Abstract
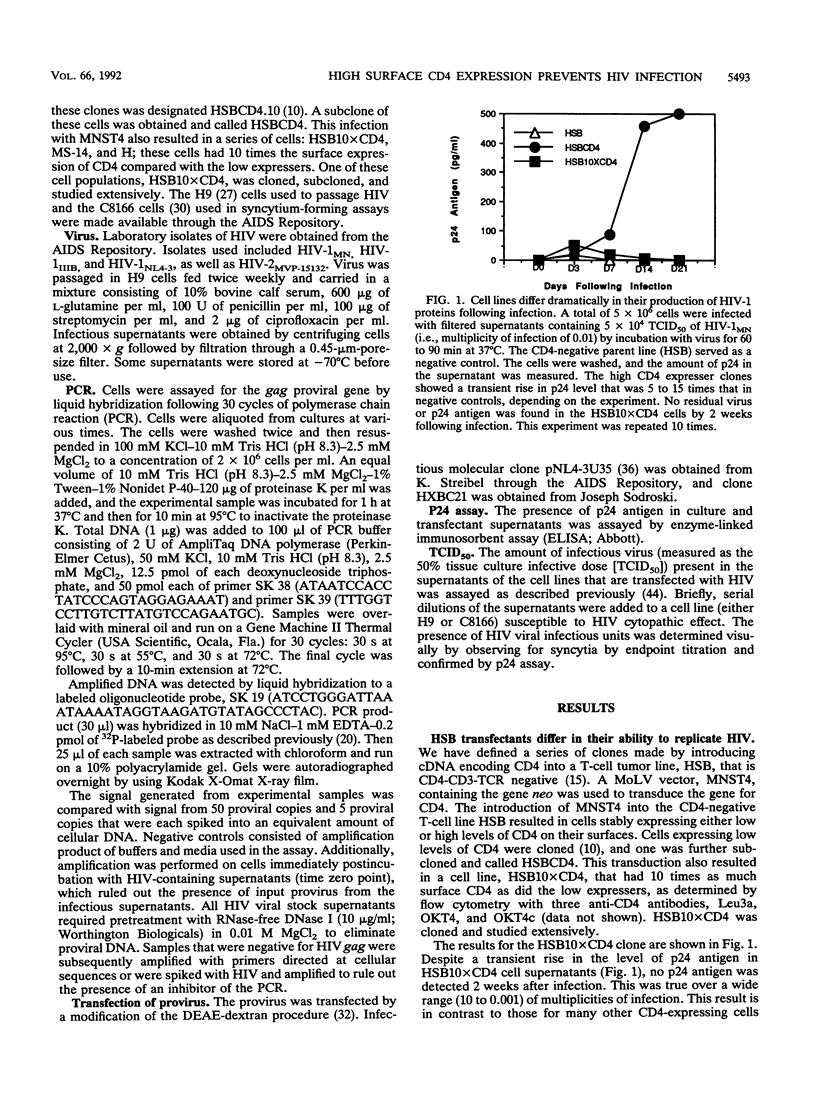
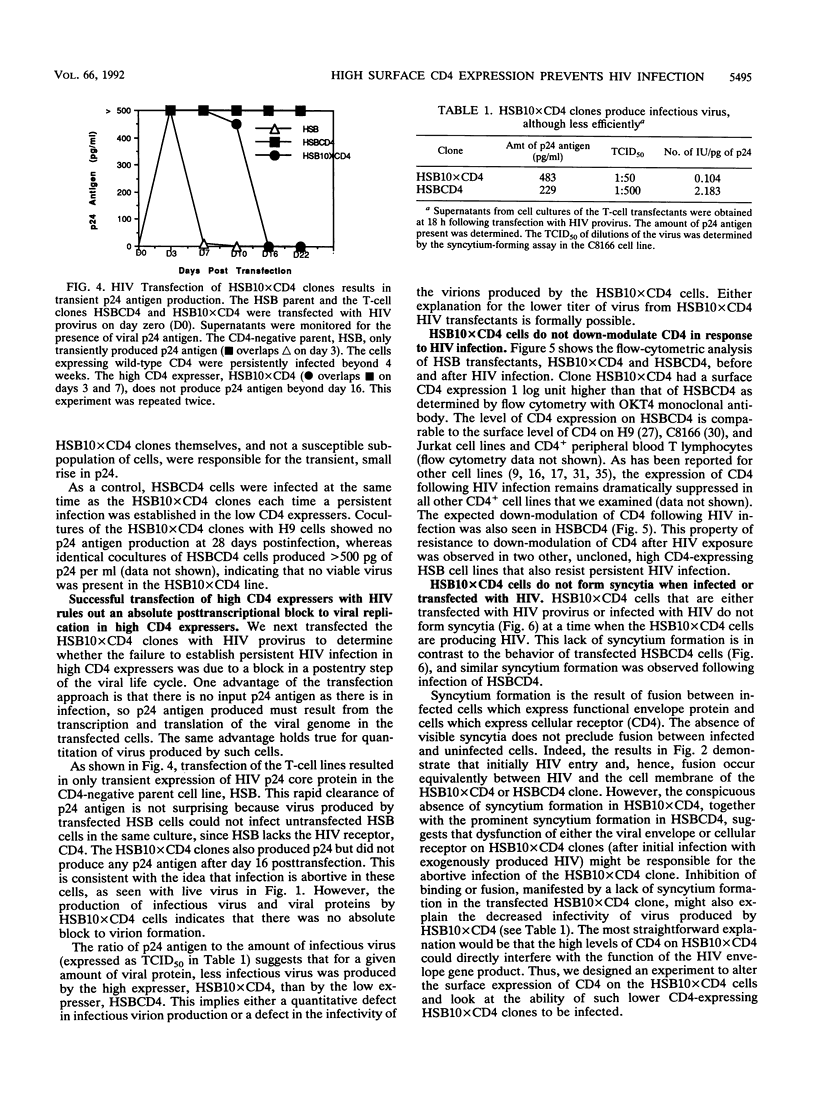
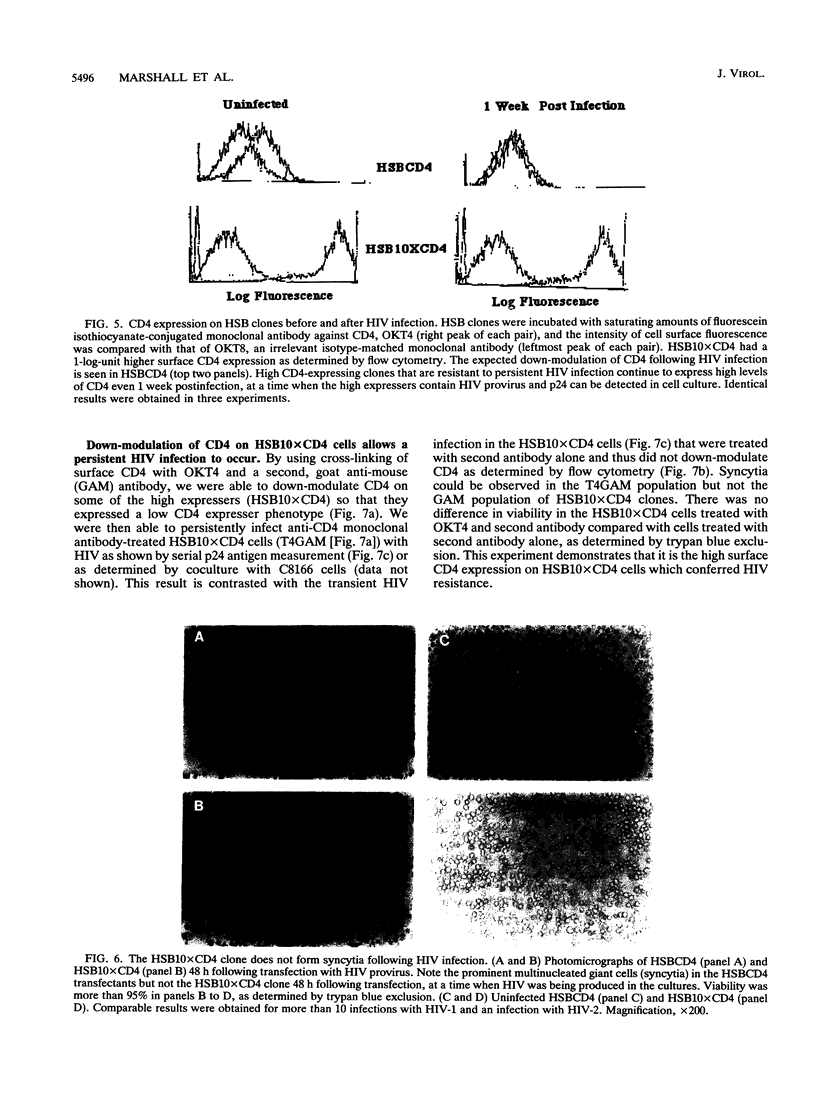
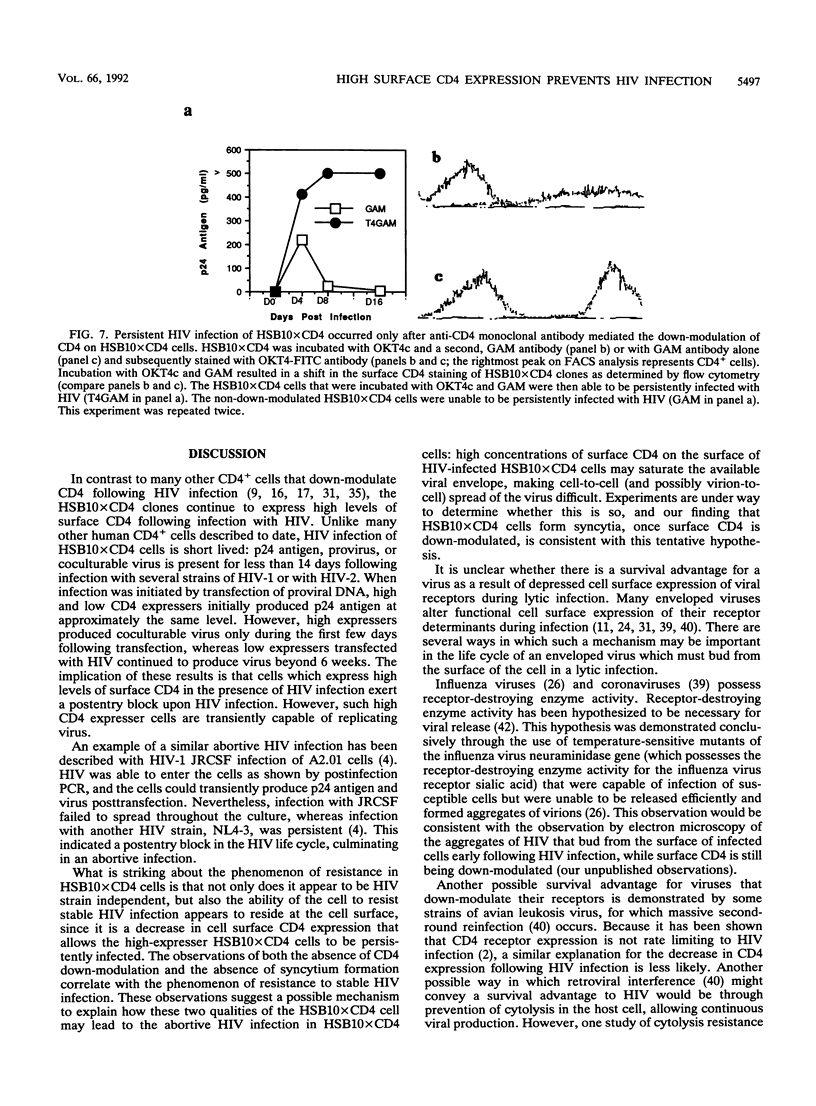
CD4 is the principal receptor for the human immunodeficiency virus (HIV). We have isolated and studied CD4-expressing tumor cell clones made by expressing CD4 in the T-cell tumor line HSB. Two clones, one designated HSBCD4, a clone expressing low levels of CD4, and the other, HSB10xCD4, a high-expresser CD4+ clone, were studied for their ability to bind and replicate HIV. In contrast to many other CD4+ cells that down-modulate CD4 following HIV infection, the HSB10xCD4 clones continued to express high levels of surface CD4 following infection with HIV. Unlike infection of HSBCD4 or many other human CD4+ cells, HIV infection of HSB10xCD4 clone was short lived: p24 antigen, provirus, or coculturable virus was present for less than 14 days following infection with several strains of HIV-1 or with HIV-2. When infection was initiated by transfection of proviral DNA, high and low CD4 expressers initially produced p24 antigen at approximately the same level. However, high CD4 expressers produced coculturable virus only during the first few days following transfection, whereas low CD4 expressers transfected with HIV continued to produce virus beyond 6 weeks. Monoclonal antibody-mediated down-modulation of CD4 surface expression on HSB10xCD4 clones permitted these formerly HIV-resistant cells to become persistently infected with HIV. Thus, high concentrations of CD4 on the surface of an HIV-infected cell prevent persistent HIV infection of CD4+ cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asjö B., Ivhed I., Gidlund M., Fuerstenberg S., Fenyö E. M., Nilsson K., Wigzell H. Susceptibility to infection by the human immunodeficiency virus (HIV) correlates with T4 expression in a parental monocytoid cell line and its subclones. Virology. 1987 Apr;157(2):359–365. doi: 10.1016/0042-6822(87)90278-9. [DOI] [PubMed] [Google Scholar]
- Besansky N. J., Butera S. T., Sinha S., Folks T. M. Unintegrated human immunodeficiency virus type 1 DNA in chronically infected cell lines is not correlated with surface CD4 expression. J Virol. 1991 May;65(5):2695–2698. doi: 10.1128/jvi.65.5.2695-2698.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue M. L., Hafler D. A., Daley J. F., Levine H., Craig K. A., Breitmeyer J. B., Schlossman S. F. Regulation of T cell clone function via CD4 and CD8 molecules. Anti-CD4 can mediate two distinct inhibitory activities. J Immunol. 1988 Jan 15;140(2):376–383. [PubMed] [Google Scholar]
- Cann A. J., Zack J. A., Go A. S., Arrigo S. J., Koyanagi Y., Green P. L., Koyanagi Y., Pang S., Chen I. S. Human immunodeficiency virus type 1 T-cell tropism is determined by events prior to provirus formation. J Virol. 1990 Oct;64(10):4735–4742. doi: 10.1128/jvi.64.10.4735-4742.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Iannello P., Shaw K., Luciw P. A., Levy J. A. Differential effects of nef on HIV replication: implications for viral pathogenesis in the host. Science. 1989 Dec 22;246(4937):1629–1632. doi: 10.1126/science.2531920. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Buller R., Portis J., Wehrly K. Failure of human immunodeficiency virus entry and infection in CD4-positive human brain and skin cells. J Virol. 1990 Jan;64(1):215–221. doi: 10.1128/jvi.64.1.215-221.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham P. R., Blanc D., Weiss R. A. Specific cell surface requirements for the infection of CD4-positive cells by human immunodeficiency virus types 1 and 2 and by Simian immunodeficiency virus. Virology. 1991 Apr;181(2):703–715. doi: 10.1016/0042-6822(91)90904-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crise B., Buonocore L., Rose J. K. CD4 is retained in the endoplasmic reticulum by the human immunodeficiency virus type 1 glycoprotein precursor. J Virol. 1990 Nov;64(11):5585–5593. doi: 10.1128/jvi.64.11.5585-5593.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- De Rossi A., Franchini G., Aldovini A., Del Mistro A., Chieco-Bianchi L., Gallo R. C., Wong-Staal F. Differential response to the cytopathic effects of human T-cell lymphotropic virus type III (HTLV-III) superinfection in T4+ (helper) and T8+ (suppressor) T-cell clones transformed by HTLV-I. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4297–4301. doi: 10.1073/pnas.83.12.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond D. C., Finberg R., Chaudhuri S., Sleckman B. P., Burakoff S. J. Human immunodeficiency virus infection is efficiently mediated by a glycolipid-anchored form of CD4. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5001–5005. doi: 10.1073/pnas.87.13.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A. G., Ratner L., Mitsuya H., Marselle L. M., Harper M. E., Broder S., Gallo R. C., Wong-Staal F. Infectious mutants of HTLV-III with changes in the 3' region and markedly reduced cytopathic effects. Science. 1986 Aug 8;233(4764):655–659. doi: 10.1126/science.3014663. [DOI] [PubMed] [Google Scholar]
- Garcia J. V., Miller A. D. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature. 1991 Apr 11;350(6318):508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- Gay D., Maddon P., Sekaly R., Talle M. A., Godfrey M., Long E., Goldstein G., Chess L., Axel R., Kappler J. Functional interaction between human T-cell protein CD4 and the major histocompatibility complex HLA-DR antigen. Nature. 1987 Aug 13;328(6131):626–629. doi: 10.1038/328626a0. [DOI] [PubMed] [Google Scholar]
- Hara J., Benedict S. H., Champagne E., Mak T. W., Minden M., Gelfand E. W. Comparison of T cell receptor alpha, beta, and gamma gene rearrangement and expression in T cell acute lymphoblastic leukemia. J Clin Invest. 1988 Apr;81(4):989–996. doi: 10.1172/JCI113453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxie J. A., Alpers J. D., Rackowski J. L., Huebner K., Haggarty B. S., Cedarbaum A. J., Reed J. C. Alterations in T4 (CD4) protein and mRNA synthesis in cells infected with HIV. Science. 1986 Nov 28;234(4780):1123–1127. doi: 10.1126/science.3095925. [DOI] [PubMed] [Google Scholar]
- Hoxie J. A., Haggarty B. S., Bonser S. E., Rackowski J. L., Shan H., Kanki P. J. Biological characterization of a simian immunodeficiency virus-like retrovirus (HTLV-IV): evidence for CD4-associated molecules required for infection. J Virol. 1988 Aug;62(8):2557–2568. doi: 10.1128/jvi.62.8.2557-2568.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga Y., Sasaki M., Nakamura K., Kimura G., Nomoto K. Intracellular distribution of the envelope glycoprotein of human immunodeficiency virus and its role in the production of cytopathic effect in CD4+ and CD4- human cell lines. J Virol. 1990 Oct;64(10):4661–4671. doi: 10.1128/jvi.64.10.4661-4671.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga Y., Sasaki M., Yoshida H., Wigzell H., Kimura G., Nomoto K. Cytopathic effect determined by the amount of CD4 molecules in human cell lines expressing envelope glycoprotein of HIV. J Immunol. 1990 Jan 1;144(1):94–102. [PubMed] [Google Scholar]
- Krone W. J., Sninsky J. J., Goudsmit J. Detection and characterization of HIV-1 by polymerase chain reaction. J Acquir Immune Defic Syndr. 1990;3(5):517–524. [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Weiss R. A., Sattentau Q. J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990 Nov 23;250(4984):1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- Muchmore E. A., Varki A. Selective inactivation of influenza C esterase: a probe for detecting 9-O-acetylated sialic acids. Science. 1987 Jun 5;236(4806):1293–1295. doi: 10.1126/science.3589663. [DOI] [PubMed] [Google Scholar]
- Neudorf S. M., Jones M. M., McCarthy B. M., Harmony J. A., Choi E. M. The CD4 molecule transmits biochemical information important in the regulation of T lymphocyte activity. Cell Immunol. 1990 Feb;125(2):301–314. doi: 10.1016/0008-8749(90)90086-7. [DOI] [PubMed] [Google Scholar]
- Palese P., Tobita K., Ueda M., Compans R. W. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology. 1974 Oct;61(2):397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Saizawa K., Rojo J., Janeway C. A., Jr Evidence for a physical association of CD4 and the CD3:alpha:beta T-cell receptor. Nature. 1987 Jul 16;328(6127):260–263. doi: 10.1038/328260a0. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Markham P. D., Wong-Staal F., Franchini G., Kalyanaraman V. S., Gallo R. C. Restricted expression of human T-cell leukemia--lymphoma virus (HTLV) in transformed human umbilical cord blood lymphocytes. Virology. 1983 Aug;129(1):51–64. doi: 10.1016/0042-6822(83)90395-1. [DOI] [PubMed] [Google Scholar]
- Salmon P., Olivier R., Riviere Y., Brisson E., Gluckman J. C., Kieny M. P., Montagnier L., Klatzmann D. Loss of CD4 membrane expression and CD4 mRNA during acute human immunodeficiency virus replication. J Exp Med. 1988 Dec 1;168(6):1953–1969. doi: 10.1084/jem.168.6.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattentau Q. J., Dalgleish A. G., Weiss R. A., Beverley P. C. Epitopes of the CD4 antigen and HIV infection. Science. 1986 Nov 28;234(4780):1120–1123. doi: 10.1126/science.2430333. [DOI] [PubMed] [Google Scholar]
- Sleckman B. P., Peterson A., Jones W. K., Foran J. A., Greenstein J. L., Seed B., Burakoff S. J. Expression and function of CD4 in a murine T-cell hybridoma. Nature. 1987 Jul 23;328(6128):351–353. doi: 10.1038/328351a0. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Campbell K., Haseltine W. A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. 1986 Jul 31-Aug 6Nature. 322(6078):470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- Stevenson M., Meier C., Mann A. M., Chapman N., Wasiak A. Envelope glycoprotein of HIV induces interference and cytolysis resistance in CD4+ cells: mechanism for persistence in AIDS. Cell. 1988 May 6;53(3):483–496. doi: 10.1016/0092-8674(88)90168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebel K., Klimkait T., Martin M. A. A novel gene of HIV-1, vpu, and its 16-kilodalton product. Science. 1988 Sep 2;241(4870):1221–1223. doi: 10.1126/science.3261888. [DOI] [PubMed] [Google Scholar]
- Sussman D. J., Milman G. Short-term, high-efficiency expression of transfected DNA. Mol Cell Biol. 1984 Aug;4(8):1641–1643. doi: 10.1128/mcb.4.8.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Samelson L. E., Bolen J. B. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck. Nature. 1989 Mar 16;338(6212):257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- Vlasak R., Luytjes W., Spaan W., Palese P. Human and bovine coronaviruses recognize sialic acid-containing receptors similar to those of influenza C viruses. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4526–4529. doi: 10.1073/pnas.85.12.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S. K., Joy A. E., Temin H. M. Correlation between cell killing and massive second-round superinfection by members of some subgroups of avian leukosis virus. J Virol. 1980 Jan;33(1):494–506. doi: 10.1128/jvi.33.1.494-506.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Maldarelli F., Martin M. A., Strebel K. Human immunodeficiency virus type 1 Vpu protein regulates the formation of intracellular gp160-CD4 complexes. J Virol. 1992 Jan;66(1):226–234. doi: 10.1128/jvi.66.1.226-234.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. A., Bates P., Willert K., Varmus H. E. Efficient incorporation of human CD4 protein into avian leukosis virus particles. Science. 1990 Dec 7;250(4986):1421–1423. doi: 10.1126/science.2175047. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]




