Abstract
Among the proteins encoded by human and simian immunodeficiency viruses (HIV and SIV) at least three, Vif, Vpu and Vpr, subvert cellular ubiquitin ligases to block the action of anti-viral defenses. This review focuses on Vpr and its HIV2/SIV counterparts, Vpx and Vpr, which all engage the DDB1•Cullin4 ubiquitin ligase complex through the DCAF1 adaptor protein. Here, we discuss the multiple functions that have been linked to Vpr expression and summarize the current knowledge on the role of the ubiquitin ligase complex in carrying out a subset of these activities.
Keywords: Vpr, DCAF1, ubiquitin ligase, cell cycle arrest, macrophage infection
1. Introduction
1.1 Proteins unique to complex retroviruses
Complex retroviruses express a number of gene products in addition to the Gag, Pol and Env proteins expressed by all retroviruses (Fig. 1). The general function of these additional proteins is to prepare infected cells for virus production. The first of these proteins encoded by HIV are expressed from highly spliced messages. Tat functions to enhance RNA polymerase II processivity of transcription from the HIV LTR [1, 2] and Rev expedites RNA export from the cell nucleus and thereby decreases the extent to which viral RNA is spliced [3]. Nef, another protein expressed early after infection, down-modulates CD4 to control super infection [4–7]. Other functions have, however also been ascribed to this protein and contribute to HIV biology [8–10]. Vpu similarly downmodulates CD4 expression at the cell surface [11] and importantly counteracts the cellular protein tetherin, which retains newly produced virions at the cell surface [12, 13]. The vif gene product redirects a cellular ubiquitin ligase to target the cellular cytidine deaminases, APOBEC3G and APOBEC3F, for proteasomal degradation [14–19]. In the absence of Vif, APOBEC3G and APOBEC3F have highly effective anti-viral activity [20–23].
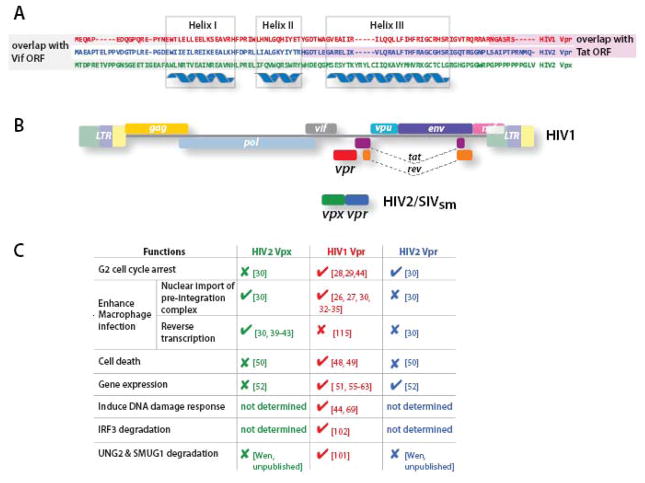
Fig. 1.
A. Amino acid sequence alignment of HIV1 Vpr and HIV2 Vpr/Vpx. HIV1 Vpr shares about 50% and 25% protein sequence identity with HIV2/SIVmac Vpr and Vpx, respectively [78, 81]. The region of HIV1 Vpr and HIV2 Vpr that overlaps with Tat is shaded in purple and the region of HIV1 Vpr and HIV2 Vpx that overlaps with Vif is shaded in grey. The amino acids that are predicted to form three alpha-helices are indicated. B. Proviral genome structure of HIV. Vpr is encoded by a reading frame in the center of the HIV1 genome that overlaps both vif and tat reading frames. HIV2 and the closely related virus SIVsmm/mac encode two vpr-like genes, vpx and vpr. C. The functions of Vpr. HIV1 Vpr is a multi-functional protein whose functions segregate to HIV2 Vpr or Vpx.
Several functions have been identified for the protein Vpr. While all of these may ultimately impact the virus, the host cells or both, identification of the primary functions that Vpr evolved will both further our understanding of HIV biology and help in the identification of new options for therapeutic intervention.
1.2 Vpr function is evolutionarily conserved among primate lentiviruses
HIV1-encoded Vpr is a 14-kDa, virion-associated protein that has two widely accepted biological effects. One is to promote infection of non-dividing cells, specifically those of the myeloid lineage [24–27], and the other is to trigger G2 cell cycle arrest in dividing cells [28, 29]. In HIV2 and SIV that infects sooty mangabey monkeys and macaques (SIVsmm and SIVmac), two separate proteins, designated Vpx and Vpr, carry out these functions respectively [30] (Fig. 1). Phylogenetic analysis, interestingly, shows that the coding sequences for the two Vpr-like proteins in HIV2 and its SIV counterparts likely arose from the duplication of a single HIV1-vpr-like precursor [31]. It is likely that the burden of carrying extra nucleic acid is offset by the functional refinement of the proteins that the duplication allowed. In addition to being a multifunctional protein HIV1 Vpr is further constrained from evolving because its coding sequences overlap vif at the amino-terminus and tat at the carboxy-terminus (Fig. 1). In HIV2/SIVsmm/mac, vpx overlaps vif at its amino-terminus and vpr overlaps tat at its carboxy-terminus (Fig. 1). Thus in each instance evolution of one end is not limited by the overlap with another reading frame. The constraints on HIV1 Vpr may prevent both further functional optimization and easy escape from immune responses or therapeutic interventions. HIV2/SIVsmm/mac Vpx, on the other hand, has evolved to reduce functional overlap and thus may have been optimized to act more efficiently and to shed any “off-target” effects that could be detrimental to the virus. The reduced functional overlap may similarly aid viral immune evasion.
2. What are the functions of Vpr and why are they important for viral replication and pathogenesis?
2.1 Vpr enhances macrophage infection
The function of Vpr relevant to viral replication has been enigmatic but clues are slowly beginning to emerge. Boosting infection of myeloid lineage-derived cells positively impacts HIV and SIV in the most obvious manner. Older experiments showed that Vpr facilitates nuclear import of viral pre-integration complexes in non-dividing cells [26, 27, 30, 32–35]. This function is shared between HIV1 Vpr and HIV2/SIVsmm/mac Vpx and was attributed to nuclear import signals which are found on both. Interestingly however, there are multiple nuclear import signals in the pre-integration complex, including one each in Gag and integrase and one in a triple-helix reverse transcription intermediate (reviewed in [36]). This of course implies that there is redundancy for the indispensable nuclear import process; however, other work [37, 38] suggests that none of these signals are required for infection of non-dividing cells.
A number of recent reports suggest that the block that restricts SIVsmm/mac and HIV2 infection in macrophages is not at the level of nuclear import [39–43]. These reports provide evidence that an as yet unidentified cellular factor interferes with efficient reverse transcription of the viral genomes. None of the work that introduced this new macrophage anti-viral factor focused specific attention on HIV1 Vpr and its role in facilitating macrophage infection. Therefore, it is not known whether HIV1 Vpr functions like HIV2/SIVsmm/mac Vpx to facilitate macrophage infection. Vpx from both SIVsmm/mac and HIV2 had such a profound effect on the infectivity of SIV and even that of HIV1 in macrophages that the previously well established role of HIV1 Vpr in promoting macrophage infection was overshadowed [42]. It is possible that the optimization of Vpx after the aforementioned gene duplication event allowed it to become functionally superior to a multifunctional HIV1 Vpr-like precursor. Intriguingly, if HIV1 Vpr blocks the function of the same macrophage anti-viral factor, its weaker action may make it easier to defeat with therapeutic interventions. Further, HIV1 Vpr may be less able to evade an immune response or a Vpr-directed therapeutic agent due to its overlapping function(s). The role of HIV1 Vpr in macrophage infection must therefore be re-examined in light of these more recent studies.
2.2 Vpr causes G2 cell cycle arrest
Expression of HIV1 or HIV2/SIVsmm/mac Vpr causes G2 cell cycle arrest [28, 29]. This function has been demonstrated with expression of Vpr alone, in the context of the virus and even in primary cells from infected patients [28, 44]. The biological significance of this G2 arrest, however, isn’t well understood.
2.2a Does G2 arrest itself benefit viral replication?
G2, the pause between the duplication of cellular chromatin and cell division, provides a favorable environment for virus production. During G2 phase, unlike in S-phase and in mitosis, chromatin is transcribed and mRNA is actively translated. This environment allows a 2–3-fold enhancement in virus production over asynchronous cell populations [45]. While this increase appears modest, the cumulative effect over several replication cycles could boost virus production significantly.
2.2b Is G2 arrest the function for which Vpr evolved or is it a by-product of another process?
Vpr appears to aid HIV replication most in terminally differentiated macrophages, which of course do not divide. Further, in a typical experiment, not every cell that is transfected with an HIV1 Vpr expression vector responds by arresting in G2. That percentage is significantly lower in cells transfected with Vpr from HIV2 (de Noronha unpublished observations and [46]). Assuming that HIV2 Vpr is less restricted than HIV1 Vpr to evolve its function, more efficient arrest is expected if that is the prime function of Vpr. Of note, HIV2 Vpr is present in smaller quantities in total cell lysates than HIV1 Vpr [46]. Finally, the HIV1 protein, Vif, when expressed at high levels, also causes cells to accumulate in G2 [47]. This effect also requires the action of an ubiquitin ligase, albeit a different one than Vpr engages. Thus, it appears possible that G2 arrest can result from the over-engagement of ubiquitin ligases rather than from a specific Vif- or Vpr-mediated function.
2.3 What are the other functions of Vpr?
2.3a Vpr causes cell death
Vpr has been linked to other functions that may be direct or indirect consequences of Vpr action. For example expression of HIV1 Vpr leads to cell death, but it’s not clear whether it is by apoptosis [48] or by necrosis [49]. Further unclear is whether cell death is a function of Vpr that is advantageous for the virus or merely again a consequence of another process; does holding cells in G2 for extended periods contribute to cell death? Neither HIV2/SIVsmm/mac Vpr nor Vpx induce apoptosis in nonhuman and human cell lines [50].
2.3b Vpr regulates gene expression
Vpr expression can modify both viral and host gene transcription. As a virion-incorporated protein, Vpr is poised to activate immediate-early HIV1 gene expression prior to Tat production. Indeed, one of the first functions attributed to Vpr was transactivation of the HIV1 long terminal repeat (LTR) [51]. This transactivation function is conserved among primate lentivirus Vpr proteins but is not shared by Vpx [52]. Subsequent studies showed that Vpr and Tat have an additive effect on HIV1 LTR activation [53, 54]. However, this function has been linked to the cell cycle, suggesting that LTR transactivation is a by-product of G2 arrest. In support of this hypothesis, expression from the HIV1 LTR is highest in cells in the G2 phase [45], and Vpr mutants that fail to induce G2 cell cycle arrest also fail to activate an HIV1 LTR luciferase reporter [45, 53]. However, Vpr has also been shown to regulate transcription from the LTR in a manner that is not linked specifically to Vpr-mediated cell cycle arrest [51].
In addition to regulating the HIV1 LTR, Vpr also modulates host gene expression. Vpr activates expression of the cyclin-dependent kinase inhibitor, p21WAF1, in a variety of cell types [55, 56]. Changes in the levels of various cytokines and chemokines have also been associated with Vpr expression. These include IL-12 [57–59], TGF-beta [58], IL-8 [60], CD28, CTLA-4, IFN-gamma [61], RANTES, MIP-1-alpha and MIP-1-beta [62, 63], and IL2, IL4, IL10, and TNF-alpha [57].
Vpr may alter gene expression directly through cellular factors that modulate transcription or by direct DNA-protein interactions. For example, Vpr physically engages transcription factors/co-activators such as SP-1 [64], the glucocorticoid receptor [65], p300/CREB-binding protein [54] and TFIIB [66]. These interactions can modify both the amplitude of virus expression as well as the host response to the virus. Further, Vpr has been shown to activate the HIV1 LTR by directly binding to specific DNA sequences within the LTR [67, 68].
2.3c Vpr signals a DNA damage response
Expression of Vpr triggers a DNA damage response that depends on the activation of ATR but not ATM [44, 69]. The signals that trigger the response have not been resolved. Vpr has been shown to cause double strand breaks in DNA [70]. Since Vpr has no apparent enzymatic function, recruitment of other proteins would be necessary. Other work demonstrated that Vpr expression causes disruptions of the nuclear envelope architecture [71]. The nuclear envelope is important for the elongation phase of DNA replication [72] and stalled replication activates signaling through ATR. The mechanism through which Vpr disrupts the nuclear envelope structure and the impact that this effect has on HIV remains to be resolved.
2.4 Insights into Vpr function in vivo
The impact of Vpr on HIV and SIV may not be fully revealed in in vitro infections. The effect of deletion of Vpr from HIV1 or deletion of Vpx from HIV2/SIV is evident in in vitro infections only in non-dividing cells whereas in dividing cells, HIV1 Vpr and HIV2/SIV Vpx seem to be dispensable for infectivity [24, 25, 30, 73, 74]. Deletion of Vpr from HIV2/SIV has little effect on in vitro infections regardless of the cell type [30]. It is clear however, that Vpr/Vpx benefits the virus in vivo. In a rhesus macaque infection experiment where the SIVmac293 Vpr start codon was mutated to TTG, three of five test animals showed reversion to ATG. The remaining two maintained low, persistent levels of virus and did not develop disease during the observation period [75]. In another study, also using SIVmac293, deletions were introduced into the Vpr or Vpx reading frames, or into both [76]. Reversion was not possible in this scenario. The most notable decrease in virus replication and pathology was in the animals infected with Vpx(−) Vpr(−) virus, followed by Vpx(−) virus [76]. Thus Vpr appeared to act synergistically with Vpx but curiously deletion of Vpr alone seemed to have little effect on viral pathology.
A third trial, using SIVsmm with deletions of Vpr, Vpx, or both in pig-tailed macaques, showed the importance of Vpr in viral spread and disease [77]. In this experiment, half of the animals infected with Vpr(−) virus survived despite first reaching peak virus levels that were comparable to those in animals that died. Possible interpretations of this observation are that the virus population was able to expand initially but was then inefficient at evading a later, more refined, immune response or that it was impaired in transitioning to a new host cell population after the first had been saturated or depleted.
The possibility that Vpr contributes to viral immune-evasion is supported by two papers showing that expression of Vpr hinders effective cellular immune responses against Vpr itself as well as against co-expressed viral antigens [78, 79]. The role of Vpr could thus be to blunt cellular immune responses directed against HIV. This function may also be carried out by HIV2/SIVsmm/mac Vpr, Vpx or both together. Cellular immune responses play an important role in keeping HIV levels in check, especially in elite HIV controllers who can maintain low viral loads without HAART therapy. CTLs however play a major role in helping to suppress virus until late in infection. Perhaps Vpr influences the quality of the CTL response that is proving to be a critical part of elite controllers’ antiviral armamentarium.
The second interpretation of the observations from the in vivo experiments is that Vpr broadens the range of cell types permissive to infection. SIV Vpx expands the cellular host-range of the virus into macrophages and perhaps also into as yet unidentified cell-types that are required to maintain the virus later in infection. Vpx may do this by defeating an antiviral host defense like that which it blocks in macrophages. This capacity may be less pronounced in HIV1.
3. Vpr and the ubiquitin ligase complex
Dissecting cause and effect for Vpr will be difficult. For example, G2 cell cycle arrest can be triggered by numerous processes that can elicit various changes within the cell. Recently our lab and others identified a DCAF1•DDB1•Cul4-containing ubiquitin ligase complex as a partner for HIV1 Vpr, HIV2 Vpr or for HIV2/SIVsmm/mac Vpx [80–86] (Fig. 2). Ubiquitin ligases attach 76-amino-acid-long ubiquitin peptide side chains to proteins, usually at lysine residues. These ubiquitinations can be simple or further branched from lysine on ubiquitin itself. The modifications can direct changes in function or subcellular distribution. Most commonly however polyubiquitination marks proteins for destruction by proteasomes.
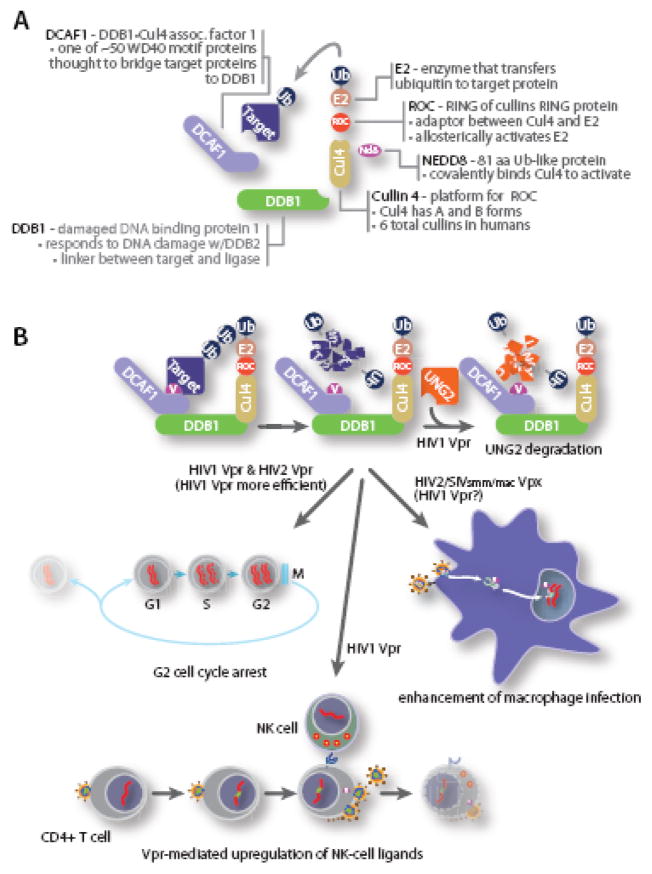
Fig. 2.
A. Overview of DCAF1•DDB1•Cul4 ubiquitin ligase complex. Cullin 4 assembles with a multi-subunit complex to ubiquitylate targeted proteins. Cul4 C terminus associates with a small RING protein, ROC, that recruits and activates an E2 enzyme, while the N terminus of Cul4 interacts with a linker protein, DDB1 that binds the adaptor protein, DCAF1 that interacts with HIV1 Vpr, HIV2 Vpr or HIV2/SIV Vpx. B. The role of the ubiquitin ligase complex in Vpr function. Evidence supports a model in which Vpr/Vpx directs the DDB1•Cul4 ubiquitin ligase complex to ubiquitinate (1) a cellular uracil N glycosylase, UNG2, (2) a cellular protein required for cell cycle progression into mitosis, (3) a cellular protein that is degraded to activate ATR signaling and increases cell surface NKG2D ligands to enhance NK cell-mediated killing, and (4) a cellular antiviral factor that inhibits viral reverse transcription in macrophages.
A number of targets have been discovered for the DDB1•Cul4-containing ubiquitin ligase complex in the absence of Vpr. The association between DDB1 and Cul4 was first described in the context of DNA damage resulting from cellular exposure to ultraviolet light [87]. More recent studies showed that DDB1 links various other proteins, either directly or indirectly to the Cul4 E3 ubiquitin ligase component. These ubiquitination targets include histones [88, 89], STAT1 [90], XPC [91, 92], Chk1 [93], CDT1 [94–96], Merlin [97], c-jun [98], TSC2 [99] and p27kip [100].
The DDB1•Cul4 ubiquitin ligase complex is required for at least three Vpr-associated phenotypes. These include (1) HIV1 and HIV2 Vpr-mediated G2 cell cycle arrest, (2) facilitation of macrophage infection by HIV2/SIVsmm/mac Vpx and (3) HIV1 Vpr-mediated degradation of the uracil-DNA glycosylases UNG2 and SMUG1 [101]. In addition, HIV1 Vpr function in triggering a DNA damage response has also been linked to the DDB1•Cul4 ubiquitin ligase. While HIV1 Vpr has also been shown to promote degradation of the interferon response factor IRF3, this particular Vpr function has not been linked to a specific ubiquitin ligase complex [102].
Several labs analyzed cellular partners of HIV1 Vpr to determine how and why this viral protein engages cellular processes (reviewed in [103]). Vpr has been shown, using mostly yeast 2-hybrid screens, to interact with a number of cellular proteins. Recent searches for Vpr partners however relied on mass-spectrometry to identify proteins that co-immunoprecipitate with Vpr. Some studies further identified proteins that co-purify with HIV2/SIVmac Vpr and Vpx, which share about 50% and 25% protein sequence identity with HIV1 Vpr respectively [80, 83].
One analysis revealed that like HIV1 Vpr, HIV2 Vpr and to a much lesser extent HIV2 Vpx also engage the DCAF1•DDB1•Cul4 ubiquitin ligase complex [80]. Another study found that HIV2 and SIVmac Vpx assembled with DCAF1 [86]. This interaction connects Vpr specifically to the DDB1•Cul4 ubiquitin ligase complex as depletion of DCAF1 reduced the quantity of DDB1 that was recovered after immunoprecipitation of HIV1 Vpr. Interestingly, the association between Vpr and DCAF1 had been made a decade ago, before contemporary technology and knowledge could allow the discoverers to appreciate the significance of this finding [104]. Of note, Angers et al., looking for adaptors between DDB1 and unspecified target proteins, found that DCAF1 is one such protein [105]. Further confirmation that DCAF1 acts as an adaptor between DDB1 and putative target proteins came through the findings of other labs [105, 106].
3.1 The role of the ubiquitin ligase in Vpr-mediated G2 arrest
The involvement of the DCAF1•DDB1•Cul4 ubiquitin ligase complex in G2 arrest was confirmed by several independent means. First, severing the connection between Vpr and the rest of the complex using siRNA or shRNA attenuated Vpr-mediated arrest in a dose-dependent manner. Disruption of the complex by depleting DDB1, over-expressing DDB1 or over-expressing a dominant negative version of cullin4A led to the same outcome. Importantly, none of the modifications to the ubiquitin ligase complex that blocked Vpr-mediated G2 arrest interfered with the cells capacity to arrest in G2 in response to chemically induced DNA damage [80]. A subsequent publication of experiments investigating the in vivo function of DCAF1 showed that cells lacking the capacity to express this protein were slowed in their transition through S-phase over a 5-day time course [107]; however, this did not appear to be the case in analyses examining Vpr function on a shorter time-scale.
3.2 The role of ubiquitin ligase in HIV1 Vpr and HIV2/SIV Vpx-mediated facilitation of macrophage infection
HIV1 Vpr is a karyophilic protein, localizes to the nuclear periphery, and associates with the viral pre-integration complex (PIC). As such, it is easy to envision a role for Vpr in nuclear import of the PIC. Support for this model was provided by the following results: mutation of nuclear localization signals (NLS) in Vpr abrogated HIV1 infection of growth-arrested cells, vpr defective PICs cannot translocate to the nucleus in an in vitro nuclear import assay, disruption of the interaction between Vpr and nuclear envelope or import factors reduces HIV1 infectivity in macrophages and vpr(−) virus resulted in lower levels of Gag transcripts but did not affect total proviral levels as measured by semi-quantitative PCR in macrophages [34, 108–113]. However, the model that Vpr is required for nuclear entry of the PIC in macrophages is controversial since redundant nuclear localization signals found in HIV integrase, matrix and in the viral DNA have been shown to be critical for nuclear import (reviewed in [114]). Furthermore, there is no evidence that Vpr participates directly in nuclear transport of the PIC in macrophages. Finally, for HIV2/SIV infection, the function of enhancing macrophage infection segregates to HIV2 Vpx [30] and recent work re-examining the role of Vpx in macrophage infection found that it acts by blocking a restriction to reverse transcription rather than by enhancing nuclear import [39, 42, 43, 115].
The most recent findings regarding Vpx function call into question the previously accepted model in which HIV2/SIVsmm Vpx, like HIV1 Vpr, enhanced macrophage infectivity by facilitating nuclear entry of the PIC [30, 116]. HIV2/SIV Vpx, like HIV1 Vpr, contains a nuclear localization signal, is incorporated into virions, and associates with the PIC. However, an experiment in which heterokaryons were formed between permissive cells and macrophages [42] show that Vpx defeats a macrophage-specific restriction. The restriction occurs during reverse transcription; real-time PCR of viral DNA at different stages early during infection showed that early, late, and post-nuclear imported transcripts are decreased in macrophages infected with HIV-2/SIV vpx(−) virus [39, 42, 43, 117]. This is supported by another study demonstrating that Vpx is critical for completion of reverse transcription in macrophages [40]. Interestingly, Vpx neutralizes a restriction to reverse transcription in macrophages of not only complex lentiviruses such as HIV2, SIV and HIV1, but also of gammaretroviruses such as murine leukemia virus (MLV) [118]. Importantly, the role of HIV2/SIVsmm/mac Vpx in counteracting macrophage restriction is dependent on its association with the same ubiquitin ligase complex that interacts with HIV1 Vpr [39, 42, 43] (Fig. 2). Experiments using siRNA directed against components of the DCAF1•DDB1•Cul4 ubiquitin ligase complex, a Vpx mutant, Q76R, that fails to interact with DCAF1, or proteasome inhibitors, decreased the capacity of Vpx to neutralize the macrophage restriction factor [39, 42, 43]. It is interesting that even though HIV1 Vpr interacts with the DCAF1•DDB1•Cul4 ubiquitin ligase complex HIV-2/SIV Vpx enhances macrophage infectivity more than HIV1 Vpr [42, 117]. This is evident from the observation that Vpx supplied in trans by either pre-infection with SIV or by co-packaging of Vpx with HIV1 virions enhances the infectivity of HIV1 in macrophages [42, 117]. Perhaps the interaction between HIV-2/SIV Vpx and the ubiquitin ligase complex is optimized to target the macrophage restriction. Alternatively, due to its multifunctional nature, HIV1 Vpr may engage more targets than HIV2/SIV Vpx and thus neutralize the macrophage restriction factor less effectively. Whether the interaction between HIV1 Vpr and the DCAF1•DDB1•Cul4 ubiquitin ligase complex is required to enhance macrophage infectivity is an issue for further study.
Finally, while there is consensus among published studies that HIV1 Vpr and HIV2/SIV Vpx function to enhance infection of human macrophages [24–27, 34, 41, 119, 120], the mechanism employed by HIV1 Vpr and HIV2/SIV Vpx remains unknown.
3.3 The role of ubiquitin ligase in HIV1 Vpr-mediated protein degradation
To date only a few proteins have been identified that are degraded in the presence of HIV1 Vpr. Okumura et al. found that expression of either HIV Vif or Vpr caused a decrease in IRF3 protein levels [102]. This was attributed to proteasomal degradation; however no specific ubiquitin ligase was implicated in targeting the degradation. The decrease in IRF3 could, in the context of a natural infection benefit the virus by thwarting host immune defenses.
The interaction between HIV1 Vpr and UNG2 was discovered over a decade ago using yeast 2-hybrid technology [121]. These experiments showed that Vpr recruits UNG2 into virions [122, 123]. This association was intriguing because non-dividing cells, like terminally differentiated macrophages, harbor skewed nucleotide pools favoring RNA synthesis rather than the DNA synthesis. Tapping into UNG2 function, particularly by recruiting it into virions, could target misincorporated uridines excision. Further, viruses that lack Vpr-like open reading frames but infect non-dividing cells minimize uracil misincorporation by encoding dUTPase as a part of the pol gene.
More recently the discovery that APOBEC3G, a cytidine deaminase, attacks the HIV genome during reverse transcription re-ignited interest in the association between Vpr and UNG2 [124]. Schroefelbauer et al. discovered that expression of Vpr rather than recruiting UNG2 into virions, promoted not only its degradation, but also that of SMUG1 [101]. They further demonstrated that in the presence of APOBEC3G, Vpr-mediated degradation of UNG2 enhanced viral infectivity. Fenard et al. recently demonstrated that UNG2 exerts a negative effect of transcription from the HIV1 LTR [125]. Work from another group however showed that UNG2, in association with integrase, is vital for HIV propagation [126]. Yet other groups showed that UNG2 has little or no effect on HIV replication [127, 128]. Finally, the link between Vpr and UNG2 can also be dissociated from G2 cell cycle arrest [129]. Thus, the significance of this function for HIV infection and pathogenesis remains unclear.
3.4 The role of the ubiquitin ligase complex in HIV1 Vpr-mediated DNA damage
Schröfelbauer et al. were the first to propose a model for Vpr-mediated DNA damage that incorporates the association between Vpr and the ubiquitin ligase complex [85]. The model suggested that by usurping the ubiquitin ligase complex Vpr hinders its normal function in DNA repair. This effect however is revealed primarily in response to UV irradiation and thus does not account for G2 cell cycle arrest that is encountered upon Vpr expression.
Regardless of how the initial DNA damage signal is generated, recent work demonstrated that activation of ATR depends on the interaction between Vpr and the ubiquitin ligase complex [130]. The work further showed that Vpr, likely through the DNA-damage signal promotes expression of NKG2D ligands on HIV-infected cells. Cell surface expression of NKG2D ligands makes infected cells targets for killing by natural killer cells [130, 131]. How expression of this cell surface molecule benefits HIV by enhancing killing of infected cells is still unclear. Like many other experimental observations, this one raises more interesting questions and holds the promise for additional means of therapeutic intervention.
4. Future directions for Vpr research
After years of study, the role of HIV1 Vpr and its HIV2 and SIV paralogs in viral infection and pathogenesis has not been fully revealed. Recent progress, including the discovery that a number of Vpr functions depend upon its assembly with an ubiquitin ligase complex that includes DCAF1, DDB1 and Cul4, has opened new opportunities to learn about and perhaps to block Vpr function.
Does Vpr target one or many proteins for ubiquitination? UNG2 assembles with HIV1 Vpr through a WXXF motif [132]. This motif is not uncommon and is also found on SMUG1. Vpr requires a tryptophan at position 54 for its interaction with UNG2, and a glutamine at position 65 for UNG2 degradation, yet the former residue is dispensable for Vpr-mediated G2 arrest [101]. This suggests despite its small size Vpr can target proteins for ubiquitination with at least two different motifs. Vpr-mediated ubiquitination can of course be modulated by the spatial and temporal availability of putative targets and the subcellular targeting signals of Vpr may further influence their distribution.
Identification of the biologically relevant substrates for Vpr-mediated ubiquitination will be a key step for revealing how and why Vpr is important for HIV infection and pathogenesis. This will help to determine, for example, whether HIV1 Vpr acts, albeit less efficiently, like HIV2/SIVsmm/mac Vpx to block an anti-viral factor. Identification of a Vpr-dependent ubiquitination substrate will also help to determine whether Vpr evolved to cause G2 cell cycle arrest or whether this is a by-product of another process. Finally, identification of the substrates may reveal new functions for this protein that were not apparent in previous studies.
Acknowledgments
We would like to thank Yong-Hiu Zheng for critical reading of this manuscript. This work was supported by a grant to C.N. from the National Institutes of Health (R01AI073178).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laspia MF, Rice AP, Mathews MB. HIV-1 Tat protein increases transcriptional initiation and stabilizes elongation. Cell. 1989;59:283–92. doi: 10.1016/0092-8674(89)90290-0. [DOI] [PubMed] [Google Scholar]
- 2.Bohan CA, Kashanchi F, Ensoli B, Buonaguro L, Boris-Lawrie KA, Brady JN. Analysis of Tat transactivation of human immunodeficiency virus transcription in vitro. Gene Expr. 1992;2:391–407. [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer U, Meyer S, Teufel M, Heckel C, Luhrmann R, Rautmann G. Evidence that HIV-1 Rev directly promotes the nuclear export of unspliced RNA. Embo J. 1994;13:4105–12. doi: 10.1002/j.1460-2075.1994.tb06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson RE, Sanfridson A, Ottinger JS, Doyle C, Cullen BR. Downregulation of cell-surface CD4 expression by simian immunodeficiency virus Nef prevents viral super infection. J Exp Med. 1993;177:1561–6. doi: 10.1084/jem.177.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariani R, Skowronski J. CD4 down-regulation by nef alleles isolated from human immunodeficiency virus type 1-infected individuals. Proc Natl Acad Sci U S A. 1993;90:5549–53. doi: 10.1073/pnas.90.12.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson S, Shugars DC, Swanstrom R, Garcia JV. Nef from primary isolates of human immunodeficiency virus type 1 suppresses surface CD4 expression in human and mouse T cells. J Virol. 1993;67:4923–31. doi: 10.1128/jvi.67.8.4923-4931.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue M, Koga Y, Djordjijevic D, Fukuma T, Reddy EP, Yokoyama MM, Sagawa K. Down-regulation of CD4 molecules by the expression of Nef: a quantitative analysis of CD4 antigens on the cell surfaces. Int Immunol. 1993;5:1067–73. doi: 10.1093/intimm/5.9.1067. [DOI] [PubMed] [Google Scholar]
- 8.Roeth JF, Collins KL. Human immunodeficiency virus type 1 Nef: adapting to intracellular trafficking pathways. Microbiol Mol Biol Rev. 2006;70:548–63. doi: 10.1128/MMBR.00042-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thoulouze MI, Sol-Foulon N, Blanchet F, Dautry-Varsat A, Schwartz O, Alcover A. Human immunodeficiency virus type-1 infection impairs the formation of the immunological synapse. Immunity. 2006;24:547–61. doi: 10.1016/j.immuni.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Pizzato M, Helander A, Popova E, Calistri A, Zamborlini A, Palu G, Gottlinger HG. Dynamin 2 is required for the enhancement of HIV-1 infectivity by Nef. Proc Natl Acad Sci U S A. 2007;104:6812–7. doi: 10.1073/pnas.0607622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willey RL, Maldarelli F, Martin MA, Strebel K. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J Virol. 1992;66:7193–200. doi: 10.1128/jvi.66.12.7193-7200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–30. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 13.Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3:245–52. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conticello SG, Harris RS, Neuberger MS. The Vif protein of HIV triggers degradation of the human antiretroviral DNA deaminase APOBEC3G. Curr Biol. 2003;13:2009–13. doi: 10.1016/j.cub.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 15.Marin M, Rose KM, Kozak SL, Kabat D. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat Med. 2003;9:1398–403. doi: 10.1038/nm946. [DOI] [PubMed] [Google Scholar]
- 16.Sheehy AM, Gaddis NC, Malim MH. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat Med. 2003;9:1404–7. doi: 10.1038/nm945. [DOI] [PubMed] [Google Scholar]
- 17.Stopak K, de Noronha C, Yonemoto W, Greene WC. HIV-1 Vif blocks the antiviral activity of APOBEC3G by impairing both its translation and intracellular stability. Mol Cell. 2003;12:591–601. doi: 10.1016/s1097-2765(03)00353-8. [DOI] [PubMed] [Google Scholar]
- 18.Yu X, Yu Y, Liu B, Luo K, Kong W, Mao P, Yu XF. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science. 2003;302:1056–60. doi: 10.1126/science.1089591. [DOI] [PubMed] [Google Scholar]
- 19.Wiegand HL, Doehle BP, Bogerd HP, Cullen BR. A second human antiretroviral factor, APOBEC3F, is suppressed by the HIV-1 and HIV-2 Vif proteins. Embo J. 2004;23:2451–8. doi: 10.1038/sj.emboj.7600246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- 21.Harris RS, Bishop KN, Sheehy AM, Craig HM, Petersen-Mahrt SK, Watt IN, Neuberger MS, Malim MH. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113:803–9. doi: 10.1016/s0092-8674(03)00423-9. [DOI] [PubMed] [Google Scholar]
- 22.Liddament MT, Brown WL, Schumacher AJ, Harris RS. APOBEC3F properties and hypermutation preferences indicate activity against HIV-1 in vivo. Curr Biol. 2004;14:1385–91. doi: 10.1016/j.cub.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 23.Zheng YH, Irwin D, Kurosu T, Tokunaga K, Sata T, Peterlin BM. Human APOBEC3F is another host factor that blocks human immunodeficiency virus type 1 replication. J Virol. 2004;78:6073–6. doi: 10.1128/JVI.78.11.6073-6076.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balliet JW, Kolson DL, Eiger G, Kim FM, McGann KA, Srinivasan A, Collman R. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology. 1994;200:623–31. doi: 10.1006/viro.1994.1225. [DOI] [PubMed] [Google Scholar]
- 25.Balotta C, Lusso P, Crowley R, Gallo RC, Franchini G. Antisense phosphorothioate oligodeoxynucleotides targeted to the vpr gene inhibit human immunodeficiency virus type 1 replication in primary human macrophages. J Virol. 1993;67:4409–14. doi: 10.1128/jvi.67.7.4409-4414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–44. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 27.Heinzinger NK, Bukinsky MI, Haggerty SA, Ragland AM, Kewalramani V, Lee MA, Gendelman HE, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci U S A. 1994;91:7311–5. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jowett JB, Planelles V, Poon B, Shah NP, Chen ML, Chen IS. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol. 1995;69:6304–13. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogel ME, Wu LI, Emerman M. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J Virol. 1995;69:882–8. doi: 10.1128/jvi.69.2.882-888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fletcher TM, 3rd, Brichacek B, Sharova N, Newman MA, Stivahtis G, Sharp PM, Emerman M, Hahn BH, Stevenson M. Nuclear import and cell cycle arrest functions of the HIV-1 Vpr protein are encoded by two separate genes in HIV-2/SIV(SM) Embo J. 1996;15:6155–65. [PMC free article] [PubMed] [Google Scholar]
- 31.Tristem M, Marshall C, Karpas A, Hill F. Evolution of the primate lentiviruses: evidence from vpx and vpr. Embo J. 1992;11:3405–12. doi: 10.1002/j.1460-2075.1992.tb05419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bukrinsky MI, Sharova N, Dempsey MP, Stanwick TL, Bukrinskaya AG, Haggerty S, Stevenson M. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci U S A. 1992;89:6580–4. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popov S, Rexach M, Ratner L, Blobel G, Bukrinsky M. Viral protein R regulates docking of the HIV-1 preintegration complex to the nuclear pore complex. J Biol Chem. 1998;273:13347–52. doi: 10.1074/jbc.273.21.13347. [DOI] [PubMed] [Google Scholar]
- 34.Popov S, Rexach M, Zybarth G, Reiling N, Lee MA, Ratner L, Lane CM, Moore MS, Blobel G, Bukrinsky M. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. Embo J. 1998;17:909–17. doi: 10.1093/emboj/17.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subbramanian RA, Kessous-Elbaz A, Lodge R, Forget J, Yao XJ, Bergeron D, Cohen EA. Human immunodeficiency virus type 1 Vpr is a positive regulator of viral transcription and infectivity in primary human macrophages. J Exp Med. 1998;187:1103–11. doi: 10.1084/jem.187.7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamashita M, Emerman M. Retroviral infection of non-dividing cells: old and new perspectives. Virology. 2006;344:88–93. doi: 10.1016/j.virol.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Riviere L, Darlix JL, Cimarelli A. Analysis of the viral elements required in the nuclear import of HIV-1 DNA. J Virol. 2009 doi: 10.1128/JVI.01952-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamashita M, Emerman M. The Cell Cycle Independence of HIV Infections Is Not Determined by Known Karyophilic Viral Elements. PLoS Pathog. 2005;1:e18. doi: 10.1371/journal.ppat.0010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergamaschi A, Ayinde D, David A, Le Rouzic E, Morel M, Collin G, Descamps D, Damond F, Brun-Vezinet F, Nisole S, Margottin-Goguet F, Pancino G, Transy C. The human immunodeficiency virus type 2 Vpx protein usurps the CUL4A-DDB1 DCAF1 ubiquitin ligase to overcome a postentry block in macrophage infection. J Virol. 2009;83:4854–60. doi: 10.1128/JVI.00187-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujita M, Otsuka M, Miyoshi M, Khamsri B, Nomaguchi M, Adachi A. Vpx is critical for reverse transcription of the human immunodeficiency virus type 2 genome in macrophages. J Virol. 2008;82:7752–6. doi: 10.1128/JVI.01003-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goujon C, Arfi V, Pertel T, Luban J, Lienard J, Rigal D, Darlix JL, Cimarelli A. Characterization of simian immunodeficiency virus SIVSM/human immunodeficiency virus type 2 Vpx function in human myeloid cells. J Virol. 2008;82:12335–45. doi: 10.1128/JVI.01181-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharova N, Wu Y, Zhu X, Stranska R, Kaushik R, Sharkey M, Stevenson M. Primate lentiviral Vpx commandeers DDB1 to counteract a macrophage restriction. PLoS Pathog. 2008;4:e1000057. doi: 10.1371/journal.ppat.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srivastava S, Swanson SK, Manel N, Florens L, Washburn MP, Skowronski J. Lentiviral Vpx accessory factor targets VprBP/DCAF1 substrate adaptor for cullin 4 E3 ubiquitin ligase to enable macrophage infection. PLoS Pathog. 2008;4:e1000059. doi: 10.1371/journal.ppat.1000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimmerman ES, Sherman MP, Blackett JL, Neidleman JA, Kreis C, Mundt P, Williams SA, Warmerdam M, Kahn J, Hecht FM, Grant RM, de Noronha CM, Weyrich AS, Greene WC, Planelles V. Human immunodeficiency virus type 1 Vpr induces DNA replication stress in vitro and in vivo. J Virol. 2006;80:10407–18. doi: 10.1128/JVI.01212-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goh WC, Rogel ME, Kinsey CM, Michael SF, Fultz PN, Nowak MA, Hahn BH, Emerman M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat Med. 1998;4:65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- 46.Kewalramani VN, Park CS, Gallombardo PA, Emerman M. Protein stability influences human immunodeficiency virus type 2 Vpr virion incorporation and cell cycle effect. Virology. 1996;218:326–34. doi: 10.1006/viro.1996.0201. [DOI] [PubMed] [Google Scholar]
- 47.DeHart JL, Bosque A, Harris RS, Planelles V. Human immunodeficiency virus type 1 Vif induces cell cycle delay via recruitment of the same E3 ubiquitin ligase complex that targets APOBEC3 proteins for degradation. J Virol. 2008;82:9265–72. doi: 10.1128/JVI.00377-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muthumani K, Choo AY, Premkumar A, Hwang DS, Thieu KP, Desai BM, Weiner DB. Human immunodeficiency virus type 1 (HIV-1) Vpr-regulated cell death: insights into mechanism. Cell Death Differ. 2005;12 (Suppl 1):962–70. doi: 10.1038/sj.cdd.4401583. [DOI] [PubMed] [Google Scholar]
- 49.Sakai K, Dimas J, Lenardo MJ. The Vif and Vpr accessory proteins independently cause HIV-1-induced T cell cytopathicity and cell cycle arrest. Proc Natl Acad Sci U S A. 2006;103:3369–74. doi: 10.1073/pnas.0509417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang LJ, Chen CH, Urlacher V, Lee TZ. Differential apoptosis effects of primate lentiviral Vpr and Vpx in mammalian cells. J Biomed Sci. 2000;7:322–33. doi: 10.1007/BF02253252. [DOI] [PubMed] [Google Scholar]
- 51.Cohen EA, Dehni G, Sodroski JG, Haseltine WA. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J Virol. 1990;64:3097–9. doi: 10.1128/jvi.64.6.3097-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Philippon V, Matsuda Z, Essex M. Transactivation is a conserved function among primate lentivirus Vpr proteins but is not shared by Vpx. J Hum Virol. 1999;2:167–74. [PubMed] [Google Scholar]
- 53.Forget J, Yao XJ, Mercier J, Cohen EA. Human immunodeficiency virus type 1 vpr protein transactivation function: mechanism and identification of domains involved. J Mol Biol. 1998;284:915–23. doi: 10.1006/jmbi.1998.2206. [DOI] [PubMed] [Google Scholar]
- 54.Kino T, Gragerov A, Slobodskaya O, Tsopanomichalou M, Chrousos GP, Pavlakis GN. Human immunodeficiency virus type 1 (HIV-1) accessory protein Vpr induces transcription of the HIV-1 and glucocorticoid-responsive promoters by binding directly to p300/CBP coactivators. J Virol. 2002;76:9724–34. doi: 10.1128/JVI.76.19.9724-9734.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chowdhury IH, Wang XF, Landau NR, Robb ML, Polonis VR, Birx DL, Kim JH. HIV-1 Vpr activates cell cycle inhibitor p21/Waf1/Cip1: a potential mechanism of G2/M cell cycle arrest. Virology. 2003;305:371–7. doi: 10.1006/viro.2002.1777. [DOI] [PubMed] [Google Scholar]
- 56.Vazquez N, Greenwell-Wild T, Marinos NJ, Swaim WD, Nares S, Ott DE, Schubert U, Henklein P, Orenstein JM, Sporn MB, Wahl SM. Human immunodeficiency virus type 1-induced macrophage gene expression includes the p21 gene, a target for viral regulation. J Virol. 2005;79:4479–91. doi: 10.1128/JVI.79.7.4479-4491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ayyavoo V, Mahboubi A, Mahalingam S, Ramalingam R, Kudchodkar S, Williams WV, Green DR, Weiner DB. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor kappa B [see comments] Nat Med. 1997;3:1117–23. doi: 10.1038/nm1097-1117. [DOI] [PubMed] [Google Scholar]
- 58.Majumder B, Venkatachari NJ, O’Leary S, Ayyavoo V. Infection with Vpr-positive human immunodeficiency virus type 1 impairs NK cell function indirectly through cytokine dysregulation of infected target cells. J Virol. 2008;82:7189–200. doi: 10.1128/JVI.01979-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mirani M, Elenkov I, Volpi S, Hiroi N, Chrousos GP, Kino T. HIV-1 protein Vpr suppresses IL-12 production from human monocytes by enhancing glucocorticoid action: potential implications of Vpr coactivator activity for the innate and cellular immunity deficits observed in HIV-1 infection. J Immunol. 2002;169:6361–8. doi: 10.4049/jimmunol.169.11.6361. [DOI] [PubMed] [Google Scholar]
- 60.Roux P, Alfieri C, Hrimech M, Cohen EA, Tanner JE. Activation of transcription factors NF-kappaB and NF-IL-6 by human immunodeficiency virus type 1 protein R (Vpr) induces interleukin-8 expression. J Virol. 2000;74:4658–65. doi: 10.1128/jvi.74.10.4658-4665.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Venkatachari NJ, Majumder B, Ayyavoo V. Human immunodeficiency virus (HIV) type 1 Vpr induces differential regulation of T cell costimulatory molecules: direct effect of Vpr on T cell activation and immune function. Virology. 2007;358:347–56. doi: 10.1016/j.virol.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 62.Muthumani K, Kudchodkar S, Papasavvas E, Montaner LJ, Weiner DB, Ayyavoo V. HIV-1 Vpr regulates expression of beta chemokines in human primary lymphocytes and macrophages. J Leukoc Biol. 2000;68:366–72. [PubMed] [Google Scholar]
- 63.Si Q, Kim MO, Zhao ML, Landau NR, Goldstein H, Lee S. Vpr- and Nef-dependent induction of RANTES/CCL5 in microglial cells. Virology. 2002;301:342–53. doi: 10.1006/viro.2002.1613. [DOI] [PubMed] [Google Scholar]
- 64.Wang L, Mukherjee S, Jia F, Narayan O, Zhao LJ. Interaction of virion protein Vpr of human immunodeficiency virus type 1 with cellular transcription factor Sp1 and trans-activation of viral long terminal repeat. J Biol Chem. 1995;270:25564–9. doi: 10.1074/jbc.270.43.25564. [DOI] [PubMed] [Google Scholar]
- 65.Kino T, Gragerov A, Kopp JB, Stauber RH, Pavlakis GN, Chrousos GP. The HIV-1 virion-associated protein vpr is a coactivator of the human glucocorticoid receptor. J Exp Med. 1999;189:51–62. doi: 10.1084/jem.189.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Agostini I, Navarro JM, Bouhamdan M, Willetts K, Rey F, Spire B, Vigne R, Pomerantz R, Sire J. The HIV-1 Vpr co-activator induces a conformational change in TFIIB. FEBS Lett. 1999;450:235–9. doi: 10.1016/s0014-5793(99)00501-3. [DOI] [PubMed] [Google Scholar]
- 67.Hogan TH, Nonnemacher MR, Krebs FC, Henderson A, Wigdahl B. HIV-1 Vpr binding to HIV-1 LTR C/EBP cis-acting elements and adjacent regions is sequence-specific. Biomed Pharmacother. 2003;57:41–8. doi: 10.1016/s0753-3322(02)00333-5. [DOI] [PubMed] [Google Scholar]
- 68.McAllister JJ, Phillips D, Millhouse S, Conner J, Hogan T, Ross HL, Wigdahl B. Analysis of the HIV-1 LTR NF-kappaB-proximal Sp site III: evidence for cell type-specific gene regulation and viral replication. Virology. 2000;274:262–77. doi: 10.1006/viro.2000.0476. [DOI] [PubMed] [Google Scholar]
- 69.Roshal M, Kim B, Zhu Y, Nghiem P, Planelles V. Activation of the ATR-mediated DNA damage response by the HIV-1 viral protein R. J Biol Chem. 2003;278:25879–86. doi: 10.1074/jbc.M303948200. [DOI] [PubMed] [Google Scholar]
- 70.Tachiwana H, Shimura M, Nakai-Murakami C, Tokunaga K, Takizawa Y, Sata T, Kurumizaka H, Ishizaka Y. HIV-1 Vpr induces DNA double-strand breaks. Cancer Res. 2006;66:627–31. doi: 10.1158/0008-5472.CAN-05-3144. [DOI] [PubMed] [Google Scholar]
- 71.de Noronha CM, Sherman MP, Lin HW, Cavrois MV, Moir RD, Goldman RD, Greene WC. Dynamic disruptions in nuclear envelope architecture and integrity induced by HIV-1 Vpr. Science. 2001;294:1105–8. doi: 10.1126/science.1063957. [DOI] [PubMed] [Google Scholar]
- 72.Moir RD, Spann TP, Herrmann H, Goldman RD. Disruption of nuclear lamin organization blocks the elongation phase of DNA replication. J Cell Biol. 2000;149:1179–92. doi: 10.1083/jcb.149.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dedera D, Hu W, Vander Heyden N, Ratner L. Viral protein R of human immunodeficiency virus types 1 and 2 is dispensable for replication and cytopathogenicity in lymphoid cells. J Virol. 1989;63:3205–8. doi: 10.1128/jvi.63.7.3205-3208.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kawamura M, Ishizaki T, Ishimoto A, Shioda T, Kitamura T, Adachi A. Growth ability of human immunodeficiency virus type 1 auxiliary gene mutants in primary blood macrophage cultures. J Gen Virol. 1994:2427–31. doi: 10.1099/0022-1317-75-9-2427. [DOI] [PubMed] [Google Scholar]
- 75.Lang SM, Weeger M, Stahl HC, Coulibaly C, Hunsmann G, Muller J, Muller HH, Fuchs D, Wachter H, Daniel MM, et al. Importance of vpr for infection of rhesus monkeys with simian immunodeficiency virus. J Virol. 1993;67:902–12. doi: 10.1128/jvi.67.2.902-912.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gibbs JS, Lackner AA, Lang SM, Simon MA, Sehgal PK, Daniel MD, Desrosiers RC. Progression to AIDS in the absence of a gene for vpr or vpx. J Virol. 1995;69:2378–83. doi: 10.1128/jvi.69.4.2378-2383.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hirsch VM, Sharkey ME, Brown CR, Brichacek B, Goldstein S, Wakefield J, Byrum R, Elkins WR, Hahn BH, Lifson JD, Stevenson M. Vpx is required for dissemination and pathogenesis of SIV(SM) PBj: evidence of macrophage-dependent viral amplification. Nat Med. 1998;4:1401–8. doi: 10.1038/3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muthumani K, Bagarazzi M, Conway D, Hwang DS, Ayyavoo V, Zhang D, Manson K, Kim J, Boyer J, Weiner DB. Inclusion of Vpr accessory gene in a plasmid vaccine cocktail markedly reduces Nef vaccine effectiveness in vivo resulting in CD4 cell loss and increased viral loads in rhesus macaques. J Med Primatol. 2002;31:179–85. doi: 10.1034/j.1600-0684.2002.02004.x. [DOI] [PubMed] [Google Scholar]
- 79.Muthumani K, Hwang DS, Dayes NS, Kim JJ, Weiner DB. The HIV-1 accessory gene vpr can inhibit antigen-specific immune function. DNA Cell Biol. 2002;21:689–95. doi: 10.1089/104454902760330237. [DOI] [PubMed] [Google Scholar]
- 80.Wen X, Duus KM, Friedrich TD, de Noronha CM. The HIV1 protein Vpr acts to promote G2 cell cycle arrest by engaging a DDB1 and Cullin4A-containing ubiquitin ligase complex using VprBP/DCAF1 as an adaptor. J Biol Chem. 2007;282:27046–57. doi: 10.1074/jbc.M703955200. [DOI] [PubMed] [Google Scholar]
- 81.Belzile JP, Duisit G, Rougeau N, Mercier J, Finzi A, Cohen EA. HIV-1 Vpr-Mediated G2 Arrest Involves the DDB1-CUL4A(VPRBP) E3 Ubiquitin Ligase. PLoS Pathog. 2007;3:e85. doi: 10.1371/journal.ppat.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan L, Ehrlich E, Yu XF. DDB1 and Cul4A are required for human immunodeficiency virus type 1 Vpr-induced G2 arrest. J Virol. 2007;81:10822–30. doi: 10.1128/JVI.01380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hrecka K, Gierszewska M, Srivastava S, Kozaczkiewicz L, Swanson SK, Florens L, Washburn MP, Skowronski J. Lentiviral Vpr usurps Cul4-DDB1[VprBP] E3 ubiquitin ligase to modulate cell cycle. Proc Natl Acad Sci U S A. 2007;104:11778–83. doi: 10.1073/pnas.0702102104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DeHart JL, Zimmerman ES, Ardon O, Monteiro-Filho CM, Arganaraz ER, Planelles V. HIV-1 Vpr activates the G2 checkpoint through manipulation of the ubiquitin proteasome system. Virol J. 2007;4:57. doi: 10.1186/1743-422X-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schrofelbauer B, Hakata Y, Landau NR. HIV-1 Vpr function is mediated by interaction with the damage-specific DNA-binding protein DDB1. Proc Natl Acad Sci U S A. 2007;104:4130–5. doi: 10.1073/pnas.0610167104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Le Rouzic E, Belaidouni N, Estrabaud E, Morel M, Rain JC, Transy C, Margottin-Goguet F. HIV1 Vpr arrests the cell cycle by recruiting DCAF1/VprBP, a receptor of the Cul4-DDB1 ubiquitin ligase. Cell Cycle. 2007;6:182–8. doi: 10.4161/cc.6.2.3732. [DOI] [PubMed] [Google Scholar]
- 87.Shiyanov P, Nag A, Raychaudhuri P. Cullin 4A associates with the UV-damaged DNA-binding protein DDB. J Biol Chem. 1999;274:35309–12. doi: 10.1074/jbc.274.50.35309. [DOI] [PubMed] [Google Scholar]
- 88.Kapetanaki MG, Guerrero-Santoro J, Bisi DC, Hsieh CL, Rapic-Otrin V, Levine AS. The DDB1-CUL4ADDB2 ubiquitin ligase is deficient in xeroderma pigmentosum group E and targets histone H2A at UV-damaged DNA sites. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0511160103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang H, Zhai L, Xu J, Joo HY, Jackson S, Erdjument-Bromage H, Tempst P, Xiong Y, Zhang Y. Histone H3 and H4 ubiquitylation by the CUL4-DDB-ROC1 ubiquitin ligase facilitates cellular response to DNA damage. Mol Cell. 2006;22:383–94. doi: 10.1016/j.molcel.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 90.Precious B, Childs K, Fitzpatrick-Swallow V, Goodbourn S, Randall RE. Simian virus 5 V protein acts as an adaptor, linking DDB1 to STAT2, to facilitate the ubiquitination of STAT1. J Virol. 2005;79:13434–41. doi: 10.1128/JVI.79.21.13434-13441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Groisman R, Kuraoka I, Chevallier O, Gaye N, Magnaldo T, Tanaka K, Kisselev AF, Harel-Bellan A, Nakatani Y. CSA-dependent degradation of CSB by the ubiquitin-proteasome pathway establishes a link between complementation factors of the Cockayne syndrome. Genes Dev. 2006;20:1429–34. doi: 10.1101/gad.378206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sugasawa K, Okuda Y, Saijo M, Nishi R, Matsuda N, Chu G, Mori T, Iwai S, Tanaka K, Tanaka K, Hanaoka F. UV-induced ubiquitylation of XPC protein mediated by UV-DDB-ubiquitin ligase complex. Cell. 2005;121:387–400. doi: 10.1016/j.cell.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 93.Leung-Pineda V, Huh J, Piwnica-Worms H. DDB1 targets Chk1 to the Cul4 E3 ligase complex in normal cycling cells and in cells experiencing replication stress. Cancer Res. 2009;69:2630–7. doi: 10.1158/0008-5472.CAN-08-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Higa LA, Banks D, Wu M, Kobayashi R, Sun H, Zhang H. L2DTL/CDT2 interacts with the CUL4/DDB1 complex and PCNA and regulates CDT1 proteolysis in response to DNA damage. Cell Cycle. 2006;5:1675–80. doi: 10.4161/cc.5.15.3149. [DOI] [PubMed] [Google Scholar]
- 95.Higa LA, Mihaylov IS, Banks DP, Zheng J, Zhang H. Radiation-mediated proteolysis of CDT1 by CUL4-ROC1 and CSN complexes constitutes a new checkpoint. Nat Cell Biol. 2003;5:1008–15. doi: 10.1038/ncb1061. [DOI] [PubMed] [Google Scholar]
- 96.Hu J, McCall CM, Ohta T, Xiong Y. Targeted ubiquitination of CDT1 by the DDB1-CUL4A-ROC1 ligase in response to DNA damage. Nat Cell Biol. 2004;6:1003–9. doi: 10.1038/ncb1172. [DOI] [PubMed] [Google Scholar]
- 97.Huang J, Chen J. VprBP targets Merlin to the Roc1-Cul4A-DDB1 E3 ligase complex for degradation. Oncogene. 2008;27:4056–64. doi: 10.1038/onc.2008.44. [DOI] [PubMed] [Google Scholar]
- 98.Wertz IE, O’Rourke KM, Zhang Z, Dornan D, Arnott D, Deshaies RJ, Dixit VM. Human De-etiolated-1 regulates c-Jun by assembling a CUL4A ubiquitin ligase. Science. 2004;303:1371–4. doi: 10.1126/science.1093549. [DOI] [PubMed] [Google Scholar]
- 99.Hu J, Zacharek S, He YJ, Lee H, Shumway S, Duronio RJ, Xiong Y. WD40 protein FBW5 promotes ubiquitination of tumor suppressor TSC2 by DDB1-CUL4-ROC1 ligase. Genes Dev. 2008;22:866–71. doi: 10.1101/gad.1624008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bondar T, Kalinina A, Khair L, Kopanja D, Nag A, Bagchi S, Raychaudhuri P. Cul4A and DDB1 associate with Skp2 to target p27Kip1 for proteolysis involving the COP9 signalosome. Mol Cell Biol. 2006;26:2531–9. doi: 10.1128/MCB.26.7.2531-2539.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schrofelbauer B, Yu Q, Zeitlin SG, Landau NR. Human immunodeficiency virus type 1 Vpr induces the degradation of the UNG and SMUG uracil-DNA glycosylases. J Virol. 2005;79:10978–87. doi: 10.1128/JVI.79.17.10978-10987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Okumura A, Alce T, Lubyova B, Ezelle H, Strebel K, Pitha PM. HIV-1 accessory proteins VPR and Vif modulate antiviral response by targeting IRF-3 for degradation. Virology. 2008;373:85–97. doi: 10.1016/j.virol.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kino T, Pavlakis GN. Partner molecules of accessory protein Vpr of the human immunodeficiency virus type 1. DNA Cell Biol. 2004;23:193–205. doi: 10.1089/104454904773819789. [DOI] [PubMed] [Google Scholar]
- 104.Zhang S, Feng Y, Narayan O, Zhao LJ. Cytoplasmic retention of HIV-1 regulatory protein Vpr by protein-protein interaction with a novel human cytoplasmic protein VprBP. Gene. 2001;263:131–40. doi: 10.1016/s0378-1119(00)00583-7. [DOI] [PubMed] [Google Scholar]
- 105.Angers S, Li T, Yi X, MacCoss MJ, Moon RT, Zheng N. Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature. 2006;443:590–3. doi: 10.1038/nature05175. [DOI] [PubMed] [Google Scholar]
- 106.Jin J, Arias EE, Chen J, Harper JW, Walter JC. A family of diverse Cul4-Ddb1-interacting proteins includes Cdt2, which is required for S phase destruction of the replication factor Cdt1. Mol Cell. 2006;23:709–21. doi: 10.1016/j.molcel.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 107.McCall CM, Miliani de Marval PL, Chastain PD, 2nd, Jackson SC, He YJ, Kotake Y, Cook JG, Xiong Y. Human immunodeficiency virus type 1 Vpr-binding protein VprBP, a WD40 protein associated with the DDB1-CUL4 E3 ubiquitin ligase, is essential for DNA replication and embryonic development. Mol Cell Biol. 2008;28:5621–33. doi: 10.1128/MCB.00232-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fouchier RA, Meyer BE, Simon JH, Fischer U, Albright AV, Gonzalez-Scarano F, Malim MH. Interaction of the human immunodeficiency virus type 1 Vpr protein with the nuclear pore complex. J Virol. 1998;72:6004–13. doi: 10.1128/jvi.72.7.6004-6013.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jacquot G, Le Rouzic E, Maidou-Peindara P, Maizy M, Lefrere JJ, Daneluzzi V, Monteiro-Filho CM, Hong D, Planelles V, Morand-Joubert L, Benichou S. Characterization of the molecular determinants of primary HIV-1 Vpr proteins: impact of the Q65R and R77Q substitutions on Vpr functions. PLoS One. 2009;4:e7514. doi: 10.1371/journal.pone.0007514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nie Z, Bergeron D, Subbramanian RA, Yao XJ, Checroune F, Rougeau N, Cohen EA. The putative alpha helix 2 of human immunodeficiency virus type 1 Vpr contains a determinant which is responsible for the nuclear translocation of proviral DNA in growth-arrested cells. J Virol. 1998;72:4104–15. doi: 10.1128/jvi.72.5.4104-4115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nitahara-Kasahara Y, Kamata M, Yamamoto T, Zhang X, Miyamoto Y, Muneta K, Iijima S, Yoneda Y, Tsunetsugu-Yokota Y, Aida Y. Novel nuclear import of Vpr promoted by importin alpha is crucial for human immunodeficiency virus type 1 replication in macrophages. J Virol. 2007;81:5284–93. doi: 10.1128/JVI.01928-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Subbramanian RA, Yao XJ, Dilhuydy H, Rougeau N, Bergeron D, Robitaille Y, Cohen EA. Human immunodeficiency virus type 1 Vpr localization: nuclear transport of a viral protein modulated by a putative amphipathic helical structure and its relevance to biological activity. J Mol Biol. 1998;278:13–30. doi: 10.1006/jmbi.1998.1685. [DOI] [PubMed] [Google Scholar]
- 113.Vodicka MA, Koepp DM, Silver PA, Emerman M. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes & Development. 1998;12:175–185. doi: 10.1101/gad.12.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fassati A. HIV infection of non-dividing cells: a divisive problem. Retrovirology. 2006;3:74. doi: 10.1186/1742-4690-3-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fujita M, Otsuka M, Nomaguchi M, Adachi A. Multifaceted activity of HIV Vpr/Vpx proteins: the current view of their virological functions. Rev Med Virol. doi: 10.1002/rmv.636. [DOI] [PubMed] [Google Scholar]
- 116.Pancio HA, Vander Heyden N, Ratner L. The C-terminal proline-rich tail of human immunodeficiency virus type 2 Vpx is necessary for nuclear localization of the viral preintegration complex in nondividing cells. J Virol. 2000;74:6162–7. doi: 10.1128/jvi.74.13.6162-6167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gramberg T, Sunseri N, Landau NR. Evidence for an Activation Domain at the Amino-terminus of SIV Vpx. J Virol. 2009 doi: 10.1128/JVI.01437-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kaushik R, Zhu X, Stranska R, Wu Y, Stevenson M. A cellular restriction dictates the permissivity of nondividing monocytes/macrophages to lentivirus and gammaretrovirus infection. Cell Host Microbe. 2009;6:68–80. doi: 10.1016/j.chom.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eckstein DA, Sherman MP, Penn ML, Chin PS, de Noronha CM, Greene WC, Goldsmith MA. HIV-1 Vpr enhances viral burden by facilitating infection of tissue macrophages but not nondividing CD4+ T cells. J Exp Med. 2001;194:1407–19. doi: 10.1084/jem.194.10.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Subbramanian RA, Cohen EA. Molecular biology of the human immunodeficiency virus accessory proteins. J Virol. 1994;68:6831–5. doi: 10.1128/jvi.68.11.6831-6835.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bouhamdan M, Benichou S, Rey F, Navarro JM, Agostini I, Spire B, Camonis J, Slupphaug G, Vigne R, Benarous R, Sire J. Human immunodeficiency virus type 1 Vpr protein binds to the uracil DNA glycosylase DNA repair enzyme. J Virol. 1996;70:697–704. doi: 10.1128/jvi.70.2.697-704.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen R, Le Rouzic E, Kearney JA, Mansky LM, Benichou S. Vpr-mediated incorporation of UNG2 into HIV-1 particles is required to modulate the virus mutation rate and for replication in macrophages. J Biol Chem. 2004;279:28419–25. doi: 10.1074/jbc.M403875200. [DOI] [PubMed] [Google Scholar]
- 123.Mansky LM, Preveral S, Selig L, Benarous R, Benichou S. The interaction of vpr with uracil DNA glycosylase modulates the human immunodeficiency virus type 1 In vivo mutation rate. J Virol. 2000;74:7039–47. doi: 10.1128/jvi.74.15.7039-7047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–50. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 125.Fenard D, Houzet L, Bernard E, Tupin A, Brun S, Mougel M, Devaux C, Chazal N, Briant L. Uracil DNA Glycosylase 2 negatively regulates HIV-1 LTR transcription. Nucleic Acids Res. 2009;37:6008–18. doi: 10.1093/nar/gkp673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Priet S, Gros N, Navarro JM, Boretto J, Canard B, Querat G, Sire J. HIV-1-associated uracil DNA glycosylase activity controls dUTP misincorporation in viral DNA and is essential to the HIV-1 life cycle. Mol Cell. 2005;17:479–90. doi: 10.1016/j.molcel.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 127.Mbisa JL, Barr R, Thomas JA, Vandegraaff N, Dorweiler IJ, Svarovskaia ES, Brown WL, Mansky LM, Gorelick RJ, Harris RS, Engelman A, Pathak VK. Human immunodeficiency virus type 1 cDNAs produced in the presence of APOBEC3G exhibit defects in plus-strand DNA transfer and integration. J Virol. 2007;81:7099–110. doi: 10.1128/JVI.00272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kaiser SM, Emerman M. Uracil DNA glycosylase is dispensable for human immunodeficiency virus type 1 replication and does not contribute to the antiviral effects of the cytidine deaminase Apobec3G. J Virol. 2006;80:875–82. doi: 10.1128/JVI.80.2.875-882.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Selig L, Benichou S, Rogel ME, Wu LI, Vodicka MA, Sire J, Benarous R, Emerman M. Uracil DNA glycosylase specifically interacts with Vpr of both human immunodeficiency virus type 1 and simian immunodeficiency virus of sooty mangabeys, but binding does not correlate with cell cycle arrest. J Virol. 1997;71:4842–6. doi: 10.1128/jvi.71.6.4842-4846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ward J, Davis Z, DeHart J, Zimmerman E, Bosque A, Brunetta E, Mavilio D, Planelles V, Barker E. HIV-1 Vpr triggers natural killer cell-mediated lysis of infected cells through activation of the ATR-mediated DNA damage response. PLoS Pathog. 2009;5:e1000613. doi: 10.1371/journal.ppat.1000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Belzile JP, Richard J, Rougeau N, Xiao Y, Cohen EA. HIV-1 Vpr Induces the K48-Linked Polyubiquitination and Proteasomal Degradation of Target Cellular Proteins to Activate ATR and Promote G2 Arrest. J Virol. doi: 10.1128/JVI.02590-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.BouHamdan M, Xue Y, Baudat Y, Hu B, Sire J, Pomerantz RJ, Duan LX. Diversity of HIV-1 Vpr interactions involves usage of the WXXF motif of host cell proteins. J Biol Chem. 1998;273:8009–16. doi: 10.1074/jbc.273.14.8009. [DOI] [PubMed] [Google Scholar]