Abstract
The classic view of estrogen actions in the brain was confined to regulation of ovulation and reproductive behavior in the female of all mamamalian species studied, including humans. Burgeoning evidence now documents profound effects of estrogens on learning, memory, and mood as well as neurodevelopmental and neurodegenerative processes. Most data derive from studies in females, but there is mounting recognition that estrogens play important roles in the male brain, where they can be generated from circulating testosterone by local aromatase enzymes or synthesized de novo by neurons and glia. Estrogen-based therapy therefore holds considerable promise for brain disorders that affect both men and women. However, as investigations are beginning to consider the role of estrogens in the male brain more carefully, it emerges that they have different, even opposite, effects as well as similar effects in male and female brains. This review focuses on these differences, including sex dimorphisms in the ability of estradiol to influence synaptic plasticity, neurotransmission, neurodegeneration, and cognition, which, we argue, are due in a large part to sex differences in the organization of the underlying circuitry. There are notable sex differences in the incidence and manifestations of virtually all central nervous system disorders, including neurodegenerative disease (Parkinson's and Alzheimer's), drug abuse, anxiety, and depression. Understanding the cellular and molecular basis of sex differences in brain physiology and responses to estrogen and estrogen mimics is, therefore, vitally important for understanding the nature and origins of sex-specific pathological conditions and for designing novel hormone-based therapeutic agents that will have optimal effectiveness in men or women.
I. Introduction
The last decade has seen a revolution in our understanding of the actions of estrogen in the body. More than 60 years ago, estrogen, produced by the ovaries, was identified as “the woman's hormone,” leading to its use as hormone replacement therapy (HRT1) for menopausal/postmenopausal symptoms (hot flashes, night sweats, and vaginal dryness and atrophy). Along the way, scores of anecdotal and retrospective case studies fuelled its reputation to combat diseases of aging (at least in women), including cardiovascular disease (Sullivan and Fowlkes, 1996), osteoporosis (Riggs and Melton, 1995), and Alzheimer's disease (Sherwin, 2002; Brinton, 2004). This spawned a billion-dollar industry in HRT and opened up the prospect that tissues other than the female reproductive tract, particularly the brain, are important targets for estrogen's actions. Our perception of the roles of estrogen in the male has also expanded with the realization that it can be synthesized locally from steroid precursors, including circulating testosterone, by aromatase enzymes in many tissues (Sharpe, 1998; Jones et al., 2006). This includes the brain, where estrogen may act via its classic nuclear receptors, which are widely distributed in the brains of males as well as females, or via rapid membrane actions (Toran-Allerand, 2005; Balthazart and Ball, 2006; Brann et al., 2007; Micevych and Dominguez, 2009).
Today estrogens remain the recommended active compound for the short-term treatment for menopausal symptoms (American College of Obstetricians and Gynecologists Women's Health Care Physicians, 2004), but links to cancer (especially breast and uterus) and the unexpected finding that current HRT regimes exacerbated rather than ameliorated susceptibility to stroke and heart attacks in postmenopausal women (Rossouw et al., 2002; Murphy et al., 2003; Wise et al., 2009) led to a precipitous fall in the rate of prescribing estrogen-based replacement therapies (Mandavilli, 2006; Lewis, 2009). However, this shock has stimulated a heightened interest in the extraordinary, cell-specific nature of the effects of estrogen, its metabolites and natural isomers in diverse tissues throughout the body. In particular, research into the actions of estrogen in the brain alone has produced an average of almost two publications a day for the last couple of years. These document the profound effects and multiple mechanisms of action of estrogen on memory, mood, mental state, and neurodevelopmental and neurodegenerative processes, providing mounting support for the views that estrogen is neurotrophic, neuroprotective, and psychoprotective (Fink et al., 1996; McEwen and Alves, 1999; Gillies et al., 2004; Craig et al., 2005, 2008; Cahill, 2006; Brann et al., 2007; Craig and Murphy, 2007a,b). Estrogen-based therapies therefore hold enormous promise for brain disorders that affect both men and women (Rochira et al., 2002; Jones et al., 2006). However, the overwhelming proportion of experimental investigations of estrogen effects in the brain have been performed in females, which contrasts starkly with the majority of basic neuroscience research that uses males (Cahill, 2006; Luine, 2007).
There is now a growing literature to suggest that, in addition to similarities between male and female brains, there are marked sex dimorphisms in brain morphology, neurochemistry, hard-wiring, and functional outcomes (De Vries and Boyle, 1998; Simerly, 2005; Cahill, 2006; Cosgrove et al., 2007). Moreover, increasing evidence suggests that estrogen can have different (sometimes opposite) effects as well as similar effects in male and female subjects, probably because of underlying brain dimorphisms that occur in some brain processes but not others. These observations come from diverse areas of the literature ranging from neuroscience and neurodegeneration to cognitive and reproductive behaviors. Therefore, the purposes of this review are as follows:
to assimilate evidence from some major brain areas, such as the hypothalamus, midbrain, hippocampus, and prefrontal cortex.
to document sex dimorphisms in the neural substrate in experimental species and humans, where it is known.
to analyze evidence that estrogen plays important roles in the male as well as female brain, with a particular focus on studies involving both male and female subjects in which the actions of estrogen have been directly compared.
to question the origin of these differences (arising developmentally or in adulthood), which has great significance for understanding the foundations of sex differences in the prevalence, progression, and/or severity of many of the common neuropsychiatric and neurodegenerative diseases, including Parkinson's disease, Alzheimer's disease, drug addiction, and schizophrenia.
These discussions constitute a strong argument for the urgent need for a better understanding of brain sex dimorphisms, as well as sex-specific responses to estrogen/estrogen mimics. Such knowledge of the physiological and pharmacological relevance of estrogen actions in the brain is essential if we are to realize the full translational potential of this ubiquitous steroid for promoting human health and wellbeing. Furthermore, it will highlight the importance of adopting a sex-specific approach to treating highly debilitating neurological and neuropsychiatric conditions, the prevalence of which is increasing (Szpir, 2006; Becker and Hu, 2008; Mayes et al., 2008; Williams et al., 2008).
II. Definitions, Concepts, and Why Brain Sex Dimorphisms Are Important
Throughout this article, the term sex will be used to distinguish male or female subjects according to the reproductive organs and functions that derive from the chromosomal complement (individual organisms bearing the male XY or female XX sex chromosomes seen in most mammals). This is distinct from the term gender, used to refer to a human subject's self-representation as male or female (Wizeman and Pardue, 2001). In addition, although male and female are traditionally used only as adjectives, they will sometimes be used as nouns to avoid convoluted language.
There are hundreds, if not thousands, of original articles in the scientific literature that address topics that pertain to this review. Therefore, we shall refer wherever possible to many excellent reviews by experts in their fields, rather than the original manuscripts, which would be too copious.
A. Sex Dimorphisms Are Widespread in Animal and Human Brains and Are Not Restricted Only to Reproductive Functions
For several decades it was a generally held belief that differences in male and female brains were the sole privilege of the hypothalamus, the brain region regulating the production of reproductive hormones and mating behaviors in all mammalian species. Early evidence for sex differences in learning and cognition (Carey, 1958) were largely attributed to environmental and sociocultural factors. However, the last decade has seen an exponential increase in evidence for structural, cellular, and molecular sex differences in the brain that can be described as true dimorphisms, defined as the occurrence of two forms in the same species. These include regions of human and animal brains that are important for cognition, memory, and affect, such as the hippocampus, amygdala, and cortex (Kelly et al., 1999; Baron-Cohen et al., 2005; McCarthy and Konkle, 2005; Cahill, 2006; Cosgrove et al., 2007; Wilson and Davies, 2007), and for regions controlling sensorimotor and reward systems (Becker, 1999; Dewing et al., 2006; Cantuti-Castelvetri et al., 2007; McArthur et al., 2007a). Indeed, post mortem studies, as well as evidence from new technologies for in vivo imaging, are adding rapidly to the view that sex differences in the human brain may be the norm rather than the exception (Madeira and Lieberman, 1995; Allen et al., 2003; Kruijver et al., 2003; Swaab et al., 2003; Luders et al., 2004; Mechelli et al., 2005; Cosgrove et al., 2007; Ishunina and Swaab, 2008; Swaab, 2008).
B. Origins of Sex Differences and Dimorphisms: Activational versus Organizational Effects and Hormonal versus Genetic Influences
1. Activational versus Organizational Effects.
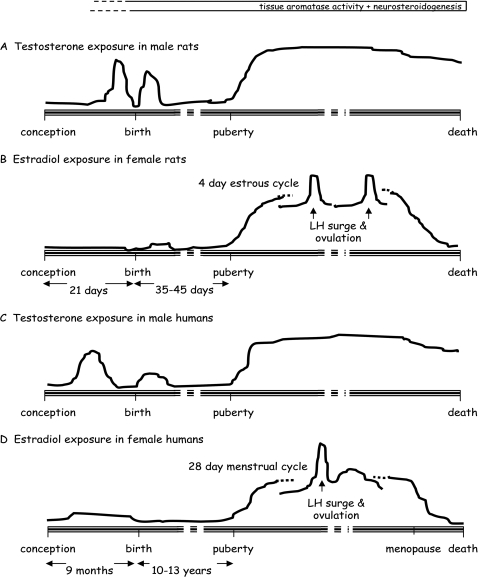
The predominant circulating gonadal sex steroid hormones after puberty are estrogens in females and testosterone in males. Thus, sex differences in a biological response could be the result of differences in the prevailing levels of gonadal hormones in adulthood, with no presumptive sex differences in the underlying biological substrate. For example, in humans and in species used in research, administration of androgens to females may induce aspects of male-typical behavior that revert to normal once hormone treatment ceases; the cyclical rise and fall in levels of ovarian hormones in women and animal species used in research also influences many behaviors (Kelly et al., 1999; Halpern and Tan, 2001; Cahill, 2006; Goldstein, 2006; Wilson and Davies, 2007). These are traditionally called activational (reversible) effects (Arnold and Breedlove, 1985; Williams, 1986) or hormonally modulated responses (McCarthy and Konkle, 2005), which dictate sex differences at molecular, cellular, and functional levels but are not in themselves true dimorphisms. However, not all features of adult brain activity that exhibit sex differences are trans-sexual; that is, they cannot be equalized if an equivalent hormonal environment is created experimentally in both sexes by the administration of sex hormones to gonadectomized animals. Estrogen treatment of adult castrated rats cannot feminize all male CNS functions and androgen treatment of adult ovariectomized rats cannot masculinize all aspects of female CNS function because of permanent (irreversible) sex-specific organization of the brain during development (see also section IV). The classic concept of sexual differentiation of the brain, originating from work on the hypothalamus, states that once formation of the fetal testes is established by the Sry gene (sex determining region of the Y chromosome), sexual differentiation of the brain is a hormone-dependent process (Arnold and Gorski, 1984; Morris et al., 2004; Simerly, 2005; McCarthy, 2008). The key factor is the masculinizing/defeminizing effect of testosterone, produced by a transitory activation of the testes during a critical developmental window, lasting from the late embryonic period to the first week of life in rats (Huhtaniemi, 1994) (Fig. 1A). Testosterone freely enters the brain and, perhaps surprisingly, in certain regions its ability to sculpt the male brain relies principally on its conversion to estradiol by local aromatase enzymes. Estrogen receptor (ER)-dependent influences on processes such as neurogenesis, apoptosis, and migration then ensue to imprint enduring sex differences in the number of cells and their distribution within specific regions or nuclei. In addition, influences on neurite extension/branching, synaptogenesis, and establishment of neurochemical phenotype establish sex differences in projection pathways, innervation density, connectivity, and neurotransmitter control in specific brain regions (Simerly, 1989; De Vries and Simerly, 2002; Simerly, 2005; Wilson and Davies, 2007; Forger, 2009; Tobet et al., 2009). In addition to producing sex dimorphisms in the “hard-wiring,” perinatal exposure to testosterone (after aromatization) can also program sexually dimorphic patterns in ER expression in selected adult brain regions, which can have profound effects on the way a cell or pathway responds to estradiol (see sections III and IV and Table 1). Many advances have been made recently in the cellular and molecular mechanisms by which testosterone/estradiol engenders a sexually differentiated brain, and both classic nuclear and non-nuclear mechanisms that are active in the adult brain play a role. These are thoroughly reviewed elsewhere (McCarthy and Konkle, 2005; Wilson and Davies, 2007; McCarthy, 2008) and are of particular interest not only for understanding the actions of estrogens in the developing brain but also for possibly providing clues about estrogenic actions in the injured brain, in which certain developmental processes may be recapitulated in attempts to protect, repair, and recover.
Fig. 1.
Patterns of hormone exposure throughout life: a biological basis for sex differences in the brain. In male rats (A) and humans (C), a transitory activation of the testes during a critical developmental window means that the brain develops in a different hormonal environment in males and females, which establishes irreversible sex dimorphisms in specific neural circuits. After puberty, the rise in gonadal steroids in males and females activates the sexually dimorphic circuitry; the rodent (A) and human (C) male brain is exposed to a relatively steady level of the main gonadal steroid, testosterone, for most of adult life. In contrast, the rodent (B) and human (D) female brain is exposed to a cyclical pattern of the main gonadal steroid, estradiol, for a certain period of adult life, until levels fall precipitously at reproductive senescence or menopause.
TABLE 1.
Summary of the major findings on ER distribution in the adult brain
The diversity of approaches taken when investigating ER distribution in the brain are illustrated. Some studies present data throughout the brain, whereas others focus on specific areas, with variations in the use of intact and gonadectomized rodents and the sex of the subjects under investigation. Along with difficulties inherent in the absolute quantification of immunoreactivity (IR) and in situ hybridization (ISH) signals, this complicates direct comparisons between studies. Overall, however, there is a strong consistency for the anatomical organization of ERs in the brain: it is clear that across species, ERα and ERβ are widely distributed in brain regions that are and are not principally associated with reproductive functions. Although overlapping in many brain regions, ERα and ERβ have distinct patterns of distribution. In humans and rodents, the hypothalamus (especially the VMN) and amygdala emerge as ERα-dominant regions (Shughrue et al., 1997; Osterlund et al., 2000a,c), providing neuroanatomical evidence for a role in regulating neuroendocrine, autonomic, emotional, affective, and motivational responses. Both ERα and ERβ are found in the hippocampus in rodents and humans, ERβ being the dominant form in the human subiculum (where information leaves the hippocampus to influence amygdala, cortical, and subcortical structures). ERs are thus well placed to influence learning and memory. The basal ganglia are notable by their relative lack of classical ERs. The distribution patterns of ERs are remarkably similar in adult male and female brains. However, sex differences are present in the relative levels of expression in hypothalamic subnuclei involved in reproductive processes, which may be determined early in life (Khünemann et al., 1994; Orikasa et al., 2002; Ikeda et al., 2003). In the human hypothalamus, sex differences were also revealed by closer analysis of their subcellular distribution to the nucleus, cytoplasm, and nerve terminals (Kruijver et al., 2002). In contrast, a lack of overall sex differences in ER expression levels was notable in the hippocampal regions, where estradiol-responsiveness is known to be sexually dimorphic (Weiland et al., 1997). Sex differences are also absent in the cortex (Kritzer, 2002), but finer analysis revealed that males and females did exhibit differences in the cytoarchitectural localization of ERs in the mesocortical neurons supplying different regions of the PFC (Kritzer and Creutz, 2008).
| Species | Protein/Immunoreactivity | mRNA (In Situ Hybridization) |
|---|---|---|
| ERα | ||
| Rat | Gonad-intact, male and female cerebral cortex: wide neuronal distribution (distinct from ERβ); no sex differences (Kritzer, 2002). | Twelve days post-OVX: exclusively in the VMN and subfornical organ; also in perikarya in cerebral cortex and hippocampus (weak compared with ERβ), as well as other brain regions, including the BNST, medial and cortical amygdaloid nuclei, POA, lateral habenula, periaqueductal gray, parabrachial nucleus, LC, NTS, spinal trigeminal nucleus, superficial laminae of the spinal cord (Shughrue et al., 1997). |
| Gonad-intact, male and female dopaminergic neurones of the mesocortical system: no overall sex differences, but sex differences revealed at cytoarchitectural level (Kritzer and Creutz, 2008). | Intact males and females; olfactory cortex, hippocampus, amygdala, septum, BNST, thalamus, POA, AVPV, SCN, ARC, PeN, SNc, NTS, LC, midbrain raphe nuclei; no sex differences (Laflamme et al., 1998). | |
| Mouse | Two weeks post-OVX; widely distributed throughout brain; predominant subtype in hippocampus, POA, and most of the hypothalamus; sparse or absent from cerebral cortex and cerebellum (Mitra et al., 2003). | CX; widely expressed throughout brain; few positive cells in striatum; none in SNc (Shughrue, 2004). |
| CX: concentrated in many brain regions, especially hypothalamus (POA, ARC, VMN), BNST, amygdala; scattered positive cells in striatum; few in lateral SN, not SNc (not located in dopamine neurons) (Shughrue, 2004). | ||
| Monkey | OVX; present in hippocampus and hypothalamus at a relatively high ERβ/ERα ratio (Register et al., 1998) | RT-PCR; widely distributed in males and females; exclusive subtype in frontal cortex, caudate nucleus and cerebellum; no sex differences (Pau et al., 1998). |
| Human | Hypothalamic region; 5 men and 5 women (20–39 years old); strong sub-regional sex differences in staining intensity and cellular location (nuclear, cytoplasmic, nerve terminals) (Kruijver et al., 2002) | Forebrain (three men, two women); abundant in amygdala and hypothalamus, lower in cerebral cortex and hippocampus; similar in monkey (two males) but differs in part from rat (Osterlund et al., 2000c). |
| Forebrain (seven men, two women) alternative ERα promoter expression in distinct forebrain populations; suggests multiple promoter usage may underlie differentiated regulation of expression (Osterlund et al., 2000a). | ||
| Dominates in amygdala, hypothalamus (Ostlund et al., 2003). | ||
| ERβ | ||
| Rat | Twelve days post-OVX brain; nuclear IR in neurons colocalizes with mRNA; includes the olfactory nuclei, laminae IV–VI of the cerebral cortex, medial septum, POA, BNST, SON, PVN, ZI, medial and cortical amygdaloid nuclei, cerebellum, NTS, VTA, and spinal trigeminal nucleus (Shughrue and Merchenthaler, 2001). | Twelve days post-OVX hypothalamus; dense expression in mPOA and BNST (similar to ERα mRNA), PVN and SON (vs. little/negligible ERα); weak in ARC, VMN (vs. abundant ERα) (Shughrue et al., 1996). |
| Gonad-intact, male and female cerebral cortex; wide neuronal distribution; distinct from ERα; no sex differences (Kritzer, 2002). | Twelve days post-OVX brain; exclusively in neurons of the olfactory bulb, SON, PVN, SCN, tuberal nuclei, ZI, VTA, cerebellum (Purkinje cells), laminae III–V, VIII, and IX of the spinal cord, and pineal gland. Also in perikarya in cerebral cortex and hippocampus, as well as other brain regions, including the BNST, medial and cortical amygdaloid nuclei, POA, lateral habenula, periaqueductal gray, parabrachial nucleus, LC, NTS, spinal trigeminal nucleus, superficial laminae of the spinal cord (Shughrue et al., 1997). | |
| Similar wide distribution in male and female rat brains, including cerebral cortex, LC (high); SN, amygdala (moderate); hypothalamic subnuclei (weak): sex differences in IR intensity in hippocampus (female dominant) and BNST, mPOA, LC (male dominant); also sex differences in intracellular (nuclear, cytoplasmic, terminal) distribution (Zhang et al., 2002). | Intact males and females; exclusive to SON and PVN magnocellular and autonomic subdivisions; also in olfactory cortex, hippocampus, amygdala, BNST, substantia inominata, POA, AVPV, ARC, SNc, NTS, cerebellum; no sex differences (Laflamme et al., 1998). | |
| VMN: females have significantly more IR cells than males at postnatal days 5–14; sex difference was not significant by P21; confirmed by ISH; remarkably higher expression levels in neonatal VMN compared with adult (Ikeda et al., 2003). | Sex differences in AVPV and mPOA from first week of birth to adulthood; confirmed by ICC (Orikasa et al., 2002). | |
| Gonad-intact-intact female brain: compared with young rats (10 weeks), numbers of ERβ mRNA-positive cells were reduced in the olfactory bulb, cerebral cortex, hippocampus, N.Acc, parts of the amygdala and raphe nuclei in middle-age (12 months), but did not decline further in aged animals (24 months); by contrast, numbers in hippocampus, striatum, claustrum, SN and cerebellum did not change by middle-age, but decreased in old rats: age-dependent changes are region specific (Yamaguchi-Shima and Yuri, 2007). | ||
| Mouse | Two weeks post-OVX; widely distributed throughout brain; primarily in cell nuclei in select regions of the brain, including the olfactory bulb, cerebral cortex, septum, POA, BNST, amygdala, PVN, thalamus, VTA, SN, dorsal raphe, LC, and cerebellum. Extranuclear-IR detected in several areas, including fibers of the olfactory bulb, areas CA3 and CA1 of the hippocampus, and the cerebellum. (Shughrue, 2004); CX males; concentration of positive cells in POA, BNST, PVN, amygdala; no positive cells in striatum or SN (Mitra et al., 2003). | CX males; widely expressed throughout brain; no positive cells in striatum or SN (Shughrue, 2004). |
| Monkey | OVX: present in hippocampus and hypothalamus at a relatively high ERβ/ERα ratio (Register et al., 1998). | RT-PCR: more widely distributed in female brains, including putamen, hippocampus and PVN, which lack mRNA in males (Pau et al., 1998). |
| Human | Five men, five women (20–39 years old); subregional sex differences in IR intensity and cellular location (nuclear, cytoplasmic, nerve terminals) (Kruijver et al., 2003). | Eight men and two women; most abundant in hippocampus, claustrum, and cerebral cortex; low in hypothalamus and amygdala (distinct from ERα) (Osterlund et al., 2000b). |
| Sex differences: 50-fold more IR neurons in the AVP-containing region of the dorsolateral SON in young women compared with men; no sex differences in ERα (Ishunina et al., 2000). | Dominates in hippocampal formation, entorhinal cortx, thalamus (Ostlund et al., 2003). | |
| GPR30 (proposed G protein-coupled receptor for estradiol) | ||
| Rat | Adult males and females; Island of Calleja, striatum (high density), PVN, SON, hippocampus, SN, brainstem autonomic nuclei (Brailoiu et al., 2007) | Adult males and females; PVN (particularly magnocellular region), SON (Hazell et al., 2009). |
| Mouse | Adult males and females; cortex, hypothalamus, hippocampus, pontine nuclei, LC, trigeminal nuclei and cerebellum; distinct from ERα and ERβ; no sex differences (Hazell et al., 2009). | Adult males and females; PVN (particularly magnocellular region), SON (Hazell et al., 2009). |
| No distinction between ERα or ERβ | ||
| Rat | Males and females; mRNA (ISH) widely distributed in hypothalamus and cortex; also in lateral septal nucleus, amygdala, hippocampus, BNST; no sex differences (Simerly et al., 1990). | |
| Gonad-intact male and female midbrain; ER-IR absent in SNc and present in subpopulations of VTA and retrorubral field; no sex differences (Kritzer, 1997). | ||
| Gonad-intact male and female hippocampal CA1 region; sex differences in estradiol responsiveness, but ER-IR levels in showed no sex differences (Weiland et al., 1997). | ||
| Quantitative in vitro autoradiography in developing rat hypothalamus; sex differences in some sub-regions are present around birth (mPOA), others emerge at 1–2 weeks (VMN), and persist into adulthood. Note well: Using ISH, sex differences in ER in mPOA disappear by postnatal day 10 (DonCarlos and Handa, 1994; Khünemann et al., 1994). | ||
| Mouse | ER transcriptional activity in the ERE-luciferase reporter mouse; no sex differences at diestrus; sex differences at proestrus (high estradiol) (Stell et al., 2008). | |
ARC, arcuate nucleus of the hypothalamus; AVPV, anteroventral paraventricular nucleus of the hypothalamus; BNST, bed nucleus of the stria terminalis; CX, castrated adult male; GPR30, proposed G protein-coupled receptor for estradiol; LC, locus ceruleus; NTS, nucleus tractus solitarius; OVX, ovariectomized adult female; PeN, periventricular nucleus of the hypothalamus; RT-PCR, reverse transcription-polymerase chain reaction; SCN, suprachiasmatic nucleus of the hypothalamus; SN, substantia nigra; SON, supraoptic nucleus of the hypothalamus; ZI, zona incerta.
Unlike the testes, activation of steroidogenesis in the ovaries is not detectable until later in development, not before 5 days after birth (Weniger et al., 1993) (Fig. 1B). In addition, α-fetoprotein sequesters circulating estrogens in early life, so systemic sources cannot access the brain (Bakker et al., 2006). Hence, male and female brains develop under the influence of very different hormonal environments; indeed, it is believed that without exposure to testosterone, animal and human brains develop along essentially female lines. Together, these forces ensure that there is a biological basis for sex differences in the brain. Once in adulthood, the sexually imprinted brain may then be further differentiated by the activational actions of gonadal steroids. Sex differences may therefore be engendered by the perinatal surge of testosterone, but they are not necessarily functionally manifest until puberty, when the relevant, preformed neurocircuitries are activated by the changing gonadal hormone environment. This is exemplified by our studies on the sexual differentiation of the hypothalamic somatostatin neuron populations in the periventricular nucleus, which control growth hormone release. From postnatal day 5, there is evidence for sex differences in the biosynthetic capacity and GABAergic regulation of these neurons, but this circuitry is not activated until the rise in pubertal hormones, when the sexually dimorphic profile of growth hormone secretion emerges to regulate not only growth patterns, which occur at a faster rate in male rats, but also sexually dimorphic expression of key liver enzymes and metabolic processes in adulthood (Simonian et al., 1998; Murray et al., 1999a,b,c).
The aromatization hypothesis of sexual differentiation of the brain is based on investigations in the hypothalamus. With increasing attention on the sexually dimorphic nature of other brain regions, it is now apparent that the critical period for hormonal influences on sex differentiation may extend later into development and may involve androgen- as well as estrogen-dependent mechanisms. In particular, pubertal hormones may exert organizational influences on structures such as the hippocampus and amygdala as well as hypothalamic regions, including the anteroventricular periventricular nucleus and sexually dimorphic nucleus of the preoptic area (POA), where sex differences in regional volumes and the addition of new cells have been identified in humans and animals used in research (Williams, 1986; Ahmed et al., 2008; Neufang et al., 2009). The full implications for the onset of many psychiatric disorders that display sex differences and emerge in adolescence, such as schizophrenia, depression, and anorexia/bulimia (Paus et al., 2008), remain to be determined, and implications for Parkinson's disease (PD) are discussed further in section V.B.2.
Although the basic processes of neural development are identical in rodents and humans, a significantly greater proportion of brain development occurs after birth in rats. The rat brain in late gestation is therefore believed to approximate the human fetal brain at mid-gestation, which also coincides with a peak in testosterone production in the male of both species (Kelly et al., 1999; Wilson and Davies, 2007) (Fig. 1C). Along with evidence from clinical conditions involving disturbances in hormonal activities during development, this supports the view that sex hormones play a significant role in masculinizing/defeminizing the brain in humans just as they do in other mammalian species (Gorski, 2002; Morris et al., 2004; Swaab, 2004). However, the extent to which this involves androgen- or estrogen-dependent mechanisms remains unclear.
2. Hormonal versus Genetic Influences.
For many years, the organizational and activational influences of gonadal hormones were thought to be the only biological factors that determine sexual differentiation of the brain and other tissues. Emerging evidence shows that genetic factors must also be incorporated into the equation, especially the influence of sex-specific genes on the sex chromosomes (De Vries et al., 2002; Arnold and Burgoyne, 2004; De Vries, 2005; Bocklandt and Vilain, 2007; Quinn et al., 2007). Sex chromosome effects may be due to a direct action of Y chromosome genes or differential expression of X chromosome genes arising either from gene dosage differences (i.e., not all genes on the second X chromosome in XX females are perfectly silenced) or sex differences in the genomic imprint of X chromosome genes (Federman, 2006; van Nas et al., 2009). Our understanding of these influences, and how they interact with gonadal hormone programming of sex dimorphisms, is in its infancy, so the focus of this review will remain on hormonal actions.
One could logically argue that, once formed, sex dimorphisms in the brain substrate signify differences in function. Although this is often true (as illustrated in section IV), recent evidence supports the emerging concept that certain sex dimorphisms may exist in an attempt to preserve critical brain functions that have an evolutionary advantage (De Vries, 2004; Cahill, 2006). For example, sex differences in the patterns of brain activity during tests of memory have been identified when there were no differences in performance of the memory task in men and women (Shaywitz et al., 1995; Grabowski et al., 2003; Piefke et al., 2005). This may not be so unexpected if one considers that in both sexes, the brain strives to achieve equally optimal performance in cognitive functions, but this has to be attained in very different hormonal and genetic environments, both during development and in adulthood, that have to exist to ensure procreation and survival of the species. The underlying sex dimorphisms in nonreproductive functions may thus enable the individual to achieve the same goal but by different mechanisms in male and female brains (De Vries and Boyle, 1998).
C. Impact of Sex Dimorphisms on Our Understanding of Brain Disorders: a Role for Estrogens
Whether the ultimate endpoint is to achieve functional differences (for reproductive success) or similarities (such as cognition), it is clear that male/female brains operate under very different constraints that may manifest at genetic, molecular, cellular, and systems levels, as discussed throughout this review. Although these biological sex differences are clearly important from a physiological point of view to maintain homeostasis, if the system is challenged by external factors, such as stress and disease, different organizations in circuitries in male and female brains will respond differently to environmental challenges (endogenous or exogenous) and emerge as different vulnerabilities to behavioral and neurological disorders. Conditions that differ markedly in their prevalence, progression, and/or severity between the sexes include PD, attention deficit/hyperactivity disorder, and schizophrenia, all of which show a greater prevalence in men and involve the midbrain dopaminergic systems (Swaab, 2004; Gillies and McArthur, 2010). In contrast, in Alzheimer's disease (AD), involving cognitive brain regions such as the hippocampus, postmenopausal women fare worse than men (Swaab, 2004). Without doubt, estrogen has been pinpointed as a critical protective factor in females that gives them the advantage in diseases prevalent in men, whereas its rapid decline after menopause may forfeit this advantage. Although there is hot debate in the literature as to why the protective effects of estrogen therapy for postmenopausal women have not yet been realized clinically (Mandavilli, 2006; Toran-Allerand, 2006; Brann et al., 2007), in theory, estrogen holds great clinical potential for CNS disorders because of its proven neuroprotective and neuroactivating properties (McEwen and Alves, 1999; Wise et al., 2001; Brann et al., 2007; Garcia-Segura, 2008). However, as discussed in detail in the remainder of this review, there is mounting evidence that estrogen may have opposite effects in male and female brains which we propose is due principally to differences in brain organization. Despite these striking, significant findings, studies that make direct comparisons of estrogenic actions in male and female brains are relatively small in number because the vast majority of studies focus solely on females, despite the fact that the diseases that they are modeling may predominate in males. Therefore, in this review, we aim to use these examples of sex differences in the actions of estrogen in the brain to highlight the importance of understanding sex differences in brain organization, which is critical if we are to develop optimal therapies for the many common brain disorders that differentially affect men and women.
III. Estrogen Synthesis and Signaling Mechanisms
A. Peripheral and Central Sites of Synthesis of Estrogens in Males and Females
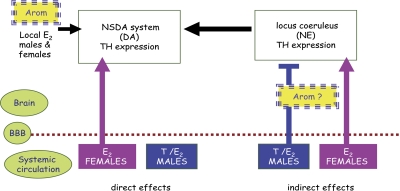
In all mammalian species, the gonads and adrenal glands synthesize and release estrogens into the general circulation in both sexes. This accounts for relatively low circulating levels, except in females in the phase lasting from the end of puberty to the beginning of reproductive senescence, during which time the ovaries synthesize and release much greater amounts of estrogens in a cyclical fashion, which maintains ovulation and reproductive capacity. In nonpregnant females, the principal and most potent circulating estrogen is 17β-estradiol; estrone and estriol are present at lower concentrations. The gonads and adrenal glands also synthesize and secrete androgens in both sexes, but the much greater levels of circulating testosterone produced by the mature testes generates and maintains the sexual phenotype in males, just as estrogens do in females. However, mounting evidence points to the importance of estrogens as the active factors in mediating many of the effects of testosterone in target tissues in males, where aromatase enzymes, encoded by the CYP19 gene, are responsible for the local synthesis of estrogens from circulating androgens (Sharpe, 1998; Jones et al., 2006). Circulating testosterone therefore acts as a precursor for estrogens, which then act in a paracrine fashion in a large number of tissues expressing aromatase in the periphery and the brain. It is not surprising, therefore, that in the small number of clinical cases that have been identified with inactivating mutations in the CYP19 gene, several physiological disturbances have been identified in men, including skeletal, metabolic, and reproductive impairments (Sharpe, 1998; Rochira et al., 2002; Jones et al., 2006). Studies in aromatase knockout (ArKO) mice generally recapitulate these sequelae in peripheral physiology and, in addition, reveal important functions of estrogens in both male and female brains, thereby highlighting the ubiquitous distribution and function of aromatase enzymes in peripheral and central tissues (Lauber et al., 1997; Simpson et al., 2002; Roselli et al., 2009).
In the adult brain, the highest levels of aromatase activity are found in the hypothalamus of all species studied, especially the POA and ventromedial nucleus (VMN), where the enzyme is regulated by gonadal steroids and found at higher levels in males than in females (Roselli et al., 2009). In rodents, this reflects the fact that the sex-specific reproductive behaviors governed by these nuclei are activated by estradiol in males (where circulating testosterone up-regulates aromatase and, hence, its own metabolism to estradiol) as well as in females. Significant levels of aromatase are also found in other brain regions, including the amygdala, hippocampus, midbrain, and cortical regions in rodents, nonhuman primates, and humans, where its expression is steroid-independent and not significantly different in males and females (MacLusky et al., 1994; Abdelgadir et al., 1997; Sasano et al., 1998; Stoffel-Wagner et al., 1999; Hojo et al., 2004; Yague et al., 2008; Roselli et al., 2009). Although this provides neuroanatomical evidence in support of a role for estrogens in regulating nonreproductive behaviors, it suggests that any sex differences are not likely to be dependent on differences in local aromatase activity.
Bilateral gonadectomy with or without hormone replacement is clearly an important experimental approach for manipulating circulating hormone levels to investigate the effects estrogen or its potential precursor, testosterone, in various tissues. Studies with ER-null mice also provide valuable insights into the roles of estrogens in males as well as females (Ogawa et al., 1997, 1998, 1999; Wang et al., 2003; Weiser et al., 2008). However, it is now known that in addition to aromatase, the brain possesses the full complement of enzymes required for the de novo synthesis of steroids from cholesterol and not just from gonadal and adrenal precursors present in the circulation (Garcia-Ovejero et al., 2005; McCarthy and Konkle, 2005; Rune and Frotscher, 2005; Balthazart and Ball, 2006; Garcia-Segura, 2008). This adds a level of complexity to interpreting the role of systemic steroids in CNS function. As discussed further below, we also know that central actions of estrogens may occur via ER-independent mechanisms, which would persist in ER-null mice. Therefore, because the ArKO mice lack the classic ability to synthesize both peripheral and central estrogens, they have provided some novel insights into the CNS roles of estrogens. These include the intriguing observations that in the absence of estrogen synthesis, apoptosis of dopaminergic neurons occurs spontaneously in the adult male, not female, hypothalamus, whereas apoptosis of pyramidal neurons in the frontal cortex occurs spontaneously in the adult female but not male brain (Hill et al., 2004, 2009). This highlights a notable sex dimorphism in the requirement and/or ability of estrogen to maintain specific neuronal populations in different brain regions. Although the underlying mechanisms and the functional consequences of these morphological changes are unknown, sex- and age-specific behavioral deficits have been identified in ArKO mice (van den Buuse et al., 2003; Hill et al., 2007) and support the concept that estrogens play a sexually dimorphic role in the CNS.
B. Nuclear and Extranuclear Mechanisms for Genomic and Rapid, Nongenomic Mechanisms of Estrogen Signaling in the Brain
The last decade or so has seen very rapid advances in our understanding of the mechanisms of action of estrogen in the brain, as evidenced by many excellent reviews (McEwen and Alves, 1999; Toran-Allerand et al., 1999; Green and Simpkins, 2000; Lee and McEwen, 2001; McEwen, 2001; Wise et al., 2001; Maggi et al., 2004; Brann et al., 2007; Raz et al., 2008; Micevych and Dominguez, 2009; Tetel, 2009). Here we summarize some basic background information on cellular signaling mechanisms and ER expression patterns where these may have bearing on sex differences in response to estrogen, which will be highlighted in later sections.
1. Classic Estrogen Receptors.
Classic ERs are located in the nucleus and cytoplasm of the cell and belong to the nuclear receptor superfamily, members of which act as nuclear ligand-gated transcription factors, binding to estrogen response elements (EREs) within specific genes to alter their rate of transcription (Mangelsdorf et al., 1995). The two known isoforms, ERα and ERβ (also termed NR3A1 and NR3A2, where NR3 has been adopted as nomenclature for steroid receptors) are coded by separate genes and are located throughout the brain, but have a differential distribution (Table 1). ERα mRNA is widely distributed in many brain regions, including the hippocampus, hypothalamus, amygdala, and brainstem nuclei, and colocalizes with ERβ mRNA in many regions. ERβ has a more restricted distribution and is found in particular abundance in human, nonhuman primate, and rodent hippocampus and selected hypothalamic nuclei, especially the supraoptic and paraventricular nuclei (PVN) (Shughrue et al., 1997, 1998; Register et al., 1998; Gundlah et al., 2000; Osterlund et al., 2000a,b; Mitra et al., 2003; Ostlund et al., 2003; Merchenthaler et al., 2004; Suzuki and Handa, 2005; González et al., 2007; Weiser et al., 2008). The two forms of ER are structurally and functionally distinct, each regulating unique sets of target genes in a tissue- and cell type-specific manner (Kian Tee et al., 2004). This may be the net effect of homo- or heterodimerization of ERα and ERβ. Steroid receptor-mediated transcription is also modulated by coregulators (activator and repressor proteins and protein complexes). There are vast numbers of these coregulator proteins, and various selective combinations associate with ERs and critically determine the region and cell-type specificity of the effects of ER ligands, as well as potential interactions of ER with other nuclear receptors, such as those for progesterone (PR), testosterone, androgen receptors (AR), and glucocorticoids (Tetel, 2009). The recent discovery in rodent and human brains of ER splice variant proteins, which alter gene transcription in a promoter- and ligand-dependent fashion, adds further diversity to ER signaling mechanisms (Chung et al., 2007; Ishunina and Swaab, 2008). Moreover, work with the ERβ2 splice variant, which is expressed in a region- and cell-specific manner, provides evidence for ligand-independent interactions with ERE, suggesting that it may be a constitutive activator of transcription (Weiser et al., 2008). As well as acting directly through EREs, ligand-activated classic ERs can also modulate gene transcription indirectly at alternative response elements by influencing the activity of other transcription factors. Specifically, estradiol can activate transcription via the activated protein-1 response element in the presence of ERα but fails to do so when liganded with ERβ (Paech et al., 1997; Kushner et al., 2000). The expression, coexpression, and ratio of ERα/ERβ and their splice variants, as well as the presence of any given combination of coregulatory proteins in any given cell, will therefore greatly influence the estrogen response.
2. Membrane Signaling.
In addition to classic genomic actions, it is now recognized that estrogens can initiate rapid signaling via actions at the cell membrane in many brain regions. Because there is no clear consensus on the molecular identity of the membrane receptors, it is not possible to define their expression patterns in the brain. However, pharmacological and emerging ultrastructural evidence demonstrates that classic “nuclear” ERα and ERβ, and probably other novel receptors (such as GPR30; Table 1), can also be localized at the cell membrane to effect rapid activation of intracellular brain signaling pathways and modulatory proteins within seconds to minutes of exposure to steroid (McEwen and Alves, 1999; Toran-Allerand et al., 2002; Gorosito et al., 2008; Kawata et al., 2008; Prossnitz et al., 2008; Raz et al., 2008; Vasudevan and Pfaff, 2008; Dennis et al., 2009; Mermelstein, 2009; Micevych and Dominguez, 2009). These include effects on calcium channels and intracellular stores to increase intracellular [Ca2+], which may lead to activation of calcium-calmodulin-dependent kinases, and activation of other protein kinases in 1) the cAMP/cAMP-dependent protein kinase pathway, 2) the mitogen-activated protein kinase (MAPK or extracellular signal-regulated kinases, ERK) pathway (also named MEK), and 3) the phosphoinositide 3-kinase (PI3K)/Akt (also termed PKB) pathway. In parallel or in series, these pathways may interact and converge, finally to affect gene transcription and protein synthesis via the rapid downstream activation of transcription factors, such as the cAMP response element binding protein (CREB) or nuclear factor κB (Boulware et al., 2005; Vasudevan and Pfaff, 2008; Mermelstein, 2009). Thus, although referred to as nongenomic mechanisms to distinguish them from the classic mode of action, it is now understood that actions initiated at the plasma membrane may also ultimately affect gene transcription.
The mechanisms by which activated membrane ERs elicit cellular responses are not yet understood, but interactions with other cell-surface receptors and their associated molecules, such as G-proteins, insulin-like growth factor 1, and metabotropic glutamate receptors (which are linked to G-proteins) have emerged as means by which membrane ERs can trigger intracellular second-messenger signaling systems and affect cellular responses (Garcia-Segura et al., 2001; Wyckoff et al., 2001; Mermelstein, 2009). Estrogen-activated signaling pathways can also increase mitochondrial efficiency and lead to a reduction in free radical generation in the brain and mitochondrial-dependent apoptosis (Nilsen et al., 2007; Brinton, 2008; Chen et al., 2009). Furthermore, membrane-initiated and genomic actions of hormones may be coupled, so the distinctions are not as clear-cut as was first thought (Vasudevan and Pfaff, 2008). It is noteworthy that most of the cellular mechanisms described for estrogen actions, especially MEK/ERK and PI3K/Akt signaling and mitochondrial function, have important roles in cell survival, apoptosis, function, and neurodevelopment and may subserve the critical neuroregulatory, neurotrophic, and neuroprotective effects of estrogens in brain physiology and pathological conditions of the brain. There is, however, no simple rule to predict which mode of estrogenic action will prevail and whether estrogens will exert positive/enhancing or negative/suppressing influences on any given signaling pathway because, notoriously, these vary between neural phenotype and brain region.
3. Potential for Brain-Selective Estrogen Receptor-Modifying Compounds.
The foregoing discussion illustrates the exceptional diversity and complexity of the mechanisms that mediate estrogenic signaling in the brain. Although estradiol has the capability to activate all these pathways, and ERα and ERβ have very similar ligand-binding domains and relative binding affinities for estradiol, other ligands have very different relative binding affinities and relative potencies in transcriptional assays (Patchev et al., 2008; Weiser et al., 2008). Likewise, the particular signaling pathway involved in any given cell type also seems to dictate the nature of response to ER ligands. For example, transfection studies indicate that tamoxifen is an antagonist of ER-mediated transcriptional activation when this occurs via ERE-dependent mechanisms, but when ER interacts with the activated protein-1 pathway, tamoxifen is an effective agonist (Kushner et al., 2000). Selective estrogen receptor-modifying compounds (SERMs), such as tamoxifen, can also activate nongenomic ER-mediated signaling pathways (Wessler et al., 2006). These observations are highly pertinent for the well known tissue-selective agonist/antagonist actions of tamoxifen in peripheral tissues. Its antiestrogenic and antiproliferative actions in breast tissue are of great clinical benefit in the treatment of breast cancer; likewise, its positive estrogenic effects in bone and the cardiovascular system in postmenopausal women are beneficial, whereas its proliferative estrogenic actions in the uterus are unwanted. Other SERMs, such as raloxifene, retain beneficial estrogenic activity in bone and lack unwanted proliferative actions on the uterus, but seem to have antiestrogenic cardiovascular actions (Cheskis et al., 2007). There are considerable precedents to fuel efforts to develop SERMs with selectivity for the brain, and not peripheral targets, which could eliminate unwanted peripheral actions of estrogens, including their feminizing actions, thereby making them accessible for men as well as women. However, how SERMs can be estrogenic in some cells and antiestrogenic in others is not clearly understood (Cheskis et al., 2007; DonCarlos et al., 2009). This will require far greater knowledge about how estrogens signal in specific pathways in the brain, and a recently proposed set of criteria for demonstrating a dissociation of CNS and systemic effects of ER ligands should aid in this goal (Patchev et al., 2008).
4. Sex Differences.
The overall distribution patterns of ERα and ERβ in the brain provide some broad neuroanatomical clues for their involvement in specific brain functions (Table 1), which may be supported by functional studies. For example, studies with ER-null mice have indicated that ERα, not ERβ, is vital for neuroendocrine reproductive function (Ogawa et al., 1998), although ERβ does have important roles in reproduction (Kudwa et al., 2006; Antal et al., 2008). On the other hand, CNS actions of ERβ are gaining interest for the improvement of mood and affect (Weiser et al., 2008; Solomon and Herman, 2009). However, an often-ignored variable is sex. Although ERα and ERβ have a similar distribution in male and female brains, there are numerous reports of sex differences in their relative expression levels in various regions (see Table 1, and sections IV–VI). It is important to note, however, that some studies used gonadectomized animals, and in many brain regions, ER expression is regulated by gonadal steroids. Although sex differences in overall mRNA or protein levels may not be apparent in some brain regions, it is also noteworthy that more subtle analyses can reveal sex differences (Table 1). For example, subcellular distributions of ER to the nucleus, cytoplasm, dendrites, and nerve terminals have been reported to be different in male and female human hypothalami (Kruijver et al., 2002). Although the functional consequences of this remain to be determined, this could indicate differential effects on processes such as neurite extension, synaptic plasticity, and mitochondrial energy regulation via mitochondrial ERs (Romeo et al., 2004; Chen et al., 2009). In addition, the distribution of ER-positive neurons (although not their overall numbers) forming the mesocortical system, which probably reflects their functional connectivity, was found to be sexually dimorphic (Kritzer and Creutz, 2008). As will be discussed in the relevant sections later, there is also evidence for sex differences in the mechanisms of intracellular signaling (Abrahám and Herbison, 2005; Swamydas et al., 2009), in the expression of coregulatory proteins (Bousios et al., 2001), and in the response of the brain ER/aromatase system to injury (Westberry et al., 2008). Together, such sex differences would theoretically have considerable influence on response to estrogens, although this remains to be investigated thoroughly.
Sex differences in ER signaling and expression, as well as aromatase expression, in the brain may also be age-dependent, different roles being played out during development, adulthood, and aging (Lauber et al., 1997; González et al., 2007; McCarthy, 2008). It is also important to consider whether estrogen signaling mechanisms are the same or different in healthy and damaged brains. In this respect, it is noteworthy that aromatase and ER expression may be induced within glial cells, but not neurons, at sites of injury in the adult CNS, whereas in the healthy brain, constitutive aromatase expression has been reported to be primarily neuronal (Garcia-Ovejero et al., 2005; Garcia-Segura, 2008). Much of our knowledge of estrogen signaling comes from studies involving cell lines, and evidence is building to corroborate these mechanisms in vivo. However, appreciation that mechanisms could be different in male and female brains requires whole-animal studies, which will be invaluable for more effective targeting of potential novel therapies.
IV. Lessons from the Hypothalamus
Sex dimorphisms were first noted in the hypothalamus, where a subregion of the rodent brain, appropriately named the sexually dimorphic nucleus, was found to be 3 to 7 times larger in males than in females (Arnold and Breedlove, 1985); an analogous region of the human brain was subsequently identified (Swaab et al., 2003). It was soon recognized that an overt difference in nuclear size was not the only salient feature to differ in male and female brains; subsequently, many more subtle dimorphisms in neuronal phenotype, fiber density, neurochemistry, and cytoarchitecture have been discovered (Kelly et al., 1999; Cahill, 2006; McArthur et al., 2006, 2007a; Cosgrove et al., 2007; Wilson and Davies, 2007). Most importantly, sexually dimorphic responses to estrogen, which are a key focus of this review, were first characterized in the hypothalamic circuitry regulating reproductive hormonal and behavior patterns. Interest in this phenomenon remains as active as when it began 3 decades ago (Raisman and Field, 1971; MacLusky and Naftolin, 1981; Naftolin et al., 2007) and continues to provide important insights into brain structure-function relationships as well as clear examples in which specific structural and functional sex differences in the brain can be linked with sexually dimorphic behaviors. Therefore, we shall first use the hypothalamus as the prototype to illustrate these dimorphisms and, where possible, consider the findings of experimental studies in context with what we know about the human hypothalamus. Subsequent sections will be devoted to male/female differences in specific neurological or psychiatric diseases and/or brain regions primarily associated with their underlying pathology and will consider the applicability of what we understand about the nature and origins of hypothalamic sex dimorphisms to the rest of the brain.
As noted in section II.B, sexual differentiation of the developing hypothalamus by testosterone (aromatized to estradiol) involves both masculinization and defeminization. These are thought to be separate processes involving distinct neuronal populations, although their precise identity remains elusive (Kudwa et al., 2006; McCarthy, 2008). It has been proposed that ERα may be predominantly responsible for masculinization and ERβ for defeminization (Kudwa et al., 2006). Masculinization has been defined as the organization of a neural substrate permissive to the expression of male sexual behavior, which is manifest in rats as mounting, thrusting/intromission, and ejaculation in the presence of a female. Defeminization involves the loss of capacity as an adult to display female sexual behavior, namely lordosis in rats, a stereotypic posture that signals receptivity to males (Pfaff and Schwartz-Giblin, 1988). Because exposure to estradiol, followed by progesterone, is essential for priming lordosis in female rats, defeminization has also been defined as the loss of capacity to respond to the activational effects of estradiol and progesterone to induce female sexual behavior (Schwarz and McCarthy, 2008). Sex differences in the ability of underlying circuitry to respond to estrogens are therefore fundamental to sexual differentiation of the brain. These definitions are clearly predicated on functions that are controlled primarily by the hypothalamus, which serves well to illustrate sex dimorphisms in the response to estradiol. Subsequent sections of this review will discuss how the concept that sexual differentiation by the perinatal hormone environment relates to brain regions outside the hypothalamus, although distinctions between masculinization and defeminization have not yet been made.
A. Estradiol Activates Specific Hypothalamic Circuitry in Female Species but Not Males
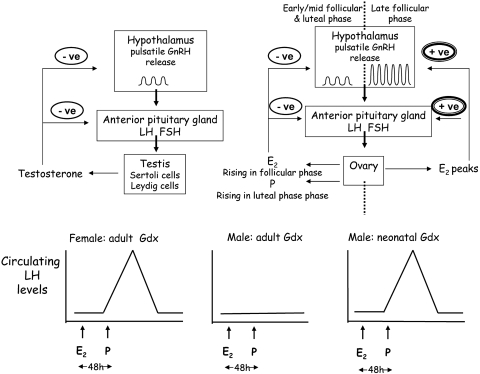
Robust functional sex differences have been identified in hypothalamic circuitry regulating reproductive function. Of particular biological importance are the neural mechanisms controlling ovulation, which exhibit unique sensitivity to estradiol. The key trigger to ovulation in a host of mammalian species, from rodents and sheep to nonhuman primates and humans, is the mid-cycle surge in luteinizing hormone (LH), a gonadotropin. The release of LH from the anterior pituitary gland (Fig. 2), in turn, is regulated by neurons scattered throughout the hypothalamus that produce gonadotropin-releasing hormone (GnRH) (Herbison, 1998; Kelly et al., 1999; Naftolin et al., 2007; Wilson and Davies, 2007). For approximately 90% of the time, circulating estradiol exerts a negative feedback on the GnRH neurons in the female hypothalamus, but this converts briefly and dramatically to a positive feedback before ovulation, leading to a massive, coordinated release of GnRH, then LH (Herbison, 1998; Naftolin et al., 2007) (Fig. 2B). An important experimental paradigm used to investigate this phenomenon involves the priming of ovariectomized female rats with an injection of estradiol followed by progesterone to mimic hormonal patterns over the first half (follicular phase) of the estrous cycle to trigger the GnRH/LH surge (Kelly et al., 1999; Wilson and Davies, 2007) (Fig. 2C). In male rats, the distribution and numbers of hypothalamic GnRH neurons are similar to those seen in females, but the pattern of LH release in males is tonic, or acyclical, leading to a steady rate of release of testosterone, which, in turn, always exerts a negative feedback on GnRH release. However, if exposed to same hormone environment as females (that is, by gonadectomy in adulthood), followed by the same priming regime with estradiol that triggers LH release in females, males fail to exhibit positive feedback and an LH surge (Fig. 2D). Alternatively, the female pattern of an LH surge can be induced in adult males by estrogen priming if gonadectomy is performed immediately after birth (Fig. 2E). Likewise, estrogen fails to elicit an LH surge in females masculinized at birth by brief exposure to exogenous testosterone/estradiol. This, along with a large body of other experimental evidence, has led to the well accepted view that the circuitry within the GnRH network is hard-wired differently in males and females, primarily as a result of the sculpting of male hypothalamic circuitry in the neonatal period by testosterone acting in the brain via ERs after aromatization to estrogen (Herbison, 1998; Kelly et al., 1999; Wilson and Davies, 2007). This explains why the GnRH/LH response to estrogen is sexually dimorphic in adulthood.
Fig. 2.
Adult sexually dimorphic circuitry is imprinted by neonatal hormone action. In the adult male HPG axis (A), GnRH is released from hypothalamic neurons in a pulsatile manner to stimulate the release of LH and follicle-stimulating hormone (FSH), which in turn stimulate testosterone (T) production and spermatogenesis. T exerts a negative feedback (-ve) at hypothalamic and pituitary levels to maintain a steady state in the hypothalamo-pituitary-gonadal axis. In females (B), estradiol (E2) and progesterone (P) produced by the ovaries also exert a negative feedback in the early follicular phase and luteal phase, respectively, of the menstrual cycle, but in the late follicular phase, as E2 levels peak, this converts to a positive feedback (+ve), which augments GnRH release and triggers an LH surge and ovulation at mid-cycle. In gonadectomized female rats (C), activation of the LH surge can be induced experimentally by the injection of E2 followed 48 h later by P. In male rats gonadectomized as adults (D), the hypothalamic circuitry, and hence the LH surge, fails to respond to hormonal priming, whereas the LH surge can be induced in adult male rats if they were gonadectomized as newborns (E). These and related studies demonstrate that early exposure to T, after its aromatization to E2, suppresses the circuitry responsible for the positive feedback of E2 on GnRH release.
Apart from the mid-cycle period discussed above, estradiol suppresses GnRH gene expression in females; in males, estrogen-dependent mechanisms also mediate testosterone negative feedback on GnRH expression (Naftolin et al., 2007). However, the mechanism of transcriptional control is different in males and females (Thanky et al., 2003). This further indicates the subtlety of the sexually dimorphic mechanisms by which estradiol can regulate gene expression in the brain.
B. Estradiol Has Sexually Dimorphic Influences on Synaptic Remodeling and Behaviors
Investigations into the mechanisms by which estradiol can trigger a GnRH/LH surge in female but not male rodents have revealed sexually dimorphic effects on synaptic and glial plasticity.
1. Arcuate Nucleus.
The estrogen positive feedback mechanism seen in females but not in males is likely to use an indirect pathway involving ERα-positive neurons projecting to ERα-immunonegative GnRH neurons (Herbison, 2008). A strong contender within this indirect pathway is the GABAergic interneuron population in the arcuate nucleus, a region of the hypothalamus known to have an important role in generating the LH surge (Herbison, 1998; McCarthy and Konkle, 2005; Parducz et al., 2006; Naftolin et al., 2007). High physiological levels of estradiol markedly reduced GABAergic axosomatic synapses (inhibitory inputs), whereas the density of dendritic spine synapses (the major site of excitatory glutamatergic inputs) is greatly increased, along with the frequency of neuronal firing (Parducz et al., 2002, 2006; Csakvari et al., 2007, 2008; Naftolin et al., 2007). This estrogen-induced disinhibition and activation of GnRH neurons contributes to the synchronized burst of GnRH release. In contrast, these responses to estradiol are not seen in adult gonadectomized males because of hormonal programming during development (Horvath et al., 1997; Parducz et al., 2006; Csakvari et al., 2008). These observations illustrate the concept that organization of the brain leads to sexually dimorphic responses to estrogen in adult neurotransmitter systems.
Glial cells have emerged as important players in the phenomenon of mid-cycle disinhibition of GnRH neurons. In female rats, the shape and hence the surface area covered by astrocytic processes varies across the estrous cycle in an estrogen-dependent manner, resulting in greater ensheathment of arcuate neurons at mid-cycle (McCarthy et al., 2002). This is thought to be instrumental in causing the loss of inhibitory synapses at times when estrogen levels are high, thereby triggering the GnRH/LH surge. In the male arcuate nucleus, and other hypothalamic regions involved in the control of reproduction, there are consistently more stellate-shaped astrocytes with longer processes and a greater degree of branching compared with females. This greater complexity of astrocyte morphology does not change with manipulations of circulating levels of androgens or estrogens in adults (McCarthy, 2008), but it is programmed as early as postnatal day 3 by testosterone after conversion to estradiol and correlates with a permanent 2-fold reduction in the number of dendritic spines, as well as fewer axospinous synapses, in the male arcuate nucleus compared with females (McCarthy et al., 2002). This early, permanent elimination of synapses in males therefore imprints a sexually dimorphic neuroarchitecture and seems to limit arcuate glial plasticity and the ability to respond to estradiol in adulthood. Consequently, the ability of estradiol to reduce GABAergic inhibitory tone on GnRH neurons in males is suppressed (Csakvari et al., 2007, 2008). This work is just beginning to reveal fascinating mechanisms that are likely to contribute region- and sex-specific synaptic patterning in many other brain regions (McCarthy, 2008).
2. Preoptic Area.
The POA, especially the medial POA (mPOA), is the major site for regulating male sexual behaviors (Meisel and Sachs, 1994). This region has 2 to 3 times more dendritic spine synapses in male rats compared with females, indicating sex differences in the excitatory input. This sex difference is imprinted by estradiol in the developing brain (Amateau and McCarthy, 2004). Moreover, estradiol produced by aromatization of circulating testosterone seems to be the main activator of certain aspects of male sexual behavior. Because estradiol is also the principal activator of lordosis in female rats, it is evident that that the same hormone elicits very dissimilar behaviors in normal adult males and females (Clancy et al., 1995), providing further evidence that sex-specific circuitry underpins sexually dimorphic responses to estradiol. On the other hand, estradiol treatment of newborn female rat pups can masculinize the pattern of dendritic spines and enable the adult female to express male sexual behavior in response to adult hormonal treatment (Amateau and McCarthy, 2004). Together, these observations clearly link structural and behavioral sex dimorphisms in response to estradiol.
3. Ventromedial Nucleus.
The ventrolateral subdivision of the VMN (vlVMN) plays a central role in regulating female sexual behavior (i.e., lordosis) (Pfaff and Schwartz-Giblin, 1988), as opposed to the dorsomedial subdivision, which is implicated in energy homeostasis (also linked to reproduction). In adult ovariectomized female rats, the estradiol priming regime induces expression of PRs in key regions that target the VMN, increases spine and synaptic density in the VMN, and induces lordosis in response to a male (Lewis et al., 1995; Kelly et al., 1999; Schwarz and McCarthy, 2008), largely via ERα-mediated effects (Musatov et al., 2006). Conversely, in adult gonadectomized males, the female hormone regimen fails to induce PR, inhibits spine and synaptic density, and cannot induce lordosis. These sex dimorphisms in response to estradiol are also due to the programming effects of testosterone (aromatized to estradiol) in the perinatal period (Lewis et al., 1995; Kelly et al., 1999; Schwarz and McCarthy, 2008) and further illustrate the concept that sex-specific organization of the brain leads to sex dimorphisms in dynamic structural and behavioral responses to estrogen in adulthood.
C. Mechanisms Underlying Sexually Dimorphic Actions of Estradiol in the Adult Hypothalamus
1. Estrogen Receptor Expression.
In addition to morphological and functional differences (discussed in sections IV.A and IV.B), sex dimorphisms in estrogenic signaling pathways may underlie male/female differences in the response to estradiol. Sex differences in expression levels of the classic ERs will clearly affect cellular responses (see section III). For example, an abundance of ERα and ERβ expression in neuronal afferents to GnRH neurons were found in the female anteroventral PVN relative to the same male brain region, and is associated with generation of the GnRH surge and ovulation (Orikasa et al., 2002; Herbison, 2008). However, the interactions between ERs are likely to be complex, and the balance of ERα and ERβ can profoundly alter the response to estradiol. This has been best studied in the mPOA, where there is a down-regulation of expression levels of ERα and an up-regulation of ERβ in male rodents relative to females from the critical neonatal period onward into adulthood (Kudwa et al., 2006). In addition, several studies suggest that ERβ normally decreases the effectiveness of ERα in peripheral and central tissues, supporting a “yin/yang relationship” between the ERs (Weihua et al., 2000; Lindberg et al., 2003; Kudwa et al., 2006). It is noteworthy that certain typically sexually dimorphic responses to estradiol treatment that are evident in gonadectomized mice, including the up-regulation of PR in the female (not male) mPOA, and the down-regulation of ERα in the male (not female) mPOA, are lost in ERβ knockout (KO) mice (Kudwa et al., 2006). This work indicates that ERβ may be involved in sexual differentiation; it also suggests that ERβ may act differently in the male and female hypothalamus and may therefore be a key factor for sexually differentiated responses to estradiol (Kudwa et al., 2006). ERα also has an important role to play, in that the ERα gene has opposite effects on aggressive behavior in male and female rats, suggesting that it, too, may act differently in males and females (Ogawa et al., 1997, 1998; Rissman et al., 1997; Wersinger et al., 1997).
The findings described above were gained largely from studies using mice lacking one or both ERs. In view of the evidence for a role for ERβ in the brain, it may at first seem surprising that male ERβ KO mice are reported to have normal fertility and sexual behavior (Krege et al., 1998; Ogawa et al., 1999). However, female ERβ KO mice are subfertile (Krege et al., 1998; Ogawa et al., 1999), and closer investigations are beginning to reveal more subtle, but nonetheless important, differences in ERβ KO mice, including altered developmental profiles for certain behaviors (aggression) and an influence of ERβ on the timing of puberty (Ogawa et al., 1999; Kudwa et al., 2006). Inevitably, the caveats of using KO strains, such as compensatory responses to the lifelong absence of a gene and genetic backgrounds, will apply to these studies. Furthermore, four independently generated mutants with null mutations in the ERβ gene do show some wide variations in phenotype. In particular, both males and females of the most recently generated mutant strain were reported to be sterile (Antal et al., 2008), and they also lacked the considerable cytoarchitectural disorganization in the somatosensory cortex reported for another ERβ KO strain (Wang et al., 2001, 2003).
2. Intracellular Signaling.
Recent data suggest that sexually differentiated intracellular signaling pathways may represent a further mechanism underlying sex-specific responses to estradiol in the brain. In the mPOA and the VMN, the numbers of cells expressing phosphorylated (activated) CREB were significantly increased within minutes of treating gonadectomized female mice with estradiol, but this effect was not seen in males (Abrahám and Herbison, 2005). These rapid membrane-signaling effects are mediated by classic ERs (Abrahám et al., 2003); therefore, sex differences in ERα expression seen in the mPOA could contribute to this effect (Herbison and Theodosis, 1992). However, ER expression levels seem to be similar in the male and female VMN, so sexually dimorphic responses to estradiol may lie downstream in the signaling pathway. Because phosphorylation of CREB is a necessary step in the estrogen-dependent generation of new dendritic spines (at least in cultured hippocampal neurons) (Murphy and Segal, 1997), sex differences in this signaling mechanism may account for sex-specific effects of estradiol on spine density in the VMN (see section IV.B.3). Sex differences in estradiol's effect on CREB phosphorylation have also been reported for a specific neuronal phenotype, namely the hypothalamic GnRH neurons, which express only ERβ (not ERα) at similar levels in both sexes(Abrahám and Herbison, 2005). It remains to be determined whether a sex difference in rapid ER signaling is due to direct effects of estradiol on GnRH neurons or to indirect effects on estrogen-sensitive inputs to the GnRH neurons. It is noteworthy that the numbers of cells expressing phosphorylated CREB in the mPOA, VMN, and GnRH neurons are higher in male rodents compared with females, and this may be traced to perinatal exposure to raised endogenous estradiol levels in males (Auger et al., 2001; Abrahám and Herbison, 2005).
3. Estradiol and Gene Expression.
There are literally thousands of reports in the literature documenting that estradiol alters the expression of a multitude of genes in the brain, but the question of which genes are responsible for the sexually dimorphic effects of the hormone on CNS physiology and behavior is only beginning to be answered. Until recently, investigations have focused invariably on single genes. In the hypothalamus, for example, the expression in the VMN of GAP-43 gene, encoding a protein important for neurite outgrowth, is regulated in a sexually dimorphic manner by estradiol, thereby linking it to sex dimorphisms in synaptic patterning and behaviors controlled by this nucleus (Lustig et al., 1991). Viewed from another perspective, sexual dimorphism in gene expression occurs on a large scale and is widespread not only in mammals but across phyla (Rinn and Snyder, 2005; Ellegren and Parsch, 2007). Although often referred to as sex-biased genes, it is recognized that it is not the genes themselves that are biased, but their expression. As a potent and pleiotropic direct and indirect regulator of transcription, this places the estradiol/ER system in an important position to create sex-biased gene expression. Yet it seems that genetic-based studies of sex-biased gene expression favor the perspective of evolutionary biology (Ellegren and Parsch, 2007), or the power to identify genes underlying complex traits (Weiss et al., 2006), with relatively little attention given to hormonal status. Because the technology for executing these studies is relatively new, even less is known about how global patterns of sex-biased gene expression change during the different stages of life from development to aging, when hormonal status can change dramatically. Although few in number, studies are emerging that aim to determine the relative contribution of gonadal hormones and chromosomes in driving sexually dimorphic gene expression. A mouse model, termed the four-core genotype mouse, in which the sex chromosomes are independent of gonadal sex, has considerable value in this respect (De Vries et al., 2002; Arnold and Burgoyne, 2004). Together with computational analyses of transcriptional networks based on microarray studies, this work reveals marked differences in gene networks and connectivity in male and female brains, with a strong role for gonadal hormones in driving sexually dimorphic gene coexpression networks. Ultimately, such investigations should provide invaluable data to help explain the basis of differential disease susceptibility between the sexes. (van Nas et al., 2009). Because of the heterogeneity of cell types, even in a very discrete brain region, another elegant approach to analyzing gene networks has used the ability to sample and amplify RNA from a single neuron, as exemplified in a study of vlVMN neurons (Devidze et al., 2005). Analysis of a small subset of genes that are known to be linked functionally and regulated by estradiol, namely ERs, the oxytocin receptor, and Ca2+/phospholipid-dependent protein kinases, revealed patterns of coexpression that were significantly different in male and female vlVMN. Such an approach promises to be fruitful in furthering our understanding of molecular pathways activated by estrogen treatment.
In summary, several lines of evidence discussed in this section, from studies on glial and synaptic plasticity to rapid intracellular signaling pathways, seem to support the unifying view that developmental exposure to testosterone (aromatized to estradiol) essentially imprints selectivity in responsiveness to estradiol in later life in specific hypothalamic processes that characterize the male. We shall next consider the possibility that similar sex dimorphisms lie outside the hypothalamus in brain regions that are also targeted by gonadal steroids. Indeed, it is known that regions of the hypothalamus with known structural, neurochemical, and functional dimorphisms project to and influence brain areas that do not have immediate, obvious connections with reproductive functions. For example, estrogen-sensitive sexually dimorphic output pathways from the hypothalamus (vlVMN) in the female brain have to recruit midbrain pathways to initiate motor output and ensure that hormonal status coordinates with hindbrain regions and motoneuron integration of spinal stretch and flexion reflexes (lordosis) (Flanagan-Cato et al., 2001). Likewise, sensory pheromonal stimuli received through the olfactory bulb signal through the olfactory cortex and medial amygdala, which may themselves send sexually differentiated pathways to activate hormone-sensitive, sex-specific mating circuits (Polston et al., 2004; Kimchi et al., 2007). Moreover, changes in higher centers, including those involved in reward and memory, are associated with lordosis and other sex-specific behaviors, such as mothers foraging for their young, where enhanced memory ensures accurate return to their nest. Sex, therefore, has repercussions throughout the whole brain.
V. Parkinson's Disease and Sex Dimorphisms in the Nigrostriatal Dopaminergic Pathway
The midbrain dopaminergic populations (Fig. 3) are implicated in a number of CNS disorders that show sex differences in their incidence, manifestation, severity, and/or progression, such as PD, schizophrenia, attention deficit/hyperactivity disorder, and autism (Aleman et al., 2003; Hennessy et al., 2004; Barnes et al., 2005; Baron-Cohen et al., 2005; Shulman and Bhat, 2006; Becker and Hu, 2008). This implies fundamental differences between men and women in the underlying pathophysiology, which in turn has implications for responsiveness to treatments (Cahill, 2006; Shulman and Bhat, 2006; Cantuti-Castelvetri et al., 2007). A better understanding of the neurobiological basis for sex differences in brain disorders, therefore, is a key goal for improving therapies for conditions for which current treatments have limited success. Inevitably, a great number of studies have focused on gonadal sex hormones as factors driving these sex differences. Here and in the remainder of the review, we argue that fundamental differences in the organization and normal physiology of certain regions in the male and female brain contribute to susceptibility to disease and probably to responsiveness to hormonal therapies, which currently are the focus of growing attention (Shulman and Bhat, 2006; Zhao and Brinton, 2006).
Fig. 3.
Simplified schema describing the main components of midbrain dopaminergic systems (circuitry), the behavioral domains they influence, and association of their malfunction with some CNS disorders. The NSDA (or mesostriatal) system has its origins in the perikarya of the SNc and projects to the dorsal striatum. This regulates locomotor activity and is involved in stereotypical behaviors (e.g., grooming and gnawing in rats). The NSDA pathway degenerates in Parkinson's disease. The mesolimbic dopaminergic system (MLDA) originates in the perikarya of the ventral tegmental area (VTA) and projects to the ventral striatum, especially the N.Acc. This pathway also has some influence on locomotor behavior and is involved primarily in regulating motivation, reward, and reinforcement. Altered activity in the MLDA is associated with addictive behaviors and drug abuse. Perikarya in the VTA also project to the PFC, forming the mesocortical dopaminergic system (MCDA) involved in higher cognitive functions, which may deteriorate in AD. Release of DA from the VTA projections to the PFC trans-synaptically attenuates subcortical/mesoaccumbens DA activity and N.Acc DA release; hypofunction in the mesocortical dopaminergic system and hyperfunction in the MLDA activity to the N.Acc is characteristic of schizophrenia.
A. Epidemiological and Clinical Studies
In PD, progressive degeneration of the dopaminergic (DA) cell bodies in the substantia nigra pars compacta (SNc) and their terminals in the dorsal striatum (the caudate and putamen) leads to disturbances in sensorimotor control (primary manifestations), often associated with depression and dementia (secondary symptoms) (Weintraub et al., 2008). Around 3% of people over the age of 65 are affected by this highly prevalent condition; in the vast majority of cases, however, the cause is unknown and PD is likely to arise from the interplay of environmental and genetic factors. Sex can be considered an environmental factor and male sex, along with age, is one of the strongest risk factors for PD, men having at least a 2-fold greater risk than women of developing the disease at all ages and for all nationalities studied (Diamond et al., 1990; Baldereschi et al., 2000; Schrag et al., 2000; Swerdlow et al., 2001; Van Den Eeden et al., 2003; Shulman and Bhat, 2006; Cantuti-Castelvetri et al., 2007; Haaxma et al., 2007). Epidemiological and clinical data suggest that protective effects of estrogen in women are likely to contribute to the female advantage (for review, see Dluzen, 2000; Dluzen and Horstink, 2003; Shulman and Bhat, 2006). For example, at the onset of menses and menopause, and after withdrawal of hormone replacement therapy, when endogenous estrogen levels are low, parkinsonian symptoms worsen (Quinn and Marsden, 1986; Sandyk, 1989). Although not unanimous (Liu and Dluzen, 2007), a significant number of studies also conclude that the symptoms and risk of developing PD are reduced by estrogen treatment (Saunders-Pullman et al., 1999; Tsang et al., 2000; Benedetti et al., 2001; Shulman and Bhat, 2006) and prolonged natural exposure to endogenous estrogens (Saunders-Pullman et al., 2009). However, the timing of postmenopausal estrogen treatment may be crucial (Strijks et al., 1999), and the potential for estrogenic compounds to act as novel neuroprotective agents (Ravina et al., 2003; Johnston and Brotchie, 2006) requires further characterization in preclinical studies. For example, positive effects in women could be due to estradiol's effects on normal dopaminergic transmission (the surviving neurons in PD), as well as its more generally acclaimed neuroprotective actions, which prevent cell loss (McEwen and Alves, 1999; Wise et al., 2001; Brann et al., 2007); as discussed below, these two processes may use distinct mechanisms. Although it is difficult to distinguish the two in the human population, it has been proposed that estrogenic modulation of DA function may underlie sex differences in motor and sensory functions and contribute to women's ability to outperform men at tests of fine motor control and speech articulation (Jennings et al., 1998). It is noteworthy that endogenous estradiol levels, but not testosterone levels, in women's blood were associated with improved motor performance, whereas neither sex hormone, endogenous or exogenous, was related to men's motor performance (Jennings et al., 1998; Siegel et al., 2008), suggesting marked sex differences in estradiol's actions in the human NSDA pathway.
Protective effects of estradiol in women could also be mediated via activation of mitochondrial ERs, which are present in many cell types, especially tissues such as the brain with high demand for mitochondrial energy metabolism (Chen et al., 2009). Along with nuclear ERα and ERβ and their coactivators, mitochondrial ERs are involved in cytoprotection from oxidative stress and regulation of apoptosis. It has been suggested that a deficiency in this estrogen-dependent mechanism might be causally related to PD pathogenesis (Chen et al., 2009). Although inherent sex differences in this system have not been reported, a recent study described a gain-of-function rare polymorphism in the X-linked gene encoding a glutamate dehydrogenase (a mitochondrial enzyme) expressed in the brain, which was associated with an earlier age of onset in PD. In hemizygous male subjects, it has been speculated that this might be due to enhanced glutamate oxidative phosphorylation in dopaminergic neurons. It is noteworthy that there was no association in heterozygous female patients with PD, which was attributable to the finding that estrogens suppress enzyme activity (Plaitakis et al., 2010).
In addition to the likely protective/prodopaminergic effects of estrogens (at least in women), evidence is now emerging for inherent sex dimorphisms in the healthy human nigrostriatal dopaminergic (NSDA) pathway, which could confer differential risk factors to disease in men and women. For example, laser capture microdissection and microarray analysis of gene expression in single SNc DA neurons from post mortem brains revealed that in healthy subjects, cells start with a sex difference in the natural pattern of gene expression, with men expressing genes implicated in PD pathogenesis (α-synuclein, PINK-1) at a higher level compared with women (Cantuti-Castelvetri et al., 2007). The same study showed that changes in gene expression in end-stage surviving neurons in subjects with PD were also sexually differentiated. Functional differences have also been identified in the NSDA pathway in healthy men and women using real-time in vivo imaging techniques. These include sex differences in amphetamine-stimulated striatal DA release (Munro et al., 2006), in basal striatal dopaminergic neuron dynamics (Pohjalainen et al., 1998; Lavalaye et al., 2000; Kaasinen et al., 2001; Mozley et al., 2001; Laakso et al., 2002; van Beilen et al., 2008), and in striatal responses while performing tests that target cognitive and motor functioning associated with DA activity (Mozley et al., 2001). Imaging DA neuron dynamics in early, unmedicated PD confirms the expected large loss in DA terminal density in the striatum, but in the prefrontal cortex a far greater accumulation of 3,4-dihydroxy-5-fluorophenylalanine (indicative of uptake mechanisms) was seen in female compared with male patients (Kaasinen et al., 2001). The precise implications of this observation are unclear, but they emphasize underlying sex differences in neurotransmission and could possibly indicate sex differences in adaptive responses, which are known to be very powerful during the early stages of PD (Bezard et al., 2003). There are also clear indicators of sex differences in the profiles and severity of PD symptoms as well as outcomes of pharmacological and surgical treatments, with implications for clinical management (Lyons et al., 1998; Fernandez et al., 2000; Rojo et al., 2003; Scaglione et al., 2005; Shulman and Bhat, 2006; Gillies and McArthur, 2010). In women, for example, PD is associated with a later onset, greater frequency of presentation with a tremor-dominant form of disease, and a slower progression compared with men (Shulman and Bhat, 2006; Haaxma et al., 2007). Secondary behavioral symptoms also show marked sex differences, with depression featuring to a greater extent in women, whereas wandering and physical abusiveness were more common in men (Fernandez et al., 2000). These observations indicate that imbalance of the NSDA system by disease results in differential instability in associated neural networks in men and women, and further supports the view that midbrain DA circuitry is sexually dimorphic.
Together, these data suggest that underlying dimorphisms in the human NSDA pathway may underlie the predisposition of men to PD, and that sex influences both the nature of its degenerative processes and response to therapy. The contributions of sex hormones to these processes clearly require further analysis. In this respect, preclinical studies, which generally support and predict the human data, have an important role to play. Therefore, the next section will consider what we know about sex dimorphisms in the NSDA system of species used in research and how this might inform our interpretation of hormonal influences in experimental parkinsonism.
B. Preclinical Evidence
1. Sex Dimorphism in the Nigrostriatal Dopaminergic Pathway and Responses to Estrogen.
The NSDA system performs essentially the same functions in male and female subjects, yet animal studies confirm the human evidence that these are likely to be attained by different mechanisms. Rodent studies show that there are significantly more DA neurons in the male SNc than in the female SNc (Murray et al., 2003; Dewing et al., 2006; McArthur et al., 2007a,b). Moreover, we have reported a notable sex differences in their topographical distribution, indicating a structural basis for sex differences in connectivity and DA transmission (Fig. 4, A and B) (McArthur et al., 2007a). Despite differences in neuronal number, there are no sex differences in striatal DA content and basal extracellular DA levels in the caudate putamen, which is thought to be due to sex differences in neuron dynamics (DA reuptake, release, terminal density, etc.) (Robinson et al., 1990; Walker et al., 2000; Murray et al., 2003; Ji and Dluzen, 2008), similar to the human data described above. Functional consequences of these differences can be seen in terms of stimulated striatal DA release and motor behaviors involving midbrain DA pathways (for review, see Becker, 1999). These are more exaggerated in females because of endogenous estrogens, but, contrary to the situation in females, estradiol treatment of gonadectomized male rats fails to affect these parameters (Robinson et al., 1990; Castner et al., 1993; Pasqualini et al., 1995; Xiao and Becker, 1998; Becker, 1999; Thompson, 1999; Walker et al., 2000; Ohtani et al., 2001). Together, these observations support our recurring theme that sex differences in the organization of brain circuitry coincide with sex dimorphisms in responses to estrogens.
Fig. 4.
Sex differences in the topographical organization of the midbrain dopaminergic neurons (mid-DAs) of the SNc and VTA. Adult male and female rat brain slices (30 μm) were processed immunocytochemically for identification of tyrosine hydroxylase immunoreactive (TH-IR) cells as a marker of dopaminergic cell bodies in the SNc (A and B) and VTA (C and D). Cavalieri's principle was used to calculate the volume delineated by TH-IR, and the total number of TH-IR cells was counted. For data analysis, the midbrain region was divided through its rostrocaudal extent into anatomically defined regions (I –IV), each separated by 300 μm, beginning at bregma −4.8; the SNc traversed all four levels, and the VTA was clearly distinguished at 3 levels (II–IV). For SNc, the total volume (I + II + III + IV) was greater in female than in male brains (A, Total) but total cell numbers were significantly greater in males (B, Total); because there were no sex differences in cell size, this suggests a greater packing density in the male brain. Analysis at each level (I–IV) showed significant differences in volume between males and females, indicating sex differences in the overall shape (A). The percentage of TH-IR cells located at each level was also calculated, and revealed a significant sex difference in the distribution of the dopaminergic cells throughout the nucleus (B). For VTA, like the SNc, the total volume (II + III + IV) was greater in the female compared with the male brain (C), but, unlike the SNc, the total cell counts were also greater in females (D). Analysis at each level (II–IV) showed a significant sex difference in volume, indicating differences in the overall shape (C), as well as a significant sex difference in the percentage of TH-IR cells located at each level, indicating male/female differences in the distribution of the dopaminergic cells throughout the nucleus (D). These results identify structural sex dimorphisms in the mid-DAs that are likely to underpin sex differences in physiology and behaviors governed by these pathways. + indicates a significant difference for male versus female, P < 0.05. Further details in McArthur et al. (2007a).
Although not as well researched as the hypothalamus, several mechanisms have been proposed that could underlie dimorphic responses to estrogens. Sex differences in ER expression would clearly have a significant impact. This is highlighted by recent ER transfection studies using the PC12 cell line, which expresses tyrosine hydroxylase (TH), the rate-limiting enzyme for DA biosynthesis, and is often used as a surrogate for DA “neurons.” Estradiol treatment of these cells led to the up-regulation of TH transcription in the presence of ERα but down-regulation in the presence of ERβ (Maharjan et al., 2005). ERs were also able to interfere with cAMP-stimulated TH transcription, illustrating that estrogens could modulate catecholaminergic responses to other inputs. How these data relate to the NSDA system remains to be seen, but ER expression in this brain region is sparse or even absent. Current evidence provides little direct support for sex differences in ER expression in the adult rat substantia nigra (Kritzer, 1997; Shughrue et al., 1997; Creutz and Kritzer, 2002). However, separate studies using the same antibody reported a lack of ERβ in the male mouse SNc (Shughrue, 2004) but weak expression in the female SNc (Mitra et al., 2003; Merchenthaler et al., 2004), raising the possibility of sex differences for this receptor isoform. The few SNc cells that were positive for ERα did not colocalize TH, suggesting that estrogens act indirectly or via nuclear receptor-independent mechanisms to influence DA neuron activity (Shughrue, 2004). The striatum seems to lack ERβ (Shughrue, 2004), whereas ERα is present at low levels, although possibly at higher levels in female compared with male mice (Rodríguez-Navarro et al., 2008).
Apart from ER expression studies, other lines of evidence suggest that sex differences in striatal DA responses to estradiol may be mediated indirectly, rather than directly on the DA neurons. The striatal interneuron population of inhibitory GABAergic medium spiny neurons (MSNs), which play a key role in regulating basal ganglia output in the healthy brain, are one possible target. To a large extent, estrogenic potentiation of stimulated DA release from the female striatum has been attributed to a suppressive action on the MSNs, which relieves their inhibitory input to the DA terminals (Hu et al., 2006; Schultz et al., 2009). This involves rapid effects mediated via ERα, which is primarily associated with the membrane but not nuclear fraction in striatal lysates (Schultz et al., 2009). Failure of male rats to respond to estradiol in a similar manner has been attributed to sex dimorphisms in the MSNs, which would have a critical impact on basal ganglia function (Hu et al., 2004, 2006). Other networks that profoundly affect striatal DA function include the mesocortical DA pathways, which themselves exhibit hormone responsiveness and sex differences in function and ER localization in their cells of origin (Kritzer and Creutz, 2008). The serotonergic system of the dorsal raphe is also an estrogen-sensitive major regulator of SNc DA neurons (Klink et al., 2002) that not only exhibits sex differences in G-protein signaling mechanisms (Loucif et al., 2006) but also has a sexually dimorphic ultrastructure in humans (Cordero et al., 2000). Together, these observations highlight the sexually dimorphic state and hormonal sensitivity of the networks interacting with and regulating the NSDA system. They also indicate a multiplicity of mechanisms that could explain sex differences in the intact, normally functioning pathway that require further investigation.
2. Gonadal Factors Are Protective in Female, but Not in Male, Experimental Parkinson's Disease.
Apart from a small percentage of PD cases that can be traced to single gene mutations, we do not know the causes of the disease. Therefore, experimental PD aims to make the best approximation possible by administering toxins selective for DA neurons, to reiterate as many features of the condition as possible, including oxidative stress, progressive loss of DA neurons and striatal DA levels, neuroinflammation, and excitotoxicity (Smeyne and Jackson-Lewis, 2005; Simola et al., 2007; Gillies and McArthur, 2010). Evidence from our own and other laboratories shows that animal models of PD reproduce the sex differences in susceptibility seen in the human population (Miller et al., 1998; Dluzen, 2000; Murray et al., 2003; Gillies et al., 2004; Liu and Dluzen, 2007; McArthur et al., 2007b; Gillies and McArthur, 2010). Both SNc and striatal lesions are significantly greater in male rats treated with 6-hydroxydopamine (6-OHDA) (Murray et al., 2003) and in male mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) or methamphetamine (MA) (Miller et al., 1998) compared with female. However, it is important to appreciate that sex differences are apparent only when creating submaximal lesions of the NSDA pathway (which mimic early, preclinical stages of PD); above a certain dose of neurotoxin, when lesion size exceeds 60%, sex differences are lost (Murray et al., 2003; Gillies and McArthur, 2010). The relative degree of neuroprotection intrinsic to the female brain would thus seem to be limited by the extent of neuronal damage, but these observations also suggest that an understanding of the factors responsible for these differences could point the way to novel therapies with potential to prevent or delay progression or even onset. The use of models with submaximal lesions, therefore, are well suited to investigating the roles that hormones play in creating these sex differences, and whether this knowledge might be exploited therapeutically.
As might be predicted from clinical observations, ovarian factors, specifically estradiol, protect against toxin-induced depletion of DA in the female striatum, and administration of physiological levels of estradiol to ovariectomized females are similarly effective against striatal damage induced by 6-OHDA in rats or MA or MPTP in mice (Miller et al., 1998; Murray et al., 2003; Bourque et al., 2009; Gillies and McArthur, 2010). However, the balance of evidence suggests that the levels of estrogen prevailing at the time of injury are critical, whereas administration after injury has been initiated fails to protect (Datla et al., 2003; Bourque et al., 2009). Moreover, very high doses of estradiol may fail to protect and may even worsen lesion size (Ramirez et al., 2003; Bourque et al., 2009). Fewer studies have been performed in male rodents, but in contrast to females, gonadal hormones exacerbate the extent of mild/moderate striatal lesions (Dluzen et al., 1994; Murray et al., 2003; Lewis and Dluzen, 2008; Gillies and McArthur, 2010). Surprisingly, replacement of physiological levels of estradiol, but not nonaromatizable androgens, reversed the effect of castration in the rat PD model (Murray et al., 2003), suggesting that circulating testosterone promotes lesion size only after aromatization to estradiol. Although estradiol has been reported to have some protective capacity in male mice of the C57BL/6 strain, which are highly sensitive to MPTP (Ekue et al., 2002; Bourque et al., 2009), reports regarding other male mouse models (CD-1 strain) indicate that systemic estradiol is not neuroprotective (Dluzen et al., 1994; Yu and Wagner, 1994; Gao and Dluzen, 2001; Lewis and Dluzen, 2008). On balance, the data suggest that both the response of the NSDA system to injury and the protective effects of estrogen are sexually dimorphic.
Despite robust evidence that systemic hormonal status differentially affects striatal loss of DA in models of preclinical PD, hormonal manipulations failed to influence DA cell survival in the SNc in either sex (Ferraz et al., 2003; Moroz et al., 2003; McArthur et al., 2007b; Ferraz et al., 2008). These results indicate that sex differences in toxin-induced cell loss are not hormonally generated and that hormone-dependent changes in striatal DA depletion can occur independently of cell survival. To understand this apparent dissociation of hormonal effects at the cell body and nerve terminals, it is important to appreciate that the damaged neurons and associated basal ganglia circuitry have a remarkable capacity for compensation both in experimental and clinical PD, such that overt motor symptoms may not be apparent until around 80% of striatal dopamine and 60% of SNc perikarya are lost (Castañeda et al., 1990; Song and Haber, 2000; Bezard et al., 2003; Bassilana et al., 2005). These adaptive mechanisms include increased synthesis, metabolism, and release of DA per impulse, which restore functionality in the partially damaged NSDA system. Circulating estradiol can promote all these parameters, as well as behavioral recovery after 6-OHDA-induced lesions in female rodents, but fails to do so in males (McDermott et al., 1994; Pasqualini et al., 1995; Becker, 1999; Ohtani et al., 2001; Serova et al., 2004; Dluzen, 2005; Tamás et al., 2005). It is also known that striatal interneurons, including the MSNs, undergo synaptic reorganization after 6-OHDA-induced injury to the NSDA system (Salin et al., 2009). We have already highlighted the evidence that this population responds to estradiol in female but not male rodents (Hu et al., 2004, 2006; Schultz et al., 2009) in an ERα-dependent manner, and studies using selective ER ligands favor a role for ERα over ERβ in mediating estrogenic neuroprotection at striatal level (Morissette et al., 2008b). Together, these observations support our proposal (McArthur et al., 2007b) that the influences of physiological levels of circulating sex hormones on the intact or partially damaged NSDA system center principally on activity, rather than survival, of the existing neurons, which clearly renders females more able to adapt to injury.
In addition to acting within the NSDA system itself, gonadal hormones and exogenous estradiol could also differentially influence adaptive responses to injury indirectly by targeting estrogen-sensitive sexually dimorphic input pathways. Noradrenergic transmission from the locus ceruleus is one possible candidate because it has positive influences on neurotransmission and adaptive responses in the injured NSDA system (Marien et al., 2004). Moreover, circulating estrogen and testosterone (after conversion to estradiol) have opposing actions in female and male mice to up- or down-regulate the expression of tyrosine hydroxylase in the locus ceruleus, respectively (Thanky et al., 2002). Likewise, sexually dimorphic influences of the MSNs (the striatal interneurons) or input from the prefrontal cortex or dorsal raphe (section V.B.1) could modify adaptations in the neurons surviving partial injury of the NSDA system. Further work is needed to explore such possibilities, and a schematic representation of some possible direct and indirect actions of gonadal steroids on the male and female NSDA system is presented in Fig. 5.
Fig. 5.
Systemic and central sex hormones: schematic representation of potential modes of action and interaction on the intact and damaged NSDA system in male and female brains. Circulating estradiol (E2) up-regulates activity in the NSDA system in females but not males (evidence discussed in section V.B.2) This could be due to sex differences in the response of the NSDA neurons to the direct effects of circulating E2 or to indirect effects on networks interacting with and regulating the system, which are differentially sensitive to E2 in males and females. The schema shows one such indirect pathway, the nor-adrenergic (norepinephrine, NE) neurons of the locus ceruleus (LC). These positively influence neurotransmission in the NSDA system and play an important role in adaptive responses within the injured NSDA system in Parkinson's disease. This influence is up-regulated by circulating E2 in females (arrows), but down-regulated in males (blocked line). This systemic E2 may promote adaptive responses to neurodegeneration in the female NSDA system, whereas in males circulating E2 or testosterone (after conversion to E2) would not have this effect and might even exacerbate lesions. However, local up-regulation of aromatase activity to promote E2 production at the site of NSDA injury has the potential to protect in brains of both sexes. See the section V.B.2 for further discussion.
The origins of sex dimorphisms in the NSDA have not been as intensively researched as those in the hypothalamus, but both organizational actions of hormones during development and genetic factors seem to play a role (Vadász et al., 1985, 1988; Gillies et al., 2004; Dewing et al., 2006). Furthermore, perinatal treatment of female rats with testosterone compromised the protective effects of estradiol against 6-OHDA-induced injury in adulthood (Moroz et al., 2003), indicating that perinatal “masculization” also interferes with the neuroprotective capacity of estrogens in later life. There is also evidence that the sensitive periods for sexual differentiation of many extrahypothalamic brain areas may fall outside the critical period as it has been defined for the hypothalamus (see section II.B). It is noteworthy that the NSDA system and associated networks retain a high degree of plasticity well into the adolescent period, when certain sex dimorphisms first emerge (Kalsbeek et al., 1988; Voorn et al., 1988; Velísková and Moshé, 2001). Moreover, late hormonal programming may be important for the neuroprotective effects of estrogens in adult mice. In female mice prepubertal ovariectomy abolished the ability of estradiol to protect against MA toxicity, whereas in males, prepubertal castration did not alter the fact that estradiol was not protective (Anderson et al., 2005). This indicates that a process of active feminization in females, as distinct from masculinization/defeminization in males, also contributes to sex dimorphisms in the NSDA system. These findings are compatible with recent reports that pubertal hormones may be associated with organizational effects in developing human and mouse brains (Ahmed et al., 2008; Neufang et al., 2009) and have important implications not only for PD but also for other conditions, such as schizophrenia and anorexia/bulimia, which often emerge around the time of puberty in a sexually dimorphic manner. Glial cells may also play an important role in mediating the neuroprotective effects of estrogens, and their role in this respect may be affected by hormonal programming in the neonatal period and, consequently, may be sexually dimorphic (Moroz et al., 2003; Morale et al., 2006).
The preceding discussion has focused on the effects of physiological levels of systemic estradiol where striatal lesions are relatively small (<50%); in such circumstances, the activational influences of sex hormones on transmission in surviving neurons, rather than effects on cell survival, seem to account for striatal “neuroprotection.” However, there is evidence that estradiol, acting by other mechanisms, can ameliorate toxin-induced loss of DA cell bodies in the SNc (Quesada and Micevych, 2004). This pertains principally to supraphysiological levels of estradiol in females, in situations where cell loss is relatively extensive and associated with almost total loss of striatal DA. This may use PI3K/Akt signaling, which may involve CREB as a downstream effector (Morissette et al., 2008a; Quesada et al., 2008), with possible effects on expression of genes for antiapoptotic, growth factor, and antioxidant activity. These might be considered nonspecific effects on processes common to neuroprotection against many forms of injury in different brain regions and are known to require concentrations of estradiol that are orders of magnitude greater than levels in the circulation, even at proestrus (Sawada et al., 1998, 2002; Green and Simpkins, 2000; Behl, 2002; Callier et al., 2002; Garcia-Ovejero et al., 2005). It remains to be determined whether these responses are sexually dimorphic.
Although much still needs to be learned about the precise mechanisms of estrogenic neuroprotection in PD, some recent work indicates that molecular mechanisms within the DA neurons themselves could be sexually dimorphic. One of the few single gene mutations that accounts for a very small percentage of cases of PD lies in the parkin gene, which codes for an E3 ubiquitin ligase. This ubiquitinates misfolded or unfolded proteins to direct them through the ubiquitin-proteasome system for regulating protein concentrations in the cell. In response to cellular stress, including oxidative damage and infections, heat shock proteins are up-regulated; they identify damaged proteins and recruit E3 ligases to promote their proteolysis within the proteasome. Dysfunction of the ubiquitin-proteasome pathway, which is a likely consequence of disruption to the parkin gene, is generally involved in PD (McNaught et al., 2001). This, in turn, may contribute to protein aggregation, leading to formation of Lewy bodies (a hallmark pathological feature of PD), which could be either cytotoxic or neuroprotective as a result of their ability to sequester cytotoxic, misfolded proteins. It is noteworthy that proteasome activity is also important for the turnover of ERα [expressed in the striatum (Kipp et al., 2006; Schultz et al., 2009)] and its associated coactivators and repressor proteins (Alarid et al., 1999); it is also required for efficient ERα-dependent transactivation and transcription (Lonard et al., 2000; Métivier et al., 2003; Reid et al., 2003). A recent study suggests that the parkin gene may play an important part in sex-specific effects on cellular neuroprotective processes within the NSDA system that are known to be responsive to estradiol. For example, as part of their general neuroprotective capabilities, estrogens increase expression of antiapoptotic proteins such as Bcl-2 (Dubal et al., 1999; Garcia-Segura et al., 2001), and there is a higher Bcl-2/Bax protein ratio in wild-type female mice that may contribute to inherent sex differences in susceptibility to neurotoxins (Rodríguez-Navarro et al., 2008). However, in parkin-null mice, the female advantage and sex difference is lost because of a reduced Bcl-2 expression in females and no change in males. In addition, expression of the 70-kDa heat shock protein levels are significantly lower and glutathione levels higher in the wild-type female striatum compared with males, further suggesting that females may be under a less severe protein and oxidative stress, but in the absence of parkin, the advantageous profile of cellular neuroprotective factors in females are suppressed. Loss of parkin also suppresses the ability of estradiol to elicit neurotrophic responses in DA neurons [which is seen only in females (Kipp et al., 2006; Schultz et al., 2009)] and Akt activation in mesencephalic cultures (Rodríguez-Navarro et al., 2008). Further understanding of the sex-specific molecular pathways involved in mediating the neuroprotective effects of estrogen will be of great value.
3. Brain Aromatase Confers Protection in Male and Female Experimental Parkinson's Disease: Complex Interactions between Steroids of Peripheral and Central Origins.
Aromatase up-regulation (principally in glial cells) and estradiol synthesis at the site of excitotoxic and ischemic brain injury is thought to be an important neuroprotective mechanism (Garcia-Segura et al., 2001, 2008; Azcoitia et al., 2005; Carswell et al., 2005; Garcia-Ovejero et al., 2005). Recent studies using either central administration of an aromatase inhibitor (McArthur et al., 2007b) or ArKO mice (Morale et al., 2008) suggest that local production of estradiol is also protective against 6-OHDA-induced toxicity in both the male and female striatum. This contrasts with the sexually dimorphic effects of systemic estradiol, which is universally protective against experimental parkinsonism in female, but not male brains. As discussed in section V.B.1, convergent evidence supports the concept that these differences may be due, at least in part, to the fact that circulating estrogens have free access to all brain regions, including sexually differentiated NSDA-associated circuitry, leading to better adaptive responses in female brains exposed to early experimental PD. By definition, up-regulation of neuronal or glial aromatase will increase estrogen levels only locally, probably to levels exceeding those in the systemic circulation (Prange-Kiel and Rune, 2006), thereby avoiding more diffuse effects and highlighting brain aromatase as an attractive therapeutic target (see also Fig. 5).
Before the potential of exploiting brain steroid biosynthesis within the brain can be realized, the precise relationship between sex hormones produced in the gonads and brain requires clarification. Although the mechanisms for regulating neurosteroid production are not certain, recent work has identified a “mini hypothalamic-pituitary-gonadal (HPG) axis” in the brain (Meethal et al., 2009). For example, neuronal GnRH receptors have been found outside the hypothalamus, including the human hippocampus and cortex, which may signal the intracellular production of LH and activation of steroidogenic acute regulatory protein, thereby providing a local regulatory mechanism for sex steroid production using the same molecules that control HPG activation (Meethal et al., 2009). Moreover, the extrahypothalamic system is responsive to ovariectomy, raising the possibility that some of the central effects of peripheral hormonal manipulations could be secondary to consequences on neurosteroidogenesis. It remains to be seen whether the central system is sexually dimorphic, which is clearly the case for the HPG axis (see section IV.A.). However, sex differences in the levels of neuroactive steroids have been reported in areas of the rat central and peripheral nervous systems both under basal conditions and after challenge with injury or disease (Cosimo Melcangi and Garcia-Segura, 2010; Pesaresi et al., 2010). Further studies are needed to clarify the significance of these sex differences both for physiology and pathology.
Although the local aromatase/ER system may confer protection within both the injured male and female NSDA system, some important sex differences have been noted in other brain regions involving different types of neuronal injury. A recent study has shown that ischemic damage in the cortex results in an up-regulation of ERα in the cortex of the female, but not male, brain (Westberry et al., 2008). This seems to be attributable to suppression of methylation of the ERα gene, thereby removing the gene-silencing influence of DNA methylation. ERα mRNA is also transiently highly expressed in the rodent cortex during neonatal development, although expression levels are very low in the normal adult cortex, possibly as a result of epigenetic modulation by methylation of the 5′-untranslated exons during brain development (Westberry et al., 2010). Together, these observations support the view that brain cells revert back to an early developmental stage after neuronal injury, and that this phenomenon may be sexually dimorphic and highly relevant to sex differences in estrogen responsiveness. In this context, it is interesting to note that astrocytes derived from neonatal female rat brains also seem to have a greater capacity for aromatization and estradiol formation than male astrocytes, which could account for the relative protection of female astrocytes from the consequences of oxygen-glucose deprivation (Liu et al., 2007). This further supports the notion that recapitulation of developmental processes during injury and repair could be different in male and female brains. Such differences could contribute to the greater lesion size observed in male brains in experimental stroke models, although, unlike PD, exogenous estrogen treatment protects in both sexes, possibly because nongenomic and/or ERβ-dependent mechanisms operate in males (Prokai and Simpkins, 2007).
C. Potential for Selective Estrogen Receptor-Modifying and Selective Aromatase-Modifying Drugs
In the case of PD, ER polymorphisms have been associated with an increased risk of developing the disease, with a bias to ERβ in early onset forms (Maraganore et al., 2002; Håkansson et al., 2005). In experimental PD using MPTP, the 17α-estradiol stereoisomer with weak classic estrogenic activity also failed to show neuroprotective capacity, and estrone and estriol (the natural metabolites of estradiol) had weak or no protective properties, respectively (Morissette et al., 2008a). Progressing to more selective agonists, initial studies suggested that ERα agonists were protective in the MPTP model, whereas ERβ-selective agonists were not. However, estradiol proved not to be protective in mice lacking either ERα or ERβ (Morissette et al., 2008a), suggesting that both isoforms may contribute to neuroprotective effects of estrogens. The realization that the effects of estrogens in the brain are region- and cell type-specific, coupled with our expanding knowledge of the multiple mechanisms through which ERs can exert their effects, opens up enormous potential for developing not only SERMs for specifically targeting the brain [perhaps more appropriately termed neuro-SERMs (Zhao et al., 2005)], but also for neuro-SERMs that may be region- or pathway-specific and, hence, disease-specific. However, possibly because of the low expression levels in the NSDA pathway, relatively few studies have been performed with clinically relevant SERMs in animal models of PD, and those that have do not present a simple picture. For example, in the mouse model, which uses MA as the NSDA neurotoxin, there are consistent data (discussed in section V.B.2) to show that estradiol protects against striatal DA depletion only in the female striatum, but there are reports that tamoxifen (a SERM with antiestrogenic activity in breast tissue but estrogenic activity in other peripheral tissues) is protective against MA toxicity in both the male and female striatum, possibly because of its antioxidant and free-radical-scavenging properties (Dluzen et al., 2001; Kuo et al., 2003; Brann et al., 2007). Looking at the other experimental PD mouse model using MPTP, where estradiol is reported to protect against striatal DA loss in both sexes, treatment with tamoxifen alone in ovariectomized female mice was not neuroprotective, but it did block the protective effect of estradiol, indicating ER involvement. In intact males, tamoxifen was again not protective but failed to antagonize the protective actions of estradiol (Morissette et al., 2008a), supporting other reports that that the actions of tamoxifen in the unlesioned NSDA pathway may be sexually dimorphic (Dluzen and Mickley, 2005). For raloxifene (a SERM with antiestrogenic actions in breast and uterus, but agonistic action on bone and cholesterol metabolism), there are reports that it may (Grandbois et al., 2000; Callier et al., 2001) or may not (Ramirez et al., 2003) be protective against MPTP. Whatever the real answers underlying these contradictions, which are likely to be attributable to the many differences in the experimental paradigms used (treatment regimens, toxins, brain penetrability of SERMs, animal strains, age, and sex), they serve to illustrate that neuro-SERMs may offer promise for the development of estrogen-based therapies with central selectivity.
In view of the protective effects of aromatase enzyme activity in both male and female rats exposed to 6-OHDA, selective aromatase-modifying drugs also offer interesting potential as neuroprotective agents. The aromatase gene is widely distributed throughout the brain, including the adult striatum (Kipp et al., 2006), where it is regulated in a highly tissue- and brain region-specific manner (Simpson et al., 2002). Its up-regulation and protective capacity in brain injury, including ischemic or excitotoxic challenge (Azcoitia et al., 2001; Carswell et al., 2005), has been well documented, but it is not yet clear to what extent this occurs in the NSDA system. However, a better understanding of the molecular mechanisms controlling tissue-specific aromatase gene expression, especially the switch from repression in quiescent glial cells to up-regulation at sites of injury or neurodegeneration, has important therapeutic potential (Saldanha et al., 2009). Alternatively, where aromatase is naturally up-regulated in injury, the local production of estradiol could be boosted by increased delivery of a substrate and precursor of estradiol synthesis, such as dehydroepiandrosterone/sulfate, which is normally secreted by the adrenal glands, and has been shown to protect against MPTP in male mice (Morissette et al., 2008a). Local aromatases therefore offer a particularly interesting area for exploitation, especially in PD, where selective enhancers of transcription, translation or activity would focus raised levels of estradiol where they are needed at the site of damage, thereby avoiding a host of unwanted peripheral actions, as well as any indirect actions of systemic estradiol on sexually dimorphic input pathways, which appear to disadvantage the male brain (Gillies and McArthur, 2010).
VI. Sex, Estrogens, and Drug Abuse
In addition to the A9 DA cell group in the SNc, the midbrain also possesses the A10 cell group in the ventral tegmental area (VTA; Fig. 3). This is a convenient structural and functional division of these DA populations, but their involvement in several processes may overlap because of the complex interconnectedness with the limbic system and cortex. The VTA DA neurons project to the ventral striatum, especially the nucleus accumbens (the mesolimbic system), and also to the prefrontal cortex (the mesocortical system). This section will focus particularly on the role of the ascending mesolimbic DA pathway in motivation, reward, and drug abuse.
A. Epidemiological and Clinical Studies
Although the overall prevalence of drug abuse is greater in men than women, emerging evidence suggests that this may have been due to sociocultural influences that are changing rapidly. The use of, and dependence on, stimulant drugs, such as cocaine and methamphetamine, is now increasing worldwide but at a much faster rate in women than in men (Lynch et al., 2002; Carroll et al., 2004; Becker and Hu, 2008), which is a growing public concern. Sex dimorphisms in patterns of drug abuse include a faster escalation of intake of psychoactive stimulants, alcohol, opioids, and marijuana and more rapid progression to addiction in women than men (for review, see Lynch et al., 2002; Carroll et al., 2004; Lynch, 2006; Becker and Hu, 2008). Recent data show that women are more sensitive to the rewarding effects of psychoactive drugs such as cocaine and amphetamine, and studies across the menstrual cycle suggest that estrogens seem to be critical for these sex differences (Becker and Hu, 2008). The behavioral effects of the psychomotor stimulants therefore seem to be sexually dimorphic and sensitive to the prevailing hormonal environment. Convergent evidence suggests that the ventral striatum and amygdala respond to predictors of reward or anticipation (motivational behavior, or the appetitive component of a behavior), rather than the reward itself (the consummatory component of a behavior); the mesolimbic DA system and DA release in the nucleus accumbens play a particularly important role in the motivational and reward network, whereas medial prefrontal cortex and the dorsal striatum are more responsive at the time of reward (O'Doherty, 2004; Becker, 2009). Although relatively little is known about hormonal influences on the DA-reward system in humans, in vivo imaging studies have demonstrated fluctuations over the menstrual cycle (Caldú and Dreher, 2007) and sex differences in striatal DA release in healthy men and women (Munro et al., 2006). These observations provide important beginnings from which to expand our understanding of the biological mechanisms underpinning drug addiction and how these differentially affect vulnerability to drug abuse in men and women.
B. Preclinical Evidence
Preclinical data provide substantial support for the clinical evidence that addictive behaviors are sexually dimorphic and hormone-responsive. Self-administration of drugs in experimental animals is used as a model of human addictive behavior, allowing separate analysis of the acquisition, maintenance, and motivation to seek reward. These data are excellently reviewed elsewhere (Lynch et al., 2002; Lynch, 2006; Becker and Hu, 2008; Becker, 2009), and the salient points are summarized here. Compared with males, female rats acquire cocaine self-administration and cocaine-induced conditioned place preference (a test to determine the rewarding effect of cocaine) faster and at a lower dose. Experiments in ovariectomized and hormone-replaced female rodents show that estradiol is an important driving factor that can be blocked by the nonselective ER antagonist, tamoxifen. Estradiol also increases the amount of drug consumed as well as the motivation to consume. In males, however, neither castration nor treatment with physiological levels of testosterone or estradiol has any effect on the acquisition of cocaine self-administration. Together, these findings suggest that an estrogen-dependent mechanism, which operates in females but not males, contributes to sex differences in drug abuse. In support of this, endogenous estradiol levels in female rats correlate positively with behavioral responses to amphetamine (locomotor activity and stereotypy), as well as basal and amphetamine-stimulated extracellular concentrations of DA in the striatum, especially the nucleus accumbens, which plays a critical role in reinforcement/rewarding processes (Fink et al., 1996; Hyman and Malenka, 2001). Estradiol also influences activity in DA and 5-hydroxytryptamine systems in key brain areas, such as the prefrontal cortex (PFC), involved in learning and memory (Fink et al., 1996; McEwen and Alves, 1999), which, in turn, may impinge on ability to store and recall cocaine-induced rewarding events (Russo et al., 2003). This is distinct from responses in males, where behavioral responses are suppressed compared with females, and castration with or without hormonal treatments affects neither locomotor responses nor striatal DA release. In this context, estradiol-dependent stimulation of DA pathways is thought to reinforce the motivation to administer cocaine in females by activating downstream pathways (Becker and Hu, 2008). It follows, therefore, that fundamental sex dimorphisms in the underlying circuitry contribute to sex differences in drug abuse and the sexually dimorphic response to estrogen. In support of that, our work demonstrates that the topographical distribution of DA neurons throughout the adult rat VTA, as well as size of the VTA dopaminergic population, is sexually dimorphic (Fig. 4, C and D) (McArthur et al., 2005, 2007a), suggesting significant male/female differences in connectivity. Although the origins of sex dimorphisms in the mesolimbic DA system has received little attention, it has been proposed that gonadal hormone-dependent processes underpin a masculinization/defeminization of midbrain pathways in the neonatal period (which suppress estradiol sensitivity in adult males, as discussed in section III) as well as a novel feminization process around puberty [which induces permanent sensitivity to estradiol in females (Becker, 2009)].
It is clear that the risk factors involved in drug abuse are not simply hormonal but are likely to involve an interaction between environmental factors (which may well be hormonal) and genetic factors. In particular, some of the genes affecting TH activity in midbrain DA systems contribute to the expression of DA-mediated behaviors. For example, a recent human functional magnetic resonance imaging study suggests that functional polymorphisms in genes that influence DA transmission, such as the DA transporter and metabolizing enzyme catechol-O-methyl transferase, will modulate reward-seeking behavior and potentially predispose to addictive behaviors and other neuropsychiatric disorders (Dreher et al., 2009). In rodents, sex chromosome-linked genes have clearly been shown to be instrumental in creating sex differences in mid-DA systems. It is noteworthy that expression of the Sry gene, uniquely present in males across species, not only determines the greater size of the midbrain DA population in male rodents but also affects male DA-dependent locomotor behaviors (Dewing et al., 2006). X-chromosome linked gene(s) also play a major role in the genetic influences on sexual dimorphism in TH activity (Vadász et al., 1985) and in food-reinforced habit formation (Quinn et al., 2007). It is noteworthy that the former seems to depend on gonadal steroid levels during a critical period of development (Vadász et al., 1988), which highlights the complexity and importance of hormonal influences on gene networks.
Whatever the underlying mechanisms, the available evidence suggests that hormone-based mechanisms offer novel targets for controlling reward systems in the brain and should be important considerations in the development of improved treatments for drug abuse. Further research is needed to define precisely the different pathways that subserve drug abuse in males and females, including the relative importance of developmental and adult influences of gonadal hormones as well as genetic influences. Although current knowledge indicates the potential for estrogen-based strategies in women, it also highlights the sex differences in underlying processes that would profoundly influence the likely efficacy of similar treatments in men. These findings further underscore the importance of considering male and female individuals separately.
VII. Learning, Memory, and Alzheimer's Disease
Complex behaviors are clearly the result of complex interactions between different brain regions, and our understanding of the contributions of specific pathways to cognition and mood are far from complete. However, substantial evidence in humans and experimental animals documents sex differences in specific cognitive and behavioral tasks (De Vries, 2004; Cahill, 2006; Cosgrove et al., 2007). Whether males or females have the advantage depends on the task. For example, men generally outperform women on visuospatial tasks, quantitative tasks, and targeted motor skills, whereas women excel in verbal skill tasks, perceptual tasks, and fine motor skills (Sherwin, 2003; Sherwin and Henry, 2008). The popular view that men are better at reading maps, whereas women talk for longer on the telephone, could clearly introduce pressures of social stereotyping into the equation (Halpern and Tan, 2001). However, a recent analysis spanning data from 40 countries to examine the effect of cultural and social factors on young adults concluded that girls outperform boys in reading scores, irrespective of societal factors, whereas the math gender gap, where girls score consistently less than the boys, could be closed by positive economic developments (Guiso et al., 2008). Whatever the reason or cause for sex differences in memory and performance on cognitive tasks, age-related decline in working and long-term memory is a hallmark of human aging and may signal impending disease (Janowsky, 2006). In each sex, performance on cognitive tasks have been correlated with circulating sex hormones in humans and rodents (Christiansen and Knussmann, 1987; Hampson, 1990; Dohanich, 2002; Sherwin, 2002, 2003; Gibbs and Gabor, 2003; Brinton, 2004, 2008; Korol, 2004; Gibbs, 2005; Luine, 2008; Sherwin and Henry, 2008; Spencer et al., 2008; Pike et al., 2009; Rosario et al., 2009), and an understanding of the roles of sex hormones and their possible exploitation therapeutically, therefore, has been very actively pursued. This is especially important for age-associated dementia and mild cognitive impairment, as well as AD, which are conditions affecting tens of millions of people worldwide, a figure that is set to escalate dramatically over the next few decades.
A. Epidemiological and Clinical Evidence for the Effect of Estrogen on Cognition in the Normal Brain and during Cognitive Decline
AD, like PD, is a multifactorial disease, the underlying causes of which are unknown; unlike PD, however, being female rather than male features among a number of risk factors, after aging, for developing the disease (Henderson et al., 2000; Pike et al., 2009). Some have suggested that this may be attributable to the longer life expectancy in women rather than sex-specific risk factors, but clear sex differences in pathological features of AD and its relationship to behavioral disturbances indicate a biological basis for the differences (for review, see Cahill, 2006; Bao et al., 2008). The dramatic loss of estrogens at menopause is generally acknowledged as a risk factor for women to develop AD (Sherwin, 2002; Brinton, 2004; Pike et al., 2009). Although there is no consensus on differences in circulating levels of estradiol in control subjects and women with AD, brain estrogens have been shown to be lower than normal in female subjects with AD (Yue et al., 2005; Rosario et al., 2009). In this context, it is interesting to note that aromatase expression is altered in AD brains (Ishunina et al., 2005), and single nucleotide polymorphisms in the CYP19 (aromatase) gene are among genetic factors associated with the risk of developing AD (Hiltunen et al., 2006), raising the possibility that the generation of protective levels of estrogens in the AD brain may be compromised. Early views that estrogens are effective in protecting against memory decline in healthy postmenopausal women and in delaying the onset of AD were largely observational in nature and lacked the relevant controls. Therefore, to establish the value of estrogen therapy for age- and menopause-related cognitive decline and AD, a number of large clinical studies have been conducted over the last decade or so, including the randomized, double-blind, placebo-controlled Women's Health Initiative Memory Study (WHIMS). These resulted in many contradictory findings, culminating in cessation of the WHIMS trial and the recommendation that “use of hormone therapy to prevent dementia or cognitive decline in women 65 years of age or older is not recommended” (Eberling, 2002; Shumaker et al., 2004). Many excellent reviews discuss these important controversies and highlight factors that are likely to account for them, such as differences in the dosing regimens, the types of estrogens used, the route of administration and, in retrospect, possible flaws in the original experimental designs (Sherwin, 2002; Brinton, 2004; Craig et al., 2005; Gleason et al., 2005). In the interim, a novel concept has emerged that seems to reconcile, but not yet explain, the positive versus negative effects of estrogens on cognition. This has been defined by two inter-related hypotheses, termed the healthy cell bias of gonadal hormone action hypothesis (Brinton, 2005, 2008) and the critical window hypothesis (Sherwin and Henry, 2008). In essence, treatment with estrogens in young women with surgically or pharmacologically induced menopause, or at the time of natural menopause (preventive mode of treatment), suggest that estrogens have a positive effect on cognition and reduce the risk of developing AD (Eberling, 2002; Zhao and Brinton, 2007; Sherwin and Henry, 2008; Rosario et al., 2009). In contrast, the use of hormone therapy, whether estrogens (conjugated equine estrogens) alone or in combination with progesterone (medroxyprogesterone acetate), initiated several years or more after menopause to women free of neurological disease, failed to show any positive effects and, if treatment was prolonged beyond 5 years, the risk of developing dementia was increased, possibly as much as 2-fold (Brinton, 2005; Gleason et al., 2005). In addition, no benefit was obtained if treatment was commenced once AD is established. Therefore, the emerging view posits that if neurons are healthy at the time of estrogen treatment (the healthy cell bias), and have not been deprived of estrogens for significant periods (the critical window), there are beneficial effects on neurological function and neuron survival in women; in contrast, prolonged exposure of unhealthy neurons to estrogens will exacerbate damaging neurological processes (Brinton, 2004, 2005; Naftolin and Malaspina, 2007; Sherwin and Henry, 2008). A corollary states that the brain looses its sensitivity to estradiol with aging (Brinton, 2008; Sherwin and Henry, 2008; Pike et al., 2009). The human brain does possess large numbers of different ER splice variants; although their roles are unclear, it is pertinent to note that their expression changes with aging and disease, such as AD (Ishunina and Swaab, 2008).
In contrast to the extensive data on women, there is a relative paucity of studies in men, probably because the age-related fall in endogenous gonadal steroids is far less dramatic. Nonetheless, it has been estimated that circulating free testosterone levels decline at the rate of 1 to 2% annually in elderly men, with 20% of men considered hypogonadal at 60 to 69 years of age, rising to 50% after 80 years of age (Matousek and Sherwin, 2010; Rosario et al., 2009). This decline in circulating testosterone has been associated with the risk for cognitive decline and AD in aging men (Sherwin, 2003; Pike et al., 2009); it has also been suggested that a higher circulating level of estradiol rather than low testosterone constitutes a risk factor (Geerlings et al., 2006), raising the possibility that estrogens may not be universally protective. In men, however, circulating estrogens are derived principally from the conversion of testosterone to estradiol by tissue aromatases, so circulating levels of both hormones in men generally follow each other (Matousek and Sherwin, 2010). This clearly makes it difficult to ascribe any effects of exogenous or endogenous testosterone to the activation of androgen- or estrogen-dependent pathways, but a role for the latter in preservation of male cognitive abilities has been recognized (Sherwin, 2003; Janowsky, 2006; Pike et al., 2009). In particular, estrogens seem to be important for certain aspects of memory in aging men, but not spatial memory, which is improved by androgenic actions (Sherwin, 2003; Cherrier et al., 2005, 2007). However, estradiol treatment failed to improve short- or long-term memory in elderly men receiving androgen blockade therapy for prostate cancer (Matousek and Sherwin, 2010), and more studies will be needed to unravel the various contributions of each hormonal pathway. It should also be considered that circulating hormone levels may not be the only factor because, just as low brain estradiol levels are linked with AD in women, so are low brain androgens, not estrogens, associated with AD in men (Rosario et al., 2009), suggesting that the profile of brain sex hormones in AD is sex-specific. Coupled with the knowledge that estrogens in women and androgens in men are generally positively associated with cognition, this adds to the view that hormonal contribution to AD pathogenesis may be sex-specific (Rosario et al., 2009).
On balance, the human studies have raised more questions than answers. Brain imaging studies will undoubtedly be an important way to address some of these issues and are already revealing that increased regional blood flow to specific brain regions, such as the prefrontal cortex and hippocampus, that subserve memory functions and decline in AD, bring to light positive associations between performance on tests of memory/cognition and estradiol concentrations at different phases of the menstrual cycle (Craig and Murphy, 2007a; Craig et al., 2008; Brinton, 2009). Imaging studies are also providing fascinating insights to suggest that even if no sex difference exists behaviorally, there are clear sex-related differential activations in various higher centers of the brain associated with memory tasks (De Vries, 2004; Piefke et al., 2005; Cahill, 2006). This indicates that the underlying neural circuitry may not be the same and that cognitive strategies are significantly different in men and women. In support of this, sex differences in specific cognitive and behavioral tasks may be correlated with structural and functional sex differences in humans and experimental animals in brain regions especially associated with learning, memory, and emotions, such as the hippocampus, amygdala, striatum, and neocortex (De Vries, 2004; Cahill, 2006; Cosgrove et al., 2007). However, as yet there is negligible information from human studies to understand the extent to which these differences are due to the activational or organizational actions of estrogens. The ability of testosterone to enhance spatial performance in men and estradiol to enhance verbal memory in women is taken as evidence for well established sex differences in cognitive functioning (Matousek and Sherwin, 2010), but is this solely because men have more testosterone and women have more estrogen, or is there a biological basis for these differences? Some studies investigating the efficacy of phytoestrogens (plant compounds resembling mammalian estrogens) in young men and women suggest there could be some underlying sex dimorphisms in response to estrogenic compounds, because following a high soy diet was significantly correlated with certain aspects of memory in both sexes, but only women benefited in tests of letter fluency and planning (Zhao and Brinton, 2007). However, the soy diet is a complex mix of compounds, and potential interactions with endogenous sex steroids cannot be eliminated in such studies, which should be interpreted cautiously. Nonetheless, it is an important goal to understand the contribution of sex hormones to sex differences in cognitive strategies and why AD pathology and dementia differ in men and women (Pike et al., 2009), because it will advance our understanding of the disease and open up avenues for sex-specific preventive strategies and therapies. An ever-expanding literature in basic neuroscience offers many insights into this subject and promises to inform the judicious use of hormone therapy in humans.
B. Preclinical Studies
1. Alzheimer's Disease Models.
The human sex differences in AD-like pathology are also present in several transgenic mouse models of AD, strengthening the view that the female brain is more vulnerable to AD pathogenesis (Pike et al., 2009). The hallmarks of AD that may be recapitulated in these studies include extracellular deposition of β amyloid peptide (Aβ) [the enzymatic cleavage product of amyloid precursor protein (APP)]; hyperphosphorylation of tau (a cytoskeletal protein), leading to the formation of neurofibrillary tangles; and neuroinflammatory responses that are due to activation of glial cells, especially microglia. Together, these processes lead to synaptic and neuronal loss. Studies using cell cultures or cell lines suggest that protective actions of estrogen may be due to an ability to interfere with all these processes. For example, estradiol can reduce Aβ accumulation either by modulating APP processing or by increasing its clearance, which could be due to effects on enzymatic degradation of Aβ and/or microglial phagocytosis of the toxic peptide; the consequent reduction in Aβ toxicity prevents the damaging chain reaction of mitochondrial dysfunction, increased production of reactive oxygen species, and neuronal apoptosis (Li et al., 2000; Yue et al., 2005; Brinton, 2008; Pike et al., 2009). Estradiol may also inhibit hyperphosphorylation of tau. In its own right, estradiol may also exert its general protective effects that are not necessarily specific to AD, including increased expression of antioxidant enzymes to lower oxidative stress and modify the expression of proapoptotic genes (as discussed in section IV.B.1). All these processes have generally been shown to depend on ERα/β using both classic genomic and nonclassic mechanisms, and available data from many experimental neurodegenerative paradigms suggest that both receptor isoforms are important, but each is likely to act against AD-related insults via unique signaling pathways (Rosario et al., 2009). Conclusions from in vitro work are generally borne out in vivo in studies using female rats, guinea pigs, and some AD transgenic mouse models. Hence, ovariectomy increases the level of soluble Aβ in the brain, which directly impairs synaptic plasticity and memory (Shankar et al., 2008; Pike et al., 2009), and this is reversed by estradiol treatment (Yue et al., 2005; Pike et al., 2009).
In contrast to females, treatment of male rats with estradiol failed to reverse elevations in brain Aβ levels induced by orchidectomy, whereas treatment with the nonaromatizable androgen DHT was successful (Ramsden et al., 2003a). These results suggest that estradiol effects are sexually dimorphic, and sex hormone protection against Aβ is due to ER-dependent mechanisms in females but AR-dependent mechanisms in males. It is noteworthy that these preclinical observations are in agreement with the view, based on clinical observations, that the contribution of estrogens and androgens to AD pathogenesis is sex-specific, as discussed above. In contrast, in vitro studies suggest that some aspects of androgen protection, including regulation of Aβ generation via APP proteolysis, may be mediated indirectly after aromatization to estradiol (Pike et al., 2009). Recent work, suggesting that a metabolite of DHT, 5α-androstane-3β,17β-diol, is active at ERβ, not ERα or AR (Lund et al., 2006; Pak et al., 2007; Handa et al., 2008), could also suggest that AR and/or ERβ, but not ERα, mediate the effects of DHT on Aβ levels in males. Further work will clearly be important to understand to what degree the direct application of hormones to cultured neurons, which are capable of producing their own steroids in vitro (Prange-Kiel and Rune, 2006), reflects the effects of systemic hormones in vivo. In male rodents, direct activation of AR-dependent pathways seems to include a suppression of Aβ accumulation, involving increased expression of the Aβ-catabolizing enzyme neprilysin as well as attenuation of oxidative stress, suppression of pro-apoptotic pathways, and activation of intracellular signaling pathways, such as MAPK/ERK. Although less well researched, androgens also seem to share the phenomenon that is characteristic of estrogens: they may be protective at physiological concentrations but harmful at pharmacological concentrations (Pike et al., 2009). It is of interest to note that androgens working through AR-dependent mechanisms seem to be the main protectors in clinical and experimental AD, which contrasts with PD, where the consensus seems to be that endogenous androgens are not protective (Yu and Wagner, 1994; Dluzen, 1996; Murray et al., 2003; Myers et al., 2003) and may even be deleterious (Murray et al., 2003). These observations serve to highlight the fact that sex hormones contribute to sex differences in disease susceptibility by very different mechanisms, depending on the nature of the underlying lesion and pathways involved.
Analysis of neuron viability after challenge with excitotoxic lesions is another experimental approach that has been used to assess the neuroprotective effects of sex hormones in the hippocampus. The literature contains evidence to suggest that estradiol is and is not protective in male rodents, but these contradictions may well be explained by differences in the experimental regimens. For example, one study found that excitotoxic lesions induced by intraperitoneal injections of kainic acid were greater in male rats that had been castrated for 4 weeks compared with control rats, and this effect was reversed by continuous replacement with physiological levels of DHT, not estradiol, for 2 weeks before lesion (Ramsden et al., 2003b; Pike et al., 2009). In contrast, a single, large dose of estradiol, not DHT, given 3 weeks after castration at the same time as an intraperitoneal injection of domoic acid, reversed the ability of gonadectomy to increase lesion size in male mice (Azcoitia et al., 2001). The same study confirmed that central aromatization of peripherally administered testosterone was required for protective action, supporting the likely involvement of ER-dependent mechanisms. Apart from differences in species and excitotoxin (which should work by identical mechanisms), analysis of the context of each of these studies supports our proposal that steady, physiological levels of sex hormones exert one type of action [in this instance, mediation of protective actions in males by AR, not ER (Ramsden et al., 2003b; Pike et al., 2009)], whereas a large bolus dose may be protective through different mechanisms [in this instance, ER, not AR, mediated protective actions in Azcoitia et al. (2001)]. Other variables in these studies include the duration between gonadectomy and hormone treatments, which the healthy cell bias and critical window hypotheses suggest is important, and also the fact that one study reported on cell loss in the hilus of the hippocampus (Azcoitia et al., 2001), whereas the other observed effects in the CA2/3 regions (Ramsden et al., 2003b). Together, these observations highlight the many factors that impinge on the nature of hormonal influences and emphasize the need for further investigations in male as well as female subjects to reconcile the many contradictions and realize the promises of our current knowledge.
2. Learning and Memory.
In addition to investigating sex hormone actions in specific models of AD and neurodegeneration, preclinical studies have also revealed notable sex differences in hormonal influences on cognitive behaviors and their underlying neurobiological correlates (such as the expression of neurotransmitters and their receptors, synaptic contacts, electrophysiological parameters, and neurogenesis) that may become dysregulated in specific brain regions affected by AD.
a. The hippocampus.
i. Behavior.
The hippocampus plays a major role in regulating learning, memory, and emotional responses (stress, fear), as well as spatial, declarative, and contextual memory in humans and in animals used in research. Spatial learning and memory are impaired in AD. Therefore, the hippocampus has become a particular research target because of its important cognitive role. In experimental mammalian species, such as rats, and in humans, males perform better than females, on average, in the acquisition of tasks involving spatial memory, which are highly dependent on the hippocampus (Luine, 2008; Mitsushima et al., 2009b). The activational influences of adult gonadal steroids play an important role in maintaining sexually differentiated cognitive behaviors. Many studies investigating only females report a positive effect of estrogens on hippocampal-dependent tasks in rats, mice, and rhesus monkeys (for review, see Foster et al., 2008; Luine, 2008; Spencer et al., 2008). Relatively few studies have made direct comparisons of males and females, but in some tests of hippocampal spatial discrimination (the Morris water-maze), hormone treatment of gonadectomized mice revealed that estradiol selectively impaired performance in females but had no effect in males (Fugger et al., 1998). Several reports suggest that functional sex differences are related to organizational influences in early development (Roof, 1993a,b; McEwen, 1999; Romeo et al., 2004). For example, exposure to high levels of estradiol during development improves (masculinizes) spatial behavior in adult female rats to levels seen in normal males (Williams and Meck, 1991). It therefore seems that sex differences in aspects of hippocampal function are determined by testosterone acting after conversion to estrogen by aromatase in a manner similar to that established for the hypothalamus, indicating that nonreproductive brain regions are subject to hormonal processes of sex differentiation similar to those in brain regions intimately associated with reproduction. It should be noted, however, that in other tests of hippocampal function (a delayed matching-to-position task), estradiol treatment of rats gonadectomized as adults enhanced task acquisition in both sexes and, although testosterone treatment of males was without effect on this component of the task, testosterone did affect delay-dependent working memory (Gibbs, 2005). On balance, it seems that estrogens and androgens influence different aspects of cognitive tasks or domains in males and females, with indications that these effects are sex-specific (Warren and Juraska, 1997; Gibbs, 2005). Indeed, it is generally thought that males and females use different strategies, underpinned by different organization of the underlying neural substrate, to solve similar spatial tasks; females tend to rely more on local cues and landmarks, whereas males rely more on the spatial relationships between two fixed points (Raber, 2008). The available evidence also suggests that hormonal influences can differ with the type of task, the aspect of the task under investigation (acquisition, consolidation, retrieval), and the degree to which the task relies on input from brain regions other than the hippocampus, such as the prefrontal cortex, which is associated more with working memory tasks requiring visual object information (Robbins, 2000; Takase et al., 2009). Therefore, at behavioral level it is very difficult to reach simple interpretations on the influences of estradiol or testosterone on cognition in males and females. In contrast, striking sexually dimorphic responses to estradiol have been reported for neuroanatomical, morphological, neurochemical, and electrophysiological correlates of learning and memory.
ii. Structural, neurochemical, and electrophysiological correlates of learning and memory.
Many studies report clear sex dimorphisms in hippocampal structure and morphology in several species (rodents, nonhuman primates, and humans) that are thought to be related to functional differences (Roof, 1993a,b; McEwen, 1999; Swaab et al., 2003; Romeo et al., 2004). In very simple terms, the hippocampal formation comprises the dentate gyrus, which receives the main hippocampal input from the entorhinal cortex; the flow of information proceeds to the CA3 region, then the CA1 region and subiculum, with the main outflow arising from the latter two regions. Sex differences are observed in the size of perikarya of CA1 and CA3 pyramidal cells, in their number of primary dendrites and degree of dendritic branching, and in the number of glial cells in the CA1 and CA3 regions (Romeo et al., 2004). In terms of sex hormone actions, particular attention has been given to the density of dendritic spines, mainly in the CA1 pyramidal cells, because these structures receive excitatory inputs that are key to synaptic plasticity and learning (Kandel, 2001; Kasai et al., 2003). The loss of dendritic spines is also a major feature of hippocampal synaptic pathology in AD that correlates strongly with cognitive decline (Scheff and Price, 2003). In female rats and monkeys, endogenous and exogenous estradiol rapidly (within minutes) increases spine density and synaptic density in the CA1 region (Woolley, 1999; Leranth et al., 2003; Cooke and Woolley, 2005; McEwen and Milner, 2007; Spencer et al., 2008), which coincides with enhanced long-term potentiation (an electrophysiological correlate of learning and memory) and some hippocampal-dependent behaviors (Gibbs and Gabor, 2003; Daniel, 2006; Luine, 2008). The female hippocampus has a relative paucity of nuclear ERs, but effects could be mediated directly and rapidly via non-nuclear ERs, especially ERα, located in the hippocampal dendritic spines themselves, or via effects on glial cells, which also express ERs (McEwen and Milner, 2007; Spencer et al., 2008). The ability of estradiol to increase NMDA receptor expression in the dendritic field of the CA1 pyramidal cells is also a necessary mechanism for estrogen-induced spine formation in female rats (Romeo et al., 2005). This, in turn, requires an increase in hippocampal acetylcholine (ACh) release that is due to estradiol-dependent activation of basal forebrain cholinergic neurons, specifically those in the medial septum and horizontal limb of the diagonal band of Broca (Luine, 1985; Leranth et al., 2000; Lâm and Leranth, 2003; Romeo et al., 2004; Cooke and Woolley, 2005; Mitsushima et al., 2008). This septo-hippocampal pathway is essential for driving long-term potentiation in the hippocampal CA1 neurons, and its destruction in female rats impairs acquisition of a spatial memory task as well as the ability of estradiol to enhance memory acquisition (Gibbs, 2002), indicating the importance of trans-synaptic mechanisms in mediating estrogenic influences on hippocampal memory and learning. Estradiol-dependent suppression of hippocampal GABAergic interneurons is a further trans-synaptic mechanism, leading to dis-inhibition of CA1 pyramidal cells and increased spine density in female rats (Murphy et al., 1998; Cooke and Woolley, 2005). This could be due to a direct effect on the GABAergic interneurons or indirect actions via the basal forebrain cholinergic neurons, both of which possess ERα (Rudick et al., 2003). Although some studies favor a role for ERα in regulating synaptic plasticity (Foster et al., 2008; Spencer et al., 2008), other work suggests that ERβ plays the dominant role in improving hippocampal-dependent cognition (Liu et al., 2008).
Studies in male rodents suggest that hippocampal synaptic plasticity is maintained by hormone-dependent mechanisms that differ markedly from those seen in females. Castration reduces CA1 spine density in males, but, unlike responses in females, estrogen administration to males fails to increase spine density (Leranth et al., 2003; Lee et al., 2004), to up-regulate choline acetyltransferase activity in basal forebrain cholinergic neurons (Luine et al., 1986), to increase ACh release in the hippocampus (Mitsushima et al., 2009a), and to up-regulate NMDA receptors (Romeo et al., 2005). Reports of sex differences in ER expression are variable (Weiland et al., 1997; Zhang et al., 2002); significantly, a relative lack of ERα in males compared with females has been noted in hippocampal dendritic spines and GABAergic interneurons. In contrast to the failure of ER activation to induce spine formation in males, activation of ARs by testosterone or DHT is effective, and this is also associated with up-regulation of NMDA receptors (Leranth et al., 2003; Romeo et al., 2005; Hajszan et al., 2008), but with little effect on cholinergic input (Romeo et al., 2004). It is noteworthy that the cellular and subcellular localization of hippocampal ARs is distinct from that of ERs, suggesting that ARs in the male hippocampus does not simply “usurp” the role of ERs in females. In particular, unlike ER-labeled terminals, AR-containing terminals in the male hippocampus almost exclusively form asymmetric synapses, suggesting their presence primarily in excitatory afferents, with relatively little influence on inhibitory tone (McEwen and Milner, 2007). Also in contrast with ER location in the female hippocampus, male GABAergic interneurons lack nuclear AR, whereas pyramidal cells have a relative wealth of nuclear and extranuclear ARs (Romeo et al., 2004; McEwen and Milner, 2007; Hajszan et al., 2008). Thus it has been proposed that the trans-synaptic influences involving the cholinergic input and GABAergic interneurons that are all-important for the estrogenic influences on synaptic plasticity in females seem to be relatively unimportant in mediating the androgenic influences in males, where direct actions on pyramidal neurons seems more likely (Romeo et al., 2004).
Although estrogens fail to increase dendritic spines in male hippocampal regions, androgens were found to be effective in females as well as males (Hajszan et al., 2008). Despite the fact that the affinities for the classic AR and the order of potency in maintaining prostate gland weight are DHT > testosterone > DHEA, all compounds were equally effective in maintaining CA1 synaptic density in castrated male rats; flutamide, an AR antagonist in the periphery, was approximately half as potent as the androgens in males and females (Hajszan et al., 2008). Because ARs are also expressed in female hippocampal pyramidal cells, this could potentially be a direct effect of androgens on these neurons. However, in females, but not males, the ability of testosterone to increase hippocampal spine synapses was blocked by inhibition of aromatase, indicating an ER-dependent mechanism. DHT was somewhat less potent in females than testosterone, and its effects were unaffected by aromatase inhibition (Hajszan et al., 2008). However, metabolites of DHT are known to have activity at ERβ (Handa et al., 2008) and are allosteric modulators of GABAA receptors (Frye et al., 1996), both of which offer alternatives to actions at AR. It is noteworthy that DHEA, which is a weak androgen in peripheral tissues but can be metabolized to several neuroactive compounds in the brain, including estradiol and GABAA receptor modulators, was equipotent with testosterone in inducing hippocampal spinogenesis in male brains, and was also effective in females (Hajszan et al., 2008). This raises the novel possibility that a compound such as DHEA, which is known to have very little hormonal activity peripherally, could be an effective pro-drug for different central processes with the same outcome in males and females, namely increased hippocampal spine synapses formed via ER/GABAergic and AR/GABAergic mechanisms in males and females, respectively.
iii. Developmental origins of sex differences.
The failure of estradiol to induce hippocampal spine formation and up-regulate transmission in the septo-hippocampal cholinergic projections in the male brain is dependent on the aromatization of testosterone and estradiol exposure in the neonatal period (Luine et al., 1986; McEwen, 1999; Mitsushima et al., 2009a). This is evidence that masculinization of the hippocampus occurs in much the same way as the hypothalamus, and predicts that responsiveness to estrogen will be suppressed in adulthood. This knowledge is particularly important because it tells us that the wealth of data on the neuroprotective effects of estradiol especially relating to hippocampal dysfunction in AD is unlikely to be beneficial in male subjects, where androgenic mechanisms are likely to be much more important. Because androgens in male individuals seem to be working on quite a different neural substrate compared with that on which estrogens act in females, this work further highlights the importance of understanding these sex-specific mechanisms more clearly to develop sex-specific medicines.
iv. Estrogen receptors and membrane signaling.
In addition to the sexually dimorphic effects of estradiol on hippocampal morphology and synaptic inputs discussed above, recent evidence suggests that rapid membrane actions of estradiol that alter intracellular signaling pathways may differ in hippocampal neurons derived from male and female rat pups. Specifically, activation of ERα using very low physiological concentrations of estradiol rapidly increased phosphorylation of CREB (pCREB) in cultures derived from female, but not male, hippocampus (Boulware et al., 2005; Mermelstein, 2009). Although the reasons for these differences are not yet clear, the positive effect on pCREB in female hippocampal neurons was dependent on activation of metabotropic glutamate receptor type 1 (mGluR1), which, along with mGluR5, comprise group 1 mGluRs; these, in turn, are linked to the G-protein Gq, leading to activation of phospholipase C/inositol triphosphate, diacylglycerol opening calcium channels, and increasing MAPK-dependent pCREB (Mermelstein, 2009). This increases NMDA activity principally at postsynaptic sites. Conversely, estradiol was also able to reduce pCREB because of an interaction of either ERα or ERβ with group II mGluRs (mGluR2 and/or mGluR3), which are coupled to Gi/o second-messenger signaling, leading to a reduction in intracellular cAMP. The pairing of ERs with specific mGluRs, and hence determination of whether downstream signaling is increased or decreased, seems to depend on which caveolin protein is available to facilitate ER coupling with various G-protein coupled receptors at sites associated with the cell membrane. This fascinating finding clearly opens up potential mechanisms that could underlie the great diversity in ER actions in the male and female brain (Mermelstein, 2009). Although it remains to be seen whether the sex-specific effects of estradiol on neonatal hippocampal neurons are present also in adult cells, it is noteworthy that ER effects were silenced in the male hippocampus at the critical time for sexual differentiation (Boulware et al., 2005), which seems to be an emerging pattern in masculinization of the brain. In addition to the classic ERs, estrogens may exert rapid and transcriptional responses via the novel transmembrane G-protein-coupled receptor GPR30 (Prossnitz et al., 2008), as well as ER-X, a membrane-associated receptor expressed in the brain during development and injury (Toran-Allerand, 2005). It is not yet known whether these receptors contribute to sex differences in the brain.
v. Neurogenesis.
Contrary to early beliefs, the birth of new neurons occurs on a daily basis at discrete loci in the adult brain of many mammalian species, including the subgranular zone of the hippocampal dentate gyrus (Leuner et al., 2006; Galea, 2008). Although there is still some debate, a body of evidence suggests that neurogenesis (the balance between cell birth and natural cell death), coupled with integration of newborn neurons into local hippocampal circuitry, is important for cognition, possibly in the formation of trace memories (Shors et al., 2001; Leuner et al., 2006). Many newborn adult neurons die within a few weeks of birth, and there is a critical time period when environmental factors, including hormones, play an important role. The finding that repeated estradiol treatment can increase hippocampal neurogenesis in female rats, therefore, is of potential significance for the use of hormonal treatments for neurodegenerative disease and cognitive decline (Barker and Galea, 2008). However, no such effects were observed in male brains. This line of investigation is relatively new, and more work will be needed to establish the functional significance of the findings. They do, however, emphasize the importance of pursuing this work in both sexes.
b. The prefrontal cortex.
The prefrontal cortex plays an important role in working or short-term memory in various mammalian species, including rats, nonhuman primates, and humans. Tests of prefrontal functions reveal many sex differences: females generally outperform males in the acquisition of tasks that rely more heavily on this region, such as visual object recognition (Silverman and Eals, 1992; Kritzer et al., 2007; Luine, 2007; Mitsushima et al., 2009b). Although the organizational and activational influences of sex hormones have not be studied as extensively in this region as the hippocampus and hypothalamus, emerging evidence suggests that both estrogens and androgens have significant influences in both sexes (Kampen and Sherwin, 1996; Gibbs, 2005). For example, estradiol promotes performance in memory tasks in women (Berman et al., 1997; Keenan et al., 2001), female rhesus monkeys (Rapp et al., 2003; Tang et al., 2004), and rats (Wallace et al., 2006), and circulating levels of both estradiol and testosterone correlate with certain spatial and mnemonic tasks in female rats (Kritzer et al., 2007). In adult male rats, gonadectomy impairs performance in various tasks of working memory and other types of cognitive tasks that are known to rely on the PFC, but the hormone responsiveness depends on the task and probably the neurotransmitter pathways involved. Hence, testosterone, but not estradiol, reversed the effects of castration on performance in tests of spatial working memory, and this correlated with the density of dopaminergic terminals in the medial PFC as well as AR expression in the mesocortical dopaminergic neurons of the ventral midbrain populations that project to the mPFC. This suggests a principal (nuclear) site of action of androgens on the mesocortical dopaminergic system, which is known to be critical for working memory (Kritzer et al., 2007). In contrast, effects of castration on performance in tests involving motivation were reversed by estradiol, not androgen, treatment, with no effects on mPFC dopaminergic axon density. Yet other aspects of PFC function involving impulsivity were unaffected by castration or hormone treatment, which also did not alter mPFC dopaminergic axon density (Kritzer et al., 2007). Using retrograde labeling, the same study reported a similar distribution of AR in VTA dopaminergic neurons projecting to the PFC in males and females, but the nondopaminergic cells projecting to the PFC were found to be ERβ-positive, not ERα-positive, in males and vice versa in females. This is likely to indicate sex-specific effects of estradiol, which merit further investigation.
Another major pathway associated with PFC function is the cholinergic input from the basal forebrain nuclei, which arises principally from the nucleus basalis of Meynert (nucleus basalis magnocellularis in rats) and the diagonal band of Broca. Both the number of cholinergic cell bodies in the nucleus basalis of Meynert and the extracellular levels of dopamine in the PFC are greater in female rats compared with males, and this may underlie the superior performance of females in certain tasks of PFC function (Mitsushima et al., 2008). This contrasts with the cholinergic neurons in the medial septum and horizontal limb of the diagonal band of Broca, which project to the dorsal hippocampus, where ACh release is greater in males than in females and is associated with the superior performance of males on hippocampus-dependent cognitive tasks (Mitsushima et al., 2009a). As discussed above, perinatal masculinization by testosterone (after aromatization) imprints these sex dimorphisms in the septo-hippocampal pathway, including the inability of the adult male pathway to respond to estradiol (Mitsushima et al., 2009b). The origins of sex differences in the PFC cholinergic projections are not known, but they are likely to be similar, because estradiol has been reported to increase choline acetyltransferase in the PFC of adult gonadectomized female, but not male, rats (Luine, 1985).
Like the hippocampus, the PFC retains the capacity for synaptic remodeling, which is also critical for learning and memory that is lost in AD (Scheff and Price, 2003). Also as in the hippocampus, NMDA receptors play a significant role in this phenomenon, as evidenced by the ability of NMDA receptor antagonists to disrupt PFC-dependent cognition and reduce the number of asymmetric spine synapses (indicative of excitatory input) in the male PFC (Hajszan et al., 2008). However, unlike the hippocampus, estradiol can increase spine synapses in the castrated male rat PFC, albeit not as potently as androgens (Hajszan et al., 2007). Estradiol also maintains dendritic spines in specific cortical regions of female nonhuman primates (Tang et al., 2004) and rats (Wallace et al., 2006), in parallel with positive effects on working memory; preliminary studies indicate that androgens may also positively affect spine synapses in the female PFC (Hajszan et al., 2008). Although work in this brain region is still at an early stage, both morphological and behavioral studies suggests that the PFC uses sex hormones in a manner different from that seen in the hippocampus and hypothalamus, suggesting that hormonal therapeutic strategies to modulate the function of each of these brain regions could be achieved by a different cocktail of hormone supplementation that might be unique to males or females. As yet, it is not clear to what extent sex differences in PFC function may be hormonally programmed; however, because aspects of estrogen sensitivity are retained in both the male and female PFC, it is interesting to speculate that this brain region is not targeted in the same manner by the early masculinizing events. The present state of knowledge also raises the possibility that androgen modulation of cortical processing in the female brain is physiologically important and clinically relevant (Hajszan et al., 2008).
VIII. Sex Dimorphisms in the Stress Response
To maintain homeostasis, individual organisms adapt continuously to changes in the internal and external environment (i.e., to physiological, physical and psychological “stressors”). The underlying processes that achieve this adaptation, termed collectively allostasis (stability through change), involve changes in the sympathetic nervous system, the hypothalamic-pituitary-adrenal (HPA) axis, and activity in limbic brain structures (hippocampus, amygdala, prefrontal cortex) (McEwen, 1998; Charmandari et al., 2005; de Kloet et al., 2005). The final common pathway whereby the brain controls this response lies in the parvocellular division of the hypothalamic PVN, where neuronal populations producing corticorelin (CRH) and arginine vasopressin (AVP) coordinate the neuroendocrine, behavioral, autonomic, and immune responses to stress (Gillies et al., 1982; Charmandari et al., 2005). Within the neuroendocrine HPA axis, CRH and AVP control anterior pituitary secretion of adrenocorticotropin, which, in turn, stimulates the adrenal cortex to produce the glucocorticoid (GC) stress steroid hormone (cortisol in humans, corticosterone in many species used in research). The GCs have potent actions throughout the body of higher organisms, including the brain, where they influence arousal, cognition, mood, and the autonomic nervous system and exert negative feedback on their own release via actions in the hypothalamus, hippocampus, and other limbic regions. GCs therefore have a critical role in orchestrating behavioral and systemic adaptive responses to stress. Central projections of neurons producing CRH, CRH-related peptides (urocortins), and AVP are also involved in many of the behavioral adaptive responses to stress, and the specific brain pathways that initiate or respond to stress will vary depending on the type of stress (Pacák and Palkovits, 2001). For efficient adaptive responses (effective coping with stress), the HPA axis reacts robustly, and its activation is terminated efficiently to prevent GC excess from damaging the body and brain. However, an excessive or prolonged response, as well as a failure to mount an adequate response, are both risk factors for developing common psychiatric conditions, especially depression, anxiety, addiction, and neurodegenerative disease, where stress has been identified as a strong risk factor (McEwen, 1998; de Kloet et al., 1999; Conrad et al., 2004b; Chrousos and Kino, 2007; Bao et al., 2008; Allen et al., 2009; Solomon and Herman, 2009). For example, in combination with genetic and environmental factors, a hyperactive HPA axis is associated with, and may even have a primary causative role in, the development of melancholic depression, whereas a hypoactive HPA axis is associated with atypical depression and post-traumatic stress disorder (Gold and Chrousos, 2002; de Kloet et al., 2005; Bao et al., 2008). It is noteworthy that the manifestations of these pathologies are sex-specific. For example, the 2-fold greater incidence of major depressive disorder in women compared with men is a considerable driving force to understand the underlying mechanisms (Solomon and Herman, 2009). Therefore, it is of particular clinical significance that neuroendocrine, autonomic, and behavioral responses to stress in humans (Chrousos and Gold, 1992; Wolf et al., 2001; Kudielka and Kirschbaum, 2005; Goldstein, 2006) and animals (Wood and Shors, 1998; Wood et al., 2001; Luine et al., 2007) are sexually dimorphic. In species used in research, there is good agreement that basal and acute stress-induced adrenocorticotropin and corticosterone levels are higher in the female circulation relative to that in males. The situation is less clear in humans. Although reports are varied, on balance it seems that the HPA axis response to psychological stressors is greater in men than women, although women reported a greater subjective experience of stress; in contrast, CRH administration, as a pharmacological test of HPA reactivity, elicited a greater pituitary/adrenal response in women (Kudielka and Kirschbaum, 2005). However, despite some species differences, stress exposure can lead to opposite or qualitatively different effects of the same stressful event in male and female subjects on emotional arousal, learning and memory in humans and species used in research (Leuner et al., 2004; Andreano and Cahill, 2006; Luine et al., 2007). Thus, although often characterized as the “fright, fight, or flight” response, this more accurately describes a male-typical response to stress, whereas “tend and befriend” has been proposed as a better description of the typical female response (Taylor et al., 2000).
The effects of stress on specific variables, such as HPA axis, autonomic and behavioral reactivity, and whether males or females have the biological advantage, may vary with the nature of response or cognitive task under investigation, as well as the type and duration (acute versus chronic) of stress (Conrad et al., 2004a; Luine et al., 2007), but sexually dimorphic responses are invariably present. Because of this complexity, conclusions on brain-stress interactions have to be qualified by the specific experimental paradigms used. However, there is broad agreement from studies using rodents that chronic stress produces deficits in hippocampal-dependent memory in male rats but enhances it in females (Bowman, 2005; Luine et al., 2007). This is associated with sex-specific hippocampal restructuring, in which apical dendritic atrophy in CA3 pyramidal neurons is seen in male but not female brains under the influence of chronic stress (Galea et al., 1997). Chronic stress also has pronounced sex-specific effects in behavioral tests of anxiety and depression, but whether this involves an increase, decrease, or no effect in males or females depends on the experimental paradigm used (Luine et al., 2007; Solomon and Herman, 2009). In addition, our recent work demonstrates that chronic stress in male, but not female rats, exacerbates lesion size in experimental PD (Allen et al., 2008, 2009). Reports of effects of acute stressors are more variable. For highly aversive tasks, stress may enhance learning in male rats but impair it in females (Wood and Shors, 1998; Shors, 2004). This effect is mirrored by changes in dendritic spine density in the hippocampal CA1 region, which is increased by acute stress in male rats but decreased in females (Bangasser and Shors, 2007). Conversely, in appetitive spatial recognition tasks and object recognition tasks, acute restraint stress may impair or have no effect on performance in males but facilitate it in females (Conrad et al., 2004a). Extensive studies in males show that stress-induced elevations in glucocorticoid levels are critical factors in determining the effects of stress on memory and learning (de Kloet et al., 1999). There are fewer data in females, but they suggest a different relationship. For example, although exposure to the same experience can have opposite effects on learning in male and female rats, a correlation with GC levels was found only in males, not females (Wood et al., 2001). In addition, similar serum corticosterone levels in response to the same acute stress have been reported in rats of both sexes, although performance on memory tasks was affected in opposite directions in males and females (Conrad et al., 2004a). Although clinical evidence for the effects of chronic stress in humans is difficult to obtain, exposure to acute stress was reported to enhance performance in a memory task in men, not women, despite raised GC levels in both sexes (Andreano and Cahill, 2006). Together, these data clearly illustrate that the relationship between HPA and behavioral responses to stress are not the same in male and female animals and supports the view that there are sex differences in the central pathways regulating the stress response.
Although the underlying mechanisms are unclear, estradiol plays an important role in generating these dimorphic effects of stress on central processes. Animal studies demonstrate that this includes both activational (feminizing) influences in adulthood of ovarian hormones (Wood and Shors, 1998; Wood et al., 2001; Conrad et al., 2004a; Luine et al., 2007), as well as organizational (masculinizing) effects in the neonatal period (Patchev and Almeida, 1998; Bowman et al., 2004; Bangasser and Shors, 2008), which program sex-specific circuitries in regions important for memory and learning, including the hippocampus, amygdala, PFC, and bed nucleus of the stria terminalis. These estrogenic influences prevail irrespective of whether the stressors are chronic or acute and whether male or female responses are impaired or facilitated. Stress-induced GC influences on memory are also modified by prevailing levels of estradiol in women (Andreano et al., 2008). It is noteworthy that the sex differences in adrenocorticotropin and cortisol responses to pharmacological tests of HPA axis responsiveness (CRH administration) and physical stress (exercise) are maintained in men and women even when circulating gonadal steroid levels are pharmacologically suppressed (Roca et al., 2005). Such sex differences in the stress response even in the absence of the activational effects of gonadal steroids, therefore, suggest fundamental sex differences in the organization of the underlying circuitry.
A further complexity for understanding stress responses is introduced by the finding that the behavioral consequences of stress or glucocorticoid exposure vary with age (Luine et al., 2007; Lupien et al., 2009). For example, although males normally outperform females in certain spatial tasks, exposure to stress prenatally impaired learning and memory in adult male rats but enhanced it in females compared with nonstressed controls (Bowman et al., 2004; Mueller and Bale, 2007). It is generally thought that males and females use different strategies for learning in spatial tasks, and it seems that these are altered by prenatal stress because of a demasculinization in males and a defeminization in females of the underlying circuitries at critical developmental stages. In accord with this, stressors and glucocorticoid treatment administered in the perinatal period are known to interfere with sexual differentiation of the brain (Ward et al., 1994). This may be due, at least in part, to the ability of GCs to oppose the action of estradiol (Uht et al., 1997), which is essential for masculinization of the developing brain (section I.B). Although little is known of the specific neural pathways involved, the midbrain dopaminergic systems are sexually dimorphic (sections IV and V), are implicated in learning, and are differentially reactive to stress in adult male and female rats (Allen et al., 2008; Dalla et al., 2008). Moreover, the sexually dimorphic cytoarchitecture of the dopaminergic neurons in the VTA and SNc is disrupted by elevations in glucocorticoid levels in the perinatal period, including a “feminization” of the pattern of distribution of the neurons throughout the region (Fig. 6) (McArthur et al., 2005, 2007a). This provides a possible mechanism and structural basis for functional change in the mesolimbic system that occurs after early-life exposure to stress (Meaney et al., 2002; Pruessner et al., 2004; Thomas et al., 2009). These observations are especially pertinent in view of the fact that exposure to traumatic experiences in early life compromises later ability to cope with stress and predisposes to development of psychiatric disorders (de Kloet et al., 2005; Glover et al., 2009) (Goldstein, 2006; Rao et al., 2010).
Fig. 6.
Sex-specific effects of antenatal glucocorticoid treatment on dopaminergic neurons in the adult rat VTA. Fetal rats were exposed to antenatal glucocorticoid treatment (AGT) by including dexamethasone (0.5 μg/ml) in the mother's drinking water between gestational days 16 and 19. The dams of control animals received normal drinking water. Offspring were allowed to grow to adulthood, when brains were processed immunocytochemically for identification of TH-IR cells as a marker of dopaminergic cell bodies in the VTA, as described in the legend to Fig. 4. Top, sex differences in the overall total number of TH-IR cells were preserved after AGT, but the total cell counts increased dramatically. Bottom, the distribution of TH-IR cells through the VTA (percentage at each level II–IV) was sexually dimorphic and AGT altered the topographical distribution in male and female brains such that more cells were located in the caudal regions. Further details in McArthur et al. (2007a). *, p < 0.05 versus male. ▴/▾ significant increase/decrease versus respective controls; p < 0.05.
Functional brain imaging studies in humans are now producing interesting data showing that men and women engage different neural substrates when experiencing mild stressful stimuli (Cahill, 2006; Wang et al., 2007) and that this is influenced by sex hormones (Goldstein et al., 2010). Together with the animal studies, these findings support the view that sex differences in the way that these circuitries adapt to stress, coupled with the actions of estradiol, are thought to contribute to the biological basis for sex dimorphisms in CNS disorders, including anxiety and depression, drug abuse, and neurodegenerative disease (Goldstein, 2006; Bao et al., 2008; Allen et al., 2009; Thomas et al., 2009). It is essential, therefore, that we have a better understanding of how male and female brains differ in their mechanisms to cope with stress and how this is influenced by gonadal hormones if we are to develop treatments for stress related disease with optimal effectiveness for both men and women.
Here we have discussed the view that stress predisposes to psychiatric disorders that predominate in women and that gonadal steroids, especially estrogens, are implicated in the underlying mechanisms. Therefore, it may seem anomalous that clinical data suggest that depression in women predominates in hypoestrogenic states, namely in premenstrual, postpartum, and perimenopausal periods (Solomon and Herman, 2009), suggesting that estrogen has a positive effect on affective behavior. Contradictions may be reconciled by recent findings in preclinical studies suggesting differential roles for ERα and ERβ, which would both be activated by estradiol (Weiser et al., 2008; Solomon and Herman, 2009). The data suggest that supraphysiological levels of estradiol or ERα agonists increase HPA axis responsiveness, depression, and anxiety-like behaviors. In contrast, physiological levels of estradiol or ERβ agonists decrease HPA axis responsiveness, depression, and anxiety-like behaviors (Weiser et al., 2008). These observations are highly pertinent in view of the facts that ERβ is highly expressed not only in the hypothalamic PVN (Table 1), which is a key regulator of HPA axis reactivity (discussed earlier in this section), but also in serotonergic neurons of the dorsal raphe, which are key targets for the actions of antidepressant drugs (Weiser et al., 2008; Solomon and Herman, 2009). These observations fuel the current interest in ERβ ligands as promising CNS-active compounds that lack ERα actions and are known to mediate some unwanted peripheral actions of estradiol, including proliferative effects in breast tissue (Patchev et al., 2008). It is noteworthy that although ERβ ligands may have anxiolytic actions in gonadectomized males and females, a recent study has shown that they do not affect anxiety-related behavior in gonad-intact male rats, indicating that efficacy may be dependent on gonadal hormone status and different in males and females (Patisaul et al., 2009).
VIII. Summary and the Way Forward
This review of the effects of estradiol in the brain has highlighted significant differences as well as similarities in males and females of humans and animals used in research. Investigations in the hypothalamus first revealed sexually dimorphic effects of estradiol on synaptic remodeling, glial plasticity, and neuronal activity (including GABAergic interneurons and glutamatergic, cholinergic, and dopaminergic populations), as well as ER expression, intracellular signaling pathways, and transcriptional control, which could be related to the neuroendocrine control of reproduction and sex-specific reproductive behaviors. However, it came as some surprise to find that similar sexually dimorphic responses to estradiol are present in brain regions that are not directly associated with reproductive success but are important for learning, memory, emotional responding, mood, and sensorimotor control, including the hippocampus, PFC, striatum, and amygdala. An emerging theme for all these brain regions identifies sex-specific organization of susceptible neural circuitries at critical stages of development as a major factor underlying the sexually dimorphic effects of estrogens in the brain. This is dependent to a large extent on a transitory surge of testosterone production in males during development, which, after its conversion to estradiol, masculinizes and defeminizes the brain; of particular significance is that the defeminizing actions result in the loss of capacity to respond to the feminizing actions of estradiol in adulthood. In the hypothalamus, this achieves the necessary sex differences in functions to secure reproductive success, but for other brain regions this may be considered a disadvantage. Indeed, learning and experience may further modify certain circuitries to achieve similar outcomes for critical functions, such as cognition and sensorimotor integration, although they are attained by different mechanisms in male and female brains (Cahill, 2006). These sex differences in connectivity and estrogen responsiveness have important implications for the different vulnerabilities of men and women to psychiatric and neurodegenerative conditions, especially under conditions of stress, where adaptive responses may result in a different degree of allostatic load in sex specific circuitries. Together, these observations highlight the urgent need for a better understanding of the nature and origins of brain sex dimorphisms to realize the full potential of hormone-based therapies. Given the depth and breadth of the evidence for differential actions of estradiol in male and female brains, it is also important to redress the fact that the majority of preclinical studies still focus on estrogenic actions in the brains of ovariectomized female rodents, whereas menopausal women are the main focus of clinical studies; hence, data relate only to half the population.
In addition to male/female differences in normal brain structure, function, and estrogen responsiveness, this review has highlighted sex differences in the nature, progression, and manifestation of neurodegenerative disease (PD and AD) and drug abuse, where hormonal contributions may be sex-specific. Despite many controversies surrounding estrogen actions in the brain, its potential as a neuroprotective agent is still actively pursued. A main goal for women's health is to find the ideal, brain-specific neuro-SERM or selective aromatase-modifying drug to determine the optimal dosing regime, including dose, route of administration, and mode of administration (continuous versus pulsatile), and to unravel the age-associated differences in efficacy, as reviewed extensively elsewhere (Brinton, 2004; Wise et al., 2005; Miller and Duckles, 2008; Sherwin and Henry, 2008; Pike et al., 2009). In considering the additional variable of sex, in the present review, we argue for the presence of two broad categories of estrogen action in the brain: one in which the actions are the same in both sexes, and one where they are different. Based on observations of neuroprotective effects in experimental PD (section IV.B.2 and Fig. 5), we hypothesize that the former can be elicited by relatively high pharmacological doses of estradiol, which prevent cell loss via nonspecific effects on processes common to neuroprotection against many forms of injury, including antiapoptotic and antioxidant activity. This has proven effective when cell loss is relatively extensive, but the use of high doses is disadvantageous. In contrast, physiological levels of estradiol seem to promote brain plasticity and adaptive responses (not cell loss) in the partially injured NSDA system in females, but not male rodents, illustrating sex differences in hormonal activation of the powerful compensatory mechanisms that preserve functionality in the striatum during early stages of NSDA degeneration. This suggests a novel mechanism for estrogen protection in early PD that would be relevant only in the female NSDA system. Like the striatum, there are also sex differences in the organization of the hippocampus, PFC, and amygdala, and in the responses of these regions to estradiol, including positive effects on synaptic remodeling in the female, but not male brain. It is therefore interesting to speculate that estradiol could also promote brain plasticity after injury and improve cognition in females.
Some major debilitating conditions in which estrogens have been implicated as likely protective agents also include stroke and multiple sclerosis, which are excellently reviewed elsewhere (Murphy et al., 2003; Gold and Voskuhl, 2009; Suzuki et al., 2009). The general view indicates that estrogens may be similarly effective in male and female brains in experimentally induced ischemia (a model of stroke) or experimental autoimmune encephalomyelitis (a model of multiple sclerosis), so we have not focused on these issues. However, it is interesting to note that, similar to our proposal for PD, a distinction has been made in the protective effects of physiological versus pharmacological estradiol treatment regimens in stroke models (Suzuki et al., 2009). This work emphasizes the need to differentiate a prophylactic mode of therapy (low physiological doses) from a mode of treatment after the event (high doses).
Although there are seemingly contradictory reports on the relative contributions of ERα and ERβ to the neuroprotective effects of estrogens in most disease models (Brann et al., 2007; Suzuki et al., 2009), evidence is emerging that both ERs have protective capacity, but they operate via different mechanisms and possibly in different time frames. For example, in ischemic brain injury and experimental autoimmune encephalomyelitis, ERα is induced early, whereas ERβ is induced later (Suzuki et al., 2002; Tiwari-Woodruff et al., 2007; Tiwari-Woodruff and Voskuhl, 2009). Considerable attention is now focused on which ER/ER signaling pathway controls various protective mechanisms. These range from antiapoptotic, neurotrophic, and neurogenic actions of ER ligands to suppression of neuroinflammation, which accompanies, and probably contributes to the progression, if not initiation, of so many pathological brain conditions, including PD, AD, stroke, and multiple sclerosis. Although clinical data on selective ERα and ERβ ligands are lacking, clinical trials are beginning to focus on the CNS effects of classic SERMs such as tamoxifen and raloxifene, which are widely used for their peripheral actions, and on natural estrogenic compounds, such as estriol, which has 5-fold potency for ERβ over ERα (Murphy et al., 2003; Gold and Voskuhl, 2009). Although this work is almost exclusively done in female subjects, a recent study reported that there are sex differences in the doses of estradiol, tamoxifen, and raloxifene required to suppress microglial activation induced by bacterial lipopolysaccharide (Tapia-Gonzalez et al., 2008). Moreover, in the healthy brain, the same study found that raloxifene has a moderate pro-inflammatory effect in female, but not male rats. This work is important in demonstrating not only that the central response to SERMs may be sexually dimorphic but also that more attention needs to be given to the CNS actions of SERMS used therapeutically for their peripheral effects. Unraveling the effects of SERMs in the brain will certainly be very complex, and a recent overview concluded that each SERM will have a unique set of clinical activities and that efficacy in one tissue cannot be assumed from effects in another (Shelly et al., 2008) or, indeed, from one sex from another.
In summary, although many questions still need to be resolved, there is substantial evidence for the therapeutic benefits of estrogens in the brain, but current evidence suggests that beneficial effects found in females are not directly transferable to males. This is due to sex dimorphisms in the brain, which, contrary to early views, seem to be the norm rather than the exception. Together, these are powerful arguments that highlight the need for a sex-specific approach to novel hormone-dependent therapies.
Acknowledgments.
This work was supported by The Wellcome Trust [Grant 086871/Z/08/Z].
This article is available online at http://pharmrev.aspetjournals.org.
doi:10.1124/pr.109.002071.
- 6-OHDA
- 6-hydroxydopamine
- Aβ
- β amyloid peptide
- ACh
- acetylcholine
- AD
- Alzheimer's disease
- AGT
- antenatal glucocorticoid treatment
- APP
- amyloid precursor protein
- AR
- androgen receptor
- ArKO
- aromatase knockout
- AVP
- arginine vasopressin
- CA
- cornu ammonis
- CNS
- central nervous system
- CREB
- cAMP response element binding protein
- CRH
- corticorelin
- DA
- dopamine/dopaminergic
- DA
- dopaminergic
- DHEA
- dehydroepiandrosterone
- DHT
- dihydrotestosterone
- ER
- estrogen receptor
- ERE
- estrogen response element
- ERK
- extracellular signal-regulated kinase
- GC
- glucocorticoid
- GnRH
- gonadotropin-releasing hormone
- HPA
- hypothalamic-pituitary-adrenal
- HPG
- hypothalamic-pituitary-gonadal
- HRT
- hormone replacement therapy
- KO
- knockout
- LH
- luteinizing hormone
- MA
- methamphetamine
- MAPK
- mitogen-activated protein kinase
- MLDA
- mesolimbic dopaminergic system
- mPFC
- medial prefrontal cortex
- mPOA
- medial preoptic area
- MPTP
- 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- N.Acc
- nucleus accumbens
- NMDA
- N-methyl-d-aspartate
- NSDA
- nigrostriatal dopaminergic
- pCREB
- phosphorylation of CREB
- PD
- Parkinson's disease
- PFC
- prefrontal cortex
- PI3K
- phosphoinositide 3-kinase
- POA
- preoptic area
- PR
- progesterone
- PVN
- paraventricular nuclei
- SERM
- selective estrogen receptor modulator
- SNc
- substantia nigra pars compacta
- TH
- tyrosine hydroxylase
- TH-IR
- tyrosine hydroxylase immunoreactive
- vlVMN
- ventrolateral subdivision of the ventromedial nucleus
- VMN
- ventromedial nucleus
- VTA
- ventral tegmental area.
References
- Abdelgadir et al., 1997.Abdelgadir SE, Roselli CE, Choate JV, Resko JA. (1997) Distribution of aromatase cytochrome P450 messenger ribonucleic acid in adult rhesus monkey brains. Biol Reprod 57:772–777 [DOI] [PubMed] [Google Scholar]
- Abrahám et al., 2003.Abrahám IM, Han SK, Todman MG, Korach KS, Herbison AE. (2003) Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci 23:5771–5777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahám and Herbison, 2005.Abrahám IM, Herbison AE. (2005) Major sex differences in non-genomic estrogen actions on intracellular signaling in mouse brain in vivo. Neuroscience 131:945–951 [DOI] [PubMed] [Google Scholar]
- Ahmed et al., 2008.Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. (2008) Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci 11:995–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarid et al., 1999.Alarid ET, Bakopoulos N, Solodin N. (1999) Proteasome-mediated proteolysis of estrogen receptor: a novel component in autologous down-regulation. Mol Endocrinol 13:1522–1534 [DOI] [PubMed] [Google Scholar]
- Aleman et al., 2003.Aleman A, Kahn RS, Selten JP. (2003) Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry 60:565–571 [DOI] [PubMed] [Google Scholar]
- Allen et al., 2003.Allen JS, Damasio H, Grabowski TJ, Bruss J, Zhang W. (2003) Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. Neuroimage 18:880–894 [DOI] [PubMed] [Google Scholar]
- Allen et al., 2009.Allen R, Buckingham J, Gillies G, Dexter D. (2009) Stress in males, but not females, may render the nigrostriatal system susceptible to toxic insults. Br Neurosci Assoc Abstr 19:29.02 [Google Scholar]
- Allen et al., 2008.Allen R, Buckingham J, Gillies GE, Dexter D. (2008) Stress, glucocorticoids and the nigrostriatal system, in 6th Forum of European Neuroscience (FENS); 12–16 Jul 2008; Geneva, Switzerland Federation of European Neuroscience Societies, Berlin, Germany [Google Scholar]
- Amateau and McCarthy, 2004.Amateau SK, McCarthy MM. (2004) Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci 7:643–650 [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists Women's Health Care Physicians, 2004.American College of Obstetricians and Gynecologists Women's Health Care Physicians (2004) Hormone therapy. Obstet Gynecol 104 (4 Suppl):1S–131S [DOI] [PubMed] [Google Scholar]
- Anderson et al., 2005.Anderson LI, Leipheimer RE, Dluzen DE. (2005) Effects of neonatal and prepubertal hormonal manipulations upon estrogen neuroprotection of the nigrostriatal dopaminergic system within female and male mice. Neuroscience 130:369–382 [DOI] [PubMed] [Google Scholar]
- Andreano et al., 2008.Andreano JM, Arjomandi H, Cahill L. (2008) Menstrual cycle modulation of the relationship between cortisol and long-term memory. Psychoneuroendocrinology 33:874–882 [DOI] [PubMed] [Google Scholar]
- Andreano and Cahill, 2006.Andreano JM, Cahill L. (2006) Glucocorticoid release and memory consolidation in men and women. Psychol Sci 17:466–470 [DOI] [PubMed] [Google Scholar]
- Antal et al., 2008.Antal MC, Krust A, Chambon P, Mark M. (2008) Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proc Natl Acad Sci USA 105:2433–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold and Breedlove, 1985.Arnold AP, Breedlove SM. (1985) Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav 19:469–498 [DOI] [PubMed] [Google Scholar]
- Arnold and Burgoyne, 2004.Arnold AP, Burgoyne PS. (2004) Are XX and XY brain cells intrinsically different? Trends Endocrinol Metab 15:6–11 [DOI] [PubMed] [Google Scholar]
- Arnold and Gorski, 1984.Arnold AP, Gorski RA. (1984) Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci 7:413–442 [DOI] [PubMed] [Google Scholar]
- Auger et al., 2001.Auger AP, Hexter DP, McCarthy MM. (2001) Sex difference in the phosphorylation of cAMP response element binding protein (CREB) in neonatal rat brain. Brain Res 890:110–117 [DOI] [PubMed] [Google Scholar]
- Azcoitia et al., 2005.Azcoitia I, Sierra A, Veiga S, Garcia-Segura LM. (2005) Brain steroidogenesis: emerging therapeutic strategies to prevent neurodegeneration. J Neural Transm 112:171–176 [DOI] [PubMed] [Google Scholar]
- Azcoitia et al., 2001.Azcoitia I, Sierra A, Veiga S, Honda S, Harada N, Garcia-Segura LM. (2001) Brain aromatase is neuroprotective. J Neurobiol 47:318–329 [DOI] [PubMed] [Google Scholar]
- Bakker et al., 2006.Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. (2006) Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci 9:220–226 [DOI] [PubMed] [Google Scholar]
- Baldereschi et al., 2000.Baldereschi M, Di Carlo A, Rocca WA, Vanni P, Maggi S, Perissinotto E, Grigoletto F, Amaducci L, Inzitari D. (2000) Parkinson's disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. ILSA Working Group. Italian Longitudinal Study on Aging. Neurology 55:1358–1363 [DOI] [PubMed] [Google Scholar]
- Balthazart and Ball, 2006.Balthazart J, Ball GF. (2006) Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci 29:241–249 [DOI] [PubMed] [Google Scholar]
- Bangasser and Shors, 2007.Bangasser DA, Shors TJ. (2007) The hippocampus is necessary for enhancements and impairments of learning following stress. Nat Neurosci 10:1401–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser and Shors, 2008.Bangasser DA, Shors TJ. (2008) The bed nucleus of the stria terminalis modulates learning after stress in masculinized but not cycling females. J Neurosci 28:6383–6387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao et al., 2008.Bao AM, Meynen G, Swaab DF. (2008) The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res Rev 57:531–553 [DOI] [PubMed] [Google Scholar]
- Barker and Galea, 2008.Barker JM, Galea LA. (2008) Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience 152:888–902 [DOI] [PubMed] [Google Scholar]
- Barnes et al., 2005.Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. (2005) Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry 62:685–691 [DOI] [PubMed] [Google Scholar]
- Baron-Cohen et al., 2005.Baron-Cohen S, Knickmeyer RC, Belmonte MK. (2005) Sex differences in the brain: implications for explaining autism. Science 310:819–823 [DOI] [PubMed] [Google Scholar]
- Bassilana et al., 2005.Bassilana F, Mace N, Li Q, Stutzmann JM, Gross CE, Pradier L, Benavides J, Ménager J, Bezard E. (2005) Unraveling substantia nigra sequential gene expression in a progressive MPTP-lesioned macaque model of Parkinson's disease. Neurobiol Dis 20:93–103 [DOI] [PubMed] [Google Scholar]
- Becker, 1999.Becker JB. (1999) Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav 64:803–812 [DOI] [PubMed] [Google Scholar]
- Becker, 2009.Becker JB. (2009) Sexual differentiation of motivation: a novel mechanism? Horm Behav 55:646–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker and Hu, 2008.Becker JB, Hu M. (2008) Sex differences in drug abuse. Front Neuroendocrinol 29:36–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl, 2002.Behl C. (2002) Oestrogen as a neuroprotective hormone. Nat Rev Neurosci 3:433–442 [DOI] [PubMed] [Google Scholar]
- Benedetti et al., 2001.Benedetti MD, Maraganore DM, Bower JH, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA. (2001) Hysterectomy, menopause, and estrogen use preceding Parkinson's disease: an exploratory case-control study. Mov Disord 16:830–837 [DOI] [PubMed] [Google Scholar]
- Berman et al., 1997.Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, Van Horn JD, Esposito G, Ostrem JL, Weinberger DR. (1997) Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci USA 94:8836–8841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard et al., 2003.Bezard E, Gross CE, Brotchie JM. (2003) Presymptomatic compensation in Parkinson's disease is not dopamine-mediated. Trends Neurosci 26:215–221 [DOI] [PubMed] [Google Scholar]
- Bocklandt and Vilain, 2007.Bocklandt S, Vilain E. (2007) Sex differences in brain and behavior: hormones versus genes. Adv Genet 59:245–266 [DOI] [PubMed] [Google Scholar]
- Boulware et al., 2005.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. (2005) Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci 25:5066–5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque et al., 2009.Bourque M, Dluzen DE, Di Paolo T. (2009) Neuroprotective actions of sex steroids in Parkinson's disease. Front Neuroendocrinol 30:142–157 [DOI] [PubMed] [Google Scholar]
- Bousios et al., 2001.Bousios S, Karandrea D, Kittas C, Kitraki E. (2001) Effects of gender and stress on the regulation of steroid receptor coactivator-1 expression in the rat brain and pituitary. J Steroid Biochem Mol Biol 78:401–407 [DOI] [PubMed] [Google Scholar]
- Bowman, 2005.Bowman RE. (2005) Stress-induced changes in spatial memory are sexually differentiated and vary across the lifespan. J Neuroendocrinol 17:526–535 [DOI] [PubMed] [Google Scholar]
- Bowman et al., 2004.Bowman RE, MacLusky NJ, Sarmiento Y, Frankfurt M, Gordon M, Luine VN. (2004) Sexually dimorphic effects of prenatal stress on cognition, hormonal responses, and central neurotransmitters. Endocrinology 145:3778–3787 [DOI] [PubMed] [Google Scholar]
- Brailoiu et al., 2007.Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. (2007) Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol 193:311–321 [DOI] [PubMed] [Google Scholar]
- Brann et al., 2007.Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. (2007) Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids 72:381–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton, 2004.Brinton RD. (2004) Impact of estrogen therapy on Alzheimer's disease: a fork in the road? CNS Drugs 18:405–422 [DOI] [PubMed] [Google Scholar]
- Brinton, 2005.Brinton RD. (2005) Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Ann NY Acad Sci 1052:57–74 [DOI] [PubMed] [Google Scholar]
- Brinton, 2008.Brinton RD. (2008) The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci 31:529–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton, 2009.Brinton RD. (2009) Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci 30:212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill, 2006.Cahill L. (2006) Why sex matters for neuroscience. Nat Rev Neurosci 7:477–484 [DOI] [PubMed] [Google Scholar]
- Caldú and Dreher, 2007.Caldú X, Dreher JC. (2007) Hormonal and genetic influences on processing reward and social information. Ann NY Acad Sci 1118:43–73 [DOI] [PubMed] [Google Scholar]
- Callier et al., 2002.Callier S, Le Saux M, Lhiaubet AM, Di Paolo T, Rostène W, Pelaprat D. (2002) Evaluation of the protective effect of oestradiol against toxicity induced by 6-hydroxydopamine and 1-methyl-4-phenylpyridinium ion (Mpp+) towards dopaminergic mesencephalic neurones in primary culture. J Neurochem 80:307–316 [DOI] [PubMed] [Google Scholar]
- Callier et al., 2001.Callier S, Morissette M, Grandbois M, Pélaprat D, Di Paolo T. (2001) Neuroprotective properties of 17beta-estradiol, progesterone, and raloxifene in MPTP C57Bl/6 mice. Synapse 41:131–138 [DOI] [PubMed] [Google Scholar]
- Cantuti-Castelvetri et al., 2007.Cantuti-Castelvetri I, Keller-McGandy C, Bouzou B, Asteris G, Clark TW, Frosch MP, Standaert DG. (2007) Effects of gender on nigral gene expression and parkinson disease. Neurobiol Dis 26:606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey, 1958.Carey GL. (1958) Sex differences in problem-solving performance as a function of attitude differences. J Abnorm Psychol 56:256–260 [DOI] [PubMed] [Google Scholar]
- Carroll et al., 2004.Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. (2004) Sex and estrogen influence drug abuse. Trends Pharmacol Sci 25:273–279 [DOI] [PubMed] [Google Scholar]
- Carswell et al., 2005.Carswell HV, Dominiczak AF, Garcia-Segura LM, Harada N, Hutchison JB, Macrae IM. (2005) Brain aromatase expression after experimental stroke: topography and time course. J Steroid Biochem Mol Biol 96:89–91 [DOI] [PubMed] [Google Scholar]
- Castañeda et al., 1990.Castañeda E, Whishaw IQ, Robinson TE. (1990) Changes in striatal dopamine neurotransmission assessed with microdialysis following recovery from a bilateral 6-OHDA lesion: variation as a function of lesion size. J Neurosci 10:1847–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castner et al., 1993.Castner SA, Xiao L, Becker JB. (1993) Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res 610:127–134 [DOI] [PubMed] [Google Scholar]
- Charmandari et al., 2005.Charmandari E, Tsigos C, Chrousos G. (2005) Endocrinology of the stress response. Annu Rev Physiol 67:259–284 [DOI] [PubMed] [Google Scholar]
- Chen et al., 2009.Chen JQ, Cammarata PR, Baines CP, Yager JD. (2009) Regulation of mitochondrial respiratory chain biogenesis by estrogens/estrogen receptors and physiological, pathological and pharmacological implications. Biochim Biophys Acta 1793:1540–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier et al., 2005.Cherrier MM, Matsumoto AM, Amory JK, Ahmed S, Bremner W, Peskind ER, Raskind MA, Johnson M, Craft S. (2005) The role of aromatization in testosterone supplementation: effects on cognition in older men. Neurology 64:290–296 [DOI] [PubMed] [Google Scholar]
- Cherrier et al., 2007.Cherrier MM, Matsumoto AM, Amory JK, Johnson M, Craft S, Peskind ER, Raskind MA. (2007) Characterization of verbal and spatial memory changes from moderate to supraphysiological increases in serum testosterone in healthy older men. Psychoneuroendocrinology 32:72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheskis et al., 2007.Cheskis BJ, Greger JG, Nagpal S, Freedman LP. (2007) Signaling by estrogens. J Cell Physiol 213:610–617 [DOI] [PubMed] [Google Scholar]
- Christiansen and Knussmann, 1987.Christiansen K, Knussmann R. (1987) Sex hormones and cognitive functioning in men. Neuropsychobiology 18:27–36 [DOI] [PubMed] [Google Scholar]
- Chrousos and Gold, 1992.Chrousos GP, Gold PW. (1992) The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 267:1244–1252 [PubMed] [Google Scholar]
- Chrousos and Kino, 2007.Chrousos GP, Kino T. (2007) Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress 10:213–219 [DOI] [PubMed] [Google Scholar]
- Chung et al., 2007.Chung WC, Pak TR, Suzuki S, Pouliot WA, Andersen ME, Handa RJ. (2007) Detection and localization of an estrogen receptor beta splice variant protein (ERbeta2) in the adult female rat forebrain and midbrain regions. J Comp Neurol 505:249–267 [DOI] [PubMed] [Google Scholar]
- Clancy et al., 1995.Clancy AN, Zumpe D, Michael RP. (1995) Intracerebral infusion of an aromatase inhibitor, sexual behavior and brain estrogen receptor-like immunoreactivity in intact male rats. Neuroendocrinology 61:98–111 [DOI] [PubMed] [Google Scholar]
- Conrad et al., 2004a.Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. (2004a) Acute stress impairs spatial memory in male but not female rats: influence of estrous cycle. Pharmacol Biochem Behav 78:569–579 [DOI] [PubMed] [Google Scholar]
- Conrad et al., 2004b.Conrad CD, Jackson JL, Wise LS. (2004b) Chronic stress enhances ibotenic acid-induced damage selectively within the hippocampal CA3 region of male, but not female rats. Neuroscience 125:759–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke and Woolley, 2005.Cooke BM, Woolley CS. (2005) Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol 64:34–46 [DOI] [PubMed] [Google Scholar]
- Cordero et al., 2000.Cordero ME, Valenzuela CY, Torres R, Rodriguez A. (2000) Sexual dimorphism in number and proportion of neurons in the human median raphe nucleus. Brain Res Dev Brain Res 124:43–52 [DOI] [PubMed] [Google Scholar]
- Cosgrove et al., 2007.Cosgrove KP, Mazure CM, Staley JK. (2007) Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry 62:847–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosimo Melcangi and Garcia-Segura, 2010.Cosimo Melcangi R, Garcia-Segura LM. (2010) Sex-specific therapeutic strategies based on neuroactive steroids: in search for innovative tools for neuroprotection. Horm Behav 57:2–11 [DOI] [PubMed] [Google Scholar]
- Craig et al., 2008.Craig MC, Fletcher PC, Daly EM, Rymer J, Brammer M, Giampietro V, Murphy DG. (2008) Physiological variation in estradiol and brain function: a functional magnetic resonance imaging study of verbal memory across the follicular phase of the menstrual cycle. Horm Behav 53:503–508 [DOI] [PubMed] [Google Scholar]
- Craig et al., 2005.Craig MC, Maki PM, Murphy DG. (2005) The Women's Health Initiative Memory Study: findings and implications for treatment. Lancet Neurol 4:190–194 [DOI] [PubMed] [Google Scholar]
- Craig and Murphy, 2007a.Craig MC, Murphy DG. (2007a) Estrogen: effects on normal brain function and neuropsychiatric disorders. Climacteric 10 (Suppl 2):97–104 [DOI] [PubMed] [Google Scholar]
- Craig and Murphy, 2007b.Craig MC, Murphy DG. (2007b) Oestrogen, cognition and the maturing female brain. J Neuroendocrinol 19:1–6 [DOI] [PubMed] [Google Scholar]
- Creutz and Kritzer, 2002.Creutz LM, Kritzer MF. (2002) Estrogen receptor-beta immunoreactivity in the midbrain of adult rats: regional, subregional, and cellular localization in the A10, A9, and A8 dopamine cell groups. J Comp Neurol 446:288–300 [DOI] [PubMed] [Google Scholar]
- Csakvari et al., 2007.Csakvari E, Hoyk Z, Gyenes A, Garcia-Ovejero D, Garcia-Segura LM, Párducz A. (2007) Fluctuation of synapse density in the arcuate nucleus during the estrous cycle. Neuroscience 144:1288–1292 [DOI] [PubMed] [Google Scholar]
- Csakvari et al., 2008.Csakvari E, Kurunczi A, Hoyk Z, Gyenes A, Naftolin F, Parducz A. (2008) Estradiol-induced synaptic remodeling of tyrosine hydroxylase immunopositive neurons in the rat arcuate nucleus. Endocrinology 149:4137–4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla et al., 2008.Dalla C, Antoniou K, Kokras N, Drossopoulou G, Papathanasiou G, Bekris S, Daskas S, Papadopoulou-Daifoti Z. (2008) Sex differences in the effects of two stress paradigms on dopaminergic neurotransmission. Physiol Behav 93:595–605 [DOI] [PubMed] [Google Scholar]
- Daniel, 2006.Daniel JM. (2006) Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol 18:787–795 [DOI] [PubMed] [Google Scholar]
- Datla et al., 2003.Datla KP, Murray HE, Pillai AV, Gillies GE, Dexter DT. (2003) Differences in dopaminergic neuroprotective effects of estrogen during estrous cycle. Neuroreport 14:47–50 [DOI] [PubMed] [Google Scholar]
- de Kloet et al., 2005.de Kloet ER, Joëls M, Holsboer F. (2005) Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6:463–475 [DOI] [PubMed] [Google Scholar]
- de Kloet et al., 1999.de Kloet ER, Oitzl MS, Joëls M. (1999) Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci 22:422–426 [DOI] [PubMed] [Google Scholar]
- De Vries, 2004.De Vries GJ. (2004) Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology 145:1063–1068 [DOI] [PubMed] [Google Scholar]
- De Vries, 2005.De Vries GJ. (2005) Sex steroids and sex chromosomes at odds? Endocrinology 146:3277–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries and Boyle, 1998.De Vries GJ, Boyle PA. (1998) Double duty for sex differences in the brain. Behav Brain Res 92:205–213 [DOI] [PubMed] [Google Scholar]
- De Vries et al., 2002.De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. (2002) A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci 22:9005–9014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries and Simerly, 2002.De Vries GJ, Simerly RB. (2002) Anatomy, development and function of sexually dimorphic neural circuits in the mammalian brain, in Hormones, Brain, and Behaviour (Pfaff DW. ed) pp 137–191, Elsevier, San Diego: [Google Scholar]
- Dennis et al., 2009.Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, et al. (2009) In vivo effects of a GPR30 antagonist. Nat Chem Biol 5:421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devidze et al., 2005.Devidze N, Mong JA, Jasnow AM, Kow LM, Pfaff DW. (2005) Sex and estrogenic effects on coexpression of mRNAs in single ventromedial hypothalamic neurons. Proc Natl Acad Sci USA 102:14446–14451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing et al., 2006.Dewing P, Chiang CW, Sinchak K, Sim H, Fernagut PO, Kelly S, Chesselet MF, Micevych PE, Albrecht KH, Harley VR, et al. (2006) Direct regulation of adult brain function by the male-specific factor SRY. Curr Biol 16:415–420 [DOI] [PubMed] [Google Scholar]
- Diamond et al., 1990.Diamond SG, Markham CH, Hoehn MM, McDowell FH, Muenter MD. (1990) An examination of male-female differences in progression and mortality of Parkinson's disease. Neurology 40:763–766 [DOI] [PubMed] [Google Scholar]
- Dluzen and Horstink, 2003.Dluzen D, Horstink M. (2003) Estrogen as neuroprotectant of nigrostriatal dopaminergic system: laboratory and clinical studies. Endocrine 21:67–75 [DOI] [PubMed] [Google Scholar]
- Dluzen et al., 1994.Dluzen D, Jain R, Liu B. (1994) Modulatory effects of testosterone on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J Neurochem 62:94–101 [DOI] [PubMed] [Google Scholar]
- Dluzen, 1996.Dluzen DE. (1996) Effects of testosterone upon MPTP-induced neurotoxicity of the nigrostriatal dopaminergic system of C57/B1 mice. Brain Res 715:113–118 [DOI] [PubMed] [Google Scholar]
- Dluzen, 2000.Dluzen DE. (2000) Neuroprotective effects of estrogen upon the nigrostriatal dopaminergic system. J Neurocytol 29:387–399 [DOI] [PubMed] [Google Scholar]
- Dluzen, 2005.Dluzen DE. (2005) Unconventional effects of estrogen uncovered. Trends Pharmacol Sci 26:485–487 [DOI] [PubMed] [Google Scholar]
- Dluzen et al., 2001.Dluzen DE, McDermott JL, Anderson LI. (2001) Tamoxifen diminishes methamphetamine-induced striatal dopamine depletion in intact female and male mice. J Neuroendocrinol 13:618–624 [DOI] [PubMed] [Google Scholar]
- Dluzen and Mickley, 2005.Dluzen DE, Mickley KR. (2005) Gender differences in modulatory effects of tamoxifen upon the nigrostriatal dopaminergic system. Pharmacol Biochem Behav 80:27–33 [DOI] [PubMed] [Google Scholar]
- Dohanich, 2002.Dohanich G. (2002) Gonadal Steroids, Learning and Memory, Academic Press, San Diego: [Google Scholar]
- DonCarlos et al., 2009.DonCarlos LL, Azcoitia I, Garcia-Segura LM. (2009) Neuroprotective actions of selective estrogen receptor modulators. Psychoneuroendocrinology 34 (Suppl 1):S113–S122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DonCarlos and Handa, 1994.DonCarlos LL, Handa RJ. (1994) Developmental profile of estrogen receptor mRNA in the preoptic are of male and female neonatal rats. Brain Res Dev Brain Res 79:283–289 [DOI] [PubMed] [Google Scholar]
- Dreher et al., 2009.Dreher JC, Kohn P, Kolachana B, Weinberger DR, Berman KF. (2009) Variation in dopamine genes influences responsivity of the human reward system. Proc Natl Acad Sci USA 106:617–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal et al., 1999.Dubal DB, Shughrue PJ, Wilson ME, Merchenthaler I, Wise PM. (1999) Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J Neurosci 19:6385–6393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberling, 2002.Eberling JL. (2002) Oestrogen has neuroprotective effects and may reduce the risk of Alzheimer's disease. Expert Opin Biol Ther 2:647–657 [DOI] [PubMed] [Google Scholar]
- Ekue et al., 2002.Ekue A, Boulanger JF, Morissette M, Di Paolo T. (2002) Lack of effect of testosterone and dihydrotestosterone compared to 17beta-oestradiol in 1-methyl-4-phenyl-1,2,3,6, tetrahydropyridine-mice. J Neuroendocrinol 14:731–736 [DOI] [PubMed] [Google Scholar]
- Ellegren and Parsch, 2007.Ellegren H, Parsch J. (2007) The evolution of sex-biased genes and sex-biased gene expression. Nat Rev Genet 8:689–698 [DOI] [PubMed] [Google Scholar]
- Federman, 2006.Federman DD. (2006) The biology of human sex differences. N Engl J Med 354:1507–1514 [DOI] [PubMed] [Google Scholar]
- Fernandez et al., 2000.Fernandez HH, Lapane KL, Ott BR, Friedman JH. (2000) Gender differences in the frequency and treatment of behavior problems in Parkinson's disease. SAGE Study Group. Systematic Assessment and Geriatric drug use via Epidemiology. Mov Disord 15:490–496 [PubMed] [Google Scholar]
- Ferraz et al., 2008.Ferraz AC, Matheussi F, Szawka RE, Rizelio V, Delattre AM, Rigon P, Hermel Edo E, Xavier LL, Achaval M, Anselmo-Franci JA. (2008) Evaluation of estrogen neuroprotective effect on nigrostriatal dopaminergic neurons following 6-hydroxydopamine injection into the substantia nigra pars compacta or the medial forebrain bundle. Neurochem Res 33:1238–1246 [DOI] [PubMed] [Google Scholar]
- Ferraz et al., 2003.Ferraz AC, Xavier LL, Hernandes S, Sulzbach M, Viola GG, Anselmo-Franci JA, Achaval M, Da Cunha C. (2003) Failure of estrogen to protect the substantia nigra pars compacta of female rats from lesion induced by 6-hydroxydopamine. Brain Res 986:200–205 [DOI] [PubMed] [Google Scholar]
- Fink et al., 1996.Fink G, Sumner BE, Rosie R, Grace O, Quinn JP. (1996) Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol 16:325–344 [DOI] [PubMed] [Google Scholar]
- Flanagan-Cato et al., 2001.Flanagan-Cato LM, Calizo LH, Daniels D. (2001) The synaptic organization of VMH neurons that mediate the effects of estrogen on sexual behavior. Horm Behav 40:178–182 [DOI] [PubMed] [Google Scholar]
- Forger, 2009.Forger NG. (2009) Control of cell number in the sexually dimorphic brain and spinal cord. J Neuroendocrinol 21:393–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster et al., 2008.Foster TC, Rani A, Kumar A, Cui L, Semple-Rowland SL. (2008) Viral vector-mediated delivery of estrogen receptor-alpha to the hippocampus improves spatial learning in estrogen receptor-alpha knockout mice. Mol Ther 16:1587–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye et al., 1996.Frye CA, Duncan JE, Basham M, Erskine MS. (1996) Behavioral effects of 3 alpha-androstanediol. II: Hypothalamic and preoptic area actions via a GABAergic mechanism. Behav Brain Res 79:119–130 [DOI] [PubMed] [Google Scholar]
- Fugger et al., 1998.Fugger HN, Cunningham SG, Rissman EF, Foster TC. (1998) Sex differences in the activational effect of ERalpha on spatial learning. Horm Behav 34:163–170 [DOI] [PubMed] [Google Scholar]
- Galea, 2008.Galea LA. (2008) Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev 57:332–341 [DOI] [PubMed] [Google Scholar]
- Galea et al., 1997.Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. (1997) Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience 81:689–697 [DOI] [PubMed] [Google Scholar]
- Gao and Dluzen, 2001.Gao X, Dluzen DE. (2001) The effect of testosterone upon methamphetamine neurotoxicity of the nigrostriatal dopaminergic system. Brain Res 892:63–69 [DOI] [PubMed] [Google Scholar]
- Garcia-Ovejero et al., 2005.Garcia-Ovejero D, Azcoitia I, Doncarlos LL, Melcangi RC, Garcia-Segura LM. (2005) Glia-neuron crosstalk in the neuroprotective mechanisms of sex steroid hormones. Brain Res Brain Res Rev 48:273–286 [DOI] [PubMed] [Google Scholar]
- Garcia-Segura, 2008.Garcia-Segura LM. (2008) Aromatase in the brain: not just for reproduction anymore. J Neuroendocrinol 20:705–712 [DOI] [PubMed] [Google Scholar]
- Garcia-Segura et al., 2001.Garcia-Segura LM, Azcoitia I, DonCarlos LL. (2001) Neuroprotection by estradiol. Prog Neurobiol 63:29–60 [DOI] [PubMed] [Google Scholar]
- Garcia-Segura et al., 2008.Garcia-Segura LM, Lorenz B, DonCarlos LL. (2008) The role of glia in the hypothalamus: implications for gonadal steroid feedback and reproductive neuroendocrine output. Reproduction 135:419–429 [DOI] [PubMed] [Google Scholar]
- Geerlings et al., 2006.Geerlings MI, Strozyk D, Masaki K, Remaley AT, Petrovitch H, Ross GW, White LR, Launer LJ. (2006) Endogenous sex hormones, cognitive decline, and future dementia in old men. Ann Neurol 60:346–355 [DOI] [PubMed] [Google Scholar]
- Gibbs, 2002.Gibbs RB. (2002) Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching-to-position T-maze task. Horm Behav 42:245–257 [DOI] [PubMed] [Google Scholar]
- Gibbs, 2005.Gibbs RB. (2005) Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm Behav 48:268–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs and Gabor, 2003.Gibbs RB, Gabor R. (2003) Estrogen and cognition: applying preclinical findings to clinical perspectives. J Neurosci Res 74:637–643 [DOI] [PubMed] [Google Scholar]
- Gillies et al., 1982.Gillies GE, Linton EA, Lowry PJ. (1982) Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature 299:355–357 [DOI] [PubMed] [Google Scholar]
- Gillies and McArthur, 2010.Gillies GE, McArthur S. (2010) Independent influences of sex steroids of systemic and central origin in a rat model of Parkinson's disease: a contribution to sex-specific neuroprotection by estrogens. Horm Behav 57:23–34 [DOI] [PubMed] [Google Scholar]
- Gillies et al., 2004.Gillies GE, Murray HE, Dexter D, McArthur S. (2004) Sex dimorphisms in the neuroprotective effects of estrogen in an animal model of Parkinson's disease. Pharmacol Biochem Behav 78:513–522 [DOI] [PubMed] [Google Scholar]
- Gleason et al., 2005.Gleason CE, Cholerton B, Carlsson CM, Johnson SC, Asthana S. (2005) Neuroprotective effects of female sex steroids in humans: current controversies and future directions. Cell Mol Life Sci 62:299–312 [DOI] [PubMed] [Google Scholar]
- Glover et al., 2009.Glover V, O'Connor TG, O'Donnell K. (2009) Prenatal stress and the programming of the HPA axis. Neurosci Biobehav Rev doi: 10.1016/j.neubiorev.2009.11.008 [DOI] [PubMed] [Google Scholar]
- Gold and Chrousos, 2002.Gold PW, Chrousos GP. (2002) Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry 7:254–275 [DOI] [PubMed] [Google Scholar]
- Gold and Voskuhl, 2009.Gold SM, Voskuhl RR. (2009) Estrogen and testosterone therapies in multiple sclerosis. Prog Brain Res 175:239–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, 2006.Goldstein JM. (2006) Sex, hormones and affective arousal circuitry dysfunction in schizophrenia. Horm Behav 50:612–622 [DOI] [PubMed] [Google Scholar]
- Goldstein et al., 2010.Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. (2010) Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci 30:431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González et al., 2007.González M, Cabrera-Socorro A, Pérez-García CG, Fraser JD, López FJ, Alonso R, Meyer G. (2007) Distribution patterns of estrogen receptor alpha and beta in the human cortex and hippocampus during development and adulthood. J Comp Neurol 503:790–802 [DOI] [PubMed] [Google Scholar]
- Gorosito et al., 2008.Gorosito SV, Lorenzo AG, Cambiasso MJ. (2008) Estrogen receptor alpha is expressed on the cell-surface of embryonic hypothalamic neurons. Neuroscience 154:1173–1177 [DOI] [PubMed] [Google Scholar]
- Gorski, 2002.Gorski RA. (2002) Hypothalamic imprinting by gonadal steroid hormones. Adv Exp Med Biol 511:57–70; discussion 70–73 [DOI] [PubMed] [Google Scholar]
- Grabowski et al., 2003.Grabowski TJ, Damasio H, Eichhorn GR, Tranel D. (2003) Effects of gender on blood flow correlates of naming concrete entities. Neuroimage 20:940–954 [DOI] [PubMed] [Google Scholar]
- Grandbois et al., 2000.Grandbois M, Morissette M, Callier S, Di Paolo T. (2000) Ovarian steroids and raloxifene prevent MPTP-induced dopamine depletion in mice. Neuroreport 11:343–346 [DOI] [PubMed] [Google Scholar]
- Green and Simpkins, 2000.Green PS, Simpkins JW. (2000) Neuroprotective effects of estrogens: potential mechanisms of action. Int J Dev Neurosci 18:347–358 [DOI] [PubMed] [Google Scholar]
- Guiso et al., 2008.Guiso L, Monte F, Sapienza P, Zingales L. (2008) Diversity. Culture, gender, and math. Science 320:1164–1165 [DOI] [PubMed] [Google Scholar]
- Gundlah et al., 2000.Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. (2000) Distribution of estrogen receptor beta (ERbeta) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Brain Res Mol Brain Res 76:191–204 [DOI] [PubMed] [Google Scholar]
- Haaxma et al., 2007.Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, Booij J, Dluzen DE, Horstink MW. (2007) Gender differences in Parkinson's disease. J Neurol Neurosurg Psychiatry 78:819–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan et al., 2007.Hajszan T, MacLusky NJ, Johansen JA, Jordan CL, Leranth C. (2007) Effects of androgens and estradiol on spine synapse formation in the prefrontal cortex of normal and testicular feminization mutant male rats. Endocrinology 148:1963–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan et al., 2008.Hajszan T, MacLusky NJ, Leranth C. (2008) Role of androgens and the androgen receptor in remodeling of spine synapses in limbic brain areas. Horm Behav 53:638–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkansson et al., 2005.Håkansson A, Westberg L, Nilsson S, Buervenich S, Carmine A, Holmberg B, Sydow O, Olson L, Johnels B, Eriksson E, et al. (2005) Interaction of polymorphisms in the genes encoding interleukin-6 and estrogen receptor beta on the susceptibility to Parkinson's disease. Am J Med Genet B Neuropsychiatr Genet 133B:88–92 [DOI] [PubMed] [Google Scholar]
- Halpern and Tan, 2001.Halpern DF, Tan U. (2001) Stereotypes and steroids: using a psychobiosocial model to understand cognitive sex differences. Brain Cogn 45:392–414 [DOI] [PubMed] [Google Scholar]
- Hampson, 1990.Hampson E. (1990) Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology 15:97–111 [DOI] [PubMed] [Google Scholar]
- Handa et al., 2008.Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. (2008) An alternate pathway for androgen regulation of brain function: activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta,17beta-diol. Horm Behav 53:741–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell et al., 2009.Hazell GG, Yao ST, Roper JA, Prossnitz ER, O'Carroll AM, Lolait SJ. (2009) Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol 202:223–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson et al., 2000.Henderson VW, Paganini-Hill A, Miller BL, Elble RJ, Reyes PF, Shoupe D, McCleary CA, Klein RA, Hake AM, Farlow MR. (2000) Estrogen for Alzheimer's disease in women: randomized, double-blind, placebo-controlled trial. Neurology 54:295–301 [DOI] [PubMed] [Google Scholar]
- Hennessy et al., 2004.Hennessy RJ, Lane A, Kinsella A, Larkin C, O'Callaghan E, Waddington JL. (2004) 3D morphometrics of craniofacial dysmorphology reveals sex-specific asymmetries in schizophrenia. Schizophr Res 67:261–268 [DOI] [PubMed] [Google Scholar]
- Herbison, 1998.Herbison AE. (1998) Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev 19:302–330 [DOI] [PubMed] [Google Scholar]
- Herbison, 2008.Herbison AE. (2008) Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev 57:277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison and Theodosis, 1992.Herbison AE, Theodosis DT. (1992) Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience 50:283–298 [DOI] [PubMed] [Google Scholar]
- Hill et al., 2009.Hill RA, Chua HK, Jones ME, Simpson ER, Boon WC. (2009) Estrogen deficiency results in apoptosis in the frontal cortex of adult female aromatase knockout mice. Mol Cell Neurosci 41:1–7 [DOI] [PubMed] [Google Scholar]
- Hill et al., 2007.Hill RA, McInnes KJ, Gong EC, Jones ME, Simpson ER, Boon WC. (2007) Estrogen deficient male mice develop compulsive behavior. Biol Psychiatry 61:359–366 [DOI] [PubMed] [Google Scholar]
- Hill et al., 2004.Hill RA, Pompolo S, Jones ME, Simpson ER, Boon WC. (2004) Estrogen deficiency leads to apoptosis in dopaminergic neurons in the medial preoptic area and arcuate nucleus of male mice. Mol Cell Neurosci 27:466–476 [DOI] [PubMed] [Google Scholar]
- Hiltunen et al., 2006.Hiltunen M, Iivonen S, Soininen H. (2006) Aromatase enzyme and Alzheimer's disease. Minerva Endocrinol 31:61–73 [PubMed] [Google Scholar]
- Hojo et al., 2004.Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, et al. (2004) Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci USA 101:865–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath et al., 1997.Horvath TL, Garcia-Segura LM, Naftolin F. (1997) Lack of gonadotropin-positive feedback in the male rat is associated with lack of estrogen-induced synaptic plasticity in the arcuate nucleus. Neuroendocrinology 65:136–140 [DOI] [PubMed] [Google Scholar]
- Hu et al., 2004.Hu M, Crombag HS, Robinson TE, Becker JB. (2004) Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology 29:81–85 [DOI] [PubMed] [Google Scholar]
- Hu et al., 2006.Hu M, Watson CJ, Kennedy RT, Becker JB. (2006) Estradiol attenuates the K+-induced increase in extracellular GABA in rat striatum. Synapse 59:122–124 [DOI] [PubMed] [Google Scholar]
- Huhtaniemi, 1994.Huhtaniemi I. (1994) Fetal testis—a very special endocrine organ. Eur J Endocrinol 130:25–31 [DOI] [PubMed] [Google Scholar]
- Hyman and Malenka, 2001.Hyman SE, Malenka RC. (2001) Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci 2:695–703 [DOI] [PubMed] [Google Scholar]
- Ikeda et al., 2003.Ikeda Y, Nagai A, Ikeda MA, Hayashi S. (2003) Sexually dimorphic and estrogen-dependent expression of estrogen receptor beta in the ventromedial hypothalamus during rat postnatal development. Endocrinology 144:5098–5104 [DOI] [PubMed] [Google Scholar]
- Ishunina et al., 2000.Ishunina TA, Kruijver FP, Balesar R, Swaab DF. (2000) Differential expression of estrogen receptor alpha and beta immunoreactivity in the human supraoptic nucleus in relation to sex and aging. J Clin Endocrinol Metab 85:3283–3291 [DOI] [PubMed] [Google Scholar]
- Ishunina and Swaab, 2008.Ishunina TA, Swaab DF. (2008) Estrogen receptor-alpha splice variants in the human brain. Gynecol Endocrinol 24:93–98 [DOI] [PubMed] [Google Scholar]
- Ishunina et al., 2005.Ishunina TA, van Beurden D, van der Meulen G, Unmehopa UA, Hol EM, Huitinga I, Swaab DF. (2005) Diminished aromatase immunoreactivity in the hypothalamus, but not in the basal forebrain nuclei in Alzheimer's disease. Neurobiol Aging 26:173–194 [DOI] [PubMed] [Google Scholar]
- Janowsky, 2006.Janowsky JS. (2006) The role of androgens in cognition and brain aging in men. Neuroscience 138:1015–1020 [DOI] [PubMed] [Google Scholar]
- Jennings et al., 1998.Jennings PJ, Janowsky JS, Orwoll E. (1998) Estrogen and sequential movement. Behav Neurosci 112:154–159 [DOI] [PubMed] [Google Scholar]
- Ji and Dluzen, 2008.Ji J, Dluzen DE. (2008) Sex differences in striatal dopaminergic function within heterozygous mutant dopamine transporter knock-out mice. J Neural Transm 115:809–817 [DOI] [PubMed] [Google Scholar]
- Johnston and Brotchie, 2006.Johnston TH, Brotchie JM. (2006) Drugs in development for Parkinson's disease: an update. Curr Opin Investig Drugs 7:25–32 [PubMed] [Google Scholar]
- Jones et al., 2006.Jones ME, Boon WC, Proietto J, Simpson ER. (2006) Of mice and men: the evolving phenotype of aromatase deficiency. Trends Endocrinol Metab 17:55–64 [DOI] [PubMed] [Google Scholar]
- Kaasinen et al., 2001.Kaasinen V, Nurmi E, Brück A, Eskola O, Bergman J, Solin O, Rinne JO. (2001) Increased frontal [(18)F]fluorodopa uptake in early Parkinson's disease: sex differences in the prefrontal cortex. Brain 124:1125–1130 [DOI] [PubMed] [Google Scholar]
- Kalsbeek et al., 1988.Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. (1988) Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol 269:58–72 [DOI] [PubMed] [Google Scholar]
- Kampen and Sherwin, 1996.Kampen DL, Sherwin BB. (1996) Estradiol is related to visual memory in healthy young men. Behav Neurosci 110:613–617 [DOI] [PubMed] [Google Scholar]
- Kandel, 2001.Kandel ER. (2001) The molecular biology of memory storage: a dialogue between genes and synapses. Science 294:1030–1038 [DOI] [PubMed] [Google Scholar]
- Kasai et al., 2003.Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. (2003) Structure-stability-function relationships of dendritic spines. Trends Neurosci 26:360–368 [DOI] [PubMed] [Google Scholar]
- Kawata et al., 2008.Kawata M, Nishi M, Matsuda K, Sakamoto H, Kaku N, Masugi-Tokita M, Fujikawa K, Hirahara-Wada Y, Takanami K, Mori H. (2008) Steroid receptor signalling in the brain—lessons learned from molecular imaging. J Neuroendocrinol 20:673–676 [DOI] [PubMed] [Google Scholar]
- Keenan et al., 2001.Keenan PA, Ezzat WH, Ginsburg K, Moore GJ. (2001) Prefrontal cortex as the site of estrogen's effect on cognition. Psychoneuroendocrinology 26:577–590 [DOI] [PubMed] [Google Scholar]
- Kelly et al., 1999.Kelly SJ, Ostrowski NL, Wilson MA. (1999) Gender differences in brain and behavior: hormonal and neural bases. Pharmacol Biochem Behav 64:655–664 [DOI] [PubMed] [Google Scholar]
- Kian Tee et al., 2004.Kian Tee M, Rogatsky I, Tzagarakis-Foster C, Cvoro A, An J, Christy RJ, Yamamoto KR, Leitman DC. (2004) Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors alpha and beta. Mol Biol Cell 15:1262–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi et al., 2007.Kimchi T, Xu J, Dulac C. (2007) A functional circuit underlying male sexual behaviour in the female mouse brain. Nature 448:1009–1014 [DOI] [PubMed] [Google Scholar]
- Kipp et al., 2006.Kipp M, Karakaya S, Pawlak J, Araujo-Wright G, Arnold S, Beyer C. (2006) Estrogen and the development and protection of nigrostriatal dopaminergic neurons: concerted action of a multitude of signals, protective molecules, and growth factors. Front Neuroendocrinol 27:376–390 [DOI] [PubMed] [Google Scholar]
- Klink et al., 2002.Klink R, Robichaud M, Debonnel G. (2002) Gender and gonadal status modulation of dorsal raphe nucleus serotonergic neurons. Part II. Regulatory mechanisms. Neuropharmacology 43:1129–1138 [DOI] [PubMed] [Google Scholar]
- Korol, 2004.Korol DL. (2004) Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem 82:309–323 [DOI] [PubMed] [Google Scholar]
- Krege et al., 1998.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. (1998) Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer, 1997.Kritzer MF. (1997) Selective colocalization of immunoreactivity for intracellular gonadal hormone receptors and tyrosine hydroxylase in the ventral tegmental area, substantia nigra, and retrorubral fields in the rat. J Comp Neurol 379:247–260 [DOI] [PubMed] [Google Scholar]
- Kritzer, 2002.Kritzer MF. (2002) Regional, laminar, and cellular distribution of immunoreactivity for ER alpha and ER beta in the cerebral cortex of hormonally intact, adult male and female rats. Cereb Cortex 12:116–128 [DOI] [PubMed] [Google Scholar]
- Kritzer et al., 2007.Kritzer MF, Brewer A, Montalmant F, Davenport M, Robinson JK. (2007) Effects of gonadectomy on performance in operant tasks measuring prefrontal cortical function in adult male rats. Horm Behav 51:183–194 [DOI] [PubMed] [Google Scholar]
- Kritzer and Creutz, 2008.Kritzer MF, Creutz LM. (2008) Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. J Neurosci 28:9525–9535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijver et al., 2002.Kruijver FP, Balesar R, Espila AM, Unmehopa UA, Swaab DF. (2002) Estrogen receptor-alpha distribution in the human hypothalamus in relation to sex and endocrine status. J Comp Neurol 454:115–139 [DOI] [PubMed] [Google Scholar]
- Kruijver et al., 2003.Kruijver FP, Balesar R, Espila AM, Unmehopa UA, Swaab DF. (2003) Estrogen-receptor-beta distribution in the human hypothalamus: similarities and differences with ER alpha distribution. J Comp Neurol 466:251–277 [DOI] [PubMed] [Google Scholar]
- Kudielka and Kirschbaum, 2005.Kudielka BM, Kirschbaum C. (2005) Sex differences in HPA axis responses to stress: a review. Biol Psychol 69:113–132 [DOI] [PubMed] [Google Scholar]
- Kudwa et al., 2006.Kudwa AE, Michopoulos V, Gatewood JD, Rissman EF. (2006) Roles of estrogen receptors alpha and beta in differentiation of mouse sexual behavior. Neuroscience 138:921–928 [DOI] [PubMed] [Google Scholar]
- Kühnemann et al., 1994.Kühnemann S, Brown TJ, Hochberg RB, MacLusky NJ. (1994) Sex differences in the development of estrogen receptors in the rat brain. Horm Behav 28:483–491 [DOI] [PubMed] [Google Scholar]
- Kuo et al., 2003.Kuo YM, Chen HH, Shieh CC, Chuang KP, Cherng CG, Yu L. (2003) 4-Hydroxytamoxifen attenuates methamphetamine-induced nigrostriatal dopaminergic toxicity in intact and gonadetomized mice. J Neurochem 87:1436–1443 [DOI] [PubMed] [Google Scholar]
- Kushner et al., 2000.Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. (2000) Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol 74:311–317 [DOI] [PubMed] [Google Scholar]
- Laakso et al., 2002.Laakso A, Vilkman H, Bergman J, Haaparanta M, Solin O, Syvälahti E, Salokangas RK, Hietala J. (2002) Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biol Psychiatry 52:759–763 [DOI] [PubMed] [Google Scholar]
- Laflamme et al., 1998.Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. (1998) Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol 36:357–378 [DOI] [PubMed] [Google Scholar]
- Lâm and Leranth, 2003.Lâm TT, Leranth C. (2003) Role of the medial septum diagonal band of Broca cholinergic neurons in oestrogen-induced spine synapse formation on hippocampal CA1 pyramidal cells of female rats. Eur J Neurosci 17:1997–2005 [DOI] [PubMed] [Google Scholar]
- Lauber et al., 1997.Lauber ME, Sarasin A, Lichtensteiger W. (1997) Sex differences and androgen-dependent regulation of aromatase (CYP19) mRNA expression in the developing and adult rat brain. J Steroid Biochem Mol Biol 61:359–364 [PubMed] [Google Scholar]
- Lavalaye et al., 2000.Lavalaye J, Booij J, Reneman L, Habraken JB, van Royen EA. (2000) Effect of age and gender on dopamine transporter imaging with [123I]FP-CIT SPET in healthy volunteers. Eur J Nucl Med 27:867–869 [DOI] [PubMed] [Google Scholar]
- Lee and McEwen, 2001.Lee SJ, McEwen BS. (2001) Neurotrophic and neuroprotective actions of estrogens and their therapeutic implications. Annu Rev Pharmacol Toxicol 41:569–591 [DOI] [PubMed] [Google Scholar]
- Lee et al., 2004.Lee SJ, Romeo RD, Svenningsson P, Campomanes CR, Allen PB, Greengard P, McEwen BS. (2004) Estradiol affects spinophilin protein differently in gonadectomized males and females. Neuroscience 127:983–988 [DOI] [PubMed] [Google Scholar]
- Leranth et al., 2003.Leranth C, Petnehazy O, MacLusky NJ. (2003) Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci 23:1588–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth et al., 2000.Leranth C, Shanabrough M, Horvath TL. (2000) Hormonal regulation of hippocampal spine synapse density involves subcortical mediation. Neuroscience 101:349–356 [DOI] [PubMed] [Google Scholar]
- Leuner et al., 2006.Leuner B, Gould E, Shors TJ. (2006) Is there a link between adult neurogenesis and learning? Hippocampus 16:216–224 [DOI] [PubMed] [Google Scholar]
- Leuner et al., 2004.Leuner B, Mendolia-Loffredo S, Shors TJ. (2004) Males and females respond differently to controllability and antidepressant treatment. Biol Psychiatry 56:964–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis and Dluzen, 2008.Lewis C, Dluzen DE. (2008) Testosterone enhances dopamine depletion by methamphetamine in male, but not female, mice. Neurosci Lett 448:130–133 [DOI] [PubMed] [Google Scholar]
- Lewis et al., 1995.Lewis C, McEwen BS, Frankfurt M. (1995) Estrogen-induction of dendritic spines in ventromedial hypothalamus and hippocampus: effects of neonatal aromatase blockade and adult GDX. Brain Res Dev Brain Res 87:91–95 [DOI] [PubMed] [Google Scholar]
- Lewis, 2009.Lewis V. (2009) Undertreatment of menopausal symptoms and novel options for comprehensive management. Curr Med Res Opin 25:2689–2698 [DOI] [PubMed] [Google Scholar]
- Li et al., 2000.Li R, Shen Y, Yang LB, Lue LF, Finch C, Rogers J. (2000) Estrogen enhances uptake of amyloid beta-protein by microglia derived from the human cortex. J Neurochem 75:1447–1454 [DOI] [PubMed] [Google Scholar]
- Lindberg et al., 2003.Lindberg MK, Movérare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsson C. (2003) Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in mice. Mol Endocrinol 17:203–208 [DOI] [PubMed] [Google Scholar]
- Liu and Dluzen, 2007.Liu B, Dluzen DE. (2007) Oestrogen and nigrostriatal dopaminergic neurodegeneration: animal models and clinical reports of Parkinson's disease. Clin Exp Pharmacol Physiol 34:555–565 [DOI] [PubMed] [Google Scholar]
- Liu et al., 2008.Liu F, Day M, Muñiz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, et al. (2008) Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci 11:334–343 [DOI] [PubMed] [Google Scholar]
- Liu et al., 2007.Liu M, Hurn PD, Roselli CE, Alkayed NJ. (2007) Role of P450 aromatase in sex-specific astrocytic cell death. J Cereb Blood Flow Metab 27:135–141 [DOI] [PubMed] [Google Scholar]
- Lonard et al., 2000.Lonard DM, Nawaz Z, Smith CL, O'Malley BW. (2000) The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation. Mol Cell 5:939–948 [DOI] [PubMed] [Google Scholar]
- Loucif et al., 2006.Loucif AJ, Bonnavion P, Macri B, Golmard JL, Boni C, Melfort M, Leonard G, Lesch KP, Adrien J, Jacquin TD. (2006) Gender-dependent regulation of G-protein-gated inwardly rectifying potassium current in dorsal raphe neurons in knock-out mice devoid of the 5-hydroxytryptamine transporter. J Neurobiol 66:1475–1488 [DOI] [PubMed] [Google Scholar]
- Luders et al., 2004.Luders E, Narr KL, Thompson PM, Rex DE, Jancke L, Steinmetz H, Toga AW. (2004) Gender differences in cortical complexity. Nat Neurosci 7:799–800 [DOI] [PubMed] [Google Scholar]
- Luine, 1985.Luine VN. (1985) Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp Neurol 89:484–490 [DOI] [PubMed] [Google Scholar]
- Luine, 2007.Luine VN. (2007) The prefrontal cortex, gonadal hormones and memory. Horm Behav 51:181–182 [DOI] [PubMed] [Google Scholar]
- Luine, 2008.Luine VN. (2008) Sex steroids and cognitive function. J Neuroendocrinol 20:866–872 [DOI] [PubMed] [Google Scholar]
- Luine et al., 2007.Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. (2007) Chronic stress and neural function: accounting for sex and age. J Neuroendocrinol 19:743–751 [DOI] [PubMed] [Google Scholar]
- Luine et al., 1986.Luine VN, Renner KJ, McEwen BS. (1986) Sex-dependent differences in estrogen regulation of choline acetyltransferase are altered by neonatal treatments. Endocrinology 119:874–878 [DOI] [PubMed] [Google Scholar]
- Lund et al., 2006.Lund TD, Hinds LR, Handa RJ. (2006) The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci 26:1448–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien et al., 2009.Lupien SJ, McEwen BS, Gunnar MR, Heim C. (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10:434–445 [DOI] [PubMed] [Google Scholar]
- Lustig et al., 1991.Lustig RH, Sudol M, Pfaff DW, Federoff HJ. (1991) Estrogenic regulation and sex dimorphism of growth-associated protein 43 kDa (GAP-43) messenger RNA in the rat. Brain Res Mol Brain Res 11:125–132 [DOI] [PubMed] [Google Scholar]
- Lynch, 2006.Lynch WJ. (2006) Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol 14:34–41 [DOI] [PubMed] [Google Scholar]
- Lynch et al., 2002.Lynch WJ, Roth ME, Carroll ME. (2002) Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 164:121–137 [DOI] [PubMed] [Google Scholar]
- Lyons et al., 1998.Lyons KE, Hubble JP, Tröster AI, Pahwa R, Koller WC. (1998) Gender differences in Parkinson's disease. Clin Neuropharmacol 21:118–121 [PubMed] [Google Scholar]
- MacLusky and Naftolin, 1981.MacLusky NJ, Naftolin F. (1981) Sexual differentiation of the central nervous system. Science 211:1294–1302 [DOI] [PubMed] [Google Scholar]
- MacLusky et al., 1994.MacLusky NJ, Walters MJ, Clark AS, Toran-Allerand CD. (1994) Aromatase in the cerebral cortex, hippocampus, and mid-brain: ontogeny and developmental implications. Mol Cell Neurosci 5:691–698 [DOI] [PubMed] [Google Scholar]
- Madeira and Lieberman, 1995.Madeira MD, Lieberman AR. (1995) Sexual dimorphism in the mammalian limbic system. Prog Neurobiol 45:275–333 [DOI] [PubMed] [Google Scholar]
- Maggi et al., 2004.Maggi A, Ciana P, Belcredito S, Vegeto E. (2004) Estrogens in the nervous system: mechanisms and nonreproductive functions. Annu Rev Physiol 66:291–313 [DOI] [PubMed] [Google Scholar]
- Maharjan et al., 2005.Maharjan S, Serova L, Sabban EL. (2005) Transcriptional regulation of tyrosine hydroxylase by estrogen: opposite effects with estrogen receptors alpha and beta and interactions with cyclic AMP. J Neurochem 93:1502–1514 [DOI] [PubMed] [Google Scholar]
- Mandavilli, 2006.Mandavilli A. (2006) Hormone in the hot seat. Nat Med 12:8–9 [DOI] [PubMed] [Google Scholar]
- Mangelsdorf et al., 1995.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. (1995) The nuclear receptor superfamily: the second decade. Cell 83:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraganore et al., 2002.Maraganore DM, Farrer MJ, McDonnell SK, Elbaz A, Schaid DJ, Hardy JA, Rocca WA. (2002) Case-control study of estrogen receptor gene polymorphisms in Parkinson's disease. Mov Disord 17:509–512 [DOI] [PubMed] [Google Scholar]
- Marien et al., 2004.Marien MR, Colpaert FC, Rosenquist AC. (2004) Noradrenergic mechanisms in neurodegenerative diseases: a theory. Brain Res Brain Res Rev 45:38–78 [DOI] [PubMed] [Google Scholar]
- Matousek and Sherwin, 2010.Matousek RH, Sherwin BB. (2010) A randomized controlled trial of add-back estrogen or placebo on cognition in men with prostate cancer receiving an antiandrogen and a gonadotropin-releasing hormone analog. Psychoneuroendocrinology 35:215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes et al., 2008.Mayes R, Bagwell C, Erkulwater J. (2008) ADHD and the rise in stimulant use among children. Harv Rev Psychiatry 16:151–166 [DOI] [PubMed] [Google Scholar]
- McArthur et al., 2005.McArthur S, McHale E, Dalley JW, Buckingham JC, Gillies GE. (2005) Altered mesencephalic dopaminergic populations in adulthood as a consequence of brief perinatal glucocorticoid exposure. J Neuroendocrinol 17:475–482 [DOI] [PubMed] [Google Scholar]
- McArthur et al., 2007a.McArthur S, McHale E, Gillies GE. (2007a) The size and distribution of midbrain dopaminergic populations are permanently altered by perinatal glucocorticoid exposure in a sex- region- and time-specific manner. Neuropsychopharmacology 32:1462–1476 [DOI] [PubMed] [Google Scholar]
- McArthur et al., 2007b.McArthur S, Murray HE, Dhankot A, Dexter DT, Gillies GE. (2007b) Striatal susceptibility to a dopaminergic neurotoxin is independent of sex hormone effects on cell survival and DAT expression but is exacerbated by central aromatase inhibition. J Neurochem 100:678–692 [DOI] [PubMed] [Google Scholar]
- McArthur et al., 2006.McArthur S, Siddique ZL, Christian HC, Capone G, Theogaraj E, John CD, Smith SF, Morris JF, Buckingham JC, Gillies GE. (2006) Perinatal glucocorticoid treatment disrupts the hypothalamo-lactotroph axis in adult female, but not male, rats. Endocrinology 147:1904–1915 [DOI] [PubMed] [Google Scholar]
- McCarthy, 2008.McCarthy MM. (2008) Estradiol and the developing brain. Physiol Rev 88:91–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy et al., 2002.McCarthy MM, Amateau SK, Mong JA. (2002) Steroid modulation of astrocytes in the neonatal brain: implications for adult reproductive function. Biol Reprod 67:691–698 [DOI] [PubMed] [Google Scholar]
- McCarthy and Konkle, 2005.McCarthy MM, Konkle AT. (2005) When is a sex difference not a sex difference? Front Neuroendocrinol 26:85–102 [DOI] [PubMed] [Google Scholar]
- McDermott et al., 1994.McDermott JL, Liu B, Dluzen DE. (1994) Sex differences and effects of estrogen on dopamine and DOPAC release from the striatum of male and female CD-1 mice. Exp Neurol 125:306–311 [DOI] [PubMed] [Google Scholar]
- McEwen, 1998.McEwen BS. (1998) Stress, adaptation, and disease. Allostasis and allostatic load. Ann NY Acad Sci 840:33–44 [DOI] [PubMed] [Google Scholar]
- McEwen, 1999.McEwen BS. (1999) Permanence of brain sex differences and structural plasticity of the adult brain. Proc Natl Acad Sci USA 96:7128–7130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen, 2001.McEwen BS. (2001) Invited review: Estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol 91:2785–2801 [DOI] [PubMed] [Google Scholar]
- McEwen and Alves, 1999.McEwen BS, Alves SE. (1999) Estrogen actions in the central nervous system. Endocr Rev 20:279–307 [DOI] [PubMed] [Google Scholar]
- McEwen and Milner, 2007.McEwen BS, Milner TA. (2007) Hippocampal formation: shedding light on the influence of sex and stress on the brain. Brain Res Rev 55:343–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaught et al., 2001.McNaught KS, Olanow CW, Halliwell B, Isacson O, Jenner P. (2001) Failure of the ubiquitin-proteasome system in Parkinson's disease. Nat Rev Neurosci 2:589–594 [DOI] [PubMed] [Google Scholar]
- Meaney et al., 2002.Meaney MJ, Brake W, Gratton A. (2002) Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology 27:127–138 [DOI] [PubMed] [Google Scholar]
- Mechelli et al., 2005.Mechelli A, Friston KJ, Frackowiak RS, Price CJ. (2005) Structural covariance in the human cortex. J Neurosci 25:8303–8310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meethal et al., 2009.Meethal SV, Liu T, Chan HW, Ginsburg E, Wilson AC, Gray DN, Bowen RL, Vonderhaar BK, Atwood CS. (2009) Identification of a regulatory loop for the synthesis of neurosteroids: a steroidogenic acute regulatory protein-dependent mechanism involving hypothalamic-pituitary-gonadal axis receptors. J Neurochem 110:1014–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel and Sachs, 1994.Meisel R, Sachs B. (1994) The physiology of male sexual behavior, in The Physiology of Reproduction, 5th ed (Knobil E, Neill J. eds) pp 3–106, Raven Press, New York: [Google Scholar]
- Merchenthaler et al., 2004.Merchenthaler I, Lane MV, Numan S, Dellovade TL. (2004) Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol 473:270–291 [DOI] [PubMed] [Google Scholar]
- Mermelstein, 2009.Mermelstein PG. (2009) Membrane-localised oestrogen receptor alpha and beta influence neuronal activity through activation of metabotropic glutamate receptors. J Neuroendocrinol 21:257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métivier et al., 2003.Métivier R, Penot G, Hübner MR, Reid G, Brand H, Kos M, Gannon F. (2003) Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115:751–763 [DOI] [PubMed] [Google Scholar]
- Micevych and Dominguez, 2009.Micevych P, Dominguez R. (2009) Membrane estradiol signaling in the brain. Front Neuroendocrinol 30:315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller et al., 1998.Miller DB, Ali SF, O'Callaghan JP, Laws SC. (1998) The impact of gender and estrogen on striatal dopaminergic neurotoxicity. Ann NY Acad Sci 844:153–165 [PubMed] [Google Scholar]
- Miller and Duckles, 2008.Miller VM, Duckles SP. (2008) Vascular actions of estrogens: functional implications. Pharmacol Rev 60:210–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra et al., 2003.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, et al. (2003) Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology 144:2055–2067 [DOI] [PubMed] [Google Scholar]
- Mitsushima et al., 2008.Mitsushima D, Takase K, Funabashi T, Kimura F. (2008) Gonadal steroid hormones maintain the stress-induced acetylcholine release in the hippocampus: simultaneous measurements of the extracellular acetylcholine and serum corticosterone levels in the same subjects. Endocrinology 149:802–811 [DOI] [PubMed] [Google Scholar]
- Mitsushima et al., 2009a.Mitsushima D, Takase K, Funabashi T, Kimura F. (2009a) Gonadal steroids maintain 24 h acetylcholine release in the hippocampus: organizational and activational effects in behaving rats. J Neurosci 29:3808–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsushima et al., 2009b.Mitsushima D, Takase K, Takahashi T, Kimura F. (2009b) Activational and organisational effects of gonadal steroids on sex-specific acetylcholine release in the dorsal hippocampus. J Neuroendocrinol 21:400–405 [DOI] [PubMed] [Google Scholar]
- Morale et al., 2008.Morale MC, L'Episcopo F, Tirolo C, Giaquinta G, Caniglia S, Testa N, Arcieri P, Serra PA, Lupo G, Alberghina M, et al. (2008) Loss of aromatase cytochrome P450 function as a risk factor for Parkinson's disease? Brain Res Rev 57:431–443 [DOI] [PubMed] [Google Scholar]
- Morale et al., 2006.Morale MC, Serra PA, L'episcopo F, Tirolo C, Caniglia S, Testa N, Gennuso F, Giaquinta G, Rocchitta G, Desole MS, et al. (2006) Estrogen, neuroinflammation and neuroprotection in Parkinson's disease: glia dictates resistance versus vulnerability to neurodegeneration. Neuroscience 138:869–878 [DOI] [PubMed] [Google Scholar]
- Morissette et al., 2008a.Morissette M, Al Sweidi S, Callier S, Di Paolo T. (2008a) Estrogen and SERM neuroprotection in animal models of Parkinson's disease. Mol Cell Endocrinol 290:60–69 [DOI] [PubMed] [Google Scholar]
- Morissette et al., 2008b.Morissette M, Le Saux M, D'Astous M, Jourdain S, Al Sweidi S, Morin N, Estrada-Camarena E, Mendez P, Garcia-Segura LM, Di Paolo T. (2008b) Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Mol Biol 108:327–338 [DOI] [PubMed] [Google Scholar]
- Moroz et al., 2003.Moroz IA, Rajabi H, Rodaros D, Stewart J. (2003) Effects of sex and hormonal status on astrocytic basic fibroblast growth factor-2 and tyrosine hydroxylase immunoreactivity after medial forebrain bundle 6-hydroxydopamine lesions of the midbrain dopamine neurons. Neuroscience 118:463–476 [DOI] [PubMed] [Google Scholar]
- Morris et al., 2004.Morris JA, Jordan CL, Breedlove SM. (2004) Sexual differentiation of the vertebrate nervous system. Nat Neurosci 7:1034–1039 [DOI] [PubMed] [Google Scholar]
- Mozley et al., 2001.Mozley LH, Gur RC, Mozley PD, Gur RE. (2001) Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry 158:1492–1499 [DOI] [PubMed] [Google Scholar]
- Mueller and Bale, 2007.Mueller BR, Bale TL. (2007) Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol Behav 91:55–65 [DOI] [PubMed] [Google Scholar]
- Munro et al., 2006.Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W, et al. (2006) Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry 59:966–974 [DOI] [PubMed] [Google Scholar]
- Murphy et al., 1998.Murphy DD, Cole NB, Greenberger V, Segal M. (1998) Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci 18:2550–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy and Segal, 1997.Murphy DD, Segal M. (1997) Morphological plasticity of dendritic spines in central neurons is mediated by activation of cAMP response element binding protein. Proc Natl Acad Sci USA 94:1482–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy et al., 2003.Murphy S, McCullough L, Littleton-Kearney M, Hurn P. (2003) Estrogen and selective estrogen receptor modulators: neuroprotection in the Women's Health Initiative era. Endocrine 21:17–26 [DOI] [PubMed] [Google Scholar]
- Murray et al., 2003.Murray HE, Pillai AV, McArthur SR, Razvi N, Datla KP, Dexter DT, Gillies GE. (2003) Dose- and sex-dependent effects of the neurotoxin 6-hydroxydopamine on the nigrostriatal dopaminergic pathway of adult rats: differential actions of estrogen in males and females. Neuroscience 116:213–222 [DOI] [PubMed] [Google Scholar]
- Murray et al., 1999a.Murray HE, Rantle CM, Simonian SX, DonCarlos LL, Herbison AE, Gillies GE. (1999a) Sexually dimorphic ontogeny of GABAergic influences on periventricular somatostatin neurons. Neuroendocrinology 70:384–391 [DOI] [PubMed] [Google Scholar]
- Murray et al., 1999b.Murray HE, Simonian SX, Herbison AE, Gillies GE. (1999b) Correlation of hypothalamic somatostatin mRNA expression and peptide content with secretion: sexual dimorphism and differential regulation by gonadal factors. J Neuroendocrinol 11:27–33 [DOI] [PubMed] [Google Scholar]
- Murray et al., 1999c.Murray HE, Simonian SX, Herbison AE, Gillies GE. (1999c) Ontogeny and sexual differentiation of somatostatin biosynthesis and secretion in the hypothalamic periventricular-median eminence pathway. J Neuroendocrinol 11:35–42 [DOI] [PubMed] [Google Scholar]
- Musatov et al., 2006.Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. (2006) RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc Natl Acad Sci USA 103:10456–10460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers et al., 2003.Myers RE, Anderson LI, Dluzen DE. (2003) Estrogen, but not testosterone, attenuates methamphetamine-evoked dopamine output from superfused striatal tissue of female and male mice. Neuropharmacology 44:624–632 [DOI] [PubMed] [Google Scholar]
- Naftolin et al., 2007.Naftolin F, Garcia-Segura LM, Horvath TL, Zsarnovszky A, Demir N, Fadiel A, Leranth C, Vondracek-Klepper S, Lewis C, Chang A, et al. (2007) Estrogen-induced hypothalamic synaptic plasticity and pituitary sensitization in the control of the estrogen-induced gonadotrophin surge. Reprod Sci 14:101–116 [DOI] [PubMed] [Google Scholar]
- Naftolin and Malaspina, 2007.Naftolin F, Malaspina D. (2007) Estrogen, estrogen treatment and the post-reproductive woman's brain. Maturitas 57:23–26 [DOI] [PubMed] [Google Scholar]
- Neufang et al., 2009.Neufang S, Specht K, Hausmann M, Güntürkün O, Herpertz-Dahlmann B, Fink GR, Konrad K. (2009) Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex 19:464–473 [DOI] [PubMed] [Google Scholar]
- Nilsen et al., 2007.Nilsen J, Irwin RW, Gallaher TK, Brinton RD. (2007) Estradiol in vivo regulation of brain mitochondrial proteome. J Neurosci 27:14069–14077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty, 2004.O'Doherty JP. (2004) Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol 14:769–776 [DOI] [PubMed] [Google Scholar]
- Ogawa et al., 1999.Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. (1999) Survival of reproductive behaviors in estrogen receptor beta gene-deficient (betaERKO) male and female mice. Proc Natl Acad Sci USA 96:12887–12892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa et al., 1998.Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. (1998) Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology 139:5070–5081 [DOI] [PubMed] [Google Scholar]
- Ogawa et al., 1997.Ogawa S, Lubahn DB, Korach KS, Pfaff DW. (1997) Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci USA 94:1476–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani et al., 2001.Ohtani H, Nomoto M, Douchi T. (2001) Chronic estrogen treatment replaces striatal dopaminergic function in ovariectomized rats. Brain Res 900:163–168 [DOI] [PubMed] [Google Scholar]
- Orikasa et al., 2002.Orikasa C, Kondo Y, Hayashi S, McEwen BS, Sakuma Y. (2002) Sexually dimorphic expression of estrogen receptor beta in the anteroventral periventricular nucleus of the rat preoptic area: implication in luteinizing hormone surge. Proc Natl Acad Sci USA 99:3306–3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund et al., 2000a.Osterlund MK, Grandien K, Keller E, Hurd YL. (2000a) The human brain has distinct regional expression patterns of estrogen receptor alpha mRNA isoforms derived from alternative promoters. J Neurochem 75:1390–1397 [DOI] [PubMed] [Google Scholar]
- Osterlund et al., 2000b.Osterlund MK, Gustafsson JA, Keller E, Hurd YL. (2000b) Estrogen receptor beta (ERbeta) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERalpha mRNA. J Clin Endocrinol Metab 85:3840–3846 [DOI] [PubMed] [Google Scholar]
- Osterlund et al., 2000c.Osterlund MK, Keller E, Hurd YL. (2000c) The human forebrain has discrete estrogen receptor alpha messenger RNA expression: high levels in the amygdaloid complex. Neuroscience 95:333–342 [DOI] [PubMed] [Google Scholar]
- Ostlund et al., 2003.Ostlund H, Keller E, Hurd YL. (2003) Estrogen receptor gene expression in relation to neuropsychiatric disorders. Ann NY Acad Sci 1007:54–63 [DOI] [PubMed] [Google Scholar]
- Pacák and Palkovits, 2001.Pacák K, Palkovits M. (2001) Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev 22:502–548 [DOI] [PubMed] [Google Scholar]
- Paech et al., 1997.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. (1997) Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science 277:1508–1510 [DOI] [PubMed] [Google Scholar]
- Pak et al., 2007.Pak TR, Chung WC, Hinds LR, Handa RJ. (2007) Estrogen receptor-beta mediates dihydrotestosterone-induced stimulation of the arginine vasopressin promoter in neuronal cells. Endocrinology 148:3371–3382 [DOI] [PubMed] [Google Scholar]
- Parducz et al., 2006.Parducz A, Hajszan T, Maclusky NJ, Hoyk Z, Csakvari E, Kurunczi A, Prange-Kiel J, Leranth C. (2006) Synaptic remodeling induced by gonadal hormones: neuronal plasticity as a mediator of neuroendocrine and behavioral responses to steroids. Neuroscience 138:977–985 [DOI] [PubMed] [Google Scholar]
- Parducz et al., 2002.Parducz A, Hoyk Z, Kis Z, Garcia-Segura LM. (2002) Hormonal enhancement of neuronal firing is linked to structural remodelling of excitatory and inhibitory synapses. Eur J Neurosci 16:665–670 [DOI] [PubMed] [Google Scholar]
- Pasqualini et al., 1995.Pasqualini C, Olivier V, Guibert B, Frain O, Leviel V. (1995) Acute stimulatory effect of estradiol on striatal dopamine synthesis. J Neurochem 65:1651–1657 [DOI] [PubMed] [Google Scholar]
- Patchev and Almeida, 1998.Patchev VK, Almeida OF. (1998) Gender specificity in the neural regulation of the response to stress: new leads from classical paradigms. Mol Neurobiol 16:63–77 [DOI] [PubMed] [Google Scholar]
- Patchev et al., 2008.Patchev VK, Bachurin SO, Albers M, Fritzemeier KH, Papadopoulos V. (2008) Neurotrophic estrogens: essential profile and endpoints for drug discovery. Drug Discov Today 13:734–747 [DOI] [PubMed] [Google Scholar]
- Patisaul et al., 2009.Patisaul HB, Burke KT, Hinkle RE, Adewale HB, Shea D. (2009) Systemic administration of diarylpropionitrile (DPN) or phytoestrogens does not affect anxiety-related behaviors in gonadally intact male rats. Horm Behav 55:319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau et al., 1998.Pau CY, Pau KY, Spies HG. (1998) Putative estrogen receptor beta and alpha mRNA expression in male and female rhesus macaques. Mol Cell Endocrinol 146:59–68 [DOI] [PubMed] [Google Scholar]
- Paus et al., 2008.Paus T, Keshavan M, Giedd JN. (2008) Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 9:947–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaresi et al., 2010.Pesaresi M, Maschi O, Giatti S, Garcia-Segura LM, Caruso D, Melcangi RC. (2010) Sex differences in neuroactive steroid levels in the nervous system of diabetic and non-diabetic rats. Horm Behav 57:46–55 [DOI] [PubMed] [Google Scholar]
- Pfaff and Schwartz-Giblin, 1988.Pfaff D, Schwartz-Giblin S. (1988) Cellular mechanisms of female reproductive behaviors, in The Physiology of Reproduction (Knobil E, Neill JD. eds) pp 1487–1568, Raven Press, New York: [Google Scholar]
- Piefke et al., 2005.Piefke M, Weiss PH, Markowitsch HJ, Fink GR. (2005) Gender differences in the functional neuroanatomy of emotional episodic autobiographical memory. Hum Brain Mapp 24:313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike et al., 2009.Pike CJ, Carroll JC, Rosario ER, Barron AM. (2009) Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinol 30:239–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaitakis et al., 2010.Plaitakis A, Latsoudis H, Kanavouras K, Ritz B, Bronstein JM, Skoula I, Mastorodemos V, Papapetropoulos S, Borompokas N, Zaganas I, et al. (2010) Gain-of-function variant in GLUD2 glutamate dehydrogenase modifies Parkinson's disease onset. Eur J Hum Genet 18:336–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohjalainen et al., 1998.Pohjalainen T, Rinne JO, Någren K, Syvälahti E, Hietala J. (1998) Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am J Psychiatry 155:768–773 [DOI] [PubMed] [Google Scholar]
- Polston et al., 2004.Polston EK, Gu G, Simerly RB. (2004) Neurons in the principal nucleus of the bed nuclei of the stria terminalis provide a sexually dimorphic GABAergic input to the anteroventral periventricular nucleus of the hypothalamus. Neuroscience 123:793–803 [DOI] [PubMed] [Google Scholar]
- Prange-Kiel and Rune, 2006.Prange-Kiel J, Rune GM. (2006) Direct and indirect effects of estrogen on rat hippocampus. Neuroscience 138:765–772 [DOI] [PubMed] [Google Scholar]
- Prokai and Simpkins, 2007.Prokai L, Simpkins JW. (2007) Structure-nongenomic neuroprotection relationship of estrogens and estrogen-derived compounds. Pharmacol Ther 114:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz et al., 2008.Prossnitz ER, Sklar LA, Oprea TI, Arterburn JB. (2008) GPR30: a novel therapeutic target in estrogen-related disease. Trends Pharmacol Sci 29:116–123 [DOI] [PubMed] [Google Scholar]
- Pruessner et al., 2004.Pruessner JC, Champagne F, Meaney MJ, Dagher A. (2004) Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci 24:2825–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada et al., 2008.Quesada A, Lee BY, Micevych PE. (2008) PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson's disease. Dev Neurobiol 68:632–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada and Micevych, 2004.Quesada A, Micevych PE. (2004) Estrogen interacts with the IGF-1 system to protect nigrostriatal dopamine and maintain motoric behavior after 6-hydroxdopamine lesions. J Neurosci Res 75:107–116 [DOI] [PubMed] [Google Scholar]
- Quinn et al., 2007.Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR. (2007) Sex chromosome complement regulates habit formation. Nat Neurosci 10:1398–1400 [DOI] [PubMed] [Google Scholar]
- Quinn and Marsden, 1986.Quinn NP, Marsden CD. (1986) Menstrual-related fluctuations in Parkinson's disease. Mov Disord 1:85–87 [DOI] [PubMed] [Google Scholar]
- Raber, 2008.Raber J. (2008) AR, apoE, and cognitive function. Horm Behav 53:706–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisman and Field, 1971.Raisman G, Field PM. (1971) Sexual dimorphism in the preoptic area of the rat. Science 173:731–733 [DOI] [PubMed] [Google Scholar]
- Ramirez et al., 2003.Ramirez AD, Liu X, Menniti FS. (2003) Repeated estradiol treatment prevents MPTP-induced dopamine depletion in male mice. Neuroendocrinology 77:223–231 [DOI] [PubMed] [Google Scholar]
- Ramsden et al., 2003a.Ramsden M, Nyborg AC, Murphy MP, Chang L, Stanczyk FZ, Golde TE, Pike CJ. (2003a) Androgens modulate beta-amyloid levels in male rat brain. J Neurochem 87:1052–1055 [DOI] [PubMed] [Google Scholar]
- Ramsden et al., 2003b.Ramsden M, Shin TM, Pike CJ. (2003b) Androgens modulate neuronal vulnerability to kainate lesion. Neuroscience 122:573–578 [DOI] [PubMed] [Google Scholar]
- Rao et al., 2010.Rao U, Chen LA, Bidesi AS, Shad MU, Thomas MA, Hammen CL. (2010) Hippocampal changes associated with early-life adversity and vulnerability to depression. Biol Psychiatry 67:357–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp et al., 2003.Rapp PR, Morrison JH, Roberts JA. (2003) Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci 23:5708–5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravina et al., 2003.Ravina BM, Fagan SC, Hart RG, Hovinga CA, Murphy DD, Dawson TM, Marler JR. (2003) Neuroprotective agents for clinical trials in Parkinson's disease: a systematic assessment. Neurology 60:1234–1240 [DOI] [PubMed] [Google Scholar]
- Raz et al., 2008.Raz L, Khan MM, Mahesh VB, Vadlamudi RK, Brann DW. (2008) Rapid estrogen signaling in the brain. Neurosignals 16:140–153 [DOI] [PubMed] [Google Scholar]
- Register et al., 1998.Register TC, Shively CA, Lewis CE. (1998) Expression of estrogen receptor alpha and beta transcripts in female monkey hippocampus and hypothalamus. Brain Res 788:320–322 [DOI] [PubMed] [Google Scholar]
- Reid et al., 2003.Reid G, Hübner MR, Métivier R, Brand H, Denger S, Manu D, Beaudouin J, Ellenberg J, Gannon F. (2003) Cyclic, proteasome-mediated turnover of unliganded and liganded ERalpha on responsive promoters is an integral feature of estrogen signaling. Mol Cell 11:695–707 [DOI] [PubMed] [Google Scholar]
- Riggs and Melton, 1995.Riggs BL, Melton LJ., 3rd (1995) The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone 17 (5 Suppl):505S–511S [DOI] [PubMed] [Google Scholar]
- Rinn and Snyder, 2005.Rinn JL, Snyder M. (2005) Sexual dimorphism in mammalian gene expression. Trends Genet 21:298–305 [DOI] [PubMed] [Google Scholar]
- Rissman et al., 1997.Rissman EF, Wersinger SR, Taylor JA, Lubahn DB. (1997) Estrogen receptor function as revealed by knockout studies: neuroendocrine and behavioral aspects. Horm Behav 31:232–243 [DOI] [PubMed] [Google Scholar]
- Robbins, 2000.Robbins TW. (2000) Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp Brain Res 133:130–138 [DOI] [PubMed] [Google Scholar]
- Robinson et al., 1990.Robinson TE, Castañeda E, Whishaw IQ. (1990) Compensatory changes in striatal dopamine neurons following recovery from injury induced by 6-OHDA or methamphetamine: a review of evidence from microdialysis studies. Can J Psychol 44:253–275 [DOI] [PubMed] [Google Scholar]
- Roca et al., 2005.Roca CA, Schmidt PJ, Deuster PA, Danaceau MA, Altemus M, Putnam K, Chrousos GP, Nieman LK, Rubinow DR. (2005) Sex-related differences in stimulated hypothalamic-pituitary-adrenal axis during induced gonadal suppression. J Clin Endocrinol Metab 90:4224–4231 [DOI] [PubMed] [Google Scholar]
- Rochira et al., 2002.Rochira V, Balestrieri A, Madeo B, Spaggiari A, Carani C. (2002) Congenital estrogen deficiency in men: a new syndrome with different phenotypes; clinical and therapeutic implications in men. Mol Cell Endocrinol 193:19–28 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Navarro et al., 2008.Rodríguez-Navarro JA, Solano RM, Casarejos MJ, Gomez A, Perucho J, de Yébenes JG, Mena MA. (2008) Gender differences and estrogen effects in parkin null mice. J Neurochem 106:2143–2157 [DOI] [PubMed] [Google Scholar]
- Rojo et al., 2003.Rojo A, Aguilar M, Garolera MT, Cubo E, Navas I, Quintana S. (2003) Depression in Parkinson's disease: clinical correlates and outcome. Parkinsonism Relat Disord 10:23–28 [DOI] [PubMed] [Google Scholar]
- Romeo et al., 2005.Romeo RD, McCarthy JB, Wang A, Milner TA, McEwen BS. (2005) Sex differences in hippocampal estradiol-induced N-methyl-D-aspartic acid binding and ultrastructural localization of estrogen receptor-alpha. Neuroendocrinology 81:391–399 [DOI] [PubMed] [Google Scholar]
- Romeo et al., 2004.Romeo RD, Waters EM, McEwen BS. (2004) Steroid-induced hippocampal synaptic plasticity: sex differences and similarities. Neuron Glia Biology 1:219–229 [DOI] [PubMed] [Google Scholar]
- Roof, 1993a.Roof RL. (1993a) The dentate gyrus is sexually dimorphic in prepubescent rats: testosterone plays a significant role. Brain Res 610:148–151 [DOI] [PubMed] [Google Scholar]
- Roof, 1993b.Roof RL. (1993b) Neonatal exogenous testosterone modifies sex difference in radial arm and Morris water maze performance in prepubescent and adult rats. Behav Brain Res 53:1–10 [DOI] [PubMed] [Google Scholar]
- Rosario et al., 2009.Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. (2009) Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer's disease. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2009.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli et al., 2009.Roselli CE, Liu M, Hurn PD. (2009) Brain aromatization: classic roles and new perspectives. Semin Reprod Med 27:207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw et al., 2002.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, et al. (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 288:321–333 [DOI] [PubMed] [Google Scholar]
- Rudick et al., 2003.Rudick CN, Gibbs RB, Woolley CS. (2003) A role for the basal forebrain cholinergic system in estrogen-induced disinhibition of hippocampal pyramidal cells. J Neurosci 23:4479–4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rune and Frotscher, 2005.Rune GM, Frotscher M. (2005) Neurosteroid synthesis in the hippocampus: role in synaptic plasticity. Neuroscience 136:833–842 [DOI] [PubMed] [Google Scholar]
- Russo et al., 2003.Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, Jenab S, Quiñones-Jenab V. (2003) Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience 120:523–533 [DOI] [PubMed] [Google Scholar]
- Saldanha et al., 2009.Saldanha CJ, Duncan KA, Walters BJ. (2009) Neuroprotective actions of brain aromatase. Front Neuroendocrinol 30:106–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin et al., 2009.Salin P, López IP, Kachidian P, Barroso-Chinea P, Rico AJ, Gómez-Bautista V, Coulon P, Kerkerian-Le Goff L, Lanciego JL. (2009) Changes to interneuron-driven striatal microcircuits in a rat model of Parkinson's disease. Neurobiol Dis 34:545–552 [DOI] [PubMed] [Google Scholar]
- Sandyk, 1989.Sandyk R. (1989) Estrogens and the pathophysiology of Parkinson's disease. Int J Neurosci 45:119–122 [DOI] [PubMed] [Google Scholar]
- Sasano et al., 1998.Sasano H, Takashashi K, Satoh F, Nagura H, Harada N. (1998) Aromatase in the human central nervous system. Clin Endocrinol (Oxf) 48:325–329 [DOI] [PubMed] [Google Scholar]
- Saunders-Pullman et al., 2009.Saunders-Pullman R, Derby C, Santoro N, Bressman S, Chiu B, Lipton RB, Wasswetheil-Smoller S. (2009) Role of endogenous and exogenous hormone exposure on the risk of Parkinson's disease: results from the Women's Health Initiative Observational Study (abstract S23.002), in 61st Annual Meeting of the American Academy of Neurology; 25 Apr–2 May 2009; Seattle, WA American Academy of Neurology, Saint Paul, MN: [Google Scholar]
- Saunders-Pullman et al., 1999.Saunders-Pullman R, Gordon-Elliott J, Parides M, Fahn S, Saunders HR, Bressman S. (1999) The effect of estrogen replacement on early Parkinson's disease. Neurology 52:1417–1421 [DOI] [PubMed] [Google Scholar]
- Sawada et al., 2002.Sawada H, Ibi M, Kihara T, Honda K, Nakamizo T, Kanki R, Nakanishi M, Sakka N, Akaike A, Shimohama S. (2002) Estradiol protects dopaminergic neurons in a MPP+Parkinson's disease model. Neuropharmacology 42:1056–1064 [DOI] [PubMed] [Google Scholar]
- Sawada et al., 1998.Sawada H, Ibi M, Kihara T, Urushitani M, Akaike A, Shimohama S. (1998) Estradiol protects mesencephalic dopaminergic neurons from oxidative stress-induced neuronal death. J Neurosci Res 54:707–719 [DOI] [PubMed] [Google Scholar]
- Scaglione et al., 2005.Scaglione C, Vignatelli L, Plazzi G, Marchese R, Negrotti A, Rizzo G, Lopane G, Bassein L, Maestri M, Bernardini S, et al. (2005) REM sleep behaviour disorder in Parkinson's disease: a questionnaire-based study. Neurol Sci 25:316–321 [DOI] [PubMed] [Google Scholar]
- Scheff and Price, 2003.Scheff SW, Price DA. (2003) Synaptic pathology in Alzheimer's disease: a review of ultrastructural studies. Neurobiol Aging 24:1029–1046 [DOI] [PubMed] [Google Scholar]
- Schrag et al., 2000.Schrag A, Ben-Shlomo Y, Quinn NP. (2000) Cross sectional prevalence survey of idiopathic Parkinson's disease and Parkinsonism in London. BMJ 321:21–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz et al., 2009.Schultz KN, von Esenwein SA, Hu M, Bennett AL, Kennedy RT, Musatov S, Toran-Allerand CD, Kaplitt MG, Young LJ, Becker JB. (2009) Viral vector-mediated overexpression of estrogen receptor-alpha in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci 29:1897–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz and McCarthy, 2008.Schwarz JM, McCarthy MM. (2008) Cellular mechanisms of estradiol-mediated masculinization of the brain. J Steroid Biochem Mol Biol 109:300–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serova et al., 2004.Serova LI, Maharjan S, Huang A, Sun D, Kaley G, Sabban EL. (2004) Response of tyrosine hydroxylase and GTP cyclohydrolase I gene expression to estrogen in brain catecholaminergic regions varies with mode of administration. Brain Res 1015:1–8 [DOI] [PubMed] [Google Scholar]
- Shankar et al., 2008.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. (2008) Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med 14:837–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe, 1998.Sharpe RM. (1998) The roles of oestrogen in the male. Trends Endocrinol Metab 9:371–377 [DOI] [PubMed] [Google Scholar]
- Shaywitz et al., 1995.Shaywitz BA, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Fletcher JM, Shankweiler DP, Katz L. (1995) Sex differences in the functional organization of the brain for language. Nature 373:607–609 [DOI] [PubMed] [Google Scholar]
- Shelly et al., 2008.Shelly W, Draper MW, Krishnan V, Wong M, Jaffe RB. (2008) Selective estrogen receptor modulators: an update on recent clinical findings. Obstet Gynecol Surv 63:163–181 [DOI] [PubMed] [Google Scholar]
- Sherwin, 2002.Sherwin BB. (2002) Estrogen and cognitive aging in women. Trends Pharmacol Sci 23:527–534 [DOI] [PubMed] [Google Scholar]
- Sherwin, 2003.Sherwin BB. (2003) Steroid hormones and cognitive functioning in aging men: a mini-review. J Mol Neurosci 20:385–393 [DOI] [PubMed] [Google Scholar]
- Sherwin and Henry, 2008.Sherwin BB, Henry JF. (2008) Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol 29:88–113 [DOI] [PubMed] [Google Scholar]
- Shors, 2004.Shors TJ. (2004) Learning during stressful times. Learn Mem 11:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors et al., 2001.Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. (2001) Neurogenesis in the adult is involved in the formation of trace memories. Nature 410:372–376 [DOI] [PubMed] [Google Scholar]
- Shughrue, 2004.Shughrue PJ. (2004) Estrogen attenuates the MPTP-induced loss of dopamine neurons from the mouse SNc despite a lack of estrogen receptors (ERalpha and ERbeta). Exp Neurol 190:468–477 [DOI] [PubMed] [Google Scholar]
- Shughrue et al., 1996.Shughrue PJ, Komm B, Merchenthaler I. (1996) The distribution of estrogen receptor-beta mRNA in the rat hypothalamus. Steroids 61:678–681 [DOI] [PubMed] [Google Scholar]
- Shughrue et al., 1997.Shughrue PJ, Lane MV, Merchenthaler I. (1997) Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol 388:507–525 [DOI] [PubMed] [Google Scholar]
- Shughrue and Merchenthaler, 2001.Shughrue PJ, Merchenthaler I. (2001) Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol 436:64–81 [PubMed] [Google Scholar]
- Shughrue et al., 1998.Shughrue PJ, Scrimo PJ, Merchenthaler I. (1998) Evidence for the colocalization of estrogen receptor-beta mRNA and estrogen receptor-alpha immunoreactivity in neurons of the rat forebrain. Endocrinology 139:5267–5270 [DOI] [PubMed] [Google Scholar]
- Shulman and Bhat, 2006.Shulman LM, Bhat V. (2006) Gender disparities in Parkinson's disease. Expert Rev Neurother 6:407–416 [DOI] [PubMed] [Google Scholar]
- Shumaker et al., 2004.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, et al. (2004) Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA 291:2947–2958 [DOI] [PubMed] [Google Scholar]
- Siegel et al., 2008.Siegel JA, Young LA, Neiss MB, Samuels MH, Roselli CE, Janowsky JS. (2008) Estrogen, testosterone, and sequential movement in men. Behav Neurosci 122:955–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman and Eals, 1992.Silverman I, Eals M. (1992) Sex differences in spatial abilities: evolutionary theory and data, in The Adapted Mind: Evolutionary Psychology and the Generation of Culture (Barkow J, Tooby J, Cosmides L. eds) pp 533–549, Oxford University Press, New York: [Google Scholar]
- Simerly, 1989.Simerly RB. (1989) Hormonal control of the development and regulation of tyrosine hydroxylase expression within a sexually dimorphic population of dopaminergic cells in the hypothalamus. Brain Res Mol Brain Res 6:297–310 [DOI] [PubMed] [Google Scholar]
- Simerly, 2005.Simerly RB. (2005) Wired on hormones: endocrine regulation of hypothalamic development. Curr Opin Neurobiol 15:81–85 [DOI] [PubMed] [Google Scholar]
- Simerly et al., 1990.Simerly RB, Chang C, Muramatsu M, Swanson LW. (1990) Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol 294:76–95 [DOI] [PubMed] [Google Scholar]
- Simola et al., 2007.Simola N, Morelli M, Carta AR. (2007) The 6-hydroxydopamine model of Parkinson's disease. Neurotox Res 11:151–167 [DOI] [PubMed] [Google Scholar]
- Simonian et al., 1998.Simonian SX, Murray HE, Gillies GE, Herbison AE. (1998) Estrogen-dependent ontogeny of sex differences in somatostatin neurons of the hypothalamic periventricular nucleus. Endocrinology 139:1420–1428 [DOI] [PubMed] [Google Scholar]
- Simpson et al., 2002.Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, Speed C, Jones M. (2002) Aromatase—a brief overview. Annu Rev Physiol 64:93–127 [DOI] [PubMed] [Google Scholar]
- Smeyne and Jackson-Lewis, 2005.Smeyne RJ, Jackson-Lewis V. (2005) The MPTP model of Parkinson's disease. Brain Res Mol Brain Res 134:57–66 [DOI] [PubMed] [Google Scholar]
- Solomon and Herman, 2009.Solomon MB, Herman JP. (2009) Sex differences in psychopathology: of gonads, adrenals and mental illness. Physiol Behav 97:250–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song and Haber, 2000.Song DD, Haber SN. (2000) Striatal responses to partial dopaminergic lesion: evidence for compensatory sprouting. J Neurosci 20:5102–5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer et al., 2008.Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. (2008) Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol 29:219–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell et al., 2008.Stell A, Belcredito S, Ciana P, Maggi A. (2008) Molecular imaging provides novel insights on estrogen receptor activity in mouse brain. Mol Imaging 7:283–292 [PMC free article] [PubMed] [Google Scholar]
- Stoffel-Wagner et al., 1999.Stoffel-Wagner B, Watzka M, Schramm J, Bidlingmaier F, Klingmüller D. (1999) Expression of CYP19 (aromatase) mRNA in different areas of the human brain. J Steroid Biochem Mol Biol 70:237–241 [DOI] [PubMed] [Google Scholar]
- Strijks et al., 1999.Strijks E, Kremer JA, Horstink MW. (1999) Effects of female sex steroids on Parkinson's disease in postmenopausal women. Clin Neuropharmacol 22:93–97 [DOI] [PubMed] [Google Scholar]
- Sullivan and Fowlkes, 1996.Sullivan JM, Fowlkes LP. (1996) Estrogens, menopause, and coronary artery disease. Cardiol Clin 14:105–116 [DOI] [PubMed] [Google Scholar]
- Suzuki et al., 2002.Suzuki A, Sugihara A, Uchida K, Sato T, Ohta Y, Katsu Y, Watanabe H, Iguchi T. (2002) Developmental effects of perinatal exposure to bisphenol-A and diethylstilbestrol on reproductive organs in female mice. Reprod Toxicol 16:107–116 [DOI] [PubMed] [Google Scholar]
- Suzuki et al., 2009.Suzuki S, Brown CM, Wise PM. (2009) Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol 30:201–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki and Handa, 2005.Suzuki S, Handa RJ. (2005) Estrogen receptor-beta, but not estrogen receptor-alpha, is expressed in prolactin neurons of the female rat paraventricular and supraoptic nuclei: comparison with other neuropeptides. J Comp Neurol 484:28–42 [DOI] [PubMed] [Google Scholar]
- Swaab, 2004.Swaab DF. (2004) Sexual differentiation of the human brain: relevance for gender identity, transsexualism and sexual orientation. Gynecol Endocrinol 19:301–312 [DOI] [PubMed] [Google Scholar]
- Swaab, 2008.Swaab DF. (2008) Sexual orientation and its basis in brain structure and function. Proc Natl Acad Sci USA 105:10273–10274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab et al., 2003.Swaab DF, Chung WC, Kruijver FP, Hofman MA, Hestiantoro A. (2003) Sex differences in the hypothalamus in the different stages of human life. Neurobiol Aging 24 (Suppl 1): S1–S16; discussion S17–S19 [DOI] [PubMed] [Google Scholar]
- Swamydas et al., 2009.Swamydas M, Bessert D, Skoff R. (2009) Sexual dimorphism of oligodendrocytes is mediated by differential regulation of signaling pathways. J Neurosci Res 87:3306–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow et al., 2001.Swerdlow RH, Parker WD, Currie LJ, Bennett JP, Harrison MB, Trugman JM, Wooten GF. (2001) Gender ratio differences between Parkinson's disease patients and their affected relatives. Parkinsonism Relat Disord 7:129–133 [DOI] [PubMed] [Google Scholar]
- Szpir, 2006.Szpir M. (2006) Tracing the origins of autism: a spectrum of new studies. Environ Health Perspect 114:A412–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takase et al., 2009.Takase K, Kimura F, Yagami T, Mitsushima D. (2009) Sex-specific 24-h acetylcholine release profile in the medial prefrontal cortex: simultaneous measurement of spontaneous locomotor activity in behaving rats. Neuroscience 159:7–15 [DOI] [PubMed] [Google Scholar]
- Tamás et al., 2005.Tamás A, Lubics A, Szalontay L, Lengvári I, Reglodi D. (2005) Age and gender differences in behavioral and morphological outcome after 6-hydroxydopamine-induced lesion of the substantia nigra in rats. Behav Brain Res 158:221–229 [DOI] [PubMed] [Google Scholar]
- Tang et al., 2004.Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, et al. (2004) Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex 14:215–223 [DOI] [PubMed] [Google Scholar]
- Tapia-Gonzalez et al., 2008.Tapia-Gonzalez S, Carrero P, Pernia O, Garcia-Segura LM, Diz-Chaves Y. (2008) Selective oestrogen receptor (ER) modulators reduce microglia reactivity in vivo after peripheral inflammation: potential role of microglial ERs. J Endocrinol 198:219–230 [DOI] [PubMed] [Google Scholar]
- Taylor et al., 2000.Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. (2000) Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol Rev 107:411–429 [DOI] [PubMed] [Google Scholar]
- Tetel, 2009.Tetel MJ. (2009) Nuclear receptor coactivators: essential players for steroid hormone action in the brain and in behaviour. J Neuroendocrinol 21:229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanky et al., 2003.Thanky NR, Slater R, Herbison AE. (2003) Sex differences in estrogen-dependent transcription of gonadotropin-releasing hormone (GnRH) gene revealed in GnRH transgenic mice. Endocrinology 144:3351–3358 [DOI] [PubMed] [Google Scholar]
- Thanky et al., 2002.Thanky NR, Son JH, Herbison AE. (2002) Sex differences in the regulation of tyrosine hydroxylase gene transcription by estrogen in the locus coeruleus of TH9-LacZ transgenic mice. Mol Brain Res 104:220–226 [DOI] [PubMed] [Google Scholar]
- Thomas et al., 2009.Thomas MB, Hu M, Lee TM, Bhatnagar S, Becker JB. (2009) Sex-specific susceptibility to cocaine in rats with a history of prenatal stress. Physiol Behav 97:270–277 [DOI] [PubMed] [Google Scholar]
- Thompson, 1999.Thompson TL. (1999) Attenuation of dopamine uptake in vivo following priming with estradiol benzoate. Brain Res 834:164–167 [DOI] [PubMed] [Google Scholar]
- Tiwari-Woodruff et al., 2007.Tiwari-Woodruff S, Morales LB, Lee R, Voskuhl RR. (2007) Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER)alpha and ERbeta ligand treatment. Proc Natl Acad Sci USA 104:14813–14818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari-Woodruff and Voskuhl, 2009.Tiwari-Woodruff S, Voskuhl RR. (2009) Neuroprotective and anti-inflammatory effects of estrogen receptor ligand treatment in mice. J Neurol Sci 286:81–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobet et al., 2009.Tobet S, Knoll JG, Hartshorn C, Aurand E, Stratton M, Kumar P, Searcy B, McClellan K. (2009) Brain sex differences and hormone influences: a moving experience? J Neuroendocrinol 21:387–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand, 2005.Toran-Allerand CD. (2005) Estrogen and the brain: beyond ER-alpha, ER-beta, and 17beta-estradiol. Ann NY Acad Sci 1052:136–144 [DOI] [PubMed] [Google Scholar]
- Toran-Allerand, 2006.Toran-Allerand CD. (2006) Reply to ‘Hormone in the hot seat’. Nat Med 12:379–380 [DOI] [PubMed] [Google Scholar]
- Toran-Allerand et al., 2002.Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr., Nethrapalli IS, Tinnikov AA. (2002) ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci 22:8391–8401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand et al., 1999.Toran-Allerand CD, Singh M, Sétáló G., Jr (1999) Novel mechanisms of estrogen action in the brain: new players in an old story. Front Neuroendocrinol 20:97–121 [DOI] [PubMed] [Google Scholar]
- Tsang et al., 2000.Tsang KL, Ho SL, Lo SK. (2000) Estrogen improves motor disability in parkinsonian postmenopausal women with motor fluctuations. Neurology 54:2292–2298 [DOI] [PubMed] [Google Scholar]
- Uht et al., 1997.Uht RM, Anderson CM, Webb P, Kushner PJ. (1997) Transcriptional activities of estrogen and glucocorticoid receptors are functionally integrated at the AP-1 response element. Endocrinology 138:2900–2908 [DOI] [PubMed] [Google Scholar]
- Vadász et al., 1985.Vadász C, Baker H, Fink SJ, Reis DJ. (1985) Genetic effects and sexual dimorphism in tyrosine hydroxylase activity in two mouse strains and their reciprocal F1 hybrids. J Neurogenet 2:219–230 [DOI] [PubMed] [Google Scholar]
- Vadász et al., 1988.Vadász C, Kobor G, Kabai P, Sziráki I, Vadász I, Lajtha A. (1988) Perinatal anti-androgen treatment and genotype affect the mesotelencephalic dopamine system and behavior in mice. Horm Behav 22:528–539 [DOI] [PubMed] [Google Scholar]
- van Beilen et al., 2008.van Beilen M, Portman AT, Kiers HA, Maguire RP, Kaasinen V, Koning M, Pruim J, Leenders KL. (2008) Striatal FDOPA uptake and cognition in advanced non-demented Parkinson's disease: a clinical and FDOPA-PET study. Parkinsonism Relat Disord 14:224–228 [DOI] [PubMed] [Google Scholar]
- van den Buuse et al., 2003.van den Buuse M, Simpson ER, Jones ME. (2003) Prepulse inhibition of acoustic startle in aromatase knock-out mice: effects of age and gender. Genes Brain Behav 2:93–102 [DOI] [PubMed] [Google Scholar]
- Van Den Eeden et al., 2003.Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. (2003) Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol 157:1015–1022 [DOI] [PubMed] [Google Scholar]
- van Nas et al., 2009.van Nas A, Guhathakurta D, Wang SS, Yehya N, Horvath S, Zhang B, Ingram-Drake L, Chaudhuri G, Schadt EE, Drake TA, et al. (2009) Elucidating the role of gonadal hormones in sexually dimorphic gene coexpression networks. Endocrinology 150:1235–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan and Pfaff, 2008.Vasudevan N, Pfaff DW. (2008) Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol 29:238–257 [DOI] [PubMed] [Google Scholar]
- Velísková and Moshé, 2001.Velísková J, Moshé SL. (2001) Sexual dimorphism and developmental regulation of substantia nigra function. Ann Neurol 50:596–601 [DOI] [PubMed] [Google Scholar]
- Voorn et al., 1988.Voorn P, Kalsbeek A, Jorritsma-Byham B, Groenewegen HJ. (1988) The pre- and postnatal development of the dopaminergic cell groups in the ventral mesencephalon and the dopaminergic innervation of the striatum of the rat. Neuroscience 25:857–887 [DOI] [PubMed] [Google Scholar]
- Walker et al., 2000.Walker QD, Rooney MB, Wightman RM, Kuhn CM. (2000) Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience 95:1061–1070 [DOI] [PubMed] [Google Scholar]
- Wallace et al., 2006.Wallace M, Luine V, Arellanos A, Frankfurt M. (2006) Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res 1126:176–182 [DOI] [PubMed] [Google Scholar]
- Wang et al., 2007.Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, McEwen BS, Detre JA. (2007) Gender difference in neural response to psychological stress. Soc Cogn Affect Neurosci 2:227–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al., 2001.Wang L, Andersson S, Warner M, Gustafsson JA. (2001) Morphological abnormalities in the brains of estrogen receptor beta knockout mice. Proc Natl Acad Sci USA 98:2792–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al., 2003.Wang L, Andersson S, Warner M, Gustafsson JA. (2003) Estrogen receptor (ER)beta knockout mice reveal a role for ERbeta in migration of cortical neurons in the developing brain. Proc Natl Acad Sci USA 100:703–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward et al., 1994.Ward IL, Ward OB, Winn RJ, Bielawski D. (1994) Male and female sexual behavior potential of male rats prenatally exposed to the influence of alcohol, stress, or both factors. Behav Neurosci 108:1188–1195 [DOI] [PubMed] [Google Scholar]
- Warren and Juraska, 1997.Warren SG, Juraska JM. (1997) Spatial and nonspatial learning across the rat estrous cycle. Behav Neurosci 111:259–266 [DOI] [PubMed] [Google Scholar]
- Weihua et al., 2000.Weihua Z, Saji S, Mäkinen S, Cheng G, Jensen EV, Warner M, Gustafsson JA. (2000) Estrogen receptor (ER) beta, a modulator of ERalpha in the uterus. Proc Natl Acad Sci USA 97:5936–5941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland et al., 1997.Weiland NG, Orikasa C, Hayashi S, McEwen BS. (1997) Distribution and hormone regulation of estrogen receptor immunoreactive cells in the hippocampus of male and female rats. J Comp Neurol 388:603–612 [DOI] [PubMed] [Google Scholar]
- Weintraub et al., 2008.Weintraub D, Comella CL, Horn S. (2008) Parkinson's disease—Part 1: Pathophysiology, symptoms, burden, diagnosis, and assessment. Am J Manag Care 14 (2 Suppl):S40–S48 [PubMed] [Google Scholar]
- Weiser et al., 2008.Weiser MJ, Foradori CD, Handa RJ. (2008) Estrogen receptor beta in the brain: from form to function. Brain Res Rev 57:309–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss et al., 2006.Weiss LA, Pan L, Abney M, Ober C. (2006) The sex-specific genetic architecture of quantitative traits in humans. Nat Genet 38:218–222 [DOI] [PubMed] [Google Scholar]
- Weniger et al., 1993.Weniger JP, Zeis A, Chouraqui J. (1993) Estrogen production by fetal and infantile rat ovaries. Reprod Nutr Dev 33:129–136 [DOI] [PubMed] [Google Scholar]
- Wersinger et al., 1997.Wersinger SR, Sannen K, Villalba C, Lubahn DB, Rissman EF, De Vries GJ. (1997) Masculine sexual behavior is disrupted in male and female mice lacking a functional estrogen receptor alpha gene. Horm Behav 32:176–183 [DOI] [PubMed] [Google Scholar]
- Wessler et al., 2006.Wessler S, Otto C, Wilck N, Stangl V, Fritzemeier KH. (2006) Identification of estrogen receptor ligands leading to activation of non-genomic signaling pathways while exhibiting only weak transcriptional activity. J Steroid Biochem Mol Biol 98:25–35 [DOI] [PubMed] [Google Scholar]
- Westberry et al., 2008.Westberry JM, Prewitt AK, Wilson ME. (2008) Epigenetic regulation of the estrogen receptor alpha promoter in the cerebral cortex following ischemia in male and female rats. Neuroscience 152:982–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westberry et al., 2010.Westberry JM, Trout AL, Wilson ME. (2010) Epigenetic regulation of estrogen receptor alpha gene expression in the mouse cortex during early postnatal development. Endocrinology 151:731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, 1986.Williams CL. (1986) A reevaluation of the concept of separable periods of organizational and activational actions of estrogens in development of brain and behavior. Ann NY Acad Sci 474:282–292 [DOI] [PubMed] [Google Scholar]
- Williams and Meck, 1991.Williams CL, Meck WH. (1991) The organizational effects of gonadal steroids on sexually dimorphic spatial ability. Psychoneuroendocrinology 16:155–176 [DOI] [PubMed] [Google Scholar]
- Williams et al., 2008.Williams JG, Allison C, Scott FJ, Bolton PF, Baron-Cohen S, Matthews FE, Brayne C. (2008) The Childhood Autism Spectrum Test (CAST): sex differences. J Autism Dev Disord 38:1731–1739 [DOI] [PubMed] [Google Scholar]
- Wilson and Davies, 2007.Wilson CA, Davies DC. (2007) The control of sexual differentiation of the reproductive system and brain. Reproduction 133:331–359 [DOI] [PubMed] [Google Scholar]
- Wise et al., 2005.Wise PM, Dubal DB, Rau SW, Brown CM, Suzuki S. (2005) Are estrogens protective or risk factors in brain injury and neurodegeneration? Reevaluation after the Women's health initiative. Endocr Rev 26:308–312 [DOI] [PubMed] [Google Scholar]
- Wise et al., 2001.Wise PM, Dubal DB, Wilson ME, Rau SW, Liu Y. (2001) Estrogens: trophic and protective factors in the adult brain. Front Neuroendocrinol 22:33–66 [DOI] [PubMed] [Google Scholar]
- Wise et al., 2009.Wise PM, Suzuki S, Brown CM. (2009) Estradiol: a hormone with diverse and contradictory neuroprotective actions. Dialogues Clin Neurosci 11:297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wizeman and Pardue, 2001.Wizeman T, Pardue ML. (2001) Exploring the Biological Contributions to Human Health: Does Sex Matter? National Academy Press, Washington DC: [PubMed] [Google Scholar]
- Wolf et al., 2001.Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. (2001) The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology 26:711–720 [DOI] [PubMed] [Google Scholar]
- Wood et al., 2001.Wood GE, Beylin AV, Shors TJ. (2001) The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav Neurosci 115:175–187 [DOI] [PubMed] [Google Scholar]
- Wood and Shors, 1998.Wood GE, Shors TJ. (1998) Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci USA 95:4066–4071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley, 1999.Woolley CS. (1999) Effects of estrogen in the CNS. Curr Opin Neurobiol 9:349–354 [DOI] [PubMed] [Google Scholar]
- Wyckoff et al., 2001.Wyckoff MH, Chambliss KL, Mineo C, Yuhanna IS, Mendelsohn ME, Mumby SM, Shaul PW. (2001) Plasma membrane estrogen receptors are coupled to endothelial nitric-oxide synthase through Galpha(i). J Biol Chem 276:27071–27076 [DOI] [PubMed] [Google Scholar]
- Xiao and Becker, 1998.Xiao L, Becker JB. (1998) Effects of estrogen agonists on amphetamine-stimulated striatal dopamine release. Synapse 29:379–391 [DOI] [PubMed] [Google Scholar]
- Yague et al., 2008.Yague JG, Wang AC, Janssen WG, Hof PR, Garcia-Segura LM, Azcoitia I, Morrison JH. (2008) Aromatase distribution in the monkey temporal neocortex and hippocampus. Brain Res 1209:115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shima and Yuri, 2007.Yamaguchi-Shima N, Yuri K. (2007) Age-related changes in the expression of ER-beta mRNA in the female rat brain. Brain Res 1155:34–41 [DOI] [PubMed] [Google Scholar]
- Yu and Wagner, 1994.Yu YL, Wagner GC. (1994) Influence of gonadal hormones on sexual differences in sensitivity to methamphetamine-induced neurotoxicity. J Neural Transm Park Dis Dement Sect 8:215–221 [DOI] [PubMed] [Google Scholar]
- Yue et al., 2005.Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R. (2005) Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer's disease animal model. Proc Natl Acad Sci USA 102:19198–19203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al., 2002.Zhang JQ, Cai WQ, Zhou DS, Su BY. (2002) Distribution and differences of estrogen receptor beta immunoreactivity in the brain of adult male and female rats. Brain Res 935:73–80 [DOI] [PubMed] [Google Scholar]
- Zhao and Brinton, 2006.Zhao L, Brinton RD. (2006) Select estrogens within the complex formulation of conjugated equine estrogens (Premarin) are protective against neurodegenerative insults: implications for a composition of estrogen therapy to promote neuronal function and prevent Alzheimer's disease. BMC Neurosci 7:24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao and Brinton, 2007.Zhao L, Brinton RD. (2007) WHI and WHIMS follow-up and human studies of soy isoflavones on cognition. Expert Rev Neurother 7:1549–1564 [DOI] [PubMed] [Google Scholar]
- Zhao et al., 2005.Zhao L, O'Neill K, Diaz Brinton R. (2005) Selective estrogen receptor modulators (SERMs) for the brain: current status and remaining challenges for developing NeuroSERMs. Brain Res Rev 49:472.to [DOI] [PubMed] [Google Scholar]