and NH4+ and supplemented with various concentrations of (NH4)2SO4 or K2SO4 as indicated. Plates were incubated at 30 °C for at least 3 d. The K+ content of agar was determined to be 500 μg g−1, which has been accounted for in the composition of the media.
Statistical analysis
Statistical analysis was performed using SAS software (SAS Institute Inc.,USA, version 9.1).
Results
Uptake and accumulation of NH4+ and K+ in barley
To characterize potential interferences in the uptake and accumulation of NH4+ and K+ in barley, seedlings were cultivated in hydroponic medium, containing NO3− but neither NH4+ nor K+. Subsequently, seedlings were transferred for 5 d to medium supplemented with various NH4+ and K+ concentrations. Fresh weight and the soluble NH4+ and K+ content were determined.
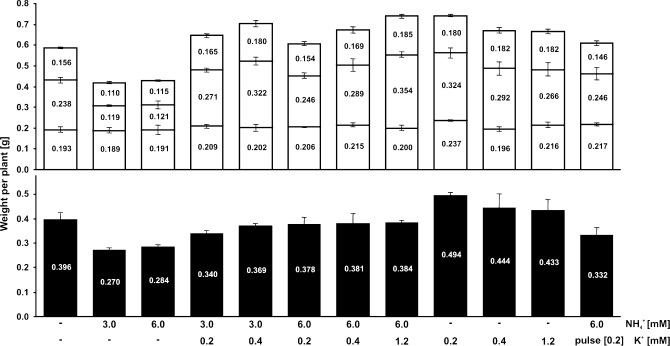
Cultivation for 5 d on high levels (3–6 mM) of NH4+ without K+, significantly decreased both root and shoot fresh weight compared with plants grown in similar media but in the absence of NH4+ (Figs 1, 2). This growth depression by NH4+ was progressively alleviated by the presence of sub-millimolar concentrations of K+. The weight of seedlings grown in the absence of K+ and NH4+ was only slightly decreased compared with plants that had received K+, but no NH4+ (Fig. 2). Three days on 6 mM NH4+ and 0.2 mM K+, followed by a 2 d K+-starvation period with unchanged NH4+ provision did not produce any significant difference in fresh weight relative to plants with constant K+ supply.
Fig. 1.
Barley plants (cv. Antonia) cultivated at various K+ and NH4+ concentrations. Plants were grown in a greenhouse in hydroponic medium with all nutrients including 1.5 mM NO3− as nitrogen source but without NH4+ or K+ for 3 d and subsequently transferred for 5 d to various K+ and NH4+ concentrations as indicated.
Fig. 2.
Fresh weight of barley plants (cv. Antonia) cultivated at various K+ and NH4+ concentrations. Plants were grown in a greenhouse in hydroponic medium with all nutrients including 1.5 mM NO3− as nitrogen source but without NH4+ or K+ for 3 d and subsequently transferred for 5 d to various K+ and NH4+ concentrations as indicated. Black bars represent root weight; white bars represent shoot weight, with old leaves (bottom), young leaves (middle), and stem (top). Data represent means ±SE of 12 plants for each measurement.
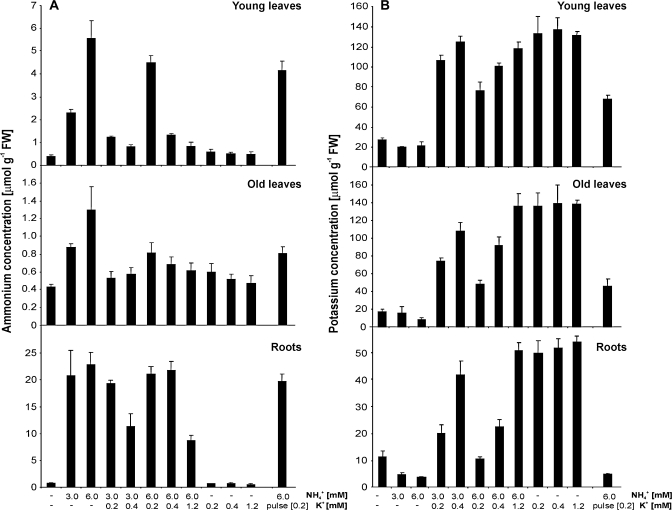
The soluble NH4+ content (Fig. 3A) of plants cultivated in the absence of NH4+ was in the range of 0.4–0.7 μmol g−1 FW in all tissues, regardless of the K+ concentration in the medium. A concentration of 0.4–0.7 μmol NH4+ g−1 FW may therefore reflect the basal NH4+ concentration resulting from nitrate reduction and processes such as protein turnover and photorespiration. The presence of NH4+ in the nutrient solution led to elevated levels of soluble NH4+ in all tissues, with maxima of 5.6 μmol g−1 FW in young leaves and 22.9 μmol g−1 FW in roots when NH4+ was supplied at 6 mM. These amounts were reduced in the presence of K+, with a higher effect in shoot than in root tissue (Fig. 3A).
Fig. 3.
NH4+ (A) and K+ (B) tissue concentration of barley plants (cv. Antonia) cultivated on different NH4+ and K+ regimes in hydroponic medium. Plants were grown in hydroponic medium including all nutrients and 1.5 mM NO3− as nitrogen source but without NH4+ or K+ for 3 d and subsequently transferred to similar medium including various K+ and NH4+ concentrations (as indicated) for an additional 5 d. Data represent means ±SE of three measurements with material from four plants per measurement.
The corresponding K+ content showed values as low as 10 μmol g−1 FW in roots and 20 μmol g−1 FW in leaves of plants that had never received any K+ other than their seed reserves (Fig. 3B). In these conditions, increasing levels of NH4+ in the growth media had a negative effect on the K+ concentrations of all the tissues tested, in line with the interpretation that NH4+ causes K+ extrusion (Szczerba et al., 2006). This reduction in K+ concentration was particularly apparent in roots, where a concentration as low as 3.9 μmol K+ g−1 FW was observed in plants that were not supplied with K+. When neither K+ nor NH4+ was supplied, the K+ concentration of roots was more than twice as high (11.5 μmol g−1 FW). In the absence of external NH4+, the addition of 0.2, 0.4 or 1.2 mM K+ resulted in comparable levels of K+, approximately 50 μmol g−1 in roots and 140 μmol g−1 in leaves. Withholding K+ during the final 2 d of a treatment with 6 mM NH4+ and 0.2 mM K+ reduced the K+ concentration in roots, but not in leaves, indicating that barley plants are very efficient in controlling and retaining K+ in leaves.
Besides the smaller size and chlorotic appearance of the young leaves of plants grown on high NH4+ concentrations, in the absence of K+, there were no clear phenotypic differences between plants in different treatments. In the presence of low K+ concentrations, the negative effects of NH4+ provision were ameliorated (Fig. 1).
Blocking K+ uptake of Arabidopsis seedlings by pharmacological inhibitors
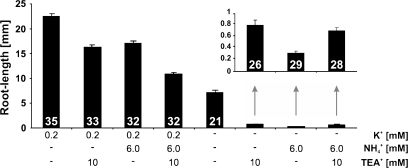
An earlier study by Cao et al. (1993) reported the dramatic depression of root growth of Arabidopsis seedlings caused by the presence of NH4+ in the absence of K+ in the growth media. To study the effect reported by Cao et al. (1993) further, Arabidopsis seeds were germinated on media containing 6 mM NH4+ in the absence of K+. Seedlings hardly developed roots (Fig. 4). The addition of 0.2 mM K+ to the 6 mM NH4+ medium restored root growth above the level measured when neither NH4+ nor K+ were present, essentially confirming the earlier results of Cao et al. (1993). To investigate whether the amelioration of NH4+ toxicity by K+ was due to competition between K+ and NH4+ at the same uptake site, 10 mM tetraethyl ammonium (TEA+), a known blocker of K+ channels (Yellen et al., 1991; Lenaeus et al., 2005), was added to the medium (Fig. 4). Addition of TEA+ generally led to a substantial, significant reduction in root growth, which was most pronounced in the absence of K+ in the growth medium. This pronounced root growth depression may be due to the inhibition of remobilization of K+, which had initially derived from seed reserves. However, growth depression by 10 mM TEA+ in the absence of K+ and NH4+ was less pronounced than growth depression by the application of 6 mM NH4+ alone, the latter of which almost completely inhibited root formation. More importantly, in the presence of 10 mM TEA+ plus 6 mM NH4+, growth was not significantly different from growth in the presence of 10 mM TEA+ alone (Figs 4, 5). In fact, in this case the addition of TEA+ significantly improved growth and completely abolished the NH4+-induced growth depression. These data suggest that K+ efficiently reduced the uptake of NH4+, but that NH4+ toxicity was not exclusively the result of competition with K+. Rather, NH4+ toxicity seemed to directly relate to an increased flux of NH4+ into the roots. Addition of Gd3+, a known blocker of NSCCs (Demidchik et al., 2002), had a minor effect and did not reverse the effects of NH4+ toxicity to the same extent as TEA+ (data not shown).
Fig. 4.
Primary root length of Arabidopsis Col-0 seedlings cultivated for 10 d on plates containing different amounts of NH4+, K+, and TEA+ as indicated. Inset: Root length at treatments with high levels of NH4+ and/or TEA+ in the absence of K+. Data represent means ±SE. Between 21 and 35 roots were measured per treatment.
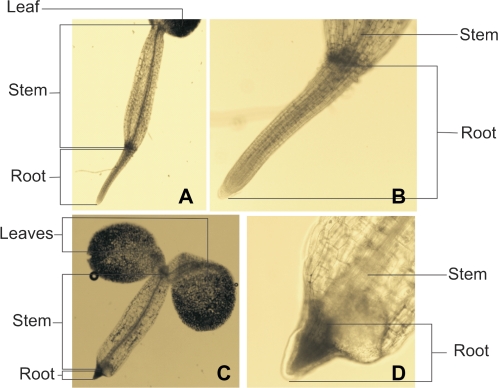
Fig. 5.
Roots of Arabidopsis Col-0 plants grown on medium without K+, but in the presence of 6 mM NH4+ (C, D) or 6 mM NH4+ plus 10 mM TEA+ (A, B). (This figure is available in colour at JXB online.)
Fluxes of NH4+ and K+ under the influence of uptake blockers in Arabidopsis
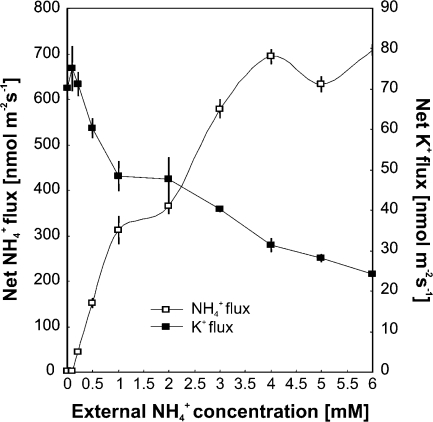
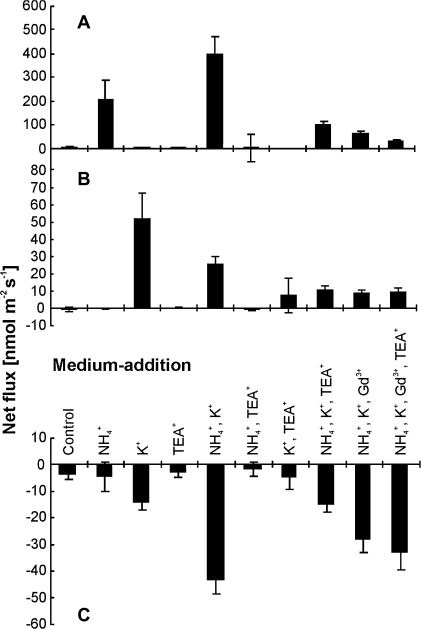
To characterize more closely the interference between NH4+ and K+ at the site of uptake at the root surface, net NH4+, K+, and H+ fluxes were determined in the mature root zone of Arabidopsis seedlings with microelectrodes using the MIFE® technique (Shabala et al., 1997; Newman, 2001). Roots of seedlings grown on agarose plates without K+ and NH4+ (as described previously), were exposed to various bathing solutions. When fluxes of NH4+ and K+ were measured at constant 0.2 mM K+ and various NH4+ concentrations (Fig. 6), an almost linear inverse correlation of fluxes for the two cations was found. Increasing NH4+ in the solution increased the NH4+ influx, while reducing the K+ influx. Addition of NH4+ and K+ separately resulted in increased NH4+ and K+ influxes, respectively (Fig. 7). Interestingly, in the presence of both K+ and NH4+, net influx of NH4+ was about double that measured upon sole NH4+ exposure. Conversely, net influx of K+ was reduced to about half of the flux measured under sole K+ exposure. Thus, K+ stimulated NH4+ influx, while NH4+ reduced the influx of K+. The high net influx of NH4+ in the presence of both ions was accompanied by a marked increase in net H+ efflux across the plasma membrane. The addition of 10 mM TEA+ and 0.1 mM Gd3+ strongly reduced both NH4+ and K+ influxes, supporting the view that stimulation of NH4+ influx by K+ and the inhibition of K+ influx by NH4+ were related to one or several common uptake systems. Application of TEA+ did not affect H+ extrusion in the absence of K+ and NH4+, but reduced H+ effluxes in solution where K+ and NH4+ were present. Net H+ efflux was less affected by Gd3+ than by TEA+ (Fig. 7).
Fig. 6.
Net fluxes of NH4+ and K+ at the root surface are dependent on the external concentration of NH4+. Arabidopsis Col-0 plants were grown on agarose plates with NO3− as nitrogen source and without K+ and NH4+. Measurements were done at constant external K+ of 0.2 mM in bathing solution (0.1 mM CaCl2).
Fig. 7.
Net fluxes of NH4+ (A), K+ (B), and H+ (C). Arabidopsis Col-0 plants were grown on agarose plates with NO3− as nitrogen source and without K+ and NH4+. Fluxes were measured for 5 min in bathing solution (0.1 mM CaCl2) 15 min after the addition of various NH4+, K+, TEA+, and Gd3+ as indicated.
NH4+/K+ interference via plant transporters expressed in yeast
The results described above suggest that certain plasma membrane transporters or channels in both Arabidopsis and barley are capable of transporting NH4+ and K+. To identify putative transporters or channels at the molecular level, several new mutants lacking multiple NH4+ and K+ transporters were generated from single deletion mutants in the yeast BY4741 background (Euroscarf): (i) an NH4+ transporter mutant Δmep1-3, devoid of all three NH4+ transporter genes MEP1, MEP2, and MEP3, (ii) a K+ transporter mutant Δtrk1,2 lacking the two K+ transporter genes TRK1 and TRK2, and (iii) a 5-fold deletion mutant Δmep1-3 Δtrk1,2 lacking all five genes. The triple mutant Δmep1-3, not expressing the NH4+ transporters Mep1p, Mep2p, and Mep3p, did not grow on media containing 10 mM NH4+ as the sole nitrogen source after 3 d of incubation at 30 °C (Fig. 8A, empty vector control). The trk1,2 double mutant did not grow at less than 10 mM K+ in the medium when proline was the sole nitrogen source (Fig. 8B, empty vector control). Deletion of all five genes in Δmep1-3 Δtrk1,2 thus provided a tool for the identification of transporters that transport both NH4+ and K+ at limiting concentrations.
Fig. 8.
Growth assays of various yeast strains transformed with cDNA encoding HvHKT2;1, ΔN62-hkt2;1, AtAKT1, or hak5L776H at various K+ and NH4+ concentrations or 0.1% proline instead of NH4+ as nitrogen source. The various yeast mutant strains were (A) Δmep1-3, (B) Δtrk1-2, and (C) Δmep1-3 Δtrk1-2. Transformants were pre-grown on SD medium containing 0.1% proline as nitrogen source and supplemented with 100 mM K+. Yeast suspensions with an OD600nm of 0.01 (upper drop) and 0.0001 (lower drop) were placed onto SG medium (1% galactose; YNB without amino acids, K+ and NH4+) with various supplementations as indicated. Control, empty vector pYES2.
The yeast mutant Δmep1-3 Δtrk1,2 was transformed with a cDNA library made from roots of barley seedlings (Pedas et al., 2008). In the initial screen, transformants were grown on media containing six different combinations of pH, [NH4+], and [K+]: pH 4.5 with 0.5 mM K+ and 5 or 10 mM NH4+, pH 4.5 with 5 mM K+ and 5 or 10 mM NH4+, pH 5.5 with 1 mM K+ and 20 mM NH4+, and pH 5.5 with 5 mM K+ and 50 mM NH4+. A total of 112 colonies from all conditions were harvested and plasmids of 24 of these transformants (four of each of the six conditions) were extracted, transformed into E. coli and subjected to DNA sequencing. A database search revealed that all 24 coding sequences were identical to HvHKT2;1. Strikingly, about half of the plasmids contained a version of HvHKT2;1 cDNA lacking either 36 or 85 nucleotides in the 5′ end when compared to full-length clones. Twenty nucleotides of this deletion represented a sequence of the 5′-UTR. An in-frame ATG codon, 186 nucleotides downstream of the original ATG start codon, probably served as a translational initiation point resulting in ΔN62-hkt2,1 in both truncated versions. HvHKT2;1 and ΔN62-hkt2;1 as well as hak5L776H and AtAKT1 from Arabidopsis were subsequently characterized in the triple mutant Δmep1-3, the double mutant Δtrk1,2 and the 5-fold mutant Δmep1-3 Δtrk1,2 on media containing various NH4+ and K+ concentrations (Fig. 8). The mutation L776H in the AtHAK5 protein was earlier shown to be important for functional expression of AtHAK5 in yeast (Rubio et al., 2000).
Full-length HvHKT2;1 and AtAKT1 very weakly increased growth of Δmep1-3 compared with the empty vector control and other transporter genes at low NH4+ (10 mM) and low K+ (0.15 and 1 mM; Fig. 8A). In all transformants of Δmep1-3, growth was suppressed by increasing K+ concentration irrespective of the NH4+ level, indicating that K+ competed with NH4+ (Fig. 8A) When transformed into Δtrk1,2, AtAKT1, HvHKT2;1, ΔN62-hkt2;1, and hak5L776H clearly complemented the growth deficiency of the mutant at low K+ concentrations (Fig. 8B). However, increasing NH4+ at low K+ caused growth repression, particularly in Δtrk1,2 transformed with ΔN62-hkt2;1, and hak5L776H but also, albeit to a smaller extent, in Δtrk1,2 transformed with HvHKT2;1 and AtAKT1 (Fig, 8B) In the 5-fold mutant lacking all yeast endogenous NH4+ and K+ transporters, only HvHKT2;1, AtAKT1 and, less markedly, hak5L776H, complemented the growth deficiency of the mutant (Fig. 8C). Interestingly, when transformed with full-length HvHKT2;1, the triple mutant Δmep1-3 and the 5-fold mutant Δmep1-3 Δtrk1,2 grew well (Fig. 8C), whereas the double mutant Δtrk1,2 did not (Fig. 8B). The opposite was the case for its N-terminally truncated counterpart ΔN62-hkt2;1, which complemented growth deficiency on low [K+] better in Δtrk1,2 than in Δmep1-3 or Δmep1-3 Δtrk1,2.
Discussion
Changes in cation composition, specifically a reduction in tissue levels of K+ have been reported in connection with NH4+ toxicity in plants (see for example Scherer et al., 1984; Vale et al., 1987, 1988; Finnemann and Schjoerring, 1999; Santa María et al., 2000; Kronzucker et al., 2003; Szczerba et al., 2006). Elevated external concentrations of K+ can alleviate the effects of NH4+ toxicity, and cause a decrease in the tissue concentrations of NH4+ (Barker et al., 1967; Cao et al., 1993; Spalding et al., 1999; Szczerba et al., 2008a, and references therein). To understand the role of cation transporters and channels in NH4+ toxicity better, plant growth, NH4+ and K+ accumulation, and the specific fluxes of NH4+, K+, and H+ in Arabidopsis and barley seedlings were examined. High NH4+ and low K+ concentrations caused chlorosis in leaves and reduced biomass and root growth of barley plants (Figs 1, 2), suggesting that these conditions caused NH4+ toxicity. All of these symptoms were alleviated by an elevated provision of K+.
In barley, plant-soluble NH4+ content was low at high external K+ concentration and vice versa (Fig. 3; see also Szczerba et al., 2006, 2008a). In young leaves, however, K+ and NH4+ concentrations were relatively constant, with NH4+ only reaching high levels when the external NH4+ concentration was very high and that of K+ very low. K+ levels in young leaves were always above 70–80 μmol g−1 FW, unless K+ was absent from the growth medium. This constant level of K+ may reflect the important role of K+ in vital processes such as stomatal opening and closure (Humble and Hsiao, 1969; Cakmak et al., 1994; Dietrich et al., 2001) and protein and starch synthesis (Marschner, 1995). In K+-deficient barley plants several transport mechanisms may be activated to enable a more efficient translocation from root to shoot, thus sustaining the K+ concentration of the latter and securing K+-dependent metabolic processes (Britto and Kronzucker, 2006).
The exact mechanism behind the interaction of K+ and NH4+ is not known, but it has been suggested that membrane passage of NH4+ via K+ channels and NSCCs could play a role (Howitt and Udvardi, 2000; Kronzucker et al., 2001; Szczerba et al., 2008a, b). Our results show that the growth inhibition by high external NH4+ was reduced in the presence of either TEA+ or Gd3+, which suggests a role for both K+-selective channels as well as NSCCs in mediating NH4+ uptake. Interestingly, alleviation of NH4+ induced growth depression by TEA+ was only seen when the growth medium was supplemented with sucrose (data from experiments without sucrose not shown). This may be explained by an inhibition of internal K+ transport by TEA+ causing a negative impact on carbohydrate and energy supply to the roots via the phloem which will be more severe in the absence of external sucrose.
To investigate more closely the transport of NH4+ and K+ at the root surface, flux measurements were undertaken, using the MIFE® technique (Newman, 2001). Net fluxes of NH4+, H+, and K+ were recorded at the mature root zone of Arabidopsis seedlings. At a constant K+ concentration of 0.2 mM, net NH4+ and K+ fluxes into the roots showed an almost linear inverse correlation with NH4+ reducing the uptake of K+, and K+ reducing the uptake of NH4+ (Fig. 6). This indicates competition for uptake sites between the two ions as also suggested by Scherer et al. (1984), Nielsen and Schjoerring (1998), and Szczerba et al. (2008a).
Although fluxes showed a reverse correlation, co-supplementing plants with K+ increased NH4+ influx (Fig. 7). This could be the result of increased channel- or transporter activity to enable K+ uptake. Indeed, both Gd3+ and TEA+ strongly reduced the NH4+ influx under these conditions with a combination of both blockers giving the largest effect. Very similar results were obtained in experiments using nutrient solution instead of CaCl2 as the bathing medium (data not shown). The inhibition of NH4+ influx by uptake blockers suggests that K+ channels/transporters as well as NSCCs are involved in membrane passage of NH4+. This result is in contrast to reports by Szczerba et al. (2008a) who observed an increased NH4+ influx at 0.1 and 40 mM K+ in the presence of TEA+, and concluded that the K+-sensitive influx of NH4+ was mainly via the NSCC pathway.
Unlike the short-term K+ stimulation of NH4+ influx in Arabidopsis, K+ provision to barley inhibited NH4+ accumulation over time (Fig. 3). The stimulation of NH4+ influx by K+ in our measurements with Arabidopsis may be related to the growth conditions: for flux measurement, plants were germinated in the absence of both K+ and NH4+. This would lead to an induction of transporters, such as HAK5, which is repressed by high NH4+ (Rubio et al., 2008) but induced by low K+ supply. It would have been relevant to measure fluxes from plants that were grown in the presence of high NH4+ and in the absence of K+, but such conditions led to root growth inhibition.
Excessive uptake of cations over anions across the plasma membrane in root cells leads to acidification of the rhizosphere (Marschner, 1995), explaining the measured proton efflux with K+, NH4+ or a combination of both ions in the media (Fig. 7C). The highest H+ efflux was consequently measured in the presence of both NH4+ and K+, with a reduction upon the co-presence of TEA+ and Gd3+, in line with the reduced uptake of NH4+ and K+. As compared with the H+ fluxes measured upon the presence of NH4+, K+, and TEA+, an unexpected large net H+ efflux was observed in the additional presence of Gd3+, a situation in which the fluxes of NH4+ and K+ were reduced. Gd3+ has been shown to inhibit H+ influx in the alga Chara corallina (McConneaughy and Falk, 1991) although the precise mechanisms behind this inhibition are unknown. A similar mechanism could lead to the external accumulation of H+ observed in this study.
Competition of NH4+ and K+ at the site of transporters
In Arabidopsis roots, AKT1 and particularly HAK5 are the main players in high affinity K+ uptake (Rubio et al., 2008). AKT1 transports K+ over a broad range of concentrations and is suggested to facilitate NH4+-insensitive K+ uptake (Hirsch et al., 1998; Angeles Martínez-Cordero et al., 2005). However, a study using insertion lines showed that transport of K+ by both AKT1 and HAK5 is sensitive to NH4+ (Rubio et al., 2008). Our results from heterologous expression of AKT1 and HAK5 in the yeast mutant Δtrk1,2 support this earlier study, although inhibition of K+ transport by NH4+ was more pronounced in yeast expressing HAK5 relative to AKT1. In the presence of NH4+, induction of HAK5 is suppressed by low K+ concentration (Qi et al., 2008; Rubio et al., 2008), suggesting that AKT1 is the main transporter for high affinity K+ uptake in the presence of NH4+ (Rubio et al., 2008) and that HAK5 does not play a role in the inhibition of K+ uptake by NH4+ in Arabidopsis. However, in the tomato variety Micro Tom, the gene encoding LeHAK5 is highly expressed independent of whether NH4+ is present or not (Nieves-Cordones et al., 2007).
HKT isoforms in various species have been shown to function as K+/Na+- or Na+/Na+ co-transporters (Rodríguez-Navarro and Rubio, 2006). Our data suggest that HvHKT2;1 and AtAKT1, when expressed in yeast, are able to transport NH4+ and K+. However, the barley cDNA library screen in yeast only retrieved HvHKT2;1 clones, although transformants were grown on various concentrations of K+ and NH4+. Other isoforms may not have been functionally expressed in our yeast heterologous expression system as was also the case for AtHAK5 in yeast (Rubio et al., 2000). Lack of functional expression may similarly explain results from a wheat library screen in yeast for the complementation of potassium transport deficiency, which only retrieved TaHKT2;1 and TaLCT1 (Schachtman et al., 1997).
The library screen resulted in the identification of both full-length and truncated versions of HvHKT2;1 with no apparent correlation as to the particular concentrations of NH4+ and K+ and the pH of the media. However, the closer characterization on media with lower galactose concentration and without succinic acid as buffer revealed profound differences between full-length HKT2;1 and ΔN62-hkt2;1. Full length HvHKT2;1 best supported growth of Δmep1-3 and Δmep1-3 Δtrk1,2, whereas ΔN62-hkt2;1 complemented better in Δtrk1,2, i.e. in the presence of MEP1-3. This may suggest a cross-regulation between HKT2;1 and Mep/AMT homologues that deserves further investigation. Haro et al. (2005) reported differences in the transport of full-length versus truncated versions of HvHKT2;1. A recent investigation by the same group suggested that it is the expression level of HKT2;1, rather than its truncation, that is responsible for variation in activity (Bañuelos et al., 2008). These differences in expression levels were due to small ORFs in artificial UTRs resulting from the plasmid. We do not know how truncation in our experiments affected the expression level of the proteins. However, we did not observe ORFs in the polylinker of our plasmid, suggesting that the level of expression of both HvHKT2;1 and ΔN62-hkt2;1 was similar.
How could NH4+ uptake through K+ transport proteins and NSCCs result in NH4+ toxicity?
Recently, it was suggested that growth depression of barley plants at high NH4+ was due to a futile cycling, involving uptake of NH4+ followed by active extrusion at the plasma membrane (Britto et al., 2001). While such a mechanism can well explain growth depression, it does not explain why plants would aim to eliminate excess NH4+. Extrusion of NH4+ should be the cellular response to avoid the accumulation of NH4+, which is critical at high concentration. Here, the cost of extrusion would represent a secondary but, potentially, rather serious problem. Alternatively, the cycling of NH4+ itself would be an unavoidable process. In such a scenario, futile cycling could indeed be the cause of NH4+ toxicity. Yet, such cycling would require uptake and extrusion mechanisms.
It is well accepted that NH4+ transporters of the AMT family are highly specific for NH4+ over K+ (Ninnemann et al., 1994). Recent studies on the structure of AmtB have suggested that discrimination of K+ against NH4+ in AMT homologues is due to a de-protonation process of NH4+ in the transporter (Khademi et al., 2004). The E. coli AmtB binds NH4+ with high affinity and, following de-protonation, NH3, and possibly H+, is transported through the channel. Thus, AmtB could be considered a catalytic channel that modifies its substrate with the aim of discriminating between NH4+ and the highly similar K+ ion. Although it is a matter of controversy if the transport via AmtB is electrogenic (Javelle et al., 2008), de-protonation at S219 appears to be a prerequisite for transport (Ishikita and Knapp, 2007) and may serve a function to discriminate between NH4+ and K+. Similar roles may apply to plant AMTs which have been shown to perform electrogenic transport (Ludewig et al., 2002). Ion transporters and channels transporting K+ do not possess such a mechanism of ion discrimination, providing an explanation as to why K+ transporters and NSCCs do take up NH4+ even when NH4+ uptake by AMTs is down-regulated. The finding that K+ transporters and channels as well as NSCCs transport NH4+ provides a plausible explanation for the uptake of NH4+, at least at low external K+, being an unavoidable process due to the physico-chemical similarities of both ions.
In plants, plasma membrane transporters for active extrusion of NH4+ as postulated by Britto et al. (2001) have not been identified. An alternative explanation for an active, energy-consuming extrusion mechanism could be passive diffusion of ammonia (NH3) out of the cytoplasm either into the vacuole or across the plasma membrane into the apoplast. The vacuole is likely to be the primary compartment trapping NH4+ due to the presence of NH3 permeable tonoplast intrinsic proteins (Jahn et al., 2004; Loque et al., 2005). Such a role for TIPs in an acid trap mechanism for NH4+ has recently been suggested (Ludewig et al., 2007). The vacuolar compartment, however, can only provide a limited volume for buffering of excess cytoplasmic NH4+. Once this buffer capacity is exhausted, excess NH4+ continuously provided from the external medium could diffuse in the form of uncharged NH3 along its concentration gradient back across the plasma membrane and into the apoplast. The concentration gradient for NH3 is maintained by the pH gradient across the plasma membrane. Thus, the continuous uptake of NH4+ into the cell versus diffusion of NH3 out of the cell would result in a futile cycling of H+ across the plasma membrane, and part of this cycle would be catalysed by the ATP-fuelled plasma membrane H+-ATPase. Obtaining unambiguous proof for such an hypothesis is challenging, as it is difficult to demonstrate the form in which NH4+/NH3 is transported in and out of the cell. The observation that the addition of TEA+ inhibited NH4+-induced H+ efflux from Arabidopsis roots (Fig. 7) however, supports the interpretation that the activity of the plasma membrane H+-ATPase represents the energy-consuming step rather than the NH4+ extrusion.
Acknowledgments
We thank Kim A Kristiansen, Michael C Hansen, Bente R Broeng, and Mette Sylvan (University of Copenhagen, Denmark) and Stefan Kol (University of Groningen, Holland) for excellent help with microscopy, ICP-OES, NH4+ measurements, and assistance in preparing figures. Daniel P Schachtman (Donald Danforth Plant Science Center, St Louis, Missouri) and Hervé Sentenac (Université Montpellier Cedex 1, France) are acknowledged for kindly providing yeast expression vectors with Arabidopsis hakL776H and AtAKT1. Financial support from the Danish Ministry of Science, Technology and Innovation by grants to TPJ (23-03-0103) and PP (274-06-0325) is gratefully acknowledged.
Glossary
Abbreviations
- FW
fresh weight
- ICP-OES
Inductively Coupled Plasma-Optical Emission Spectrometry
- MIFE
Microelectrode Ion Flux Estimation
- NSCC
non-selective cation channel
- ORF
open reading frame
- TEA
tetraethyl ammonium
- UTR
untranslated region
- YNB
yeast nitrogen base
References
- Allen S, Raven JA. Intracellular pH regulation in Ricinus communis grown with ammonium or nitrate as N sources: the role of long-distance transport. Journal of Experimental Botany. 1987;38:580–596. [Google Scholar]
- Angeles Martínez-Cordero M, Martínez V, Rubio F. High-affinity K+ uptake in pepper plants. Journal of Experimental Botany. 2005;56:1553–1562. doi: 10.1093/jxb/eri150. [DOI] [PubMed] [Google Scholar]
- Babourina O, Leonova T, Shabala S, Newman I. Effect of sudden salt stress on ion fluxes in intact wheat suspension cells. Annals of Botany. 2000;85:759–767. [Google Scholar]
- Balleza D, Gómez-Lagunas F, Sánchez F, Quinto C. A high conductance cationic channel from Phaseolus vulgaris roots incorporated into planar lipid bilayers. Biochemistry and Biophysics. 2005;438:88–92. doi: 10.1016/j.abb.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Bañuelos MA, Haro R, Fraile-Escanciano A, Rodríguez-Navarro A. Effects of polylinker uATGs on the function of grass HKT1 transporters expressed in yeast cells. Plant and Cell Physiology. 2008;49:1128–1132. doi: 10.1093/pcp/pcn088. [DOI] [PubMed] [Google Scholar]
- Barker AV. Ammonium accumulation and ethylene evolution by tomato infected with root-knot nematode and grown under different regimes of plant nutrition. Communications in Soil Science and Plant Analysis. 1999;30:175–182. [Google Scholar]
- Barker AV, Maynard DN, Lachman WH. Induction of tomato stem and leaf lesions, and potassium deficiency, by excessive ammonium nutrition. Soil Science. 1967;103:319–325. [Google Scholar]
- Bertl A, Andersson JA, Slayman CL, Gaber RF. Use of Saccharomyces cerevisiae for patch-clamp analysis of heterologous membrane proteins: characterization of Kat1, an inward-rectifying K+channel from Arabidopsis thaliana, and comparison with endogenous yeast channels and carriers. Proceedings of the National Academy of Sciences, USA. 1995;92:2705–2710. doi: 10.1073/pnas.92.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto DT, Siddiqi MY, Glass ADM, Kronzucker HJ. Futile transmembrane NH4+ cycling: a cellular hypothesis to explain ammonium toxicity in plants. Proceedings of the National Academy of Sciences, USA. 2001;98:4255–4258. doi: 10.1073/pnas.061034698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto DT, Kronzucker HJ. Futile cycling at the plasma membrane: a hallmark of low-affinity nutrient transport. Trends in Plant Science. 2006;11:529–534. doi: 10.1016/j.tplants.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Buurman ET, Pennock J, Tempest DW, Teixeira de Mattos J, Neijssel OM. Replacement of potassium ions by ammonium ions in different micro-organisms grown in potassium-limited chemostat culture. Archives of Microbiology. 1989;152:58–63. doi: 10.1007/BF00447012. [DOI] [PubMed] [Google Scholar]
- Buurman ET, Teixeira de Mattos J, Neijssel OM. Futile cycling of ammonium ions via the high affinity potassium uptake system (kdp) of Escherichia coli. Archives of Microbiology. 1991;155:391–395. doi: 10.1007/BF00243460. [DOI] [PubMed] [Google Scholar]
- Cakmak I, Hengeler C, Marschner H. Changes in phloem export of sucrose in leaves in response to phosphorus, potassium and magnesium deficiency in bean plants. Journal of Experimental Botany. 1994;45:1251–1257. [Google Scholar]
- Cao Y, Glass ADM, Crawford NM. Ammonium inhibition of Arabidopsis root growth can be reversed by potassium and by auxin resistance mutants aux1, axr1, and axr2. Plant Physiology. 1993;102:983–989. doi: 10.1104/pp.102.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Ward JM, Kelly WB, Ichida AM, Gaber RF, Andersson JA, Uozumi N, Schroeder JI, Crawford NM. Multiple genes, tissue specificity, and expression-dependent modulation contribute to the functional diversity of potassium channels in Arabidopsis thaliana. Plant Physiology. 1995;109:1093–1106. doi: 10.1104/pp.109.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czempinski K, Zimmermann S, Erhardt T, Müller-Röber B. New structure and function in plant K+channels: KCO1, an outward rectifier with a steep Ca2+dependency. EMBO Journal. 1997;16:2565–2575. doi: 10.1093/emboj/16.10.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport RJ, Tester M. A weakly voltage-dependent, non-selective cation channel mediates toxic sodium influx in wheat. Plant Physiology. 2000;122:823–834. doi: 10.1104/pp.122.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Davenport RJ, Tester M. Non-selective cation channels in plants. Annual Review of Plant Biology. 2002;53:67–107. doi: 10.1146/annurev.arplant.53.091901.161540. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Tester M. Sodium fluxes through non-selective cation channels in the plasma membrane of protoplasts from Arabidopsis roots. Plant Physiology. 2002;128:379–387. doi: 10.1104/pp.010524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich P, Sanders D, Hedrich R. The role of ion channels in light-dependent stomatal opening. Journal of Experimental Botany. 2001;52:1959–1967. doi: 10.1093/jexbot/52.363.1959. [DOI] [PubMed] [Google Scholar]
- Finnemann J, Schjoerring JK. Translocation of NH4+ in oilseed rape plants in relation to glutamine synthetase isogene expression and activity. Physiologia Plantarum. 1999;105:469–477. [Google Scholar]
- Gassmann W, Schroeder JI. Inward-rectifying K+ channels in root hairs of wheat. Plant Physiology. 1994;105:1399–1408. doi: 10.1104/pp.105.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerendás J, Zhu ZJ, Bendixen R, Ratcliffe RG, Sattelmacher B. Physiological and biochemical processes related to ammonium toxicity in higher plants. Zeitschrift für Pflanzenernährung und Bodenkunde. 1997;160:239–251. [Google Scholar]
- Haro R, Bañuelos MA, Senn ME, Barrero-Gil J, Rodríguez-Navarro A. HKT1 mediates sodium uniport in roots. Pitfalls in the expression of HKT1 in yeast. Plant Physiology. 2005;139:1495–1506. doi: 10.1104/pp.105.067553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DC, Lu W, Rabinowitz JD, Botstein D. Ammonium toxicity and potassium limitation in yeast. PLoS Biology. 2006;4 doi: 10.1371/journal.pbio.0040351. e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch RE, Lewis BD, Spalding EP, Sussman MR. A role for the AKT1 potassium channel in plant nutrition. Science. 1998;280:918–921. doi: 10.1126/science.280.5365.918. [DOI] [PubMed] [Google Scholar]
- Howitt SM, Udvardi MK. Structure, function and regulation of ammonium transporters in plants. BBA Biomembranes. 2000;1465:152–170. doi: 10.1016/s0005-2736(00)00136-x. [DOI] [PubMed] [Google Scholar]
- Humble GD, Hsiao TC. Specific requirement of potassium for light-activated opening of stomata in epidermal strips. Plant Physiology. 1969;44:230–234. doi: 10.1104/pp.44.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husted S, Hebbern CA, Mattsson M, Schjoerring JK. A critical experimental evaluation of methods for determination of NH4+ in plant tissue, xylem sap and apoplastic fluid. Physiologia Plantarum. 2000;109:167–179. [Google Scholar]
- Ishikita H, Knapp EW. Protonation states of ammonia/ammonium in the hydrophobic pore of ammonia transporter protein AmtB. Journal of the American Chemical Society. 2007;129:1210–1215. doi: 10.1021/ja066208n. [DOI] [PubMed] [Google Scholar]
- Jahn TP, Møller ALB, Zeuthen T, Holm LM, Klaerke DA, Mohsin B, Kühlbrandt W, Schjoerring JK. Aquaporin homologues in plants and mammals transport ammonia. FEBS Letters. 2004;574:31–36. doi: 10.1016/j.febslet.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Javelle A, Lupo D, Ripoche P, Fulford T, Merick M, Winkler FK. Substrate binding, deprotonation, and selectivity at the periplasmic entrance of Escherichia coli ammonia channel AmtB. Proceedings of the National Academy of Sciences, USA. 2008;105:5040–5045. doi: 10.1073/pnas.0711742105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy KW. Ammonia, glutamine, and asparagines: a carbon nitrogen interface. Canadian Journal of Botany. 1988;66:2103–2109. [Google Scholar]
- Kassir Y, Simchen G. Monitoring meiosis and sporulation in Saccharomyces cerevisiae. Methods in Enzymology. 1991;194:94–110. doi: 10.1016/0076-6879(91)94009-2. [DOI] [PubMed] [Google Scholar]
- Khademi S, O'Connell J III, Remis J, Robles-Colmenares Y, Miercke LJ, Stroud RM. Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35Å. Science. 2004;305:1573–1574. doi: 10.1126/science.1101952. [DOI] [PubMed] [Google Scholar]
- Knowles A, Shabala S. Overcoming the problem of non-ideal liquid ion exchanger selectivity in microelectrode ion flux measurements. Journal of Membrane Biology. 2004;202:51–59. doi: 10.1007/s00232-004-0719-2. [DOI] [PubMed] [Google Scholar]
- Kronzucker HJ, Schjoerring JK, Erner Y, Kirk GJD, Siddiqi MY, Glass ADM. Dynamic interactions between root NH4+ influx and long distance N translocation in rice: insights into feedback processes. Plant and Cell Physiology. 1998;39:1287–1293. [Google Scholar]
- Kronzucker HJ, Britto DT, Davenport RJ, Tester M. Ammonium toxicity and the real cost of transport. Trends in Plant Science. 2001;6:335–337. doi: 10.1016/s1360-1385(01)02022-2. [DOI] [PubMed] [Google Scholar]
- Kronzucker HJ, Szczerba MW, Britto DT. Cytosolic potassium homeostasis revisited: 32K-tracer analysis in Hordeum vulgare L. reveals set-point variations in [K+] Planta. 2003;217:540–546. doi: 10.1007/s00425-003-1032-5. [DOI] [PubMed] [Google Scholar]
- Lenaeus MJ, Vamvouka M, Focia PJ, Gross A. Structural basis of TEA blockade in a model potassium channel. Nature Structural and Molecular Biology. 2005;12:454–459. doi: 10.1038/nsmb929. [DOI] [PubMed] [Google Scholar]
- Loqué D, Ludewig U, Yuan L, von Wirén N. Tonoplast intrinsic proteins AtTIP2;1 and AtTIP2;3 facilitate NH3 transport into the vacuole. Plant Physiology. 2005;137:671–680. doi: 10.1104/pp.104.051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig U, Neuhäuser B, Dynowski M. Molecular mechanism of ammonium transport and assimilation in plants. FEBS Letters. 2007;581:2301–2308. doi: 10.1016/j.febslet.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Ludewig U, von Wirén N, Frommer WB. Uniport of NH4+ by the root hair plasma membrane ammonium transporter LeAMT1;1. Journal of Biological Chemistry. 2002;277:13548–13555. doi: 10.1074/jbc.M200739200. [DOI] [PubMed] [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. London: Academic Press; 1995. [Google Scholar]
- McConnaughey TA, Falk RH. Calcium–proton exchange during algal calcification. The Biological Bulletin. 1991;180:185–195. doi: 10.2307/1542440. [DOI] [PubMed] [Google Scholar]
- Moroni A, Bardella L, Thiel G. The impermeant ion methylammonium blocks K+ and NH4+ currents through KAT1 channel differently: evidence for ion interaction in channel permeation. Journal of Membrane Biology. 1998;163:25–35. doi: 10.1007/s002329900367. [DOI] [PubMed] [Google Scholar]
- Mäser P, Thomine S, Schroeder JI, et al. Phylogenetic relationships within cation transporter families in Arabidopsis. Plant Physiology. 2001;126:1646–1667. doi: 10.1104/pp.126.4.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman IA. Ion transport in roots: measurements of fluxes using ion-selective microelectrodes to characterize transporter function. Plant, Cell and Environment. 2001;24:1–14. doi: 10.1046/j.1365-3040.2001.00661.x. [DOI] [PubMed] [Google Scholar]
- Nielsen KH, Schjoerring JK. Regulation of apoplastic NH4+ concentration in leaves of oilseed rape. Plant Physiology. 1998;118:1361–1368. doi: 10.1104/pp.118.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieves-Cordones M, MillerAJ Alemán F, Martínez V, Rubio F. A putative role for the plasma membrane potential in the control of the expression of the gene encoding the tomato high-affinity potassium transporter HAK5. Plant Molecular Biology. 2007;68:521–532. doi: 10.1007/s11103-008-9388-3. [DOI] [PubMed] [Google Scholar]
- Ninnemann O, Jauniaux JC, Frommer WB. Identification of a high affinity NH4+ transporter from plants. EMBO Journal. 1994;13:3464–3471. doi: 10.1002/j.1460-2075.1994.tb06652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedas P, Ytting CK, Fuglsang AT, Jahn TP, Schjoerring JK, Husted S. Manganese efficiency in barley: identification and characterization of the metal ion transporter HvIRT1. Plant Physiology. 2008;148:455–466. doi: 10.1104/pp.108.118851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putrich GS, Barker AV. Structure and function of tomato leaf chloroplasts during ammonium toxicity. Plant Physiology. 1967;42:1229–1238. doi: 10.1104/pp.42.9.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Hampton CR, Shin R, Barkla BJ, White PJ, Schachtman DP. The high affinity K+ transporter AtHAK5 plays a physiological role in planta at very low K+ concentrations and provides a cesium uptake pathway in Arabidopsis. Journal of Experimental Botany. 2008;59:595–607. doi: 10.1093/jxb/erm330. [DOI] [PubMed] [Google Scholar]
- Raven JA, Smith FA. Nitrogen assimilation and transport in the vascular land plants in relation to intracellular pH regulation. New Phytologist. 1976;76:415–431. [Google Scholar]
- Rodríguez-Navarro A, Rubio F. High-affinity potassium and sodium transport systems in plants. Journal of Experimental Botany. 2006;57:1149–1160. doi: 10.1093/jxb/erj068. [DOI] [PubMed] [Google Scholar]
- Roosta HR, Schjoerring JK. Effects of ammonium toxicity on nitrogen metabolism and elemental profile of cucumber plants. Journal of Plant Nutrition. 2007;30:1933–1951. [Google Scholar]
- Rubio F, Santa-Maria GE, Rodriguez-Navarro A. Cloning of Arabidopsis and barley cDNAs encoding HAK potassium transporters in root and shoot cells. Physiologia Plantarum. 2000;109:34–43. [Google Scholar]
- Rubio F, Nieves-Cordones M, Alemán F, Martínez V. Relative contribution of AtHAK5 and AtAKT1 to K+ uptake in the high-affinity range of concentration. Physiologia Plantarum. 2008;134:598–608. doi: 10.1111/j.1399-3054.2008.01168.x. [DOI] [PubMed] [Google Scholar]
- Rufty TW Jr, Jackson WA, Raper CD. Inhibition of nitrate assimilation in roots in the presence of ammonium: the moderating influence of potassium. Journal of Experimental Botany. 1982;33:1122–1137. [Google Scholar]
- Santa-María GE, Danna CH, Czibener C. High-affinity potassium transport in barley roots. Ammonium-sensitive and -insensitive pathways. Plant Physiology. 2000;123:297–306. doi: 10.1104/pp.123.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Schroeder JI, Lucas WJ, Andersson JA, Gaber RF. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science. 1992;258:1654–1658. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- Schachtman DP, Kumar R, Schroeder JI, Marsh EL. Molecular and functional characterization of a novel low-affinity cation transporter (LCT1) in higher plants. Proceedings of the National Academy of Sciences, USA. 1997;94:11079–11084. doi: 10.1073/pnas.94.20.11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer HW, Mackown CT, Everett Leggett J. Potassium-ammonium uptake interactions in tobacco seedlings. Journal of Experimental Botany. 1984;35:1060–1070. [Google Scholar]
- Schjoerring JK, Husted S, Mack G, Mattsson M. The regulation of ammonium translocation in plants. Journal of Experimental Botany. 2002;53:883–890. doi: 10.1093/jexbot/53.370.883. [DOI] [PubMed] [Google Scholar]
- Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon JM, Gaymard F, Grignon C. Cloning and expression in yeast of a plant potassium ion transport system. Science. 1992;256:663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- Shabala SN, Newman IA, Morris J. Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiology. 1997;113:111–118. doi: 10.1104/pp.113.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S. Ionic and osmotic components of salt stress specifically modulate net ion fluxes from bean leaf mesophyll. Plant, Cell and Environment. 2000;23:825–837. [Google Scholar]
- Shabala L, Ross T, Newman I, McMeekin T, Shabala S. Measurements of net fluxes and extracellular changes of H+, Ca2+, K+ and NH4+ in Escherichia coli using ion-selective microelectrodes. Journal of Microbiological Methods. 2001;46:119–129. doi: 10.1016/s0167-7012(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Spalding EP, Hirsch RE, Lewis DR, Qi Z, Sussman MR, Lewis BD. Potassium uptake supporting plant growth in the absence of AKT1 channel activity. Inhibition by ammonium and stimulation by sodium. Journal of General Physiology. 1999;113:909–918. doi: 10.1085/jgp.113.6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczerba MW, Britto DT, Balkos K, Kronzucker HJ. Alleviation of rapid, futile ammonium cycling at the plasma membrane by potassium reveals K+-sensitive and -insensitive components of NH4+ transport. Journal of Experimental Botany. 2008a;59:303–313. doi: 10.1093/jxb/erm309. [DOI] [PubMed] [Google Scholar]
- Szczerba MW, Britto DT, Kronzucker HJ. Rapid, futile K+ cycling and pool-size dynamics define low-affinity potassium transport in barley. Plant Physiology. 2006;141:1494–1507. doi: 10.1104/pp.106.082701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczerba MW, Britto DT, Shabana AA, Balkos KD, Kronzucker HJ. NH4+-stimulated and -inhibited components of K+ transport in rice (Oryza sativa L.) Journal of Experimental Botany. 2008b;59:3415–3423. doi: 10.1093/jxb/ern190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uozumi N, Gassmann W, Cao Y, Schroeder JI. Identification of strong modifications in cation selectivity in an Arabidopsis inward rectifying potassium channel by mutant selection in yeast. Journal of Biological Chemistry. 1995;270:24276–24281. doi: 10.1074/jbc.270.41.24276. [DOI] [PubMed] [Google Scholar]
- Van Beusichem ML, Kirkby EA, Baas R. Influence of nitrate and ammonium nutrition on the uptake, assimilation, and distribution of nutrients in Ricinus communis. Plant Physiology. 1988;86:914–921. doi: 10.1104/pp.86.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale FR, Jackson WA, Volk RJ. Potassium influx into maize root systems: influence of root potassium concentration and ambient ammonium. Plant Physiology. 1987;84:1416–1420. doi: 10.1104/pp.84.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale FR, Volk RJ, Jackson WA. Simultaneous influx of ammonium and potassium into maize roots: kinetics and interactions. Planta. 1988;173:424–431. doi: 10.1007/BF00401031. [DOI] [PubMed] [Google Scholar]
- Véry A-A, Sentenac H. Cation channels in the Arabidopsis plasma membrane. Trends in Plant Science. 2002;7:168–175. doi: 10.1016/s1360-1385(02)02262-8. [DOI] [PubMed] [Google Scholar]
- Walch-Liu P, Neumann G, Bangerth F, Engels C. Rapid effects of nitrogen form on leaf morphogenesis in tobacco. Journal of Experimental Botany. 2000;51:227–237. doi: 10.1093/jexbot/51.343.227. [DOI] [PubMed] [Google Scholar]
- Wall ME. The role of potassium in plants. l. Effects of varying amounts of potassium on nitrogenous, carbohydrate and mineral metabolism in the tomato plant. Soil Science. 1939;47:143–161. [Google Scholar]
- Wang MY, Siddiqi MY, Glass ADM. Interactions between K+ and NH4+: effects on ion uptake by rice roots. Plant, Cell and Environment. 1996;19:1037–1046. [Google Scholar]
- White PJ. The permeation of ammonium through a voltage-independent K+ channel in the plasma membrane of rye roots. Journal of Membrane Biology. 1996;152:89–99. doi: 10.1007/s002329900088. [DOI] [PubMed] [Google Scholar]
- Yellen G, Jurman ME, Abramson T, MacKinnon R. Mutations affecting internal TEA blockade identify the probable pore-forming region of a K+ channel. Science. 1991;251:939–942. doi: 10.1126/science.2000494. [DOI] [PubMed] [Google Scholar]