Abstract
Immune-based self-recognition and failure to modulate this response are believed to contribute to the debilitating autoimmune pathology observed in multiple sclerosis (MS). Studies from its murine model, experimental autoimmune encephalomyelitis (EAE), have shown that neuroantigen-specific CD4+ T cells are capable of inducing disease, while their immune sibling, the CD8+ T cells, have largely been ignored. To understand their role in autoimmune demyelination, we first confirmed that, similar to our observations in human MS, there is robust induction of neuroantigen-reactive CD8+ T cells in several models, including MOG35–55/CFA-induced EAE. However, MOG35–55-specific CD8+ T-cells, when purified, were unable to adoptively transfer disease into naïve mice (in contrast to CD4+ T-cells). In fact, we observed that the transfer of these neuroantigen-specific CD8+ T cells was able to suppress the induction of EAE and to inhibit ongoing EAE. These regulatory CD8+ T cells produced IFN-γ and perforin and were able to kill MOG loaded CD4+ T-cells as well as CD4-depleted APC, suggesting a cytotoxic/suppressor mechanism. Inhibition of EAE was associated with both the modulation of APC function as well as decreased MOG-specific CD4+ T cell responses. Our studies reveal a novel and unexpected immune regulatory function for neuroantigen-specific CD8+ T cells and have interesting biologic and therapeutic implications.
Keywords: EAE, Multiple sclerosis, CD8, T cells, Regulatory
1. Introduction
Multiple sclerosis (MS) is an immune-mediated demyelinating disease of the central nervous system (CNS), characterized by an inflammatory infiltrate that includes both CD4+ and CD8+ T cells, among other mononuclear cells [1]. Several studies from others and us provide evidence for the involvement of CD8+ T cells in the immune pathogenesis and/or regulation of MS [2–5]. In fact, CD8+ T cells comprise the majority of lymphocytes found in MS lesions with oligoclonal expansion, indicating their active participation in the lesion [4,6–12]. However, there is a relative paucity of knowledge regarding their functional role in MS, likely due to the focus on CD4+ T cells as the putative pathogenic and regulatory cells in MS and its animal model, experimental autoimmune encephalomyelitis.
EAE is a well-established animal model of MS that can be induced in several strains of mice by immunization with myelin-related antigens or by adoptive transfer of neuroantigen-specific CD4+ T cells. Prior EAE studies using CD8(−/−) mice, β2-microglobulin(−/−) mice or antibody depletion of CD8+ T cells in vivo are suggestive of both a pathogenic [13–17] as well as a regulatory role [16,18–20] for these CD8+ T cells. However, the antigenic specificity of immune regulatory CD8+ T cells remains somewhat of a mystery. In recent studies, CNS-specific CD8+ T cells were capable of inducing EAE in certain models, suggesting a pathogenic role for these CNS-targeted cells. For example, myelin basic protein (MBP)-specific CD8+ T cells in C3H mice [14] or myelin oligodendrocyte glycoprotein (MOG)35–55-specific CD8+ T cells in C57BL/6 (B6) mice could induce, augment or transfer disease in certain settings [14,17,21].
In this study, we attempt to further elucidate the role of myelin-specific CD8+ T cells in the context of wildtype EAE. We demonstrate the presence of neuroantigen-specific CD8+ T cell responses in several models of EAE. Importantly, using adoptive transfer approaches in wildtype immune-replete mice, we show that these cells do not display pathogenecity. Rather, we provide the first evidence that neuroantigen-reactive CD8+ T cells inhibit EAE, through suppression of CD4+ T cell and/or antigen-presenting cell function, mediated in part, by direct killing in an antigen-specific manner. These studies unveil an important and novel aspect of immune regulation during ongoing autoimmunity.
2. Materials and methods
2.1. Mice
C57BL/6 (B6) female mice were purchased from Taconic (Hudson, NY) and the UT Southwestern Mouse Breeding Core Facility (Dallas, TX). SJL/J female mice were purchased from National Cancer Institute (Bethesda, MD). B10.PL mice were purchased from Taconic. B6.129 CD8−/− mice were purchased from Jackson Laboratory (Bar Harbor, ME). All mice were housed and bred in the UT Southwestern Medical Center Animal Resource Center and used according to approved IACUC protocols.
2.2. Active EAE induction and evaluation
Six to 8 week-old C57BL/6 mice were immunized subcutaneously at two injection sites with 200 μg MOG35–55 (MEVGWYRSPFSRVVHLYRNGK, UT Southwestern Protein Chemistry Technology Center) or whole bovine myelin basic protein (MBP, Sigma) emulsified in CFA supplemented with 4 mg/ml Mycobacterium tuberculosis (MTB, H37Ra, Difco). SJL/J mice were immunized with proteolipid protein peptide 139–151 (PLP139–151, HSLGKWLGHPDKF) emulsified in CFA while B10.PL mice were immunized with MBP Ac1–11 (ASQKRPSQRSK) emulsified in CFA. In some experiments, EAE was induced in B6 mice using 200 μg PLP178–191 in CFA. Ovalbumin peptide 323–339 (OVA323–339, ISQAVHAAHAEINEAGR) was used as a peptide control. On days 0 and 2 post-immunization, 250 ng of pertussis toxin (PTX, List Biological Laboratories) was administered intraperitoneally in 100 μl of phosphate buffered saline (PBS). Disease severity was monitored daily and EAE clinical disease scored according to the following scale: 0, no paralysis; 1, loss of tail tonicity; 2, hind limb weakness; 3, partial hind limb paralysis; 4, complete hind limb paralysis; 5, hind limb paralysis and forelimb weakness/moribund state; 6, death. In all experiments with multiple groups, all groups were represented across multiple cages and all scoring was performed in a blinded manner. In mice that showed signs of EAE (clinical score ≥1), incidence, mortality, disease onset, time to peak, peak score, cumulative score (sum of all scores from disease onset to day 20), outcome (final score at day 20), and grade of remission (difference between peak score and outcome) data were recorded.
2.3. Adoptive EAE
B6 mice were immunized with MOG35–55/CFA. Lymph node cells and/or splenocytes were harvested at either 10 or 20–25 days post-immunization. These cells were then incubated for 72 h at 37 °C in Dulbecco's Modified Eagle's Medium supplemented with 10% fetal calf serum (FCS), L-glutamine, penicillin, streptomycin, HEPES buffer, non-essential amino acids, sodium pyruvate and β-mercaptoethanol, stimulated with MOG35–55 (20 μg/ml) and mouse rIL-2 (10 pg/ml). Live cells were then separated using a Ficoll gradient. CD4+ and/or CD8+ cells (purity >90%) were positively selected using magnetic beads (Miltenyi Biotech, Germany). A total of 5–15 × 106 CD4+, CD8+ or bulk splenocytes were injected intravenously into naïve, wildtype B6 female mice and monitored for disease according to the previously outlined scale. To test for the ability of CD8+ T cells to transfer EAE, culture conditions were modified across several experiments by inclusion of murine IL-12, IL-23 or varying cell and stimulating peptide concentrations. In some experiments, 250 ng pertussis toxin was also administered intraperitoneally on days 0 and 2.
2.4. Protective adoptive transfer experiments
Female B6 mice were immunized with MOG35–55/CFA or OVA323–339/CFA. A total of 20–25 days post-immunization, splenocytes were stimulated with either MOG35–55 or OVA323–339 (20 μg/ml) respectively and murine rIL-2 (10 pg/ml) for 72 h at 37 °C at a concentration of 7.5 × 106 cells/ml. Live cells were then isolated on a Ficoll gradient. CD8+ cells in PBS were then injected into naïve, wildtype B6 via tail vein. The next day, these mice were immunized with MOG35–55/CFA for active EAE induction and monitored for disease.
2.5. Tritiated thymidine proliferation assay
Draining lymph node cells and/or splenocytes from immunized mice were harvested. CD8+ cells were magnetically separated using a negative selection protocol (Miltenyi Biotech, Germany) to recover “untouched” CD8+ cells (>90%). Cultures were set up in triplicates with either 4 × 105 bulk or 5 × 104 CD8+ cells per well. When necessary, irradiated splenocytes from naïve or immunized mice were used as APC at a ratio of 1:5 (CD8+ T cells:APC). After 72 h in culture, cells were pulsed with 0.5 μCi/well of 3H-thymidine for 18–20 h. Cells were then harvested on glass fibers mats and counted using a Betaplate counter (Wallac, Gaithersburg, MD).
2.6. CFSE-based proliferation assays
Proliferation assays were performed using a carboxyfluorescein succinimidyl ester (CFSE)-dilution assay as described previously [22]. Splenocytes were harvested and used either in bulk proliferation assays or as a source for CD8+ cells, which were isolated using a negative selection magnetic bead protocol. Bulk splenocytes or “untouched” CD8+ cells were suspended at 1 × 106 cells/ml in PBS and incubated at 37 °C for 7 min with 0.25 μM CFSE, followed by addition of serum and two PBS washes. Subsequently, CD8+ cells were suspended at 0.5–1 × 106/ml of media. Irradiated, CD8-depleted splenocytes (3500 rads) from mice immunized with OVA323–339/CFA or from naïve mice were used as APC at a ratio of 1:5. On day 5, cells were washed with staining buffer and labeled with phycoerythrin (PE)-conjugated-anti-CD8 and allophycocyanin (APC)-conjugated-anti-CD4 antibodies (Caltag/Invitrogen, Carlsbad, CA). After a short incubation at 4 °C, cells were washed and fixed in 1% paraformaldehyde (PFA, Electron Microscopy Sciences, Hattfield, PA). Flow cytometric data were acquired on a BD FACSort using BD CellQuest Software or BD LSR II flow cytometer using FACSDiva software. For analysis, FlowJo (Treestar, Ashland, OR) software was used to gate on lymphocytes and further on the CD4+ CD8− or CD8+ CD4− T cell subsets. The mean background proliferation was calculated based on the proliferating fractions (PF) in media alone. Proliferation was considered significant if the delta proliferation fraction (ΔPF = % sample proliferation – %mean background proliferation) was >1 and the stimulation index(SI = % sample proliferation ÷ % mean background proliferation) was >2
2.7. In vivo killing assays
Splenocytes from naïve female B6 mice were harvested for use as target cells. These cells were stained with 0.25 μM CFSE (CFSE high) or 0.05 μM CFSE (CFSE low) and stimulated overnight with Con A (10 μg/ml), with concomitant loading with either MOG35–55 or OVA323–339 (20 μg/ml). In some experiments, an additional dye (CMTPX) was used in order to distinguish four target populations. A portion of CFSE high and CFSE low cells were further stained with CMTPX (Invitrogen, Eugene, OR) according to manufacturer's protocol and loaded with alternative antigen. Subsequently, populations bearing distinct antigens were mixed in equal proportions and 20 × 106 cells were injected via tail vein into naïve, PBS/CFA-immunized or MOG/CFA-immunized mice at 25 days post-immunization. A total of 48 h after injection, mice were sacrificed, splenocytes harvested, fixed in 1% PFA and collected using a BD LSR II. Data were analyzed using FlowJo software. Killing was measured as a difference in ratio between the MOG/CFA immunized mice and either naïve or PBS/CFA-immunized mice as controls.
For specific target cell populations, CD4+ cells were purified via positive selection using magnetic beads. CD4+ and CD4-depleted subsets were then stained separately according to the procedure described above. Analysis showed CD4+ populations to be >90% pure and CD4-depleted populations to contain <10% CD4+ cells.
2.8. Quantitative polymerase chain reaction
Total RNA was extracted from purified CD8+ cells derived from in vitro-activation cultures using RNeasy Mini Kit (Qiagen, Valencia, CA) and reversed transcribed to cDNA using Ready-To-Go T-primed First Strain Kit (Amerisham, Piscataway, NJ) per manufacturer's protocol. The following primer pairs were used to evaluate relative expression level of each mRNA: β-actin: F:GTG GGC CGC TCT AGG CAC CAA; R:CTC TTT GAT GTC ACG CAC GAT TTC, IL-10: F:CAG AGC CAC ATG CTC CTA; R:GGA GTC GGT TAG CAG TAT G, TNF-α: F:CAT CTT CTC AAA ATT CGA GTG ACA A; R:TGG GAG TAG ACA AGG TAC AAC CC, IL-4: F:ACA GGA GAA GGG ACC CCA T; R:GAA GCC CTA CAG ACG AGC TCA, Perforin: F:AAC TCC CTA ATG AGA GAC GCC; R:CCA CAC GCC AGT CGT TAT TGA. All primers were purchased from Invitrogen (Carlsbad, CA). Quantitative PCR assays were performed in 25 μl reaction volumes using Brilliant SYBR Green QPCR Master Mix (Stratagene, La Jolla, CA) on an MX3000p thermocycler (Stratagene, La Jolla, CA) as described previously [2,23]. The following thermocycler conditions were used: 10 min at 95 °C, followed by 40 cycles of 45 s at 95 °C, 1 min at 72 °C followed by an extension phase of 3 min at 95 °C and 5 min at 55 °C, and completed with a product dissociation cycle. The SYBRGreen fluorescence was read during the extension phase of the reaction. The specificity of the product was confirmed by dissociation curve analysis as well as visualization of product size on 3% agarose gels. The data were normalized to β-actin (assigned a value of 1) using the standard ΔCT method and expressed as the mean relative expression (+SEM) of the indicated molecule.
2.9. Data analysis
In all experiments with multiple experimental groups, differences in clinical scores, peak scores, disease onset and remission indices were evaluated using two-tailed Student's t-test. Statistical significance of proliferation studies were evaluated by using twoway ANOVA analysis using Graphpad Prism Software. Differences were considered significant if p ≤ 0.05.
3. Results
3.1. Neuroantigen-specific, autoreactive CD8+ T cell responses are induced in several models of EAE
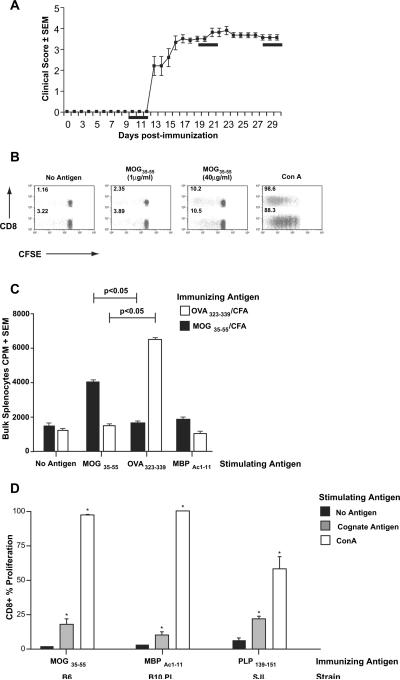
In previously published studies, it has been shown that immunization with neuronal antigens can result in the induction in CD8+ T cell responses in some models of EAE [19,21]. As a first step toward studying their role during autoimmune demyelination, we investigated whether the presence of such responses was unique to specific models of EAE. Using established protocols, we immunized C57BL/6 (B6) mice with MOG35–55/CFA, SJL/J mice with PLP139–151/CFA and B10.PL mice with MBP Ac1–11/CFA [24]. Lymph node and spleen cells were harvested at various time points post-immunization (day 10; days 20–25; days 30–35). As seen in Fig. 1A (example of chronic EAE, typical of B6 mice), these time points correspond to the pre-disease, acute and chronic disease phases of MOG35–55-induced EAE. Bulk cells were stained with CFSE and cultured in the presence of no antigen, immunizing antigen, irrelevant antigen (such as OVA323–339) or concanavalin A (Con A). Following 5 days of culture, CD4+ and CD8+ proliferation was quantified by CFSE dilution, showing not only the expected CD4+ T cell response, but also robust dose-dependent CD8+ T cell proliferation (Fig. 1B). Parallel 96-well cultures were performed for traditional tritiated thymidine-based proliferation assays and confirmed robust antigen-specific proliferation in bulk lymph node cells (not shown), as well as splenocyte cultures (Fig. 1C). In flow cytometric assays, CD4+ T cell responses were detected at pre, acute and chronic phases of disease, while CD8+ responses showed slightly delayed kinetics, being consistently detected by day 20 post-immunization, suggesting the possibility that they may not play a central role in initiating disease. Of note, CD8+ T cell responses to the immunizing antigen were not unique to the B6/MOG35–55 EAE model on the H-2KbDb background. As shown in Fig. 1D, CD8+ responses could be detected following immunization with various neuroantigenic peptides in different strains of mice. In each case, the expected CD4+ T cell responses could also be detected (data not shown).
Fig. 1.
Neuroantigen-specific CD8+ T cell responses in EAE. (A) EAE was induced in wildtype female B6 mice with MOG35–55/CFA immunization. A representative disease course is depicted showing mean clinical score ± SEM. The black bars represent the time frames for evaluation of antigen-specific CD4+ and CD8+ T cell responses in various experiments. (B) Bulk splenocytes from MOG-immunized mice (day 20 post-immunization) were labeled with CFSE and cultured for 5 days with the indicated stimuli. Cells were washed, stained and analyzed by flow cytometry. Gated T cells are shown as CD8 vs. CFSE. The CD8(−) population represents specifically gated CD4+ cells. The numbers express the percentage of proliferating cells (% proliferation) within the specific subpopulation (i.e. CFSE dilute CD8+ cells (green)/total CD8+ cells (green and gray)). These data are representative of over 10 independent experiments. (C) The bars show CPM ± SEM from 3H-thymidine-based proliferation assays on bulk splenocytes from either OVA323–339/CFA (white bars) or MOG35–55/CFA-immunized mice (black bars). The in vitro stimulating antigens are shown on the X-axis. (D) Female B6 mice were immunized with MOG35–55, B10.PL with MBPAc1–11 and SJL with PLP139–151. Day 25 post-immunization, CFSE-based proliferation assays were performed on splenocytes, using media alone (no antigen), appropriate stimulating antigen (cognate antigen) or Con A. The bars show CD8+ proliferative responses. Proliferation responses to cognate antigens and Con A were significantly greater than background (*p < 0.01). These results are representative of two to three independent experiments in each strain.
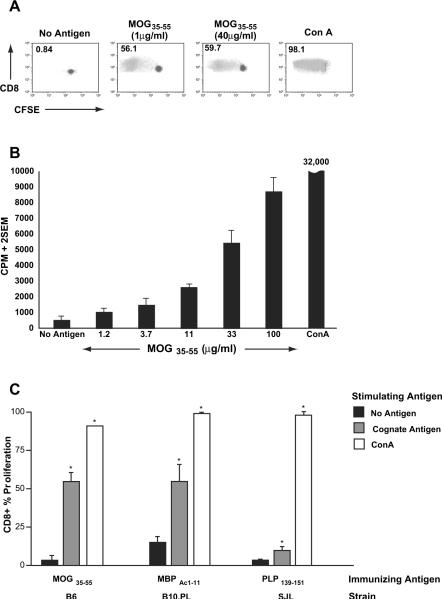
In order to confirm that this CD8+ T cell proliferation was indeed antigen-specific and not a bystander phenomenon in a bulk culture, we isolated pure populations of CD8+ T cells from the spleens of immunized mice using magnetic bead sorting (>90% purity), stained them with CFSE and cultured them in the presence (or absence) of various stimuli. To ascertain absence of cytokine secretion by APC, we used irradiated, CD8-depleted splenocytes from OVA323–339-immunized mice as APC in these cultures. Flow cytometric evaluation on day 5 confirmed the antigen specificity of the CD8 T cell response (Fig. 2A). Parallel thymidine-based assays using purified CD8+ T cells also showed dose-response pattern to the appropriate stimulating antigen (Fig. 2B) and a lack of response to irrelevant antigens (not shown), confirming antigenic specificity. Such antigen-specific CD8+ T cell responses were detected in several different models of EAE, as indicated in Fig. 2C.
Fig. 2.
Purified CD8+ T cells confirm neuroantigen-specific reactivity. Untouched CD8+ T cells were purified from bulk splenocytes of MOG-immunized B6 mice and subjected to either CFSE-based proliferation assays (A) or 3H-thymidine-based proliferation assays (B). In each case, purified CD8+ T cells were incubated with irradiated, CD8-depleted splenocytes from OVA323–339-immunized mice. The numbers in panel A represent % proliferation and demonstrate robust MOG35–55-specific CD8+ T cell responses. Panel B demonstrates dose-response to MOG35–55. Results are representative of over 10 independent experiments. Panel C shows CFSE-based proliferation assays conducted on splenocytes from B6, B10.PL and SJL mice immunized with MOG35–55/CFA (B6), MBPAc1–11/CFA (B10.PL) or PLP139–151/CFA (SJL). The bars represent % proliferation of purified CD8+ T cells and are representative of two to three independent experiments. Proliferation responses to cognate antigens and Con A were significantly greater than background (*p < 0.01).
3.2. MOG35–55-reactive CD8+ T cells downregulate EAE
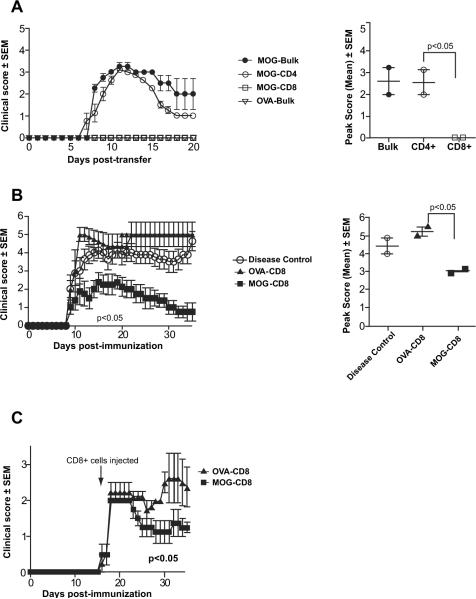
Since previous publications have suggested that MOG35–55 immunization may induce a pathogenic population of T cells [13,17], we decided to focus the remainder of our studies on this specific model. A potential pathogenic role for these cells has been shown through adoptive transfer of in vitro stimulated cells into either wildtype B6 mice or T cell deficient scid mice [13,17]. To investigate the function of MOG35–55-specific CD8+ T cells in wildtype disease, we asked whether a magnetically sorted population of in vitro stimulated, highly pure CD8+ T cells could transfer EAE to naïve mice. Using in vitro protocols similar to published methods, we stimulated splenocytes from MOG35–55-immunized mice for 72 h. Following this activation, we transferred either bulk splenocytes or magnetically sorted CD4+ or CD8+ T cells from these cultures into naïve wildtype B6 mice via tail vein injections. We observed that either bulk splenocytes or sorted CD4+ T cells could robustly transfer EAE to naïve mice. In contrast, MOG-stimulated CD8+ T cells were found to be consistently incapable of adoptively transferring disease (Fig. 3A). Analysis of average peak score and disease incidence, suggested that CD4+ T cells were the major contributors to disease induction. Thus, over multiple experiments, none of the mice receiving MOG-stimulated CD8+ T cells showed signs of EAE (0/32), whereas MOG-stimulated CD4+ T cells were able to transfer robust disease, as published previously (p < 0.05).
Fig. 3.
MOG35–55-specific CD8+ T cells show a regulatory, but not pathogenic, role in wildtype B6 EAE. (A) Splenocytes from MOG35–55/CFA-immunized female B6 mice were harvested at day 25 post-immunization and stimulated in vitro for 72 h with MOG35–55 and mIL-2. Following incubation, either bulk splenocytes (MOG-Bulk) or magnetically purified CD4+ (MOG-CD4+) or CD8+ (MOG-CD8+) cells were injected intravenously into naïve wildtype B6 mice. As controls, splenocytes were harvested from OVA323–339/CFA-immunized mice (OVA-Bulk) and similarly adoptively transferred following in vitro activation. Adoptive EAE was monitored and is depicted as mean clinical score ± SEM (left panel). Analyses of mean peak scores showed no difference between MOG-CD4+ and MOG-Bulk cells, although MOG-specific-CD8 cells were consistently incapable of transferring disease (right panel). These results are representative of over 8 independent experiments. (B) Splenocytes from MOG35–55-or OVA323–339-immunized mice were harvested at day 25 post-immunization and were cultured in vitro with their respective antigens. CD8+ T cells were purified by magnetic sorting and injected intravenously into naïve wildtype B6 mice (“day 1”). One day later (“day 0”), EAE was induced with MOG35–55/CFA. EAE scores for the various groups are shown on the left. Analyses of mean peak scores showed significant differences between the mean peak scores of OVA-CD8 and MOG-CD8 groups. These results are representative of five independent experiments. (C) EAE was induced in B6 mice with MOG35–55/CFA. Mice were split into two groups at day 13 of disease (randomized based on disease scores) and received either in vitro activated OVA323–339- or MOG35–55-stimulated CD8+ T cells. Mice were then monitored for disease (observation period of 40 days) in a blinded manner. These results are representative of three independent experiments.
Although we clearly observed failure of CD8-mediated EAE induction, we could not completely exclude the possibility that peptide manufacturing, culture media variations or cytokine supplementation may be affecting the results, hence we repeatedly attempted disease transfer by using various protocols that differed in number of cells transferred, pertussis toxin administration, method of T cell subset isolation (negative vs. positive selection) and the addition of various cytokines to the in vitro culture condition (IL-2, IL-12, or IL-23). In each of these attempts, highly purified MOG35–55-specific CD8+ T cells were not able to confer disease when adoptively transferred to naïve mice. Thus, we concluded that MOG-stimulated CD8+ T cells were not pathogenic by themselves in wildtype mice.
We next tested whether these cells may contribute to the pathogenesis of the disease in the context of CD4+ stimulation/help. It has been proposed that MOG-reactive CD8+ pathogenecity may be augmented by the addition of MOG-reactive CD4+ T cells in order to induce disease [21]. Thus, we first stimulated splenocytes from MOG-immunized mice in the manner described above and separately stimulated splenocytes from OVA323–339-immunized mice with OVA323–339 as controls. Of note, the magnitude of MOG- and OVA-specific CD8+ T cell responses was comparable (data not shown). CD8+ T cells were isolated from these cultures and injected into naïve, wildtype mice via tail vein (designated as day −1). On day 0, mice were immunized with MOG35–55/CFA in order to induce active EAE. These experiments demonstrated that MOG-specific CD8+ T cells did not result in worsening of disease. Importantly and in contrast to their purported pathogenic role, we observed that MOG-specific CD8+ T cells, in fact, significantly and consistently abrogated active EAE, as shown in Fig. 3B (representative experiment) and Table 1 (cumulative data). Amelioration of EAE paralysis was found to affect both the acute and chronic phases of the disease as evidenced by the decrease in peak score and increase grade of remission of MOG-induced paralysis. In vitro-stimulated (pre-activated and expanded) CD8+ T cells (Fig. 3B; Table 1) had a stronger protective effect than those that were obtained ex vivo (data not shown).
Table 1.
Clinical evaluation of EAE induced after transfer of in vitro-activated MOG35–55-specific CD8+T cells (cumulative data from three experiments).
| Condition | Experiment duration (day) | Incidence | Mortality | Onset (day) | Time to peak (day) | Peak score | Cumulative score | Average clinical score | Outcome | Grade of remission |
|---|---|---|---|---|---|---|---|---|---|---|
| Disease control | 30 | 13/13 | 2/13 | 9.69 | 11.38 | 3.77 | 65.15 | 2.20 | 3.27 | 0.50 |
| OVA-CD8+ | 30 | 12/12 | 2/12 | 9.92 | 13.08 | 4.38 | 78.46 | 2.53 | 3.42 | 0.96 |
| MOG-CD8+ | 30 | 11/11 | 0/11 | 11.91 | 13.09 | 2.77 * | 36.73 * | 1.20 * | 1.00 * | 1.77 * |
MOG-CD8+ clinical data was statistically compared to OVA-CD8+ data or disease control.
p ≤ 0.05.
Although we observed a clear decrease in disease severity upon pre-immunization adoptive transfer, there was a possibility that these CNS-reactive CD8+ T cells may still exhibit a pathogenic role in the context of ongoing EAE. Thus, active EAE was induced by MOG35–55 immunization, followed by transfer of MOG- or OVA-activated CD8+ T cells at the peak of the EAE course. Again, instead of exacerbating the disease, injection of these MOG-reactive CD8+ T cells resulted in a rapid and sustained decrease in disease severity, as shown in Figure 3C (representative experiment) and Table 2 (cumulative data).
Table 2.
Clinical evaluation of the effect of MOG35–55-CD8+ T cells on ongoing EAE (cumulative data from three experiments).
| Condition | Experiment duration (day) | Incidence | Mortality | Onset (day) | Peak score | Cumulative score | Average clinical score | Outcome | Grade of remission |
|---|---|---|---|---|---|---|---|---|---|
| OVA-CD8+ | 36 | 12/12 | 0/0 | 15.67 | 2.98 | 50.37 | 1.39 | 2.62 | 0.37 |
| MOG-CD8+ | 36 | 12/12 | 0/0 | 15.67 | 2.25 * | 36.04 * | 1.00 * | 1.73 * | 0.53 |
MOG-CD8+ clinical data were statistically compared to OVA-CD8+ data.
p ≤ 0.05.
3.3. MOG35–55-specific CD8+ T cells kill MOG35–55-loaded immune targets in vivo
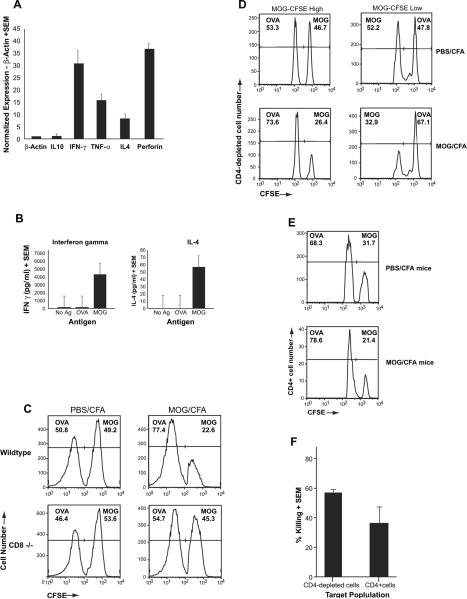
Following the unexpected discovery that neuroantigen-specific CD8+ T cells were immune regulatory in function, we next sought to characterize the mechanism whereby these cells exert their function. Using QPCR, we quantified the relative level of effector cytokines on an aliquot of these in vitro stimulated MOG-reactive CD8+ T cells, which we had observed to be protective during an adoptive transfer. These cells showed significant expression of IFN-γ, IL-4, TNF-α, and perforin, suggesting both cytotoxic functionality as well as the ability for cytokine-mediated immune modulation (Fig. 4A). Supernatant cytokine levels were also measured and confirmed the robust production of IFN-γ and small but significant amount of IL-4 (Fig. 4B), although IL-10 levels were not significant above background (data not shown).
Fig. 4.
Cytokine expression and cytotoxic function of MOG-specific CD8+ T cells. (A) Total RNA was extracted from in vitro stimulated MOG35–55-specific CD8+ T cells. Relative expression of IL-10, IFN-γ, TNF-α, IL-4 and Perforin were assessed using a quantitative PCR (qPCR) assay and normalize using the housekeeping gene β-actin. (B) CD8+ T cells were purified by negative selection from splenocytes of MOG35–55-immunized mice at day 20 post-immunization. These cells were cultured with irradiated APC and stimulated with the indicated antigen. Supernatants were collected at 72 h of culture and assayed for IFN-γ or IL-4 by ELISA. (C) Splenocytes from naïve B6 mice were harvested and stained for different levels of CFSE: CFSE-high and CFSE-low. Following staining, the cells were incubated with Con A (10 μg/ml) in the presence of either MOG35–55 (20 μg/ml) or OVA323–339 (20 μg/ml) for 18–20 h. Cells from each group were then counted and mixed at a 1:1 ratio and injected into either wildtype or CD8(−/−) mice that had been immunized 25 days prior with MOG35–55/CFA or PBS/CFA. A total of 2 days after injection of target cells, splenocytes harvested and analyzed by flow cytometry. This figure is a representative of three experiments and shows disappearance of the MOG-loaded target cells only in wildtype MOG-immunized mice. (D & E) Target cells were split into either CD4-depleted APC (D) or CD4+ T cells (E) after loading with either control antigen (OVA323–339) or MOG35–55. These cells were injected into PBS/CFA- or MOG/CFA-immunized mice at day 20 post-immunization and in vivo killing was evaluated and normalized to the ratios of the injected cells in the CFA-immunized mice. (E) Cumulative graphic representation of percent killing determined by comparing ratio of CFSE-low and CFSE-high cells in MOG-immunized mice to that of CFA control mice.
Next, we directly tested whether these cells were able to kill antigen-loaded immune target cells. For this, we used a previously described in vivo killing assay [25], taking advantage of the ability to detect pre-marked cell populations using intracellular dyes. Target cells where prepared by labeling naïve B6 splenocytes with high or low levels of CFSE. In some experiments, aliquots of each population were then stained with a different dye, CMTPX, allowing us to look at four target populations in a single mouse. These trackable populations were then loaded with different antigens (MOG35–55 or OVA323–339) by incubating overnight in the presence of Con A (to generate antigen-loaded “Con A blasts”). Next, these cells were washed, mixed together at a 1:1 ratio and injected intravenously into PBS/CFA- or MOG/CFA-immunized mice. Either wildtype or CD8(−/−) mice were used to ascertain the role of CD8+ T cells. Two days post-injection, splenocytes were harvested and CFSE-high and CFSE-low populations were quantified by flow cytometry. As shown in Fig. 4C, when OVA- and MOG-loaded cells were injected into wildtype B6 mice immunized with PBS/CFA (top left panel), the ratio of the two populations remained constant (~50%). In contrast, the number of MOG-loaded cells was significantly diminished in the MOG/CFA-immunized mouse (top right panel), indicating antigen-specific killing. Moreover, this killing was almost completely abrogated in MOG-immunized CD8(−/−) mice (bottom panels), confirming that it is predominantly mediated by MOG-specific CD8+ T cells and eliminating the possibility that preferential trafficking of MOG-loaded cells would account for their disappearance.
These results were confirmed in multiple replicates, by using different permutations of CFSE- and CMTPX-stained cells for loading control vs. MOG antigen. Thus, killing was observed only on MOG-loaded target cells, regardless of whether these represented the CFSE high or low peaks or the CMTPX (+) or (−) peaks (data not shown). Importantly, the target cells in these assays were Con A blasts which were loaded with no antigen, OVA or MOG peptide. Thus, the observed killing is not non-specific killing of activated cells; rather, a MOG-specific killing of MOG-loaded target cells by MOG-specific CD8+ T cells.
To further delineate the preferred immune target population for MOG-specific killing by CD8+ T cells, we separated our antigen-loaded target cells into a CD4+ fraction or a CD4-depleted fraction. In repeat and reciprocal experiments, we were able to observe killing of both MOG-loaded CD4+ cells, as well as MOG-loaded CD4-depleted APC populations (Figs. 4D,E, cumulative data shown in Fig. 4F).
3.4. MOG-reactive CD8+ T cells ameliorate EAE by modulating APC function and CD4+ T cell responses
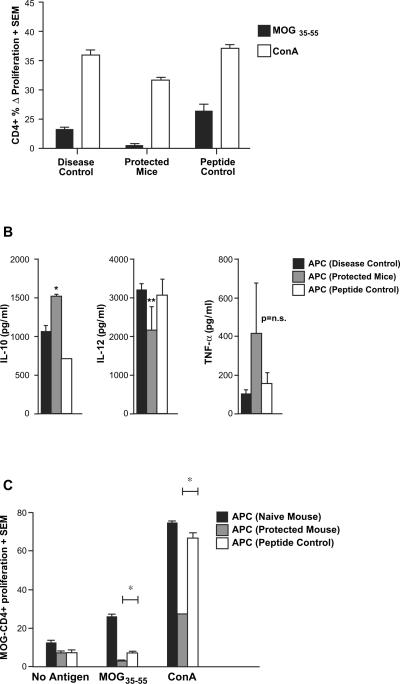
Our observations suggest that the immune regulatory effect of MOG-reactive CD8+ T cells is mediated, at least in part, by affecting APC as well as by killing potentially pathogenic CD4+ T cells. To address these hypotheses, we next performed two sets of related experiments. In the first set, we asked whether MOG-specific CD4+ T cell responses were affected in mice as a result of adoptive transfer of MOG-reactive CD8+ T cells. As seen in Fig. 5A, mice that were protected from EAE by adoptively transferred MOG35–55-stimulated CD8+ T cells had significantly diminished MOG-specific CD4+ Tcell responses, compared to mice that received either no CD8+ Tcells or OVA323–339-reactive CD8+ Tcells.
Fig. 5.
MOG35–55-specific CD8+ T cells modulate MOG-specific CD4+ T cell responses as well as APC function. In vitro-activated MOG- (protected mice) or OVA-specific (peptide control) CD8+ T cells mice were injected into naïve B6 mice, followed by immunization with MOG35–55/CFA (similar to experiments in Fig. 3B). A third group of mice only received MOG35–55/CFA immunization without any transfer of cells (disease control). At day 15 post-immunization, lymph node cells as well as splenocytes were harvested. (A) MOG-specific CD4+ T cell responses were quantified by CFSE-based proliferation assays and are depicted as Δ% proliferating fraction (“no antigen” background subtracted), showing significant inhibition (p < 0.05) of these responses in mice receiving MOG-specific CD8+ T cells. (B) T-depleted antigen presenting cells (APC) from the protected mice [APC (protected mice)], OVA control mice [APC (peptide control)] and no transfer control [APC (disease control)] were stimulated with LPS. Supernatants from these cultures were extracted at various time points and tested by ELISA for the indicated cytokines. APC from protected mice showed significantly higher IL-10 secretion (*p < 0.05) and a trend toward lower IL-12 secretion (p = 0.07). TNF-α differences were not statistically significant. (C) APC from the three groups of mice were also used to stimulate purified CD4+ cells from MOG35–55-immunized mice (used as responders). The magnitudes of proliferation from a CFSE-based assay are depicted. All results are representative of two replicate experiments.
In the next set of experiments, we evaluated the function of APC obtained from mice that were protected by the transfer of MOG-reactive CD8+ T cells. First, T cell-depleted splenocytes, from protected and control mice, were exposed to lipopolysaccharide (LPS) and evaluated for their cytokine secretion by ELISA. As shown in Fig. 5B, APC derived from protected mice showed significantly higher levels of IL-10 than controls and tended to show lower levels of IL-12 production, suggesting an anti-inflammatory phenotype. Differences in TNF-α secretion did not reach statistical significance. Next, APC from protected or control mice were used in proliferation assays using MOG-specific CD4+ T cells isolated from MOG-immunized mice as responders. Cultures were stimulated with no antigen, MOG35–55 or Con A. As shown in Fig. 5C, APC from MOG-CD8-protected mice showed a significant decrease in their ability to stimulate an antigen-specific T cell response as well as their ability to assist in Con A-mediated stimulation. Thus, immune regulatory MOG-reactive CD8+ T cells resulted in modulation of APC function and reduction in pathogenic CD4+ T cell responses.
4. Discussion
The role of CD8+ T cells in the process of autoimmune pathology has been both understudied and controversial. While it is known that CD8+ T cells represent the predominant T cell in an MS lesion and are oligoclonally expanded at the site of pathology, the antigenic specificity of these cells and their role is not known. There have been multiple reports showing an immune regulatory role for CD8+ T cells as early as the 1970s [26,27]. After a period of disfavor, the place of the regulatory CD8+ T cell as an important contributor to peripheral tolerance is undergoing a revival [5,20,25,28–32]. While not as well characterized as the CD4+CD25+FOXP3+ Tregs [33–36], it is becoming clear that CD8+ T cells play a role in the peripheral regulation of autoimmune pathology [5,28,37–42]. However, in most cases, the antigenic specificity of these regulatory T cells is unclear. Qa-1-restricted cytotoxic/suppressor CD8+ T cells have been shown to have specificity for TCR-derived peptides presented by pathogenic CD4+ T cells [5,23,30,38–40,42]. We have similarly shown copolymer therapy-induced, antigen-specific HLA-E-restricted CD8+ T cells in patients with MS [5,23,30]. In contrast, the role of CNS-specific CD8+ T cells is still incompletely understood.
It makes intuitive sense that a CNS-targeted, MHC Class I-restricted CD8+ T cell response would likely have a pathogenic role in disease and, perhaps, the oligoclonally-expanded CD8+ T cells in MS lesions represent such a response. In fact, there are reports demonstrating MHC-restricted in vitro cytotoxic killing of oligodendrocytes by CNS-specific CD8+ T cells from MS patients [43,44] While these studies show the potential of a pathogenic role, the in vivo role of these cells remains poorly understood. It is entirely possible that specificity for certain antigens in the correct MHC context may allow for CD8+ T cell-mediated CNS pathogenesis. Previous studies in certain models of EAE also suggest a pathogenic role of CNS-specific CD8+ T cells, such as MBP-specific cells in a C3H mouse [14,15] and MOG35–55-specific CD8+ cells in the B6 mouse [13,17]. In addition, recent mouse models based on transgenically expressed CNS-sequestered antigens combined with TCR-transgenic CD8+ T cells or HLA-transgenic mice also suggest that these cells may have pro-inflammatory potential [45–47]. However, most of these studies involved either induced homeostatic expansion of CD8+ T cells in T cell-deficient mice or the use of transgenic manipulation.
In the current study, we have directly addressed the role of MOG35–55-induced CD8+ T cells in a classic model of EAE using wildtype B6 mice. Interestingly, while attempting to replicate their purported pathogenic role, we generated the first evidence for a novel and unexpected regulatory role for neuroantigen-specific CD8+ T cells in EAE. In multiple attempts that included varying in vitro and in vivo conditions, we were unable to transfer disease with MOG-activated CD8+ T cells. In fact, these cells attenuated the induction of EAE and were able to reduce the severity of ongoing EAE. Thus, it seems that while one can generate models wherein CD8+ T cells can target myelin-expressing cells and cause CNS inflammation, innately these cells play a predominate regulatory role in MOG35–55/CFA-induced EAE. This corroborates with the fact that MOG35–55-induced EAE is more severe in CD8−/− B6 mice [20], especially at suboptimal doses of inducing antigen.
In the usual setting of MOG35–55-induced EAE, the generation of a pathogenic CD4+ T cell response preceded the detection of MOG35–55-specific CD8+ T cell responses, as the CD8+ T cell responses were reproducibly detected after days 20–25 post-immunization. Thus, the dynamics of pathogenic vs. regulatory mechanisms clearly give the upper hand to the pathogenesis. Moreover, the numeric balance between the two populations also appears to be important, as we did not see significant enhanced adoptive EAE from preparations that contained only CD4+ T cells (as compared to bulk transfers). However, when an adequate number of pre-primed CD8+ T cells were transferred, they could effectively inhibit EAE.
The mechanisms of this inhibition appear to depend, at least in part, on the ability of the CD8+ Tcells to modulate APC function and CD4+ Tcell responses. These cells produce cytokines that may effect such immune modulation. For example, even IFN-γ may not be a purely pathogenic cytokine and could mediate down modulation of EAE [37,48–54]. Moreover, MOG35–55-specific CD8+ T cells also have direct cytotoxicity towards MOG35–55-loaded target cells, but not towards a non-specifically activated T cell. In that regard, these regulatory CD8+ Tcells are distinct from previously described Qa-1-restricted cytotoxic/suppressor T cells, which are capable of killing activated CD4+ T cells by recognizing heat shock proteins or other self antigens loaded in the context of Qa-1. In those examples, once activated, the suppressor cells appear to suppress in a non-antigenspecific manner, i.e. with disregard to the priming antigen. In contrast, MOG-reactive CD8+ T cells maintain their antigenic specificity, appearing to require MOG presentation. We have observed in recent experiments that MOG35–55-induced CD8+ Tcells were unable to inhibit PLP178–191-induced EAE in B6 mice and were also unable to inhibit MOG35–55-induced EAE in the absence of MHC Class I (unpublished observations). This is compatible with a model wherein these CD8+ T cells could directly kill proinflammatory populations of APC or CD4+ T cells, provided these can cross-present MOG in the context of their class I MHC. It is still unclear whether such presentation involves Class Ia or Ib molecules and what effector molecules are utilized for suppression. These issues will be the focus of future experimentation. While we have clearly demonstrated the ability of these cells to regulate immune responses in the periphery, it also remains to be seen whether these immunologic effects may be exerted directly at the site of pathology through CNS trafficking.
Thus far, our studies have not revealed a specific immunophenotypic feature of these CD8+ T cells that might allow us to sort them prior to antigenic stimulation. Thus, we do not know whether these cells might belong to the varied ex vivo CD8+ Treg population described before, such as CD8+ CD25+ [55], CD8+ CD28− [20,56] CD8+CD122+ [57], CD8+CD75s+ [58] or CD8aa [59]. Our experiments have relied on their antigenic specificity as a mode of stimulating and expanding these cells in vitro. It is likely that CD8+ Tregs, as a whole, may represent distinct populations, depending on the context in which they are derived, similar to the varying phenotypes of CD4+ Tregs in distinct disease settings (CD4+CD25+FOXP3+ vs. Tr1 vs. Th3 vs. Th2).
Finally, there may be important therapeutic as well as biological implications for these observations. While using autoreactive T cells as a model of therapy may seem unorthodox, there is a clear drive to generate autoantigen-reactive regulatory T cells for adoptive immunotherapy, especially with regard to CD4+CD25+FOXP3+ Tregs. Other forms of autoreactive CD4+ Tregs (Tr1, Th3) have also shown promise in animal models and are being considered as potential strategies. The role of human neuroantigen-specific CD8+ T cells in the context of MS, is of yet, unknown. We have shown that there is a high prevalence of CNS-specific CD8+ T cell responses in both healthy individuals and MS patients. If these cells, in fact, have immune regulatory functions, one may be able to harness this for therapeutic benefit in a very targeted manner.
In terms of the biological implications of such a response, it is tempting to speculate that this may represent an underappreciated arm of immune tolerance. Hence, in the setting of an ongoing robust CD4+ T cell response (such as that induced by antigen/CFA immunization), one could envision the generation of a CD8+ T cell response specific to the same inducing antigen, with the inherent function of controlling the expansion of the initial response. It remains to be seen whether such a cross-talk is inherent during non-pathogenic immune responses and what types of presentation requirements are needed for its generation.
To summarize, our studies demonstrate a novel role for neuro-antigen-specific CD8+ T cells, revealing a potential pathway of intrinsic immune regulation during ongoing inflammation. This aspect of immune modulation may have implications in human MS as well as other immune-mediated disorders and these studies should hopefully pave the way for intensified investigations in these settings.
5. Conclusions
This report provides the first evidence that CNS-specific autoreactive CD8+ T cells may play an immune regulatory role during autoimmune demyelination. Thus, purified MOG35–55-specific CD8+ T cells derived from wildtype B6 mice were incapable of transferring EAE to naïve mice and, in fact, resulted in the amelioration of disease when transferred either prior to EAE induction or during ongoing disease. These cells appear to operate through a cytotoxic/suppressor mechanism, likely resulting in both the direct cytotoxic killing of pro-inflammatory cells as well as modulation of APC.
Acknowledgements
The authors would like to thank Maycie M. Garibay and Dr. Chris L. Ayers for discussions and manuscript review. This work was supported, in part, by grant awards from the National Institutes of Health (R01AI053439, R01AI065463, K24AI079272) and National Multiple Sclerosis Society (Harry Weaver Neuroscience Scholar Award JF2118-A-2).
References
- [1].Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- [2].Crawford MP, Yan SX, Ortega SB, Mehta RS, Hewitt RE, Price DA, et al. High prevalence of autoreactive, neuroantigen-specific CD8+ T cells in multiple sclerosis revealed by novel flow cytometric assay. Blood. 2004;103:4222–31. doi: 10.1182/blood-2003-11-4025. [DOI] [PubMed] [Google Scholar]
- [3].Neumann H, Medana IM, Bauer J, Lassmann H. Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends Neurosci. 2002;25:313–9. doi: 10.1016/s0166-2236(02)02154-9. [DOI] [PubMed] [Google Scholar]
- [4].Skulina C, Schmidt S, Dornmair K, Babbe H, Roers A, Rajewsky K, et al. Multiple sclerosis: brain-infiltrating CD8+ T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc Natl Acad Sci U S A. 2004;101:2428–33. doi: 10.1073/pnas.0308689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tennakoon DK, Mehta RS, Ortega SB, Bhoj V, Racke MK, Karandikar NJ. Therapeutic induction of regulatory, cytotoxic CD8+ T cells in multiple sclerosis. J Immunol. 2006;176:7119–29. doi: 10.4049/jimmunol.176.11.7119. [DOI] [PubMed] [Google Scholar]
- [6].Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, et al. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hauser SL, Bhan AK, Gilles F, Kemp M, Kerr C, Weiner HL. Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Ann Neurol. 1986;19:578–87. doi: 10.1002/ana.410190610. [DOI] [PubMed] [Google Scholar]
- [8].Jacobsen M, Cepok S, Quak E, Happel M, Gaber R, Ziegler A, et al. Oligoclonal expansion of memory CD8+ T cells in cerebrospinal fluid from multiple sclerosis patients. Brain. 2002;125:538–50. doi: 10.1093/brain/awf059. [DOI] [PubMed] [Google Scholar]
- [9].Junker A, Ivanidze J, Malotka J, Eiglmeier I, Lassmann H, Wekerle H, et al. Multiple sclerosis: T-cell receptor expression in distinct brain regions. Brain. 2007;130:2789–99. doi: 10.1093/brain/awm214. [DOI] [PubMed] [Google Scholar]
- [10].Monteiro J, Hingorani R, Pergolizzi R, Apatoff B, Gregersen PK. Clonal dominance of CD8+ T-cell in multiple sclerosis. Ann N Y Acad Sci. 1995;756:310–2. doi: 10.1111/j.1749-6632.1995.tb44529.x. [DOI] [PubMed] [Google Scholar]
- [11].Traugott U, Reinherz EL, Raine CS. Multiple sclerosis: distribution of T cell subsets within active chronic lesions. Science. 1983;219:308–10. doi: 10.1126/science.6217550. [DOI] [PubMed] [Google Scholar]
- [12].Woodroofe MN, Bellamy AS, Feldmann M, Davison AN, Cuzner ML. Immunocytochemical characterisation of the immune reaction in the central nervous system in multiple sclerosis. Possible role for microglia in lesion growth. J Neurol Sci. 1986;74:135–52. doi: 10.1016/0022-510x(86)90100-0. [DOI] [PubMed] [Google Scholar]
- [13].Ford ML, Evavold BD. Specificity, magnitude, and kinetics of MOG-specific CD8+ T cell responses during experimental autoimmune encephalomyelitis. Eur J Immunol. 2005;35:76–85. doi: 10.1002/eji.200425660. [DOI] [PubMed] [Google Scholar]
- [14].Huseby ES, Liggitt D, Brabb T, Schnabel B, Ohlen C, Goverman J. A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis. J Exp Med. 2001;194:669–76. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ji Q, Goverman J. Experimental autoimmune encephalomyelitis mediated by CD8+ T cells. Ann N Y Acad Sci. 2007;1103:157–66. doi: 10.1196/annals.1394.017. [DOI] [PubMed] [Google Scholar]
- [16].Koh DR, Fung-Leung WP, Ho A, Gray D, Acha-Orbea H, Mak TW. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8−/− mice. Science. 1992;256:1210–3. doi: 10.1126/science.256.5060.1210. [DOI] [PubMed] [Google Scholar]
- [17].Sun D, Whitaker JN, Huang Z, Liu D, Coleclough C, Wekerle H, et al. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J Immunol. 2001;166:7579–87. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- [18].Jiang H, Zhang SI, Pernis B. Role of CD8+ T cells in murine experimental allergic encephalomyelitis. Science. 1992;256:1213–5. doi: 10.1126/science.256.5060.1213. [DOI] [PubMed] [Google Scholar]
- [19].Linker RA, Rott E, Hofstetter HH, Hanke T, Toyka KV, Gold R. EAE in beta-2 microglobulin-deficient mice: axonal damage is not dependent on MHC-I restricted immune responses. Neurobiol Dis. 2005;19:218–28. doi: 10.1016/j.nbd.2004.12.017. [DOI] [PubMed] [Google Scholar]
- [20].Najafian N, Chitnis T, Salama AD, Zhu B, Benou C, Yuan X, et al. Regulatory functions of CD8+ CD28− T cells in an autoimmune disease model. J Clin Invest. 2003;112:1037–48. doi: 10.1172/JCI17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bettini M, Rosenthal K, Evavold BD. Pathogenic MOG-reactive CD8+ T cells require MOG-reactive CD4+ T cells for sustained CNS inflammation during chronic EAE. J Neuroimmunol. 2009;213:60–8. doi: 10.1016/j.jneuroim.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Quah BJ, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc. 2007;2:2049–56. doi: 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
- [23].Biegler BW, Yan SX, Ortega SB, Tennakoon DK, Racke MK, Karandikar NJ. Glatiramer acetate (GA) therapy induces a focused, oligoclonal CD8+ T-cell repertoire in multiple sclerosis. J Neuroimmunol. 2006;180:159–71. doi: 10.1016/j.jneuroim.2006.07.015. [DOI] [PubMed] [Google Scholar]
- [24].Miller SD, Karpus WJ. Experimental autoimmune encephalomyelitis in the mouse. Curr Protoc Immunol. 2007 doi: 10.1002/0471142735.im1501s77. Chapter 15: Unit 15 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ingulli E. Tracing tolerance and immunity in vivo by CFSE-labeling of administered cells. Methods Mol Biol. 2007;380:365–76. doi: 10.1007/978-1-59745-395-0_23. [DOI] [PubMed] [Google Scholar]
- [26].Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18:723–37. [PMC free article] [PubMed] [Google Scholar]
- [27].Cantor H, Shen FW, Boyse EA. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976;143:1391–1340. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chess L, Jiang H. Resurrecting CD8+ suppressor T cells. Nat Immunol. 2004;5:469–71. doi: 10.1038/ni0504-469. [DOI] [PubMed] [Google Scholar]
- [29].Jiang H, Braunstein NS, Yu B, Winchester R, Chess L. CD8+ T cells control the TH phenotype of MBP-reactive CD4+ T cells in EAE mice. Proc Natl Acad Sci U S A. 2001;98:6301–6. doi: 10.1073/pnas.101123098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Karandikar NJ, Crawford MP, Yan X, Ratts RB, Brenchley JM, Ambrozak DR, et al. Glatiramer acetate (Copaxone) therapy induces CD8(+) T cell responses in patients with multiple sclerosis. J Clin Invest. 2002;109:641–9. doi: 10.1172/JCI14380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sercarz E, Maverakis E, van den Elzen P, Madakamutil L, Kumar V. Seven surprises in the TCR-centred regulation of immune responsiveness in an autoimmune system. Novartis Found Symp. 2003;252:165–71. doi: 10.1002/0470871628.ch12. discussion 171–176, 203–210. [DOI] [PubMed] [Google Scholar]
- [32].Zozulya AL, Wiendl H. The role of CD8 suppressors versus destructors in autoimmune central nervous system inflammation. Hum Immunol. 2008;69:797–804. doi: 10.1016/j.humimm.2008.07.014. [DOI] [PubMed] [Google Scholar]
- [33].Gavin M, Rudensky A. Control of immune homeostasis by naturally arising regulatory CD4+ T cells. Curr Opin Immunol. 2003;15:690–6. doi: 10.1016/j.coi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- [34].Kohm AP, Carpentier PA, Miller SD. Regulation of experimental autoimmune encephalomyelitis (EAE) by CD4+ CD25+ regulatory T cells. Novartis Found. 2003;252:45–52. discussion 52–54, 106–114. [PubMed] [Google Scholar]
- [35].Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, et al. Foxp3 + CD25 + CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- [36].Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- [37].Balashov KE, Khoury SJ, Hafler DA, Weiner HL. Inhibition of T cell responses by activated human CD8+ Tcells is mediated by interferon-gamma and is defective in chronic progressive multiple sclerosis. J Clin Invest. 1995;95:2711–9. doi: 10.1172/JCI117973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jiang H, Kashleva H, Xu LX, Forman J, Flaherty L, Pernis B, et al. T cell vaccination induces T cell receptor Vbeta-specific Qa-1-restricted regulatory CD8 (+) T cells. Proc Natl Acad Sci U S A. 1998;95:4533–7. doi: 10.1073/pnas.95.8.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Li J, Goldstein I, Glickman-Nir E, Jiang H, Chess L. Induction of TCR Vbeta-specific CD8+ CTLs by TCR Vbeta-derived peptides bound to HLA–E. J Immunol. 2001;167:3800–8. doi: 10.4049/jimmunol.167.7.3800. [DOI] [PubMed] [Google Scholar]
- [40].Sarantopoulos S, Lu L, Cantor H. Qa-1 restriction of CD8+ suppressor T cells. J Clin Invest. 2004;114:1218–21. doi: 10.1172/JCI23152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Smith TR, Kumar V. Revival of CD8+ Treg-mediated suppression. Trends Immunol. 2008;29:337–42. doi: 10.1016/j.it.2008.04.002. [DOI] [PubMed] [Google Scholar]
- [42].Tang X, Maricic I, Purohit N, Bakamjian B, Reed-Loisel LM, Beeston T, et al. Regulation of immunity by a novel population of Qa-1-restricted CD8alphaalpha + TCRalphabeta + T cells. J Immunol. 2006;177:7645–55. doi: 10.4049/jimmunol.177.11.7645. [DOI] [PubMed] [Google Scholar]
- [43].Jurewicz A, Biddison WE, Antel JP. MHC class I-restricted lysis of human oligodendrocytes by myelin basic protein peptide-specific CD8 T lymphocytes. J Immunol. 1998;160:3056–9. [PubMed] [Google Scholar]
- [44].Niland B, Banki K, Biddison WE, Perl A. CD8+ T cell-mediated HLA-A*0201-restricted cytotoxicity to transaldolase peptide 168–176 in patients with multiple sclerosis. J Immunol. 2005;175:8365–78. doi: 10.4049/jimmunol.175.12.8365. [DOI] [PubMed] [Google Scholar]
- [45].Mars LT, Bauer J, Gross DA, Bucciarelli F, Firat H, Hudrisier D, et al. CD8 T cell responses to myelin oligodendrocyte glycoprotein-derived peptides in humanized HLA-A*0201-transgenic mice. J Immunol. 2007;179:5090–8. doi: 10.4049/jimmunol.179.8.5090. [DOI] [PubMed] [Google Scholar]
- [46].Na SY, Cao Y, Toben C, Nitschke L, Stadelmann C, Gold R, et al. Naive CD8 T-cells initiate spontaneous autoimmunity to a sequestered model antigen of the central nervous system. Brain. 2008;131:2353–65. doi: 10.1093/brain/awn148. [DOI] [PubMed] [Google Scholar]
- [47].Saxena A, Bauer J, Scheikl T, Zappulla J, Audebert M, Desbois S, et al. Cutting edge: multiple sclerosis-like lesions induced by effector CD8 T cells recognizing a sequestered antigen on oligodendrocytes. J Immunol. 2008;181:1617–21. doi: 10.4049/jimmunol.181.3.1617. [DOI] [PubMed] [Google Scholar]
- [48].Adorini L. Immunotherapeutic approaches in multiple sclerosis. J Neurol Sci. 2004;223:13–24. doi: 10.1016/j.jns.2004.04.014. [DOI] [PubMed] [Google Scholar]
- [49].Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:123–8. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Correale J, Villa A. Isolation and characterization of CD8+ regulatory T cells in multiple sclerosis. J Neuroimmunol. 2008;195:121–34. doi: 10.1016/j.jneuroim.2007.12.004. [DOI] [PubMed] [Google Scholar]
- [51].Duong TT, Finkelman FD, Singh B, Strejan GH. Effect of anti-interferon-gamma monoclonal antibody treatment on the development of experimental allergic encephalomyelitis in resistant mouse strains. J Neuroimmunol. 1994;53:101–7. doi: 10.1016/0165-5728(94)90069-8. [DOI] [PubMed] [Google Scholar]
- [52].Duong TT, Louis J, Gilbert JJ, Finkelman FD, Strejan GH. Effect of anti-interferon-gamma and anti-interleukin-2 monoclonal antibody treatment on the development of actively and passively induced experimental allergic encephalomyelitis in the SJL/J mouse. J Neuroimmunol. 1992;36:105–15. doi: 10.1016/0165-5728(92)90042-j. [DOI] [PubMed] [Google Scholar]
- [53].Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- [54].Myers L, Croft M, Kwon BS, Mittler RS, Vella AT. Peptide-specific CD8 T regulatory cells use IFN-gamma to elaborate TGF-beta-based suppression. J Immunol. 2005;174:7625–32. doi: 10.4049/jimmunol.174.12.7625. [DOI] [PubMed] [Google Scholar]
- [55].Mahic M, Henjum K, Yaqub S, Bjornbeth BA, Torgersen KM, Tasken K, et al. Generation of highly suppressive adaptive CD8(+)CD25(+)FOXP3(+) regulatory T cells by continuous antigen stimulation. Eur J Immunol. 2008;38:640–6. doi: 10.1002/eji.200737529. [DOI] [PubMed] [Google Scholar]
- [56].Filaci G, Suciu-Foca N. CD8+ T suppressor cells are back to the game: are they players in autoimmunity? Autoimmun Rev. 2002;1:279–83. doi: 10.1016/s1568-9972(02)00065-4. [DOI] [PubMed] [Google Scholar]
- [57].Lee YH, Ishida Y, Rifa'i M, Shi Z, Isobe K, Suzuki H. Essential role of CD8+ CD122+ regulatory T cells in the recovery from experimental autoimmune encephalomyelitis. J Immunol. 2008;180:825–32. doi: 10.4049/jimmunol.180.2.825. [DOI] [PubMed] [Google Scholar]
- [58].Zimring JC, Kapp JA. Identification and characterization of CD8+ suppressor T cells. Immunol Res. 2004;29:303–12. doi: 10.1385/IR:29:1-3:303. [DOI] [PubMed] [Google Scholar]
- [59].Kumar V, Sercarz E. An integrative model of regulation centered on recognition of TCR peptide/MHC complexes. Immunol Rev. 2001;182:113–21. doi: 10.1034/j.1600-065x.2001.1820109.x. [DOI] [PubMed] [Google Scholar]