Abstract
Stomatal closure during water stress is a major plant mechanism for reducing the loss of water through leaves. The opening and closure of stomata are mediated by endomembrane trafficking. The role of the vacuolar trafficking pathway, that involves v-SNAREs of the AtVAMP71 family (formerly called AtVAMP7C) in stomatal movements, was analysed. Expression of AtVAMP711–14 genes was manipulated in Arabidopsis plants with sense or antisense constructs by transformation of the AtVAMP711 gene. Antisense plants exhibited decreased stomatal closure during drought or after treatment with abscisic acid (ABA), resulting in the rapid loss of leaf water and tissue collapse. No improvement was seen in plants overexpressing the AtVAMP711 gene, suggesting that wild-type levels of AtVAMP711 expression are sufficient. ABA treatment induced the production of reactive oxygen species (ROS) in guard cells of both wild-type and antisense plants, indicating that correct hormone sensing is maintained. ROS were detected in nuclei, chloroplasts, and vacuoles. ABA treatment caused a significant increase in ROS-containing small vacuoles and also in plastids and nuclei of neighbouring epidermal and mesophyll cells. Taken together, our results show that VAMP71 proteins play an important role in the localization of ROS, and in the regulation of stomatal closure by ABA treatment. The paper also describes a novel aspect of ROS signalling in plants: that of ROS production in small vacuoles that are dispersed in the cytoplasm.
Keywords: Abiotic stress, oxidative stress, phosphatidylinositol, SNAREs, vesicle
Introduction
The ability of guard cells to respond to environmental changes and close when water availability is limited is one of the major mechanisms that govern water loss in plants (Schroeder et al., 2001; Sirichandra et al., 2009). The primary plant response towards water loss and dehydration is the reduction of transpiration from the leaves, primarily by closing stomatal pores on the leaf surface. During stomatal opening and closure cycles, guard cell volume changes by a factor of two or more, far beyond the ability of membranes to stretch. The delivery of endomembranes tonoplast and plasma membrane, which is governed by the vesicle trafficking machinery was shown to play a role in these volume changes (Blatt, 2000). These membrane dynamics involve the tonoplast that controls the vacuolar volume. The size of stomatal guard cells is rapidly reversible and is crucial for repetitive stomatal cycling (Shope et al., 2003).
Guard cells respond to the plant stress hormone, abscisic acid (ABA), which regulates ion channels in the tonoplast and plasma membranes, allowing movement of water in and out of the cell. This enables the necessary volume changes for opening and closing the stomatal pores (Fan et al., 2004). The process is regulated by a complex signal transduction network, involving protein kinases, phosphatases, and secondary messengers, such as inositol 1,4,5-trisphosphate (InsP3), calcium, hydrogen peroxide, and nitric oxide (Pei et al., 2000; Fan et al., 2004). In addition, the potassium and calcium channels are regulated by heterotrimeric G proteins (Wang et al., 2001; Neill et al., 2008; Wang and Zhang, 2008). In vivo analysis of ABA signalling elements during drought stress in Nicotiana tabacum identified a syntaxin protein encoded by NtSyr1 (Leyman et al., 1999). Nt-Syr1 is a t-SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) that resides in the plasma membrane and participates in the induction of ABA-dependent ion currents. Overexpression of an NtSyr1 fragment in transgenic plants compromised the SNARE-mediated secretory trafficking between the Golgi apparatus and the plasma membrane that was rescued by overexpression of the full length gene, suggesting that NtSyr1 functions in vesicle trafficking to the plasma membrane (Geelen et al., 2002).
Vacuolar membrane trafficking has been shown to play an important role in stomatal movements (Gao et al., 2005). The major components of the vesicle trafficking machinery include phospholipids and integral membrane proteins, such as VAMP (vesicle associated membrane proteins). VAMP proteins constitute the major component of SNARE complexes, which facilitate vesicle fusion with target membranes (Surpin and Raikhel, 2004; Sutter et al., 2006). During the fusion process VAMPS form a biochemically stable trans-SNARE complex with homologous t-SNARE (syntaxin) proteins that are located in the target membrane. Plants contain an especially large set of SNAREs (Sanderfoot et al., 2000), which are thought to be associated with different vacuole types. (Bassham and Raikhel, 2000; Jurgens, 2004). Vesicle trafficking has traditionally been viewed as a housekeeping process, but recent findings in plant, yeast, and animal cells showed that it also plays an important role in the regulation of stress responses (Cavalli et al., 2001; Kargul et al., 2001; Levine et al., 2001; Mazel et al., 2004).
Studies are presented here demonstrating the function of the tonoplast-specific v-SNAREs (AtVAMP71/AtVAMP7C) in the plant's response to water deficit. The focus is on Arabidopsis thaliana guard cells and it is shown that members of the AtVAMP71 complex play an important role in the proper ROS localization, regulating stomatal closure after ABA treatment.
Materials and methods
Plant material and growth conditions
Transgenic Arabidopsis plants with altered AtVAMP711 (At4g32150) expression were engineered as described in Leshem et al. (2006). Seedlings were selected on kanamycin, transferred to pots with soil mixture and grown in a growth chamber (120 μE light in short-day conditions, 8 h light). Drought was induced by preventing irrigation from 3-month-old plants.
Water loss measurements
All plants were placed in the same tray for concurrent watering and drying. The weights of the pots were measured daily and recorded. For leaf water loss, 3–4 rosette leaves from 3–4 individual 2-month-old plants were detached from the plants, placed in weighing dishes, and incubated on the laboratory bench. Loss of fresh weight was monitored by weighing at the indicated times and expressing it as the percentage of the initial fresh weight as described by Wang et al. (2001).
Stomatal closure measurements
Measurements were performed as described by Wang et al. (2001) with minor changes. Briefly, 2–3-d-old cotyledons or rosette leaf discs (∼ 3 mm2) from 2–3-week-old plants were harvested and floated with the cuticle side up in Petri dishes containing 20 mM KCl, 1 mM CaCl2, 5 mM MES-KOH, pH 6.15, at 20 °C for 2 h under 200 μmol m−2 s−1 white light. The leaf sections were then treated in 20 μM ABA for 3 h, as described in Suhita et al. (2004), or 1 M sorbitol for 1 h. Stomatal closure was determined by measuring 15 apertures from four different paradermal sections of the abaxial epidermis with a microscope optical micrometer. Each experiment was repeated three times.
Confocal and light microscopy
Arabidopsis leaf discs were analysed in a MRC-1024 confocal microscope (Bio-Rad, USA) with a ×40 oil immersion objective (N.A. 1.3). Excitation was from an Argon laser. All images were scanned using identical conditions (laser power was 5% and 3% for green and red filters, respectively; gain of PMT was 700; iris size was 1.0; zoom=5). H2O2 was detected using 10 μm H2DCFDA probe (Molecular Probes-Invitrogen, Eugene OR). The ROS-sensitive dye was loaded for 10 min, washed twice in the same buffer and analysed by confocal microscopy using 485±10 nm/535±10 nm excitation/emission. Intracellular membranes were detected using a membrane potential independent membrane dye MitoFluor™ Red 589 (Molecular Probes, Invitrogen, Eugene, OR), described in Cerveny and Jensen (2003) and Kondo-Okamoto et al. (2003). The dye was loaded at 6 μM final concentration for 30 min, then washed twice in the same medium and analysed by confocal microscopy using 588±10 nm/622±10 nm excitation/emission filters (see Supplementary Fig. S2 at JXB online). Intracellular membranes were also detected using the styryl dye FM4-64 (Molecular Probes, Invitrogen) as described in Leshem et al. (2006) and in Supplementary Fig. S4 at JXB online. Both dyes stain membranes in a non-specific manner, but MitoFluor™ Red 589 produced better staining of the guard cells. Nuclei of guard and pavement epidermal cells were visualized using 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI), which was applied at 5 mg ml−1 for 10 min followed by a double wash as described by Ashtamker et al. (2007) and analysed using 358±10 nm/461±10 nm excitation/emission wavelengths.
All confocal images were processed using ImagePro Plus software package (Media Cybernetics, Bethesda, MD). Quantification of whole guard cell ROS accumulation in the different genotypes and treatments was performed by projecting the complete guard cells Z-stack (8–10 sections, 1 μm thick) of the H2DCFDA separate filter in each guard cell, and analysing the mean signal intensity of the integrated image by ImagePro program.
The light microscope (Olympus IX70) with an optical micrometer was used for stomatal aperture experiments. For vacuole detection, Neutral Red was loaded at a final concentration of 0.1% for 30 min followed by two washes prior to sorbitol treatment. Pictures were taken with a Coolpix 4500 camera (Nikon, Japan) using identical exposure settings for each image set.
Results
Loss of water from leaves of wild-type and antisense AtVAMP711 plants
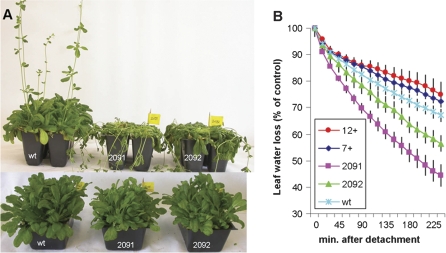
To test the role of the vacuolar vesicle trafficking pathway in drought stress transgenic Arabidopsis plants were prepared with altered expression of the major vacuolar v-SNARE, AtVAMP7C/ATVAMP71 complex, which is composed of four homologous genes, AtVAMP711–14 (Sanderfoot et al., 2000). It has been shown previously that the antisense approach resulted in strong suppression of AtVAMP711, AtVAMP713, and AtVAMP714 genes in line 2091 and a weaker inhibition in line 2095 (see Leshem et al., 2006, for details). Here the expression of all AtVAMP711–14 was analysed genes in the antisense line 2092. All genes showed decreased expression, including AtVAMP712 that was not tested previously (see Supplementary Fig. S1 at JXB online). The wild-type and mutant seedlings were grown in small pots in soil in short-day conditions (8 h light). All pots were placed in the same tray to ensure identical irrigation conditions. Plants were watered regularly for 8 weeks after which the irrigation was stopped. The pots were weighed daily to measure soil drying. No major difference in plant appearance was seen between the wild type and the transgenic lines that over-expressed the AtVAMP711 gene (data not shown). However, the antisense lines exhibited greatly increased sensitivity to drought, resulting in their rapid collapse (Fig. 1A). Similar results were also obtained in plants grown under long-day (18 h light) conditions (see Supplementary Fig. S2B at JXB online).
Fig. 1.
Drought sensitivity and water loss in wild-type and AtVAMP711 transgenic plants. (A) Increased drought sensitivity of antisense AtVAMP711 transgenic lines. Irrigation was stopped in 3-month-old plants, grown under a short-day light regime. The picture was taken after 10 d without water. The upper row shows a representative picture from a triplicate experiment (line 2091, middle pot; 2092, right pot). The lower row, shows plants that remained to be irrigated. The phenotype was also observed in other antisense line (2096, data not shown). (B) Water loss measurements in detached leaves of wild-type and transgenic plants. Rosette leaves of AtVAMP711 antisense (lines 2091 and 2092) or overexpressing (line 7+, 12+), were detached from irrigated, 2-month-old plants and placed in weighing dishes. Loss of fresh weight was monitored at the indicated times, as described in the Materials and methods. Shown is a representative triplicate experiment (n=4 leaves from four individual plants ±SE).
Measurements of water loss in detached leaves from irrigated plants showed faster water loss in the antisense lines, as compared to wild-type, while leaves from the overexpressing plants exhibited a slightly slower transpiration rate (Fig. 1B). Despite the reduced evaporation rate, no improvement was observed in drought tolerance in the overexpressing lines, suggesting that the expression level of the AtVAMP711 gene in the wild-type plants is sufficient for efficient stomatal closure during normal drought conditions.
ABA-induced stomatal closure in wild-type and antisense plants
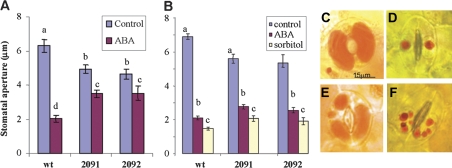
The water status in leaves is primarily controlled by the opening and closing of stomatal pores. Since ABA regulates stomatal activity (Hetherington, 2001), the stomatal response to ABA was examined in the transformed lines. Parallel examination of young mature rosette leaves from the wild-type and antisense transgenic lines showed a significant reduction in the ABA induced stomatal closure in the antisense plants (Fig. 2A). A similarly reduced response was also observed in the cotyledons of the transgenic antisense lines (data not shown). Importantly, in the absence of ABA the stomata of the antisense plants were usually less open.
Fig. 2.
Induction of stomatal closure by ABA and sorbitol. (A) Induction of stomatal closure by ABA in wild-type and antisense VAMP711 lines. Leaf discs from young rosette leaves were treated with ABA (20 μM) for 3 h. Stomatal aperture was measured by the microscope optical micrometer as described in the Materials and methods. Shown are representative triplicate experiments. The different small letters denote statistical significance after F test (P <0.05), N=60 ±SE. (B) Stomatal closure by osmotic conditions. Young rosette leaves were detached and loaded with Neutral Red as a vacuolar stain. Leaf discs were incubated in 1 M sorbitol for 1 h. ABA was applied as above in (A). Stomatal aperture was observed and measured using light microscopy as in (A). Different small letters within columns denote statistical significance after F test (P <0.05), N=60 ±SE. (C–F) Representative images from (B), above, showing control and sorbitol-treated stomata of wild-type (C, D, respectively) and antisense AtVAMP711 line 2091 plants (E, F).
To test whether the stomata of antisense plants were mechanically able to close their pores, their leaves were incubated under osmotic stress conditions equivalent to treatment with 0.5 M NaCl. 1 M sorbitol was chosen as osmoticum, rather than NaCl in order to avoid the ionic stress that is produced by salt treatment (Leshem et al., 2007). The osmotic treatment forced the closure of guard cells slightly beyond the ABA-induced response in both wild-type and antisense plants (Fig. 2B–F).
To visualize the vacuolar morphology and the endomembranes of guard cells several dyes were used that bind to lipids and/or are sensitive to acidic pH in the vacuole. Under control conditions, a single central vacuole was detected in the wild-type plants by staining with the classic vacuolar probe, Neutral Red (Diekmann et al., 1993). However, in the antisense lines a range of different sized vacuoles was observed (compare Fig. 2C with E; Table 1). The osmotic stress intensified the phenotype in the antisense plants, resulting in a multitude of vacuoles (compare Fig. 2D with F).
Table 1.
Number of vacuoles, percentage of ROS containing vacuoles, and vacuolar diameter in guard cells of wild-type and VAMP711 antisense line 2091
| Number of vacuoles |
% of Vacuoles with ROS |
Vacuolar diameter (μm) |
||||
| Wt | 2091 | Wt | 2091 | Wt | 2091 | |
| Control | 12.3 a | 18.2 b | 56.2 a | 69.2 a | 0.95 a | 1.01 a |
| ABA | 14.7 a | 25.3 b* | 54.2 a | 81.2 b* | 0.99 a | 1.23 b* |
The data are derived from experiments presented in Fig. 3B. Analysis was performed on complete Z stack images of six individual guard cells. Different letters within rows indicate statistical significance after t test at P <0.05. Asterisks within columns indicate statistical significance after F test at P <0.05.
Multiple vacuoles in the antisense guard cells were also observed after ABA treatment, by using FM4-64 dye (see Supplementary Fig. S5 at JXB online), or by MitoFluor Red 589, which also stains the membranes (see Supplementary Fig. S3 at JXB online). Interestingly, the multivacuole phenotype was also observed in the neighbouring epidermal pavement cells in the 2091 antisense line (see Supplemental Fig. S5 at JXB online).
Subcellular localization of ABA-induced ROS in guard cells
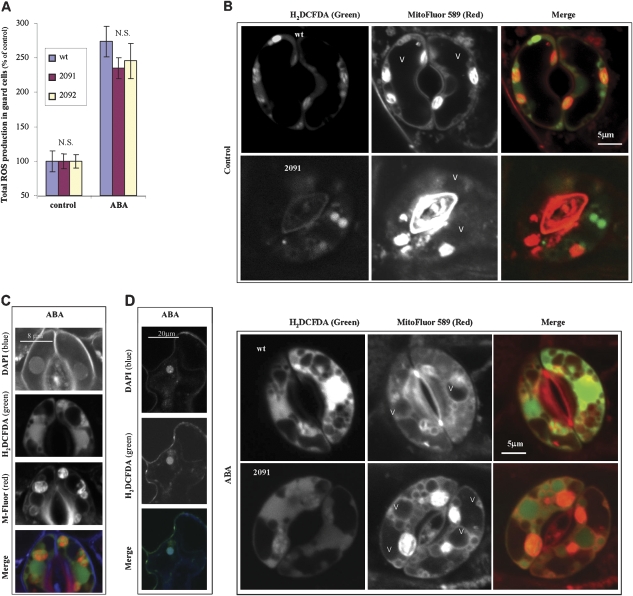
One of the earliest cellular responses to ABA is the induction of reactive oxygen species (ROS) in the guard cells (Kwak et al., 2003; Suhita et al., 2004). To see whether the reduced stomatal closure in the antisense plants was due to impaired sensing of ABA or downstream signal transduction, the accumulation ROS in the guard cells was analysed. ABA treatment caused only a small decrease in ROS accumulation in the transgenic plants, suggesting an intact signalling response to ABA in the antisense plants (Fig. 3A).
Fig. 3.
Induction and localization of intracellular ROS in guard cells after treatment with ABA. (A) Total ROS accumulation in guard cells. Leaf discs of wild-type or antisense plants were treated with ABA as in Fig. 2. ROS were detected by 2′,7′-dichlorodihydrofluorescein diacetate as described in the Materials and methods. Confocal Z-sections of each guard cell (compiled 8-10 sections, each 1 μm thick) were projected to quantify the mean intensity of H2DCFDA in a whole guard cell. Z section projections and fluorescence measurements were done using the ImagePro Plus program. Different letters within column denote statistical significance after F test (P <0.05, NS, not significant), N=15 ±SE. (B) Intracellular localization of ROS induced by ABA in wild-type and antisense AtVAMP711 guard cells. Simultaneous staining of ROS by H2DCFDA (green filter) and intracellular membranes by MitoFluor Red 589 (red filter) in guard cells of wild-type and antisense plants (line 2091). Leaf discs were treated with ABA for 2 h as in (A). Loading of dyes and fluorescence detection are described in the Materials and methods. Shown are representative confocal images of a single section in guard cells of control (top panel), or ABA-treated plants (lower panel). For a complete Z stack of each image see Supplementary Fig. S2 at JXB online. Similar results were obtained in the other VAMP711 antisense lines (data not shown). (C, D) Nuclear localization of ABA-induced ROS in wild-type guard cells and epidermal cells. Simultaneous staining of nuclei (DAPI, blue), ROS (H2DCFDA, green) (D) and intracellular membranes [MitoFluo Red 589 (C)] in guard cells (C) and epidermal pavement cells (D). Leaf discs of wild-type and antisense plants (line 2091) were treated with ABA for 2 h as in (A). Shown are representative confocal images (C, D). Control images are presented in the top panel of (B). Similar results were obtained in the antisense lines (data not shown).
To analyse the localization of ABA-induced ROS, the guard cells were double stained with (i) a ROS-specific dye, H2DCFDA (Murata et al., 2001; Leshem et al., 2006), and with (ii) two membrane dyes that are independent of membrane potential, MitoFluor™ Red 589 (Cerveny and Jensen, 2003; Kondo-Okamoto et al., 2003) and FM4-64 (Bolte et al., 2004; Leshem et al., 2006). ROS were detected in nuclei, plastids, and vacuoles in both wild-type and antisense plants (Fig. 3B–D). ABA treatment increased the number and size of vacuoles, as well as the percentage of ROS producing vacuoles in the antisense plants, but not in the wild-type plants (compare the columns in Table 1).
Interestingly, in addition to ROS accumulation in the nuclei of guard cells (Fig. 3C), the ABA treatment caused ROS accumulation in the neighbouring epidermal cells in both wild-type (Fig. 3D) and antisense plants (data not shown).
Discussion
The effect of endomembrane trafficking on stomatal functioning
ABA signalling constitutes one of the most critical pathways in plant responses to changing environmental conditions. Analysis of the different ABA signalling elements in tobacco guard cells led to the isolation of Nt-Syr, a syntaxin t-SNARE protein that is involved in stomatal closure (Leyman et al., 1999). The pairing of cognate v-SNAREs (VAMPs) with t-SNAREs (syntaxins) plays a central role in intracellular vesicle trafficking, fusion, and secretion. The role of the Arabidopsis vacuolar v-SNARE, ATVAMP71, complex in stomatal functioning, was analysed using an antisense approach to reduce the expression of the ATVAMP71 genes in transgenic plants.
It is shown that suppression of the ATVAMP71 genes impaired the control of water loss from the plant (Fig. 1). These results are in agreement with the ABA-dependent regulation of stomatal functioning by the t-SNAREs, such as SYP61 and Nt-Syr1, which are localized in the trans-Golgi network and the plasma membrane, respectively (Geelen et al., 2002; Zhu et al., 2002). The increased ATVAMP711 gene expression in the overexpressing lines, however, did not improve drought tolerance, suggesting the existence of a certain expression threshold for proper membrane fusion. Alternatively, it is possible that subtle differences in water stress (Fig. 1B) were missed by our use of simple visual observation to determine drought-induced changes (Fig. 1A).
Trafficking of large membrane material during stomatal aperture and closure is necessary to accommodate the changes in cellular volume (Gao et al., 2005; Shope and Mott, 2006). The plasma membrane surface area of guard cells has to undergo rapid adjustments in order to respond to the osmotically induced volume changes. It is noteworthy that while the Nt-Syr1 protein is located in the plasma membrane, the AtVAMP71 complex is associated with vacuoles, underscoring the close continuum between the plasma and the vacuolar membranes in stomatal functioning.
Interestingly, a similar stomatal aperture phenotype was recently described in plants with suppressed expression of the AtVSR3 gene that encodes a vacuolar sorting receptor (Avila et al., 2008). A link between vacuole fusion and stomatal movements was also described for plants mutated in SGR3/VAM3 that code for syntaxin located in the prevacuole and tonoplast (Gao et al., 2005). The AtVAM3 t-SNARE interacts with AtVTI11 v-SNARE, together forming the SNvti11 complex in the vacuole (Sanderfoot et al., 2001). The sgr3 mutants exhibited reduced vacuolar fusion and stomatal functioning (Gao et al., 2005). Taken together, these results show that flawed vacuolar fusion is associated with stomatal malfunctioning, resulting in slower and incomplete stomatal opening and closing.
The guard cells of the AtVAMP711 antisense plants displayed varying size vacuoles (Fig. 3B; Table 1). Interestingly, the impaired stomatal closure of the antisense plants was associated with increased vacuole number and size in both control and ABA treated plants (Table 1). The increased number of vacuoles is in line with the disruption of vacuolar fusion in the antisense lines; thus, multiple vesicles remain floating in the cytosol. The increase in vacuole number and size, however, did not correlate with the reduced stomatal aperture in the 2091 and 2092 plants (Fig. 2A, B), suggesting the existence of alternative pathways, independent of AtVAMP71, possibly by altered endocytotic activity, and by fusion between the vesicles rather than fusion to main vacuole. Nonetheless, despite their different subcellular structures, the sorbitol treatment showed that the antisense plants can execute a nearly complete stomatal closure (Fig. 2B), suggesting impaired signal transduction, rather than the presence of multiple large vacuoles to prevent closing. Interestingly, a rise in the Neutral Red staining was observed in the antisense lines, suggesting a possible link between vesicle trafficking and the control of intravesicular pH.
ROS localization and stomatal closure
A central question related to the observed reduction in ABA-induced stomatal closure in the antisense plants is whether the reduced expression of AtVAMP711–14 genes affected the ABA sensing or its downstream signalling. Genetic and biochemical studies of ABA-induced signal transduction in guard cells have shown that ROS induction by ABA constitutes a central step in stomatal closure (Pei et al., 2000; Kwak et al., 2003; Apel and Hirt, 2004). The insignificant reduction in ABA-induced ROS accumulation in the antisense guard cells (Fig. 3A) argue against impaired ABA sensing by reduced expression of ATVAMP71 genes. Alternatively, the impaired response to ABA may be due to differential ROS distribution within the cells. In the antisense lines, ABA significantly increased the size of vacuoles and the percentage of ROS-containing vacuoles. The localization of ROS within the differently sized vacuoles may affect downstream signalling differently.
Although ROS were traditionally considered as purely damaging agents, recent data show that ROS may also have important signalling functions in plants as well (Foyer and Noctor, 2005; Mullineaux et al., 2006; Desikan et al., 2007; Leshem et al., 2007). It should be noted that ABA mainly affected the ROS distribution within the cell, without significantly reducing the total ROS level, suggesting that the localization of ROS is important for the ROS signal transduction (Fig. 3B).
ROS imaging beyond the guard cells showed that ROS accumulation was not limited to guard cells, but spread into the neighbouring epidermal (Fig. 3D) and mesophyll cells (see Supplementary Fig. S6 at JXB online). Thus, our results suggest that ABA-induced ROS have additional far-reaching effects on whole shoot functioning. Indeed, ABA functions in multiple pathways associated with environmental stresses and even in the control of flowering (Schroeder and Kuhn, 2006).
Another far-reaching result of the ROS localization experiments is the increase in ABA-induced ROS accumulation in the nucleus (Fig. 3C, D; see Supplementary Fig. S6 at JXB online). Although the presence of ROS in guard cell nuclei and in plastids, has been suggested previously, it had not been demonstrated experimentally (Suhita et al., 2004). Our results show unambiguously that ROS accumulate in these organelles, as well as in vacuoles.
In summary, it is shown that ATVAMP71 genes play an important role in stomatal functioning during drought stress. The results underscore the multiple roles of the v-SNARE proteins in abiotic stress responses that are mediated by ABA signalling. In addition to the primary role of v-SNAREs in membrane trafficking, they also participate in ROS localization and ROS trafficking to different cellular compartments.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Expression of the AtVAMP711–14 genes in wild type and antisense plants
Supplementary Fig. S2. (A) Moisture content of pots during drought treatment; (B) drought in wild-type and antisense plants grown under long day conditions
Supplementary Fig. S3. Liposome membrane staining with MitoFluor Red 589
Supplementary Fig. 4. Complete Z stack of guard cells presented in Fig. 3B
Supplementary Fig. 5. Staining of stomata (top row) with epidermal pavement cells (bottom row) with FM 4-64
Supplementary Fig. 6. Nuclear localization of ROS in ABA treated mesophyll cells
Supplementary Material
Acknowledgments
We thank Dr Naomi Melamed-Book for assistance with confocal microcopy and J Manor and S Arkin for preparation of liposomes. We thank the Canadian Friends of the Hebrew University of Jerusalem for financial aid to YG and YK. The research was supported by a grant from the Israel Science Foundation.
Author Disclosure Statement: No competing financial interests exist.
References
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Ashtamker C, Kiss V, Sagi M, Davydov O, Fluhr R. Diverse subcellular locations of cryptogein-induced reactive oxygen species production in tobacco Bright Yellow-2 Cells. Plant Physiology. 2007;143:1817–1826. doi: 10.1104/pp.106.090902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila EL, Brown M, Pan S, Desikan R, Neill SJ, Girke T, Surpin M, Raikhel NV. Expression analysis of Arabidopsis vacuolar sorting receptor 3 reveals a putative function in guard cells. Journal of Experimental Botany. 2008;59:1149–1161. doi: 10.1093/jxb/ern025. [DOI] [PubMed] [Google Scholar]
- Bassham DC, Raikhel NV. Unique features of the plant vacuolar sorting machinery. Current Opinion in Cell Biology. 2000;12:491–495. doi: 10.1016/s0955-0674(00)00121-6. [DOI] [PubMed] [Google Scholar]
- Blatt MR. Cellular signaling and volume control in stomatal movements in plants. Annual Review of Cell Developmental Biology. 2000;16:221–241. doi: 10.1146/annurev.cellbio.16.1.221. [DOI] [PubMed] [Google Scholar]
- Bolte S, Talbot C, Boutte Y, Catrice O, Read ND, Satiat-Jeunemaitre B. FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. Journal of Microscopy. 2004;214:159–173. doi: 10.1111/j.0022-2720.2004.01348.x. [DOI] [PubMed] [Google Scholar]
- Cavalli V, Vilbois F, Corti M, Marcote MJ, Tamura K, Karin M, Arkinstall S, Gruenberg J. The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI: Rab5 complex. Molecular Cell. 2001;7:421–432. doi: 10.1016/s1097-2765(01)00189-7. [DOI] [PubMed] [Google Scholar]
- Cerveny KL, Jensen RE. The WD-repeats of Net2p interact with Dnm1p and Fis1p to regulate division of mitochondria. Molecular Biology of the Cell. 2003;14:4126–4139. doi: 10.1091/mbc.E03-02-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Hancock J, Neill S. Reactive oxygen species as signalling molecules. In: Smirnoff N, editor. Antioxidants and reactive oxygen species in plants. Oxford: Blackwell Publishing Ltd; 2007. pp. 169–196. [Google Scholar]
- Diekmann W, Hedrich R, Raschke K, Robinson DG. Osmocytosis and vacuolar fragmentation in guard-cell protoplasts: their relevance to osmotically-induced volume changes in guard-cells. Journal of Experimental Botany. 1993;44:1569–1577. [Google Scholar]
- Fan L-M, Zhao Z, Assmann SM. Guard cells: a dynamic signaling model. Current Opinion in Plant Biology. 2004;7:537–546. doi: 10.1016/j.pbi.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant, Cell and Environment. 2005;28:1056–1071. [Google Scholar]
- Gao X-Q, Li C-G, Wei P-C, Zhang X-Y, Chen J, Wang X- C. The dynamic changes of tonoplasts in guard cells are important for stomatal movement in. Vicia faba. Plant Physiology. 2005;139:1207–1216. doi: 10.1104/pp.105.067520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geelen D, Leyman B, Batoko H, Di Sansabastiano GP, Moore I, Blatt MR. The abscisic acid-related SNARE homolog NtSyr1 contributes to secretion and growth: Evidence from competition with its cytosolic domain. The Plant Cell. 2002;14:387–406. doi: 10.1105/tpc.010328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AM. Guard cell signaling. Cell. 2001;107:711–714. doi: 10.1016/s0092-8674(01)00606-7. [DOI] [PubMed] [Google Scholar]
- Jurgens G. Membrane trafficking in plants. Annual Review of Cell and Developmental Biology. 2004;20:481–504. doi: 10.1146/annurev.cellbio.20.082503.103057. [DOI] [PubMed] [Google Scholar]
- Kargul J, Gansel X, Tyrrell M, Sticher L, Blatt MR. Protein-binding partners of the tobacco syntaxin NtSyr1. FEBS Letters. 2001;508:253–258. doi: 10.1016/s0014-5793(01)03089-7. [DOI] [PubMed] [Google Scholar]
- Kondo-Okamoto N, Shaw JM, Okamoto K. Mmm1p spans both the outer and inner mitochondrial membranes and contains distinct domains for targeting and foci formation. Journal of Biological Chemistry. 2003;278:48997–49005. doi: 10.1074/jbc.M308436200. [DOI] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO Journal. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem Y, Melamed-Book N, Cagnac O, Ronen G, Nishri Y, Solomon M, Cohen G, Levine A. Suppression of Arabidopsis v-SNARE expression inhibited fusion of H2O2-containing vesicles with tonoplast and increased salt tolerance. Proceedings of the National Academy of Sciences, USA. 2006;103:18008–18013. doi: 10.1073/pnas.0604421103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem Y, Seri L, Levine A. Induction of phosphatidylinositol 3-kinase-mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance. The Plant Journal. 2007;51:185–197. doi: 10.1111/j.1365-313X.2007.03134.x. [DOI] [PubMed] [Google Scholar]
- Levine A, Belenghi B, Damari-Weisler H, Granot D. Vesicle associated membrane protein of Arabidopsis suppresses Bax-induced apoptosis in yeast downstream of oxidative burst. Journal of Biological Chemistry. 2001;276:46284–46289. doi: 10.1074/jbc.M107375200. [DOI] [PubMed] [Google Scholar]
- Leyman B, Geelen D, Quintero FJ, Blatt MR. A tobacco syntaxin with a role in hormonal control of guard cell ion channels. Science. 1999;283:537–540. doi: 10.1126/science.283.5401.537. [DOI] [PubMed] [Google Scholar]
- Mazel A, Leshem Y, Tiwari BS, Levine A. Induction of salt and osmotic stress tolerance by overexpression of an intracellular vesicle trafficking protein AtRab7 (AtRabG3e) Plant Physiology. 2004;134:118–128. doi: 10.1104/pp.103.025379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux PM, Karpinski S, Baker NR. Spatial dependence for hydrogen peroxide-directed signaling in light-stressed plants. Plant Physiology. 2006;141:346–350. doi: 10.1104/pp.106.078162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Pei ZM, Mori IC, Schroeder J. Abscisic acid activation of plasma membrane Ca2+channels in guard cells requires NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. The Plant Cell. 2001;13:2513–2523. doi: 10.1105/tpc.010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I. Nitric oxide, stomatal closure, and abiotic stress. Journal of Experimental Botany. 2008;59:165–176. doi: 10.1093/jxb/erm293. [DOI] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- Sanderfoot AA, Assaad FF, Raikhel NV. The Arabidopsis genome. An abundance of soluble N-ethylmaleimide-sensitive factor adaptor protein receptors. Plant Physiology. 2000;124:1558–1569. doi: 10.1104/pp.124.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot AA, Kovaleva V, Bassham DC, Raikhel NV. Interactions between syntaxins identify at least five SNARE complexes within the Golgi/prevacuolar system of the Arabidopsis cell. Molecular Biology of the Cell. 2001;12:3733–3743. doi: 10.1091/mbc.12.12.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D. Guard cell signal transduction. Annual Review of Plant Physiology and Plant Moleculat Biology. 2001;52:627–658. doi: 10.1146/annurev.arplant.52.1.627. [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Kuhn JM. Plant biology: abscisic acid in bloom. Nature. 2006;439:277–278. doi: 10.1038/439277a. [DOI] [PubMed] [Google Scholar]
- Shope JC, DeWald DB, Mott KA. Changes in surface area of intact guard cells are correlated with membrane internalization. Plant Physiology. 2003;133:1314–1321. doi: 10.1104/pp.103.027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shope JC, Mott KA. Membrane trafficking and osmotically induced volume changes in guard cells. Journal of Experimental Botany. 2006;57:4123–4131. doi: 10.1093/jxb/erl187. [DOI] [PubMed] [Google Scholar]
- Sirichandra C, Wasilewska A, Vlad F, Valon C, Leung J. The guard cell as a single-cell model towards understanding drought tolerance and abscisic acid action. Journal of Experimental Botany. 2009;60:1439–1463. doi: 10.1093/jxb/ern340. [DOI] [PubMed] [Google Scholar]
- Suhita D, Raghavendra AS, Kwak JM, Vavasseur A. Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiology. 2004;134:1536–1545. doi: 10.1104/pp.103.032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surpin M, Raikhel N. Traffic jams affect plant development and signal transduction. Nature Reviews Molecular and Cell Biology. 2004;5:100–109. doi: 10.1038/nrm1311. [DOI] [PubMed] [Google Scholar]
- Sutter JU, Campanoni P, Blatt MR, Paneque M. Setting SNAREs in a different wood. Traffic. 2006;7:627–638. doi: 10.1111/j.1600-0854.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- Wang X-F, Zhang D- P. Abscisic acid receptors: multiple signal-perception sites. Annals of Botany. 2008;101:311–317. doi: 10.1093/aob/mcm284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-Q, Ullah H, Jones AM, Assmann SM. G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science. 2001;292:2070–2072. doi: 10.1126/science.1059046. [DOI] [PubMed] [Google Scholar]
- Zhu JH, Gong ZZ, Zhang CQ, Song CP, Damsz B, Inan G, Koiwa H, Zhu JK, Hasegawa PM, Bressan RA. OSM1/SYP61: a syntaxin protein in Arabidopsis controls abscisic acid-mediated and non-abscisic acid-mediated responses to abiotic stress. The Plant Cell. 2002;14:3009–3028. doi: 10.1105/tpc.006981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.