Abstract
Purpose
Phase I of Radiation Therapy Oncology Group (RTOG) 0117 determined that 74 Gy was the maximum-tolerated dose with concurrent weekly carboplatin/paclitaxel chemotherapy for inoperable non–small-cell lung cancer (NSCLC). Phase II results are reported here.
Patients and Methods
Patients with unresectable stages I-III NSCLC were eligible. Chemotherapy consisted of weekly paclitaxel at 50 mg/m2 and carboplatin at area under the curve 2 mg/m2. The radiation dose was 74 Gy given in 37 fractions. Radiation therapy volumes included those of the gross tumor and involved nodes. The volume of lung at or exceeding 20 Gy (V20) was mandated to be ≤ 30%.
Results
Of the combined phase I/II enrollment, a total of 55 patients received 74 Gy, of whom 53 were evaluable. The median follow-up was 19.3 months (range, 0.9 to 57.9 months) for all patients and 25.4 months (range, 13.1 to 57.9 months) for those still alive. The median survival for all patients was 25.9 months. The percentage surviving at least 12 months was 75.5% (95% CI, 65.7% to 85.2%). The median overall survival (OS) and progression-free survival (PFS) times for stage III patients (n = 44) were 21.6 months and 10.8 months, respectively. OS and PFS rates at 12 months were 72.7% and 50.0%, respectively. Twelve patients experienced grade ≥ 3 lung toxicity (two patients had grade 5 lung toxicity).
Conclusion
The median survival time and OS rate at 12 months for this regimen are encouraging. These results serve as projection expectations for the high-dose radiation arms of the current RTOG 0617 phase III intergroup trial.
INTRODUCTION
The currently accepted standard of care for patients with unresectable, locally advanced non–small-cell lung cancer (NSCLC) is concurrent chemotherapy and radiation therapy. In recent years, most research has focused on which chemotherapy drugs to use and how to integrate them with radiation therapy. Moreover, little attention has been given to optimizing the radiation therapy component. In particular, the nationally accepted standard radiation prescription dose has remained at the same level (60-63 Gy) for more than 30 years.1 It has been demonstrated that biopsy-proven local disease control using conventionally fractionated doses in this range is only 15%.2 Curing patients with unresectable NSCLC is not possible without local disease control. Therefore, the Radiation Therapy Oncology Group (RTOG) initiated a series of radiation dose-escalation trials (RTOG 9311, 0117, and 0617) with the intent of improving local control and subsequent survival.3 Our ongoing intergroup phase III trial (RTOG 0617) is testing whether the overall survival (OS) rate is improved with 74 Gy versus 60 Gy in patients with unresectable stage III NSCLC.
Phase I results of RTOG 0117 have been reported, determining that 74 Gy is the maximum-tolerated dose (MTD) in the setting of concurrent weekly paclitaxel and carboplatin chemotherapy.3 This article reports the results of the phase II portion of RTOG 0117, giving 74 Gy with concurrent weekly paclitaxel and carboplatin.
PATIENTS AND METHODS
Patients and Treatment
Eligible patients had histologically proven stages I-IIIB NSCLC, Zubrod performance status 0 to 1, ≤ 5% weight loss within the past 6 months, a forced expiratory volume at 1 second of ≥ 1 L, and atelectasis, if present, must be less than one lung. Based on conformal treatment planning, the volume of lung at or exceeding 20 Gy (V20) must have been ≤ 30%, the mean esophagus dose ≤ 34 Gy, and the volume of esophagus exceeding 55 Gy (V55) ≤ 30%. Exclusion criteria included prior radiation therapy to the thorax, prior chemotherapy or biologic cancer therapy for lung cancer within the past 2 years, prior or concurrent malignancy (except nonmelanoma skin cancer) unless disease-free for one or more years, supraclavicular lymph node metastasis, pleural or pericardial effusions, and superior vena cava syndrome. The metastatic work-up included pulmonary function testing, chest x-ray, computed tomography (CT) of the chest and upper abdomen, either magnetic resonance imaging or CT of the brain, a bone scan, complete blood counts, electrolyte and alkaline phosphatase levels, and liver function tests. Positron emission tomography (PET) was not required because it was not routine at the outset of the study.
Treatment consisted of fractionated radiation therapy given with concurrent weekly chemotherapy consisting of paclitaxel at 50 mg/m2 over 1 hour on days 1, 8, 15, 22, 29, 36, and 43 followed by carboplatin at area under the serum concentration-time curve at 2 mg/m2 over 30 minutes on days 1, 8, 15, 22, 29, 36, and 43. Adjuvant systemic chemotherapy was optional following completion of radiation therapy.
Radiation therapy was delivered using three-dimensional conformal techniques to a cumulative dose of 74 Gy, the MTD from the phase I portion of this trial. Radiation doses were prescribed to the isocenter using water-based calculations. Heterogeneity corrections were not used to calculate the radiation dose to the patient. However, two plans were generated for each patient for the purpose of data collection, with and without tissue density corrections. Gross tumor volume was defined as the primary tumor and any lymph nodes exceeding 1 cm in greatest diameter. The gross tumor volume was expanded by 1 to 1.5 cm to achieve the planning target volume (PTV). No clinical target volume was specifically delineated. Elective nodal volumes were not included within the PTV. The protocol was designed to be stringent with respect to radiation dose to the normal lung and esophagus. Patients must have met V20 ≤ 30%, mean esophagus dose ≤ 34 Gy, and esophageal V55 ≤ 30%. V20 was calculated by using total lung volume minus PTV as the normal lung volume. The radiation treatment plan for each patient was stored centrally at the Image-Guided Therapy Center (ITC) and scored for compliance by the principal investigator.
Statistical Design
The primary end point of the phase II portion of this study was to estimate the percentage of patients who survive at least 12 months using three-dimensional conformal radiation treatment concurrently with paclitaxel and carboplatin chemotherapy. The three secondary end points were to (1) evaluate the toxicity of this regimen, (2) determine the relationship between higher than normal tissue doses to lung and esophagus and encountered radiotherapy toxicity, and (3) estimate complete response rate as defined by diagnostic CT performed at 3 months after completion of all therapy.
The historical control for this study is the best arm of RTOG 9410, arm 2.4 In RTOG 9410, the proportion of patients surviving at least 12 months was 62%. Using a one-group χ2 test with α = .10, a sample size of 50 gives 87% power to detect a 25% or greater relative increase or an absolute increase of at least 0.1558 in the proportion of patients surviving at least 12 months (null hypothesis: P ≤ .6233; alternative hypothesis: P ≥ .7791). We estimated that 46 patients were required for the phase II portion of this trial.
Statistical Analysis Method
The primary end point hypotheses, the percentage of patients who survive at least 12 months, will be tested with a Fleming single-stage phase II procedure5 using a one-sided 90% normal approximation confidence interval on 62.33%. If the point estimate for 12-month survival is greater than 0.71113 (the upper bound), then the conclusion is that the 12-month survival rate significantly improved from 62.33% under the new treatment at α = .10. A failure event for OS was considered a death due to any cause, and OS rates were calculated using the Kaplan-Meier method.6 A failure event for progression-free survival (PFS) was considered the first of either local, regional, or distant progression. Patients without treatment failure were censored at the date of death or last follow-up.
Frequency tables with counts and percentages were used to describe pretreatment characteristics and toxicities for each arm. Acute radiation therapy and chemotherapy toxicities were graded using Common Toxicity Criteria (CTC) v 2.0 criteria. Late radiation therapy toxicities were reported using the RTOG/EORTC (European Organisation for Research and Treatment of Cancer) Late Toxicity Criteria. Results for all eligible and evaluable patients from the phase II portion are reported.
RESULTS
Between February 19, 2003, and January 13, 2004, nine patients were accrued to arm 2 (74 Gy) for the phase I portion of this trial, and 74 Gy was determined to be the MTD. The phase II portion opened to accrual August 3, 2004, and closed November 26, 2007, after 46 patients were accrued to the phase II portion of the trial. These 46 patients have been combined with the nine patients from arm 2 for use in the phase II analysis. Forty-four patients were eligible from the phase II only arm; therefore, 53 patients were eligible and analyzable for the analysis of the phase II portion of this trial. This provides 89% statistical power for the primary end point of OS at 12 months.
The median follow-up time was 19.3 months (range, 0.9 to 57.9 months) for all patients and 25.4 months (range, 13.1 to 57.9 months) for those still alive. Two patients were excluded from the analysis: one withdrew consent and the other was ineligible because of the presence of a synchronous prostate cancer. All patient treatment plans were centrally reviewed. Ninety-four percent of radiation therapy plans were per protocol or acceptable and 6% had unacceptable deviations.
Table 1 contains the pretreatment characteristics of patients. The median age was 67 years (range, 43 to 83 years), 72% of patients were male, and 83% of patients entered onto the trial had stage IIIA/B NSCLC. Patients on RTOG 0117 were slightly older and with earlier stage disease compared with patients on the arm of the RTOG 9410 trial that used concurrent delivery beginning with day 1 (Table 1).
Table 1.
Pretreatment Characteristics of Patients in RTOG 0117 and RTOG 9410 Trials
| Characteristic | RTOG 0117 (n = 53) |
RTOG 9410 CON-QD (n = 195) |
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | |||||
| Median | 67 | 60 | < .001 | ||
| Range | 43-83 | 33-79 | |||
| Sex | |||||
| Male | 38 | 72 | 125 | 64 | .30 |
| Female | 15 | 28 | 70 | 36 | |
| Zubrod/KPS | |||||
| 0/90-100 | 35 | 66 | 148 | 71 | .15 |
| 1/70-80 | 18 | 34 | 57 | 29 | |
| Stage | |||||
| IB | 3 | 6 | 0 | 0 | |
| IIA/B | 6 | 11 | 3 | 2 | |
| I/IIA/B | 9 | 17 | 3 | 2 | < .001 |
| IIIA | 38 | 72 | 84 | 43 | |
| IIIB | 6 | 11 | 108 | 55 | |
| Histology | |||||
| Squamous cell carcinoma | 21 | 40 | 75 | 38 | NA† |
| Adenocarcinoma | 17 | 32 | 73 | 37 | |
| Large cell lung cancer | 4 | 8 | 27 | 14 | |
| Carcinoma NOS | 4 | 8 | 18 | 9 | |
| Other | 7 | 13 | 2 | 1 | |
NOTE. Radiation Therapy Oncology Group (RTOG) 0117 collected Zubrod performance status and used American Joint Committee on Cancer Staging, 5th edition. RTOG 9410 collected Karnofsky performance status and used International Union Against Cancer -American Joint Committee on Cancer Staging, 1988.
Abbreviations: CON QD, concurrent delivery beginning with day 1; KPS, Karnofsky performance score; NA, not applicable; NOS, not otherwise specified.
Continuous: t test; categorical: χ2 test.
Insufficient cell counts.
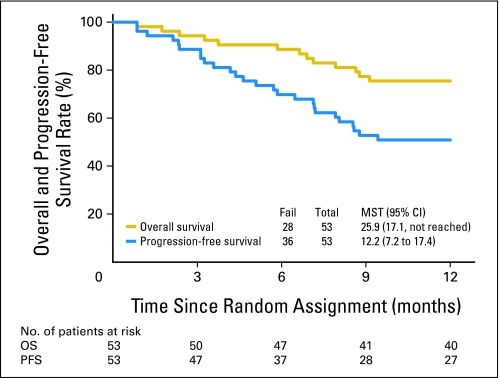
There were 40 patients who survived at least 12 months among the 53 eligible patients (75.5%; 95% CI, 65.7% to 85.2%; Table 2). The median OS was 25.9 months (95% CI, 17.1 months to not reached). When designing this trial, we used arm 2 of RTOG 9410, in which the 12-month survival rate was 62%, to hypothesize what 12-month survival rate was predetermined as successful. This survival rate at 12 months is 0.755, which is statistically improved relative to the projected rate from the designated historical control cohort. The OS curve at 12 months by Kaplan-Meier estimation is shown in Figure 1.
Table 2.
Overall and Progression-Free Survival Outcomes (n = 53)
| Survival Time | RTOG 0117 Overall Survival |
RTOG 0117 Progression-Free Survival |
RTOG 9410 CON-QD Arm Overall Survival |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Alive | 95% CI | No. at Risk | No. Dead/No. Alive | % Alive | 95% CI | No. at Risk | No. Dead/No. Alive | % Alive | 95% CI | No. at Risk | No. Dead/No. Alive | |
| Time, months | ||||||||||||
| 0 | 100 | 53 | 100 | 53 | 100 | 195 | ||||||
| 3 | 94.3 | 83.5 to 98.1 | 50 | 88.7 | 76.5 to 94.7 | 47 | 95.4 | 91.3 to 97.6 | 186 | |||
| 6 | 88.7 | 76.5 to 94.7 | 47 | 69.8 | 55.5 to 80.3 | 37 | 84.1 | 78.2 to 88.5 | 164 | |||
| 9 | 77.4 | 63.6 to 86.5 | 41 | 52.8 | 38.6 to 65.2 | 28 | 74.4 | 67.6 to 79.9 | 145 | |||
| 12 | 75.5 | 61.5 to 85.0 | 40 | 50.9 | 36.9 to 63.4 | 27 | 62.1 | 54.8 to 68.4 | 120 | |||
| Median survival time, months | 25.9 | 17.1 to NA | 12.2 | 7.2 to 17.4 | 17 | 14.0 to 20.2 | ||||||
| No. of patients surviving at end of study | 28/53 | 36/53 | 185/195 | |||||||||
Abbreviations: RTOG, Radiation Therapy Oncology Group; CON QD, concurrent delivery beginning with day 1; NA, not applicable.
Fig 1.
Overall survival (OS) and progression-free survival (PFS) rates for patients enrolled on trial. MST, mean survival time.
The PFS at 12 months was 50.9% (95% CI, 36.9% to 63.4%) and the median PFS time was 12.2 months (95% CI, 7.2 to 17.4 months; Table 2 and Fig 1). The median OS and PFS times for patients with stage III disease (n = 44) were 21.6 months and 10.8 months, respectively; OS and PFS rates at 12 months were 72.7% and 50.0%, respectively. As shown, this compares favorably with the arm of RTOG 9410 trial that used concurrent delivery beginning with day 1.
Acute and late toxicity are summarized in Table 3. There were two (4%) grade 5 acute toxicities reported. One was due to bilateral pneumonia and one was due to hemoptysis. There was one grade 5 late radiation therapy lung toxicity reported, which was due to pulmonary hemorrhage that occurred 146 days after the start of treatment. There were 30 patients (57%) with grade 3 to 4 acute nonhematologic toxicities and 10 patients (20%) with grade 3 to 4 late radiation therapy toxicities.
Table 3.
Toxicity for Patients With Grades 1-5 Lung Toxicity
| Treatment and Toxicity | Grade |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
5 |
||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Chemotherapy and acute radiotherapy toxicity (n = 53) | ||||||||||
| Allergy/immunology | 1 | 0 | 1 | 1 | 0 | |||||
| Auditory/hearing | 1 | 2 | 0 | 0 | 0 | |||||
| Blood/bone marrow | 7 | 10 | 24 | 3 | 0 | |||||
| Cardiovascular (arrhythmia) | 0 | 3 | 4 | 0 | 0 | |||||
| Cardiovascular (general) | 2 | 2 | 7 | 0 | 0 | |||||
| Coagulation | 2 | 0 | 2 | 0 | 0 | |||||
| Constitutional symptoms | 16 | 21 | 3 | 0 | 0 | |||||
| Dermatology/skin | 20 | 9 | 1 | 0 | 0 | |||||
| Gastrointestinal | 9 | 26 | 12 | 0 | 0 | |||||
| Hemorrhage | 5 | 0 | 1 | 0 | 1 | |||||
| Hepatic | 9 | 4 | 0 | 0 | 0 | |||||
| Infection febrile neutropenia | 0 | 1 | 6 | 0 | 0 | |||||
| Metabolic/laboratory | 8 | 4 | 5 | 0 | 0 | |||||
| Musculoskeletal | 1 | 1 | 0 | 0 | 0 | |||||
| Neurology | 12 | 7 | 5 | 0 | 0 | |||||
| Pain | 10 | 12 | 6 | 0 | 0 | |||||
| Pulmonary | 10 | 11 | 8 | 1 | 1 | |||||
| Renal/genitourinary | 5 | 2 | 2 | 0 | 0 | |||||
| Sexual/reproductive function | 1 | 0 | 0 | 0 | 0 | |||||
| Worst nonhematologic | 4 | 8 | 16 | 30 | 28 | 53 | 2 | 4 | 2 | 4 |
| Worst hematologic | 1 | 2 | 8 | 15 | 37 | 70 | 5 | 9 | 2 | 4 |
| Late radiotherapy toxicity (n = 50) | ||||||||||
| Bone | 1 | 0 | 0 | 0 | 0 | |||||
| Esophagus | 6 | 1 | 4 | 0 | 0 | |||||
| Heart | 0 | 3 | 1 | 0 | 0 | |||||
| Lung | 11 | 9 | 6 | 1 | 1 | |||||
| Other | 4 | 4 | 0 | 0 | 0 | |||||
| Skin (within the irradiated field) | 5 | 0 | 0 | 0 | 0 | |||||
| Spinal cord | 1 | 0 | 0 | 0 | 0 | |||||
| Subcutaneous tissue | 2 | 0 | 0 | 0 | 0 | |||||
| Worst overall | 13 | 26 | 10 | 20 | 9 | 18 | 1 | 2 | 1 | 2 |
Table 4 shows the dose-volume RT values for lung (V20 and mean lung dose [MLD]) and esophagus (mean esophagus dose [MED]) for plans with doses corrected for tissue heterogeneity. The mean V20 value, MLD, and MED were 23.9% (range, 9.9% to 35.7%), 15.0 Gy (range, 5.7 to 21.8 Gy), and 22.4 Gy (range, 7.1 to 34.9 Gy), respectively. Table 4 also lists the Gy dose given to 95% of the PTV (D95) values for both heterogeneity corrected and uncorrected plans.
Table 4.
RT Summary (with heterogeneity corrections) Based on RT Planning Data Submitted to the Image-Guided Therapy Center
| Therapy Summary | Normal Tissue Dose |
||
|---|---|---|---|
| Lung V20 (n = 51) | Mean Lung Dose (Gy) (n = 51) | Mean Esophageal Dose (Gy) (n = 52) | |
| Minimum | 9.9 | 5.7 | 7.1 |
| 25th percentile | 19.0 | 12.1 | 18.5 |
| Median | 24.4 | 15.2 | 22.1 |
| 75th percentile | 29.4 | 17.8 | 26.4 |
| Maximum | 35.7 | 21.8 | 34.9 |
| Mean | 23.9 | 15.0 | 22.4 |
| Standard deviation | 6.1 | 3.6 | 6.4 |
| D95* | Target Dose |
|
|---|---|---|
| Heterogeneity Corrected (n = 55) | Heterogeneity Uncorrected (n = 55) | |
| Median | 73.1 | 72.2 |
| Minimum | 61.8 | 66.6 |
| Maximum | 82.1 | 76.5 |
| P (paired t test)† | .003 | |
Abbreviations: RT, radiotherapy; V20, volume of lung at or exceeding 20 Gy; PTV, planning target volume.
D95, Gy dose given to 95% of the PTV.
Calculated with 55 patients with both corrected and uncorrected data. A total of 59 patients had uncorrected data (median dose, 72.2 Gy; range, 66.6-78 Gy).
Table 5 summarizes the dosimetric parameters for patients with or without grade ≥ 3 lung or grade ≥ 2 esophagus toxicities. There were 12 patients with RT data who experienced grade ≥ 3 lung toxicity. There were 21 patients with V20 and MLD data and 22 patients with MED data who experienced grade ≥ 2 esophageal toxicity. For the 12 patients developing grade ≥ 3 pneumonitis, the mean V20 or MLD values were similar to the values of patients who did not develop these toxicities. Likewise, for the 22 patients who developed grade ≥ 2 esophagitis, there was no difference detected in the MED (P > .05). Thirty-three patients (62%) experienced partial or complete response at 3 months post-RT. Four patients (8%) progressed, and five patients (9%) died before the 3 month post-RT time point (data not shown).
Table 5.
Radiotherapy (based on radiotherapy planning data submitted to ITC) and Esophagitis and Lung Toxicity at Any Time With Heterogeneity Corrections (n = 53)
| Radiotherapy | Grade < 3 Lung Toxicity (n = 39) |
Grade ≥ 3 Lung Toxicity (n = 12) |
P | Grade < 2 Esophagitis (n = 30) |
Grade ≥ 2 Esophagitis (n = 21) |
P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |||
| Lung V20 | ||||||||||
| Median | 24.1 | 26.3 | .62* | 22.4 | 26.3 | .22* | ||||
| Range | 9.9-35.7 | 15.6-33.3 | 9.9-35.7 | 14.0-33.3 | ||||||
| < 25% | 13 | 33 | 5 | 42 | .73† | |||||
| ≥ 25% | 27 | 68 | 7 | 58 | ||||||
| ≤ 30% | 34 | 85 | 12 | 100 | .32† | |||||
| > 30% | 6 | 15 | 0 | 0 | ||||||
| Mean lung dose, Gy | ||||||||||
| Median | 15.2 | 17.2 | .30‡ | 14.5 | 16.9 | .17‡ | ||||
| Range | 5.7-21.8 | 11.5-18.9 | 5.7-21.8 | 9.9-20.6 | ||||||
| ≤ 20 | 37 | 95 | 12 | 100 | 1.00† | |||||
| > 20 | 2 | 5 | 0 | 0 | ||||||
| Mean esophageal dose, Gy (n = 22) | ||||||||||
| Median | 21.0 | 24.6 | .08‡ | |||||||
| Range | 7.1-34.9 | 16.2-33.7 | ||||||||
| ≤ 30 | 27 | 90 | 19 | 86 | .69† | |||||
| > 30 | 3 | 10 | 3 | 14 | ||||||
Abbreviations: ITC, Image-Guided Therapy Center; V20, volume of lung at or exceeding 20 Gy.
Wilcoxon rank sum test.
t test.
Fisher's exact test.
DISCUSSION
Phase I results of RTOG 0117 determined that 74 Gy was the MTD to the mediastinum in the setting of concurrent weekly carboplatin and paclitaxel chemotherapy.3 Patients in cohort 1 were treated with 75.25 Gy in 2.15-Gy daily fractions, and six (75%) of eight patients developed grade ≥ 3 toxicity. Patients in cohort 2 received 74 Gy in 2-Gy daily fractions, and only one of seven developed grade 3 toxicity. On the basis of these data, we proceeded to phase II of RTOG 0117. A median survival time of 25.9 months for the overall population and 21.6 months for patients with stage III disease is among the best observed for RTOG studies.4,7,8 Likewise, the 1-year OS and PFS rates of 75.5% and 50.9%, respectively, are encouraging.
The RTOG has conducted a series of trials designed to increase radiation dose with the aim of improving local tumor control since the early 1990s. RTOG 9311 was a prospective dose-escalation trial for patients with stage I-III NSCLC in which radiation was given alone in doses ranging from 70.9 to 90 Gy. That trial determined that 84 Gy was likely the MTD for radiation given to mediastinal structures in settings without concurrent chemotherapy. During the same time interval, patients were also enrolling onto RTOG 9410,4 a three-arm phase III trial designed to test two hypotheses. The first hypothesis was that concurrent chemotherapy would provide a survival benefit compared with sequential chemoradiotherapy. The second hypothesis was that hyperfractionated RT to 69.6 Gy with concurrent chemotherapy would provide an even greater survival benefit compared with doses of 63 Gy with chemotherapy. While the first hypothesis proved true, the second showed that dose escalation with concurrent chemotherapy via hyperfractionation was not feasible because of increased toxicity. When data initially reported that the use of concurrent chemotherapy provided a survival benefit, RTOG 9311 was closed.9 RTOG 0117 was then opened with the aims of determining the MTD of daily fractionated RT with concurrent weekly chemotherapy (phase I) and subsequently, the efficacy of that dose (phase II).
Over the past several years, three other groups have prospectively tested the tolerability and efficacy of 74 Gy given with weekly paclitaxel and carboplatin chemotherapy. All three groups independently reached a similar conclusion: 74 Gy is likely the MTD in this setting. The North Central Cancer Treatment Group (NCCTG) has reported prospective phase I results for 13 patients treated to 70, 74, or 78 Gy.10 In their study, 70 and 74 Gy were well tolerated and 78 Gy was not. With a median follow-up of 28 months for the 13 patients on NCCTG N0028, the median survival time was 37 months. The Cancer and Leukemia Group B (CALGB) has also reported results of a randomized phase II comparison of two different chemotherapy regimens given with 74 Gy.11 Patients either received induction paclitaxel and carboplatin for two cycles followed by the same weekly chemotherapy or they received induction carboplatin and gemcitabine followed by twice weekly gemcitabine during RT. The trial enrolled 69 patients and had a median follow-up of 16.4 months at last report. The median survival was 24.2 months on the paclitaxel/carboplain arm. The gemcitibine arm was closed early because 13% of the patients had grade 5 pulmonary events. On the basis of these two trials as well as RTOG 0117, the NCCTG and the CALGB have joined efforts with the RTOG to support RTOG 0617 as an intergroup trial.
North Carolina investigators have reported the results of four sequential prospective phase I/II studies to assess the safety and feasibility of high-dose (74-90 Gy) three-dimensional conformal radiation treatment in the setting of concurrent weekly carboplatin and paclitaxel chemotherapy.12–15 These investigators also delivered two cycles of carboplatin and paclitaxel neoadjuvantly before concurrent chemoradiotherapy. In total, 112 patients were accrued, with a median follow-up of 4.9 years for surviving patients. The median survival was 24 months (range, 18 to 31 months). The 1-, 3-, and 5-year OS rates were 69% (range, 60% to 77%), 36% (range, 27% to 45%), and 24% (range, 16% to 33%), respectively. The relatively longer follow-up duration of this population provides information about late complication risks.16 Late complications—defined as grade ≥ 3 occurring > 90 days after RT—occurred in 22% (25 of 112 patients). Patients with complications appear to have longer survival than the overall group (P = .007). Late complications included bronchial stenosis (n = 3), fatal hemoptysis (n = 2), tracheoesophageal fistula (n = 1), esophageal stricture (n = 7), myocardial infarction (n = 5), pericardial disease (n = 4), and bone fracture (n = 6).
The relatively lower rates of severe toxicity seen in RTOG 0117 could be attributed to smaller volumes of radiation therapy. Unlike the other studies noted above, dosimetric constraints for RTOG 0117 required total lung V20 ≤ 30%. Furthermore, to minimize toxicity while escalating radiation dose, elective nodal irradiation (ENI) was not delivered in RTOG 0117. The ENI failure rate in the preceding RTOG trial (9311) was only 8%.9 This is in contrast to the CALGB and North Carolina studies noted above.11,13 These two groups continued to deliver ENI to a dose of 45 to 50 Gy while escalating the radiation dose to 74 Gy. Our grade ≥ 3 acute pulmonary toxicity rate was 10% for 74 Gy, likely a result of the V20 restriction and lack of ENI. As for late toxicity, the follow-up for RTOG 0117 patients is still too short to be comprehensive. The North Carolina data show that late toxicities continue to occur with longer follow-up.
Some investigators have criticized the use of carboplatin and paclitaxel given weekly with radiation therapy for NSCLC. CALGB 39801 was a randomized comparison of weekly carboplatin and paclitaxel with or without two cycles of induction chemotherapy for patients with stage III NSCLC.17 This study showed no advantage to induction chemotherapy and reasoned that the median survival results in both arms were lower than expected because of the weekly dosing schedule. Alternatives to that reasoning could be that patients on the CALGB study had more advanced disease. Nearly 50% of enrolled patients had stage IIIB cancers and 26% had weight loss exceeding 5%. Only 11% of patients enrolled on RTOG 0117 had stage IIIB disease and none had weight loss exceeding 5%. Furthermore, arm A of CALGB 30105 delivered weekly doses of carboplatin and paclitaxel and reported a median survival of 24.3 months.11 In addition to the RTOG 0117 results reported here, arm A of CALGB 30105 served as a basis for weekly dosing with these agents in the current follow-up phase III study, RTOG 0617.
A potential weakness of this study is the relative lack of [18F]fluorodeoxyglucose PET (FDG-PET) staging. The use of FDG-PET for staging patients with NSCLC has become routine over the past 10 years and was increasing over the duration of accrual for RTOG 0117. At Washington University, the use of FDG-PET diagnostic imaging for NSCLC increased from 28% in 2000 to 46% in 2004.18 As a result, nearly 10% of patients with presumed stage III NSCLC were found to have stage IV. Other investigators have shown similar effects of stage migration as a result of FDG-PET.19,20 Future studies requiring PET staging could report higher survival rates for chemoradiotherapy for the stage III NSCLC population simply because patients with occult metastases have been excluded by FDG-PET. Thus, the median survival of 21.6 months for stage III patients in RTOG 0117 is likely to increase, using the same chemoradiotherapy regimen, in subsequent trials employing FDG-PET staging.
In conclusion, the median survival time and OS rate at 12 months for RTOG 0117 is encouraging. A radiation dose of 74 Gy given with concurrent weekly carboplatin and paclitaxel appears to be worthy of further investigation within a phase III trial. These survival and toxicity results serve as the projection expectations for the high-dose radiation arms of the current intergroup trial (RTOG 0617).
Supplementary Material
Footnotes
Supported by Grants No. RTOG U10 CA21661, CCOP U10 CA37422, and ITC U24 CA081647 from the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00023673.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Jeffrey D. Bradley, Mary V. Graham, Roger Byhardt, Ramaswamy Govindan, Jack Fowler, James A. Purdy
Financial support: Hak Choy
Administrative support: Kyounghwa Bae, Jeff M. Michalski
Provision of study materials or patients: Jeffrey D. Bradley, Ramaswamy Govindan, Elizabeth Gore, Hak Choy
Collection and assembly of data: Jeffrey D. Bradley, Kyounghwa Bae
Data analysis and interpretation: Jeffrey D. Bradley, Kyounghwa Bae, Jeff M. Michalski
Manuscript writing: Jeffrey D. Bradley, Kyounghwa Bae,Roger Byhardt, Ramaswamy Govindan
Final approval of manuscript: Jeffrey D. Bradley, Kyounghwa Bae, Mary V. Graham, Roger Byhardt, Ramaswamy Govindan, Jack Fowler, James A. Purdy, Jeff M. Michalski, Elizabeth Gore, Hak Choy
REFERENCES
- 1.Perez CA, Stanley K, Rubin P, et al. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat cell carcinoma of the lung: Preliminary report by the Radiation Therapy Oncology Group. Cancer. 1980;45:2744–2753. doi: 10.1002/1097-0142(19800601)45:11<2744::aid-cncr2820451108>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Le Chevalier T, Arriagada R, Quoix E, et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in nonresectable non-small-cell lung cancer: First analysis of a randomized trial in 353 patients. J Natl Cancer Inst. 1991;83:417–423. doi: 10.1093/jnci/83.6.417. [DOI] [PubMed] [Google Scholar]
- 3.Bradley J, Graham M, Swann S, et al. Phase I results of RTOG L-0117: A phase I/II dose intensification study using 3DCRT and concurrent chemotherapy for patients with inoperable NSCLC. J Clin Oncol. 2005;23(suppl):636s. abstr 7063. [Google Scholar]
- 4.Curran W, Scott C, Langer C, et al. Long-term benefit is observed in a phase III comparison of sequential vs concurrent chemo-radiation for patients with unresected stage III NSCLC: RTOG 9410. Proc Am Soc Clin Oncol. 2003;22:621. abstr 2499. [Google Scholar]
- 5.Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38:143–151. [PubMed] [Google Scholar]
- 6.Kaplan E, Meier P. Nonparameteric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 7.Blumenschein G, Moughan J, Curran W. A phase II study of cetuximab (C225) in combination with chemoradiation (CRT) in patients (pts) with stage III A/B non-small cell lung cancer (NSCLC): An interim report of the RTOG 0324 trial. J Clin Oncol. 2007;25(suppl):392s. abstr 7531. [Google Scholar]
- 8.Movsas B, Scott C, Langer C, et al. Phase III study of amifostine in patients with locally advanced non-small cell lung cancer (NSCLC) receiving chemotherapy & hyperfractionated radiation (chemo/HFxRT): Radiation Therapy Oncology Group (RTOG) 98-01. Proc Am Soc Clin Oncol. 2003;22:636. doi: 10.1200/JCO.2005.07.167. abstr 2559. [DOI] [PubMed] [Google Scholar]
- 9.Bradley J, Graham MV, Winter K, et al. Toxicity and outcome results of RTOG 9311: A phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:318–328. doi: 10.1016/j.ijrobp.2004.06.260. [DOI] [PubMed] [Google Scholar]
- 10.Schild SE, McGinnis WL, Graham D, et al. Results of a phase I trial of concurrent chemotherapy and escalating doses of radiation for unresectable non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65:1106–1111. doi: 10.1016/j.ijrobp.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 11.Socinski MA, Blackstock AW, Bogart JA, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol. 2008;26:2457–2463. doi: 10.1200/JCO.2007.14.7371. [DOI] [PubMed] [Google Scholar]
- 12.Rosenman JG, Halle JS, Socinski MA, et al. High-dose conformal radiotherapy for treatment of stage IIIA/IIIB non-small-cell lung cancer: Technical issues and results of a phase I/II trial. Int J Radiat Oncol Biol Phys. 2002;54:348–356. doi: 10.1016/s0360-3016(02)02958-9. [DOI] [PubMed] [Google Scholar]
- 13.Socinski MA, Kies M, Schell MJ, et al. Duration of therapy in stage IIIB/IV non-small cell lung cancer (NSCLC): A multi-institutional phase III trial. Proc Am Soc Clin Oncol. 2001;20:309a. abstr 1232. [Google Scholar]
- 14.Socinski MA, Morris DE, Halle JS, et al. Induction and concurrent chemotherapy with high-dose thoracic conformal radiation therapy in unresectable stage IIIA and IIIB non-small-cell lung cancer: A dose-escalation phase I trial. J Clin Oncol. 2004;22:4341–4350. doi: 10.1200/JCO.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Stinchcombe TE, Lee CB, Moore DT, et al. Long-term follow-up of a phase I/II trial of dose escalating three-dimensional conformal thoracic radiation therapy with induction and concurrent carboplatin and paclitaxel in unresectable stage IIIA/B non-small cell lung cancer. J Thorac Oncol. 2008;3:1279–1285. doi: 10.1097/JTO.0b013e31818b1971. [DOI] [PubMed] [Google Scholar]
- 16.Lee CB, Stinchcombe TE, Moore DT, et al. Late complications of high-dose (>/= 66 Gy) thoracic conformal radiation therapy in combined modality trials in unresectable stage III non-small cell lung cancer. J Thorac Oncol. 2009;4:74–79. doi: 10.1097/JTO.0b013e3181915028. [DOI] [PubMed] [Google Scholar]
- 17.Vokes EE, Herndon JE, 2nd, Kelley MJ, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol. 2007;25:1698–1704. doi: 10.1200/JCO.2006.07.3569. [DOI] [PubMed] [Google Scholar]
- 18.Morgensztern D, Goodgame B, Baggstrom M, et al. The effect of FDG-PET on the stage distribution of non-small cell lung cancer. J Thorac Oncol. 2008;3:135–139. doi: 10.1097/JTO.0b013e3181622c2c. [DOI] [PubMed] [Google Scholar]
- 19.Hicks RJ, Kalff V, MacManus MP, et al. (18)F-FDG PET provides high-impact and powerful prognostic stratification in staging newly diagnosed non-small cell lung cancer. J Nucl Med. 2001;42:1596–1604. [PubMed] [Google Scholar]
- 20.Pieterman RM, van Putten JW, Meuzelaar JJ, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med. 2000;343:254–261. doi: 10.1056/NEJM200007273430404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.