Abstract
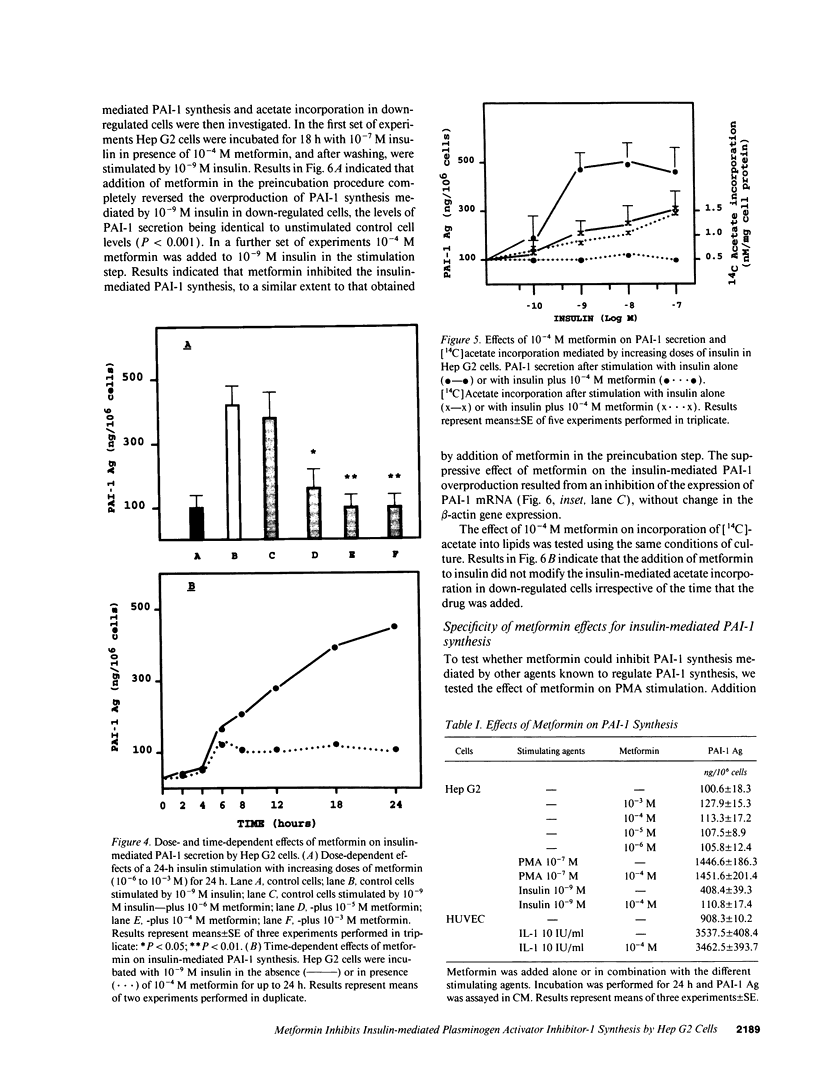
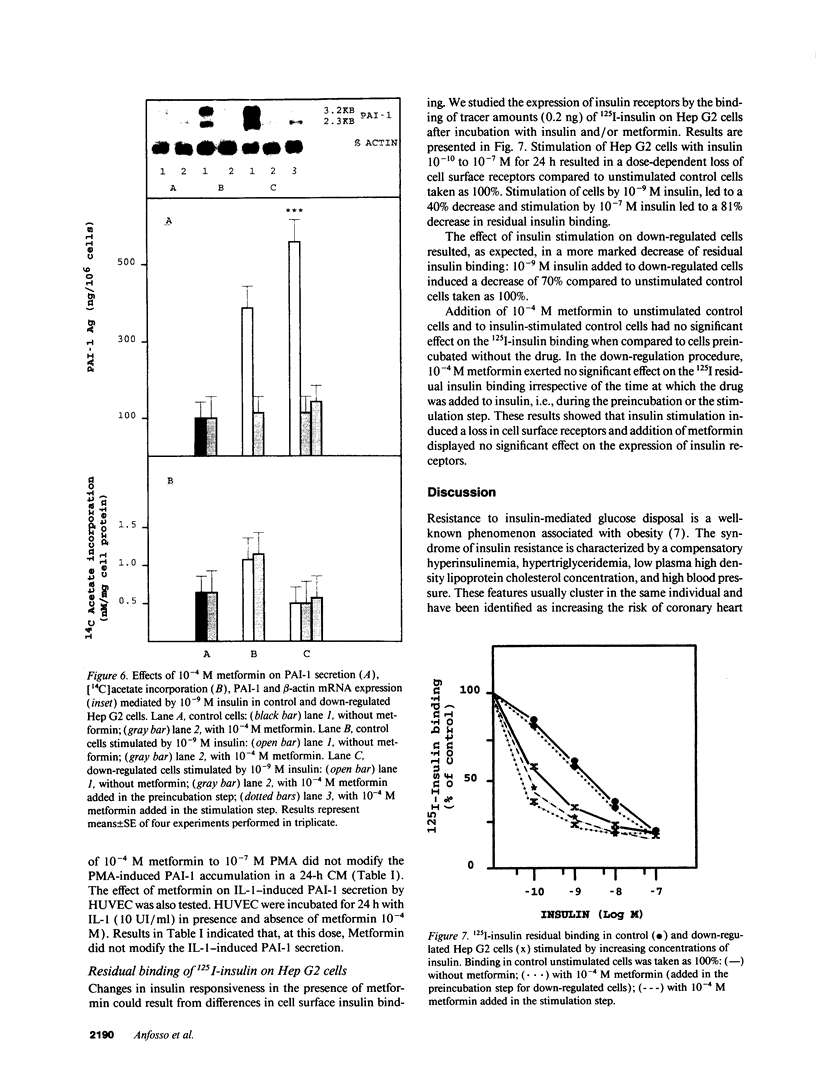
High plasma plasminogen activator inhibitor-1 (PAI-1) activity is associated with insulin resistance and is correlated with hyperinsulinemia. The cellular origin of plasma PAI-1 in insulin resistance is not known. The hepatoma cell line Hep G2 has been shown to synthesize PAI-1 in response to insulin. The aim of this study was to analyze the insulin-mediated response of PAI-1 and lipid synthesis in Hep G2 cells after producing an insulin-resistant state by decreasing insulin receptor numbers. The effect of metformin, a dimethyl-substituted biguanide, known to lower plasma insulin and PAI-1 levels in vivo was concomitantly evaluated. Preincubation by an 18-h exposure of Hep G2 cells to 10(-7) M insulin aimed at reducing the number of insulin receptors, was followed by a subsequent 24-h stimulation with 10(-9) M insulin. The decrease in insulin receptors was accompanied as expected, by a reduction in [14C]acetate incorporation, an index of lipid synthesis, whereas PAI-1 secretion and PAI-1 mRNA expression were enhanced. The addition of metformin did not modify the effect of insulin on insulin receptors or [14C]acetate incorporation. In contrast, the drug (10(-4) M) inhibited insulin-mediated PAI-1 synthesis. The results indicate that PAI-1 synthesis in presence of insulin is markedly increased in down-regulated cells, and that metformin inhibits this effect by acting at the cellular level. These in vitro data are relevant with those found in vivo in insulin-resistant patients. Hep G2 cells may be a suitable model to study PAI-1 regulation in response to hyperinsulinemia.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alengrin F., Grossi G., Canivet B., Dolais-Kitabgi J. Inhibitory effects of metformin on insulin and glucagon action in rat hepatocytes involve post-receptor alterations. Diabete Metab. 1987 Nov-Dec;13(6):591–597. [PubMed] [Google Scholar]
- Alessi M. C., Juhan-Vague I., Kooistra T., Declerck P. J., Collen D. Insulin stimulates the synthesis of plasminogen activator inhibitor 1 by the human hepatocellular cell line Hep G2. Thromb Haemost. 1988 Dec 22;60(3):491–494. [PubMed] [Google Scholar]
- Arsenis G., Livingston J. N. Alterations in the tyrosine kinase activity of the insulin receptor produced by in vitro hyperinsulinemia. J Biol Chem. 1986 Jan 5;261(1):147–153. [PubMed] [Google Scholar]
- Benzi L., Trischitta V., Ciccarone A., Cecchetti P., Brunetti A., Squatrito S., Marchetti P., Vigneri R., Navalesi R. Improvement with metformin in insulin internalization and processing in monocytes from NIDDM patients. Diabetes. 1990 Jul;39(7):844–849. doi: 10.2337/diab.39.7.844. [DOI] [PubMed] [Google Scholar]
- Bosma P. J., Kooistra T. Different induction of two plasminogen activator inhibitor 1 mRNA species by phorbol ester in human hepatoma cells. J Biol Chem. 1991 Sep 25;266(27):17845–17849. [PubMed] [Google Scholar]
- CHAKRABARTI R., HOCKING E. D., FEARNLEY G. R. FIBRINOLYTIC EFFECT OF METFORMIN IN CORONARY-ARTERY DISEASE. Lancet. 1965 Aug 7;2(7406):256–259. doi: 10.1016/s0140-6736(65)92383-4. [DOI] [PubMed] [Google Scholar]
- Caro J. F., Dohm L. G., Pories W. J., Sinha M. K. Cellular alterations in liver, skeletal muscle, and adipose tissue responsible for insulin resistance in obesity and type II diabetes. Diabetes Metab Rev. 1989 Dec;5(8):665–689. doi: 10.1002/dmr.5610050804. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991 Mar;14(3):173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- Declerck P. J., Alessi M. C., Verstreken M., Kruithof E. K., Juhan-Vague I., Collen D. Measurement of plasminogen activator inhibitor 1 in biologic fluids with a murine monoclonal antibody-based enzyme-linked immunosorbent assay. Blood. 1988 Jan;71(1):220–225. [PubMed] [Google Scholar]
- Ducimetiere P., Eschwege E., Papoz L., Richard J. L., Claude J. R., Rosselin G. Relationship of plasma insulin levels to the incidence of myocardial infarction and coronary heart disease mortality in a middle-aged population. Diabetologia. 1980 Sep;19(3):205–210. doi: 10.1007/BF00275270. [DOI] [PubMed] [Google Scholar]
- Duerden J. M., Bartlett S. M., Gibbons G. F. Regulation of very-low-density-lipoprotein lipid secretion in hepatocyte cultures derived from diabetic animals. Biochem J. 1989 Aug 15;262(1):313–319. doi: 10.1042/bj2620313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrington P. N., Newton R. S., Weinstein D. B., Steinberg D. Effects of insulin and glucose on very low density lipoprotein triglyceride secretion by cultured rat hepatocytes. J Clin Invest. 1982 Jul;70(1):63–73. doi: 10.1172/JCI110604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeis J. J., Kooistra T. Interleukin 1 and lipopolysaccharide induce an inhibitor of tissue-type plasminogen activator in vivo and in cultured endothelial cells. J Exp Med. 1986 May 1;163(5):1260–1266. doi: 10.1084/jem.163.5.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fantus I. G., Brosseau R. Mechanism of action of metformin: insulin receptor and postreceptor effects in vitro and in vivo. J Clin Endocrinol Metab. 1986 Oct;63(4):898–905. doi: 10.1210/jcem-63-4-898. [DOI] [PubMed] [Google Scholar]
- Fontbonne A. M., Eschwège E. M. Insulin and cardiovascular disease. Paris Prospective Study. Diabetes Care. 1991 Jun;14(6):461–469. doi: 10.2337/diacare.14.6.461. [DOI] [PubMed] [Google Scholar]
- Frayn K. N., Adnitt P. I. Effects of metformin on glucose uptake by isolated diaphragm from normal and diabetic rats. Biochem Pharmacol. 1972 Dec 1;21(23):3153–3162. doi: 10.1016/0006-2952(72)90142-6. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Olefsky J. M., Marshall S. Insulin induces progressive insulin resistance in cultured rat adipocytes. Sequential effects at receptor and multiple postreceptor sites. Diabetes. 1986 Mar;35(3):258–267. doi: 10.2337/diab.35.3.258. [DOI] [PubMed] [Google Scholar]
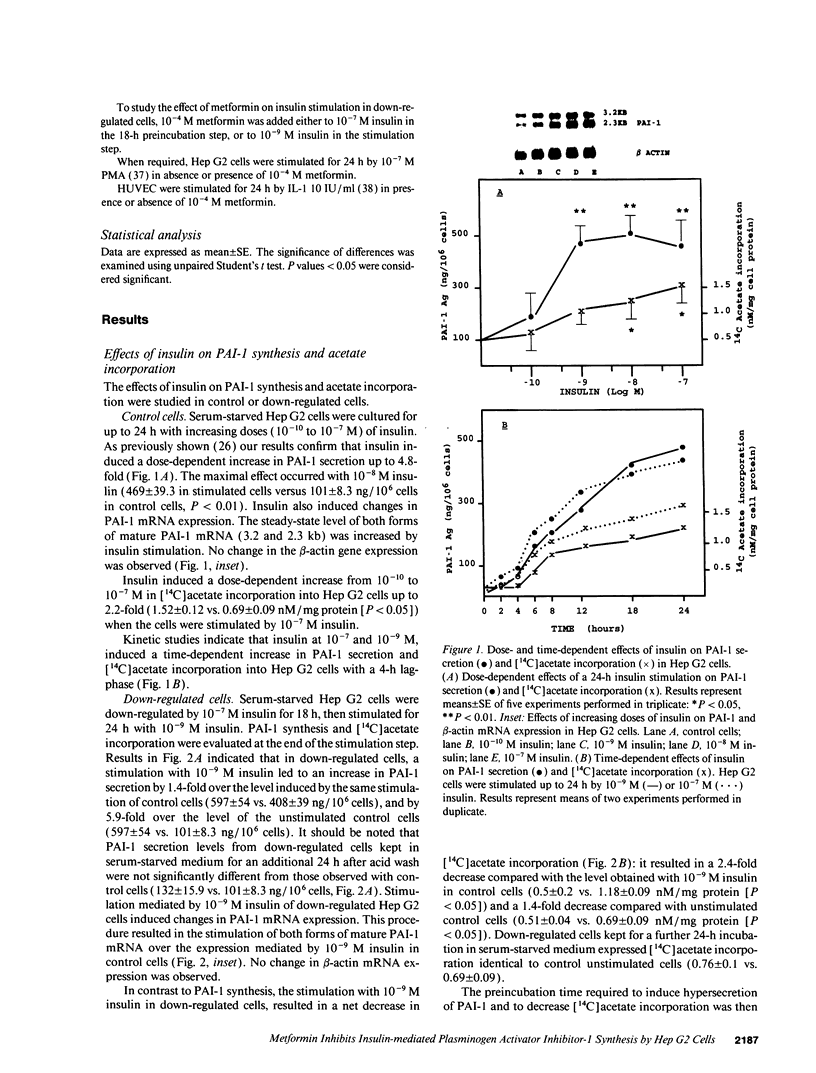
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P. J., Stickland M. H., Booth N. A., Prentice C. R. Metformin causes a reduction in basal and post-venous occlusion plasminogen activator inhibitor-1 in type 2 diabetic patients. Diabet Med. 1991 May;8(4):361–365. doi: 10.1111/j.1464-5491.1991.tb01610.x. [DOI] [PubMed] [Google Scholar]
- Grigorescu F., Laurent A., Chavanieu A., Capony J. P. Cellular mechanism of metformin action. Diabete Metab. 1991 May;17(1 Pt 2):146–149. [PubMed] [Google Scholar]
- Groop L. C., Bonadonna R. C., DelPrato S., Ratheiser K., Zyck K., Ferrannini E., DeFronzo R. A. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989 Jul;84(1):205–213. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hah J., Jo I., Chakrabarti R., Jung C. Y. Demonstration of an insulin-insensitive storage pool of glucose transporters in rat hepatocytes and HepG2 cells. J Cell Physiol. 1992 Jul;152(1):56–63. doi: 10.1002/jcp.1041520108. [DOI] [PubMed] [Google Scholar]
- Hamsten A., de Faire U., Walldius G., Dahlén G., Szamosi A., Landou C., Blombäck M., Wiman B. Plasminogen activator inhibitor in plasma: risk factor for recurrent myocardial infarction. Lancet. 1987 Jul 4;2(8549):3–9. doi: 10.1016/s0140-6736(87)93050-9. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D. E., Brown R., Navarro J., Pilch P. F. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1988 May 12;333(6169):183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- Juhan-Vague I., Alessi M. C., Joly P., Thirion X., Vague P., Declerck P. J., Serradimigni A., Collen D. Plasma plasminogen activator inhibitor-1 in angina pectoris. Influence of plasma insulin and acute-phase response. Arteriosclerosis. 1989 May-Jun;9(3):362–367. doi: 10.1161/01.atv.9.3.362. [DOI] [PubMed] [Google Scholar]
- Juhan-Vague I., Alessi M. C., Vague P. Increased plasma plasminogen activator inhibitor 1 levels. A possible link between insulin resistance and atherothrombosis. Diabetologia. 1991 Jul;34(7):457–462. doi: 10.1007/BF00403280. [DOI] [PubMed] [Google Scholar]
- Klip A., Leiter L. A. Cellular mechanism of action of metformin. Diabetes Care. 1990 Jun;13(6):696–704. doi: 10.2337/diacare.13.6.696. [DOI] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Kooistra T., Bosma P. J., Töns H. A., van den Berg A. P., Meyer P., Princen H. M. Plasminogen activator inhibitor 1: biosynthesis and mRNA level are increased by insulin in cultured human hepatocytes. Thromb Haemost. 1989 Sep 29;62(2):723–728. [PubMed] [Google Scholar]
- Livingston J. N., Purvis B. J., Lockwood D. H. Insulin-dependent regulation of the insulin-sensitivity of adipocytes. Nature. 1978 Jun 1;273(5661):394–396. doi: 10.1038/273394a0. [DOI] [PubMed] [Google Scholar]
- Maegawa H., Olefsky J. M., Thies S., Boyd D., Ullrich A., McClain D. A. Insulin receptors with defective tyrosine kinase inhibit normal receptor function at the level of substrate phosphorylation. J Biol Chem. 1988 Sep 5;263(25):12629–12637. [PubMed] [Google Scholar]
- Marshall S., Olefsky J. M. Effects of insulin incubation on insulin binding, glucose transport, and insulin degradation by isolated rat adipocytes. Evidence for hormone-induced desensitization at the receptor and postreceptor level. J Clin Invest. 1980 Oct;66(4):763–772. doi: 10.1172/JCI109914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthaei S., Hamann A., Klein H. H., Benecke H., Kreymann G., Flier J. S., Greten H. Association of Metformin's effect to increase insulin-stimulated glucose transport with potentiation of insulin-induced translocation of glucose transporters from intracellular pool to plasma membrane in rat adipocytes. Diabetes. 1991 Jul;40(7):850–857. doi: 10.2337/diab.40.7.850. [DOI] [PubMed] [Google Scholar]
- McClain D. A., Maegawa H., Lee J., Dull T. J., Ulrich A., Olefsky J. M. A mutant insulin receptor with defective tyrosine kinase displays no biologic activity and does not undergo endocytosis. J Biol Chem. 1987 Oct 25;262(30):14663–14671. [PubMed] [Google Scholar]
- Medcalf R. L., Van den Berg E., Schleuning W. D. Glucocorticoid-modulated gene expression of tissue- and urinary-type plasminogen activator and plasminogen activator inhibitor 1 and 2. J Cell Biol. 1988 Mar;106(3):971–978. doi: 10.1083/jcb.106.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meheus L. A., Van Beeumen J. J., Coomans A. V., Vanfleteren J. R. Age-specific nuclear proteins in the nematode worm Caenorhabditis elegans. Biochem J. 1987 Jul 1;245(1):257–261. doi: 10.1042/bj2450257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melin B., Cherqui G., Blivet M. J., Caron M., Lascols O., Capeau J., Picard J. Dual effect of metformin in cultured rat hepatocytes: potentiation of insulin action and prevention of insulin-induced resistance. Metabolism. 1990 Oct;39(10):1089–1095. doi: 10.1016/0026-0495(90)90171-8. [DOI] [PubMed] [Google Scholar]
- Misbin R. I., Merimee T. J., Lowenstein J. M. Insulin removal by isolated perfused rat liver. Am J Physiol. 1976 Jan;230(1):171–177. doi: 10.1152/ajplegacy.1976.230.1.171. [DOI] [PubMed] [Google Scholar]
- Okabayashi Y., Maddux B. A., McDonald A. R., Logsdon C. D., Williams J. A., Goldfine I. D. Mechanisms of insulin-induced insulin-receptor downregulation. Decrease of receptor biosynthesis and mRNA levels. Diabetes. 1989 Feb;38(2):182–187. doi: 10.2337/diab.38.2.182. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. The insulin receptor. A multifunctional protein. Diabetes. 1990 Sep;39(9):1009–1016. doi: 10.2337/diab.39.9.1009. [DOI] [PubMed] [Google Scholar]
- Pentikäinen P. J., Neuvonen P. J., Penttilä A. Pharmacokinetics of metformin after intravenous and oral administration to man. Eur J Clin Pharmacol. 1979 Sep;16(3):195–202. doi: 10.1007/BF00562061. [DOI] [PubMed] [Google Scholar]
- Purrello F., Gullo D., Buscema M., Pezzino V., Vigneri R., Goldfine I. D. Metformin enhances certain insulin actions in cultured rat hepatoma cells. Diabetologia. 1988 Jun;31(6):385–389. doi: 10.1007/BF02341508. [DOI] [PubMed] [Google Scholar]
- Pyöräla K. Relationship of glucose tolerance and plasma insulin to the incidence of coronary heart disease: results from two population studies in Finland. Diabetes Care. 1979 Mar-Apr;2(2):131–141. doi: 10.2337/diacare.2.2.131. [DOI] [PubMed] [Google Scholar]
- Reaven G. M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988 Dec;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Rohilla A. M., Anderson C., Wood W. M., Berhanu P. Insulin downregulates the steady-state level of its receptor's messenger ribonucleic acid. Biochem Biophys Res Commun. 1991 Mar 15;175(2):520–526. doi: 10.1016/0006-291x(91)91595-4. [DOI] [PubMed] [Google Scholar]
- Rothenberg P. L., Kahn C. R. Insulin inhibits pertussis toxin-catalyzed ADP-ribosylation of G-proteins. Evidence for a novel interaction between insulin receptors and G-proteins. J Biol Chem. 1988 Oct 25;263(30):15546–15552. [PubMed] [Google Scholar]
- Russell D. S., Gherzi R., Johnson E. L., Chou C. K., Rosen O. M. The protein-tyrosine kinase activity of the insulin receptor is necessary for insulin-mediated receptor down-regulation. J Biol Chem. 1987 Aug 25;262(24):11833–11840. [PubMed] [Google Scholar]
- Schneider D. J., Sobel B. E. Augmentation of synthesis of plasminogen activator inhibitor type 1 by insulin and insulin-like growth factor type I: implications for vascular disease in hyperinsulinemic states. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9959–9963. doi: 10.1073/pnas.88.22.9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J., Erren T., Zöfel P., Kaffarnik H. Metformin-induced changes in serum lipids, lipoproteins, and apoproteins in non-insulin-dependent diabetes mellitus. Atherosclerosis. 1990 May;82(1-2):97–103. doi: 10.1016/0021-9150(90)90148-c. [DOI] [PubMed] [Google Scholar]
- Sinha M. K., Taylor L. G., Pories W. J., Flickinger E. G., Meelheim D., Atkinson S., Sehgal N. S., Caro J. F. Long-term effect of insulin on glucose transport and insulin binding in cultured adipocytes from normal and obese humans with and without non-insulin-dependent diabetes. J Clin Invest. 1987 Oct;80(4):1073–1081. doi: 10.1172/JCI113163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengers E. D., Princen H. M., Kooistra T., van Hinsbergh V. W. Inhibition of plasminogen activators by conditioned medium of human hepatocytes and hepatoma cell line Hep G2. J Lab Clin Med. 1985 Jun;105(6):751–758. [PubMed] [Google Scholar]
- Sung C. K. Insulin receptor signaling through non-tyrosine kinase pathways: evidence from anti-receptor antibodies and insulin receptor mutants. J Cell Biochem. 1992 Jan;48(1):26–32. doi: 10.1002/jcb.240480106. [DOI] [PubMed] [Google Scholar]
- Vague P., Juhan-Vague I., Alessi M. C., Badier C., Valadier J. Metformin decreases the high plasminogen activator inhibition capacity, plasma insulin and triglyceride levels in non-diabetic obese subjects. Thromb Haemost. 1987 Jun 3;57(3):326–328. [PubMed] [Google Scholar]
- Wang S. R., Pessah M., Infante J., Catala D., Salvat C., Infante R. Lipid and lipoprotein metabolism in Hep G2 cells. Biochim Biophys Acta. 1988 Aug 12;961(3):351–363. doi: 10.1016/0005-2760(88)90082-3. [DOI] [PubMed] [Google Scholar]
- Welborn T. A., Wearne K. Coronary heart disease incidence and cardiovascular mortality in Busselton with reference to glucose and insulin concentrations. Diabetes Care. 1979 Mar-Apr;2(2):154–160. doi: 10.2337/diacare.2.2.154. [DOI] [PubMed] [Google Scholar]
- Williams J. F., Olefsky J. M. Defective insulin receptor function in down-regulated HepG2 cells. Endocrinology. 1990 Oct;127(4):1706–1717. doi: 10.1210/endo-127-4-1706. [DOI] [PubMed] [Google Scholar]
- Wiman B., Hamsten A. The fibrinolytic enzyme system and its role in the etiology of thromboembolic disease. Semin Thromb Hemost. 1990 Jul;16(3):207–216. doi: 10.1055/s-2007-1002671. [DOI] [PubMed] [Google Scholar]
- Wollen N., Bailey C. J. Metformin potentiates the antigluconeogenic action of insulin. Diabete Metab. 1988 Mar-Apr;14(2):88–91. [PubMed] [Google Scholar]
- Wu M. S., Johnston P., Sheu W. H., Hollenbeck C. B., Jeng C. Y., Goldfine I. D., Chen Y. D., Reaven G. M. Effect of metformin on carbohydrate and lipoprotein metabolism in NIDDM patients. Diabetes Care. 1990 Jan;13(1):1–8. doi: 10.2337/diacare.13.1.1. [DOI] [PubMed] [Google Scholar]