Abstract
High-risk human papillomaviruses cause certain anogenital and head and neck cancers. E6, one of three potent HPV oncogenes that contribute to the development of these malignancies, is a multifunctional protein with many biochemical activities. Among these activities are its ability to bind and inactivate the cellular tumor suppressor p53, induce expression of telomerase, and bind to various other proteins including Bak, E6BP1, E6TP1, and proteins that contain PDZ domains such as hScrib and hDlg. Many of these activities are thought to contribute to E6’s role in carcinogenesis. E6’s interaction with many of these cellular proteins, including p53, leads to their destabilization. This property is mediated at least in part through E6’s ability to recruit the ubiquitin ligase, E6AP into complexes with these cellular proteins resulting in their ubiquitin–mediated degradation by the proteasome. In this study, we address the requirement for E6AP in mediating E6's acute and oncogenic phenotypes, including induction of epithelial hyperplasia, abrogation of DNA damage response and induction of cervical cancer. Loss of E6AP had no discernable effect on E6's ability to induce hyperplasia or abrogate DNA damage responses, akin to what we had earlier observed in the mouse epidermis. Nevertheless, in cervical carcinogenesis studies, there was a complete loss of E6’s oncogenic potential in mice nulligenic for E6AP. Thus, E6AP is absolutely required for E6 to cause cervical cancer.
Keywords: E6AP, E6, p53, HPV and Cervical cancer
Introduction
Human papillomaviruses (HPVs) are etiologically associated with cancers of the head and neck and the anogenital tract, which includes the cervix. Infection with HPVs is attributed with almost all cases of cervical cancer (1, 2). Cervical cancer accounts for approximately 5% of the world’s total tumor burden (3) and is the second leading cause of cancer-related deaths among women (4, 5). Approximately 70% of all cervical cancers are attributed to infection with the “high-risk” HPV types −16 or −18. In cervical cancers, two HPV genes, E6 and E7 are expressed (6, 7). HPV E6 and E7 have transforming and oncogenic properties in tissue culture (8, 9) and in animal models (10–12). E6 and E7 are primarily known for their abilities to target the tumor suppressors, p53 (13, 14) and pRb (15) respectively, for inactivation and ubiquitin-mediated degradation.
High-risk E6 is known to target p53 for ubiquitin-mediated degradation through its interaction with the E3 ligase, E6-associated protein (E6AP). Similarly, E6 has been found to induce the degradation of other cellular proteins and in many cases it is mediated through interactions with E6AP (16, 17). While the majority of data support the requirement of E6AP in E6-mediated degradation of p53 (18, 19), our previous studies in the mouse skin indicate that HPV16 E6 is able to target p53 for inactivation and degradation in the absence of E6AP (20). Results from other groups using either in vitro or in vivo systems also suggest the existence of E6AP-independent means for high-risk HPV E6 to target p53 for ubiquitin-mediated degradation (21, 22). Therefore, it remained unclear what role E6AP would have in E6-mediated acute and oncogenic phenotypes in the context of the cervix.
In mice, E6 and E7 cooperate to induce cervical carcinogenesis (23, 24). HPV E6 induces cervical cancer both through its ability to inactivate p53 and other cellular proteins including PDZ containing proteins that interact directly with E6 (24, 25). Similar requirements were needed for E6 to induce skin cancers in mice (26). Because HPV16 E6 can target p53 for inactivation in the skin through E6AP-independent means (20), it was of interest to determine the importance of E6AP in cervical carcinogenesis.
Further interest fueled our pursuits of E6AP in the cervix. In addition to its function as an E3 ubiquitin ligase, E6AP also functions as a steroid receptor coactivator (27) and may be differentially regulated in cancers of the breast and prostate (28, 29). Recent evidence demonstrate that the nuclear hormone receptor, ERα, is a necessary cofactor in HPV-mediated cervical carcinogenesis (30). Given the involvement of E6AP in the regulation of nuclear hormone receptors, its importance in cervical cancer warranted further investigation.
To determine E6AP’s role in the oncogenic effects of E6, we generated HPV transgenic mice on an FRAP-null (E6AP−/−) background. Our results demonstrate that in the cervix, loss of E6AP ablates E6’s oncogenic activity. We conclude that E6AP is a critical mediator of HPV16 E6’s oncogenic activities in the cervix.
Materials and Methods
Mice
The mouse lines, K14E6WT (31), K14E7WT (10) and E6AP−/− (20, 32) were maintained on the inbred FVB/n background. All mice were bred and maintained in the American Association for Accreditation of Laboratory Animal Care-approved McArdle Laboratory Animal Care Facility in accordance with an institutionally approved animal protocol.
Estrogen treatment and analysis of reproductive tracts
For acute studies, 5-week old virgin female mice were treated with 17β-estradiol for six weeks to achieve a state of persistent estrus (24, 25). Mice acutely treated with estrogen were either exposed to 10Gy of ionizing radiation from a 137Cs source or left untreated and sacrificed 24 hours later. One hour prior to sacrifice, the nucleotide analog, 5-bromo-2’-deoxyuridine (BrdUrd, 12.5mg/mL in PBS) was injected intraperitoneally at 10µl /1g body weight to label any newly synthesized DNA.
For acute studies that require ovariectomy, 8-week old virgin female mice were ovariectomized and were allowed to finish the remaining cycle and recover for 10 days. The mice were then injected with either estrogen (30µg/kg weight) or ethanol (EtOH) vehicle and sacrificed 24 hours later. Animals were perfused with 4% paraformaldehyde and reproductive tracts were harvested for analysis.
For long-term cancer studies, five-week old virgin female mice were either untreated or treated with 17β-estradiol for a period of six to nine months after which, the reproductive tracts were harvested and fixed according to previously described methods (23) and sectioned. Every tenth, 5-µm section was stained with hematoxylin and eosin and evaluated histopathologically, with the worst lesion scored as the final diagnosis. The severity of disease was evaluated by taking the spectrum of lesions within a given group and given a score of 0–5. Specifically, a diagnosis of hyperplasia is given a value of 0; CIN1 (cervical intraepithelial neoplasia 1) a value of 1 and increases progressively with each higher lesion grade to a score of 5 for LIC (large invasive cancer).
Quantification of DNA synthesis and DNA damage response
To quantify the levels of DNA synthesis in the epithelium of the cervix, basal and suprabasal BrdUrd-positive cells were counted and divided by the total number of cells and multiplied by 100 to determine the percentage. To quantify the DNA damage response, the total number of BrdUrd-positive cells were counted and divided by the total number of cells in the epithelium and multiplied by 100. BrdUrd was counted in at least 8, 40× microscopic fields per mouse, with a total of at least 3 or more mice per genotype group.
Statistical Analysis
A two-sided Wilcoxon-Rank Sum test was used for determining statistical significance in the quantification of DNA synthesis, tumor multiplicity, tumor burden and severity of disease. A two-sided Fisher’s Exact test was used in determining significance for cancer incidence.
Immunohistochemistry and Immunofluorescence
Conditions for IHC or IF for the following antibodies BrdUrd (Calbiochem, #NA20), p53 (Novacastra, CM5), MCM7 (NeoMarkers, # ab47DC141) and ERα (Santa Cruz, MC20) were previously described (20, 24, 25).
Results
E6-induced cervical epithelial hyperplasia is not dependent on E6AP
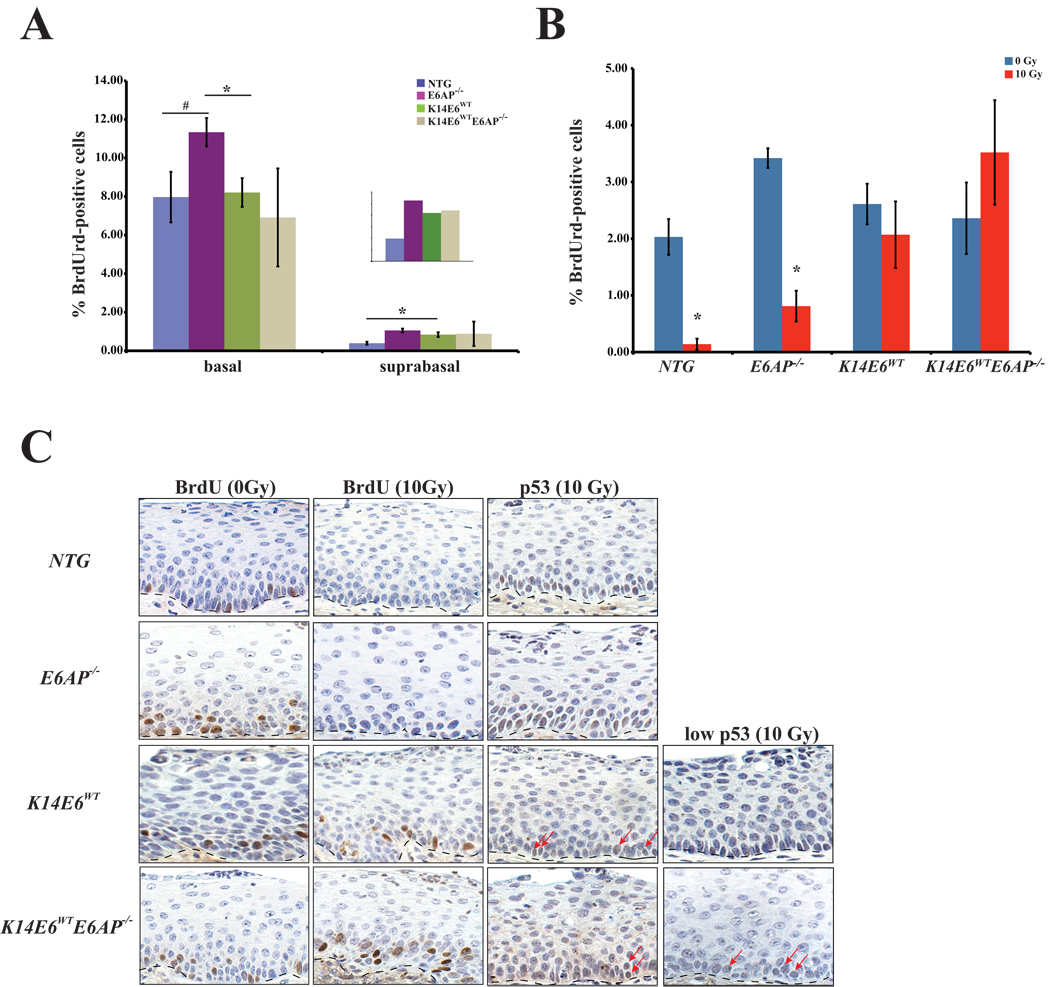
Previous results indicate that the ubiquitin ligase, E6AP is not required for E6-induced epithelial hyperplasia in the skin (20). To determine whether E6AP is required for E6-induced cervical epithelial hyperplasia, we crossed K14E6WT transgenic mice onto an E6AP−/− background and monitored for proliferation in young female mice treated acutely with estrogen. Acute treatment with estrogen is necessary to avoid variability in the thickness of the cervical epithelium and synchronizes all mice. Animals were injected with the nucleotide analog, bromodeoxyuridine (BrdUrd) one-hour prior to sacrifice. Proliferation was subsequently measured by quantifying the incorporation of BrdUrd into nascently synthesized DNA via BrdUrd-specific immunohistochemistry (IHC). Similar to previous results, K14E6WT transgenic mice were able to induce hyperplasia in the cervical epithelium as indicated by higher levels of suprabasal DNA synthesis relative to nontransgenic (NTG) mice (0.9% vs 0.4% respectively, p= 0.03, Fig. 1A inset). Loss of E6AP in K14E6WT transgenic mice (K14E6WTE6AP−/−) did not result in a loss of epithelial hyperplasia (0.9%) and was not different than K14E6WT mice (p=1). Surprisingly, the loss of E6AP in the absence of HPV E6 (E6AP−/−) also resulted in significantly higher levels of suprabasal DNA synthesis relative to NTG mice (1.0% vs. 0.4% respectively, p=0.02). Thus E6AP is not the mediator of HPV-E6 induced hyperplasia.
Figure 1. Examination of acute effects in HPV E6 transgenic mice on an E6AP-deficient background in the cervix.
A. The graph shows the quantification of DNA synthesis in the cervical epithelium of female mice acutely treated with estrogen to synchronize mice in estrus. Groups of three or more mice per genotype were injected with BrdUrd one hour prior to sacrifice. Formalin fixed, paraffin embedded (FFPE) tissue was subjected to BrdUrd-specific immunohistochemistry and the percentage of BrdUrd-positive cells were counted both in the basal and suprabasal layers in at least eight 40× frames/mouse tissue, as a measure of DNA synthesis. Epithelial hyperplasia is defined by an increase in the percentage of suprabasal BrdUrd-positive cells. E6AP−/− mice have a marginal increase in the number of basal BrdUrd-positive cells relative to NTG (p=0.08, #) mice and is significantly different than K14E6WT mice (p=0.03, *). The percentage of suprabasal BrdUrd-positive cells was significantly higher in both E6AP−/− and K14E6WT groups (p<0.05) relative to NTG mice. Loss of E6AP in the presence of HPV E6 did not prevent the induction of epithelial hyperplasia (p=1). The inset is a magnification of the number of the BrdUrd-positive cells in the suprabasal layers.
B. The graph shows the quantification of DNA synthesis in the cervical epithelium of females acutely treated with estrogen and either given 10Gy of ionizing radiation or not treated. Groups of three or more mice per genotype were injected with BrdUrd 23 hours post irradiation and one hour later tissue was harvested. Like size groups of age matched mice not exposed to irradiation served as controls. Percentage BrdUrd-positive cells was quantified as described in A. Following irradiation, significant reductions in the percentage of cells supporting DNA synthesis were observed in both NTG (*, p=0.01) and E6AP−/− (*, p=0.03) groups. Both K14E6 WT and K14E6 WTE6AP−/− groups were abrogated in this DNA damage response, maintaining the levels of DNA synthesis seen in the control groups for each genotype (both p>0.05).
C. Shown are sections of cervical epithelium from mice acutely treated with exogenous estrogen and stained for BrdUrd or p53 by IHC. Mice were either unirradiated or irradiated and sacrificed 24hours later. Dotted lines in each picture demarcate the cervical epithelium from the underlying stroma. DNA synthesis, as represented by BrdUrd positive staining nuclei, is reduced upon radiation in nontransgenic mice on either an E6AP-sufficient or insufficient background (first and second columns). In contrast, both K14E6WT and K14E6WTE6AP−/− retain the ability to synthesize DNA. p53 is undetectable in the absence of DNA damage (data not shown), however is induced in NTG and E6AP−/− mice upon radiation (third column). K14E6WT and K14E6WTE6AP−/− mice display variable levels of p53 upon irradiation. Arrows point to examples of p53-positive cells. Examples of a strong inhibition of the DNA damage response as exemplified by either a lack of (K14E6WT) or a few (K14E6WTE6AP−/−) p53-positive cells in the epithelium are also shown (fourth column).
A trivial explanation for our observation that E6AP is not required for E6-induced cell proliferation is that E6 is itself causing lower steady state levels of E6AP. To determine if this is the case, we performed E6AP-specific western analysis. We observed no obvious differences in the levels of E6AP in NTG versus K14E6WT, K14E7WT or K14E6WTE7WT transgenic tissues (Fig. S1). Thus in our mouse model E6 does not alter levels of E6AP.
Inhibition of the DNA damage response by HPV E6 in the absense of E6AP
Previous results demonstrate that HPV E6 transgenic mice retain the ability to inhibit DNA damage responses in response to ionizing radiation in the epidermis of the mouse skin despite the absence of E6AP (20). Given these results, we wanted to determine whether HPV E6 maintains the ability to inhibit the DNA damage response in the absence of E6AP in the cervical epithelium. Mice acutely treated with estrogen were either given 10 Gy of ionizing radiation or left untreated and harvested 24-hours later. One hour prior to sacrifice, all of the mice were injected with BrdUrd to label nascently synthesized DNA. We assessed two different responses to DNA damage: (1) the ability to cease DNA synthesis and (2) the induction and subsequent accumulation of p53. As expected, DNA synthesis was significantly reduced in both NTG (2.0% to 0.3%, p=0.01) and E6AP−/− (3.4% to 0.8%, p=0.03) mice upon radiation (Fig. 1B, C). Similar to previous results observed in the skin, DNA synthesis in K14E6WT transgenic mice was not significantly changed upon radiation (2.6% vs 2.1%, p=0.09). Interestingly, K14E6WTE6AP−/− transgenic mice did not show a reduction in DNA synthesis upon DNA damage (2.3% vs 3.5%, p=0.16), suggesting that in the cervical epithelium, E6AP is not required for E6’s targeted inactivation of p53 in inhibiting the DNA damage response. This result is in kind to that observed previously in the skin (20).
To address whether E6 can prevent the accumulation of p53 after DNA damage in the absence of E6AP, IHC for p53 was performed on sections from both unirradiated and irradiated mice. In the absence of any DNA damage, p53 was undetectable in all genotypes (data not shown). As expected, p53 was highly induced in the cervical epithelium of NTG and E6AP−/− mice upon radiation (Fig. 1C). In contrast to skin where E6 is able to largely prevent induction of detectable p53 following irradiation, p53 levels in the irradiated K14E6WT cervix varied from nil to modest levels, with positive cells found predominantly in the basal layer. Thus in the cervix, E6 partially prevents the accumulation of p53 in response to irradiation, even though it efficiently prevents DNA damage-associated growth arrest (Fig. 1B). The cervical epithelia of irradiated K14E6WTE6AP−/− transgenic mice also displayed low to modest levels of p53 protein, similar to K14E6WT mice, indicating that E6AP is not required for E6's ability to prevent accumulation of p53 following irradiation. This is similar to what was observed in the skin of E6AP-null mice (20).
The acute response to estrogen is affected in mice without E6AP
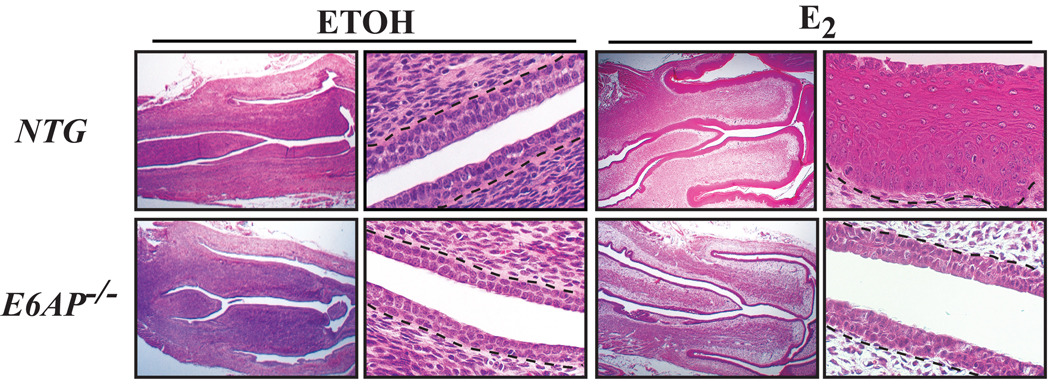
In addition to its function as an ubiquitin ligase, E6AP also functions as a steroid receptor coactivator (27). E6AP has been reported to target various nuclear hormone receptors (29) including estrogen receptor alpha (ERα) for ubiquitin-mediated degradation (33). Because E6AP is reported to play a role in estrogen signaling, we investigated whether loss of E6AP has any effect in the cervix, where estrogen signaling plays an important role. Effects from estrogen signaling can be observed quickly, and thus the acute response to estrogen was assessed in groups of NTG and E6AP−/− mice. Mice were ovariectomized to eliminate endogenous levels of estrogen and treated 10 days later with either vehicle or estrogen for 24-hours and after which the reproductive tracts were harvested. Ovariectomy results in a thin epithelium throughout the reproductive tract due to a lack of proliferation signals from ovarian hormones. The normal response to estrogen is an increase in uterine wet weight (34) and the induction of epithelial hyperplasia. The epithelium from NTG and E6AP−/− groups treated with ETOH vehicle were hypoplastic. Upon estrogen treatment, the majority of reproductive tracts from NTG mice were overtly (data not shown) and histologically thickened (Fig. 2). Acute treatment of E6AP−/− mice with estrogen, on the contrary, did not result in either an overt or a histologically thickened epithelium. It is not clear why this is the case, because at the time of sacrifice, the proliferative index in the cervical epithelium of these mice was similar to NTG mice as assessed by BrdUrd incorporation over a one hour period prior to sacrifice, and there was no detectable programmed cell death (data not shown). One possible explanation is that there is a slight delay in the response of the E6AP-null tissue to estrogen, given that E6AP-null tissue does undergo hyperplasia when treated long term with estrogen (see below).
Figure 2. Acute responses to estrogen partially require E6AP.
Shown are H/E sections of cervix from mice that were ovariectomized and treated either with ETOH or estrogen (E2) for 24hours. The first and third columns are sections taken at 1.25× magnification, while the second and fourth columns are sections taken at 40× magnification. Note the relative lack of epithelial hyperplasia in E6AP−/− mice relative to NTG mice upon treatment with estrogen (far right column).
E6-induced cervical carcinogenesis is dependent upon E6AP
Given that ERα signaling is affected in E6AP−/− mice, we investigated whether loss of E6AP would result in a reduced ability of HPV E6 to induce cervical cancer. We therefore compared mice nulligenic for E6AP and bred them to K14E6WT mice. K14E6WT mice develop cervical cancer after treatment with physiological levels of estrogen for nine months (24). Cervical lesions that arise in these mice are histopathologically similar to that found in HPV16-infected women. Specifically, we observe a progressive disease that includes the development of cervical intraepithelial neoplasia (CIN) grades 1–3, followed by the onset of frank cancer of the cervix. We therefore compared the severity of cervical disease by comparing the complete spectrum of lesions observed within any given group (see Methods for details). Similar to previous findings, K14E6WT mice have an increase in the severity of disease, which was significantly different from NTG mice (p≪0.001). K14E6WT transgenic mice have an increase in tumor incidence, multiplicity and tumor burden (as defined as the average of area of tumor mass) relative to NTG mice (Suppl. Fig. 2, Table 1 & Table 2). In K14E6WTE6AP−/− transgenic mice, however, the severity of disease was significantly reduced (p≪0.001) when compared to K14E6WT. Specifically, K14E6WTE6AP−/− mice have reductions in the incidence of cervical cancer (6.3%, p=0.04), tumor multiplicity (0.13, p=0.06) and tumor burden (p=0.04). Indeed, the incidence of cervical disease in the K14E6 WTE6AP−/− mice was not significantly different from that observed in nontransgenic mice. These results indicate that E6’s capacity to induce cervical cancer was completely abrogated on an E6AP-null background.
Table 1.
Summary of Disease in the Cervix and Vagina of Mice After 9 Months with Estrogen
| Mouse Genotype |
Severity of Diseasea |
||||||
|---|---|---|---|---|---|---|---|
| H | CIN1 | CIN2 | CIN3 | MIC | LIC | ||
| cervix | NTG | 17 | 3 | 1 | 2 | ||
| E6AP−/− | 2 | 11 | 2 | 1 | 3 | ||
| K14E6WTb | 3 | 9 | 7 | 8 | 5 | 7 | |
| K14E6WTE6AP−/−c | 1 | 12 | 2 | 1 | |||
| vagina | H | VAIN1 | VAIN2 | VAIN3 | MIC | LIC | |
| NTG | 16 | 7 | |||||
| E6AP−/− | 1 | 18 | |||||
| K14E6WTb | 9 | 12 | 15 | 2 | 1 | ||
| K14E6WTE6AP−/−c | 5 | 9 | 2 | ||||
Cohorts of female mice of the indicated genotypes were treated with estrogen for 9 months and the reproductive tracts from every mouse was scored for the worst state (severity) of cervical (top half) or vaginal (bottom half of table) disease. The overall severity of disease between different genotypes was compared using a two-sided Wilcoxon-rank sum test. Abbreviations: H, hyperplasia; CIN, cervical intraepithelial hyperplasia; MIC, microinvasive cancer; LIC, large invasive cancer; VAIN, vaginal intraepithelial neoplasia.
The shift in the severity of disease in K14E6WT transgenic mice is highly significant relative to NTG and E6AP−/− mice (p ≪ 0.001).
Loss of E6AP results in a highly significant reduction in the severity of disease in K14E6WTE6AP−/− in the cervix (p≪0.001) and a significant reduction in the vagina (p = 0.05) relative to K14E6WT mice.
Table 2.
Summary of Tumor Characteristics in the Cervix and Vagina
| Genotype | Cancer Incidence# (%) |
LIC Incidence (%) |
Tumor Multiplicity (tumors/ mouse) |
Tumor Burden (Area of Tumor Invasion/Mouse) (mm2) |
|---|---|---|---|---|
| NTG (n=23) | 8.7 | 0 | 0.09 | 5 × 10−3 |
| E6AP−/− (n=19) | 15.8 | 0 | 0.21 | 2 × 10−3 |
| K14E6WT (n=39) | 30.8 | 17.9 b | 0.51d | 0.39 f |
| K14E6WTE6AP−/− (n=16) | 6.3 a | 0 b | 0.13 e | 3 × 10−3g |
| NTG (n=7) | 0 | 0 | 0 | 0 |
| K14E7WT (n=22) | 50 | 9.1 | 1.8 | 0.17 |
| K14E7WTE6AP−/− (n=7) | 57.1 | 14.3 c | 2.3 | 0.38 |
| K14E6WTE7WT (n=17) | 82.4 | 41 | 5.6 | 1.31 |
| K14E6WTE7WTE6AP−/− (n=6) | 50 | 0 | 3.2 | 0.15 h |
NOTE: Animals from the top half of the table (above double line) were treated with estrogen for 9 months, while the bottom half of the table shows animals from a 6-month treatment. All statistical tests were two-sided.
Cancer incidence was defined as the incidence of cancer that occurred in the cervix and the lower reproductive tract combined.
Loss of E6AP resulted in a reduction in cancer incidence in K14E6WTE6AP−/− and was significantly different than K14E6WT transgenic mice (p=0.04).
Loss of E6AP resulted in a marginally significant reduction in the formation of LIC in K14E6WTE6AP−/− relative to K14E6WT transgenic mice (p=0.09).
Loss of E6AP did not result in a reduction in the percentage of LIC in K14E7WTE6AP−/− and was not different than K14E7WT transgenic mice (p > 0.05).
Tumor multiplicity is significantly higher in K14E6WT transgenic mice relative to NTG mice (p <0.05).
Loss of E6AP results in a reduction in tumor multiplicity in K14E6WTE6AP−/− relative to K14E6WT transgenic mice (p=0.06).
K14E6WT transgenic mice have significantly larger tumors than NTG mice (p <0.05).
Loss of E6AP results in a significant reduction in tumor size in K14E6WTE6AP−/− relative to K14E6WT transgenic mice (p < 0.05).
Loss of E6AP in K14E6WTE7WTE6AP−/− transgenic mice resulted in smaller tumors relative to K14E6WTE7WT transgenic mice (p=0.07).
In human cervical cancers, E6 is always expressed with E7, a second HPV oncogene. HPV16 E7 is the more potent oncogene in the mouse cervix, as K14E7WT mice develop cervical cancers after only treatment with six months of 17β-estradiol (23). In comparison, K14E6WT mice only develop cervical cancers when treated for nine months (24). Double transgenic mice (K14E6WTE7WT) expressing both HPV16 E6 and E7, develop larger cancers than K14E7WT mice when treated for six months with 17β-estradiol (23). To assess the importance of E6AP in cervical carcinogenesis in the presence of both E6 and E7, we assessed the severity and incidence of cervical disease in K14E6WTE7WT mice on an E6AP-sufficent or nulligenic background after treatment with estrogen for six months (Suppl. Fig. 2, Table 2 & Table 3). Histopathological diagnosis revealed that in the absence of E6AP (K14E6WTE7WTE6AP−/−), the severity of disease is significantly reduced in the cervix of HPV mice (p=0.03). Moreover, K14E6WTE7WTE6AP−/− transgenic mice did not show any incidence of large invasive cancers, as opposed to an incidence of 41% in K14E6WTE7WT mice on the E6AP-sufficient background. To determine if E6AP status had any influence on carcinogenesis when only E7 is expressed, we compared the severity and incidence of cervical disease in K14E7WT mice on an E6AP-sufficent or nulligenic background (Table 3). No significant differences in cervical disease were observed. Based upon these results we conclude that E6AP is specifically a critical cofactor for HPV16 E6 in cervical carcinogenesis.
Table 3.
Summary of Disease State in the Cervix and Vagina of Mice After 6 Months with Estrogen
| Mouse Genotype | Severity of Diseasea |
||||||
|---|---|---|---|---|---|---|---|
| H | CIN1 | CIN2 | CIN3 | MIC | LIC | ||
| cervix | NTG | 5 | 1 | 1 | |||
| K14E7WTb | 1 | 1 | 2 | 7 | 9 | 2 | |
| K14E7WTE6AP−/−b, c | 2 | 1 | 3 | 1 | |||
| K14E6WTE7WTb, d | 3 | 8 | 6 | ||||
| K14E6WTE7WTE6AP−/− b,e | 1 | 2 | 3 | ||||
| vagina | H | VAIN1 | VAIN2 | VAIN3 | MIC | LIC | |
| NTG | 5 | 1 | 1 | ||||
| K14E7WTb | 2 | 4 | 7 | 1 | 8 | ||
| K14E7WTE6AP−/− b,c | 2 | 4 | 1 | ||||
| K14E6WTE7WT b,d | 1 | 2 | 2 | 11 | 1 | ||
| K14E6WTE7WTE6AP−/−b, e | 1 | 2 | 2 | 1 | |||
Cohorts of female mice of the indicated genotypes were treated with estrogen for 6 months and the reproductive tracts from every mouse was scored for the worst state (severity) of cervical (top half) or vaginal (bottom half of table) disease. The overall severity of disease between different genotypes was compared using a two-sided Wilcoxon-rank sum test. Abbreviations: H, hyperplasia; CIN, cervical intraepithelial hyperplasia; MIC, microinvasive cancer; LIC, large invasive cancer; VAIN, vaginal intraepithelial neoplasia.
Regardless of E6AP status, the shift in the severity of disease in transgenic mice expressing HPV E7 in the presence or absence of E6 were all highly significant relative to NTG mice (p ≪ 0.001).
Loss of E6AP did not result in a reduction in the severity of disease in K14E7WTE6AP−/− and was not significantly different than K14E7WT.
The severity of disease is significantly increased in K14E6WTE7WT transgenic mice in the cervix (p=0.001) and in the lower reproductive tract (p=0.07) relative to K14E7WT mice.
Loss of E6AP resulted in a reduction in the severity of disease in K14E6WTE7WTE6AP−/− transgenic mice both in the cervix (p=0.006) and lower reproductive tract (p=0.01) relative to K14E6WTE7WT mice; the resulting severity of disease is similar to K14E7WTE6AP−/− mice (p >0.05).
A progressive disease leading to cancer is also observed in the vaginal cavity or lower reproductive tract (LRT) in our HPV transgenic mouse model for cervical carcinogenesis, consistent with the role of high-risk HPVs in vaginal cancers (35, 36). We therefore assessed the influence of E6AP on vaginal carcinogenesis. Similar findings were observed as in the cervix. In K14E6WT mice treated with estrogen for nine months, the absence of E6AP led to a complete abrogation of E6-dependent carcinogenesis in the vagina (Supp. Fig. 2, Table 1). Likewise, the contribution of E6 was lost in K14E6WTE7WT mice treated with estrogen for six months in K14E6WTE7WTE6AP−/− mice (Supp. Fig. 2, Table 3).
It is interesting to note that in our analysis of the influence of E6AP-status on the acute response to estrogen in the cervical epithelia, we observed a defect in hyperplasia induced by estrogen in the E6AP-deficient cervix of ovariectomized mice. Nevertheless, in our 9-month estrogen treated E6AP−/− mice we observed hyperplasia, dysplasia and even cancer at a low frequency, akin to what we observed in E6AP-sufficent mice (Table 1). These results indicate that any effect of E6AP-deficiency on estrogen's function in this tissue is not manifested in the long-term, and could simply reflect a slight delay in the acute response.
Reduced incidence of cervical cancer is not due to a lack of ERα expression
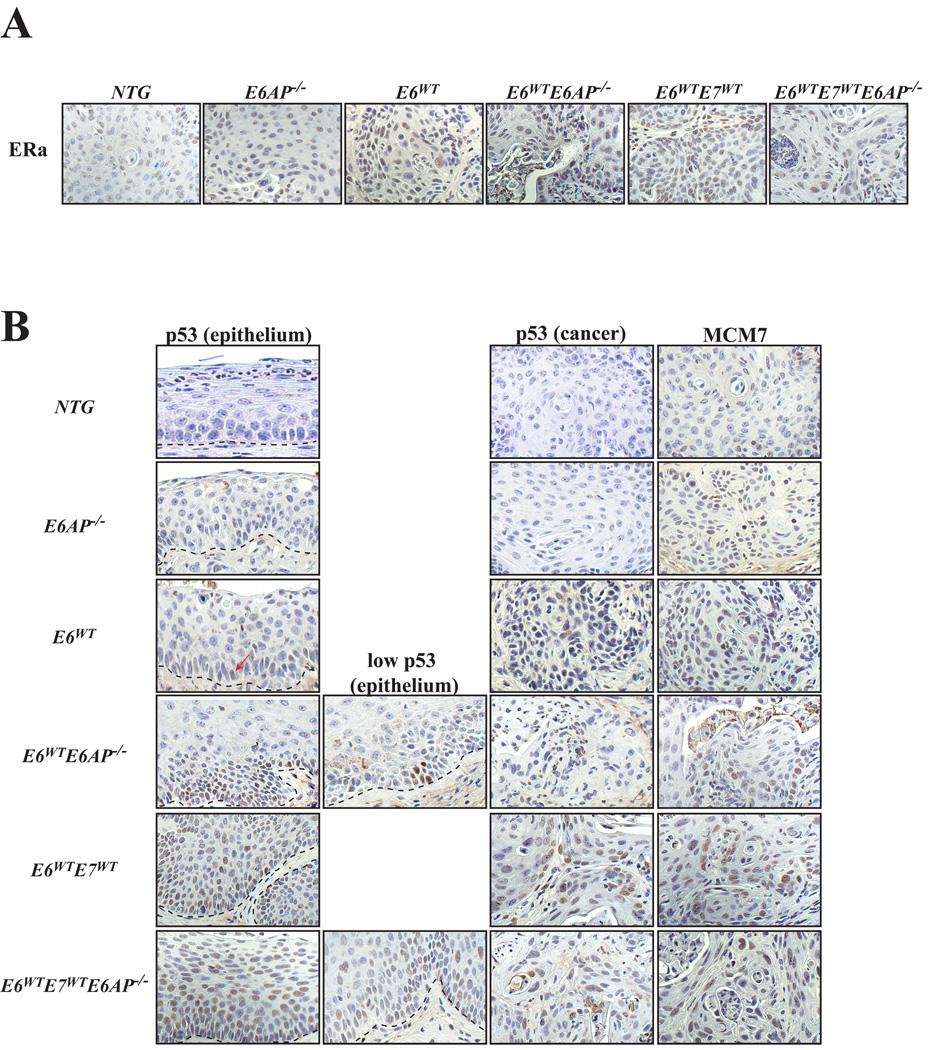
Given the acute and long-term results observed in mice on the E6AP−/− background, we wanted to determine whether the reduced incidence of cancer in K14E6WTE6AP−/− mice is due to a lack of ERα expression. Previous findings show that ERα is a necessary cofactor in cervical carcinogenesis in our estrogen-dependent HPV model (30). Thus IHC for ERα was performed on long-term treated mice. There were varying levels of ERα in reproductive tract epithelium across all genotypes (data not shown). No differences were observed in the expression of ERα between NTG and E6AP−/− cancers. In the presence of either HPV E6 or E7, the loss of E6AP resulted in minor reductions in the expression of ERα, however, the cancers still expressed appreciable levels of ERα (Figure 3A).
Figure 3. Immunohistochemical characterization of tumors from the reproductive tract.
A. Shown are sections of cancers taken from the reproductive tract from mice of the indicated genotypes and stained for ERα by IHC. All of the cancers from the various genotypes express ERα. All of the images are 40× magnification.
B. Shown are sections of epithelium (first & second column) or cancers (third column) taken from the reproductive tract from mice of the indicated genotypes and stained by IHC for either p53 or MCM7. Dotted lines in each picture demarcate the cervical epithelium from the underlying stroma. In the epithelium, p53 is not often detected, however, loss of E6AP in K14E6WTE6AP−/− mice results in the induction of p53 at either low or higher (second column) levels. p53 is detected both in the epithelium and cancers of K14E6WTE7WT and K14E6WTE7WTE6AP−/− as p53 is stabilized by HPV E7. MCM7 (fourth column) is expressed at low levels in cancers from NTG and E6AP−/− mice. Levels of MCM7 are increased in cancers that express HPV oncogenes, E6 and/or E7. All of the images are 40× magnification.
Reduced incidence of cancer is not a result of reduced proliferation or expression of the HPV biomarker, MCM7
To confirm that the reduction in the severity of disease and cancer incidence in HPV transgenic mice on the E6AP−/− background is not due to an inability to proliferate, IHC for BrdUrd was performed on NTG, E6AP−/−, K14E6WT, K14E6WE6AP−/−, K14E7WT, K14E7WTE6AP−/−, K14E6WTE7WT and K14E6WTE7WTE6AP−/− mice treated with estrogen for either six or nine months (data not shown). As expected, proliferation was low in NTG mice. Similar to acute studies reported above, E6AP−/− mice had higher levels of proliferation. No differences in the levels of BrdUrd were observed in comparing HPV transgenic (K14E6WT or K14E6WTE7WT) mice either on an E6AP-sufficient or insufficient background.
Mcm7 is a biomarker for HPV-positive lesions both in clinical human and murine samples (37). Cancers from the above genotypes were evaluated for the expression of MCM7. Cancers from NTG and E6AP−/− mice expressed low but detectable levels of MCM7. Mice expressing either HPV E6 and/or E7 generally had increased levels of MCM7 expression, driven mostly by E7 oncogene’s inactivation of pRb (38) or E6’s dysregulation of p16/pRb pathway (24, 39), both of which can lead to subsequent activation of E2F-target genes. Indeed, there were no significant differences in the expression of MCM7 between HPV transgenic mice on an E6AP-sufficient versus insufficient background (Fig 3B).
Loss of E6AP results in an increased frequency of detectable p53 in the reproductive tract
Due to the reduction in the incidence of cancer and the severity of disease in K14E6WTE6AP−/− mice, we wanted to determine whether the inability of E6 to degrade p53 accounted for these observations. To investigate this question, IHC for p53 was performed on mice treated with estrogen. Cancers from NTG, E6AP−/−, K14E6WT and K14E6WTE6AP−/− mice have little to no detectable p53 (Fig. 3B). However, focal expression of p53 is detectable in the reproductive epithelium of E6AP−/− and K14E6WT mice, with an appreciable increase in K14E6WTE6AP−/− mice. Expression of p53 was more likely to be detected in the epithelium of the LRT and thus may explain the rare occurrence of cancers in this region in mice that only express E6 and not the E7 transgene [(24, 36) and author’s observation]. Mice expressing the E7 transgene have detectable levels of p53 both in the dysplastic epithelium and cancers, likely due to E7’s ability to cause increases in the levels of p53 (40, 41). Accordingly, K14E6WTE7WTE6AP−/− transgenic mice have an appreciable increase in the expression of p53 over that of K14E6WE6AP−/− mice, which is evident both in the epithelium and in the cancers. In sum, the loss of E6AP results in an increased frequency of detectable p53 in the reproductive tract that correlated with decreases in cancer incidence and the severity of disease in E6 transgenic mice.
Discussion
In this study, we found that the loss of E6AP had little effect on acute activities of E6 in the cervix, yet had dramatic effects on the ability of E6 to synergize with estrogen to form cancers in the reproductive tract, in the presence or absence of E7. The loss of E6AP resulted in reductions in both cancer incidence and the severity of disease. In sum, this study demonstrates that E6AP plays a critical role in mediating HPV16 E6’s contributions to cervical cancer.
E6AP is not required for HPV E6-induced acute effects
Similar to previous observations in the skin of HPV E6 transgenic mice (20), E6AP was found to be required neither for the induction of epithelial hyperplasia nor for the inactivation of the DNA damage response in the reproductive tract. E6AP is thought to potentially mediate E6-induced hyperplasia due to its predicted ability to target the PDZ partners of E6, Scribble and Dlg for degradation. Loss of either of these tumor suppressor genes in Drosophila results in the development of epithelial hyperplasia, loss of cell-cell contacts and cancer development (42–44). In human cervical cancers, hScrib and hDlg becomes mislocalized and their levels progressively reduced (45, 46). Observations from the skin and the reproductive tract suggest that E6AP is either not the mediator of E6-induced epithelial hyperplasia and/or that in its absence there is alternative ubiquitin ligase that can target these proteins for degradation. Evidence from others suggests that the latter hypothesis may be the case. Work from Massimi and colleagues show that E6 maintains the ability to target both Scribble and Dlg for ubiquitin-mediated degradation in the absence of E6AP (22) in contrast to reports that argue for a dependence of E6AP (16). Moreover, the ability of E6 to inhibit the DNA damage response in both the skin and reproductive tract (this study) in the absence of E6AP similarly suggests that there are alternative means to inactivating p53. Alternatively proposed mechanisms by which E6 inactivates p53 include repression of transactivation of p53 target genes either by acetylation (47) or by E6’s interaction with CBP/p300 (48), which may be relevant here.
Implications of our findings
Our studies provide clear evidence for an essential role of E6AP in mediating HPV16 E6’s oncogenic activity, but leave unanswered the mechanism underlying its requirement. Specifically, those acute phenotypes of E6 that were measured were retained on the E6AP-deficient background despite the complete loss of neoplastic disease. Our results suggest that E6AP's role in mediating E6's activities has yet to be fully appreciated.
Supplementary Material
Acknowledgements
We thank Denis Lee for assistance with immunohistochemistry as well as Dr. Elaine Alarid and members of the Lambert laboratory for insightful discussions. This research was supported by grants from the National Cancer Institute (CA098428, CA022443, and CA009135).
References
- 1.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.zur Hausen H. Papillomavirus infections--a major cause of human cancers. Biochim Biophys Acta. 1996;1288:F55–F78. doi: 10.1016/0304-419x(96)00020-0. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24 Suppl 3 doi: 10.1016/j.vaccine.2006.05.111. S3/11–25. [DOI] [PubMed] [Google Scholar]
- 5.Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol. 2006;20:207–225. doi: 10.1016/j.bpobgyn.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Durst M, Kleinheinz A, Hotz M, Gissmann L. The physical state of human papillomavirus type 16 DNA in benign and malignant genital tumours. J Gen Virol. 1985;66(Pt 7):1515–1522. doi: 10.1099/0022-1317-66-7-1515. [DOI] [PubMed] [Google Scholar]
- 7.Jeon S, Lambert PF. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: implications for cervical carcinogenesis. Proc Natl Acad Sci U S A. 1995;92:1654–1658. doi: 10.1073/pnas.92.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63:4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawley-Nelson P, Vousden KH, Hubbert NL, Lowy DR, Schiller JT. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989;8:3905–3910. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herber R, Liem A, Pitot H, Lambert PF. Squamous epithelial hyperplasia and carcinoma in mice transgenic for the human papillomavirus type 16 E7 oncogene. J Virol. 1996;70:1873–1881. doi: 10.1128/jvi.70.3.1873-1881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arbeit JM, Howley PM, Hanahan D. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc Natl Acad Sci U S A. 1996;93:2930–2935. doi: 10.1073/pnas.93.7.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song S, Liem A, Miller JA, Lambert PF. Human papillomavirus types 16 E6 and E7 contribute differently to carcinogenesis. Virology. 2000;267:141–150. doi: 10.1006/viro.1999.0106. [DOI] [PubMed] [Google Scholar]
- 13.Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 14.Huibregtse JM, Scheffner M, Howley PM. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991;10:4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyson N, Howley PM, Munger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto Y, Nakagawa S, Yano T, et al. Involvement of a cellular ubiquitin-protein ligase E6AP in the ubiquitin-mediated degradation of extensive substrates of high-risk human papillomavirus E6. J Med Virol. 2006;78:501–507. doi: 10.1002/jmv.20568. [DOI] [PubMed] [Google Scholar]
- 17.Kuhne C, Banks L. E3-ubiquitin ligase/E6-AP links multicopy maintenance protein 7 to the ubiquitination pathway by a novel motif, the L2G box. J Biol Chem. 1998;273:34302–34309. doi: 10.1074/jbc.273.51.34302. [DOI] [PubMed] [Google Scholar]
- 18.Huibregtse JM, Scheffner M, Howley PM. E6-AP directs the HPV E6-dependent inactivation of p53 and is representative of a family of structurally and functionally related proteins. Cold Spring Harb Symp Quant Biol. 1994;59:237–245. doi: 10.1101/sqb.1994.059.01.028. [DOI] [PubMed] [Google Scholar]
- 19.Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 20.Shai A, Nguyen ML, Wagstaff J, Jiang YH, Lambert PF. HPV16 E6 confers p53-dependent and p53-independent phenotypes in the epidermis of mice deficient for E6AP. Oncogene. 2007;26:3321–3328. doi: 10.1038/sj.onc.1210130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camus S, Menendez S, Cheok CF, Stevenson LF, Lain S, Lane DP. Ubiquitin-independent degradation of p53 mediated by high-risk human papillomavirus protein E6. Oncogene. 2007;26:4059–4070. doi: 10.1038/sj.onc.1210188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massimi P, Shai A, Lambert P, Banks L. HPV E6 degradation of p53 and PDZ containing substrates in an E6AP null background. Oncogene. 2008;27:1800–1804. doi: 10.1038/sj.onc.1210810. [DOI] [PubMed] [Google Scholar]
- 23.Riley RR, Duensing S, Brake T, Munger K, Lambert PF, Arbeit JM. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003;63:4862–4871. [PubMed] [Google Scholar]
- 24.Shai A, Brake T, Somoza C, Lambert PF. The human papillomavirus E6 oncogene dysregulates the cell cycle and contributes to cervical carcinogenesis through two independent activities. Cancer Res. 2007;67:1626–1635. doi: 10.1158/0008-5472.CAN-06-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shai A, Pitot HC, Lambert PF. p53 Loss synergizes with estrogen and papillomaviral oncogenes to induce cervical and breast cancers. Cancer Res. 2008;68:2622–2631. doi: 10.1158/0008-5472.CAN-07-5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simonson SJ, Difilippantonio MJ, Lambert PF. Two distinct activities contribute to human papillomavirus 16 E6's oncogenic potential. Cancer Res. 2005;65:8266–8273. doi: 10.1158/0008-5472.CAN-05-1651. [DOI] [PubMed] [Google Scholar]
- 27.Nawaz Z, Lonard DM, Smith CL, et al. The Angelman syndrome-associated protein, E6-AP, is a coactivator for the nuclear hormone receptor superfamily. Molecular & Cellular Biology. 1999;19:1182–1189. doi: 10.1128/mcb.19.2.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sivaraman L, Nawaz Z, Medina D, Conneely OM, O'Malley BW. The dual function steroid receptor coactivator/ubiquitin protein-ligase integrator E6-AP is overexpressed in mouse mammary tumorigenesis. Breast Cancer Res Treat. 2000;62:185–195. doi: 10.1023/a:1006410111706. [DOI] [PubMed] [Google Scholar]
- 29.Gao X, Mohsin SK, Gatalica Z, Fu G, Sharma P, Nawaz Z. Decreased expression of e6-associated protein in breast and prostate carcinomas. Endocrinology. 2005;146:1707–1712. doi: 10.1210/en.2004-1198. [DOI] [PubMed] [Google Scholar]
- 30.Chung SH, Wiedmeyer K, Shai A, Korach KS, Lambert PF. Requirement for estrogen receptor alpha in a mouse model for human papillomavirus-associated cervical cancer. Cancer Res. 2008;68:9928–9934. doi: 10.1158/0008-5472.CAN-08-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song S, Gulliver GA, Lambert PF. Human papillomavirus type 16 E6 and E7 oncogenes abrogate radiation-induced DNA damage responses in vivo through p53-dependent and p53-independent pathways. Proc Natl Acad Sci U S A. 1998;95:2290–2295. doi: 10.1073/pnas.95.5.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang YH, Armstrong D, Albrecht U, et al. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998;21:799–811. doi: 10.1016/s0896-6273(00)80596-6. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Li Z, Howley PM, Sacks DB. E6AP and calmodulin reciprocally regulate estrogen receptor stability. J Biol Chem. 2006;281:1978–1985. doi: 10.1074/jbc.M508545200. [DOI] [PubMed] [Google Scholar]
- 34.Hewitt SC, Deroo BJ, Hansen K, et al. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol. 2003;17:2070–2083. doi: 10.1210/me.2003-0146. [DOI] [PubMed] [Google Scholar]
- 35.Ferreira M, Crespo M, Martins L, Felix A. HPV DNA detection and genotyping in 21 cases of primary invasive squamous cell carcinoma of the vagina. Mod Pathol. 2008;21:968–972. doi: 10.1038/modpathol.2008.91. [DOI] [PubMed] [Google Scholar]
- 36.Koyamatsu Y, Yokoyama M, Nakao Y, et al. A comparative analysis of human papillomavirus types 16 and 18 and expression of p53 gene and Ki-67 in cervical, vaginal, and vulvar carcinomas. Gynecol Oncol. 2003;90:547–551. doi: 10.1016/s0090-8258(03)00401-3. [DOI] [PubMed] [Google Scholar]
- 37.Brake T, Connor JP, Petereit DG, Lambert PF. Comparative analysis of cervical cancer in women and in a human papillomavirus-transgenic mouse model: identification of minichromosome maintenance protein 7 as an informative biomarker for human cervical cancer. Cancer Res. 2003;63:8173–8180. [PubMed] [Google Scholar]
- 38.Balsitis S, Dick F, Dyson N, Lambert PF. Critical Roles for Non-pRb Targets of Human Papillomavirus Type 16 E7 in Cervical Carcinogenesis. Cancer Res. 2006;66:9393–9400. doi: 10.1158/0008-5472.CAN-06-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malanchi I, Accardi R, Diehl F, et al. Human papillomavirus type 16 E6 promotes retinoblastoma protein phosphorylation and cell cycle progression. J Virol. 2004;78:13769–13778. doi: 10.1128/JVI.78.24.13769-13778.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seavey SE, Holubar M, Saucedo LJ, Perry ME. The E7 oncoprotein of human papillomavirus type 16 stabilizes p53 through a mechanism independent of p19(ARF) J Virol. 1999;73:7590–7598. doi: 10.1128/jvi.73.9.7590-7598.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eichten A, Westfall M, Pietenpol JA, Munger K. Stabilization and functional impairment of the tumor suppressor p53 by the human papillomavirus type 16 E7 oncoprotein. Virology. 2002;295:74–85. doi: 10.1006/viro.2002.1375. [DOI] [PubMed] [Google Scholar]
- 42.Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403:676–680. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- 43.Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- 44.Humbert P, Russell S, Richardson H. Dlg, Scribble and Lgl in cell polarity, cell proliferation and cancer. Bioessays. 2003;25:542–553. doi: 10.1002/bies.10286. [DOI] [PubMed] [Google Scholar]
- 45.Lin HT, Steller MA, Aish L, Hanada T, Chishti AH. Differential expression of human Dlg in cervical intraepithelial neoplasias. Gynecol Oncol. 2004;93:422–428. doi: 10.1016/j.ygyno.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 46.Nakagawa S, Yano T, Nakagawa K, et al. Analysis of the expression and localisation of a LAP protein, human scribble, in the normal and neoplastic epithelium of uterine cervix. Br J Cancer. 2004;90:194–199. doi: 10.1038/sj.bjc.6601465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas MC, Chiang CM. E6 oncoprotein represses p53-dependent gene activation via inhibition of protein acetylation independently of inducing p53 degradation. Mol Cell. 2005;17:251–264. doi: 10.1016/j.molcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 48.Zimmermann H, Koh CH, Degenkolbe R, et al. Interaction with CBP/p300 enables the bovine papillomavirus type 1 E6 oncoprotein to downregulate CBP/p300-mediated transactivation by p53. J Gen Virol. 2000;81(Pt 11):2617–2623. doi: 10.1099/0022-1317-81-11-2617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.