Abstract
Increased oxidative stress in the placenta has been associated with preeclampsia (PE), a clinical syndrome involving placental pathology. The enzymatic sources of reactive oxygen species in the human placenta are as yet unidentified. We hypothesized that NADPH oxidase is a main source of reactive oxygen species in the placenta and its expression may change in PE. Employing RTPCR, we have amplified a novel NADPH oxidase isoform Nox1 from human choriocarcinoma BeWo cells. Using polyclonal anti-peptide antiserum recognizing unique Nox1 peptide sequences, we identified by immunohistochemistry and cell fractionation that Nox1 protein localizes in the BeWo cell membrane structures. Immunohistochemistry of normal placental tissues showed that Nox1 was localized in syncytiotrophoblasts, in villous vascular endothelium, and in some stromal cells. At the immunohistochemical level Nox1 expression was significantly increased in syncytiotrophoblast and endothelial cells in placentas from patients with preeclampsia as compared to gestational age-matched controls. Western blot analysis of whole placental homogenate confirmed this increase. Our data suggests that increased Nox1 expression is associated with the increased oxidative stress found in these placentas.
Keywords: Oxidative stress, NADPH oxidase, Placenta, Trophoblast, Preeclampsia
INTRODUCTION
Approximately 7% of all pregnancies in the United States are affected by preeclampsia (PE), which is the leading cause of fetal growth restriction, premature delivery, and maternal death. Preeclampsia is characterized by poor perfusion of the maternal and fetal circulations of the placenta, and therefore affects fetal growth and development. The pathophysiology of preeclampsia involves shallow trophoblast invasion and abnormal maternal immunoreactivity [1,2]. In addition, oxidative stress, described as an imbalance between production of reactive oxygen species (ROS) and scavenging by antioxidants, with global or localized ROS accumulation, is observed in the placenta in preeclampsia and intrauterine growth restriction [3]. As ROS play an important role in placental cellular growth, differentiation and apoptosis, events which are of critical importance in determining the outcome of pregnancy [4], we reasoned there might be changes in expression of enzymes generating ROS in the placenta that are associated with preeclampsia.
NADPH oxidase has been shown to be the main source of ROS in neutrophils and macrophages [5]. The phagocytic enzyme is composed of several subunits including the membrane-bound core enzyme gp91phox and a membrane-bound regulatory subunit p22phox as well as cytosolic regulatory subunits p47phox, p67phox, p40phox and rac-GTPase. It has been found that gp91phox contains the entire catalytic machinery of NADPH oxidase [6]. It is a protein composed of 5–6 trans-membrane helices containing two heme-binding domains, and NADPH/FAD binding domains at the C-terminus. Recently, a family of genes that are homologous to the human gp91phox core unit has been discovered by molecular cloning from various somatic cell types. These include Nox1, 3, 4, 5 and Duox1 and Duox2, with gp91phox being termed as Nox2 [7-15]. The novel non-phagocytic Nox/Duox proteins contain highly conserved binding sites for NADPH, FAD and heme. However, their functions are less well known when compared to Nox2 [5,16,17].
Nox1 was cloned from a human colon cDNA library and rat vascular smooth muscle cells [7]. It is a 564 amino acid peptide and appears to be specifically expressed in non-phagocytic cells. Nox1 causes transformational changes when it is overexpressed in NIH 3T3 cells [15]. Further, Nox1 has been found to be activated by growth factors such as epidermal growth factor (EGF) and angiotensin II and is involved in vascular growth and remodeling, and therefore, plays an important role in vascular pathophysiology such as hypertension and atherosclerosis [18-20].
A NADPH oxidase activity in the human placenta has been detected with cytochemistry and chemiluminescence methods [21,22]. We have previously detected expression of the phagocytic Nox2 protein in the stroma of villous tissue with immunohistochemistry [4]. Increased NADPH oxidase activity has been suspected to be associated with preeclampsia both in maternal cells and in placental cells [21-25]. Recently, an enzyme consisting of a 58 kDa and a 33 kDa subunit has been purified by Manes from placental villous tissue. This placental NADPH oxidase was constitutively active and differs from the phagocytic enzyme in several biochemical properties [26]. However, there has not been any information on the gene structure of the catalytic Nox subunits in placental cell types, despite identification of several cytosolic regulatory subunits of the phagocytic NADPH oxidase, i.e., p22phox, p47phox and p67phox, in trophoblast and placental vessels [25]. Therefore, we investigated if and where Nox1 is expressed in specific placental cells and whether its expression is altered in preeclampsia.
MATERIALS AND METHODS
Cell culture
BeWo choriocarcinoma cells and HEK293 fibroblasts were obtained from ATCC. BeWo cells were cultured in F12-K media supplemented with 4 mM glutamine and 10% fetal bovine serum and 1× antibiotic–antimycotic solution. HEK293 cells were grown in DMEM supplemented with 10% fetal bovine serum and 1× antibiotic–antimycotic. The standard culture condition was 5% CO2 in air at 37 °C.
Total RNA isolation, reverse transcription and PCR cloning
Total RNA was extracted from cultured cells (≈1 × 107) with the RNeasy total RNA isolation kit (Qiagen). One microgram of RNA, as determined by calculation from the absorbance at 260 nm, was treated with 1 unit RNase–free DNase I (Invitrogen) to eliminate genomic DNA by reacting in a 10 μl volume for 15 min at room temperature. The reaction was stopped by adding EDTA and heating for 10 min at 65 °C. The first strand cDNA was synthesized by utilizing oligo(dT)12–18 primer and the SuperScript II™ reverse transcriptase (Invitrogen) in a 20 μl reaction which contains DTT and dNTP. Three microliters of product was subjected to PCR reaction which included 2 μl gene-specific primer pairs (5 μM) and 45 μl Platinum PCR Supermix (Invitrogen) on an Eppendorf MasterCycler for 35 cycles. The primers are: GCCAGTGAGGATGTTTTCCAGTATG (Nox1 forward) and CCCAAAGGAGGTTTTCTGTTTCAG (Nox1 reverse). The PCR product was subcloned to topoisomerase-activated pCR®4.0-TOPO vector (Invitrogen) and was transformed into One Shot™ competent Escherichia coli. The plasmid carrying PCR product was purified with plasmid mini kit (Qiagen) and sequenced in the University of Cincinnati DNA Core Lab.
Preparation of anti-Nox1 peptide antibody
Two peptides corresponding to specific sequences in Nox1 were designed according to their hydrophilicity, antigenicity and accessibility as follows: (Nox1a) RGQTEESMNESHPRKC and (Nox1b) CLDPRKVQFYFNKENF. The sequences were unique to Nox1 as determined by Blast analysis against Genbank. The peptides were each synthesized and conjugated with KLH and the antibody was raised in rabbits in a commercial resource (Alpha Diagnostics) by following a standard 63-day protocol where both the peptides were injected into the rabbit. The generated antiserum was tested with ELISA and dot blot against each peptide to determine sensitivity. Data suggest a range of 1:100–1:1000 as working dilutions. Immunoglobulin was then purified with Millipore Montage kit according to the manufacturer's instruction.
NADPH oxidase assay
A modification of a previously published method was used [27]. BeWo cells at 70–90% confluency were washed twice with Hank's balanced salt solution containing no calcium or magnesium. They were harvested by trypsinization and pelleted by centrifugation at 1000 × g for 5 min. Cells were resuspended in assay buffer containing 130 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.5 mM CaCl2, 35 mM phosphoric acid and 20 mM HEPES, pH 7.4, and stored on ice until use. The following experiments were performed at room temperature. To start the assay, lucigenin (final concentration 250 μM) was added to 5 × 105 cells with or without pre-incubation in 50 μM diphenyleneiodonium (DPI) followed by stimulation with or without 10 ng/ml EGF in total volume of 1 ml assay buffer. Photoemission in terms of raw light unit (RLU) was measured every minute for 5 min.
Cellular protein fractionation and western blot analysis
Cultured BeWo cells (≈5 × 106) were collected by scraping in 0.25 M sucrose solution containing 50 mM HEPES and a 1:50 dilution of the protease inhibitor cocktail (Sigma). Cells were homogenized with 30 strokes on ice using a type B dounce homogenizer and were centrifuged at 1000 × g for 15 min. The supernatant was centrifuged at 100,000 × g for 1 h. The supernatant was collected as the cytosolic fraction and the protein concentration was determined by the BCA assay according to the manufacturer's instructions (Pierce). The pellet was dissolved in Laemmli sample buffer to give the membrane fraction. Fifteen micrograms cytosolic protein and one-fourth of the membrane fraction were resolved by 10% SDS-PAGE and then transferred to a polyvinylidene difluoride membrane by electroblotting at 200 mA overnight. The membrane was immunoblotted with 1:500 dilution of anti-Nox1 antibody for 2 h, 1:2000 dilution of HRPconjugated goat anti-rabbit IgG for 1 h, with three washes in between incubations at room temperature. The immunoreaction was detected with enhanced chemiluminescence (Amersham Pharmacia).
Fluorescent immunohistochemistry
BeWo cells grown in slide chambers were washed once with PBS and fixed with 4% paraformaldehyde for 10 min at room temperature. After washing with PBS, the cells were incubated with or without a 1:200 dilution of anti-Nox1 antibody overnight at 4 °C. After three washes in PBS for 5 min each at room temperature, the cells were incubated in 1:3000 diluted FITC-conjugated mouse anti-rabbit IgG at 37 °C for 1 h. After three washes in PBS for 5 min each, cells were stained in 0.1 μM DAPI for 20 s followed by one PBS wash. The slides were mounted in 1:9 PBS/glycerol and viewed on a Zeiss digital camera with the images captured using AxioVision software.
Collection of human placenta
Placentas from preeclamptic and gestational age-matched normotensive control patients were collected from the Labor and Delivery Unit of the University Hospitals of Cincinnati following an Institutional Review Board approved protocol. Placentas from patients with known medical complications other than preeclampsia, e.g. infection, diabetes, and hyper-cholesterolemia, were excluded from this study. Mild preeclampsia is defined as a systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg on two occasions 6 h apart on or after 20 weeks of gestation, together with a diagnosis of proteinuria (≥ 300 mg in a 24 h urine specimen, or 2+ dipstick, or ≥ 30 mg/dl), and with resolution of hypertension in the postpartum period. Severe preeclampsia is defined as a systolic blood pressure ≥ 160 mm Hg or diastolic blood pressure ≥ 110 mm Hg plus diagnosis of proteinuria, or mild preeclampsia with severe proteinuria (≥ 5 g in a 24 h urine specimen), with or without edema. Preterm placentas were collected from deliveries on and before 34 weeks + 4 days.
Placental samples were obtained from 33 pregnant women with 21 being normotensive controls and 12 diagnosed with preeclampsia (Table 1).
Table 1.
General clinical characteristics of patients
| Normotensive (n = 21) |
PE (n = 12) |
|
|---|---|---|
| Age (years) | ||
| Range | 15–39 | 20–34 |
| Mean ± SE | 24 ± 6 | 27 ± 7 |
| Gestational age (weeks) | ||
| Range | 26–41 | 26–41 |
| Mean ± SE | 35 ± 4 | 31 ± 4* |
| Diastolic blood pressure (mm Hg) |
108 ± 10 | |
| Mode of delivery (C-section/vaginal delivery) |
14 (67%)/7 (33%) 10 | (83%)/2 (17%) |
Grading scale: 0 = absent; 3 = most intense.
p ≤ 0.05.
Placenta immunohistochemistry
Placental villous tissue from 12 preeclamptic patients between 26 and 41 weeks of gestational age and from 21 gestational age-matched control patients was dissected from beneath the chorionic plate and flash frozen in liquid nitrogen and stored at −80 °C. The frozen tissue blocks were embedded in Tissue-Tek O.C.T. Embedding Compound and sectioned at 7 μm on a cryostat. Tissue sections were stored at −80 °C.
All tissue sections were immunostained at the same time. Sections were air dried for 1 h at room temperature and rehydrated in PBS for 20 min. The sections were then fixed with 4% paraformaldehyde for 10 min at room temperature followed by 0.3% hydrogen peroxide treatment for 5 min. They were incubated with normal blocking serum at 4 °C overnight and then incubated either with 1:100 diluted primary Nox1 antibody or the blocking serum (as negative control) overnight at 4 °C. The Vectastain Elite ABC staining kit was employed according to the manufacturer's instruction (Vector Laboratories Inc.). Aminoethyl carbazole, which forms a red precipitate, was used as the peroxidase substrate and the reaction time was 2 min. Slides were then counterstained in hematoxylin and mounted in 1:9 PBS/glycerol.
The localization and intensity of staining was determined by three people blinded to the identities of the tissue. For intensity of immunostaining, a scale of 0–3 was assigned with 0 being the absence of staining (no primary antibody) and 3 being the most intense staining.
Preparation of placental (or cellular) homogenate and microvillous membrane for Western blot analysis
Placental tissues stored at −80 °C were powdered into small pieces in liquid N2. Lysates were made by homogenizing with a tissue grinder on ice in lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% NP-40, 1 mM Na3VO4, 1 mM NaF, 1 mM EGTA, and 1:50 diluted Protease Inhibitor Cocktail). The samples were centrifuged at 10,000 × g for 15 min. Placental microvillous plasma membrane was prepared by differential centrifugation and sucrose gradient fractionation as described [28]. Protein content in the samples was determined by the BCA method and samples (10 μg/each) were subjected to Western blot analysis as described above. For peptide blocking, aliquots of 5 μl anti-Nox1 antibody were mixed with or without 10 μg competing peptide in 0.25 ml buffer at room temperature for 20 min and were employed in final dilution of 1:1000 in Western blot.
Data analysis
For immunohistochemistry, grading data was expressed as mean ± standard error. To compare intensity between different tissues, the non-parametric Mann–Whitney U-test was performed on ranked grading data between control and preeclampsia groups. Student's t-test was employed to statistical analysis when appropriate. A p value of less than 0.05 was considered statistically significant.
RESULTS
It has been shown previously that Nox1 is expressed in several cell types including HEK293 fibroblasts [15]. PCR primers were designed using the DNAMAN program for Nox1 sequence (ACC# AF127763). These primers were used to amplify Nox1 from total RNA isolated from either cultured HEK293 cell or BeWo cells. We amplified by RT-PCR a 357 bp cDNA from either HEK293 or BeWo cells (Figure 1A). Sequence analysis has confirmed that these fragments correspond to nucleotides 1368–1724 of human Nox1.
Figure 1.
(A) RT-PCR of Nox1 from HEK293 (Lane 1) and BeWo choriocarcinoma (Lane 2) cells. L, molecular weight ladder. (B) Chemiluminescence assessment of NADPH oxidase activity in BeWo cells stimulated with 10 ng/ml EGF with or without DPI (50 μM) pretreatment.
Since Nox1 has been shown to be activated by growth factor stimulation [18-20], the effect of EGF on BeWo cells was examined by employing lucigenin chemiluminescence, an established method in this laboratory [27]. EGF (10 ng/ml) stimulated chemiluminescence with a peak activation at 2 min (Figure 1B). Pre-treating BeWo cells for 10 min with 50 μM DPI, a non-selective inhibitor of flavoprotein enzymes [29], significantly blocked the effect of EGF (p < 0.05, n = 2). DPI also abolished the chemiluminescence in the control sample, which is considered as basal Nox-like activity (data not shown). Together these data suggest that there is functional Nox1 expression in the BeWo cells.
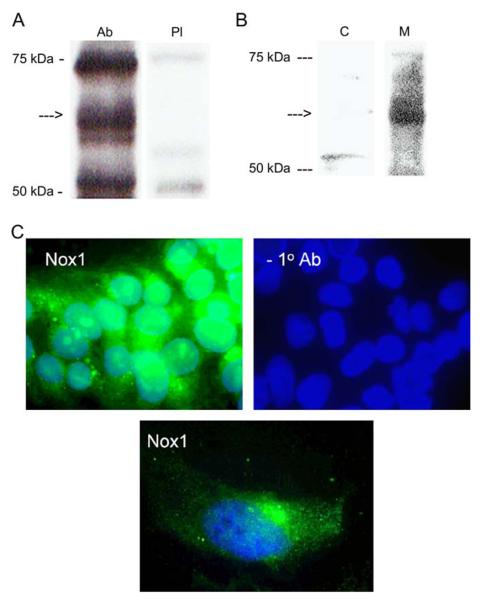
Western blot analysis showed that the anti-Nox1 peptide serum, but not the pre-immune serum, recognized a protein of about 56 kDa in BeWo cell lysate (Figure 2A). However, two unknown proteins of apparent molecular weight of 52 kDa and 70 kDa were present in the total lysate, which were also recognized by the pre-immune serum. When BeWo cell fractionation was performed, these unknown proteins were negligible in both the membrane and cytosolic fractions. In addition, the 56 kDa Nox1 protein was present only in the membrane fraction. The result strongly suggests that Nox1 is membrane-bound (Figure 2B). As shown in Figure 2C, expression of Nox1 in BeWo cells was detected as green fluorescence by immunohistochemistry using anti-Nox1 peptide antibody as primary and FITC-anti-rabbit IgG antibody as secondary antibody (upper left). There was no immunostaining when the primary antibody was omitted and only nuclear staining with DAPI was seen (upper right). A punctuate Nox1 distribution in the single BeWo cell (lower) plus condensation near cell nuclei was observed, confirming that Nox1 is a membrane protein.
Figure 2.
(A) Western blot of Nox1 (arrow) in BeWo cell lysate employing anti-Nox1 serum (Ab) or pre-immune serum (PI). (B) Western blot of Nox1 (arrow) in BeWo cell protein fractions. C, cytosolic protein; M, membrane protein. (C) Fluorescent immunohistochemistry of Nox1 expression in BeWo cells detected by FITC-conjugated anti-rabbit IgG. Cell nucleuses were stained with DAPI.
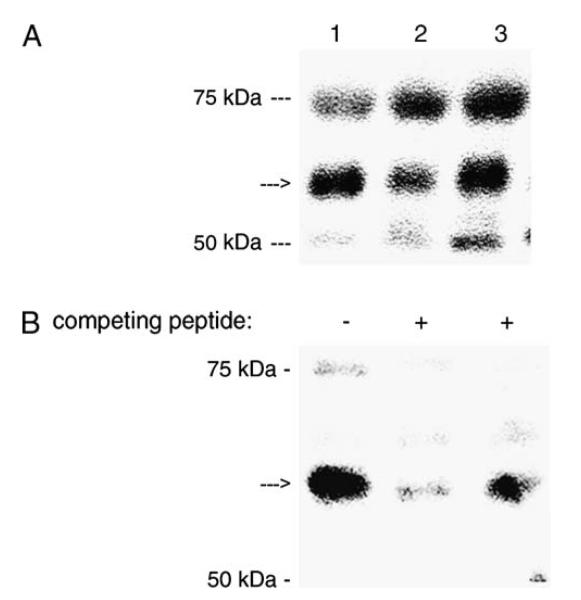
The anti-Nox1 peptide IgG also recognized a ~56 kDa protein from placental homogenates or microvillous membrane fraction obtained from normal pregnant women (Figure 3A and B). Again the unknown proteins of 52 kDa and 70 kDa were present in the total lysate but were considered to be artifacts due to the preparation method. Furthermore, pre-incubation with competing peptides Nox1a or Nox1b blocked the recognition of Nox1 with the polyclonal antibody which proved the specific recognition of the antibody (Figure 3B).
Figure 3.
(A) Western blot of Nox1 in placental homogenates from three normal patients. (B) Western blot of Nox1 in microvillous membrane in the absence or presence of competing peptides.
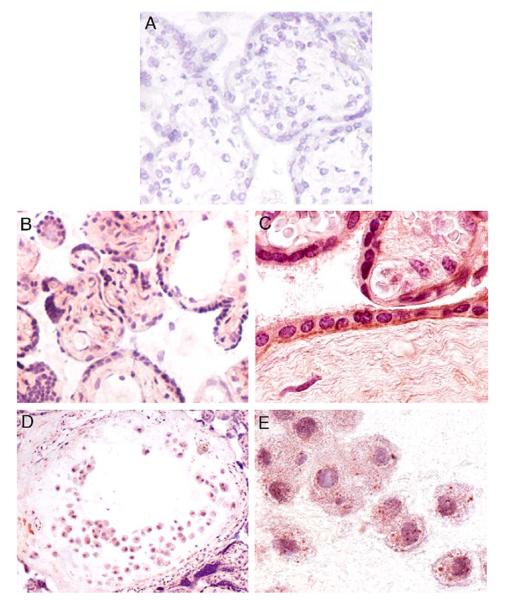
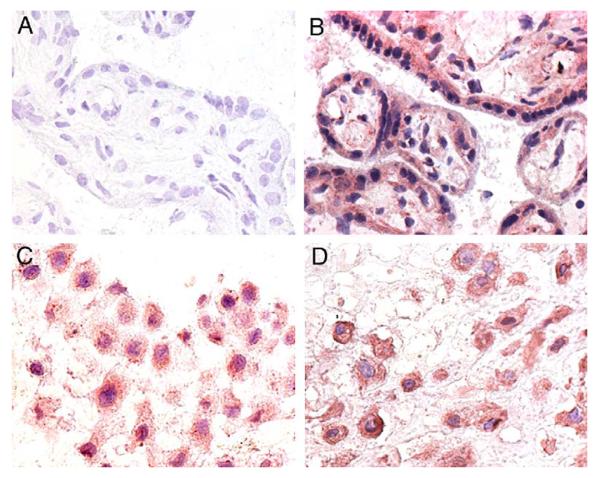
Immunohistochemistry was then employed to localize Nox1 enzyme expression in human placentas. In normal term placenta (Figure 4), it was found that Nox1 was intensely expressed in the syncytiotrophoblasts, in extravillous trophoblasts (EVT), in vascular endothelial cells and in the stromal cells (Figure 4B). Immunostaining of the vasculature was mainly found in stem villous vessels but not in the terminal villous vessels (not shown). Examination of paraffin-fixed tissue sections at higher power showed that Nox1 expression was localized to the syncytiotrophoblast apical and basolateral membranes (Figure 4C). However, Nox1 expression in the EVT had a characteristic staining which is similar to that seen in BeWo cells (Figure 4D and E). In addition, Nox1 expression in the placentas of normal preterm subjects showed a similar pattern to that of the term placentas (Figure 5). Positive identification of EVT was made using cytokeratin 18 (Figure 5C). In contrast, no staining was seen on sections when primary antibody was omitted (Figures 4A and 5A).
Figure 4.
Immunohistochemistry (frozen sections except C) of normal term placental tissue. (A) Control staining without primary antibody (200×). (B) Villous tissue Nox1 staining (200×). (C) Villous Nox1 staining, paraffin-fixed section (600×). (D) Anchoring villous EVT Nox1 staining (200×). (E) EVT Nox1 staining (600×).
Figure 5.
Immunohistochemistry of normal preterm placental tissue (400× for all). (A) Control staining without primary antibody. (B) Villous tissue Nox1 staining. (C) EVT cytokeratin 18 staining. (D) EVT Nox1 staining.
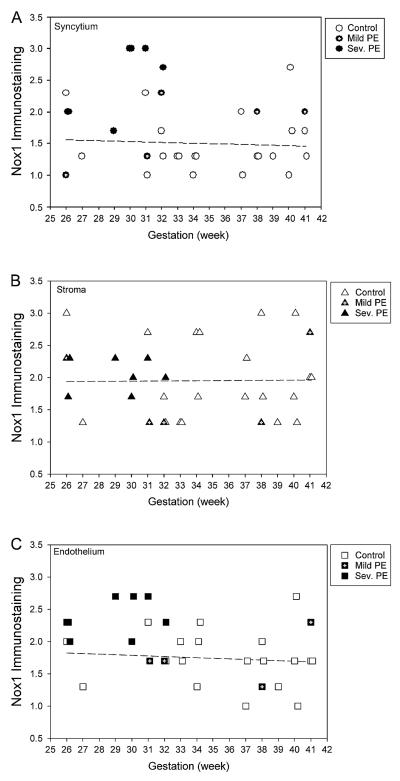
As an increase of oxidative stress has been reported in preeclampsia, it was of interest to determine if Nox1 expression was altered in the preeclamptic placentas. Thus, we evaluated the expression of Nox1 using immunohisto-chemistry on placentas obtained from either normotensive control (n = 21) or preeclamptic patients (n = 12). As shown in Figure 6, in normal placentas no apparent change in Nox1 expression was found in syncytium (Figure 6A), stroma (Figure 6B), and vascular endothelium (Figure 6C) between 26 and 41 weeks of gestational age. However, a statistically significant increase (by Mann–Whitney U-test) in the expression of Nox1 was found in preeclamptic placenta when compared to normal control placentas in the syncytium (2.2 ± 0.6 vs. 1.5 ± 0.5, p < 0.01) and in the vascular endothelium (2.2 ± 0.4 vs. 1.7 ± 0.4, p < 0.05), but not in the stroma (1.9 ± 0.5 vs. 2.0 ± 0.6, p = 0.878). The biggest difference was found in the placentas of patients with delivery at earlier gestational ages. Figure 7 shows representative immunohistochemistry staining of Nox1 in these preterm placentas.
Figure 6.
Nox1 expression in syncytium (A), stroma (B), and endothelium (C) from normotensive (open symbols), mild preeclamptic (closed symbols with center hairline), and severe preeclamptic (closed symbols) patients. Regression line was calculated for normotensive patients.
Figure 7.
Immunohistochemistry (400×) of Nox1 in three normotensive control and three preeclamptic patients. Representative staining with no primary antibody were shown on top.
A quantitative analysis of Nox1 expression was performed using Western blot analysis (Figure 8). The result showed that Nox1 was higher in placental homogenates and in microvillous membrane of preeclamptic patients (Figure 8A). As shown in Figure 8B, there was a significantly (p = 0.034) higher Nox1 expression in placental homogenates obtained from preterm preeclamptic patients (n = 3, 30–31 weeks) as compared to controls (n = 4, 31–33 weeks), delivered due to motor vehicle accident, advanced cervical dilation, and premature membrane rupture.
Figure 8.
(A) Comparison of Nox1 expression in placental homogenates or microvillous membrane preparations from control and preeclamptic patients. (B) Quantization of Nox1 expression in placental homogenates obtained from gestational age-match preterm control (n = 4) and preeclamptic (n = 3) patients.
We also hypothesized that changes in Nox1 expression may be associated with the severity of preeclampsia. When comparing to normotensive subjects, there was a gradual increase of Nox1 expression in mild and severe preeclamptic placentas in both the syncytium and the endothelium, but not in the stroma (Table 2). However, no statistically significant change of Nox1 expression was found between mild and severe preeclamptic placental tissue types from gestational age 26–41 weeks. Although Nox1 expression was not increased in mild preeclamptic tissues as compared to normotensive subjects, we observed, on the other hand, a more significantly increased Nox1 expression in severe preeclamptic (n = 7) syncytial and endothelial tissues than those in gestational age-matched (≤ 34 weeks, n = 11) normotensive controls (2.5 ± 0.5 vs. 1.5 ± 0.4, p = 0.003 and 2.4 ± 0.3 vs. 1.8 ± 0.3, p = 0.006, respectively).
Table 2.
Grading of Nox1 immunostaining in normotensive, mild and severe preeclamptic placentas
| Normotensive (n = 21) |
Mild PE (n = 5) |
Severe PE (n = 7) |
Significance (severe vs. mild) |
|
|---|---|---|---|---|
| Syncytium | 1.5 ± 0.5 | 1.7 ± 0.5 | 2.5 ± 0.5 | p = 0.080 |
| Stroma | 2.0 ± 0.6 | 1.8 ± 0.6 | 2.0 ± 0.2 | p = 0.403 |
| Endothelium | 1.7 ± 0.4 | 1.9 ± 0.4 | 2.4 ± 0.3 | p = 0.055 |
Grading scale: 0 = absent; 3 = most intense.
DISCUSSION
Preeclampsia is a complex pregnancy-associated syndrome. A two-stage model in the development of preeclampsia has been proposed which involves impaired trophoblast invasion during early pregnancy and an abnormal maternal inflammatory reaction at later gestational ages [30]. Early onset (preterm) preeclampsia is more likely to be severe and to be associated with poor or absent trophoblast invasion, i.e., a placental pathology [31]. Late onset (term) preeclampsia is mostly mild and may represent the maternal component of the disease. Moreover, a failure of placenta in handling hypoxia/reoxygenation-induced oxidative stress is also proposed to involve the pathophysiology [1,2]. Numerous lines of evidence indicate that oxidative stress in the placenta is a key element. However, the mechanism underlying this oxidative stress remains elusive due to a lack of knowledge of the enzyme(s) that is responsible for generation of ROS.
NADPH oxidase has been shown to be the main source of hypoxia/reoxygenation-induced ROS production in vascular smooth muscle cells [32], pulmonary artery endothelial cells [33], hepatocytes [34], and microglial cells [35]. NADPH oxidase was found to be hyperactive in maternal granulocytes and monocytes from patients with preeclampsia [23]. A recent study on immortalized maternal lymphocytes from either preeclamptic or normotensive pregnant women has shown a predisposed higher NADPH oxidase activity in preeclampsia [36]. In the placenta, existence of a de novo NADPH oxidase was suggested with cytochemical detection [21]. An enzyme consisting of a 58 kDa and a 33 kDa subunit has been isolated from term human placenta, presumably from syncytiotrophoblast [26]. In addition, an autoimmune antibody against angiotensin II type 1 receptor has been shown to present in patients with preeclampsia and it activates the NADPH oxidase in isolated human trophoblast and smooth muscle cells [25,37]. It was also found that there is an increased expression of cytosolic subunits belonging to the phagocytic NADPH oxidase in the preeclamptic placentas which correlated with increased reactive oxygen species generation [25]. However, employing cytochemical method with cerium chloride as substrate for detecting placental microvillous NADPH oxidase-like activities, Matsubara and Sato failed to see any changes in either the intensity or the localization of this enzyme in the villous tissue by comparing normotensive and preeclamptic patients. This study, however, employed a small sample number and a relatively low sensitivity method [21]. Employing chemiluminescence method, no statistically significant changes in NADPH oxidase-like activity was observed in placental homogenates obtained from preeclamptic patients as compared with normal controls [22]. This could be due to the heterogeneity of the samples. The authors, however, observed a significantly increased enzyme activity in placental homogenate obtained from severe early onset preeclampsia when comparing with late onset preeclampsia. Therefore, the association of the placental NADPH oxidase with preeclampsia warrants analysis of specific enzyme isoforms in specific cell types.
Our data shows that Nox1, a gene coding a novel NADPH oxidase isoform, is expressed in BeWo choriocarcinoma cells. EGF, a known stimulant of Nox1, was found to induce chemiluminescence in BeWo cells and its effect was inhibited by DPI. Although DPI was found to inhibit all FAD-binding enzymes, including nitric oxide synthase [38] and P450 enzymes [39] at higher concentrations, a relatively lower concentration (e.g., 30 μM, not shown) needed for EGF-induced activation suggests that an NADPH oxidase was involved. Previous studies employing Western blot analysis has shown multiple bands of Nox1 in vascular smooth muscle cells [40] and in Cos-7 cells transfected with Nox1 expression plasmid [41]. Our data agree with these investigators that a major Nox1 isoform is localized in BeWo cells in the membrane fraction. In addition, Hilenski et al. has shown that Nox1 co-localized with caveolin in the membrane, which may explain the punctate surface distribution pattern of Nox1 in BeWo cells [40]. Furthermore, a basal Nox-like activity appears to be maintained in BeWo cells (not shown) and it is stimulated by EGF with yet unknown mechanisms for full activation.
Existence of Nox1 in trophoblast was shown by immunoblot of microvillous membrane and immunohistochemistry of placental villous tissue. Several cellular components of the phagocytic NADPH oxidase (p22phox, p47phox, and p67phox) have been identified in trophoblasts and villous vessels, and these proteins were found to be increased in trophoblast and villous vessels from preeclamptic patients [25]. On the other hand, novel cytosolic components of Nox1 have also been identified in non-placental cell types [42,43]. Therefore, the mechanism of Nox1 activation in placental cells needs further study.
At the immunohistochemical level, Nox1 protein was found to be expressed in placental syncytiotrophoblasts, extravillous trophoblasts, vascular endothelial cells, and stromal cells. It appears that Nox1 expression in the normal placenta does not change throughout gestation, at least from 26 to 41 weeks, in these cell types. A statistically significant increase in Nox1 protein expression was observed in placental syncytiotrophoblasts and villous endothelial cells from patients with preeclampsia as compared to normotensive controls (Figure 6). In addition, the protein expression level also seems to correlate with the severity of preeclampsia in syncytium and villous endothelium, in that a gradual increment was seen in mild and severe preeclampsia (Table 2). Particularly, a more significantly increased Nox1 expression was observed by immunohistochemistry in syncytiotrophoblast and endothelial cells in preterm severe preeclampsia as compared to normotensive subjects. If considering those cases of preeclampsia with early onset are more severe and most likely resulted from a placental pathology, the elevation of Nox1 expression in syncytium may suggest an etiologic role. Nevertheless, our data show for the first time expression and localization of a novel NADPH oxidase isoform in the placenta, and the association of increased expression of this enzyme core unit with preeclampsia. Therefore, Nox1 may be a key enzyme involved in the increased oxidative stress seen in the placenta with preeclampsia.
ACKNOWLEDGMENTS
The authors wish to thank Drs. Nelson Horseman and James Ball for advice on peptide design; Mr. Brad Pitzer for technique assistance in immunohistochemistry; Ms. Katherine Recht for assistance in placenta collection. This work was supported by National Institutes of Health HL075297 to LM and University of Cincinnati Spring URC Award to XLC.
Abbreviations
- ROS
reactive oxidative species
- EVT
extravillous trophoblasts
REFERENCES
- 1.Waite LL, Atwood AK, Taylor RN. Preeclampsia, an implantation disorder. Rev Endocr Metab Disord. 2002;3:151–8. doi: 10.1023/a:1015411113468. [DOI] [PubMed] [Google Scholar]
- 2.Redman CW, Sargent IL. The pathogenesis of pre-eclampsia. Gynecol Obstet Fertil. 2001;29:518–22. doi: 10.1016/s1297-9589(01)00180-1. [DOI] [PubMed] [Google Scholar]
- 3.Bilodeau JF, Hubel CA. Current concepts in the use of antioxidants for the treatment of preeclampsia. J Obstet Gynaecol Can. 2003;25:742–50. doi: 10.1016/s1701-2163(16)31003-9. [DOI] [PubMed] [Google Scholar]
- 4.Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol. 2004;122:369–82. doi: 10.1007/s00418-004-0677-x. [DOI] [PubMed] [Google Scholar]
- 5.Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–4. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 6.Yu L, Quinn MT, Cross AR, Dinauer MC. Gp91(phox) is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc Natl Acad Sci U S A. 1998;95:7993–8. doi: 10.1073/pnas.95.14.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 8.Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A. 2000;97:8010–4. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dupuy C, Ohayon R, Valent A, Noel-Hudson MS, Deme D, Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. J Biol Chem. 1999;274:37265–9. doi: 10.1074/jbc.274.52.37265. [DOI] [PubMed] [Google Scholar]
- 10.Dupuy C, Pomerance M, Ohayon R, Noel-Hudson MS, Deme D, Chaaraoui M, et al. Thyroid oxidase (THOX2) gene expression in the rat thyroid cell line FRTL-5. Biochem Biophys Res Commun. 2000;277:287–92. doi: 10.1006/bbrc.2000.3671. [DOI] [PubMed] [Google Scholar]
- 11.De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, et al. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem. 2000;275:23227–33. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- 12.Kikuchi H, Hikage M, Miyashita H, Fukumoto M. NADPH oxidase subunit, gp91(phox) homologue, preferentially expressed in human colon epithelial cells. Gene. 2000;254:237–43. doi: 10.1016/s0378-1119(00)00258-4. [DOI] [PubMed] [Google Scholar]
- 13.Yang S, Madyastha P, Bingel S, Ries W, Key L. A new superoxide-generating oxidase in murine osteoclasts. J Biol Chem. 2001;276:5452–8. doi: 10.1074/jbc.M001004200. [DOI] [PubMed] [Google Scholar]
- 14.Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, et al. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem. 2001;276:37594–601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 15.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–40. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 16.Griendling KK. Novel NAD(P)H oxidases in the cardiovascular system. Heart. 2004;90:491–3. doi: 10.1136/hrt.2003.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones RD, Hancock JT, Morice AH. NADPH oxidase: a universal oxygen sensor? Free Radic Biol Med. 2000;29:416–24. doi: 10.1016/s0891-5849(00)00320-8. [DOI] [PubMed] [Google Scholar]
- 18.Griendling KK, Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept. 2000;91:21–7. doi: 10.1016/s0167-0115(00)00136-1. [DOI] [PubMed] [Google Scholar]
- 19.Munzel T, Hink U, Heitzer T, Meinertz T. Role for NADPH/NADH oxidase in the modulation of vascular tone. Ann N Y Acad Sci. 1999;874:386–400. doi: 10.1111/j.1749-6632.1999.tb09253.x. [DOI] [PubMed] [Google Scholar]
- 20.Meyer JW, Schmitt ME. A central role for the endothelial NADPH oxidase in atherosclerosis. FEBS Lett. 2000;472:1–4. doi: 10.1016/s0014-5793(00)01397-1. [DOI] [PubMed] [Google Scholar]
- 21.Matsubara S, Sato I. Enzyme histochemically detectable NAD(P)H oxidase in human placental trophoblasts: normal, preeclamptic, and fetal growth restriction-complicated pregnancy. Histochem Cell Biol. 2001;116:1–7. doi: 10.1007/s004180100301. [DOI] [PubMed] [Google Scholar]
- 22.Raijmakers MT, Peters WH, Steegers EA, Poston L. NAD(P)H oxidase associated superoxide production in human placenta from normotensive and pre-eclamptic women. Placenta. 2004;25(Suppl A):S85–9. doi: 10.1016/j.placenta.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Gervasi MT, Chaiworapongsa T, Pacora P, Naccasha N, Yoon BH, Maymon E, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol. 2001;185:792–7. doi: 10.1067/mob.2001.117311. [DOI] [PubMed] [Google Scholar]
- 24.Lee VM, Quinn PA, Jennings SC, Ng LL. Neutrophil activation and production of reactive oxygen species in pre-eclampsia. J Hypertens. 2003;21:395–402. doi: 10.1097/00004872-200302000-00032. [DOI] [PubMed] [Google Scholar]
- 25.Dechend R, Viedt C, Muller DN, Ugele B, Brandes RP, Wallukat G, et al. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107:1632–9. doi: 10.1161/01.CIR.0000058200.90059.B1. [DOI] [PubMed] [Google Scholar]
- 26.Manes C. Human placental NAD(P)H oxidase: solubilization and properties. Placenta. 2001;22:58–63. doi: 10.1053/plac.2000.0589. [DOI] [PubMed] [Google Scholar]
- 27.Cui XL, Douglas JG. Arachidonic acid activates c-jun N-terminal kinase through NADPH oxidase in rabbit proximal tubular epithelial cells. Proc Natl Acad Sci U S A. 1997;94:3771–6. doi: 10.1073/pnas.94.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riquelme G, Llanos P, Tischner E, Neil J, Campos B. Annexin 6 modulates the maxi-chloride channel of the apical membrane of syncytiotrophoblast isolated from human placenta. J Biol Chem. 2004;279:50601–8. doi: 10.1074/jbc.M407859200. [DOI] [PubMed] [Google Scholar]
- 29.Brar SS, Kennedy TP, Sturrock AB, Huecksteadt TP, Quinn MT, Whorton AR, et al. An NAD(P)H oxidase regulates growth and transcription in melanoma cells. Am J Physiol Cell Physiol. 2002;282:C1212–24. doi: 10.1152/ajpcell.00496.2001. [DOI] [PubMed] [Google Scholar]
- 30.Page NM. The endocrinology of pre-eclampsia. Clin Endocrinol (Oxf) 2002;57:413–23. doi: 10.1046/j.1365-2265.2002.01626.x. [DOI] [PubMed] [Google Scholar]
- 31.Myatt L. Role of placenta in preeclampsia. Endocrine. 2002;19:103–11. doi: 10.1385/ENDO:19:1:103. [DOI] [PubMed] [Google Scholar]
- 32.Sorescu D, Somers MJ, Lassegue B, Grant S, Harrison DG, Griendling KK. Electron spin resonance characterization of the NAD(P)H oxidase in vascular smooth muscle cells. Free Radic Biol Med. 2001;30:603–12. doi: 10.1016/s0891-5849(00)00507-4. [DOI] [PubMed] [Google Scholar]
- 33.Zulueta JJ, Yu FS, Hertig IA, Thannickal VJ, Hassoun PM. Release of hydrogen peroxide in response to hypoxia-reoxygenation: role of an NAD(P)H oxidase-like enzyme in endothelial cell plasma membrane. Am J Respir Cell Mol Biol. 1995;12:41–9. doi: 10.1165/ajrcmb.12.1.7529030. [DOI] [PubMed] [Google Scholar]
- 34.Caraceni P, Ryu HS, van Thiel DH, Borle AB. Source of oxygen free radicals produced by rat hepatocytes during postanoxic reoxygenation. Biochim Biophys Acta. 1995;1268:249–54. doi: 10.1016/0167-4889(95)00077-6. [DOI] [PubMed] [Google Scholar]
- 35.Spranger M, Kiprianova I, Krempien S, Schwab S. Reoxygenation increases the release of reactive oxygen intermediates in murine microglia. J Cereb Blood Flow Metab. 1998;18:670–4. doi: 10.1097/00004647-199806000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Lee VM, Quinn PA, Jennings SC, Ng LL. NADPH oxidase activity in preeclampsia with immortalized lymphoblasts used as models. Hypertension. 2003;41:925–31. doi: 10.1161/01.HYP.0000062021.68464.9D. [DOI] [PubMed] [Google Scholar]
- 37.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, et al. Patients with preeclampsia develop agonistic autoanti-bodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–52. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendes AF, Carvalho AP, Caramona MM, Lopes MC. Diphenyleneiodonium inhibits NF-kappaB activation and iNOS expression induced by IL-1beta: involvement of reactive oxygen species. Mediators Inflamm. 2001;10:209–15. doi: 10.1080/09629350120080401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGuire JJ, Anderson DJ, McDonald BJ, Narayanasami R, Bennett BM. Inhibition of NADPH-cytochrome P450 reductase and glyceryl trinitrate biotransformation by diphenyleneiodonium sulfate. Biochem Pharmacol. 1998;56:881–93. doi: 10.1016/s0006-2952(98)00216-0. [DOI] [PubMed] [Google Scholar]
- 40.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–83. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 41.Geiszt M, Lekstrom K, Leto TL. Analysis of mRNA transcripts from the NAD(P)H oxidase 1 (Nox1) gene. Evidence against production of the NADPH oxidase homolog-1 short (NOH-1S) transcript variant. J Biol Chem. 2004;279:51661–8. doi: 10.1074/jbc.M409325200. [DOI] [PubMed] [Google Scholar]
- 42.Banfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 43.Cheng G, Lambeth JD. NOXO1, regulation of lipid binding, localization, and activation of Nox1 by the Phox homology (PX) domain. J Biol Chem. 2004;279:4737–42. doi: 10.1074/jbc.M305968200. [DOI] [PubMed] [Google Scholar]