Abstract
In C4 plants, water deficit may decrease photosynthetic CO2 assimilation independently of changes in stomatal conductance, suggesting decreased turnover by ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco). The activity and biochemistry of Rubisco was studied in three different C4 grasses: Paspalum dilatatum, Cynodon dactylon, and Zoysia japonica. The objectives were to characterize the C4 Rubisco in these species and to identify factors associated with decreased photosynthetic rates caused by drought. Rubisco isolated from each of the three C4 grasses was characterized by smaller specificity factors (SC/O), larger Michaelis–Menten constants for CO2 (Kc) and O2 (Ko), and larger maximum carboxylation velocities (Vc) than Rubisco from wheat, which can be rationalized in terms of the CO2-rich environment of C4 Rubisco in the bundle sheath. During leaf dehydration the quantity and maximum activity of Rubisco remained unchanged but the initial and total activities declined slightly, possibly due to increased inhibition. Tight-binding inhibitors were present in the light but were more abundant in the dark, especially in Z. japonica, and increased in quantity with drought stress. The inhibitor from darkened leaves of Z. japonica was identified as 2-carboxyarabinitol-1-phosphate (CA1P). Consistent with the presence of CA1P, the total activity of Rubisco was decreased after 12 h darkness in Z. japonica. Ribulose-1,5-bisphosphate (RuBP) in the leaves decreased with drought stress, to quantities approximating those of Rubisco catalytic sites. The magnitude of the decrease in RuBP suggested that, at least in C. dactylon and Z. japonica, it could contribute to the drought-induced decrease in photosynthesis.
Keywords: CA1P, Cynodon dactylon, kinetic constants, Paspalum dilatatum, Rubisco, RuBP, water deficit, Zoysia japonica
Introduction
The presence of a CO2-concentrating mechanism in the leaves of C4 plants results in improved water use efficiency compared to C3 plants (Long, 1999) and is generally seen as a potential advantage for areas with increased aridity. Stomatal closure is amongst the earliest plant responses to decreasing water availability and, in C3 plants, constitutes a major limitation to photosynthetic CO2 assimilation under such conditions. In those plants, metabolic or non-stomatal limitations to photosynthesis may also be observed under mild to moderate water-deficit conditions, concomitantly with stomatal limitation, but their relative contribution to decreased CO2 assimilation increases with the severity of stress (Lawlor, 2002). C4 photosynthesis saturates at much lower CO2 concentrations and is therefore unlikely to be affected by stomatal closure in the same fashion as C3 photosynthesis. Increased leakiness of the bundle sheath may cause decreased CO2 concentrations at the site of carboxylation (Saliendra et al., 1996; Williams et al., 2001). However, in several C4 grasses subjected to water deficit, raising CO2 concentrations to physiologically high levels—which would suppress the effect of decreased stomatal conductance and increased leakiness—did not result in the recovery of net photosynthetic rates to those of well-watered plants (Carmo-Silva et al., 2008; Ghannoum, 2009), supporting the presence of non-stomatal limitations of photosynthesis. The limited number of studies with C4 species suggests higher susceptibility of the C3 than the C4 cycle enzymes to impairment by water deficit (reviewed by Ghannoum, 2009).
Ribulose-1,5-bisphosphate (RuBP) carboxylase/oxygenase (Rubisco) is crucial in the regulation of photosynthesis in fully-hydrated leaves of the C4 species Flaveria bidentis (Furbank et al., 1997), but the importance of this most abundant of leaf proteins in the limitation of C4 photosynthesis under drought conditions is not well understood. Flexas and Medrano (2002) suggested that, in C3 plants, Rubisco capacity was of little importance in the drought-induced limitation of photosynthesis, whilst decreased capacity for RuBP regeneration—possibly as a result of decreased ATP synthesis or impaired Calvin cycle enzyme activities (Lawlor, 2002)—would constitute a major metabolic limitation. However, decreased activity and/or quantity of Rubisco has been observed in several different C3 and C4 species exposed to water deficit (Majumdar et al., 1991; Du et al., 1996; Lal and Edwards, 1996; Parry et al., 2002; Tezara et al., 2002; Bota et al., 2004; Marques da Silva and Arrabaça, 2004; Carmo-Silva et al., 2007; Soares-Cordeiro et al., 2009).
Rubisco activity is regulated by the extent of carbamylation of a lysyl residue within the catalytic site (Lorimer and Miziorko, 1980) and is affected by various chloroplast metabolites (Hatch and Jensen, 1980; Badger and Lorimer, 1981; Jordan et al., 1983) and by naturally occurring tight-binding inhibitors (Pearce and Andrews, 2003; Kim and Portis, 2004). At night, Rubisco may be inhibited by a specific tight-binding inhibitor, 2-carboxyarabinitol-1-phosphate (Gutteridge et al., 1986; Berry et al., 1987; Moore et al., 1992), which is present in some species (Vu et al., 1984; Seemann et al., 1985; Servaites et al., 1984, 1986; Holbrook et al., 1992; Sage and Seemann, 1993). The main contender for the inhibition of Rubisco in the light (Kane et al., 1998), D-glycero-2,3-diulose-1,5-bisphosphate (PDBP), is too labile for detailed study, and the occurrence of inhibitors during daytime (Keys et al., 1995) has become associated with misfire products formed during enolization and oxygenation of RuBP catalysed by Rubisco (Pearce and Andrews, 2003; Kim and Portis, 2004). It is generally accepted that removal of all these inhibitors requires the ATP-dependent action of Rubisco activase. Tight-binding inhibitors such as CA1P protect Rubisco from proteolytic breakdown (Khan et al., 1999) and may play an important role in regulating the enzyme under stress conditions (Parry et al., 2008), as well as during darkness and at low irradiance (Kobza and Seemann, 1989a, b).
Photorespiration is initiated by the oxygenase activity of Rubisco and decreases both the rate and efficiency of CO2 assimilation in C3 plants. Because CO2 and O2 are competing substrates (Bowes and Ogren, 1972; Laing et al., 1974), the high CO2 concentration in the bundle sheath of C4 plants means that the oxygenase activity is much decreased. This situation could be compromised under conditions of water deficit since the concomitant reduction in stomatal aperture leads to decreased CO2 availability. However, photorespiration has been shown neither to increase nor contribute to the limitation of photosynthesis in C4 grasses under drought stress (Carmo-Silva et al., 2008). From a consideration of the kinetics of the carboxylation and oxygenation of RuBP catalysed by Rubisco, the specificity factor (SC/O) defines the relative reactivity towards the two gaseous substrates and is given by VcKo/VoKc, where Vc and Vo represent the maximum velocities of the carboxylase and oxygenase reactions, respectively, and Kc and Ko the Michaelis–Menten constants for CO2 and O2. The individual kinetic constants are known for relatively few types of Rubisco, mostly from C3 species. Michaelis–Menten constants of Rubisco from C3 and C4 grasses for CO2 and RuBP have been reported by Yeoh et al. (1980, 1981) and the Rubisco kinetic parameters have recently been compared in eudicot plants with the C3 and C4 photosynthetic pathways (Kubien et al., 2008). In the sequence of studies described by Carmo-Silva et al. (2007, 2008, 2009) and Soares-Cordeiro et al. (2009) on the responses of three different C4 grasses to water deficit, it became important to add details of the kinetic constants for the Rubiscos peculiar to these three species.
The objectives of this investigation were to study the biochemistry of Rubisco and to determine the effects of water deficit on the regulation of the enzyme in three C4 grasses of different metabolic subtypes: Paspalum dilatatum Poir. (NADP-malic enzyme, NADP-ME), Cynodon dactylon (L.) Pers (NAD-malic enzyme, NAD-ME), and Zoysia japonica Steudel (phosphoenolpyruvate carboxykinase, PEPCK). The previous studies showed that net photosynthesis was decreased in these three grasses by both slowly and rapidly induced drought stress (Carmo-Silva et al., 2007, 2008). The purpose of the present study was to investigate the relationship to drought of decreased carboxylation of RuBP (photosynthesis), the activation state of Rubisco due to carbamylation, the presence of inhibitors, and the quantity of RuBP in the leaves.
Materials and methods
General methods
Rubisco was purified from wheat as described by Keys and Parry (1990). RuBP prepared as in Wong et al. (1980), 2-carboxyarabinitol-1,5-bisphosphate (CABP) prepared from RuBP by reaction with KCN or [14C]-KCN followed by hydrolysis of the cyanohydrins, and CA1P made from CABP by partial dephosphorylation using alkaline phosphatase (Gutteridge et al., 1989) were all purified by anion-exchange chromatography. Radioactivity of 14C labelled compounds, typically in a volume of 0.4–0.45 ml of aqueous solution, was measured after mixing with 3.6 ml Ultima Gold Scintillation cocktail in a Liquid Scintillation Analyser (Perkin-Elmer, USA). Specific mixtures of N2 and O2 were prepared using a gas divider (Signal Group, UK) and concentrations of O2 in solution were calculated by taking the solubility at 25 °C in water as 257.5 μM in a standard atmosphere at 100% relative humidity and correcting for the atmospheric pressure during measurements [using the formula 257.5×(P–11589)/(101 325–11589), where P is the observed pressure, 11589 Pa is the saturated H2O vapour pressure at 25 °C, and 101325 Pa is the standard atmospheric pressure]. Concentrations of CO2 in solution in equilibrium with HCO3− were calculated assuming a pKa1 for carbonic acid at 25 °C of 6.11 and using accurate measures of the pH of each buffer solution. Values of Michaelis–Menten constants and maximum velocities were estimated using EnzFitter (Biosoft: Software for Science, UK).
Plant growth and drought stress induction
The C4 grasses Paspalum dilatatum Poir. cv. Raki (NADP-ME), Cynodon dactylon (L.) Pers var. Shangri-Lá (NAD-ME), and Zoysia japonica Steudel ‘Jacklin Sunrise Brand’ (produced by Jacklin Seed Company, USA) (PEPCK) were grown from seeds in trays or pots with peat-free compost in a greenhouse, as previously described by Carmo-Silva et al. (2008, 2009). Artificial light was provided whenever the natural light was below a photosynthetic photon flux density (PPFD) of 500 μmol m−2 s−1 during a 16 h photoperiod. The temperature was set not to fall below 25 °C during the day or below 18 °C during the night. Pots, containing five seedlings each, were well-watered until the beginning of the drought stress treatment and then placed according to a rectangular split-plot design, where each column of pots was a main plot of a particular species and the sampling-days and treatments (control and drought stress) were randomized in the rows (split-plots). Each pot corresponded to one independent sample. When appropriate, the number of samples was duplicated and organized in two blocks to allow the imposition of a light/dark regime, by restricted randomization. Several batches of plants were grown, to obtain samples for the different measurements. Because the growing conditions were not fully controlled, batches of plants varied slightly in the extent of drought stress achieved.
Water deficit was imposed by ceasing to provide water, consecutively, with 1 d intervals, to the plants of C. dactylon, then Z. japonica and last P. dilatatum. Samples composed of young fully expanded leaves were taken from all three species for four consecutive days at the end of the drought period (after the soil water content in the pots had fallen below 10%; see Carmo-Silva et al., 2009). Five-week-old plants of P. dilatatum and C. dactylon and 9-week-old plants of the slow-growing Z. japonica were analysed simultaneously. On each occasion, two leaf samples were taken from each pot: the first was quickly frozen in liquid nitrogen (LN2) and then stored at –80 °C for biochemical assays and the second was used to determine the leaf relative water content (RWC; Catsky, 1960). The leaf samples were collected in the growth environment under fully illuminated conditions, 4 h after the beginning of the photoperiod, or in the dark, after placing the plants in a dark environment for a period of 12 h (overnight).
Rubisco activity and concentration in leaf crude extracts
Rubisco was extracted from leaves by grinding frozen samples (0.1–0.4 g fresh weight, FW) in a cold mortar with quartz sand, 1% (w/v) insoluble polyvinylpyrrolidone (PVP) and 10 (P. dilatatum and C. dactylon) or 15 vols (Z. japonica) of ice-cold extraction medium containing 50 mM Bicine-KOH pH 8.0, 1 mM EDTA, 5% (w/v) PVP25000, 6% (w/v) polyethylene glycol 4000 (PEG4000), 10 mM dithiothreitol (DTT), 50 mM 2-mercaptoethanol, and 1% (v/v) protease inhibitor cocktail (Sigma, USA). After taking aliquots for total chlorophyll determination, the remaining homogenate was centrifuged for 3 min at 16000 g and 4 °C and the supernatant immediately used for measuring the activities and quantities of Rubisco, with two analytical replicates for each measurement.
The activities of Rubisco were determined by the incorporation of 14CO2 into acid-stable products at 25 °C (Parry et al., 1997). The reaction mixture (final volume 0.5 ml) contained 100 mM Bicine-NaOH pH 8.2, 20 mM MgCl2, 10 mM NaH14CO3 (18.5 kBq μmol−1), and 0.4 mM RuBP. The initial activity was determined by adding 25 μl of crude extract and quenching the reaction after 60 s with 0.2 ml 10 M HCOOH. Total activity was measured after incubating 25 μl of the same extract for 3 min with all the components except RuBP, to allow carbamylation of all available Rubisco catalytic sites, and then starting the reaction by adding RuBP. Maximal activity was measured after the removal of Rubisco tight-binding inhibitors by incubating 250 μl of crude extract with 200 mM Na2SO4, 10 mM NaHCO3, and 20 mM MgCl2 for 30 min at 4 °C. Protein was precipitated with 20% (w/v) PEG4000 and 20 mM MgCl2 and then washed three times with 20% PEG4000 in 100 mM Bicine-NaOH pH 8.2, 20 mM MgCl2, 10 mM NaHCO3, and 50 mM 2-mercaptoethanol. The final precipitate was dissolved in extraction buffer and assayed for total activity. The quenched reaction mixtures were completely dried at 100 °C and the residues rehydrated before 14C determination.
Rubisco in leaf crude or inhibitor-free extracts was quantified by the [14C]-CABP binding assay (Parry et al., 1997). For this purpose, 100 μl of extract was incubated with 100 mM Bicine-NaOH pH 8.0, 20 mM MgCl2, 10 mM NaHCO3, 50 mM 2-mercaptoethanol, 100 mM Na2SO4, and 75 μM [14C]-CABP (37 kBq μmol−1) for 15 min at 4 °C. Rubisco was precipitated by the addition of PEG4000 to a final concentration of 25% and the precipitate washed three times with 20% PEG4000 in 100 mM Bicine-NaOH pH 8.2, 20 mM MgCl2, 10 mM NaHCO3, and 50 mM 2-mercaptoethanol, and then redissolved in 0.5 ml of 1% (v/v) Triton X-100. A subsample (0.45 ml) was used to measure the radioactivity due to [14C]-CABP bound to Rubisco catalytic sites.
Quantities of RuBP and Rubisco tight-binding inhibitors
RuBP and Rubisco tight-binding inhibitors were extracted by grinding frozen leaf samples (0.1–0.4 g FW) to a fine powder in LN2 and then adding 0.45 M trifluoroacetic acid (TFA; 0.25 ml per 0.1 g FW). The mixture was ground further during thawing. Duplicate subsamples (20 μl) were taken for chlorophyll determination as phaeophytin and the remaining homogenate was centrifuged for 5 min at 16000 g and 4 °C.
For the estimation of RuBP, an aliquot (50 μl) of the acid extract was dried in a glass vial under high vacuum over anhydrous CaCl2 and NaOH pellets. The residue was redissolved in 50 μl H2O, dried down, and redissolved in 50 μl H2O again. The RuBP contained in each vial was converted to [14C]-phosphoglycerate by incubating at room temperature for 45 min in a reaction mixture (0.5 ml) containing 100 mM Bicine-NaOH pH 8.2, 20 mM MgCl2, 8 mM NaH14CO3 (18.5 kBq μmol−1), and 20 μg of pure, activated wheat Rubisco. The reaction was quenched with 0.1 ml 10 M HCOOH. The mixture was dried down and the residue rehydrated for liquid scintillation counting.
Rubisco inhibitors in 20 μl of the acid extracts were measured by comparison to inhibition of the enzyme by known quantities of CA1P (in 20 μl of 0.45 M TFA). Each standard and sample extract solution was mixed with 230 μl of (final concentrations) 100 mM Bicine-NaOH pH 8.2, 20 mM MgCl2, 10 mM NaH12CO3, and 10 μg of activated wheat Rubisco, and incubated for 5 min to hydrolyse lactones of CA1P and allow the binding to Rubisco. Rubisco activity was measured by adding 250 μl of 100 mM Bicine-NaOH pH 8.2, 20 mM MgCl2, 10 mM NaH14CO3 (18.5 kBq μmol−1) and 0.4 mM RuBP, and the reaction was quenched after 2.5 min with 0.1 ml 10 M HCOOH. The mixture was dried down and the residue rehydrated before scintillation counting.
Identification of CA1P by HPLC
Frozen leaf samples (0.1–0.2 g FW) were ground to a fine powder in LN2 and extracted with 1.0 ml of 0.45 M TFA after the addition of 490 Bq (240 pmol) [14C]-CA1P. After centrifugation, the extracts were purified by passage through 0.5 g Solid Phase Extraction columns (C18-E, Phenomenex, USA). The eluate was evaporated to dryness in vacuo over anhydrous CaCl2 and NaOH pellets. The residue was resuspended in H2O, passed through a column of Dowex 50 H+ (0.5 ml), and the eluate evaporated to dryness as before. The residue was dissolved in 0.25 ml H2O and mixed with 50 μl 1 M TRIS base. The resulting, mildly alkaline, solution was stored at –20 °C before fractionation by HPLC. The fractionation was conducted on a Dionex PA1 column (4× 250 mm) using a DX500 chromatography system operated at 1 ml min−1. The eluent was 100 mM CO2-free NaOH containing varying concentrations of sodium acetate: 100 mM from 0 to 5 min increasing to 800 mM at 30 min, to 900 mM at 35 min, and decreasing back to 100 mM at 40 min. In this system the retention times (min) were 8.9 for glucose 1-phosphate, 15.1 for fructose 6-phosphate, 16.0±0.2 for CA1P, and 20.9 for fructose 1,6-bisphosphate. Fractions of 0.5 ml were collected and each mixed with 75 μl of 0.2 M Bicine-NaOH pH 8.0 and 1 M HCl. Fractions containing CA1P were identified by the presence of 14C and frozen at –20 °C.
The CA1P was restricted to two HPLC fractions and the total in each sample was obtained by the addition of the quantities estimated for each fraction. CA1P in 100 μl aliquots was measured by the inhibition of Rubisco activity by comparison to CA1P standards, making appropriate allowance for the presence of the small quantity of added [14C]-CA1P. Reaction mixtures (0.5 ml) contained 100 mM Bicine-NaOH pH 8.0, 20 mM MgCl2, 10 mM NaH14CO3 (18.5 kBq μmol−1), 0.4 mM RuBP, and 10 μg of activated Rubisco. Samples and standard solutions were preincubated with the reaction mixture without RuBP for 7 min before adding the RuBP to start the reaction. The reaction was stopped after a further 7 min by adding 0.1 ml 10 M HCOOH. The acidified reaction mixtures were dried and 14C measured in the residues after rehydration. Corrections for losses of CA1P during the isolation process were made, based upon the recovery of the added [14C]-CA1P.
Total chlorophyll determination
The total chlorophyll content in the leaf homogenates was determined spectrophotometrically, as either chlorophyll or phaeophytin, after extraction in ethanol (Wintermans and de Mots, 1965). The total chlorophyll content (relative to the leaf area and turgid mass) remained unchanged with water deficit in the three C4 grasses (data not shown) and provided a good basis to normalize the data of the different measurements.
Rubisco kinetic constants
Seedlings of Triticum aestivum L. cv. Riband (C3 species used as the reference) and of the three C4 grasses were grown in trays for the determination of Rubisco kinetic parameters using young leaves. Leaf samples (0.5 g FW) were quickly frozen in LN2 and used within one day. Rubisco was extracted by grinding the leaves in a cold mortar with quartz sand, 2.5% (w/v) insoluble PVP, and 5 vols of ice-cold extraction medium containing 100 mM Bicine-KOH pH 8.2, 0.1 mM EDTA, 6% (w/v) PEG4000, 10 mM DTT, 50 mM 2-mercaptoethanol, 2 mM MgCl2, 10 mM NaHCO3, 1 mM benzamidine, 1 mM ϵ-aminocaproic acid, and 1% (v/v) protease inhibitor cocktail (Sigma, USA). After grinding to produce a fine suspension, the homogenate was centrifuged for 4 min at 16000 g and 4 °C. Low molecular weight proteins and salts present in the leaf crude extracts were removed by passage of supernatant (1 ml) through a Sephadex G-200 (GE Healthcare, USA) column (20 ml bed volume, 1.5×11.5 cm) pre-equilibrated and eluted with desalt buffer (100 mM Bicine-KOH pH 8.2, 0.5 mM EDTA, 1 mM KH2PO4, 20 mM MgCl2, 10 mM NaHCO3, 1 mM benzamidine, and 1 mM ϵ-aminocaproic acid). This treatment was introduced to remove potentially interfering enzymes of mass ≤150 kDa. Fractions of 0.5 ml were collected and, after measuring the soluble protein content (Bradford, 1976), the three fractions containing most protein were combined and the protease inhibitor cocktail added to a final concentration of 2.5% (v/v). The mixture was divided into different aliquots, some of which were immediately frozen in LN2 (for later measurement of Rubisco quantity and for appropriate control assays).
All measurements for the determination of kinetic parameters were conducted at 25 °C. The Michaelis–Menten constant (Km) for CO2 (Kc) was measured essentially as described by Bird et al. (1982), but Km for O2 (Ko) was estimated by measuring Kc apparent at several O2 concentrations (0, 21, 60, and 100%, balanced with N2). The carboxylation activity of Rubisco was determined at several CO2 concentrations for each gas mixture. The assay buffer was pretreated by sparging with the appropriate gas mixture. Septum-sealed vials with stirring magnets were connected in series through the septa using butyl rubber transfer tubes fitted at each end with hypodermic needles and flushed with the appropriate CO2-free gas mixtures at 20 ml min−1 for at least 30 min. Flushing of the vials was discontinued after the addition of the appropriate buffer but before the addition of NaH14CO3. The reaction mixtures (1 ml) contained (final concentrations) 100 mM Bicine-NaOH pH 8.2, 20 mM MgCl2, 10 μg ml−1 carbonic anhydrase (freshly dissolved), 0.4 mM RuBP, and six different concentrations of NaH14CO3 (0–10 mM; 37 kBq μmol−1). The reactions were started at 30 s intervals by adding 20 μl of the partially purified leaf extract previously activated by incubation with 10 mM NaH14CO3 (37 kBq μmol−1) for 30 min. The reactions were quenched after 2 min with 0.1 ml 10 M HCOOH. Changes in the activity of Rubisco through the course of the assays were monitored by the use of replicates of the same vial at staggered time points. The acidified mixtures were dried down and the residues rehydrated for scintillation counting.
Several control assays were performed in order to ensure that 14C incorporation by Rubisco occurred in the conditions of the assay at saturating CO2 and low O2 concentrations (positive control), and that 14C incorporation did not occur (a) in the absence of the substrate RuBP; (b) when RuBP had been replaced by phosphoglycerate; or (c) when the Rubisco preparation had been preincubated with CABP to block the catalytic sites (negative controls). The quantity of Rubisco in the partially purified extracts was determined essentially as described above, using [14C]-CABP.
Rubisco specificity factor
Rubisco was purified from fresh and young leaves of each of the three C4 grasses to determine the specificity factor (SC/O) by total consumption of RuBP in the oxygen electrode (Hansatech Instruments, UK) as described by Parry et al. (1989). The purification was essentially as described by Haslam et al. (2005) and consisted of precipitation of Rubisco from the leaf extracts with 20% PEG4000, step-elution from an anion-exchange column, and desalting.
Statistical analysis
All the analyses were made using GenStat® 9.2, 2005 (Lawes Agricultural Trust, UK). Regression analysis was applied to model the variation of the different measurements with RWC. Non-significantly different parameters (t tests, P >0.05) in the significant model terms of the regression (F-tests, P <0.05) were amalgamated in order to attain parsimony. The residuals were checked and found to conform to the assumptions of the analysis. The resulting best regression models were plotted and the parameter estimates (intercepts and slopes) with their respective standard errors (se), the percentage of variance accounted for by the model (R2), the residuals mean square (s2), and the degrees of freedom (df) are given with the figures.
Results
Rubisco activities
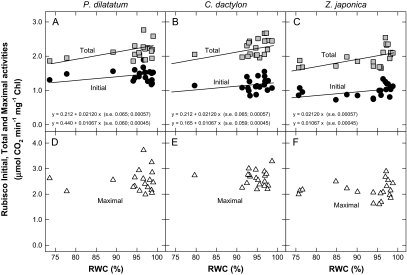
The initial and total activities of Rubisco declined slightly with decreasing RWC but maximal activities of the enzyme were not significantly affected (P >0.05) by leaf dehydration (Fig. 1). Initial activities were approximately 60% of maximal activities for P. dilatatum and about 40% of maximal activities for C. dactylon and Z. japonica, indicating that Rubisco activity was down-regulated by the lack of carbamylation of many catalytic sites under the growing conditions. Total activities were also less than maximal activities, especially at decreased RWC, consistent with further down-regulation by the presence of inhibitors in all three species.
Fig. 1.
Rubisco initial, total and maximal carboxylation activities (μmol CO2 min−1 mg−1 chlorophyll) as a function of the relative water content (RWC, %) in the leaves of Paspalum dilatatum (A, D), Cynodon dactylon (B, E), and Zoysia japonica (C, F). Initial activities (A–C, black symbols) were determined immediately after extraction, total activities (A–C, grey symbols) after activation in the presence of CO2 and Mg2+, and maximal activities (D–F, open symbols) after removal of tight-binding inhibitors with sulphate. Each data point corresponds to one sample, with seven (P. dilatatum and Z. japonica) or eight (C. dactylon) control (well-watered) and 12 non-watered samples per species. Regression lines were fitted when the RWC effect was significant (F-test; Initials: R2=50.5%, s2=0.033, df=55, P <0.001; Totals: R2=32.5%, s2=0.052, df=56, P <0.001; Maximals: no regression on RWC, P >0.05).
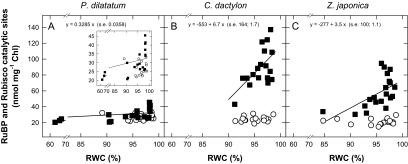
Quantities of Rubisco and RuBP
The concentration of Rubisco catalytic sites mg−1 chlorophyll in leaves of the three species was not significantly affected (P >0.05) by water deficit and the average values for P. dilatatum, C. dactylon, and Z. japonica were 26.6±0.8, 24.4±0.8, and 21.0±0.8 nmol mg−1 Chl, respectively. In all three species, as the RWC fell, the quantities of RuBP decreased to values approaching the catalytic site concentration (Fig. 2), suggesting that the rate of carboxylation could become increasingly limited by the availability of the CO2 acceptor substrate as the leaves suffered water deficit. The quantities of RuBP present in the fully-hydrated leaves of C. dactylon and Z. japonica were greater, but the decrease with water deficit was more marked than in P. dilatatum.
Fig. 2.
Quantities of RuBP (Rt, filled symbols) and Rubisco catalytic sites (Et, open symbols) (nmol mg−1 chlorophyll) as a function of the relative water content (RWC, %) in the leaves of Paspalum dilatatum (A), Cynodon dactylon (B), and Zoysia japonica (C). Each data point corresponds to one sample, with seven or eight samples from control (well-watered) and 12 samples from non-watered plants per species. The insert in (A) represents a scale-up of the plot. Regression lines were fitted when the RWC effect was significant (F-tests; RuBP: R2=75.6%, s2=216.7, df=54; P <0.001; Rubisco catalytic sites: no regression on RWC, P >0.05).
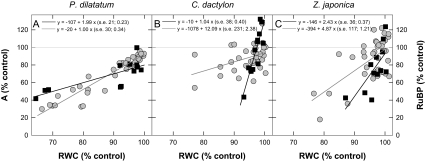
In order to compare the effects of water deficit on RuBP quantities (this study) and on net CO2 assimilation (Carmo-Silva et al., 2008), values obtained for drought-stressed plants of each of the three species were expressed as a percentage of average values for the corresponding control plants. Both the relative rates of net CO2 assimilation and RuBP quantities declined with decreasing RWC in the leaves of the three species (Fig. 3). Notably, in C. dactylon and Z. japonica, but not in P. dilatatum, the decrease in RuBP was apparently more marked than the decrease in the net photosynthetic rates.
Fig. 3.
Effect of leaf dehydration on the quantity of RuBP (black squares) and net CO2 assimilation rate (A, grey circles) of Paspalum dilatatum, Cynodon dactylon, and Zoysia japonica. The RuBP quantities (μmol m−2) and rates of photosynthesis (μmol m−2 s−1; measured at 25±2 °C, a PPFD of 850±50 μmol m−2 s−1, and ambient concentrations of CO2 and O2; Carmo-Silva et al., 2008) obtained for non-watered plants were expressed as a percentage of average values for corresponding control (well-watered) plants, to allow a comparison of drought effects in two independent batches of plants of the three species grown under identical conditions. The relative values of RuBP and photosynthesis were plotted against the corresponding RWC of each drought-stressed sample (also expressed as a percentage of average control values). Each data point corresponds to one sample. Regression lines were fitted when the RWC effect was significant (F-tests; RuBP: R2=65.5%, s2=220.4, df=30; P <0.001; A: R2=59.2%, s2=198.6, df=105; P <0.001).
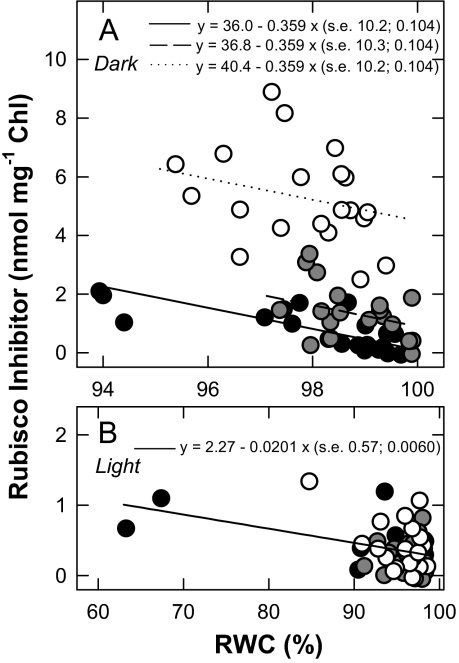
Evidence for tight-binding inhibitors
Tight-binding inhibitors were quantified by the inhibition of purified Rubisco by acid extracts (Fig. 4). In illuminated leaves these inhibitors were present in very low quantities and appeared to increase only marginally with leaf dehydration (Fig. 4B), up to the equivalent of ∼5% of the Rubisco catalytic sites (Fig. 2). Due to a non-significant interaction between species and treatment (P >0.05), the same regression with RWC fitted the data for the three C4 grasses in the light (Fig. 4B). Larger quantities of tight-binding inhibitors were found in dark-adapted than in illuminated leaves, especially in Z. japonica (Fig. 4A). Because RWC was partly re-established during the night, through decreased evaporation from the leaves and continued water absorption from the soil, samples collected from non-watered pots at the end of the 12 h dark period had higher RWC values, and corresponded to a narrower RWC range, than samples collected in the light period (compare Fig. 4A with Fig. 4B). Even so, and despite the large scatter, a significant (P <0.001) increase in the quantity of inhibitor with increasing water deficit was observed. In the dark, the inhibitor content increased with decreasing RWC to the equivalent of ∼8% of the Rubisco catalytic sites in P. dilatatum and C. dactylon and to ∼28% of the catalytic sites in Z. japonica.
Fig. 4.
Quantities of tight-binding inhibitor(s) of Rubisco activity (nmol mg−1 chlorophyll) in leaf acid extracts of Paspalum dilatatum (black symbols), Cynodon dactylon (grey symbols), and Zoysia japonica (open symbols) as a function of the leaf relative water content (RWC, %). Samples were either harvested after 12 h of darkness (Dark, A) or from illuminated plants (Light, B). Each data point corresponds to one sample, with seven or eight samples from control (well-watered) and 12 samples from non-watered plants for each species for each light regime. Regression lines were fitted when the RWC effect was significant (F-test; Dark: R2=79.1%, s2=1.138, df=56, P <0.001; Light: R2=15.0%, s2=0.0805, df=56, P=0.002).
Further studies attempted to characterize the inhibitor in Z. japonica. A component was isolated from acid extracts of the dark-adapted leaves of this species, which inhibited purified Rubisco and had a retention time in HPLC identical to that of authentic CA1P. Quantification of this inhibitory component utilized a trace of [14C]-CA1P—added at the time of tissue homogenization—to correct for losses during extraction and HPLC separation. Leaves of plants not subjected to drought were used to establish the occurrence of CA1P after a prolonged period of darkness. The quantities of CA1P estimated after HPLC (Table 1) were in the range of values obtained by the direct estimates of inhibitor in crude acid extracts shown in Fig. 4. Therefore, it is likely that CA1P is the predominant inhibitor present in the dark in Z. japonica.
Table 1.
Quantity of CA1P (nmol mg−1 chlorophyll) in light- and dark-adapted leaves of Zoysia japonica
| Pre-treatment | Z. japonica |
| CA1P (nmol mg−1 Chl) | |
| Dark | 5.97±1.76 |
| Light | 0.53±0.39 |
Samples were taken from well-watered plants after a period of 12 h in darkness (Dark) or 4 h after the beginning of the subsequent photoperiod (Light). The inhibitor was extracted with trifluoroacetic acid and measured after HPLC separation. Extracts were spiked with tracer quantities of [14C]-CA1P to allow correction for losses in processing the samples (average 56.4%). The retention time in the chromatographic system was the same as authentic CA1P and consistent with the radioactive spike. Values are means of four biological replicates ±standard errors.
In agreement with the presence of a tight-binding inhibitor in the dark in Z. japonica, the total activities of Rubisco in dark-adapted leaves of this species (after the 12 h dark period) were ∼74% of the total activities in leaves harvested in the light (Table 2), meaning that inhibitors were bound to ∼26% of the catalytic sites in the dark. This result was independent of water deficit and was observed only for Z. japonica. No significant (P >0.05) differences in Rubisco total activities were observed between the light- and dark-adapted leaves of P. dilatatum and C. dactylon and therefore no evidence was shown of a bound inhibitor for these two species. In neither light nor dark was there evidence of an increase in tight-binding inhibitor with drought stress, so these data did not support the slight trend shown in Figs 1 and 4.
Table 2.
Total activity of Rubisco (μmol min−1 mg−1 Rubisco) extracted from light- or dark-adapted leaves of well watered (control, C) and non-watered (drought stress, S) plants of Paspalum dilatatum, Cynodon dactylon, and Zoysia japonica
| Treatment |
P. dilatatum | C. dactylon | Z. japonica | |
| Rubisco total activity (mmol min−1 mg−1 Rubisco) | ||||
| Dark | C | 2.03±0.06 | 1.83±0.10 | 1.74±0.07 |
| S | 1.96±0.08 | 1.81±0.10 | 1.74±0.03 | |
| Light | C | 2.11±0.02 | 1.73±0.25 | 2.36±0.04 |
| S | 2.14±0.16 | 1.79±0.07 | 2.39±0.05 | |
| RWC (%) | ||||
| Dark | C | 98.2±0.4 | 97.1±0.3 | 97.5±0.4 |
| S | 96.4±0.4 | 95.7±0.6 | 93.8±3.2 | |
| Light | C | 98.6±0.1 | 97.0±1.2 | 96.9±1.5 |
| S | 80.2±4.6 | 87.9±4.1 | 76.1±0.3 | |
Samples were taken after a period of 12 h in darkness (Dark) or 4 h after the beginning of the photoperiod (Light). Corresponding leaf relative water contents (RWC, %) are also given. Values are means of three biological replicates ±standard errors.
Rubisco kinetic parameters
Rubisco purified from each of the three C4 grasses, P. dilatatum, C. dactylon, and Z. japonica, was characterized by lower SC/O than in wheat (C3 species used as reference) (Table 3). The Michaelis–Menten constants of Rubisco for CO2 and O2 (Kc and Ko) estimated for each of the C4 species were in the same range and were higher than the corresponding values estimated for wheat. The maximum Rubisco carboxylation activity (Vc) was also higher in the C4 than in the C3 species, and among the grasses Vc was higher in Z. japonica than in C. dactylon and lowest in P. dilatatum.
Table 3.
Kinetic parameters of Rubisco from wheat (Triticum aestivum) and from the three C4 grasses (Paspalum dilatatum, Cynodon dactylon, and Zoysia japonica) at 25 °C
| Species | SC/O (VcKo/VoKc) | Kc (μM) | Ko (μM) | Vc (μmol min−1 mg−1 Rubisco) | Vo (μmol min−1 mg−1 Rubisco) |
| T. aestivum | 100.0±9.2 | 10.9±0.9 | 341±33 | 2.54±0.07 | 0.79±0.03 |
| P. dilatatum | 88.0±7.1 | 19.9±0.8 | 415±5 | 3.11±0.04 | 0.71±0.03 |
| C. dactylon | 89.2±9.0 | 21.0±1.3 | 402±27 | 3.41±0.18 | 0.73±0.06 |
| Z. japonica | 84.1±7.7 | 18.5±1.2 | 403±27 | 3.78±0.08 | 0.98±0.08 |
For the Michaelis–Menten constants of Rubisco for CO2 and O2 (Kc and Ko, μM) and the maximum Rubisco carboxylation and oxygenation activities (Vc and Vo, μmol min−1 mg−1 Rubisco) the mean values and respective standard errors were calculated from measurements taken with three biological and three analytical replicates. The specificity factor (SC/O) was determined in Rubisco purified from each species using a minimum of five analytical replicates.
Attempts were made to measure both Vc and Vo by an HPLC method based on that of Yaguchi et al. (1996) using partially purified extracts of leaves from wheat and each of the three C4 grasses. These attempts failed and Vo was subsequently determined indirectly by solving the equation SC/O=VcKo/VoKc. However, the variation in the measurements of SC/O is too high to allow much precision in the estimation of Vo. The estimated Vo was highest in Z. japonica, the species with lowest specificity towards CO2.
Discussion
In the three C4 grasses, Paspalum dilatatum, Cynodon dactylon, and Zoysia japonica, Rubisco initial and total activities decreased slightly with water deficit, but maximal activity and Rubisco quantity remained unchanged, consistent with commonly observed drought effects (Ghannoum, 2009; Lawlor and Tezara, 2009). In all three species, Rubisco initial activity was lower than total activity and both initial and total activities were lower than maximal activity, and suggested increasing down-regulation with leaf dehydration (decreasing RWC). Rubisco down-regulation can be explained by a combination of decreased carbamylation of the active site lysine and the presence of competitive and tight-binding inhibitors. Carbamylation may be impeded by negative effectors (Hatch and Jensen, 1980; Badger and Lorimer, 1981; Jordan et al., 1983) or by RuBP bound into the non-carbamylated sites (Brooks and Portis, 1988), but these effects will not necessarily affect the activity in extracts because of dilution. The proportion of initial to total activity was not affected by leaf dehydration (see Supplementary Fig. S1 at JXB online), whereas both initial and total activities showed a similar decrease in relation to maximal activity with increasing water deficit, suggesting a possible increase in Rubisco tight-binding inhibitors.
Parry et al. (2002) showed that Rubisco activity was inhibited in tobacco under drought stress. In that study, the inhibitory component in acid extracts of illuminated leaves was assumed to be D-glycero-2,3-diulose-1,5-bisphosphate (PDBP). Figure 4 shows that the quantities of tight-binding inhibitors in the leaves of P. dilatatum, C. dactylon, and Z. japonica were only marginally increased with water deficit. The maximum quantities in the light were estimated to be, at the most, ∼5% of the Rubisco catalytic sites and, therefore, insufficient to explain entirely the decrease in total activity with respect to maximal activity. It can be argued that some Rubisco inhibitors are unstable in the acid extracts and this merits further investigation.
The quantity of Rubisco inhibitor in the acid extracts from leaves after 12 h in the dark was most in Z. japonica and least in P. dilatatum. The first evidence of CA1P in leaves was the observed modulation of Rubisco activity by light and dark transitions (Vu et al., 1983, 1984; Besford, 1984; Servaites et al., 1984). Sage and Seemann (1993) reported regulation of Rubisco in Z. japonica by darkness and considered that this might be due to the accumulation of CA1P. The removal of CA1P from Rubisco catalytic sites in the light depends on the action of Rubisco activase and the inhibitor is then degraded by CA1P phosphatase (Robinson and Portis, 1988). The effects of CA1P may continue at the beginning of the light period, at low light intensities, and in long periods of low light intensity due either to dense cloud coverage (Sage et al., 1993) or to shading by neighbouring leaves (Kobza and Seemann, 1989a, b).
The concentration of inhibitor in acid extracts from dark-adapted leaves was approximately 28%, in Z. japonica, and 5%, in P. dilatatum and C. dactylon, of the quantity of Rubisco catalytic sites in each species. For Z. japonica, the difference in the total Rubisco activity between extracts of leaves harvested in the light and after 12 h in the dark was consistent with the quantity of inhibitor measured, but there were no consistent differences between total Rubisco activity in the light- and dark-adapted leaves for P. dilatatum and C. dactylon. These results corroborate the observations of Sage and Seemann (1993), suggesting the presence of CA1P in Z. japonica, but are not conclusive about the presence/absence of this inhibitor in P. dilatatum and C. dactylon. To confirm the identity of the inhibitor occurring in Z. japonica in the dark, the putative CA1P was analysed by HPLC after isolation from light- and dark-adapted (12 h dark period) leaves. Rubisco activity was inhibited by components in the acid extracts that were recovered in the same HPLC fractions as authentic CA1P and the quantities of inhibitor estimated by this method were in the range found by the direct estimates on crude leaf acid extracts, thus providing definitive evidence for the presence of CA1P in the leaves of Z. japonica. The presence of inhibitors is likely to protect Rubisco from proteolysis, especially in stressed leaves (Parry et al., 2008).
The RuBP content was greater in fully hydrated leaves of C. dactylon and Z. japonica than in P. dilatatum. In vivo, this potential disadvantage for P. dilatatum may be partly counter-balanced by a greater Rubisco activation state, as indicated by the higher initial activity in this species than in C. dactylon or Z. japonica. Leaf dehydration caused a much larger decrease in the quantity of RuBP in C. dactylon and Z. japonica than in P. dilatatum. Decreased RuBP availability with water deficit has been observed in several studies (Gimenez et al., 1992; Gunasekera and Berkowitz, 1993; Tezara et al., 1999; Wingler et al., 1999; Parry et al., 2002; Bota et al., 2004). Because RuBP consumption and production are interdependent, it is difficult to assess whether decreased RuBP availability may constitute a limitation to or be a consequence of decreased CO2 assimilation. Comparison of relative effects of drought stress revealed a more marked decrease in RuBP quantities than in net photosynthetic rates by C. dactylon and Z. japonica, suggesting that the decline in substrate availability could constitute a factor contributing to limit carboxylation in these two C4 grasses. In P. dilatatum the moderate relative decrease in RuBP with water deficit is more likely to represent a consequence of decreased CO2 assimilation (and thus decreased 3-phosphoglycerate production), rather than constitute a limiting factor per se.
Decreased capacity for RuBP regeneration may be a consequence of impairment of ATP synthesis, of Calvin cycle enzymes activity, or both. Whilst some studies on tobacco and barley indicate decreased activity of Calvin cycle enzymes (Gunasekera and Berkowitz, 1993; Wingler et al., 1999), in sunflower, although the decrease in ATP was less dramatic than the decrease in RuBP, it was concluded that the latter was a consequence of decreased ATP synthesis through its effect on RuBP regeneration (Tezara et al., 1999; Lawlor, 2002). Decreased ATP synthesis, due to impaired photophosphorylation by chloroplastic ATP synthase, in association with increased concentrations of Mg2+, has been suggested as a major metabolic limitation to photosynthesis under water deficit (Lawlor, 2002; Lawlor and Tezara, 2009). An alternative view is that ATP availability may decrease as a result of increased consumption by alternative sinks under drought stress. For instance, increased bundle sheath leakage, as observed in sorghum and sugarcane when subjected to drought (Saliendra et al., 1996; Williams et al., 2001), decreases the efficiency of the C4 cycle, and accumulation of compounds associated with drought tolerance, such as proline, as observed for the three C4 grasses under study (Carmo-Silva et al., 2009), would increase the usage of ATP.
The quantities, on a chlorophyll basis, of both RuBP (Rt) and Rubisco catalytic sites (Et) were included in Fig. 2 to allow easy comparison. In P. dilatatum, Rt was only marginally above Et, even in well-watered plants. Even though only some 60–70% of the catalytic sites were active in this species, the ratio Rt/Et was less than 2 and so approaching the range where RuBP becomes limiting in C3 species (below ∼1.5–1.8; von Caemmerer, 2000; Lawlor, 2002). The modelling of the situation where the substrate concentration is similar to the catalytic site concentration is described by von Caemmerer (2000). The Rt in C. dactylon and Z. japonica was much higher in control plants, corresponding to Rt/Et ratios of 3.5 and 2.9, respectively, but the values fell steeply as RWC decreased, giving ratios similar to those in P. dilatatum at about 85% RWC. Thus, the quantities of RuBP in the leaves of all three species under drought stress were close to the critical value for RuBP-dependence of C3 photosynthesis (Lawlor, 2002).
Rubisco activase removes inhibitors from carbamylated catalytic sites and facilitates the removal of RuBP bound to non-carbamylated sites. Rubisco activase requires ATP for activation of Rubisco but is inhibited by ADP (Portis, 2003). If the decreased capacity for regeneration of RuBP under drought is a consequence of decreased ATP synthesis, a further consequence could be more Rubisco-bound inhibitors and fewer carbamylated Rubisco catalytic sites (Lawlor, 2002; Lawlor and Tezara, 2009) – both resulting from the decline in activase activity caused by a fall in the ratio ATP/ADP. Inactivation of Rubisco catalytic sites with increasing water deficit will decrease both carboxylation and oxygenation and, therefore, photosynthesis and photorespiration, as previously predicted by the modelling of carbon assimilation under drought (Carmo-Silva et al., 2008).
Rubiscos isolated from leaves of the three C4 grasses had lower specificity factors (SC/O), higher Michaelis–Menten constants for CO2 (Kc) and higher maximum carboxylase activities (Vc) than wheat Rubisco, which is in agreement with previous reports on the kinetics of Rubisco from C3 and C4 species (Yeoh et al., 1980, 1981; Tcherkez et al., 2006; Kubien et al., 2008). The lower specificity for CO2 of Rubisco from C4 compared with C3 monocots is associated with specific differences in the large subunits (Christin et al., 2008) and may be a consequence of diminished selection pressure resulting from increased CO2 concentrations in the vicinity of the enzyme (Jordan and Ogren, 1981). Tcherkez et al. (2006) suggested evolutionary optimization of Rubiscos from different species to the subcellular environment in which the enzyme functions. In the present study, the relatively high Vc would support rapid assimilation in the CO2-rich environment of the bundle sheath, consistent with the high photosynthetic performance of the three C4 grasses (Carmo-Silva et al., 2008).
The Michaelis–Menten constant for oxygen (Ko) of Rubisco from different photosynthetic organisms is largely unknown. The few values published (see von Caemmerer, 2000; Kubien et al., 2008) suggest that Ko may be highly variable between different species, even when they are closely related and represent the same photosynthetic pathway. The need to determine Ko for each species is therefore justified. The estimates of Ko obtained here for the three C4 grasses were similar and provide a valuable contribution for future modelling of photosynthesis.
In view of the effects of drought on the quantities of RuBP in the leaves, a measure of the Michaelis–Menten constant for RuBP (KRuBP) would be a valuable addition to the kinetic constants determined for the three grasses. Values for KRuBP for several C4 species have been published (Yeoh et al., 1981) and ranged from 15 μM to 100 μM which also suggests that measurements are needed for each species for which the objective is the mechanistic modelling of photosynthesis. The much lower concentration of RuBP in fully-hydrated leaves of P. dilatatum (NADP-ME) than in C. dactylon (NAD-ME) may be associated with a lower average KRuBP in NADP-ME than in NAD-ME C4 grasses (calculated from data in Yeoh et al., 1981). Estimation of the effective concentration of RuBP at the catalytic site of Rubisco is problematic because of the presence of competitive inhibitors and the formation of complexes with Mg2+ (von Caemmerer, 2000).
The results presented here show that the decline in the quantity of RuBP in the leaves as water deficit increased was of the same order of magnitude as the decline in photosynthetic rates reported previously (Carmo-Silva et al., 2008). Concomitant measurements of ATP and potential competitive inhibitors of RuBP binding to Rubisco, including intermediate metabolites of the Calvin cycle, particularly 3-phosphoglycerate, would improve understanding of factors limiting C4 photosynthesis under water deficit. The marginal increase in Rubisco tight-binding inhibitors under water deficit is unlikely to have a relevant contribution to the decreased photosynthetic rates. The presence of CA1P in dark-adapted leaves of Z. japonica may confer protection against degradation of Rubisco by proteolysis, but the mechanism by which Rubisco is down-regulated in the three C4 grasses under water deficit merits further study.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Rubisco activation state in relation to RWC in the three C4 grasses
Supplementary Material
Acknowledgments
AE Carmo-Silva acknowledges Fundação para a Ciência e a Tecnologia, Portugal, for financial support (PhD grant SFRH/BD/13730/2003). Rothamsted Research is a grant-aided institute of The Biotechnology and Biological Sciences Research Council, UK. The authors thank AgResearch, Margot Forde Forage Germplasm Centre, New Zealand, and Alípio Dias & Irmão, Lda, Portugal, for providing grass seeds; and Dr Michael E Salvucci, US Arid-Land Agricultural Research Center, USA, for helpful comments on the manuscript.
References
- Badger MR, Lorimer GH. Interaction of sugar phosphates with the catalytic site of ribulose-1,5-bisphosphate carboxylase. Biochemistry. 1981;20:2219–2225. doi: 10.1021/bi00511a023. [DOI] [PubMed] [Google Scholar]
- Berry JA, Lorimer GH, Pierce J, Seemann JR, Meek J, Freas S. Isolation, identification, and synthesis of 2-carboxyarabinitol 1-phosphate, a diurnal regulator of ribulose-bisphosphate carboxylase activity. Proceedings of the National Academy of Sciences, USA. 1987;84:734–738. doi: 10.1073/pnas.84.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besford RT. Some properties of ribulose bisphosphate carboxylase from tomato leaves. Journal of Experimental Botany. 1984;153:495–504. [Google Scholar]
- Bird IF, Cornelius MJ, Keys AJ. Affinity of RuBP carboxylases for carbon dioxide and inhibition of the enzymes by oxygen. Journal of Experimental Botany. 1982;33:1004–1013. [Google Scholar]
- Bota J, Medrano H, Flexas J. Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytologist. 2004;162:671–681. doi: 10.1111/j.1469-8137.2004.01056.x. [DOI] [PubMed] [Google Scholar]
- Bowes G, Ogren WL. Oxygen inhibition and other properties of soybean ribulose 1,5-diphosphate carboxylase. Journal of Biological Chemistry. 1972;247:2171–2176. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brooks A, Portis AR. Protein-bound ribulose bisphosphate correlates with deactivation of ribulose bisphosphate carboxylase in leaves. Plant Physiology. 1988;87:244–249. doi: 10.1104/pp.87.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Silva AE, Francisco A, Powers SJ, Keys AJ, Lia A, Parry MAJ, Arrabaça MC. Grasses of different C4 subtypes reveal leaf traits related to drought tolerance in their natural habitats: changes in structure, water potential and amino acid content. American Journal of Botany. 2009;96:1222–1235. doi: 10.3732/ajb.0800224. [DOI] [PubMed] [Google Scholar]
- Carmo-Silva AE, Powers SJ, Keys AJ, Arrabaça MC, Parry MAJ. Photorespiration in C4 grasses remains slow under drought conditions. Plant, Cell and Environment. 2008;31:925–940. doi: 10.1111/j.1365-3040.2008.01805.x. [DOI] [PubMed] [Google Scholar]
- Carmo-Silva AE, Soares AS, Marques da Silva J, Bernardes da Silva A, Keys AJ, Arrabaça MC. Photosynthetic responses of three C4 grasses of different metabolic subtypes to water deficit. Functional Plant Biology. 2007;34:204–213. doi: 10.1071/FP06278. [DOI] [PubMed] [Google Scholar]
- Catsky J. Determination of water deficit in discs cut out from leaf blades. Biologia Plantarum. 1960;2:76–77. [Google Scholar]
- Christin PA, Salamin N, Muasya AM, Roalson EH, Russier F, Besnard G. Evolutionary switch and genetic convergence on rbcL following the evolution of C4 photosynthesis. Molecular Biology and Evolution. 2008;25:2361–2368. doi: 10.1093/molbev/msn178. [DOI] [PubMed] [Google Scholar]
- Du YC, Kawamitsu Y, Nose A, Hiyane S, Murayama S, Wasano K, Uchida Y. Effects of water stress on carbon exchange rate and activities of photosynthetic enzymes in leaves of sugarcane (Saccharum sp.) Australian Journal of Plant Physiology. 1996;23:719–726. [Google Scholar]
- Flexas J, Medrano H. Drought-inhibition of photosynthesis in C3 plants: stomatal and non-stomatal limitations revisited. Annals of Botany. 2002;89:183–189. doi: 10.1093/aob/mcf027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbank RT, Chitty JA, Jenkins CLD, Taylor WC, Trevanion SJ, von Caemmerer S, Ashton AR. Genetic manipulation of key photosynthetic enzymes in the C4 plant. Flaveria bidentis. Australian Journal of Plant Physiology. 1997;24:477–485. [Google Scholar]
- Ghannoum O. C4 photosynthesis and water stress. Annals of Botany. 2009;103:635–644. doi: 10.1093/aob/mcn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez C, Mitchell VJ, Lawlor DW. Regulation of photosynthetic rate of two sunflower hybrids under water stress. Plant Physiology. 1992;98:516–524. doi: 10.1104/pp.98.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekera D, Berkowitz GA. Use of transgenic plants with ribulose-1,5-bisphosphate carboxylase oxygenase antisense DNA to evaluate the rate limitation of photosynthesis under water stress. Plant Physiology. 1993;103:629–635. doi: 10.1104/pp.103.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge S, Parry MAJ, Burton S, Keys AJ, Mudd A, Feeney J, Servaites JC, Pierce J. A nocturnal inhibitor of carboxylation in leaves. Nature. 1986;324:274–276. [Google Scholar]
- Gutteridge S, Reddy GS, Lorimer GH. The synthesis and purification of 2’-carboxy-D-arabinitol 1-phosphate, a natural inhibitor of ribulose 1,5-bisphosphate carboxylase, investigated by 31P NMR. Biochemical Journal. 1989;260:711–716. doi: 10.1042/bj2600711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam RP, Keys AJ, Andralojc PJ, Madgwick PJ, Andersson I, Grimsrud A, Eilertsen HC, Parry MAJ. Specificity of diatom Rubisco. In: Omasa K, Nouchi I, DeKok LJ, editors. Plant responses to air pollution and global change. Tokyo: Springer-Verlag; 2005. pp. 157–164. [Google Scholar]
- Hatch AL, Jensen RG. Regulation of ribulose-1,5-bisphosphate carboxylase from tobacco: changes in pH response and affinity for CO2 and Mg2+ induced by chloroplast intermediates. Archives of Biochemistry and Biophysics. 1980;205:587–594. doi: 10.1016/0003-9861(80)90142-3. [DOI] [PubMed] [Google Scholar]
- Holbrook GP, Turner JA, Polans NO. Dark inhibition of ribulose-1,5-bisphosphate carboxylase oxygenase in legumes: a biosystematic study. Photosynthesis Research. 1992;32:37–44. doi: 10.1007/BF00028796. [DOI] [PubMed] [Google Scholar]
- Jordan DB, Chollet R, Ogren WL. Binding of phosphorylated effectors by active and inactive forms of ribulose-1,5-bisphoaphate carboxylase. Biochemistry. 1983;22:3410–3418. [Google Scholar]
- Jordan DB, Ogren WL. Species variation in the specificity of ribulose bisphosphate carboxylase/oxygenase. Nature. 1981;291:513–515. [Google Scholar]
- Kane HJ, Wilkin JM, Portis AR, Andrews TJ. Potent inhibition of ribulose-bisphosphate carboxylase by an oxidised impurity in ribulose-1,5-bisphosphate. Plant Physiology. 1998;117:1059–1069. doi: 10.1104/pp.117.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys AJ, Major I, Parry MAJ. Is there another player in the game of Rubisco regulation? Journal of Experimental Botany. 1995;46:1245–1251. [Google Scholar]
- Keys AJ, Parry MAJ. Ribulose bisphosphate carboxylase/oxygenase and carbonic anhydrase. In: Lea PJ, editor. Enzymes of primary metabolism. London: Academic Press; 1990. pp. 1–14. [Google Scholar]
- Khan S, Andralojc PJ, Lea PJ, Parry MAJ. 2'-Carboxy-D-arabitinol 1-phosphate protects ribulose 1,5-bisphosphate carboxylase/oxygenase against proteolytic breakdown. European Journal of Biochemistry. 1999;266:840–847. doi: 10.1046/j.1432-1327.1999.00913.x. [DOI] [PubMed] [Google Scholar]
- Kim K, Portis AR. Oxygen dependent H2O2 production by Rubisco. FEBS Letters. 2004;571:124–128. doi: 10.1016/j.febslet.2004.06.064. [DOI] [PubMed] [Google Scholar]
- Kobza J, Seemann JR. Light-dependent kinetics of 2-carboxyarabinitol 1-phosphate metabolism and ribulose-1,5-bisphosphate carboxylase activity. in vivo. Plant Physiology. 1989a;89:174–179. doi: 10.1104/pp.89.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobza J, Seemann JR. Regulation of ribulose-1,5-bisphosphate carboxylase activity in response to diurnal changes in irradiance. Plant Physiology. 1989b;89:918–924. doi: 10.1104/pp.89.3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubien DS, Whitney SM, Moore PV, Jesson LK. The biochemistry of Rubisco in Flaveria. Journal of Experimental Botany. 2008;59:1767–1777. doi: 10.1093/jxb/erm283. [DOI] [PubMed] [Google Scholar]
- Laing WA, Ogren WL, Hageman RH. Regulation of soybean net photosynthetic CO2 fixation by interaction of CO2, O2 and ribulose 1,5-diphosphate carboxylase. Plant Physiology. 1974;54:678–685. doi: 10.1104/pp.54.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A, Edwards GE. Analysis of inhibition of photosynthesis under water stress in the C4 species Amaranthus cruentus and Zea mays: electron transport, CO2-fixation and carboxylation capacity. Australian Journal of Plant Physiology. 1996;23:403–412. [Google Scholar]
- Lawlor DW. Limitation to photosynthesis in water-stressed leaves: stomata vs. metabolism and the role of ATP. Annals of Botany. 2002;89:871–885. doi: 10.1093/aob/mcf110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DW, Tezara W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Annals of Botany. 2009;103:561–579. doi: 10.1093/aob/mcn244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP. Environmental responses. In: Sage RF, Monson RK, editors. C4 plant biology. New York: Academic Press; 1999. pp. 215–249. [Google Scholar]
- Lorimer GH, Miziorko HM. Carbamate formation on the amino group of a lysine residue as the basis for the activation of RuBP carboxylase. Biochemistry. 1980;19:5321–5328. doi: 10.1021/bi00564a027. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Ghosh S, Glick BR, Dumbroff EB. Activities of chlorophyllase, phosphoenolpyruvate carboxylase and ribulose-1,5-bisphosphate carboxylase in the primary leaves of soybean during senescence and drought. Physiologia Plantarum. 1991;81:473–480. [Google Scholar]
- Marques da Silva J, Arrabaça MC. Photosynthetic enzymes of the C4 grass Setaria sphacelata under water stress: a comparison between rapidly and slowly imposed water deficit. Photosynthetica. 2004;42:43–47. doi: 10.1078/0176-1617-01109. [DOI] [PubMed] [Google Scholar]
- Moore BD, Sharkey TD, Kobza J, Seemann JR. Identification and levels of 2'-carboxyarabinitol in leaves. Plant Physiology. 1992;99:1546–1550. doi: 10.1104/pp.99.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry MAJ, Andralojc PJ, Khan S, Lea PJ, Keys AJ. Rubisco activity: effects of drought stress. Annals of Botany. 2002;89:833–839. doi: 10.1093/aob/mcf103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry MAJ, Andralojc PJ, Parmar S, Keys AJ, Habash D, Paul MJ, Alred R, Quick WP, Servaites JC. Regulation of Rubisco by inhibitors in the light. Plant, Cell and Environment. 1997;20:528–534. [Google Scholar]
- Parry MAJ, Keys AJ, Gutteridge S. Variation in the specificity factor of C3 higher plant Rubisco determined by the total consumption of ribulose-P2. Journal of Experimental Botany. 1989;40:317–320. [Google Scholar]
- Parry MAJ, Keys AJ, Madgwick PJ, Carmo-Silva AE, Andralojc PJ. Rubisco regulation: a role for inhibitors. Journal of Experimental Botany. 2008;59:1569–1580. doi: 10.1093/jxb/ern084. [DOI] [PubMed] [Google Scholar]
- Pearce FG, Andrews TJ. The relationship between side reactions and the slow inhibition of ribulose-bisphosphate carboxylase revealed by a loop 6 mutant of the tobacco enzyme. Journal of Biological Chemistry. 2003;278:32526–32536. doi: 10.1074/jbc.M305493200. [DOI] [PubMed] [Google Scholar]
- Portis AR. Rubisco activase: Rubisco's catalytic chaperone. Photosynthesis Research. 2003;75:11–27. doi: 10.1023/A:1022458108678. [DOI] [PubMed] [Google Scholar]
- Robinson SP, Portis AR. Release of the nocturnal inhibitor, carboxyarabinitol-1-phosphate, from ribulose bisphosphate carboxylase/oxygenase by Rubisco activase. FEBS Letters. 1988;233:413–416. [Google Scholar]
- Sage RF, Reid CD, Moore Bd, Seemann JR. Long-term kinetics of the light-dependent regulation of ribulose-1,5-bisphosphate carboxylase/oxygenase activity in plants with and without 2-carboxyarabinitol 1-phosphate. Planta. 1993;191:222–230. [Google Scholar]
- Sage RF, Seemann JR. Regulation of ribulose-1,5-bisphosphate carboxylase oxygenase activity in response to reduced light-intensity in C4 plants. Plant Physiology. 1993;102:21–28. doi: 10.1104/pp.102.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliendra NZ, Meinzer FC, Perry M, Thom M. Associations between partitioning of carboxylase activity and bundle sheath leakiness to CO2, carbon isotope discrimination, photosynthesis, and growth in sugarcane. Journal of Experimental Botany. 1996;47:907–914. [Google Scholar]
- Seemann JR, Berry JA, Freas SM, Krump MA. Regulation of ribulose bisphosphate carboxylase activity in vivo by a light modulated inhibitor of catalysis. Proceedings of the National Academy of Sciences, USA. 1985;82:8024–8028. doi: 10.1073/pnas.82.23.8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites JC, Parry MAJ, Gutteridge S, Keys AJ. Species variation in the predawn inhibition of ribulose-1,5-bisphosphate carboxylase oxygenase. Plant Physiology. 1986;82:1161–1163. doi: 10.1104/pp.82.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites JC, Torisky RS, Chao SF. Diurnal changes in ribulose 1,5-bisphosphate carboxylase activity and activation state in leaves of field-grown soybeans. Plant Science Letters. 1984;35:115–121. [Google Scholar]
- Soares-Cordeiro AS, Carmo-Silva AE, Bernardes da Silva A, Marques da Silva J, Keys AJ, Arrabaça MC. Effects of rapidly imposed water deficit on photosynthetic parameters of three C4 grasses. Photosynthetica. 2009;47:304–308. [Google Scholar]
- Tcherkez G, Farquhar GD, Andrews TJ. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proceedings of the National Academy of Sciences, USA. 2006;103:7246–7251. doi: 10.1073/pnas.0600605103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezara W, Mitchell VJ, Driscoll SD, Lawlor DW. Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature. 1999;401:914–917. [Google Scholar]
- Tezara W, Mitchell V, Driscoll SP, Lawlor DW. Effects of water deficit and its interaction with CO2 supply on the biochemistry and physiology of photosynthesis in sunflower. Journal of Experimental Botany. 2002;53:1781–1791. doi: 10.1093/jxb/erf021. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S. Biochemical models of leaf photosynthesis. Collingwood: CSIRO Publishing; 2000. [Google Scholar]
- Vu CV, Allen LH, Bowes G. Effects of light and elevated atmospheric CO2 on the ribulose bisphosphate carboxylase activity and ribulose bisphosphate level of soybean leaves. Plant Physiology. 1983;73:729–734. doi: 10.1104/pp.73.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu CV, Allen LH, Bowes G. Dark/light modulation of ribulose bisphosphate carboxylase activity in plants from different photosynthetic categories. Plant Physiology. 1984;76:843–845. doi: 10.1104/pp.76.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DG, Gempko V, Fravolini A, Leavitt SW, Wall GW, Kimball BA, Pinter PJ, LaMorte R, Ottman M. Carbon isotope discrimination by Sorghum bicolor under CO2 enrichment and drought. New Phytologist. 2001;150:285–293. [Google Scholar]
- Wingler A, Quick WP, Bungard RA, Bailey KJ, Lea PJ, Leegood RC. The role of photorespiration during drought stress: an analysis utilizing barley mutants with reduced activities of photorespiratory enzymes. Plant, Cell and Environment. 1999;22:361–373. [Google Scholar]
- Wintermans JFGM, De Mots A. Spectrophotometric characteristics of chlorophylls a and b and their phaeophytins in ethanol. Biochimica et Biophysica Acta. 1965;109:448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- Wong C-H, McCurry SD, Whitesides GM. Practical enzymatic syntheses of ribulose 1,5-bisphosphate and ribose 5-phosphate. Journal of the American Chemical Society. 1980;102:7938–7939. [Google Scholar]
- Yaguchi T, Oguni A, Ouchiyama N, Igarashi Y, Kodama T. A non-radioisotopic anion-exchange chromatographic method to measure the CO2/O2 specificity factor for ribulose bisphosphate carboxylase/oxygenase. Bioscience, Biotechnology, and Biochemistry. 1996;60:942–944. [Google Scholar]
- Yeoh H-H, Badger MR, Watson L. Variations in Km(CO2) of ribulose-1,5-bisphosphate carboxylase among grasses. Plant Physiology. 1980;66:1110–1112. doi: 10.1104/pp.66.6.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh H-H, Badger MR, Watson L. Variations in kinetic properties of ribulose-1,5-bisphosphate carboxylases among plants. Plant Physiology. 1981;67:1151–1155. doi: 10.1104/pp.67.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.