Abstract
The explanation of higher neural processes requires an understanding of the dynamics of complex, spiking neural networks. So far, modeling studies have focused on networks with linear or sublinear dendritic input summation. However, recent single-neuron experiments have demonstrated strongly supralinear dendritic enhancement of synchronous inputs. What are the implications of this amplification for networks of neurons? Here, I show numerically and analytically that such networks can generate intermittent, strong increases of activity with high-frequency oscillations; the models developed predict the shape of these events and the oscillation frequency. As an example, for the hippocampal region CA1, events with 200-Hz oscillations are predicted. I argue that these dynamics provide a plausible explanation for experimentally observed sharp-wave/ripple events. High-frequency oscillations can involve the replay of spike patterns. The models suggest that these patterns may reflect underlying network structures.
Keywords: network dynamics, nonlinear dendrites, hippocampus, ripples
During the last few years, experiments have shown that several inputs that arrive simultaneously (or with a temporal difference of at most few milliseconds) at a dendrite can cooperatively trigger a dendritic spike mediated by voltage-gated sodium channels (1–5). This spike generates a rapid depolarization in the soma, which has a rise time constant in the submillisecond range and typically is larger than the sum of the depolarizations the individual inputs would generate. If a somatic spike is generated by such a depolarization, this generation happens with high temporal precision; variations in the somatic spike response times are in the submillisecond range, as are the differences between response times of different neurons (1, 3, 5).
Theoretical studies on active dendrites mainly considered single neurons. Simulations of neuron models incorporating details of channel spatial distribution and dendritic morphology showed dendritic spike generation in agreement with experiments (1, 2, 4, 6). For neurons with comparatively slow NMDA receptor-dependent dendritic spikes (7), which are largely insensitive to temporal coincidence of inputs and generate somatic depolarizations with rise times of tens of milliseconds, firing rate models have been developed (6). Based on these models, the computational abilities of simple circuits have been studied (7–9). In ref. 10, networks of bursting neurons were examined, where the bursts can be explained by slow dendritic spikes. Active dendrites generating fast dendritic sodium spikes were studied in a two-neuron circuit and in a simple feed-forward structure (11), and model neurons incorporating such dendritic spikes were used as an output layer in simulations of hippocampal network models (12).
This article considers the implications of supralinear dendritic interactions as mediated by fast dendritic spikes in larger recurrent neural networks. How does a mechanism leading to strong enhancement of synchronous input and to responses with high temporal precision affect the dynamics of a neural network with complex topology? First, I develop a method of incorporating supralinear dendritic interactions in neural network models that allows an analytical approach. The next section shows that the networks generate events of transiently enhanced activity and high-frequency oscillations, and yields a quantitative understanding of these dynamics. Thereafter, I introduce a more detailed network model to ensure that previous modeling assumptions are not essential for the generation of such events and to gain insight into more detailed dynamical properties. In particular, I highlight that the spiking activity can reflect underlying network structure. Finally, I show that the models’ dynamics provide a plausible explanation for sharp wave/ripples (SPW/Rs) in the hippocampus.
Results
An Analytically Tractable Model (Model 1).
The level of abstraction appropriate for this study of recurrent neural networks with complex topology is the level of integrate-and-fire neurons. These models capture essential properties of cortical neurons but also allow investigation of the underlying mechanisms of network dynamics without obscuring them by a many-variable, many-parameter single-neuron description (e.g., ref. 14). How can we account for supralinear dendritic interactions in networks of single-compartment neurons? The cooperative effects between inputs occur for highly synchronous inputs (1–5). I model this occurrence by allowing supralinear amplification for precisely synchronous inputs (Methods). Accordingly, the differences in conduction delays are neglected, so synchronous presynaptic spiking can be amplified. For simplicity, I replace the steep rise of the membrane potential following an amplification by a jump-like rise, and I assume that nonamplified inputs also generate small jumps in the postsynaptic potential and that all responses occur at a delay τ after presynaptic spiking. Slow inputs are integrated in a constant, suprathreshold input current I0, and slow internal currents are neglected. In conventional models, the effects of several simultaneous inputs are simply summed up linearly. I account for nonlinear amplification by applying a nonlinear function σ to the sum of simultaneous excitatory inputs. Interestingly, this dendritic modulation function can be obtained directly from experimental results (SI Text, Section C) (1, 2, 15, 16): σ has a sigmoidal shape, it equals the identity for small argument, increases steeply or in a jump-like manner above some threshold, and then reaches the saturation level. I model this shape by a piecewise linear function (Fig. 1B Inset) with parameters chosen according to experimental data (1).
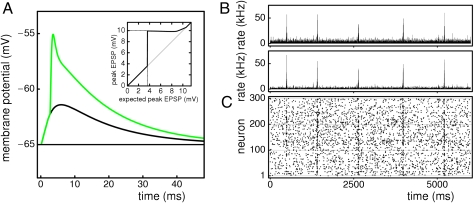
Fig. 1.
Random networks with supralinear dendritic interactions generate transiently increased activity and high-frequency oscillations with frequency 1/τ. (A) A section of the dynamics of a supralinearly coupled network with a synchronous pulse of size g′0 = 45 generated at time t0 = 300 ms by external stimulation. This pulse initiates a short-lived chain of propagating, enhanced synchrony. (Bottom) The spiking activity of 200 neurons. Spikes within the chain are marked red. (Middle) The network's spike rate (bin size 1 ms). (Top) The size of synchronous spike pulses within the chain. (B) The propagation of synchrony can be understood quantitatively in Markovian approximation. The chain evolution is characterized by the transition matrix (gray shaded). The dots indicate the mean response pulse sizes derived numerically (green), semianalytically (red), and analytically (blue). Between G1 and G2 a range with high probability of amplification exists. The gray dashed line shows the evolution of the event in A. (Inset) The dendritic modulation function σ (black line) mapping the peak excitatory postsynaptic potential (EPSP) expected from linear input summation to the effective peak EPSP.
Transient Propagation of Synchrony.
In this section, I study the evolution of synchronous activity in network model 1. A typical ground state of the linear (σ equal to the identity) and the nonlinear (σ sigmoidal) model is irregular asynchronous spiking activity (Fig. 1 and Figs. S1 and S2) (17–19), which constitutes a background activity. If external stimulation excites a group of g′0 neurons to synchronous firing at time t0, a pulse of synchronous spikes is generated, and synchronous spikes arrive at time t0 + τ at all neurons postsynaptic to neurons in the initial group. A subgroup of g′1 ≥ 0 of these neurons receives inputs that are strong enough to raise the membrane potential above threshold (suprathreshold excitation). Because of the infinitesimal rise time of the membrane potential, these neurons send their spikes immediately (and synchronously) at t0 + τ. Their spikes in turn evoke a pulse of g′2 synchronous spikes at t0 + 2τ and so on: Synchrony propagates through the recurrent network against a background of irregular spiking activity.
In agreement with previous studies (20, 21), I find that in linearly coupled networks the pulse size decays quickly, and the chain extinguishes (Figs. S1 and S2 B and D). In networks with supralinear dendritic interactions, chains initiated by small groups, about the size of spontaneously synchronized groups, also die out quickly. With increasing group size, however, the number of postsynaptic neurons receiving supralinearly enhanced inputs increases strongly. For larger group sizes, there is a strong overall amplification of the effect of excitation. A sufficiently large initial group thus can excite a larger second group of neurons to synchronous spiking, and subsequent synchronous pulses in the chain can grow further. If a pulse is too large, however, it results in many refractory neurons and in saturation of excitatory inputs to individual neurons, while inhibition still increases. The subsequent pulses then are too small to be amplified, and the chain extinguishes (Fig. 1 and Fig. S2 A and C). This short-lived, enhanced propagation of synchrony generates, in the presence of repeated stimulation or intrinsic trigger mechanisms, a pattern of intermittent enhanced activity and high-frequency oscillations. It relies on a sufficiently large ratio between excitation and inhibition. If the ratio is too large, epileptic activity emerges (Fig. S2). For the hippocampal region CA1, a delay time constant of τ ≈ 5 ms can be determined by adding the axonal and synaptic delays and the latencies of the dendritic spike and the somatic action potential generation (SI Text, Section B) or can be inferred directly by taking into account only the dendritic spike timing (1). This delay leads to an oscillation frequency of ~200 Hz.
For a quantitative understanding of chain evolution, we can approximate it by a stationary discrete Markov process. The transition matrix from the initial group size g′0 to the next group size g′1 characterizes the chain evolution. In networks with supralinear dendritic interactions, a range of group sizes exists where amplification of group sizes is highly probable (Fig. 1B, between G1 and G2). Such a range is absent in linearly coupled networks (Fig. S1B). If a group becomes too large (larger than G3), subsequent groups are small (smaller than G1), and the chain dies out. This extinguishing is supported by a decrease of background activity. I derived the transition matrices and the mean response sizes numerically, analytically, and semianalytically (Methods), all with very similar results.
A Model with a Finite Interaction Window (Model 2).
Are modeling assumptions such as amplification only of precisely synchronous activity, homogeneous delay distribution, or jump-like postsynaptic responses essential for the generation of intermittent high-frequency oscillations? A more detailed network model shows that they are not. In a simple multicompartmental model, we would describe the dendrite as a single compartment with resistive coupling to the soma. If a sufficiently strong input arrived at the dendrite, a dendritic spike would be elicited, and the resulting depolarization would cause a current pulse in the somatic compartment. I use a single-compartment model and include this current pulse (Fig. 2). If the total strength of the excitatory input, which arrives within a short time window Δt, exceeds the threshold for dendritic spiking, a current pulse is injected after a fixed delay (1–4). The neuron and network parameters for this phenomenological model are chosen according to experimental findings in CA1 (1–3, 5, 22) and neighboring regions (Methods and SI Text, Sections B and C). In particular, coupling delays are inhomogeneous, and recurrent connections of the excitatory population are very sparse and show supralinear dendritic interactions (1, 23).
Fig. 2.
Supralinear dendritic enhancement of inputs within a finite temporal interaction window leads to spontaneous, intermittent increases in the network activity. (A) Comparison of the depolarizations caused by several simultaneous inputs in a conventional neuron (black) and in a neuron with supralinear dendritic interactions (model 2; green). (Inset) Modulation function σ. (B and C) The dynamics of a network incorporating supralinear dendritic interactions. (B) The spike rate (bin size 1 ms) of the inhibitory (Upper) and of the excitatory (Lower) neuron population. (C) The spiking activity of 300 of the excitatory neurons. The dynamics are characterized by irregular spiking interrupted by spontaneous, intermittent increases of activity involving both the inhibitory and the excitatory neuron population. During such an event a larger fraction (about one third) of the neurons in the excitatory population sends a spike, and almost every inhibitory neuron sends usually several spikes. The event around t = 1,400 ms is depicted in Fig. 3A.
Intermittent Increases of Activity with High-Frequency Oscillations.
Network model 2 generates irregular, asynchronous background activity and, in irregular intervals, spontaneous increases of activity with a duration of ~50 ms. These events consist of several subsequent pulses of highly synchronous spiking activity in the excitatory and inhibitory neuron populations (Fig. 3). Pulses in these two populations have ~5 ms temporal distance and are shifted in time with respect to each other. Accordingly, the spectrogram of the spike rates shows at events a maximum at ~200 Hz. The occurrence of events and the frequency of the oscillations depend on parameters such as the coupling strengths and the size of the interaction window, but both occurrence and frequency remain remarkably stable when the network parameters are varied within the biologically plausible range (SI Text, Section B). Occasionally, events with weak or undetectable oscillations occur. Also, events can be evoked by stimulation, where the level of synchrony depends on the width and amplitude of the stimulation. On increase of excitation and decrease of inhibition, the activity approaches a nearly uninterrupted series of events, and the size of events grows, suggesting a possible transition from healthy events to epileptiform activity (SI Text, Section B) (24).
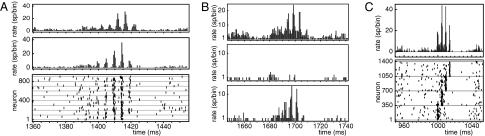
Fig. 3.
Single events in the network dynamics of model 2 consist of several subsequent pulses of highly synchronous spiking activity with temporal distance of about 5 ms. (A) An event in a random network. (Top and Middle) The spike rates (number of spikes per bin, all bin sizes 0.5 ms) of the inhibitory and of the excitatory population, respectively. (Bottom) The spiking activity of the excitatory population. (B) An event generated by a network in which only the recurrent connections of a subpopulation of the excitatory neurons allow supralinear dendritic interactions. This subpopulation (spike rate of 500 neurons; Bottom) and the inhibitory population (spike rate; Top) participate in events. Other excitatory neurons essentially do not participate (spike rate of 500 neurons; Middle). (C) An event in a network with a feed-forward structure in the excitatory population created by the presence of supralinear dendritic interactions (group sizes: 350 neurons). (Upper) The event has the usual profile (rate of the excitatory population). (Lower) The spiking activity of the excitatory population reflects the feed-forward structure in the underlying network. See main text and Fig. S4 for details.
The structural similarities to model 1 as well as the prevalence and the timing of excitatory inputs and dendritic spikes during events (Fig. 3 and Fig. S3) suggest that the events are based on subsequent excitation of synchronous neuron groups caused by supralinearly enhanced excitation by dendritic spikes. This suggestion is confirmed by the direct dependence of the interpulse intervals on the timing properties of the dendritic spikes and by their insensitivity to other parameters such as the delay of connections between the excitatory and the inhibitory population or the response properties of the inhibitory neurons (SI Text, Section B and Fig. S5).
The mechanism leading to events in model 2 can be described as follows: Spontaneous fluctuations, slow, not too strong oscillations in the network activity or external stimulation, lead to mildly enhanced synchronous spiking activity in the population of excitatory neurons. This activity enhances dendritic spiking in postsynaptic excitatory neurons. The dendritic spikes promote somatic spikes or directly generate them with high temporal precision. Together with conventional inputs, they evoke a better synchronized, larger pulse of response spikes in the excitatory population. This pulse then evokes a third one, and so on. At first, because of nonlinearly enhanced feedback within the excitatory population, the increase of activity is not sufficiently suppressed by increased activity in the inhibitory neurons, despite their faster response properties. The pulse size and thus the overall activity increase. After larger pulses, however, a substantial fraction of excitatory neurons is refractory, and, with time, the impacts of strong inhibition accumulate. Both effects limit the pulse sizes, the inhibition finally dominates the excitation, the overall activity decreases, and the event ends (Fig. S3).
Structured Networks.
The spiking activity during events can reflect underlying network structure. I demonstrate this ability by means of two model 2-type networks (“network I” and “network II”) with random topology. A single simple modification introduces specific structure: Only selected subsets of the existing couplings support supralinear dendritic enhancement. Inputs from these couplings to a neuron can cooperatively trigger dendritic spikes, whereas other inputs to the neuron do not contribute to supralinear amplification; i.e. the neuron has several dendrites or several dendritic compartments. In network I, the recurrent couplings of a subpopulation of the excitatory neurons are selected to allow supralinear enhancement. Simulations show that this subpopulation supports the intermittent events, whereas other excitatory neurons do not participate significantly. The spiking activity during an event thus reflects the network structure (Fig. 3B and Fig. S4 A and B). In network II, a sequence of neuron groups is chosen within the excitatory neuron population. The couplings from one group to its successor are selected to support supralinear enhancement, yielding a feed-forward structure that is reflected by the spiking activity during events (Fig. 3C and Fig. S4 C–F). The models thus suggest a simple explanation for replay of spike patterns during high-frequency oscillations (25–27): The network structure might have been modified in a previous learning phase to generate an experience-associated activity pattern which then is replayed during the events. The activity-dependent learning might have changed synaptic strengths or, as shown above, have determined which inputs support supralinear amplification (5). The structure and plasticity of external inputs also are likely to play an important role.
A Model for Sharp Wave/Ripples.
For hippocampal region CA1 (and for regions which are similar with respect to their single-neuron and network properties), the model predicts events of increased activity with high-frequency oscillations of ~200 Hz (SI Text, Section B). Indeed, in CA1 and neighboring regions, SPW/Rs, intermittent strong increases in network activity (sharp waves) with high-frequency oscillations (ripples) of ~200 Hz, have been found (28–31). Does the model provide a plausible explanation for SPW/Rs?
I first consider anatomical evidence and note that recurrent excitatory connectivity in CA1 is mediated by axon-to-basal dendrite synapses (23) and that these basal dendrites generate the dendritic sodium spikes incorporated in the model (1). The recurrent excitatory connectivity in CA1 is sparse but significant, and individual couplings are strong (23). A comparison between the number of inputs expected during SPW/Rs from the estimated connectivity and those expected from CA3 afferents that generate dendritic spikes (32) indicates that already global unstructured connectivity might lead to dendritic spiking. Sparsity is compensated by large numbers of neurons participating in SPW/Rs. Anatomical findings and a comparison with neocortex suggest a locally enhanced connectivity. Sparsity might further be compensated by nonrandom connectivity (23) and other network features (SI Text, Section C).
I now examine and compare the characteristics of events in the model and of SPW/Rs. (i) They agree in the frequency of the high-frequency oscillations. Ripples in in vitro slice preparations (30, 31, 33) generally have a higher oscillation frequency than those detected in vivo (29, 34). The model suggests that this higher oscillation frequency is caused by the reduction of longer-range connections during slice preparation and suggests a decrease in the oscillation frequency with increasing slice thickness (Fig. S6, SI Text, Section B, Fig. S7). This, together with the broader delay distribution in networks with long-range connections, also might explain why there are only weak lower-frequency ripples (if any) in the globally connected region CA3 in vivo (22, 29, 34), whereas there are marked high-frequency ripples in thin-slice preparations of CA3 (30, 31, 33). (ii) The events in the model and SPW/Rs have similar shape (rate profile) and duration. (iii) They both are associated with increased inputs to the excitatory and the inhibitory neuron populations. (iv) The model predicts spiking in basal dendrites of CA1 pyramidal neurons during SPW/Rs. So far, this spiking has been observed in apical dendrites, which are more easily accessible experimentally (32). (v) The firing characteristics of the individual neuron populations agree: The excitatory neuron population and the inhibitory neuron population show increased activity. A larger fraction (but not all) of the neurons of the excitatory population contributes usually one spike to an event. Inhibitory neurons of the types participating during SPW/R events fire at high frequency, often around 200 Hz (29, 35). (vi) The phase of the oscillation of the inhibitory neuron population is delayed with respect to the phase of the excitatory population. If the interneuron parameters in the model are chosen to reproduce the fast-response properties of participating interneurons (35, 36), even quantitative agreement can be achieved. In biological neural networks, additional effects might promote synchronous and early firing of interneurons (Fig. S5) (29, 37). (vii) During SPW/Rs, patterns of spiking activity from previous learning phases are repeated (25–27). The model suggests that this repetition might be explained by the reflection of underlying network structure in the spiking activity. Further studies should consider learning, different topologies, and the influence of plastic inputs from CA3; such investigations might lead to an explicit modeling of the replay of spike patterns at the experimentally observed frequencies.
Finally, I consider the pharmacological findings of direct importance. (i) The fast recurrent excitatory interactions in the regions considered are mediated by AMPA receptors. AMPA antagonists abolish the SPW/R activity in both CA1 and in CA3 (30). Although SPW/R events usually are initiated in CA3, they also are found in the functionally disconnected region CA1 (30, 31). This data indicates that the recurrent excitatory connectivity is essential for their generation in both regions, as assumed in the model. The model reproduces the behavior of the network dynamics under blocking of excitation (SI Text, Section B). (ii) Blocking of GABAA-mediated inhibition can lead to large, epileptiform events that have a shape similar to SPW/Rs and ~200-Hz ripple frequency (30). Upon reduction of inhibition, the event size increases in the model as well (SI Text, Section B). (iii) There is experimental indication that SPW/Rs also depend on electrical coupling (29–31). This dependence might be explained by electrical synapses which increase the tendency of the neurons to synchronize and compensate for sparse coupling (38, 39). The application of gap junction blockers then should reduce the incidence of SPW/Rs and the ripple strength but leave the oscillation frequency invariant, as is observed in experiments (29–31). Under certain conditions such as strong external chemical stimulation, high-frequency oscillations and patterns similar to SPW/Rs can be generated, which are mediated by electric couplings (31, 40–42). It is unclear if similar mechanisms underlie native SPW/Rs; recent experimental findings might indicate they do not (24, 43).
Taken together, the model is substantiated by hippocampal anatomy and by data concerning nonlinear dendrites. Further, the events in the model and SPW/Rs share essential properties, and the model is compatible with the neurophysiological findings on SPW/Rs. It can thus be concluded that the model yields a plausible explanation for SPW/Rs in the hippocampus.
Discussion
This article assesses the implications of supralinear dendritic interactions as found in recent experiments (1–5) on the dynamics of complex, spiking neural networks. I develop a method to incorporate supralinear dendritic interactions, such that the network dynamics can be studied numerically and, despite the high nonlinearity and complex network structure, even analytically. The proposed method is very general. It is restricted neither to neurons of the integrate-and-fire type nor to single-compartment neurons, and it can be used to model other types of nonlinear dendritic interactions. Also, electrical coupling is well compatible with the studied concept of nonlinear integration. The models show that networks incorporating fast dendritic spikes can generate a robust emergent phenomenon: events of intermittently enhanced activity with high-frequency oscillations. The underlying mechanisms can be quantitatively understood, and it can be shown that they are based on propagation of synchrony. This mechanism is particularly remarkable, because in networks of conventional neurons, propagation of synchrony is difficult to realize and requires highly structured networks, especially if the networks are sparse (20, 21).
For hippocampal region CA1 and related regions, the models predict the occurrence of phases of enhanced activity with high-frequency oscillations of ~200 Hz. This prediction agrees with the prominent SPW/R pattern observed in these regions (28–31). Two main modeling approaches for SPW/Rs have been proposed so far. The first approach (here referred to as “model IN”), assumes in its basic form that a sharp wave from CA3 excites interneurons to oscillate (29, 44, 45). Their fast inhibitory connections synchronize them (46), and the resulting periodic inhibition entrains the population of excitatory neurons to oscillatory activity. The second approach (“model GJ”) is based on the assumption that axo-axonal gap junctions connect the pyramidal neurons of region CA1 to a sparse network (41, 42, 47). Simulations show that for coupling strengths in the physiological range, spikes can pass from axon to axon. A depolarizing sharp wave input from CA3 allows spontaneous axonal ectopic spikes to propagate, to multiply in the axonal bulk, and to excite pyramidal cells and interneurons after antidromic and orthodromic propagation. This process leads to bursts of rhythmic network activity (41, 42). The predicted axo-axonic gap junctions have been found experimentally (38, 48), and they most likely underlie high-frequency oscillations and patterns resembling SPW/Rs which can be evoked in CA1 networks in vitro after blocking of chemical transition (31, 40–42). This finding supports model GJ as an explanation for native SPW/Rs.
Synchronization in model IN is mediated by recurrent inhibitory connectivity. In model GJ, an ectopic spike generates a pulse of spikes in the axonal plexus. Its width depends on the network topology. In my model, synchronization is mediated by supralinear dendritic amplification, inhibition serving as a balancing mechanism (Figs. S5, S8 and S9). In model IN, the frequency of oscillations depends on strengths and delays of recurrent connectivity, external stimulation, and single-cell properties. In model GJ, the period of the oscillations is approximately the product of the average mean path length in the network and the time needed for spikes to cross between axons. In my model, it depends on the timing properties of dendritic spikes and the delays of excitatory-to-excitatory connections (SI Text, Section B). Both model GJ and my model predict spikelets (32, 40). In model GJ, a spike in one axon induces a spike in a coupled axon (“one:one” propagation). In a model proposed for cerebellar cortex (49), several synchronous spikes are necessary (“many:one” nonlinearity), conceptually related to my model. Both models IN and GJ suggest a more prominent role of structured input from CA3 during replay of spiking activity (25–27), whereas the present model suggests an emphasis on recurrent connectivity. The present model explains the overall increase of activity associated with SPW/Rs and the oscillations by the same phenomenon, enhanced propagation of synchrony. Further, it suggests explanations for various phenomena associated with SPW/Rs, in particular for the experimentally observed different frequencies in different hippocampal regions and under different experimental conditions. Given the current knowledge of SPW/Rs, all three modeling approaches are biologically plausible; combinations also are possible and should be investigated.
The contribution of the present work can be seen as threefold: It introduces methods to incorporate supralinear dendritic interactions in neural network models and to study their dynamics, it shows that networks incorporating supralinear dendritic interactions give rise to an interesting emergent pattern of activity, and it proposes a model for SPW/Rs in the hippocampus.
The study suggests a number of directions for future research. The methods presented allow the investigation of the dynamics of networks incorporating various types of dendritic nonlinearities in various types of neurons and neuron models and might be applied to networks coupled by gap junctions. For σ equaling the identity, model 1 simplifies to a standard model which has been employed to explain the dynamics of neural networks as well as of earthquakes and of populations of flashing fireflies (14, 19, 50, 51). The proposed models thus might find straightforward application in other fields. The models might apply to other kinds of high-frequency oscillations in neural networks, e.g., to those accompanying the P1/N1 complex in somatosensory cortex and to pathological ripples (24) (SI Text, Section B). Several of the models' predictions can be directly tested experimentally, such as the suggested relationship between slice thickness and oscillation frequency and the occurrence of dendritic spikes. How does the spiking dynamics during intermittent high-frequency oscillations change the network structure? Answers to such questions might shed light on the function of SPW/Rs and could change our view of the role of CA1 and the other hippocampal regions in learning and memory.
Methods
Network Models.
Model 1.
I consider networks of N current-based leaky integrate-and-fire neurons in the limit of short synaptic currents (14, 19, 50, 51). The networks have the topology of an Erdös-Rényi random graph, i.e., directed couplings are independently present with probability p0. An existing coupling is excitatory with probability pEx and inhibitory with probability 1 − pEx; in particular, there are no separate excitatory and inhibitory populations. The neurons sending at time tf excitatory and inhibitory inputs to neuron l are gathered in the sets MEx,l(f) and MIn,l(f), respectively. They induce at tf + τ a jump-like response in neuron l’s membrane potential,
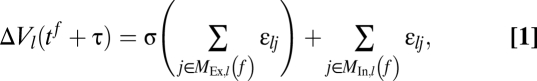 |
where εlj denotes the coupling strength from neuron j to neuron l. (Details are given in SI Text, Section C.)
Model 2.
I consider networks of N conductance-based leaky integrate-and-fire neurons with 90% excitatory and 10% inhibitory neurons (22). Excitatory and inhibitory interactions are mediated by AMPA and GABAA synapses, respectively. If the excitatory input strength arriving at an excitatory neuron within time window Δt is larger than a threshold gΘ, the generation of a dendritic spike is initiated, which, after time τDS, generates a current pulse at the soma. (Details are given in SI Text, Section C.) In ref. 13 I introduced another network model with a degree of abstraction between models 1 and 2, which generates similar intermittent events. This result further emphasizes the robustness of the dynamical mechanisms.
Numerical Methods.
Model 1.
Network simulations were implemented in phase representation using an event-based algorithm (51). Chains of propagating synchrony can be defined recursively as consisting of the spikes sent at time t0 (initiation time) and of those generated by suprathreshold excitation caused by a pulse within the chain. These pulses are sent at t0 + nτ (pulse size g′n), n ∈ ℕ0. Spike times of background activity deviate at least slightly. Fig. 1B and Fig. S1B show the relative frequencies and the mean values of pulse size g′1 for a preceding group of size g′0 (g′0 ∈ {1, …, 351}, 400 simulations for each value) approximating the transition matrix (conditional probability) P(g1 = g′1|g0 = g′0) and the mean response pulse sizes (conditional expectation) E(g1|g0 = g′0). Here, gn is the random variable describing the nth pulse size. This transition matrix also is an approximation for later stages of propagation and determines the chain evolution.
Model 2.
Network simulations were implemented with a Runge-Kutta-fourth-order method with fixed step sizes of 0.02 ms. Simulations and analyses were implemented in programs written in C using the GNU scientific library, in R and in Mathematica.
Analytical Methods.
Model 1.
The transition matrix P(g1 = g′1|g0 = g′0) and the mean response pulse sizes E(g1|g0 = g′0) were computed analytically and semianalytically. In the purely analytical approach, the probability distribution of the membrane potentials P(V) of the background activity is derived in diffusion approximation (18). To eliminate resulting errors, I determined P(V) in the semianalytical approach numerically. Based on P(V) and on the statistics of the underlying network and taking into account the refractoriness of neurons that already have spiked, I derived the probabilities that g′1 neurons respond to a pulse sent by g′0 neurons, i.e., the transition matrix, and the mean response sizes. For the detailed derivation, see ref. 13. The transition matrix characterizes the chain evolution under Markovian and stationarity assumptions. The validity of the assumptions depends on the network parameters and was numerically confirmed. The critical pulse sizes determining the evolution of larger pulses are G1 and G2, the intersections of the interpolated conditional expectations with the diagonal, i.e., Gα ≈ E(g1|g0 ≈ Gα), α ∈ {1, 2},  , and G3, given by G1 ≈ E(g1|g0 ≈ G3). Explicit computations were implemented in Mathematica.
, and G3, given by G1 ≈ E(g1|g0 ≈ G3). Explicit computations were implemented in Mathematica.
Supplementary Material
Acknowledgments
For fruitful discussions and suggestions, I thank Margarida Agrochão, Martin Both, Yoram Burak, György Buzsáki, Markus Diesmann, Andreas Draguhn, Kai Gansel, Theo Geisel, Caroline Geisler, Harold Gutch, Sven Jahnke, Adam Kampff, Christoph Kirst, Anna Levina, Jeffrey Magee, Nikolaus Maier, Georg Martius, Abigail Morrison, Eran Mukamel, Gordon Pipa, Alon Polsky, Susanne Reichinnek, Jackie Schiller, Dietmar Schmitz, Wolf Singer, Anton Sirota, Tatjana Tchumatchenko, Alex Thomson, Marc Timme, Roger Traub, Annette Witt, and Fred Wolf. The work was supported by the Max Planck Society, by a grant from the Swartz Foundation, and by Grant 01GQ0430 from the German Federal Ministry of Education and Research via the Bernstein Center for Computational Neuroscience, Göttingen.
Footnotes
The author declares no conflict of interest.
Parts of the results presented in this article were derived and published in ref. 13 and have been published in abstract form.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0909615107/-/DCSupplemental.
References
- 1.Ariav G, Polsky A, Schiller J. Submillisecond precision of the input-output transformation function mediated by fast sodium dendritic spikes in basal dendrites of CA1 pyramidal neurons. J Neurosci. 2003;23:7750–7758. doi: 10.1523/JNEUROSCI.23-21-07750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gasparini S, Migliore M, Magee J. On the initiation and propagation of dendritic spikes in CA1 pyramidal neurons. J Neurosci. 2004;24:11046–11056. doi: 10.1523/JNEUROSCI.2520-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gasparini S, Magee J. State-dependent dendritic computation in hippocampal CA1 pyramidal neurons. J Neurosci. 2006;26:2088–2100. doi: 10.1523/JNEUROSCI.4428-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nevian T, Larkum M, Polsky A, Schiller J. Properties of basal dendrites of layer 5 pyramidal neurons: A direct patch-clamp recording study. Nat Neurosci. 2007;10:206–214. doi: 10.1038/nn1826. [DOI] [PubMed] [Google Scholar]
- 5.Losonczy A, Makara J, Magee J. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452:436–441. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- 6.Poirazi P, Brannon T, Mel B. Pyramidal neuron as two-layer network. Neuron. 2003;37:989–999. doi: 10.1016/s0896-6273(03)00149-1. [DOI] [PubMed] [Google Scholar]
- 7.London M, Häusser M. Dendritic computation. Annu Rev Neurosci. 2005;28:503–532. doi: 10.1146/annurev.neuro.28.061604.135703. [DOI] [PubMed] [Google Scholar]
- 8.Morita K. Possible role of dendritic compartmentalization in the spatial working memory circuit. J Neurosci. 2008;28:7699–7724. doi: 10.1523/JNEUROSCI.0059-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhodes P. Recoding patterns of sensory input: Higher order features and the function of nonlinear dendritic trees. Neural Comput. 2008;20:2000–2036. doi: 10.1162/neco.2008.04-07-511. [DOI] [PubMed] [Google Scholar]
- 10.Traub R, Wong R. Cellular mechanism of neuronal synchronization in epilepsy. Science. 1982;216:745–747. doi: 10.1126/science.7079735. [DOI] [PubMed] [Google Scholar]
- 11.Poznanski R. Dendritic integration in a recurrent network. J Integr Neurosci. 2002;1:69–99. doi: 10.1142/s0219635202000050. [DOI] [PubMed] [Google Scholar]
- 12.Katz Y, Kath W, Spruston N, Hasselmo M. Coincidence detection of place and temporal context in a network of spiking hippocampal neurons. PLoS Comput Biol. 2007;3:2432–2445. doi: 10.1371/journal.pcbi.0030234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Memmesheimer RM. Göttingen, Germany: Georg-August Univ. of Göttingen; 2008. Precise spike timing in complex neural networks. PhD thesis. [Google Scholar]
- 14.Burkitt A. A review of the integrate-and-fire neuron model: I. Homogeneous synaptic input. Biol Cybern. 2006;95:1–19. doi: 10.1007/s00422-006-0068-6. [DOI] [PubMed] [Google Scholar]
- 15.Polsky A, Mel B, Schiller J. Computational subunits in thin dendrites of pyramidal cells. Nat Neurosci. 2004;7:621–627. doi: 10.1038/nn1253. [DOI] [PubMed] [Google Scholar]
- 16.Losonczy A, Magee J. Integrative properties of radial oblique dendrites in hippocampal CA1 pyramidal neurons. Neuron. 2006;50:291–307. doi: 10.1016/j.neuron.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 17.van Vreeswijk C, Sompolinsky H. Chaos in neuronal networks with balanced excitatory and inhibitory activity. Science. 1996;274:1724–1726. doi: 10.1126/science.274.5293.1724. [DOI] [PubMed] [Google Scholar]
- 18.Brunel N. Dynamics of sparsely connected networks of excitatory and inhibitory spiking neurons. J Comput Neurosci. 2000;8:183–208. doi: 10.1023/a:1008925309027. [DOI] [PubMed] [Google Scholar]
- 19.Jahnke S, Memmesheimer RM, Timme M. Stable irregular dynamics in complex neural networks. Phys Rev Lett. 2008;100:048102. doi: 10.1103/PhysRevLett.100.048102. [DOI] [PubMed] [Google Scholar]
- 20.Vogels T, Abbott L. Signal propagation and logic gating in networks of integrate-and-fire neurons. J Neurosci. 2005;25:10786–10795. doi: 10.1523/JNEUROSCI.3508-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A, Rotter S, Aertsen A. Conditions for propagating synchronous spiking and asynchronous firing rates in a cortical network model. J Neurosci. 2008;28:5268–5280. doi: 10.1523/JNEUROSCI.2542-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J. The Hippocampus Book. Oxford, UK: Oxford Univ Press; 2007. [Google Scholar]
- 23.Deuchars J, Thomson A. CA1 pyramid-pyramid connections in rat hippocampus in vitro: Dual intracellular recordings with biocytin filling. Neuroscience. 1996;74:1009–1018. doi: 10.1016/0306-4522(96)00251-5. [DOI] [PubMed] [Google Scholar]
- 24.Engel J, Bragin A, Staba R, Mody I. High-frequency oscillations: What is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- 25.Wilson M, McNaughton B. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 26.Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsáki G. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci. 1999;19:9479–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee A, Wilson M. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- 28.Buzsáki G, Horváth Z, Urioste R, Hetke J, Wise K. High frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- 29.Ylinen A, et al. Sharp wave-associated high-frequency oscillation (200Hz) in the intact hippocampus: Network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maier N, Nimmrich V, Draguhn A. Cellular and network mechanisms underlying spontaneous sharp wave-ripple complexes in mouse hippocampal slices. J Physiol. 2003;550:873–887. doi: 10.1113/jphysiol.2003.044602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nimmrich V, Maier N, Schmitz D, Draguhn A. Induced sharp wave-ripple complexes in the absence of synaptic inhibition in mouse hippocampal slices. J Physiol. 2005;563:663–670. doi: 10.1113/jphysiol.2004.079558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamondi A, Acsádi L, Buzsáki G. Dendritic spikes are enhanced by cooperative network activity in the intact hippocampus. J Neurosci. 1998;18:3919–3928. doi: 10.1523/JNEUROSCI.18-10-03919.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Both M, Bähner F, von Bohlen und Halbach O, Draguhn A. Propagation of specific network patterns through the mouse hippocampus. Hippocampus. 2008;18:899–908. doi: 10.1002/hipo.20446. [DOI] [PubMed] [Google Scholar]
- 34.Csicsvari J, Hirase H, Czurkó A, Mamiya A, Buzsáki G. Fast network oscillations in the hippocampal CA1 region of the behaving rat. J Neurosci. 1999;19:RC20:1–4. doi: 10.1523/JNEUROSCI.19-16-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klausberger T, et al. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- 36.Geiger J, Lübke J, Roth A, Frotschner M, Jonas P. Submillisecond AMPA receptor signaling at a principal neuron-interneuron synapse. Neuron. 1997;18:1009–1023. doi: 10.1016/s0896-6273(00)80339-6. [DOI] [PubMed] [Google Scholar]
- 37.Csicsvari J, Hirase H, Czurkó A, Mamiya A, Buzsáki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J Neurosci. 1999;19:274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitz D, et al. Axo-axonal coupling: A novel mechanism for ultrafast neuronal communication. Neuron. 2001;31:831–840. doi: 10.1016/s0896-6273(01)00410-x. [DOI] [PubMed] [Google Scholar]
- 39.Mercer A, Bannister A, Thomson A. Electrical coupling between pyramidal cells in adult cortical regions. Brain Cell Biol. 2006;35:13–27. doi: 10.1007/s11068-006-9005-9. [DOI] [PubMed] [Google Scholar]
- 40.Draguhn A, Traub R, Schmitz D, Jefferys J. Electrical coupling underlies high frequency oscillations in the hippocampus in vitro. Nature. 1998;394:189–192. doi: 10.1038/28184. [DOI] [PubMed] [Google Scholar]
- 41.Traub R, Schmitz D, Jefferys J, Draguhn A. High frequency population oscillations are predicted to occur in hippocampal pyramidal neuronal networks interconnected by axoaxonal gap junctions. Neuroscience. 1999;92:407–426. doi: 10.1016/s0306-4522(98)00755-6. [DOI] [PubMed] [Google Scholar]
- 42.Traub R, Bibbig A. A model of high frequency ripples in the hippocampus based on synaptic coupling plus axon-axon gap junctions between pyramidal neurons. J Neurosci. 2000;20:2086–2093. doi: 10.1523/JNEUROSCI.20-06-02086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vandecasteele M, Menzies A, Creese I, Paul D, Buzsáki G. Persistence of hippocampal oscillations in connexin 36, 45 double knock-out mice. Soc Neurosci Abstr. 2008;435:4. [Google Scholar]
- 44.Buzsáki G, Chrobak J. Temporal structures in spatially organized neuronal ensembles: A role for interneuronal networks. Curr Opin Neurobiol. 1995;5:504–519. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 45.Geisler C, Brunel N, Wang XJ. Contributions of intrinsic membrane dynamics to fast network oscillations with irregular neuronal discharges. J Neurophysiol. 2005;94:4344–4361. doi: 10.1152/jn.00510.2004. [DOI] [PubMed] [Google Scholar]
- 46.Whittington M, Traub R, Jefferys J. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- 47.Maex R, De Schutter E. Mechanism of spontaneous and self-sustained oscillations in networks connected through axo-axonal gap junctions. J Neurosci. 2007;25:3347–3358. doi: 10.1111/j.1460-9568.2007.05593.x. [DOI] [PubMed] [Google Scholar]
- 48.Hamzei-Sichani F, et al. Gap junctions on hippocampal mossy fiber axons demonstrated by thin-section electron microscopy and freeze fracture replica immunogold labeling. Proc Natl Acad Sci USA. 2007;104:12548–12553. doi: 10.1073/pnas.0705281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Traub R, Middleton S, Knöpfel T, Whittington M. Model of very fast (>75Hz) network oscillations generated by electrical coupling between the proximal axons of cerebellar Purkinje cells. Eur J Neurosci. 2008;28:1603–1616. doi: 10.1111/j.1460-9568.2008.06477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mirollo R, Strogatz S. Synchronization of pulse coupled biological oscillators. SIAM J Appl Math. 1990;50:1645–1662. [Google Scholar]
- 51.Ernst U, Pawelzik K, Geisel T. Synchronization induced by temporal delays in pulse-coupled oscillators. Phys Rev Lett. 1995;74:1570–1573. doi: 10.1103/PhysRevLett.74.1570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.