Abstract
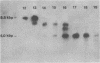
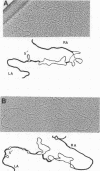
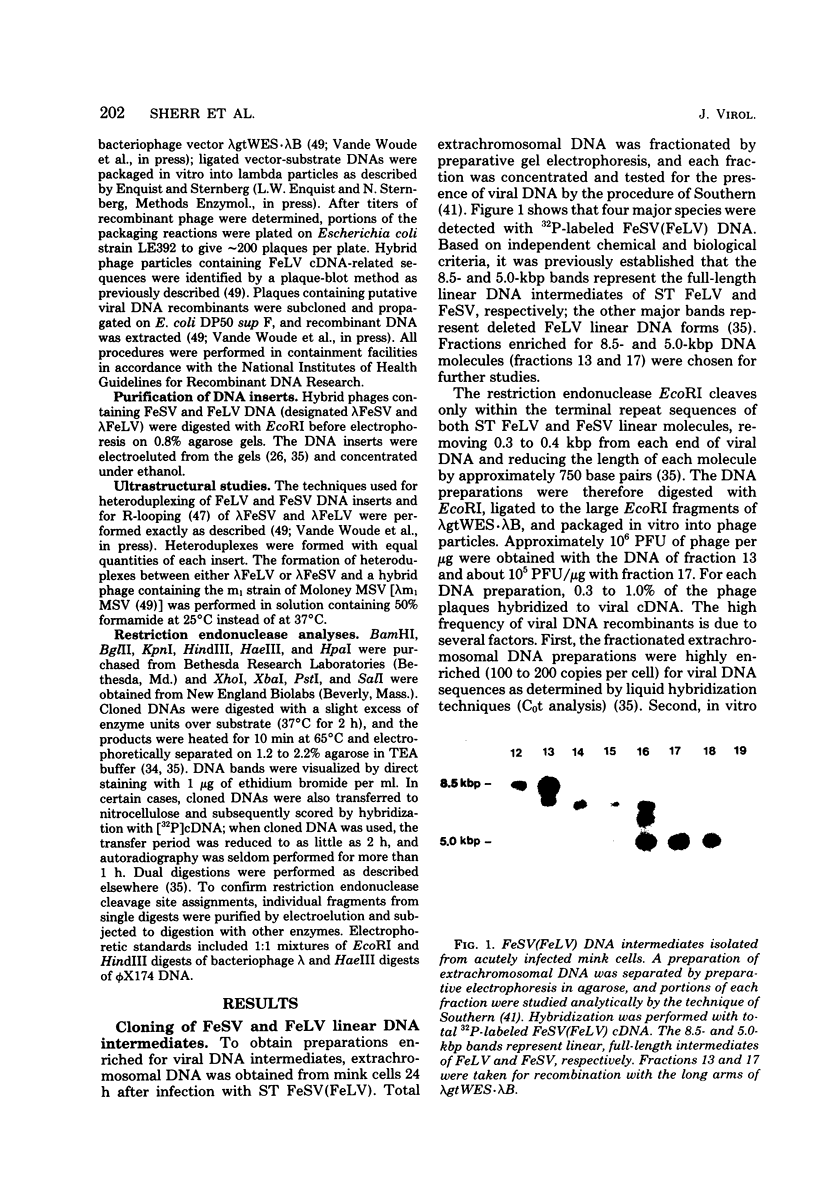
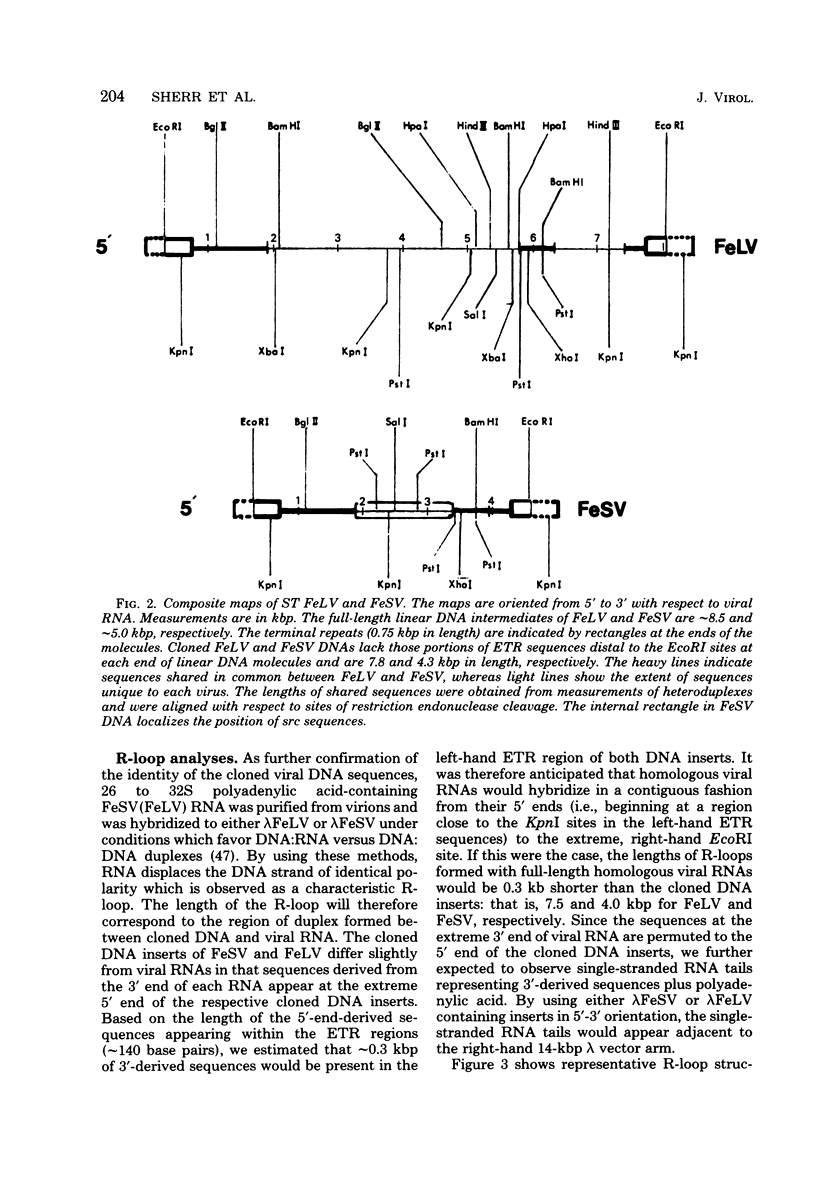
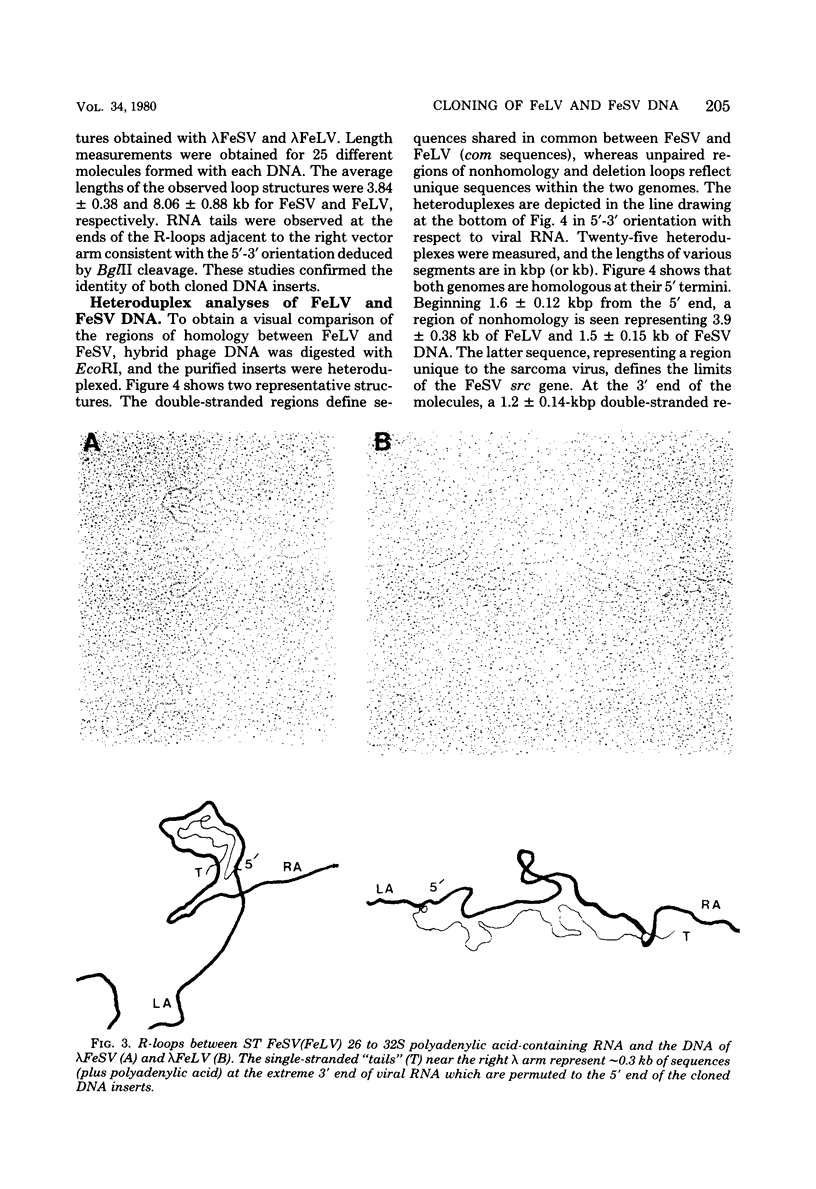
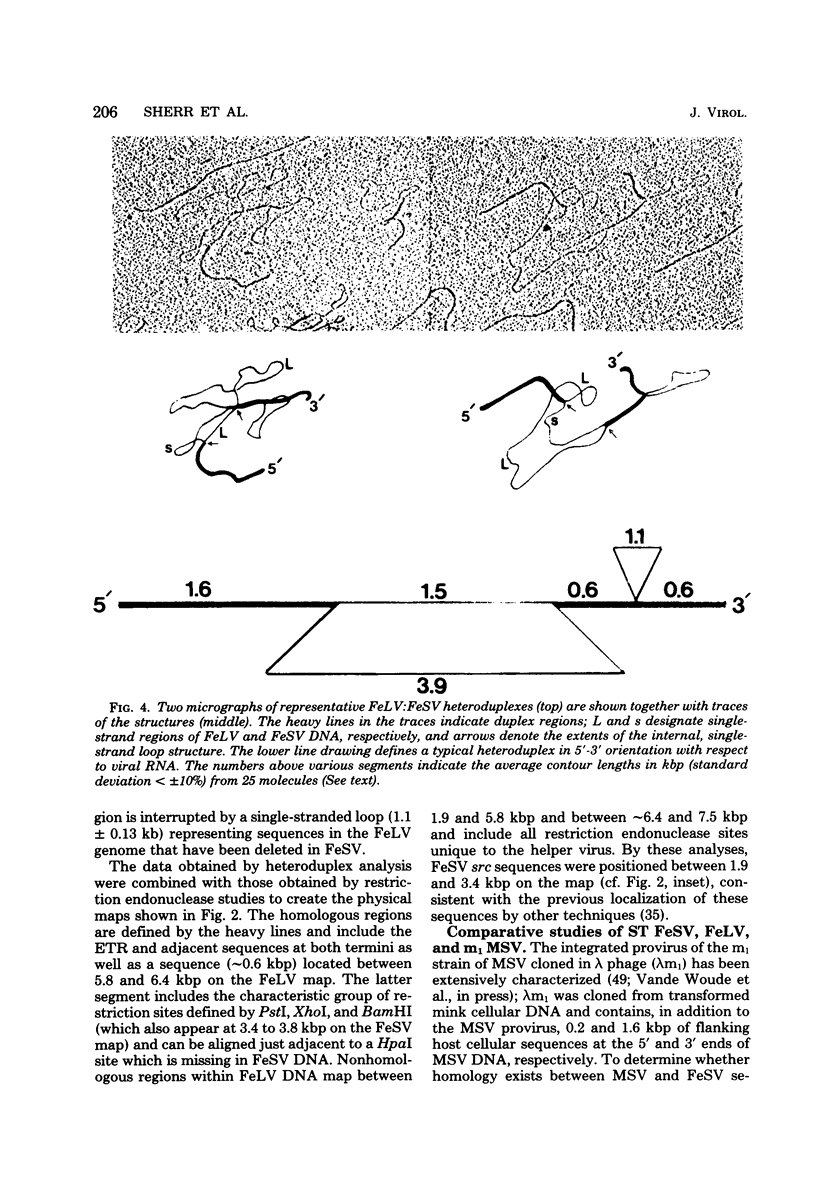
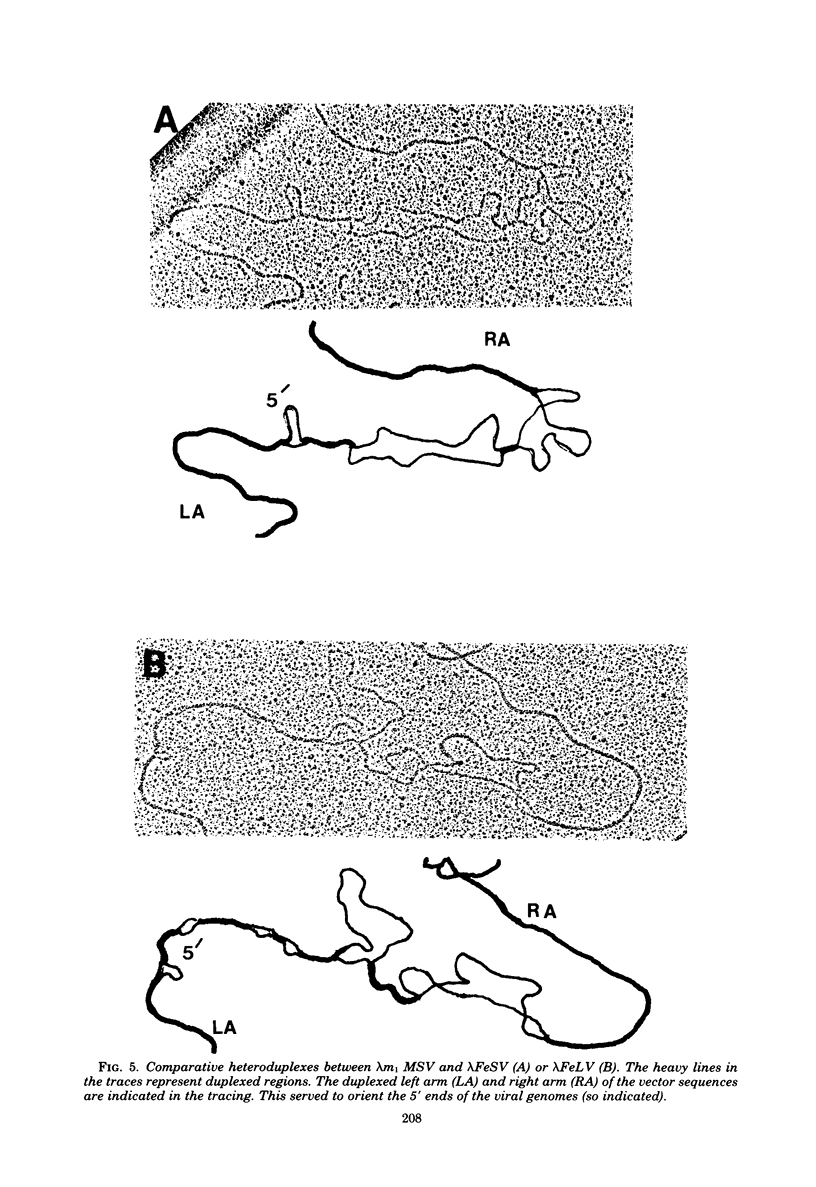
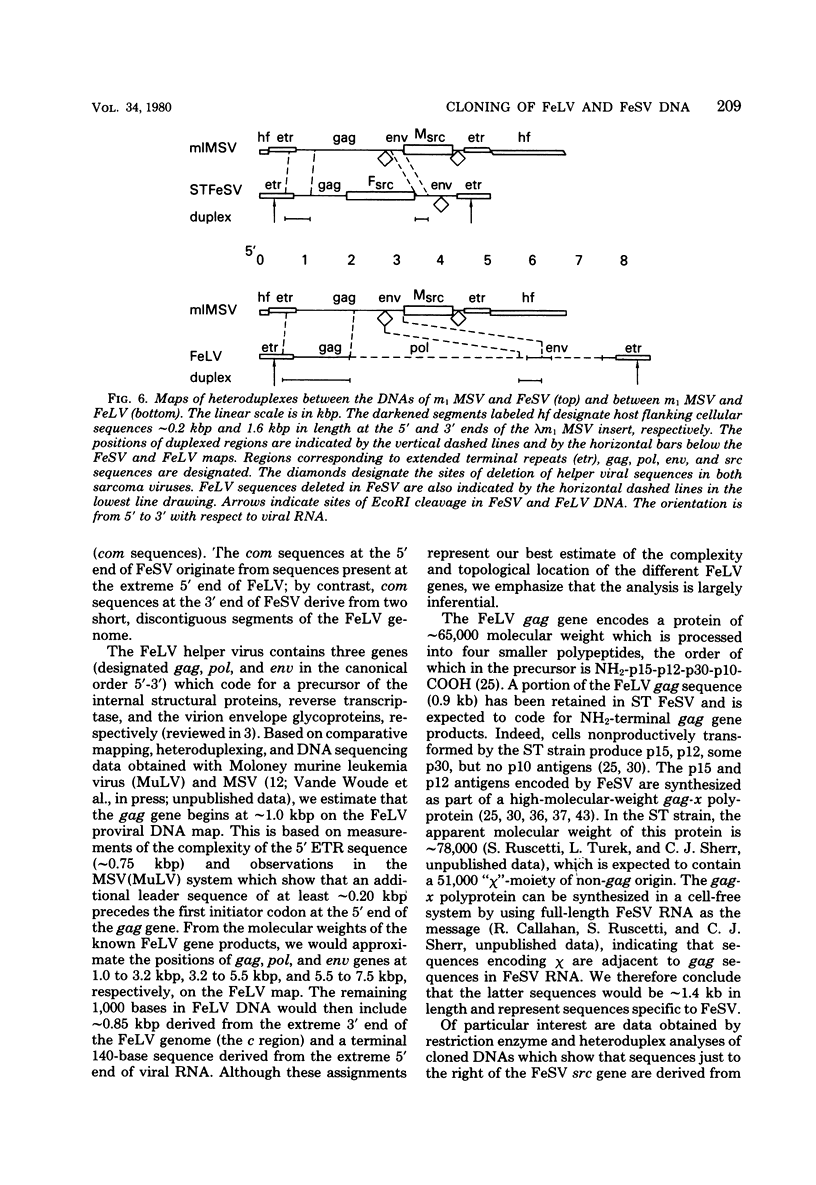
Extrachromosomal DNA obtained from mink cells acutely infected with the Snyder-Theilen (ST) strain of feline sarcoma virus (feline leukemia virus) [FeSV(FeLV)] was fractionated electrophoretically, and samples enriched for FeLV and FeSV linear intermediates were digested with EcoRI and cloned in lambda phage. Hybrid phages were isolated containing either FeSV or FeLV DNA "inserts" and were characterized by restriction enzyme analysis, R-looping with purified 26 to 32S viral RNA, and heteroduplex formation. The recombinant phages (designated lambda FeSV and lambda FeLV) contain all of the genetic information represented in FeSV and FeLV RNA genomes but lack one extended terminally redundant sequence of 750 bases which appears once at each end of parental linear DNA intermediates. Restriction enzyme and heteroduplex analyses confirmed that sequences unique to FeSV (src sequences) are located at the center of the FeSV genome and are approximately 1.5 kilobase pairs in length. With respect to the 5'-3' orientation of genes in viral RNA, the order of genes in the FeSV genome is 5'-gag-src-env-c region-3'; only 0.9 kilobase pairs of gag and 0.6 kilobase pairs of env-derived FeLV sequences are represented in ST FeSV. Heteroduplex analyses between lambda FeSV or lambda FeLV DNA and Moloney murine sarcoma virus DNA (strain m1) were performed under conditions of reduced stringency to demonstrate limited regions of base pair homology. Two such regions were identified: the first occurs at the extreme 5' end of the leukemia and both sarcoma viral genomes, whereas the second corresponds to a 5' segment of leukemia virus "env" sequences conserved in both sarcoma viruses. The latter sequences are localized at the 3' end of FeSV src and at the 5' end of murine sarcoma virus src and could possibly correspond to regions of helper virus genomes that are required for retroviral transforming functions.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando T. A nuclease specific for heat-denatured DNA in isolated from a product of Aspergillus oryzae. Biochim Biophys Acta. 1966 Jan 18;114(1):158–168. doi: 10.1016/0005-2787(66)90263-2. [DOI] [PubMed] [Google Scholar]
- Baltimore D., Gilboa E., Rothenberg E., Yoshimura F. Production of a discrete, infectious, double-stranded DNA by reverse transcription in virions of Moloney murine leukemia virus. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):869–874. doi: 10.1101/sqb.1979.043.01.093. [DOI] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Baltimore D. Tumor viruses: 1974. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1187–1200. doi: 10.1101/sqb.1974.039.01.137. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Heinemann R., Wilson G. L., Callahan R., Todaro G. J. Detection of baboon type C viral sequences in various primate tissues by molecular hybridization. J Virol. 1974 Jul;14(1):56–67. doi: 10.1128/jvi.14.1.56-67.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz E. W., Jr, Dina D. Moloney murine sarcoma virions synthesize full-genome-length double-stranded DNA in vitro. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3294–3298. doi: 10.1073/pnas.76.7.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Hayman M. J., Vogt P. K. Defectiveness of avian myelocytomatosis virus MC29: isolation of long-term nonproducer cultures and analysis of virus-specific polypeptide synthesis. Virology. 1977 Oct 15;82(2):431–448. doi: 10.1016/0042-6822(77)90017-4. [DOI] [PubMed] [Google Scholar]
- Bister K., Löliger H. C., Duesberg P. H. Oligoribonucleotide map and protein of CMII: detection of conserved and nonconserved genetic elements in avian acute leukemia viruses CMII, MC29, and MH2. J Virol. 1979 Oct;32(1):208–219. doi: 10.1128/jvi.32.1.208-219.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Cordell B., Weiss S. R., Varmus H. E., Bishop J. M. At least 104 nucleotides are transposed from the 5' terminus of the avian sarcoma virus genome to the 5' termini of smaller viral mRNAs. Cell. 1978 Sep;15(1):79–91. doi: 10.1016/0092-8674(78)90084-3. [DOI] [PubMed] [Google Scholar]
- Donoghue D. J., Sharp P. A., Weinberg R. A. An MSV-specific subgenomic mRNA in MSV-transformed G8-124 cells. Cell. 1979 May;17(1):53–63. doi: 10.1016/0092-8674(79)90294-0. [DOI] [PubMed] [Google Scholar]
- Donoghue D. J., Sharp P. A., Weinberg R. A. Comparative study of different isolates of murine sarcoma virus. J Virol. 1979 Dec;32(3):1015–1027. doi: 10.1128/jvi.32.3.1015-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist L. W., Madden M. J., Schiop-Stanley P., Vande Woude G. F. Cloning of herpes simplex type 1 DNA fragments in a bacteriophage lambda vector. Science. 1979 Feb 9;203(4380):541–544. doi: 10.1126/science.216076. [DOI] [PubMed] [Google Scholar]
- Essex M. Horizontally and vertically transmitted oncornaviruses of cats. Adv Cancer Res. 1975;21:175–248. doi: 10.1016/s0065-230x(08)60973-2. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Blevins C. S., Nomura S. Simple, quantitative assay for both xenotropic murine leukemia and ecotropic feline leukemia viruses. J Virol. 1974 Jul;14(1):177–179. doi: 10.1128/jvi.14.1.177-179.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. E., Gilbert J. H., Porzig K. J., Scolnick E. M., Aaronson S. A. Nature and distribution of feline sarcoma virus nucleotide sequences. J Virol. 1979 Jun;30(3):821–827. doi: 10.1128/jvi.30.3.821-827.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Rongey R. W., Arnstein P., Estes J. D., Sarma P., Huebner R. J., Rickard C. G. Experimental transmission of feline fibrosarcoma to cats and dogs. Nature. 1970 May 30;226(5248):807–809. doi: 10.1038/226807a0. [DOI] [PubMed] [Google Scholar]
- Gilboa E., Goff S., Shields A., Yoshimura F., Mitra S., Baltimore D. In vitro synthesis of a 9 kbp terminally redundant DNA carrying the infectivity of Moloney murine leukemia virus. Cell. 1979 Apr;16(4):863–874. doi: 10.1016/0092-8674(79)90101-6. [DOI] [PubMed] [Google Scholar]
- Hager G. L., Chang E. H., Chan H. W., Garon C. F., Israel M. A., Martin M. A., Scolnick E. M., Lowy D. R. Molecular cloning of the Harvey sarcoma virus closed circular DNA intermediates: initial structural and biological characterization. J Virol. 1979 Sep;31(3):795–809. doi: 10.1128/jvi.31.3.795-809.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Kleid D. G., Panet A., Rothenberg E., Baltimore D. Ordered transcription of RNA tumor virus genomes. J Mol Biol. 1976 Sep 5;106(1):109–131. doi: 10.1016/0022-2836(76)90303-x. [DOI] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I. C., Lieber M. M., Todaro G. J. Mink cell line Mv 1 Lu (CCL 64). Focus formation and the generation of "nonproducer" transformed cell lines with murine and feline sarcoma viruses. Virology. 1974 Jul;60(1):282–287. doi: 10.1016/0042-6822(74)90386-9. [DOI] [PubMed] [Google Scholar]
- Hsu T. W., Sabran J. L., Mark G. E., Guntaka R. V., Taylor J. M. Analysis of unintegrated avian RNA tumor virus double-stranded DNA intermediates. J Virol. 1978 Dec;28(3):810–818. doi: 10.1128/jvi.28.3.810-818.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. S., Lai M. M., Vogt P. K. Genome of avian myelocytomatosis virus MC29: analysis by heteroduplex mapping. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1265–1268. doi: 10.1073/pnas.76.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Stephenson J. R. Feline leukemia virus: biochemical and immunological characterization of gag gene-coded structural proteins. J Virol. 1977 Sep;23(3):599–607. doi: 10.1128/jvi.23.3.599-607.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- McDonough S. K., Larsen S., Brodey R. S., Stock N. D., Hardy W. D., Jr A transmissible feline fibrosarcoma of viral origin. Cancer Res. 1971 Jul;31(7):953–956. [PubMed] [Google Scholar]
- Mellon P., Pawson A., Bister K., Martin G. S., Duesberg P. H. Specific RNA sequences and gene products of MC29 avian acute leukemia virus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5874–5878. doi: 10.1073/pnas.75.12.5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson M. K., Robey W. G., Harris C. L., Fischinger P. J., Haapala D. K., Vande Woude G. F. A p60 polypeptide in the feline leukemia virus pseudotype of Moloney sarcoma virus with murine leukemia virus p30 antigenic determinants. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2380–2384. doi: 10.1073/pnas.72.6.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porzig K. J., Barbacid M., Aaronson S. A. Biological properties and translational products of three independent isolates of feline sarcoma virus. Virology. 1979 Jan 15;92(1):91–107. doi: 10.1016/0042-6822(79)90217-4. [DOI] [PubMed] [Google Scholar]
- Robbins K. C., Barbacid M., Porzig K. J., Aaronson S. A. Involvement of different exogenous feline leukemia virus subgroups in the generation of independent feline sarcoma virus isolates. Virology. 1979 Aug;97(1):1–11. doi: 10.1016/0042-6822(79)90367-2. [DOI] [PubMed] [Google Scholar]
- Rothenberg E., Donoghue D. J., Baltimore D. Analysis of a 5' leader sequence on murine leukemia virus 21S RNA: heteroduplex mapping with long reverse transcriptase products. Cell. 1978 Mar;13(3):435–451. doi: 10.1016/0092-8674(78)90318-5. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Log T. Subgroup classification of feline leukemia and sarcoma viruses by viral interference and neutralization tests. Virology. 1973 Jul;54(1):160–169. doi: 10.1016/0042-6822(73)90125-6. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Donner L., Turek L. P. Restriction endonuclease mapping of unintegrated proviral DNA of Snyder-Theilen feline sarcoma virus: localization of sarcoma-specific sequences. J Virol. 1979 Dec;32(3):860–875. doi: 10.1128/jvi.32.3.860-875.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Sen A., Todaro G. J., Sliski A., Essex M. Pseudotypes of feline sarcoma virus contain an 85,000-dalton protein with feline oncornavirus-associated cell membrane antigen (FOCMA) activity. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1505–1509. doi: 10.1073/pnas.75.3.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Todaro G. J., Sliski A., Essex M. Characterization of a feline sarcoma virus-coded antigen (FOCMA-S) by radioimmunoassay. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4489–4493. doi: 10.1073/pnas.75.9.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Sen A. A rapid method for the purification of extrachromosomal DNA from eukaryotic cells. J Biol Chem. 1978 Oct 10;253(19):6654–6656. [PubMed] [Google Scholar]
- Sliski A. H., Essex M., Meyer C., Todaro G. Feline oncornavirus-associated cell membrane antigen: expression in transformed nonproducer mink cells. Science. 1977 Jun 17;196(4296):1336–1339. doi: 10.1126/science.194310. [DOI] [PubMed] [Google Scholar]
- Snyder S. P., Theilen G. H. Transmissible feline fibrosarcoma. Nature. 1969 Mar 15;221(5185):1074–1075. doi: 10.1038/2211074a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stacey D. W., Allfrey V. G., Hanafusa H. Microinjection analysis of envelope-glycoprotein messenger activities of avian leukosis viral RNAs. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1614–1618. doi: 10.1073/pnas.74.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg N., Tiemeier D., Enquist L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene. 1977 May;1(3-4):255–280. doi: 10.1016/0378-1119(77)90049-x. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Thomas M., Cameron J. R., Davis R. W. Viable molecular hybrids of bacteriophage lambda and eukaryotic DNA. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4579–4583. doi: 10.1073/pnas.71.11.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., White R. L., Davis R. W. Hybridization of RNA to double-stranded DNA: formation of R-loops. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2294–2298. doi: 10.1073/pnas.73.7.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Coffin J. M. Recombination between the defective component of an acute leukemia virus and Rous associated virus O, an endogenous virus of chickens. Proc Natl Acad Sci U S A. 1979 Jun;76(6):3001–3005. doi: 10.1073/pnas.76.6.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vande Woude G. F., Oskarsson M., Enquist L. W., Nomura S., Sullivan M., Fischinger P. J. Cloning of integrated Moloney sarcoma proviral DNA sequences in bacteriophage lambda. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4464–4468. doi: 10.1073/pnas.76.9.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M. Genome organization of RNA tumor viruses. I. In vitro synthesis of full-genome-length single-stranded and double-stranded viral DNA transcripts. J Virol. 1978 Jun;26(3):615–629. doi: 10.1128/jvi.26.3.615-629.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Baltimore D. Preparation of syngeneic tumor regressor serum reactive with the unique determinants of the Abelson murine leukemia virus-encoded P120 protein at the cell surface. J Virol. 1979 Sep;31(3):776–784. doi: 10.1128/jvi.31.3.776-784.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Paskind M., Shields A., Baltimore D. Identification of an Abelson murine leukemia virus-encoded protein present in transformed fibroblast and lymphoid cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2488–2492. doi: 10.1073/pnas.75.5.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]