Summary
Secondary lymphoid organs are strategically placed to recruit locally activated antigen presenting cells (APCs) as well as naïve, recirculating T and B cells. The structure of secondary lymphoid organs - separated B and T zones, populations of specialized stromal cells, high endothelial venules and lymphatic vessles - has also evolved to maximize encounters between APCs and lymphocytes and to facilitate the expansion and differentiation of antigen-stimulated T and B cells. Many of the general mechanisms that govern the development and organization of secondary lymphoid organs have been identified over the last decade. However, the specific cellular and molecular interactions involved in the development and organization of each secondary lymphoid organ are slightly different and probably reflect the cell types available at that time and location. Here we review the mechanisms involved in the development, organization and function of local lymphoid tissues in the respiratory tract, including Nasal Associated Lymphoid Tissue (NALT) and inducible Bronchus Associated Lymphoid Tissue (iBALT).
Keywords: Nasal Associated Lymphoid Tissue (NALT), inducible Bronchus Associated Lymphoid Tissue (iBALT), homeostatic chemokines, secondary lymphoid organs, lymphotoxin (LT), respiratory immunity
INTRODUCTION
The respiratory tract is constantly exposed to inhaled environmental antigens and pathogens. In turn, the immune system must respond appropriately to eliminate pathogens without triggering excessive inflammatory responses that may damage the lung or permanently alter normal breathing physiology. The respiratory tract uses a variety of mechanisms and tissues, including specialized lymphoid tissues in the nose and lung, to detect inhaled antigens, promote pathogen clearance and limit the magnitude of local immune responses. Two important tissues that are strategically located to confer protection against aerial pathogens are the Nasal Associated Lymphoid Tissue (NALT) and the Bronchus Associated Lymphoid Tissue (BALT). The cellular organization of NALT and BALT is similar to that of conventional lymphoid organs, such as lymph nodes (LNs) and Peyer’s patches, suggesting that the molecular mechanisms involved in maintaining the architecture of these organs are shared with other mucosal lymphoid tissues. However, the development of NALT and BALT differs substantially from that of both LNs and Peyer’s patches, suggesting that these organs may also use unique mechanisms to initiate immune responses that protect the respiratory tract.
NALT
Structure of NALT
NALT was first identified in rodent species as a paired mucosal lymphoid organ, located on the roof of the soft palate just at the entrance of the pharyngeal duct (1–3). NALT is structurally similar to and was thought to be the rodent equivalent of Waldeyer’s ring in humans, which includes the palatine tonsil. However a mucosal lymphoid tissue more similar to NALT was recently identified in the nasal passages of humans (4). Thus, NALT in rodents probably serves the same function as several mucosal lymphoid tissues in the nasal pharyngeal passages of humans.
In cross-section, NALT appears to contain a single B cell follicle surrounded by a smaller T cell zone directly underneath the nasal epithelium (5, 6). However NALT is actually composed of a series of B cell follicles that extend along the nasal passages. These follicles are surrounded and separated by interfollicular T cell areas. Like other mucosal lymphoid organs, NALT is covered by a follicle-associated epithelium (FAE) that contains specialized membranous (M) cells that sample antigens and pathogens in the lumen of the nasal passages (7–10). In fact, several pathogens, including group A Streptococcus (7, 11), use M cells to gain entry into the body. The FAE of NALT consists of ciliated cells intermixed with clusters or individual M cells and a few goblet cells (3, 8, 12). In addition, a population of subepithelial dendritic cells (DCs) is positioned directly beneath the FAE of NALT (5, 13), where they are poised to receive antigens and pathogens that are transported by M cells from the lumen of the nasal passages. As defined in other mucosal lymphoid tissues (6, 14–17), these subepithelial DCs are often in direct contact with the M cells in an M cell “pocket”. Although the DC populations in NALT have not been rigorously characterized, the T cell zone contains CD11c+ as well as CD11b+Ia+ DCs (18) and there is a population of CD11c+ subepithelial DCs (5, 13). Thus, NALT has the ability to sample antigens from the nasal passages and initiate immune responses.
The B cell follicles in the NALT of naïve mice primarily contain IgD+IgMlo follicular B cells centered on networks of follicular dendritic cells (FDCs)(5, 13, 19). However, NALT often contains germinal center B cells after intranasal immunization or infection (13, 19–22). Like other mucosal lymphoid organs, the B cells in NALT predominantly switch to IgA (23–27), which is an ideal isotype to protect mucosal surfaces due to its ability to bind the polymeric Ig receptor and to be transported across the mucosal epithelium (28, 29). The ability to promote IgA responses is ideal for intranasal vaccines against mucosal pathogens. For example, live attenuated influenza vaccines administered intranasally are highly effective, yet elicit relatively little neutralizing IgG in the serum (30–33). Instead they are thought to elicit primarily IgA responses in the upper respiratory tract.
T cells in NALT are predominantly CD4 T cells (6) and some CD8 cells, including CD8αβ+ and CD8αα+ T cells (6) as well as CD8γδ+ T cells (34). NALT also contains regulatory T cells that express toll like receptor 2 (TLR2)(35). These cells are thought to maintain tolerance to innocuous inhaled antigens, but are repressed in the presence of TLR2 agonists to allow responses to pathogens (35). Depending on the types of adjuvants used with intranasal antigens, NALT can support the differentiation of naïve CD4 T cells into Th0, Th1 or Th2 cells. For example, bacterial cell wall components administered together with cholera toxin typically induce type 2 responses (18, 25, 36), which promote IgA production in nasal passages as well as in distal mucosal sites, including the respiratory, intestinal and genito-urinary tracts (37, 38). In contrast, intranasal vaccination with Mycobacterium bovis bacillus Calmette-Guerin (rBCG) expressing foreign antigens induces a classic Th1 polarization (39).
Despite the obvious similarities of NALT with other mucosal lymphoid organs, particularly Peyer’s patches (6, 14), the high endothelial venules (HEVs) in NALT primarily express the peripheral lymph node addressin (PNAd) rather than Mucosal Addressin Cell Adhesion Molecule (MAdCAM)(40), which is expressed on HEVs in Peyer’s patches and mesenteric lymph nodes (41, 42). In fact, T cells primarily use CD62L (the receptor for PNAd) to home to NALT (40). This raises the question of whether the function of NALT can truly be extrapolated based on our knowledge of Peyer’s patches.
Another interesting issue is the excellent communication between NALT and distal mucosal sites. Several studies have shown that vaccination through the intranasal route elicits antigen-specific IgA responses in the pulmonary and vaginal tract (43, 44). This extraordinary connection between mucosal sites is due to the characteristic expression of CCR10 and α4β1 integrin on IgA-producing cells that are attracted to sites in the respiratory and genito-urinary tract by the local production of the corresponding ligands, CCL28 and VCAM-1 (45, 46). The homing properties of T and B cells in Peyer’s patches are imprinted by Peyer’s patch DCs (47, 48) and it is likely that DCs in NALT will have similar functions. This makes intranasal vaccination an ideal way to elicit immunity to mucosal pathogens.
Development of NALT
The development of most LNs and Peyer’s patches is dependent on the lymphotoxin (LT) signaling pathway (49–51). Despite this, NALT develops in the absence of LTα, LTβ and TNFα as well as the receptors TNFR1 and LTβR (5, 52). Moreover, NALT develops normally in mice treated in utero with both soluble LTβR and soluble TNFR1 (52), even though these mice do not develop any LNs or Peyer’s patches (53). These data argue that none of the ligands that bind to either the LTβR or the TNFR1, including the LTα homotrimer, the LTαβ heterotrimer, TNFα or LIGHT, are essential for NALT development.
TNFR and LTβR are coupled to the NFκB signaling pathway (54) and as a result, animals that have defects in the NFkB pathway also have defects in the development of secondary lymphoid organs (55). However, mice with mutations in the NFkB signaling pathway, including aly/aly, Nfkb1−/−, Nfkb2−/− and Relb−/− mice, all have NALT to some degree (52, 55). These data suggest that the NFkB signaling pathway is also not essential for the development of NALT, even though it is essential for the development of LNs and Peyer’s patches.
The current model for LN and Peyer’s patch development maintains that Lymphoid Tissue inducer (LTi) cells are instrumental for the initiation of lymphoid organ development (56–58). LTi cells are derived from the fetal liver and can be distinguished from other lymphocytes based on their surface phenotype (CD3−CD4+CD45+IL-7R+c-Kit+)(59). LTi cells also express the chemokine receptors, CCR7 and CXCR5 (60), which promote their homing to sites of future lymphoid organ development and lead to their interactions with local mesenchymal VCAM+ICAM+ lymph node organizer cells (61–67). LTi cells express LT and trigger the LTβR on the lymph node organizer cells. In turn, the local mesenchymal cells produce homeostatic chemokines, including CCL19, CCL21 and CXCL13, which accelerates the recruitment of LTi cells as well as other lymphocytes and promotes the formation of lymphoid architecture, including the differentiation of stromal cells and high endothelial venules (HEVs) as well as the formation of segregated T and B cell zones (59, 61, 68).
CD4+CD3− LTi cells are among the first to appear at the site of NALT organogenesis just after birth, suggesting that they are likely to be involved in the initiation of NALT formation (21, 43). However, the role of LTi cells in NALT development remains unresolved. For example, the importance of LTi cells in the development of secondary lymphoid organs is illustrated in gene-targeted mice that lack the transcription factors, retinoic acic related orphan receptor γ (RORγ) (58, 69, 70) or inhibitor of DNA binding 2 (Id2) (71). These transcription factors are necessary for the development of LTi cells and, as a result, mice lacking these factors lack LTi cells and fail to develop LNs and Peyer’s patches (69, 71). Despite this, Rorc−/− mice have NALT (5), suggesting that LTi cells may not be required for NALT development. In contrast, Id2−/− mice completely lack NALT (52). Moreover, the adoptive transfer of LTi cells from normal mice into Id2−/− mice during the first week after birth partially restores NALT formation (52), suggesting that at least some types of LTi cells are required for NALT development. Alternatively, the difference in NALT development in Rorc−/− and Id2−/− mice may reflect a requirement for additional cell types to promote NALT development. For example, Id2−/− mice lack some populations of DCs as well as NK cells (72, 73) and these cell types may play important, although currently undefined, roles in NALT development. This would be consistent with other data showing that NK cells participate in the stabilization of LNs during development (74).
The discrepancy between Rorc−/− and Id2−/− mice may also reflect diversity in the LTi population -with some LTi cells being formed independently of RORγ. Consistent with this idea, the LTi cells found in NALT express very low levels of the chemokine receptors, CCR7 and CXCR5, relative to LTi cells from Peyer’s patches (21). These receptors are normally required for the recruitment of LTi cells to sites of LN and Peyer’s patch development and as a result, mice lacking these receptors or their chemokine ligands are unable to form most LNs and Peyer’s patches (60, 75). In contrast, NALT develops in the absence of CXCL13, CCL19 and CCL21 and normal numbers of LTi cells migrate to the NALT of Cxcl13−/− or plt/plt mice just after birth ((13, 21) and unpublished data). These data demonstrate that conventional LTi cells are probably not involved in NALT development and further suggest that additional, as yet undefined chemokines attract LTi cells to the NALT anlage.
Additional evidence suggesting that LTi cells may not be required for NALT development is that NALT is formed independently of IL-7R and TRANCE receptor signaling (5). IL-7 and TRANCE provide survival and proliferation signals for LTi cells (76, 77). Interestingly, LNs and Peyer’s patches are selectively dependent on either IL-7 or TRANCE (60, 65, 67, 76). As a result, LNs fail to form in TRANCE−/− mice (76), even though Peyer’s patch development is normal. Conversely, the development of Peyer’s patches is much more dependent on IL-7 than on TRANCE (60, 65, 67). Interestingly, NALT develops in TRANCE−/− mice and even appears hyperplastic (5). This may be due to the altered bone structure in TRANCE−/− mice, which lack osteoclasts (78, 79), resulting in unusual skull morphology including alterations to structure of the nasal passages surrounding NALT. Although the development of NALT in TRANCE−/− mice may not be surprising, since these mice form Peyer’s patches, NALT also develops in IL-7R−/− mice, which lack Peyer’s patches (67). Despite its ability to form in IL-7R−/− mice, the structure of NALT is abnormal, since IL-7R−/− mice have impaired T and B cell development resulting in lymphopenia (5). Regardless, NALT development can occur in the apparent absence of LTi cells or factors that promote LTi cell survival.
One potential explanation for why NALT development may occur independently of LTi cells is that NALT forms in the postnatal period in the first couple of weeks after birth (21, 43, 52). In contrast, most LNs develop during embryogenesis between days E11 and E19 (53, 80). Since LTi cells are one of the few cells that express LT during embryogenesis, they are essential to initiate the development of LNs and Peyer’s patches. However, conventional lymphocytes also express LT and begin to circulate after birth. Thus, these cells may replace the function of LTi cells. Given that LT is not required for the development of NALT, the expression of LT on LTi cells or other cells may be relatively unimportant compared to other signals delivered by LTi cells. For example, agonistic antibodies to the LTβR do not promote LN development in Rorc−/− mice (58, 69), suggesting that LT is not the only signal provided by LTi cells. Thus, any cell type that may substitute for LTi cells in the developmental program of NALT would likely have to deliver this unknown signal as well.
LT and the maintenance of lymphoid architecture
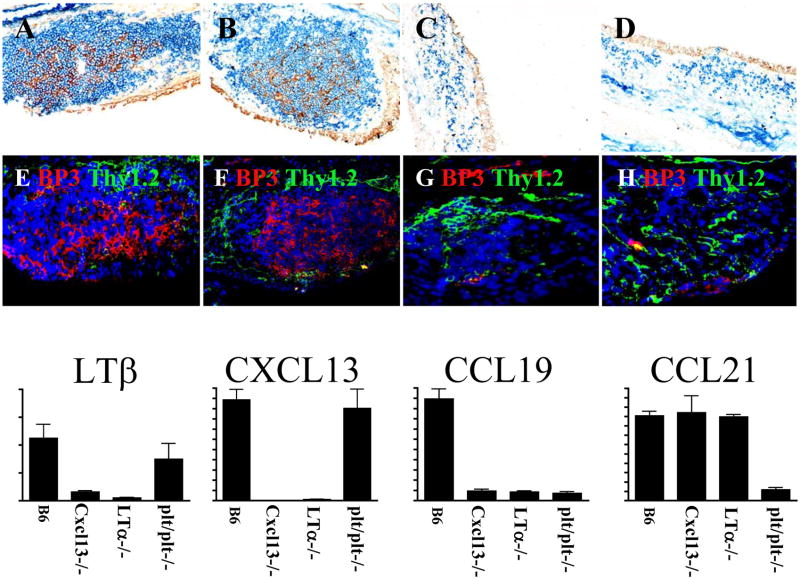
In addition to the development of lymph nodes and Peyer’s patches, LT signaling is also important for the maintenance of lymphoid architecture (81–84). For example, the spleens of Lta−/− mice are unable to form segregated B and T cell zones (81), lack marginal zones (51, 85) and cannot form germinal centers (83, 86). LT is also important for the differentiation of stromal cell populations, including FDC (87), gp38+ stromal cells and BP3+ stromal cells (88, 89). Local LT production is also important to maintain steady state number of DCs in secondary lymphoid organs (90). Furthermore, expression of peripheral lymph node addressin (PNAd) and mucosal addressin cell adhesion molecule 1 (MAdCAM-1) on blood vessels from SLO is totally dependent on LT (91, 92). Consistent with these results, the few B and T cells present in the NALT of Lta−/− mice are not segregated into separate areas (5). Moreover, the NALT of Lta−/− mice lack FDCs and BP3+ stromal cells (Figure 1)(5, 13). Moreover, the development of functional HEVs is impaired in the absence of LT. For example, LT is crucial for the induction of the high endothelial cell sulfotransferase (HEC-6ST), which is needed for the expression of PNAd on HEVs (43). LT is also required for the expression of the protein GlyCAM-1, which is modified on sialyl Lewis × by HEC-6ST to generate PNAd (43, 93). Although PNAd-expressing HEVs appear nearly normal in the NALT of Cxcl13−/− and plt/plt mice (13), they have reduced chemokine expression, particularly CCL21, and are somewhat smaller than those in normal mice, possibly due to reduced trafficking of cells. Thus, even though NALT is formed in the absence of LT, LT is still necessary for the maintenance of NALT architecture.
Fig 1. LT expression in NALT is important for stromal cell differentiation and HC production.
FDC differentiation is compromised in LT−/− (C) and Cxcl13 −/− mice (D) (CD21: Brown, B220: Blue). BP3+ stromal cells do not develop in the absence of LT (G) and CXCL13 (H). FDC- and BP3 stromal cell networks are only detected in NALT from B6- (A, E) and plt/plt mice (B, F). mRNA expression measured by quantitative PCR. CXCL13 is important for LT expression in NALT and LT is required for production of CXCL13 and CCL19.
For the most part, the structural defects in the NALT of Lta−/− mice are not developmental and can be repaired by the reconstitution of Lta−/− mice with normal bone marrow (BM)(5). For example, BM reconstitution restores the structure of B cell follicles, FDCs, PNAd+ HEVs and even subepithelial DCs (5). This points out an important difference between NALT and other lymphoid organs, since Peyer’s patches and LNs are never restored in Lta−/− mice, even after BM transfer (not shown and (53, 86)). BM reconstition also restores the function of NALT (5). In fact, antigen-specific CD8 T cells and germinal center B cells are formed at normal frequencies and antigen-specific IgM and IgG are produced at normal levels after intranasal immunization or infection of Lta−/− mice that have been reconstituted with normal BM.
Homeostatic chemokines and the structure of NALT
The architecture of most secondary lymphoid organs is maintained by homeostatic chemokines, including CCL19, CCL21 and CXCL13, which are constitutively expressed in secondary lymphoid organs and direct the recruitment and placement of lymphocytes and DCs (75, 94–97). These chemokines are also expressed in the NALT and their expression pattern fits with a role for these molecules in organizing the structure of NALT (13). For example, CXCL13 is located in the center of the B cell follicle where it can recruit B cells, CCL21 is located around the follicle in the T cell area as well as on HEVs, where it can attract recirculating B and T cells and CCL20 is expressed in the dome epithelium, where it can attract subepithelial DCs (13). As in other lymphoid organs, LT controls the expression of these homeostatic chemokines (13). In fact, the structure of NALT in Cxcl13−/− × plt/plt mice (which lack CXCL13, CCL19 and CCL21) appears very similar to the structure of NALT in Lta−/− mice, with very few B or T cells and no FDCs or germinal centers (Figure 1)(13).
In fact, there is a positive feedback relationship between LT expression on lymphocytes and homeostatic chemokine expression, particularly CXCL13 expression, by stromal cells (75). Consistent with this, Lta−/− and Cxcl13−/− mice lack well-organized CD21+ and BP3+ stromal cell networks (13), suggesting that interactions between LT expressing cells and CXCL13 producing cells is crucial for differentiation of stromal cell populations and the maintenance of well-populated B cell zones in the NALT. For example, the overall structure of NALT is similarly compromised in the absence of either LTα or CXCL13 (13). In addition, both LTα and CXCL13 are necessary for the differentiation of FDCs as well as BP3+ and ERTR7+ stromal cells in NALT (13). This is consistent with the idea that CXCL13 is necessary for primary follicle formation and FDC differentiation under steady state conditions in the spleen as well as the idea that CXCL13 is involved in a positive feedback loop with LTαβ and TNFα (75, 89). In fact, the stromal cell defects in the NALT of Lta−/− and Cxcl13−/− mice are most likely due to the interruption of this loop and the failure of LTαβ and TNFα to promote the differentiation of stromal cell precursors. Thus, the structural defects in the NALT of Lta−/− mice and Cxcl13−/− appear to be linked by the co-dependent expression of CXCL13 and LTαβ.
Although the expression of CCL19 and to a lesser extent, CCL21 is also controlled by LT signaling in NALT (13), the loss of these chemokines in plt/plt mice has less dramatic consequences for the structure of NALT than does the loss of CXCL13 in Cxcl13−/− mice (13). In part, this is because the large central B cell follicle of NALT including the FDC network, is minimally affected by the loss of CCL19/CCL21. However, T cell recruitment is substantially impaired in the NALT of plt/plt mice (Figure 1)(13, 21).
CCL20 should also be considered a homeostatic chemokine, as it is constitutively expressed in the FAE of mucosal lymphoid organs (15, 17, 98). In Peyer’s patches, CCL20 is thought to control the recruitment and placement of subepithelial DCs (15). This is a critical function, as subepithelial DCs are believed to process antigen that is transported across the epithelium by M cells (15, 99, 100). The current model is that M cells in Peyer’s patches express CCL20 and possibly other chemokines, like CXCL16 and CCL9 (15, 17, 101, 102) and that these chemokines attract CCR6+ sub-populations of DCs and lymphocytes to the area below the dome epithelium, where they are positioned to receive antigen from the M cells and to interact with naïve lymphocytes entering Peyer’s patches via HEVs. Cells in the FAE of NALT also express CCL20 (13), although it is not known whether they also express CXCL16 and CCL9. The expression of CCL20 in NALT, like the expression of CXCL13, CCL19 and CCL21, is also dependent on LTα(13). Although the role of CCL20 in NALT development and recruiting LTi cells has not been directly tested, Epithelial cells overlaying the NALT of Lta−/− mice do not express CCL20 and DCs are not recruited to the subepithelial dome region of Lta−/− NALT.
The relationship between LT and homeostatic chemokine expression was clarified by the demonstration that LTβR uses both the canonical and an alternative NFκB signaling pathway (54). The canonical NFκb pathway culminates with the translocation of p50/relA or p52/RelA and leads to the expression of inflammatory chemokines like macrophage induced protein 1 (MIP 1β) and macrophage induced protein 2 (MIP2)(54, 55). In contrast, the alternative NFκB signaling cascade goes through NFκB inducing kinase (NIK) and leads to the translocation of p52/RelB, which promotes the production of the homeostatic chemokines, CCL19, CCL21 and CXCL13 (54). Alymphoplasia or aly/aly mice have a mutation in NIK, which leads to altered stromal cell differentiation and abnormal production of homeostatic chemokines and ultimately to the failure of LN and Peyer’s patch development (103).
Chemokines and the generation of local immune responses in NALT
The function of homeostatic chemokines in fully developed secondary lymphoid organs is to promote immune responses by recruiting naïve B and T cells from the blood and facilitating their interactions with activated DCs and with each other (94). In the T cell zone, CCL19 and CCL21 bring together mature DCs and naïve T cells to initiate T cell priming (94, 104). In the B cell area, CXCL13 coordinates the interaction of B cells and T follicular helper (TFH) cells and promotes B cell differentiation that culminates with the production of antigen-specific, antibody producing plasma cells (95, 97, 105–108).
The function of NALT is similarly dependent on chemokines. For example, LTα and CXCL13 are both required for the formation of germinal centers in NALT after influenza infection (5, 13, 21). Although a lack of germinal centers would normally lead to impaired B cell responses, isotype switching to IgA does occur in the NALT of both Lta−/− and Cxcl13−/− mice after influenza infection, albeit at reduced levels (13). Despite this reduction, IgA-secreting influenza-specific plasma cells are observed in the nasal mucosa of Cxcl13−/− and Lta−/− mice at normal or above normal numbers (13). These results are surprising, given that switching to IgA is thought to be impaired in Lta−/− mice (109–111). However, the production of influenza-specific IgA in serum is normal in Lta−/− mice (5), suggest that Lta−/− mice actually make more IgA than normal mice in the NALT and nasal mucosa after influenza infection. Thus, not all IgA responses are dependent on LTα. Consistent with this, the administration of LTβR-Ig reduced, but did not prevent, IgA responses to tetanus toxin in the intestine (112), demonstrating that LT signaling, FDCs and germinal centers are not required for IgA switching. Interestingly, previous studies suggested that switching to IgA may occur via unusual mechanisms, such as the direct development of IgA cells from bone marrow (113, 114), the differentiation of IgA cells outside of lymphoid organs (115–117) and the production of IgA independently of cognate interactions with T cells (118). However, other studies showed that IgA switching occurs only in lymphoid tissues, such as NALT, Peyer’s patches and isolated lymphoid follicles (119). Thus, it appears that functional lymphoid tissues are important to facilitate switching to IgA, however LTα is not essential for this function in NALT.
LTα is also important for CD8 T cell responses in NALT, as CD8 T cell responses to influenza are delayed in the NALT of Lta−/− mice (13, 120). In part, this is due to the poor expression of CCL21 and CCL19 in the NALT of Lta−/− mice, since CD8 T cell responses are also impaired in the NALT of plt/plt mice (13). However, the delayed T cell responses in Lta−/− mice are probably due to more than just the impaired expression of CCR7 ligands. In fact, it is likely that the impaired expression of CCL20 in the dome epithelium of Lta−/− NALT disrupts the recruitment of sub-epithelial DCs to the follicular dome of the NALT (13). Therefore, the process of antigen acquisition from the nasal lumen is likely to be impaired in the NALT of Lta−/− mice as a consequence of a reduced CCL20 production by epithelial cells. In addition, it was recently demonstrated that LTα is required for homeostatic proliferation of DCs in the spleen (90). Thus, the delayed activation of influenza specific CD8 T cells in the NALT of Lta−/− mice may result from a combination of deficiencies in DC activation and antigen retrieval from the dome epithelium of the NALT.
BALT
Structure of BALT
BALT was first defined by Bienenstock in the lungs of pigs and rabbits as a mucosal lymphoid tissue located just beneath the bronchial epithelium and adjacent to a pulmonary artery (121–125). Like NALT, BALT is structurally similar to Peyer’s patches, with prominent B cell follicles and interfollicular T cell areas underneath a specialized FAE (121–125). BALT also has a population of subepithelial DCs beneath the FAE that can be identified with antibodies against S-100 and MHC class II antigens (126). T cells are also found in this same environment (126). The strategic positioning of these cells in the dome structure suggests that the subepithelial DCs are ready to receive antigens obtained by M cells from the luminal side of the epithelium and to present these antigens to local T and B cells (126). Unlike Peyer’s patches, the HEVs of BALT express PNAd and not MAdCAM (127, 128), suggesting that NALT and BALT are more closely related to each other than to lymphoid tissues of the intestine. Unike HEVs in conventional lymphoid organs, HEVs in BALT also express VCAM-1 (128). In fact, lymphocytes use L-selectin/PNAd, α4β1 integrin/VCAM-1 and LFA-1 to enter BALT (128). The use of this combination of receptors is unique among secondary lymphoid tissues and may recruit specific populations of effector or memory cells to the BALT. Thus, BALT probably functions as a mucosal lymphoid tissue in the upper bronchi of the lungs that primes and expands T cells that are specialized to protect the lung.
BALT can be detected in several species, such as rabbits, rats, pigs and guinea pigs, under normal physiological conditions and without antigenic stimulation (129). However, it is absent in most healthy cats, mice and humans (130). Moreover, BALT is not found in the lungs of pigs living in pathogen free conditions or humans who died from non-pulmonary causes (130). In fact, the sporadic appearance of BALT-like areas in humans and mice has led some investigators to doubt whether iBALT is an important secondary lymphoid tissue in these species (131). Despite this concern, it is clear that tissues structurally similar to BALT are formed in the lungs of mice and humans in response to inflammation and infection (127, 130, 132–134). We have termed these tissues inducible BALT or iBALT (127). Unlike classically defined BALT, which develops in during embryogenesis and is generated and maintained in the absence of antigen (121–124), iBALT appears to be formed only after infection or inflammation in both mice and humans (127, 130, 132, 135, 136) and is found in numerous places along the upper and lower bronchi and even in the interstitial areas of the lung (137). In addition, BALT is detected upon autopsy of human fetal and infant lungs, mainly in episodes of amnionitis or intrauterine pneumonia without any signs of infection (138). Moreover, transgenic mice that express various cytokines in the lung develop iBALT (139, 140), as do mice and humans prone to autoimmune diseases (128, 141) or humans with recurrent or chronic respiratory infections (126, 130, 141, 142). These areas of iBALT are consistent with Sminia’s description of multiple Bronchus Associated Lymphoid Units (BALUs) (125). The areas of iBALT are also similar to tertiary lymphoid tissues detected at peripheral locations in several autoimmune and chronic inflammatory diseases (143) (144) (145) (146) (147) (148, 149). A common component of each of these models is chronic inflammation, which is implicated in the development of ectopic or tertiary lymphoid areas in other sites, such as rheumatoid joints (150–153). Interestingly, the most well organized iBALT (with well-defined B cell follicles, T cell areas, FDCs and HEVs) is observed in mice in which respiratory inflammation is present from birth (51, 128, 139, 140). These data suggest the possibility that the development of iBALT, like the development of other lymphoid organs, may be preferentially initiated during a specific developmental window during the neonatal period.
One difference between classically defined BALT and iBALT is the presence of the FAE. Classic BALT is a true mucosal lymphoid organ underneath a specialized FAE that contains M cells for the transport of antigen (154–156). In contrast, most examples of iBALT do not have a clearly visible FAE and in many cases they are in perivascular areas or parenchymal areas of the lung with no discernable access to antigens from the lumen of the respiratory tract (127, 141). Despite the apparent lack of access to antigen, most iBALT follicles are surrounded by lymphatic vessels (141). However, it is unclear whether these are afferent or efferent lymphatics. Afferent lymphatics could bring antigen from distal parts of the lung to the iBALT, while efferent lymphatics would connect iBALT with the draining lymph node. Transport of antigen and DCs through afferent lymphatics can have a positive impact in T- and B cell priming in the LN. Also, the presence of efferent lymphatics can be associated to the exclusive stimulation of lymphocytes in tissue domains that produce self-antigens (141). This is an important issue that needs to be resolved.
Although most reports of iBALT in humans have associated the presence of this tissue in the lungs with local pathology (126, 130, 135, 141, 157–159), it is clear that iBALT also participates in lymphocyte priming and differentiation, formation of germinal centers, B cell isotype switching, recruitment of effector cells during infection and maintenance of local memory cells in mice (127, 136, 137, 160–166). In these cases, iBALT appears to be beneficial and can even reduce the immunopathology associated with influenza infection (127, 137). In contrast, iBALT hypertrophy in humans is often associated with persistent viral infections, such as HIV, EBV or KSHV (135, 157), and is also associated with autoimmune diseases, such as Systemic Lupus Erythematosus, Rheumatoid Arthritis and Sjogren Syndrome (126, 167). In addition, iBALT is associated with various forms of interstitial lung disease, such as chronic obstructive pulmonary disease (157, 168), which leads to decreased lung function. In these cases, it appears that the formation of iBALT and the recruitment of lymphocytes to the lung result in pathology (141, 157, 168). Thus, iBALT functions like many other secondary lymphoid organs and promotes beneficial immune responses that help clear infection, but also serves to amplify pathologic immune responses against autoantigens or chronic infections.
Development of iBALT
It is becoming increasingly clear that iBALT in mice and humans is not a constitutive secondary lymphoid organ, but is an ectopic or tertiary lymphoid tissue that is formed in response to antigen or inflammation (127, 130, 138). The development of tertiary lymphoid tissues is termed lymphoid neogenesis (150, 153) and is thought to use mechanisms similar to those used during the programmed development of secondary lymphoid organs. For example, the ectopic expression of LT or homeostatic chemokines in the pancreas leads to the development of a fully formed tertiary lymphoid tissue in the pancreas (153, 169). Given the central importance of LT in lymphoid organ development it seems that LT should be involved in iBALT development as well. However, this issue is not been fully resolved. For example, Lta−/− mice form large iBALT-like areas in the lungs (51, 170). These iBALT areas are not fully developed as they lack segregated B and T cell zones, FDCs and PNAd+ HEVs (51, 127). However, they do seem capable of promoting DC/T cell interactions and priming naïve T cells (171, 172). Lta−/− mice are also capable of generating primary T and B cell responses to influenza and other respiratory viruses (120, 127, 173) and at least some of the response appears to be initiated directly in the lung. Interestingly the B and T cell responses are delayed in Lta−/− mice (120), suggesting that although the iBALT areas in these mice are functional, they are not particularly efficient. This poor efficiency probably reflects the poor organization of iBALT in Lta−/− mice. However, Lta−/− mice do eventually accumulate large numbers of B cells in the lung, many of which had germinal center characteristics (120). Again, this is surprising, as germinal centers are not formed in the spleens of Lta−/− mice (51, 84, 86, 120). Like it does for NALT, the reconstitution of Lta−/− mice with normal BM restores the structure of iBALT - including segregated B and T cell zones, networks of FDCs and PNAd+ HEVs (127). These areas of organized iBALT are much more efficient at inducing immune responses to influenza than the disorganized lymphoid accumulations in Lta−/− mice. In fact, splenectomized, BM-reconstituted Lta−/− mice generated B and T cell responses to influenza with normal kinetics (127). These data argue that although LT is clearly involved in the organization and efficient function of iBALT, it is not absolutely required for the development of iBALT.
Areas of iBALT that are formed in response to influenza infection contain large B cell zones with a central FDC networks and germinal center B cells (127). Consistent with its ability to organize follicular structures, CXCL13 is found in the center of B cell follicle in a reticular pattern that appears to co-localize with the FDC network (127). CCL21 is detected in the T cell zone in a reticular pattern and more strongly on PNAd+ HEVs (127). The spatial location of chemokine expression in iBALT suggests that these molecules are central for the organization of iBALT. The role of homeostatic chemokines in the lung is not restricted to simply organizing iBALT. CCL21 expression in the lung helps to recruit activated T cells to sites of inflammation (174) and CCL21 expression on lymphatics helps to retrieve CCR7+ T cells from the lung and return them to the draining LNs (175, 176). Thus, homeostatic chemokine expression in the lung serves numerous functions.
Based on the paradigm for conventional lymphoid organs, the expression of these chemokines should be dependent on LT. This would make sense, since iBALT is disorganized in Lta−/− mice (51, 127). However, CXCL13, CCL19 and CCL21 are all inducibly expressed in the lung during influenza infection in the complete absence of LTα and TNF (127). This suggests that there may be other TNF family members, such as LIGHT, that can compensate for the absence of LT and TNFα and induce the expression of homeostatic chemokines (177). Alternatively, there may be very different mechanisms that control the expression of homeostatic chemokines under conditions of acute inflammation. If this is the case, it may explain how the development of ectopic lymphoid follicles is initiated. An initial infection or inflammation could trigger the acute expression of CXCL13, CCL21 and CCL19, which would lead to an influx of lymphocytes. The lymphocytes would then express LT and promote the differentiation of local stromal cell precursors into mature lymphoid stromal cells. Chemokine expression by these stromal cells would then be maintained under homeostatic conditions by LT.
Clues to the type of inflammation that is required for iBALT formation can be found in the literature. For example, mice deficient in the NAD(P)H/NRH:quinone oxidoreductases, NQO1 and NQO2, develop iBALT (178), suggesting that oxidative stress may be involved in iBALT formation. Conversely, mice lacking iNOS do not develop iBALT (179). The effects of oxidative stress could be direct, via activation of local mesenchymal cells, or indirect via the production of cytokines like TNF, IL-6, and IL-1β (178). Supporting a role for cytokines is the recent observation that Tregs are instrumental in the negative regulation of iBALT (180). However, it is not clear whether Tregs are acting to suppress other T cells from making cytokines or whether Tregs are acting on APCs, such as macrophages, and suppressing the production of inflammatory mediators.
One open question is whether LTi cells are involved in the development of ectopic lymphoid follicles, such as iBALT. Given that CXCL13 is expressed in the lungs just a few days after influenza infection (127) and that inflamed lungs also express VCAM (128), it seems likely that LTi cells expressing CXCR5 and α4β1 integrin could home to sites of inflammation in the lung and be involved in the formation of iBALT. However, it is not clear whether LTi cells exist in adults or if they do exist, whether they are functionally similar to those in the developing embryo (181, 182). Moreover, LTi cells are not required for the development of ectopic lymphoid follicles in an experimental model of thyroiditis (183, 184). In this model, interactions between infiltrating DCs and activated CD4 T cells trigger formation of ectopic follicles (183). This seems reasonable because dendritic cells express the LTβR and can express CCL19, setting up the conditions for the attraction for populations of CCR7+ B and T cells that can continue with the compartmentalization of the ectopic follicle. Again, LT may not be the primary inducer of iBALT, since some aspects of iBALT can be found in Lta−/− mice (51). Thus, the cellular and molecular events that initiate iBALT formation are still incompletely understood.
Once iBALT is generated during acute inflammation or infection, the structure is maintained for several months. For example, iBALT is found in murine lungs at least 90 days after influenza infection (137). However, iBALT progressively becomes smaller over time in the absence of continued stimulation (137). However, it is not clear if iBALT ever completely resolves since a subsequent infection seems to rapidly replenish the areas of iBALT (unpublished data). Moreover, iBALT probably serves as a niche to maintain and reactivate antigen specific memory cells that recirculate through the lung (137). Thus, there is a dynamic process of recirculation of cells in and out of iBALT that probably helps to maintain local immune surveillance.
The role of iBALT in lung pathology
Data from our laboratory show that B and T cell immune responses to influenza can be initiated directly in iBALT without involvement of conventional lymphoid organs(127). This suggests that iBALT can serve a protective function. In fact, mice lacking conventional lymphopid organs survive higher doses of influenza significantly better than normal mice(127). These data are very surprising and suggest that priming in iBALT may elicit a different type of immune response than priming in draining LN. This would be consistent with other data showing that T cells primed in situ in the lung are skewed towards a Th2 response (171, 172). Although Th2 responses are not normally associated with anti-viral immunity, the lymphocytes responding to influenza in iBALT may produce anti-inflammatory cytokines or accelerate B cell responses that promote viral clearance without excess pathology. This would fit with the idea that iBALT is a mucosal tissue that generates immune responses specialized to protect the lung. These possibilities are currently being investigated.
IBALT is also formed in response to other infectious pulmonary diseases (185) and is prominent in tuberculosis (186). Pulmonary infection with tuberculosis leads to the formation of granulomas, which consist predominantly of lymphocytes and macrophages. Granulomas are highly organized and have characteristics of iBALT, including B cell follicles, HEVs, FDCs and expression of the homeostatic chemokines CXCL13 and CCL19 (186). The CCR7 ligands, CCL19 and CCL21, contribute to the formation and effectiveness of tuberculosis granulomas and iBALT (186). Again, the proper formation of iBALT functions protectively in response to tuberculosis.
On the other hand, iBALT in humans is most often associated with pulmonary pathology. For example, iBALT has been identified in humans with a variety of interstitial lung diseases (187), including hypersensitivity pneumonitis (159, 188), desquamative pneumonia (135), nonspecific interstitial pneumonia, bronchioalveolar carcinoma and particularly in patients with autoimmune diseases such as rheumatoid arthritis and Sjogren syndrome (141). Interestingly, iBALT was never observed in patients with idiopathic pulmonary fibrosis (141), suggesting that particular types of disease preferentially lead to the formation of iBALT. Given the extensive formation of iBALT in patients with tuberculosis or autoimmune disease (141, 186), we believe that prolonged exposure to antigen from pathogens or even self-antigens in the lung triggers iBALT formation (132). In patients with rheumatoid arthritis and Sjogren syndrome, there is a general association between the progression of autoimmunity and the complexity in the organization of iBALT (141). Again, this suggests that antigen may be the driving force behind the maintenance of iBALT.
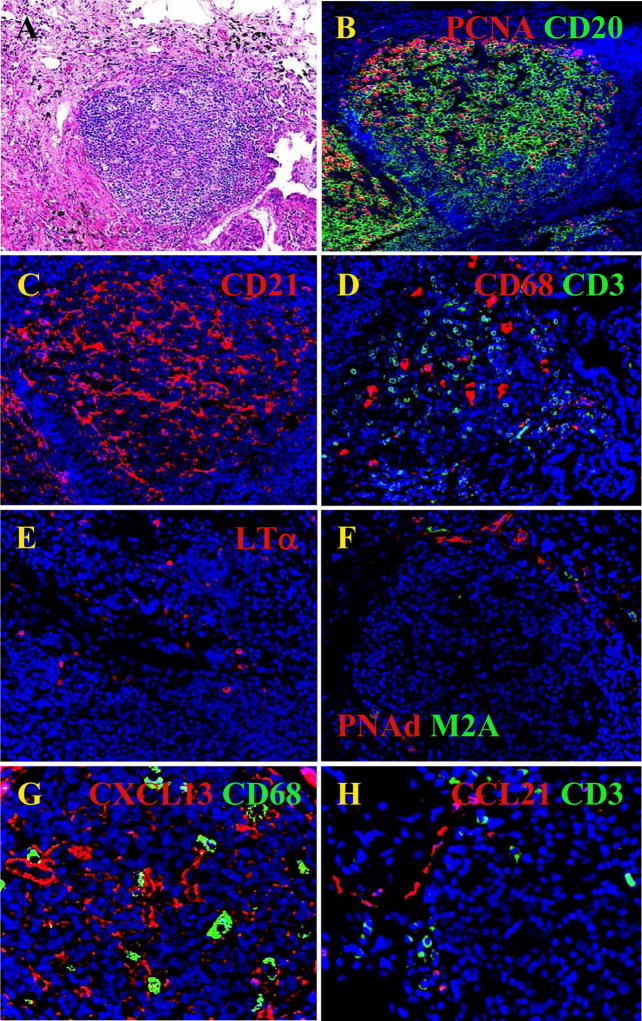
The structure of iBALT in patients with autoimmune diseases resembles iBALT associated with infectious disease - with separated B and T cell zones, FDCs, germinal centers, HEVs and lymphatics (Figure 2)(126, 141). Interestingly, LT-expressing T cells can be found in the T cell zone, suggesting that activated T cells may contribute to the organization of T cell zone in BALT by triggering the LTβR on stromal cells and inducing production of CCL21 and CCL19. LT-expressing T cells may also promote the differentiation of HEVs (91). LT is also expressed on B cells and is crucial for lymphangiogenesis in reactive LNs (189), making the traffic of antigen and DCs into the LN more efficient. Thus, it is likely that antigen-activated B and T cells maintain the structure of iBALT via LT expression.
Fig. 2. Cellular and molecular features of human iBALT.
A) H&E stain of a lymphoid follicle like structure in a lung biopsy from a RA patient. B) Proliferating B cells (PCNA/CD20). C) FDC network (CD21). D) Parafollicular area (PFA) containing APCs (CD68) and T cells (CD3). E) LT producing cells are found in PFA. F) Specialized vasculature: HEVs (PNAd) and lymphatics (M2A) develops in PFA. G) FDC like cells are producing CXCL13 in the middle of a germinal center (CXCL13/CD68). H) CCL21 is produced by vessels outside the B cell follicle and close to T cell zone (CCL21/CD3).
In addition to supporting the structure of iBALT, the activated B and T cells in the lungs of autoimmune patients may also contribute to disease. For example, the lungs of rheumatoid arthritis patients contain increased levels of peptidyl arginine deiminase 2 (PADI2)(141), an enzyme involved in the postranslational transformation of arginine into citrulline (190, 191). Since citrullinated proteins are a primary autoantigen in rheumatoid arthritis (190, 192), this enzyme probably contributes to pathology. In fact, citrullinated proteins are easily detected in the lungs of rheumatoid arthritis patients surrounding areas of iBALT (141). Moreover, plasma cells producing autoantibodies against citrullinated fibrinogen are also found surrounding areas of iBALT in the lungs of rheumatoid arthritis patients (141). Finally, there is a positive correlation between iBALT complexity and the production of anti-cyclic citrullinated peptide antibodies in the bronchial alveolar lavage (141), suggesting that autoantibodies are produced locally in the lung. Since immune complexes lead to the release of inflammatory mediators that can activate fibroblasts, the local production of autoantibodies is likely to lead to collagen deposition and pulmonary fibrosis. Thus, iBALT may be contributing to pulmonary pathology in patients with autoimmune disease.
Autoimmunity is not the only disease associated with the formation of iBALT. For example, iBALT formation is also associated with the most severe stages of chronic obstructive pulmonary disease (168). Moreover, iBALT if formed in the lungs of heavy smokers (133, 157, 193). Thus, some types of pulmonary inflammation, particularly inflammation associated with oxidative stress (178), lead to iBALT formation and pathology in the absence of easily identified antigens or autoantigens.
SUMMARY AND CONCLUSIONS
Like other lymphoid organs, NALT and iBALT have evolved to promote the interactions of lymphocytes and APCs and to efficiently elicit antigen-specific B and T cell responses to infectious agents. In this regard the roles of lymphotoxin and the homeostatic chemokines play similar, if not identical roles in all secondary lymphoid organs. However, the details of the development and function of NALT and iBALT differ from those of conventional lymphoid organs. This probably reflects the specialized requirements of the respiratory tract to promote immunity without excessive inflammation. However, in pathological conditions like autoimmunity, LT-induced homeostatic chemokines participate in the organization of iBALT and have a detrimental impact in lung physiology, promoting formation of local cellular niches with favorable conditions for the expansion and activation of autoreactive and bystander lymphocytes and attraction of additional cell populations that can contribute to the induction of severe lung pathology.
Given the positive impact of iBALT on immunity to infectious agents and the negative impact of iBALT on respiratory function in chronic pulmonary diseases, it is important to discover the mechanisms that regulate the formation and function of these tissues. However, many important questions about iBALT remain open. For example: do adult LTi cells participate in iBALT formation? What molecular pathways initiate homeostatic chemokine expression in the lung and how do these mechanims impact iBALT formation? Finally, can we manipulate the mechanisms of iBALT formation and function to promote immunity to respiratory infections and prevent pulmonary disease associated with autoimmunity? The answers to these questions will require research in both murine and human systems and should allow us to intervene in a variety of pulmonary diseases.
Acknowledgments
This work was funded by the Trudeau Institute, the Sandler Program for Asthma Research and NIH grant HL-69409.
References
- 1.Heritage PL, Brook MA, Underdown BJ, McDermott MR. Intranasal immunization with polymer-grafted microparticles activates the nasal-associated lymphoid tissue and draining lymph nodes. Immunology. 1998;93:249–256. doi: 10.1046/j.1365-2567.1998.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuper CF, Koornstra PJ, Hameleers DM, Biewenga J, Spit BJ, Duijvestijn AM, van Breda Vriesman PJ, Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13:219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 3.Spit BJ, Hendriksen EG, Bruijntjes JP, Kuper CF. Nasal lymphoid tissue in the rat. Cell Tissue Res. 1989;255:193–198. doi: 10.1007/BF00229081. [DOI] [PubMed] [Google Scholar]
- 4.Debertin AS, Tschernig T, Tonjes H, Kleemann WJ, Troger HD, Pabst R. Nasal-associated lymphoid tissue (NALT): frequency and localization in young children. Clin Exp Immunol. 2003;134:503–507. doi: 10.1111/j.1365-2249.2003.02311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harmsen A, Kusser K, Hartson L, Tighe M, Sunshine MJ, Sedgwick JD, Choi Y, Littman DR, Randall TD. Cutting edge: organogenesis of nasal-associated lymphoid tissue (NALT) occurs independently of lymphotoxin-alpha (LT alpha) and retinoic acid receptor-related orphan receptor-gamma, but the organization of NALT is LT alpha dependent. J Immunol. 2002;168:986–990. doi: 10.4049/jimmunol.168.3.986. [DOI] [PubMed] [Google Scholar]
- 6.Heritage PL, Underdown BJ, Arsenault AL, Snider DP, McDermott MR. Comparison of murine nasal-associated lymphoid tissue and Peyer’s patches. Am J Respir Crit Care Med. 1997;156:1256–1262. doi: 10.1164/ajrccm.156.4.97-03017. [DOI] [PubMed] [Google Scholar]
- 7.Park HS, Francis KP, Yu J, Cleary PP. Membranous cells in nasal-associated lymphoid tissue: a portal of entry for the respiratory mucosal pathogen group A streptococcus. J Immunol. 2003;171:2532–2537. doi: 10.4049/jimmunol.171.5.2532. [DOI] [PubMed] [Google Scholar]
- 8.Jeong KI, Suzuki H, Nakayama H, Doi K. Ultrastructural study on the follicle-associated epithelium of nasal-associated lymphoid tissue in specific pathogen-free (SPF) and conventional environment-adapted (SPF-CV) rats. J Anat. 2000;196 (Pt 3):443–451. doi: 10.1046/j.1469-7580.2000.19630443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giannasca PJ, Boden JA, Monath TP. Targeted delivery of antigen to hamster nasal lymphoid tissue with M-cell-directed lectins. Infect Immun. 1997;65:4288–4298. doi: 10.1128/iai.65.10.4288-4298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimura Y. Evidence of M cells as portals of entry for antigens in the nasopharyngeal lymphoid tissue of humans. Virchows Arch. 2000;436:560–566. doi: 10.1007/s004289900177. [DOI] [PubMed] [Google Scholar]
- 11.Park HS, Costalonga M, Reinhardt RL, Dombek PE, Jenkins MK, Cleary PP. Primary induction of CD4 T cell responses in nasal associated lymphoid tissue during group A streptococcal infection. Eur J Immunol. 2004;34:2843–2853. doi: 10.1002/eji.200425242. [DOI] [PubMed] [Google Scholar]
- 12.Jeong KI, Uetsuka K, Nakayama H, Doi K. Glycoconjugate expression in follicle-associated epithelium (FAE) covering the nasal-associated lymphoid tissue (NALT) in specific pathogen-free and conventional rats. Exp Anim. 1999;48:23–29. doi: 10.1538/expanim.48.23. [DOI] [PubMed] [Google Scholar]
- 13.Rangel-Moreno J, Moyron-Quiroz J, Kusser K, Hartson L, Nakano H, Randall TD. Role of CXC chemokine ligand 13, CC chemokine ligand (CCL) 19, and CCL21 in the organization and function of nasal-associated lymphoid tissue. J Immunol. 2005;175:4904–4913. doi: 10.4049/jimmunol.175.8.4904. [DOI] [PubMed] [Google Scholar]
- 14.Kiyono H, Fukuyama S. NALT- versus Peyer’s-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwasaki A, Kelsall BL. Localization of distinct Peyer’s patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J Exp Med. 2000;191:1381–1394. doi: 10.1084/jem.191.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park MK, Amichay D, Love P, Wick E, Liao F, Grinberg A, Rabin RL, Zhang HH, Gebeyehu S, Wright TM, et al. The CXC chemokine murine monokine induced by IFN-gamma (CXC chemokine ligand 9) is made by APCs, targets lymphocytes including activated B cells, and supports antibody responses to a bacterial pathogen in vivo. J Immunol. 2002;169:1433–1443. doi: 10.4049/jimmunol.169.3.1433. [DOI] [PubMed] [Google Scholar]
- 17.Zhao X, Sato A, Dela Cruz CS, Linehan M, Luegering A, Kucharzik T, Shirakawa AK, Marquez G, Farber JM, Williams I, et al. CCL9 is secreted by the follicle-associated epithelium and recruits dome region Peyer’s patch CD11b+ dendritic cells. J Immunol. 2003;171:2797–2803. doi: 10.4049/jimmunol.171.6.2797. [DOI] [PubMed] [Google Scholar]
- 18.Porgador A, Staats HF, Itoh Y, Kelsall BL. Intranasal immunization with cytotoxic T-lymphocyte epitope peptide and mucosal adjuvant cholera toxin: selective augmentation of peptide-presenting dendritic cells in nasal mucosa-associated lymphoid tissue. Infect Immun. 1998;66:5876–5881. doi: 10.1128/iai.66.12.5876-5881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuper CF, Hameleers DM, Bruijntjes JP, van der Ven I, Biewenga J, Sminia T. Lymphoid and non-lymphoid cells in nasal-associated lymphoid tissue (NALT) in the rat. An immuno- and enzyme-histochemical study. Cell Tissue Res. 1990;259:371–377. doi: 10.1007/BF00318460. [DOI] [PubMed] [Google Scholar]
- 20.Ohshima K, Hiramatsu K. Distribution of T-cell subsets and immunoglobulin-containing cells in nasal-associated lymphoid tissue (NALT) of chickens. Histol Histopathol. 2000;15:713–720. doi: 10.14670/HH-15.713. [DOI] [PubMed] [Google Scholar]
- 21.Fukuyama S, Nagatake T, Kim DY, Takamura K, Park EJ, Kaisho T, Tanaka N, Kurono Y, Kiyono H. Cutting edge: Uniqueness of lymphoid chemokine requirement for the initiation and maturation of nasopharynx-associated lymphoid tissue organogenesis. J Immunol. 2006;177:4276–4280. doi: 10.4049/jimmunol.177.7.4276. [DOI] [PubMed] [Google Scholar]
- 22.Zuercher AW, Coffin SE, Thurnheer MC, Fundova P, Cebra JJ. Nasal-associated lymphoid tissue is a mucosal inductive site for virus-specific humoral and cellular immune responses. J Immunol. 2002;168:1796–1803. doi: 10.4049/jimmunol.168.4.1796. [DOI] [PubMed] [Google Scholar]
- 23.Carr RM, Lolachi CM, Albaran RG, Ridley DM, Montgomery PC, O’Sullivan NL. Nasal-associated lymphoid tissue is an inductive site for rat tear IgA antibody responses. Immunol Invest. 1996;25:387–396. doi: 10.3109/08820139609055728. [DOI] [PubMed] [Google Scholar]
- 24.Tamura S, Iwasaki T, Thompson AH, Asanuma H, Chen Z, Suzuki Y, Aizawa C, Kurata T. Antibody-forming cells in the nasal-associated lymphoid tissue during primary influenza virus infection. J Gen Virol. 1998;79 (Pt 2):291–299. doi: 10.1099/0022-1317-79-2-291. [DOI] [PubMed] [Google Scholar]
- 25.Asanuma H, Aizawa C, Kurata T, Tamura S. IgA antibody-forming cell responses in the nasal-associated lymphoid tissue of mice vaccinated by intranasal, intravenous and/or subcutaneous administration. Vaccine. 1998;16:1257–1262. doi: 10.1016/s0264-410x(98)00048-6. [DOI] [PubMed] [Google Scholar]
- 26.Shimoda M, Nakamura T, Takahashi Y, Asanuma H, Tamura S, Kurata T, Mizuochi T, Azuma N, Kanno C, Takemori T. Isotype-specific selection of high affinity memory B cells in nasal-associated lymphoid tissue. J Exp Med. 2001;194:1597–1607. doi: 10.1084/jem.194.11.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imai Y, Ishikawa T, Tanikawa T, Nakagami H, Maekawa T, Kurohane K. Production of IgA monoclonal antibody against Shiga toxin binding subunits employing nasal-associated lymphoid tissue. J Immunol Methods. 2005;302:125–135. doi: 10.1016/j.jim.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Shimada S, Kawaguchi-Miyashita M, Kushiro A, Sato T, Nanno M, Sako T, Matsuoka Y, Sudo K, Tagawa Y, Iwakura Y, et al. Generation of polymeric immunoglobulin receptor-deficient mouse with marked reduction of secretory IgA. J Immunol. 1999;163:5367–5373. [PubMed] [Google Scholar]
- 29.Monteiro RC, Van De Winkel JG. IgA Fc receptors. Annu Rev Immunol. 2003;21:177–204. doi: 10.1146/annurev.immunol.21.120601.141011. [DOI] [PubMed] [Google Scholar]
- 30.Ashkenazi S, Vertruyen A, Aristegui J, Esposito S, McKeith DD, Klemola T, Biolek J, Kuhr J, Bujnowski T, Desgrandchamps D, et al. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J. 2006;25:870–879. doi: 10.1097/01.inf.0000237829.66310.85. [DOI] [PubMed] [Google Scholar]
- 31.Belshe RB. Current status of live attenuated influenza virus vaccine in the US. Virus Res. 2004;103:177–185. doi: 10.1016/j.virusres.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 32.King JC, Jr, Lagos R, Bernstein DI, Piedra PA, Kotloff K, Bryant M, Cho I, Belshe RB. Safety and immunogenicity of low and high doses of trivalent live cold-adapted influenza vaccine administered intranasally as drops or spray to healthy children. J Infect Dis. 1998;177:1394–1397. doi: 10.1086/517822. [DOI] [PubMed] [Google Scholar]
- 33.Treanor JJ, Kotloff K, Betts RF, Belshe R, Newman F, Iacuzio D, Wittes J, Bryant M. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine. 1999;18:899–906. doi: 10.1016/s0264-410x(99)00334-5. [DOI] [PubMed] [Google Scholar]
- 34.Sosa GA, Roux ME. Development of T lymphocytes in the nasal-associated lymphoid tissue (NALT) from growing Wistar rats. Clin Dev Immunol. 2004;11:29–34. doi: 10.1080/10446670410001670463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rharbaoui F, Bruder D, Vidakovic M, Ebensen T, Buer J, Guzman CA. Characterization of a B220+ lymphoid cell subpopulation with immune modulatory functions in nasal-associated lymphoid tissues. J Immunol. 2005;174:1317–1324. doi: 10.4049/jimmunol.174.3.1317. [DOI] [PubMed] [Google Scholar]
- 36.Matsuo K, Iwasaki T, Asanuma H, Yoshikawa T, Chen Z, Tsujimoto H, Kurata T, Tamura SS. Cytokine mRNAs in the nasal-associated lymphoid tissue during influenza virus infection and nasal vaccination. Vaccine. 2000;18:1344–1350. doi: 10.1016/s0264-410x(99)00401-6. [DOI] [PubMed] [Google Scholar]
- 37.Hiroi T, Iwatani K, Iijima H, Kodama S, Yanagita M, Kiyono H. Nasal immune system: distinctive Th0 and Th1/Th2 type environments in murine nasal-associated lymphoid tissues and nasal passage, respectively. Eur J Immunol. 1998;28:3346–3353. doi: 10.1002/(SICI)1521-4141(199810)28:10<3346::AID-IMMU3346>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 38.Kurono Y, Yamamoto M, Fujihashi K, Kodama S, Suzuki M, Mogi G, McGhee JR, Kiyono H. Nasal immunization induces Haemophilus influenzae-specific Th1 and Th2 responses with mucosal IgA and systemic IgG antibodies for protective immunity. J Infect Dis. 1999;180:122–132. doi: 10.1086/314827. [DOI] [PubMed] [Google Scholar]
- 39.Hiroi T, Goto H, Someya K, Yanagita M, Honda M, Yamanaka N, Kiyono H. HIV mucosal vaccine: nasal immunization with rBCG-V3J1 induces a long term V3J1 peptide-specific neutralizing immunity in Th1- and Th2-deficient conditions. J Immunol. 2001;167:5862–5867. doi: 10.4049/jimmunol.167.10.5862. [DOI] [PubMed] [Google Scholar]
- 40.Csencsits KL, Jutila MA, Pascual DW. Nasal-associated lymphoid tissue: phenotypic and functional evidence for the primary role of peripheral node addressin in naive lymphocyte adhesion to high endothelial venules in a mucosal site. J Immunol. 1999;163:1382–1389. [PubMed] [Google Scholar]
- 41.Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 42.Sikorski EE, Hallmann R, Berg EL, Butcher EC. The Peyer’s patch high endothelial receptor for lymphocytes, the mucosal vascular addressin, is induced on a murine endothelial cell line by tumor necrosis factor-alpha and IL-1. J Immunol. 1993;151:5239–5250. [PubMed] [Google Scholar]
- 43.Ying X, Chan K, Shenoy P, Hill M, Ruddle NH. Lymphotoxin plays a crucial role in the development and function of nasal-associated lymphoid tissue through regulation of chemokines and peripheral node addressin. Am J Pathol. 2005;166:135–146. doi: 10.1016/S0002-9440(10)62239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanagita M, Hiroi T, Kitagaki N, Hamada S, Ito HO, Shimauchi H, Murakami S, Okada H, Kiyono H. Nasopharyngeal-associated lymphoreticular tissue (NALT) immunity: fimbriae-specific Th1 and Th2 cell-regulated IgA responses for the inhibition of bacterial attachment to epithelial cells and subsequent inflammatory cytokine production. J Immunol. 1999;162:3559–3565. [PubMed] [Google Scholar]
- 45.Kunkel EJ, Kim CH, Lazarus NH, Vierra MA, Soler D, Bowman EP, Butcher EC. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. J Clin Invest. 2003;111:1001–1010. doi: 10.1172/JCI17244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazarus NH, Kunkel EJ, Johnston B, Wilson E, Youngman KR, Butcher EC. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J Immunol. 2003;170:3799–3805. doi: 10.4049/jimmunol.170.7.3799. [DOI] [PubMed] [Google Scholar]
- 47.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 48.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 49.Mariathasan S, Matsumoto M, Baranyay F, Nahm MH, Kanagawa O, Chaplin DD. Absence of lymph nodes in lymphotoxin-alpha(LT alpha)-deficient mice is due to abnormal organ development, not defective lymphocyte migration. J Inflamm. 1995;45:72–78. [PubMed] [Google Scholar]
- 50.Banks TA, Rouse BT, Kerley MK, Blair PJ, Godfrey VL, Kuklin NA, Bouley DM, Thomas J, Kanangat S, Mucenski ML. Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol. 1995;155:1685–1693. [PubMed] [Google Scholar]
- 51.Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 52.Fukuyama S, Hiroi T, Yokota Y, Rennert PD, Yanagita M, Kinoshita N, Terawaki S, Shikina T, Yamamoto M, Kurono Y, et al. Initiation of NALT organogenesis is independent of the IL-7R, LTbetaR, and NIK signaling pathways but requires the Id2 gene and CD3(−)CD4(+)CD45(+) cells. Immunity. 2002;17:31–40. doi: 10.1016/s1074-7613(02)00339-4. [DOI] [PubMed] [Google Scholar]
- 53.Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS. Surface lymphotoxin alpha/beta complex is required for the development of peripheral lymphoid organs. J Exp Med. 1996;184:1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li ZW, Karin M, Ware CF, Green DR. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17:525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 55.Weih F, Caamano J. Regulation of secondary lymphoid organ development by the nuclear factor-kappaB signal transduction pathway. Immunol Rev. 2003;195:91–105. doi: 10.1034/j.1600-065x.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 56.Cupedo T, Mebius RE. Cellular interactions in lymph node development. J Immunol. 2005;174:21–25. doi: 10.4049/jimmunol.174.1.21. [DOI] [PubMed] [Google Scholar]
- 57.Cupedo T, Kraal G, Mebius RE. The role of CD45+CD4+CD3− cells in lymphoid organ development. Immunol Rev. 2002;189:41–50. doi: 10.1034/j.1600-065x.2002.18905.x. [DOI] [PubMed] [Google Scholar]
- 58.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 59.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3− LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 60.Luther SA, Ansel KM, Cyster JG. Overlapping roles of CXCL13, interleukin 7 receptor alpha, and CCR7 ligands in lymph node development. J Exp Med. 2003;197:1191–1198. doi: 10.1084/jem.20021294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishikawa S, Honda K, Vieira P, Yoshida H. Organogenesis of peripheral lymphoid organs. Immunol Rev. 2003;195:72–80. doi: 10.1034/j.1600-065x.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 62.Yoshida H, Kawamoto H, Santee SM, Hashi H, Honda K, Nishikawa S, Ware CF, Katsura Y, Nishikawa SI. Expression of alpha(4)beta(7) integrin defines a distinct pathway of lymphoid progenitors committed to T cells, fetal intestinal lymphotoxin producer, NK, and dendritic cells. J Immunol. 2001;167:2511–2521. doi: 10.4049/jimmunol.167.5.2511. [DOI] [PubMed] [Google Scholar]
- 63.Hashi H, Yoshida H, Honda K, Fraser S, Kubo H, Awane M, Takabayashi A, Nakano H, Yamaoka Y, Nishikawa S. Compartmentalization of Peyer’s patch anlagen before lymphocyte entry. J Immunol. 2001;166:3702–3709. doi: 10.4049/jimmunol.166.6.3702. [DOI] [PubMed] [Google Scholar]
- 64.Honda K, Nakano H, Yoshida H, Nishikawa S, Rennert P, Ikuta K, Tamechika M, Yamaguchi K, Fukumoto T, Chiba T, et al. Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer’s patch organogenesis. J Exp Med. 2001;193:621–630. doi: 10.1084/jem.193.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoshida H, Honda K, Shinkura R, Adachi S, Nishikawa S, Maki K, Ikuta K, Nishikawa SI. IL-7 receptor alpha+ CD3(−) cells in the embryonic intestine induces the organizing center of Peyer’s patches. Int Immunol. 1999;11:643–655. doi: 10.1093/intimm/11.5.643. [DOI] [PubMed] [Google Scholar]
- 66.Nishikawa S, Honda K, Hashi H, Yoshida H. Peyer’s patch organogenesis as a programmed inflammation: a hypothetical model. Cytokine Growth Factor Rev. 1998;9:213–220. doi: 10.1016/s1359-6101(98)00014-8. [DOI] [PubMed] [Google Scholar]
- 67.Adachi S, Yoshida H, Honda K, Maki K, Saijo K, Ikuta K, Saito T, Nishikawa SI. Essential role of IL-7 receptor alpha in the formation of Peyer’s patch anlage. Int Immunol. 1998;10:1–6. doi: 10.1093/intimm/10.1.1. [DOI] [PubMed] [Google Scholar]
- 68.Finke D, Acha-Orbea H, Mattis A, Lipp M, Kraehenbuhl J. CD4+CD3− cells induce Peyer’s patch development: role of alpha4beta1 integrin activation by CXCR5. Immunity. 2002;17:363–373. doi: 10.1016/s1074-7613(02)00395-3. [DOI] [PubMed] [Google Scholar]
- 69.Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 70.Kurebayashi S, Ueda E, Sakaue M, Patel DD, Medvedev A, Zhang F, Jetten AM. Retinoid-related orphan receptor gamma (RORgamma) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc Natl Acad Sci U S A. 2000;97:10132–10137. doi: 10.1073/pnas.97.18.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, Gruss P. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 72.Hacker C, Kirsch RD, Ju XS, Hieronymus T, Gust TC, Kuhl C, Jorgas T, Kurz SM, Rose-John S, Yokota Y, et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol. 2003;4:380–386. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]
- 73.Ikawa T, Fujimoto S, Kawamoto H, Katsura Y, Yokota Y. Commitment to natural killer cells requires the helix-loop-helix inhibitor Id2. Proc Natl Acad Sci U S A. 2001;98:5164–5169. doi: 10.1073/pnas.091537598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coles MC, Veiga-Fernandes H, Foster KE, Norton T, Pagakis SN, Seddon B, Kioussis D. Role of T and NK cells and IL7/IL7r interactions during neonatal maturation of lymph nodes. Proc Natl Acad Sci U S A. 2006;103:13457–13462. doi: 10.1073/pnas.0604183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ansel KM, Ngo VN, Hyman PL, Luther SA, Forster R, Sedgwick JD, Browning JL, Lipp M, Cyster JG. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 76.Kim D, Mebius RE, MacMicking JD, Jung S, Cupedo T, Castellanos Y, Rho J, Wong BR, Josien R, Kim N, et al. Regulation of peripheral lymph node genesis by the tumor necrosis factor family member TRANCE. J Exp Med. 2000;192:1467–1478. doi: 10.1084/jem.192.10.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cupedo T, Vondenhoff MF, Heeregrave EJ, De Weerd AE, Jansen W, Jackson DG, Kraal G, Mebius RE. Presumptive lymph node organizers are differentially represented in developing mesenteric and peripheral nodes. J Immunol. 2004;173:2968–2975. doi: 10.4049/jimmunol.173.5.2968. [DOI] [PubMed] [Google Scholar]
- 78.Wong BR, Josien R, Choi Y. TRANCE is a TNF family member that regulates dendritic cell and osteoclast function. J Leukoc Biol. 1999;65:715–724. doi: 10.1002/jlb.65.6.715. [DOI] [PubMed] [Google Scholar]
- 79.Kim N, Kadono Y, Takami M, Lee J, Lee SH, Okada F, Kim JH, Kobayashi T, Odgren PR, Nakano H, et al. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J Exp Med. 2005;202:589–595. doi: 10.1084/jem.20050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rennert PD, James D, Mackay F, Browning JL, Hochman PS. Lymph node genesis is induced by signaling through the lymphotoxin beta receptor. Immunity. 1998;9:71–79. doi: 10.1016/s1074-7613(00)80589-0. [DOI] [PubMed] [Google Scholar]
- 81.Chaplin DD. Regulation of spleen white pulp structure and function by lymphotoxin. Adv Exp Med Biol. 2002;512:49–56. doi: 10.1007/978-1-4615-0757-4_7. [DOI] [PubMed] [Google Scholar]
- 82.Fu YX, Molina H, Matsumoto M, Huang G, Min J, Chaplin DD. Lymphotoxin-alpha (LTalpha) supports development of splenic follicular structure that is required for IgG responses. J Exp Med. 1997;185:2111–2120. doi: 10.1084/jem.185.12.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fu YX, Huang G, Matsumoto M, Molina H, Chaplin DD. Independent signals regulate development of primary and secondary follicle structure in spleen and mesenteric lymph node. Proc Natl Acad Sci U S A. 1997;94:5739–5743. doi: 10.1073/pnas.94.11.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsumoto M, Fu YX, Molina H, Chaplin DD. Lymphotoxin-alpha-deficient and TNF receptor-I-deficient mice define developmental and functional characteristics of germinal centers. Immunol Rev. 1997;156:137–144. doi: 10.1111/j.1600-065x.1997.tb00965.x. [DOI] [PubMed] [Google Scholar]
- 85.Amiot F, Bellkaid Y, Lebastard M, Ave P, Dautry F, Milon G. Abnormal organisation of the splenic marginal zone and the correlated leukocytosis in lymphotoxin-alpha and tumor necrosis factor alpha double deficient mice. Eur Cytokine Netw. 1996;7:733–739. [PubMed] [Google Scholar]
- 86.Matsumoto M, Mariathasan S, Nahm MH, Baranyay F, Peschon JJ, Chaplin DD. Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science. 1996;271:1289–1291. doi: 10.1126/science.271.5253.1289. [DOI] [PubMed] [Google Scholar]
- 87.Endres R, Alimzhanov MB, Plitz T, Futterer A, Kosco-Vilbois MH, Nedospasov SA, Rajewsky K, Pfeffer K. Mature follicular dendritic cell networks depend on expression of lymphotoxin beta receptor by radioresistant stromal cells and of lymphotoxin beta and tumor necrosis factor by B cells. J Exp Med. 1999;189:159–168. doi: 10.1084/jem.189.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ngo VN, Cornall RJ, Cyster JG. Splenic T zone development is B cell dependent. J Exp Med. 2001;194:1649–1660. doi: 10.1084/jem.194.11.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ngo VN, Korner H, Gunn MD, Schmidt KN, Riminton DS, Cooper MD, Browning JL, Sedgwick JD, Cyster JG. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med. 1999;189:403–412. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kabashima K, Banks TA, Ansel KM, Lu TT, Ware CF, Cyster JG. Intrinsic lymphotoxin-beta receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity. 2005;22:439–450. doi: 10.1016/j.immuni.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 91.Browning JL, Allaire N, Ngam-Ek A, Notidis E, Hunt J, Perrin S, Fava RA. Lymphotoxin-beta receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity. 2005;23:539–550. doi: 10.1016/j.immuni.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 92.Stopfer P, Obermeier F, Dunger N, Falk W, Farkas S, Janotta M, Moller A, Mannel DN, Hehlgans T. Blocking lymphotoxin-beta receptor activation diminishes inflammation via reduced mucosal addressin cell adhesion molecule-1 (MAdCAM-1) expression and leucocyte margination in chronic DSS-induced colitis. Clin Exp Immunol. 2004;136:21–29. doi: 10.1111/j.1365-2249.2004.02402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Drayton DL, Bonizzi G, Ying X, Liao S, Karin M, Ruddle NH. I kappa B kinase complex alpha kinase activity controls chemokine and high endothelial venule gene expression in lymph nodes and nasal-associated lymphoid tissue. J Immunol. 2004;173:6161–6168. doi: 10.4049/jimmunol.173.10.6161. [DOI] [PubMed] [Google Scholar]
- 94.Cyster JG. Chemokines and the homing of dendritic cells to the T cell areas of lymphoid organs. J Exp Med. 1999;189:447–450. doi: 10.1084/jem.189.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Okada T, Ngo VN, Ekland EH, Forster R, Lipp M, Littman DR, Cyster JG. Chemokine requirements for B cell entry to lymph nodes and Peyer’s patches. J Exp Med. 2002;196:65–75. doi: 10.1084/jem.20020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci U S A. 2000;97:12694–12699. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5:943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- 98.Williams IR. CCR6 and CCL20: partners in intestinal immunity and lymphorganogenesis. Ann N Y Acad Sci. 2006;1072:52–61. doi: 10.1196/annals.1326.036. [DOI] [PubMed] [Google Scholar]
- 99.Iwasaki A, Kelsall BL. Unique functions of CD11b+, CD8 alpha+, and double-negative Peyer’s patch dendritic cells. J Immunol. 2001;166:4884–4890. doi: 10.4049/jimmunol.166.8.4884. [DOI] [PubMed] [Google Scholar]
- 100.Fleeton M, Contractor N, Leon F, He J, Wetzel D, Dermody T, Iwasaki A, Kelsall B. Involvement of dendritic cell subsets in the induction of oral tolerance and immunity. Ann N Y Acad Sci. 2004;1029:60–65. doi: 10.1196/annals.1309.008. [DOI] [PubMed] [Google Scholar]
- 101.Hase K, Murakami T, Takatsu H, Shimaoka T, Iimura M, Hamura K, Kawano K, Ohshima S, Chihara R, Itoh K, et al. The membrane-bound chemokine CXCL16 expressed on follicle-associated epithelium and M cells mediates lympho-epithelial interaction in GALT. J Immunol. 2006;176:43–51. doi: 10.4049/jimmunol.176.1.43. [DOI] [PubMed] [Google Scholar]
- 102.Hase K, Ohshima S, Kawano K, Hashimoto N, Matsumoto K, Saito H, Ohno H. Distinct gene expression profiles characterize cellular phenotypes of follicle-associated epithelium and m cells. DNA Res. 2005;12:127–137. doi: 10.1093/dnares/12.2.127. [DOI] [PubMed] [Google Scholar]
- 103.Shinkura R, Kitada K, Matsuda F, Tashiro K, Ikuta K, Suzuki M, Kogishi K, Serikawa T, Honjo T. Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-kappa b-inducing kinase. Nat Genet. 1999;22:74–77. doi: 10.1038/8780. [DOI] [PubMed] [Google Scholar]
- 104.Tang HL, Cyster JG. Chemokine Up-regulation and activated T cell attraction by maturing dendritic cells. Science. 1999;284:819–822. doi: 10.1126/science.284.5415.819. [DOI] [PubMed] [Google Scholar]
- 105.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. 2001;193:1373–1381. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 109.Wang J, Anders RA, Wu Q, Peng D, Cho JH, Sun Y, Karaliukas R, Kang HS, Turner JR, Fu YX. Dysregulated LIGHT expression on T cells mediates intestinal inflammation and contributes to IgA nephropathy. J Clin Invest. 2004;113:826–835. doi: 10.1172/JCI20096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chin R, Wang J, Fu YX. Lymphoid microenvironment in the gut for immunoglobulin A and inflammation. Immunol Rev. 2003;195:190–201. doi: 10.1034/j.1600-065x.2003.00066.x. [DOI] [PubMed] [Google Scholar]
- 111.Kang HS, Chin RK, Wang Y, Yu P, Wang J, Newell KA, Fu YX. Signaling via LTbetaR on the lamina propria stromal cells of the gut is required for IgA production. Nat Immunol. 2002;3:576–582. doi: 10.1038/ni795. [DOI] [PubMed] [Google Scholar]
- 112.Davis IA, Knight KA, Rouse BT. The spleen and organized lymph nodes are not essential for the development of gut-induced mucosal immune responses in lymphotoxin-alpha deficient mice. Clin Immunol Immunopathol. 1998;89:150–159. doi: 10.1006/clin.1998.4601. [DOI] [PubMed] [Google Scholar]
- 113.Macpherson AJ, Lamarre A, McCoy K, Harriman GR, Odermatt B, Dougan G, Hengartner H, Zinkernagel RM. IgA production without mu or delta chain expression in developing B cells. Nat Immunol. 2001;2:625–631. doi: 10.1038/89775. [DOI] [PubMed] [Google Scholar]
- 114.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 115.Fagarasan S, Kinoshita K, Muramatsu M, Ikuta K, Honjo T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 2001;413:639–643. doi: 10.1038/35098100. [DOI] [PubMed] [Google Scholar]
- 116.Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol. 2003;3:63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- 117.Fagarasan S, Honjo T. Regulation of IgA synthesis at mucosal surfaces. Curr Opin Immunol. 2004;16:277–283. doi: 10.1016/j.coi.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 118.Sangster MY, Riberdy JM, Gonzalez M, Topham DJ, Baumgarth N, Doherty PC. An early CD4+ T cell-dependent immunoglobulin A response to influenza infection in the absence of key cognate T-B interactions. J Exp Med. 2003;198:1011–1021. doi: 10.1084/jem.20021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shikina T, Hiroi T, Iwatani K, Jang MH, Fukuyama S, Tamura M, Kubo T, Ishikawa H, Kiyono H. IgA class switch occurs in the organized nasopharynx- and gut-associated lymphoid tissue, but not in the diffuse lamina propria of airways and gut. J Immunol. 2004;172:6259–6264. doi: 10.4049/jimmunol.172.10.6259. [DOI] [PubMed] [Google Scholar]
- 120.Lund FE, Partida-Sanchez S, Lee BO, Kusser KL, Hartson L, Hogan RJ, Woodland DL, Randall TD. Lymphotoxin-alpha-deficient mice make delayed, but effective, T and B cell responses to influenza. J Immunol. 2002;169:5236–5243. doi: 10.4049/jimmunol.169.9.5236. [DOI] [PubMed] [Google Scholar]
- 121.Bienenstock J, Johnston N, Perey DY. Bronchial lymphoid tissue. I. Morphologic characteristics. Lab Invest. 1973;28:686–692. [PubMed] [Google Scholar]
- 122.Bienenstock J, Johnston N, Perey DY. Bronchial lymphoid tissue. II. Functional characterisitics. Lab Invest. 1973;28:693–698. [PubMed] [Google Scholar]
- 123.Bienenstock J, Befus D. Gut- and bronchus-associated lymphoid tissue. Am J Anat. 1984;170:437–445. doi: 10.1002/aja.1001700316. [DOI] [PubMed] [Google Scholar]
- 124.Bienenstock J, McDermott MR. Bronchus- and nasal-associated lymphoid tissues. Immunol Rev. 2005;206:22–31. doi: 10.1111/j.0105-2896.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 125.Sminia T, van der Brugge-Gamelkoorn GJ, Jeurissen SH. Structure and function of bronchus-associated lymphoid tissue (BALT) Crit Rev Immunol. 1989;9:119–150. [PubMed] [Google Scholar]
- 126.Sato A, Hayakawa H, Uchiyama H, Chida K. Cellular distribution of bronchus-associated lymphoid tissue in rheumatoid arthritis. Am J Respir Crit Care Med. 1996;154:1903–1907. doi: 10.1164/ajrccm.154.6.8970384. [DOI] [PubMed] [Google Scholar]
- 127.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 128.Xu B, Wagner N, Pham LN, Magno V, Shan Z, Butcher EC, Michie SA. Lymphocyte homing to bronchus-associated lymphoid tissue (BALT) is mediated by L-selectin/PNAd, alpha4beta1 integrin/VCAM-1, and LFA-1 adhesion pathways. J Exp Med. 2003;197:1255–1267. doi: 10.1084/jem.20010685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tai DY. Bronchoscopy in the intensive care unit (ICU) Ann Acad Med Singapore. 1998;27:552–559. [PubMed] [Google Scholar]
- 130.Tschernig T, Pabst R. Bronchus-associated lymphoid tissue (BALT) is not present in the normal adult lung but in different diseases. Pathobiology. 2000;68:1–8. doi: 10.1159/000028109. [DOI] [PubMed] [Google Scholar]
- 131.Pabst R, Gehrke I. Is the bronchus-associated lymphoid tissue (BALT) an integral structure of the lung in normal mammals, including humans? Am J Respir Cell Mol Biol. 1990;3:131–135. doi: 10.1165/ajrcmb/3.2.131. [DOI] [PubMed] [Google Scholar]
- 132.Delventhal S, Hensel A, Petzoldt K, Pabst R. Effects of microbial stimulation on the number, size and activity of bronchus-associated lymphoid tissue (BALT) structures in the pig. Int J Exp Pathol. 1992;73:351–357. [PMC free article] [PubMed] [Google Scholar]
- 133.Escolar Castellon JD, Escolar Castellon A, Roche Roche PA, Minana Amada C. Bronchial-associated lymphoid tissue (BALT) response to airway challenge with cigarette smoke, bovine antigen and anti-pulmonary serum. Histol Histopathol. 1992;7:321–328. [PubMed] [Google Scholar]
- 134.Holt PG. Development of bronchus associated lymphoid tissue (BALT) in human lung disease: a normal host defence mechanism awaiting therapeutic exploitation? Thorax. 1993;48:1097–1098. doi: 10.1136/thx.48.11.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brodie SJ, de la Rosa C, Howe JG, Crouch J, Travis WD, Diem K. Pediatric AIDS-associated lymphocytic interstitial pneumonia and pulmonary arterio-occlusive disease: role of VCAM-1/VLA-4 adhesion pathway and human herpesviruses. Am J Pathol. 1999;154:1453–1464. doi: 10.1016/S0002-9440(10)65400-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 136.Rodriguez F, Fernandez A, Oros J, Ramirez AS, Luque R, Ball HJ, Sarradell J. Changes in lymphocyte subsets in the bronchus-associated lymphoid tissue of goats naturally infected with different Mycoplasma species. J Vet Med B Infect Dis Vet Public Health. 2001;48:259–266. doi: 10.1046/j.1439-0450.2001.00434.x. [DOI] [PubMed] [Google Scholar]
- 137.Moyron-Quiroz JE, Rangel-Moreno J, Hartson L, Kusser K, Tighe MP, Klonowski KD, Lefrancois L, Cauley LS, Harmsen AG, Lund FE, et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25:643–654. doi: 10.1016/j.immuni.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 138.Ersch J, Tschernig T, Stallmach T. Frequency and potential cause of bronchus-associated lymphoid tissue in fetal lungs. Pediatr Allergy Immunol. 2005;16:295–298. doi: 10.1111/j.1399-3038.2005.00269.x. [DOI] [PubMed] [Google Scholar]
- 139.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, Brenneise IE, Horton MA, Haczku A, Gelfand EW, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Goya S, Matsuoka H, Mori M, Morishita H, Kida H, Kobashi Y, Kato T, Taguchi Y, Osaki T, Tachibana I, et al. Sustained interleukin-6 signalling leads to the development of lymphoid organ-like structures in the lung. J Pathol. 2003;200:82–87. doi: 10.1002/path.1321. [DOI] [PubMed] [Google Scholar]
- 141.Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, Randall TD. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest. 2006;116:3183–3194. doi: 10.1172/JCI28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Luhrmann A, Tschernig T, Pabst R. Stimulation of bronchus-associated lymphoid tissue in rats by repeated inhalation of aerosolized lipopeptide MALP-2. Pathobiology. 2002;70:266–269. doi: 10.1159/000070740. [DOI] [PubMed] [Google Scholar]
- 143.Magliozzi R, Columba-Cabezas S, Serafini B, Aloisi F. Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148:11–23. doi: 10.1016/j.jneuroim.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 144.Xanthou G, Polihronis M, Tzioufas AG, Paikos S, Sideras P, Moutsopoulos HM. “Lymphoid” chemokine messenger RNA expression by epithelial cells in the chronic inflammatory lesion of the salivary glands of Sjogren’s syndrome patients: possible participation in lymphoid structure formation. Arthritis Rheum. 2001;44:408–418. doi: 10.1002/1529-0131(200102)44:2<408::AID-ANR60>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 145.Amft N, Curnow SJ, Scheel-Toellner D, Devadas A, Oates J, Crocker J, Hamburger J, Ainsworth J, Mathews J, Salmon M, et al. Ectopic expression of the B cell-attracting chemokine BCA-1 (CXCL13) on endothelial cells and within lymphoid follicles contributes to the establishment of germinal center-like structures in Sjogren’s syndrome. Arthritis Rheum. 2001;44:2633–2641. doi: 10.1002/1529-0131(200111)44:11<2633::aid-art443>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 146.Salomonsson S, Jonsson MV, Skarstein K, Brokstad KA, Hjelmstrom P, Wahren-Herlenius M, Jonsson R. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjogren’s syndrome. Arthritis Rheum. 2003;48:3187–3201. doi: 10.1002/art.11311. [DOI] [PubMed] [Google Scholar]
- 147.Armengol MP, Juan M, Lucas-Martin A, Fernandez-Figueras MT, Jaraquemada D, Gallart T, Pujol-Borrell R. Thyroid autoimmune disease: demonstration of thyroid antigen-specific B cells and recombination-activating gene expression in chemokine-containing active intrathyroidal germinal centers. Am J Pathol. 2001;159:861–873. doi: 10.1016/S0002-9440(10)61762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Armengol MP, Cardoso-Schmidt CB, Fernandez M, Ferrer X, Pujol-Borrell R, Juan M. Chemokines determine local lymphoneogenesis and a reduction of circulating CXCR4+ T and CCR7 B and T lymphocytes in thyroid autoimmune diseases. J Immunol. 2003;170:6320–6328. doi: 10.4049/jimmunol.170.12.6320. [DOI] [PubMed] [Google Scholar]
- 149.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 150.Ruddle NH. Lymphoid neo-organogenesis: lymphotoxin’s role in inflammation and development. Immunol Res. 1999;19:119–125. doi: 10.1007/BF02786481. [DOI] [PubMed] [Google Scholar]
- 151.Hjelmstrom P. Lymphoid neogenesis: de novo formation of lymphoid tissue in chronic inflammation through expression of homing chemokines. J Leukoc Biol. 2001;69:331–339. [PubMed] [Google Scholar]
- 152.Hjelmstrom P, Fjell J, Nakagawa T, Sacca R, Cuff CA, Ruddle NH. Lymphoid tissue homing chemokines are expressed in chronic inflammation. Am J Pathol. 2000;156:1133–1138. doi: 10.1016/S0002-9440(10)64981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med. 1996;183:1461–1472. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tango M, Suzuki E, Gejyo F, Ushiki T. The presence of specialized epithelial cells on the bronchus-associated lymphoid tissue (BALT) in the mouse. Arch Histol Cytol. 2000;63:81–89. doi: 10.1679/aohc.63.81. [DOI] [PubMed] [Google Scholar]
- 155.Roy MJ, Ruiz A, Varvayanis M. A novel antigen is common to the dome epithelium of gut- and bronchus-associated lymphoid tissues. Cell Tissue Res. 1987;248:635–644. doi: 10.1007/BF00216494. [DOI] [PubMed] [Google Scholar]
- 156.Plesch BE, Gamelkoorn GJ, van de Ende M. Development of bronchus associated lymphoid tissue (BALT) in the rat, with special reference to T- and B-cells. Dev Comp Immunol. 1983;7:179–188. doi: 10.1016/0145-305x(83)90066-6. [DOI] [PubMed] [Google Scholar]
- 157.Bosken CH, Hards J, Gatter K, Hogg JC. Characterization of the inflammatory reaction in the peripheral airways of cigarette smokers using immunocytochemistry. Am Rev Respir Dis. 1992;145:911–917. doi: 10.1164/ajrccm/145.4_Pt_1.911. [DOI] [PubMed] [Google Scholar]
- 158.Sato A, Chida K, Iwata M, Hayakawa H. Study of bronchus-associated lymphoid tissue in patients with diffuse panbronchiolitis. Am Rev Respir Dis. 1992;146:473–478. doi: 10.1164/ajrccm/146.2.473. [DOI] [PubMed] [Google Scholar]
- 159.Suda T, Chida K, Hayakawa H, Imokawa S, Iwata M, Nakamura H, Sato A. Development of bronchus-associated lymphoid tissue in chronic hypersensitivity pneumonitis. Chest. 1999;115:357–363. doi: 10.1378/chest.115.2.357. [DOI] [PubMed] [Google Scholar]
- 160.Eskenasy A. On the development and immune reactivity of the so-called bronchus-associated lymphoid tissue (BALT) in rabbits and humans: experimental and anatomoclinical observations. Morphol Embryol (Bucur) 1978;24:51–63. [PubMed] [Google Scholar]
- 161.Gregson RL, Davey MJ, Prentice DE. The response of rat bronchus-associated lymphoid tissue to local antigenic challenge. Br J Exp Pathol. 1979;60:471–482. [PMC free article] [PubMed] [Google Scholar]
- 162.Marquez MG, Sosa GA, Roux ME. Developmental study of immunocompetent cells in the bronchus-associated lymphoid tissue (BALT) from Wistar rats. Dev Comp Immunol. 2000;24:683–689. doi: 10.1016/s0145-305x(00)00003-3. [DOI] [PubMed] [Google Scholar]
- 163.Sminia T, van de Ende MB, Jeurissen SH. Specific antibody formation in mucosa associated lymphoid tissue after intratracheal and intra-intestinal administration of TNP-KLH; differences between BALT and GALT. Adv Exp Med Biol. 1987;216B:981–987. [PubMed] [Google Scholar]
- 164.van der Brugge-Gamelkoorn GJ, Plesch BE, Sminia T, Langevoort HL. Specific antibody-forming cells in bronchus-associated lymphoid tissue (BALT) and lung of the rat after intratracheal challenge with horseradish peroxidase. Virchows Arch B Cell Pathol Incl Mol Pathol. 1985;49:269–276. doi: 10.1007/BF02912104. [DOI] [PubMed] [Google Scholar]
- 165.van der Brugge-Gamelkoorn GJ, Claassen E, Sminia T. Anti-TNP-forming cells in bronchus-associated lymphoid tissue (BALT) and paratracheal lymph node (PTLN) of the rat after intratracheal priming and boosting with TNP-KLH. Immunology. 1986;57:405–409. [PMC free article] [PubMed] [Google Scholar]
- 166.van der Brugge-Gamelkoorn GJ, Plesch BE, Sminia T, Langevoort HL. Histological changes in rat bronchus-associated lymphoid tissue after administration of five different antigens. Respiration. 1985;48:29–36. doi: 10.1159/000194795. [DOI] [PubMed] [Google Scholar]
- 167.Weyand CM, Goronzy JJ. Ectopic germinal center formation in rheumatoid synovitis. Ann N Y Acad Sci. 2003;987:140–149. doi: 10.1111/j.1749-6632.2003.tb06042.x. [DOI] [PubMed] [Google Scholar]
- 168.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 169.Drayton DL, Ying X, Lee J, Lesslauer W, Ruddle NH. Ectopic LT alpha beta directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med. 2003;197:1153–1163. doi: 10.1084/jem.20021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chin RK, Lo JC, Kim O, Blink SE, Christiansen PA, Peterson P, Wang Y, Ware C, Fu YX. Lymphotoxin pathway directs thymic Aire expression. Nat Immunol. 2003;4:1121–1127. doi: 10.1038/ni982. [DOI] [PubMed] [Google Scholar]
- 171.Constant SL, Brogdon JL, Piggott DA, Herrick CA, Visintin I, Ruddle NH, Bottomly K. Resident lung antigen-presenting cells have the capacity to promote Th2 T cell differentiation in situ. J Clin Invest. 2002;110:1441–1448. doi: 10.1172/JCI16109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Constant SL, Lee KS, Bottomly K. Site of antigen delivery can influence T cell priming: pulmonary environment promotes preferential Th2-type differentiation. Eur J Immunol. 2000;30:840–847. doi: 10.1002/1521-4141(200003)30:3<840::AID-IMMU840>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 173.Lee BJ, Santee S, Von Gesjen S, Ware CF, Sarawar SR. Lymphotoxin-alpha-deficient mice can clear a productive infection with murine gammaherpesvirus 68 but fail to develop splenomegaly or lymphocytosis. J Virol. 2000;74:2786–2792. doi: 10.1128/jvi.74.6.2786-2792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lo JC, Chin RK, Lee Y, Kang HS, Wang Y, Weinstock JV, Banks T, Ware CF, Franzoso G, Fu YX. Differential regulation of CCL21 in lymphoid/nonlymphoid tissues for effectively attracting T cells to peripheral tissues. J Clin Invest. 2003;112:1495–1505. doi: 10.1172/JCI19188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 176.Ebert LM, Schaerli P, Moser B. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol Immunol. 2005;42:799–809. doi: 10.1016/j.molimm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 177.Lee Y, Chin RK, Christiansen P, Sun Y, Tumanov AV, Wang J, Chervonsky AV, Fu YX. Recruitment and activation of naive T cells in the islets by lymphotoxin beta receptor-dependent tertiary lymphoid structure. Immunity. 2006;25:499–509. doi: 10.1016/j.immuni.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 178.Das A, Kole L, Wang L, Barrios R, Moorthy B, Jaiswal AK. BALT development and augmentation of hyperoxic lung injury in mice deficient in NQO1 and NQO2. Free Radic Biol Med. 2006;40:1843–1856. doi: 10.1016/j.freeradbiomed.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 179.Karupiah G, Chen JH, Mahalingam S, Nathan CF, MacMicking JD. Rapid interferon gamma-dependent clearance of influenza A virus and protection from consolidating pneumonitis in nitric oxide synthase 2-deficient mice. J Exp Med. 1998;188:1541–1546. doi: 10.1084/jem.188.8.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kocks JR, Davalos-Misslitz AC, Hintzen G, Ohl L, Forster R. Regulatory T cells interfere with the development of bronchus-associated lymphoid tissue. J Exp Med. 2007 doi: 10.1084/jem.20061424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lane PJ, Kim MY, Gaspal FM, McConnell FM. CD4+CD3− cells regulate the organization of lymphoid tissue and T-cell memory for antibody responses. Int J Hematol. 2006;83:12–16. doi: 10.1532/IJH97.05117. [DOI] [PubMed] [Google Scholar]
- 182.Lane PJ, Gaspal FM, Kim MY. Two sides of a cellular coin: CD4(+)CD3− cells regulate memory responses and lymph-node organization. Nat Rev Immunol. 2005;5:655–660. doi: 10.1038/nri1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Marinkovic T, Garin A, Yokota Y, Fu YX, Ruddle NH, Furtado GC, Lira SA. Interaction of mature CD3+CD4+ T cells with dendritic cells triggers the development of tertiary lymphoid structures in the thyroid. J Clin Invest. 2006;116:2622–2632. doi: 10.1172/JCI28993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Furtado GC, Marinkovic T, Martin AP, Garin A, Hoch B, Hubner W, Chen BK, Genden E, Skobe M, Lira SA. Lymphotoxin beta receptor signaling is required for inflammatory lymphangiogenesis in the thyroid. Proc Natl Acad Sci U S A. 2007;104:5026–5031. doi: 10.1073/pnas.0606697104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chen HD, Fraire AE, Joris I, Welsh RM, Selin LK. Specific history of heterologous virus infections determines anti-viral immunity and immunopathology in the lung. Am J Pathol. 2003;163:1341–1355. doi: 10.1016/S0002-9440(10)63493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kahnert A, Hopken UE, Stein M, Bandermann S, Lipp M, Kaufmann SH. Mycobacterium tuberculosis triggers formation of lymphoid structure in murine lungs. J Infect Dis. 2007;195:46–54. doi: 10.1086/508894. [DOI] [PubMed] [Google Scholar]
- 187.Wallace WA, Howie SE, Krajewski AS, Lamb D. The immunological architecture of B-lymphocyte aggregates in cryptogenic fibrosing alveolitis. J Pathol. 1996;178:323–329. doi: 10.1002/(SICI)1096-9896(199603)178:3<323::AID-PATH467>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 188.Charavaryamath C, Janardhan KS, Townsend HG, Willson P, Singh B. Multiple exposures to swine barn air induce lung inflammation and airway hyper-responsiveness. Respir Res. 2005;6:50. doi: 10.1186/1465-9921-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liao S, Ruddle NH. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J Immunol. 2006;177:3369–3379. doi: 10.4049/jimmunol.177.5.3369. [DOI] [PubMed] [Google Scholar]
- 190.Yamada R, Suzuki A, Chang X, Yamamoto K. Citrullinated proteins in rheumatoid arthritis. Front Biosci. 2005;10:54–64. doi: 10.2741/1506. [DOI] [PubMed] [Google Scholar]
- 191.Suzuki A, Yamada R, Chang X, Tokuhiro S, Sawada T, Suzuki M, Nagasaki M, Nakayama-Hamada M, Kawaida R, Ono M, et al. Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat Genet. 2003;34:395–402. doi: 10.1038/ng1206. [DOI] [PubMed] [Google Scholar]
- 192.Yamada R. Peptidylarginine deiminase type 4, anticitrullinated peptide antibodies, and rheumatoid arthritis. Autoimmun Rev. 2005;4:201–206. doi: 10.1016/j.autrev.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 193.Richmond I, Pritchard GE, Ashcroft T, Avery A, Corris PA, Walters EH. Bronchus associated lymphoid tissue (BALT) in human lung: its distribution in smokers and non-smokers. Thorax. 1993;48:1130–1134. doi: 10.1136/thx.48.11.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]